Abstract
The ACTH receptor (melanocortin 2 receptor – MC2R) is the smallest G protein coupled receptor which, when activated by the peptide hormone, adrenocorticotropin (ACTH), stimulates cAMP production and adrenal steroidogenesis. Receptor expression is dependent on a specific membrane trafficking process involving an accessory protein (Melanocortin 2 receptor accessory protein - MRAP) and other unidentified components. In an attempt to discover novel receptor interacting proteins, the C-terminal tail of the MC2R was used to screen a mouse adrenal Y6 cell cDNA library using the bacterial 2-hybrid system. This identified the nucleoporin, Nup 50 (Npap60), as the major full length interacting protein. Interaction was confirmed by a GST pull-down assay and by coimmunoprecipitation in human H295R cells (which express both proteins endogenously). Deletion analysis identified the region between residues 143 and 466 in Nup50 as being required for interaction with the MC2R. Stimulation of H295R cells with ACTH (10−6M) was followed by a gradual translocation of the Nup50-MC2R complex from the membrane to the nucleus after 30min. This time course is most consistent with MC2R internalisation dynamics and may suggest a novel role for Nup50.
Keywords: ACTH, Melanocortin receptor, Nucleoporin
INTRODUCTION
The pituitary hormone adrenocorticotropin (ACTH) is the principal regulator of adrenal steroidogenesis and is essential for life. ACTH acts through a cell surface G protein-coupled receptor (GPCR) to stimulate the intracellular production of cAMP by activating adenylate cyclase [1]. The ACTH receptor is a member of the melanocortin receptor subtype and is known as the melanocortin 2 receptor (MC2R) [2]. MC2R is primarily expressed in the adrenal cortex [2-4] although lower levels of expression are present in adipose tissue [5], pituitary [6], skin [7] and sympathetic ganglia [8].
Although the major role of ACTH in the adrenal is to stimulate steroidogenesis, a function which appears to be largely dependent on its ability to stimulate cAMP generation, additional signaling functions of this receptor also exist, but remain less well defined. In particular these include a number of observations on its ability to stimulate calcium influx which may have a synergistic effect with cAMP on steroidogenesis [9-13]. ACTH may also stimulate arachidonic acid metabolism [14] and activate protein kinase C [15], in addition to effects in certain models on MAP kinase activation [16]. The influence of ACTH on adrenal cell growth is also highly dependent on the experimental paradigm and cell or tissue type and origin under investigation, and both proliferative (mainly in vivo studies) and anti-proliferative actions (mainly in in vitro cell culture models) are described. Recent work has identified the role of the Cdk inhibitor p27kip1 in the anti-proliferative response [17].
The MC2R requires the presence of an accessory factor, melanocortin 2 receptor accessory protein (MRAP), for trafficking to the cell surface and generation of a functional response [18,19]. We wished to explore the hypothesis that additional factors are required to interact with this receptor in order for it to achieve its full range of functions, and for this purpose we sought to identify additional proteins that might interact with it using bacterial two-hybrid (B2H) screening of cDNA libraries. In the studies reported here we used the C-terminal tail of the mouse MC2R as a ‘bait’ in the B2H system to screen a mouse Y6 adrenocortical cell line cDNA library [20].
We show that the murine MC2R interacts with the nucleoporin Nup50 previously known as Npap60 [21]. Nup50 a 60 kDa vertebrate nuclear protein was originally identified as part of the nuclear pore complex [21-23] and has since been shown to be mobile, shuttling between cytoplasm and nucleoplasm [24]. Our data suggests that ACTH stimulates this shuttling to the nucleus, raising the possibility of a novel mechanism of action for this receptor.
METHODS
Bacterial 2 Hybrid Screens
The C-terminal end of the mMC2 receptor (residues 273-296) was used as a ‘bait’ attached by a (Gly4Ser)3 repeat flexible linker to the λCI protein, taking care to ensure the reading frame was maintained. The complete sequence and frame of the construct were verified by DNA sequencing. This was used to screen a cDNA library constructed in the BacterioMatch™ target vector pTRG (Stratagene, Amsterdam) using Y6 cell cDNA. The Y6 cell is a mouse adrenocortical cell line related to the Y1 cell line, but which fails to express the MC2R gene and which shows no response to ACTH [26]. Transfection of the MC2R into this cell restores ACTH signalling. This library had an amplified titre of 1 × 109 cfu/ml and an estimated background of <0.6% non-recombinants. The average insert size was 1.13 kb and the insert range 0.7-1.9 kb. 150,000 clones/plate in 15 250×250 mm agar plates containing 10 mg/ml tetracycline were screened.
Three independent library screens were performed using tetracycline (10mg/ml) and chlorapmhenicol (10mg/ml) with kanamycin (10mg/ml) and carbenicillin (10mg/ml) as selective agents in the first screen, or 3-amino-1,2,4-triazole (3-AT; 5 mM) and streptomycin (12mg/ml) (in the latter two screens). Two plasmids (pBT-LGF2 and pTRG-Gal11) served as a positive interaction control. As negative controls the pBT MC2R tail and the pTRG empty vector and the pTRG Y6 cDNA library co-transformed with empty pBT vector were used.
