Abstract
Premise of the study
The early Cenozoic was a key period of evolutionary radiation in Fagaceae. The common notion is that species thriving in the modern Mediterranean climate of California originated in climates with summer rain during the Paleogene.
Methods
We investigated in situ and dispersed pollen of Fagaceae from the uppermost Eocene Florissant Fossil Beds, Colorado, using a combined light and scanning electron microscopy approach (single grain method).
Key results
Pollen types of Castaneoideae with affinities to modern Castanea, Lithocarpus, and Castanopsis are recognized. Pollen of the extinct genus Fagopsis represents a derived type of Castaneoideae pollen. Infrageneric groups of Quercus are well represented, including pollen of Group Protobalanus. The flora indicates a climatic setting for Florissant, located in the Front Range of the Rocky Mountains, which corresponds with the modern-day situation in the Coastal Ranges. Continental climatic conditions and seasonality may have triggered the evolution of sclerophyllous leaves and adaptive radiation in Quercus and other taxa confined today to Mediterranean vegetation of California and Mexico.
Conclusions
The Florissant plant assemblage indicates that modern vegetation belts of the Coastal Ranges (Chaparral, nemoral conifer forest) were established in the Front Range by the late Eocene. The constituent taxa demonstrate that typical elements found in the Mediterranean climate of Pacific North America (Group Protobalanus, Notholithocarpus, Torreya, and Calocedrus) did not undergo dramatic ecological shifts. They opportunistically migrated into their modern ranges. This is in stark contrast to the evolution and migration patterns of their western Eurasian Mediterranean counterparts (Quercus Group Ilex).
Keywords: Castaneoideae, Eocene-Oligocene boundary, Front Range, laurel forest, nemoral conifer forest, origin of Mediterranean vegetation, Quercus Group Cyclobalanopsis, Quercus Group Quercus/Lobatae, Quercus Group Protobalanus, sclerophyllous forest
Introduction
The family Fagaceae is the most diverse tree family in the northern temperate regions and comprises about 650 to 750 (Flora of North America Editorial Committee, 1997; Wu and Raven, 1999) or 1000 species (Govaerts and Frodin, 1998). Fagaceae play an important role in broadleaved forests across the northern hemisphere and commonly form mono-dominant forests. Modern centers of diversity for the family are in Mexico and in Southeast Asia (Govaerts and Frodin, 1998). A number of genera or infrageneric groups that are at present confined to North America (Quercus Group Lobatae) or East Asia (Castanopsis, Lithocarpus, Quercus Group Cyclobalanopsis) were widely distributed across the northern hemisphere during the Cenozoic and the present centers of diversity may not reflect the regions of origin of modern groups (Denk and Grimm, 2009b). The Eocene was a key epoch for the earliest appearance of modern genera of Fagaceae (e.g. Castanea, Crepet and Daghlian, 1980; Fagus, Manchester and Dillhoff, 2004; Quercus, Kvaček and Walther, 1989; Manchester, 1994; McIver and Basinger, 1999) while extinct lineages still played important roles in plant communities (e.g. Manchester and Crane, 1983; Jones and Dilcher, 1988; Denk et al., 2012). For many groups within Fagaceae, however, it is difficult to establish generic affinities based on leaf imprints without preserved epidermal features (e.g. Kvaček and Walther, 1981, 1988) or based on light microscopy (LM) investigations of pollen (Denk and Grimm, 2009a). Using scanning electron microscopy (SEM) for the investigation of in situ and dispersed pollen grains has opened up new vistas in the assessment of fossil pollen grains for evolutionary and paleoecological studies (Crepet and Daghlian, 1980; Friis et al, 1988; Walther and Zetter, 1993; Kohlmann-Adamska and Ziembínska-Tworzydło, 2000, 2001; Denk et al., 2010; 2011, 2012). Here, we use a combined LM and SEM investigation of in situ and dispersed pollen grains in order to evaluate the taxonomic diversity of Fagaceae in the uppermost Eocene of the Florissant Formation, Colorado, USA. The Florissant Fossil Beds have yielded one of the most diverse fossil floras and faunas worldwide (Meyer, 2003). Fossil plants have been treated monographically by MacGinitie (1953; revised by Manchester, 2001), who described leaves, flowers, fruits, and seeds. Palynological accounts were published by Leopold and Clay-Poole (2001) and Wingate and Nichols (2001). Two genera of Fagaceae have previously been recognized on the basis of leaf fossils and partly pollen: the extinct genus Fagopsis (Manchester and Crane, 1983) and Quercus (MacGinitie, 1953). MacGinitie attributed several leaf types to white oaks (Quercus Group Quercus), red oaks (Quercus Group Lobatae), golden cup oaks (Quercus Group Protobalanus), and cycle cup oaks (Quercus Group Cyclobalanopsis). Furthermore, one leaf type was compared to the enigmatic European fossil taxon Quercus cruciata.
In the present study we investigated pollen of Fagaceae from the Florissant Fossil Beds with LM and SEM to achieve high taxonomic resolution. The observed diversity of pollen types was then compared to the previously reported diversity of leaf types. The diversity of Fagaceae in the latest Eocene of western North America was viewed in a wider northern hemispheric context. Trends of morphological/ecological adaptive radiation due to the alleged increased seasonality in parts of western North America are discussed and biogeographic links of the Fagaceae of Florissant assessed. Furthermore, we use the revised plant fossil record of Florissant to comment on the paleovegetation and paleoecology during the deposition of the Florissant Fossil Beds.
MATERIAL AND METHODS
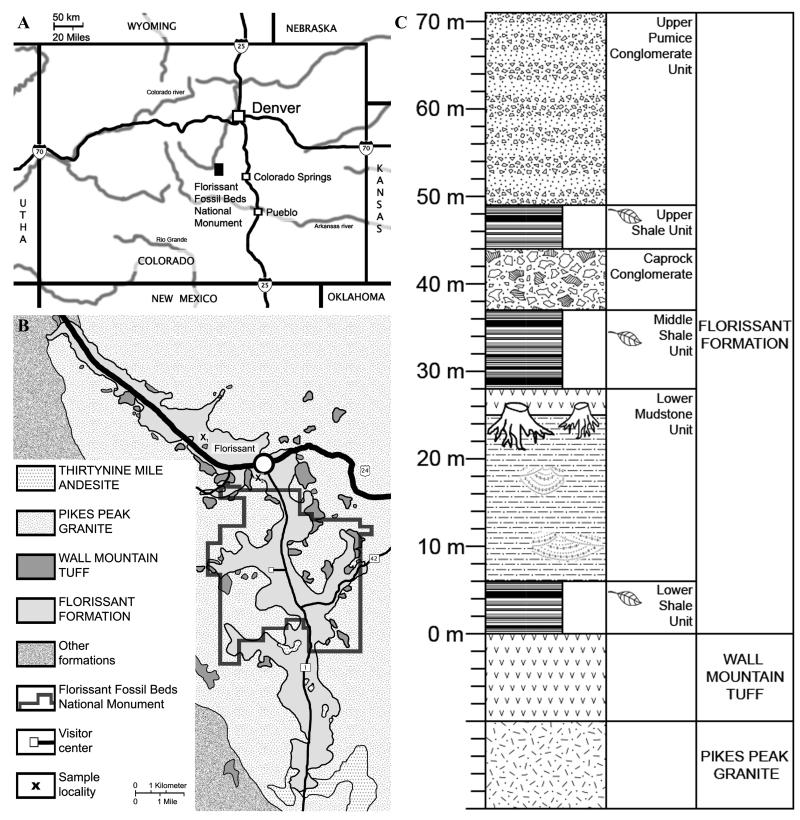
The plant material investigated for the present study originates from the Florissant Fossil Beds in the Southern Rocky Mountains of Colorado (Front Range). Today, the area is located at an elevation of 2500 to 2600 m a.s.l. (Meyer, 2003) with the adjacent highest peaks to the northwest reaching more than 4000 m a.s.l. In the area of Florissant, four formal rock units can be distinguished, the Pikes Peak Granite, the Wall Mountain Tuff, the Florissant Formation, and breccias of the Thirtynine Mile Andesite (Evanoff et al., 2001; Fig. 1.A).
Figure 1.
A-C. Geography, geologic setting, and stratigraphy of the Florissant Formation A. Map showing the geographical position of the Florissant Fos sil Beds National Monument in Colorado, USA. B. Geological map indicating Florissant Formation and surrounding formations. X1 = sample UF15880–7285 locality, X2 = sample S151454 locality. Map based upon and modified after U.S.G.S. Map 1-1044 (Wobus and Epis, 1978). C. Generalized stratigraphy of the Florissant Formation, modified after Evanoff (2001).
The Florissant Formation is a heterolithic accumulation of shale, tuffaceous mudstone and siltstone, tuff, arkosic and volcaniclastic sandstone and conglomerate (Evanoff et al., 2001). The formation consists of six informal units: the Lower Shale, the Lower Mudstone, the Middle Shale, the Caprock Conglomerate, the Upper Shale, and the Upper Pumice Conglomerate.
The paper shales of the Lower, Middle and Upper Shale unit are the main fossil-bearing strata, which yield the exceptionally well-preserved insect, plant, fish and bird fossils. An individual of the genus Herpetotherium (an extinct small opossum) has been recovered from these strata. All the stratigraphically relevant mammal fossils were found in the lower mudstone unit. Two species of Lipotyphla, six species of Rodentia, two species of Lagomorpha, four species of Artiodactyla and two species of Perissodactyla are known to come from this unit and suggest a middle to late Chadronian age (NALMA) (Lloyd et al., 2008) or Priabonium age (ICS). This corresponds with the 40Ar/39Ar weighted mean age of 34.07 ± 0.10 Ma of pumice samples obtained from the upper parts of the Florissant Formation (Evanoff et. al., 2001).
The sediment samples investigated for the present study were taken from slab pieces of the paleobotany and palynology collection of the Florida Museum of Natural History, Gainesville, labeled as UF15880 – 7285 and the paleobotany collection of the Naturhistoriska Riksmuseet, Stockholm, labeled as S151454. UF15880 – 7285 was collected on the private property of family Stoll, west of Florissant (Fig. 1. B, X1), near the old Denver Museum Locality (MacGinitie, 1953). S151454 was collected from the Claire Quarry locality south of Florissant (Fig. 1. B, X2) Sedimentary rock was processed following the protocol described in Grímsson et al. (2008) and the same pollen grains were investigated with light microscopy (LM) and electron scanning microscopy (SEM; single grain method, Zetter, 1989). The compressed staminate inflorescence of Fagopsis longifolia with in situ pollen grains is from the collection of the University of California Museum of Paleontology in Berkeley, labelled as UCMP200/254999. The sample was macerated in Danclor™ and acetolysed; individual pollen grains were isolated with a micromanipulator.
LM micrographs were taken with a Nikon Eclipse 80i microscope equipped with a Samsung Digimax V70 digital camera. SEM micrographs were taken with a Joel JSM 6400 scanning electron microscope and a Hitachi S-4300 cold field emission scanning electron microscope. In most cases, specimens were investigated and photographed immediately after sputter coating with gold. The terminology for pollen morphology followed Punt et al. (2007) and Hesse et al. (2009). The term vermiculate is used to describe winding features in a general way.
