Common multi-allelic copy-number variants (CNVs) appear enriched for phenotypic associations compared to their di-allelic counterparts1-4. Here we investigated the influence of gene-dosage effects on adiposity through a CNV association study of gene expression levels in adipose tissue. We identified significant association of a multi-allelic CNV encompassing the salivary amylase gene (AMY1) with body mass index and obesity, and replicated this finding in 6,200 subjects. Increased AMY1 copy-number was positively associated with both amylase gene expression (P=2.31×10−14) and serum enzyme levels (P<2.20×10−16), while reduced AMY1 copy-number was associated with increased BMI (per-estimated-copy:β=−0.15[0.02]kg/m2;P=6.93×10−10) and obesity risk (per-estimated-copy:OR=1.19[1.13-1.26]95%CI;P=1.46×10−10). The OR of 1.19 per-copy of AMY1 translates to about an eight-fold difference in risk of obesity between subjects in the top (CN>9) and bottom (CN<4) 10% of the copy-number distribution. Our study provides a first genetic link between carbohydrate metabolism and BMI and demonstrates the power of integrated genomic approaches beyond genome-wide association studies.
We designed a gene-centric association study (GCAS) to identify common CNVs overlapping genes and inducing a dosage effect on gene expression, hypothesising that these might be enriched for physiologically-relevant CNVs. To achieve this, we conducted a family-based association analysis of signal intensity data from DNA arrays (log R ratio and B-allele frequency) with transcriptomic data from adipose tissue using famCNV5 in 149 Swedish families ascertained through siblings discordant for obesity6 (Table 1;Figure 1;Supplementary Figure 1). A total of 76 probes located within putative CNVs showed a dosage effect on gene expression at 1% FDR (Supplementary Table 1). Of these probes, only cnvi0020639, located within a CNV overlapping the amylase gene cluster (including the AMY1 salivary and the AMY2 pancreatic amylase genes expression probeset 208498_s_at; FDR=6.88×10−3), was also associated with adiposity [both BMI (P=3.86×10−4) and fat mass (P=3.11×10−4)] (Supplementary Figures 2-4). Reduced signal intensity at this probe was associated with increased adiposity levels (Table 2;Figure 2;Supplementary Figures 2-4).
Table 1.
Summary information on subjects included in this study.
| Sample | Total | Male | Female | Median age | 1st – 3rd quartiles | Median BMI | 1st – 3rd quartiles |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Swedish | 342 | 98 | 244 | 37 | (33 - 43) | 27.9 | (22.6 - 36.5) |
| TwinsUK | 1,479* | - | 1,479 | 53 | (45 - 60) | 26.0 | (22.8 - 28.4) |
| DESIR | 2,137 | 942 | 1,195 | 52 | (44 - 61) | 24.6 | (22.2 - 26.6) |
| AOB | 563 | 160 | 403 | 35 | (32 - 39) | - | - |
| Cases | 205 | 39 | 166 | 36 | (29 - 41) | 46.2 | (42.5 - 51.3) |
| Controls | 358 | 121 | 237 | 35 | (33 - 38) | 21.6 | (20.3 - 22.4) |
| SP2 | 658 | 237 | 421 | 46 | (37 - 52) | - | - |
| Cases** | 333 | 139 | 194 | 47 | (40 - 54) | 27.1 | (25.9 - 28.9) |
| Controls | 325 | 98 | 227 | 44 | (34 - 51) | 18.4 | (17.5 - 19.1) |
| ABOS | 468 | 122 | 346 | 43 | (33 - 51) | 46.2 | (41.7 - 52.3) |
Consisting of 334 dizygotic and 193 monozygotic twin pairs and 425 singletons.
Including 136 obese (BMI ≥ 28 kg/m2) and 197 overweight (23 kg/m2 ≤ BMI < 28 kg/m2) Singaporean Chinese subjects.
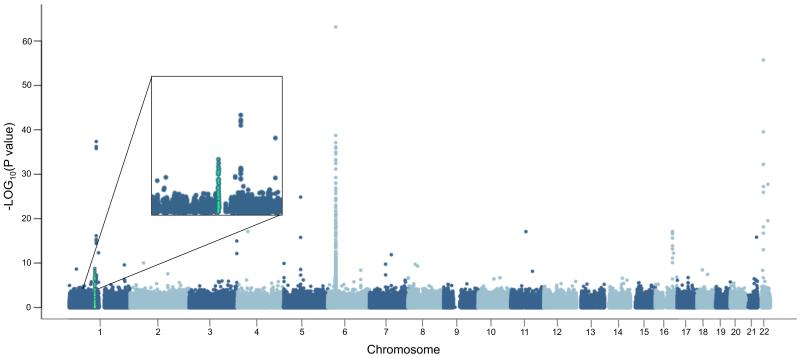
Figure 1. Manhattan plot of gene-centric CNV association study (GCAS) results with gene expression levels in subcutaneous adipose tissue from the Swedish sib-pair dataset.
Chromosomal location for each probe is given on the horizontal axis for each of the 22 autosomes, while minus log10 (P) of the association between probe signal intensity and gene expression levels is shown on the vertical axis. The probes tested against the amylase genes transcriptional levels are shown in green.