The initial screen involved plating the co-transformants on selective media plates for 24 hours at 30 °C and then for a further 16 hours to detect weak interactors. All interacting clones were picked and placed on an enrichment plate and incubated at 37 °C for a further 24 hours. Following that they were streaked on plates containing selective agents tetracycline and chloramphenicol as a source for glycerol stocks and on plates containing streptomycin and 3-AT (5mM) as a secondary screen to verify positive interactors.
All positive interactors went through a scoring system to identify the strength of the interaction and possible false positives. This involved sequencing miniprep DNA to ascertain the length of the translated product of the cDNA clone and whether it was in frame with the RNA polymerase-α protein. Interactors that were out of frame and/or contained in frame stop codons were treated as false positives and discarded from further investigations.
Construction of Plasmids
Positive interactors were amplified using primers designed against the pTRG plasmid in order to be subcloned into the pGEMT-easy vector (Promega). The forward primer was designed against the end of the RNA polymerase α sequence of the pTRG target plasmid and contained an ATG codon to ensure translation in frame in later stages. All constructs were verified via PCR and sequencing. (Primer sequences and PCR conditions are available on request).
The C-terminal end of the MC2R together with the flexible linker that made up the ‘bait’ used in the B2H screens was digested out of the pBT vector using EcoRI and XhoI and subcloned into the pGEX-4T-3 vector (Amersham Biosciences) in frame with the glutathione S-transferase sequence on the vector. The correct insertion of the fragment in frame with the GST protein sequence was verified by DNA sequencing.
Construction of the plasmids containing the Nup50 fragments for in vitro transcription translation was achieved by amplifying various regions of the cDNA and subcloning these into pGEMT-easy (Primer sequences and PCR conditions are available on request).
GST Pull-down assay
A single colony of E.coli containing the recombinant pGEX-mMC2R tail plasmid with the GST- fusion protein was cultured and induced with Isopropyl β-D-1-thiogalactopyranoside (IPTG;1 mM). The pellet was lysed, sonicated and centrifuged and the supernatant was used to harvest the expressed protein. All extractions were performed in the presence of protease and phosphatase inhibitor cocktails. Extracted proteins were visualised following SDS-PAGE. Typical yields of the expressed protein were 0.8 to 1 mg/ml for a 400 ml starting culture.
The pGEMT-easy plasmid constructs containing the Nup50 fragments were sequenced and used for in vitro transcription-translation in the presence of 35S methionine-cysteine (Amersham Biosciences #AGQ0080). 500 ng of the expressed mMC2R tail-GST protein were incubated with 200 μl of ice cold PBS (binding buffer), 50 μl of the appropriate 35S-labelled Nup50 fragment and 50 μl of prepared Glutathione Sepharose 4B beads (Amersham) overnight at 4°C. The beads were then washed 4 times for 20 minutes with ice cold PBS before adding 2X SDS Laemmli buffer. Samples were boiled at 95°C for 5 minutes before SDS-PAGE electrophoresis. Results were visualised after standard autoradiography.
Cell Culture
H295R cells (ATCC) were maintained in 50% Dulbecco’s modifies Eagle’s medium (DMEM) (Sigma), 50% F12 Ham medium (Invitrogen) supplemented with 10% Ultraser SF, 1% Pen/Strep solution containing 100 U/ml penicillin and 0.1mg/ml streptomycin (Sigma), 1% ITS (0.1 mg/ml insulin, 5.5 μg/ml transferrin and 0.05 μg/ml sodium selenite) (Sigma) at 37°C in a humidified atmosphere containing 5% CO2.
Immunoprecipitations and immunoblotting
H295R cells were grown to 70% confluency before treatment and harvesting. Cells were left overnight in serum free medium before treatment with ACTH (10−6M) for various intervals. Cells were fractionated as described [27]. Briefly, cells were harvested, then resuspended in 1 ml of Buffer A (10mM Hepes pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.1 % NP40, 0.5 mM DTT, 25 u μg /ml leupeptin, 0.2 mM Na3VO4) and left to stand on ice for 20 minutes. 200 μl of this was retained as Fraction 1 (total cell lysate). The remainder was centrifuged for 20 minutes at 1000g at 4°C. The supernatant was retained as Fraction 2 (cellular and membrane fraction) and the pellet resuspended in 500 μl of Buffer B (0.3 M Hepes pH 7.9, 1.5 M KCl, 0.03 M MgCl2, 25 μg /ml leupeptin, 0.2 mM Na3VO4) and left to stand on ice for 20 minutes (crude nuclear fraction). This was centrifuged for 15 minutes at 13,000 rpm at 4°C, and the pellet resuspended in 200 μl of Buffer C (20 mM Na-Hepes pH 7.9, 25% v/v glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 mM DTT, 25 μg/ml leupeptin, 0.2 mM Na3VO4). This last fraction (Fraction 4) was the enriched nuclear fraction.
These fractions were used for immunoprecipitations and western blot analysis. For western blot analysis 30 μl of sample from each fraction was used. 3X SDS loading dye was added and the samples heated to 95 °C for 5 min. Samples were loaded onto 10% polyacrylamide gels (Biorad) and electrophoresed at 120V in Tris-glycine buffer. Gels were blotted onto PVDF membranes (Amersham) which were subsequently blocked for 1 hour in 5% Marvel and probed with anti-Nup50 followed by anti-goat-HRP. Blots were developed using the ECLplus chemiluminescence system (Amersham).
