Abstract
Background
Scrub typhus, an important cause of acute fever in Asia, is caused by Orientia tsutsugamushi, an obligate intracellular bacterium. Antibiotics currently used to treat scrub typhus include tetracyclines, chloramphenicol, macrolides, and rifampicin.
Objectives
To assess and compare the effects of different antibiotic regimens for treatment of scrub typhus.
Search methods
We searched the following databases up to 8 January 2018: the Cochrane Infectious Diseases Group specialized trials register; CENTRAL, in the Cochrane Library (2018, Issue 1); MEDLINE; Embase; LILACS; and the metaRegister of Controlled Trials (mRCT). We checked references and contacted study authors for additional data. We applied no language or date restrictions.
Selection criteria
Randomized controlled trials (RCTs) or quasi‐RCTs comparing antibiotic regimens in people with the diagnosis of scrub typhus based on clinical symptoms and compatible laboratory tests (excluding the Weil‐Felix test).
Data collection and analysis
For this update, two review authors re‐extracted all data and assessed the certainty of evidence. We meta‐analysed data to calculate risk ratios (RRs) for dichotomous outcomes when appropriate, and elsewhere tabulated data to facilitate narrative analysis.
Main results
We included six RCTs and one quasi‐RCT with 548 participants; they took place in the Asia‐Pacific region: Korea (three trials), Malaysia (one trial), and Thailand (three trials). Only one trial included children younger than 15 years (N = 57). We judged five trials to be at high risk of performance and detection bias owing to inadequate blinding. Trials were heterogenous in terms of dosing of interventions and outcome measures. Across trials, treatment failure rates were low.
Two trials compared doxycycline to tetracycline. For treatment failure, the difference between doxycycline and tetracycline is uncertain (very low‐certainty evidence). Doxycycline compared to tetracycline may make little or no difference in resolution of fever within 48 hours (risk ratio (RR) 1.14, 95% confidence interval (CI) 0.90 to 1.44, 55 participants; one trial; low‐certainty evidence) and in time to defervescence (116 participants; one trial; low‐certainty evidence). We were unable to extract data for other outcomes.
Three trials compared doxycycline versus macrolides. For most outcomes, including treatment failure, resolution of fever within 48 hours, time to defervescence, and serious adverse events, we are uncertain whether study results show a difference between doxycycline and macrolides (very low‐certainty evidence). Macrolides compared to doxycycline may make little or no difference in the proportion of patients with resolution of fever within five days (RR 1.05, 95% CI 0.99 to 1.10; 185 participants; two trials; low‐certainty evidence). Another trial compared azithromycin versus doxycycline or chloramphenicol in children, but we were not able to disaggregate date for the doxycycline/chloramphenicol group.
One trial compared doxycycline versus rifampicin. For all outcomes, we are uncertain whether study results show a difference between doxycycline and rifampicin (very low‐certainty evidence). Of note, this trial deviated from the protocol after three out of eight patients who had received doxycycline and rifampicin combination therapy experienced treatment failure.
Across trials, mild gastrointestinal side effects appeared to be more common with doxycycline than with comparator drugs.
Authors' conclusions
Tetracycline, doxycycline, azithromycin, and rifampicin are effective treatment options for scrub typhus and have resulted in few treatment failures. Chloramphenicol also remains a treatment option, but we could not include this among direct comparisons in this review.
Most available evidence is of low or very low certainty. For specific outcomes, some low‐certainty evidence suggests there may be little or no difference between tetracycline, doxycycline, and azithromycin as treatment options. Given very low‐certainty evidence for rifampicin and the risk of inducing resistance in undiagnosed tuberculosis, clinicians should not regard this as a first‐line treatment option. Clinicians could consider rifampicin as a second‐line treatment option after exclusion of active tuberculosis.
Further research should consist of additional adequately powered trials of doxycycline versus azithromycin or other macrolides, trials of other candidate antibiotics including rifampicin, and trials of treatments for severe scrub typhus. Researchers should standardize diagnostic techniques and reporting of clinical outcomes to allow robust comparisons.
11 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (8 Jan, 2018) were included and four ongoing studies have been identified (see 'Characteristics of ongoing studies' section)
Plain language summary
Antibiotics for treating scrub typhus
What is the aim of this review?
The aim of this Cochrane Review is to find out whether certain antibiotics are more effective in treating scrub typhus. We collected and analysed all relevant studies to answer this question and included seven studies.
Key messages
Tetracycline, doxycycline, azithromycin, and rifampicin are effective antibiotics for scrub typhus treatment that have led to few treatment failures. For specific outcomes, some low‐certainty evidence suggests there may be little or no difference between tetracycline, doxycycline, and azithromycin. Healthcare workers should not use rifampicin as a first‐line treatment. Researchers should standardize the way they diagnose and assess scrub typhus.
What was studied in the review?
Scrub typhus is an important cause of fever in Asia. We studied people with scrub typhus diagnosed by health professionals and confirmed by laboratory tests. We compared different antibiotic treatments. We looked at whether choice of antibiotic made a difference in the number of people who experienced failed treatment, and we determined the proportions who had resolution of fever at 48 hours.
What are the main results of the review?
We found seven relevant studies. Only one study included children younger than 15 years.
We are uncertain whether doxycycline compared to tetracycline affects treatment failure, as the certainty of the evidence is very low. Studies looked at resolution of fever within five days. Doxycycline compared to tetracycline may make little or no difference in the proportion of patients with resolution of fever within 48 hours and in time to defervescence. Studies did not formally report serious adverse events.
We are uncertain whether macrolides compared to doxycycline affect treatment failure, resolution of fever within five days, time to defervescence, or serious adverse events, as the certainty of the evidence is very low. Macrolides compared to doxycycline may make little or no difference in the proportion of patients with resolution of fever within five days.
We are uncertain whether rifampicin compared to doxycycline affects treatment failure, proportion of patients with resolution of fever within 48 hours, or time to defervescence, as the certainty of evidence is very low. The single study that performed this comparison did not look at resolution of fever within five days and did not formally report serious adverse events.
How up‐to‐date is this review?
We searched for studies that had been published up to 8 January 2018.
Summary of findings
Background
Description of the condition
Scrub typhus is an important cause of acute fever in Asia. It is caused by Orientia tsutsugamushi (formerly Rickettsia tsutsugamushi), an obligate intracellular bacterium in the order Rickettsia. This bacterium is transmitted in the bite of larvae of the Leptotrombidium mite, commonly called chiggers, which form the reservoir. Clinical features are non‐specific and include fever, headache, and myalgia (Watt 2003). An eschar, an ulcerated lesion with a black crust, may develop at the site of the bite. The frequency of eschar formation varies across populations from 7% to 80%. Scrub typhus may lead to pneumonia, shock, meningoencephalitis, renal failure, or myocarditis (Griffith 2014). Disease severity appears to be related to the virulence of the O tsutsugamushi strain, patient age, patient genetic factors, and previous infections, but literature regarding prognostic factors is limited (Rajapakse 2012). For untreated scrub typhus, median mortality is 6% (range 0 to 70%) (Taylor 2015). With treatment, median mortality is 1.4% (range 0 to 33.3%) (Bonell 2017).
As O tsutsugamushi is intracellular, it cannot be isolated via standard bacterial culture but instead requires cell culture. Therefore, the main modalities for diagnosis of scrub typhus are nucleic acid amplification tests (for example, polymerase chain reaction (PCR)) and serology (including immunofluorescence assays (IFAs), rapid diagnostic tests (RDTs), and enzyme‐linked immunosorbent assay (ELISA)). The historical mainstay of diagnosis has been indirect immunofluorescence assay, but this is limited by subjectivity and a requirement for paired acute and convalescent sera. Increasingly, ELISA tests are replacing IFAs, as they are more sensitive, specific, and reproducible. Real‐time PCR on blood or eschar biopsy is helpful in diagnosing early‐stage infection (Paris 2016). In some resource‐limited settings, the only serological test available is the Weil‐Felix test, a non‐specific antibody agglutination test that cannot distinguish O tsutsugamushi from other Rickettsial infections (Koralur 2018).
Scrub typhus is endemic to Asia‐Pacific. Most cases occur in a region traditionally known as the ‘tsutsugamushi triangle’, which extends from Japan to India, and to Northern Australia. Incidence varies across the region, ranging from 1.2 to 17.7 per 100,000 per year. Seroprevalence similarly varies, ranging from 9.3% to 27.9% (Bonell 2017). In the Mekong region, scrub typhus represents the second most common cause of non‐malarial febrile illness after dengue (Acestor 2012). Infection classically occurs when humans encroach upon 'mite islands' ‐ discrete areas where infected chiggers are found. For this reason, cases often occur in association with land clearing, logging, or military operations; in rice fields; and during outdoor travel activities (Watt 2003).
Description of the intervention
Antibiotics currently recommended to treat scrub typhus include the following (CDC 2017).
Tetracyclines: doxycycline 100 mg twice per day for one week. In clinical practice, this is favoured over tetracycline owing to convenience of the dosing schedule.
Chloramphenicol.
Macrolides: azithromycin.
Rifampicin.
A previous version of this review also identified fluoroquinolones as a possible alternative treatment (Liu 2002).
How the intervention might work
Doxycycline historically has been the mainstay of treatment across the rickettsial diseases, including scrub typhus. Given the difficulties associated with cell culture, there is a relative paucity of in vitro susceptibility data needed to provide a theoretical basis for its use. Chloramphenicol is the traditional second‐line treatment and was one of the first drugs found to be effective (Smadel 1950).
Several reports have indicated suspected doxycycline resistance inferred by treatment failures in cohort studies such as Thipmontree 2016, or due to acquisition of scrub typhus during doxycycline malaria prophylaxis (Corwin 1999). However, few studies have correlated clinical evidence suggesting drug resistance with in vitro data, possibly because of the difficulty involved in culturing O tsutsugamushi. Watt 1996 studied 19 patients with scrub typhus and through mouse fibroblast cell culture identified one isolate as doxycycline‐resistant and another isolate as showing partial resistance; these findings correlated with attenuated therapeutic response. Whole‐genome sequencing has indicated the presence of putative resistance genes, but evaluating their potential to mediate resistance is challenging (Kelly 2017). Overall, we found uncertain evidence to support the existence or clinical significance of doxycycline resistance in O tsutsugamushi, and this remains a public health concern.