Table 2.
Association of relative copy-number in the amylase region with obesity and measures of adiposity. Copy-number estimates used in the association analyses for both the “Population samples” and the “Obesity case-control” samples were derived by qPCR.
| DNA Array-based CNV analysis# | N | Trait | Associated probe | P | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Swedish families | 342 | BMI | cnvi0020639 | 3.86×10−4 | ||||
| Swedish families | 331 | Fat mass | cnvi0020639 | 3.11×10−4 | ||||
| TwinsUK | 972 | BMI | cnvi0022844 | 1.13×10−3 | ||||
|
| ||||||||
| Population samples ‡ | N | BMI * | Age * | β (SE) § | P | |||
|
| ||||||||
| TwinsUK | 1,479 | 26.0 (22.8-28.4) | 53 (45-60) | −0.18 (0.05) | 5.91×10−4 | |||
| DESIR | 2,137 | 24.6 (22.2-26.6) | 52 (44-61) | −0.14 (0.03) | 2.49×10−7 | |||
| N | β (SE) § | P | Het P ** | |||||
|
| ||||||||
| Meta-analysis | 3,616 | −0.15 (0.02) | 6.93×10−10 | 0.54 | ||||
|
| ||||||||
| Cases | Controls | |||||||
| Obesity case-control ‡ | N | Age * | N | Age * | β (SE) § | P | OR (95%CI) § | |
|
| ||||||||
| TwinsUK | 251 | 53 (47-60) | 711 | 51 (44-59) | −0.26 (0.09) | 3.61×10−3 | 1.30 (1.08-1.55) | |
| DESIR | 137 | 55 (47-64) | 1267 | 51 (42-59) | −0.16 (0.04) | 7.47×10−5 | 1.18 (1.09-1.27) | |
| AOB | 205 | 36 (29-41) | 358 | 35 (33-38) | −0.17 (0.04) | 4.44×10−5 | 1.19 (1.10-1.29) | |
| SP2 | 136 | 47 (37-54) | 325 | 44 (34-51) | −0.15 (0.05) | 3.73×10−3 | 1.17 (1.05-1.29) | |
| N | N | β (SE) § | P | OR (95%CI) § | Het P ** | |||
|
| ||||||||
| Meta-analysis*** | 593 | 2,336 | −0.18 (0.03) | 1.46×10−10 | 1.19 (1.13-1.26) | 0.62 | ||
The listed numbers of samples are those which passed quality control and were used in the association analyses.
Obesity case-control analyses in TwinsUK and DESIR were conducted using a subset (subjects with BMI < 25kg/m2 and those with BMI ≥ 30kg/m2) of those subjects included in the quantitative trait analysis in the population samples category.
“DNA Array-based” denotes signal intensity data from Illumina SNP genotyping arrays
median (1st, 3rd quartiles)
Heterogeneity P-value
Estimates calculated using integer AMY1 copy-numbers inferred from the underlying continuous distribution.
Obesity case-control meta-analysis was limited to European samples.
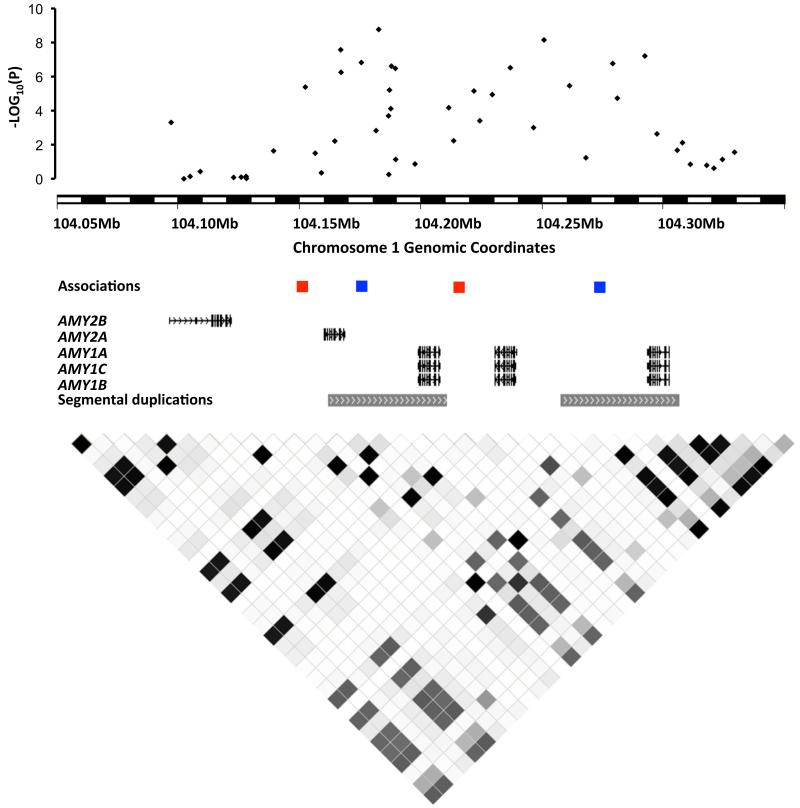
Figure 2. The amylase region in detail.