Antibodies
Anti-Nup 50 antibody (Abcam, #ab4005) and anti-β-actin antibody (Abcam, #ab8227), Anti-GRP78/BiP (Abcam #ab2902), and anti-nucleoporin 62 (Transduction Laboratories # N43620) were all used at a concentrations of 1:1000. MC2R antibodies H70 (rabbit) and C-16 (goat) raised against residues 64 – 133 and the C-terminal tail of the human receptor respectively (Santa Cruz, Heidelberg) were used at 1:1000. Peroxidase labelled anti-mouse antibodies (Cell Signalling Technology) anti-rabbit antibodies (Amersham) and anti-goat (Jackson Immunochemicals) were used at a concentration of 1: 10,000.
RESULTS
Identification of Nup 50 as an MC2R interacting protein
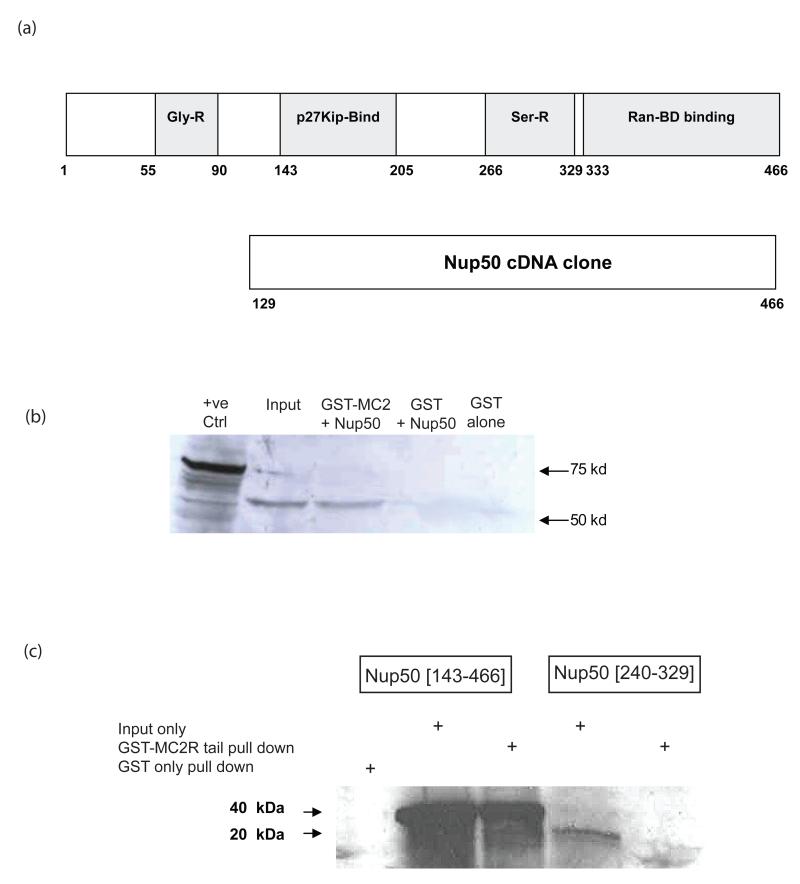
The C-terminal tail of the murine MC2R, was subcloned in frame with the λCI protein in pBT and this was used to screen a Y6 mouse adrenocortical cell cDNA library. Three independent screens of approximately 106 clones each were performed and led to the isolation on two separate occasions of the same cDNA fragment of 1.4 kb size. DNA sequencing revealed this to be identical to the cDNA encoding Nup50. This cDNA encoded a protein fragment extending from residue 129 of the translated protein to the C-terminus at residue 466 (Figure 1a).
Figure 1.
GST-pull down of Nup 50. (a) depicts the domain structure of the full length Nup50 protein and the fragment of this protein encoded by the cloned cDNA identified in bacterial two-hybrid screening. (b) GST-pull down of full length Nup50 [1-466]. 35S-labelled in vitro translated Nup50 input (lane 2) was incubated with GST-MC2R tail, washed and the retained fraction separated on SDS-PAGE (lane 3). GST alone failed to pull down any 35S-labelled protein (lane 4) (c) The Nup50 [143-466] fragment is pulled down efficiently in this system (lanes 2 & 3), although little or no pull down of the smaller serine-rich domain (Nup50 [240-329]) (lanes 4 & 5) is detected.
The MC2R:Nup50 interaction was further confirmed using the mMC2R C-tail coupled to Glutathione-S-Transferase (GST) in conjunction with in vitro transcribed and translated full length Nup50 protein labelled with 35S-Methionine. Combination of recombinant GST-mMC2R C-tail and in vitro transcribed and translated Nup501-466 protein with GST beads confirmed the interaction in vitro (Figure 1b). Two additional fragments – Nup50143-466 (containing the p27kip1-binding domain, the serine-rich domain and the Ran-BD-binding domain) and Nup50240–329 (containing the serine-rich domain alone) were also tested and demonstrated interaction of GST-mMC2R C-tail with the former but not the latter (Figure 1c). As a negative control, the GST protein alone was unable to pull down the Nup50143-466 fragment. (Figure 1c).
