Abstract
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 1, 2003.
Tonsillectomy continues to be one of the most common surgical procedures performed worldwide. Despite advances in anesthetic and surgical techniques, post‐tonsillectomy morbidity remains a significant clinical problem.
Objectives
To assess the clinical efficacy of a single intraoperative dose of dexamethasone in reducing post‐tonsillectomy morbidity.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; ISRCTN; and additional sources for published and unpublished trials. The date of the most recent search was 29 October 2010, following a previous search in September 2002.
Selection criteria
Randomized, double‐blind, placebo‐controlled trials of a single dose of intravenous, intraoperative corticosteroid for pediatric patients (age < 18 years) who underwent tonsillectomy or adenotonsillectomy.
Data collection and analysis
The first author extracted data regarding the primary outcome measures and measurement tools from the published studies. The first author also recorded data regarding study design, patient ages, procedures performed, dose of corticosteroid and method of delivery, as well as methodological quality. When data were missing from the original publications, we contacted the authors for more information. We performed data analysis with a random‐effects model, using the RevMan 5.1 software developed by the Cochrane Collaboration.
Main results
We included 19 studies (1756 participants). We selected only randomized, placebo‐controlled, double‐blinded studies to minimize inclusion of poor quality studies. However, the risk of bias in the included studies was not formally assessed. Children receiving a single intraoperative dose of dexamethasone (dose range = 0.15 to 1.0 mg/kg) were half as likely to vomit in the first 24 hours compared to children receiving placebo (risk ratio (RR) 0.49; 95% confidence interval (CI) 0.41 to 0.58; P < 0.00001). Routine use in five children would be expected to result in one less patient experiencing post‐tonsillectomy emesis (risk difference (RD) ‐0.24; 95% CI ‐0.32 to ‐0.15; P < 0.00001). Children receiving dexamethasone were also more likely to advance to a soft/solid diet on post‐tonsillectomy day one (RR 1.45; 95% CI 1.15 to 1.83; P = 0.001) than those receiving placebo. Finally, postoperative pain was improved in children receiving dexamethasone as measured by a visual analog scale (VAS, 0 to 10) (MD ‐1.07; 95% CI ‐1.73 to ‐0.41; P = 0.001), which correlates clinically to a reduction in pain (on a VAS of 0 to 10) from 4.72 to 3.65. No adverse events were noted in the included studies.
Authors' conclusions
The evidence suggests that a single intravenous dose of dexamethasone is an effective, safe and inexpensive treatment for reducing morbidity from pediatric tonsillectomy.
Keywords: Adolescent; Child; Humans; Adenoidectomy; Adenoidectomy/adverse effects; Antiemetics; Antiemetics/therapeutic use; Convalescence; Dexamethasone; Dexamethasone/therapeutic use; Glucocorticoids; Glucocorticoids/therapeutic use; Pain, Postoperative; Pain, Postoperative/prevention & control; Postoperative Nausea and Vomiting; Postoperative Nausea and Vomiting/prevention & control; Time Factors; Tonsillectomy; Tonsillectomy/adverse effects; Treatment Outcome
Steroids for improving recovery following tonsillectomy in children
After children have a tonsillectomy or adenotonsillectomy (surgery to remove the adenoids and/or tonsils), pain, nausea, vomiting and delays to return to eating are common. The corticosteroid drug dexamethasone is sometimes given in a single intravenous dose (through the veins) during surgery to try to prevent vomiting after the operation. We included 19 randomized controlled trials in the review, with a total of 1756 patients. The review of trials found that a dose of corticosteroid during tonsillectomy or adenotonsillectomy can prevent vomiting for one out of every five children who gets the drug. Children also return to a normal diet more quickly and they have less pain after surgery.
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 1, 2003.
Tonsillectomy continues to be one of the most common surgical procedures performed worldwide. Despite advances in anesthetic and surgical techniques, post‐tonsillectomy morbidity (emesis, poor oral intake, pain and bleeding) remains a significant clinical problem for the patient, family and physician (Randall 1998).
During the past 40 years, investigators have studied the effects of systemic corticosteroids in reducing post‐tonsillectomy morbidity (Papangelou 1972). Results of randomized, placebo‐controlled studies of single intravenous steroid dosing have been conflicting; some demonstrating benefit and others showing no benefit (Aouad 2001; April 1996; Catlin 1991; Ohlms 1995; Pappas 1998; Splinter 1996; Tom 1996; Volk 1993; Vosdoganis 1999). For this reason, a Cochrane systematic review was completed in 2003 (Steward 2003), which showed that a single dose of intraoperative, intravenous steroids reduced postoperative emesis by half and children were more likely to return to a soft/solid diet by day one. Although pain was a predefined outcome measure in the 2003 review, due to inconsistencies in pain measurement we could not analyze this outcome, although a systematic review focusing specifically on pain was performed in 2006 (Afman 2006). Additionally, multiple randomized, double‐blind, placebo‐controlled trials have been performed during the intervening years since the initial review in 2003, which has created the need for an update of the current literature.
In an attempt to consolidate current research findings for emesis, return to diet and pain outcomes, and to update the Cochrane Review performed in 2003, we performed a systematic overview of published clinical trials using established meta‐analysis techniques with a predetermined protocol (Boissel 1989; Saks 1987). Our hypothesis that a single intraoperative dose of dexamethasone reduces morbidity following pediatric tonsillectomy or adenotonsillectomy is based upon clinical experience and published randomized studies demonstrating reduction in post‐tonsillectomy morbidity. Our goals were to determine if a meta‐analysis of randomized studies would still statistically support this hypothesis and to determine if a statistically significant result would have clinical relevance.
Objectives
To assess the clinical efficacy of a single intraoperative dose of dexamethasone in reducing post‐tonsillectomy morbidity.
Methods
Criteria for considering studies for this review
Types of studies
Randomized, double‐blind, placebo‐controlled trials.
Types of participants
Pediatric patients (age < or = 18 years) who underwent tonsillectomy or adenotonsillectomy.
Types of interventions
Single dose of intravenous, intraoperative corticosteroid.
Types of outcome measures
The number of children experiencing emesis during the first 24 hours after surgery.
The number of children returning to a soft or solid diet by postoperative day one.
Pain at 24 hours as measured by a visual analog scale (VAS) normalized to a 0 to 10 range (0 = least pain, 10 = most pain).
Note: in the Cochrane Review of 2003, return to diet by post‐tonsillectomy day three was also analyzed. In that review, only two studies (Catlin 1991; Volk 1993) met the inclusion criteria for day three analysis, and the results did not show any statistical significance between the steroid group and placebo (Steward 2003). Since 2003, return to diet by day three has not been measured with any consistency. For this reason, day three diet was eliminated from the current review. Additionally, several studies have measured time to first oral intake (hours). This outcome measure was not included in the 2003 review, and an improvement in this measure was felt to add little additional information separate from the ‘return to diet by day one’ measure. Therefore, this measure was not included in the current review.
Search methods for identification of studies
We conducted systematic searches for randomized controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 29 October 2010, following a previous search in September 2002.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 4); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; CNKI; ISRCTN; ClinicalTrials.gov; ICTRP; and Google.
We modeled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomized controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase, NHS Evidence ‐ ENT and Audiology, and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. In previous searches in 2002, we contacted leading experts in the fields of pediatric otolaryngology and pediatric anesthesiology for information on any relevant unpublished data.
Data collection and analysis
Selection of studies
We reviewed the titles and abstracts of all studies obtained by the search and two authors independently selected trials meeting the eligibility criteria. We obtained the full texts of the articles if there was insufficient information to make a decision and arranged translations where necessary. We resolved any disagreements by discussion until a consensus was reached. We documented our justification for the exclusion of studies in the Characteristics of excluded studies table.
Data extraction and management
The first author extracted data regarding the primary outcome measures and measurement tools from the published studies. The first author also recorded data regarding study design, patient ages, procedures performed, dose of corticosteroid and method of delivery, as well as methodological quality. When data were missing from the original publications, we contacted the authors for more information.
Assessment of risk of bias in included studies
We specifically designed our protocol to select only randomized, placebo‐controlled, double‐blinded studies to minimize inclusion of poor quality studies. While we did not rank studies based upon quality, the authors did assess the methodological quality of included studies. Two of the authors (DLS, JG) worked independently to assess trials for methodological quality using the following criteria: allocation concealment (blinding of randomization), blinding of intervention, completeness of follow up and blinding of outcome measurement/assessment. We added information about allocation concealment to the table Characteristics of included studies under the 'Risk of bias' tables.
Data synthesis
We performed all meta‐analyses of the included trials using the RevMan 5.1 software developed by the Cochrane Collaboration (RevMan 2011). We performed all data analyses with random‐effects models and reported Mantel‐Haenszel risk ratios for dichotomous outcomes. For continuous outcomes, we derived a pooled risk difference from a random‐effects model. When a study collected but did not report data regarding one of the three primary outcome measures, we excluded that study from the meta‐analysis of that particular outcome measure. When a measurement tool for an outcome from one study differed from other studies in such a way as to prevent pooling of data, we excluded that study and provided the reasons in the results section. Lastly, we excluded studies that did not collect data regarding a particular outcome measure from analysis of that outcome measure. We made decisions regarding the exclusion of studies from analysis based on the above criteria with the investigators blinded to the results of the studies. RevMan calculates measures of heterogeneity with the Chi2 test and I2 statistic, which measures the inconsistency of effects across interventions. We performed sensitivity analyses to assess statistically the impact of study exclusion due to missing data or different measurement tools (Boissel 1989). We also performed sensitivity analysis to assess the potential impact of publication bias or missed studies (Rosenthal 1979).