Top to bottom: famCNV association results between signal intensity at probes within 30kb of the amylase cluster and amylase expression levels (probeset 208498_s_at) in adipose tissue (black dots) in the Swedish family discovery study, with chromosomal coordinates given on the horizontal axis and minus log10(P) on the vertical axis; locations of probes showing association between signal intensity and BMI: cnvi0020639 (blue; Swedish family discovery study), cnvi0022844 (red; TwinsUK); gene content in the amylase region based on the human reference sequence (hg19; RefSeq), depicting AMY2B, AMY2A and the AMY1A/B/C genes, as well as two high sequence similarity segmental duplications in the region; LD between HapMap markers (release 23) calculated with HaploView46 (darker shading corresponds to higher r2 value). Because of the repetitive nature of this region, which contains six paralogs (including one pseudogenized copy) in the reference genome (Supplementary Figure 13), the cnvi0020639 and the cnvi0022844 probes were found to map to two locations within the amylase gene cluster.
This inverse association between copy-number in the amylase region and BMI was first replicated using signal intensity data from DNA arrays in 972 subjects from TwinsUK (Table 1)7. The strongest association was observed at cnvi0022844 (P=1.13×10−3;Table 2), which showed significant association with BMI after Bonferroni correction. When multiple probes were considered through principal component analysis, the BMI association signal actually extended over a region between cnvi0022844 and cnvi0016754 (P=1.32×10−3), which overlapped the cnvi0020639 probe associated with adiposity in the Swedish discovery families. These results, although supportive of the association in the amylase region, did not permit us to distinguish which of the salivary or pancreatic amylases was driving the association with adiposity, necessitating use of a non-array-based method of copy-number measurement.
Consequently, we estimated copy-number at AMY1 and AMY2 in 481 subjects from the Swedish families (Table 1) using quantitative real-time PCR (qPCR). This approach generates a continuous intensity distribution from which integer copy-numbers can be inferred by comparison to a reference sample of known copy-number (Supplementary Information). Given the many technical challenges inherent in copy-number measurement at multi-allelic loci2,8-11, we treated these discretised measurements as relative estimates or surrogates correlated with the true underlying copy-number state, as opposed to absolute copy-number genotypes.
Only three estimated copy-number states (one to three) were detected for the pancreatic amylase (AMY2) gene, and these were not associated with either BMI or fat mass (Supplementary Table 2). In contrast, copy-number estimates at AMY1 ranged from two to fourteen, and showed association with both BMI (P=8.08×10−3) and fat mass (P=8.53×10−3) confirming our previous DNA-array based analysis (Supplementary Table 2). We found greater correlation between signal intensity at cnvi0020639 and AMY1 (r=0.73; P<2.20×10−16) than AMY2 copy-number (r=0.35; P=1.25×10−8), suggesting that the GCAS association was mainly capturing copy-number variation at AMY1 as opposed to AMY2, justifying follow-up of the former. Furthermore, we validated accuracy of the AMY1 qPCR assay by using AMY1 copy-number estimates derived using whole-genome shotgun-sequencing data from the 1000 Genomes Project12, and observed a correlation of 0.94 (P<2.20×10−16) between AMY1 copy-number estimates derived by qPCR and sequencing (Supplementary Figures 5-8; Supplementary Table 3). To further validate the AMY1 qPCR assay, we also compared the copy-number measured by qPCR in 96 samples from the DESIR cohort13 with AMY1 copy-number measured by digital PCR in the same samples, obtaining high correlation between the two methods (r=0.95; P<2.20×10−16; Supplementary Figure 9). Analogously, high correlation (r=0.98; P<2.20×10−16; Supplementary Figure 9) was also observed between copy-numbers measured using the qPCR assay used in this study and those obtained using a different qPCR assay on the same 96 DESIR samples.
To replicate the observed association in a larger sample, we next estimated AMY1 copy-number by qPCR in an additional sample of 1,479 female subjects from TwinsUK14 and 2,137 male and female subjects from DESIR13 (Table 1). The two population samples showed a similar copy-number distribution (Wilcoxon test P>0.05) with estimated median copy-number of six, ranging from one to eighteen (Supplementary Figure 10; Supplementary Tables 4-5). Meta-analysis of AMY1 effects in TwinsUK and DESIR (total n=3,616) showed significant association between reduced AMY1 copy-number and increased BMI (per copy-number β=−0.15[0.02]kg/m2;P=6.93×10−10; Table 2;Figure 3;Supplementary Tables 6-9). Results of associations assessed using both the qPCR intensity signal as a continuous measure, as well as discretised using an unsupervised clustering approach (k-means), were concordant with those generated using integer copy-numbers (Supplementary Information).
