Abstract
Purpose
Digital PCR is a highly accurate method of determining DNA concentration. We adapted digital PCR to determine the presence of oncogenic amplification through non-invasive analysis of circulating free plasma DNA, and exemplify this approach by developing a plasma DNA digital PCR assay for HER2 copy number.
Experimental design
The reference gene for copy number assessment was assessed experimentally and bioinformatically. Chromosome 17 peri-centromeric probes were demonstrated to be suboptimal, and EFTUD2 at chromosome position 17q21.31 was selected for analysis. Digital PCR assay parameters were determined on plasma samples from a development cohort of 65 patients, and assessed in an independent validation cohort of plasma samples from 58 patients with metastatic breast cancer. The Sequential Probability Ratio Test was used to assign the plasma DNA digital PCR test as being HER2 positive or negative in the validation cohort.
Results
In the development cohort, the HER2:EFTUD2 plasma DNA copy number ratio had a receiver operator curve AUC 0.92 (95% CI 0.86 to 0.99, P=0.0003). In the independent validation cohort, 64% (7/11) of patients with HER2 amplified cancers were classified as plasma digital PCR HER2 positive, and 94% (44/47) of patients with HER2 non-amplified cancers were classified as digital PCR HER2 negative, with a positive and negative predictive value of 70% and 92% respectively.
Conclusion
Analysis of plasma DNA with digital PCR has the potential to screen for the acquisition of HER2 amplification in metastatic breast cancer. This approach could potentially be adapted to the analysis of any locus amplified in cancer.
Keywords: Breast Cancer, HER2, plasma DNA, digital PCR
Introduction
Genomic amplifications present important therapeutic targets as demonstrated by the efficacy of the HER2 targeting antibody trastuzumab in patients with HER2 (ERBB2) amplified breast and gastric cancers (1, 2). In routine clinical practice, the presence of an amplification is determined by analysis of a tumour biopsy at initial diagnosis. However, amplifications can be ‘acquired’, and lost, through tumour progression and prior treatment (3, 4), and this presents a substantial challenge to the concept of personalized cancer therapy. For example, HER2 amplification is ‘acquired’ in ~2-5% of metastatic breast cancers that originally had HER2 non-amplified primary cancers (4), MET amplification may be ‘acquired’ as a mechanism of resistance to EGFR inhibitor therapy in non-small cell lung cancer (5), and amplification of c-MYC may be a common mechanism of resistance to many targeted therapies (6). The underlying biology behind ‘acquisition’ of amplifications at least in part reflect intra-tumour heterogeneity and clonal selection (7).
To optimally deliver targeted therapy, repeated sampling of a tumour is therefore required to determine whether the genetic profile of a cancer has altered following prior therapy. In current practice this would require repeated biopsies of recurrent and metastatic cancers, yet this approach has limitations. Biopsy has associated risks and may be technically challenging depending on the site(s) of relapsed cancer. Biopsy usually samples only a single area of tumour, and in heterogeneous tumours may underestimate the array of genetic aberrations present (8). Ideally, to overcome these limitations, and to allow repeated sampling, the presence of amplification could be diagnosed non-invasively.
DNA arising from tumour cells is found in the plasma of patients with cancer and this represents a potential source of non-invasively analyzing tumour DNA (9). Indeed, high sensitivity assays of coding mutations on plasma DNA, also referred to as cell free DNA or circulating free DNA, have reported high concordance with cancer mutational status (10, 11). Assays of plasma DNA are non-invasive, can be repeated at multiple occasions throughout the disease course, and potentially may assess the full heterogeneity of mutations present. Analysis of plasma DNA requires an assay of high sensitivity as DNA is frequently present at only low concentration in plasma and tumour cell derived DNA may be only a small fraction of the total plasma DNA (9, 12), with the remainder being derived from somatic cells.
Digital PCR has the potential to highly accurately quantify the concentration of nucleic acids in a sample, to a much greater degree than traditional quantitative PCR, through counting individual DNA molecules (13). We investigated whether digital PCR could be adapted to detect small increases in plasma DNA gene copy number that accompany a cancer specific amplification, in a similar fashion to the diagnosis of foetal aneuploidy on the basis of maternal plasma DNA analysis (14). To examine the potential of digital PCR for amplification detection we developed an assay for HER2, ultimately demonstrating that digital PCR has high concordance with tumour derived HER2 status in an independent validation set.
