Abstract
Objectives
Lower muscle strength is associated with a range of adverse health outcomes in later life. The variation in muscle strength between individuals is only partly accounted for by factors in adult life such as body size and physical activity. The aim of this review was to assess the strength of the association between intrauterine development (indicated by birth weight) and subsequent muscle strength.
Design
Systematic review and meta-analysis of studies that assessed the association between birth weight and subsequent muscle strength.
Results
Nineteen studies met inclusion criteria with 17 studies showing that higher birth weight was associated with greater muscle strength. Grip strength was used as a single measure of muscle strength in 15 studies. Meta-analysis (13 studies, 20 481 participants, mean ages 9.3 to 67.5) showed a 0.86 kg (95% CI 0.58, 1.15) increase in muscle strength per additional kilogram of birth weight, after adjustment for age, gender and height at the time of strength measurement.
Conclusion
This review has found consistent evidence of a positive association between birth weight and muscle strength which is maintained across the lifecourse. Future work will be needed to elucidate the biological mechanisms underlying this association, but it suggests the potential benefit of an early intervention to help people maintain muscle strength in later life.
Keywords: Birth weight, Muscle strength, Sarcopenia, Muscle development, Early origins of health and disease hypothesis
Introduction
Lower handgrip strength is associated with a range of negative outcomes in later life, including greater risk of functional impairment (1), falls (2), impaired glucose tolerance and diabetes (3), and higher all cause mortality rates (4). Factors known to affect muscle strength include age, gender, body size and physical activity but these do not fully account for the variation in muscle strength between individuals. Evidence from several observational epidemiological studies (5-9) suggests that poor intrauterine growth (as assessed by birth weight) is associated with lower handgrip strength, even after adjustment for potential modifying and confounding factors such as height. A lasting effect of poor early growth on muscle tissue is suggested by the findings of altered muscle morphology in humans of lower birth weight (10,11) and in sheep with poor prenatal nutrition (12).
A previous review published in 2008 found an unadjusted effect size based on six studies of a 2.06 kg (95% CI 1.77, 2.35) increase in grip strength per additional kilogram of birth weight (13). However this review was not a systematic review and did not synthesise all the relevant literature. Added to that, a number of studies have been published since. Thus a systematic review is now warranted. We aimed to assess the strength of the evidence for the association between poor early growth and lower muscle strength, taking into account important modifying and confounding factors (height, gender and age). We contacted authors asking for results in a standardised form, allowing us to limit the possibility that differences between studies could be attributed to differences in analytical strategy. This paper reports the results related to the measure of early growth utilised most frequently, birth weight.
Methods
We used the methods recommended by the Centre for Reviews and Dissemination (CRD), University of York (14) and followed the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines (15) and the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (16).
Literature search and eligibility criteria
Eligible observational studies were those which reported a relationship between birth weight, size or growth in the first two years of life and a measure of later muscle strength (full review protocol available on request; exposure limited to birth weight only in this report). We limited our search to articles published in English and excluded animal studies. We searched MEDLINE and EMBASE for articles which included terms related to early growth and muscle strength (for a complete list of search terms used, see Appendix 1) from January 1980 to October 2011.
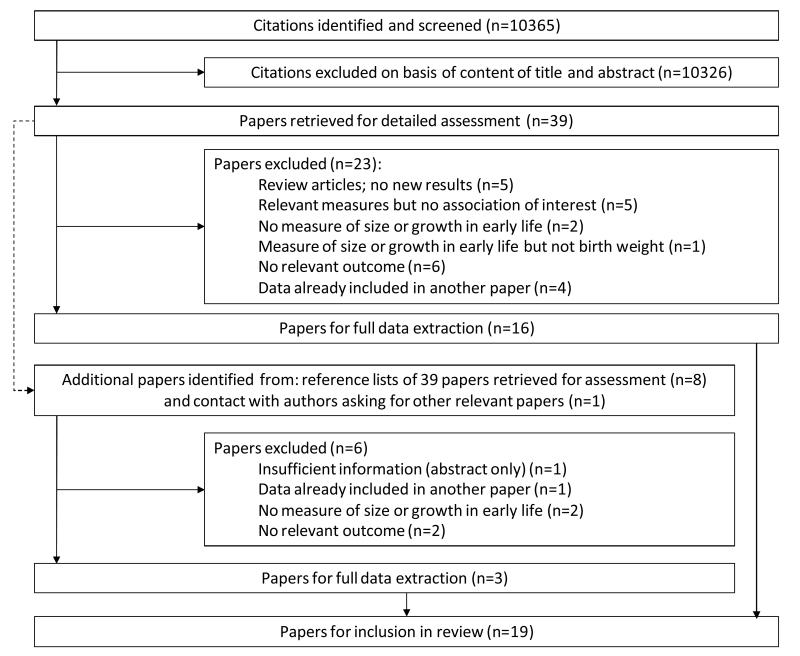
Figure 1 shows how studies were identified. Two authors (RD and HD) independently screened all 10 365 abstracts and assessed the text of 48 papers (including their reference lists for any further potentially relevant articles), leading to the identification of 19 papers which were eligible for inclusion.
Figure 1. Flow diagram showing the selection of studies for inclusion in the review.
We also screened abstracts for studies from selected birth cohorts (see Appendix 2), although this did not yield any further papers.
Data extraction and assessment of risk of bias
Working independently, RD and HD extracted relevant data from eligible papers using a standardised form (available on request). This included information about the study setting, design and population; the selection and baseline characteristics; the exposure measurement; statistical methods used and potential modifying and confounding factors adjusted for; as well as the relevant study results.