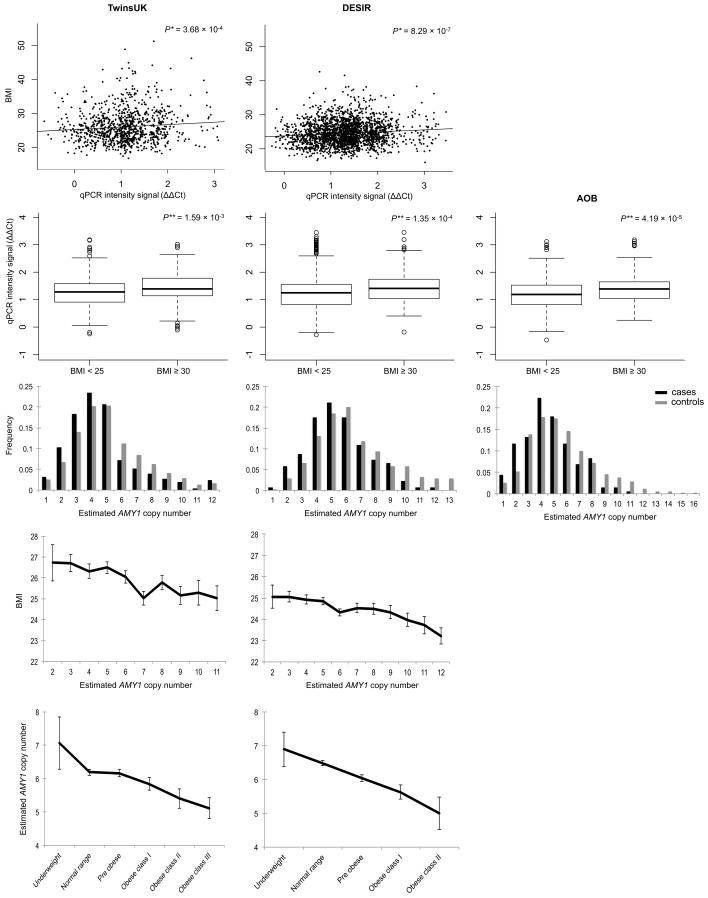
Figure 3.
Effect of estimated AMY1 copy-number on obesity and BMI. A: Scatter plots of raw qPCR signal intensity (ΔΔCt) plotted against BMI for the TwinsUK and DESIR samples. B: Boxplots of ΔΔCt in normal weight (BMI<25kg/m2) and obese (BMI≥30kg/m2) subjects in the TwinsUK, DESIR and AOB samples. For plots A and B, low ΔΔCt values correspond to high AMY1 copy-numbers. C: relative copy-number distribution in obese cases (BMI≥30 kg/m2; black bars) versus normal weight controls (BMI<25 kg/m2; grey bars) in the TwinsUK, DESIR, and AOB studies. Estimated copy-numbers higher than 13 (showing frequency < 2.5%) were collapsed together into a single category. D and E: BMI at different estimated AMY1 copy-numbers and AMY1 copy-number estimates by BMI categories in the TwinsUK and DESIR population samples. WHO BMI classification: Underweight (<18.5); Normal range (18.50 – 24.99); Pre obese (25.00 – 29.99); Obese class I (30.00 – 34.99); Obese class II (35.00 – 39.99). Error bars represent the standard error of the mean. *Association between BMI and qPCR ΔΔCt intensity signal, corrected for age, sex (DESIR), family (TwinsUK) and genotyping plate. **Wilcoxon rank sum test.
We then assessed the effect of AMY1 copy-number on obesity susceptibility by selecting obese cases (BMI≥30kg/m2) and normal-weight controls (BMI<25kg/m2) from TwinsUK and DESIR and by measuring AMY1 copy-number by qPCR in an additional 205 severely obese cases and 358 age-matched controls from the AOB15 study (Table 1;Supplementary Information). In these European samples, subjects with lower estimated AMY1 copy-number showed significantly increased risk of obesity in each of the three samples (per-estimated AMY1 copy-number meta-analysis: OR=1.19[1.13-1.26]95%CI;P=1.46×10−10;Table 2;Figure 3). The AMY1 copy number distribution in our sample ranged from one to eighteen copies, with approximately 10% of subjects carrying fewer than four copies of AMY1, and 10% of subjects with an AMY1 copy number greater than nine (Table 2). Given the multi-allelic nature of the AMY1 CNV, this OR of 1.19 per copy of AMY1 translates to about an eight-fold difference in risk of obesity between subjects in the top (CN>9) and bottom (CN<4) decile of the estimated AMY1 copy-number distribution (OR=7.67[3.92-14.99]95%CI;P=2.52×10−9;Supplementary Table 10). Using a multi-factorial liability threshold model16, we estimated the proportion of total variance of obesity explained by estimated AMY1 copy-number to lie between 1.73-7.94%[95%CI] (Supplementary Table 11). Therefore, based on an estimated heritability of 40-70%17,18, copy-number variation at AMY1 may account for 2.47-19.86% of the total genetic variation of obesity. Analogously, we estimated that between 0.66% and 4.40% of the proportion of genetic variance of BMI could be explained by inferred AMY1 copy-number in these European samples.
As all the samples included in our analyses were of European origin, we reasoned that replication in a sample of different ethnicity and under differing environmental influences on obesity would provide greater support for its physiological role. We therefore selected a Singaporean Chinese case-control sample from SP219. A total of 136 obese and 197 overweight subjects were identified among the 2,431 Chinese subjects included in the SP2 cohort, with 325 matched lean Chinese SP2 normal-weight controls. AMY1 copy-number was measured by qPCR in all 658 subjects. Median copy-number in SP2 normal-weight subjects was 6 (ranging from 2 to 16), similar to our French DESIR and UK TwinsUK populations, and in line with previous observations by Perry et al20. Case-control association analysis in the Chinese sample showed reduced AMY1 estimated copy number to be associated with increased risk of obesity (per copy-number OR=1.17[1.05-1.29]95%CI;P=3.73×10−3). Extending the case sample to include the 197 overweight subjects further confirmed the results (per copy-number OR=1.13[1.06-1.21]95%CI;P=3.52×10−4).
