Abstract
Background
Early stage multiple myeloma (MM) represents about 20% of MM. Most of the patients are asymptomatic. Thus, it is far less dramatic than advanced disease and may require different treatment strategies. For these patients, it is not clear whether it is better to start chemotherapy right after the diagnosis or to delay the treatment until symptoms become obvious as the disease progresses.
Objectives
To identify and synthesize all available research evidence on whether early treatment intervention results in improved clinical outcomes when compared with observation alone. The main outcomes of interest that were examined included mortality, disease progression, response rate, and toxicity of early treatment.
Search methods
Searches of the following electronic databases were undertaken: MEDLINE, EMBASE, CANCERLIT, LILLIACS and Cochrane Database of RCTs. We have recently compiled a comprehensive database of RCTs in myeloma. This search was updated and supplemented by hand‐search of abstracts from main society meetings such as the ASH (American Society of Hematology), ASCO (American Society of Clinical Oncology), and EHA (European Haematology Association) . In addition, we compared our list with a list of RCTs maintained by the Oxford Clinical Trial Service Unit.
Selection criteria
Randomized controlled trials (RCT) with a parallel design that compared early versus deferred treatment of patients with early stage multiple myeloma based on Durie‐Salmon (D‐S) staging system. We also considered those trials that did not define early stage myeloma according to D‐S staging system, but enrolled patients according to clinical uncertainty about the benefits of immediate intervention.
Data collection and analysis
Data synthesis was performed for all studies and according to the defined quality criteria. The first reviewer and the contact reviewer of this proposal independently extracted data. Disagreement was resolved by consensus. Revman software (4.1) was used to combine results from all studies and expressed as an overall odds ratio or Peto's Odds Ratio, with 95% confidence interval.
Main results
Three trials were included with a total of 131 patients in each of the early treatment and deferred treatment groups. Early MM is asymptomatic stage I in these trials. All trials used standard Melphalan treatment but not stem cell transplantation. No statistically significant heterogeneity among the studies was detected. Beneficial effects of early treatment were seen in delay of myeloma progression (Peto's OR = 0.16, 95% CI: 0.09 to 0.29), and reduced vertebral compression (OR = 0.18, 95%CI: 0.02 to 1.59, NNT = 23, 95% CI: an NNT of 11, via infinity, to an NNH of 50). No significant effects on mortality and response rate were seen (Peto's OR = 1.11, 95% CI: 0.67 to 1.84, and OR = 0.63, 95% CI: 0.33 to 1.23, respectively). Early treatment may increase the risk of acute leukemia (Peto's OR = 3.20, 95% CI: 0.55 to 18.73, NNH = 44, 95% CI: an NNT of 63, via infinity, to an NNH of 15).
Authors' conclusions
Early treatment of early stage multiple myeloma inhibits disease progression, and may reduce vertebral compression. However, early treatment may increase the risk of acute leukemia. However, the data on vertebral compression and leukemic transformation may not be interpretable due to very small numbers. Based on the current evidence, mortality and response rate are not significantly affected by introducing early treatment in the progression of myeloma. However, it is quite possible that the lack of beneficial effects of early intervention in myeloma is a false negative result due to the paucity of the existing evidence. In addition, data on quality of life and toxicity were sparsely reported adding to additional difficulties about management decisions in early stage myeloma.
Keywords: Humans, Disease Progression, Multiple Myeloma, Multiple Myeloma/pathology, Multiple Myeloma/therapy, Randomized Controlled Trials as Topic, Time Factors
Plain language summary
Early treatment for early stage multiple myeloma may slow the disease progression but does not appear to improve survival
Multiple myeloma (MM) is cancer of the bone marrow. It causes bone destruction that leads to pain, spinal cord compression and fractures.In early stages, most people do not show any symptoms of MM. It is not clear whether it is better to start treatment with cancer drugs straight after diagnosis, or to wait until symptoms of the disease appear. The review of trials found that early treatment slows the progression of the disease. However, there is not enough evidence, due to too few studies conducted in patients with early stage myeloma to show that early treatment improves the survival of people with MM.