Results
Description of studies
Results of the search
From the update searches in 2010, we retrieved a total of 514 references: 302 of these were removed in first‐level screening (i.e. removal of duplicates and clearly irrelevant references), leaving 101 references for further consideration. The searches conducted in 2002 had yielded over 180 titles and abstracts for review.
We reviewed all titles and abstracts for possible inclusion. We evaluated foreign language publications based upon available English translations or translated them for evaluation. We then reviewed any potential studies in full. Of these, 10 met the above inclusion criteria, which we combined with the studies included in the 2003 review (see Characteristics of included studies). Other searches, using the 'clinical query' filter of the PubMed (MEDLINE) database, The Cochrane Library database and other search terms (CORTICOSTEROIDS, DEXAMETHASONE and MORBIDITY), yielded no additional studies meeting the selection criteria. Likewise, no other studies meeting the selection criteria were identified through cross‐referencing, nor through contacting experts in the field.
Included studies
We included 19 studies in the current review (1756 participants). Ten of these studies were new for the current update and nine were included from the previous version of the review (Steward 2003). A summary of the included studies is as follows:
Design
All included studies were randomized, placebo‐controlled and double‐blinded.
Setting
Studies were all performed in academic health centers from international locations.
Participants
Only children were included and ages ranged from nine months to 18 years.
Interventions
The studied intervention was a single, intraoperative dose of corticosteroid (dexamethasone), ranging from 0.15 mg/kg to 1.0 mg/kg.
Outcomes
Measured outcomes included:
the number of children experiencing emesis during the first 24 hours after surgery;
return to a soft or solid diet by postoperative day one; and
pain at 24 hours as measured by a visual analog scale (VAS) normalized to a 0 to 10 range (0 = least pain, 10 = most pain).
Excluded studies
Forty‐three studies addressing steroid treatment to reduce post‐tonsillectomy morbidity failed to meet the selection criteria and were excluded (see Characteristics of excluded studies). Thirteen studies did not meet the allocation requirement (not randomized, placebo‐controlled or double‐blinded). Twenty‐three studies did not involve intravenous administration of steroids. Eleven studies were not limited to children. Fifteen studies did not limit the experimental group to steroids only. Two studies included procedures other than tonsillectomy/adenoidectomy (uvulopalatopharyngoplasty in both cases). One study did not report any of the outcomes included in this review, and one study was terminated early.
Risk of bias in included studies
To address problems of quality in meta‐analysis, some authors have suggested methods to assign relative value to studies based upon assessment of quality (Chalmers 1981). However, this technique has not gained universal acceptance (Boissel 1989). We specifically designed our protocol to select only randomized, placebo‐controlled, double‐blinded studies to minimize inclusion of poor quality studies.
While we did not rank studies based upon quality, the authors did assess the methodological quality of included studies. Two of the authors (DLS, JG) worked independently to assess trials for methodological quality using the following criteria: allocation concealment (blinding of randomization), blinding of intervention, completeness of follow up, and blinding of outcome measurement/assessment. We added information on allocation concealment to the table Characteristics of included studies in the 'Risk of bias' tables.
Only six studies reported adequate allocation concealment (April 1996; Catlin 1991; Elhakim 2003; Giannoni 2002; Hanasono 2004; Volk 1993), while the remaining studies were unclear on this point. Performance bias included surgical and anesthetic techniques, which were controlled in nine of the studies (Aouad 2001; Bhattacharya 2009; Catlin 1991; Elhakim 2003; Giannoni 2002; Hanasono 2004; Kaufmann 2006; Ohlms 1995; Pappas 1998). Surgical technique was not controlled in four studies (Kaan 2006; Khani 2009; Samarkandi 2004; Splinter 1996), while anesthetic technique was not controlled in six studies (Al Shehri 2007; April 1996; Splinter 1996; Tom 1996; Volk 1993; Vosdoganis 1999), one of which used blocked randomization to address this issue (Splinter 1996). Attrition bias did not appear to be significant within any of the studies and all studies reported the number of subjects excluded from analysis. Outcome measurements differed amongst studies and are discussed specifically in the results section for each outcome measure. Reporting bias (not reporting data with no statistical significance) was present in one study for the emesis outcome (Catlin 1991), two studies for dietary outcomes (Catlin 1991; Volk 1993) and four studies for pain outcomes (April 1996; Catlin 1991; Tom 1996; Volk 1993). Based on this analysis, the authors feel that the risk of bias is low, however we did not perform a formal assessment.
Effects of interventions
Emesis
We evaluated emesis by analyzing the number of patients who experienced an emetic event during the first 24 hours following tonsillectomy. For this outcome measure, we pooled the data from 15 studies (1273 participants). The result of the meta‐analysis suggests a statistically significant risk ratio, favoring steroids (RR 0.49; 95% confidence interval (CI) 0.41 to 0.58; P < 0.00001) (Analysis 1.1). In addition, patients who received steroids had lower rate of emesis (21%) compared to patients who received placebo (48%). The pooled risk difference for this analysis was statistically significant (RD ‐0.24; 95% CI ‐0.32 to ‐0.15; P < 0.00001) (Analysis 1.2). No significant heterogeneity regarding the risk ratio was noted in this meta‐analysis (I2 = 14%; P = 0.3), although significant heterogeneity was present regarding the risk difference (I2 = 71%; P < 0.0001).
Analysis 1.1.
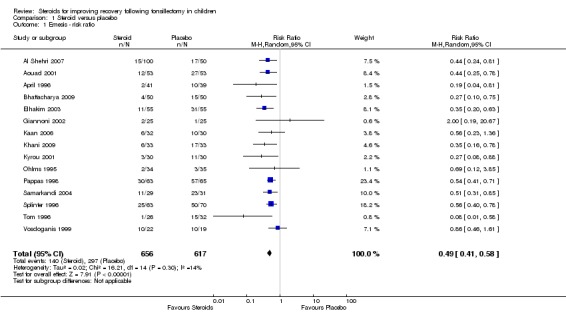
Comparison 1 Steroid versus placebo, Outcome 1 Emesis ‐ risk ratio.
Analysis 1.2.
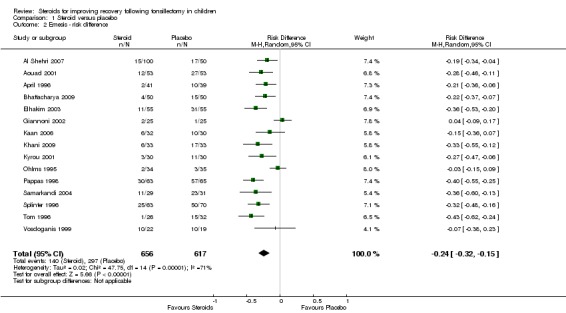
Comparison 1 Steroid versus placebo, Outcome 2 Emesis ‐ risk difference.
Sensitivity analysis (emesis)
We checked the sensitivity of this analysis to potential reporting bias (not reporting data with a null result) first by deleting and then by assuming a null result for the Giannoni et al study (Giannoni 2002). The results were not significantly different for either the deleted (RR 0.48; 95% CI 0.40 to 0.58; P < 0.00001) or null assumption analyses (RR 0.50; 95% CI 0.41 to 0.60; P < 0.00001). We also conducted sensitivity analysis by assuming a null result for the Catlin et al study (Catlin 1991). Again, the results were not different including Catlin et al with a null assumption (RR 0.49; 95% CI 0.41 to 0.59; P < 0.00001). To check the sensitivity of the emesis analysis to possible publication bias (not publishing studies with a null result) and/or studies missed by the search strategy, we calculated the fail‐safe N as described by Rosenthal (Rosenthal 1979). The result of this analysis suggests that 129 studies with a null risk ratio (i.e. RR = 1) would be required to raise the overall risk ratio to statistical non‐significance.
Diet (day one)
We evaluated diet by analyzing the number of patients that advanced to a soft or solid diet during post‐tonsillectomy day one.
We pooled the data from five studies (452 participants) in the day one diet analysis. The result of this meta‐analysis suggests statistical significance using both risk ratio (RR 1.45; 95% CI 1.15 to 1.83; P = 0.001) (Analysis 1.3) and risk difference (RD 0.21; 95% CI 0.10 to 0.31; P < 0.0001) (Analysis 1.4). Although moderate heterogeneity was noted regarding both the risk ratio (I2 = 46%; P = 0.12) and the risk difference (I2 = 44%; P = 0.13), neither was statistically significant.
Analysis 1.3.
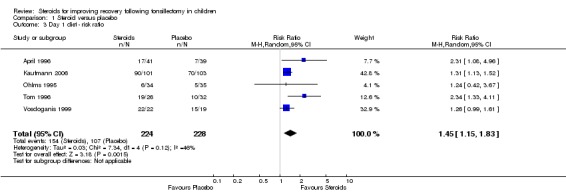
Comparison 1 Steroid versus placebo, Outcome 3 Day 1 diet ‐ risk ratio.
Analysis 1.4.