To validate our AMY1 genomic copy-number data at the protein level, we investigated the effect of copy-number variation at AMY1 and AMY2 on serum amylase enzyme levels, and their relationship with BMI using 468 French morbidly obese subjects from the ABOS study (Table 1;Supplementary Table 12). On average, salivary and pancreatic amylase proportions were approximately equal in serum (52% and 48%, respectively) and their levels showed close positive association with copy-number variation at their respective genes (P<2.20×10−16 and P=1.04×10−11, respectively; Supplementary Figure 11). BMI was inversely associated with serum salivary amylase (β=−0.23[0.04]kg/m2;P=2.26×10−7;Supplementary Figure 12) and to a lesser extent serum pancreatic amylase (β=−0.23[0.06]kg/m2;P=2.29×10−4;Supplementary Figure 12), likely reflecting the physiological correlation between the levels of the two enzymes (r=0.21;P=4.29×10−6).
Salivary amylase catalyses hydrolysis of the α-1,4-glycosidic bonds of starch, initiating carbohydrate digestion in the oral cavity. While individual salivary amylase levels vary in response to environmental factors including psychological stress21, they are genetically influenced by and directly correlated with the highly variable copy-number at AMY120,22. Increased gene copy-numbers at this locus are believed to have evolved in the human lineage as a consequence of a shift to a starch-rich diet23. Human populations traditionally consuming a high proportion of carbohydrates in their diet show higher copy-numbers and salivary amylase activity than those consuming a low-starch diet20,24. Both the salivary glands and pancreas contribute similarly to determine overall levels of serum amylase25, although enzyme activity is also detectable in other organs, including adipose tissue26,27. Indeed amylase was among the 30% most-highly expressed genes in adipose tissue in both our discovery sample and publicly-available data from the general population, thus suggesting that this gene is actively expressed in adipose tissue (Supplementary Information). Whether adipose tissue is functionally involved in the link between AMY1 copy-number and obesity, or whether this link implicates a different tissue in which AMY1 is also actively transcribed warrants further investigation.
Decreased blood amylase levels have been observed in both obese humans28 and rats29, and have recently been associated with increased risk of metabolic abnormalities30,31 and reduced pre-absorptive insulin release32. Furthermore, a recent study in mice fed a high fat/high sugar diet suggested association between the amylase locus and weight gain33. In these mice, this locus was also shown to be associated with the proportion of Enterobacteriaceae in the gut microbiota33, which have been previously correlated with obesity in humans34.
Rare copy-number variants have recently been implicated in highly-penetrant forms of obesity35,36 and severe thinness37, through a gene dosage effect. Common bi-allelic CNVs have also been associated with BMI38-41, however, since most of these are reliably tagged by surrounding SNPs42, they share the same properties of small effect sizes and limited predictive value for obesity risk. In contrast, complex multi-allelic CNVs show decreased linkage disequilibrium with surrounding SNPs (Supplementary Table 13) and are consequently less detectable by SNP-based GWAS43. Surprisingly, FTO is the most-replicated obesity susceptibility gene identified through GWAS41, yet in our analyses estimated AMY1 copy-number appeared to show stronger association with BMI than FTO SNPs (Supplementary Tables 14-15). It is conceivable that high structural variability in the amylase region and subsequent low SNP coverage (Supplementary Figures 13-14) may have hampered previous SNP-based GWAS attempts to detect association between the amylase cluster and adiposity. Indeed, examination of data from the most recent BMI meta-analysis conducted by the GIANT consortium41 revealed a large gap in SNP coverage across the locus encompassing the salivary amylase gene (Supplementary Figure 14).
Present DNA high-throughput methods for CNV assessment, including array-, PCR- and sequencing-based approaches, are all affected by a wide number of variables including DNA source, extraction methods, quality and concentration, as well as experimental factors inducing batch effects10,11,44. These factors complicate copy-number measurement at multi-allelic CNVs and hinder pooling of data from multiple centres. The observed association of AMY1 with obesity may rekindle interest in the role of multi-allelic CNVs in common disease, driving development of novel technological approaches for accurate and high-throughput measurement of absolute copy-number at such loci. These technological improvements will enable high-quality association analyses at such loci in larger sample sizes similar to those included in SNP association studies, and are mandatory for disease risk-assessment at the individual level, paving the way towards personalized medicine.
Our study provides a first genetic link between carbohydrate metabolism and obesity, with low copy-number at AMY1 resulting in decreased salivary amylase levels and a higher risk of obesity. This finding provides intriguing insight into some of the biological mechanisms underlying obesity, as well as a novel rationale for the investigation of innovative obesity treatments based on manipulation of digestive enzyme levels.