Background
Multiple myeloma (MM) is a disease characterized by the neoplastic proliferation of a clone of plasma cells secreting immunoglobulins (Alexanian 1994; Hussein 1994). It represents about 10% of hematological malignancies and is the ninth leading cause of cancer deaths in African Americans (Landis 1998). Currently, myeloma is considered an incurable disease. The goal of treatment and prognosis in myeloma depends, to the large extent, on the stage of the disease at presentation (Alexanian 1994; Anderson 1998). In patients who are symptomatic and present in advanced stages, such as a stage IIA to IIIB according to Durie‐Salmon (D‐S) staging system (Durie 1975), the goal of treatment is effective palliation and prolongation of survival. In these patients, a treatment with combined chemotherapy was found not to be superior to conventional chemotherapy of melphalan‐prednisone (MPH‐P) (Myeloma 1998). In recent years, aggressive high‐dose chemotherapy with stem‐cell transplant has emerged as the treatment of choice for advanced, symptomatic myeloma (Attal 1996).
Early stage multiple myeloma, i.e. stage I according to D‐S staging system, represents about 20% of MM (Riccardi 2000). Most of the patients with stage I MM are asymptomatic. Thus, it is far less dramatic than advanced disease and may require different treatment strategies. For patients with stage I MM, it is not clear whether it is better to start chemotherapy right after the diagnosis or to delay the treatment until symptoms become obvious as disease progresses.
Several retrospective studies have suggested that early MM patients do not benefit from aggressive chemotherapies, while more advanced patients do (Harley 1979; Salmon 1983; Cooper 1986). Currently, treatment is not recommended for asymptomatic patients in early stage disease according to D‐S staging system. This recommendation is based on data derived from several small randomized controlled trials. Two randomized studies (Hjorth 1993; Riccardi 1994) have suggested that delayed treatment had no influence on survival as compared with early treatment. Another study has demonstrated that deferring treatment may be a reasonable alternative to immediate chemotherapy, and immediate treatment does not prolong long‐term survival compared with treatment at the disease progression (Riccardi 2000).
To provide the most reliable assessment of existing evidence to guide practitioners regarding optimal therapeutic approach, a research synthesis of the total available evidence on the management of early stage myeloma is needed but has never been performed. It is necessary to perform a systematic review of the available randomized controlled trials to obtain conclusive evidence as to whether early treatment intervention results in improved clinical outcomes for early stage MM patients, when compared with deferred treatment. Based on the totality of the available evidence, better recommendations for practice can be made.
Objectives
To identify and synthesize all available research evidence which attempts to answer the question whether early treatment intervention results in improved clinical outcomes when compared with observation alone. The main outcomes of interest to be examined in this project include mortality, progression, response rate, and toxicity of early treatment.
Methods
Criteria for considering studies for this review
Types of studies
Included: randomized controlled trials (RCT) with a parallel design that compared early versus deferred treatment of patients with early stage multiple myeloma based on Salmon‐Durie (D‐S) staging system. We also considered those trials that did not define early stage myeloma according to D‐S staging system, but enrolled the patients according to clinical uncertainty about the benefits of immediate intervention. No such studies were identified. Patients had to be initially randomized either to observation or to up‐front treatment with chemotherapy. Only studies that included clinical outcomes, such as overall survival, progression‐free survival and/or toxicity of treatment were eligible for our meta‐analysis.
Types of participants
Patients with early stage myeloma according to Salmon‐Durie (D‐S) staging system (stage I), or where there is uncertainty about the benefit of immediate treatment.
Types of interventions
Experimental group: Treatment intervention at early stage of myeloma.
Control group: observation (deferred treatment until progression of the disease).
Types of outcome measures
Primary outcomes
Overall mortality
Progression
Response rate
Secondary outcomes
Toxicity of early treatment
Effects of alkylator therapy (e.g. MDS, and acute leukemia)
Quality of life (pain, fatigue, anxiety)
Search methods for identification of studies
Electronic searches
To identify RCTs of interest, searches of the following electronic databases were undertaken:
MEDLINE,
EMBASE,
CANCERLIT,
LILLIACS and
Cochrane Database of RCTs.