Co-immunoprecipitation of MC2R/Nup50 in H295R cells
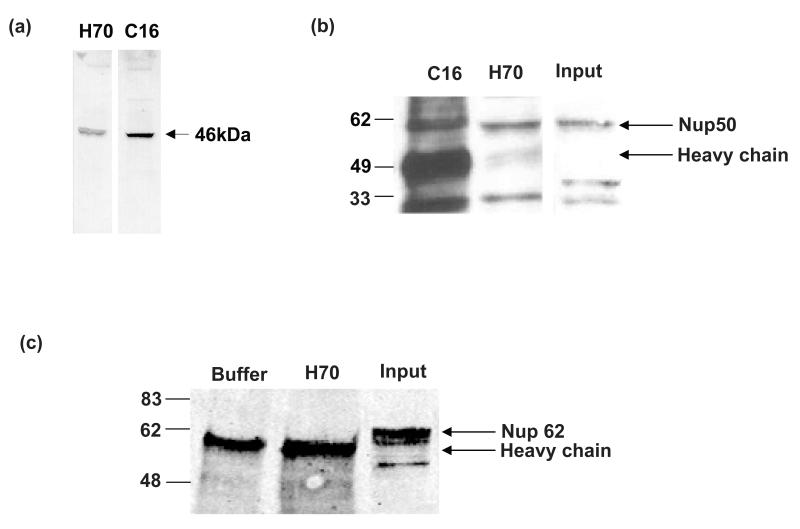
In order to attempt to demonstrate the physiological nature of this interaction we used the human adrenocortical H295R cell line which expresses both the MC2R and Nup50 endogenously. Two antibodies that immunoprecipitate the MC2R are available – C-16, raised against an epitope in the C-terminal tail, and H-70, raised against a fragment extending from the second to the third transmembrane domain of the MC2R. Both antibodies precipitate a band of a similar size in 35S-methionine labelled mouse Y1 cells (Figure 2a). Immunoprecipitates from H295R cells were separated on SDS-PAGE and immunoblotted with Nup50 antibody, revealing a strong coimmunoprecipitation (Figure 2b). The reverse co-immunoprecipitation using the Nup50 antibody to precipitate receptor was attempted, but both MC2R antibodies failed to work conclusively in these precipitates or on immunoblotting of cell lysates. This is consistent with a general observation that this receptor, in common with many other G protein-coupled receptors, resolves poorly on SDS-PAGE. The possibility that Nup50 co-precipitation was non-specific was addressed using an antibody to another nucleoporin, Nup62. No evidence of Nup62 co-precipitation was found in these cells (Figure 2c).
Figure 2.
Co-immunoprecipitation of MC2R-Nup50 complex in H295R cells. (a) Both the C16 and H-70 antibodies raised against the MC2R precipitate a similar sized 35S-methionine-labelled band in mouse Y1 cells. (b) Using both antibodies it was possible to co-immunoprecipitate Nup50 in H295R cells. (c) Nup 62 does not interact with MC2R. Immunoprecipitation of buffer alone (lane 1) or H295R lysate (lane 2) with the H70 MC2R antibody failed to precipitate Nup 62. Nup 62 is readily detectable in non-immunoprecipitated cell lysates (lane 3).
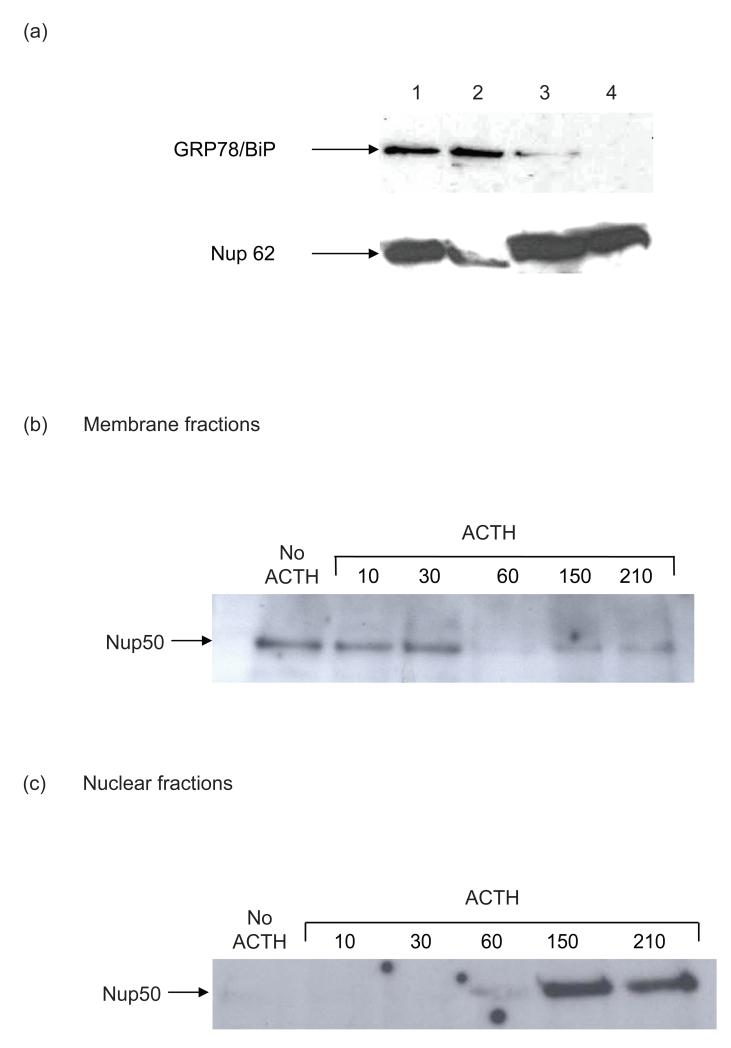
In view of the uncertain subcellular localization of the MC2R-Nup50 complex, cells were fractionated so as to enrich both membranous and nuclear fractions. The quality of the fractionation was demonstrated by immunoblotting using GRP78/ BiP, an endoplasmic reticulum (ER) marker, and Nup62, as a nuclear envelope marker. (Figure 3a). This supports the notion that the purified nuclear fraction (Fraction 4) is free of endoplasmic reticulum contamination, although some nuclear contamination persists in the membrane fraction (Fraction 2). In the membrane fraction Nup50 coimmunoprecipitates with MC2R in the unstimulated cell. However stimulation of cells with ACTH leads to a decline in the presence of the complex by 60 min (Figure 3b). In contrast, in the enriched nuclear fraction no co-precipitated Nup50 is detectable at rest or until 60 min after ACTH stimulation, following which there is a significant accumulation of the MC2R-Nup50 complex. (Figure 3c).