Materials and Methods
Patient cohort
Blood samples were obtained from a consecutive prospective series of patients with metastatic breast cancer treated at the Royal Marsden Hospital between 2010 and 2012. All patients had recently progressed following prior therapy. Patients were allowed to be taking maintenance therapies such as hormone therapy or trastuzumab at the time of plasma sampling. ER, PR, and HER2 were assessed in a single laboratory at the Royal Marsden Hospital Histopathology department. A tumour was considered to be HER2 positive if 3+ positive by Hercept® test, or 2+ positive with a FISH/SISH HER2:CEP17 ratio ≥ 2.2 (15). For patients who had biopsy of recurrent cancer, pathology of the recurrent cancer biopsy was compared with digital PCR, and for the other patients the pathology of the original primary cancer was used. Patients who presented primary breast cancer simultaneously with metastatic disease were recorded as having biopsy of recurrent cancer. Research was approved by the Royal Marsden Hospital Research Ethics Committee.
Identification of reference region on chromosome 17
We utilised microarray comparative genomic hybridisation data from 311 invasive breast cancers, 65 HER2 amplified and 246 HER2 non-amplified, to identify an optimal chromosome 17 copy number reference region(16). The copy number ratio between the mean of all probes covering ERBB2 (HER2) and every BAC probe on chromosome 17 was assessed for each cancer. For each BAC probe the sensitivity comparing HER2 amplified and non-amplified cancers was calculated, as was the statistical significance of the difference between HER2 amplified and non-amplified cancers with the Student’s T test. The sensitivity was assessed as the proportion of HER2 amplified cancers that had a copy number ratio higher than the maximum ratio of the HER2 non-amplified cancers. All genomic positions were according to genome version hg19. TCGA data was from SNP pipeline 3.0 data from the BROAD institute (http://www.broadinstitute.org/tcga/).
Plasma DNA collection and quantification
Plasma was collected in CPT tubes (BD Biosciences) and centrifuged within 2 hours of venesection. DNA was extracted with QIAamp MinElute virus spin kit (Qiagen) essentially according to manufacturers instruction and quantified as described in Supplementary methods.
Digital PCR
Digital PCR for HER2: UBBP4 was performed in 384 well format as discussed in supplementary methods. Digital PCR for HER2 : EFTUD2 was performed with the Bio-Rad QX100 system using custom primers against HER2 and EFTUD2 reference. DNA was diluted to aim for ~400 copies per well and partitioned into ~14,000 droplets as per manufacturer instructions. PCR reactions were run on G-Storm GS4 thermal cycler incubating the plates at 95 °C for 10 min followed by 40 cycles of 95°C for 15 sec, 60 °C for 60 sec, followed by 10 min incubation at 98 °C. Plates were read on a Bio-Rad QX100 droplet reader using QuantaSoft v1.2.10.0 software from Bio-Rad to assess the number of droplets positive for HER2, EFTUD2, both or neither.
Digital PCR analysis development cohort
Assessment of peri-centromeric UBBP4 and TUFML probes is described in supplementary methods. HER2 : EFTUD2 copy number ratio droplet digital PCR was analysed in the development cohort by calculating the copies per droplet from the Poisson distribution. We aimed for at least 400 droplets positive for EFTUD2 to accurately assess the ratio. The development cohort was analysed with a receiver operator curve.
Digital PCR analysis of independent validation cohort with Sequential Probability Ratio Test
The validation cohort was evaluated using the Sequential Probability Ratio Test (SPRT) with a likelihood ratio of 8, as previously reported with modifications (14, 17). The thresholds for the SPRT were assessed from the development cohort prior to prospective analysis of the validation cohort. For the SPRT only informative droplets were analyzed, those droplets positive for either HER2 alone or control probe alone. Analysis with SPRT is discussed in detail in supplementary methods. Samples were assessed blinded to HER2 amplification status.
