Summary
Iconic examples of insect pollination have emphasized narrowly specialized pollinator mutualisms such as figs and fig wasps [1] and yuccas and yucca moths [2]. However, recent attention by pollination ecologists has focused on the broad spectra of pollinated plants by generalist pollinators such as bees. Bees have great impact for formulating hypotheses regarding specialization vs. generalization in pollination mutualisms [3,4]. We report the pollination biology of six northern European species of an extinct tribe of pollen-basket-bearing apine bees, Electrapini, of early–middle Eocene age, examined from two deposits of 48 and 44 million years in age [5]. These bees exhibit a pattern of generalized, incidental pollen occurring randomly on their heads, thoraces, and abdomens, obtained from diverse, nectar-bearing plants. By contrast, a more restricted suite of pollen was acquired for metatibial pollen baskets (corbiculae) of the same bee taxa from a taxonomically much narrower suite of arborescent, evergreen hosts with uniform flower structure. The stereotyped plant sources of the specialist strategy of pollen collection consisted of pentamerous, radially symmetrical flowers with a conspicuous gynoecium surrounded by prominent nectar rewards, organized in structurally similar compound inflorescences. Pollen specialization in bees occurs not for efficient pollination, but rather in the corbiculate Electrapini as food for bee larvae (brood) and involves packing corbiculae with moistened pollen that rapidly looses viability with age. This specialist strategy was a well-developed preference by the early Eocene, providing a geochronologic midpoint assessment of bee pollen-collection strategies.
Results
Pollination is vital to ecosystem health not only because it promotes the reproduction of seed plants, but also for its crucial role in intraspecific genetic exchange within and among populations, and thus enhancing the health of terrestrial ecosystems. This vital feature of pollination biology is as true in the distant past as it is for the modern world. Given this context, we have identified pollen found on the bodies of eleven individuals from six bee species of the tribe Electrapini from the floristically and entomologically well-known Eckfeld and Messel sites, of middle Eocene age in west-central Germany [5-7] (Fig. S1). These associational data uniquely provide an ideal opportunity to test hypotheses about the evolution of bee pollen-collection strategies. Based on the elevated diversity of electrapine bees – 31% of all 60 described Eocene bees are electrapines [3, 8] – and the prominence in which modern apine bees serve in studies of generalized versus specialized pollen-collection studies [9], Eckfeld and Messel electrapines are ideally suited to explore patterns of foraging specialization among bees in the fossil record. Our results indicate two patterns of pollen acquisition that are important features in the pollination biology of extant apine bees.
Incidental pollen acquisition
The first pattern consists of a broad spectrum of contact with pollen sources, in which a diverse assemblage of pollen representing a broad distribution of habitats was incidentally picked up on the body of the bees as they encountered a variety of flower morphologies that included the Anacardiaceae, Araliaceae, Elaeocarpaceae, Fagaceae, Iridaceae, Lythraceae, Olacaceae, and Sapotaceae (Fig. 1) [10]. Twelve pollen taxa from these eight families represent a generalized mode of entomophily, of which four of the pollen taxa also included strategies of vertebrate pollination. These pollen taxa were found on the heads, thoraces, and abdomens of the electrapine bees, but largely excluded the legs (Fig. 1). The pollen distributed on these body regions, minus the legs, generally are not actively collected in the absence of specialized setae modified for such collection, and no such setae have yet been documented on any species of Electrapini [6, 11]. Instead, such pollen is often picked up as a byproduct when bees visit flowers for nectar for their own consumption while out of the nest, or other purposes during foraging or scouting bouts [12]. By contrast, pollen also is actively collected as a source of food for the developing brood, but such pollen in electrapines and other corbiculate bees is placed in the metatibial corbiculae (pollen baskets) for transport back to the nest (Fig. 2, Fig. S1). The presence of a diverse array of incidentally-acquired pollen on multiple individuals demonstrates that the surrounding environment included diverse floral sources that were visited by the bees at some time during their flights, but were not purposefully collected by the bees for their food provisions as corbicular pollen was of significantly different floral composition.
