Abstract
Background
Damage caused by lung overdistension (volutrauma) has been implicated in the development of bronchopulmonary dysplasia (BPD). Modern neonatal ventilation modes can target a set tidal volume as an alternative to traditional pressure‐limited ventilation (PLV) using a fixed inflation pressure. Volume‐targeted ventilation (VTV) aims to produce a more stable tidal volume in order to reduce lung damage and stabilise the partial pressure of carbon dioxide (pCO2).
Objectives
To determine whether VTV compared with PLV leads to reduced rates of death and death or BPD in newborn infants and to determine whether use of VTV affected outcomes including air leak, cranial ultrasound findings and neurodevelopment.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 12), MEDLINE via PubMed (1966 to 13 January 2017), Embase (1980 to 13 January 2017) and CINAHL (1982 to 13 January 2017). We also searched clinical trials databases, conference proceedings and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials. We contacted the principal investigators of studies to obtain supplementary information.
Selection criteria
Randomised and quasi‐randomised trials comparing VTV versus PLV in infants of less than 44 weeks' postmenstrual age and reporting clinically relevant outcomes.
Data collection and analysis
We assessed risk of bias for each trial using Cochrane methodology. We evaluated quality of evidence for each outcome using GRADE criteria. We tabulated mortality, rates of BPD, short‐term clinical outcomes and long‐term developmental outcomes.
Statistics: for categorical outcomes, we calculated typical estimates for risk ratios (RR), risk differences (RD) and number needed to treat for an additional beneficial outcome (NNTB). For continuous variables, we calculated typical estimates for mean differences (MD). We used 95% confidence intervals (CI) and assumed a fixed‐effect model for meta‐analysis.
Main results
Twenty randomised trials met our inclusion criteria; 16 parallel trials (977 infants) and four cross‐over trials (88 infants). No studies were blinded and the quality of evidence for outcomes assessed varied from moderate to low.
We found no difference in the primary outcome, death before hospital discharge, between VTV modes versus PLV modes (typical RR 0.75, 95% CI 0.53 to 1.07; low quality evidence). However, there was moderate quality evidence that the use of VTV modes resulted in a reduction in the primary outcome, death or BPD at 36 weeks' gestation (typical RR 0.73, 95% CI 0.59 to 0.89; typical NNTB 8, 95% CI 5 to 20) and the following secondary outcomes: rates of pneumothorax (typical RR 0.52, 95% CI 0.31 to 0.87; typical NNTB 20, 95% CI 11 to 100), mean days of mechanical ventilation (MD ‐1.35 days, 95% CI ‐1.83 to ‐0.86), rates of hypocarbia (typical RR 0.49, 95% CI 0.33 to 0.72; typical NNTB 3, 95% CI 2 to 5), rates of grade 3 or 4 intraventricular haemorrhage (typical RR 0.53, 95% CI 0.37 to 0.77; typical NNTB 11, 95% CI 7 to 25) and the combined outcome of periventricular leukomalacia with or without grade 3 or 4 intraventricular haemorrhage (typical RR 0.47, 95% CI 0.27 to 0.80; typical NNTB 11, 95% CI 7 to 33). VTV modes were not associated with any increased adverse outcomes.
Authors' conclusions
Infants ventilated using VTV modes had reduced rates of death or BPD, pneumothoraces, hypocarbia, severe cranial ultrasound pathologies and duration of ventilation compared with infants ventilated using PLV modes. Further studies are needed to identify whether VTV modes improve neurodevelopmental outcomes and to compare and refine VTV strategies.
Plain language summary
A comparison of volume‐targeted ventilation modes with traditional pressure‐limited ventilation modes for newborn babies
Review question: Does ventilator therapy of infants using a strategy targeting inflation volume rather than inflation pressure lead to lower rates of death or lung damage (or both) among these infants?
Background: Preterm babies may need help to breathe. The risk of lung problems increases with increasing immaturity (the earlier the babies are born). For some babies, the assistance of a ventilator (breathing machine) can be life saving; however, ventilators may also injure the infant's immature lungs. Traditionally, ventilators for infants have been used in a pressure‐limited mode of ventilation, where the pressure leads to variable amount of air entering the lungs. New volume‐targeted methods of ventilation have been developed which aim to reduce lung injury by controlling the amount of air entering the lungs with each inflation.
Study characteristics: In a search updated to January 2017, review authors identified 20 studies for inclusion in the review. Sixteen studies (977 infants) compared two separate groups of infants treated with a volume‐targeted mode of ventilation compared with a pressure‐limited mode of ventilation. In four studies (84 infants), the infants were treated with both modes of ventilation in a cross‐over design (where infants had ventilation with one method and were then swapped over to the second method). Most of the studies were of moderate to low quality and none of them were blinded to those who assessed therapy. The most important results from this review were based on data from eight to 12 studies including 584 to 771 infants.
Key results: Babies ventilated using volume‐targeted modes of ventilation were more likely to survive free of lung damage. They needed ventilator assistance for a shorter duration and were less likely to develop pneumothorax (a condition when air escapes from the lung into the chest). They had more stable carbon dioxide levels in the blood, and had fewer brain ultrasound abnormalities. There was no evidence that volume‐targeted modes were more likely to harm the infant than traditional pressure‐limited modes. More research is needed to understand whether volume‐targeted modes also lead to improvements in the development of movement and intellect. More research is also needed comparing different volume‐targeting techniques.
Quality of evidence: Low to moderate quality as none of the studies were blinded and there were issues with study design in some of the studies.
Summary of findings
Summary of findings for the main comparison. Volume‐targeted compared to pressure‐limited ventilation: main findings.
| Volume‐targeted ventilation compared to pressure‐limited ventilation | ||||||
| Patient or population: neonates up to 44 weeks' postmenstrual age Setting: neonatal intensive care unit Intervention: VTV Comparison: PLV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with PLV | Risk with VTV | |||||
| Death before discharge from hospital | Study population | RR 0.75 (0.53 to 1.07) | 771 (11 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Imprecision of estimates. 95% CI < 0.75. | |
| 163 per 1000 | 122 per 1000 (86 to 175) | |||||
| Death or BPD (36 weeks) | Study population | RR 0.73 (0.59 to 0.89) | 584 (8 RCTs) | ⊕⊕⊕⊝ Moderate | Unblinded studies. | |
| 458 per 1000 | 334 per 1000 (270 to 408) | |||||
| Duration of positive pressure ventilation (days) | MD of positive pressure ventilation (days); PLV group 0 | MD 1.35 lower (1.83 lower to 0.86 lower) in VTV group | ‐ | 736 (12 RCTs) | ⊕⊕⊕⊝ Moderate | Unblinded studies. |
| Pneumothorax | Study population | RR 0.52 (0.31 to 0.87) | 825 (13 RCTs) | ⊕⊕⊕⊝ Moderate | Unblinded studies. | |
| 88 per 1000 | 46 per 1000 (27 to 77) |
|||||
| IVH grade 3‐4 | Study population | RR 0.53 (0.37 to 0.77) |
712 (10 RCTs) |
⊕⊕⊕⊝ Moderate | Unblinded studies. | |
| 184 per 1000 | 97 per 1000 (68 to 141) |
|||||
| IVH grade 3‐4 or PVL | Study population | RR 0.47 (0.27 to 0.80) |
441 (6 RCTs) | ⊕⊕⊕⊝ Moderate | Unblinded studies. | |
| 164 per 1000 | 77 per 1000 (44 to 131) |
|||||
| BPD (supplemental oxygen at 36 weeks) | Study population | RR 0.68 (0.53 to 0.87) | 620 (9 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Possible publication bias based on funnel plot. | |
| 346 per 1000 | 235 per 1000 (183 to 301) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPD: bronchopulmonary dysplasia; CI: confidence interval; IVH: intraventricular haemorrhage; MD: mean difference; PLV: pressure‐limited ventilation; PVL: periventricular leukomalacia; RCT: randomised controlled trial; RR: risk ratio; VTV: volume‐targeted ventilation. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 2. Volume‐targeted compared to pressure‐limited ventilation: additional findings.
| Volume‐targeted ventilation compared to pressure‐limited ventilation | ||||||
| Patient or population: neonates up to 44 weeks' postmenstrual age Setting: neonatal intensive care unit Intervention: VTV Comparison: PLV | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with PLV | Risk with VTV | |||||
| Death or BPD (28 days) | Study population | RR 0.87 (0.64 to 1.18) | 149 (3 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 536 per 1000 | 467 per 1000 (343 to 633) |
|||||
| Failure of mode of ventilation | Study population | RR 0.69 (0.48 to 1.00) | 445 (5 RCTs) | ⊕⊕⊕⊝ Moderate | Unblinded studies. | |
| 242 per 1000 | 167 per 1000 (116 to 242) | |||||
| Addition of neuromuscular paralysis where previously not paralysed | Study population | RR 0.32 (0.07 to 1.40) | 75 (2 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Small numbers of participants. | |
| 179 per 1000 | 57 per 1000 (13 to 251) | |||||
| Duration of positive pressure ventilation (log data) | The mean duration of IPPV (log data) was 0 | MD 0.08 lower (0.16 lower to 0) | ‐ | 381 (5 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Heterogeneity of study results. |
| Inspired oxygen concentration % (study definition) | The mean difference in inspired oxygen concentration %; PLV group 0 |
The mean inspired oxygen concentration % was 0.92 lower (2.08 lower to 0.24 higher) in VTV group | ‐ | 324 (7 RCTs) | ⊕⊕⊕⊝ Moderate | Unblinded studies. |
| Any pH < 7.25 | Study population | RR 0.80 (0.52 to 1.23) | 98 (3 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 380 per 1000 | 304 per 1000 (198 to 467) | |||||
|
Hypocarbia pCO2 < 35 mmHg/4.7 kPa |
Study population | RR 0.49 (0.33 to 0.72) | 98 (3 RCTs) | ⊕⊕⊕⊝ Moderate | Unblinded studies. Small trials, but large effect and biologically plausible. | |
| 720 per 1000 | 353 per 1000 (238 to 518) |
|||||
|
Respiratory acidosis pH < 7.25 and pCO2 > 60 mmHg/8 kPa |
Study population | RR 0.93 (0.51 to 1.70) | 98 (3 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 240 per 1000 | 223 per 1000 (122 to 408) | |||||
| Either hypocarbia or respiratory acidosis | Study population | RR 0.68 (0.42 to 1.10) | 37 (2 RCTs) | ‐ | No quality assessment possible. | |
| 1889 per 1000 | 1000 per 1000 (793 to 1000) | |||||
| Patent ductus arteriosus | Study population | RR 0.95 (0.80 to 1.12) | 754 (10 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Variable diagnostic practices employed. | |
| 391 per 1000 | 371 per 1000 (313 to 438) | |||||
| Air leak (any) | Study population | RR 0.79 (0.44 to 1.43) | 374 (5 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. Few participants. | |
| 117 per 1000 | 92 per 1000 (51 to 167) | |||||
| Pulmonary interstitial emphysema | Study population | RR 1.21 (0.63 to 2.30) | 430 (6 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. | |
| 65 per 1000 | 79 per 1000 (41 to 150) | |||||
| Any IVH | Study population | RR 0.82 (0.62 to 1.08) | 445 (5 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. | |
| 342 per 1000 | 281 per 1000 (212 to 370) | |||||
| PVL | Study population | OR 0.43 (0.19 to 0.98) |
508 (7 RCTs) | ⊕⊕⊕⊝ Moderate | Unblinded studies. | |
| 71 per 1000 | 32 per 1000 (14 to 69) |
|||||
| Any IVH or PVL | Study population | RR 0.83 (0.58 to 1.18) | 298 (3 RCTs) | ⊕⊕⊕⊝ Moderate | Unblinded studies. | |
| 308 per 1000 | 256 per 1000 (179 to 364) | |||||
| BPD (supplemental oxygen at 28 days) | Study population | RR 0.91 (0.64 to 1.30) | 206 (4 RCTs) | ⊕⊕⊝⊝ Low | Unblinded studies. Imprecision of estimates, 95% CI < 0.75. | |
| 354 per 1000 | 322 per 1000 (226 to 460) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPD: bronchopulmonary dysplasia; CI: confidence interval; IPPV: intermittent positive pressure ventilation; IVH: intraventricular haemorrhage; MD: mean difference; pCO2: partial pressure of carbon dioxide; PLV: pressure‐limited ventilation; PVL: periventricular leukomalacia; RCT: randomised controlled trial; RR: risk ratio; VTV: volume‐targeted ventilation. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
Background
Description of the condition
Mechanical ventilation remains an essential tool in the care of critically sick and very preterm infants, despite improvements in perinatal care including increased use of antenatal steroids and non‐invasive respiratory support. The Neonatal Research Network in the USA reported for the year 2012 that 82% of infants born before 29 weeks' gestation received conventional mechanical ventilation during their stay in the neonatal intensive care unit (Stoll 2015). The main indications for mechanical ventilation in preterm infants are respiratory distress syndrome (RDS), lung immaturity and poor respiratory drive. Although the respiratory difficulties resolve in most of these infants, studies show that around 40% of surviving infants 28 weeks' or less gestational age (GA) develop bronchopulmonary dysplasia (BPD) with oxygen dependency at 36 weeks' postmenstrual age (Stoll 2015; Stensvold 2017). The resulting burden of BPD includes increased duration of respiratory support and hospital stay, need for home oxygen, impaired neurodevelopmental outcome, more readmissions to hospital and increased mortality.
BPD was first reported in 1967 in a group of preterm infants who developed chronic lung disease after receiving ventilation and high oxygen concentration for RDS (Northway 1967). More recently, BPD has been defined as the requirement for supplemental oxygen at either 28 days' postnatal age (NIH 1979) or at 36 weeks' postmenstrual age (Shennan 1988). The current definition takes into account total duration of oxygen supplementation, need for positive pressure ventilation (PPV) or nasal continuous positive airway pressure (CPAP) and GA, in addition to oxygen dependency at 36 weeks' postmenstrual age (Jobe 2001).
BPD is characterised by the histopathological findings of impaired alveolarisation, altered pulmonary microvasculature and pulmonary fibrosis. The development of BPD has been linked to lung immaturity, intrauterine growth restriction (Bardin 1997; Gortner 1999), infection (Hannaford 1999), oxidant stress (Warner 1998), in‐utero inflammation (Watterberg 1996), and mechanical ventilation (Coalson 1999; Clark 2000). Ventilation strategies have been identified as potentially modifiable cause of BPD, and research has been devoted to developing ventilation strategies which avoid the overdistension, atelectasis and shear stresses that are thought to lead to lung injury and consequently BPD. The fact that lung injury in a preterm lamb model was demonstrated following only six large inflations immediately after birth (Bjorklund 1997), highlights the potential importance of early use of protective ventilation strategies in the neonate.
Description of the intervention
Mechanical ventilation is primarily used because an infant is failing to breathe adequately, leading to CO2 retention. CO2 removal is determined by tidal volume (VT) and respiratory or ventilator rate. Volume‐targeted ventilation (VTV) strategies aim to deliver a consistent VT. TV‐oriented modes have been in use in paediatric and adult practice for many years. However, the technological limitations of older ventilators precluded their use in preterm infants because they were unable to accurately deliver the small VT required when ventilating small preterm infants. Modern microprocessor‐controlled neonatal ventilators with flow sensors permit accurate measurement and delivery of a set VT. Earlier designs included a flow sensor built into the ventilator, however, with this design, VT measurements are affected by the compliance of the ventilator circuit. Newer designs include sensors that can be placed at the Wye piece between the ventilator circuit and the endotracheal tube (ETT). With appropriate software, the ventilators measure and control ventilator parameters to target the delivered VT, and reduce VT variability delivery compared with pressure‐limited ventilation (PLV) modes (Abubakar 2001).
When using a ventilator in a VTV mode, the clinician sets a target VT. Different VTV modes measure inflation VT, expired VT or both to control VT delivery. Expired VT is less affected by ETT leaks, and measuring both inspired and expired VT enables ETT leak to be quantified. There are many different forms of VTV. Depending on the ventilator design and the mode selected, the ventilator adjusts one or more of the peak inflation pressure (PIP), inflation time and inflation flow. Some ventilators offer more than one VTV mode. Thus, there are some differences between the mechanisms or algorithms by which different ventilators control and modify VT, but they all provide a similar volume‐targeted approach to newborn mechanical ventilation.
How the intervention might work
Traditionally, neonatologists treating infants with severe respiratory conditions have employed continuous flow, time‐cycled, PLV. In PLV mode, the assistance provided by the ventilator is controlled in two ways. The magnitude of each inflation is determined by the change in airway pressure (i.e. the difference between PIP and the baseline or positive end‐expiratory pressure (PEEP)). The VT for any inflation depends on both this pressure difference, which drives gas movement, and the lung compliance. Although VT is indirectly determined by the clinician when the PIP and PEEP are set, VT may not be consistent when the infant breathes, cries, splints, is apnoeic or when compliance and resistance change. For example, following administration of artificial surfactant, improved compliance may result in the delivery of increased VT if the PIP is not reduced.
In the past, there was concern about lung damage caused by high pressures ('barotrauma'). However, several studies have indicated that lung collapse and overdistension (or atelectasis and 'volutrauma') are the major instigators of inflammation in the preterm lung (Dreyfuss 1993; Dreyfuss 1998). This is supported by animal studies comparing high PIP in an animal model where a cast was used to reduce chest wall compliance and hence VT (Hernandez 1989). Histological examination demonstrated a significant reduction in lung inflammation in the animals that were protected from high VT. Further support came from a randomised controlled trial (RCT) comparing two ventilation strategies, high VT (12 mL/kg) versus low VT (6 mL/kg), in adults with acute lung injury. This study was stopped prematurely when interim analysis revealed a significant reduction in both mortality and duration of ventilation in low‐VT group (ARDS Network 2000). Lung compliance changes rapidly and substantially during the evolution and treatment of RDS (Hentschel 2002; Wheeler 2009). Ventilation strategies that adapt to these changes may enhance stability and reduce lung injury. Furthermore, avoiding rapid changes in the partial pressure of carbon dioxide in arterial blood (PaCO2) by maintaining stable minute volume ventilation may stabilise cerebral blood perfusion and reduce brain damage.
There is a paucity of information regarding the optimal VT for preterm infants. An observational study of VT values in infants weighing less than 800 g ventilated using VTV during the first three weeks of life reported obtaining acceptable blood gases using target VT of 5 mL/kg to 6 mL/kg with the Drager Babylog 8000plus (Keszler 2009). Other studies have suggested that a VT of 4 mL/kg or less may increase lung inflammation and work of breathing (Lista 2006; Patel 2009; Patel 2010; Chowdhury 2012). When selecting target VT for devices which measure VT at the ventilator (rather than at the Wye piece), allowance must be made for the additional compressible gas volume and compliance of the ventilator circuit (Cannon 2000; Al‐Majed 2004).
Why it is important to do this review
The uptake of VTV varies between countries and continents. Surveys have shown that 5% to 63% of neonatal units in Europe, Australia and New Zealand routinely use VTV modes and perceptions vary as to whether the use of VTV modes leads to improved outcomes (Sharma 2007; Klingenberg 2011; Van Kaam 2010). It is important to understand how outcomes of infants ventilated using VTV modes compare with those of infants ventilated using PLV modes.
Objectives
To determine whether VTV compared with PLV leads to reduced rates of death and death or BPD in newborn infants and to determine whether use of VTV affected outcomes including air leak, cranial ultrasound findings and neurodevelopment.
Methods
Criteria for considering studies for this review
Types of studies
We included only RCTs and quasi‐RCTs.
Types of participants
Participants were intubated newborn infants being mechanically ventilated with PPV at the time of study entry. Infants of all GAs up to 44 weeks' postmenstrual age and both muscle‐relaxed and non‐muscle‐relaxed infants were eligible.
Types of interventions
The review only included studies comparing ventilation using VTV modes versus ventilation using PLV modes.
Types of outcome measures
Primary outcomes
-
Death, defined in two ways:
death before discharge from the primary hospital;
death before two years' corrected age.
-
Death or BPD (BPD defined as need for supplemental oxygen requirement), assessed at two time points:
BPD at 28 days or death prior to 28 days;
BPD at 36 weeks' postmenstrual age or death prior to 36 weeks' postmenstrual age.
Secondary outcomes
Failure of mode of ventilation (clinical decision to change to different mode of ventilation).
Addition of neuromuscular paralysis where previously not paralysed.
-
Ventilation data:
days of PPV;
days of non‐invasive respiratory support;
total duration of respiratory support in days.
-
Markers of gas exchange as shown on arterial or capillary blood gas sampling:
any pH less than 7.25;
any episode of hypocarbia (partial pressure of carbon dioxide (pCO2) less than 35 mmHg/4.7 kPa);
any episode of respiratory acidosis (pH less than 7.25 with pCO2 greater than 60 mmHg/8 kPa).
Inspired oxygen concentrations (FiO2).
Patent ductus arteriosus (PDA).
-
Incidence of air leak:
overall incidence of air leak;
incidence of pneumothorax;
incidence of pulmonary interstitial emphysema (PIE).
-
Growth:
days to regain birth weight (BW);
grams weight gain per week until discharge.
-
Intracranial pathology:
all cranial ultrasound abnormalities (intraventricular haemorrhage (IVH) and periventricular leukomalacia (PVL)).
IVH;
cystic PVL.
