Abstract
Background
Most patients with acute myeloid leukaemia are older, with many unsuitable for conventional chemotherapy. Low dose Ara-C (LDAC) is superior to best supportive care but is still inadequate. The combination of arsenic trioxide (ATO) and LDAC showed promise in an un-randomised study. We report a randomised trial of LDAC versus LDAC+ATO.
Methods
Patients with AML according to WHO criteria or myelodysplastic syndrome with >10% blasts, considered unfit for conventional chemotherapy, were randomised between subcutaneous ara-c (20mg b.d. for 10 days) and the same LDAC schedule with ATO (0.25mg/kg) on days 1-5,9,11, for at least 4 courses every 4 to 6 weeks. Overall 166 patients were entered; the trial was terminated on the advice of the DMC since the projected benefit was not observed.
Results
Overall 14% of patients achieved complete remission (CR) and 7% CRi. Median survival was 5.5 months, and 19 months for responders (CR: not reached; CRi: 14 months; non-responders: 4 months). There were no differences in response or survival between the arms. Grade 3/4 cardiac and liver toxicity, and supportive care requirements were greater in the ATO arm.
Conclusions
This randomised comparison demonstrates that adding ATO to LDAC provides no benefit for older patients with AML.
AML is a disease most frequently seen in older patients, many of whom are not considered likely to benefit from, or do not wish to receive conventional chemotherapy1,2. In recent years there has been increasing interest in developing better treatments for this patient group while recognising the limitations determined by age and comorbidity. In order to develop a basic “standard of care” for such patients we previously showed in a randomised fashion that low dose Ara-C (LDAC) could be effective in a minority of patients providing a complete remission rate of 18%, when compared with best supportive care and Hydroxycarbamide to control the peripheral white count3. Although there was acceptable benefit for the minority who achieved CR, with a median duration of CR of 15 months, there was no apparent benefit for patients who did not achieve CR. However LDAC did not increase supportive care requirement, episodes of sepsis, or hospitalisation. In that study 24% of patients given LDAC had poor risk cytogenetics, none of whom entered CR. We concluded from that study that achievement of CR was a useful surrogate for clinical benefit. On this basis, we developed a strategic approach to treatment development in this population by incorporating a randomised “pick a winner” approach – the operational characteristics of which will be discussed elsewhere – whereby several novel treatment options could be simultaneously compared to LDAC, and those which significantly improved the remission rate could continue to be assessed with overall survival as the principal endpoint.
A combination of LDAC and Arsenic Trioxide was developed by Roboz and colleagues4 for the treatment of this AML patient population and for patients with Myelodysplastic syndrome, and showed an encouraging 34% rate of complete remission and 5 month median survival in an unrandomised phase 2 trial. This appeared to be superior to what is seen with LDAC alone, while ATO as a single agent was ineffective in a previous small study5. While dramatically effective in preclinical and clinical treatment of acute promyelocytic leukaemia, there is also some preclinical evidence that AML cell lines have sensitivity to ATO6.
For these reasons we decided to incorporate this combination as one of the options in our “pick a winner” design where the independent data monitoring and ethics committee's remit was to terminate the trial early if the aspiration to improve survival by improving the remission rate to over 30% was unlikely to be achieved. To progress beyond 50 assessable patients per arm, an improvement in remission rate of at least 2.5% was required; to progress beyond 100 patients per arm, the necessary improvement was set at 7.5%.
Materials and Methods
Patient Population
Between May 2007 and May 2009, 166 patients entered the randomisation. There were no a priori restrictions in relation to co-morbidity, in particular, to cardiac function. The median age was 74 years, and the demographic characteristics were balanced between the arms (table 1). Contemporaneously, patients and investigators could have entered the intensive chemotherapy arm of the AML16 trial. The reason why they did not opt for intensive treatment and details of co-morbidity (using the Sorror index7 components) were collected at entry. In 64% of cases patients chose or were allocated non-intensive treatment on the grounds of age; 60% gave fitness as a reason, and 15% gave other reasons (mainly comorbidity). In 29% and 26% of cases respectively were age and fitness chosen as the only reasons. For patients 75 years and older, the primary reasons were age (76%), fitness (64%) and other reasons (9%), with 45% of patients specifying both age and fitness, while in patients <75 years the primary reasons were age (51%), fitness (56%), and other reasons (21%); here 20% of patients specified both age and fitness. The distribution of patients by the multi-parameter risk score (Wheatley score8) over and under 75 years was 4% vs 2% favourable: 42% vs 45% intermediate ; and 55% vs 52% poor risk. This validated score predicted a 12 month survival of 36%, 42% and 14% for LDAC in the three risk groups. Of the co-morbidities listed, most frequent were those described as cardiac, in 25% of patients. Diagnosis and response definitions were designated by the local investigator. Cytogenetic and immunophenotypic characterisation was carried out in regional reference laboratories who participate in national quality assurance schemes. Patients were required to give written consent and the trial was approved by the Wales Research Ethics Committee.