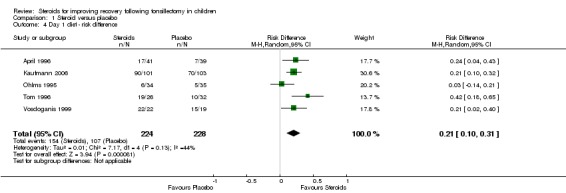
Comparison 1 Steroid versus placebo, Outcome 4 Day 1 diet ‐ risk difference.
Sensitivity analyses (day one diet)
To determine the impact of potential reporting bias, we performed sensitivity analysis assuming a null result for the two studies with missing data (Catlin 1991; Volk 1993). The missing proportions in each group for each study were set equal to the observed average over all studies (0.58). The risk ratio is reduced, but still suggests statistical significance (RR 1.35; 95% CI 1.13 to 1.61; P = 0.001). To assess the impact of possible selection bias resulting from exclusion of the studies by Pappas et al, Aouad et al and Kaan et al, we performed sensitivity analysis by also including these studies (Aouad 2001; Kaan 2006; Pappas 1998). The result suggests statistical significance (RR 1.52; 95% CI 1.18 to 1.97; P = 0.001) even when including the null assumption for the missing data as described above (RR 1.23; 95% CI 1.04 to 1.46; P = 0.01).
Pain
Eight studies (652 participants) were included in this analysis (Elhakim 2003; Giannoni 2002; Hanasono 2004; Kaan 2006; Khani 2009; Ohlms 1995; Volk 1993; Vosdoganis 1999). The result of this meta‐analysis suggests statistical difference in the mean difference regarding mean pain scores (mean difference (MD) ‐1.07; 95% CI ‐1.73 to ‐0.41; P = 0.001) (Analysis 1.5). We excluded three studies because they did not report pain as an outcome (Aouad 2001; Kyrou 2001; Splinter 1996). Six studies (Al Shehri 2007; April 1996; Bhattacharya 2009; Catlin 1991; Kaufmann 2006; Samarkandi 2004) that evaluated pain as an outcome measure did not publish enough data for analysis, and thus were excluded. Pappas et al reported analgesic use as a pain outcome measure and Tom et al reported the frequency of pain between study groups; both studies were also excluded (Pappas 1998; Tom 1996). Significant heterogeneity was noted in this meta‐analysis (I2 = 86%; P < 0.00001).
Analysis 1.5.
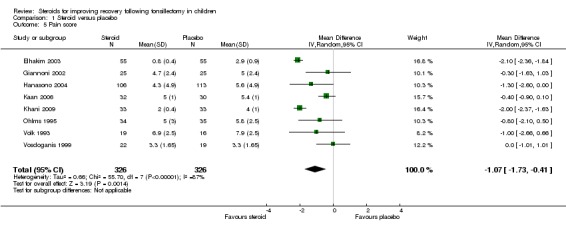
Comparison 1 Steroid versus placebo, Outcome 5 Pain score.
Sensitivity analysis (pain)
To determine the impact of potential reporting bias, we performed sensitivity analysis assuming a null result for the six studies that did record pain scores, but did not adequately report results for inclusion (Al Shehri 2007; April 1996; Bhattacharya 2009; Catlin 1991; Kaufmann 2006; Samarkandi 2004). This was done by assuming a null result for the additional studies and recalculating the pooled difference in means. Including the additional studies reduced the pooled mean difference, but the results remained significant (MD ‐0.59; 95% CI ‐1.18 to 0.0; P = 0.05).
We detected significant heterogeneity in the meta‐analysis of pain scores, therefore we also performed a sensitivity analysis to determine whether the heterogeneity was due to the presence of one or two outlying studies. Each study was subsequently removed from the analysis and the pooled results were recalculated to determine which study(ies) contributed the most to the heterogeneity. Results indicated that both of the studies by Elhakim et al (Elhakim 2003) and Khani et al (Khani 2009) contributed the most to the I2. After excluding these two studies, the I2 was 0% (P = 0.67) (MD ‐0.48; 95% CI ‐0.85, ‐0.10; P = 0.01).
Discussion
Meta‐analysis
With certain limitations, meta‐analysis is a valuable tool that can provide a more objective evaluation of a group of studies than a traditional narrative review (Alsarraf 2000). Limitations of meta‐analysis are well described and can be grouped into problems of combinability, selection bias and the quality of the original studies (Sharpe 1997). Combinability problems result from 'mixing apples and oranges'. In our study this relates to pooling data from studies with different surgical techniques, surgical procedures, anesthetic techniques, dexamethasone dosages, patient populations and outcome measurement tools. To address this problem, we accepted the inherent heterogeneity amongst the trials and utilized a random‐effects model for analysis, rather than a fixed‐effect model. This has the advantage of allowing for variability within the patient populations and also for variability in treatment effect (Boissel 1989).
To address problems of selection bias in our meta‐analysis, we performed sensitivity analyses to determine the impact on our results due to exclusion of studies: potentially missed or unpublished; with missing data; or with a significantly different measurement tool (Boissel 1989; Rosenthal 1979). The results for the emesis and day one diet outcome measures remained significant even after the sensitivity analysis. For the pain outcome, the pooled mean difference (MD) changed from ‐1.07 (95% confidence interval (CI) ‐1.73 to ‐0.41; P = 0.001) to ‐0.59 (95% CI ‐1.18 to 0.0; P = 0.05).
To address problems of quality in meta‐analysis, some authors have suggested methods to assign relative value to studies based upon assessment of quality (Chalmers 1981). However, this technique has not gained universal acceptance (Boissel 1989). We specifically designed our protocol to select only randomized, placebo‐controlled, double‐blinded studies to minimize inclusion of poor quality studies. As such, we think it unlikely that quality issues significantly impact the result of our analyses.
Emesis
Our results suggest a statistically significant reduction in chance of experiencing episodes of post‐tonsillectomy emesis during the first 24 hours with dexamethasone versus placebo for pediatric patients.
The mechanism by which dexamethasone may exert an anti‐emetic effect remains unknown (Brunton 1996). However, dexamethasone has been shown to be an effective anti‐emetic in randomized trials with emetogenic chemotherapy (Hesketh 1994; Research 1995). Additionally, randomized studies of a serotonin antagonist plus either dexamethasone or placebo have shown a statistically significant reduction in emesis after pediatric tonsillectomy in the dexamethasone‐treated group (Fujii 1996; Holt 2000). Thus, despite the fact that the mechanism is not yet understood, an anti‐emetic effect of corticosteroids is supported by other studies (Goldman 2000; Henzi 2000) and widely accepted (Schimmer 1996).
The number needed to treat (NNT = 1/risk difference) is a statistical inference of the size of therapeutic effect one might expect in clinical practice. The NNT of 4.17 predicts that routine use of a single intravenous dose of dexamethasone in approximately five patients would result in one less patient experiencing post‐tonsillectomy emesis. This suggests therapeutic benefit with a clinically relevant effect size. This therapeutic benefit is consistent with the previous review in 2003. In clinical practice, whether or not an individual patient responds will likely be dependent upon patient factors, anesthetic technique, narcotic use and possibly the amount of blood swallowed during tonsillectomy.
Diet
An earlier return to a regular diet following tonsillectomy as a result of dexamethasone therapy could be the result of mood elevation, appetite stimulation, anti‐emetic effect or a combination of these (Schimmer 1996). Our results of the day one diet outcome measure demonstrate a statistically significant increase in the number of patients returning to a soft/solid diet during the first 24 hours. In 2003, the systematic review also demonstrated a statistically significant difference (RR 1.69, P = 0.04). The current review reduces the chance of a type I error from 4% to 0.1%.
The NNT of 4.76 suggests that routine use of dexamethasone in five children would result in one more child advancing to a soft/solid diet on post‐tonsillectomy day one. This suggests a clinically significant effect size. These results are consistent with and support the findings of the 2003 systematic review (Steward 2003).
Pain
In the 2003 Cochrane Review, pain was listed as a predefined outcome, but there were insufficient data to analyze the results. Since that time, eight studies have rigorously measured postoperative pain using a visual analog scale (VAS). (Elhakim 2003; Giannoni 2002; Hanasono 2004; Kaan 2006; Khani 2009; Ohlms 1995; Volk 1993; Vosdoganis 1999). The results of meta‐analysis of these studies demonstrate a statistically significant improvement in postoperative pain for patients in the steroid group (MD ‐1.07; 95% CI ‐1.73 to ‐0.41; P = 0.001). These data are consistent with the results of a systematic review devoted specifically to this issue (Afman 2006).
The clinical significance of this observed pain reduction must be considered. Each study measured pain on a visual analog scale (VAS) with 0 corresponding to 'least pain' and 10 corresponding to 'most pain' (studies with different pain scales were normalized to a 0 to 10 scale). The mean score in the placebo group was 4.72 and the steroid group was reduced to 3.65. The sensitivity analysis decreased this pain reduction benefit (MD ‐0.59; 95% CI ‐1.18 to 0.0; P = 0.05). Therefore, while dexamethasone was associated with reduction in pain, the clinical significance of this finding should be interpreted with caution. The effect size of the estimated benefit on postoperative pain appeared to be, in part, driven by specific studies.
Safety
One of the excluded studies was terminated prematurely because the steroid group demonstrated significant postoperative hemorrhage compared to placebo (Czarnetzki 2008). Several methodological questions have been raised regarding this study, including non‐standardization of diagnosis, administration of ibuprofen, non‐standard surgical technique and attributing primary hemorrhage to dexamethasone use (Gunter 2009). Indeed, four of eight patients requiring surgical re‐intervention had bleeding on the day of surgery. The use of ibuprofen perioperatively is also controversial. Ibuprofen is known to have antiplatelet effects and some practice guidelines discourage its perioperative use (Douketis 2008), although a recent prospective study comparing tonsillectomy patients recovered with ibuprofen did not observe increased bleeding rates compared to placebo (Yaman 2011).