We assessed the risk of bias of each study in relation to our review question using a set of 11 criteria. These addressed areas including study design, whether the exposure and outcome measurements were reliably obtained, losses to follow-up and the appropriateness of analyses presented. RD and HD independently carried out the quality assessment of each paper, and any discrepancies in scoring were resolved by discussion with JB acting as a third reviewer. An overall risk of bias (low / medium / high) was assigned to each study based on both the quality score and reviewer judgment.
Contact with authors
We contacted the corresponding authors of all 19 included studies, requesting results from analyses performed in a standard way for inclusion in meta-analyses. We asked authors to analyse the association between birth weight and later muscle strength, preferably using linear regression models to produce regression coefficients for the increase in muscle strength per 1 kg increase in birth weight. We asked authors to perform three separate models: (1) unadjusted; (2) age adjusted and; (3) age and height adjusted, on men and women separately and on men and women combined with an adjustment for sex included in models 2 and 3 when men and women were combined. We chose to ask for adjustment for these covariates (sex, age and height), as these have been shown to be important determinants of adult grip strength (17). We sent one reminder to authors who did not respond initially. We received responses to 17 of the 19 requests; 13 authors provided results and 4 were unable to do so within the period requested. We excluded one of the 13 sets of results received as the small sample sizes (< 25 in each of the two birth weight groups) meant that the confidence intervals were too wide to include in our meta-analysis (18).
We also asked for information on any other relevant published research and this yielded one further paper (19) which had not been indexed by either of the databases searched.
Statistical methods
RD and GN separately collated regression coefficients for the dataset. We then carried out meta-analyses using Stata version 11.0 software, to derive pooled estimates of regression coefficients and 95% CIs for the relationship between birth weight and grip strength. We anticipated significant heterogeneity between studies and so used a random effects model.
Sensitivity analyses were performed to explore the potential influences of various prespecified factors on our findings. We tested for a gender difference in our results by performing a meta-analysis of the differences in the sex-specific regression coefficients for each study after adjustment for age and height. We ran separate meta-analyses of the studies stratified by mean age at time of strength measurement (<21, >20 and <41, and >40 years), study setting (developing or developed country) and risk of bias with regard to the review question. We also re-ran our analysis with each study removed in turn to check that no one study was significantly contributing to the heterogeneity between studies. We produced funnel plots and used the tests proposed by Egger and colleagues to check for publication bias (20).
Results
Nineteen studies met review inclusion criteria (Table 1). The majority (n=16) were based in developed countries, the others were conducted in India (19), Guatemala (21) and the Philippines (22). All studies included birth weight as an independent exposure variable except Duppe et al who used a combined measure of birth weight and length (23).
Table 1. Characteristics of studies included in the review.
| First author, year |
Source* | Study name and country | Gender | Characteristics of study population: n Mean (SD) age (y) |
Mean (SD) birth weight (kg) (unless otherwise stated) |
Measure of muscle strength Brief description of instrument and protocol used (if stated) Mean (SD) |
Risk of bias score** |
|---|---|---|---|---|---|---|---|
| Barr, 2010 (19) |
Both | Mysore Parthenon Study, India |
Grip strength (kg)
Jamar handgrip dynamometer Three readings from each hand alternately, maximum of all six used |
+5 Low |
|||
| Male | 275 9.33 (0.14) |
2.90 (0.46) | 12.7 (2.2) | ||||
| Female | 299 9.35 (0.13) |
2.83 (0.43) | 11.0 (2.0) | ||||
| Duppe, 1997 (23) |
PO | Kirseberg Public Health Project in Malmö, Sweden |
Birth weight and
birth length (units not given) |
Quadriceps strength
(units not given) Biodex isokinetic muscle force meter |
0 High |
||
| Male | 48 15.1 (0.3) |