Figure 3.
Subcellular fractionation of the MC2R-Nup50 complex. H295R cells were fractionated as described [27] into four fractions (whole cell lysates, membrane, crude nuclear, and purified nuclear fractions). (a) Demonstrates the contents of subcellular fraction for an ER marker (GRP78/BiP) and nuclear membranes (Nup62) by immunoblotting. (Fraction 1, whole cell lysate; fraction 2, membranes; fraction 3, crude nuclear extract; fraction 4, purified nuclear extract). (b) In membrane fractions the MC2R-Nup50 complex is demonstrated by co-immunoprecipitation using the MC2R C-16 antibody at rest and following stimulation by ACTH (10−6M) for the initial 30 minutes. Some reappearance of the complex occurs by 150 minutes. (c) in purified nuclear fractions the MC2R-Nup50 complex is not detectable at rest, but begins to appear by 60 minutes and is prominent at 150 and 210 minutes.
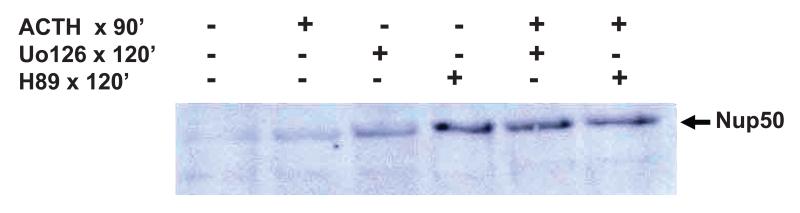
In view of the dependency of the nuclear translocation of the MC2R-Nup50 complex on ACTH stimulation the role of established signalling pathways in mediating this process was investigated. ACTH acting via the MC2R in the adrenal cortex stimulates steroidogenesis by activating adenylate cyclase to make cAMP, which in turn activates protein kinase A as its second messenger. However in the H295R cell ACTH stimulation normally only generates a very weak cAMP response. Use of the widely recognised protein kinase A inhibitor, H89, has no effect in blocking the translocation of MC2R-Nup50 to the nucleus at 90 min (Figure 4). H295R cells generate a fairly robust MAP kinase response to ACTH stimulation. However use of the MAP kinase inhibitor UO126 to block this action also was without effect on nuclear translocation.
Figure 4.
Effect of inhibition of signal transduction. Nuclear fractions of H295R cells stimulated with or without ACTH (10−6M) for 90 min with prior exposure to either the protein kinase A inhibitor (H89) or the MAP kinase inhibitor (UO126) were used to coimmunoprecipitate the MC2R-Nup50 complex. Neither inhibitor appears to significantly reduce appearance of the complex.
DISCUSSION
Characterisation of the MC2R has been severely impaired as a result of difficulties in expressing a functional receptor protein in transfected cells. We previously demonstrated that transfected MC2R was translated, but that this protein accumulated in the endoplasmic reticulum and Golgi, and we postulated that an accessory factor was required for the receptor to traffic to the cell surface in the adrenal, its principal physiological site of expression [18]. A genetic approach based on ACTH resistant patients ultimately led to the identification of MRAP [19]. However alternative strategies were initiated to identify MC2R interacting factors which included the B2H approach reported here. As we report, the principal cDNA identified in this screen was the nuclear protein Nup50.
The MC2R-Nup50 interaction was confirmed using a GST pull down assay with the full length Nup50 protein as well as with a smaller fragment that contained the putative p27kip1 and RanBD binding domains. The physiological relevance of this interaction was subsequently confirmed by co-immunoprecipitation in the H295R cell line, a human adrenocortical cell line that expresses modest amounts of endogenous MC2R and Nup50. Furthermore the MC2R-Nup50 complex appears to translocate from the membrane to the nucleus in a relatively delayed response to ACTH stimulation. One possibility is that endoplasmic reticulum may co-purify with the nucleus. However, this is unlikely in view of the demonstration of the complete absence of GRP78/Bip, a well recognized marker of endoplasmic reticulum in the purified nuclear fraction.