In MEDLINE published studies were identified using comprehensive search strategies for identification of randomized controlled trials (RCTs) described by Dickersin, et al (Dickersin 1994) (1966‐2001) and ( Robinson 2002) for year 2002. This methodologic search strategy was combined with added terms (see Appendix 1).
Cochrane Controlled Trials Register was searched (all years, latest issue 02/2002) using key words (see Appendix 2).
LILACS (1982 June 2002) according to the optimal search strategy described by Castro et al (Castro 1997), with the added terms ((MYELOMA OR MIELOMA) AND (MULTIPLO OR MULTIPLE)).
EMBASE (1974 ‐ December 2000) using the search strategy kindly provided by Julie Glanville of the NHS Centre for Reviews and Dissemination, University of York, UK (combined with added terms related to multiple myeloma, see ):
Searching other resources
All relevant references in each article were also scanned.
Additional strategies used were to contact researchers in the field and handsearched abstracts from the meetings of
ASH (American Society of Hematology),
ASCO (American Society for Clinical Oncology) from 1993 to 2001 and
EHA (European Haematology Association) from 1993 to 2001.
The authors of each selected paper were also contacted. In addition, we compared our list with a list of RCTs maintained by the Oxford Clinical Trial Service Unit.
This search formed a basis for creation of a comprehensive database of RCTs in myeloma (Djulbegovic 2002). This database currently contains information on 165 RCTs in myeloma and 3 meta‐analysis. From this database, 3 trials met our eligibility criteria and were included in our analysis (see below).
We believe that our search has been most comprehensive attempt to date to identify all RCTs in myeloma (Djulbegovic 2001). A comprehensive search is very important because failure to include all studies ‐ published or unpublished ‐ may result in biased results (Clarke 1999).
Data collection and analysis
Quality assessment of the trials and data extraction: To reliably and accurately evaluate any health care intervention, studies have to be of the highest quality. A number of the quality dimensions in the design and conduct of a trial have been described which, if violated, may lead to biased assessment. In general, assessment of the quality revolves about evaluation for possible effect of bias and random error in the design of trials, which will affect the internal and external validity of the trials (Juni 2001; Egger 2001). The most important quality criteria are the appropriateness of randomization, allocation concealment, blinding of assessment of outcomes of interest, intention to treat analysis, pre‐specified b‐error (power analysis), and pre‐specified a‐error (Altman 2001; Verhagen 1998). The effect of random errors is minimized by pooling data in meta‐analysis. A method of Jadad, which combines some of these quality dimensions in a reproducible quality score was also used to complement a component‐based approach in the quality assessment (Jadad 1996; Jadad 1998). We should note that Jadad's scale pays particular attention to the effect of blinding, which in our systematic review is unimportant since the main outcome of interest is death.
Each selected study was assessed according to these quality criteria. Data synthesis was performed for all studies and according to each quality criterion. This allows detection of any bias in the analysis. The first reviewer and the contact reviewer of this proposal independently extracted data. Disagreement was resolved by consensus.
Statistical methods: Since main outcomes of interest are overall mortality and progression, the hazard ratio was extracted for each study using methods described by Parmar et al (Parmar 1998). Revman software (4.1) was used to combine results from all studies and express as an overall odds ratio or Peto's Odds Ratio (Clarke 1999), with 95% confidence interval, where OR = 1.0 indicates no difference between observation and early treatment. Peto's method was used to test for heterogeneity between studies combined in the final analysis, following the RevMan handbook (Clarke 1999). Toxicity was extracted and calculated as the number of patients experiencing a given event using the standard odds ratio (Clarke 1999). After data were extracted, they were then sent to the authors of each identified study for verification and/or update. However, we did not hear back from these authors and thus the data remained the same as were initially extracted.
Results
Description of studies
In our list of 156 RCTs on multiple myeloma, we identified three studies on early versus deferred treatment for early stage disease. 6 additional RCTs were identified in Oxford's database that were not in our list, but none were eligible for our meta‐analysis (Those six RCTs will be supplemented to our RCT list). New updated search of MEDLINE and Cochrane Database of RCTs from 2000 to present identified 5 additional RCTs on multiple myeloma, but none of them were eligible for our analysis either.