Descriptives not provided |
Descriptives not provided |
||||
| Female | 39 15.1 (0.4) |
||||||
| Ericson, 1998 (24) |
Au | Data from National Service Enrolment Register, Sweden |
Male | 802 17.7 (0.5) |
2.42 (0.99) |
Grip strength (N) 591.5 (95.8) |
+3 Medium |
| Ford, 1988 (18) |
Both | Data from Royal Women’s Hospital, Melbourne, Australia |
Grip strength (N)
Harpenden handgrip dynamometer Three or four readings of both hands together and then of each hand separately |
−4 High |
|||
| VLBW Male |
9 5.2 (0.21) |
1.22 (0.17) | 90.22 (20.67) | ||||
| VLBW Female |
15 5.20 (0.15) |
1.13 (0.19) | 96.73 (20.63) | ||||
| NBW Male |
13 5.10 (0.06) |
3.58 (0.46) | 117.61 (34.91) | ||||
| NBW Female |
5 5.10 (0.09) |
3.18 (0.30) | 96.2 (21.36) | ||||
| Inskip, 2007 (5) |
Both | Southampton Women’s Survey, UK |
Female | 1352 30.6 (3.8) |
3.24 (0.56) |
Grip strength (kg) Jamar handgrip dynamometer Three readings from each hand, maximum of all six used 32.3 (5.9) |
+7 Low |
| Kuh, 2002 (7) |
Both | MRC National Survey of Health and Development, UK |
Grip strength (kg)
Electronic handgrip dynamometer Two readings from each hand after a practice, maximum value used in analysis |
+8 Low |
|||
| Male | 1398 53 (n/a) |
3.47 (0.53) | 47.68 (12.20) | ||||
| Female | 1432 53 (n/a) |
3.33 (0.48) | 27.74 (7.93) | ||||
| Kuzawa, 2010 (22) |
Both | Cebu Longitudinal Health and Nutrition Survey, Philippines |
Grip strength (kg)
Handgrip dynamometer Three readings taken and the average used in analysis |
+4 Medium |
|||
| Male | 907 20.95 (0.33) |
3.03 (0.43) | 73.49 (22.54) | ||||
| Female | 815 20.94 (0.35) |
2.99 (0.42) | 43.99 (16.97) | ||||
| All | 1722 20.94 (0.34) |
3.01 (0.42) | 59.53 (24.91) | ||||
| Martorell, 1998 (21) |
PO | Institute of Nutrition of Central America and Panama Longitudinal Study, Guatemala |
Birth weight
means for 3 groups (kg): |
Mean grip strength by group** (kg) Handgrip dynamometer |
+4 Medium |
||
| Male | 169 14.9 (1.5) |
IUGR: 2.31 Middle: 2.79 Upper: 3.43 |
IUGR: 23.8 Middle: 26.9 Upper: 26.3 |
||||
| Female | 162 14.8 (1.5) |
IUGR: 2.36 Middle: 2.81 Upper: 3.38 |
IUGR: 17.3 Middle: 20.7 Upper: 20.1 |
||||
| Martorell , 1998 (21) (2002-04 follow-up data, unpublished) |
Au | Human Capital Study, 2002-04, Guatemala |
Grip strength (kg) | N/A | |||
| Male | 227 29.75 (2.37) |
3.10 (0.50) | 41.72 (7.30) | ||||
| Female | 268 29.59 (2.30) |
3.02 (0.43) | 26.94 (5.03) | ||||
| All | 495 29.66 (2.33) |
3.06 (0.46) | 33.72 (9.61) | ||||
| Ortega, 2009 (25) |
PO | AVENA (Food and Assessment of the Nutritional Status of Adolescents) Study, Spain |
Grip strength (kg)
TKK 5101 Grip D Takey handgrip dynamometer Two attempts from each hand and the average of the better score from each hand used in analysis |
+5 Low |
|||
| Male | 818 15.3 (1.3) |
3.5 (0.5) | 35.0 (8.0) | ||||
| Female | 983 15.4 (1.3) |
3.3 (0.5) | 25.5 (4.1) | ||||
| Pitcher, 2009 (28) |
PO | Adelaide Family Heart Study, Australia |
All | 35 (19 male) 28 (no SD given) |
Range: 1.47-4.71 |
Grip strength Handgrip dynamometer Three attempts from each hand and mean value for each hand used in analysis Descriptives not provided |
−3 High |
| Ridgway, 2009 (29) |
Both | Northern Finland Birth Cohort, Finland |
Grip strength (kg) Newtest handgrip dynamometer Three attempts from dominant hand and maximum value used in analysis |
+7 Low |
|||
| Male | 2061 31 (n/a) |
3.60 (0.50) | 49.7 (8.7) | ||||
| Female | 2212 31 (n/a) |
3.47 (0.47) | 28.2 (6.5) | ||||
| Ridgway, 2011 (30) |
Both | East Flanders Prospective Twin Survey, Belgium |
Grip strength (kg) Jamar hand grip dynamometer One attempt from dominant hand following familiarisation |
+5 Low |
|||
| Male | 382 25.7 (4.7) |
2.60 (0.48) | 41.4 (7.0) | ||||
| Female | 401 25.5 (4.7) |
2.49 (0.49) | 25.6 (4.6) | ||||
| Robinson, 2008 (33) |
Both | Hertfordshire Cohort Study, UK |
Grip strength (kg)
Jamar handgrip dynamometer Three readings from each hand alternately, maximum of all six used |
+7 Low |
|||
| Male | 1569 65.7 (2.9) |
3.50 (0.54) | 44.0 (7.5) | ||||
| Female | 1414 66.6 (2.7) |
3.35 (0.50) | 26.5 (5.7) | ||||
| Rogers, 2005 (26) |
PO | Follow-up of ELBW ( here <800g) children admitted to Neonatal ICU in British Columbia, Canada |
Mean (range) |
Grip strength***
(kg) A handgrip dynamometer Readings from both hands taken |
−1 High |
||
| Male and female |
ELBW individuals 53 17.3 (range 16.3- 19.7) |
0.72 (0.52-0.80) | Males: 36.27 Females: 25.79 |
||||
| NBW individuals 31 17.8 (range 16.5- 19.0) |