Fluoroquinolones such as ciprofloxacin have been used; however, one in vitro study indicates that O tsutsugamushi may be intrinsically resistant to these antibiotics (Tantibhedhyangkul 2010).
Why it is important to do this review
This is an update of a Cochrane Review first published in 2000 (Panpanich 2000), and later updated (Liu 2002), which identified seven small trials and presented several limited conclusions.
The review authors concluded that rifampicin seemed to be more effective than doxycycline in areas where scrub typhus responds poorly to standard drugs; they based these conclusions on data from one study (Watt 2000). A recent non‐Cochrane systematic review performed an analysis of the same study and reached a different conclusion (Wee 2017), cautioning against interpreting the results in favour of rifampicin. The disagreement marked an important reason to update this review. Since the last update in 2002, the review process has become more sophisticated. This updated review is improved by GRADE methods and 'Summary of findings' tables, which enable more conclusions and provide clear indications to the reader regarding the certainty of evidence presented. Wee 2017 did not use GRADE methods.
Scrub typhus remains an important cause of morbidity in endemic areas, and choice of antibiotic remains a topical clinical question.
Objectives
To assess and compare the effects of different antibiotic regimens for treatment of scrub typhus.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all relevant randomized controlled trials (RCTs) and quasi‐RCTs. We define quasi‐RCTs as those using an allocation method that is not truly random (for example, based on date of birth).
Types of participants
Trials had to include people with a diagnosis of scrub typhus based on clinical symptoms and compatible laboratory tests, including the following.
Serology (IFA, ELISA, RDT).
Nucleic acid amplification (PCR).
Isolation (cell culture).
Given the poor specificity of the Weil‐Felix test, we excluded studies that used this as the sole measure to confirm the diagnosis.
Types of interventions
Interventions
Anti‐rickettsial antibiotics, irrespective of route of administration, dose, dose frequency, or course duration.
Controls
Other anti‐rickettsial antibiotics. We excluded studies comparing interventions versus placebo or no drug as it is clear antibiotics are effective. We planned to include trials that provided additional interventions to all treatment arms, but we did not encounter such trials.
Types of outcome measures
Primary outcomes
Treatment failure, defined as persistence of symptoms at the end of the treatment course.
Resolution of fever within 48 hours.
Secondary outcomes
Resolution of fever within five days.
-
Time to defervescence
Defined as the time interval between administration of the first dose of antibiotic and the first time at which temperature was less than 37.5°C and was thereafter maintained for > 48 hours.
Serious adverse events.
Frequency and types of reported adverse events.
Search methods for identification of studies
We performed a comprehensive search to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and ongoing) up to 8 January 2018.
Electronic searches
We searched the following databases using the search terms and strategy detailed in Table 4.
1. Detailed search strategy.
| Search set | CIDG SRa | CENTRAL | MEDLINEb | Embaseb | LILACSb |
| 1 | Scrub typhus | SCRUB TYPHUS | SCRUB TYPHUS | SCRUB TYPHUS | Scrub typhus |
| 2 | Rickettsia tsutsugamushi | Scrub typhus [ti, ab] | Scrub typhus [ti, ab] | Scrub typhus [ti, ab] | Rickettsia tsutsugamushi |
| 3 | Orientia tsutsugamushi | Orientia tsutsugamushi [ti, ab] | Orientia tsutsugamushi [ti, ab] | Orientia tsutsugamushi [ti, ab] | Orientia tsutsugamushi |
| 4 | 1 or 2 or 3 | Rickettsia tsutsugamushi [ti, ab] | Rickettsia tsutsugamushi [ti, ab] | Rickettsia tsutsugamushi [ti, ab] | 1 or 2 or 3 |
| 5 | ¬ | ORIENTIA TSUTSUGAMUSHI | ORIENTIA TSUTSUGAMUSHI | ORIENTIA TSUTSUGAMUSHI | ¬ |
| 6 | ¬ | 1 or 2 or 3 or 4 or 5 | 1 or 2 or 3 or 4 or 5 | 1 or 2 or 3 or 4 or 5 | ¬ |
| 7 | ¬ | ¬ | Limit 6 to humans | Limit 6 to humans | ¬ |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by Cochrane; upper case: MeSH or EMTREE heading; lower case: free text term.
Cochrane Infectious Diseases Group Specialized Register.
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library (2018, Issue 1).
MEDLINE (1966 to January 2018).
Embase (1980 to January 2018).
Latin American Caribbean Health Sciences Literature (LILACS) (1982 to January 2018).
We searched the metaRegister of Controlled Trials (mRCT) using ''scrub typhus'' OR ''orientia tsutsugamushi'', "antibiotics" OR ''antimicrobial therapy'' as search terms. The search strategy remains unchanged since the previous version of the protocol was prepared.
Searching other resources
Reference lists
To identify additional published, unpublished, and ongoing studies, we checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
For this update, two review authors (IES and IW) independently screened search results to identify potentially relevant trials and obtained the full‐text reports of these trials. For English language studies, IES and IW then used a standard eligibility form to assess newly identified studies and to re‐assess previously included studies against inclusion and exclusion criteria. For Chinese language studies, IW and QL followed the same process. We resolved disagreements by discussion with a third review author (PH). We documented reasons for excluding trials in the Characteristics of excluded studies table. We scrutinized each trial report to ensure that we did not include multiple publications from the same trial.
Data extraction and management
Three review authors (IES, QL, and IW) independently extracted data onto a data extraction form (modified from previous versions of this review). We extracted information on study design, setting, population, diagnostic criteria, antibiotic regimen (dose, route, duration, timing, and frequency), total numbers randomized, number of participants in each group, numbers lost to follow‐up, duration of follow‐up, dates of the study, funding source, and withdrawals from each group. We encountered no disagreements.
For dichotomous data, we extracted the number of participants who experienced the event of interest and the number of participants randomized and analysed in each group.
For continuous outcomes, we extracted mean values, standard deviations, and number of participants in each group for whom the outcome was assessed. When medians were reported, we extracted ranges or interquartile ranges. When data were incomplete, we contacted trial authors to request additional data.
Assessment of risk of bias in included studies
Three review authors (IES, QL, and IW) independently assessed potential biases of included studies using a prepared form and the Cochrane ‘Risk of bias’ tool (Higgins 2011). For each domain, we described what trial authors reported and made a subjective judgement for each domain as having ‘high, low, or unclear’ risk of bias. We resolved discrepancies by discussion and reached agreement. We included all assessments in a 'Risk of bias' graph and a 'Risk of bias' summary figure. We provided in the Results section a narrative description of our risk of bias conclusions for each domain of all included studies.
We assessed the following domains for each study: sequence generation, allocation concealment, blinding or masking, incomplete outcome data, selective outcome reporting, and other sources of bias.
Sequence generation
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We considered trials as having low risk of bias if the investigator described a random component of sequence generation (for example, a random number table, a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing lots, minimization); high risk of bias if sequence generation was non‐random (for example, sequence generated by odd or even date of birth, some rule based on date (or day) of admission, some rule based on hospital or clinic record number); or unclear risk of bias if the randomization process was not clearly described.
Allocation concealment
We assessed whether participants and investigators enrolling participants could foresee assignment. We judged this domain as having low risk of bias if the investigator used central allocation (including telephone‐based, web‐based, and pharmacy‐controlled randomization), sequentially numbered drug containers of identical appearance, or sequentially numbered opaque, sealed envelopes to conceal allocation; high risk of bias if the allocation process was not concealed (for example, open randomization, unsealed or non‐opaque envelopes); or unclear risk of bias if study authors did not describe the process of concealing allocation sufficiently to permit a judgement.
Blinding of participants or personnel
We described whether blinding was done and who was blinded. We regarded a trial as having low risk of performance bias if blinding was done; high risk of bias if blinding was not done and this was likely to affect the results; or unclear risk of bias if study authors did not clearly describe blinding.
Blinding of outcome assessment
We described knowledge of allocated interventions by outcome assessors. All primary and secondary outcomes in our review are subjective. So, we judged trials as having low risk of detection bias if blinding of outcome assessment was ensured and it was unlikely that the blinding could have been broken; high risk of detection bias if no blinding of outcome assessment was performed; or unclear risk of detection bias if study authors did not adequately describe this domain to allow a judgement of ‘low risk’ or ‘high risk’.
Incomplete outcome data
We assessed the proportions of missing outcome data between different groups. We judged trials as having a low level of attrition bias if the proportion of participants lost to follow‐up was < 10%, or if missing outcome data were balanced in numbers across intervention groups, with similar reasons provided for missing data across groups. We regarded trials as having high risk of attrition bias if the proportion of participants lost to follow‐up was > 10%, or if reasons for missing outcome data were likely to be related to true outcomes, with imbalance in numbers or reasons for missing data across intervention groups. We judged trials as having unclear risk of attrition bias if study authors did not adequately describe this domain to permit a judgement of ‘low risk’ or ‘high risk’.
Selective outcome reporting
We determined that if published reports included all expected outcomes, including those prespecified in the Methods section, then those trials had low risk of bias. We considered trials to have high risk of bias if not all of the study’s prespecified primary outcomes were reported; if one or more primary outcomes were reported through measurement or analysis methods that were not prespecified; or if one or more reported primary outcomes were not prespecified.
Other sources of bias
We assessed other potential sources of bias related to the specific study design used, baseline imbalance, and deviation from the trial protocol.
Measures of treatment effect
We calculated the risk ratio (RR) for dichotomous outcomes. We presented all measures with corresponding 95% confidence intervals (CIs). When we assessed the data, we regarded time to defervescence as a time‐to‐event outcome, as we were not certain whether all participants experienced this outcome. We therefore decided it was inappropriate to analyse time to defervescence using methods for continuous outcomes. We were unable to extract the log hazard ratio and its standard error from Cox proportional hazards models; therefore we did not combine this outcome in the meta‐analysis but instead presented a narrative analysis.
Unit of analysis issues
We did not encounter unit of analysis issues.
Dealing with missing data
We extracted data to allow an intention‐to‐treat analysis in which all randomized participants were analysed in the groups to which they were originally assigned, outcome data were provided on all participants, and all randomized participants were included in the analysis. For three included studies (Brown 1978; Song 1995; Watt 2000), we tried to contact the study authors to request missing data. We emailed the corresponding author for one study (Watt 2000), but we did not receive a reply. We did not find email addresses for corresponding authors for two included studies (Brown 1978; Song 1995). We then conducted a complete‐case analysis and included in the analysis only participants with a recorded outcome.