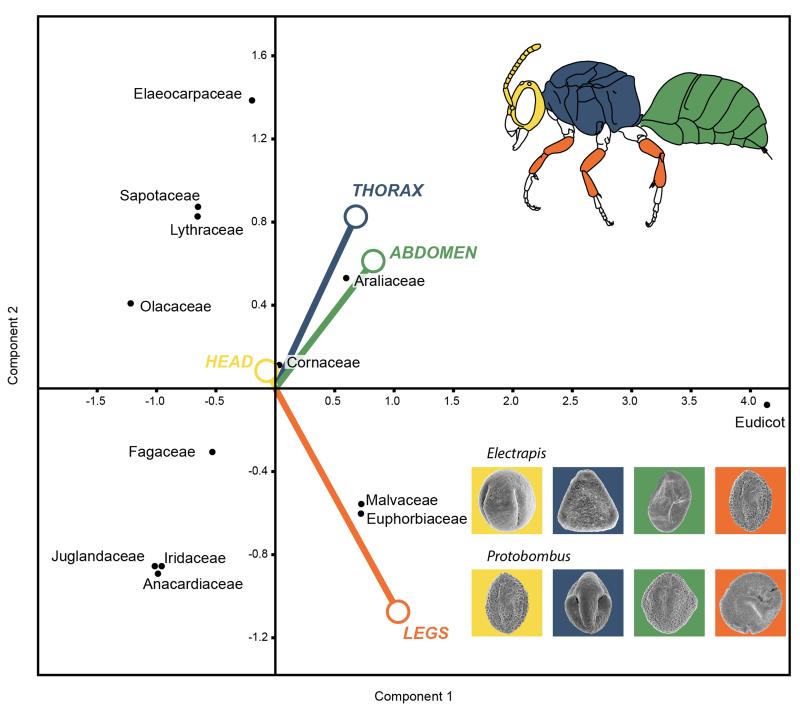
Figure 1. Plot of the first two significant components (Component 1 vs. Component 2).
Obtained from a principal components analysis resulting from pollen load variables of the plant families vs. bee body regions (Table S1). Examples of pollen grains obtained from specimens of multiple species of the electrapine genera Electrapis and Protobombus are depicted. (Refer to supplementary text.). See Tables S2-S4 for summary statistics.
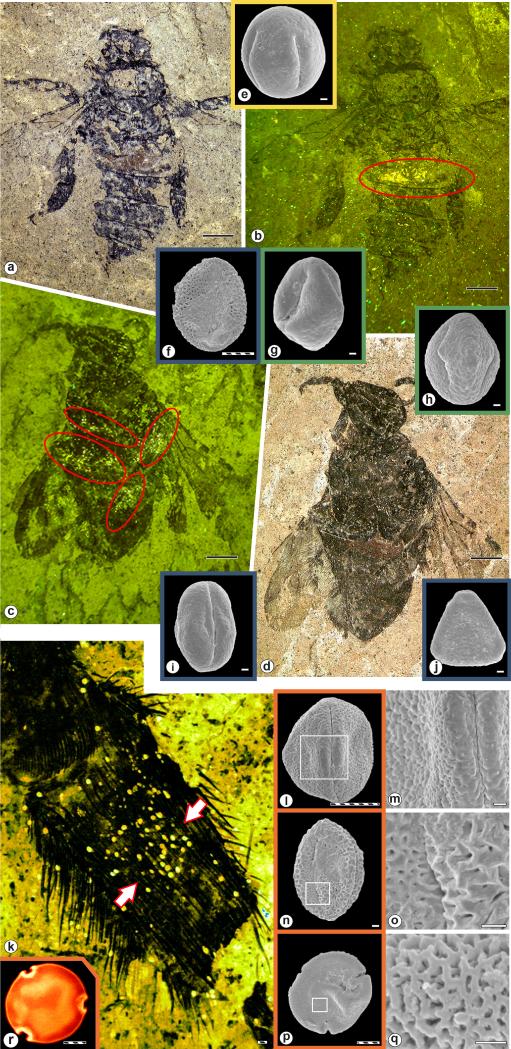
Figure 2. Representative pollen load and taxa in the Eocene bee specimens examined for this study.
(A-J) Spectrum of pollen found on heads (yellow square), thoraces (blue squares), abdomens (green squares), and legs (orange squares) of the studied fossil electrapine bees. (A) Electrapis prolata Engel & Wappler (PE 2000/847a,b.LS; holotype), female (worker caste). (B) Autofluorescence image of (A); red circle indicates the position of the pollen cluster on the specimen. (C) Autofluorescence image of (D); red circles indicate the position of pollen clusters. (D) Protobombus messelensis Engel & Wappler (FIS Me 6388; holotype). (E) Pouteria sp. pollen in equatorial view. (F) Iridaceae gen. et sp. indet. pollen (G) Elaeocarpus sp. 1 pollen grain in equatorial view. (H) Decodon sp. pollen. (I) Castanopsis/Lithocarpus sp. pollen in equatorial view. (J) Olax sp. pollen in polar view. (K-Q) Spectrum of pollen found on metatibial pollen baskets from the same taxa. (K) Autofluorescence image of the metatibial pollen baskets of Electrapis sp. (FIS MeI 3300); arrows indicate the position of Tilioideae pollen between distinct rows of stiff, apically-directed setae. (L) Nyssa sp. pollen in equatorial view; detail of tectum surface in (M). (N) Euphorbiaceae gen. et sp. indet. 1 pollen in equatorial view; detail of tectum surface in (O). (P) Tilioideae pollen in polar view; detail of tectum surface in (Q). (R) CLMS image (orange-red spectrum) of fluorescent exine of a Tilioideae pollen from (K). Scale bars: black, 1 mm; back-slashed, 10 μm; white, 1 μm.