3.51 (3.07-4.20) | Males: 46.62 Females: 27.39 |
|||||
| Saigal, 2007 (34) |
PO | Follow up of ELBW (here 501-1000g) survivors born between 1977-1982 in central-West Ontario, Canada |
Grip strength (kg)
A handgrip dynamometer Reading from dominant hand used |
+4 Medium |
|||
| ELBW Male |
149 (67 male) 23.3 (1.2) |
0.84 (0.12) | 41 (9) | ||||
| ELBW Female |
25 (5) | ||||||
| NBW Male |
133 (60 male) 23.6 (1.1) |
3.38 (0.49) | 47 (9) | ||||
| NBW Female |
31 (5) | ||||||
| Small, 1998 (27) |
PO |
Knee extension
& flexion (both Nm) Kin Com isokinetic dynamometer Three sets of extension- flexion cycles were performed with the right leg; highest values used in analysis |
−1 High |
||||
| ELBW Male |
8 13.3 (1.8) |
0.82 (0.13) | 380.3 (139.8) 200.8 (63.1) |
||||
| ELBW Female |
9 13.8 (1.7) |
0.83 (0.14) | 395.0 (103.2) 193.0 (45.2) |
||||
| NBW Male |
8 13.5 (2.1) |
3.65 (0.42) | 442.6 (165.7) 236.1 (86.1) |
||||
| NBW Female |
9 14.0 (1.5) |
3.54 (0.53) | 451.4 (148.2) 206.2 (55.3) |
||||
| Sayer, 1998 (6) |
Both | Hertfordshire Ageing Study, UK |
Grip strength (kg)
Harpenden handgrip dynamometer |
+3 Medium |
|||
| Male | 411 67.5 (2.4) |
3.53 (0.50) | 38.26 (7.14) | ||||
| Female | 306 67.5 (2.2) |
3.41 (0.48) | 22.49 (2.23) | ||||
| te Velde, 2004 (32) |
Both | Amsterdam Growth and Health Longitudinal Study, Holland |
Static arm pull (kg)
Bettendorf dynamometer Two attempts in best arm and higher value used in analysis |
+2 Medium |
|||
| Male | 119 36.5 (0.59) |
3.55 (0.47) | 71.5 (13.4) | ||||
| Female | 154 36.6 (0.67) |
3.42 (0.51) | 38.7 (7.5) | ||||
| Yliharsila, 2007 (9) |
Both | Helsinki Birth Cohort, Finland |
Grip strength (kg)
Newtest Grip Force dynamometer Three attempts in dominant hand and maximum value used in analysis |
+4 Medium |
|||
| Male | 928 61.5 (2.8) |
3.48 (0.50) | 40.23 (9.45) | ||||
| Female | 1075 61.5 (3.0) |
3.35 (0.47) | 22.92 (6.29) |
Abbreviations. ELBW, extremely low birth weight (definition varies; for range of included birth weights see individual study entry). ICU, intensive care unit. IUGR, intrauterine growth retardation. NBW, normal birth weight. VLBW, very low birth weight (<1500g).
Source column: PO, paper only, all results available in published paper. Au, all results from request to corresponding author. Both, some results from published paper and some from author.
Risk of bias score, range −11 to +11: low risk of bias (>4), medium risk of bias (>0 and <5), high risk of bias (<1).
Average of left and right hand figures shown.
Age at follow-up and outcome measure
The mean age of participants varied between studies and was 20 or below (range 5.1 to 17.7) in eight studies (18,19,21,23-27), between 21 and 40 (range 20.9 to 36.5) in seven studies (5,22,28-32) and 41 or above (range 53.0 to 67.5) in the remaining four studies (6,7,9,33). Grip strength was used as a single measure of muscle strength in the majority of studies (n=15). The instruments and protocols used to measure grip strength varied between studies as outlined in Table 1. Other muscle strength measurements included static arm pull and high jump (32), strength of knee movements (23,27) and a calculated estimate of muscle strength based on a combination of grip strength, knee extension strength and elbow flexion strength (24).
The association of birth weight with muscle strength
Nineteen studies analysed the association of birth weight with later muscle strength and 13 sets of results were available for inclusion in meta-analysis. In the remaining six studies, there was not sufficient information in the paper or analyses available from the relevant author to allow inclusion.
Meta-analysis results
In the meta-analysis of age-adjusted results there was a positive association between birth weight and later muscle strength with a pooled estimate of 2.07kg (95% CI 1.47, 2.66) increase in muscle strength per kilogram increase of birth weight in men and 1.59kg (95% CI 1.25, 1.93) in women.
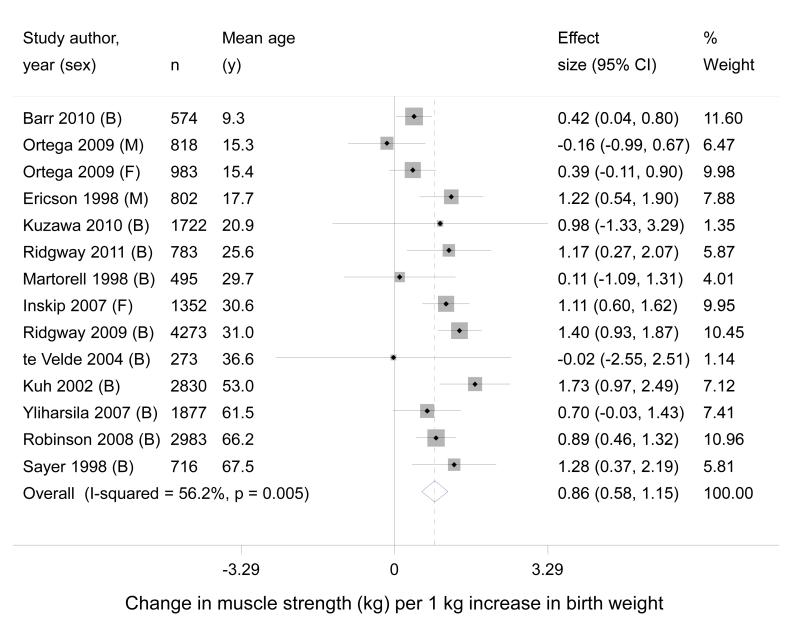
Meta-analysis of the differences between sex-specific regression coefficients (from the 11 studies which included both men and women) in the age and height adjusted model did not reveal evidence of an overall gender difference, so we pooled results for men and women in this analysis. There was still evidence of an association after adjustment for age and height, although the pooled estimate was attenuated; 0.86kg (95% CI 0.58, 1.15) increase in muscle strength per kilogram increase of birth weight (Figure 2).