-
Adverse neurosensory sequelae at two years:
cerebral palsy;
blindness;
deafness;
moderate to severe developmental delay as assessed on performance in formal neurodevelopmental testing (Bayley score, Wechsler Preschool and Primary Scale of Intelligence (WIPPSI), etc.).
-
Surviving infants with BPD:
BPD (supplemental oxygen requirement) at 28 days after birth;
BPD (supplemental oxygen requirement) at 36 weeks' postmenstrual age.
Modifications of these outcome measures (post hoc after viewing the available data):
Duration of PPV: measure was calculated in survivors only.
Failure of ventilatory mode: was clarified as a change from the assigned mode of ventilation within the study intervention period.
Days of non‐invasive respiratory support. The outcome was originally defined as days of CPAP. However, none of the included studies reported this consistently in an extractable fashion. Therefore, this outcome was not analysed.
IVH: collected outcomes for both total incidence of IVH and incidence of IVH grade 3 or 4.
Cystic PVL: most studies did not specify PVL. We included outcomes for studies reporting any PVL.
Data from studies reporting BPD rates were included for all participants when data on survivors were unavailable.
BPD at 28 days after birth: only four trials reported BPD at 28 days after birth (Piotrowski 1997; Lista 2004; Piotrowski 2007; Chowdhury 2013). The vast majority of infants included in these four trials were born before 32 weeks' gestation (Characteristics of included studies table). The definition of BPD (Jobe 2001) was based on diagnostic criteria, and it was only recommended to assess an infant for BPD at 28 days of age if the infant was born at or after 32 weeks' gestation. Thus, we did not find it appropriate to report BPD at 28 days in this updated review.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register). We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 12) in the Cochrane Library; MEDLINE via PubMed (1966 to 13 January 2017); Embase (1980 to 13 January 2017) and CINAHL (1982 to 13 January 2017) using the following search terms: (ventilation OR ventilator OR artificial respiration OR respiratory support) AND volume, plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization's International Trials Registry and Platform www.whoint/ictrp/search/en/, and the ISRCTN Registry). The previous search was performed using the standard strategy of the Neonatal Review Group of the Cochrane Collaboration. MEDLINE (1966 to January 2010) was searched using the MeSH terms: infant, newborn and respiration, artificial and the text word: volume. These terms were also used in a search of the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, 2015, Issue 4) and CINAHL. there were no language restrictions. A review (1981 to 2015) of abstracts published by the Society for Pediatric Research and the European Society for Pediatric Research completed the literature search. This was combined with cross‐referencing of previous reviews, use of expert informants and newer additional resources such as ClinicalTrials.gov.
Data collection and analysis
We used the standard methods of the Neonatal Review Group of Cochrane. Two review authors (KW and CK) independently performed trial searches, assessments of methodology and extraction of data with comparison and resolution of any differences found at each stage.
For each included study, we collected information regarding method of randomisation, blinding, intervention, stratification and whether the trial was a single‐centre or multi‐centre study. We noted information regarding trial participants, including GA criteria, BW criteria and other inclusion or exclusion criteria. We analysed information on clinical outcomes, including death and BPD and other relevant secondary outcomes. We contacted trial authors to obtain supplementary data and to clarify issues.
Quality of evidence
We used the GRADE approach to assess the quality of evidence, as outlined in the GRADE Handbook (Schünemann 2013). Three review authors (CK, NM and KW) independently assessed the quality of the evidence for each of the primary and secondary outcomes. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create 'Summary of findings' tables to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect;
very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Selection of studies
We included all RCTs and quasi‐RCTs fulfilling the selection criteria. All review authors reviewed results of the search, separately selected studies for inclusion and resolved disagreements by discussion.
Data extraction and management
Two review authors (KW and CK) independently extracted, assessed and coded all data for each study, for studies newly added to this version of the review, using the same categories as in the previous version. We replaced any standard error of the mean reported with the corresponding standard deviation (SD) and resolved disagreements by discussion.
For each study, one review author (KW) entered final data into Review Manager 5 (RevMan 2014), and the other review authors (CK, NM, CM, PGD) checked the data. All review authors reviewed the analysis and draft manuscript.
Assessment of risk of bias in included studies
Three review authors (CK, KW, NM) independently assessed the risk of bias (low, high or unclear) of all included trials using the Cochrane 'Risk of bias' tool (Higgins 2011) for the following eight domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Intervention bias (other differences in ventilator management than purely VTV versus PLV).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved any disagreements by discussion or by discussion with a fourth review author (PGD). See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed meta‐analysis using Review Manager 5 (RevMan 2014). We analysed categorical data using risk ratio (RR), risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH). We analysed continuous data using mean difference (MD). We reported 95% confidence intervals (CIs) on all estimates. We applied the fixed‐effect model.
Unit of analysis issues
We combined individually randomised trials in a single meta‐analysis using the generic inverse variance method. We identified no cluster randomised trials.
Dealing with missing data
We requested supplemental information from authors when data were missing or unclear.
Assessment of heterogeneity
We estimated treatment effects reported by individual trials and examined heterogeneity among trials by inspecting forest plots and quantifying the impact of heterogeneity using the I² statistic. We graded the degree of heterogeneity as less than 25% (no heterogeneity), 25% to 49% (low heterogeneity), 50% to 75% (moderate heterogeneity) or greater than 75% (substantial heterogeneity), as recommended by the Cochrane Neonatal group. When there was evidence of apparent or moderate to substantial heterogeneity (I² greater than 50%), we explored possible causes using sensitivity analysis (e.g. differences between strict and hybrid studies), looking for evidence of bias or methodological differences between trials.
Assessment of reporting biases
Studies included in this review were performed between 1994 and 2014 (published between 1997 and 2016), across the three eras of when prospective trial registrations were not available, was suggested and is now mandatory. We requested possible trial registration number from authors of all studies. In studies where no prospective trial registration was available we defined the risk of reporting bias as unclear.
Data synthesis
We used the Mantel‐Haenszel method for estimates of typical RR, RD, NNTB and NNTH. When we judged meta‐analysis to be inappropriate, we analysed and interpreted outcomes from trials separately.
Subgroup analysis and investigation of heterogeneity
Three subgroup analyses were originally planned based on:
-
mode of VTV: in view of the differences between VTV modes, subgroups were defined according to:
volume‐controlled (VC) ventilation;
volume‐guaranteed (VG) ventilation;
-
age at recruitment into study: in view of possible differences in outcomes according to postnatal age at time of study recruitment, subgroups were defined according to:
early recruitment (i.e. commencement of ventilation strategy at birth or within the first four hours of life);
late recruitment (i.e. beyond four hours of age). This subgroup included trials in which VTV was tested as a rescue strategy);
-
maturity/BW of the infants: in view of the increased risk of BPD in the smallest/most immature infants, subgroups were defined according to:
BW, with a cut‐off of 1000 g;
GA, with a cut‐off of 30 weeks' gestation.
Modifications of these subgroup analyses
Subgroup analysis based on VTV mode was not performed. Since the original protocol was written (McCallion 2002), the range of available VTV modes has changed, and the suggested subgroup classification was not appropriate. We analysed all VTV modes together without attempting to subdivide them into different modes.
Subgroup analysis based on postnatal age at time of study recruitment was not performed as the vast majority of parallel studies all had early recruitment.
Subgroup analysis based on BW was performed.
Sensitivity analysis
For major outcomes, we performed sensitivity analyses by running the meta‐analysis both with strict studies (13 RCTs), in which volume targeting was the only difference in ventilator strategy or ventilator use between groups and hybrid studies (seven RCTs), in which the ventilators used or ventilator triggering modes were different in the two groups. We decided that there was a higher risk of bias (intervention bias) associated with hybrid studies. See further description of strict and hybrid studies under Results and Characteristics of included studies table. In outcomes with moderate to high heterogeneity, we performed analyses both with and without outlying studies as part of a sensitivity analysis.
Results
Description of studies
Results of the search
The previous version of this review identified 12 RCTs. Updated searches revealed 1877 records after duplicates were removed; after screening of 40 full‐text articles/studies, review authors identified eight new RCTs for inclusion in the review (Figure 1).
1.
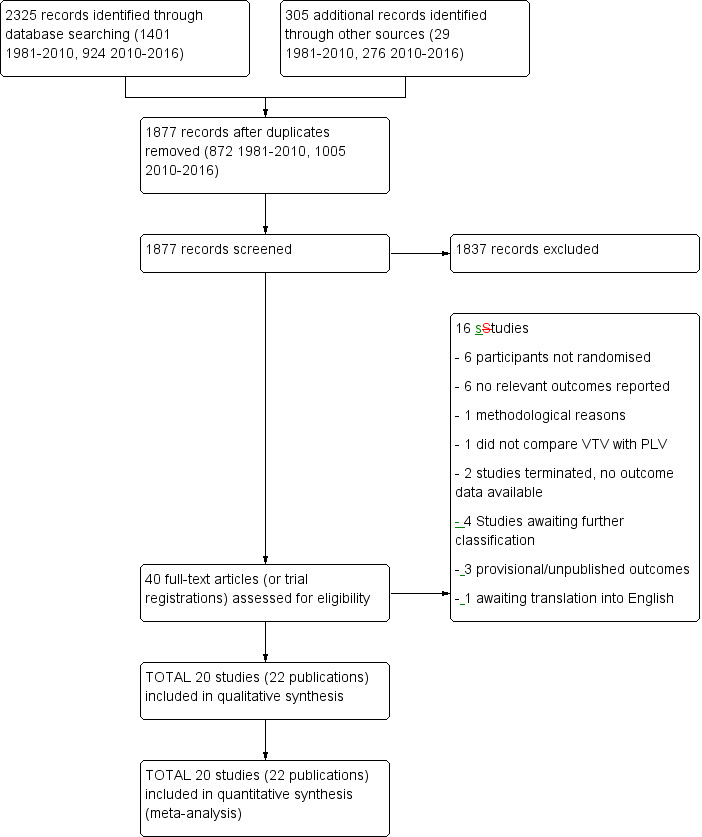
Study flow diagram: review update. PLV: pressure‐limited ventilation; VTV: volume‐targeted ventilation.
Included studies
Twenty RCTs (22 publications) met our inclusion criteria and reported one or more outcomes defined in our protocol. Sixteen were parallel studies, resulting in 18 publications (Piotrowski 1997; Sinha 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Nafday 2005; Singh 2006 (including two separate publications of follow‐up data from the inception cohort: Swamy 2008; Singh 2009); Cheema 2007; Piotrowski 2007; Zhou 2007; Liu 2011; Duman 2012; Chowdhury 2013; Guven 2013; Erdemir 2014; Bhat 2016). Four were within‐participant cross‐over studies (Herrera 2002; Hummler 2006; Polimeni 2006; Jain 2016). Details on all 20 included studies are described in the Characteristics of included studies table. In brief, we presented the following general information about the 20 studies.
Study sample size: ranging from 15 infants (Hummler 2006) to 212 infants (D'Angio 2005).
Inclusion criteria: varied from studies which included only smaller infants (less than 1200 g BW or less than 32 weeks' GA), larger infants (1200 g or greater), any infant less than 2500 g), or infants born at or near term.
Time of recruitment: 17 trials recruited infants in the early neonatal period (first three days after birth) (Piotrowski 1997; Sinha 1997; Herrera 2002; Keszler 2004a; Lista 2004; D'Angio 2005; Nafday 2005; Singh 2006; Cheema 2007; Piotrowski 2007; Zhou 2007; Liu 2011; Duman 2012; Chowdhury 2013; Guven 2013; Erdemir 2014; Bhat 2016). Three cross‐over trials enrolled preterm infants with mean postnatal age between 33 and 37 days (Hummler 2006; mean (SD) 33 (13) days of age; Polimeni 2006; mean (SD) 37 (17) days of age; Jain 2016; mean (SD) 33 (22) days of age). All 20 trials studied infants at less than 44 weeks' postmenstrual age.
Duration of intervention: for the parallel trials, the duration of intervention ranged from median 95 minutes (Cheema 2007) up to almost the full period of mechanical ventilation. For the cross‐over trials, duration of intervention period ranged from 60 minutes (Herrera 2002) up to 24 hours (Jain 2016).
Exclusion criteria: these were similar across trials and included the following: lethal congenital anomalies, muscle relaxation, suspected sepsis, severe IVH, asphyxia, pneumothorax and meconium aspiration. Some studies specified lack of arterial access or treatment with narcotics as additional exclusion criteria. Erdemir 2014 specifically noted that an ETT leak less than 20% as an exclusion criterion. We tried to clarify this but have not been able to verify whether this was an error. Antenatal steroids and surfactant were available in all participating units, although, in the studies by Zhou 2007 and Liu 2011, financial considerations influenced access.
Ventilators used in the trials: the VTV group used a range of ventilators, including the VIP Bird and Bird Gold, Siemens Servo 300, Draeger Babylog 8000plus, Stephanie Infant ventilator, AVEA CareFusion and SLE 5000. The ventilation settings were not always well described in each trial. Further details are shown in the Characteristics of included studies table.
We decided post hoc to define some of the studies as 'hybrid studies' (also used in the 2010 version of this review) with increased risk of bias based on the fact that in some trials volume targeting was not the only difference between study groups. The differences detected were:
different ventilators: three studies used different ventilators in each groups (Piotrowski 1997; Piotrowski 2007; Liu 2011). This is a potential source of bias;
different use of triggering:the use of triggering in one arm of the trial but not in the other is a potential source of bias (Greenough 2008). In the trial of Piotrowski 1997, the PLV group received non‐triggered intermittent mandatory ventilation, whereas the VTV groups received triggered ventilation. Sinha 1997 used an assist‐control (AC) mode in both arms, but the volume control arm used pressure‐triggering and the pressure limited arm used flow‐triggering;
different trigger modes: two studies used a mode where all inflations were triggered in the VTV group (pressure‐regulated volume control (PRVC) mode) and synchronised intermittent mandatory ventilation (SIMV) in the PLV group (D'Angio 2005; Piotrowski 2007). This difference in trigger modes is a potential source of bias (Greenough 2008); however, when the inflation rate in the SIMV mode is high (i.e. 50/minute to 60/minute), the difference between the two modes becomes less clinically important;
flow termination: in the studies by Nafday 2005 and Erdemir 2014, the VTV group received pressure support ventilation (PSV) with flow termination and the PLV group received SIMV without flow termination.
In view of these differences, we performed a sensitivity analysis of strict studies (both groups initially ventilated with similar modes/ventilators with VTV being to the only difference) versus hybrid studies (other differences between the groups; different ventilators, different use of triggering and different trigger modes).
Supplemental information: we requested raw data and supplemental information to clarify randomisation procedures, outcomes, permit more detailed analysis of duration of ventilation and facilitate subgroup analysis of infants weighing less than 1000 g. The review authors are grateful to the authors for making supplemental information available, details of which are described in the Characteristics of included studies table.
Excluded studies
We excluded 16 studies for the following reasons (see Characteristics of excluded studies table):
not randomised (Lista 2000; Abubakar 2001; Wach 2003; Abd El‐Moneim 2005; Shah 2013; Stefanescu 2015);
randomised, but did not report any of the outcomes specified in the protocol (Olsen 2002; Dotta 2004; Keszler 2004b; Ramirez‐Del Valle 2006; Colnaghi 2006; Sinha 2008);
randomised, but the PIP setting was the same in both arms, which may have interfered with the ventilator's capacity to deliver the set VT and hence affected the outcomes (Cheema 2001);
compared two different modes of VTV, and did not include a comparison with PLV (Unal 2014);
randomised, but later the studies were terminated (NCT00157989; NCT00295230).
Two studies are awaiting classification (Liu 2016; Miracle 2016; see Characteristics of studies awaiting classification table), and two studies are ongoing (ACTRN12609000986279; Salvia 2006; see Characteristics of ongoing studies table).
Risk of bias in included studies
Details of each study appear in the Characteristics of included studies table and in Figure 2 and Figure 3.
2.
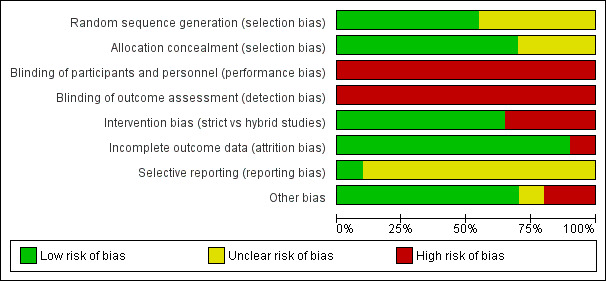
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
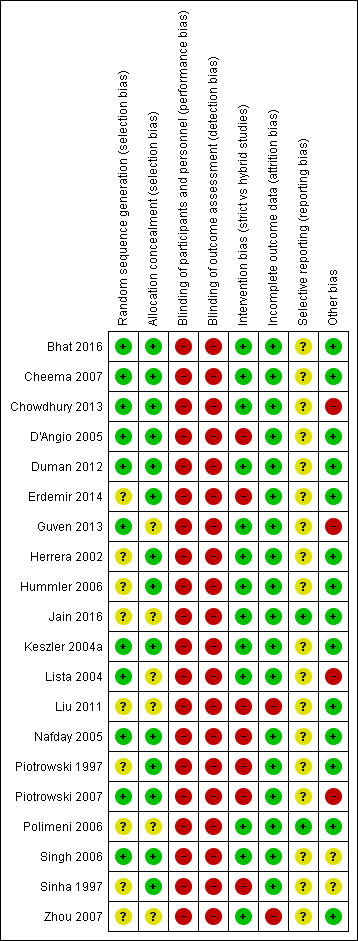
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation (selection bias): eight studies used block randomisation (Sinha 1997; Nafday 2005; D'Angio 2005; Singh 2006; Cheema 2007 (supplemental data); Piotrowski 2007 (supplemental data); Duman 2012; Guven 2013). Some trials used stratification by BW (D'Angio 2005; Nafday 2005; Singh 2006; Cheema 2007), GA (Lista 2004; Piotrowski 2007), centre (Lista 2004; D'Angio 2005) or a combination of these (D'Angio 2005; Lista 2004). Five trials did not specify the pattern of randomisation (Piotrowski 1997; Keszler 2004a; Zhou 2007; Liu 2011; Jain 2016).
Allocation concealment (selection bias): the studies by Piotrowski 1997; Sinha 1997; Herrera 2002; Keszler 2004a; Nafday 2005; D'Angio 2005; Hummler 2006; Singh 2006; Cheema 2007 (supplemental information); Duman 2012; Chowdhury 2013; Erdemir 2014; and Bhat 2016 used sealed envelopes for blinding of randomisation. Five trials did not specify blinding of randomisation (Lista 2004; Polimeni 2006; Zhou 2007; Liu 2011; Guven 2013).
Blinding
Blinding of participants and personnel (performance bias): none of the studies included in this review attempted to mask the carers to the intervention/group assignment.
Blinding of outcome assessment (detection bias): in the majority of studies, the allocated treatment method of each participant was known to those assessing the trial outcomes. In Sinha 1997, severity of lung disease was assessed by a radiographer blinded to the treatment assignment. In Singh 2006, information regarding masking during interpretation of cranial imaging was not reported. A questionnaire was used to determine neurodevelopmental follow‐up. The questionnaire administrator was masked to the original intervention group. D'Angio 2005 reported neurodevelopment outcomes at six to 18 months as assessed by a paediatric neurologist who was blinded to the treatment assignment (supplemental information).
Intervention bias (strict versus hybrid studies)
Some study designs had potential to be biased as they included comparisons between different ventilator devices and ventilator modes (triggering) in the VTV and the PLV groups. These studies are termed 'hybrid studies' in contrast to studies where this was not a problem ('strict studies'). Sensitivity analyses were performed exploring strict and hybrid studies separately.
Incomplete outcome data
Piotrowski 1997 excluded three out of 60 enrolled infants after randomisation, two who did not fulfil enrolment criteria and one for whom the allocated ventilator was unavailable. Outcome assessment was otherwise complete.
In the study by Lista 2004, there was an uneven distribution of participants between the VTV (30 infants) and PLV (23 infants) groups. Postrandomisation, seven infants were withdrawn because placental histology confirmed chorioamnionitis (supplemental data).
D'Angio 2005 randomised 213 infants, but one infant was erroneously enrolled without consent and immediately withdrawn from the study at the request of the parents. Follow‐up in the hospital was complete for the other 212 infants. However, data on brain ultrasound beyond the first week of life were not available for all infants, and PVL was assessed in only 173 infants. Neurodevelopmental follow‐up data at six to 18 months of age were available in 128 infants (64 from each group). These 128 infants represented 83% of the 154 participants that survived to discharge in one of the two study centres.
Singh 2006 randomised 110 infants, but one infant (randomised to the PLV mode) was excluded postrandomisation following diagnosis of a major congenital anomaly (trisomy 13). Follow‐up in the hospital was complete for the other 109 infants. Of the 94 infants who survived to discharge, mortality and follow‐up data at median age of 22 months were reported on 47/52 (90%) infants in the VTV group and 41/42 (98%) infants in the PLV group.
In the studies by Zhou 2007 and Liu 2011, the denominators for some outcomes were unclear due to inadequately reported completeness of follow‐up (unreported numbers of infant transfers to other hospitals). As the denominators were unclear in these studies, only outcomes reported during the intervention period were included in the meta‐analyses.