Table 1.
Patient Demographics
| LDAC + ATO | LDAC | p-value | |
|---|---|---|---|
| Number of patients | 84 | 82 | |
| Age group | 0.6* | ||
| <60 | 2 | 1 | |
| 60-64 | 3 | 3 | |
| 65-69 | 13 | 12 | |
| 70-74 | 25 | 25 | |
| 75-79 | 28 | 25 | |
| 80+ | 13 | 16 | |
| Median (range) | 74 (36-86) | 74.5 (58-89) | |
| Sex | 1.0 | ||
| Female | 32 | 31 | |
| Male | 52 | 51 | |
| Type of disease | 1.0 | ||
| de Novo AML | 52 | 50 | |
| Secondary AML | 19 | 19 | |
| High risk MDS | 13 | 13 | |
| Performance Status | 0.13* | ||
| Grade 0 | 22 | 27 | |
| Grade 1 | 48 | 47 | |
| Grade 2 | 8 | 5 | |
| Grade 3 | 5 | 3 | |
| Grade 4 | 1 | 0 | |
| White blood count (×109/l) | 0.8* | ||
| 0-9.9 | 51 | 49 | |
| 10-49.9 | 22 | 24 | |
| 50-99.9 | 8 | 8 | |
| 100+ | 3 | 1 | |
| Median (range) | 6.0 (0.6-154.0) | 4.1 (0.6-170) | |
| Cytogenetics | 1.0* | ||
| Favourable | 1 | 2 | |
| Intermediate | 51 | 38 | |
| Adverse | 17 | 15 | |
| Unknown | 15 | 27 | |
| Wheatley Index | 0.9* | ||
| Good | 3 | 2 | |
| Standard | 35 | 37 | |
| Poor | 46 | 43 | |
|
Reason for non-intensive treatment |
|||
| Age | 52/80 (65%) | 50/80 (63%) | 0.7 |
| Fitness | 50/80 (63%) | 46/80 (58%) | 0.4 |
| Other | 11/80 (14%) | 13/80 (16%) | 0.7 |
| “Patient preference” | 5 | 8 | |
| “Clinician choice” | 1 | 1 | |
| Secondary disease | 1 | 0 | |
| Adverse prognostic factors | 1 | 0 | |
| Previous BMT | 1 | 0 | |
| Previous CABG | 1 | 1 | |
| Previous cancer | 0 | 1 | |
| Severe RA | 0 | 1 | |
| Site policy | 0 | 0 | |
| Not known | 1 | 1 | |
|
Presence of comorbidities (number with known answer) |
|||
| Arrhythmia | 6/76 | 8/77 | 0.6 |
| Cardiac | 24/81 | 17/80 | 0.2 |
| Cerebrovascular | 6/81 | 5/79 | 0.8 |
| Diabetes | 7/81 | 10/80 | 0.4 |
| Mild Hepatic | 2/80 | 3/80 | 0.7 |
| Moderate/Severe Hepatic | 2/80 | 0/80 | 0.16 |
| Heart valve disease | 2/81 | 3/80 | 0.6 |
| Inflammatory bowel disease | 3/80 | 1/79 | 0.3 |
| Infection | 8/82 | 10/80 | 0.6 |
| Obesity | 5/82 | 6/80 | 0.7 |
| Peptic ulcer | 1/80 | 2/80 | 0.6 |
| Prior solid tumour | 5/81 | 9/80 | 0.3 |
| Psychiatric disturbance | 2/80 | 3/79 | 0.6 |
| Severe pulmonary | 3/80 | 1/80 | 0.3 |
| Moderate/severe renal | 4/80 | 2/80 | 0.4 |
| Rheumatolgical | 5/81 | 5/80 | 1.0 |
| Other | 23/78 | 27/70 | 0.2 |
Mantel-Haenszel test for trend; chi-squared test otherwise
Treatment
The treatment schedule for LDAC was 20mgs b.i.d for 10 days (20 doses) given subcutaneously frequently at home by the patients or their carer, which was repeated after an interval of 4 to 6 weeks, with the intention to deliver four courses. For the ATO arm the same schedule of LDAC was given. ATO was given in a dose of 0.25 mg/kg on days 1-5, 9 and 11. The aim was to deliver four courses. Patients were permitted to receive more than four courses of either treatment if they were deriving benefit.