Figure 2. Forest plot of studies assessing the association between birth weight (kg) and later muscle strength (kg), after adjustment for age and height.
Studies ordered by mean age at time of strength measurement. B = both males and females; M = males only; F = females only included in study.
There was evidence of significant heterogeneity between studies (P value from Q statistic = 0.005 and I2 = 56.2% for the adjusted results). To explore if differences in the magnitude of muscle strength measurements between different study populations accounted for this heterogeneity, we repeated the meta-analysis using Z-scores for muscle strength but this did not greatly impact on the level of heterogeneity (P value = 0.05, I2 = 41.7% in the age and height adjusted models). We also stratified the results of our meta-analysis to explore other potential sources of heterogeneity (Table 2). We found greater heterogeneity among the male participants and in those studies with a mean age under 21 years. Results from studies in developing countries or those from studies judged to have a medium risk of bias did not appear to account for the heterogeneity in our combined results.
Table 2. Stratified results from meta-analyses of birth weight to grip strength relationship (age and height adjusted model used in all cases).
| Stratification | No. of data points* | Increase in grip strength (kg) per 1 kg increase in birth weight (95% CI) |
I2 (%) | P value** |
|---|---|---|---|---|
| None | 14 | 0.86 (0.58 to 1.15) | 56.2 | 0.005 |
| Gender | ||||
| Female | 12 | 0.81 (0.59 to 1.02) | 3.0 | 0.415 |
| Male | 12 | 0.96 (0.49 to 1.44) | 58.8 | 0.005 |
| Mean age (years) | ||||
| < 21 | 4 | 0.48 (0.05 to 0.92) | 57.1 | 0.072 |
| 21 – 40 | 6 | 1.16 (0.85 to 1.46) | 0.0 | 0.436 |
| > 40 | 4 | 1.09 (0.67 to 1.51) | 36.7 | 0.192 |
| Study setting | ||||
| Developing | 3 | 0.41 (0.05 to 0.77) | 0.0 | 0.788 |
| Developed | 11 | 0.96 (0.66 to 1.26) | 54.4 | 0.016 |
| Risk of bias | ||||
| Low | 8 | 0.86 (0.49 to 1.24) | 72.6 | <0.001 |
| Medium | 6 | 0.92 (0.52 to 1.31) | 0.0 | 0.553 |
Note there are 14 data points in gender-adjusted models as for one study (Ortega et al) we only had separate results for males and females.
From Q-statistic
We also repeated the meta-analysis removing each study in turn and this did not suggest that any one study was unduly influencing heterogeneity. Inspection of funnel plots did not suggest any clear evidence of publication bias. The tests proposed by Egger and colleagues (20) supported this finding, with p values of 0.46 or greater.
Other results
Of the six studies not included in the meta-analysis (Table 3), four of these were studies comparing outcomes, including muscle strength, of cases of low birth weight individuals with normal birth weight controls (18,26,27,34). All but one (27) found that the lower birth weight group had reduced muscle strength when compared with the normal birth weight group. In the fifth study, Pitcher et al showed a positive correlation between birth weight and grip strength in an Australian birth cohort, although the relationship attenuated and was no longer found when placental weight was included in the model (28). In the final study in this group, Duppe et al examined the association between a combined measure of weight and length at birth and quadriceps strength in adolescence (23). They found no association although this study was considered to have a high risk of bias in relation to our review question, mainly due to its small sample size (87 participants) and the limited information given on the method used for strength measurement.
Table 3. Summary of results for studies that examined the relationship between birth weight and later muscle strength that were not included in the meta-analysis.
| First author, year |
Main findings | Adjustments | ||
|---|---|---|---|---|
| Duppe, 1997(23) |
Combined weight and height at 6 months in females and 1 year in males correlated with quadriceps strength measured at mean age 15.1 years. |
Height Weight Total body BMC and BMD Femoral neck BMC and BMD |
||
| Birth |
Males 0.27 (NS) |
Females 0.31 (NS) |
||
| Ford, 1988(18) | Grip strength in combined total of right and left hands for significantly higher in NBW individuals (178.9N) than VLBW individuals (150.7N). P=0.019 for difference. |
None | ||
| Martorell, 1998(21)* |
Compared to the middle birth weight group (2500-3000g), those in the IUGR group (<1500g) had significantly lower right hand grip strength (females) and significantly lower left hand grip (males). |
Age Gestational age |
||
| Pitcher, 2009(28) |
Birth weight correlated with grip strength in left (r=0.42, p = 0.01) and right (r=0.43, p = 0.01) hands. However, in a combined model of birth weight and placenta weight, only placenta weight predicted grip strength. |
Gestational age Maternal size, ethnicity and parity |
||
| Rogers, 2005(26) |
ELBW individuals had significantly lower combined left and right hand grip strength than normal birth weight controls (p = 0.0001). Males in the study had higher grip strength than females (p = 0.0001). Group × gender interactions were also found (p = 0.009). |
None | ||
| Saigal, 2007(34) | Overall, mean grip strength was −6.4kg (95% CI −9.1, −3.7) lower in ELBW group compared to NBW controls. |
None | ||
| Small, 1998(27) | Isokinetic muscle extension and flexion did not differ between birth weight or gender groups, nor was there any interaction between group and gender. |
None | ||
Based on published data for this cohort at age 15 years; subsequent follow-up at mean age 30 years (unpublished) included in the meta-analysis.
