Abstract
Vibrio cholerae is an aquatic bacterium that causes the human disease cholera as well as milder forms of diarrhea. V. cholerae is found in the environment in association with a variety of aquatic animals, including vertebrate fish. Here we describe the use of zebrafish (Danio rerio) as a model for the pathogenic life cycle of V. cholerae. Being that fish are natural hosts for V. cholerae, the model provides several significant advantages over existing mammalian models that are not natural hosts.
Keywords: Cholera, Vibrio cholera, Diarrhea, Zebrafish, Infectious disease transmission
1. Introduction
The zebrafish, Danio rerio, has been used extensively in biological research over the past four decades. Due to the transparency of embryos, zebrafish development was initially a fruitful area of study. Zebrafish have also been used in studies of nearly every other aspect of biology, including neuroscience, behavior, cancer, immunology, and infectious diseases, among others [1–3].
Vibrio cholerae is a particularly pertinent human pathogen that has recently been studied using zebrafish as a model host [4]. V. cholerae is an aquatic bacterium that is primarily known for causing the deadly human diarrheal disease cholera [5]. Cholera has been endemic in southern Asia throughout recorded history. The native habitat of zebrafish is also southern Asia, so V. cholerae and D. rerio have likely interacted in their aquatic habitats for thousands of years. Recent work has described several species of vertebrate fish as apparently natural V. cholerae hosts [6–9]. However, it is unknown whether V. cholerae causes any pathology in these fish. Zebrafish were explored as a potential natural host model and found to recapitulate the V. cholerae pathogenic life cycle: (1) fish are infected simply by exposure in water; (2) fish are specifically colonized in the intestine; (3) colonization leads to diarrhea; (4) and V. cholerae excreted by infected fish transmit the infection to naïve fish [4].
Here we describe the details behind this model system, with specific information about colonization, dissection, bacterial counts, and transmission experiments.
2. Materials
All dissection tools were obtained from Fine Science Tools unless otherwise specified.
Two Dumont #5 forceps.
Student Vannas spring scissors.
# 3 scalpel handle.
Insect pins, size 1.
# 11 scalpel blades, sterile.
Kelly hemostats.
Cling wrap for hemostats.
Plastic spoons.
Dissecting mat or flat piece of Styrofoam.
2 mL tubes with O-ring caps.
1 mm glass beads.
Mini-Beadbeater-24 (BioSpec Products, Inc.).
2.1. Infection/Tank Water
Tap water is passed through reverse osmosis (RO) and then conditioned with 60 mg/L Instant Ocean salt. Infection water is autoclaved before use in experiments.
2.2. Tricaine
Tricaine (ethyl 3-aminobenzoate methanesulfonate salt; Sigma A5040) stock solution is 4 mg/mL in RO water, pH adjusted to 7.0 with 1 M Tris–HCl, pH 9.0.
2.3. Zebrafish
Wild-type, outbred zebrafish, aged 6 months to 1 year, are used for intestinal colonization and bacterial transmission experiments. For anesthesia, zebrafish are placed in 100 mL of 168 μg/mL tricaine solution for approximately 1–2 min or until gill movement slows and fish stop swimming. Fish are recovered from anesthesia by removing them from the Tricaine solution and immediately placing them in fresh infection water. For euthanasia of zebrafish, the dose of tricaine is doubled, and fish remain in the solution for at least 25 min. All animal protocols were approved by the Wayne State University IACUC committee.
3. Methods
3.1. Inoculation of Zebrafish via Immersion
Bacterial cultures are grown with aeration in LB broth at 37 °C for 16–18 h. Cells are subsequently washed once with sterile 1× PBS and then diluted to the correct concentration in sterile 1× PBS. Bacterial cell densities should range from 107 to 1010 per beaker depending on the desired multiplicity of infection (~5 × 104−5 × 107 cfu/mL). Four to five zebrafish are placed into a 400 mL beaker containing 200 mL of sterile infection water (Fig. 1). 1000 μL of bacterial inoculum is then added to the beaker with fish, and the beaker is covered with a perforated lid. Each beaker is labeled and placed into a glass-front incubator set at 28 °C for the duration of the experiment.
Fig. 1.

Incubation of infected zebrafish
3.2. Dissection
Scoop a fish out of the tricaine solution with a plastic spoon and place on the dissecting surface. Position the fish with its ventral side facing upward, and pin it through the lower jaw, angled away from the body lengthwise (Fig. 2a). Place another pin just posterior to the anus, also angled away from the body lengthwise (Fig. 2b). Swab the ventral surface with a Kimwipe soaked in 70% ethanol and flame sterilize the scalpel and Vannas scissors. Use the scalpel to make a small incision in the belly, only penetrating just under the skin (Fig. 2c). Using the scissors, extend the incision along the length of the body, being careful to cut no deeper than skin level and avoiding the anus (Fig. 2d). Pin the skin on each side of the incision to the dissecting surface, angling the pins out away from the body (Fig. 2e). Flame sterilize the forceps, then use them to remove the length of the intestinal tract (Fig. 2f), and place it into a homogenization tube, containing glass beads (see below) and 1000 μL of 1× PBS (Fig. 2g), on ice.
Fig. 2.