Assessment of heterogeneity
We visually inspected forest plots that displayed overlapping confidence intervals for two or more studies as an indicator of clinical heterogeneity. We assessed statistical heterogeneity using Chi² and I² tests. We considered a Chi² test P value < 0.1 and an I² statistic value > 75% as indicating substantial heterogeneity.
Assessment of reporting biases
We planned to investigate potential publication bias by using a funnel plot if at least 10 studies met the inclusion criteria of the review.
Data synthesis
We analysed the data using Review Manager 5 (RevMan 5) (RevMan 2014). We used a fixed‐effect model and Mantel‐Haenszel methods if we noted no heterogeneity. Otherwise, we used a random‐effects model and Mantel‐Haenszel methods for significant heterogeneity.
Certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach (Schünemann 2013). We appraised the certainty of evidence in relation to the following criteria.
Study design.
Risk of bias.
Inconsistency.
Indirectness.
Imprecision.
Other considerations (including publication bias).
We used GRADEpro GDT software to create 'Summary of findings' tables for comparisons included in the review (GRADEpro 2015). We included our primary outcomes and used these tables to guide our conclusions.
Subgroup analysis and investigation of heterogeneity
We planned to investigate heterogeneity by conducting prespecified subgroup analyses for primary outcomes, according to the following potential sources.
Geographical setting, which may influence antibiotic susceptibility.
Date of the study (before or after antibiotic resistance was first reported).
Participant age (children versus adults).
Dose, frequency, and duration of treatment.
However, the number of included studies for each comparison was not sufficient to permit subgroup analysis.
Sensitivity analysis
We planned to assess the robustness of summary estimates by restricting analysis to studies with low risk of bias, especially in terms of allocation concealment and low incomplete follow‐up (< 10%), but the number of included studies was not sufficient.
Results
Description of studies
Results of the search
The previous version of this review included seven studies. We re‐screened these according to our eligibility criteria and excluded one previously included study, as those researchers used only the Weil‐Felix test for laboratory diagnosis (Sheehy 1973).
Through the updated literature search on 8 January 2018, we identified 128 references. We excluded 36 duplicate records and excluded 81 of the remaining 92 references after title and abstract screening. We assessed 12 full‐text articles for eligibility, from which we excluded 10 articles (see Characteristics of excluded studies). One new study ‐ Chanta 2015 ‐ met the inclusion criteria, in addition to six previously included studies (see Characteristics of included studies), and two new ongoing trials met these criteria (see Characteristics of ongoing studies). In total, seven studies (in seven publications) met the inclusion criteria of this review (Figure 1).
1.
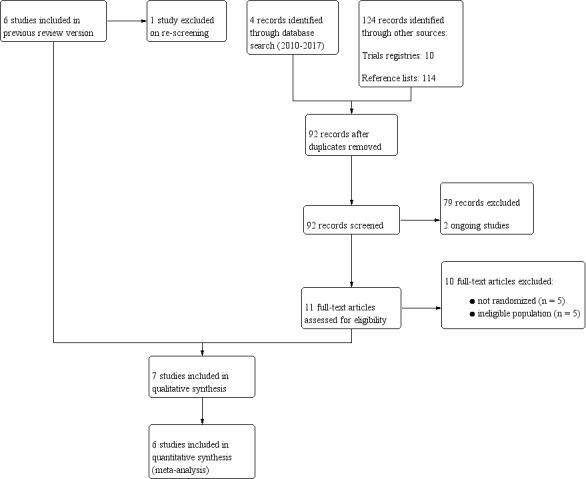
PRISMA flow diagram.
Included studies
Seven studies met our inclusion criteria (see Characteristics of included studies).
Six were RCTs (Brown 1978; Chanta 2015; Kim 2004; Phimda 2007; Song 1995; Watt 2000), and one was a quasi‐RCT (Kim 2007). These studies contributed 548 participants to this review.
All studies took place within hospital settings, located in Korea (three trials), Malaysia (one trial), and Thailand (three trials).
The point at which participants were randomized varied between studies.
One study randomized patients with acute undifferentiated fever (Phimda 2007).
Four studies randomized patients with clinically suspected scrub typhus (Brown 1978; Kim 2004; Kim 2007; Song 1995).
Two studies randomized patients after a positive RDT (Chanta 2015; Watt 2000).
The number of randomized participants ranged from 57 in Chanta 2015, to 296 in Phimda 2007. Five studies recruited adults only (n = 434); one study recruited people aged 14 and over (n = 57; Phimda 2007); and one study recruited children under the age of 15 (n = 57; Chanta 2015). All studies recruited males and females.
Four studies confirmed diagnosis using IFA only (Kim 2004; Kim 2007; Phimda 2007; Song 1995); one study confirmed diagnosis using IFA, agglutination testing, or isolation (Brown 1978); one study used only screening RDT for laboratory confirmation (Chanta 2015); and another study used screening RDT followed by confirmatory indirect immunoperoxidase (Watt 2000).
Two trials compared doxycycline and tetracycline (Brown 1978; Song 1995); and four trials compared doxycycline versus a macrolide (Chanta 2015; Kim 2004; Kim 2007; Phimda 2007). Chanta 2015 investigated children and used chloramphenicol in place of doxycycline for children under the age of eight but did not report results for chloramphenicol and doxycycline separately. One trial compared doxycycline alone, rifampicin alone at low and high doses, and doxycycline and rifampicin in combination (Watt 2000).
Included studies reported a variety of outcome measures and used different terminology to incorporate similar categories. With respect to our primary outcomes:
treatment failure: six studies provided data (Chanta 2015; Kim 2004; Kim 2007; Phimda 2007; Song 1995; Watt 2000). Definitions of treatment failure varied according to the persistence of fever and/or symptoms at time points including 48 hours, 72 hours, and after treatment completion; and
resolution of fever within 48 hours: two studies provided data (Brown 1978; Watt 2000).
With respect to our secondary outcomes:
resolution of fever within five days: three studies provided data (Kim 2004; Kim 2007; Phimda 2007);
time to defervescence: six studies provided data (Chanta 2015; Kim 2004; Kim 2007; Phimda 2007; Song 1995; Watt 2000); and
adverse events: all studies provided data.
Excluded studies
We excluded 13 studies (see Characteristics of excluded studies) for the following reasons: five studies were retrospective and confirmed laboratory diagnosis via the Weil‐Felix test only; four studies had an unclear study design and confirmed laboratory diagnosis via the Weil‐Felix test only; two studies assessed antibiotics for preventing rather than treating scrub typhus; and two studies confirmed laboratory diagnosis via the Weil‐Felix test only, one of which was included in the previous version of this review (Sheehy 1973).
Risk of bias in included studies
See Figure 2 for a summary of the ‘Risk of bias' assessments. Further details are available in the Characteristics of included studies table.
2.
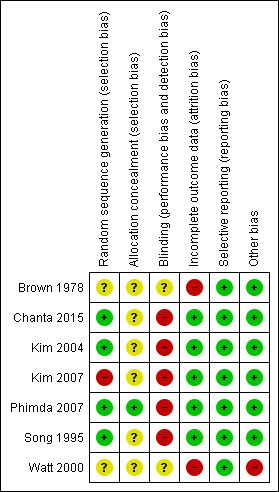
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged four studies to have low risk of selection bias because they adequately described generation of allocation sequences (Chanta 2015; Kim 2004; Phimda 2007; Song 1995). We judged one study as having high risk of selection bias because researchers randomized participants according to the last digit of a registration number (Kim 2007). We judged two studies to have unclear risk of selection bias as study authors did not provide sufficient information (Brown 1978; Watt 2000).
Allocation concealment was unclear in all trials except one, which used an opaque and numbered envelope for allocation concealment (Phimda 2007).
Blinding
Five studies were open‐label and provided inadequate blinding of participants and providers. We judged these studies as having high risk of performance and detection bias (Chanta 2015; Kim 2004; Kim 2007; Phimda 2007; Song 1995). Description of blinding was unclear in Brown 1978 and Watt 2000.
Incomplete outcome data
We assessed five trials to be at low risk of attrition bias (Chanta 2015; Kim 2004; Kim 2007; Phimda 2007; Song 1995). Chanta 2015 and Kim 2007 had no missing data; in Phimda 2007, missing data were balanced between different groups; and in Kim 2004 and Song 1995, small percentages of participants were lost to follow‐up.
We considered two trials as having high risk of attrition bias because large numbers of participants from both intervention and control arms were lost to follow‐up (> 10%) (Brown 1978; Watt 2000).
Selective reporting
We judged all trials as having low risk of reporting bias. These trials adequately reported all prespecified primary and secondary outcomes.
Other potential sources of bias
All trials reported comparable baseline characteristics between groups of participants. We judged one trial to have high risk of bias owing to deviation from the protocol (Watt 2000); in one arm, participants were initially randomized to receive combined doxycycline and rifampicin therapy; and treatment failure occurred in three of eight participants receiving combination therapy.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Doxycycline compared to tetracycline for treating scrub typhus.
| Doxycycline compared to tetracycline for treating scrub typhus | ||||||
|
Patient or population: adults with scrub typhus Settings: hospitals in endemic areas Intervention: doxycycline 200 mg single oral dose (Brown 1978), doxycycline oral 100 mg 12‐hourly for 3 days (Song 1995) Comparison: tetracycline 500 mg 6‐hourly for 7 days (Brown 1978), tetracycline oral 500 mg 12‐hourly for 7 days (Song 1995) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with tetracycline | Risk with doxycycline | |||||
| Treatment failure | 0 events in 50 participants | 4 events in 66 participants | RR 6.85 (0.38 to 124.38) |
116 (1 RCT) | ⊕⊝⊝⊝
VERY LOWa,b Due to risk of bias and imprecision |
We are uncertain whether doxycycline compared to tetracycline affects treatment failure, as the certainty of the evidence is very low. |
| Resolution of fever within 48 hours | 792 per 1000 | 902 per 1000 (713 to 1000) |
RR 1.14 (0.90 to 1.44) |
55 (1 RCT) | ⊕⊕⊝⊝
LOWc,d Due to risk of bias and imprecision |
Doxycycline compared to tetracycline may make little or no difference in the proportions of patients with resolution of fever within 48 hours. |
| Resolution of fever within 5 days | Not reported | Neither of the studies looked at resolution of fever within 5 days. | ||||
| Time to defervescence | Mean 37 hours, SD 26.6 hours | Mean 34 hours, SD 26.5 hours | ‐ | 116 (1 RCT) | ⊕⊕⊝⊝
LOWa,e Due to risk of bias and imprecision |
Doxycycline compared to tetracycline may make little or no difference in time to defervescence. |
| Serious adverse events | Not formally reported | |||||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low certainty: we are very uncertain about the estimate | ||||||
aDowngraded by 1 due to serious risk of bias. Song 1995 had unclear allocation concealment and was not blinded. bDowngraded by 2 due to very serious imprecision. Sample size and number of events were small and did not meet optimal information size. cDowngraded by 1 due to serious risk of bias. Brown 1978had high risk of attrition bias, although this was not likely to differentially affect treatment groups, and unclear risk of bias from sequence generation, allocation concealment, and blinding. dDowngraded by 1 due to serious imprecision. The 95% CI overlaps no effect (that is, CI includes RR of 1.0), and the CI fails to exclude appreciable benefit. eDowngraded by 1 for serious imprecision: Song 1995 was underpowered to detect this effect.