It is clear from our data that the MC2R-Nup 50 complex is present in unstimulated cells at the plasma membrane. Stimulation of receptor with ACTH leads to translocation of the receptor-Nup50 complex to the nucleus with a time course which is not dissimilar to that of MC2R internalization observed in the mouse Y1 cell line [28] or the H295R cell line (unpublished observations). Inhibition of PKA or MAPK signalling in this cell line does not interfere significantly with either internalisation (unpublished data) or MC2R-Nup50 nuclear translocation. If, as we speculate, translocation follows receptor internalisation, it is possible that a process of endosomal trafficking from the plasma to the nuclear membrane is taking place. Attempts to visualise this using immunofluorescent studies have been unsuccessful owing to the poor specificity of both the Nup 50 and MC2R antibodies when used in this way.
Some uncertainty exists over the function of Nup50. It was originally found to be one of the nucleoporins that form the nuclear pore basket [22] and to be nucleoplasmically orientated [21]. Subsequent work suggested that it was not a structural component of the nuclear pore complex, but a Ran binding protein and a co-factor for importin α:β mediated import [24]. The same study reported that endogenous Nup50 was mobile and could shuttle across the nuclear membrane. The N-terminal domain has been shown to bind with high affinity to importin-α and the crystallographic structure of this complex has recently been reported [25]. Matsuura & Stewart have used this data together with a stopped-flow FRET assay to argue that Nup50 functions to undo the importin-α-NLS cargo complex on the nucleoplasmic side of the nuclear membrane, thereby enhancing the rate of nuclear import [25].
It has also been shown that Nup50 can interact with p27kip1, a member of the Cip-Kip family of the cyclin-dependent kinase (Cdk) inhibitors that binds to cyclin-Cdk complexes and inhibits their catalytic activity in response to anti-proliferative stimuli [23]. This is of particular interest since there is substantial data to show that ACTH activates anti-mitogenic mechanisms in the Y1 mouse adrenocortical cell line and that this is mediated through p27kip1 protein induction [17, 29]. These studies did not examine nuclear:cytoplasmic distribution of p27kip1, and this is likely to offer a further level of regulation of p27kip1 activity. Deletion of p27kip1 in mice leads to adrenal tumour development (in addition to other tumours) [30]. However we were unable to convincingly demonstrate co-precipitation of p27kip1 with the MC2R-Nup50 complex in H295R cells (data not shown).
The presence of GPCRs in the nucleus or at the nuclear membrane has been observed in several previous studies with a range of GPCRs. Amongst these, some of the earliest observations were with the AT1 angiotensin receptors which used ligand binding and immunoelectron microscopy techniques to demonstrate the presence of this receptor on nuclear membranes or in the nucleus, probably in a G protein-coupled state. Other receptors apparently expressed in this location include prostaglandin EP3 and EP4 receptors in porcine cerebral microvascular endothelial cells and in stably transfected HEK293 cells [31]. This study used mainly indirect immunofluorescence and confocal microscopy but also demonstrated functional G protein coupling and a potential role in regulation of genes such as eNOS [32,33]. In the same way, nuclear metabotropic glutamate receptors bound radiolabeled quisqualate and regulated nuclear oscillations of Ca++ with consequent effects on gene expression [34]. In the case of the endothelin receptors, a yeast two hybrid approach [35] revealed an interaction between the C-terminal tail of the ETA receptor and the nuclear proteins Tip60 and HDAC7. In the absence of ET-1 the receptor was mainly expressed at the cell surface and Tip60 and HDAC7 were found mainly in the nucleus whereas in the presence of ET-1 there was a marked shift of Tip60 and HDAC7 to the perinuclear region where the ETA receptor could also be detected. A further study demonstrated nuclear endothelin receptors were capable of specific ligand binding, induction of a transient increase in nuclear cisternal Ca++ content and stimulation of nuclear protein kinase activity [36].
The physiological functions of GPCRs on nuclear membranes are at present largely unknown. It is noteworthy that several proteins that have been strongly associated with the cell surface functions of GPCRs have also been localised in the nuclear region. These include heterotrimeric G proteins [37], adenylyl cyclase [38], phospholipase C [39] and β-arrestin 1 [40]. Many questions about a possible nuclear role for GPCRs remain to be answered such as identifying a mechanism for their translocation to the nucleus, but the mounting evidence suggests this is an underexplored aspect of the function of this class of receptors.
ACKNOWLEDGEMENTS
We would like to thank Professor Bernard Schimmer, University of Toronto, Canada, for the gift of the Y6 cell line. This work was funded by a project grant (C15456) from the BBSRC and the MRC.