Results
Pericentromeric probes are suboptimal for HER2 digital PCR assessment
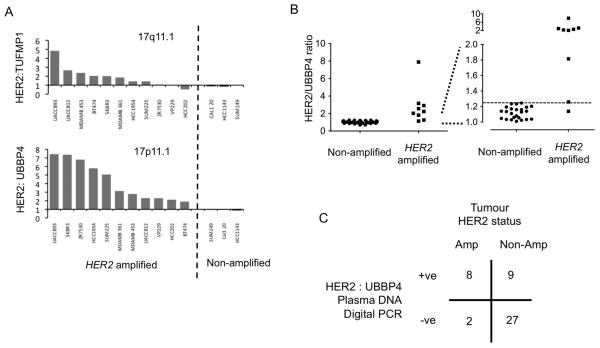
To assess the potential of plasma DNA digital PCR we optimized an assay for HER2 copy number. HER2 copy number in digital PCR is assessed relative to a reference gene, and we initially assessed two potential pericentromeric genes adjacent to the chromosome 17 centromere (CEP17), centromeric DNA itself consisting predominantly of highly repetitive alpha-satellite DNA not suited to the design of a specific PCR amplicon. We designed and optimized a set of custom primer-probes using TaqMan MGB chemistry (Applied Biosystems), with a HER2 probe labeled with FAM and reference probes labeled with VIC. We selected reference probes through avoidance of known single nucleotide polymorphisms and regions of normal copy number variation (as discussed in supplementary methods). The 17q pericentromeric gene TUFML was rejected as a reference probe due to co-amplification of 17q peri-centromeric DNA in some HER2 amplified cancers (Figure 1A), and we therefore assessed the 17p centromeric gene UBBP4.
Figure 1. Digital PCR assessment with chromosome 17 peri-centromeric probes.
A. Assessment of reference probes for digital PCR. HER2:reference copy number ratio from a single 384 well digital PCR assessment of DNA from 11 HER2 amplified cell lines and 3 non-amplified cell lines. The HER2:UBBP4 (17p11.1) ratio is substantially elevated in all amplified cancer cell lines, but the HER2:TUFMP1 is not raised in 3 HER2 amplified cell lines due to co-amplification of 17q peri-centromeric DNA (Supplementary Figure 1).
B. Development cohort for UBBP4 reference probe. Plasma DNA digital PCR HER2:UBBP4 ratio from 9 patients with HER2 positive and 35 patients with HER2 negative cancers. Left full data-set and Right expanded y axis for ratios 1.0-2.0, dashed line indicates a ratio of 1.25.
C. Assessment of HER2:UBBP4 in an independent validation cohort. Tabulated results of plasma DNA digital PCR analysed by SPRT on plasma samples from 10 patients with HER2 amplified cancer and 36 patients with HER2 non-amplified cancer. p=0.003 Fishers Exact test.
For the assessment of HER2:UBBP4 ratio we used 384 well microplate digital PCR (Supplementary Figures 1 and 2). We first determined the optimal threshold to define an elevated plasma DNA Digital PCR HER2:UBBP4 ratio in a development set of plasma samples taken from patients with metastatic breast cancer. Although a HER2:CEP17 ratio of ≥2.0 is used when directly analyzing tumour DNA, for example by fluorescent in situ hybridization, a lower threshold is required in the analysis of plasma DNA as tumour derived DNA is only a small fraction of total plasma DNA, with the majority being derived from stromal/normal genomic DNA (12). In the development set of 44 patients (described in Supplementary Table 1) the digital PCR HER2:UBBP4 ratio in patients with HER2 amplified cancers (median 2.231, range 1.138-7.89) was significantly elevated compared to HER2 non-amplified cancers (median 1.046, range 0.70-1.245, p<0.001 Mann Whitney U test) (Figure 1B), with an ROC curve AUC 0.967 (95% CI 0.585-0.997). A threshold to define a HER2 positive cancer was selected as 1.25 in digital PCR to accommodate a small decrease in specificity.
We assessed the assay in an independent validation cohort of 46 patients (Supplementary table 1). In the validation set we utilized the sequential probability ratio test (SPRT) to assign a sample as being from a patient with HER2 amplified (HER2 positive) or non-amplified cancer (HER2 negative). The SPRT uses Bayesian likelihood methods to assess whether after each round of digital PCR the results should be assigned as HER2 positive, HER2 negative, or unassigned requiring further rounds of digital PCR to determine the HER2 status (Supplementary Figure 1). We utilized only informative wells in the SPRT, as the informative wells ratio modestly amplifies small ratios (14). The SPRT parameters were set with a likelihood ratio of 8 to differentiate between a ratio of 1.3 as HER2 positive and 1.2 as HER2 negative (corresponding to the 1.25 threshold, Supplementary methods). In the independent validation cohort, of the patients with HER2 amplified cancers 80% (8/10) were classified as digital PCR HER2 positive, and with HER2 non-amplified cancers 75% (27/36) were classified as digital PCR HER2 negative (Figure 1C, p=0.003 Fisher’s exact test) providing a highly significant proof of principle. However, the specificity of 75% and positive predictive value of 47%, questioned the potential clinical usefulness of such an assay based on the UBBP4 peri-centromeric probe. Loss of chromosome arm 17p occurs in ~10-15% of HER2 non-amplified cancers as a result of genomic chromosomal instability (16, 18),. The resulting loss of one copy of UBBP4 in the tumour may cause a false positive due to elevation of the HER2:UBBP4 ratio not because of HER2 gain but due to UBBP4 loss.