The findings published by Czarnetski et al resulted in several retrospective chart reviews representing 3318 patients receiving intraoperative, intravenous dexamethasone (Brigger 2010; Shakeel 2010). These studies did not show any increase in bleeding rates associated with steroid use. None of the other included or excluded studies for this review demonstrated any increased bleeding risk. It should also be considered that the findings of Czarnetski et al were a type I error. Duplication under more appropriate study conditions should be performed before serious consideration can be made of their claim.
Other risks to patients of single‐dose dexamethasone appear minimal. A single dose of corticosteroid, even a large one, has been considered to be virtually without harmful effects (Schimmer 1996). The most concerning potential risks of corticosteroid therapy include immune system suppression resulting in severe systemic infection and avascular joint necrosis. Disseminated varicella infection (chicken pox) is described in patients undergoing immune suppressive steroid therapy. A systematic review of avascular joint necrosis found that while risk is associated with increased daily steroid dosage, no increased risk was observed with single bolus dosing (Felsen 1987). While neither complication has been reported as a consequence of a single perioperative dose during tonsillectomy, this does not imply that these complications are not possible. Appropriate discussion of the risks and benefits of therapy should occur between patient, family and physician.
The cost of dexamethasone is relatively low, which makes routine use seem reasonable. This modest increase in cost may be offset by a reduction in cost resulting from rescue anti‐emetic therapy, prolonged hospitalization or both. However, its cost‐effectiveness remains to be studied.
A previously published version of this meta‐analysis suggested improved anti‐emetic effect with increasing dexamethasone dose (Steward 2001). Though meta‐analysis lends itself to subset analysis (Boissel 1989; Saks 1987), caution should be used in evaluating this result as these studies were not designed to assess dose effect and patients were not randomized to different dosages. Additionally, because both mg/kg dose and the maximum dose given varied amongst these studies, statistical results of regression analysis of only the former are questionable. Therefore, the question of appropriate dosing remains unanswered and final recommendations must await randomized dose‐control trials
Generalizability
As this analysis excluded studies with adult patients, these results cannot be generalized to the adult population. However, dexamethasone has been shown to be an effective anti‐emetic in adult patients undergoing emetogenic chemotherapy, suggesting a role for further study in adult tonsillectomy patients. Previous randomized, placebo‐controlled studies of adult patients have focused primarily on pain as an outcome measure (Carr 1999; Tewary 1993).
Authors' conclusions
Tonsillectomy is one of the most commonly performed surgical procedures worldwide. Despite changes in anesthetic and surgical techniques, however, postoperative morbidity remains a significant clinical problem. The results of our meta‐analysis suggest a statistically significant reduction in postoperative morbidity with single‐dose dexamethasone therapy given during pediatric tonsillectomy or adenotonsillectomy. Specifically, our results suggest a statistically significant reduction in post‐tonsillectomy emesis events during the first 24 hours and an increase in the number of patients advancing to a soft/solid diet on postoperative day one. Postoperative pain is also reduced with dexamethasone use. Moreover, single‐dose dexamethasone therapy is inexpensive and, if harmful effects occur, they are exceptionally rare. Given the frequency with which tonsillectomy is performed; the low cost and safety of single‐dose intravenous dexamethasone; and the benefit from emesis reduction, return to a soft/solid diet and pain reduction, the evidence suggests that routine use would significantly reduce morbidity from pediatric tonsillectomy.
Further study is still warranted to determine optimum dexamethasone dosing. Additionally, further study is suggested to determine possible benefit in the adult tonsillectomy patient. Lastly, any suggestion that single‐dose dexamethasone increases bleeding risk needs to be substantiated with further studies.
Acknowledgements
Statistical support was provided by the Center for Epidemiology and Biostatistics at Cincinnati Children's Medical Center, Cincinnati, OH.
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | EMBASE (Ovid) | CINAHL (EBSCO) |
| #1 MeSH descriptor Tonsillectomy explode all trees #2 MeSH descriptor Palatine Tonsil explode all trees with qualifier: SU #3 MeSH descriptor Palatine Tonsil explode all trees #4 MeSH descriptor Tonsillitis explode all trees #5 (tonsil* OR adenotonsil*) #6 (#3 OR #4 OR #5) #7 MeSH descriptor Surgical Procedures, Operative explode all trees #8 (surg* or excis* or extract* or remov):ti #9 (#7 OR #8) #10 (#6 AND #9) #11 (tonsillectom* OR tonsilectom* OR adenotonsillectom* OR adenotonsilectom* OR tonsillotom* OR tonsilotom*) #12 (#1 OR #2 OR #10 OR #11) #13 MeSH descriptor Steroids explode all trees #14 MeSH descriptor Adrenal Cortex Hormones explode all trees #15 MeSH descriptor Anti‐Inflammatory Agents explode all trees #16 MeSH descriptor Anti‐Inflammatory Agents, Non‐Steroidal explode all trees #17 (#15 AND NOT #16) #18 (steroid* or corticosteroid* or glucocorticoid* or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone) #19 (#13 OR #14 OR #17 OR #18) #20 (#12 AND #19) | #1 “Tonsillectomy” [Mesh] OR “Palatine Tonsil/surgery” [Mesh] OR tonsillectom* [tiab] OR tonsilectom* [tiab] OR adenotonsillectom* [tiab] OR adenotonsilectom* [tiab] OR tonsillotom* [tiab] OR tonsilotom* [tiab] #2 (“Tonsillitis” [Mesh] OR “Palatine Tonsil” [Mesh] OR tonsil [tiab] OR adenotonsil* [tiab]) AND (“Surgery” [Mesh] OR surg* [ti] OR excis* [ti] OR extract* [ti] OR remov* [ti]) #3 #1 OR #2 #4 “STEROIDS” [Mesh] OR “Adrenal Cortex Hormones” [Mesh] OR “Adrenal Cortex Hormones” [Pharmacological Action] OR (“ANTI‐INFLAMMATORY AGENTS” [Mesh] NOT "Anti‐Inflammatory Agents, Non‐Steroidal"[Mesh]) #5 steroid* [tiab] OR corticosteroid* [tiab] OR glucocorticoid* [tiab] OR beclomethasone [tiab] OR betamethasone [tiab] OR budesonide [tiab] OR cortisone [tiab] OR dexamethasone [tiab] OR flunisolide [tiab] OR fluticasone [tiab] OR fludrocortisone [tiab] OR hydrocortisone [tiab] OR cortisol [tiab] OR methylprednisolone [tiab] OR mometasone [tiab] OR prednisolone [tiab] OR prednisone [tiab] OR triamcinolone [tiab] #6 #4 OR #5 #7 #3 AND #6 | 1 exp tonsillectomy/ 2 (tonsillectom* OR tonsilectom* OR adenotonsillectom* OR adenotonsilectom* OR tonsillotom* OR tonsilotom*).tw. 3 (tonsil* or adenotonsil*).tw. 4 exp tonsil/ or tonsillitis/ 5 exp surgery/ 6 (surg* or excis* or extract* or remov*).ti. 7 3 OR 4 8 5 OR 6 9 7 AND 8 10 1 OR 2 OR 9 11 exp Steroid/ 12 exp Antiinflammatory Agent/ not exp Nonsteroid Antiinflammatory Agent/ 13 (steroid* or corticosteroid* or glucocorticoid* or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone).tw. 14 11 OR 12 OR 13 15 10 AND 14 | S1 (MH "Tonsillectomy") OR TX tonsillectom* OR tonsilectom* OR adenotonsillectom* OR adenotonsilectom* OR tonsillotom* OR tonsilotom* S2 (MH "Tonsil") OR (MH "Tonsillitis") S3 TX tonsil* OR adenotonsil* S4 S2 or S3 S5 surg* or excis* or extract* or remov* S6 S4 and S5 S7 S1 or S6 S8 (MH "Steroids+") S9 (MH "Adrenal Cortex Hormones+") S10 (MH "Antiinflammatory Agents, Steroidal+") S11 (MH "Antiinflammatory Agents, Non‐Steroidal+") S12 S10 NOT S11 S13 TX steroid* or corticosteroid* or glucocorticoid* or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone |
| Web of Science/BIOSIS Previews | Cochrane Ear, Nose and Throat Disorders Group Trials Register | CAB Abstracts (Ovid) | ISRCTN (mRCT) |
| #1 TS=(tonsillectom* OR adenotonsillectom*) #2 TI=((tonsil* OR adenotonsil*) AND (surg* or excis* or extract* or remov*)) #3 #2 OR #1 #4 TS=(steroid* or corticosteroid* or glucocorticoid* or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone) #5 #3 AND #4 | (tonsil* OR adenotonsil* OR posttonsil*) AND (steroid* or corticosteroid* or glucocorticoid* or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone) | 1 (tonsillectom* OR tonsilectom* OR adenotonsillectom* OR adenotonsilectom* OR tonsillotom* OR tonsilotom*).tw. 2 (tonsil* or adenotonsil*).tw. 3 exp tonsil/ or tonsillitis/ 4 exp surgery/ 5 (surg* or excis* or extract* or remov*).ti. 6 2 OR 3 7 4 OR 5 8 6 AND 7 9 1 OR 8 10 (steroid* or corticosteroid* or glucocorticoid* or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone).tw. 11 9 AND 10 | (tonsil% OR adenotonsil%) AND (steroid% or corticosteroid% or glucocorticoid% or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone) |
Data and analyses
Comparison 1.