Toxicity
Adverse events and toxicity was recorded as defined by the NCI CRC version 3
Definitions of endpoints
The protocol defined complete remission (CR) as a normocellular bone marrow aspirate containing <5% leukaemic blasts and showing evidence of normal maturation of other marrow elements. Persistence of myelodysplastic features did not preclude the diagnosis of CR. Although not in the original protocol, in this report, to achieve CR patients required neutrophil recovery to 1.0×109/l and platelets to 100×109/l, without evidence of extramedullary disease. Patients who achieved CR according to the protocol, but without recovery are denoted here as CRi.
Following the international guidelines9 , overall survival (OS) is defined as the time from randomization to death. For remitters, survival from CR is defined as the time from CR/CRi (first report) until death. Survival percentages are quoted at 1 year.
Statistical methods
All analyses are by intention-to-treat. Surviving patients were censored at 1st January 2010 with complete follow-up on all but 6 patients. Patients lost to follow-up are censored at the date last known to be alive. Median follow-up for survival is 19 months (longest survivor 27 months).
Categorical endpoints (e.g. CR rates) were compared using Mantel-Haenszel tests, giving Peto odds ratios and confidence intervals. Continuous variables were analysed by parametric (t-test) or non-parametric (Wilcoxon rank sum) tests as appropriate. Time-to-event outcomes were analysed using the log-rank test, with Kaplan-Meier survival curves. Odds/hazard ratios (OR/HR) less than 1 indicate benefit for investigational therapy.
In addition to overall analyses, subgroup analyses were performed by the randomization stratification parameters (age, performance status, white blood cell count, and type of disease) and other important variables (e.g. cytogenetics, other treatments randomised), with suitable tests for interaction.
Trial Design
The “Pick A Winner” design aims to rapidly sift new treatments based on the assumption that small to moderate differences in outcome are unlikely to be worthwhile in this patient population. Its design is effectively a special case of the “Multi Arm Multi Stage” (MAMS) design of Royston et al.10 Briefly, patients entering the trial are randomised between LDAC (as standard of care) and a number of experimental treatment arms. Some patients may not be eligible for certain arms of the trial – they will only be randomised between those arms for which they are eligible. All novel treatments are compared against a standard of care (LDAC) in randomised comparisons. Under the rules of the “pick-a-winner” design, the DMEC examined data once CR information was complete for 100 patients; recruitment was not stopped in the interim. If the treatment does not look sufficiently promising it is discarded. Only those treatments which pass the interim analyses continue to a full trial of 200 patients per arm with overall survival as primary endpoint.
AML16 was designed based on the need to see a doubling of remission rates 15% to 30% in order for there to be a sufficiently large improvement in survival. The effect of different cut-offs for success at 50 and 100 patients was simulated using 150,000 simulated clinical trials of 400 patients each. In order to balance losing power by being too strict on one hand, and continuing with worthless treatments if too lax on the other, atrade off between power and sample size was accepted at a 2.5% improvement in CR rate at 50 patients per arm, and a 7.5% improvement in CR rate after 100 patients per arm. This provides power to detect a doubling of remission rates of 85% at p<0.05; a worthless treatment would recruit on average 74 patients.
Results
A total of 166 patients (LDAC n=82; LDAC+ATO n=84) were recruited from 69 centres. At this point, the independent DMEC recommended closure because the necessary 2.5% improvement in ORR had not been observed on the first 100 evaluable patients.