Identification of a superior chromosome 17 reference probe
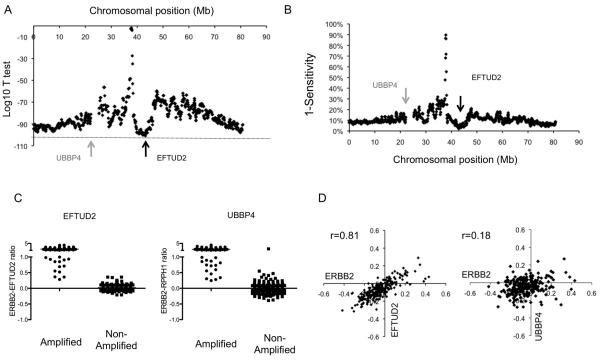
We bio-informatically examined for a potentially superior copy number reference region on chromosome 17. An optimal reference region would be one that in HER2 amplified cancers is never co-amplified with HER2, and in non-amplified cancers robustly has the same stable copy number as HER2. We examined publically available microarray comparative genomic hybridization (array CGH) profiles of 311 invasive breast cancers (16). We assessed the ERBB2 (HER2) to reference copy number ratio for every genomic position across chromosome 17, and examined for the genomic region that gave the most significantly different copy number ratios between HER2 amplified and non-amplified cancers, and that had the highest sensitivity (Figure 2A and 2B).
Figure 2. Identification of an optimal chromosome 17 reference probe for digital PCR assay.
A. Analysis of microarray CGH data from 311 primary breast cancers (16). For each cancer the ERBB2 (HER2) to reference copy number ratio was calculated for every genomic position along chromosome 17. The ERBB2 to reference copy number ratio of HER2 amplified cancers was compared with HER2 negative cancers using Student’s T test. Displayed is the log 10 p value for each genomic position, with arrow indicating the locus of EFTUD2 with the most significant difference between amplified and non-amplified cancers.
B. The corresponding sensitivity for each reference genomic position was assessed for each genomic position.
C. Comparison of ERBB2 : EFTUD2 and ERBB2 : UBBP4 copy number ratios in HER2 amplified and non-amplified cancers from the same micro-array CGH series(16).
D. Correlation of ERBB2 and EFTUD2, along with ERBB2 and UBBP4, copy number in the 246 HER2 non-amplified cancers. The copy number of ERBB2 and EFTUD2 are highly correlated as low level gain or loss of ERBB2 extends to EFTUD2. EFTUD2 therefore is predicted to generate stable copy number ratios in the analysis of non-amplified cancers.
A region on chromosome 17q21.31 from ~42.2Mb to ~43.9Mb was identified as the optimal region by this assessment, ~5Mb telomeric of the ERBB2 (HER2) locus. This region was co-amplified with the ERBB2 locus in none of the 65 HER2 amplified cancers in the series. The EFTUD2 gene was selected in this region (42.93-42.98Mb) as not being subject to normal copy number variation (Supplementary methods). In HER2 non-amplified cancers the EFTUD2 locus had a highly stable copy number ratio with the ERBB2 locus (Figure 2C and D), to a substantially greater extent than UBBP4 (Figure 2C and D), and therefore EFTUD2 would be anticipated to have more consistent copy number ratio in non-amplified cancers. We analysed SNP copy number data from the TCGA data set, and confirmed that EFTUD was co-amplified with ERBB2 in none of the 110 cancers with focal ERBB2 amplification (19). Therefore considering both data sets EFTUD2 was co-amplified with ERBB2 in none of 175 HER2 amplified cancers.
Digital PCR with EFTUD2 control probe has high diagnostic accuracy
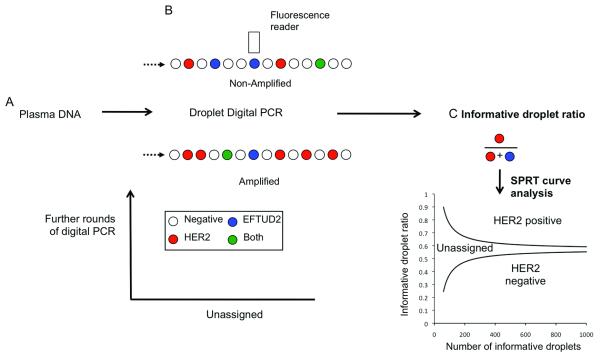
We determined the parameters of a HER2 : EFTUD2 digital PCR assay. For this we switched to a specific digital PCR platform, due to the development of relatively inexpensive dedicated platforms, that partition a PCR reaction into ~14,000 droplets (20). Following PCR each droplet is individually assessed by a fluorescent reader to effectively assay ~14,000 individual PCR reactions (Figure 3 and Supplementary Figure 3). The assay parameters for a plasma HER2 : EFTUD2 assay were determined in a development set of 65 patients (described in Table 1). Analysis of the development set by ROC had an AUC 0.92 (95% CI 0.86 to 0.99, P=0.0003) (Figure 4A). Assessing the 58 patients with HER2 non-amplified cancer in the development set we selected a cut-off of 1.25 as this was estimated to give a specificity of ~95%.