Steroid versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Emesis ‐ risk ratio | 15 | 1273 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.41, 0.58] |
| 2 Emesis ‐ risk difference | 15 | 1273 | Risk Difference (M‐H, Random, 95% CI) | ‐0.24 [‐0.32, ‐0.15] |
| 3 Day 1 diet ‐ risk ratio | 5 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.15, 1.83] |
| 4 Day 1 diet ‐ risk difference | 5 | 452 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [0.10, 0.31] |
| 5 Pain score | 8 | 652 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.73, ‐0.41] |
What's new
| Date | Event | Description |
|---|---|---|
| 1 February 2011 | New search has been performed | New searches run October 2010. |
| 1 February 2011 | New citation required and conclusions have changed | The current review differs from the 2003 review in several ways. Two authors (JA Welge and CM Myer) were no longer involved and two new authors (JJ Grisel and J Meinzen‐Derr) joined. We included 10 new studies and excluded 17 additional studies. This review does not include diet at day three as an outcome measure (included in 2003), but it does now include pain as measured by a visual analog scale. Children in the steroid group were two times less likely to vomit than children in the placebo group. They were also more likely to advance to a diet by day one and less likely to experience pain. These results strengthen the results found in the 2003 review. |
History
| Date | Event | Description |
|---|---|---|
| 27 October 2008 | Amended | Converted to new review format. |
Differences between protocol and review
There was no preceding protocol for this review. Changes from the previous version of the review: we have added details of study selection; day three diet has been eliminated as an outcome measure; and we have been able to analyze pain as an outcome.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 150 patients enrolled age 2 to 6 years undergoing tonsillectomy with or without adenoidectomy (electrodissection) | |
| Interventions | Dexamethasone 0.5 mg/kg (max dose 8 mg) IV or dexamethasone 1.0 mg/kg (max dose 16 mg) IV or 2 mL normal saline | |
| Outcomes | Incidence of early (PACU) and late (ward) vomiting; total incidence Time to first oral intake (hours) Postoperative pain score (recorded but not reported) |
|
| Notes | Anesthetic not controlled; surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 110 patients enrolled age 2 to 12 years undergoing tonsillectomy or adenotonsillectomy (electrosurgery) 106 patients completed (53 placebo and 53 steroid) |
|
| Interventions | Dexamethasone 0.5 mg/kg (max dose 8 mg) IV or normal saline | |
| Outcomes | Emesis 24 hours Day 1 diet (rated based upon child requesting food, accepts when offered, accepts when coaxed, refuses) Time to first oral intake Outcomes not included: day 3 diet; pain |
|
| Notes | Anesthesia and surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 80 patients age 3 to 15 years undergoing tonsillectomy or adenotonsillectomy (electrosurgery) (41 steroid and 39 placebo) | |
| Interventions | Dexamethasone 0.5 mg/kg (max dose 8 mg) IV or normal saline | |
| Outcomes | Emesis 6 hours Day 1 diet Pain (scale and analgesic use) recorded but not reported Outcomes not included: day 3 diet |
|
| Notes | Anesthesia not controlled; surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 100 patients enrolled age 6 to 15 years undergoing tonsillectomy (no electrocautery) | |
| Interventions | Dexamethasone 8 mg IV or normal saline Diclofenac patch applied to all participants |
|
| Outcomes | Emesis 24 hours Day 1 diet (obtained by communication) Pain score (VAS) (obtained by communication) |
|
| Notes | Anesthesia and surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 25 patients age 3 to 12 years undergoing tonsillectomy or adenotonsillectomy (cold) (10 steroid and 15 placebo) | |
| Interventions | Dexamethasone 1.0 mg/kg (max dose 16 mg) IV or normal saline | |
| Outcomes | Emesis 24 hours recorded but not reported Day 1 diet recorded but not reported Day 3 diet Pain (score and analgesic use) recorded but not reported |
|
| Notes | Anesthesia and surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 120 patients age 4 to 11 years undergoing tonsillectomy or adenotonsillectomy (electrocautery) | |
| Interventions | Dexamethasone 0.5 mg/kg (max dose 8 mg) IV or normal saline | |
| Outcomes | Emesis 24 hours (steroid 20% versus placebo 56%) Time to first oral intake Pain score 24 hours (VAS) |
|
| Notes | Anesthesia and surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 50 patients age 3 to 15 years undergoing tonsillectomy or adenotonsillectomy (electrocautery) | |
| Interventions | Dexamethasone 1.0 mg/kg (max dose 16 mg) IV or normal saline | |
| Outcomes | Emesis 24 hours (recorded but not reported) Pain score 24 hours (VAS) Quality of life survey |
|
| Notes | Surgery and anesthesia controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 219 patients age 9 months to 12 years undergoing tonsillectomy (62 electrocautery and steroids, 44 cold and steroids, 56 electrocautery and placebo, 57 cold and placebo) | |
| Interventions | Dexamethasone 1 mg/kg IV or placebo | |
| Outcomes | Episodes of emesis Percentage of oral intake Patient related pain score (VAS) |
|
| Notes | Surgery and anesthesia controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 66 patients age 4 to 12 years undergoing tonsillectomy or adenotonsillectomy | |
| Interventions | Dexamethasone 0.5 mg/kg (max dose 8 mg) or saline | |
| Outcomes | Emesis at at least 8 hours Time to first oral intake Number of patients requesting oral intake or accepting when offered Pain (VAS), rated 1 to 5 and multiplied by 2 to get a 10‐point scale; steroid 2.0 (SD 0.4) versus placebo 4.0 (SD 1.0) |
|
| Notes | Anesthesia controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, placebo‐controlled (does not report blinding) | |
| Participants | 230 children ages 2 to 16 years undergoing tonsillectomy or adenotonsillectomy (combination hot/cold technique); 204 completed study (steroid 101 versus placebo 103) | |
| Interventions | Dexamethasone 0.5 mg/kg (max dose 10 mg) IV versus no treatment | |
| Outcomes | Emesis 24 hours: only recorded number of patients with more than 1 episode Day 1 diet Pain score (VAS): recorded but not reported |
|
| Notes | Surgery and anesthesia controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 66 patients age 4 to 12 years undergoing tonsillectomy or adenotonsillectomy (method unclear) | |
| Interventions | Dexamethasone 0.5 mg/kg (max dose 8 mg) IV or saline | |
| Outcomes | Emesis 24 hours: only measured at 8 hours Day 1 diet: only measured as time to first oral intake Pain score (VAS): recorded but not reported |
|
| Notes | Anesthesia controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 60 patients age 4 to 14 years undergoing tonsillectomy or adenotonsillectomy | |
| Interventions | Dexamethasone 0.2 mg/kg IV AND metoclopramide 0.15 mg/kg IV or metoclopramide 0.15 mg/kg IV | |
| Outcomes | Emesis 24 hours (steroid 10% versus placebo 37%) | |
| Notes | Anesthesia and surgery control not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 69 patients age 3 to 18 years undergoing tonsillectomy or adenotonsillectomy (cold) (34 steroid and 35 placebo) | |
| Interventions | Dexamethasone 0.5 mg/kg (max dose 12 mg) IV or normal saline | |
| Outcomes | Emesis 24 hours Day 1 diet Day 3 diet Pain score Analgesic doses |
|
| Notes | Anesthesia and surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 130 patients age 2 to 12 years undergoing tonsillectomy or adenotonsillectomy (electrosurgery) (63 steroid and 65 placebo) | |
| Interventions | Dexamethasone 1.0 mg/kg (max dose 25 mg) IV or normal saline | |
| Outcomes | Emesis 24 hours ("retch" or emesis) Day 1 diet (rated based upon child requesting food, accepts when offered, accepts when coaxed, refuses) Pain: analgesic use in PACU Outcomes not included: day 3 diet |
|
| Notes | Anesthesia and surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 60 patients age 2 to 12 years undergoing tonsillectomy (electrocautery) | |
| Interventions | Dexamethasone 0.5 mg/kg IV or saline (steroid 29 versus placebo 31) | |
| Outcomes | Emesis 24 hours (steroid 38% versus steroid 74%); data collected only until time of discharge Pain (VAS) recorded but not reported Oral intake recorded only as number of patient taking oral intake within first 3 postoperative hours |
|