Phylogenetic framework
For a phylogenetic framework the studies by Manos et al. (2001) and Denk and Grimm (2009, 2010) were used. These studies identified six infrageneric groups and showed that the classical subdivision into Quercus subgenus Quercus and Quercus subgenus Cyclobalanopsis is unnatural. We follow the concept of Denk and Grimm (2010) who introduced informal names for the six groups within Quercus. The nomenclature for these groups is as follows: 1. Quercus Group Cerris (Eurasia), 2. Quercus Group Cyclobalanopsis (Asia), 3. Quercus Group Ilex (Eurasia), 4. Quercus Group Lobatae (North America), 5. Quercus Group Quercus (Northern Hemisphere), and 6. Quercus Group Protobalanus (western North America). For previously used partly synonymous names, see Denk and Grimm (2010). For practical reasons, we use the subfamilial name Castaneoideae Oersted to denote the genera Castanea, Castanopsis, Lithocarpus, Chrysolepis, and Notholithocarpus. These genera form a grade in recent molecular phylogenetic studies (Manos et al., 2008; Oh and Manos, 2008; Denk and Grimm, 2010).
SYSTEMATIC PALEOBOTANY
Family
Fagaceae Dumortier
Genus
Fagopsis Hollick
Species
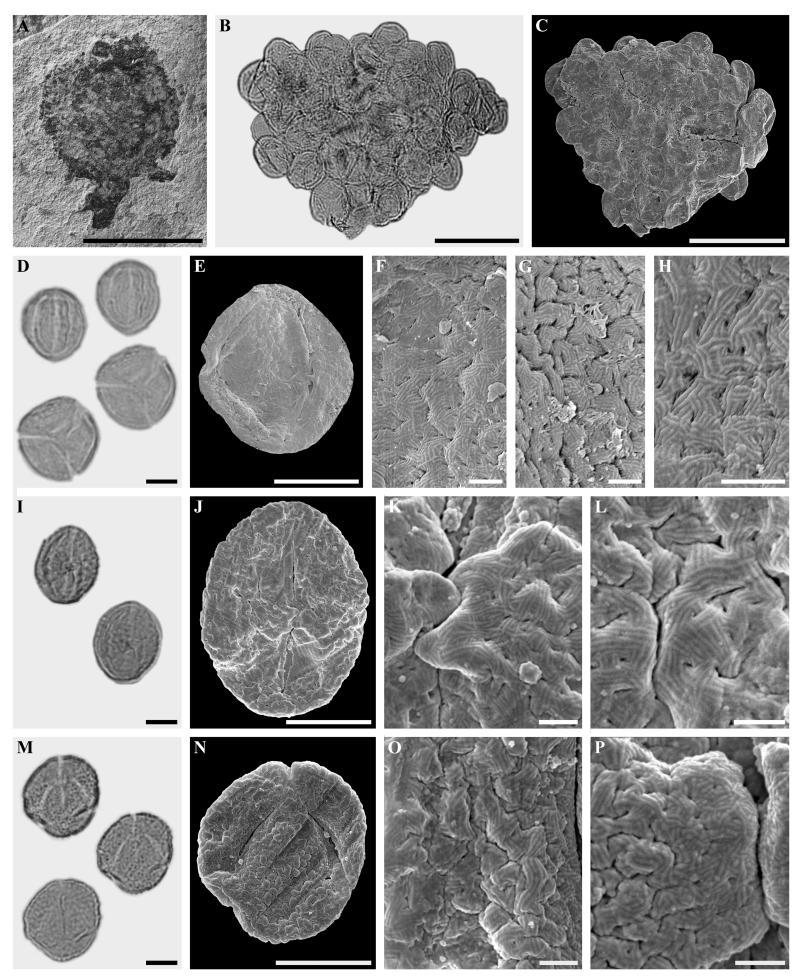
Fagopsis longifolia (Lesquereux) (Fig. 2 A-P, 3 A-L)
Figure 2.
A-P. Staminate inflorescence, in situ pollen, and dispersed pollen of Fagopsis longifolia.
A. Staminate inflorescence (UCMP200/254999).
B-C. Pollen agglomeration from staminate inflorescence in LM and SEM.
D-H. Isolated pollen from staminate inflorescence. D. LM, overview E. SEM, overview. F-H. SEM, details.
I.-P. Dispersed pollen grains. I-L. LM and SEM micrographs from the same pollen grain. I. LM, overview. J. SEM, overview. K-L. SEM, details.
M-P. LM and SEM pictures from the same pollen grain. M. LM, overview. N. SEM, overview. O-P. SEM, details.
Scale bars = 1 cm in Fig. A. 50 μm in Figs. B, C. 10 μm in Figs D, E, I, J, M and N. 1 μm in Figs. F-H, K, L, O and P
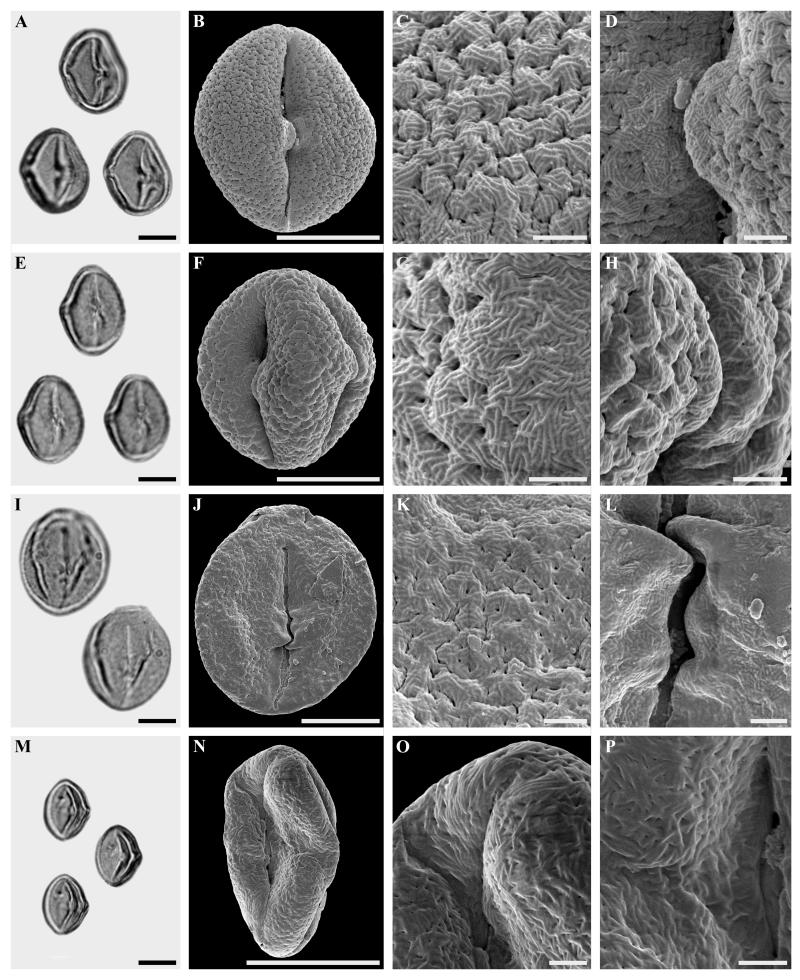
Figure 3.
Fagopsis longifolia and Castaneoideae gen. et sp. indet., sp. 1. A-L. Dispersed Fagopsis longifolia pollen.
A-D. LM and SEM micrographs from the same pollen grain. A. LM, overview. B. SEM, overview. C-D. SEM, details.
E-H. LM and SEM micrographs from the same pollen grain. E. LM, overview. F. SEM, overview. G-H. SEM, details.
I-L. LM and SEM micrographs from the same pollen grain. I. Pollen in equatorial view, LM. J. Pollen in equatorial view, SEM. K-L. Pollen details, SEM.
M-P Castaneoideae gen. et sp. indet., sp. 1. LM and SEM micrographs from the same pollen grain. M. LM, overview. N. SEM, overview. O-P. SEM, details.
Scale bars = 10 μm in Figs A, B, E, F, I, J, M and N. 1 μm in Figs. C, D, G, H, K, L, O and P
Description
Pollen, monad, shape prolate to spheroidal, elliptic in equatorial view; in situ pollen: polar axis 22-27 μm (LM), 19-22 μm (SEM) [18-20 μm according to Manchester and Crane, 1983], equatorial diameter 19-21 (LM), 17-20 μm (SEM); dispersed pollen: polar axis 22-28 μm (LM), 21-25 (SEM) equatorial diameter 19-26 μm (LM), 17-25 μm (SEM); eutectate, exine 1-1.5 μm thick (LM), tricolporate, bridge present (Fig. 2 I-K, M, N, P. 3 A, B, D-F, H-J, L), colpus length 18-22 μm (SEM); sculpturing scabrate (LM), micro-rugulate (to rarely rugulate), perforate-fossulate in non-apertural region (SEM), several parallel running micro-rugulae forming larger rugulae (vermiculate pattern), 0.5-≤1 μm long and wide (Fig. 2 F-H, K, L, O, P, 3 C, D, G, H, K; secondary-striate sculpture according to Praglowski, 1984); micro-rugulae (“striae”) connected by short perpendicular elements (Fig. 2 H, 3 C), in some of the dispersed pollen grains no such connecting elements visible between micro-rugulae (Fig. 2 K, L, O, P); sculpturing in apertural region and bridge without fossulae, perforations can be present (Fig. 2 J, K); abundance: common.
Remarks
The extinct fagaceous genus Fagopsis is known from the early Eocene Republic flora of Washington (Wolfe and Wehr, 1987), the late Eocene Florissant Formation of Colorado (MacGinitie, 1953; Manchester and Crane, 1983), and the Oligocene Ruby River Basin flora of Montana (Becker, 1961). Manchester and Crane (1983) described attached leaves, inflorescences and fruits of Fagopsis longifolia. From staminate inflorescences, pollen was described and figured, but only to a level of magnification that did not reveal its fine ornamentation. Manchester and Crane (1983) suggested that the pollen of Fagopsis is most similar to Quercus among modern Fagaceae. The present study shows that the tectum sculpturing is consistent with the one found in extant species of Castaneoideae. The micro-sculpturing in Fagopsis is not detectable at lower magnification (2000-5000 x), but is crucial for fingerprinting dispersed pollen of this type. This type of micro-sculpturing is present in some extant and Quaternary Castanopsis pollen (Miyoshi, 1983; Praglowski, 1984). In Castanopsis, rugulate pollen typical of Castaneoideae is found in most extant species, while a few species, Castanopsis cuspidata (Thunberg) Schottky and C. sieboldii (Makino) Hatusima have micro-rugulae forming larger rugulae (cf. Praglowski, 1984, fig. 4, C) as seen in Fagopsis. Moreover, the same type of short perpendicular elements connecting the micro-rugulae in pollen of Fagopsis, have been reported in pollen of Castanopsis cuspidata from modern plants and from Quaternary sediments (Miyoshi, 1983). In fully mature dispersed pollen, secondary sporopollenin can be masking the connecting elements (cf. Rowley, 1996).