REFERENCES
- 1.Halkerston IDK. Cyclic AMP and adrenocortical function. Adv. Cycl. Nucleot. Res. 1975;6:100–136. [PubMed] [Google Scholar]
- 2.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 3.Cammas FM, Pullinger GD, Barker S, Clark AJL. The mouse ACTH receptor gene: cloning and characterization of its promoter and evidence for a role for the orphan nuclear receptor SF-1. Mol. Endocrinol. 1997;11:867–876. doi: 10.1210/mend.11.7.9938. [DOI] [PubMed] [Google Scholar]
- 4.Naville D, Barjhoux L, Jaillard C, Lebrethon MC, Saez JM, Begeot M. Characterization of the transcription start site of the ACTH receptor gene: presence of an intronic sequence in the 5′-flanking region. Mol Cell Endocrinol. 1994;106:131–5. doi: 10.1016/0303-7207(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 5.Boston BA, Cone RD. Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinol. 1996;137:2043–50. doi: 10.1210/endo.137.5.8612546. [DOI] [PubMed] [Google Scholar]
- 6.Morris DG, Kola B, Borboli N, Kaltsas GA, Gueorguiev M, McNicol AM, Ferrier R, Jones TH, Baldeweg S, Powell M, et al. Identification of adrenocorticotropin receptor messenger ribonucleic acid in the human pituitary and its loss of expression in pituitary adenomas. J Clin Endocrinol Metab. 2003;88:6080–7. doi: 10.1210/jc.2002-022048. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–9. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 8.Nankova BB, Kvetnansky R, Sabban EL. Adrenocorticotropic hormone (MC-2) receptor mRNA is expressed in rat sympathetic ganglia and up-regulated by stress. Neurosc. Lett. 2003;344:149–152. doi: 10.1016/s0304-3940(03)00361-6. [DOI] [PubMed] [Google Scholar]
- 9.Kojima I, Kojima K, Rasmussen H. Role of calcium and cAMP in the action of adrenocorticotropin on aldosterone secretion. J Biol Chem. 1985;260:4248–56. [PubMed] [Google Scholar]
- 10.Durroux T, Gallo-Payet N, Payet MD. Effects of adrenocorticotropin on action potential and calcium currents in cultured rat and bovine glomerulosa cells. Endocrinol. 1991;129:2139–47. doi: 10.1210/endo-129-4-2139. [DOI] [PubMed] [Google Scholar]
- 11.Enyeart JJ. Biochemical and Ionic signaling mechanisms for ACTH-stimulated cortisol production. Vitam Horm. 2005;70:265–279. doi: 10.1016/S0083-6729(05)70008-X. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki T, Kimoto T, Higuchi K, Ohta Y, Kawato S, Kominami S. Calcium ion as a second messenger for o-nitrophenylsulfenyl-adrenocorticotropin (NPS-ACTH) and ACTH in bovine adrenal steroidogenesis. Endocrinol. 1998;139:4765–71. doi: 10.1210/endo.139.12.6338. [DOI] [PubMed] [Google Scholar]
- 13.Chorvatova A, Gendron L, Bilodeau L, Gallo-Payet N, Payet MD. A Ras-dependent chloride current activated by adrenocorticotropin in rat adrenal zona glomerulosa cells. Endocrinol. 2000;141:684–92. doi: 10.1210/endo.141.2.7328. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Walsh LP, Reinhart A, Stocco DM. The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J Biol Chem. 2000;275:20204–9. doi: 10.1074/jbc.M003113200. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe G, Pena P, Albanese C, Wilsbacher LD, Young JB, Pestell RG. Adrenocorticotropin induction of stress-activated protein kinase in the adrenal cortex in vivo. J Biol Chem. 1997;272:20063–9. doi: 10.1074/jbc.272.32.20063. [DOI] [PubMed] [Google Scholar]
- 16.Le T, Schimmer BP. The regulation of MAPKs in Y1 mouse adrenocortical tumor cells. Endocrinol. 2001;142:4282–4287. doi: 10.1210/endo.142.10.8441. [DOI] [PubMed] [Google Scholar]
- 17.Forti FL, Schwindt TT, Moraes MS, Eichler CB, Armelin HA. ACTH promotion of p27(Kip1) induction in mouse Y1 adrenocortical tumor cells is dependent on both PKA activation and Akt/PKB inactivation. Biochemistry. 2002;41:10133–40. doi: 10.1021/bi0258086. [DOI] [PubMed] [Google Scholar]
- 18.Noon LA, Franklin JM, King PJ, Goulding NJ, Hunyady L, Clark AJL. Failed export of the adrenocorticotropin receptor from the endoplasmic reticulum in non-adrenal cells: evidence in support of a requirement for a specific adrenal accessory factor. J. Endocrinol. 2002;174:17–25. doi: 10.1677/joe.0.1740017. [DOI] [PubMed] [Google Scholar]
- 19.Metherell LA, Chapple JP, Cooray S, David A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg P, et al. Mutations in MRAP, encoding a novel interacting partner of the ACTH receptor, cause Familial Glucocorticoid Deficiency Type 2. Nat. Genet. 2005;37:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 20.Dove SL, Hochschild A. A bacterial two-hybrid system based on transcription activation. Methods Mol Biol. 2004;261:231–46. doi: 10.1385/1-59259-762-9:231. [DOI] [PubMed] [Google Scholar]