| Notes | Anesthetic controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 133 patients age 2 to 12 years undergoing tonsillectomy or adenotonsillectomy (mixed technique) (63 steroid and 70 placebo) | |
| Interventions | Dexamethasone 0.5 mg/kg (max dose 8 mg) IV or normal saline | |
| Outcomes | Emesis 24 hours Outcomes not included: diet; pain |
|
| Notes | Anesthesia controlled; surgery not controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 58 patients age 1 to 18 years undergoing adenotonsillectomy (electrosurgery) (26 steroid and 32 placebo) | |
| Interventions | Dexamethasone 0.15 mg/kg (max dose 8 mg) IV or normal saline | |
| Outcomes | Emesis 24 hours Day 1 diet Day 3 diet Pain Analgesic doses recorded but not reported |
|
| Notes | Anesthesia not controlled; surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 49 patients age 4 to 12 years undergoing tonsillectomy or adenotonsillectomy (cold) (24 steroid and 25 placebo) | |
| Interventions | Dexamethasone 10 mg IV or normal saline | |
| Outcomes | Day 1 diet mean scores Day 3 diet mean scores Pain score Analgesic Outcomes not included: emesis |
|
| Notes | Anesthesia not controlled; surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Methods | Randomized, double‐blind, placebo‐controlled | |
| Participants | 41 patients age 2 to 12 years undergoing tonsillectomy or adenotonsillectomy (cold with electrosurgical hemostasis) (22 steroid and 19 placebo) | |
| Interventions | Dexamethasone 0.4 mg/kg (max dose 8 mg) IV or normal saline | |
| Outcomes | Emesis 24 hours Day 1 diet (by personal communication) Time (hours) to first solid diet Pain score Analgesic Outcomes not included: day 3 diet |
|
| Notes | Anesthesia not controlled; surgery controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not used |
IV: intravenous PACU: post‐anesthesia care unit VAS: visual analog scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alajmi 2008 | Allocation: not double‐blinded |
| Alavoine 1969 | Allocation: not randomized, double‐blinded and placebo‐controlled |
| Ammar 2009 | Interventions: not intraoperative IV administration |
| Anderson 1975 | Participants: not limited to children Interventions: not intravenous route (tonsil fossa injection) |
| Atherino 1966 | Interventions: not corticosteroid only (corticosteroid + antibiotic) Not intravenous route (tonsil fossa injection) |
| Bonaccorsi 1965 | Interventions: not corticosteroid only (steroid + antihistamine) |
| Carr 1999 | Participants: not limited to children |
| Celiker 2004 | Allocation: not placebo‐controlled |
| Cho 1998 | Interventions: not corticosteroid only (steroid + propofol or enflurane) |
| Cupero 2003 | Participants: not limited to children Not intravenous route (tonsil fossa injection) |
| Czarnetzki 2008 | Study was terminated for bleeding in steroid group; patients were recovered with ibuprofen |
| Del 1970 | Interventions: not corticosteroid only (estriol) Not intravenous route (topical) |
| Egeli 1997 | Participants: not limited to children Interventions: not intravenous route (tonsil fossa injection) |
| El Sabiee 2004 | Interventions: not corticosteroid only (steroid + ketamine versus droperidol + ketamine) |
| Ewah 2006 | Allocation: not randomized, double‐blind or placebo‐controlled |
| Fazel 2007 | Outcomes: none of the outcomes of interest were reported |
| Fujii 1996 | Interventions: not corticosteroid only (granisetron versus granisetron + steroid) |
| Gunter 2006 | Allocation: not placebo‐controlled |
| Hirunwiwatkul 2001 | Allocation: not randomized, double‐blinded and placebo‐controlled Participants: not limited to children Not tonsillectomy or adenotonsillectomy only (UP3) Interventions: not intravenous route (tonsil fossa injection) |
| Holt 2000 | Interventions: not corticosteroid only (tropisetron versus tropisetron + steroid) |
| Inci 2009 | Interventions: not corticosteroid only; not intravenous route (topical and systemic) |
| Karaman 2009 | Allocation: not double‐blinded |
| Kaygusuz 2003 | Interventions: not intravenous route (tonsil fossa injection) |
| Kerekhanjanarong 01 | Allocation: not randomized, double‐blinded and placebo‐controlled Interventions: not intravenous route (tonsil fossa injection) |
| Kim 2007 | Allocation: not placebo‐controlled |
| King 1967 | Interventions: not corticosteroid only Not intravenous route (tonsil fossa injection) |
| Liu 1996 | Allocation: not randomized, double‐blinded and placebo‐controlled Participants: not limited to children Interventions: not intravenous route (topical) |
| Malde 2005 | Participants: not limited to children |
| McKenna 1972 | Interventions: not corticosteroid only (antibiotic + anesthetic + steroid) Not intravenous route (tonsil fossa injection) |
| Mohamed 2009 | Interventions: not intravenous route (IV and tonsil fossa injection) |
| Montazeri 2009 | Interventions: not intravenous route (tonsil fossa injection) |
| Palme 2000 | Interventions: not single intravenous route of corticosteroid (oral) Participants: not limited to children |
| Papangelou 1972 | Allocation: not randomized, double‐blinded and placebo‐controlled Participants: not limited to children Interventions: not single intravenous route of corticosteroid (oral) |
| Pedersen 1967 | Interventions: not corticosteroid only (estriol) Not intravenous route (topical) |
| Rundle 1967 | Interventions: not corticosteroid only (antibiotic + anesthetic + steroid) Not intravenous route (tonsil fossa injection) |
| Schlorhaufer 1965 | Interventions: not intravenous route (terra cortyl spray) |
| Skvirskaia 1980 | Interventions: not corticosteroid only (oxycyclosol) Not intravenous route (topical) |
| Smith 1964 | Interventions: not corticosteroid only Not intravenous route (tonsil fossa injection) |
| Splinter 1997 | Allocation: not placebo‐controlled (perphenazine versus dexamethasone) |
| Stol'tser 1971 | Allocation: not randomized, double‐blinded and placebo‐controlled |
| Tewary 1993 | Participants: not limited to children |
| Vilar 1967 | Interventions: not corticosteroid only (estriol) Not intravenous route (topical) |
| Williams 1999 | Participants: not limited to children Not tonsillectomy or adenotonsillectomy only (UP3) |
IV: intravenous UP3: uvulopalatopharyngoplasty
Contributions of authors
Drs Steward and Grisel designed the study and developed the protocol according to the methods outlined in the 2003 Cochrane Review. Dr Grisel extracted the data from the original studies. Drs Steward, Grisel and Meinzen‐Derr made decisions regarding study inclusion or exclusion for each outcome measure. Dr Meinzen‐Derr performed the statistical analysis and wrote the results section. Drs Steward and Grisel wrote the discussion and conclusion based upon the results of the review.
Sources of support
Internal sources
Center for Epidemiology and Biostatistics at Cincinnati Children's Medical Center, Cincinnati, OH, USA.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Al Shehri AM. Prophylactic use of dexamethasone in tonsillectomy among children. Current Pediatric Research 2007;11(1‐2):3‐7. [Google Scholar]
- Aouad MT, Siddik SS, Rizk LB, Zaytoun GM, Baraka AS. The effect of dexamethasone on postoperative vomiting after tonsillectomy. Anesthesia and Analgesia 2001;92:636‐40. [DOI] [PubMed] [Google Scholar]
- April MM, Callan ND, Nowak DM, Hausdorff MA. The effect of intravenous dexamethasone in pediatric adenotonsillectomy. Archives of Otolaryngology ‐ Head & Neck Surgery 1996;122:117‐20. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D, Mazumdar S, Chowdhury S, Basu S, Saha S. Single dose IV dexamethasone with preemptive transdermal diclofenac patch reduces opioid requirement and postoperative morbidity following tonsillectomy. Journal of Anaesthesiology and Clinical Pharmacology 2009;25(1):29‐32. [Google Scholar]
- Catlin FI, Grimes WJ. The effect of steroid therapy on recovery from tonsillectomy in children. Archives of Otolaryngology ‐ Head & Neck Surgery 1991;117:649‐52. [DOI] [PubMed] [Google Scholar]
- Elhakim M, Naglaa MA, Rashed I, Riad MK, Refat M. Dexamethasone reduces postoperative vomiting and pain after pediatric tonsillectomy. Canadian Journal of Anaesthesia 2003;50(4):392‐7. [DOI] [PubMed] [Google Scholar]
- Giannoni C, White S, Enneking FK. Does dexamethasone with preemptive analgesia improve pediatric tonsillectomy pain. Otolaryngology ‐ Head and Neck Surgery 2002;126:307‐15. [DOI] [PubMed] [Google Scholar]
- Hanasono MM, Lalakea ML, Mikulec AA, Shepard KG, Wellis V, Messner AH. Perioperative steroids in tonsillectomy using electrocautery and sharp dissection techniques. Archives of Otolaryngology ‐ Head and Neck Surgery 2004;130(8):917‐21. [DOI] [PubMed] [Google Scholar]