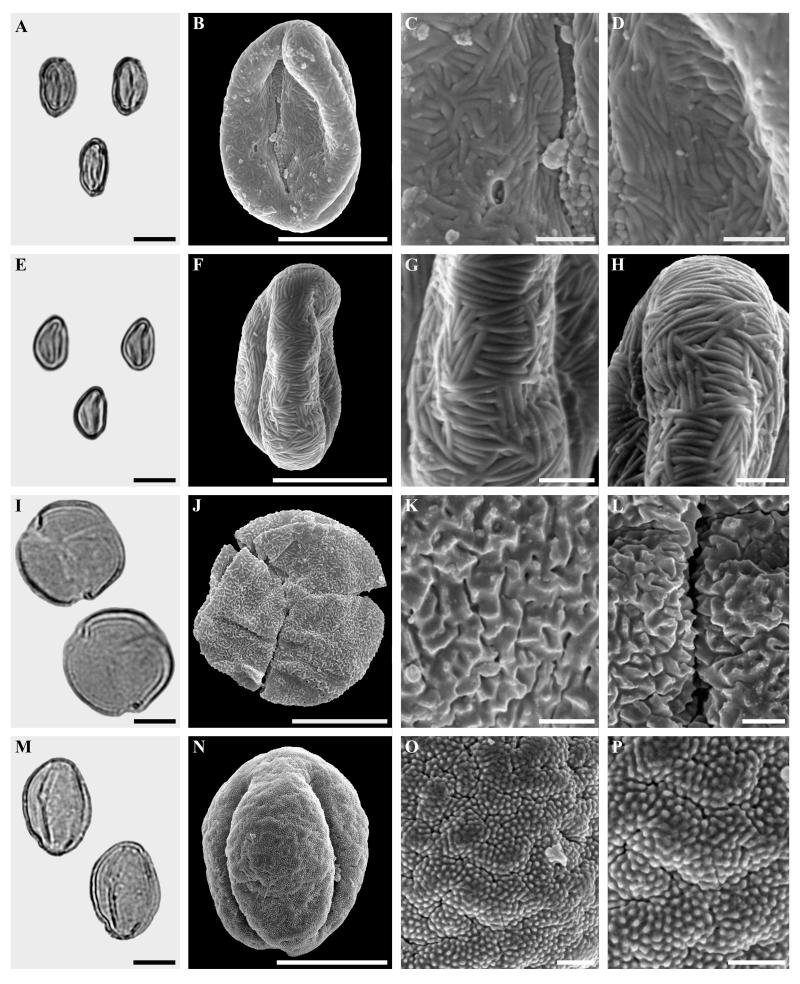
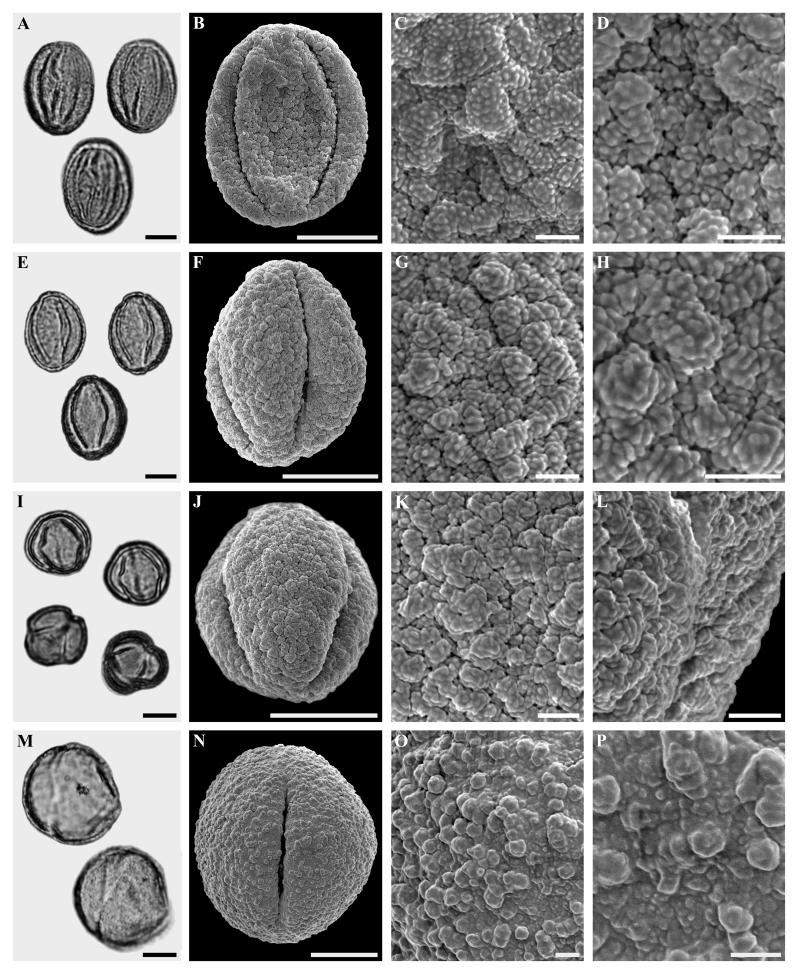
Figure 4.
Castaneoideae gen. et sp. indet., spp. 2 and 3, Quercus Group Cyclobalanopsis sp. and Quercus Group Protobalanus sp.
A-D. Castaneoideae gen. et sp. indet., sp. 2. LM and SEM micrographs from the same pollen grain. A. LM, overview. B. SEM, overview. C-D. SEM, details.
E-H. Castaneoideae gen. et sp. indet., sp. 3. LM and SEM micrographs from the same pollen grain. E. LM, overview. F. SEM, overview. G-H. SEM, details.
I-L Quercus Group Cyclobalanopsis sp., LM and SEM micrographs from the same pollen grain. I. LM, overview. J. SEM, overview. K-L. SEM, detail.
M-P. Quercus Group Protobalanus sp., LM and SEM micrographs from the same pollen grain. M. LM, overview. J. SEM, overview. K-L. SEM, detail.
Scale bars = 10 μm in Figs A, E, I, J, M and N. 5 μm in Figs. B and F. 1 μm in Figs. C, D, G, H, K, L, O and P
Also pollen ultrastructure of Fagopsis corresponds to the pattern seen in Castaneoideae. The foot layer in genera of Castaneoideae is homogeneous, relatively thick and the ratio foot layer to tectum is about 1. Manchester and Crane (1983) reported a footlayer:tectum ratio of 1.3 for Fagopsis.
Subfamily
Castaneoideae (paraphyletic)
Species
Castaneoideae gen. et sp. indet., sp. 1, aff. Castanopsis (Fig. 3 M-P)
Description
Pollen, monad, shape prolate, elliptic in equatorial view, polar axis 14-16 μm (LM), 12-14 μm (SEM), equatorial diameter 9-11 μm (LM), 7.5-9 μm (SEM); eutectate, exine 1 μm thick (LM), tricolporate, colpus length 6-8 μm long (SEM); sculpturing scabrate (LM), micro-rugulate-striate, indistinctly perforate-fossulate (SEM), rugulae 0.1-0.2 μm wide, micro-rugulae well developed, micro-rugulae show parallel running micro-striate suprasculpturing in polar areas (SEM); abundance: rare.
Species
Castaneoideae gen. et sp. indet., sp. 2, aff. Castanea (Fig. 4 A-D)
Description
Pollen, monad, shape prolate, elliptic in equatorial view, polar axis 14-16 μm (LM), 12-14 μm (SEM), equatorial diameter 9-11 μm (LM), 7.5-9 μm (SEM); eutectate, exine 1 μm thick (LM), tricolporate, colpus length 6-8 μm long (SEM); sculpturing scabrate (LM), micro-rugulatestriate, indistinctly perforate-fossulate (SEM), rugulae 0.1-0.2 μm wide, micro-rugulae well developed in equatorial area, most distinct in apertural region, in polar areas micro-rugulae are coarser and their boundaries masked by sporopollenin resulting in a weak relief (SEM); abundance: rare.
Remarks
Pollen of genera of the Castanoideae are markedly uniform and difficult if not impossible to distinguish in LM (Praglowski, 1984). Also in SEM pollen morphological variability overlaps to a large degree between different genera. Castanea differs from Lithocarpus and Castanopsis by the weak rugulation and almost smooth tectum in the polar regions. Therefore, we tentatively suggest a closer relationship of this pollen type with the genus Castanea. Modern species with very similar pollen are C. dentata (Marshall) Borkhausen and C. floridana (Sargent) Ashe, among others.
Earliest definite castaneoid inflorescences are known from the “middle” Eocene of Tennessee, USA (Crepet and Daghlian, 1980). Pollen produced from these inflorescences and described as Castaneoidea puryearensis Crepet et Daghlian, closely corresponds with the here described pollen. This pollen shows well developed rugulate, perforate-fossulate sculpturing in the mesocolpium, which decreases in the polar areas.
Leaf fossils described as Castanea dolichophylla are known from Florissant but their generic identity is in need of review (Manchester, 2001). Although the asymmetric leaf base, dentition, type and number of secondary veins are also found in Castanea, the strong intersecondary veins of C. dolichophylla are not encountered in extant species of Castanea. Such intersecondary veins are occasionally present in extinct fagaceous foliage of Berryphyllum warmanense J. H. Jones et Dilcher from the Eocene of southeastern USA (Jones and Dilcher, 1988). Similarities are also encountered with the extinct European genus Eotrigonobalanus (Mai and Walther, 1985; Kvaček and Walther, 1989) and with the Oligocene European Castaneophyllum lonchitiforme (Kvaček and Walther, 2010). In addition, the leaf base, intersecondary veins, and type of dentition of C. dolichophylla resemble the extinct Engelhardoideae Oreoroa (Engelhardia) orsbergensis (P. Wessel et Weber) Dilcher et Manchester (Manchester, 1987, fig. 21). Therefore,”Castanea” dolichophylla may represent an extinct taxon in the Fagales.
Species
Castaneoideae gen. et sp. indet., sp. 3, aff. Lithocarpus (Fig. 4 E-H)
Description
Pollen, monad, shape prolate, elliptic in equatorial view, polar axis 11-13 μm (LM), 8-11 μm (SEM), equatorial diameter 8-10 μm (LM), 5-7 μm; eutectate, exine 1.0 μm thick (LM), tricolporate; sculpturing scabrate (LM), micro-rugulate/rugulate, approaching striate (SEM), rugulae 0.1-0.2 μm wide (SEM), groups of several parallel micro-rugulae forming angles of 90° to 45° to each other, “patchwork”-like; in colpus area striae parallel with colpus; abundance: rare.
Remarks
Pollen of the extant Lithocarpus jordanae (Laguna) Rehder and L. kostermansii Soepadmo resembles the fossil pollen by its relatively long micro-rugulae and the patchwork-like appearance of the pollen sculpture (Praglowski, 1984).
Possible fossil acorn cups of Lithocarpus are known from the Eocene Green River Formation, Colorado, USA (MacGinitie, 1969). Manchester (1994) described cupules and nuts of Castanopsis crepetii from the lower Lutetian Nut Beds locality of the Clarno Formation, Oregon. When discussing taxonomic affinities of the fossil species, he clearly stated that the genera Castanopsis and Lithocarpus have overlapping morphological variability and cannot clearly be separated based on the cupule/nut complex. The same is true for pollen morphology.
Genus
Quercus Linné
Species
Quercus Group Cyclobalanopsis sp. (Fig. 4, I-L)
Description
Pollen, monad, circular in polar view, pollen diameter 24-26 μm wide (LM), 21-23 μm (SEM); eutectate, exine 1.0-1.5 μm thick (LM), nexine thinner than sexine, tricolpate to tricolporoidate; sculpturing scabrate (LM), platy verrucate, perforate (SEM); abundance: rare.
Remarks
This type of pollen shows sculpturing corresponding to extant pollen of Quercus glauca, Q. hondae and Q. acuta, figured in Makino et al. (2009), which are part of Quercus Group Cyclobalanopsis (Denk and Grimm, 2010).