Abbreviations. BMC, bone mineral content. BMD, bone mineral density. ELBW, extremely low birth weight (definition varies; for range of included birth weights see individual study entry). IUGR, intrauterine growth retardation. NBW, normal birth weight. NS, not significant. VLBW, very low birth weight (<1500g).
In the meta-analysis we included unpublished results from a Guatemalan cohort at a mean age of 30 years. The original paper contained results for the same cohort at mean age 15 years divided into three birth weight groups. This showed reduced muscle strength in the low compared to the normal birth weight group (21).
Discussion
This systematic review has shown strong and consistent evidence of a positive association between birth weight and muscle strength (typically assessed by grip) even after adjustment for the important covariates, age, gender and height. The studies included were conducted in a range of settings and included participants with mean ages from 9 to 68 years. The effect size was larger in studies with a mean age of participants greater than 20 years.
Although most studies found evidence of associations acting in the same direction there was evidence of significant heterogeneity in the size of associations between studies which likely relates to a range of factors. Studies varied markedly in their mean age; results were adjusted for age but this was only across the narrow age range of any one study (the maximum standard deviation for age for any study population was 3.8 years). The birth weight to strength relationship had lower heterogeneity in the group of studies with participants between 21 and 40 years of age, perhaps reflecting a particular influence of early growth on the peak strength obtained in adulthood.
We found greater heterogeneity between the results for male study participants than females. There is evidence that physical activity is more strongly associated with grip strength among men than women (35,36), so it may be that varying levels of physical activity among the populations studied partly account for the higher level of heterogeneity that we have observed in the meta-analysis of male participants.
Another likely source of variation between studies was the method of strength measurement. This varied widely in terms of number of trials and which hand was used in the case of grip strength (Table 1). There is evidence that variation in approach can affect the values recorded (37). There was also typically limited reporting of the protocol used by each study for strength measurement; only 3 of the 19 included studies were judged to have explained this clearly and therefore to have had low risk of bias in this area.
A previous review combining the results of six studies found a pooled estimate of a 2.06 kg unadjusted increase in grip per kilogram of birth weight, for men and women (13). Our updated meta-analysis includes five of these studies (with the sixth, a conference abstract, not meeting our review inclusion criteria) as well as eight cohorts not analysed previously. Our unadjusted value was similar across this larger number of studies and by contacting authors for standardised results we have also been able to confirm that adjustment for age and height attenuates, but does not remove, the birth weight to grip strength relationship. There is also evidence found in men from the Fels Longitudinal Study that increasing birth weight is associated with a higher rate of increase of grip and higher peak grip strength in adult life (38).
There is evidence that an adverse intrauterine environment can affect muscle histology in animals (12,39). There have been far fewer studies in human muscle and the results have not been consistent although this may reflect the different groups studied. A study of middle-aged women showed no relationship between birth weight and muscle morphology (40). However a study of young men demonstrated altered skeletal muscle fiber composition in those with low birth weight (10). More recently, a study of older men participating in the Hertfordshire Sarcopenia Study showed that low birth weight was associated with a reduced muscle fibre score and suggested that this might underlie the association between lower birth weight and reduced muscle strength (11).
Strengths and limitations
We conducted a rigorous systematic review following the CRD recommendations and the MOOSE and PRISMA guidelines, including the use of two reviewers at each stage of the review process. The majority of our included papers were assessed to have a low or medium risk of bias (n=14) and none of those included in our meta-analysis were high risk. We attempted to minimise publication bias by contact with study authors, receiving two unpublished sets of regression analyses and a further ten sets which had been re-analysed using our standardised models; the tests performed on the meta-analysis data suggest that significant publication bias is unlikely. Limitations of this review include the fact that we did not search for non-English language publications. We asked authors to perform analyses on their data using three standardised models, with sex, age and height included in the third model. There are of course other possible covariates, but in order for the adjustments to be made across all studies, we chose the factors that would have been routinely collected, and which we know influence grip strength (17). It is possible that other factors might confound the association between birth weight and grip strength. However, by combining evidence from a wide range of study populations which are likely to have different confounding structures and despite this, finding consistent evidence of association suggests that confounding is unlikely to fully explain the associations shown.
This systematic review has shown strong and consistent evidence for a positive association between birth weight and muscle strength in men and women across the lifecourse. Our findings underline the importance of recognising the influence of early growth and development on childhood and adult muscle function. The next stage is to elucidate the mechanisms that underlie this association with the ultimate aim of developing beneficial interventions to minimise the detrimental effects of muscle loss in later life.
Supplementary Material
Acknowledgements
We are grateful to the following people who provided data for the review: Avan Aihie Sayer (MRC Lifecourse Epidemiology Unit, University of Southampton, UK), Lex Doyle (The Royal Women’s Hospital, University of Melbourne, Australia), Ulf Ekelund (MRC Epidemiology Unit, Institute of Metabolic Science, Addenbrookes Hospital, Cambridge, UK), Johan Eriksson (Department of Epidemiology and Health Promotion, National Public Health Institute, Helsinki, Finland), Hazel Inskip (MRC Lifecourse Epidemiology Unit, University of Southampton, UK), Bengt Källén (Tornblad Institute, Lund University, Sweden), Han Kemper (EMGO Institute, VU University Medical Center, Amsterdam, The Netherlands), G. Krishnaveni (Epidemiology Research Unit, Holdsworth Memorial Hospital, Mysore, India), Diana Kuh (MRC Unit for Lifelong Health and Ageing, Department of Epidemiology and Public Health, University College London, UK), Chris Kuzawa (Institute for Policy Research, Northwestern University, Evanston, IL, USA), Reynaldo Martorell (Hubert Department of Global Health, Emory University, Atlanta, GA, USA), Charlotte Ridgway (MRC Epidemiology Unit, Institute of Metabolic Science, Cambridge, United Kingdom) and Sian Robinson (MRC Lifecourse Epidemiology Unit, University of Southampton, UK).