Figure 3. Digital PCR assay for HER2 copy number assessment by droplet digital PCR.
A. Plasma is separated within 2 hours of venepuncture and stored at −80°C before extraction of free circulating DNA.
B. Droplet Digital PCR with a FAM labeled HER2 probe and VIC labeled EFTUD2 (reference) probe. DNA is partitioned into ~14,000 droplets per reaction. After single molecule PCR droplets are assessed by a fluorescent reader. The concentration of DNA in each sample can be quantified from the number of wells positive using the Poisson distribution.
C. Validation cohort: Analysis of digital PCR with Sequential Probability Ratio Test (SPRT) using informative droplets, those droplets positive for HER2 or EFTUD2 alone, and not those positive for both or neither. The SPRT assesses whether the proportion of informative wells positive for HER2, informative wells ratio, is elevated as data accumulates. SPRT defines two boundaries, with a ratio above the upper boundary being considered HER2 positive and below the lower boundary considered HER2 negative. A ratio between the two boundaries is considered as unassigned, and the sample is subjected to further rounds of digital PCR until the result is above or below the boundaries.
Table 1.
Clinicopathological characteristics of patients included in study
| All patients | Development Set |
Validation set | |
|---|---|---|---|
| n | 123 | 65 | 58 |
| Median Age | 60 (30-83) | 61 (30-83) | 59 (33-80) |
| Pathology | |||
| IDC | 105 | 56 | 49 |
| ILC | 12 | 6 | 6 |
| Other | 3 | 3 | 0 |
| Histological Grade | |||
| 1 | 4 | 3 | 1 |
| 2 | 50 | 25 | 25 |
| 3 | 59 | 30 | 29 |
| unknown | 10 | 7 | 3 |
| ER positive | 86 | 48 | 38 |
| HER2 positive | 20 | 9 | 11 |
| HER2 testing site | |||
| Primary | 54 | 35 | 19 |
| Recurrent | 69 | 30 | 39 |
| Sites metastatic cancer visceral |
88 | 46 | 42 |
| non visceral | 35 | 19 | 16 |
| Adjuvant Chemotherapy | 39 | 31 | |
| Adjuvant Tamoxifen | 37 | 30 | |
| No prior metastatic chemo | |||
| 0 | 33 | 26 | |
| 1 | 15 | 12 | |
| 2 | 11 | 12 | |
| 3 | 5 | 5 | |
| 4 | 1 | 3 | |
| Prior HER2 directed therapy | 9 | 11 |
IDC – invasive ductal carcinoma, ILC invasive lobular carcinoma. No prior metastatic chemo – Number of prior courses of chemotherapy in the metastatic setting
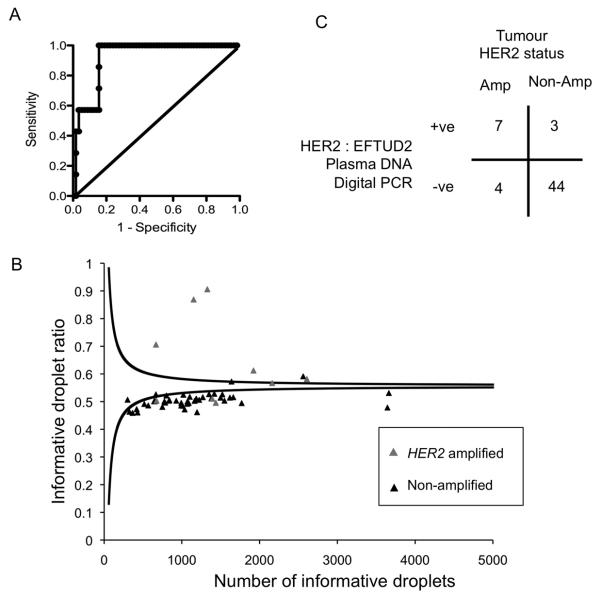
Figure 4. Plasma DNA digital PCR with the EFTUD2 reference probe has high diagnostic accuracy in an independent cohort.