Selective pollen acquisition
The second and more biologically significant pattern was pollen taxa collected in the metatibial corbiculae. Generally, corbiculate bees use their proboscis and probasitarsi to remove pollen from the anthers of target flowers, with subsequent scraping motions by the foreleg employed to remove pollen from the mouthparts and head for transfer to the hind legs [13,14]. Such pollen is almost invariably moistened with nectar. Pollen is then transferred to the mesobasitarsi and subsequently packed into the corbicula, either directly (likely homologous with the transfer of pollen to the metatibial scopa in non-corbiculate bees), or is placed between the metatibia and metabasitarsal joint and on a posterior basal projection of the metabasitarsus (auricle, or ‘pollen press’; apomorphically lacking in Meliponini) [13,14]. Compression of the metatibia–metabasitarsal joint then pushes the pollen into the base of the corbicula, with repeated compressions gradually forcing progressively more of the mass onto the corbicular surface and until filled [13,14]. Throughout the process, the bee may use its legs to help shape the pollen mass. Given different floral morphologies, varied behaviors are needed to loosen pollen from the anthers, and it is likely that electrapines were capable of the full spectrum of those behaviors observed among modern corbiculate bees, such as biting or buzzing anthers. While incidental pollen acquisition may be effective for the pollination of the floral species in question, the corbicular pollen is collected for the purpose of brood provisioning and its moistening by the bees during collection impairs pollen viability [15]. Accordingly, moistened corbicular pollen, important for the nutrition of the bee’s offspring, may not serve a reproductive role for the plant and the ecological service of corbiculate bees is seemingly provided from incidental contact of unaltered pollen on their bodies.
The pollen contained in the corbiculae of different individuals of multiple electrapine species were distinctive and are attributable to specific plant taxa affiliated at the generic level for the Euphorbiaceae (spurges), Malvaceae (Tilioideae, lindens), and probably Nyssa (tupelo) of the Cornaceae (dogwoods) that collectively bore a distinctive type of flower [Grímsson et al., in review]. Although many groups of angiosperms often possess pollen morphotypes that only approximately delimit their taxonomic affinities to source-plant taxa [16], the coarsest level of affiliation that typically occurs is that of the family [17], occasionally higher [18]. Fortunately other plant taxa, such as those in Euphorbiaceae [19], Malvaceae [20,21], and Nyssa [17], are highly distinctive and referable to their source plants at the generic level. It is for this reason that the pollen morphotypes identified in the bee corbiculae are assignable to Euphorbiaceae, Malvaceae, and Nyssa, and also exhibit a common, distinctive floral morphology.
The pollen found in the bee corbiculae were produced by evergreen shrubs or trees whose distinctive flowers were small to medium size. These flowers were radially symmetric with pentamerous petals, bore superior ovaries that had prominent stigmas, and possessed tricolporate or triporate pollen prominently exposed on anthers that opened longitudinally by slits or terminally by pores. In addition to pollen, a major reward was nectar, produced either from a centrally placed nectary disk or as tufts of secretory glandular trichomes. The flowers were organized into compound cymes, compact umbels or short racemes. Common features of the source flowers thus included a similar growth form, flowers with very similar anatomy (including pollen structure) and an accessible nectar reward. Other parallel data indicate that there was an extensive food web of Messel plant– pollinator interactions [22], including monolectic entomophilous pollination by beetles [23] and oligolectic pollination by a nectarivorous bird [24]. The restriction of pollen taxa within the corbiculae, despite visitation of numerous other flowers that also were good nectar sources, demonstrates a selective preference for these floral lineages during acquisition of food for nest provisioning.