Selective reporting
There was only one trial with prospective trial registration (Jain 2016). Four other trials were registered retrospectively in a trial registry (Polimeni 2006; Cheema 2007; Chowdhury 2013; Bhat 2016). No other trials were registered.
Other potential sources of bias
There were imbalances in the study by Piotrowski 2007 in FiO2 in the first six hours of life, and surfactant use. In the published report, Piotrowski 2007 adjusted for this difference, but in this review, we used the unadjusted outcomes.
There were imbalances in the study by Chowdhury 2013 with regard to BW, GA and antenatal steroid use. Participants in the PLV group had lower median GA/BW than participants in the VTV group (median: GA/BW 26 weeks/856 g with PLV versus 28 weeks/1016 g with VTV).
The study by Guven 2013 randomised 90 participants; however, postrandomisation they excluded 15 participants in the PLV group and three participants in the VTV group. After contact with the authors, it seems that randomisation occurred before they had considered exclusion criteria and before parents had given consent.
Weaning strategies: in two trials, both arms were weaned using a PLV mode (Sinha 1997; Singh 2006).
Effects of interventions
The 16 randomised parallel trials recruited 977 infants who were included in the meta‐analysis. Two trials including 74 participants had an intervention period of 24 hours or less (Nafday 2005; Cheema 2007). The mean duration of mechanical ventilation reported by other studies in this review ranged from one to 26 days. We believed that the short duration of intervention in the trials of Cheema 2007 and Nafday 2005 meant that these trials had a reduced ability to detect differences in longer‐term outcomes such as BPD, compared to trials that maintained the two treatment groups for a least 72 hours. Therefore, we only included these two trials in pooled analysis of outcomes that occurred during the intervention period (e.g. blood gas analysis). The study by Bhat 2016 included infants born at or near term and duration of ventilation was short. From this study, we included only duration of ventilation and blood gas data. As noted in the 'Risk of bias' section, for the trials of Zhou 2007 and Liu 2011, we included only outcomes which occurred during the intervention period in the meta‐analysis. The Erdemir 2014 trial primarily investigated VTV versus PLV in the weaning phase. However, as the weaning phase contributed more than 80% of the total duration of ventilation in both groups, we decided to include longer‐term outcomes such as BPD and mortality.
We included outcomes from 11/16 parallel trials (including 771 participants) in meta‐analysis of outcomes beyond the intervention period (Piotrowski 1997; Sinha 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Singh 2006; Piotrowski 2007; Duman 2012; Chowdhury 2013; Guven 2013).
Four cross‐over trials recruited 88 infants (Herrera 2002; Hummler 2006; Polimeni 2006; Jain 2016). The only prespecified outcome able to be assessed from these studies was inspired oxygen concentration.
There was no disagreement between assessors regarding inclusion/exclusion of studies, quality assessment or data extraction. We pooled and analysed available data as listed below.
A planned subgroup analysis based on age at enrolment (planned before/after four hours) was not performed as the only study with exclusively early recruitment of all infants only studied infants until their first blood gas (Cheema 2007). Age at enrolment varied in the other trials. In the parallel trials, study enrolment mainly occurred within first 24 hours of life. In the cross‐over trials, Herrera 2002 studied participants at mean (range) five (two to nine) days of age. The other three cross‐over trials enrolled preterm infants with mean postnatal ages between 33 and 37 days (Hummler 2006; Polimeni 2006; Jain 2016).
Piotrowski 1997 and Singh 2006 reported outcomes for subgroup of infants weighing less than 1000 g. Authors of all parallel trials were approached for supplemental data of outcomes in infants weighing less than 1000 g. Data from Keszler 2004a; Lista 2004; and D'Angio 2005 were also included in this subgroup meta‐analysis.
Primary outcomes
Death before discharge from the primary hospital (outcomes 1.1 and 2.1)
Eleven trials (771 participants) provided data for death before discharge from the primary hospital (Sinha 1997; Piotrowski 1997; Keszler 2004a (supplemental data); Lista 2004; D'Angio 2005; Singh 2006; Piotrowski 2007; Duman 2012; Chowdhury 2013 (supplemental data); Guven 2013; Erdemir 2014). No individual study demonstrated a difference in mortality between VTV and PLV groups and the pooled analysis also showed no significant difference (typical RR 0.75, 95% CI 0.53 to 1.07; Analysis 1.1). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates). There was no significant difference in mortality for infants weighing less than 1000 g (typical RR 0.71, 95% CI 0.42 to 1.21; Analysis 2.1).
1.1. Analysis.
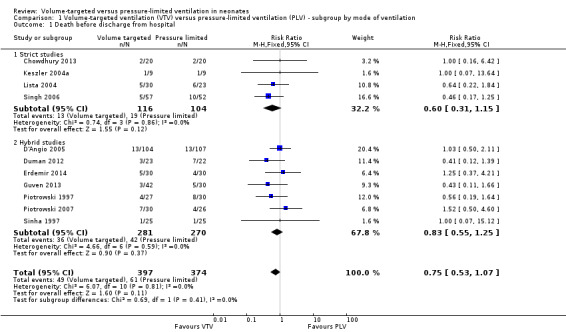
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 1 Death before discharge from hospital.
2.1. Analysis.
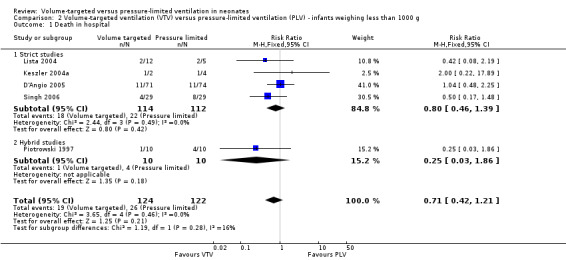
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 1 Death in hospital.
Death before two years' corrected age
None of the trials formally reported death before two years' corrected age, although Singh 2006 (reported in Singh 2009) reported mortality from discharge to follow‐up at a median age of 22 months. Overall, there were seven (12%) deaths in the VTV group versus 11 (21%) deaths in the PLV group (odds ratio 0.5, 95% CI 0.1 to 1.4; P = 0.13) (calculated by Singh 2009).
Death or bronchopulmonary dysplasia at 28 days age
Only four trials reported BPD at 28 days after birth (Piotrowski 1997; Lista 2004; Piotrowski 2007; Chowdhury 2013). However, none of them reported age of death (days) in those not surviving to discharge. Thus, we cannot report data for this outcome.
Death or bronchopulmonary dysplasia at 36 weeks' postmenstrual age (outcomes 1.2 and 2.2)
Eight trials (584 participants) reported the combined outcome of death or BPD at 36 weeks' postmenstrual age (Sinha 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Singh 2006; Duman 2012; Chowdhury 2013 (all supplemental data); Erdemir 2014). The trial by Duman 2012 was an outlier and reported a significant reduction in this outcome in the VTV group. No other individual trial reported a difference between groups. However, pooled meta‐analysis revealed a reduction in the combined outcome (typical RR 0.73, 95% CI 0.59 to 0.89; typical RD ‐0.12, 95% CI ‐0.20 to ‐0.05; typical NNTB 8, 95% CI 5 to 20; Analysis 1.2). There was moderate heterogeneity (I2 = 52%) between hybrid trials, but no heterogeneity between strict trials. Sensitivity analysis revealed that exclusion of the Duman 2012 study reduced heterogeneity between hybrid studies (I2 = 30%), but the overall RR of the remaining seven studies remained in favour of VTV (typical RR 0.77, 95% CI 0.62 to 0.95). We graded the quality of evidence for this outcome as moderate (unblinded intervention).
1.2. Analysis.
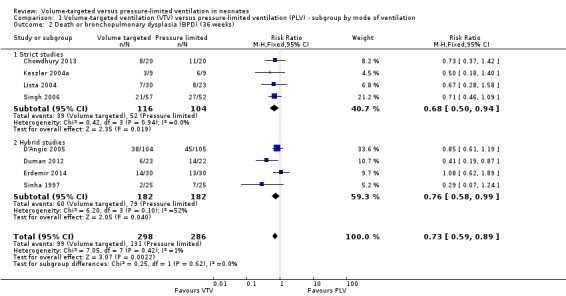
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 2 Death or bronchopulmonary dysplasia (BPD) (36 weeks).
For infants weighing less than 1000 g, there was no difference between groups (typical RR 0.79, 0.62 to 1.01; Analysis 2.2).
2.2. Analysis.
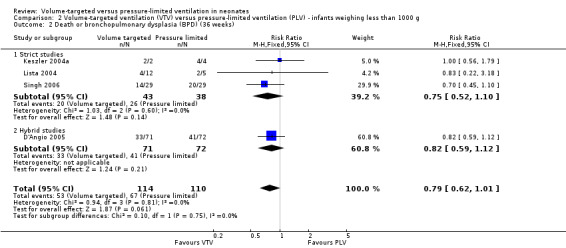
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 2 Death or bronchopulmonary dysplasia (BPD) (36 weeks).
Secondary outcomes
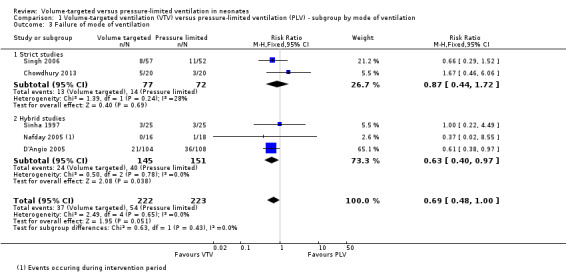
Failure of mode of ventilation (outcome 1.3)
Five trials (445 participants) provided data for failure of ventilatory mode (Sinha 1997; D'Angio 2005; Nafday 2005 (only data from the 24‐hour intervention period); Singh 2006; Chowdhury 2013). Cheema 2007 reported that no infants needed to be rescued with high‐frequency ventilation during the intervention period, but these data were not included in meta‐analysis due to the short intervention period (median 95 minutes, and before a blood gas analysis was available for the treating physician). Overall, there was no difference between groups, but there was a 'trend' towards less failure of primarily assigned ventilatory mode in the VTV group (typical RR 0.69, 95% CI 0.48 to 1.00; Analysis 1.3). Subgroup analysis for infants weighing less than 1000 g could not be performed. We graded the quality of evidence for this outcome as moderate (unblinded intervention).
1.3. Analysis.
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 3 Failure of mode of ventilation.
Addition of neuromuscular paralysis where previously not paralysed (outcome 1.4)
Two trials reported addition of new neuromuscular paralysis (Piotrowski 1997; Keszler 2004a). Overall, there was no difference between groups (Analysis 1.4). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates).
1.4. Analysis.
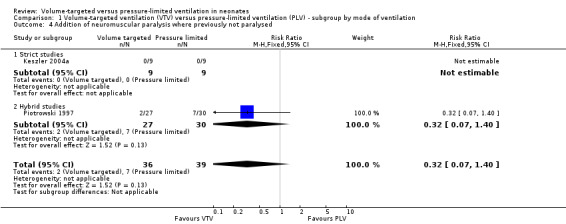
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 4 Addition of neuromuscular paralysis where previously not paralysed.
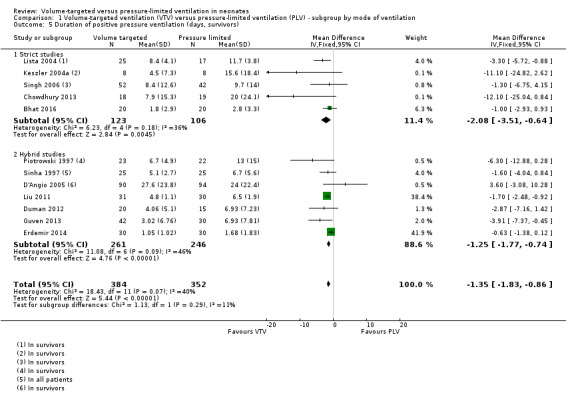
Duration of positive pressure (endotracheal) ventilation (outcomes 1.5, 1.6, 2.3 and 2.4)
Twelve trials (736 participants) provided data for the duration of positive pressure (endotracheal) ventilation (Sinha 1997; Liu 2011; Guven 2013; and Erdemir 2014 presented data in their publications; supplemental data obtained from Piotrowski 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Singh 2006; Duman 2012; Chowdhury 2013; Bhat 2016).
The trials analysed data in survivors only, with the exception of Sinha 1997 and Liu 2011, where this information was unavailable. In the trial by Sinha 1997, only one participant died in each arm, and the results are likely to be similar. Methods of meta‐analysis assume normally distributed values, but reported data on duration of PPV were skewed. Meta‐analysis performed on the skewed data gave a mathematical MD of ‐1.35 days (95% CI ‐1.83 to ‐0.86) of reduced duration of ventilation using VTV (Analysis 1.5). There was low heterogeneity between studies. The trial by D'Angio 2005 was the only trial that reported a non‐significant increase in mean duration of ventilation in the VTV group. Sensitivity analysis revealed that exclusion of the D'Angio 2005 trial did not change the level of heterogeneity and did not affect the result of the meta‐analysis. We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates).
1.5. Analysis.
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 5 Duration of positive pressure ventilation (days, survivors).
Geometrically normally distributed data were achieved by log transformation of supplemental raw data from five trials (381 participants) provided by Piotrowski 1997; Keszler 2004a; Lista 2004; D'Angio 2005; and Singh 2006 (Analysis 1.6). Using this method, after untransforming the log meta‐analysis (MD ‐0.08, 95% CI ‐0.16 to 0), the MD for ventilation with the VTV modes was 0.8 days (95% CI 0.7 to 1.0) shorter. There was evidence of heterogeneity (I2 = 52%) (strict studies 20%, hybrid studies 62%) in the subset of trials where this analysis was possible.
1.6. Analysis.
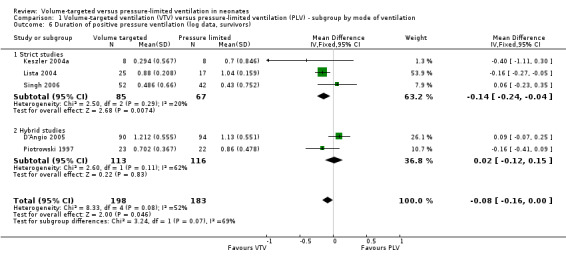
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 6 Duration of positive pressure ventilation (log data, survivors).
For infants weighing less than 1000 g, meta‐analysis of skewed data from the five trials (199 participants) did not show a statistically significant difference between groups (MD ‐0.82 days, 95% CI ‐4.43 to 2.80 days; Analysis 2.3). In this analysis, there was evidence of heterogeneity, particularly in the hybrid studies (I2 = 78%) (strict 0%, hybrid 92%). Using log transformed data, the MD corresponded to ‐0.01 (95% CI ‐0.12 to 0.10) fewer days of ventilation with VTV (Analysis 2.4; I2 = 83%) (strict 0%, hybrid 94%).
2.3. Analysis.
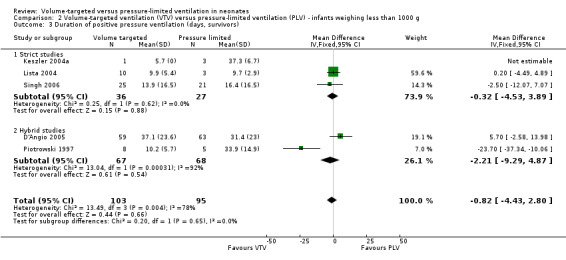
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 3 Duration of positive pressure ventilation (days, survivors).
2.4. Analysis.
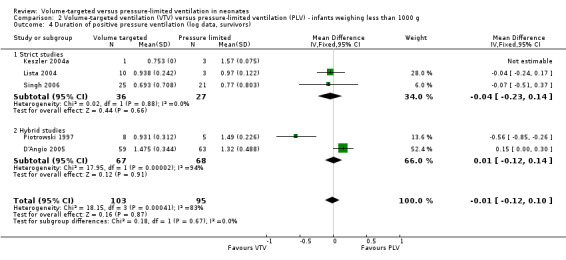
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 4 Duration of positive pressure ventilation (log data, survivors).
Other ventilation data including abnormal blood gas measurements (1.7 to 1.11 and 2.5 to 2.8)
Inspired oxygen concentration
Three parallel studies (Singh 2006 (reported in Swamy 2008); Cheema 2007; Zhou 2007) and four cross‐over studies (Herrera 2002; Hummler 2006; Polimeni 2006; Jain 2016) representing 332 infants reported inspired oxygen concentration. The oxygen targeting strategies varied; however, no trials reported a difference between groups. The study by Herrera 2002 used the same participant for multiple comparisons (VTV 3.0 mL/kg and 4.5 mL/kg versus PLV). Meta‐analysis was only performed using the measurements from the 4.5 mL/kg group (nine infants), and the eight infants in the 3.0 mL/kg were excluded from meta‐analysis, leaving 324 infants for meta‐analysis. Polimeni 2006 used different groups of participants for the comparisons of VTV 4.5 mL/kg with PLV, and VTV 6.0 mL/kg with PLV. During meta‐analysis, any statistical power gained by using an infant as their own control in a cross‐over trial is lost. Meta‐analysis showed no difference between groups (Analysis 1.7). We graded the quality of evidence for this outcome as moderate (unblinded intervention).
1.7. Analysis.
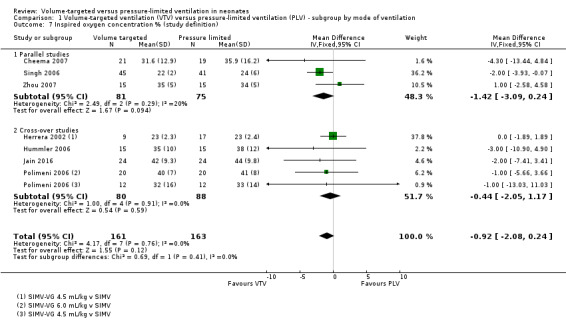
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 7 Inspired oxygen concentration % (study definition).
Blood gas data
We requested supplemental blood gas data for four trials (Keszler 2004a; Cheema 2007; Chowdhury 2013; Bhat 2016).
Keszler 2004a reported the frequency of blood gases falling outside the target range using the number of blood gases as the denominator. He found a reduced rate of hypocarbia (pCO2 less than 35 torr = mmHg) in blood gases from the VTV group versus PLV group (16/77 vs 29/80, p < 0.05). Supplemental data were analysed using the participant as the denominator (a participant event was defined as any out of range result).
Cheema 2007 reported the incidence of out of range PaCO2 (PaCO2 less than 5 kPa or PaCO2 greater than 7 kPa) and of hypocarbia (PaCO2 less than 5 kPa) on the first blood gas of 40 enrolled infants. Comparison of all infants showed no statistically significant difference, but for a post hoc subgroup analysis of infants at 26 to 33 weeks of gestation, there was a reported reduction for both outcomes. Supplemental data were analysed to identify the incidence of out of range CO2 by the criteria in this protocol (hypocarbia CO2 less than 35 mmHg, 4.7 kPa, hypercarbia CO2 greater than 60 mmHg, 8 kPa).
Chowdhury 2013 reported the number of episodes of hypocarbia per participant (PaCO2 less than 4.5 kPa) and reported fewer episodes of hypocarbia in the VTV group. Although the definition of hypocarbia used in the review is 4.7 kPa, we have included the data in the meta‐analysis.
Bhat 2016 (40 participants) reported that in the VTV group, there was a 'median of 1.5 (range 0.8 [sic]) episodes of hypocarbia' compared to a median of 4 (range 1 to 13) episodes of hypocarbia in the PLV group (P = 0.005), but data were not extractable for meta‐analysis. We did not receive raw data for meta‐analysis.
Any pH less than 7.25
Three trials (98 participants) reported data for any pH less than 7.25 (Keszler 2004a; Cheema 2007; Chowdhury 2013). Meta‐analysis showed no difference between groups (Analysis 1.8). Likewise, for infants weighing less than 1000 g, there was no difference between groups (Analysis 2.5). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates).
1.8. Analysis.
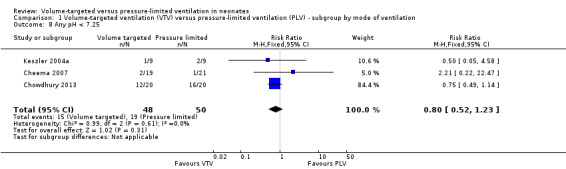
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 8 Any pH < 7.25.
2.5. Analysis.
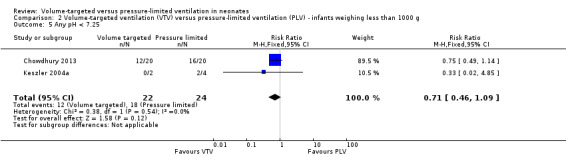
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 5 Any pH < 7.25.
Hypocarbia (any PaCO2 less than 35 mmHg/4.7 kPa)
Three trials (98 participants) reported extractable data on hypocardia for meta‐analysis (Keszler 2004a; Cheema 2007; Chowdhury 2013). There was a significant reduction in rates of hypocarbia in the VTV group (typical RR 0.49, 95% CI 0.33 to 0.72; typical RD ‐0.38, 95% CI ‐0.54 to ‐0.22; typical NNTB 3, 95% CI 2 to 5; Analysis 1.9). For the subgroup of infants weighing less than 1000 g, there was no statistically significant difference between groups (Analysis 2.6). We graded the quality of evidence for this outcome as moderate (unblinded intervention, small trials but large effect and biologically plausible).