- Kaan MN, Odabasi O, Gezer E, Daldal A. The effect of preoperative dexamethasone on early oral intake, vomiting and pain after tonsillectomy. International Journal of Pediatric Otorhinolaryngology 2006;70(1):73‐9. [DOI] [PubMed] [Google Scholar]
- Kaufmann M, Deutsch E, Hamouri H. The effect of steroid therapy on post adenotonsillectomy recovery. Harefuah 2006;145(8):577‐80. [PubMed] [Google Scholar]
- Khani A, Jaafarpour M, Khajavikhan J, Dyrekvandmogadam A. The effect of dexamethasone on morbidity related to vomiting, pain and oral intake in children after tonsillectomy. Journal of Clinical and Diagnostic Research 2009;3(4):1641‐6. [Google Scholar]
- Kyrou C, Papageorgiou‐Brousta M, Livanios S, Davou F, Malissiova A. Prevention of PONV with IV administration of metoclopramide‐dexamethasone in tonsillectomies and adenoidectomies in children. 2nd World Congress of Otorhinolaryngologic Allergy Endoscopy and Laser Surgery 2001;1:333‐7. [Google Scholar]
- Ohlms LA, Wilder RT, Weston B. Use of intraoperative corticosteroids in pediatric tonsillectomy. Archives of Otolaryngology ‐ Head & Neck Surgery 1995;121:737‐42. [DOI] [PubMed] [Google Scholar]
- Pappas AL, Sukhani R, Hotaling AJ, Mikat‐Stevens M, Javorski JJ, Donzelli J, et al. The effect of preoperative dexamethasone on the immediate and delayed postoperative morbidity in children undergoing adenotonsillectomy. Anesthesia and Analgesia 1998;87:57‐61. [DOI] [PubMed] [Google Scholar]
- Samarkandi AH, Shaikh MA, Ahmad RA, Alammar AY. Use of dexamethasone to reduce postoperative vomiting and pain after pediatric tonsillectomy procedures. Saudi Medical Journal 2004;25(11):1636‐9. [PubMed] [Google Scholar]
- Splinter WM, Roberts DJ. Dexamethasone decreases vomiting by children after tonsillectomy. Anesthesia and Analgesia 1996;83:913‐6. [DOI] [PubMed] [Google Scholar]
- Tom LW, Templeton JJ, Thompson ME, Marsh RR. Dexamethasone in adenotonsillectomy. International Journal of Pediatric Otorhinolaryngology 1996;37:115‐20. [DOI] [PubMed] [Google Scholar]
- Volk MS, Martin P, Brodsky L, Stanievich JF, Ballou M. The effects of preoperative steroids on tonsillectomy patients. Otolaryngology ‐ Head and Neck Surgery 1993;109:726‐30. [DOI] [PubMed] [Google Scholar]
- Vosdoganis F, Baines DB. The effect of single dose intravenous dexamethasone in tonsillectomy in children. Anaesthesia and Intensive Care 1999;27:489‐92. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Alajmi MA, Al Noumas HS, Al‐Abdulhadi KA, Kavitha G. Steroids for reducing post‐tonsillectomy morbidity. Kuwait Medical Journal 2008;40(3):211‐5. [Google Scholar]
- Alavoine J, Graber A. Extra‐capsular tonsillectomy under general anesthesia. Apropos of 100 cases [L'amygdalectomie extra‐capsulaire sous anasthesie generale. A propos de 100 cas]. Annales d'Oto‐Laryngologie et de Chirurgie Cervico‐Faciale 1969;86:305‐10. [PubMed] [Google Scholar]
- Ammar G, Wang Z. Effect of dexamethasone on posttonsillectomy pain. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2009;23(20):936‐8. [PubMed] [Google Scholar]
- Anderson HA, Rice BJ, Cantrell RW. Effects of injected deposteroid on posttonsillectomy morbidity: a double‐blind study. Archives of Otolaryngology 1975;101:86‐8. [DOI] [PubMed] [Google Scholar]
- Atherino T. Results obtained with a new antibiotic‐antiphlogistic‐anesthetic combination in the postoperative period following amygdalectomy [Resultados obtidos com uma nova associacao antibiotica, antiflogistica e anestesica, no pos‐operatorio de amidalectomias]. Hospital (Rio de Janeiro, Brazil) 1966;69:737‐42. [PubMed] [Google Scholar]
- Bonaccorsi P. The combination of corticosteroids with antihistaminics in tonsillectomies and adenotonsillectomies [Associazione di corticosteroidi con antistaminici nelle tonsillectomie e adenotonsillectomie]. Archivio Italiano di Otologia, Rinologia e Laringologia 1965;76:764‐81. [PubMed] [Google Scholar]
- Carr MM, Williams JG, Carmichael L, Nasser JG. Effect of steroids on posttonsillectomy pain in adults. Archives of Otolaryngology ‐ Head & Neck Surgery 1999;125:1361‐4. [DOI] [PubMed] [Google Scholar]
- Celiker V, Celebi N, Cathbay O, Basguel E, Aypar U. Minimum effective dose of dexamethasone after tonsillectomy. Paediatric Anaesthesia 2004;14(8):666‐9. [DOI] [PubMed] [Google Scholar]
- Cho SY, Cho SH, Kim KH, Kim DW, Jun JH, Kim KS. Dexamethasone administration and propofol anesthesia prevent postoperative nausea and vomiting. Korean Journal of Anesthesiology 1998;34(3):630‐5. [Google Scholar]
- Cupero TM, Kim SY, Silva AB. The effects of a preoperative steroid/anesthetic injection on post‐tonsillectomy pain. Ear, Nose and Throat Journal 2003;82(4):305‐8. [PubMed] [Google Scholar]
- Czarnetzki C, Elia N, Lysakowski C, Dumont L, Landis BN, Giger R, et al. Dexamethasone and risk of nausea and vomiting and postoperative bleeding after tonsillectomy in children: a randomized trial. JAMA 2008;300(22):2621‐30. [DOI] [PubMed] [Google Scholar]
- Villar R, Bejar I. Succinil estriol in bleeding due to adenoidectomy and tonsillectomy. Eye, Ear, Nose and Throat Monthly 1970;49:234‐5. [PubMed] [Google Scholar]
- Egeli E, Akkaya S. The effect of peritonsillar corticosteroid infiltration in tonsillectomy. Auris, Nasus, Larynx 1997;24:179‐83. [DOI] [PubMed] [Google Scholar]
- Sabiee SA. Dexamethasone‐low dose ketamine combination versus droperidol low dose ketamine. A comparative study for their effect on postoperative nausea and vomiting and swallowing evoked pain after paediatric adenotonsillectomy. Scientific Journal of Al‐Azhar Medical Facility (Girls) 2004;25(1):1775‐80. [Google Scholar]
- Ewah B, Robb PJ, Raw M. Postoperative pain, nausea and vomiting following paediatric day‐case tonsillectomy. Anaesthesia 2006;61(2):116‐22. [DOI] [PubMed] [Google Scholar]
- Fazel MR, Yegane MA, Forghani Z, Aghadoost D, Mahdian M, Fakharian E. The effect of dexamethasone on postoperative vomiting and oral intake after adenotonsillectomy. International Journal of Pediatric Otorhinolaryngology 2007;71(8):1235‐8. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Tanaka H, Toyooka H. Granistron and dexamethasone provide more improved prevention of postoperative emesis than granisetron alone in children. Canadian Journal of Anaesthesia 1996;43:1229‐32. [DOI] [PubMed] [Google Scholar]
- Gunter JB, McAuliffe JJ, Beckman EC, Wittkugel EP, Spaeth JP, Varughese AM. A factorial study of ondansetron, metoclopramide and dexamethasone for emesis prophylaxis after adenotonsillectomy in children. Paediatric Anaesthesia 2006;16(11):1153‐65. [DOI] [PubMed] [Google Scholar]
- Hirunwiwatkul P. Pain‐relieving effect of local steroid injection in uvulopalatopharyngoplasty. Journal of the Medical Association of Thailand 2001;84(Suppl 1):S384‐90. [PubMed] [Google Scholar]
- Holt R, Rask P, Coulthard KP, Sinclair M, Roberts G, Walt J, et al. Tropisetron plus dexamethasone is more effective than tropisetron alone for the prevention of postoperative nausea and vomiting in children undergoing tonsillectomy. Paediatric Anaesthesia 2000;10:181‐8. [DOI] [PubMed] [Google Scholar]
- Inci N, Basut O, Kasapoglu F, Coskun H. Management of pain after tonsillectomy: a prospective, randomized clinical study. Kulak Burun Bogaz Ihtisas Dergisi 2009;19(1):1‐8. [PubMed] [Google Scholar]
- Karaman M, Ilhan AE, Dereci G, Tek A. Determination of optimum dosage of intraoperative single dose dexamethasone in pediatric tonsillectomy and adenotonsillectomy. International Journal of Pediatric Otorhinolaryngology 2009;73(11):1513‐5. [DOI] [PubMed] [Google Scholar]
- Kaygusuz I, Susaman N. The effects of dexamethasone, bupivacaine and topical lidocaine spray on pain after tonsillectomy. International Journal of Pediatric Otorhinolaryngology 2003;67(7):737‐42. [DOI] [PubMed] [Google Scholar]
- Kerekhanjanarong V, Tang‐On N, Supiyaphun P, Sastarasadhit V. Tonsillar fossa steroid injection for reduction of the post‐tonsillectomy pain. Journal of the Medical Association of Thailand 2001;84(Suppl 1):S391‐5. [PubMed] [Google Scholar]
- Kim MS, Cote CJ, Cristoloveanu C, Roth AG, Vornov P, Jennings MA, et al. There is no dose‐escalation response to dexamethasone (0.0625‐1.0 mg/kg) in pediatric tonsillectomy or adenotonsillectomy patients for preventing vomiting, reducing pain, shortening time to first liquid intake, or the incidence of voice change. Anesthesia and Analgesia 2007;104(5):1052‐8. [DOI] [PubMed] [Google Scholar]