Summary of findings 2. Macrolides compared to doxycycline for treating scrub typhus.
| Macrolides compared to doxycycline for treating scrub typhus | ||||||
|
Patient or population: adults and adolescents with scrub typhus Settings: hospitals in endemic areas Intervention: doxycycline 200 mg per day for 7 days (Kim 2004; Phimda 2007); doxycycline 200 mg per day for 5 days (Kim 2007) Comparison: azithromycin 500 mg single oral dose (Kim 2004); telithromycin 800 mg daily for 5 days (Kim 2007); azithromycin 1 g daily for 3 days, followed by 500 mg daily for 2 days (Phimda 2007) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with doxycycline | Risk with macrolides | |||||
| Treatment failure | Assumed risk: 19 per 1000a |
51 per 1000 (2 to 1000) | RR 2.71 (0.12 to 63.84) | 242 (3 RCTs) | ⊕⊝⊝⊝
VERY LOWb,c Due to risk of bias and imprecision |
We are uncertain whether macrolides compared to doxycycline affect treatment failure, as the certainty of the evidence is very low. |
| Resolution of fever within 48 hours | 671 per 1000 | 544 per 1000 (215 to 1000) | RR 0.81 (0.32 to 2.03) | 150 (2 RCTs) | ⊕⊝⊝⊝
VERY LOWb,d Due to risk of bias, imprecision, and inconsistency |
We are uncertain whether macrolides compared to doxycycline affects the proportion of patients with resolution of fever within 48 hours. |
| Resolution of fever within 5 days | 956 per 1000 | 1000 per 1000 (946 to 1000) | RR 1.05 (0.99 to 1.10) | 185 (2 RCTs) | ⊕⊕⊝⊝
LOWb,e Due to risk of bias and inconsistency |
Macrolides compared to doxycycline may make little or no difference in the proportion of patients with resolution of fever within 5 days. |
| Time to defervescence | Each included study detected no significant difference between groups. | 242 (3 RCTs) | ⊕⊝⊝⊝
VERY LOWb,d Due to risk of bias and inconsistency |
We are uncertain whether macrolides compared to doxycycline affect time to defervescence, as the certainty of the evidence is very low. | ||
| Serious adverse events | No included trial reported serious adverse events. | 242 (3 RCTs) | ⊕⊝⊝⊝
VERY LOWb,c Due to risk of bias and imprecision |
We are uncertain whether macrolides compared to doxycycline affects serious adverse events, as the certainty of the evidence is very low. | ||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDerived from risk across all included trials in patients treated with doxycycline (four events in 212 patients). bDowngraded by 1 due to serious risk of bias: all three included trials were open‐label; Kim 2007 was quasi‐randomized. cDowngraded by 2 due to very serious imprecision: sample size and number of events were small, and confidence intervals cross the line of no effect. Two trials reported no events in either treatment arm, so they do not contribute to the risk ratio. dDowngraded by 2 due to very serious inconsistency: data show quantitative and qualitative inconsistency between trials. eDowngraded by 1 due to serious inconsistency: Kim 2004 gave azithromycin as a single oral dose; Kim 2007 gave telithromycin for five days.
Summary of findings 3. Rifampicin compared to doxycycline for treating scrub typhus.
| Rifampicin compared to doxycycline for treating scrub typhus | ||||||
|
Patient or population: adults with scrub typhus Settings: hospitals in endemic areas Intervention: rifampicin Comparison: doxycycline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| doxycycline | rifampicin | |||||
| Treatment failure | The included reported no treatment failures. | ‐ | 78 (1 RCT) | ⊕⊝⊝⊝
VERY LOWa,b Due to risk of bias and imprecision |
We are uncertain whether rifampicin compared to doxycycline affects treatment failure, as the certainty of the evidence is very low. | |
| Resolution of fever within 48 hours | 464 per 1000 | 780 per 1000 (510 to 1000) | RR 1.68 (1.10 to 2.57) |
78 (1 RCT) | ⊕⊝⊝⊝
VERY LOWa,c Due to risk of bias and imprecision |
We are uncertain whether rifampicin compared to doxycycline affects the proportion of patients with resolution of fever within 48 hours, as the certainty of the evidence is very low. |
| Resolution of fever within 5 days | Not reported | The study did not look at resolution of fever within 5 days. | ||||
| Time to defervescence | Study authors report that time to defervescence was less with rifampicin. | ‐ | 78 (1 RCT) | ⊕⊝⊝⊝
VERY LOWa,c Due to risk of bias and imprecision |
We are uncertain whether rifampicin compared to doxycycline affects time to defervescence, as the certainty of the evidence is very low. | |
| Serious adverse events | Not formally reported | |||||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low certainty: we are very uncertain about the estimate | ||||||
aDowngraded by 2 due to very serious risk of bias. In Watt 2000, sequence generation, allocation concealment, and blinding were unclear; risk of attrition bias with incomplete follow‐up was high (67.8%), as was risk of other bias due to deviation from the trial protocol. bDowngraded by 2 due to very serious imprecision. Number of events is very small and does not meet optimum information size (< 300 events), and the sample size is small. cDowngraded by 1 due to serious imprecision. The sample size is small.
Comparison 1: doxycycline versus tetracycline
Two trials compared doxycycline with tetracycline (Brown 1978; Song 1995).
One trial reported treatment failure (Song 1995); results show no significant differences between doxycycline and tetracycline (116 participants, 1 trial; Analysis 1.1).
1.1. Analysis.
Comparison 1 Doxycycline versus tetracycline, Outcome 1 Treatment failure.
One trial reported the proportion of participants with resolution of fever within 48 hours (Brown 1978); data show little or no difference between doxycycline and tetracycline (risk ratio (RR) 1.14, 95% confidence interval (CI) 0.90 to 1.44; 55 participants, 1 trial; Analysis 1.2).
1.2. Analysis.
Comparison 1 Doxycycline versus tetracycline, Outcome 2 Resolution of fever within 48 hours.
Neither trial reported proportions of participants with resolution of fever within five days.
One trial reported mean time to defervescence (Song 1995). Results show a mean of 37 hours (standard deviation (SD) ± 26.6 hours) for the tetracycline group and a mean of 34 hours (SD ± 26.5 hours) for the doxycycline group. Study authors reported that the difference was non‐significant via the log‐rank test.
Both trials discussed adverse events but did not formally report the presence or absence of serious adverse events. Gastrointestinal symptoms occurred more frequently with doxycycline than with tetracycline in both trials (not meta‐analysed; Table 5).
2. Adverse events (non‐severe).
| Study | Intervention | Comparison |
| Doxycycline | Tetracycline | |
| Brown 1978 | Vomiting (8/35) Rash (1/35) |
None reported |
| Song 1995 | Gastrointestinal reactions (33/66) | Gastrointestinal reactions (10/50) |
| Study | Doxycycline | Macrolides |
| Kim 2004 | Nausea (4/47) Diarrhoea (2/47) Abdominal discomfort (1/47) Raised ALT (5/47) Thrombocytopaenia (1/47) |
(Azithromycin) Nausea (6/46) Vomiting (3/46) Raised ALT (4/46) |
| Kim 2007 | Nausea (2/45) Vomiting (1/45) Diarrhoea (1/45) Abdominal discomfort (2/45) Elevated ALT (2/45) Skin rash (2/45) Oesophageal candidiasis (1/45) |
(Telithromycin) Abdominal discomfort (3/47) Elevated ALT (4/47) |
| Phimda 2007 | Nausea (3/145) Vomiting (22/145) Nausea and vomiting (10/145) Diarrhoea (1/145) Abdominal pain (1/145) Rash (1/145) Dizziness (1/145) |
(Azithromycin) Nausea (1/151) Vomiting (10/151) Nausea and vomiting (1/151) Diarrhoea (1/151) Rash (3/151) |
| Study | Doxycycline | Rifampicin |
| Watt 2000 | Rash and eosinophilia (1/28) "Severe gastrointestinal (GI) side effects" (2/28) "Mild GI side effects" (14/28) |
Rash and eosinophilia (7/50) "Mild GI side effects" (18/50) Red‐orange discolouration of urine (50/50) |
Abbreviations: ALT: alanine aminotransferase; GI: gastrointestinal.
Comparison 2: macrolides versus doxycycline
Three trials compared macrolide antibiotics with doxycycline (Kim 2004; Kim 2007; Phimda 2007).
All three trials reported treatment failure; meta‐analysis of this outcome revealed no significant differences between macrolides and doxycycline (242 participants, 3 RCTs; Analysis 2.1). In two of these trials (Kim 2004; Kim 2007), data show no treatment failures in either arm, and these data did not contribute to the risk ratio in meta‐analysis.
2.1. Analysis.
Comparison 2 Macrolides versus doxycycline, Outcome 1 Treatment failure.
Two trials reported the proportion of participants with resolution of fever within 48 hours (Kim 2007; Phimda 2007). Meta‐analysis revealed no differences between macrolides and doxycycline but showed quantitative and qualitative heterogeneity between trials (150 participants, 2 RCTs; Analysis 2.2).
2.2. Analysis.
Comparison 2 Macrolides versus doxycycline, Outcome 2 Resolution of fever within 48 hours.
Two trials reported the proportion of participants with resolution of fever within five days (Kim 2004; Kim 2007); results show little or no difference between groups (RR 1.05, 95% CI 0.99 to 1.10; 185 participants, 2 RCTs; Analysis 2.3; Figure 3).