From the early Lutetian (ca. 48 Ma) old Nut Beds Locality, Clarno Formation, Manchester (1994) described the oldest cupules and nuts of Quercus Group Cyclobalanopsis. Today the evergreen small trees of Cyclobalanopsis are native from West to East Asia (Menitsky, 2005).
Leaves of Q. scudderi were compared to modern species of Quercus Group Cyclobalanopsis by MacGinitie (1953). The leaf morphology of Q. scudderi does not unambiguously place this fossil taxon within Quercus Group Cyclobalanopsis. Members of this group of oaks commonly have secondary veins that are typically bent towards the apex, secondaries are arranged more densely in the apical part of leaves, and the basal part of the lamina never is dentate.
Species
Quercus Group Protobalanus sp. (Fig. 4, M-P)
Description
Pollen, monad, shape prolate, elliptic in equatorial view, polar axis 20-24 μm (LM), 19-22 μm (SEM), equatorial diameter 17-20 μm (LM), 15-17 μm (SEM); eutectate, exine 1.0-1.5 μm thick (LM), tricolpate; sculpturing scabrate (LM), weakly verrucate, perforate-fossulate (SEM), suprasculpture micro-echinate, the micro-echinae being the apical parts of rodlets; abundance: rare.
Remarks
The weak relief showing verrucae that unite to form a vermiculate pattern separated by fossulae is characteristic of pollen of the few modern members of Quercus Group Protobalanus (Denk and Grimm, 2009a). MacGinitie (1953) described the leaf taxon Quercus predayana and compared it to species of Group Protobalanus (Q. chrysolepis and allies). Although members of white oaks may have very similar leaves, virtually identical leaves are encountered in the modern Q. chrysolepis.
Species
Quercus Group Quercus/Lobatae sp. (Fig. 5, A-L)
Figure 5.
A-H Quercus Group Quercus/Lobatae sp., and Quercus Group Quercus †I-P.
A-D. Quercus Group Quercus/Lobatae sp., LM and SEM micrographs from the same pollen grain. A. LM, overview. B. SEM, overview. C-D. SEM, details.
E-H. Quercus Group Quercus/Lobatae sp., LM and SEM micrographs from the same pollen grain. E. LM, overview. F. SEM, overview. G-H. SEM, details.
I-L. Quercus Group Quercus/Lobatae sp., LM and SEM micrographs from the same pollen grain. I. LM, overview. J. SEM, overview. K-L. SEM, details.
M-P. Quercus Group Quercus, LM and SEM micrographs from the same pollen grain. M. LM, overview. N. SEM, overview. O-P. SEM, details.
Scale bars = 10 μm in Figs A, B, E, F, I, J, M and N. 1 μm in Figs. C, D, G, H, K, L, O and P
Description
Pollen, monad, shape prolate to spheroidal, circular to elliptic in equatorial view, polar axis 20-30 μm (LM), 18-25 (SEM), equatorial diameter 19-24 μm (LM) 17-21 (SEM); eutectate, exine 1.0-1.5 μm thick, nexine thinner than sexine, tricolpate, colpus length 13-22 μm (LM); sculpturing scabrate (LM), rugulate-verrucate, fossulate, perforate (SEM), rugulae and verrucae show a cauliflower-like, micro-echinate suprasculpture (SEM); abundance: common.
Remarks
This pollen belongs to Quercus Group Quercus/Lobatae, the white and red oaks (Denk and Grimm, 2009a, 2010). The pollen shows exine sculpturing corresponding to several extant North American oaks. Red oaks with very similar pollen ornamentation are e.g. Quercus myrtifolia Willdenow, Q. velutina Lamarck, Q. nuttallii E.J.Palmer, and Q. marilandica Muenchhausen (Solomon, 1983b; figs. 6c-d, 7b-d, 13b-d, and 19b-c). North American members of white oaks with this type of pollen ornamentation are Q. virginiana Miller and its varieties Q. virginiana var. minima Sargent and var. geminata (Small) Sargent (Solomon, 1983a; figs. 24b-c, 25a-b, and 26a-b). Among Eurasian white oaks, the East Asian Q. serrata Murray has a highly variable pollen ornamentation that covers the one encountered in Quercus Group Quercus/Lobatae sp. 1 and sp. 2 (Makino et al., 2009).
Species
Quercus Group Quercus sp. (Fig. 5. M-P)
Description
Pollen, monad, shape spheroidal to prolate, circular in equatorial view, circular to elliptic in polar view, polar axis 29-32 μm (LM), 28-30 μm (SEM), equatorial diameter 28-31 μm (LM), 27-29 μm (SEM); eutectate, exine 1.0-1.5 μm thick (LM), nexine thinner than sexine, tricolpate, colpus length 18-20 μm (LM), bridge present (LM and SEM); sculpturing scabrate (LM), micro-verrucate, weakly perforate (SEM), micro-verrucae weakly sculptured, smoothly rounded (SEM); abundance: common.
Remarks
This pollen type is commonly found in Eurasian species of Quercus Group Quercus and differs from the previous type by more clearly defined micro-verrucae, which appear smoother due to more embedded sporopollenin. Sculpturing of this type can be found, for example, in extant pollen of the East Asian Quercus mongolica var. grosseserrata Rehder et Wilson and Q. aliena Blume (Makino et al., 2009), and of western Eurasian white oaks (cf. Denk and Grimm, 2009a; fig. 3A, B).
MacGinitie (1953) reported eight oak species based on foliage, which he included within Quercus Groups Quercus, Lobatae, Protobalanus, and Cyclobalanopsis (Table 1). Some taxa that MacGinitie (1953) compared to modern white oaks or red oaks cannot unambiguously be referred to either the one or the other group (Q. dumosoides, Q. mohavensis). A number of sclerophyllous species among red and white oaks produce similar leaves, in particular when juvenile leaves are considered. Quercus lyratiformis shows similarities with modern deciduous white oaks with deeply lobed leaves, whereas Q. peritula resembles most closely modern sclerophyllous red oaks. Quercus predayana appears to be most similar to modern species of Quercus Group Protobalanus. The remaining three leaf taxa either belong to extinct Fagaceae or are cannot unambiguously be assigned to this family. Furthermore, Q. balaninorum may represent oak foliage but is based on a single fragmentary specimen.
Table 1.
Fagaceae diversity in the latest Eocene of Florissant and potential modern analogues (PMA) of fossil taxa. PMA of previously described macrofossils are partly revised.
|
Pollen Taxon [potential modern analogues] present study |
Macro Fossils [potential modern analogues] MacGinitie (1953) |
Remarks to previously identified macro fossils |
|---|---|---|
| †Fagopsis longifolia Lesquereux [aff. Castaneoideae] | †Fagopsis longifolia Lesquereux [Betulaceae] | Placed into Fagaceae by Manchester and Crane (1983) |
| Castaneoideae gen. et spec. indet., sp. 1 [aff. Castanopsis] | ||
| Castaneoideae gen. et spec. indet., sp. 2 [aff. Castanea] | ||
| Castaneoideae gen. et spec. indet., sp. 3 [aff. Lithocarpus] | ||
| “Castanea” dolichophylla Cockerell [Castanea seguinii Dode Castanea henryi Rehder et Wilson] | Similarities with Castanea, extinct Eotrigonobalanus (Fagaceae), and extinct Oreoroa (Juglandaceae) | |
| Quercus Group Cyclobalanopsis [Q. acuta Thunberg, Q. glauca Thunberg, Q. hondae Makino] | ||
| Quercus Group Protobalanus | ||
| Quercus Group Quercus/Lobatae | ||
| Quercus Group Quercus | ||
| Quercus dumosoides MacGinitie (L) [Q. dumosa Nutall] | Quercus Group Quercus/Lobatae [Q. agrifolia Née; Q. invaginata Trelease; Q. john-tuckeri Nixon et C.H. Muller] | |
| Quercus knowltoniana Cockerell (R) | Genus Quercus | |
| Quercus lyratiformis Cockerell (L) [Q. lyrata Walter, Q. alba L.] | Quercus Group Quercus, deciduous | |
| Quercus mohavensis Axelrod (L) [Q. brandegei Goldman, Q. fusiformis Small] | Quercus Group Quercus/Lobatae [Q. depressa Bonpland; Q. emoryi Torrey; Q. galeanensis C.H.Muller; Q. minima Small; Q. peninsularis Trelease] | |
| Quercus peritula Cockerell [Q. incarnata Trelease, syn. of Q. sideroxyla Bonpland, Q. wislizeni A.DC.] | Quercus Group Lobatae [Q. coahuilensis Nixon et C.H. Muller] | |
| Quercus predayana MacGinitie [Q. wilcoxii Rydberg syn. of Q. chrysolepis Liebmann, Q. palmeri Engelmann] | Quercus Group Protobalanus | |
| Quercus scottii (Lesquereux) MacGinitie | Extinct fagaceous leaf type aff. Eotrigonobalanus, Castaneophyllum lonchitiforme Kvaček et Walther | |
| Quercus scudderi Knowlton [Q. glauca Thunberg, Q. cornea Loureiro syn. of Lithocarpus corneus, Q. myrsinifolia Blume] | Quercus-like Doubtful whether Quercus Group Cyclobalanopsis | |
| Quercus orbata MacGinitie [Q. undulata Torrey-hybrid] | Compared to extinct European “Q.” cruciata A.Braun No Fagaceae |
(L), leaf; (R), reproductive structure
DISCUSSION
Systematic affinity of the extinct genus Fagopsis
The genus Fagopsis has previously been placed into various families (Ulmaceae, Betulaceae, Fagaceae; reviewed in Manchester and Crane, 1983). Manchester and Crane (1983) pointed out the marked differences between the fruiting structures of Fagopsis and extant Fagaceae and compared this to the situation in Juglandaceae, where Platycarya and Juglans have strikingly different fruiting structures, and to Betulaceae, where the same eye-catching differences are seen in Alnus (many, tiny samaras dispersed per infructescence) and Corylus (relatively large nuts, subtended by involucre of bracts). The infructescence of Fagopsis is more reminiscent of the cones of Alnus and Platycarya than of extant Fagaceae and suggests that the structural diversity in reproductive organs is as high in Fagaceae as in Betulaceae and Juglandaceae. The pollen of Fagopsis clearly is of the Castaneoideae type. It has recently been suggested that this pollen type based on its outer morphology and ultra-structure represents the basic type within Fagaceae (Denk and Tekleva, in press). However, the complex vermiform sculpture formed by individual micro-rugulae as seen in Fagopsis and in a few extant taxa of Castanopsis clearly is derived in Castaneoideae. Most of the modern members of Castaneoideae have a simple micro-rugulate pollen sculpturing (Praglowski, 1984; cf. Castaneoideae pollen types 1 to 3 of the present study). Overall, the character combination seen in Fagopsis suggests that it represents an extinct lineage that is distant to all modern and extinct types of Fagaceae.