Discussion
The notion that specialization in plant–pollinator interactions is the norm was advanced by several classic examples of intricate, presumably interlocking mutualisms such as figs and fig wasps [1] and yuccas and yucca moths [2]. Subsequent examinations of some of these studies have provided a more nuanced interpretation of the one-for-one model of specialization as an explanation for these associations [25,26]. Simultaneously, attention became focused on the ubiquity of generalization in pollination systems [3]. Although specification of the term is often nebulous, the best operable definition of generalization is the collection of a broad variety of pollen taxa by a foraging pollinator, referred to as polylecty [27]. By contrast, specialization, at least in its pure form, is the collection of a single pollen type from a plant-host species, known as monolecty [27]. The vast transitional zone between these two end-members is oligolecty, which ranges from broadly oligolectic pollinators that approach polylecty, to narrowly oligolectic forms that merge into monolecty [27]. The prevalence of these three major modes of pollen acquisition by foraging insect pollinators has been a contentious issue since the mid 1990’s [3,28], and currently forms a central exploratory program in pollination ecology [3].
The existence of specialized pollination has not been borne out in some re-examinations of the original systems [3,26]. Other studies, however, have validated or otherwise supported the existence of specialized pollination syndromes [25,29,30]. (A pollination syndrome is a set of adaptive morphological features by a pollinator to forage on plant rewards while simultaneously the pollinated plants evolve floral traits to display such rewards [31].) Although the presence of specialized pollination systems was established exclusively for the modern flora [31], isolated examples of specialized pollination syndromes have been documented sporadically in the fossil record [32,33]. Nevertheless, there are no previous examples from the fossil record that have identified specialized floral morphotypes based on: (i), multiple plant-host taxa with similar floral morphologies as evidenced by single aggregations of pollen grains collected by a single pollinator species; (ii), documentation of specialized types of pollen collected during the same foraging event; and (iii), knowledge of the specific plant-community context from which the pollinated plants and pollinating insects originate.
The family Apidae currently is the most diverse lineage of bees, consists of ecologically important pollinators, and comprises familiar taxa such as carpenter bees, digger bees, and cuckoo bees, but also includes the clade of corbiculate bees: the four tribes of Euglossini (orchid bees), Bombini (bumble bees), Meliponini (stingless bees), and Apini (honey bees) [34]. The corbiculate bees include notable eusocial lineages [35,36] and some of the most prominent of agricultural pollinators. All corbiculate bees possess a specialized expansion of the metatibia to form a characteristic ‘pollen basket’, or corbicula, for transport of actively-collected pollen back to the nest for use in provisioning brood cells [34]. There are three additional tribes of corbiculate bees not currently known in the modern fauna: Electrobombini, Melikertini, and the aforementioned Electrapini [6], the latter of which represent a diverse, entirely-Eocene group of advanced eusocial bees closely related to the stingless bees (Meliponini), honey bees (Apini), and Melikertini [6]. Electrapines are known from Eocene deposits ranging from northern Europe to southern Asia [5,6].
The earliest pattern of pollen collecting within the Apidae has been suggested to be generalist foraging, based on the pollen-collecting spectra of extant, corbiculate stingless bees [37], of which the latest Cretaceous (70 Ma) Cretotrigona prisca, a meliponine, is the oldest definitive bee [38]. Meliponines are derived within the Apidae [6,39], and therefore may not be indicative of the groundplan floral-visitation habits of apid bees. Nonetheless, this general conclusion is buttressed by the presence of Paleocene (60 Ma) anthophorine (digger) bees from France [11], most modern taxa of which are polylectic, as are putatively primitive apids such as many carpenter bees [34]. This pattern is not supported by narrowly oligolectic or monolectic bee-pollination modes, attributed to pollination by inferred Apidae and Megachilidae (leafcutter bees), from several specialized flower morphotypes during the mid Late Cretaceous (92 Ma) of New Jersey, USA [40]. Interestingly, given the reduced viability of corbicular pollen [15], the electrapines at Eckfeld and Messel were perhaps more effective pollinators to those floral species which they visited for nectar or their own consumption of pollen, as compared to the narrowed suite of plants visited for the purposes of brood provisions that likely received minimal pollination service from such foraging bouts.
For more encompassing, deeper-time clades that include all bee lineages, there are two hypotheses that postulate particular, dominant patterns of pollen-collecting behavior. The first hypothesis states that the earliest bees were a mix of host-plant generalists, consisting of polylectic or perhaps oligolectic foragers [41]. The second hypothesis indicates that the earliest bees, presumably occurring during the mid Cretaceous contemporaneous with early angiosperm diversification, were pollen-collection specialists. This pattern is inferred from the biologies of closely related apoid wasps [6,11], and the phylogenetic relationships among many primitive bee lineages whose members currently are pollen-host specialists [38,42]. Further support for early bee pollen specialization is the common observation that monolecty or narrow oligolecty is a plesiomorphic condition that gives rise to broad oligolecty and then to polylecty in later bee lineages [43]. Supportive paleobotanical evidence consists of clumps of sticky eudicot pollen preserved as coprolites and sourced to particular flower morphologies that tentatively indicate specialized pollination by bees during the mid-Cretaceous at 102 Ma [44].