1.9. Analysis.
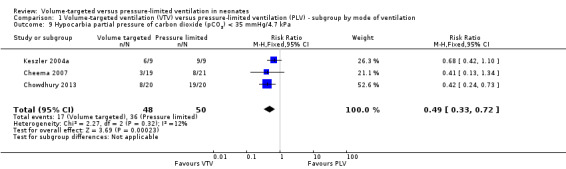
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 9 Hypocarbia partial pressure of carbon dioxide (pCO2) < 35 mmHg/4.7 kPa.
2.6. Analysis.
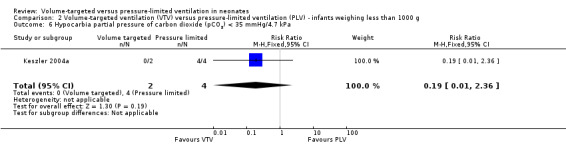
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 6 Hypocarbia partial pressure of carbon dioxide (pCO2) < 35 mmHg/4.7 kPa.
Respiratory acidosis (pH less than 7.25 and pCO2 greater than 60 mmHg/8 kPa)
Three trials (98 participants) reported data on respiratory acidosis (Keszler 2004a; Cheema 2007; Chowdhury 2013). There was no significant difference between groups (Analysis 1.10). Likewise, for the subgroup of infants weighing less than 1000 g, there was no difference between groups (Analysis 2.7). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates).
1.10. Analysis.
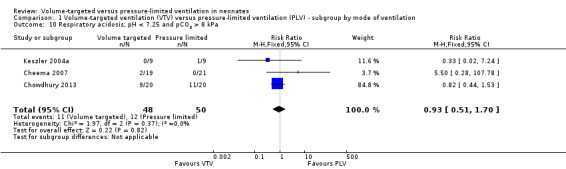
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 10 Respiratory acidosis; pH < 7.25 and pCO2 > 8 kPa.
2.7. Analysis.
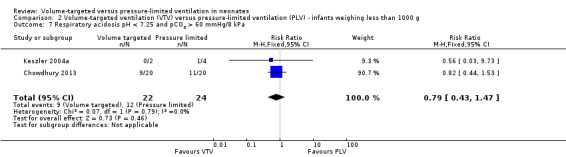
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 7 Respiratory acidosis pH < 7.25 and pCO2 > 60 mmHg/8 kPa.
Either hypocarbia or respiratory acidosis
Two trials (37 participants) reported data on either hypocarbia or respiratory acidosis (Keszler 2004a; Cheema 2007). There was no difference between groups (Analysis 1.11). Likewise, for infants weighing less than 1000 g, there was no statistically significant difference between groups (Analysis 2.8). There was no quality assessment done as analysis included only 37 participants.
1.11. Analysis.
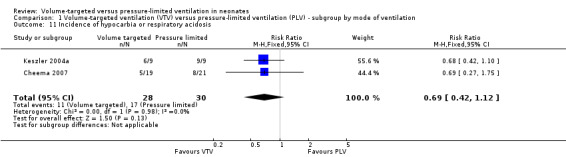
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 11 Incidence of hypocarbia or respiratory acidosis.
2.8. Analysis.
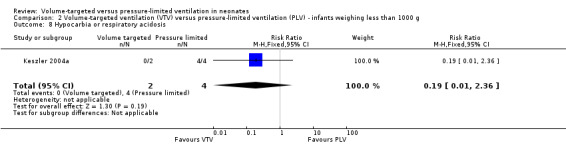
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 8 Hypocarbia or respiratory acidosis.
Patent ductus arteriosus (outcomes 1.12 and 2.9)
Ten trials (754 participants) reported data on PDA (Piotrowski 1997; Sinha 1997; Lista 2004; D'Angio 2005; Singh 2006; Piotrowski 2007; Duman 2012; Chowdhury 2013; Guven 2013; Erdemir 2014). The definition of PDA was not consistent, and the reported incidence varied between studies. There was no statistically significant difference in any of the individual trials or the pooled analysis (Analysis 1.12). Likewise, there was no difference for infants weighing less than 1000 g (Analysis 2.9). We graded the quality of evidence for this outcome as low (unblinded intervention, variable and unvalidated diagnostic criteria employed).
1.12. Analysis.
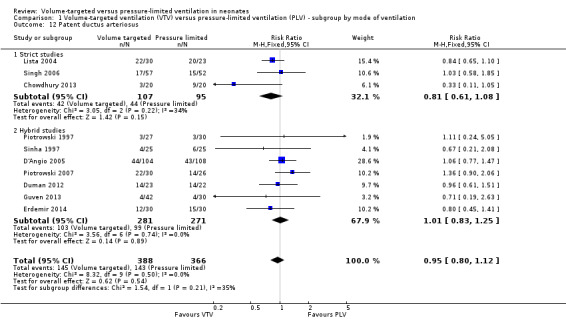
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 12 Patent ductus arteriosus.
2.9. Analysis.
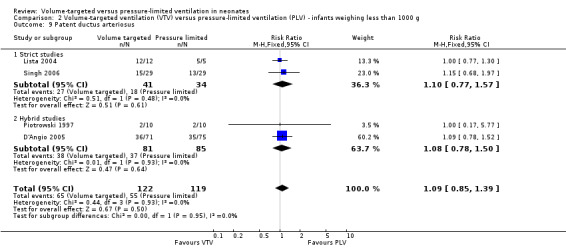
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 9 Patent ductus arteriosus.
Air leak (outcomes 1.13 to 1.15 and 2.10 to 2.12)
Overall incidence of air leak (pneumothorax or pulmonary interstitial emphysema, or both)
Five trials (374 infants) reported data for overall incidence of any air leak (Piotrowski 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Nafday 2005 (supplemental data, only including events during intervention period)). Pooled data showed no statistically significant difference between groups (Analysis 1.13). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates). Likewise, for infants weighing less than 1000 g, there was no difference between groups (Analysis 2.10).
1.13. Analysis.
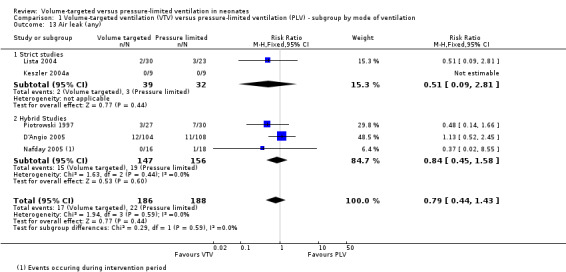
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 13 Air leak (any).
2.10. Analysis.
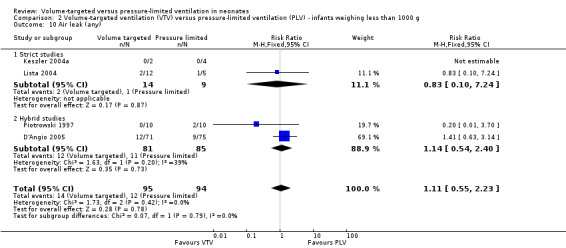
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 10 Air leak (any).
Incidence of pneumothorax
Thirteen trials (575 infants) reported data on incidence of pneumothorax (Piotrowski 1997; Sinha 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Nafday 2005 (supplemental data, only including events during intervention period); Singh 2006; Piotrowski 2007; Zhou 2007; Liu 2011; Duman 2012; Chowdhury 2013; Erdemir 2014). There was a significant reduction in infants ventilated using VTV modes (typical RR 0.52, 95% CI 0.31 to 0.87; typical RD ‐0.05, 95% CI ‐0.08 to ‐0.01; typical NNTB 20, 95% CI 12 to 100; Analysis 1.14). There were no concerns regarding heterogeneity between trials. We graded the quality of evidence for this outcome as moderate (unblinded intervention). In the subgroup of infants weighing less than 1000 g, there was no significant difference (Analysis 2.11) (Piotrowski 1997 (supplemental data); Lista 2004 (supplemental data)).
1.14. Analysis.
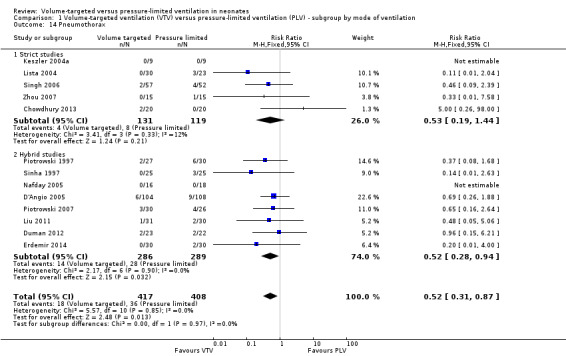
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 14 Pneumothorax.
2.11. Analysis.
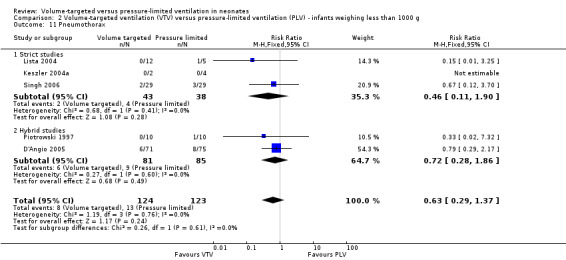
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 11 Pneumothorax.
Incidence of pulmonary interstitial emphysema
Six trials (430 infants) reported incidence of PIE (Piotrowski 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Nafday 2005 (supplemental analysis of events occurring during intervention period); Piotrowski 2007). There was no difference for any study or for overall pooled data (Analysis 1.15). We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates). Likewise, for infants weighing less than 1000 g, there was no difference between groups (Analysis 2.12).
1.15. Analysis.
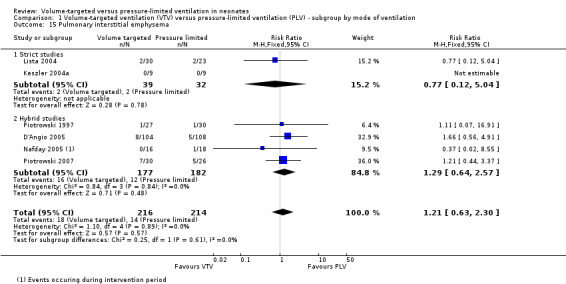
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 15 Pulmonary interstitial emphysema.
2.12. Analysis.
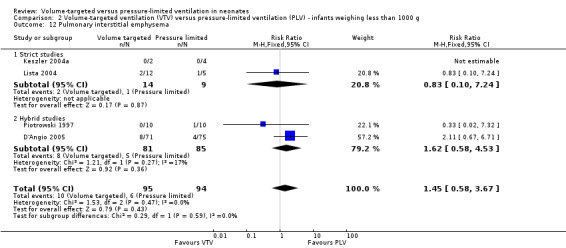
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 12 Pulmonary interstitial emphysema.
Growth
None of the studies assessed time taken to regain BW or weight gain.
Intracranial pathology (outcomes 1.16 to 1.20 and 2.13 to 2.17)
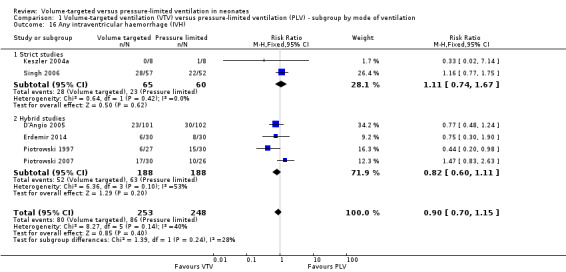
Any intraventricular haemorrhage
Six trials (501 participants) reported data on any IVH (Piotrowski 1997; Keszler 2004a (supplemental data); D'Angio 2005; Singh 2006; Piotrowski 2007; Erdemir 2014). Sinha 1997 reported only the combined outcome of large IVH or PVL (or both) and Lista 2004 reported only grade 3 or 4 IVH; the outcomes from these trials are not included in meta‐analysis of 'any IVH' but are included in the relevant meta‐analyses below. None of the individual studies showed any difference between groups and the meta‐analysis did not show a difference in any IVH between groups (Analysis 1.16).
1.16. Analysis.
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 16 Any intraventricular haemorrhage (IVH).
There was moderate heterogeneity (I2 = 53%) between hybrid trials, no heterogeneity between strict trials and low heterogeneity (I2 = 40%) for all trials included. Piotrowski 2007 reported a non‐significant increase in 'any IVH' in the VTV group. However, in this study, participants in the VTV group had increased oxygen requirements at enrolment and increased surfactant use compared with the PLV group. This indicates that infants in the VTV group were unlikely to have been at equal inception risk. Sensitivity analysis revealed that exclusion of the Piotrowski 2007 study reduced heterogeneity between hybrid studies (I2 = 0%). The overall RR of the remaining five trials still showed no difference in 'any IVH' between groups. We graded the quality of evidence for this outcome as low (unblinded intervention and imprecision of estimates).
For infants weighing less than 1000 g, there was no statistically significant difference between groups (Analysis 2.13).
2.13. Analysis.
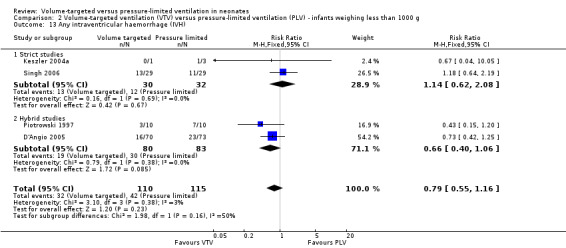
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 13 Any intraventricular haemorrhage (IVH).
Cystic periventricular leukomalacia
Seven trials (508 participants) reported data on cystic PVL (Keszler 2004a (supplemental data); Lista 2004; D'Angio 2005; Singh 2006; Duman 2012; Chowdhury 2013; Guven 2013). No individual study showed a difference between groups. However, meta‐analysis showed a significant reduction in PVL in the VTV group (typical RR 0.45, 95% CI 0.21 to 0.98; typical RD ‐0.04, 95% CI ‐0.08 to ‐0.00; Analysis 1.17). We graded the quality of evidence for this outcome as moderate (unblinded intervention). For infants weighing less than 1000 g, there was no significant difference between groups (Analysis 2.15).
1.17. Analysis.

Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 17 Periventricular leukomalacia (PVL).
2.15. Analysis.
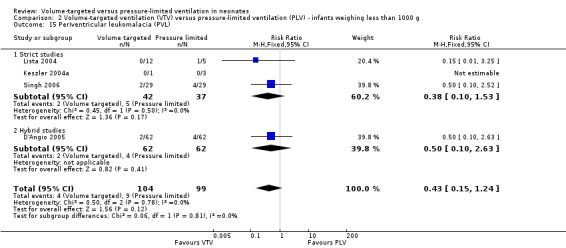
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 15 Periventricular leukomalacia (PVL).
Severe intraventricular haemorrhage grade 3 or 4
Ten trials (712 participants) reported data on severe IVH grade 3 or 4 (Piotrowski 1997; Keszler 2004a (supplemental data); Lista 2004; D'Angio 2005; Singh 2006; Piotrowski 2007; Liu 2011; Duman 2012; Chowdhury 2013; Guven 2013). No individual study showed a difference between groups. However meta‐analysis showed a significant reduction in IVH grade 3 or 4 in the VTV group (typical RR 0.53, 95% CI 0.37 to 0.77; typical RD ‐0.09, 95% CI ‐0.14 to ‐0.04; typical NNTB 11, 95% CI 7 to 25; Analysis 1.18). In addition, there was evidence of heterogeneity in the 'hybrid studies' (I2 = 49%). As for 'any IVH,' Piotrowski 2007 reported a non‐significant increase in 'IVH grade 3 or 4' in the VTV group, but infants in the VTV group were unlikely to have been at equal inception risk. Sensitivity analysis revealed that exclusion of the Piotrowski 2007 study eliminated heterogeneity, both between hybrid studies and overall (I2 = 0%). The overall RR of the remaining nine trials showed an even stronger reduction in IVH grade 3 or 4 in the VTV group (typical RR 0.42, 95% CI 0.28 to 0.65). We graded the quality of evidence for this outcome as moderate (unblinded intervention).
1.18. Analysis.
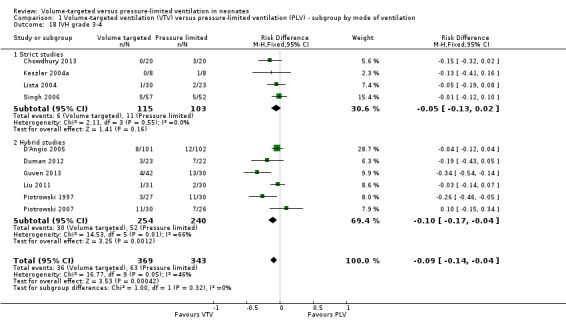
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 18 IVH grade 3‐4.
There was no significant difference for infants weighing less than 1000 g (typical RR 0.53, 95% CI 0.27 to 1.04; Analysis 2.14). There was evidence of heterogeneity in the subgroup of hybrid studies (overall I2 = 10%; strict studies 0%; hybrid studies 57%).
2.14. Analysis.
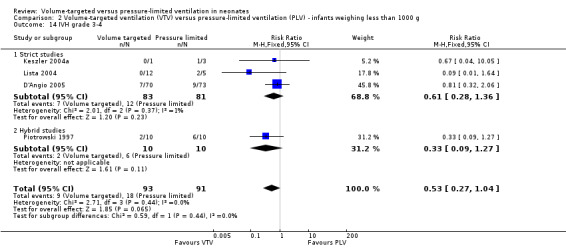
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 14 IVH grade 3‐4.
Any intraventricular haemorrhage or periventricular leukomalacia
Three trials (298 participants) reported data on any IVH or PVL (Keszler 2004a (supplemental data); D'Angio 2005; Singh 2006). Pooled analysis showed no statistically significant difference between groups (Analysis 1.19). Likewise, for infants weighing less than 1000 g, there was no difference between groups (Analysis 2.16).
1.19. Analysis.
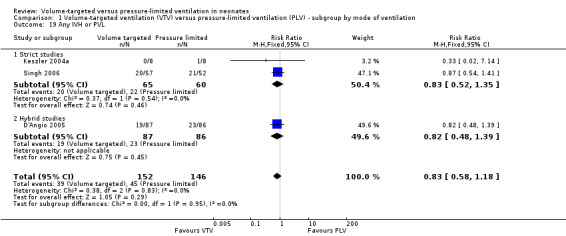
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 19 Any IVH or PVL.
2.16. Analysis.
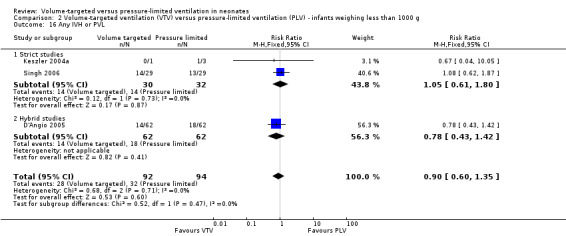
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 16 Any IVH or PVL.
Intraventricular haemorrhage grade 3 or 4 or periventricular leukomalacia
Six trials (441 participants) reported data on IVH grade 3 or 4 or PVL (Sinha 1997; Keszler 2004a (supplemental data); Lista 2004; D'Angio 2005; Singh 2006; Chowdhury 2013 (supplemental data)). No individual study showed a difference between groups. However, meta‐analysis showed a significant reduction in IVH grade 3 or 4 or PVL in the VTV group (typical RR 0.47, 95% CI 0.27 to 0.80; typical RD ‐0.09, 95% CI ‐0.15 to ‐0.03; typical NNTB 11, 95% CI 7 to 33; Analysis 1.20). We graded the quality of evidence for this outcome as moderate (unblinded intervention).
1.20. Analysis.
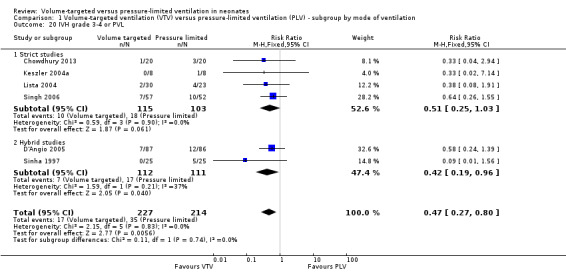
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 20 IVH grade 3‐4 or PVL.
In the subgroup of infants weighing less than 1000 g, there was also a statistically significant reduction in the VTV group (typical RR 0.44, 95% CI 0.20 to 0.99; typical RD ‐0.12, 95% CI ‐0.24 to ‐0.01; typical NNTB 8, 95% CI 4 to 100; Analysis 2.17). For this outcome, there was overall moderate heterogeneity between studies (I2 = 69%). This was primarily caused by infants weighing less than 1000 g from the Lista 2004 study where there was a marked difference in this outcome (VTV group 0/12 infants and PLV group 3/5 infants). Sensitivity analysis revealed that exclusion of the Lista 2004 trial eliminated heterogeneity (I2 = 0%), but also changed the significance level of this outcome in infants weighing less than 1000 g (typical RR 0.56, 95% CI 0.20 to 1.57).
2.17. Analysis.
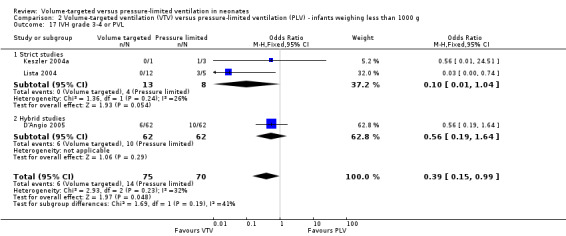
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 17 IVH grade 3‐4 or PVL.