Characteristics of included studies shows the characteristics of included studies, and their quality assessment.
Risk of bias in included studies
Two studies got a Jadad score of 3 out of 5 (Riccardi 1994; Riccardi 2000), and the other got a score of 2 (Hjorth 1993). Randomization seems to be adequate in all three studies, while allocation concealment is not well described in one study (Hjorth 1993). Withdrawals and dropouts were described in all three studies. None of the studies described power analysis. The most recent study (Riccardi 2000) had a sample size larger than the others, but overall, sample sizes were very small in those trials.
Effects of interventions
In the three trials eligible for meta‐analysis, a total of 131 patients with early stage multiple myeloma were treated at the diagnosis, compared with 131 patients with same disease treated at the disease progression. Mortality was extractable from all three studies. Progression and response data were incomplete (only available for one group, either the early treatment group or the deferred treatment group) for Hjorth 1993 and Riccardi 1994, respectively, and thus only two studies were combined for those outcomes. There were no extractable data on hematological, gastrointestinal, or renal toxicity (reported by fewer than 2 trials); two trials reported vertebral compression (Hjorth 1993; Riccardi 2000). All three trials reported acute leukemia development.
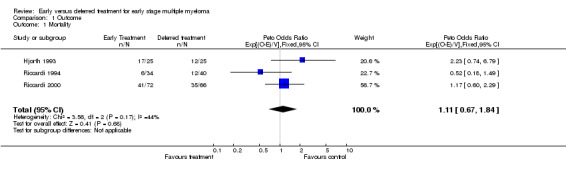
Mortality There were 64 deaths among 131 patients with early treatment, and 59 deaths among 131 patients with deferred treatment. The Peto's OR is 1.11 (95% CI: 0.67 to 1.84, Figure 1). No heterogeneity was detected by the Chi‐square test (Chi‐square = 3.56, df = 2, P = 0.17). Results indicate that there is no evidence of a beneficial effect of early treatment on mortality in patients with early stage multiple myeloma. However, it is possible that this is a false‐negative result (see discussion).
1.
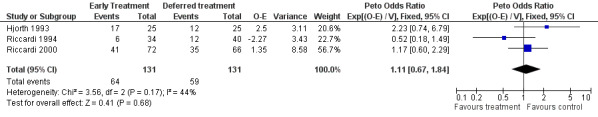
Forest plot of comparison: 1 Outcome, outcome: 1.1 Mortality.
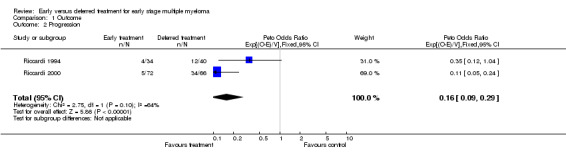
Progression Two studies were included (Riccardi 1994; Riccardi 2000), with a total of 106 patients in the early treatment group and 106 patients in the deferred treatment group. 9 patients progressed after treatment at diagnosis while 46 did when treatment was deferred, resulting in a Peto's OR of 0.16 (95% CI: 0.09 to 0.29, Figure 2), indicating a statistically significant improvement ( P < 0.00001) associated with early treatment. No significant heterogeneity between the two studies was detected (Chi‐square = 2.75, df = 1, P = 0.097).
2.
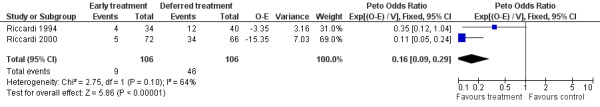
Forest plot of comparison: 1 Outcome, outcome: 1.2 Progression.
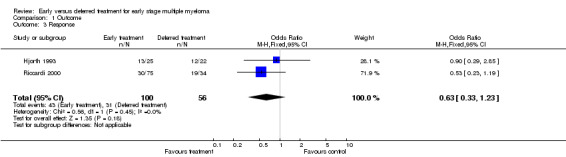
Response Rate Two studies were included (Hjorth 1993; Riccardi 2000), with 100 patients treated at the disease diagnosis and 56 patients treated because of the disease progression. 43 among the 100 and 31 among the 56 responded to treatment, corresponding to an OR of 0.63 (95% CI: 0.33 to 1.23, Figure 3). The difference is not significant, neither is the heterogeneity between the two studies (Chi‐square = 0.56, df = 1, P = 0.45).