A. Development cohort for EFTUD2 reference probe. Plasma DNA digital PCR HER2:EFTUD2 ratio was assessed in plasma samples from 65 patients with metastatic breast cancer, consisting of 7 with HER2 amplified cancer and 58 with HER2 non-amplified cancer. ROC analysis with an AUC 0.92 (95% CI 0.86 to 0.99, P=0.0003). A cut-off of 1.25 was selected to define HER2 amplification in the validation series.
B. Assessment of HER2:EFTUD2 in an independent validation cohort. Plasma DNA digital PCR results analysed by SPRT on plasma samples from 11 patients with HER2 amplified cancers and 47 patients with HER2 non-amplified cancers. Grey triangle indicates patients with HER2 amplified tumours and black triangle HER2 non-amplified tumours. The displayed SPRT decision boundaries are for illustrative purposes only, as the exact level varies according to the EFTUD2 control probe concentration (MEFTUD2), with the displayed boundaries calculated with MEFTUD2=0.025. Cases with a number of informative droplets > 5000 are not displayed.
C. Tabulated results of Plasma DNA digital PCR analysed by SPRT on independent validation cohort. p=0.0001 Fishers Exact test.
The HER2 : EFTUD2 plasma DNA test was assessed in an independent prospectively collected validation cohort of 58 patients (Supplementary Figure 4). The SPRT was again utilized to analyse the validation set, with a likelihood ratio of 8 to differentiate between a HER2 : EFTUD2 ratio of 1.3 as HER2 positive and 1.2 as HER2 negative (corresponding to the 1.25 threshold). Of the patients with HER2 amplified cancers 64% (7/11) were classified as plasma DNA digital PCR HER2 positive, and of the patients with HER2 non-amplified cancer 94% (44/47) were classified as plasma DNA digital PCR HER2 negative (p=0.0001 Fisher’s Exact test, Figure 4B and C). The positive predictive value was 70% and negative predictive value 92%.
Discussion
We have demonstrated that analysis of plasma DNA with digital PCR has high accuracy in the determination of HER2 status. An assay based on the plasma DNA HER2 : EFTUD2 ratio had a high concordance rate of 90% with tumour derived HER2 status in an independent validation set. This degree of concordance is similar to that reported between local and central laboratories for routine clinical HER2 testing on biopsy material (21), and would potentially be sufficient to use this test to screen for cancers that have acquired HER2 amplification in the metastatic setting. The potential clinical utility of such a test would be in assessing patients who have not had biopsy of recurrent breast cancer as part of their routine care. Although biopsy of recurrent disease is a standard of care, to confirm diagnosis and reassess hormone receptor and HER2 status, recurrent disease is in routine clinical practice frequently not biopsied when such a biopsy is technically challenging and there is no diagnostic doubt that relapse has occurred. A non-invasive test to screen for acquisition of HER2 amplification in such circumstances would have potentially high clinical utility.
Breast cancers are frequently chromosomally unstable and this presents a challenge to the development of a plasma DNA digital PCR assay for HER2. To achieve high accuracy, HER2 positive cancers with a low fraction of tumour derived DNA in plasma (plasma DNA highly diluted with normal germline DNA) must be discriminated from aneuploid HER2 negative cancers with a high fraction of tumour derived DNA in the plasma. The factors that are required to discriminate between these two scenarios are a reference probe that has a highly stable copy number with HER2 in non-amplified cancers, and yet is robustly not amplified with HER2. In assessing patients with non-amplified cancers, a reference probe with a highly stable copy number to HER2 will robustly have a copy number ratio of 1 regardless of the fraction of tumour derived DNA in the plasma. We describe an approach to bioinformatically identify a reference probe with these characteristics, exploiting the well-described genomic landscape of breast cancer, and demonstrate improved specificity of the identified EFTUD2 region over peri-centromeric probes (Supplementary Figure 5 and 6). RNase P (RPPH1) on chromosome 14q11.2 is frequently used as a reference probe in assays of normal copy number variation, as it is not subject to normal copy number variation. However, utilizing a control probe from a different chromsome will not deliver optimal characteristics for plasma DNA analysis in aneuploid cancers where highly accurate discrimination in copy number is required (Supplementary Figure 5).