2.3. Analysis.
Comparison 2 Macrolides versus doxycycline, Outcome 3 Resolution of fever within 5 days.
3.
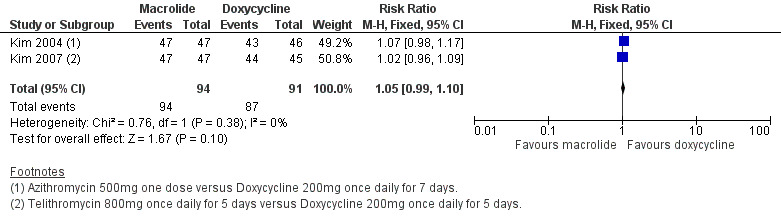
Forest plot of comparison: 2 Macrolides versus doxycycline, outcome: 2.3 Resolution of fever within five days.
All three trials reported time to defervescence as median times with ranges, which we have presented in Table 6. Between studies, median times ranged from 18 to 45 hours; within studies, ranges were also wide. The included studies detected no statistically significant differences between groups.
3. Time to defervescence: macrolides versus doxycycline.
| Study | Doxycycline | Macrolide | ||
| Median | Range | Median | Range | |
| Kim 2004 | 29 hours | 4 to 176 hours | 21 hours | 1 to 120 hours |
| Kim 2007 | 18 hours | 4 to 105 hours | 18 hours | 4 to 176 hours |
| Phimda 2007 | 45 hours | 8 to 118 hours | 40 hours | 8 to 136 hours |
All three trials reported that no serious adverse events occurred across treatment arms (242 participants, 3 RCTs; Analysis 2.4). Gastrointestinal symptoms occurred more frequently with doxycycline than with macrolides in all three trials (not meta‐analysed; Table 5).
2.4. Analysis.
Comparison 2 Macrolides versus doxycycline, Outcome 4 Serious adverse events.
Subgroup analysis restricted to macrolide subtypes (azithromycin, telithromycin) did not indicate significant differences between findings (Analysis 5.1 to Analysis 6.3).
5.1. Analysis.
Comparison 5 Macrolide subgroup: azithromycin versus doxycycline, Outcome 1 Treatment failure.
6.3. Analysis.
Comparison 6 Macrolide subgroup: telithromycin versus doxycycline, Outcome 3 Serious adverse events.
Comparison 2a: macrolides versus doxycycline/chloramphenicol
In addition to the three trials comparing macrolide antibiotics with doxycycline, one trial compared azithromycin with doxycycline or chloramphenicol (Chanta 2015). We were unable to include this trial in the meta‐analysis as study authors included doxycycline or chloramphenicol as a single arm within the trial and did not report disaggregated data.
For treatment failure, trial authors reported one case in the azithromycin group and zero cases in the doxycycline or chloramphenicol group. We were unable to extract data for resolution of fever at five days or at 48 hours. The median time to defervescence was 36 hours (interquartile range (IQR) 20 to 68 hours) in the azithromycin group and 30 hours (IQR 21 to 48 hours) in the doxycycline/chloramphenicol group. Study authors determined that the difference was non‐significant via the log‐rank test. Adverse event reporting was unclear in this trial.
Comparison 3: rifampicin versus doxycycline
One trial compared rifampicin with doxycycline (Watt 2000). For this analysis, we combined standard‐ and high‐dose rifampicin arms into one group.
Researchers detected no treatment failure in either group (78 participants, 1 trial; Analysis 3.1).
3.1. Analysis.
Comparison 3 Rifampicin versus doxycycline, Outcome 1 Treatment failure.
For resolution of fever within 48 hours, a higher proportion of participants had resolved fever with rifampicin compared to doxycycline (RR 1.68, 95% CI 1.10 to 2.57; 78 participants, 1 trial; Analysis 3.2).
3.2. Analysis.
Comparison 3 Rifampicin versus doxycycline, Outcome 2 Resolution of fever within 48 hours.
Trial authors did not report the proportions of participants with resolution of fever within five days.
Results show time to defervescence as median times with ranges: 52 hours (range 4 to 108 hours) with doxycycline, 27.5 hours (range 4 to 84 hours) with 600 mg rifampicin, and 22.5 hours (range 3 to 76 hours) with 900 mg rifampicin. Study authors used the Kruskal‐Wallis test to determine that the difference between the doxycycline group and the other two groups was significant.
Researchers did not formally report the presence or absence of serious adverse events, instead stating that there were no "serious complications". The trial excluded two participants from the doxycycline arm owing to gastrointestinal side effects. "Mild" gastrointestinal side effects occurred more commonly with doxycycline than with rifampicin. Rash and eosinophilia occurred more commonly with rifampicin than with doxycycline (Table 5).
As previously discussed, this trial deviated from the protocol after three of eight patients receiving doxycycline and rifampicin combination therapy experienced treatment failure.
Comparison 4: high‐dose rifampicin versus standard‐dose rifampicin
One trial compared 900 mg rifampicin versus 600 mg rifampicin, implementing a change to the original protocol (Watt 2000). Data show no treatment failure in either rifampicin arm (50 participants, 1 trial; Analysis 4.1). For proportions of participants with resolution of fever within 48 hours, results show little or no difference between high‐dose and standard‐dose rifampicin (RR 1.03, 95% CI 0.77 to 1.38; 50 participants, 1 trial; Analysis 4.2). Researchers did not report the proportions of participants with resolution of fever within five days. We were unable to compare time to defervescence using available data. Trial authors did not formally report the presence or absence of serious adverse events.
4.1. Analysis.
Comparison 4 High rifampicin dose versus standard rifampicin dose, Outcome 1 Failure.
4.2. Analysis.
Comparison 4 High rifampicin dose versus standard rifampicin dose, Outcome 2 Resolution of fever within 48 hours.
Discussion
See Table 1, Table 2, and Table 3.
Summary of main results
Across the trials included in this review, treatment failure rates were low.
Two trials compared doxycycline versus tetracycline (Table 1). For treatment failure, the difference between doxycycline and tetracycline is uncertain (very low‐certainty evidence). Doxycycline compared to tetracycline may make little or no difference in resolution of fever within 48 hours (low‐certainty evidence) or in time to defervescence (low‐certainty evidence). We were unable to extract data for other outcomes.
Three trials compared doxycycline versus macrolides (azithromycin and telithromycin; Table 2). For most outcomes, including treatment failure, resolution of fever within 48 hours, time to defervescence, and serious adverse events, we are uncertain whether results show a difference between doxycycline and macrolides (very low‐certainty evidence). Macrolides compared to doxycycline may make little or no difference in the proportion of patients with resolution of fever within five days (low‐certainty evidence). Another trial compared azithromycin to doxycycline or chloramphenicol in children, but we were unable to disaggregate data for the doxycycline/chloramphenicol group.
One trial compared doxycycline to rifampicin (Table 3). For all outcomes, we are uncertain whether results show a difference between doxycycline and rifampicin (very low‐certainty evidence). Of note, this trial deviated from the protocol after three out of eight patients who received doxycycline and rifampicin combination therapy experienced treatment failure.
Across trials, mild gastrointestinal side effects appeared to be more common with doxycycline than with comparator drugs; this finding does not derive from meta‐analysis but from narrative analysis of Table 5. When reported, serious adverse events were few.
Overall completeness and applicability of evidence
All included studies were reported from Malaysia, Thailand, and Korea; we excluded several studies from China because they were not RCTs. Given that there may be geographical variation in antibiotic susceptibility across the 'tsutsugamushi triangle', applicability of findings may be limited.
Factors specific to the antibiotics included in this review may influence the applicability of evidence. In general, doxycycline is preferred over tetracycline because of its more convenient dosing schedule. Production of the macrolide antibiotic telithromycin was discontinued by the manufacturer in 2016, and before this, safety warnings had been issued; therefore this agent no longer represents a viable treatment option. In tuberculosis endemic areas, rifampicin monotherapy carries the risk of inducing rifampicin resistance in undiagnosed tuberculosis. The dose schedules of included antibiotics varied across trials.
Findings with respect to rifampicin have emerged from only one trial (Watt 2000), which provided low‐certainty evidence of very limited applicability. Of concern, doxycycline and rifampicin combination therapy resulted in a high rate of treatment failures, but the reasons for this are unclear and are not discussed further by the study author team. Although study authors suggest that rifampicin should be considered in cases where Orientia tsutsugamushi is resistant to doxycycline, it is unclear epidemiologically whether such resistance is clinically significant.
The included trials employed a variety of diagnostic techniques and recruited patients at different points within the diagnostic pathway. Some of the participants included in this review may represent misdiagnoses, which also may limit the applicability of findings.
Certainty of the evidence
We have included in this update seven trials, which represented 548 participants, with dates ranging from 1978 to 2015. Most trials were open‐label and did not report clear allocation concealment or randomization techniques. Results were imprecise owing to the small numbers of participants included for each comparison. Therefore, the body of evidence in relation to treatment of scrub typhus is of low or very low certainty.
Potential biases in the review process
We attempted to minimize bias in the review process by conducting a comprehensive search of all published and non‐published literature with no language restrictions. Two review authors assessed the eligibility of studies, extracted data, and independently judged risk of bias. We resolved disagreements by consensus and by consultation with the fourth review author. We altered our inclusion criteria for this updated review. In particular, we excluded studies that used the Weil‐Felix test as the sole measure of laboratory confirmation, given poor specificity. As a result, we excluded one trial that had been included in the previous review (Sheehy 1973).
Agreements and disagreements with other studies or reviews
Our findings are in agreement with those of a recently published meta‐analysis (Wee 2017), which concluded that evidence is insufficient to support recommending one drug over the others examined here. However, authors of the meta‐analysis included trials that employed the Weil‐Felix test as the sole diagnostic tool, and the poor specificity associated with this test may raise the level of heterogeneity amongst pooled participants.
Authors' conclusions
Implications for practice.
Tetracycline, doxycycline, azithromycin, and rifampicin are effective treatment options for scrub typhus that have been associated with few treatment failures. Chloramphenicol remains a treatment option, but we could not include this agent in direct comparisons for this review.
For specific outcomes, low‐certainty evidence suggests there may be little or no difference between tetracycline, doxycycline, and azithromycin as treatment options. In the light of very low‐certainty evidence for rifampicin and the risk of inducing resistance in undiagnosed tuberculosis, clinicians should not regard this agent as a first‐line treatment option but should consider it as a second‐line treatment option after exclusion of active tuberculosis.