Systematic affinities of other Fagaceae in Florissant
We found three distinct types of Castaneoideae pollen. Pollen morphology suggests that the three taxa belong to modern genera of Castaneoideae but it is difficult to explicitly ascribe dispersed pollen to particular modern genera. Pollen of modern members of Castaneoideae shows little morphological differentiation even if some genera are conspicuously species-rich (Praglowski, 1984; Govaerts and Frodin, 1998). Based on the observation that certain pollen types are more frequent in particular genera of Castaneoideae we tentatively assigned pollen types to modern genera (Table 1). Pollen of Castaneoideae gen. et spec. indet. 2 (aff. Castanea) in the present study is indistinguishable from in situ pollen of castaneoid staminate inflorescences described from the “middle” Eocene Claiborne Formation, Tennessee (Crepet and Daghlian, 1980). The presence of Castanea-like spiny cupules in the same sediments and of foliage of Castaneophyllum tennesseense (Berry) J.H.Jones et Dilcher further would appear to support the presence of the genus Castanea in the middle Eocene of North America (cf. Manchester, 1999) and during the late Eocene of Florissant. Manchester (1999, p. 482) accepted the middle Eocene record from Tennessee as earliest for the genus Castanea. Nevertheless, Castaneoideae pollen similar to modern species of Castanea and foliage of “Castanea” dolichophylla from the Florissant Fossil Beds cannot unequivocally be attributed to Castanea. The same is true for foliage of “Castanea” from nearly contemporaneous sediments of southwestern Montana (Lielke et al., 2012). Castaneoideae gen. et spec. indet. 1 and 3 resemble particular modern species of Castanopsis and Lithocarpus but pollen morphology cannot unequivocally be used to discriminate between these genera and Castanea. Nevertheless, the pollen record demonstrates that Castaneoideae, although rare or growing at far distance from the lake, were diverse in Florissant. Virtually identical pollen of a general Castaneoideae type can be traced back until the early Late Cretaceous (early Coniacian) attached to flowers of fagalean affinity, described as Archaefagacea, which are among the earliest records of Fagaceae (Takahashi et al., 2008). This demonstrates how morphologically conserved pollen of Castaneoideae is. As outlined in the previous section, pollen of Fagopsis also displays closest similarities with extant pollen of Castaneoideae, but the pollen of this extinct lineage is more derived within Castaneoideae.
From Florissant, nine different Quercus species have been distinguished based mainly on leaf fossils and in a single case on reproductive structures (MacGinitie, 1953; Manchester, 2001; Table 1), and quercoid pollen has been reported but without further differentiation (Leopold and Clay-Poole, 2001; Wingate and Nichols, 2001). Leaf fossils are mostly sclerophyllous except for Q. lyratiformis which is a lobed, deciduous type of oak strongly resembling species of Quercus Group Quercus. Among the remaining leaf species, Q. peritula shows closest similarities with modern members of Quercus Group Lobatae, and Q. predayana matches leaves of modern species of Group Protobalanus. Quercus dumosoides and Q. mohavensis resemble modern members of both Group Quercus and Group Lobatae. In addition, two species, Q. scottii and Q. scudderi cannot unambiguously be assigned to Quercus and probably represent extinct lineages of Fagaceae. Quercus orbata was compared by MacGinitie to the European fossil species Quercus cruciata A.Braun, which was later transferred to the genus Pungiphyllum with unknown botanical affinities (Kvaček and Walther, 1981). The pollen types of Quercus partly complement the macro fossil record. Quercus Group Quercus, Cyclobalanopsis, Protobalanus and Lobatae/Quercus are unambiguously recorded in the palynological record and white and/or red oak pollen is fairly common in the counted pollen samples, while pollen of Group Cyclobalanopsis and Group Protobalanus is very rare. In contrast to Castaneoideae, pollen of Quercus is highly diagnostic at the intrageneric level.
Inferred paleovegetation of Florissant
The plant taxa recovered from the Florissant Beds are strongly indicative of four major forest/vegetation types: (1) Sclerophyllous forest (Mediterranean chaparral of the Californian/Mexican type) with extensions to tropical and subtropical dry woodlands, (2) nemoral coniferous forest, (3) laurel forest, and (4) broad-leaved deciduous forest according to Schroeder (1998; see table 23 in Velitzelos et al., 2014; Appendix S1). In addition, the fossil plant assemblage includes riparian/aquatic elements and lianas (Table 2, Appendix S1). Of 155 taxa/pollen types recovered from the Florissant Beds, 52 are at present found in summer dry Mediterranean sclerophyllous forests and woodlands extending to BS and Cw climates with summer rain; of these, 16 are not common in any other of the four vegetation types (e.g. Athyana, Cercocarpus, Schmaltzia [=Rhus sect. Lobadium], Vauquelinia). Typically, the sclerophyllous oaks of Group Protobalanus, Quercus and Lobatae fall within this vegetation type; they are well represented by foliage and pollen.
Table 2.
Total plant diversity in the latest Eocene of Florissant including an updated palynological record (Bouchal, 2013; this study).
| Class/Clade/Family | Genus | Macro Fossils |
Pollen | Source | Remarks |
|---|---|---|---|---|---|
| Ginkgopsida | |||||
| Ginkgoaceae | Ginkgo | x | 5 | ||
| Coniferopsida incl. Gnetales | |||||
| Cupressaceae | Chamaecyparis | C, L | 3 | ||
| Sequoia | C, B, W | x | 1, 2, 3, 5 | ||
| Taxaceae | Torreya | L | 3 | ||
| Pinaceae | Abies | S | x | 1, 2, 3, 5 | |
| Cathaya | x | 5 | |||
| Picea | S | x | 1, 2, 3, 5 | ||
| Pinus sect. Strobus | C, S, L | x | 1, 2, 3, 5 | 2 spp. (MF) | |
| Pinus sect. Pinus | C, S, L | x | 1, 2, 3 | 3 spp. (MF) | |
| Tsuga | x | 1, 2, 5 | |||
| Ephedraceae | Ephedra | B | x | 1, 2, 3, 5 | 2 types (P) |
| Angiosperms | |||||
| Nymphaceae | x | 1, 2 | |||
| Magnoliids | |||||
| Lauraceae | L | 3 | 2 spp. (MF) | ||
| Monocots | |||||
| Potamogetonaceae | Potamogeton | L?, Fr | 3 | ||
| Dioscoreaceae | Dioscorea | Fr | 3 | ||
| Liliaceae | x | 1, 2 | |||
| Smilacaceae | Smilax? | L | 3 | ||
| Commelinids | |||||
| Arecaceae | L | x | 1, 2, 3, 5 | ||
| Cyperaceae | Cyperacites | L | x | 2, 3 | |
| Poaceae | x | 1, 2 | |||
| Stipa | Fr | x | 3, 5 | ||
| Typhaceae | Sparganium | x | 1, 2, 5 | ||
| Typha | L | x | 1, 2, 3, 5 | ||
| Eudicots | |||||
| Buxaceae | x | 2, 5 | |||
| Platanaceae | Platanus | L | x | 1, 2?, 3, 5 | |
| Menispermaceae | x | 5 | |||
| Berberidaceae | Mahonia | L | 1, 3 | 2-3 spp. (MF) | |
| Ranunculaceae | x | 2?, 5 | |||
| Trochodendraceae | Tetracentron | x | 5 | ||
| Core Eudicots | |||||
| Amaranthaceae | x | 1, 2, 5 | 3-4 types (P) | ||
| Cercidiphyllaceae | x | 2, 5 | |||
| Grossulariaceae | Ribes | L | 3 | 6 | |
| Hamamelidaceae | x | 2?, 3?, 5 | |||
| Polygonaceae | x | 2 | |||
| Rosids | |||||
| Vitaceae | x | 1, 5 | 2 types (P) | ||
| Vitis | L | x | 3 | ||
| Eurosids | |||||
| Fabaceae | indet. | Ll | x | 1, 2, 3 | 4 spp. (MF) |
| Caesalpinia | Ll | x | 2, 3? | ||
| Cercis | L, Fr | 3 | |||
| ?Prosopis | L | 3 | |||
| Robinia | L, W | 3?, 4 | |||
| Vicia | L | 3 | |||
| Betulaceae | Paracarpinus/Asterocarpinus † | Fr, L | x | 1, 3 | |
| Alnus | x | 2 | |||
| Betula | x | 1, 5 | |||
| Ostrya/Carpinus | x | 1 | |||
| Fagaceae | Castaneoideae | L? | x | 1, 2, 3, 5, 6 | 3 types (P) |
| aFagopsis † | L, Fl, Fr | x | 3, 6 | ||
| Quercus | L, Fr | x | 1, 2, 3, 5, 6 | 8 spp. (MF) 4 types (P) |
|
| Juglandaceae | Carya | L, Ll, Fl, Fr | x | 1, 2, 3, 5 | |
| Cyclocarya | x | 1 | |||
| Engelhardoideae | 1, 2, 5 | 2 types. (P) | |||
| Juglans | S? | x | 1, 2, 3, 5 | ||
| Rhoipteleaceae | x | 1, 2 | |||
| Salicaceae | Populus | L, Fr | 3 | ||
| Salix | L | x | 1, 2, 3, 5 | 4 spp. (MF) | |
| Euphorbiaceae | L | x | 1, 2, 3, 5 | ||
| Elaeagnaceae | x | 1, 2 | |||
| Cannabaceae | Humulus | L | 3 | ||
| Moraceae | Morus | L | 3 | ||
| Rosaceae | x | 1, 2, 5 | 4 types (P) | ||
| Amelanchier | L | 3 | |||
| Cerocarpus | L, Fr | 3 | |||
| Crataegus | L, Fr | x | 2, 3, 5 | 3 spp.(MF) | |
| Holodiscus | L | 3 | |||
| Malus | L | 3 | |||
| Rosa | L, Ll | 3 | |||
| Rubus | L | 3 | |||
| Vauquelin[i]a | L | 3 | 2 spp. (MF) | ||
| Ulmaceae | W | x | 1, 2, 4, 5 | ||
| Celtis | L | 1, 3 | |||
| Cedrelospermum † | L, S | x | 3, 5 | ||
| Ulmus | L, F | x | 1, 2, 3, 5 | ||
| Zelkova-type | x | 1 | |||
| Malvids | |||||
| Malvaceae | indet. | x | 1, 2, 5 | at least 6 types (P) | |
| Florissantia † | Fl, Fr | x | 1, 2, 3, 5 | ||
| Tilia? | L | x | 2, 3 | ||
| Thymeleaceae | indet. | x | 5 | ||
| Daphne | L | x | 2, 3, 5 | ||
| Onagraceae | indet. | Fl | x | 1, 2, 3, 5 | 2 types (P) |
| Sapindaceae | Acer | L, Fr | x | 1, 2, 3, 5 | 3 spp. (MF) 3 types (P) |
| Athyana | L | x | 3 | ||
| Dipteronia | L, Fr | 1, 3 | |||
| Koelreuteria | L?, Fr, W | 1, 3, 4 | |||
| Simaroubaceae | Ailanthus | Fr | x | 2, 3 | |
| Chaneya tenuis † | Fr | 3 | |||
| Meliaceae | Cedrela or Toona | S | x? | 2, 3 | |
| Anacardiaceae | L | x | 5 | ||
| Cotinus | L | 3 | |||
| Rhus | L | 1, 3 | 3 spp. (MF) | ||
| Schmaltzia [=Rhus sect. Lobadium] | L | 3 | |||
| Rutaceae | S? | x | 1, 3, 5 | ||
| Asterids | |||||
| Cornaceae | Alangium | x | 5 | ||
| Hydrangeaceae | L, Fl | 3 | |||
| Ebenaceae | Diospyros | x | 5 | ||
| Ericaceae | x | 1, 2, 5 | |||
| Euasterids I (Lamiids) | |||||
| Apocynaceae | S | 1, 3 | |||
| Tabernaemontana | x | 1, 5 | |||
| Eucommiaceae | Eucommia | Fr | x | 1, 2, 3, 5 | |
| Oleaceae | Osmanthus? | L, Fr | x | 1, 3 | |
| Fraxinus | x | 2, 5 | |||
| Euasterids II (Campanulids) | |||||
| Asteraceae | x | 1, 2, 5 | |||
| Adoxaceae | Sambucus | L, Ll | x | 1, 2, 3 | |
| Viburnum | x | 1, 5 | |||
| Caprifoliaceae | Diplodipelta † | Fr | 3 | ||
| Lonicera | x | 2, 5 | |||
| Araliaceae | x? | 2 | |||
| Oreopanax | L | 3 | |||
The wood taxon Chadronoxylon possibly belongs to the plant that produced foliage and reproductive structures of Fagopsis (Meyer, 2003).