ONLINE METHODS
Further detailed methods are provided in the Supplementary Information. Associations were assessed using linear mixed effects models, including plate as a random effect and family structure as an additional random effect where appropriate. Age and sex were included as covariates.
Discovery
The discovery sample included 149 Swedish families (342 subjects) ascertained through an obesity-discordant sib-pair (BMI difference>10kg/m2)6. Gene expression for 29,546 transcripts (16,563 Ensembl genes) was measured in subcutaneous adipose tissue using the Affymetrix Human Genome U133 Plus 2.0 microarray. GWAS signal intensity data from Illumina 610K-Quad arrays were available for 348,150 probes lying within each transcript plus 30kb upstream and downstream to encompass the coding regions and their internal and nearby regulatory regions.
Quantitative real-time PCR (qPCR) was carried out to infer relative copy-number measurements reflecting the underlying copy-number distribution at AMY1 and AMY2, respectively, using the TaqMan assays Hs07226362_cn and Hs04204136_cn on an Applied Biosystems 7900HT Real-Time PCR System. Association analyses were carried out for 481 subjects with complete data on BMI and dual-energy X-ray absorptiometry (DEXA)-derived fat mass.
Replication
In-silico replication of the BMI association was conducted using 972 female subjects from the UK adult twin registry (TwinsUK) cohort14 using intensity signals from Illumina 610K-Quad arrays7. Association with BMI and obesity was analysed in two population samples using qPCR estimates of AMY1 copy-number for 1,479 female twins from TwinsUK14 and 2,137 subjects from the French Data from the Epidemiological Study on the Insulin Resistance syndrome (DESIR)13 cohort. Obesity association with qPCR data was also assessed in an additional case-control sample of 205 obese cases and 358 age-matched controls from the French Adult Obesity study (AOB)15. An additional case-control sample was extracted from the Singapore Prospective Study Program (SP2) cohort, a population-based study including 2,431 adult Chinese Singaporean subjects19. Obesity in the Chinese population was defined as BMI≥28kg/m2 and normal-weight as BMI<23kg/m2, based on criteria set by the Working Group on Obesity in China45 and the WHO expert consultation for Asia46. Accordingly, a total of 136 obese and 197 overweight subjects were identified among the 2,431 Chinese subjects of the SP2 cohort, with 325 matched lean SP2 subjects selected as normal-weight controls.
In order to avoid any potential population stratification impacting on our association analyses resulting from the known differences in AMY1 copy number distribution between populations traditionally consuming high versus low starch diets20, we carried out genotype principal component analysis using genome-wide SNP array data to ensure that samples included in each analysis were of the same ethnicity and genetic background. Furthermore, AMY1 association analyses were conducted separately in each of the study populations and then combined by meta-analysis using METAL47 rather than pooling.
Protein levels
‘Atlas Biologique de l’Obésité Sévère’ (ABOS) is a French cohort comprised of candidates for bariatric surgery. Serum pancreatic and total amylase levels for 468 patients were measured by an enzymatic colorimetric assay with an autoanalyzer (CoBAS Icobas® 8000 modular analyser series; kits AMYL2-03183742122 and AMY-P-20766623322, Hoffman-La Roche Ltd). Serum salivary amylase levels were calculated by subtracting serum pancreatic amylase levels from total serum amylase levels.
Supplementary Material
Acknowledgements
This work was supported by grants from the Wellcome Trust (grant ref 079534/z/06/z and 085555), the Medical Research Council (K2010-55X-11285-13), the Swedish Research Council, the Swedish foundation for Strategic Research to Sahlgrenska Center for Cardiovascular and Metabolic Research, the Swedish Diabetes foundation and the Swedish federal government under the LUA/ALF agreement, the European Community’s Seventh Framework Programme (FP7/2007-2013) EUROCHIP project. Mario Falchi is supported by the Medical Research Council (MR/K01353X/1), the British Skin Foundation (5044i), Qatar Foundation (GEQATDIAB) and the Commission of the European Communities (115005). Philippe Froguel is supported by the Medical Research Council (G1002084/1), the Imperial College Healthcare NHS Trust Biomedical Research Centre (P46304 WMDI), the Commission of the European Communities (294785) and Qatar Foundation (GEQATDIAB).
The DESIR study has been supported by INSERM contracts with CNAMTS, Lilly, Novartis Pharma and Sanofi-Aventis; by INSERM (Réseaux en Santé Publique, Interactions entre les déterminants de la santé), Cohortes Santé TGIR, the Association Diabète Risque Vasculaire, the Fédération Française de Cardiologie, La Fondation de France, ALFEDIAM, ONIVINS, Ardix Medical, Bayer Diagnostics, Becton Dickinson, Cardionics, Merck Santé, Novo Nordisk, Pierre Fabre, Roche, Topcon.
The DESIR Study Group. INSERM U780: B. Balkau, MA Charles, P. Ducimetière, E. Eschwège; INSERM U367: F. Alhenc-Gelas; CHU D’Angers: Y. Gallois, A. Girault; Bichat Hospital: F. Fumeron, M. Marre, R Roussel; CHU Rennes:F Bonnet; CNRS UMR8090, Lille: S. Cauchi, P. Froguel; Centres d’Examens de Santé: Alençon, Angers, Blois, Caen, Chartres, Chateauroux, Cholet, Le Mans, Orleans Tours; Institute de Recherche Médecine Générale: J. Cogneau; General practitioners of the region; Institute inter-Regional pour la Santé: C. Born, E. Caces, M. Cailleau, N Copin, O Lantieri, J.G. Moreau, F. Rakotozafy, J. Tichet, S. Vol.