Discovery of additional Late Cretaceous body-fossil specimens would resolve the pollen collection patterns for either the Apidae or the entire bee clade (Anthophila). Current evidence is available from five sources of data: (i) pollen spectra found on fossil bee bodies [11]; (ii), the pollen-collecting habits of extant lineages that are known descendants of fossil bees [3]; (iii), floral morphologies of Paleogene and Late Cretaceous flowers [40]; (iv), pollen-laden coprolites associated with particular flower structures [44]; and (v), inferences regarding the ancestral states of pollen collection (polylecty, oligolecty or monolecty) arising from phylogenetic analyses [6,38]. Integration of these five approaches coupled with additional pollen data from fossil bee bodies would provide more definitive answers to the early ecological history of bee foraging and pollination.
Experimental Procedures
The specimens are housed in the Naturhistorisches Museum Mainz, Landessammlung für Naturkunde Rheinland-Pfalz (NHMM) and the Forschungsinstitut und Naturmuseum Senckenberg, Frankfurt am Main (SNG). The studied insect fossils are preserved in the collections by immersing the slabs of oil shale in glycerine to prevent damage by desiccation [45]. The specimens were examined using a Leica MZ 9.5 Stereomicroscope equipped with standard incident ultraviolet (UV) illumination to locate the pollen load on the bee specimens. The fossil pollen grains were then non-destructively extracted from the bee specimens with the help of wax sticky pads mounted at the end of a preparation needle. The fossil pollen grains were investigated both by LM and SEM, using the single grain technique [46,47]. Normal photographs were taken with a Leica MZ 16 Stereomicroscope, and either a JVC (model KY-F70B) or a Nikon Coolpix 4500 digital camera. All photographs were processed using Adobe Photoshop CS6 (Adobe Systems). Statistical analyses were done using the statistical environment R 2.0-2 [48] (see Supplemental Information for additional details, including discussing of tests, test results, and tables expressing test results).
Supplementary Material
Table 1. Pollen data collected from Eckfeld and Messel bees*.
| Pollen parent plant: Classification & taxon** |
Life-form | Pollen body site |
Locality | Palynological Information figure*** |
|---|---|---|---|---|
|
Iridaceae Gen. et sp. indet. |
Evergreen or deciduous herbaceous perennial | leg | Eckfeld | Figs. 8, B–F, H, K–N, Q; 9, A–E; G–L, S |
|
Elaeocarpaceae Elaeocarpus sp. 1 |
Evergreen tree or shrub | thorax, leg | Eckfeld | Fig. 7, B–H |
|
Elaeocarpaceae Elaeocarpus sp. 2 |
Evergreen tree or shrub | thorax, abdomen | Eckfeld | Fig. 15, B–M |
|
Euphorbiaceae Gen. et sp. indet. 1 |
Evergreen tree or shrub | leg | Eckfeld | Fig. 7, O–R |
|
Euphorbiaceae Gen. et sp. indet. 2 |
Evergreen tree or shrub | leg | Eckfeld | Figs. 13, L–O; 14, A–N |
|
Euphorbiaceae Gen. et sp. indet. 3 |
Evergreen tree or shrub | body | Eckfeld | Fig. 15, N–P |
|
Fagaceae
Castanopsis / Lithocarpus |
Evergreen tree | body, thorax, leg | Eckfeld, Messel | Fig. 5, B–J; 5, I, J, L, N |
|
Juglandaceae Gen. et sp. indet. |
Deciduous or evergreen tree | leg | Eckfeld | Fig. 14, O–Q |
|
Lythraceae Decodon sp. |
Perennial shrub | thorax, abdomen | Eckfeld | Fig. 10, O–Q |
|
Malvaceae Mortoniodendron sp. |
Evergreen shrub or tree | leg, head | Messel | Fig. 5, K–Q |
|
Malvaceae Gen. et sp. indet. |
Small to large tree, possibly deciduous | leg, thorax, abdomen | Messel | Fig. 3, K; 4, A–O |
|
Anacardiaceae Gen. et sp. indet. |
Evergreen or deciduous tree or shrub | thorax, leg | Messel | Fig. 3, K; 4, A–O |
|
Olacaceae Olax sp. |
Evergreen shrub or small tree | thorax | Eckfeld | Fig. 6, H–O |
|
Cornaceae Gen. et sp. indet. |
Evergreen tree | thorax, abdomen | Eckfeld | Fig. 16, B–J |
|
Cornaceae Nyssa sp. |
Small to large evergreen tree, possibly deciduous | leg | Messel | Fig. 2, B–N |