- King JT. Alleviation of pain and prevention of infection after tonsillectomy. Transactions ‐ Indiana Academy of Ophthalmology and Otolaryngology 1967;50:25‐8. [PubMed] [Google Scholar]
- Liu CM, Su CY. Post‐operative pain control with topical steroid injection after hot dissection tonsillectomy. Journal of Laryngology and Otology 1996;110:1038‐40. [DOI] [PubMed] [Google Scholar]
- Malde AD, Sonawane VS, Jagtap SR. Effect of dexamethasone on post tonsillectomy morbidities. Indian Journal of Anesthesia 2005;49(3):202‐7. [Google Scholar]
- McKenna EL. Distress after tonsillectomy. A clinical trial of injected penicillin anesthetic steroid mixture. Transactions ‐ Pennsylvania Academy of Ophthalmology and Otolaryngology 1972;25:109‐12. [PubMed] [Google Scholar]
- Mohamed SK, Ibraheem AS, Abdelraheem MG. Preoperative intravenous dexamethasone combined with glossopharyngeal nerve block: role in pediatric postoperative analgesia following tonsillectomy. European Archives of Otolaryngology 2009;266(11):1815‐9. [DOI] [PubMed] [Google Scholar]
- Montazeri K, Okhovat A, Honarmand A, Safavi MR, Ashrafy L. Pre‐incisional infiltration of tonsils with dexamethasone dose not reduce posttonsillectomy vomiting and pain in children. Saudi Journal of Anesthesia 2009;3(2):53‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme CE, Tomasevic P, Pohl DV. Evaluating the effects of oral prednisolone on recovery after tonsillectomy: a prospective, double‐blind, randomized trial. Laryngoscope 2000;110:2000‐4. [DOI] [PubMed] [Google Scholar]
- Papangelou L. Steroid therapy in tonsillectomy. Laryngoscope 1972;82:297‐301. [DOI] [PubMed] [Google Scholar]
- Pedersen OT, Nielsen PH. Oestriol succinate. Its effect on the haemorrhage in adenotonsillectomy and tonsillectomy. Acta Oto‐Laryngologica 1967;64:65‐71. [DOI] [PubMed] [Google Scholar]
- Rundle FW. Post‐tonsillectomy morbidity: a clinical trial of a local penicillin‐steroid‐anesthetic mixture. Annals of Otology, Rhinology, and Laryngology 1967;76:1060‐6. [DOI] [PubMed] [Google Scholar]
- Schlorhaufer W, Seeber F, Semenitz E. Studies on the postoperative course of tonsillectomy. (Based on clinical and bacteriological principles and using Terra‐Cortyl sprays) [Untersuchungen zum postoperativen Verlauf nach Tonsillektomie. (Nach klinischen uns bakteriologischen Gesichtspunkten und unter Verwendung des Terra‐Cortril‐Sprays)]. Monatsschrift fur Ohrenheilkunde und Laryngo‐Rhinologie 1965;99:309‐20. [PubMed] [Google Scholar]
- Skvirskaia AA, Mamon MV, Guslistyi AV. Local use of oxycyclosol after tonsillectomy [Mestnoe primenenie oksitsiklozolia posle tonzillektomii]. Zhurnal Ushnykh, Nosovykh i Gorlovykh Boleznei 1980;1:66‐7. [PubMed] [Google Scholar]
- Smith J, King J, Gershon N. Alleviation of pain and prevention of infection after tonsillectomy: a controlled clinical study of a novel injectable combination. Transactions ‐ American Academy of Ophthalmology and Otolaryngology 1964;68:65‐9. [PubMed] [Google Scholar]
- Splinter W, Roberts DJ. Prophylaxis for vomiting by children after tonsillectomy: dexamethasone versus perphenazine. Anesthesia and Analgesia 1997;85:534‐7. [DOI] [PubMed] [Google Scholar]
- Stol'tser IM. Tonsillectomy in patients treated with corticosteroids [Tonzillektomiia u bol'nykh, lechennykh kortikosteroidami]. Vestnik Otorinolaringologii 1971;33:52‐7. [PubMed] [Google Scholar]
- Tewary A, Cable H, Barr G. Steroids and control of post‐tonsillectomy pain. Journal of Laryngology and Otology 1993;107:605‐6. [DOI] [PubMed] [Google Scholar]
- Vilar P. Action of estriol on postoperative hemorrhage in tonsillectomy and adenotonsillectomy [Accion del estriol sobre la hemorragia peroperatoria en la migdalectomia y en la adenoamigdalectomia]. Acta Oto‐Rino‐Laringologica Ibero‐Americana 1967;18:223‐31. [PubMed] [Google Scholar]
- Williams PM, Strome M, Eliachar I, Lavertu P, Wood BG, Vito KJ. Impact of steroids on recovery after uvulopalatopharyngoplasty. Laryngoscope 1999;109:1941‐6. [DOI] [PubMed] [Google Scholar]
Additional references
- Alsarraf R, Alsarraf N, Kato M, Goldman N. Meta‐analysis in otolaryngology. Archives Otolaryngology ‐ Head & Neck Surgery 2000;126:711‐6. [DOI] [PubMed] [Google Scholar]
- Boissel J, Blanchard J, Panak E, Peyrieux J, Sacks H. Considerations for the meta‐analysis of randomized clinical trials: summary of a panel discussion. Controlled Clinical Trials 1989;10:254‐81. [DOI] [PubMed] [Google Scholar]
- Brigger MT, Cunningham MJ, Hartnick CJ. Dexamethasone administration and postoperative bleeding risk in children undergoing tonsillectomy. Archives of Otolaryngology ‐ Head and Neck Surgery 2010;136(8):766‐72. [DOI] [PubMed] [Google Scholar]
- Brunton L. Agents affecting gastrointestinal water flux and motility. In: Hardman J, Limbird L editor(s). Goodman and Gilman's The Pharmacological Basis of Therapeutics. McGraw‐Hill, 1996:931. [Google Scholar]
- Chalmers T, Smith Jr H, Blackburn B, Silverman B, Schroeder B, Reitman D, et al. A method for assessing the quality of a randomized control trial. Controlled Clinical Trials 1981;2:31‐49. [DOI] [PubMed] [Google Scholar]
- Douketis JD, Berger PB, Dunn AS for the American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest 2008;133(Suppl 6):299S‐339S. [DOI] [PubMed] [Google Scholar]
- Felson DT, Anderson JJ. Across‐study evaluation of association between steroid dose and bolus steroid and avascular necrosis of bone. Lancet 1987;18(1):902‐6. [DOI] [PubMed] [Google Scholar]
- Goldman AC, Govindaraj S, Rosenfeld RM. A meta‐analysis of dexamethasone use with tonsillectomy. Otolaryngology ‐ Head and Neck Surgery 2000;123:682‐6. [DOI] [PubMed] [Google Scholar]
- Gunter JB, Willging JP, Myer CM 3rd. Dexamethasone and postoperative bleeding after tonsillectomy in children. JAMA 2009;301(17):1765‐6. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesthesia and Analgesia 2000;90:186‐94. [DOI] [PubMed] [Google Scholar]
- Hesketh P, Harvey W, Harker W, Beck TM, Ryan T, Bricker LJ, et al. A randomized, double‐blind comparison of intravenous ondansetron alone and in combination with intravenous dexamethasone in the prevention of high‐dose cisplatin‐induced emesis. Journal of Clinical Oncology 1994;12:596‐600. [DOI] [PubMed] [Google Scholar]
- Randall D, Hoffer M. Complications of tonsillectomy and adenoidectomy. Otolaryngology ‐ Head and Neck Surgery 1998;118:61‐8. [DOI] [PubMed] [Google Scholar]
- Research TIGfA. Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. New England Journal of Medicine 1995;332:1‐5. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
- Rosenthal R. The "file drawer problem" and tolerance for null results. Psychological Bulletin 1979;86:638‐41. [Google Scholar]
- Saks H, Berrier J, Reitman D, Ancona‐Berk V, Chalmers T. Meta‐analyses of randomized controlled trials. New England Journal of Medicine 1987;316:450‐5. [DOI] [PubMed] [Google Scholar]
- Schimmer B, Parker K. ACTH; Adrenocortical steroids and their synthetic analogs. In: Hardman J, Limbird L editor(s). Goodman and Gilman's The Pharmacological Basis of Therapeutics. McGraw‐Hill, 1996:1459‐85. [Google Scholar]
- Shakeel M, Trinidade A, Al‐Adhami A, Karamchandani D, Engelhardt T, Ah‐See KW, et al. Intraoperative dexamethasone and the risk of secondary posttonsillectomy hemorrhage. Journal of Otolaryngology ‐ Head and Neck Surgery 2010;39(6):732‐6. [PubMed] [Google Scholar]
- Sharpe D. Of apples and oranges, file drawer and garbage: why validity issues in meta‐analysis will not go away. Clinical Psychology Review 1997;17:881‐901. [DOI] [PubMed] [Google Scholar]
- Steward D, Chung S. The role of adjuvant therapies and techniques in tonsillectomy. Current Opinion in Otolaryngology & Head and Neck Surgery 2000;8:186‐92. [Google Scholar]
- Yaman H, Belada A, Yilmaz S. The effect of ibuprofen on postoperative hemorrhage following tonsillectomy in children. European Archives of Otorhinolaryngology 2011;268(4):615‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Afman CE, Welge JA, Steward DL. Steroids for post‐tonsillectomy pain reduction: meta‐analysis of randomized controlled trials. Otolaryngology ‐ Head and Neck Surgery 2006;134(2):181‐6. [DOI] [PubMed] [Google Scholar]
- Steward DL, Welge JA, Myer CM. Do steroids reduce morbidity of tonsillectomy? Meta‐analysis of randomized trials. Laryngoscope 2001;111:1712‐8. [DOI] [PubMed] [Google Scholar]
- Steward DL, Welge JA, Myer CM. Steroids for improving recovery following tonsillectomy in children. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003997] [DOI] [PubMed] [Google Scholar]