Treatment compliance
Patients allocated to LDAC received a median of 2.5 (range 0-8) courses, and those allocated to LDAC and ATO received 2.0 (range 0-8) courses. The number of courses received for LDAC and ATO was 0= 5;1=32; 2=16; 3=6; 4=20; 5=1; 6=3 ; 7=0; 8=1; and for LDAC was 0=8; 1=25 ; 2=8; 3=6; 4=21; 5=1; 6=7; 7=1; 8=5: there was no statistically significant difference between groups (p=0.19 by Wilcoxon rank sum test).
Outcome data
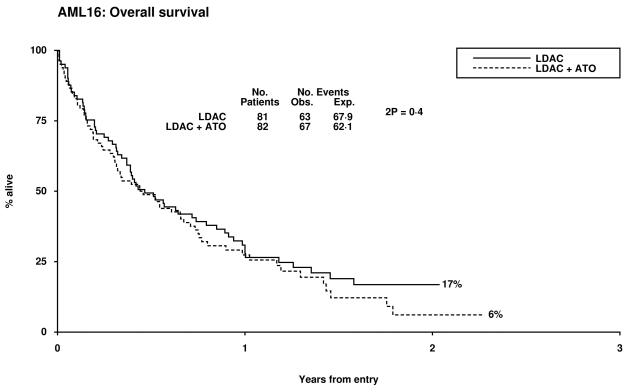
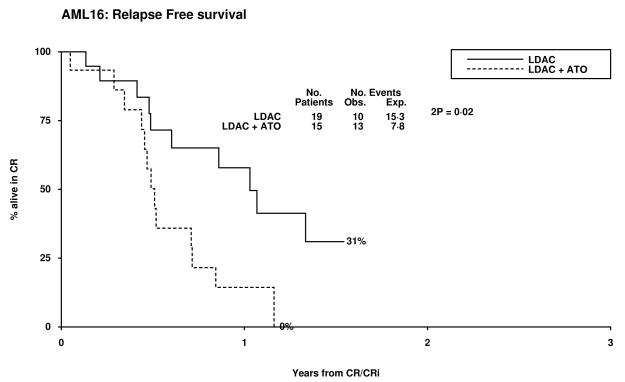
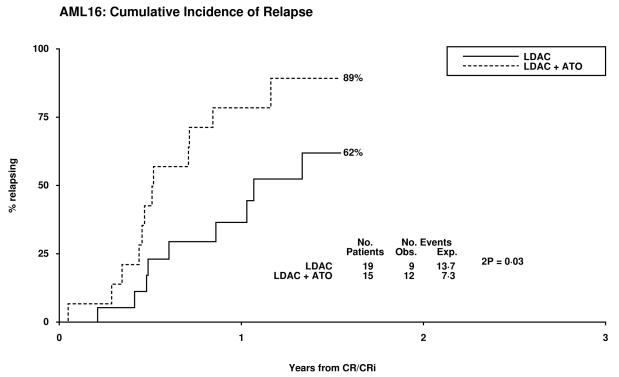
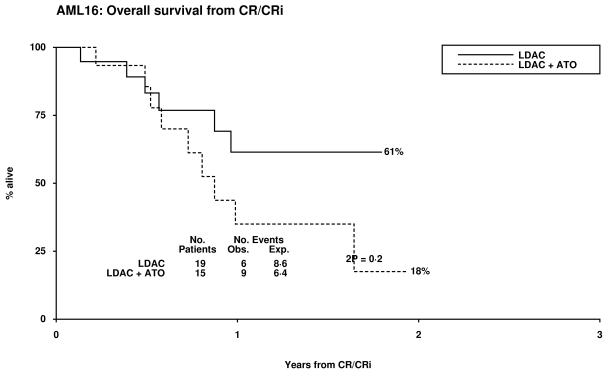
Overall 14% of patients achieved CR and 7% a CRi, resulting in an ORR of 21%. The median survival of the patients who responded was 19 months (CR median not reached and 14 months for CRi). Two of 32 patients with adverse cytogenetics obtained a CR, both in the LDAC alone arm. There was no significant difference between the treatment arms with respect to CR, CRi, 30 day and 8 week all cause mortality, (Table 2). There was a trend for poorer survival at 12 months in the LDAC + ATO arm which was also noted in the survival for patients who entered CR (Table 3, Figure 1). This appeared to be due to an increased risk of relapse (79% v 37% at 12 months, p=0.02). Overall survival at 12 months was inferior in patients whose best recorded response was CRi compared with CR (53% vs 85% p=0.001). When assessed within various subsets there was no evidence of heterogeneity of response or overall survival (Supplementary figures 1-3).