|
Sapotaceae Pouteria sp. |
Small to large evergreen tree | head, leg | Eckfeld | Fig. 13, A–K |
|
Araliaceae Gen. et sp. indet. 1 |
Probably an evergreen tree or shrub | leg, thorax, abdomen | Messel | Fig. 3, B–J |
|
Araliaceae Gen. et sp. indet. 2 |
Probably an evergreen tree or shrub | abdomen | Messel | Fig. 6, B–F |
|
Family indet. Gen. et sp. indet. 1 |
Unknown | leg | Eckfeld | Fig. 7, I–M |
|
Family indet. Gen. et sp. indet. 2 |
Unknown | abdomen, leg | Eckfeld | Figs. 8, B–G, K–M, O–Q; 9, M–R; 10, B–N; 11, A–O |
|
Family indet. Gen. et sp. indet. 3 |
Unknown | leg | Eckfeld | Figs. 8, D–F; 9, A, B, D–F |
|
Family indet. Gen. et sp. indet. 4 |
Unknown | thorax, abdomen | Eckfeld | Fig. 12, B–L |
|
Family indet. Gen. et sp. indet. 5 |
Unknown | thorax, abdomen | Eckfeld | Fig. 12,B, M–P |
Acknowledgments
We thank J. Rust and two anonymous reviewers for their constructive comments. H. Lutz and S. Wedmann allowed access to the collections under their curation. G. Heumann provided assistance, and F. Marsh developed the figures. T.W. is supported by the German Research Foundation (WA 1496/6-1, Heisenberg grant WA 1496/8-1), and F.G. is supported by the Austrian Science Fund (FWF), grant (P24427-B25). This is Contribution 305 of the Evolution of Terrestrial Ecosystems Consortium at the National Museum of Natural History, in Washington, D.C., and a contribution of the Division of Entomology, University of Kansas Natural History Museum.
Footnotes
Supplemental Information includes, Supplemental Geological and Paleobiological Context Information on the Eckfeld and Messel Deposits, Supplemental Experimental Procedures, Supplemental Information on the Bees of Eckfeld and Messel,, and one figure.
References
- 1.Rønsted N, Weiblen GD, Cook JM, Salamin N, Machado CA, Savolainen V. 60 million years of co-divergence in the fig-wasp symbiosis. Proc. R. Soc. Biol. Sci. 2005;272:2593–2599. doi: 10.1098/rspb.2005.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pellmyr O. Yuccas, Yucca moth, and coevolution: A review. Ann. Mo. Bot. Gard. 2003;90:35–55. [Google Scholar]
- 3.Waser NM, Ollerton J. Plant-pollinator interactions: from specialization to generalization. University of Chicago Press; Chicago and London: 2006. [Google Scholar]
- 4.Ollerton J, Killick A, Lamborn E, Watts S, Whiston M. Multiple meanings and modes: on the many ways to be a generalist flower. Taxon. 2007;56:717–728. [Google Scholar]
- 5.Wappler T, Engel MS. The Middle Eocene bee faunas of the Eckfeld Maar and Messel, Germany (Hymenoptera: Apoidea) J. Paleont. 2003;77:908–921. [Google Scholar]
- 6.Engel MS. A monograph of the Baltic Amber bees and evolution of the Apoidea (Hymenoptera) Bull. Am. Mus. Nat. Hist. 2001;259:1–192. [Google Scholar]
- 7.Wappler T, Labandeira CC, Rust J, Frankenhäuser H, Wilde V. Testing for the Effects and Consequences of Mid Paleogene Climate Change on Insect Herbivory. PLoS ONE. 2012;7:e40744. doi: 10.1371/journal.pone.0040744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel MS. A new interpretation of the oldest fossil bee (Hymenoptera: Apidae) Am. Mus. Novit. 2000;3296:1–11. [Google Scholar]
- 9.Cane JH, Sipes S. Characterizing floral specialization by bees: analytical methods and a revised lexicon for oligolecty. In: Waser NM, Ollerton J, editors. Plant-pollinator interactions: from specialization to generalization. University of Chicago Press; Chicago and London: 2006. pp. 99–122. [Google Scholar]
- 10.Grímsson F, Zetter R, Labandeira CC, Engel MS, Wappler T. Taxonomic description of in-situ bee pollen from the middle Eocene of Germany. Grana: (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michez D, Vanderplanck M, Engel MS. Fossil bees and their plant associates. In: Patiny S, editor. Evolution of Plant–Pollinator Relationships. Cambridge University Press; Cambridge: 2012. pp. 103–164. [Google Scholar]