- 21.Guan T, Kehlenbach RH, Schirmer EC, Kehlenbach A, Fan F, Clurman BE, Arnheim N, Gerace L. Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export. Mol Cell Biol. 2000;20:5619–30. doi: 10.1128/mcb.20.15.5619-5630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan F, Liu CP, Korobova O, Heyting C, Offenberg HH, Trump G, Arnheim N. cDNA cloning and characterization of Npap60: a novel rat nuclear pore-associated protein with an unusual subcellular localization during male germ cell differentiation. Genomics. 1997;40:444–53. doi: 10.1006/geno.1996.4557. [DOI] [PubMed] [Google Scholar]
- 23.Smitherman M, Lee K, Swanger J, Kapur R, Clurman BE. Characterization and targeted disruption of murine Nup50, a p27(Kip1)-interacting component of the nuclear pore complex. Mol Cell Biol. 2000;20:5631–42. doi: 10.1128/mcb.20.15.5631-5642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay ME, Plafker K, Smith AE, Clurman BE, Macara IG. Npap60/Nup50 is a tri-stable switch that stimulates importin-alpha:beta-mediated nuclear protein import. Cell. 2002;110:349–60. doi: 10.1016/s0092-8674(02)00836-x. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura Y, Stewart M. Nup50/Npap60 function in nuclear protein import complex disassembly and importin recycling. EMBO J. 2005;24:3681–9. doi: 10.1038/sj.emboj.7600843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schimmer BP, Kwan WK, Tsao J, Qiu R. Adrenocorticotropin-resistant mutants of the Y1 adrenal cell line fail to express the adrenocorticotropin receptor. J Cell Physiol. 1995;163:164–171. doi: 10.1002/jcp.1041630119. [DOI] [PubMed] [Google Scholar]
- 27.Jo Y, Stocco DM. Regulation of steroidogenesis and steroidogenic acute regulatory protein in R2C cells by DAX-1 (dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene-1) Endocrinol. 2004;145:5629–37. doi: 10.1210/en.2004-0941. [DOI] [PubMed] [Google Scholar]
- 28.Baig AH, Szaszák M, King PJ, Hunyady L, Clark AJL. Agonist activated adrenocorticotropin receptor internalizes via a clathrin-mediated G protein receptor kinase dependent mechanism. Endocr. Res. 2002;28:281–289. doi: 10.1081/erc-120016798. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi H, Kambe F, Imai T, Hibi Y, Kikumori T, Ohmori S, Nakao A, Seo H. Differential expression of cyclin-dependent kinase inhibitors, p27Kip1 and p57Kip2, by corticotropin in rat adrenal cortex. J.Endocrinol. 2006;189:671–9. doi: 10.1677/joe.1.06419. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–20. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya M, Peri KG, Almazan G, Ribeiro-da-Silva A, Shichi H, Durocher Y, Abramovitz M, Hou X, Varma DR, Chemtob S. Nuclear localization of prostaglandin E2 receptors. Proc Natl Acad Sci U S A. 1998;95:15792–7. doi: 10.1073/pnas.95.26.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gobeil F, Jr., Dumont I, Marrache AM, Vazquez-Tello A, Bernier SG, Abran D, Hou X, Beauchamp MH, Quiniou C, Bouayad A, et al. Regulation of eNOS expression in brain endothelial cells by perinuclear EP(3) receptors. Circ Res. 2002;90:682–9. doi: 10.1161/01.res.0000013303.17964.7a. [DOI] [PubMed] [Google Scholar]
- 33.Cieslik K, Abrams CS, Wu KK. Up-regulation of endothelial nitric-oxide synthase promoter by the phosphatidylinositol 3-kinase gamma /Janus kinase 2/MEK-1-dependent pathway. J Biol Chem. 2001;276:1211–9. doi: 10.1074/jbc.M005305200. [DOI] [PubMed] [Google Scholar]
- 34.O’Malley KL, Jong YJ, Gonchar Y, Burkhalter A, Romano C. Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J Biol Chem. 2003;278:28210–9. doi: 10.1074/jbc.M300792200. [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ, Chun M, Kandror KV. Tip60 and HDAC7 interact with the endothelin receptor a and may be involved in downstream signaling. J Biol. Chem. 2001;276:16597–600. doi: 10.1074/jbc.C000909200. [DOI] [PubMed] [Google Scholar]
- 36.Boivin B, Chevalier D, Villeneuve LR, Rousseau E, Allen BG. Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. J Biol Chem. 2003;278:29153–63. doi: 10.1074/jbc.M301738200. [DOI] [PubMed] [Google Scholar]
- 37.Willard FS, Crouch MF. Nuclear and cytoskeletal translocation and localization of heterotrimeric G-proteins. Immunol Cell Biol. 2000;78:387–94. doi: 10.1046/j.1440-1711.2000.00927.x. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto S, Kawamura K, James TN. Intracellular distribution of adenylate cyclase in human cardiocytes determined by electron microscopic cytochemistry. Microsc Res Tech. 1998;40:479–87. doi: 10.1002/(SICI)1097-0029(19980301)40:6<479::AID-JEMT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 39.Faenza I, Matteucci A, Manzoli L, Billi AM, Aluigi M, Peruzzi D, Vitale M, Castorina S, Suh PG, Cocco L. A role for nuclear phospholipase Cbeta 1 in cell cycle control. J Biol Chem. 2000;275:30520–4. doi: 10.1074/jbc.M004630200. [DOI] [PubMed] [Google Scholar]
- 40.Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, et al. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–47. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]