Table 2.
Grade 3 or 4 Toxicities
| Toxicity | Course | % Grade 3 or 4 | p-value for difference in grade |
|
|---|---|---|---|---|
| LDAC + ATO | LDAC | |||
| Nausea/Vomiting | 1 | 7% | 1% | 0.08 |
| 2 | 2% | 0 | 0.8 | |
| Diarrhoea | 1 | 1% | 3% | 0.01 |
| 2 | 0 | 2% | 1.0 | |
| Oral | 1 | 4% | 0 | 0.8 |
| 2 | 2% | 0 | 0.18 | |
| Cardiac | 1 | 9% | 6% | 0.001 |
| 2 | 2% | 0 | 0.01 | |
| Liver AST | 1 | 10% | 3% | 0.02 |
| 2 | 0 | 0 | 0.16 | |
| Liver ALT | 1 | 4% | 1% | 0.04 |
| 2 | 0 | 0 | 0.7 | |
| Liver Bilirubin | 1 | 4% | 1% | 0.6 |
| 2 | 0 | 2% | 1.0 | |
Table 3.
Clinical Outcome Data
| Outcome | LDAC+ATO | LDAC | OR/HR and 95% confidence interval |
p-value |
|---|---|---|---|---|
| CR | 12% | 15% | 1.25 (0.51-3.06) | 0.6 |
| CRi | 6% | 9% | ||
| CR/CRi | 18% | 23% | 1.36(0.64-2.90) | 0.4 |
| 30-day mortality | 16% | 15% | ||
| 8-week mortality | 24% | 25% | ||
| 12 month OS | 27% | 38% | 1.17 (0.83-1.65) | 0.4 |
| 12 month survival from CR |
35% | 61% | 2.07 (0.74-5.82) | 0.17 |
| 12 month Relapse Free Survival |
14% | 58% | 2.95 (1.21-7.18) | 0.02 |
| 12 month cumulative incidence of relapse |
79% | 37% | 3.20 (1.25-8.16) | 0.02 |
| 12 month cumulative incidence of death in CR |
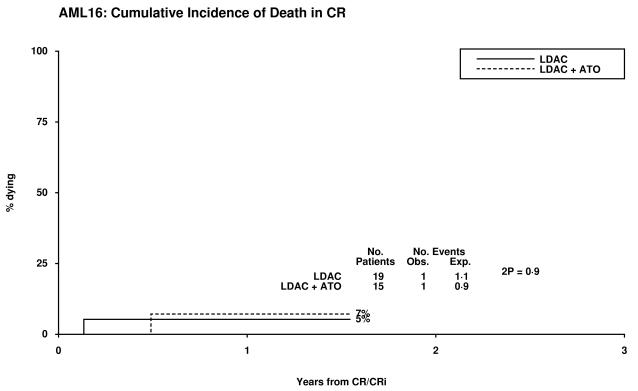
7% | 5% | 1.44 (0.09-24.0) | 0.8 |
Figure 1.
Patient Outcomes by Random Allocation: (a) Overall Survival, (b) Relapse Free Survival, (c) Cumulative Incidence of Relapse, (d) Cumulative Incidence of Death without Relapse, (e) Survival from CR/CRi
Grade 3 and 4 cardiac and liver toxicity was significantly greater in the ATO arm, the development of which was unrelated to the pre-treatment co-morbidity (Table 2). Requirements for supportive care were increased in the ATO arm particularly after course 2 (Table 4). It is not possible to say whether the excess days in hospital on the ATO arm was due to increased complexity of treatment or due to excess toxicity.
Table 4.