- 12.Pellmyr O. Pollination by animals. In: Herrera CM, Pellmyr O, editors. Plant animal interactions: an evolutionary approach. John Wiley & Sons; Oxford: 2002. pp. 157–184. [Google Scholar]
- 13.Jander R. Grooming and pollen manipulation in bees (Apoidea): the nature and evolution of movements involving the foreleg. Physiol. Entomol. 1976;1:179–194. [Google Scholar]
- 14.Michener CD, Winston ML, Jander R. Pollen manipulation and related activities and structures in bees of the family Apidae. Univ. Kans. Sci. Bull. 1978;51:575–601. [Google Scholar]
- 15.Parker A, Tran J, Ison J, Bai J, Weis A, Thomson J. Pollen packing affects the function of pollen on corbiculate bees but not non-corbiculate bees. Arth.–Plant Int. 2015;9:197–203. [Google Scholar]
- 16.Sowunmi MA. Pollen morphology of the Palmae and its bearing on taxonomy. Rev. Palaeobot. Palynol. 1972;13:1–80. [Google Scholar]
- 17.Traverse A. Nomenclature and taxonomy: systematics. A rose by any other name would be very confusing. In: Jansonius J, McGregor DS, editors. Palynology: principles and applications. Vol. 1. American Association of Stratigraphic Palynologists Foundation; Dallas, Texas: 1996. pp. 11–28. [Google Scholar]
- 18.Jarzen DM, Nichols DJ. Pollen. In: Jansonius J, McGregor DS, editors. Palynology: principles and applications. Vol. 1. American Association of Stratigraphic Palynologists Foundation; Dallas, Texas: 1996. pp. 261–291. [Google Scholar]
- 19.Punt W. A survey of pollen morphology in Euphorbiaceae with special reference to Phyllanthus. Biol. J. Linn. Soc. 1984;94:127–142. [Google Scholar]
- 20.Chung RCK, Soepadmo E, Lim AL. The significance of pollen morphology in the taxonomy of Grewia and Microcos (Tiliaceae) in peninsular Malaysia and Borneo. Gard. Bull. Singapore. 2003;55:239–256. [Google Scholar]
- 21.El Naggar SM. Pollen morphology of Egyptian Malvaceae: An assessment of taxonomic value. Turk. J. Bot. 2004;28:227–240. [Google Scholar]
- 22.Dunne JA, Labandeira CC, Williams RJ. Highly resolved early Eocene food webs show development of modern trophic structure after the end-Cretaceous extinction. Proc. R. Soc. Biol. Sci. 2014;281:20133280. doi: 10.1098/rspb.2013.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaarschmidt F. The vegetation: fossil plants as witnesses of a warm climate. In: Schaal S, Ziegler W, editors. Messel. An insight into the history of life and of the Earth. Clarendon Press; Oxford: 1992. pp. 27–52. [Google Scholar]
- 24.Mayr G, Wilde V. Eocene fossil is earliest evidence of flower-visiting by birds. Biol. Lett. 2014;10:20140223. doi: 10.1098/rsbl.2014.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armbruster WS. Evolutionary and ecological aspects of specialized pollination: views from the arctic to the tropics. In: Waser NM, Ollerton J, editors. Plant-pollinator interactions: from specialization to generalization. University of Chicago Press; Chicago and London: 2006. pp. 260–282. [Google Scholar]
- 26.Cruaud A, Cook J, Da-Rong Y, Genson G, Jabbour-Zahab R, Kjellberg F, Pereira RAS. Fig-fig wasp mutualism: the fall of the strict cospeciation paradigm? In: Patiny S, editor. Evolution of Plant–Pollinator Relationships. Cambridge University Press; Cambridge: 2012. pp. 68–102. [Google Scholar]
- 27.Cane JH, Sipes S. Characterizing floral specialization by bees: analytical methods and a revised lexicon for oligolecty. In: Waser NM, Ollerton J, editors. Plant-pollinator interactions: from specialization to generalization. University of Chicago Press; Chicago and London: 2006. pp. 99–122. [Google Scholar]
- 28.Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. [Google Scholar]
- 29.Johnson SD, Steiner KE. Generalization versus specialization in plant pollination systems. Trends Ecol. Evol. 2000;15:140–143. doi: 10.1016/s0169-5347(99)01811-x. [DOI] [PubMed] [Google Scholar]