Neurodevelopmental outcome (outcomes 3.1 and 2.2)
No studies reported neurodevelopmental outcome as defined by the review criteria.
Two trials (209 participants) reported neurological follow‐up using their own definition (D'Angio 2005; Singh 2006). We performed a post‐hoc meta‐analysis on these outcomes using the individual study criteria. There was no statistically significant difference between groups (typical RR 0.86, 95% CI 0.47 to 1.59; typical RD ‐0.02, 95% CI ‐0.12 to 0.08; Analysis 3.1).
3.1. Analysis.
Comparison 3 Miscellaneous post hoc analyses, Outcome 1 Severe disability (any definition).
One study (109 participants) also reported the combined outcome of death or severe disability (Singh 2006). There was no statistically significant difference between groups (typical RR 0.54, 95% CI 0.27 to 1.06; typical RD ‐0.15, 95% CI ‐0.31 to 0.01; Analysis 3.2). This study had unequal postdischarge follow‐up, which could be a potential source of bias.
3.2. Analysis.
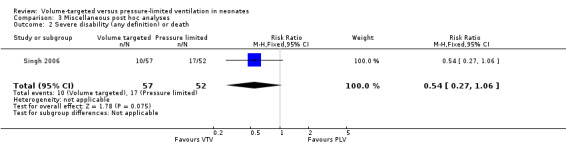
Comparison 3 Miscellaneous post hoc analyses, Outcome 2 Severe disability (any definition) or death.
One study (128 participants) reported gross motor delay (D'Angio 2005). The results from this single trial demonstrated no statistically significant difference between groups (typical RR 1.00, 95% CI 0.47 to 2.14; typical RD 0.00, 95% CI ‐0.13 to 0.13; Analysis 3.3).
3.3. Analysis.
Comparison 3 Miscellaneous post hoc analyses, Outcome 3 Gross motor developmental issue (any definition).
Surviving infants with bronchopulmonary dysplasia (outcomes 1.21 and 2.18)
Nine trials (620 participants) reported data on BPD at 36 weeks' postmenstrual age (Sinha 1997; Keszler 2004a; Lista 2004; D'Angio 2005; Singh 2006; Duman 2012; Chowdhury 2013; Guven 2013; Erdemir 2014). The trial by Guven 2013 reported a significant reduction in 36 weeks' postmenstrual age in the VTV group. No other individual trial reported a difference between groups. However, pooled meta‐analysis revealed a reduction in BPD at 36 weeks' postmenstrual age (typical RR 0.68, 95% CI 0.53 to 0.87; typical RD ‐0.11, 95% CI ‐0.18 to ‐0.04; typical NNTB 9, 95% CI 6 to 25; Analysis 1.21). We graded the quality of evidence for this outcome as low (unblinded intervention and possible publication bias with asymmetrical funnel plot). Subgroup analysis for infants weighing less than 1000 g showed no difference between groups (Analysis 2.18).
1.21. Analysis.
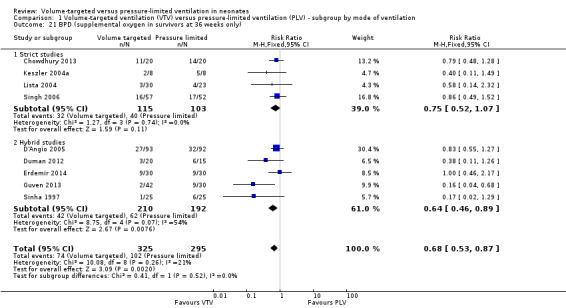
Comparison 1 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation, Outcome 21 BPD (supplemental oxygen in survivors at 36 weeks only).
2.18. Analysis.
Comparison 2 Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g, Outcome 18 BPD (supplemental oxygen in survivors at 36 weeks).
Post hoc analyses on related outcomes (outcomes 3.4 and 3.5)
One trial (203 participants) reported data on postnatal glucocorticoids for treating BPD (D'Angio 2005). There was no statistically significant difference between groups (typical RR 0.93, 95% CI 0.65 to 1.31; typical RD ‐0.03, 95% CI ‐0.16 to 0.10; Analysis 3.4).
3.4. Analysis.
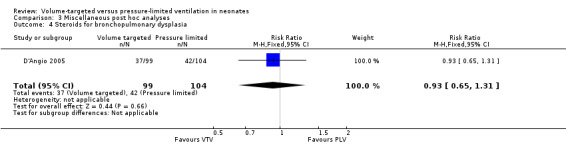
Comparison 3 Miscellaneous post hoc analyses, Outcome 4 Steroids for bronchopulmonary dysplasia.
Two trials (270 participants) reported data on need for home oxygen (D'Angio 2005; Singh 2006). Neither individual studies nor pooled analysis showed a statistically significant difference between groups (pooled analysis: typical RR 0.64, 95% CI 0.30 to 1.36; typical RD ‐0.04, 95 % CI ‐0.11 to 0.03; Analysis 3.5). Only supplemental data from D'Angio 2005 were available for the subgroup of infants weighing less than 1000 g. There was no statistically significant difference between groups (RR 0.75, 95% CI 0.25 to 2.23; RD ‐0.03, 95% CI ‐0.13 to 0.08; Analysis 3.6).
3.5. Analysis.
Comparison 3 Miscellaneous post hoc analyses, Outcome 5 Need for home oxygen (survivors).
3.6. Analysis.
Comparison 3 Miscellaneous post hoc analyses, Outcome 6 Need for home oxygen (survivors weighing < 1000 g).
Subgroup analyses
We reported subgroup analysis in infants weighing less than 1000 g, where possible, alongside the results for primary and secondary outcomes.
Discussion
Summary of main results
Meta‐analyses performed for this updated review showed that use of VTV, compared with PLV, reduced rates of death or BPD, BPD alone, pneumothorax, severe cranial ultrasound pathologies (IVH grade 3 or 4, or PVL, or both), mean duration of mechanical ventilation and hypocarbia. It is plausible that VTV modes, by controlling VT and avoiding volutrauma, may contribute to a reduction in BPD and death. Additionally, by improving the stability of blood gas parameters and reducing hypocarbia, these modes may stabilise cerebral perfusion and reduce neonatal brain injury. Studies to date have not been powered to assess longer‐term neurodevelopmental outcomes. We found no evidence of harm associated with the use of VTV modes.
Overall completeness and applicability of evidence
Most modern ventilators offer VTV modes targeting expired VT. VTV modes have been implemented as a strategy to avoid lung injury due to overinflation or underinflation. However, the target VT set for the whole lung is based on the infant's weight. Regional distribution of VT may vary depending on lung disease. In non‐homogeneous lung disease, using a VTV mode does not eliminate the regional risk of lung injury from local volutrauma or shear stress. Strategies to manage these local variations in lung mechanics may be important.
Quality of the evidence
The overall quality of evidence for outcomes in this review varied from moderate to low. Most researchers and ethics committees agree that neonatal ventilation studies should not be blinded from the clinical personnel. Using the GRADE assessment tool, 'moderate' is the highest quality assessment possible for evidence from well‐conducted randomised but unblinded studies.
Because of the nature of the interventions, blinding of carers to allocated treatment is impossible. There were no major methodological limitations with seven out of 20 studies (Herrera 2002; Keszler 2004a; Hummler 2006; Cheema 2007; Duman 2012; Bhat 2016; Jain 2016). In seven other studies, the ventilators or the triggering modes/devices used (or both) were different in the VTV and the PLV arm, leading to potential intervention bias (defined as hybrid studies) (Piotrowski 1997; Sinha 1997; D'Angio 2005; Nafday 2005; Piotrowski 2007; Liu 2011; Erdemir 2014). However, sensitivity analysis revealed similar results both when we analysed separately studies using the same ventilator and patient triggering modes/device in the VTV and PLV arms, and when we analysed 'hybrid studies.' In the trials by Piotrowski 2007 and Chowdhury 2013, despite randomisation, there was an imbalance in participant characteristics between the VTV and PLV groups. This may have introduced a bias. The studies by Lista 2004 and Guven 2013 excluded a substantial number of randomised participants (Lista 2004: 7/60; Guven 2013: 15/90) after randomisation. The trials by Sinha 1997 and Singh 2006 weaned both arms using PLV mode. Three trials had potential selection bias (Polimeni 2006; Zhou 2007; Liu 2011), and two of these trials also had other biases (Zhou 2007; Liu 2011), thus reducing their quality.
Most trials included were small, single‐ or dual‐centre studies, and some used ventilation modes that are no longer used in modern neonatal ventilators. For some outcomes, with small numbers of participants, there was evidence of imprecision. We did not identify any large multi‐centre studies which were adequately powered to address important long‐term outcomes. Some clinicians will require evidence from such trials before changing practice.
For the vast majority of outcomes, there was no evidence of publication bias. However, the funnel plot for the outcome BPD at 36 weeks' GA in survivors was asymmetrical. We have no evidence to believe that there was publication bias, but based on the funnel plot this cannot be excluded.
A further limitation with this review is that VT delivery in the VTV group was different depending on the ventilators used and additional ventilator modes. Moreover, studies started at different time points after birth and had different weaning approaches. Studies were also conducted in many different countries (England, Germany, Poland, Turkey, the USA and China). However, these factors also represent a strength of this review. Even with a substantial mixture of trial design and inclusion of infants from three different continents, there was little evidence of heterogeneity, the results clearly favour use of VTV and should be generalisable for a wide population of neonates.
We received supplementary data permitting more extensive subgroup analysis of infants weighing less than 1000 g. However, the relatively low numbers of infants weighing less than 1000 g (247) in this subgroup limit the power to identify differences between interventions.
Agreements and disagreements with other studies or reviews
Three previous systematic reviews have compared the efficacy of VTV versus PLV; two previous Cochrane reviews (McCallion 2005; Wheeler 2010), and the review by Peng 2014. The first Cochrane Review included four RCTs with 178 infants (McCallion 2005). There was no significant difference for death by the time of hospital discharge, and no trials reported the combined outcome of death or BPD. However, there were reduced rates of pneumothorax and IVH grade 3 or 4, and there was reduced days of ventilation in the VTV group. The second Cochrane Review included 12 RCTs with 693 infants (Wheeler 2010). There was no significant difference for death by the time of hospital discharge. However, the use of VTV resulted in reduced rates of death or BPD, pneumothorax, days of ventilation, hypocarbia and IVH grade 3 or 4 or PVL. Peng 2014 included 18 RCTs with 954 infants. The authors reported no difference in incidence of death. However, the use of VTV resulted in reduced rates of BPD, duration of ventilation, any IVH, IVH grade 3 or 4, PVL, pneumothorax, failure of primary mode of ventilation, hypocarbia, mean airway pressure and days of supplemental oxygen administration.
In this updated review in 2017, with 20 RCTs and 1065 infants, we confirm the previously reported beneficial effects of VTV with reductions in clinically relevant outcomes. There was no disagreement with previous reports.
Very few observational trials have compared VTV and PLV, and reported relevant clinical outcomes. Stefanescu 2015 studied two cohorts of participants treated with PLV (pressure controlled ventilation) and VTV (PSV with volume guarantee), both modes were provided by the Drager Babylog 8000 plus. Both cohorts included 135 participants. In this trial, the VTV cohort had significantly lower mortality, and lower rates of PIE and hypotension. However, there were no differences in neurodevelopmental impairment at 18 months of age.
Authors' conclusions
Implications for practice.
Since the previous review, more studies evaluating short‐ and longer‐term outcomes of volume‐targeted ventilation (VTV) have been published. In this updated systematic review, we found stronger evidence favouring the use of VTV strategies. Use of VTV compared with pressure‐limited ventilation (PLV) reduces the incidence of death or bronchopulmonary dysplasia (BPD), BPD alone, rates of pneumothorax and rates of severe cranial ultrasound pathology. Moreover, it reduces duration of mechanical ventilation and leads to less hypocarbia. This review identified no increase in any adverse outcomes associated with VTV compared with PLV. Increasing experience with VTV means that volume targeting in neonatal intensive care is no longer experimental. However, careful education is important in units considering using VTV.
Implications for research.
Further multi‐centre randomised controlled trials, powered to assess effects on important clinical outcomes, are still required, although these will be increasing difficult to conduct as increasing numbers of clinicians lose equipoise. Further research may be best conducted by units that currently do not use volume targeting as their main ventilation modality. Future research should also compare different volume‐targeting strategies. We note that some data have been presented at conferences and urge researchers to complete and publish these studies. Ventilator manufacturers can assist researchers and clinicians by making all specifications of their VTV modes freely available.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2017 | New citation required but conclusions have not changed | A total of eight new studies (8 publications: Zhou 2007; Liu 2011; Duman 2012; Guven 2013; Chowdhury 2013; Erdemir 2014; Bhat 2016; Jain 2016) were added. Moreover, additional outcomes from a publication based on one study (Singh 2006) included in the previous review were added. The conclusions are not substantially changed. |
| 31 March 2017 | New search has been performed | This updates the review "Volume‐targeted versus pressure‐limited ventilation in the neonate" first published in the Cochrane Database of Systematic Reviews, Issue 3, 2005 (McCallion 2005), and second published version in the Cochrane Database of Systematic Reviews, Issue 4, 2010 (Wheeler 2010). Searches were conducted in January 2017. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 27 September 2010 | New search has been performed | This updates the review "Volume‐targeted versus pressure‐limited ventilation in the neonate" published in the Cochrane Database of Systematic Reviews, Issue 3, 2005 (McCallion 2005). The searches were conducted in January 2010. At total of six new trials (seven publications) were added: Piotrowski 2007, Singh 2006 and 2009, D'Angio 2005, Polimeni 2006, Hummler 2006, Cheema 2007. Supplemental data from authors has been included to facilitate analysis of duration of ventilation and outcomes of infants < 1000 g. Pooled meta‐analysis identified a statistically significant reduction in the primary combined outcome of death and bronchopulmonary dysplasia favouring volume targeted ventilation. The conclusions have been revised. |
| 27 September 2010 | New citation required and conclusions have changed | Wheeler K, Klingenberg C added to authorship. The conclusions have been revised. |
| 1 April 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
KI Wheeler was supported in part by a Monash International Postgraduate Research Scholarship. PG Davis was supported in part by an Australian National Health and Medical Research Council Practitioner Fellowship. The project was supported by Australian National Health and Medical Research Council Program Grant No. 384100.
We acknowledge the assistance of Roberto Chiletti and Hania Czerwinska who freely gave their time to assist with translating articles published in Italian and Polish.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. 'Risk of bias' tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomisation, and the blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as low, high or unclear risk. Two review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the 'Characteristics of included studies' table. We evaluated the following issues and entered the findings into the 'Risk of bias' table:
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors;
unclear risk for outcome assessors.
Intervention bias (other differences in ventilator management than purely volume‐targeted ventilation versus pressure‐limited ventilation).
For each included study, we described whether the only difference between the intervention (ventilator management) was volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV). If there were no other obvious differences in the intervention we defined these studies as 'strict studies.' In contrast, for some studies there were also other differences between the VTV group and the PLV group such as use of different ventilators between the groups and use of different triggering modes/devices between groups. These studies were termed as 'hybrid studies.'
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we reincluded missing data in the analyses. We categorised the methods as:
low risk (less than 20% missing data);
high risk (20% or greater missing data);
unclear risk.
Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ subgroup by mode of ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death before discharge from hospital | 11 | 771 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.53, 1.07] |
| 1.1 Strict studies | 4 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.31, 1.15] |
| 1.2 Hybrid studies | 7 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.55, 1.25] |
| 2 Death or bronchopulmonary dysplasia (BPD) (36 weeks) | 8 | 584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.59, 0.89] |
| 2.1 Strict studies | 4 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.94] |
| 2.2 Hybrid studies | 4 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.58, 0.99] |
| 3 Failure of mode of ventilation | 5 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 1.00] |
| 3.1 Strict studies | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.44, 1.72] |
| 3.2 Hybrid studies | 3 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.97] |
| 4 Addition of neuromuscular paralysis where previously not paralysed | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.40] |
| 4.1 Strict studies | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Hybrid studies | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.40] |
| 5 Duration of positive pressure ventilation (days, survivors) | 12 | 736 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐1.83, ‐0.86] |
| 5.1 Strict studies | 5 | 229 | Mean Difference (IV, Fixed, 95% CI) | ‐2.08 [‐3.51, ‐0.64] |
| 5.2 Hybrid studies | 7 | 507 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐1.77, ‐0.74] |
| 6 Duration of positive pressure ventilation (log data, survivors) | 5 | 381 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.16, ‐0.00] |
| 6.1 Strict studies | 3 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.24, ‐0.04] |
| 6.2 Hybrid studies | 2 | 229 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.12, 0.15] |
| 7 Inspired oxygen concentration % (study definition) | 7 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐2.08, 0.24] |
| 7.1 Parallel studies | 3 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐3.09, 0.24] |
| 7.2 Cross‐over studies | 4 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐2.05, 1.17] |
| 8 Any pH < 7.25 | 3 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.23] |
| 9 Hypocarbia partial pressure of carbon dioxide (pCO2) < 35 mmHg/4.7 kPa | 3 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.33, 0.72] |
| 10 Respiratory acidosis; pH < 7.25 and pCO2 > 8 kPa | 3 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.70] |
| 11 Incidence of hypocarbia or respiratory acidosis | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.42, 1.12] |
| 12 Patent ductus arteriosus | 10 | 754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.12] |
| 12.1 Strict studies | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.61, 1.08] |
| 12.2 Hybrid studies | 7 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.25] |
| 13 Air leak (any) | 5 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.44, 1.43] |
| 13.1 Strict studies | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.09, 2.81] |
| 13.2 Hybrid Studies | 3 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.45, 1.58] |
| 14 Pneumothorax | 13 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.31, 0.87] |
| 14.1 Strict studies | 5 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.19, 1.44] |
| 14.2 Hybrid studies | 8 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.94] |
| 15 Pulmonary interstitial emphysema | 6 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.63, 2.30] |
| 15.1 Strict studies | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.12, 5.04] |
| 15.2 Hybrid studies | 4 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.64, 2.57] |
| 16 Any intraventricular haemorrhage (IVH) | 6 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.15] |
| 16.1 Strict studies | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.74, 1.67] |
| 16.2 Hybrid studies | 4 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.11] |
| 17 Periventricular leukomalacia (PVL) | 7 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.98] |
| 17.1 Strict studies | 4 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.65] |
| 17.2 Hybrid studies | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.14, 1.14] |
| 18 IVH grade 3‐4 | 10 | 712 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.14, ‐0.04] |
| 18.1 Strict studies | 4 | 218 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.13, 0.02] |
| 18.2 Hybrid studies | 6 | 494 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.10 [‐0.17, ‐0.04] |
| 19 Any IVH or PVL | 3 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.58, 1.18] |
| 19.1 Strict studies | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.52, 1.35] |
| 19.2 Hybrid studies | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.48, 1.39] |
| 20 IVH grade 3‐4 or PVL | 6 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.80] |
| 20.1 Strict studies | 4 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.25, 1.03] |
| 20.2 Hybrid studies | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.96] |
| 21 BPD (supplemental oxygen in survivors at 36 weeks only) | 9 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| 21.1 Strict studies | 4 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.52, 1.07] |
| 21.2 Hybrid studies | 5 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.89] |
Comparison 2. Volume‐targeted ventilation (VTV) versus pressure‐limited ventilation (PLV) ‐ infants weighing less than 1000 g.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death in hospital | 5 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.21] |
| 1.1 Strict studies | 4 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.46, 1.39] |
| 1.2 Hybrid studies | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.86] |
| 2 Death or bronchopulmonary dysplasia (BPD) (36 weeks) | 4 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.01] |
| 2.1 Strict studies | 3 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.52, 1.10] |
| 2.2 Hybrid studies | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.12] |
| 3 Duration of positive pressure ventilation (days, survivors) | 5 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐4.43, 2.80] |
| 3.1 Strict studies | 3 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐4.53, 3.89] |
| 3.2 Hybrid studies | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐2.21 [‐9.29, 4.87] |
| 4 Duration of positive pressure ventilation (log data, survivors) | 5 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] |
| 4.1 Strict studies | 3 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.23, 0.14] |
| 4.2 Hybrid studies | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.12, 0.14] |
| 5 Any pH < 7.25 | 2 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.46, 1.09] |
| 6 Hypocarbia partial pressure of carbon dioxide (pCO2) < 35 mmHg/4.7 kPa | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 2.36] |
| 7 Respiratory acidosis pH < 7.25 and pCO2 > 60 mmHg/8 kPa | 2 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.43, 1.47] |
| 8 Hypocarbia or respiratory acidosis | 1 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 2.36] |
| 9 Patent ductus arteriosus | 4 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.39] |
| 9.1 Strict studies | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.77, 1.57] |
| 9.2 Hybrid studies | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.78, 1.50] |
| 10 Air leak (any) | 4 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.23] |
| 10.1 Strict studies | 2 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.10, 7.24] |
| 10.2 Hybrid studies | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.54, 2.40] |
| 11 Pneumothorax | 5 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.29, 1.37] |
| 11.1 Strict studies | 3 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.11, 1.90] |
| 11.2 Hybrid studies | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.28, 1.86] |
| 12 Pulmonary interstitial emphysema | 4 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.58, 3.67] |
| 12.1 Strict studies | 2 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.10, 7.24] |
| 12.2 Hybrid studies | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.58, 4.53] |
| 13 Any intraventricular haemorrhage (IVH) | 4 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.55, 1.16] |
| 13.1 Strict studies | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.62, 2.08] |
| 13.2 Hybrid studies | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.40, 1.06] |
| 14 IVH grade 3‐4 | 4 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.27, 1.04] |
| 14.1 Strict studies | 3 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.28, 1.36] |
| 14.2 Hybrid studies | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.27] |
| 15 Periventricular leukomalacia (PVL) | 4 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.15, 1.24] |
| 15.1 Strict studies | 3 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.10, 1.53] |
| 15.2 Hybrid studies | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.63] |
| 16 Any IVH or PVL | 3 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.60, 1.35] |
| 16.1 Strict studies | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.61, 1.80] |
| 16.2 Hybrid studies | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.42] |
| 17 IVH grade 3‐4 or PVL | 3 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.15, 0.99] |
| 17.1 Strict studies | 2 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.04] |
| 17.2 Hybrid studies | 1 | 124 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.64] |
| 18 BPD (supplemental oxygen in survivors at 36 weeks) | 4 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.12] |
| 18.1 Strict studies | 3 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.49, 1.50] |
| 18.2 Hybrid studies | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.18] |
Comparison 3. Miscellaneous post hoc analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe disability (any definition) | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.47, 1.59] |
| 2 Severe disability (any definition) or death | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.27, 1.06] |
| 3 Gross motor developmental issue (any definition) | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.47, 2.14] |
| 4 Steroids for bronchopulmonary dysplasia | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.65, 1.31] |
| 5 Need for home oxygen (survivors) | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.30, 1.36] |
| 6 Need for home oxygen (survivors weighing < 1000 g) | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.25, 2.23] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhat 2016.
| Methods | Single‐centre randomised trial. | |
| Participants | 40 infants. Inclusion criteria: ≥ 34 weeks of GA and mechanically ventilated < 24 h in the first 2 weeks of life. Exclusion criteria: > 2 weeks of age, ventilated > 24 h with or without supported by high‐frequency ventilation or diagnosed with congenital diaphragmatic hernia. |
|
| Interventions | Ventilator: SLE5000 (software 4.3). Both groups initially: inflation time 0.3‐0.4 sec, inflation rate 40‐60/min, PEEP not reported.