This study makes use of data generated by the Genome Structural Variation Consortium (PIs Nigel Carter, Matthew Hurles, Charles Lee and Stephen Scherer) whom we thank for pre-publication access to their CNV discovery [and/or] genotyping data, made available through the websites http://www.sanger.ac.uk/humgen/cnv/42mio/ and http://projects.tcag.ca/variation/ as a resource to the community. Funding for the project was provided by the Wellcome Trust [Grant No. 077006/Z/05/Z], Canada Foundation of Innovation and Ontario Innovation Trust, Canadian Institutes of Health Research, Genome Canada/Ontario Genomics Institute, the McLaughlin Centre for Molecular Medicine, Ontario Ministry of Research and Innovation, the Hospital for Sick Children Foundation, the Department of Pathology at Brigham and Women’s Hospital and the National Institutes of Health grants HG004221 and GM081533.
TwinsUK was funded by the Wellcome Trust; the authors acknowledge the funding of the GWAS with the support of the Wellcome Trust Sanger Centre and the National Eye Institute via an NIH/CIDR genotyping project grant (PI: Terri Young); also “European Community’s Seventh Framework Programme (FP7/2007-2013), ENGAGE project grant agreement HEALTH-F4-2007-201413. The study also receives support from the National Institute for Health Research (NIHR) BioResource Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The project also received support from a Biotechnology and Biological Sciences Research Council (BBSRC) project grant (G20234). The ABOS study was partially supported by Lille University Hospital and INSERM (Centre d’Investigation Clinique de Lille), and grants from Conseil Regional Nord-Pas de Calais (ARCIR Obésité).
The authors would like to thank the study participants in each of the studies, as well as the field investigators for the recruitment and examinations of study subjects. The authors would also like to thank Patrice Maboudou and Thierry Brousseau from the CHRU de Lille, Pôle de Biologie-Pathologie-Génétique, UF Biochimie automatisée, for measurement of serum amylase levels in the ABOS study subjects, and Patrick Gele, Centre de Ressources Biologiques, CHRU Lille, for handling of ABOS samples. The authors thank the Genotyping Facility at the Wellcome Trust Sanger Institute for generating the Twins UK SNP array data. The authors also thank Enrico Petretto, Marco Manca, Lachlan Coin, Toby Andrew, Inga Prokopenko, Teresa Norat and Sylvia Richardson for helpful discussions, and Laurent Arnalsteen, Helene Verkindt, Carole Eberle, and Marie France Six for their contribution to the ABOS study, Marianne Deweider and Frederic Allegaert for lab processing of the DESIR samples, as well as Simon Burbidge and Matt Harvey of the Imperial College High Performance Computing service for their assistance: http://www.imperial.ac.uk/ict/services/teachingandresearchservices/highperformancecomputing
Francesco Pesce was supported by a fellowship from the European Renal Association - European Dialysis and Transplant Association (ERA-EDTA ALTF 72-2010). MNA is supported by a PhD scholarship from Research Division of Qatar Foundation. RS is a recipient of a Chercheur-boursier award from the Fonds de la recherche en santé du Québec and a New Investigator Award from CIHR. PD is supported by the Wellcome Trust. TDS is an NIHR senior Investigator and ERC Senior Researcher.
Footnotes
Accession number
Gene expression microarray data for the complete Swedish discordant sib-pair study sample have been deposited at GEO-NCBI under accession number GSE27916.