The results we report show substantially higher concordance with tumour derived HER2 status compared to prior reports of non-invasive approaches. Quantitative real-time PCR assessment of HER2 copy number in plasma DNA has only low concordance (22). Similarly, studies assessing HER2 status by immunofluoresece or FISH on circulating tumour cells (CTCs) have reported a substantial positive CTC rate in originally HER2 negative cancers (23, 24), although more recent studies with strict cut-offs report a higher level of concordance (25). No studies have yet compared CTC HER2 assessment with digital PCR to establish which is the more robust way of identifying cancers that may derive benefit from HER2 targeting in the metastatic setting. Other techniques such as massive parallel sequencing of circulating free DNA may present another potential method for detection of amplifications (26), although the application of such technology to plasma DNA is at an early stage. BEAMing, which has been used for mutation detection in plasma (10, 27) could also be adapted for copy number detection. Although tumour derived DNA can be detected in the plasma of patients with early breast cancer, or without apparently overt metastatic disease (28), in the majority of patients insufficient plasma DNA is present to allow for the formal digital PCR analysis we describe (data not shown).
For the cases of discordance between tumour derived HER2 status and plasma DNA digital PCR we reassessed HER2 status by in situ hybridization on the corresponding tumour samples (Supplementary Table 2). Of the four patients that were originally defined as HER2 amplified in the cancer, but were HER2 negative by plasma DNA digital PCR (Figure 4C), one tumour was not HER2 amplified on re-testing (Supplementary Table 2). This suggested that this case may have been correctly called negative by digital PCR assay and therefore that the positive predictive value of the test may be higher than reported in the validation cohort. Interesting a further false negative patient had a positive but low level of HER2 amplification (Supplementary Table 2).
It will be interesting in future research to ascertain the reasons for discordance between tumour HER2 status and plasma DNA digital PCR status. The three patients with apparent false positive plasma digital PCR tests in the validation set may represent genuine false positive tests due to low level loss of EFTUD2 combined with a high fraction of tumour cell derived DNA in the plasma. Alternatively, these patients may possibly also have acquired HER2 amplification. Interestingly in the development set one HER2 non-amplified cancer was classified as unequivocally digital PCR HER2 positive, with a HER2:EFTUD2 ratio of 16.9 (Supplementary Figure 6). This was confirmed on a separate plasma sample from the patient with the same result (data not shown). For this patient, tumour HER2 status had been determined on her original primary cancer, as her metachronous metastatic cancer had not been not biopsied as part of her routine care.
Considering the three patients with apparently false negative digital PCR tests, again the discordance might reflect a genuine change in HER2 status. All patients with apparently false negative plasma DNA tests had received prior HER2 directed therapy in the metastatic setting, that might in tumours with intra-tumoural heterogeneity select for outgrowth of a non-amplified clone(29). However, a potential other explanation would be a very low percentage of tumour derived DNA content in plasma DNA, combined with a relatively low level of HER2 amplification, causing a genuine false negative test.
In this study we demonstrate that digital PCR of plasma DNA has high accuracy in the determination of HER2 status. This approach could be adapted to the assessment of any amplified locus in cancer, and in particular may be a useful strategy screening for potentially rare acquisition events in response to therapy, such as acquisition of MET amplification following EGFR targeting therapy in lung cancer (5). Combined with sensitive coding mutation assays, our data suggest that it may be possible to replace routine repeat biopsies of metastatic cancer for the optimal delivery of targeted therapies.
Supplementary Material
Statement of translational relevance.
We show that cancer HER2 status can be estimated with high accuracy through analysis of free circulating plasma DNA. The particular utility of this test would be in the identification of originally HER2 negative primary cancers that relapse with HER2 positive metastatic cancer where biopsy of recurrent cancer had not been performed. The approach we develop could be adapted to detect the amplification of potentially any locus that is acquired during tumour progression or secondary to prior therapy.
Acknowledgements
This work was supported by grants from Dr. Mildred Scheel foundation for Cancer Research and Cancer Research UK. Dr Nicholas Turner is a CRUK Clinician Scientist. We acknowledge NHS funding to the NIHR Biomedical Research Centre. We thank Katarzyna Tomczyk and Nicholas Orr for technical assistance..
Financial Support: Dr. Mildred Scheel foundation for Cancer Research and Cancer Research UK CRUK_A10038
Footnotes
The authors have no conflicts of interest.