Both groups: predefined weaning strategy either VTV mode or PLV mode. Not mentioned whether synchronisation used during weaning. Duration of intervention: until extubation. |
|
| Outcomes | Primary outcome: time to extubation. Secondary outcomes: physiological measurements including work of breathing (assessed by transdiaphragmatic pressure time product), blood gas analyses and failure of ventilation mode. |
|
| Supplemental data | Data on duration of ventilation presented as mean (SD). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table generation (unspecified). |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sequential sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to end of intervention. Secondary postintervention outcomes reported during period of primary admission. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration submitted after completion of the study. |
| Other bias | Low risk | |
Cheema 2007.
| Methods | Dual‐centre randomised trial. | |
| Participants | 40 infants. Inclusion criteria: GA < 34 weeks and ventilated for RDS. Exclusion criteria: major surgical or congenital anomalies. |
|
| Interventions | Ventilator: both groups used Drager Babylog 8000plus in SIPPV (AC) mode.
Duration of intervention: from onset of mechanical ventilation after admission to neonatal unit to first blood gas result (median duration 95 min). |
|
| Outcomes | Data only collected from time between onset of ventilation and until first blood gas analysis. Primary: PaCO2 and proportion of infants with PaCO2 within target range (5‐7 kPa). Others: first pH, PaO2. Post hoc subgroup analysis 23‐25, 26‐33 weeks. |
|
| Supplemental data | Blood gas data and data on randomisation procedure. Information about trial registration. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted blocks within strata (< 1250 g and > 1250 g blocks). |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No (outcome measure defined end of intervention period). |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up complete to discharge. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered. |
| Other bias | Low risk | |
Chowdhury 2013.
| Methods | Single‐centre randomised trial. | |
| Participants | 40 infants. Inclusion criteria: < 34 weeks GA, mechanically ventilated in the first week after birth. Exclusion criteria: major congenital anomalies, ventilated > 24 h or supported by high‐frequency ventilation or both. |
|
| Interventions | Ventilator: SLE5000 (software 4,3). Both groups: inflation time 0.3‐0.4 sec, inflation rate 40‐60/min, PEEP not reported.
Both groups: predefined weaning strategy; underlying trigger mode changed from SIMV to AC. Duration of intervention: until extubation. |
|
| Outcomes | Primary: time to reach specified weaning criteria. Other: survival to discharge, BPD at 28 days, IVH grade 3 or 4, cystic PVL, PDA treated (medication/ligation), pneumothorax, postnatal steroids, duration of ventilation, failure of initial ventilation mode, blood gas analyses and work of breathing (assessed by transdiaphragmatic pressure time product). |
|
| Supplemental data | Mortality, BPD at 36 weeks, detailed blood gas, duration of ventilation presented as mean (SD). | |
| Notes | Imbalance with regard to BW, GA and antenatal steroid use despite randomisation. Participants in the PLV group had lower median GA/BW than participants in the VTV group (median GA/BW 26 weeks/856 g vs 28 weeks/1016 g). In the published report, authors adjusted for this difference, but this review used the unadjusted outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table generation. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to end of intervention. Secondary postintervention outcomes reported during period of primary admission. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration submitted after completion of study. |
| Other bias | High risk | Despite randomisation, there was imbalance with regard to BW, GA and antenatal steroid use. Participants in the PLV group had lower median GA/BW than participants in the VTV group (median GA/BW 26 weeks/856 g vs 28 weeks/1016 g). |
D'Angio 2005.
| Methods | Dual‐centre randomised trial. | |
| Participants | 213 infants enrolled, but 1 infant immediately withdrawn, see below. Data on 212 infants. Inclusion criteria: BW 500‐1249 g, GA ≥ 24 weeks and in need of mechanical ventilation. Enrolled before 6 h of age. Exclusion criteria: not specified. |
|
| Interventions | Ventilator: both groups used primarily the Siemens Servo 300 ventilator. However, participants in the SIMV group were changed over to a VIP Bird ventilator (SIMV mode) if requiring a ventilator rate > 40/min. Target: PaO2 (mmHg): 45‐60 (GA 24‐26 weeks), 50‐70 (GA 27‐28 weeks), 60‐80 (GA > 28 weeks). PaCO2: 45‐55 mmHg (all GAs).
Duration of intervention: remained on randomised method until extubated, died or met failure criteria (hypoxia, hypercapnia or hypocapnia, or decision of clinical team). |
|
| Outcomes | Primary: proportion of infants alive and extubated at 14 days. Other: FiO2, ventilator rate, PIP, VT, PaCO2, PaO2, oxygenation index, AaDO2, proportion alive and extubated at 28 days or 36 weeks, proportion died before discharge, age at final extubation, proportion extubated at 14 days without requiring subsequent reintubation. |
|
| Supplemental data | BW, age of death in non‐survivors, BPD, duration of ventilation, pneumothorax, PIE, PVL, IVH. Information regarding blinding of assessors. Study protocol. |
|
| Notes | 1 infant in VTV group enrolled in error and immediately withdrawn from study. No data collected on this participant. As such, study reported data on 212 infants; 104 in the VTV group and 108 in the PLV group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (8 participants per block). Stratified by centre and BW. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: Sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | High risk | Hybrid study: different trigger modes in VTV and PLV groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to discharge. Long‐term follow‐up: 64 infants in each group were also assessed at 6‐18 months' corrected age (neurodevelopmental outcome). |
| Selective reporting (reporting bias) | Unclear risk | Study protocol sent to review authors. Study not registered. |
| Other bias | Low risk | |
Duman 2012.
| Methods | Single‐centre randomised trial. | |
| Participants | 45 infants. Inclusion criteria: GA < 32 weeks and mechanical ventilation for severe RDS for > 24 h. Exclusion criteria: major congenital anomalies and mechanical ventilation < 24 h. |
|
| Interventions | Ventilator: both groups used Drager Babylog 8000plus. Initially in SIPPV(AC) mode and then switched to SIMV mode during weaning. Inflation time 0.3‐0.4 sec and PEEP 4‐6 cmH2O. During weaning, respiratory rate was gradually reduced to 18/min. Clear protocol for ventilation and weaning. Target: PaCO2 40‐60 mmHg.
Duration of intervention: until extubation. |
|
| Outcomes | Primary: duration of ventilation (median and IQR). Other: mortality, BPD, PDA, IVH grade 3 or 4, PVL, pneumothorax. |
|
| Supplemental data | Duration of ventilation (mean and SD), clarification about the randomisation process and inclusions/exclusions. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation with random block sizes. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to end of intervention. Secondary postintervention outcomes reported during period of primary admission. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration. |
| Other bias | Low risk | |
Erdemir 2014.
| Methods | Single‐centre randomised trial. | |
| Participants | 60 infants. Inclusion criteria: GA < 33 weeks or BW < 1500 g (or both); ventilated for RDS. Exclusion criteria: admission at > 6 h of age; congenital cardiac, respiratory or CNS malformations; congenital metabolic diseases, congenital pneumonia, sepsis, perinatal asphyxia and leak < 20% around the ETT. |
|
| Interventions | Ventilator: Babylog 8000plus. All participants initially ventilated using SIPPV (AC) mode (PEEP 4 cmH2O, inflation time 0.4 sec). Intervention started in weaning phase when FiO2 < 0.40, inflation rate < 60/min, PIP 16 cmH2O and PEEP 4 cmH2O with blood gas values within targeted area. Infants then switched to 2 randomised 'weaning modes.'
Duration of intervention: From "start of weaning" until extubation. |
|
| Outcomes | Primary: "Reduction in ventilator‐associated lung injury." Other: mortality, BPD, pneumothorax, IVH (any), PDA and duration of ventilation. |
|
| Supplemental data | ||
| Notes | Initial ventilation with SIPPV, before start of weaning, lasted 7.8 h in PLV group and 4.4 h in VTV group. Weaning phase lasted 32.4 h in PLV group and 21.1 h in VTV group. Intervention mode consisted of 80% of total duration of ventilation in PLV group and 83% of total duration of ventilation in VTV group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation strategy not described. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | High risk | Hybrid study: different modes of flow termination. In PSV mode, inflation times varies (flow termination). In SIMV, there is a fixed inflation time. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes complete. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration available. |
| Other bias | Low risk | |
Guven 2013.
| Methods | Single‐centre randomised trial. | |
| Participants | 72 infants. Inclusion criteria: GA < 32 weeks or BW < 1500 g (or both), and admitted with RDS and given surfactant within first 2 h of life. Exclusion criteria: major congenital anomalies, perinatal asphyxia and meconium aspiration. |
|
| Interventions | Ventilator: both groups used Drager Babylog 8000plus in SIMV mode.
Duration of intervention: until extubation. |
|
| Outcomes | Primary outcome: duration of ventilation (days) and need of total oxygen supplementation. Secondary outcomes: survival to discharge, air leak, BPD (36 weeks), IVH grade 3 or 4, PVL, PDA, ROP and NEC. |
|
| Supplemental data | Information about postrandomisation loss sought from authors, see "Other bias." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation with random block sizes. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to end of intervention. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration. |
| Other bias | High risk | Reported in paper that 90 participants were randomised. However, postrandomisation 15 participants were excluded in PLV group and 3 participants excluded in VTV group. After contact with authors, it seemed that randomisation occurred before they had considered exclusion criteria and before parents had given consent. |
Herrera 2002.
| Methods | Single‐centre randomised cross‐over study. | |
| Participants | 17 infants. Inclusion criteria: appropriate for GA infants of 600‐1200 g, ventilated for RDS, > 48 h of age and clinically stable. Exclusion criteria: congenital malformations, sepsis, pneumothorax, other air leak, meconium aspiration and terminal state. |
|
| Interventions | Ventilator: both groups used Draeger Babylog 8000plus. Prestudy settings, SIMV rate 16/min, PIP 15 cmH2O. Cross‐over study:
Duration of intervention: 1 + 1 h. |
|
| Outcomes | Airflow, pressure, FiO2, TcCO2, minute volume. | |
| Supplemental data | ||
| Notes | Last 8 infants (of 17) randomised to an additional third VTV epoch of SIMV‐VG 3.0 mL/kg. For meta‐analysis, only SIMV‐VG 4.5 mL/kg (9 infants) vs SIMV data of all 17 infants used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no further information about randomisation procedure. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data complete. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable for review. |
| Other bias | Low risk | |
Hummler 2006.
| Methods | Single‐centre randomised cross‐over study. | |
| Participants | 15 infants. Inclusion criteria: infants ≤ 1500 g. Ventilator dependent with a ventilator rate ≥ 10/min and having recurrent hypoxaemic episodes (study definition). |
|
| Interventions | Ventilator: Stephanie infant ventilator. Pressure controlled SIMV prior to study. Target: SpO2 82‐90%. Standardised protocols for FiO2 adjustment. Cross‐over study:
Duration of intervention: 4 + 4 h. |
|
| Outcomes | Primary: time with SpO2 < lower limit of target range (80‐92%). Other: time with SpO2 above/within target range, incidence/duration/severity of desaturation episodes, FiO2, number of FiO2 adjustment necessary to target SpO2, VT, compliance, resistance. |
|
| Supplemental data | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no further information about randomisation procedure. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data complete. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable for review. |
| Other bias | Low risk | |
Jain 2016.
| Methods | Single‐centre randomised cross‐over study. | |
| Participants | 24 infants. Inclusion criteria: < 32 weeks' GA receiving PLV > 20/min, ≥ 4 episodes of hypoxaemia (SpO2 < 75%) in 8 h prior to study. Exclusion criteria: major congenital abnormalities, inotropes, sepsis or air leak within previous 72 h prior to study. |
|
| Interventions | Ventilator: AVEA, CareFusion. Infants remained on mode of ventilation set by clinical team before study. Volume targeting was only difference between groups. Cross‐over study:
Duration of intervention: 24 + 24 h. |
|
| Outcomes | Primary outcomes: proportion of time spent with arterial SpO2 < 75%. Secondary outcomes: number and characteristics of hypoxaemic episodes; FiO2 median and IQR, VT and minute ventilation. |
|
| Supplemental data | FiO2, mean and SD. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence: not specified. |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation: not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to end of intervention. |
| Selective reporting (reporting bias) | Low risk | Trial registered: clinicaltrials.gov NCT01727505. |
| Other bias | Low risk | |
Keszler 2004a.
| Methods | Single‐centre randomised trial. | |
| Participants | 18 infants. Inclusion criteria: < 34 weeks' GA, ventilated for RDS before 6 h of age. Exclusion criteria: congenital cardiac, respiratory or CNS anomalies, paralysis or sedation or ETT leak > 30%. |
|
| Interventions | Ventilator: both groups used Drager Babylog 8000 plus with set backup rate 40/min. Target: PaCO2 of 35‐45 torr (mmHg).
Duration of intervention: 72 h or until extubation. |
|
| Outcomes | Blood gas results, pneumothorax, PIE, mortality, cranial ultrasound scan. | |
| Supplemental data | BW, age of death in non‐survivors, BPD, duration of ventilation, pneumothorax, PIE, PVL, IVH, blood gas data. | |
| Notes | Requested trial registration. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used to randomise participants. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable for review. |
| Other bias | Low risk | |
Lista 2004.
| Methods | Dual‐centre randomised trial. | |
| Participants | 53 infants. Inclusion criteria: 25‐32 weeks' GA, received ≥ 1 course of antenatal steroids, ventilated for RDS in first 24 h, treated with surfactant within 3 h. Exclusion criteria: lethal anomalies, receiving muscle relaxants at entry, IVH grade ≥ 2, actual or suspected sepsis. |
|
| Interventions | Ventilator: both groups used Draeger Babylog 8000plus with set backup rate 40/min, PEEP 3.5‐4 cmH2O. Mean inflation time 0.4‐0.5 sec (upper limit in PSV mode). Target: FiO2 to maintain SpO2 90‐96%, pH > 7.25, PaO2 50‐75 mmHg, PaCO2 40‐65 mmHg.
Duration of intervention: until extubation. |
|
| Outcomes | Lung inflammatory markers. Other outcomes reported: death in hospital, PDA, BPD/receiving oxygen at 28 days and 36 weeks, IVH, PVL, ROP, PIE, PVL, need for postnatal steroids. |
|
| Supplemental data | BW, age of death in non‐survivors, BPD, duration of ventilation, pneumothorax, PIE, PVL, IVH, postrandomisation loss. | |
| Notes | Mean GA in PLV group was 29 weeks and mean GA in VTV group was 28 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequencing, stratified by GA (25‐28 weeks and 29‐32 weeks) and centre. |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation: not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to discharge. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available for review. |
| Other bias | High risk | Imbalance in numbers between the PLV and VTV groups. Postrandomisation, 7 infants excluded because placental histology identified chorioamnionitis (supplemental data), which could have influenced the primary outcome of this study. After postrandomisation exclusions, data from 30 infants in VTV group and 23 infants in PLV group were reported. In post hoc subgroup of infants weighing < 1000 g identified from supplemental data, 12/30 (40%) participants in VTV group were < 1000 g compared with 5/23 (22%) in PLV group. |
Liu 2011.
| Methods | Single‐centre randomised trial. | |
| Participants | 84 infants allocated to 3 ventilation groups: VTV (n = 31), PLV (n = 30) and high‐frequency ventilation (n = 23). Only data from the 61 infants ventilated with PLV and VTV are included in meta‐analysis. Inclusion criteria: neonatal RDS, defined blood gas/oxygenation criteria, age < 12 h, consent to surfactant. Exclusion criteria: congenital respiratory/cardiac malformations, pulmonary haemorrhage/gas leak/congenital pneumonia/meconium aspiration/wet lung/congenital heart disease/IVH grade III‐IV. |
|
| Interventions | Ventilator: Draeger Babylog 8000 (VTV group) and VIP Bird (PLV group).
Duration of intervention: not stated. |
|
| Outcomes | No clearly reported primary outcome.
|
|
| Supplemental data | Protocol and clarification on methods and results sought, but not received. | |
| Notes | Denominators of outcomes beyond intervention periods were unclear due to challenges following up participants who had transferred to other hospitals or who were withdrawn from active clinical management for financial reasons. Only outcomes which occurred during intervention period were included in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random number table used, method not specified. Unequal allocation to 3 groups (PLV, VTV and high‐frequency ventilation), and overall substantially more boys (n = 57) than girls (n = 27) included. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | High risk | Hybrid study: different ventilators. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completeness of follow‐up not stated. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration. |
| Other bias | Low risk | |
Nafday 2005.
| Methods | Single‐centre randomised trial. | |
| Participants | 34 infants. Inclusion criteria: BW < 1500 g, clinical and radiographic RDS, < 12 h old, about to receive surfactant. Exclusion criteria: major congenital malformations, congenital heart disease, confirmed/suspected sepsis/pneumonia, pneumothorax, other air leak, requiring paralysis/heavy sedation, moribund. |
|
| Interventions | Ventilator: both groups used Drager Babylog 8000plus. Ventilator rate adjusted to target blood gas values. Target: pH 7.25‐7.35, PaCO2 45‐55 mmHg, PaO2 50‐70 mmHg, SpO2 88‐95%.
Duration of intervention: 24 h. |
|
| Outcomes | Primary: ventilatory pressures during first 24 h after surfactant administration or randomisation. Others: survival to discharge, BPD (36 weeks), IVH, PDA (requiring indomethacin or ligation), NEC (Bell ≥ 2), air leak (PIE, pneumothorax, pneumomediastinum). |
|
| Supplemental data | BW, failure of assigned mode, pneumothorax, PIE, IVH. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, stratified by weight (500‐750 g, 751‐1000 g, 1001‐1250 g, 1251‐1500 g). |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | High risk | Hybrid study: different modes of flow termination. In PSV mode, inflation times varies (flow termination). In SIMV, there is a fixed inflation time. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to discharge. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable for review. |
| Other bias | Low risk | |
Piotrowski 1997.
| Methods | Single‐centre randomised trial. | |
| Participants | 57 infants. Inclusion criteria: BW < 2500 g, postnatal age < 72 h, and need for mechanical ventilation for lung disease at randomisation and Servo ventilator available. Exclusion criteria: sepsis/pneumonia, congenital malformation, pneumothorax or any other air leak, meconium aspiration. |
|
| Interventions | Ventilator: different ventilators used for experimental group (Siemens Sevo 300 ventilator) and control group (Bear Cub or Sechrist ventilator). Both groups ventilated using PEEP 3‐5 cmH2O and inflation time 0.5 sec. Target: SpO2 88‐95%, pCO2 < 55 mmHg. Infants extubated once ventilator rate < 12/min, FiO2 < 0.25, and after 30‐60 min trial of ETT‐CPAP.