- 30.Fenster CB, Armbruster WS, Wilson PA, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 2004;35:375–403. [Google Scholar]
- 31.Faegri K, Van der Pijl L. The principles of pollination ecology. Pergamon Press; Toronto: 1966. [Google Scholar]
- 32.Labandeira CC. Fossil history of the Diptera and their associations with plants. In: Yeates DK, Wiegmann BM, editors. The Evolutionary Biology of Flies. Columbia University Press; New York: 2005. pp. 217–273. [Google Scholar]
- 33.Peñalver E, Arillo A, Pérez-de la Fuente R, Riccio ML, Delclòs X, Barrón E, Grimaldi DA. Long-Proboscid Flies as Pollinators of Cretaceous Gymnosperms. Curr. Biol. 2015;25:1917–1923. doi: 10.1016/j.cub.2015.05.062. [DOI] [PubMed] [Google Scholar]
- 34.Michener CD. The bees of the world. 2nd Edition JHU Press; Baltimore, Maryland: 2007. [Google Scholar]
- 35.Cardinal S, Packer L. Phylogenetic analysis of the corbiculate Apinae based on morphology of the sting apparatus (Hymenoptera: Apidae) Cladistics. 2007;23:99–118. doi: 10.1111/j.1096-0031.2006.00137.x. [DOI] [PubMed] [Google Scholar]
- 36.Schultz TE, Engel MS, Ascher JS. Evidence for the origin of eusociality in corbiculate bees (Hymenoptera: Apidae) J. Kans. Entomol. Soc. 2001;74:10–16. [Google Scholar]
- 37.Ramalho MV, Imperatriz-Fonseca I, Kleinert-Giovannini A, Cortopassi-Laurino M. Exploitation of floral resources by Plebeia remota Holmberg (Apidae, Meliponinae) Apidologie. 1985;16:307–330. [Google Scholar]
- 38.Danforth BN, Sipes S, Fang J, Brady SG. The history of early bee diversification based on five genes plus morphology. Proc. Natl. Acad. Sci U.S.A. 2006;103:15118–15123. doi: 10.1073/pnas.0604033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardinal S, Straka J, Danforth BN. Comprehensive phylogeny of apid bees reveals the evolutionary origins and antiquity of cleptoparasitism. Proc. Natl. Acad. Sci. U.S.A. 2010;107:16207–16211. doi: 10.1073/pnas.1006299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crepet WL. Timing in the evolution of derived floral characters: Upper Cretaceous (Turonian) taxa with tricolpate and tricolpate-derived pollen. Rev. Palaeobot. Palynol. 1996;90:339–359. [Google Scholar]
- 41.Alexander BA, Michener CD. Phylogenetic studies of the families of short-tongued bees (Hymenoptera–Apoidea) Univ. Kans. Sci. Bull. 1995;55:377–424. [Google Scholar]
- 42.Patiny S, Michez D, Danforth B. Phylogenetic relationships and host–plant evolution within the basal clade of Halictidae (Hymenoptera, Apoidea) Cladistics. 2008;24:255–269. [Google Scholar]
- 43.Larkin LL, Neff JL, Simpson BB. The evolution of a pollen diet: Host choice and diet breadth of Andrena bees (Hymenoptera: Andrenidae) Apidologie. 2008;39:133–145. [Google Scholar]
- 44.Hu S, Dilcher DL, Taylor DW. Pollen evidence for the pollination biology of early flowering plants. In: Patiny S, editor. Evolution of Plant–Pollinator Relationships. Cambridge University Press; Cambridge: 2012. pp. 165–236. [Google Scholar]
- 45.Ackermann M, Habersetzer J, Schaarschmidt F. From Excavation to Exhibition Piece. In: Schaal S, Ziegler W, editors. Messel. An insight into the history of life and of the Earth. Clarendon Press; Oxford: 1992. pp. 277–284. [Google Scholar]
- 46.Zetter R. Methodik und Bedeutung einer routinemäßig kombinierten lichtmikroskopischen und rasterelektronenmikroskopischen Untersuchung fossiler Mikrofloren. Cour. Forsch.-Inst. Senckenberg. 1989;109:41–50. [Google Scholar]
- 47.Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S. Pollen terminology: an illustrated handbook. Springer Wien; New York: 2009. [Google Scholar]
- 48.Oksanen J, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, Simpson G, Solymos P, Henry H. Vegan: Community Ecology Package. 2011 R package version 2.0–2. http://CRAN.R-project.org/package=vegan.
- 49.APG III An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009;161:128–131. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.