Implications for research.
Further research should include adequately powered trials of doxycycline versus azithromycin or other macrolides, trials of other candidate antibiotics including rifampicin, and trials of treatment of severe scrub typhus. Researchers should standardize diagnostic techniques and reporting of clinical outcomes to allow robust comparisons.
What's new
| Date | Event | Description |
|---|---|---|
| 20 September 2018 | New citation required and conclusions have changed | We updated the literature search to 8 January 2018, included one new trial (Chanta 2015), and excluded one previously included trial (Sheehy 1973). The conclusions changed to reflect certainty of evidence and to present more guarded conclusions about rifampicin. |
| 20 September 2018 | New search has been performed | The review author team changed. The review author team revised the protocol, which was approved by the CIDG editorial team on 16 March 2018 (see Appendix 1). We reworded the objectives: "To assess and compare the effects of different antibiotic regimens for treatment of scrub typhus", replaces "To evaluate antibiotic regimens for treating scrub typhus". We assessed the certainty of the evidence using the GRADE approach. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 27 May 2010 | New search has been performed | New search performed and new studies added. Primary outcomes amended |
| 8 June 2009 | Amended | Review converted to new review format |
| 3 January 2007 | New citation required and conclusions have changed | Substantive amendments made |
Notes
REVIEW HISTORY (started 4 March 2002). 4 March 2002: updated review received by editorial base: included a new trial (Watt 2000); responded to comments received from Assistant Editor and statistician: (1) made slight change to the objective; (2) changed adverse outcomes from "Number and seriousness of side effects" to "Number of adverse events"; and (3) used risk ratio for binary outcomes (previously Peto odds ratio).
June 2010: updated review received by editorial base: (1) amended primary outcomes so no longer include "death" as a primary outcome; and (2) added new trials.
Acknowledgements
The Academic Editor of this review update was Dr Geraint Davies.
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
Appendices
Appendix 1. Prespecified changes for review update 2018
| Protocol section | Refreshed protocol |
| Background and research question |
|
| |
| Methods |
|
This table was approved by the CIDG editorial team on 16 March 2018.
Data and analyses
Comparison 1. Doxycycline versus tetracycline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.85 [0.38, 124.38] |
| 2 Resolution of fever within 48 hours | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.90, 1.44] |
Comparison 2. Macrolides versus doxycycline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 3 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.12, 63.84] |
| 2 Resolution of fever within 48 hours | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.32, 2.03] |
| 3 Resolution of fever within 5 days | 2 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.99, 1.10] |
| 4 Serious adverse events | 3 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Rifampicin versus doxycycline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Resolution of fever within 48 hours | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.10, 2.57] |
| 3 Serious adverse events | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.3. Analysis.
Comparison 3 Rifampicin versus doxycycline, Outcome 3 Serious adverse events.
Comparison 4. High rifampicin dose versus standard rifampicin dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Resolution of fever within 48 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.77, 1.38] |
| 3 Serious adverse events | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.3. Analysis.
Comparison 4 High rifampicin dose versus standard rifampicin dose, Outcome 3 Serious adverse events.
Comparison 5. Macrolide subgroup: azithromycin versus doxycycline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.12, 63.84] |
| 2 Resolution of fever within 48 hours | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.32, 2.03] |
| 3 Resolution of fever within 5 days | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.98, 1.17] |
| 4 Serious adverse events | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.2. Analysis.
Comparison 5 Macrolide subgroup: azithromycin versus doxycycline, Outcome 2 Resolution of fever within 48 hours.
5.3. Analysis.
Comparison 5 Macrolide subgroup: azithromycin versus doxycycline, Outcome 3 Resolution of fever within 5 days.
5.4. Analysis.
Comparison 5 Macrolide subgroup: azithromycin versus doxycycline, Outcome 4 Serious adverse events.
Comparison 6. Macrolide subgroup: telithromycin versus doxycycline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Resolution of fever within 5 days | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.96, 1.09] |
| 3 Serious adverse events | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
Comparison 6 Macrolide subgroup: telithromycin versus doxycycline, Outcome 1 Treatment failure.
6.2. Analysis.
Comparison 6 Macrolide subgroup: telithromycin versus doxycycline, Outcome 2 Resolution of fever within 5 days.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brown 1978.
| Methods | RCT Duration: 11 months (September 1976 to July 1977) Adverse event monitoring: patient report |
|
| Participants | Adults with suspected scrub typhus (randomized before confirmed diagnosis) Number randomized: 149 Inclusion criteria: adults ≥ 18 years with febrile illness Exclusion criteria: previous tetracycline and chloramphenicol; history of allergy to tetracycline; jaundice; pregnancy; clinical and laboratory evidence of non‐rickettsial disease Diagnosis: isolation of Rickettsia tsutsugamushi; OR a 4‐fold or greater rise in IFA titre to at least 1:200 or in static titre of 1:800 or more; OR a 4‐fold or greater rise in Proteus OXK agglutination test titre to at least 1:200 |
|
| Interventions |
*Clinicians gave additional treatment at their discretion if no improvement within 48 hours, or if clinical and laboratory evidence of alternative diagnosis |
|
| Outcomes |
|
|
| Notes | Country: Malaysia Setting: district hospital Date: September 1976 to July 1977 Funding: US Army Medical Research and Development Command, Washington, DC, and Ministry of Health, Malaysia Follow‐up: 14 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Volunteers were randomly assigned"; no further details |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 149 randomized before confirmed diagnosis. 65 with confirmed diagnosis of scrub typhus. 10 excluded (mixed infection). 55 included in final analysis (84.6% of participants with a confirmed diagnosis) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes adequately reported |
| Other bias | Low risk | No obvious other sources of bias |
Chanta 2015.
| Methods | RCT Duration: 2 years, 11 months (June 2010 to May 2013) Adverse event monitoring: patient report |
|
| Participants | Children with positive scrub typhus RDT Number randomized: 57 Inclusion criteria: hospitalized children ≤ 15 years of age; clinical manifestations compatible with scrub typhus; confirmatory laboratory tests Exclusion criteria: allergy to study drug; severe clinical complications (hypotension, coma, respiratory failure, acute renal failure with renal replacement therapy); anti‐microbial therapy < 7 days pre‐admission Laboratory diagnosis: dipstick RDT (SD Bioline Tsutsugamushi test) |
|
| Interventions |
*Changed to "standard treatment" if clinical failure †Children under 8 received chloramphenicol; children 8 and older received doxycycline |
|
| Outcomes |
*Temperature < 37.3°C maintained for > 48 hours |
|
| Notes | Country: Thailand Setting: tertiary hospital, paediatrics unit Funding: Chiangrai Prachanukroh Hospital fund Follow‐up: 1 month after discharge |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization; no further details |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Open‐label"; no further details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 57 randomized after RDT positive diagnosis. No missing data (57/57) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes adequately reported. Adverse events defined as those "related to the administration of the antibiotic" |
| Other bias | Low risk | No obvious other sources of bias |
Kim 2004.
| Methods | RCT Duration: 1 year, 2 months (September 2002 to November 2003) Adverse event monitoring: patient report |
|
| Participants | Adults with suspected scrub typhus (randomized before confirmed diagnosis) Number randomized: 99 Inclusion criteria: fever (oral temperature ≥ 38°C); eschar or a maculopapular skin rash with 2 of: headache, generalized weakness, myalgia, abdominal discomfort, coughing, or nausea Exclusion criteria: hypersensitivity to study drugs; pregnancy; severe complications (shock requiring vasopressor therapy for > 1 hour, disturbed consciousness level, respiratory failure, and renal failure with immediate dialysis); antibiotics with potential anti‐rickettsial activity within previous 2 days Laboratory diagnosis: IFA with specific IgM ≥ 1:10; OR> 4‐fold increased titres in paired serum specimens |
|
| Interventions |
|
|
| Outcomes |
*Temperature < 37.3°C maintained for > 48 hours |
|
| Notes | Country: Republic of Korea Setting: tertiary hospital Funding: Chungnam National University Hospital (Daejeon, South Korea) Follow‐up: 1 month after discharge |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequences |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Open‐label"; no further details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 99 randomized before confirmed diagnosis. 6 excluded after randomization (combined infection, vomiting, medication error). 93 completed treatment and included in final analysis. 75 with laboratory‐confirmed diagnosis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes adequately reported |
| Other bias | Low risk | No obvious other sources of bias |
Kim 2007.
| Methods | Quasi‐RCT Duration: 4 months (September to December 2005) Adverse event monitoring: patient report |
|
| Participants | Adults with suspected scrub typhus (randomized before confirmed diagnosis) Number randomized: 92 Inclusion criteria: fever > 37.5°C; eschar or a maculopapular skin rash with 2 of: headache, malaise, myalgia, coughing, nausea, or abdominal discomfort Exclusion criteria: unable to take oral medications; pregnancy; hypersensitivity to trial drugs, antibiotics with potential anti‐rickettsial activity within previous 2 days; severe scrub typhus (shock requiring vasopressor therapy for longer than 1 hour, a stuporous or comatose level of consciousness, respiratory failure requiring mechanical ventilation, or renal failure requiring immediate dialysis); mixed infection Laboratory diagnosis: IFA with specific IgM > 1:80; OR > 4‐fold increased titres in paired serum specimens |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Country: Republic of Korea Setting: university hospital and 2 community hospitals Funding: Sanofi‐Aventis Korea Co., Ltd.; The Clinical Medicine Research Institute at Chosun University Hospital Follow‐up: 4 weeks after discharge |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized by last digit of resident registration number (odd numbers assigned to doxycycline, even numbers assigned to telithromycin) |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Open‐label"; no further details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 92 randomized before confirmed diagnosis. 92 patients included in final analysis. 76 with laboratory‐confirmed diagnosis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes adequately reported |
| Other bias | Low risk | No obvious other sources of bias |
Phimda 2007.