Duration of intervention: until extubation. |
|
| Outcomes | Death in hospital, oxygen at 28 days, any air leak, pneumothorax, PIE, any IVH, IVH grade 3‐4, PDA, sepsis, use of muscle relaxants, duration of ventilation. | |
| Supplemental data | BW, age of death in non‐survivors, duration of ventilation. | |
| Notes | Mean GA in PLV group was 29 weeks and mean GA in VTV group was 30 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no further information about randomisation procedure. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | High risk | Hybrid study; the VTV group and PLV group used different ventilator models, modes and synchronisation settings. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable for review. |
| Other bias | Low risk | |
Piotrowski 2007.
| Methods | Single‐centre randomised trial. | |
| Participants | 56 infants. Inclusion criteria: GA 24‐32 weeks with RDS, requiring ventilation for ≥ 24 h. Exclusion criteria: severe congenital malformation, lack of parental consent and pulmonary air leak on admission. |
|
| Interventions | Ventilator: VTV group (PRVC) used Siemens Servo 300. PLV group (SIMV) used 1 of the 4 different ventilators (depending on availability): Bear Cub (CEM)/Bear 750 PSV, Sechrist Millenium, Draeger Babylog 8000 plus or SLE 5000. Both groups: inflation time 0.4 sec, inflation rate 40/min, PEEP 3‐5 cmH2O.
Duration of intervention: until extubation. |
|
| Outcomes | Primary outcome: ≥ 12 h with "effective ventilation" (SpO2 > 90 %, PaCO2 < 50 mmHg) with FiO2 < 0.23 and PIP < 15 cmH2O. Secondary outcomes: time to extubation, BPD (28 days), air leak, IVH and PDA. |
|
| Supplemental data | Results translated into English. Information regarding stratification sought. | |
| Notes | Despite randomisation, there were imbalances between VTV and PLV groups in FiO2 in first 6 h of life and surfactant use (higher FiO2 and more surfactant used in the VTV group). In published report, authors adjusted for this difference, but in this review the unadjusted outcomes were used. Median GA in PLV group was 28 weeks and median GA in VTV group was 28 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequential numbers. Stratified by GA (24‐28 weeks and 29‐33 weeks). |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | High risk | Hybrid study: different ventilators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to discharge. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable for review. |
| Other bias | High risk | Although randomised, infants in the VTV (PRVC) group had increased surfactant use and increased FiO2 in first 6 h after admission. |
Polimeni 2006.
| Methods | Single‐centre randomised cross‐over study. | |
| Participants | 32 Infants. Inclusion criteria: BW <1500 g, recovered from RDS, presenting with hypoxaemic episodes. |
|
| Interventions | Ventilator: both groups used Draeger Babylog 8000plus. Cross‐over study: 12 infants with expired VTtarget 4.5 mL/kg. 20 infants with expired VTtarget 6.0 mL/kg. 1st group (n = 12)
2nd group (n = 20)
Duration of intervention: 2 + 2 h. |
|
| Outcomes | Primary: frequency and severity of hypoxaemic episodes. Other: PIP, distribution of VT, frequency and duration of hypoxaemia (SpO2 < 88%, < 75%), FiO2. |
|
| Supplemental data | Study protocol. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no further information about randomisation procedure. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data complete. |
| Selective reporting (reporting bias) | Low risk | Study protocol sent to review authors. Study retrospectively registered. |
| Other bias | Low risk | |
Singh 2006.
| Methods | Dual‐centre randomised trial. | |
| Participants | Initially 2 centres, but reduced to one; 109 infants. Inclusion criteria: BW 600‐1500 g, GA 24‐31 weeks with RDS requiring mechanical ventilation. Exclusion criteria: severe congenital malformations. All participants included as intention to treat. Some analyses only performed for participants from main centre. |
|
| Interventions | Ventilator: both groups used VIP Bird Gold.
Duration of intervention: until infants were recovering from their acute respiratory illness. At that point, the ventilatory mode was changed to SIMV with pressure support ("weaning mode") for participants in both groups. |
|
| Outcomes | Primary outcome criteria: time from entry into the study until achievement of either AaDO2 < 13 kPa for > 12 h or MAP < 8.0 cmH2O for > 12 h. Other: total duration of mechanical ventilation, duration of MV + CPAP, survival to discharge, frequency of complications: BPD (36 weeks), IVH, PVL, PDA (requiring treatment), NEC (Bell grade ≥ 2), FiO2 (data from Swamy 2008). Follow‐up (Singh 2009): need for home oxygen, cough, wheeze, inhaler use, rate of hospital readmission, rate of respiratory readmission, neurodisability (cerebral palsy, deaf, behavioural problems, blindness) by questionnaire. |
|
| Supplemental data | BW, age of death in non‐survivors, BPD, duration of ventilation, pneumothorax, PIE, PVL, IVH, PDA. | |
| Notes | 109 infants enrolled in Singh 2006, of whom 94 survived to discharge. 3 infants died post‐discharge. Follow‐up studies: Singh 2009: 85/91 (93%) infants eligible for follow‐up assessed at median of 22 months' corrected age; 45 in VTV group and 40 in PLV group (Singh 2009). Reported on pulmonary morbidities and gross neurodevelopmental outcomes and mortality Swamy 2008: reported on respiratory parameters |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random block randomisation. Stratified by BW. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurements to discharge: no. Investigators involved in long‐term follow‐up were blinded to original treatment modality. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete to discharge. 85/91 (93%) infants eligible for follow‐up were assessed at a median of 22 months' corrected age. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol unavailable for review. |
| Other bias | Unclear risk | Both arms weaned using PLV mode. |
Sinha 1997.
| Methods | Single‐centre randomised trial. | |
| Participants | 50 infants. Inclusion criteria: BW > 1200 g and had RDS requiring mechanical ventilation. Exclusion criteria: confirmed/suspected sepsis/pneumonia, congenital malformation or lack of arterial access. |
|
| Interventions | Ventilator: both groups used VIP Bird ventilator in AC mode with inflation time at 0.3‐0.5 sec. Target: pH 7.27‐7.40, PaCO2 4.5 to 6 kPa, PaO2 8‐11 kPa.
Duration of intervention: until weaning from ventilation. |
|
| Outcomes | "Success" criteria outcome: time from entry into study until achievement of either AaDO2< 13 kPa for > 12 h or MAP < 8.0 cmH2O for > 12 h or extubation. Other outcome criteria: death in hospital, failed allocated treatment, IVH or PVL (not reported separately), BPD (in oxygen at 36 weeks), pneumothorax, PDA. |
|
| Supplemental data | Requested study protocol/trial registration. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no further information about randomisation procedure. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Was done for chest x‐ray findings, but not for other outcome. |
| Intervention bias (strict vs hybrid studies) | High risk | Hybrid study: VTV mode was pressure triggered. PLV mode was flow triggered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: complete. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. |
| Other bias | Unclear risk | Both arms weaned using a PLV mode. |
Zhou 2007.
| Methods | Single‐centre randomised trial. | |
| Participants | 30 infants. Inclusion criteria: very low BW (≤ 1500 g, ≤ 32 weeks GA) infants with hyaline membrane disease. Exclusion criteria: perinatal infection, positive lower respiratory tract culture, blood in ETT secretion or pulmonary haemorrhage, severe congenital malformation. |
|
| Interventions | Ventilator: both groups used Drager Babylog 8000plus in SIMV mode.
Rest of ventilator parameters regulated according to blood gases analysis. Duration of intervention: not described. |
|
| Outcomes | Primary: proinflammatory cytokines in bronchoalveolar lavage fluid. Other: FiO2, pneumothorax (data included in meta‐analysis). BPD and IVH (incomplete information), death (data not included in meta‐analysis). |
|
| Supplemental data | Protocol and clarification on methods and results sought, but not received. | |
| Notes | Denominators of outcomes beyond intervention periods were unclear due to challenges following up participants who had transferred to other hospitals or who were withdrawn from active clinical management for financial reasons. Only outcomes which occurred during intervention period were included in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no further information about randomisation procedure. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No. |
| Intervention bias (strict vs hybrid studies) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Postintervention outcomes report on 25/30 (83%) participants. Stated reasons included financial dropouts and withdrawal from study due to IVH. |
| Selective reporting (reporting bias) | Unclear risk | Review authors requested study protocol, but received no response. |
| Other bias | Low risk | |
AaDO2: alveolar to arterial oxygen pressure difference; AC: assist control; BPD: bronchopulmonary dysplasia; BW: birth weight; CPAP: continuous positive airway pressure; CNS: central nervous system; ETT: endotracheal tube; FiO2: fraction of inspired oxygen; GA: gestational age; h: hour; IQR: interquartile range; IMV: intermittent mandatory ventilation; IVH: intraventricular haemorrhage; MAP: mean airway pressure; min: minute; MV: mandatory ventilation; n: number of infants; NEC: necrotising enterocolitis; Pmax: maximum peak inflation pressure; pCO2: partial pressure of carbon dioxide; PaCO2: partial pressure of carbon dioxide in arterial blood; PaO2: partial pressure of oxygen in arterial blood; PDA: patent ductus arteriosus; PEEP: positive end‐expiratory pressure; PIE: pulmonary interstitial emphysema; PIP: peak inflation pressure; PLV: pressure‐limited ventilation; Pmax: maximum peak inflation pressure; PRVC: pressure‐regulated volume control; PSV: pressure support ventilation; PVL: periventricular leukomalacia; RDS: respiratory distress syndrome; ROP: retinopathy of prematurity; SD: standard deviation; sec: second; SIMV: synchronised intermittent mandatory ventilation; SIPPV: synchronised intermittent positive pressure ventilation (same as AC); SpO2: blood oxygen saturation level; TcCO2: transcutaneous carbon dioxide; Timax: maximal inspiratory time; TTV: targeted tidal volume; VG: volume guarantee; VTtarget: target tidal volume; VTV: volume‐targeted ventilation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd El‐Moneim 2005 | Journal publication. Cross‐over study, but not randomised. |
| Abubakar 2001 | Journal publication. Order of ventilatory modes not randomised in this cross‐over study which means that an effect of fatigue cannot be excluded. Additionally, study did not report outcomes specified in this Cochrane Review. |
| Abubakar 2006 | Abstract presentation. Study investigating time to recovery after ETT suction in infants randomised to ventilation with/without VG mode. Study did not report outcomes specified in this Cochrane Review. |
| Cheema 2001 | Journal publication. Short‐term cross‐over study did not address any of the outcomes of this Cochrane Review. Also, the cross‐over was made from PLV to VG mode without changing Pmax, which may have interfered with the ventilator's capacity to deliver the set VT and hence affected the outcomes. |
| Colnaghi 2006 | Abstract presentation. Randomised trial comparing 3 groups ventilated with Draeger Babylog 8000plus: group 1: PSV; group 2: PSV + VG; group 3: AC + VG. However, outcomes were biochemical assays of inflammatory markers in serum and tracheal aspirates. Study did not report outcomes specified in this Cochrane Review. Despite randomisation, there were inception differences in study group characteristics. We attempted to contact authors for further information. |
| Dotta 2004 | Abstract presentation. Randomised study, but authors did not report outcomes specified in this Cochrane Review. |
| Keszler 2004b | Abstract presentation. Abstract did not report whether interventions randomised. Study outcomes did not include those specified in this Cochrane Review. |
| Lista 2000 | Journal publication (in Italian). A non‐randomised study. |
| NCT00157989 | Randomised study, but later the studies were terminated. |
| NCT00295230 | Study designed to compare effects of VG with pressure supported vs synchronised intermittent mandatory ventilation in very low birth weight infants. Study started in 2006, but later participant recruitment was suspended due to suboptimal enrolment after 18 months, and study was terminated. |
| Olsen 2002 | Journal publication. Cross‐over study that did not discuss outcome measurements of this Cochrane Review. |
| Ramirez‐Del Valle 2006 | Abstract presentation. Randomised study, but authors did not report the outcomes specified in this Cochrane Review. |
| Shah 2013 | Journal publication. Non‐randomised study. |
| Sinha 2008 | Abstract presentation. Outcomes did not include those specified in this Cochrane Review. |
| Stefanescu 2015 | Journal publication. Non‐randomised study. |
| Unal 2014 | Abstract presentation. Comparing 2 VTV modes, no comparison with PLV mode. |
| Wach 2003 | Abstract presentation. No information in the abstract whether intervention was randomised. Outcomes did not include those specified in this Cochrane Review. |
AC: assist control; ETT: endotracheal tube; Pmax: maximum peak inflation pressure; PLV: pressure‐limited ventilation; PSV: pressure support ventilation; VG: volume guarantee; VT: tidal volume; VTV: volume‐targeted ventilation.
Characteristics of studies awaiting assessment [ordered by study ID]
Liu 2016.
| Methods | Article published in Chinese. We have not been able to obtain a translated version. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Translated manuscript sought. |
Miracle 2016.
| Methods | Randomised parallel study. |
| Participants | 35 preterm infants 25‐32 weeks' gestation. |
| Interventions | SIMV, PSV + VG |
| Outcomes | |
| Notes | Final published data awaited. Published as abstract only. |
PSV: pressure support ventilation; SIMV: synchronised intermittent mandatory ventilation; VG: volume guarantee.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12609000986279.
| Trial name or title | A Randomised Controlled Trial of Modes of Ventilatory Support in Preterm Infants from Point of Delivery to the Neonatal Intensive Care Unit. |
| Methods | Randomisation (sealed enveloped). |
| Participants | Preterm infants < 32 weeks, ventilated in delivery room. |
| Interventions | VTV group: triggered VG mode. PLV group: IMV. |
| Outcomes | PaCO2, PaO2, cerebral blood flow, IVH grade 3‐4, PVL, BPD, neurodevelopmental impairment at 1 and 3 years. |
| Starting date | 29 November 2006. |
| Contact information | Dr Mark Tracey. The ACTRN12609000986279 trial of VTV from the point of delivery was registered with the Australian New Zealand Clinical Trials Registry. Recruitment commenced November 2006 and has now closed. At this stage, no outcomes have been reported. |
| Notes | We have attempted to contact the authors for further information. |
Salvia 2006.
| Trial name or title | Effect of VG Combined with SIMV vs SIMV in the Extremely Premature Infant. |
| Methods | Randomisation (unspecified). |
| Participants | 60 very low birth weight infants. |
| Interventions | VTV group: SIMV + VG. PLV group: SIMV. |
| Outcomes | PIP, MAP, VT, CO2, FiO2/SpO2. Duration of mechanical ventilation, oxygen therapy, duration of admission, PDA, IVH, PVL, BPD, 2‐year follow‐up data. |
| Starting date | Not reported. |
| Contact information | Dr Salvia. |
| Notes | Studied from 30 min after first surfactant dose. Study is ongoing and collecting 2‐year follow‐up data. Hitherto only short‐term outcomes have been presented in abstract form. Longer‐term follow‐up is in progress (information from author), and the final published data are awaited. We have attempted to contact the authors for further information. |
BPD: bronchopulmonary dysplasia; FiO2: fraction of inspired oxygen; IMV: intermittent mandatory ventilation; IVH: intraventricular haemorrhage; MAP: mean airway pressure; PaCO2: partial pressure of carbon dioxide in arterial blood; PaO2: partial pressure of oxygen in arterial blood; PDA: patent ductus arteriosus; PIP: peak inflation pressure; PLV: pressure‐limited ventilation; PVL: periventricular leukomalacia; SIMV: synchronised intermittent mandatory ventilation; SpO2: blood oxygen saturation level; VG: volume guarantee; VT: tidal volume; VTV: volume‐targeted ventilation.
Differences between protocol and review
The original protocol limited PLV to time‐cycled modes. In view of development of modern PLV modes that may be flow cycled (e.g. PSV mode with the Draeger Babylog Plus ventilator), we have chosen to include all trials comparing VTV with PLV, independent of PLV being provided in a time‐cycled or flow‐cycled manner.
The following subgroup analysis was performed, which was not specified in the initial protocol.
Subgroup analysis for strict versus hybrid trial designs, within Analyses 1 and 2 where applicable.
The following outcomes included above were not included in the original protocol for this review.
Analysis 3.1 Severe disability (arbitrary).
Analysis 3.2 Severe disability (arbitrary definition) or death.
Analysis 3.3 Gross motor developmental issue (arbitrary definition).
Analysis 3.4 Steroids for BPD.
Analysis 3.5 Need for home oxygen (survivors).
Analysis 3.6 Need for home oxygen (survivors weighing less than 1000 g).
We also added the methodology and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol or previous versions of the review.
Contributions of authors
NMC: wrote the protocol with assistance from CJM and PGD. For the 2005 review, NMC wrote the review with assistance from PGD and CJM.
For the 2010 update, KIW and CK performed the search, assessed articles, liaised with study authors regarding supplemental information, extracted and analysed data. PGD supervised the research. CJM and NMC assisted with reviewing the manuscript.
For the 2017 update, KIW and CK performed the search, assessed articles, liaised with study authors regarding supplemental information, extracted and analysed data. CK and KIW wrote the review. PGD, CJM and NMC assisted with reviewing the manuscript.
Sources of support
Internal sources
Royal Women's Hospital Foundation, Melbourne, Australia.
Murdoch Children's Research Institute, Melbourne, Australia.
External sources
No sources of support supplied
Declarations of interest
CJM: has acted as a consultant to Drager Medical and Acutronic Medical Instruments, both manufacturers of neonatal ventilators. The companies had no involvement with the funding, design or conduct of this review.