3.
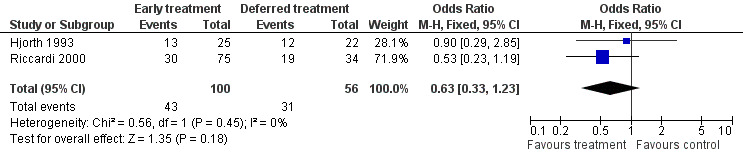
Forest plot of comparison: 1 Outcome, outcome: 1.3 Response.
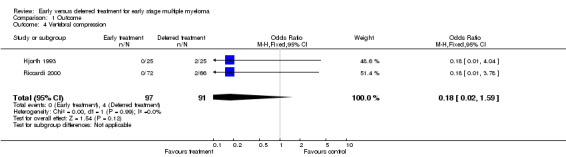
Vertebral compression Two studies reported vertebral compression in the deferred treatment group (Hjorth 1993; Riccardi 2000). None of the 97 patients with early treatment developed a vertebral compression , while 4 out of 91 patients in the deferred treatment group suffered from the disease. OR takes a value of 0.18 (95%CI: 0.02 to 1.59, Figure 4), favoring the early treatment, but without significant difference at conventional statistical level (P = 0.12). The NNT for vertebral compression is 23, with 95% CI from an NNT of 11, via infinity, to an NNH of 50. No heterogeneity was detected (Chi‐square = 1.54, df = 1, P = 0.99).
4.
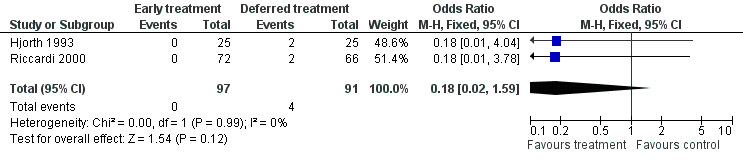
Forest plot of comparison: 1 Outcome, outcome: 1.4 Vertebral compression.
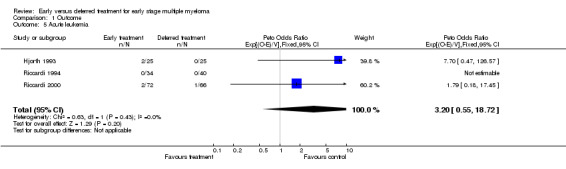
Acute leukemia All three studies provided data on acute leukemia. 4 out of 131 patients in the early treatment group and 1 out of 131 in the deferred treatment group developed acute leukemia, with a Peto's OR of 3.20 (95% CI: 0.55 to 18.73, Figure 5). Results suggest that early treatment may cause more risk for acute leukemia, although the difference does not have enough statistical significance. The NNH for acute leukemia is 44, with 95% CI from an NNT of 63, via infinity, to an NNH of 15. No heterogeneity was found between the two included studies (Chi‐square = 0.63, df = 1, P = 0.43).
5.
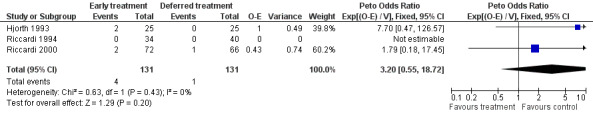
Forest plot of comparison: 1 Outcome, outcome: 1.5 Acute leukemia.
Additional information is shown in Table 1, Table 2, Table 3.