| Methods | RCT Duration: 1 year, 6 months (July 2003 to January 2005) Adverse event monitoring: patient report |
|
| Participants | Adults and adolescents with acute undifferentiated fever (subsequent diagnoses included leptospirosis, scrub typhus, murine typhus, mixed infections) Number randomized: 296 (57 patients with subsequent diagnosis of scrub typhus) Inclusion criteria: age > 14 years; oral temperature ≥ 38°C for < 15 days; no obvious focus of infection Exclusion criteria: inability to take oral medications; pregnancy/breastfeeding; allergy to study drugs; concurrent infection; anti‐rickettsial drugs < 48 hours before enrolment Laboratory diagnosis: IFA (microimmunofluorescence) with specific IgM and/or IgG > 1:400; OR > 4‐fold increased titres in paired serum specimens |
|
| Interventions |
|
|
| Outcomes |
*Temperature < 37.5°C maintained for > 2 measurements without anti‐pyretics |
|
| Notes | Country: Thailand Setting: 4 hospitals Funding: Thailand Research Fund, Ministry of Public Health, Thailand, and the Welcome Trust of Great Britain Follow‐up: 15 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent, computer‐generated, simple random allocation sequences |
| Allocation concealment (selection bias) | Low risk | Central randomization; sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label. Outcome assessment "independent". Statistician blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 296 randomized. 43 excluded after randomization owing to prior antibiotics. 89 lost to follow‐up (uncertain diagnosis). 296 included in final analysis; of these 57 participants had confirmed scrub typhus. Missing data balanced between final diagnosis groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes adequately reported |
| Other bias | Low risk | No obvious other sources of bias |
Song 1995.
| Methods | RCT Duration: 1 year, 1 month (October 1991 to November 1992) Adverse event monitoring: patient report. |
|
| Participants | Adults with positive scrub typhus IFA Number randomized: 129 Inclusion criteria: age ≥ 18 years; acute febrile illness with high fever, rash, and eschar Exclusion criteria: subsequent unconfirmed diagnosis; allergy to study drugs; received study drugs within last 72 hours; raised serum creatinine; pregnancy or lactation Laboratory diagnosis: IFA with specific IgG > 1:80; OR > 4‐fold increased titres in paired serum specimens |
|
| Interventions |
|
|
| Outcomes |
*Temperature < 37.3°C maintained for > 48 hours |
|
| Notes | Country: Korea. Setting: 8 branch hospitals Funding: Asian Institute for Life Science Follow‐up: 4 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally computer‐generated random orders |
| Allocation concealment (selection bias) | Unclear risk | ''Central randomisation''; no further details |
| Blinding (performance bias and detection bias) All outcomes | High risk | Non‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 129 randomized. 13 excluded owing to negative or indeterminate diagnosis. 116 included in final analysis (90% of those randomized) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes adequately reported |
| Other bias | Low risk | No obvious other sources of bias |
Watt 2000.
| Methods | RCT Duration: no details Adverse event monitoring: patient report |
|
| Participants | Adults with positive scrub typhus RDT Number: 126 Inclusion criteria: adults; ambulatory mild scrub typhus; ability to take oral medication Exclusion criteria: severe disease (hypotension, shock, impaired consciousness, or pulmonary dysfunction); coinfection or other febrile illness; vomiting; serum bilirubin > 25.7 μmol/L; serum ALT > 100 U/L; anti‐rickettsial drugs < 48 hours before enrolment Laboratory diagnosis: screened with dot‐blot ELISA rapid test. Confirmed with indirect immunoperoxidase test: IgM > 1:400, IgG > 1:1600 |
|
| Interventions |
*In a change to the protocol, arm 4 (450 mg rifampicin) replaced arm 3 (combined therapy) after 1 year owing to protracted fever. |
|
| Outcomes |
*Temperature < 37.3°C maintained for > 48 hours without anti‐pyretics |
|
| Notes | Country: Thailand Setting: tertiary hospital Funding source: US Army Research and Material Command and the Royal Thai Army Follow‐up: 1 month during first year of study; 2 weeks in following years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned"; no further details |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Describes efforts to "mask" investigators from evidence of drug allocation in the form of red discolouration of body fluids but provides limited details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 357 of 2090 with positive RDT; 231 excluded before randomization as "ineligible"; 126 seropositive and randomized. 32 excluded after randomization; 5 excluded as diagnosis not confirmed. 78 completed protocol and were analysed (67.8% of those randomized) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes adequately reported |
| Other bias | High risk | Deviation from the study protocol. Participants initially randomized to receive combined doxycycline and rifampicin therapy; 3 of 8 participants receiving combination therapy experienced failed treatment. Combined therapy arm changed to high‐dose rifampicin arm |
Abbreviations: ALT: alanine aminotransferase; ELISA: enzyme‐linked immunosorbent assay; IFA: immunofluorescence assay; IgG: immunoglobulin G; IgM: immunoglobulin M; RCT: randomized controlled trial; RDT: rapid diagnostic test.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2007 | A retrospective analysis, not an RCT. Diagnosis based only on Weil‐Felix reaction |
| Chen 2008 | A retrospective analysis, not an RCT. Diagnosis based only on Weil‐Felix reaction |
| Feng 2009 | A retrospective analysis, not an RCT. Diagnosis based only on Weil‐Felix reaction |
| Li 2004 | A retrospective analysis, not an RCT. Diagnosis based only on Weil‐Felix reaction |
| Li 2007 | Diagnosis based only on Weil‐Felix reaction Unclear study design. Participants were randomly allocated to 5 treatment groups, but study authors did not describe the randomization process. |
| Olson 1980 | Assessed efficacy for prevention rather than for treatment |
| Quan 2002 | Diagnosis based only on Weil‐Felix reaction Unclear study design. Participants were randomly allocated to 2 treatment groups, but study authors did not describe detailed randomization methods. Whether it is a real RCT needs clarification. |
| Sheehy 1973 | Diagnosis based only on Weil‐Felix reaction |
| Twartz 1982 | Assessed efficacy for prevention rather than for treatment |
| Wei 2004 | Diagnosis based only on Weil‐Felix reaction Unclear study design. Participants were randomly allocated to 2 treatment groups, but study authors did not describe detailed randomization methods. Whether it is a real RCT needs clarification. |
| Wu 2006 | Diagnosis based only on Weil‐Felix reaction Unclear study design. Participants were randomly allocated to 2 treatment groups, but study authors did not describe detailed randomization methods. |
| Yang 2005 | Diagnosis based only on Weil‐Felix reaction A retrospective analysis, not an RCT |
| Zhong 1996 | Diagnosis based only on Weil‐Felix reaction |
Abbreviations: RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ISRCTN47812566.
| Trial name or title | Oral Doxycycline Versus Oral Azithromycin in the Treatment of Scrub and Murine Typhus in Laos |
| Methods | Randomized controlled trial (RCT) |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | 4 August 2003 End of follow‐up: 31 December 2009 |
| Contact information | Dr. Paul Newton paul@tropmedres.ac; Ministry of Health, Mahosot Hospital, Mahosot Road, Vientiane, Laos |
| Notes | Location: Mahosot Hospital, Vientiane, Laos Registration number: ISRCTN47812566 Source of funding: The Wellcome Trust (UK) (grant ref: 066828) |
NCT00351182.
| Trial name or title | Controlled Trial: 5‐Day Course of Telithromycin Versus Doxycycline for the Treatment of Mild to Moderate Scrub Typhus |
| Methods | Multi‐centre randomized open‐label clinical trial |
| Participants |
Inclusion criteria Adult patients aged 18 years and older presenting with fever ≥ 37.5°C, eschar, or maculopapular rash and with at least 2 of the following: headache, malaise, myalgia, coughing, nausea, and abdominal discomfort Exclusion criteria
|
| Interventions | 5‐day course of telithromycin versus doxycycline |
| Outcomes | Fever clearance time |
| Starting date | September 2005 Last update posted: 12 July 2006 |
| Contact information | Prof. Dong‐Min Kim drongkim@chosun.ac.kr; Chosun University Hospital, Republic of Korea |
| Notes | Location: Chosun University Hospital, Republic of Korea Registration number: Telit_L_00276 Sources of funding: Chosun University Hospital |
NCT00568711.
| Trial name or title | Controlled Trial: 5‐Day Course of Rifampin Versus Doxycycline for the Treatment of Mild to Moderate Scrub Typhus |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2006 Expected completion: December 2009 Unknown recruitment status |
| Contact information | Prof. Dong‐Min Kim drongkim@chosun.ac.kr; Chosun University Hospital, Kwangju, Jeollanamdo, South Korea |
| Notes | Location: Chosun University Hospital, or one of its 2 community‐based affiliated hospitals, all of which are located in southwestern Korea Study ID number: NCT00568711 |
NCT03083197.
| Trial name or title | Scrub Typhus Antibiotic Resistance Trial (START) |
| Methods | Prospective, open‐label, RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
Primary
Secondary
|
| Starting date | 17 March 2017. Last update posted: 14 December 2017 Expected completion time: October 2019 |
| Contact information | Assoc. Prof. Daniel Paris parigi@tropmedres.ac; Mahidol Oxford Tropical Medicine Research Unit, Bangkok, Thailand |
| Notes | Location: Shoklo Malaria Research Unit (SMRU), Chiangrai Prachanukroh Hospital, Thailand Study ID number: START Source of funding: University of Oxford, Shoklo Malaria Research Unit and Chiangrai Prachanukroh Hospital |
Abbreviations: BP: blood pressure; HAART: highly active anti‐retroviral therapy; HIV: human immunodeficiency virus; IgM: immunoglobulin M; LP: lumbar puncture; PCR: polymerase chain reaction; RDT: rapid diagnostic test; SLE: systemic lupus erythematosus; TB: tuberculosis; TNF: tumour necrosis factor.
Differences between protocol and review
The review author team revised the protocol, which was approved by the CIDG editorial team on 16 March 2018 (see Appendix 1). Owing to small numbers of trials per each comparison, we did not perform subgroup analysis according to geographical setting (which may influence antibiotic susceptibility), dates of the study (before or after antibiotic resistance was first reported), participant age (children versus adults), or dose, frequency, and duration of treatment. We also did not perform sensitivity analysis to assess robustness of our results after restricting analysis to studies with low risk of bias. Otherwise, we fulfilled all proposed changes as shown in Appendix 1.
Upon receiving editorial feedback on the first draft of this review, we changed 'resolution of fever within five days' from a primary to a secondary outcome.
Contributions of authors
Iman El Sayed: prepared initial drafts of background and methods; selected studies; extracted data; synthesized data in RevMan 2014; and prepared initial drafts of results and 'Summary of findings' tables.
Qin Liu: selected studies and extracted data; assessed risk of bias; contributed to 'Summary of findings' tables; and contributed to the discussion.