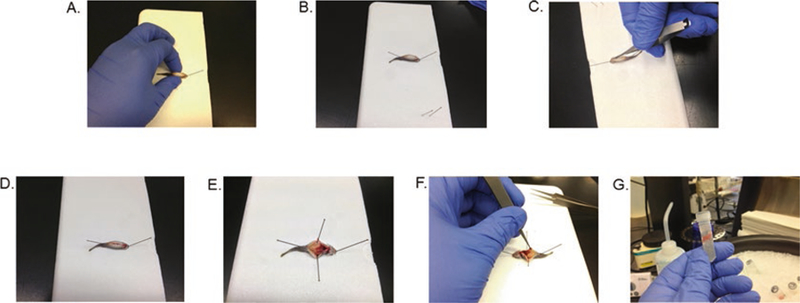
Dissection of zebrafish intestine (a) Pin fish through lower jaw. (b) Place second pin posterior to anus. (c) Use scalpel to make small incision just posterior to gills. (d) Use scissors to extend incision to anal area. (e) Pin skin on either side of fish to expose internal organs. (f) Use forceps to remove entire length of intetsinal tract. (g) Place intestine in screw cap homogenization tube for further processing
3.3. Homogenization
Homogenization tubes are prepared as follows: 2 mL tubes are filled with 1.5 g of 1 mm glass beads (approximately half-way), O-ring caps are loosely screwed on, and then tubes are autoclaved for 20 min. Once cooled, caps should be screwed on tightly and tubes can be stored until needed.
Once zebrafish intestines have been added to the tubes, the caps should be screwed on very tightly and then secured in the beadbeater. Samples are homogenized for 1 min on the maximum setting and then cooled on ice for 1–2 min, before repeating the homogenization cycle once more.
3.4. Intestinal Colonization Experiments
At designated time points, fish are removed from the beaker and euthanized in a beaker of tricaine solution as described in Subheading 2.3. Intestines are aseptically removed and homogenized as described in Subheadings 3.2–3.3. Serial dilutions of the homogenate in LB are made (typically tenfold dilutions) and plated on LB agar plates containing 100 μg/mL streptomycin and 40 μg/ mL X-gal. Plates are incubated at 30 °C for 16–18 h, after which V. cholerae colonies are counted (see Note 1). V. cholerae typically produces pale blue colonies.
3.5. Transmission Experiments
A group of four to five zebrafish, anesthetized and fin-clipped for later identification (see Note 2), are placed in a 600 mL beaker with 300 mL sterile infection water. A second group of four to five zebrafish are exposed to 107–109 V. cholerae in 200 mL sterile infection water as described above for 2–3 h to establish colonization. If a control for colonization at this time point is desirable, one or two fish can be sacrificed and dissected and intestinal homogenates plated as described above. The remaining infected fish are then transferred to another beaker of fresh infection water two times sequentially to remove external V. cholerae. The rinsed, infected fish are placed in the beaker already holding the fin-clipped naïve zebrafish. After ~24 h all the fish are sacrificed, and intestinal V. cholerae are enumerated as described above, separating the fin-clipped and unclipped fish.
Acknowledgments
This work was supported by NIH grants R21AI095520 and R01AI127390 and funding from Wayne State University. We also thank Dr. Melody Neely and her laboratory, who helped in developing this model.
Footnotes
Notes
If multiple colony morphologies are observed, this is likely due to natural streptomycin resistant microbiota. To verify bona fide V. cholerae, patch onto TCBS plates, on which V. cholerae produces large yellow colonies. Usually with a bit of experience, the V. cholerae colony morphology is easy to distinguish; however, different V. cholerae strains have different colony morphologies, so it is best to test each morphological variant on TCBS when first performing an experiment. Non-O1/ O139 V. cholerae can be particularly tricky to identify without significant experience.
For fin clipping, fish are anesthetized as described in Subheading 2.3, and the tail fin is clipped with scissors that have been flamed to sterilize. Just enough tissue is removed to make identification clear from unclipped fish. Clipped fish are then returned to the beaker of water to recover.
References
- 1.Allen JP, Neely MN (2010) Trolling for the ideal model host: zebrafish take the bait. Future Microbiol 5(4):563–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe HM, Withey JH, Neely MN (2014) Zebrafish as a model for zoonotic aquatic pathogens. Dev Comp Immunol 46(1): 96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan C, Kim CH (2008) Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol 25(4):341–350 [DOI] [PubMed] [Google Scholar]
- 4.Runft DL et al. (2014) Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Appl Environ Microbiol 80(5):1710–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sack DA et al. (2004) Cholera. Lancet 363(9404):223–233 [DOI] [PubMed] [Google Scholar]
- 6.Senderovich Y, Izhaki I, Halpern M (2010) Fish as reservoirs and vectors of Vibrio cholerae. PLoS One 5(1):e8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traore O et al. (2014) Occurrence of Vibrio cholerae in fish and water from a reservoir and a neighboring channel in Ouagadougou, Burkina Faso. J Infect Dev Ctries 8(10):1334–1338 [DOI] [PubMed] [Google Scholar]
- 8.Torres-Vitela MR et al. (1997) Incidence of Vibrio cholerae in fresh fish and ceviche in Guadalajara, Mexico. J Food Prot 60(3): 237–241 [DOI] [PubMed] [Google Scholar]
- 9.Kiiyukia C et al. (1992) Vibrio cholerae non-O1 isolated from ayu fish (Plecoglossus altivelis) in Japan. Appl Environ Microbiol 58(9): 3078–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]