Funding sources This work was supported by the Medical Research Council; the University of Southampton; the National Institute for Health Research (Academic Clinical Fellowship to R.D.) and the Healthy Ageing across the Life Course (HALCyon) programme, funded by the New Dynamics of Ageing (RES-353-25-0001).
Reference List
- 1.Al Snih S, Markides KS, Ottenbacher KJ, Raji MA. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging Clin Exp Res. 2004 Dec;16:481–6. doi: 10.1007/BF03327406. [DOI] [PubMed] [Google Scholar]
- 2.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Anderson FH, Cooper C. Falls, sarcopenia, and growth in early life: findings from the Hertfordshire cohort study. Am J Epidemiol. 2006 Oct 1;164:665–71. doi: 10.1093/aje/kwj255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care. 2005 Oct;28:2541–2. doi: 10.2337/diacare.28.10.2541. [DOI] [PubMed] [Google Scholar]
- 4.Cooper R, Kuh D, Hardy R, the Mortality Review Group Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inskip HM, Godfrey KM, Martin HJ, Simmonds SJ, Cooper C, Sayer AA, Southampton Women’s Survey Study Group Size at birth and its relation to muscle strength in young adult women. J Intern Med. 2007 Sep;262:368–74. doi: 10.1111/j.1365-2796.2007.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayer AA, Cooper C, Evans JR, Rauf A, Wormald RP, Osmond C, Barker DJ. Are rates of ageing determined in utero? Age Ageing. 1998 Sep;27:579–83. doi: 10.1093/ageing/27.5.579. [DOI] [PubMed] [Google Scholar]
- 7.Kuh D, Bassey J, Hardy R, Sayer AA, Wadsworth M, Cooper C. Birth weight, childhood size, and muscle strength in adult life: evidence from a birth cohort study. Am J Epidemiol. 2002 Oct 1;156:627–33. doi: 10.1093/aje/kwf099. [DOI] [PubMed] [Google Scholar]
- 8.Sayer AA, Syddall HE, Gilbody HJ, Dennison EM, Cooper C. Does sarcopenia originate in early life? Findings from the Hertfordshire cohort study. J Gerontol A Biol Sci Med Sci. 2004 Sep;59:M930–M934. doi: 10.1093/gerona/59.9.m930. [DOI] [PubMed] [Google Scholar]
- 9.Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG. Birth size, adult body composition and muscle strength in later life. Int J Obes (Lond) 2007 Sep;31:1392–9. doi: 10.1038/sj.ijo.0803612. [DOI] [PubMed] [Google Scholar]
- 10.Jensen CB, Storgaard H, Madsbad S, Richter EA, Vaag AA. Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J Clin Endocrinol Metab. 2007 Apr;92:1530–4. doi: 10.1210/jc.2006-2360. [DOI] [PubMed] [Google Scholar]
- 11.Patel HP, Jameson KA, Syddall HE, Martin HJ, Stewart CE, Cooper C, Sayer AA. Developmental Influences, Muscle Morphology, and Sarcopenia in Community-Dwelling Older Men. J Gerontol A Biol Sci Med Sci. 2011 Feb 28; doi: 10.1093/gerona/glr020. published online February 28, 2011 doi:10.1093/gerona/glr020. [DOI] [PubMed] [Google Scholar]
- 12.Costello PM, Rowlerson A, Astaman NA, Anthony FE, Sayer AA, Cooper C, Hanson MA, Green LR. Peri-implantation and late gestation maternal undernutrition differentially affect fetal sheep skeletal muscle development. J Physiol. 2008 May 1;586:2371–9. doi: 10.1113/jphysiol.2008.150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008 Aug;12:427–32. doi: 10.1007/BF02982703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centre for Reviews and Dissemination [Accessed 01/10/2009];Systematic reviews: CRD’s guidance for undertaking reviews in health care 2009. 2009 http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf.