Macro fossils: L-leaf, Ll-leaflet, B-branch, C-cone, Fr-fruit, S-seed, W-wood, Fl-flower.
Sources: 1-Leopold and Clay-Poole (2001) and Leopold et al. (2008, Table 1); 2-Wingate and Nichols (2001); 3-Manchester (2001); 4-Wheeler (2001); 5-Bouchal (2013); 6-present study.
= extinct; Remarks: MF-macro fossils, P-pollen.
Mediterranean sclerophyllous forests are closely connected to nemoral coniferous forests and replaced by them at higher elevations and at higher latitudes. Several taxa encountered from Florissant, therefore, are not confined to sclerophyllous forests but are also thriving in nemoral coniferous forests, often in the second tree layer (e.g. some Castaneoideae [Notholithocarpus], Quercus Group Protobalanus, Quercus/Lobatae). Fifty-five taxa belong to genera that occur in nemoral coniferous forests, of which six are restricted to this forests type (Appendix S1). Naturally, most of the conifer taxa recorded for Florissant are characteristic elements of nemoral coniferous forests at present (Tsuga, Chamaecyparis, Sequoia, Torreya). Of these, Chamaecyparis, Sequoia, and Torreya probably had a wide ecological amplitude. Today, they are typical element of the Pacific Coast Ranges. Torreya californica Torrey grows scattered along mountain streams and in moist canyons, commonly co-occurring with Sequoia, Acer spp., Platanus and Alnus. Furthermore, it occurs in coastal chaparral. Its altitudinal range is from near sea level to 2500 m (Farjon, 2013). Chamaecyparis lawsoniana (A. Murray bis) Parlatore has a similar range but is at present absent at higher elevations (0 to 1500 m; Flora of North America Editorial Committee, 1993).
The succession from Mediterranean vegetation to nemoral conifer forest encountered in the fossil plant assemblage of Florissant matches perfectly the modern situation in the Mediterranean climate of California. Major constituents of these vegetation types are well represented in modern-day California and the Eocene Florissant flora, despite the considerable geographic distance between the Coastal Ranges and the Front Range.
In addition, a relatively high number of taxa recorded from Florissant are elements of fully humid laurel forests (24 taxa, of which four are confined to laurel forests; Appendix S1). Today, comparable vegetation is found in the northern parts of the Coastal Ranges. Eocene taxa typical of this vegetation type introduce an exotic element to the fossil flora of Florissant, represented by the East Asian Rhoipteleaceae, Cyclocarya, Tetracentron, Eucommia, Cercidiphyllaceae), and possibly by some of the Castaneoideae type pollen taxa and undetermined fagaceous foliage. Humid environments are also indicated by the conifer Cathaya, another East Asian element with a wide northern hemispheric distribution throughout the Cenozoic (e.g Liu and Basinger, 2000; Grímsson and Zetter, 2012), and by Torreya (see above). Elements of laurel forests are commonly also found in broad-leaved deciduous forests (Appendix S1).
A relatively large number of riparian and aquatic elements were associated with the Florissant lake and rivers and torrents flowing into the lake. Most prominent riparian element of the Florissant paleoflora are the extinct Fagaceae Fagopsis based on the abundant occurrence of leafy branches with attached staminate and pistillate inflorescences and fruits, and Cedrelospermum (Manchester and Crane, 1984; Manchester, 2001). Other riparian elements are Ginkgo, Ailanthus, Alnus, Fraxinus, Populus, and others.
From the present, revised taxon list of Florissant (Bouchal, 2013; Table 2, Appendix S1) it appears that several of the taxa typical of and confined to laurel forest, nemoral conifer forest and broadleaved deciduous forests did not grow in close vicinity of the paleo-lake because they are represented by pollen only. In contrast, taxa resembling modern Mediterranean species are commonly represented by foliage and fruits (Athyana, Cercocarpus, Quercus spp.) and may have grown on slopes facing the lake, while laurel forest and broad-leaved deciduous forest may have grown higher up or in micro-climatically humid areas (humid valleys and ravines, aspect-wise humid slopes). The same may have applied to moisture-loving plants such as the conifers Cathaya and Torreya. This mosaic of dry and moist forest vegetation would have been followed by nemoral conifer forest with an admixture of evergreen oaks and broad-leaved deciduous elements. The western slopes of the central Cascades, Oregon, support vegetation comprising Tsuga, Taxus, Acer, Mahonia nervosa which can serve as a modern analogue to the plant assemblage from Florissant. These forests grow under a mild Mediterranean climate with cyclonic winter rains plus considerable amounts of humidity from fog precipitation (Dawson, 1998).
The inferred vegetation types are indicative of complex landscapes reflecting different vertical vegetation belts, and different aspects of slopes and canyons in an intermontane setting. According to Meyer (2003) the succession from the valley floor to the slopes of the surrounding volcano involved riparian vegetation and groves of Sequoia and Chamaecyparis and deciduous trees, followed by dry vegetation and upland coniferous forests.
Earlier concepts about paleoecology and climate of Florissant
Leopold and Clay-Poole (2001) suggested close similarities with the modern vertical vegetation zonation in Tamaulipas, Mexico (El Cielo biosphere; Hernández et al., 1951). The isolated occurrence of humid temperate vegetation in Tamaulipas occurs in a montane setting situated above the winter-dry tropical lowlands and receiving plenty of humidity from the Gulf of Mexico during the growing season. Relict stands of Fagus and Liquidambar among others occur in a warm temperate, winter-dry to fully humid climate. Fagus was reported from Florissant by Leopold and Clay-Poole (2001) but not by Leopold et al. (2008), and the presence of Fagus could not be confirmed by the present study. Liquidambar has never been reported from Florissant. This suggests that the coastal range of northeastern Mexico may not be a suitable analogue to the situation in Florissant.
DeVore and Pigg (2010, p. 114) interpreted the Florissant fossil assemblage as “savanna – woodland, tropical – dry”. The significant taxa listed by these authors are Fagopsis, Cedrelospermum, Florissantia, and Rosaceae. It is unclear how they arrived at the paleoecological interpretation based on this taxon set and the presented description of the Florissant flora (DeVore and Pigg, 2010, pp. 120, 121).
In contrast, Lielke et al. (2012) suggested a pronounced summer drying trend (Mediterranean climate) for the Eocene-Oligocene floras of southwestern Montana and Florissant. Correctly emphasizing the presence of xeric woodland elements comprising Quercus spp., Cercocarpus, and Mahonia in the Eocene-Oligocene Ruby Flora of southwestern Montana along with dry conifer woodland elements of leeward slopes of mountains these authors inferred a “highly seasonal, summer dry climate” (Lielke et al., 2012, p. 345) for the Northern Rocky Mountains and extending further south to Florissant.
Paleoclimatic setting, origin of the modern Mediterranean flora of western North America
The paleovegetation of Florissant is in conflict with traditional views that ancestors of the modern western North American sclerophyllous flora evolved in summer wet climates (Axelrod, 1973; Leopold and Clay-Poole, 2001). Based on the modern distribution of regional climates when moving from the southeastern to the southwestern USA (Appendix S2) and assuming topographic barriers similar to today during the late Eocene, it is likely that the climate in the Florissant valley was a boundary climate between Cs and BS (see Appendix S2, climate station Pocatello and Salt Lake City). From the tectonic history (Bryant et al., 1981; Mix et al., 2011; Frisch et al., 2011) it can be assumed that climatic conditions in the Front Range at the Eocene-Oligocene boundary were as complex as today. Today, summer rain decreases from Atlantic North America to the west. Therefore, the fully humid climates of the east (Cfa climate according to Köppen; Kottek et al., 2006) are replaced by dry climates with summer rain in the Great Plains (BS climates). Western North America receives cyclonic rains during the winter months and is characterized by a summer dry Mediterranean climate (Cs climates). To the north and at higher elevations, snow climates occur (Df climates). Because of complex north south running mountain ranges, the Rocky Mountains show a great variety of local climates (Lieth et al., 1999; Appendix S2). These include both summer rain and winter rain climates and it is well possible that, locally, Mediterranean climates were established during the Eocene-Oligocene boundary also in the eastern Rocky Mountains (Montana, Front Range). Comparable complex climate patterns are encountered on the southern foothills of the Hindu Kush Mountains and the lower Himalayas of Kashmir (Schroeder, 1998, p. 362; Menitsky, 2005, p. 360; Kottek et al., 2006) where fully humid (Cf), summer dry (Cs), and winter dry (Cw) climates co-occur as complex mosaic. Here, sclerophyllous oaks and Q. glauca (Quercus Group Cyclobalanopsis) co-occur (600 to 1800 m), and higher up, sclerophyllous oaks are part of nemoral conifer forests (to > 3500 m).
Convergent evolution of sclerophyllous leaf traits, early adaptive radiation in Quercus in the Paleogene of the Northern Hemisphere
Eocene floras in western North America comprise sclerophyllous fagaceous leaves and pollen that can unambiguously be assigned to modern groups of Quercus (e.g. Axelrod, 1966; MacGinitie, 1953, 1969; present study). In contrast, lobed, deciduous foliage of Quercus occurred in the middle Eocene floras of Arctic North America (McIver and Basinger, 1999). Eocene and early Oligocene floras of Europe and East Asia are dominated by extinct members of Fagaceae (Eotrigonobalanus and castanoids) which closely resemble North American (extinct) taxa, but these floras essentially lack modern genera of Fagaceae (Tanai and Takahashi, 1994; Tanai, 1995; Denk et al., 2012). Sclerophyllous oaks resembling the modern Mediterranean members of Quercus Group Ilex are not known in western Eurasia before the late early Oligocene (pollen) and the early Miocene (leaves; Mai, 1995; Denk et al., 2012) and appear to be advanced within a larger group of Eurasian sclerophyllous oaks, most of which are found in fully humid temperate Cfa climates and in winter-dry monsoon climates (Cw climates; Menitsky, 2005). In general, there is no paleobotanical evidence for summer dry, Mediterranean conditions in the Mediterranean region prior to the Pleistocene (Suc, 1984; Velitzelos et al., 2014) although many lineages comprising Mediterranean elements are phylogenetically old (e.g. Smilax aspera L., Qi et al., 2013; Quercus Group Ilex, Denk and Grimm, 2009, 2010).