References
- 1.Gonzalez E, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–40. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 2.Hollox EJ, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–5. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fellermann K, et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet. 2006;79:439–48. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aitman TJ, et al. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature. 2006;439:851–5. doi: 10.1038/nature04489. [DOI] [PubMed] [Google Scholar]
- 5.Eleftherohorinou H, et al. famCNV: copy number variant association for quantitative traits in families. Bioinformatics. 2011;27:1873–5. doi: 10.1093/bioinformatics/btr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walley AJ, et al. Differential coexpression analysis of obesity-associated networks in human subcutaneous adipose tissue. Int J Obes (Lond) 2012;36:137–47. doi: 10.1038/ijo.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hysi PG, et al. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet. 2010;42:902–5. doi: 10.1038/ng.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field SF, et al. Experimental aspects of copy number variant assays at CCL3L1. Nat Med. 2009;15:1115–7. doi: 10.1038/nm1009-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollox EJ. The Challenges of Studying Complex and Dynamic Regions of the Human Genome. In: Feuk L, editor. Genomic Structural Variants. Vol. 838. Springer; New York: 2012. pp. 187–207. [DOI] [PubMed] [Google Scholar]
- 10.Aldhous MC, et al. Measurement methods and accuracy in copy number variation: failure to replicate associations of beta-defensin copy number with Crohn’s disease. Hum Mol Genet. 2010;19:4930–8. doi: 10.1093/hmg/ddq411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter D, Walker S, Prescott N, Schalkwijk J, Armour JA. Accuracy and differential bias in copy number measurement of CCL3L1 in association studies with three auto-immune disorders. BMC Genomics. 2011;12:418. doi: 10.1186/1471-2164-12-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudmant PH, et al. Diversity of human copy number variation and multicopy genes. Science. 2010;330:641–6. doi: 10.1126/science.1197005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balkau B, Eschwege E, Tichet J, Marre M. Proposed criteria for the diagnosis of diabetes: evidence from a French epidemiological study (D.E.S.I.R.) Diabetes Metab. 1997;23:428–34. [PubMed] [Google Scholar]
- 14.Spector TD, Williams FM. The UK Adult Twin Registry (TwinsUK) Twin research and human genetics : the official journal of the International Society for Twin Studies. 2006;9:899–906. doi: 10.1375/183242706779462462. [DOI] [PubMed] [Google Scholar]
- 15.Meyre D, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41:157–9. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 16.So HC, Gui AH, Cherny SS, Sham PC. Evaluating the heritability explained by known susceptibility variants: a survey of ten complex diseases. Genet Epidemiol. 2011;35:310–7. doi: 10.1002/gepi.20579. [DOI] [PubMed] [Google Scholar]
- 17.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–51. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 18.Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. Jama. 1986;256:51–4. [PubMed] [Google Scholar]
- 19.Wen W, et al. Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet. 2012;44:307–11. doi: 10.1038/ng.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry GH, et al. Diet and the evolution of human amylase gene copy number variation. Nat Genet. 2007;39:1256–60. doi: 10.1038/ng2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterton RT, Jr., Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol. 1996;16:433–48. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 22.Park KS. Heritability of urine and plasma amylase activity. Jinrui Idengaku Zasshi. 1977;22:79–88. doi: 10.1007/BF01874272. [DOI] [PubMed] [Google Scholar]
- 23.Marques-Bonet T, et al. A burst of segmental duplications in the genome of the African great ape ancestor. Nature. 2009;457:877–81. doi: 10.1038/nature07744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squires BT. Human salivary amylase secretion in relation to diet. J Physiol. 1953;119:153–6. doi: 10.1113/jphysiol.1953.sp004835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mifflin TE, Hortin G, Bruns DE. Electrophoretic assays of amylase isoenzymes and isoforms. Clin Lab Med. 1986;6:583–99. [PubMed] [Google Scholar]
- 26.Mirski A. Metabolism of adipose tissue in vitro. Biochem J. 1942;36:232–41. doi: 10.1042/bj0360232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bingham SA, et al. Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public health nutrition. 2001;4:847–58. doi: 10.1079/phn2000102. [DOI] [PubMed] [Google Scholar]
- 28.Kondo T, Hayakawa T, Shibata T, Sato Y, Toda Y. Serum levels of pancreatic enzymes in lean and obese subjects. Int J Pancreatol. 1988;3:241–8. doi: 10.1007/BF02788453. [DOI] [PubMed] [Google Scholar]
- 29.Schneeman BO, Inman MD, Stern JS. Pancreatic enzyme activity in obese and lean Zucker rats: a developmental study. J Nutr. 1983;113:921–5. doi: 10.1093/jn/113.4.921. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima K, et al. Low serum amylase in association with metabolic syndrome and diabetes: A community-based study. Cardiovasc Diabetol. 2011;10:34. doi: 10.1186/1475-2840-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima K, Muneyuki T, Munakata H, Kakei M. Revisiting the cardiometabolic relevance of serum amylase. BMC Res Notes. 2011;4:419. doi: 10.1186/1756-0500-4-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandel AL, Breslin PA. High Endogenous Salivary Amylase Activity Is Associated with Improved Glycemic Homeostasis following Starch Ingestion in Adults. J Nutr. 2012 doi: 10.3945/jn.111.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parks BW, et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell metabolism. 2013;17:141–52. doi: 10.1016/j.cmet.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson CL, et al. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity. 2012;20:2257–61. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 35.Walters RG, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463:671–5. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bochukova EG, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–70. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacquemont S, et al. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature. 2011;478:97–102. doi: 10.1038/nature10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willer CJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarick I, et al. Novel common copy number variation for early onset extreme obesity on chromosome 11q11 identified by a genome-wide analysis. Hum Mol Genet. 2011;20:840–52. doi: 10.1093/hmg/ddq518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sha BY, et al. Genome-wide association study suggested copy number variation may be associated with body mass index in the Chinese population. J Hum Genet. 2009 doi: 10.1038/jhg.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speliotes EK, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarroll SA, et al. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet. 2008;40:1166–74. doi: 10.1038/ng.238. [DOI] [PubMed] [Google Scholar]
- 43.Locke DP, et al. Linkage disequilibrium and heritability of copy-number polymorphisms within duplicated regions of the human genome. Am J Hum Genet. 2006;79:275–90. doi: 10.1086/505653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes C, et al. A robust statistical method for case-control association testing with copy number variation. Nat Genet. 2008;40:1245–52. doi: 10.1038/ng.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomedical and environmental sciences : BES. 2002;15:83–96. [PubMed] [Google Scholar]
- 46.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 47.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.