CK: None
KIW: None
PGD: None
NMC: None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bhat 2016 {published and unpublished data}
- Bhat P, Chowdhury O, Shetty S, Hannam S, Rafferty GF, Peacock J, et al. Volume‐targeted versus pressure‐limited ventilation in infants born at or near term. European Journal of Pediatrics 2016;175(1):89‐95. [DOI: 10.1007/s00431-015-2596-3; PUBMED: 26239663] [DOI] [PubMed] [Google Scholar]
Cheema 2007 {published and unpublished data}
- Cheema IU, Sinha AK, Kempley ST, Ahluwalia JS. Impact of volume guarantee ventilation on arterial carbon dioxide tension in newborn infants: a randomised controlled trial. Early Human Development 2007;83(3):183‐9. [DOI: 10.1016/j.earlhumdev.2006.05.013; PUBMED: 16815649] [DOI] [PubMed] [Google Scholar]
Chowdhury 2013 {published and unpublished data}
- Chowdhury O, Patel DS, Hannam S, Lee S, Rafferty GF, Peacock JL, et al. Randomised trial of volume‐targeted ventilation versus pressure‐limited ventilation in acute respiratory failure in prematurely born infants. Neonatology 2013;104(4):290‐4. [DOI: 10.1159/000353956; PUBMED: 24107474] [DOI] [PubMed] [Google Scholar]
D'Angio 2005 {published and unpublished data}
- D'Angio CT, Chess PR, Kovacs SJ, Sinkin RA, Phelps DL, Kendig JW, et al. Pressure‐regulated volume control ventilation vs synchronized intermittent mandatory ventilation for very low‐birth‐weight infants: a randomized controlled trial. Archives of Pediatrics and Adolescent Medicine 2005;159(9):868‐75. [DOI: 10.1001/archpedi.159.9.868; PUBMED: 16143747] [DOI] [PubMed] [Google Scholar]
Duman 2012 {published and unpublished data}
- Duman N, Tuzun F, Sutcuoglu, Yesilirmak CD, Kumral A, Ozkan H. Impact of volume guarantee on synchronized ventilation in preterm infants: a randomized controlled trial. Intensive Care Medicine 2012;38(8):1358‐64. [DOI: 10.1007/s00134-012-2601-5; PUBMED: 22618094] [DOI] [PubMed] [Google Scholar]
Erdemir 2014 {published data only}
- Erdemir A, Kahramaner Z, Turkoglu E, Cosar H, Sutcuoglu S, Ozer E. Effects of synchronized intermittent mandatory ventilation versus pressure support plus volume guarantee ventilation in the weaning phase of preterm infants. Pediatric Critical Care Medicine 2014;15(3):236‐41. [DOI: 10.1097/PCC.0b013e3182a5570e; PUBMED: 24608494] [DOI] [PubMed] [Google Scholar]
Guven 2013 {published and unpublished data}
- Guven S, Bozdag S, Saner H, Cetinkaya M, Yazar AS, Erguven M. Early neonatal outcomes of volume guaranteed ventilation in preterm infants with respiratory distress syndrome. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(4):396‐401. [DOI: 10.3109/14767058.2012.733778; PUBMED: 23039373] [DOI] [PubMed] [Google Scholar]
Herrera 2002 {published data only}
- Herrera CM, Gerhardt T, Claure N, Everett R, Musante G, Thomas C, et al. Effects of volume‐guaranteed synchronized intermittent mandatory ventilation in preterm infants recovering from respiratory failure. Pediatrics 2002;110(3):529‐33. [PUBMED: 12205255] [DOI] [PubMed] [Google Scholar]
Hummler 2006 {published data only (unpublished sought but not used)}
- Hummler HD, Engelmann A, Pohlandt F, Franz AR. Volume‐controlled intermittent mandatory ventilation in preterm infants with hypoxemic episodes. Intensive Care Medicine 2006;32(4):577‐84. [DOI: 10.1007/s00134-006-0079-8; PUBMED: 16501947] [DOI] [PubMed] [Google Scholar]
Jain 2016 {published and unpublished data}
- Jain D, Claure N, D'Ugard C, Bello J, Bancalri E. Volume guarantee ventilation: effect on preterm infants with frequent hypoxemia episode. Neonatology 2016;110(2):129‐34. [DOI: 10.1159/000444844; PUBMED: 27088487] [DOI] [PubMed] [Google Scholar]
Keszler 2004a {published and unpublished data}
- Keszler M, Abubakar K. Volume guarantee: stability of tidal volume and incidence of hypocarbia. Pediatric Pulmonology 2004;38(3):240‐5. [DOI: 10.1002/ppul.20063; PUBMED: 15274104] [DOI] [PubMed] [Google Scholar]
Lista 2004 {published and unpublished data}
- Lista G, Colnaghi M, Castoldi F, Condo V, Reali R, Compagnoni G, et al. Impact of targeted‐volume ventilation on lung inflammatory response in preterm infants with respiratory distress syndrome (RDS). Pediatric Pulmonology 2004;37(6):510‐4. [DOI: 10.1002/ppul.10458; PUBMED: 15114551] [DOI] [PubMed] [Google Scholar]
Liu 2011 {published data only (unpublished sought but not used)}
- Liu CQ, Cui Z, Xia YF, Ma L, Fan LL. Randomized controlled study of targeted tidal volume ventilation for treatment of severe neonatal respiratory distress syndrome. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2011;13(9):1‐8. [PUBMED: 21924013] [PubMed] [Google Scholar]
Nafday 2005 {published and unpublished data}
- Nafday SM, Green RS, Lin J, Brion LP, Ochshorn I, Holzman IR. Is there an advantage of using pressure support ventilation with volume guarantee in the initial management of premature infants with respiratory distress syndrome? A pilot study. Journal of Perinatology 2005;25(3):193‐7. [DOI: 10.1038/sj.jp.7211233; PUBMED: 15674409] [DOI] [PubMed] [Google Scholar]
Piotrowski 1997 {published and unpublished data}
- Piotrowski A, Sobala W, Kawczynski P. Patient‐initiated, pressure‐regulated, volume‐controlled ventilation compared with intermittent mandatory ventilation in neonates: a prospective, randomised study. Intensive Care Medicine 1997;23(9):975‐81. [PUBMED: 9347370] [DOI] [PubMed] [Google Scholar]
Piotrowski 2007 {published and unpublished data}
- Piotrowski A, Bernas S, Fendler W. A randomised trial comparing two synchronised ventilation modes in neonates with respiratory distress syndrome. Anestezjologia Intensywna Terapia 2007;39(2):58‐63. [EMBASE: 2007370596] [Google Scholar]
Polimeni 2006 {published and unpublished data}
- Polimeni V, Claure N, D'Ugard C, Bancalari E. Effects of volume‐targeted synchronized intermittent mandatory ventilation on spontaneous episodes of hypoxemia in preterm infants. Biology of the Neonate 2006;89(1):50‐5. [DOI: 10.1159/000088198; PUBMED: 16155386] [DOI] [PubMed] [Google Scholar]
Singh 2006 {published and unpublished data}
- Singh J, Sinha SK, Alsop E, Gupta S, Misra A, Donn SM. Long term follow‐up of very low birthweight infants from a neonatal volume versus pressure mechanical ventilation trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(5):F360‐2. [DOI: 10.1136/adc.2008.150938; PUBMED: 19321507] [DOI] [PubMed] [Google Scholar]
- Singh J, Sinha SK, Clarke P, Byrne S, Donn SM. Mechanical ventilation of very low birth weight infants: is volume or pressure a better target variable?. Journal of Pediatrics 2006;149(3):308‐13. [DOI: 10.1016/j.jpeds.2006.01.044; PUBMED: 16939738] [DOI] [PubMed] [Google Scholar]
- Swamy R, Gupta S, Singh J, Donn SM, Sinha SK. Tidal volume delivery and peak inspiratory pressure in babies receiving volume targeted or time cycled, pressure limited ventilation: a randomized controlled trial. Journal of Neonatal‐Perinatal Medicine 2008;1(4):239‐43. [Google Scholar]
Sinha 1997 {published data only}
- Sinha SK, Donn SM, Gavey J, McCarty M. Randomised trial of volume controlled versus time cycled, pressure limited ventilation in preterm infants with respiratory distress syndrome. Archives of Disease in Childhood. Fetal and Neonatal Edition 1997;77(3):F202‐5. [PUBMED: 9462190 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2007 {published data only (unpublished sought but not used)}
- Zhou XJ, Zhou YR, Hu DY, Chen XW, Jin YM, Lu ZQ. Effects of different ventilation modes on the lung injury in infants with very low birth weight. Chinese Journal of Emergency Medicine 2007;16(7):703‐5. [Google Scholar]
References to studies excluded from this review
Abd El‐Moneim 2005 {published data only}
- Abd El‐Moneim ES, Fuerste HO, Krueger M, Elmagd AA, Brandis M, Schulte‐Moenting J, et al. Pressure support ventilation combined with volume guarantee versus synchronized intermittent mandatory ventilation: a pilot crossover trial in premature infants in their weaning phase. Pediatric Critical Care Medicine 2005;6(3):286‐92. [DOI: 10.1097/01.PCC.0000161071.47031.61; PUBMED: 15857526 ] [DOI] [PubMed] [Google Scholar]
Abubakar 2001 {published data only}
- Abubakar KM, Keszler M. Patient‐ventilator interactions in new modes of patient‐triggered ventilation. Pediatric Pulmonology 2001;32(1):71‐5. [PUBMED: 11416879] [DOI] [PubMed] [Google Scholar]
Abubakar 2006 {published data only}
- Abubakar KM, Montazami S, Keszler M. Volume guarantee accelerates recovery from endotracheal tube suctioning in ventilated preterm infants. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco (CA) 2006. [E‐PAS2006:5560.343]
Cheema 2001 {published data only}
- Cheema IU, Ahluwahlia JS. Feasibility of tidal volume‐guided ventilation in newborn infants: a randomised crossover trial using the volume guarantee modality. Pediatrics 2001;107(6):1323‐8. [PUBMED: 11389251] [DOI] [PubMed] [Google Scholar]
Colnaghi 2006 {published data only}
- Colnaghi M, Weissmann G, Ciralli F, Matassa PG, Condo V, Messina D, et al. Volume‐targeted ventilation and lung inflammatory injury in preterm infants with RDS. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco (CA) 2006. [E‐PAS2006:5506.340]
Dotta 2004 {published data only}
- Dotta A, Crescenzi F, Braguglia A, Campi F, Rechichi J, Orzalesi M. Cerebral haemodynamics in preterm infants during synchronized intermittent mandatory ventilation (SIMV) and synchronized intermittent positive pressure ventilation (SIPPV), with and without volume guarantee (VG). Pediatric Academic Societies' Annual Meeting; 2004 May 1‐4; San Francisco (CA). 2004. [PAS2004:3061]
Keszler 2004b {published data only}
- Keszler M, Abubakar KM. Volume guarantee accelerates recovery from forced exhalation episodes. Pediatric Academic Societies' Annual Meeting; 2004 May 1‐4, 2004; San Francisco (CA). 2004. [PAS2004:3092]
Lista 2000 {published data only}
- Lista G, Marangione P, Azzali A, Castoldi F, Pogliani L, Compagnoni G. The "guaranteed volume" in pressure support ventilation reduces the risk of barotrauma in premature children with severe respiratory syndrome. Acta Bio‐medica de l'Ateneo Parmense 2000;71(Suppl 1):453‐6. [PUBMED: 11424787] [PubMed] [Google Scholar]
NCT00157989 {unpublished data only}
- NCT00157989. Study to assess safety and feasibility of resuscitation of preterm infants with controlled volume of air/oxygen. clinicaltrials.gov/show/NCT00157989 Date first received: 8 September 2005.
NCT00295230 {published and unpublished data}
- NCT00295230. Effects of volume guarantee with pressure supported vs. synchronized intermittent mandatory ventilation in VLBW infants. clinicaltrials.gov/show/NCT00295230 Date first received: 21 February 2006.
Olsen 2002 {published data only}
- Olsen SL, Thibeault DW, Truog WE. Crossover trial comparing pressure support with synchronized intermittent ventilation. Journal of Perinatology 2002;22(6):461‐6. [DOI: 10.1038/sj.jp.7210772; PUBMED: 12168123] [DOI] [PubMed] [Google Scholar]
Ramirez‐Del Valle 2006 {published data only}
- Ramirez‐Del Valle JO, Villa‐Guillen M, Reyes A, Murguia‐de Sierra T. Tidal volume (VT) delivery and stability of different ventilatory parameters during synchronized mechanical ventilation with or without volume guarantee (VG). Is VT stability always associated to peak inspiratory changes?. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco (CA). 2006. [E‐PAS2006:4843.186]
Shah 2013 {published data only}
- Shah S, Kaul A. Volume targeted ventilation and arterial carbon dioxide in extremely preterm infants. Journal of Neonatal and Perinatal Medicine 2013;6(4):339‐44. [DOI: 10.3233/NPM-1372713] [DOI] [PubMed] [Google Scholar]
Sinha 2008 {published data only}
- Sinha AK, Kempley ST. A randomised trial comparing the effects of volume guided ventilation and synchronised intermittent positive pressure ventilation on the cerebral and mesenteric circulation following surfactant administration. 2nd Congress of the European Academy of Paediatrics; 2008 Oct 24‐28; Nice (France). 2008.
Stefanescu 2015 {published data only}
- Stefanescu BM, Frewan N, Slaughter JC, O'Shea TM. Volume guarantee pressure support ventilation in extremely preterm infants and neurodevelopmental outcome at 18 months. Journal of Perinatology 2015;35(6):419‐23. [DOI: 10.1038/jp.2014.228; PUBMED: 25569681] [DOI] [PubMed] [Google Scholar]
Unal 2014 {unpublished data only}
- Unal S, Ergenekon E, Aktas S, Altuntas N, Beken S, Kazanci E, et al. Evaluation of ventilatory parameters, short and long term morbidities in preterms ventilated with either PSV+VG or SIMV+VG. 5th Congress of the European Academy of Paediatric Societies; 2014 Oct 17‐21 October; Barcelona (Spain) 2014. [NCT01514331]
Wach 2003 {published data only}
- Wach RA, Osiovich HC. Can assist control plus volume guarantee (AC+VG) avoid large tidal volumes (TV) in ventilated spontaneously breathing infants with BPD?. Pediatric Academic Societies' Annual Meeting; 2003 May 3‐6; Seattle (Washington). 2003. [PAS2003:2896]
References to studies awaiting assessment
Liu 2016 {unpublished data only}
- Liu CZ, Huang BY, Tan BY, Guan HF, Xu XH, Guo QY. Efficacy of volume‐targeted ventilation for the treatment of neonatal respiratory distress syndrome. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2016;18(1):6‐9. [PUBMED: 26781404] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miracle 2016 {published data only}
- Miracle X, Salvia MD, Figueras J, Rodriguez JM, Carbonell X. Effects of pressure support plus volume guarantee ventilation versus synchronized intermittent mandatory ventilation in extremely low birth weight infants with respiratory distress syndrome: a prospective, randomized study. Journal of Maternal‐Fetal and Neonatal Medicine 2016;29(Supple 1):236‐7. [DOI: 10.1080/14767058.2016.1191212; PUBMED: 27633796] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12609000986279 {unpublished data only}
- ACTRN12609000986279. A randomized controlled trial of modes of ventilatory support in preterm babies from point of delivery to the neonatal intensive care unit. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=308342&isReview=true Date first received: 9 July 2009.
Salvia 2006 {published and unpublished data}
- Salvia MD, Figueras J, Miracle X, Rodríguez‐Miguélez JM, Carbonell X. Effect of volume guarantee combined with synchronized intermittent mandatory ventilation vs synchronized intermittent mandatory ventilation in the extremely premature. European Journal of Pediatrics 2006;165(Suppl 1):376. [DOI: 10.1007/s00431-006-0349-z] [DOI] [Google Scholar]
Additional references
Al‐Majed 2004
- Al‐Majed SI, Thompson JE, Watson KF, Randolph AG. Effect of lung compliance and endotracheal tube leakage on measurement of tidal volume. Critical Care 2004;8(6):R398‐402. [DOI: 10.1186/cc2954; PUBMED: 15566583] [DOI] [PMC free article] [PubMed] [Google Scholar]
ARDS Network 2000
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine 2000;342(2):1301‐8. [DOI: 10.1056/NEJM200005043421801; PUBMED: 10793162] [DOI] [PubMed] [Google Scholar]
Bardin 1997
- Bardin C, Zelkowitz P, Papageorgiou A. Outcome of small‐for‐gestational age and appropriate‐for‐gestational age infants born before 27 weeks of gestation. Pediatrics 1997;100(2):E4. [PUBMED: 9233975] [DOI] [PubMed] [Google Scholar]
Bjorklund 1997
- Bjorklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O, et al. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatric Research 1997;42(3):348‐55. [DOI: 10.1203/00006450-199709000-00016; PUBMED: 9284276] [DOI] [PubMed] [Google Scholar]
Cannon 2000
- Cannon ML, Cornell J, Tripp‐Hamel DS, Gentile MA, Hubble CL, Meliones JN, et al. Tidal volumes for ventilated infants should be determined with a pneumotachometer placed at the endotracheal tube. American Journal of Respiratory and Critical Care Medicine 2000;162(6):2109‐12. [DOI: 10.1164/ajrccm.162.6.9906112; PUBMED: 11112123] [DOI] [PubMed] [Google Scholar]
Chowdhury 2012
- Chowdhury O, Rafferty GF, Lee S, Hannam S, Milner AD, Greenough A. Volume‐targeted ventilation in infants born at or near term. Archives of Diseases in Childhood Fetal and Neonatal Edition 2012;97:F264.266. [DOI: 10.1136/archdischild-2011-301041; PUBMED: 22194469] [DOI] [PubMed] [Google Scholar]
Clark 2000
- Clark RH, Slutsky AS, Gerstmann DR. Lung protective strategies of ventilation in the neonate: what are they?. Pediatrics 2000;105(1 Pt 1):112‐4. [PUBMED: 10617711] [DOI] [PubMed] [Google Scholar]
Coalson 1999
- Coalson JJ, Winter VT, Siler‐Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. American Journal of Respiratory and Critical Care Medicine 1999;160(4):1333‐46. [DOI: 10.1164/ajrccm.160.4.9810071; PUBMED: 10508826] [DOI] [PubMed] [Google Scholar]
Dreyfuss 1993
- Dreyfuss D, Saumon G. Role of tidal volume, FRC and end‐inspiratory volume in the development of pulmonary edema following mechanical ventilation. American Review of Respiratory Disease 1993;148(5):1194‐203. [DOI: 10.1164/ajrccm/148.5.1194; PUBMED: 8239153] [DOI] [PubMed] [Google Scholar]
Dreyfuss 1998
- Dreyfuss D, Saumon G. Ventilator‐induced lung injury: lessons from experimental studies. American Journal of Respiratory and Critical Care Medicine 1998;157(1):294‐323. [DOI: 10.1164/ajrccm.157.1.9604014; PUBMED: 9445314] [DOI] [PubMed] [Google Scholar]
Gortner 1999
- Gortner L, Wauer RR, Stock GJ, Reiter HL, Reiss I, Jorch G, et al. Neonatal outcome in small for gestational age infants: do they really do better?. Journal of Perinatal Medicine 1999;27(6):484‐9. [DOI: 10.1515/JPM.1999.065; PUBMED: 10732308] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- Grade Working Group, McMaster University. GRADEpro GDT. Version accessed 14 September 2016. Hamilton (ON): Grade Working Group, McMaster University, 2014.
Greenough 2008
- Greenough A, Dimitriou G, Prendergast M, Milner AD. Synchronised mechanical ventilation for respiratory support in newborn infants. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000456.pub3] [DOI] [PubMed] [Google Scholar]
Hannaford 1999
- Hannaford K, Todd DA, Jeffery H, John E, Byth K, Gilbert G. Role of ureaplasma urealyticum in lung disease of prematurity. Archives of Disease in Childhood. Fetal and Neonatal Edition 1999;81(3):F162‐7. [PUBMED: 10525015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hentschel 2002
- Hentschel R, Brune T, Franke N, Harms E, Jorch G. Sequential changes in compliance and resistance after bolus administration or slow infusions of surfactant in preterm infants. Intensive Care Medicine 2002;28(5):622‐8. [DOI: 10.1007/s00134-002-1277-7; PUBMED: 12029412] [DOI] [PubMed] [Google Scholar]
Hernandez 1989
- Hernandez LA, Peevy KJ, Moise AA, Parker JC. Chest wall restriction limits high airway pressure‐induced lung injury in young rabbits. Journal of Applied Physiology 1989;66(5):2364‐8. [PUBMED: 2745302] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary Dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723‐9. [DOI: 10.1164/ajrccm.163.7.2011060; PUBMED: 11401896] [DOI] [PubMed] [Google Scholar]
Keszler 2009
- Keszler M, Nassabeh‐Montazami S, Abubakar K. Evolution of tidal volume requirement during the first 3 weeks of life in infants <800 g ventilated with volume guarantee. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(4):F279‐82. [DOI: 10.1136/adc.2008.147157; PUBMED: 19060010] [DOI] [PubMed] [Google Scholar]
Klingenberg 2011
- Klingenberg C, Wheeler KI, Owen LS, Kaaresen PI, Davis PG. An international survey on neonatal volume‐targeted ventilation. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(2):F146‐8. [DOI: 10.1136/adc.2009.181081; PUBMED: 20584800] [DOI] [PubMed] [Google Scholar]
Lista 2006
- Lista G, Castoldi F, Fontana P, Reali R, Reggiani A, Bianchi S, et al. Lung inflammation in preterm infants with respiratory distress syndrome: effects of ventilation with different tidal volumes. Pediatric Pulmonology 2006;41(4):357‐63. [DOI: 10.1002/ppul.20363; PUBMED: 16477653] [DOI] [PubMed] [Google Scholar]
McCallion 2002
- McCallion N, Davis PG, Morley CJ. Volume‐targeted versus pressure‐limited ventilation in the neonates. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD003666.pub2] [DOI] [PubMed] [Google Scholar]
NIH 1979
- National Institutes of Health. Report of workshop on bronchopulmonary dysplasia. NIH Publication No 80‐1660 1979.
Northway 1967
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline‐membrane disease: bronchopulmonary dysplasia. New England Journal of Medicine 1967;276(7):357‐68. [DOI: 10.1056/NEJM196702162760701; PUBMED: 5334613] [DOI] [PubMed] [Google Scholar]
Patel 2009
- Patel DS, Sharma A, Prendergast M, Rafferty GF, Greenough A. Work of breathing and different levels of volume‐targeted ventilation. Pediatrics 2009;123(4):e679‐84. [DOI: 10.1542/peds.2008-2635; PUBMED: 19254970] [DOI] [PubMed] [Google Scholar]
Patel 2010
- Patel D, Rafferty G, Lee S, Hannam S, Greenough A. Work of breathing and volume targeted ventilation in respiratory distress. Archives of Disease in Childhood Fetal and Neonatal Edition 2010;95(6):F443‐6. [DOI: 10.1136/adc.2009.176875; PUBMED: 20688862] [DOI] [PubMed] [Google Scholar]
Peng 2014
- Peng W, Zhu H, Shi H, Liu E. Volume‐targeted ventilation is more suitable than pressure‐limited ventilation for preterm infants: a systematic review and meta‐analysis. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(2):F158‐65. [DOI: 10.1136/archdischild-2013-304613; PUBMED: 24277660] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s), GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. gdt.gradepro.org/app/handbook/handbook.html Updated October 2013.
Sharma 2007
- Sharma A, Greenough A. Survey of neonatal respiratory support strategies. Acta Paediatrica 2007;96(8):1115‐7. [DOI: 10.1111/j.1651-2227.2007.00388.x; PUBMED: 17590191] [DOI] [PubMed] [Google Scholar]
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527‐32. [PUBMED: 3174313] [PubMed] [Google Scholar]
Stensvold 2017
- Stensvold HJ, Klingenberg C, Stoen R, Moster D, Braekke K, Guthe HJ, et al. Neonatal morbidity and 1‐year survival of extremely preterm infants. Pediatrics 2017;139(3):e20161821. [DOI: 10.1542/peds.2016-1821; PUBMED: 28228499] [DOI] [PubMed] [Google Scholar]
Stoll 2015
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993‐2012. JAMA 2015;314(10):1039‐51. [DOI: 10.1001/jama.2015.10244; PUBMED: 26348753] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Kaam 2010
- Kaam AH, Rimensberger PC, Borensztajn D, Jaegere AP. Ventilation practices in the neonatal intensive care unit: a cross‐sectional study. Journal of Pediatrics 2010;157(5):767‐71. [DOI: 10.1016/j.jpeds.2010.05.043; PUBMED: 20619854] [DOI] [PubMed] [Google Scholar]
Warner 1998
- Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. American Journal of Physiology 1998;275(1 Pt 1):L110‐7. [PUBMED: 9688942] [DOI] [PubMed] [Google Scholar]
Watterberg 1996
- Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97(2):210‐5. [PUBMED: 8584379] [PubMed] [Google Scholar]
Wheeler 2009
- Wheeler KI, Davis PG, Kamlin CO, Morley CJ. Assist control volume guarantee ventilation during surfactant administration. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(5):F336‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
McCallion 2005
- McCallion N, Davis PG, Morley C. Volume‐targeted versus pressure‐limited ventilation in the neonate. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003666.pub2] [DOI] [PubMed] [Google Scholar]
Wheeler 2010
- Wheeler K, Klingenberg C, McCallion N, Morley CJ, Davis PG. Volume‐targeted versus pressure‐limited ventilation in the neonate. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD003666.pub3] [DOI] [PubMed] [Google Scholar]