Resource Usage and Supportive Care
| Randomisation |
||||
|---|---|---|---|---|
| Type of Care | Course | LDAC+ ATO |
LDAC | 2p- value* |
| Blood (mean units) | 1 | 7.3 | 6.2 | 0.4 |
| 2 | 6.4 | 5.2 | 0.3 | |
| Platelets (mean units) | 1 | 6.4 | 3.1 | 0.02 |
| 2 | 3.3 | 2.3 | 0.19 | |
| Antibiotics (mean days) | 1 | 10.6 | 6.1 | 0.005 |
| 2 | 6.6 | 2.5 | 0.008 | |
| Day visits to hospital (mean) | 1 | 3.7 | 3.5 | 0.6 |
| 2 | 5.2 | 4.8 | 0.7 | |
| Nights in hospital (mean) | 1 | 20.2 | 13.0 | 0.003 |
| 2 | 15.5 | 6.9 | 0.002 | |
Discussion
Since the majority of patients with AML are older, and are poorly represented in collaborative group trials, it is important to develop treatment options beyond best supportive care. An unresolved dilemma is how to distinguish patients who are in this category rather than candidates for conventional chemotherapy. It is important first of all to distinguish between patients who are not considered fit because of some parameter of “frailness”, as opposed to patients who are unlikely to benefit because of adverse disease related features, such as poor risk cytogenetics. While there will inevitably be some overlap between these patient definitions, patients in this trial where not entered on the basis of cytogenetics. Scoring systems have evolved which help to characterise patients and give an indication of expected treatment outcome7,8,11. In this trial over 50% of patients were identified as being in the poorest group based on our validated risk score, and would have had an expected 12 month survival of 14%. Contemporaneously, investigators entered patients in to an intensive treatment option of whom 38% were in the poorest risk group.
The lack of benefit of adding ATO to low dose Ara-C is clear in this study, with no additional remissions, or benefit in overall survival. Additionally, subgroup analyses identified no subgroups of patients who might derive benefit from ATO therapy. Because of the well-known dangers of subgroup analysis, these were interpreted cautiously. In our previous trial LDAC no patient with poor risk chromosomes entered remission and therefore did not benefit which likely contributes to the reluctance to adopt LDAC as a standard of care in this patient group. In this trial 32 patients had poor risk cytogenetics of whom 6% had a overall response (CR=3%: CRi=3%). Of 276 patients recruited to alI options of our pick a winner programme who received LDAC during the same time period with cytogenetic information, 71 had poor risk cytogenetics of whom 14% responded. While the outcome using LDAC remains unsatisfactory, there are no ethical concerns about its use. We previously showed that the addition of tipifarnib to LDAC did not improve outcome12, and additional comparisons against LDAC + gemtuzumab ozogamicin, or versus low dose clofarabine were recommended by the DMC for trial expansion, and are nearing completion. In a subgroup analysis of the azacitidine trial involving AML patients in the 20 – 30% marrow blast subgroup, azacytidine resulted in a superior outcome at 2 years when compared with “doctor's choice”13, however when the options pre-specified by the investigator where compared, azacytidine was only superior to those who would have received best supportive care. The difference versus LDAC was not significant due to lack of numbers.
It is not clear why our response rates are inferior to those demonstrated in the Roboz study. There was a modest difference in the LDAC dosing. We prescribed a fixed dose of 20mg s.c bid, compared with 10mg/m2 s.c. bid. But this is unlikely to have resulted in much difference in the dose received. Similarly the schedules of ATO differed only in days 8 to 12 when patients would receive 2 days at 0.25mg/kg in our study compared with 0.25mg/kg on days 8 to 12 inclusive in the Roboz trial. In fact due to the dose finding part of the Roboz study several patients received less ATO. Neither do the characteristics of the patients included in each study differ substantially, although that cannot be completely discounted.
Treatment for this patient group remains an area of significant unmet need. Several new treatments are becoming available that could be suitable, but one of the experiences, of this and other attempts is the absolute requirement that this is undertaken in a randomised manner.