- 15.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000 Apr 19;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009 Aug 18;151:264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Syddall H, Cooper C, Martin F, Briggs R, Aihie SA. Is grip strength a useful single marker of frailty? Age Ageing. 2003 Nov;32:650–6. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- 18.Ford GW, Kitchen WH, Doyle LW. Muscular strength at 5 years of children with a birthweight under 1500 g. Aust Paediatr J. 1988 Oct;24:295–6. doi: 10.1111/j.1440-1754.1988.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 19.Barr JG, Veena SR, Kiran KN, Wills AK, Winder NR, Kehoe S, Fall CHD, Sayer AA, Krishnaveni GV. The relationship of birthweight, muscle size at birth and post-natal growth to grip strength in 9-year-old Indian children: findings from the Mysore Parthenon study. Journal of Developmental Origins of Health and Disease. 2010;1:329–37. doi: 10.1017/S2040174410000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martorell R, Ramakrishnan U, Schroeder DG, Melgar P, Neufeld L. Intrauterine growth retardation, body size, body composition and physical performance in adolescence. [Review] [25 refs] Eur J Clin Nutr. 1998 Jan;52(Suppl 1):S43–52. [PubMed] [Google Scholar]
- 22.Kuzawa CW, McDade TW, Adair LS, Lee N. Rapid weight gain after birth predicts life history and reproductive strategy in Filipino males. Proc Natl Acad Sci U S A. 2010 Sep 28;107:16800–5. doi: 10.1073/pnas.1006008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duppe H, Cooper C, Gardsell P, Johnell O. The relationship between childhood growth, bone mass, and muscle strength in male and female adolescents. Calcif Tissue Int. 1997 May;60:405–9. doi: 10.1007/s002239900253. [DOI] [PubMed] [Google Scholar]
- 24.Ericson A, Kallen B. Very low birthweight boys at the age of 19. Arch Dis Child Fetal Neonatal Ed. 1998 May;78:F171–F174. doi: 10.1136/fn.78.3.f171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortega FB, Labayen I, Ruiz JR, Martin-Matillas M, Vicente-Rodriguez G, Redondo C, Warnberg J, Gutierrez A, Sjostrom M, et al. Are muscular and cardiovascular fitness partially programmed at birth? Role of body composition. J Pediatr. 2009 Jan;154:61–6. doi: 10.1016/j.jpeds.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 26.Rogers M, Fay TB, Whitfield MF, Tomlinson J, Grunau RE. Aerobic capacity, strength, flexibility, and activity level in unimpaired extremely low birth weight (<= 800 g) survivors at 17 years of age compared with term-born control subjects. Pediatrics. 2005 Jul;116:E58–E65. doi: 10.1542/peds.2004-1603. [DOI] [PubMed] [Google Scholar]
- 27.Small E, Bar-Or O, Van ME, Saigal S. Muscle function of 11- to 17-year-old children of extremely low birthweight. Pediatric Exercise Science. 1998;10:327–36. [Google Scholar]
- 28.Pitcher JB, Robertson AL, Cockington RA, Moore VM. Prenatal growth and early postnatal influences on adult motor cortical excitability. Pediatrics. 2009 Jul;124:e128–e136. doi: 10.1542/peds.2008-1638. [DOI] [PubMed] [Google Scholar]
- 29.Ridgway CL, Ong KK, Tammelin T, Sharp SJ, Ekelund U, Jarvelin M-R. Birth size, infant weight gain, and motor development influence adult physical performance. Medicine and Science in Sports and Exercise. 2009 Jun;41:1212–21. doi: 10.1249/MSS.0b013e31819794ab. [DOI] [PubMed] [Google Scholar]
- 30.Ridgway CL, Sharp SJ, Derom C, Beunen G, Fagard R, Vlietinck R, Ekelund U, Loos RJ. The contribution of prenatal environment and genetic factors to the association between birth weight and adult grip strength. PLoS One. 2011;6:e17955. doi: 10.1371/journal.pone.0017955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saigal S, Szatmari P, Rosenbaum P, Campbell D, King S. Cognitive abilities and school performance of extremely low birth weight children and matched term control children at age 8 years: a regional study. J Pediatr. 1991 May;118:751–60. doi: 10.1016/s0022-3476(05)80043-5. [DOI] [PubMed] [Google Scholar]
- 32.te Velde SJ, Twisk JW, van MW, Kemper HC. Birth weight and musculoskeletal health in 36-year-old men and women: results from the Amsterdam Growth and Health Longitudinal Study. Osteopor International. 2004 May;15:382–8. doi: 10.1007/s00198-003-1554-5. [DOI] [PubMed] [Google Scholar]
- 33.Robinson SM, Jameson KA, Batelaan SF, Martin HJ, Syddall HE, Dennison EM, Cooper C, Sayer AA, Hertfordshire Cohort Study Group Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc. 2008 Jan;56:84–90. doi: 10.1111/j.1532-5415.2007.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saigal S, Stoskopf B, Boyle M, Paneth N, Pinelli J, Streiner D, Goddeeris J. Comparison of current health, functional limitations, and health care use of young adults who were born with extremely low birth weight and normal birth weight. Pediatrics. 2007 Mar;119:e562–e573. doi: 10.1542/peds.2006-2328. [DOI] [PubMed] [Google Scholar]
- 35.Cooper R, Mishra G, Kuh D. Physical activity across adulthood and physical performance in mid-life: Findings from a British birth cohort study. Am J Prev Med. 2011;41:376–84. doi: 10.1016/j.amepre.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuh D, Bassey EJ, Butterworth S, Hardy R, Wadsworth ME. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. J Gerontol A Biol Sci Med Sci. 2005 Feb;60:224–31. doi: 10.1093/gerona/60.2.224. [DOI] [PubMed] [Google Scholar]
- 37.Roberts HC, Denison HJ, Martin HJ, Syddall HE, Patel HP, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–9. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 38.Nahhas RW, Choh AC, Lee M, Chumlea WM, Duren DL, Siervogel RM, Sherwood RJ, Towne B, Czerwinski SA. Bayesian longitudinal plateau model of adult grip strength. Am J Hum Biol. 2010 Sep;22:648–56. doi: 10.1002/ajhb.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehfeldt C, Kuhn G. Consequences of birth weight for postnatal growth performance and carcass quality in pigs as related to myogenesis. J Anim Sci. 2006 Apr;84(Suppl):E113–E123. doi: 10.2527/2006.8413_supple113x. [DOI] [PubMed] [Google Scholar]
- 40.Thompson CH, Sanderson AL, Sandeman D, Stein C, Borthwick A, Radda GK, Phillips DI. Fetal growth and insulin resistance in adult life: role of skeletal muscle morphology. Clin Sci (Lond) 1997 Mar;92:291–6. doi: 10.1042/cs0920291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.