1. Type and content of reporting in RCTs on early stage multiple myeloma (A).
| Study ID | Mortality | Progression | Response | Relapse | Quality of life | Pain |
| Hjorth 1993 | Yes | Incomplete | Yes | No | No | Incomplete |
| Riccardi 1994 | Yes | Yes | Yes | No | No | No |
| Riccardi 2000 | Yes | Yes | Yes | No | No | No |
| Extractable | 3 | 2 | 3 | 0 | 0 | 0 |
2. Type and content of reporting in RCTs on early stage multiple myeloma (B).
| Study ID | Bone disease | Hypercalcemia | Vertebral compress | Hematological | Gastrointestinal | Renal | Leukemia |
| Hjorth 1993 | Incomplete | Incomplete | Yes | No | No | Incomplete | Yes |
| Riccardi 1994 | No | No | No | No | No | No | Yes |
| Riccardi 2000 | Yes | Yes | Yes | No | No | Yes | Yes |
| Extractable | 1 | 1 | 2 | 0 | 0 | 1 | 3 |
3. Outcome criteria.
| Study ID | Progression | Response |
| Hjorth 1993 | Increasing serum M protein IgG>50g/l, IgA>30g/l, or Bence Jones proteinuria >4g/l, bone pains and osteolytic bone lesions or hypercalcemia, anemia or rising serum creatinine | Reduction in M protein of >50% |
| Riccardi 1994 | >25% increase in MC (mono‐clonal component) and/or an increase in BMPC (bone marrow plasma cell) of at least 20% and/or worsening of laboratory parameters (hemoglobin, serum calcium, and blood urea nitrogen) and/or of skeletal lytic lesions | Reduction in MC, drop in BMPC of <20%, 2g/dl rise in Hb, normal serum calcium, serum albumin >3g/dl |
| Riccardi 2000 | Increase in MC, appearance/enlargement of bone lesions, anemia (Hb<10g/dl), hypercalcemia, renal failure | Reduction in MC, drop in BMPC of <20%, 2g/dl rise in Hb, normal serum calcium, serum albumin >3g/dl |
Discussion
The goal of this systematic review and meta‐analysis is to synthesize all available data on the effect of early treatment for early stage multiple myeloma. There are several interesting findings in our meta‐analysis.
First of all, the mortality is not significantly affected with early treatment or with deferred treatment. However, when we compared the progression rate, we see a considerable benefit of early treatment, indicating that early treatment inhibits the disease progression. Similarly, more patients experienced a vertebral compression in the deferred treatment group than those in the early treatment group did (4 out of 91 versus 0 out of 97). The difference is not statistically significant, either because there actually is no difference or due to small sample sizes. Out analysis showed that whether the treatment is administrated right after the diagnosis or at the disease progression did not affect the response rate. However, interpretation needs to be made carefully due to the wide range of the confidence interval. We should also keep in mind that only patients who got the treatment in the deferred group (i.e. those with disease progression) contributed to the analysis. Since they are a selected group out of the whole deferred arm, comparison with the whole early treatment arm may not be informative. Based on these results, early treatment seems to have the beneficial effect in terms of inhibiting disease progression, but other beneficial effects remain to be further analyzed.
We had planned to extract data on toxicity to determine the adverse effect of early treatment. However, due to the lack of adequate information provided by the three papers, only leukemia development data could be extracted. In terms of leukemia development, we see that early treatment may increase the risk of developing acute leukemia, a potential harm associated with early treatment, which is the only adverse effect that we see in our meta‐analysis. We should note here that the number of events is small and the results still can be explained by chance. This is one of the reasons that we requested long‐term follow‐up data from the investigators to examine if the results presented here would still hold. Unfortunately, to date we have not received a response from them.
Other toxicity data were not extractable and we were not sure whether or not it was because no toxicity was seen in the trials, or the investigators did not report treatment related hazards. In terms of other patient‐oriented outcomes, we were not able to extract any quality of life data, and no conclusion can be made in this respect.
While assessing the quality of the studies, we noticed that all three papers, especially the early two, had small sample sizes, and were seriously under‐powered. No power analysis was predetermined in any of these trials. The meta‐analysis based on the three studies may also have low power to detect treatment effects, and may explain why we see no significant difference between the two groups in most of the outcomes that we examined. Given the fact that highly significant early treatment effect was detected in delaying progression of the disease, one has to wonder if the negative results in the mortality reduction are true‐negative or false‐negative results.