Supplementary Material
Complete Remission rate stratified by baseline parameters
Overall Survival stratified by baseline parameters
Overall Survival by other treatments in Randomised Comparison
Acknowledgments
We thank the staff of the Birmingham Clinical Trials Unit, Cancer Research UK for research funding, Cephalon for the provision of Arsenic Trioxide and the investigators who participated in the trial:
Aalborg Hospital: Dr Mette Skov Holm; Aberdeen Royal Infirmary: Dr D J Culligan; Addenbrooke's Hospital: Dr C Crawley, Dr J Craig; Barnet General Hospital: Dr M Treacy; Belfast City Hospital: Dr F Jones, Dr Mary Frances McMullin, Dr R J G Cuthbert; Birmingham Heartlands Hospital: Prof D W Milligan, Dr R Lovell; Blackpool Victoria Hospital: Dr P A Cahalin, Dr P. R. Kelsey; Borders General Hospital: Dr J Tucker; Bristol Haematology & Oncology Centre: Dr R Evely; Christie Hospital: Dr M Dennis; Colchester General Hospital: Dr G Campbell, Dr Ti Maboreke; Countess Of Chester Hospital: Dr S Tueger; Crosshouse Hospital: Dr P Maclean; Falkirk And District Royal Infirmary: Dr R F Neilson; Gartnavel General Hospital: Dr M Drummond, Dr P McKay; Gloucestershire Royal Hospital: Dr J Ropner; Hereford County Hospital: Dr L G Robinson; Hillingdon Hospital: Dr K Patel; Hull Royal Infirmary: Dr S Ali; James Paget Hospital: Dr S Sadullah; Kent & Canterbury Hospital: Dr C F E Pocock, Dr K Saied, Dr V Ratnayake; King George Hospital: Dr I Grant; Leicester Royal Infirmary: Dr A E Hunter; Lincoln County Hospital: Dr K Saravanamuttu; Medway Maritime Hospital: Dr A Eden, Dr M Aldouri, Dr V E Andrews; Monklands Hospital: Dr J A Murphy; Ninewells Hospital: Dr K Gelly; Norfolk & Norwich University Hospital: Dr M Lawes; Northwick Park Hospital: Dr L Yung; Nottingham University Hospitals NHS Trust - City Hospital Campus: Dr E Das-Gupta, Dr J L Byrne, Professor N H Russell; Pilgrim Hospital: Dr V Tringham; Pinderfields General Hospital: Dr J Ashcroft, Dr P Moreton; Pontefract General Infirmary: Dr D Wright; Poole General Hospital: Dr A J Bell; Queen Alexandra Hospital: Dr M Ganczakowski, Dr R Corser, Dr T Cranfield; Queen Elizabeth Hospital (Kings Lynn): Dr N Curtin; Queen's Hospital, Romford: Dr I Grant; Rigshospitalet University Hospital: Dr J Jurlander, Dr L Kjeldsen; Royal Bournemouth General Hospital: Dr J Chacko; Royal Cornwall Hospital (Treliske): Dr J Blundell; Royal Free Hospital: Dr P Kottaridis; Royal United Hospital Bath: Dr C Knechtli, Dr S Wexler; Sandwell General Hospital: Dr F Wandroo, Dr J Gillson; Scunthorpe General Hospital: Dr S Jalihal; Singleton Hospital: Dr H Sati; Southampton General Hospital: Dr D Richardson; Southport & Formby District General Hospital: Dr D O'Brien; St Bartholomew's Hospital: Dr H Oakervee; St Helier Hospital: Dr J Mercieca, Dr M Clarke, Dr S Knowles; Stoke Mandeville Hospital: Dr A M O'Hea, Dr H Eagleton; The Alexandra Hospital: Dr T Skibbe; The Great Western Hospital: Dr A Sternberg; The James Cook University Hospital: Dr A Wood, Dr C Millar; The Royal Bolton Hospital: Dr J Jip; The Royal Oldham Hospital: Dr A Allameddine, Dr V Sen; Trafford General Hospital: Dr P Carrington; University College Hospital: Dr A Khwaja, Dr K Yong; University Hospital Aintree: Dr R Salim, Dr W Sadik; University Hospital Coventry (Walsgrave): Dr B Harrison, Dr M Narayanan; University Hospital Of North Tees: Dr P Mounter; University Hospital Of Wales: Dr C Rowntree, Dr J Kell, Dr S Knapper; Victoria Hospital: Dr K Davidson, Dr S Rogers; West Middlesex University Hospital: Dr M Sekhar, Dr M Al-Obaidi; Western General Hospital: Dr P R E Johnson; Whiston Hospital: Dr Toby Nicholson; Worcestershire Royal Hospital: Dr N Pemberton, Dr S Shafeek; Wycombe General Hospital: Dr R Aitchison; Ysbyty Glan Clwyd: Dr C Hoyle, Dr E Heartin, Dr M J Goodrick; Ysbyty Gwynedd : Dr D R Edwards, Dr J Seale.