Ian Wee: selected studies and extracted data; assessed risk of bias; and contributed to results and discussion sections.
Paul Hine: completed drafts of background, methods, results, 'Summary of findings' tables, discussion, and conclusions.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Project number 300342‐104
Declarations of interest
IES has no known conflicts of interest. QL has no known conflicts of interest. IW has no known conflicts of interest. PH has no known conflicts of interest.
Unchanged
References
References to studies included in this review
Brown 1978 {published data only}
- Brown GW, Saunders JP, Singh SL, Haxsoll DL, Shirai A. Single dose doxycycline therapy for scrub typhus. Transactions of the Royal Society of Tropical Medicine and Hygiene 1978;72(4):412‐6. [DOI] [PubMed] [Google Scholar]
Chanta 2015 {published data only}
- Chanta C, Phloenchaiwanit P. Randomized controlled trial of azithromycin versus doxycycline or chloramphenicol for treatment of uncomplicated pediatric scrub typhus. Journal of the Medical Association of Thailand 2015;98(8):756‐60. [PubMed] [Google Scholar]
Kim 2004 {published data only}
- Kim YS, Yun HJ, Shim SK, Koo SH, Kim SY, Kim S. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clinical Infectious Diseases 2004;39(9):1329‐35. [DOI] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim DM, Yu KD, Lee JH, Kim HK, Lee SH. Controlled trial of a 5‐day course of telithromycin versus doxycycline for treatment of mild to moderate scrub typhus. Antimicrobial Agents and Chemotherapy 2007;51(6):2011‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phimda 2007 {published data only}
- Phimda K, Hoontrakul S, Suttinont C, Chareonwat S, Losuwanaluk K, Chueasuwanchai S, et al. Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrobial Agents and Chemotherapy 2007;51(9):3259‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 1995 {published data only}
- Song JH, Lee C, Chang LW, Choi SW, Choi JE, Kim YS, et al. Short‐course doxycycline treatment versus conventional tetracycline therapy for scrub typhus: a multiple randomized trial. Clinical Infectious Diseases 1995;21(3):506‐10. [DOI] [PubMed] [Google Scholar]
Watt 2000 {published data only}
- Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D. Doxycycline and rifampicin for mild scrub typhus infections in northern Thailand: a randomised trial. Lancet 2000;356(9235):1057‐61. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chen 2007 {published data only}
- Chen SX, Lin XM, Shen HF, Zhang LJ, Sun ZM. Treatment of scrub typhus with macrolide antibiotic. Journal of Tropical Medicine 2007;7:783‐84. [Google Scholar]
Chen 2008 {published data only}
- Chen SX, Shen HF, Zhang LJ, Xu CX, Sun ZM. Effect of antibiotics in the treatment of scrub typhus in South China Sea area. Journal of Preventive Medicine of Chinese People's Liberation Army 2008;26:59. [Google Scholar]
Feng 2009 {published data only}
- Feng X. Treatment of scrub typhus with several antibiotics. Journal of Practical Medicine 2009;25:526. [Google Scholar]
Li 2004 {published data only}
- Li Y. Macrolide antibiotic to cure child scrub typhus. Journal of Pediatric Pharmacy 2004;10:36. [Google Scholar]
Li 2007 {published data only}
- Li RC, Pang L, Lu XH. Effect of antibiotics in the treatment of scrub typhus. Modernizing Medicine's Healthcare 2007;23:1936‐7. [Google Scholar]
Olson 1980 {published data only}
- Olson JG, Bourgeois AL, Fang RCY, Coolbaugh JC, Dennes DT. Prevention of scrub typhus: prophylactic administration of doxycycline in a randomized double blind trial. Journal of Tropical Medicine and Hygiene 1980;29(5):989‐97. [PubMed] [Google Scholar]
Quan 2002 {published data only}
- Quan B. The third generation of quinolone therapy for scrub typhus. Journal of Tropical Medicine 2002;2:163‐4. [Google Scholar]
Sheehy 1973 {published data only}
- Sheehy TW, Hazlett D, Turk RE. Scrub typhus. A comparison of chloramphenicol and tetracycline in its treatment. Archives of Internal Medicine 1973;132:77‐80. [DOI] [PubMed] [Google Scholar]
Twartz 1982 {published data only}
- Twartz JC, Selvarju SG, Saunders JP, Huxsoll DL, Groves MG. Doxycycline prophylaxis for human scrub typhus. Journal of Infectious Diseases 1982;146(6):811‐8. [DOI] [PubMed] [Google Scholar]
Wei 2004 {published data only}
- Wei MX, Lin SJ, Yan R, Guo WZ. A comparison of azithromycin versus chloramphenicol for treatment of pediatric scrub typhus. Journal of Applied Clinical Pediatrics 2004;19:66. [Google Scholar]
Wu 2006 {published data only}
- Wu X, Xiao H, Zhu DJ. Therapeutic effect of azithromycin on children with tsutsugamushi disease. Chinese Journal of Pediatrics 2006;24:769‐70. [Google Scholar]
Yang 2005 {published data only}
- Yang YF, Li B, Liu XM, Zhang LP. Effect of roxithromycin in the treatment of scrub typhus. Journal of Pediatric Pharmacy 2005;11:32. [Google Scholar]
Zhong 1996 {published data only}
- Zhong ZY, Zhang WY, Wu YX. Scrub typhus. A comparison of doxycycline and chloramphenicol in its treatment. Journal of Guangdong Medical College 1996;14:354‐5. [Google Scholar]
References to ongoing studies
ISRCTN47812566 {published data only}
- ISRCTN47812566. Oral doxycycline versus oral azithromycin in the treatment of scrub and murine typhus in Laos. isrctn.com/ISRCTN47812566 (date applied 7 December 2005).
NCT00351182 {published data only}
- NCT00351182. 5‐day course of telithromycin versus doxycycline for the treatment of mild to moderate scrub typhus. ClinicalTrials.gov/show/NCT00351182 (first posted 12 July 2006).
NCT00568711 {published data only}
- NCT00568711. Controlled trial: 5‐day course of rifampin versus doxycycline for the treatment of mild to moderate scrub typhus. clinicaltrials.gov/ct2/show/NCT00568711 (first posted 6 December 2007).
NCT03083197 {unpublished data only}
- NCT03083197. Scrub typhus antibiotic resistance trial (START). clinicaltrials.gov/show/NCT03083197 (first posted 17 March 2017).
Additional references
Acestor 2012
- Acestor N, Cooksey R, Newton PN, Ménard D, Guerin PJ, Nakagawa J, et al. Mapping the aetiology of non‐malarial febrile illness in Southeast Asia through a systematic review ‐ terra incognita impairing treatment policies. PLoS One 2012;7(9):e44269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonell 2017
- Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: a systematic review. PLoS Neglected Tropical Diseases 2017;11(9):e0005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2017
- Centers for Disease Control and Prevention. Scrub typhus. www.cdc.gov/typhus/healthcare‐providers/index.html (accessed 13 March 2018).
Corwin 1999
- Corwin A, Soderquist R, Suwanabun N, Sattabongkot J, Martin L, Kelly D, et al. Scrub typhus and military operations in Indochina. Clinical Infectious Diseases 1999;29(4):940‐1. [DOI] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- GRADEpro GDT. GRADEpro Guideline Development Tool. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Griffith 2014
- Griffith M, Peter JV, Karthik G, Ramakrishna K, Prakash JA, Kalki RC, et al. Profile of organ dysfunction and predictors of mortality in severe scrub typhus infection requiring intensive care admission. Indian Journal of Critical Care Medicine 2014;18(8):497‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kelly 2017
- Kelly D, Fuerst P, Richards A. The historical case for and the future study of antibiotic‐resistant scrub typhus. Tropical Medicine and Infectious Disease 2017;2(4):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koralur 2018
- Koralur M, Singh R, Varma M, Shenoy S, Acharya V, Kamath A, et al. Scrub typhus diagnosis on acute specimens using serological and molecular assays ‐ a three‐year prospective study. Diagnostic Microbiology and Infectious Disease 2018;91(2):112‐7. [DOI: 10.1016/j.diagmicrobio.2018.01.018] [DOI] [PubMed] [Google Scholar]
Paris 2016
- Paris DH, Dumler JS. State of the art of diagnosis of rickettsial diseases: the use of blood specimens for diagnosis of scrub typhus, spotted fever group rickettsiosis, and murine typhus. Current Opinion in Infectious Diseases 2016;29(5):433‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajapakse 2012
- Rajapakse S, Rodrigo C, Fernando D. Scrub typhus: pathophysiology, clinical manifestations and prognosis. Asian Pacific Journal of Tropical Medicine 2012;5(4):261‐4. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. Introduction to GRADE HandbookHandbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed 18 April 2018).
Smadel 1950
- Smadel JE, Traub R, Frick LP, Dierck FH, Bailey CA. Chloramphenicol (chloromycetin) in the chemoprophylaxis of scrub typhus (Tsutsugamushi disease). American Journal of Hygiene 1950;51:216‐28. [Google Scholar]
Tantibhedhyangkul 2010
- Tantibhedhyangkul W, Angelakis E, Tongyoo N, Newton PN, Moore CE, Phetsouvanh R, et al. Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. International Journal of Antimicrobial Agents 2010;35(4):338‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2015
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Neglected Tropical Diseases 2015;9(8):e0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thipmontree 2016
- Thipmontree W, Tantibhedhyangkul W, Silpasakorn S, Wongsawat E, Waywa D, Suputtamongkol Y. Scrub typhus in Northeastern Thailand: eschar distribution, abnormal electrocardiographic findings, and predictors of fatal outcome. American Journal of Tropical Medicine and Hygiene 2016;95(4):769‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watt 1996
- Watt G, Chouriyagune C, Ruangweerayud T, Watcharapichat P, Phulsuksombati D, Jongasakul K, et al. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet 1996;348:86‐9. [DOI] [PubMed] [Google Scholar]
Watt 2003
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Current Opinion in Infectious Disease 2003;16(5):429‐36. [DOI] [PubMed] [Google Scholar]
Wee 2017
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta‐analysis of controlled clinical trials. Transactions of the Royal Society of Tropical Medicine and Hygiene 2017;111(8):336‐44. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Liu 2002
- Liu Q, Panpanich R. Antibiotics for treating scrub typhus. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002150] [DOI] [PubMed] [Google Scholar]
Panpanich 2000
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002150] [DOI] [PubMed] [Google Scholar]