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 3.Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS) Breast Cancer Res. 2010;12:R92. doi: 10.1186/bcr2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houssami N, Macaskill P, Balleine RL, Bilous M, Pegram MD. HER2 discordance between primary breast cancer and its paired metastasis: tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res Treat. 2011;129:659–74. doi: 10.1007/s10549-011-1632-x. [DOI] [PubMed] [Google Scholar]
- 5.Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilic N, Utermark T, Widlund HR, Roberts TM. PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc Natl Acad Sci U S A. 2011;108:E699–708. doi: 10.1073/pnas.1108237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–13. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson PJ, Lo YM. Plasma nucleic acids in the diagnosis and management of malignant disease. Clin Chem. 2002;48:1186–93. [PubMed] [Google Scholar]
- 10.Li M, Diehl F, Dressman D, Vogelstein B, Kinzler KW. BEAMing up for detection and quantification of rare sequence variants. Nat Methods. 2006;3:95–7. doi: 10.1038/nmeth850. [DOI] [PubMed] [Google Scholar]
- 11.Board RE, Wardley AM, Dixon JM, Armstrong AC, Howell S, Renshaw L, et al. Detection of PIK3CA mutations in circulating free DNA in patients with breast cancer. Breast Cancer Res Treat. 2010;120:461–7. doi: 10.1007/s10549-010-0747-9. [DOI] [PubMed] [Google Scholar]
- 12.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogelstein B, Kinzler KW. Digital PCR. Proc Natl Acad Sci U S A. 1999;96:9236–41. doi: 10.1073/pnas.96.16.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo YM, Lun FM, Chan KC, Tsui NB, Chong KC, Lau TK, et al. Digital PCR for the molecular detection of fetal chromosomal aneuploidy. Proc Natl Acad Sci U S A. 2007;104:13116–21. doi: 10.1073/pnas.0705765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 16.Natrajan R, Mackay A, Wilkerson PM, Lambros MB, Wetterskog D, Arnedos M, et al. Functional characterization of the 19q12 amplicon in grade III breast cancers. Breast Cancer Res. 2012;14:R53. doi: 10.1186/bcr3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou W, Galizia G, Lieto E, Goodman SN, Romans KE, Kinzler KW, et al. Counting alleles reveals a connection between chromosome 18q loss and vascular invasion. Nat Biotechnol. 2001;19:78–81. doi: 10.1038/83572. [DOI] [PubMed] [Google Scholar]
- 18.Andre F, Job B, Dessen P, Tordai A, Michiels S, Liedtke C, et al. Molecular characterization of breast cancer with high-resolution oligonucleotide comparative genomic hybridization array. Clin Cancer Res. 2009;15:441–51. doi: 10.1158/1078-0432.CCR-08-1791. [DOI] [PubMed] [Google Scholar]
- 19.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83:8604–10. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Press MF, Sauter G, Bernstein L, Villalobos IE, Mirlacher M, Zhou JY, et al. Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res. 2005;11:6598–607. doi: 10.1158/1078-0432.CCR-05-0636. [DOI] [PubMed] [Google Scholar]
- 22.Page K, Hava N, Ward B, Brown J, Guttery DS, Ruangpratheep C, et al. Detection of HER2 amplification in circulating free DNA in patients with breast cancer. Br J Cancer. 2011;104:1342–8. doi: 10.1038/bjc.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehm T, Muller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010;124:403–12. doi: 10.1007/s10549-010-1163-x. [DOI] [PubMed] [Google Scholar]
- 24.Ignatiadis M, Rothe F, Chaboteaux C, Durbecq V, Rouas G, Criscitiello C, et al. HER2-positive circulating tumor cells in breast cancer. PLoS One. 2011;6:e15624. doi: 10.1371/journal.pone.0015624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pestrin M, Bessi S, Puglisi F, Minisini AM, Masci G, Battelli N, et al. Final results of a multicenter phase II clinical trial evaluating the activity of single-agent lapatinib in patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells. A proof-of-concept study. Breast Cancer Res Treat. 2012;134:283–9. doi: 10.1007/s10549-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 26.Leary RJ, Sausen M, Kinde I, Papadopoulos N, Carpten JD, Craig D, et al. Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing. Sci Transl Med. 2012;4:162ra54. doi: 10.1126/scitranslmed.3004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diehl F, Li M, He Y, Kinzler KW, Vogelstein B, Dressman D. BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat Methods. 2006;3:551–9. doi: 10.1038/nmeth898. [DOI] [PubMed] [Google Scholar]
- 28.Shaw JA, Page K, Blighe K, Hava N, Guttery D, Ward B, et al. Genomic analysis of circulating cell-free DNA infers breast cancer dormancy. Genome Res. 2012;22:220–31. doi: 10.1101/gr.123497.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13:e178–85. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.