A contrasting picture emerges for the late Eocene of western North America. The presence of sclerophyllous oaks belonging to two to three infrageneric groups of Quercus, along with taxa belonging to genera that are today confined to seasonally dry climates of Pacific North America (summer dry Cs climates and winter dry BS climates) suggest that these lineages might have originated as early as during Eocene times in a climatic setting similar to the modern one in the southwestern U.S. For example, members of Quercus Group Protobalanus are at present confined to southwestern USA and NW Mexico (Manos, 1997) where they occur from ca. 200 to 2800 m. The presence of pollen and foliage in Florissant suggests that they were established in western North America by the late Eocene. Phylogenetically, Quercus Group Protobalanus is an old group, representing the ancestral lineage in one of two major clades of Quercus (Manos et al., 2001; Denk and Grimm, 2009, 2010). Cercocarpus (Rosaceae) foliage and fruits from Florissant are closely similar to the modern Cercocarpus montanus Rafinesque species complex (southwestern USA; Manchester, 2001) and the genus is basal within all modern Spiroideae (van den Heuvel, 2002; Potter et al., 2007). Another Rosaceae, Vauquelinia with two modern species in southwestern USA and northwestern Mexico, forms an early diverging branch within subtribe Pyrinae (Campbell et al., 2007). Similarly, Schmaltzia (syn. Rhus sect. Lobadium, Anacardiaceae) with a modern distribution in southwestern USA, Mexico and northern Central America is basal within one of two major clades of the genus Rhus (Yi et al., 2004). In a wider biogeographic context, Athyana, a monotypic genus of Sapindaceae, forms an early diverging branch within the Paullinia group of Sapindoideae (Buerki et al., 2009). At present, Athyana occurs from Peru to Argentina in seasonal BS, Cw, and Cf climates.
Two evolutionary patterns are evident from the comparison of the fossil records of Fagaceae sclerophyllous foliage in the Old World and the New World. In western Eurasia and East Asia sclerophyllous Fagaceae are found among Quercus Group Ilex and Group Cerris. Mediterranean representatives of these groups are derived. In the New World, sclerophyllous Fagaceae are found in Quercus Group Protobalanus, Group Quercus, and Group Lobatae and are taxonomically and phylogenetically unrelated to their ecological counterparts in the Old World. This is an example of convergent evolution. Second, Axelrod (1975, p.280) assumed that “by the middle Eocene broadleaved evergreen sclerophyllous taxa occupied a subhumid belt across much of North America-Eurasia”; he termed this vegetation belt “Madrean-Tethyan sclerophyll vegetation”. Phylogenetic relationships of the constituents of this ancient vegetation clearly show that the evolutionary patterns seen in the Eurasian and North American parts of this vegetation belt were entirely unrelated and that climate conditions in Eurasia were fully humid throughout most of the Paleogene (laurel forests with extinct Fagaceae and Lauraceae; see, e.g. Mai, 1995) in contrast to the situation in western North America (appearance of modern Mediterranean lineages under markedly seasonal climates; Lielke et al., 2012). In the mountains of western North America, climatic differences owing to strong topographic relief and slope aspect might have caused high niche diversity and triggered ecological radiation in Fagaceae and other plant lineages during the Paleogene. The major climate types (Cs, Bs, Df) and vegetation types were essentially the same in the Paleogene as today. Old lineages (Quercus Group Protobalanus) occur from 200 to 2800 m in different altitudinal vegetation belts. The same is true for Notholithocarpus.
Conclusions
The Florissant paleoflora represents the earliest record of proto-Mediterranean vegetation, a vegetation adapted to seasonal drought, in North America. Thus, it challenges the traditional view by Axelrod (1973, 1975) that “sclerophyllous plants that now typify the area [i.e. California, Pacific Coast Range] are survivors of a richer flora that persisted here as summer rainfall gradually disappeared in the late Cenozoic.” In contrast, this area must have been colonized by drought-tolerant vegetation, already in place in intermontane basins and valleys of western North America, after tropical, lowland vegetation retreated. The modern-day Pacific coast region functions as a refuge for a numbers of ancestral lineages such as Quercus Group Protobalanus, Notholithocarpus, Torreya, and Calocedrus, which are typical elements of the Chaparral–nemoral conifer forest successions. This demonstrates the fundamental difference between “Mediterranean” vegetation in western North America and western Eurasia. In western Eurasia, the ecological shift from fully humid to summer-dry environments is well documented in a number of plant lineages including Quercus (Mai, 1995; Denk et al., 2014; Velitzelos et al., 2014).
Supplementary Material
Appendix S1: Taxa/plant organs reported from Florissant and inferred vegetation types. Med/Chap Cal/Mex = Mediterranean, chaparral; California, Mexico.
Appendix S2: Distribution of climate types along an east west transect from the Midwest to the Central Rocky Mountains. Representative climate diagrams illustrate the change from fully humid summer-rain climates (Cfa) to dry summer-rain climates (BS) to dry winter-rain climates (Mediterranean variant of BS climates) to Mediterranean climates (Csa).
Köppen climate types from Kottek et al. (2006) using a kmz file (http://koeppen-geiger.vuwien.ac.at/data/Koeppen-Geiger-GE.kmz) for Google Earth. Climate diagrams from Lieth et al. (1999).
Acknowledgments
The authors thank Diane Erwin, Berkeley, and Steven Manchester, Gainesville for providing material. This work was supported by a grant of the Swedish Research Council to TD and by a FWF grant to FG. Guido Grimm is thanked for helpful comments on the manuscript.
LITERATURE CITED
- Axelrod DI. The Eocene Copper Basin flora of northeastern Nevada. University of California Publications Geological Sciences. 1966;59:1–125. [Google Scholar]
- Axelrod DI. History of the Mediterranean ecosystem in California. In: Castri F, Mooney H, editors. Mediterranean Type Ecosystems. Springer; Berlin, Heidelberg: 1973. pp. 225–277. [Google Scholar]
- Axelrod DI. Evolution and biogeography of Madrean-Tethyan sclerophyll vegetation. Annals of the Missouri Botanical Garden. 1975;62:280–334. [Google Scholar]
- Becker HF. Oligocene Plants from the Upper Ruby River Basin, Southwestern Montana. Geological Society of America Memoirs. 1961;82:1–122. [Google Scholar]
- Bouchal JM. Master Thesis. University of Vienna; Austria: 2013. The microflora of the uppermost Eocene (Priabonian) Florissant Formation, a combined method approach.http://othes.univie.ac.at/27541/ [Google Scholar]
- Bryant B, Marvin RF, Naeser CW, Mehnert HH. Ages of the igneous rocks in the South-Park-Breckenridge region, Colorado, and their relation to the tectonic history of the Front Range uplift. Geological Survey Professional Paper. 1981;1199 A-E:15–35. [Google Scholar]
- Buerki S, Forest F, Acevedo-Rodríguez P, Callmander MW, Nylander JAA, Harrington M, Sanmartín I, Küpfer P, Alvarez N. Plastid and nuclear DNA markers reveal intricate relationships at subfamilial and tribal levels in the soapberry family (Sapindaceae) Molecular Phylogenetics and Evolution. 2009;51:238–258. doi: 10.1016/j.ympev.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Campbell CS, Evans RC, Morgan DR, Dickinson TA, Arsenault MP. Phylogeny of subtribe Pyrinae (formerly the Maloideae, Rosaceae): limited resolution of a complex evolutionary history. Plant Systematics and Evolution. 2007;266:119–145. [Google Scholar]
- Crepet WL, Daghlian CP. Castaneoid inflorescences from the middle Eocene of Tennessee and the diagnostic value of pollen (at the subfamily level) in the Fagaceae. American Journal of Botany. 1980;67:739–757. [Google Scholar]
- Dawson TE. Fog in the California redwood forest: ecosystem inputs and use by plants. Oecologia. 1998;117:476–485. doi: 10.1007/s004420050683. [DOI] [PubMed] [Google Scholar]
- Denk T, Grimm GW. Significance of pollen characteristics for infrageneric classification and phylogeny in Quercus (Fagaceae) International Journal of Plant Sciences. 2009a;170:926–940. [Google Scholar]
- Denk T, Grimm GW. The biogeographic history of beech trees. Review of Palaeobotany and Palynology. 2009b;158:83–100. [Google Scholar]
- Denk T, Grimm GW. The oaks of western Eurasia: Traditional classifications and evidence from two nuclear markers. Taxon. 2010;59:351–366. [Google Scholar]
- Denk T, Tekleva MV. Pollen morphology and ultrastructure of Quercus with focus on Group Ilex (= Quercus Subgenus Heterobalanus (Oerst.) Menitsky): implications for oak systematics and evolution. Grana. 2014 (in press) [Google Scholar]
- Denk T, Grímsson F, Zetter R. Fagaceae from the Early Oligocene of Central Europe: persisting New World and emerging Old World biogeographic links. Review of Palaeobotany and Palynology. 2012;169:7–20. [Google Scholar]
- Denk T, Güner HT, Grimm GW. From mesic to arid: Leaf epidermal features suggest preadaptation in Miocene dragon trees (Dracaena) Review of Palaeobotany and Palynology. 2014;200:211–228. [Google Scholar]
- Denk T, Grímsson F, Zetter R, Simonarson LA. Late Cainozoic floras of Iceland – 15 million years of vegetation and climate history in the northern North Atlantic. Springer; 2011. [Google Scholar]
- Devore ML, PIGG KB. Floristic composition and comparison of middle Eocene to late Eocene and Oligocene floras in North America. Bulletin of Geosciences. 2010;85:111–134. [Google Scholar]
- Evanovff E, Mcintosh WC, Murphey PC. Stratigraphic summary and 40Ar/39Ar geochronology of the Florissant formation, Colorado. Proceedings of the Denver Museum of Nature and Science, Series. 2001;4(1):1–16. [Google Scholar]
- Farjon A. IUCN 2013. IUCN Red List of Threatened Species. Torreya californica. Version 2013.2. 2013. www.iucnredlist.org Downloaded on 04 March 2014.