Footnotes
Conflict of interest: none to declare
References
- 1.Menzin J, Lang K, Earle C, Kerney D, Mallick R. The outcomes and costs of Acute Myeloid Leukemia among the elderly. Arch Intern Med. 2002;162:1597–1603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- 2.Juliusson G, Billström R, Gruber A, Hellström-Lindberg E, Höglund M, Karlsson K, Stockelberg D, Wahlin A, Åström M, Arnesson C, Brunell-Abrahamsson U, Carstensen J, Fredriksson E, Holmberg E, Nordenskjöld K, Wiklund F. Attitude towards remission induction for elderly patients with acute myeloid leukemia influences survival. Leukemia. 2006;20(1):42–47. doi: 10.1038/sj.leu.2404004. [DOI] [PubMed] [Google Scholar]
- 3.Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, Wheatley K. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 4.Roboz GJ, Ritchie EK, Curcio T, Provenzano J, Carlin R, Samuel M, Wittenberg B, Mazumdar M, Christos PJ, Mathew S, Allen-Bard S, Feldman EJ. Arsenic trioxide and low-dose cytarabine in older patients with untreated acute myeloid leukemia, excluding acute promyelocytic leukemia. Cancer. 2008;113(9):2504–2511. doi: 10.1002/cncr.23855. [DOI] [PubMed] [Google Scholar]
- 5.Parmar S, Rundhaugen LM, Boehlke L, Riley M, Nabhan C, Raji A, Frater JL, Tallman MS. Phase II trial of arsenic trioxide in relapsed and refractory acute myeloid leukemia, secondary leukemia and/or newly diagnosed patients at least 65 years old. Leukemia Research. 2004;9:909–919. doi: 10.1016/j.leukres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Rojewski MT, Baldus C, Knauf W, Thiel E, Schrezenmeier H. Dual effects of arsenic trioxide (As2O3) on non-acute promyelocytic leukaemia myeloid cell lines: induction of apoptosis and inhibition of proliferation. Br.J.Haematol. 2002;116(3):555–563. doi: 10.1046/j.0007-1048.2001.03298.x. [DOI] [PubMed] [Google Scholar]
- 7.Sorror ML, Giralt S, Sandmaier BM, De Lima M, Shahjahan M, Maloney DG, Deeg HJ, Appelbaum FR, Storer B, Storb R. Hematopoietic cell transplantation-specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110(13):4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG, Moorman AV, Burnett AK. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br.J.Haematol. 2009;145(5):598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendation of the International Working Group for diagnosis standardisation, of response criteria treatment outcomes and reporting standards for therapeutic trials in acute myeloid leukaemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Royston P, Parmar MKB, Qian W. Novel designs for multi-arm clinical trials with survival outcomes with an application in ovarian cancer. Stat Med. 2003;22(14):2239–2256. doi: 10.1002/sim.1430. [DOI] [PubMed] [Google Scholar]
- 11.Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, Wierda W, Ferrajoli A, Kornblau S, Pierce S, Albitar M, Cortes J, Kantarjian H. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Brit.J.Haematol. 2007;136(4):624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 12.Burnett AK, Hills RK, Milligan DW, Kell WJ, Wheatley K, Virchis AE. Low dose Ara-C versus Low dose Ara-C and Tipifarnib: Result of the UK NCRI AML16 “Pick a Winner” comparison. Blood (ASH Annual Meeting Abstracts) 2008;112:2962. [Google Scholar]
- 13.Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR. Azacitidine Prolongs Overall Survival Compared With Conventional Care Regimens in Elderly Patients With Low Bone Marrow Blast Count Acute Myeloid Leukemia. Journal of Clinical Oncology. 2010;28(4):562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete Remission rate stratified by baseline parameters
Overall Survival stratified by baseline parameters
Overall Survival by other treatments in Randomised Comparison