- Flora of North America Editorial Committee . Pteridophytes and Gymnosperms. Vol. 2. Oxford University Press; New York and Oxford: 1993. Flora of North America, North of Mexico. [Google Scholar]
- Flora of North America Editorial Committee . Magnoliophyta: Magnoliidae and Hamamelidae. Vol. 3. Oxford University Press; New York and Oxford: 1997. Flora of North America, North of Mexico. [Google Scholar]
- Friis EM, Crane PR, Pedersen KR. Reproductive structures of Cretaceous Platanaceae. Biologiske Skrifter. 1988;31:1–55. [Google Scholar]
- Frisch W, Meschede M, Blakey R. Continental Drift and mountain building. Springer; Berlin, Heidelberg: 2011. Plate tectonics. [Google Scholar]
- Govaerts R, Frodin DG. World checklist and bibliography of Fagales: Betulaceae, Corylaceae, Fagaceae and Ticodendraceae. Royal Botanic Gardens, Kew: 1998. [Google Scholar]
- Grímsson F, Zetter R. Combined LM and SEM study of the Middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part II. Pinophyta (Cupressaceae, Pinaceae and Sciadopityaceae) Grana. 2012;50:262–310. doi: 10.1080/00173134.2019.1696400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández E, Crum H, Fox W, Sharp AJ. A unique vegetational area in Tamaulipas. Bulletin Torrey Botanical Club. 1951;78:458–463. [Google Scholar]
- Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S. Pollen terminology – An illustrated handbook. Springer, Wien; New York: 2009. [Google Scholar]
- Jones JH, Dilcher DL. A study of the “Dryophyllum” leaf forms from the Paleogene of Southeastern North America. Palaeontographica B. 1988;208:53–80. [Google Scholar]
- Kohlman-Adamska A, Ziembińska-Tworzydło M. Morphological variability and botanical affinity of some species of the genus Tricolporopollenites Pf. et Thoms. from the Middle Miocene Lignite association at Lubstów (Konin region – Central Poland) Acta Palaeobotanica. 2000;40:49–71. [Google Scholar]
- Kohlman-Adamska A, Ziembínska-Tworzydło M. Morphological variability and botanical affinity of Fususpollenites Kedves 1978 (LM and SEM investigations) Acta Palaeobotanica. 2001;41:147–159. [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift. 2006;15:259–263. [Google Scholar]
- Kvaček Z, Walther H. Studium über “Quercus” cruciata Al. Braun und analoge Blattformen aus dem Tertiär Europas. Acta Palaeobotanica. 1981;21:77–100. [Google Scholar]
- Kvaček Z, Walther H. Paleobotanical studies in Fagaceae of European Tertiary. Plant Systematics and Evolution. 1989;162:213–229. [Google Scholar]
- Kvaček Z, Walther H. European Tertiary Fagaceae with chinquapin-like foliage and leaf epidermal characteristics. Feddes Repertorium. 2010;121:248–267. [Google Scholar]
- Leopold EB, Clay Poole ST. Florissant leaf and pollen floras of Colorado compared: climatic implications. Proceedings of the Denver Museum of Nature and Science, Series. 2001;4(1):17–70. [Google Scholar]
- Leopold EB. Phytogeography of the late Eocene Florissant flora reconsidered. Paleontology of the Upper Eocene Florissant Formation, Colorado. The Geological Society of America, Special Paper. 2008;435:53–70. [Google Scholar]
- Lielke K, Manchester S, Meyer H. Reconstructing the environment of the northern Rocky Mountains during the Eocene/Oligocene transition: constraints from the palaeobotany and geology of south-western Montana, USA. Acta Palaeobotanica. 2012;52:317–358. [Google Scholar]
- Lieth H, Berlekamp J, Fuest S, Riedinger S. Climate Diagram World Atlas on CD. Backhuys Publishers; Leiden: 1999. [Google Scholar]
- Liu YS, Basinger JF. Fossil Cathaya (Pinaceae) pollen from the Canadian high Arctic. International Journal of Plant Sciences. 2000;161:829–847. [Google Scholar]
- Lloyd KJ, Worley-Georg MP, Eberle JJ. The Chadronian mammalian fauna of the Florissant Formation, Florissant Fossil Beds National Monument, Colorado. Paleontology of the Upper Eocene Florissant Formation, Colorado. The Geological Society of America, Special Paper. 2008;435:117–126. [Google Scholar]
- MacGinitie HD. Fossil plants of the Florissant beds, Colorado. Carnegie Institute of Washington Publication. 1953;599:1–198. [Google Scholar]
- MacGinitie HD. The Eocene Green River Formation of northwestern Colorado and northeastern Utah. Univeristy of California Publications in Geological Science. 1969;83:1–203. [Google Scholar]
- Mai DH, Walther H. Die obereozänen Floren des Weisselster-Beckens und seiner Randgebiete. Abhandlungen des Staatlichen Museums für Mineralogie und Geologie Dresden. 1985;33:1–220. [Google Scholar]
- Mai DH. Tertiäre Vegetationsgeschichte Europas. Gustav Fischer Verlag; Jena: 1995. [Google Scholar]
- Makino M, Hayashi R, Takahara H. Pollen morphology of the genus Quercus by scanning electron microscope. Scientific Reports of Kyoto Prefectural University, Life and Environmental Sciences. 2009;61:53–81. [Google Scholar]
- Manchester SR, Crane PR. Attached leaves, inflorescences, and fruits of Fagopsis, an extinct genus of fagaceous affinitiy from the Oligocene Florissant Flora of Colorado, U.S.A. American Journal of Botany. 1983;70:1147–1164. [Google Scholar]
- Manchester SR. The fossil history of the Juglandaceae. Monographs Systematic Botany. 1987;21:1–137. [Google Scholar]
- Manchester SR. Fruits and seeds of the middle Eocene Nut Beds flora, Clarno Formation, Oregon. Palaeontographica Americana. 1994;58:1–205. [Google Scholar]
- Manchester SR. Biogeographical relationships of North American Tertiary floras. Annals of the Missouri Botanical Garden. 1999;86:472–522. [Google Scholar]
- Manchester SR. Update on the megafossil flora of Florissant Colorado. Proceedings of the Denver Museum of Nature and Science, Series. 2001;4(1):137–161. [Google Scholar]
- Manchester SR, Dillhoff RM. Fagus (Fagaceae) fruits, foliage, and pollen from the Middle Eocene of Pacific Northwestern North America. Canadian Journal of Botany. 2004;82:1509–1517. [Google Scholar]
- Manos PS. Quercus Linnaeus sect. Protobalanus (Trelease) A. Camus, Chênes 1: 157. 1938. Intermediate oaks, golden-cup oaks. In: Flora of North America Editorial Committee, editor. Flora of North America North of Mexico. ume 3, Magnoliophyta: Magnoliidae and Hamamelidae. Oxford University Press; New York: 1997. pp. 468–471. [Google Scholar]
- Manos PS, Zhou Z-K, Cannon CH. Systematics of Fagaceae: Phylogenetic tests of reproductive trait evolution. International Journal of Plant Sciences. 2001;162:1361–1379. [Google Scholar]
- Manos PS, Cannon CH, Oh SH. Phylogenetic relationships and taxonomic status of the paleoendemic Fagaceae of western North America: recognition of a new genus, Notholithocarpus. Madroño. 2008;55:181–190. [Google Scholar]
- McIver EE, Basinger JF. Early Tertiary floral Evolution in the Canadian High Arctic. Annals of the Missouri Botanical Garden. 1999;86:523–545. [Google Scholar]
- Menitsky YL. Oaks of Asia. Science Publishers; Enfield, NH: 2005. [Google Scholar]
- Meyer HW. The fossils of Florissant. Smithonian books; Washington, London: 2003. [Google Scholar]
- Mix HT, Mulch A, Kent-Corson ML, Chamberlain CP. Cenozoic migration of topography in the North American Cordillera. Geology. 2011;39:87–90. [Google Scholar]
- Miyoshi N. Pollen morphplogy of the genus Castanopsis (Fagaceae) in Japan. Grana. 1983;22:19–21. [Google Scholar]
- Oh SH, Manos PS. Molecular phylogenetics and cupule evolution in Fagaceae as inferred from nuclear CRABS CLAW sequences. Taxon. 2008;57:434–451. [Google Scholar]
- Potter D, Eriksson T, Evans RC, Oh S, Smedmark JEE, Morgan DR, Kerr M, Robertson KR, Arsenault M, Dickinson TA, Campbell SC. Phylogeny and classification of Rosaceae. Plant Systematics and Evolution. 2007;266:5–43. [Google Scholar]
- Praglowski J. Fagaceae Dumort.: Castaneoideae Oerst. World Pollen and Spore Flora. 1984;13:1–21. [Google Scholar]
- Punt W, Hoen PP, Blackmore S, Nilsson S, Thomas AL. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology. 2007;143:1–81. [Google Scholar]
- Qi Z, Cameron KM, Li P, Zhao Y, Chen S, Chen G, Fu C. Phylogenetics, character evolution, and distribution patterns of the greenbriers, Smilacaceae (Liliales), a near-cosmopolitan family of monocots. Botanical Journal of the Linnean Society. 2013;173:535–548. [Google Scholar]
- Rowley JR. Exine origin, development and structure in pteridophytes, gymnosperms and angiosperms. Chapter 14D. In: Jansonius J, McGregor DC, editors. Palynology: principles and applications. Vol. 1. American Association of Stratigraphic Palynologists Foundation; Dallas: 1996. pp. 443–462. [Google Scholar]
- Schroeder FG. Lehrbuch der Pflanzengeographie. Quelle und Meyer Verlag; Wiesbaden: 1998. [Google Scholar]
- Solomon AM. Pollen morphology and plant taxonomy of white oaks in eastern North America. American Journal of Botany. 1983a;70:481–494. [Google Scholar]
- Solomon AM. Pollen morphology and plant taxonomy of red oaks in eastern North America. American Journal of Botany. 1983b;70:495–507. [Google Scholar]
- Suc JP. Origin and evolution of the Mediterranean vegetation and climate in Europe. Nature. 1984;307:429–432. [Google Scholar]
- Takahashi M, Friis EM, Herendeen PS, Crane PR. Fossil flowers of Fagales from Kamikitaba Locality (Early Coniacian; Late Cretaceous) of Northeastern Japan. International Journal of Plant Sciences. 2008;169:899–907. [Google Scholar]
- Tanai T, Uemura K. Lobed oak leaves from the Tertiary of East Asia with reference to the oak phytogeography of the Northern Hemisphere. Transactions and Proceedings of the Palaeontological Society of Japan, New Series. 1994;173:343–365. [Google Scholar]
- Tanai T. Fagaceous leaves from the Paleogene of Hokkaido, Japan. Bulletin of the National Science Museum, Tokyo, Series C. 1995;21:71–101. [Google Scholar]
- van den Heuvel BD. PhD Thesis. University of Texas; Austin: 2002. Molecular Systematics of Cercocarpus H.B.K. (Rosaceae) [Google Scholar]
- Velitzelos D, Bouchal JM, Denk T. Review of the Cenozoic vegetation and floras of Greece. Review of Palaeobotany and Palynology. 2014 doi: 10.1016/j.revpalbo.2014.02.006. [DOI]
- Walther H, Zetter R. Zur Entwicklung der paläogenen Fagaceae Mitteleuropas. Palaeontographica B. 1993;230:183–194. [Google Scholar]
- Wheeler E. Fossil dicotyledonous woods from Florissant Fossil Beds, National Monument, Colorado. Proceedings of the Denver Museum of Nature and Science, Series. 2001;4(1):187–203. [Google Scholar]
- Wingate FH, Nichols DJ. Palynology of the uppermost Eocene lacustrine deposits at Florissant Fossil Beds National Monument, Colorado. Proceedings of the Denver Museum of Nature and Science, Series. 2001;4(1):71–135. [Google Scholar]
- Wobus RA, Epis RC. B Geologic map of the Florissant fifteen minute quadrangle, Park and Teller Counties, Colorado. United States Geological Survey Map. 1978:1–1044. [Google Scholar]
- Wolfe JA, Wehr W. Middle Eocene dicotyledonous plants from Republic, northeastern Washington. U.S. Geological Survey Bulletin. 1987;1597:1–25. [Google Scholar]
- Wu ZY, Raven PH, editors. Flora of China. Vol. 4 (Cycadaceae through Fagaceae) Science Press, Beijing, and Missouri Botanical Garden Press; St. Louis: 1999. [Google Scholar]
- Yi T, Miller AJ, Wen J. Phylogenetic and biogeographic diversification of Rhus (Anacardiaceae) in the Northern Hemisphere. Molecular Phylogenetics and Evolution. 2004;33:861–879. doi: 10.1016/j.ympev.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Zetter R. Methodik und Bedeutung einer routinemäßigen kombinierten lichtmikroskopischen und rasterelektronenmikroskopischen Untersuchung fossiler Mikrofloren. Courier Forschungs-Institute Senckenberg. 1989;109:41–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Taxa/plant organs reported from Florissant and inferred vegetation types. Med/Chap Cal/Mex = Mediterranean, chaparral; California, Mexico.
Appendix S2: Distribution of climate types along an east west transect from the Midwest to the Central Rocky Mountains. Representative climate diagrams illustrate the change from fully humid summer-rain climates (Cfa) to dry summer-rain climates (BS) to dry winter-rain climates (Mediterranean variant of BS climates) to Mediterranean climates (Csa).
Köppen climate types from Kottek et al. (2006) using a kmz file (http://koeppen-geiger.vuwien.ac.at/data/Koeppen-Geiger-GE.kmz) for Google Earth. Climate diagrams from Lieth et al. (1999).