For example, we calculated sample size based on the formula provided by Pogue and Yusuf (Pogue 1997). To reliably detect 15% mortality difference between two treatment groups (with significance level alpha = 0.05), the meta‐analysis should at least include 350 patients (as opposed to 262 patients in the current analysis). Our meta‐analysis had power of 70% to detect this difference. This means that there is 30% of chance that the conclusion that the early intervention does not change mortality in multiple myeloma could be a false‐negative result. If we assume more realistic treatment difference of about 10% reduction between the two therapeutic arms, then we would have to enroll 800 patients in the study. To detect such a difference, our meta‐analysis with 262 patients will only have a power of 51%, which means that the chance to get a false‐negative result is 49%. Despite the fact that we believe that we identified all trials that have ever investigated the issue of early intervention in myeloma, it appears that the totality of available evidence is simply inadequate to help us draw reliable conclusions about the role of chemotherapy in early stage myeloma.
In short, there is clear evidence that early therapy delays progression but no evidence that this leads to better survival. Data on vertebral compression and leukemia development are not interpretable because of the very small numbers of events. Future trials need to be performed about other aspects of the treatment such as cost and patient preferences, supplemented by more complete information about toxicity and quality of life, to help make decision on whether to treat early stage multiple myeloma at the disease diagnosis or wait until the disease progression. Much more important though is the need for further much larger trials to address this question reliably.
Authors' conclusions
Implications for practice.
Early treatment of early stage multiple myeloma clearly inhibits disease progression, but does not improve survival. Results on vertebral compression and acute leukemia need to be verified by additional trials with larger sample size.
Implications for research.
It is possible that the results in terms of the effect of early intervention on mortality of myeloma are false‐negative. Further analysis of the effect of early treatment of early stage multiple myeloma should be conducted, based on a larger sample size, and more complete outcome data, including toxicity and quality of life. Such an analysis should help demonstrate the cost‐effectiveness of early treatment, and provide recommendations for treatment of early stage multiple myeloma.
What's new
| Date | Event | Description |
|---|---|---|
| 13 March 2012 | Amended | Additional tables linked to text. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 15 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to acknowledge the help of Ambuj Kumar, M.D., with the initial RCT identification process. Thilo Kober (Cochrane Hematological Malignancies Group, Cologne, Germany) provided important support in all phases of the registration, preparation and submission of title, protocol and review.
The editorial base of the Cochrane Haematological Malignancies Group is funded, as part of the Competence Network Malignant Lymphomas, by the German Ministry of Education and Research (BMBF).
Appendices
Appendix 1. MEDLINE search strategy
MYELOMA ,
MYELOM*,
MULTIPLE MYELOMA,
PLASMACYTOMA,
PLASMOCYTOM* and PLASM?CYTOM* (free text and MESH)
Appendix 2. CENTRAL search strategy
MYELOMA,
MYELOM*;
MULTIPLE MYELOMA,
PLASMACYTOM*;
PLASMOCYTOM*.
Appendix 3. EMBASE search strategy
1 explode "clinical‐trial"/ all subheadings 2 "double‐blind‐procedure"/ all subheadings 3 "single‐blind‐procedure"/ all subheadings 4 "crossover‐procedure"/ all subheadings 5 "evaluation"/ all subheadings 6 "follow‐up"/ all subheadings 7 "prospective‐study"/ all subheadings 8 "clinical‐article"/ all subheadings 9 "major‐clinical‐study"/ all subheadings 10 "prospective‐study"/ all subheadings 11 "placebo"/ all subheadings 12 "randomization"/ all subheadings 13 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or#12 14 explode "comparative‐study"/ all subheadings 15 "meta‐analysis"/ all subheadings 16 #14 or #15 17 ((intervention or clinical*) near (trial* or study or studies)) in ti,ab 18 (random* or placebo* or rct*) in ti,ab 19 ((singl* or doubl* or trebl* or tripl*) with (blind* or mask*)) in ti,ab 20 explode "controlled‐study"/ all subheadings 21 ((control or controls or controlled) with (trial* or study or studies)) in ti,ab 22 ((multi or multic*) with (trial* or study or studies)) in ti,ab 23 ((cross over or crossover or evaluation or prospectiv*) with (trial* or study or studies)) in ti,ab 24 ((follow or follow‐up or followup) with (studies or study or trial*)) in ti,ab 25 #13 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24
Data and analyses
Comparison 1. Outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 262 | Peto Odds Ratio (95% CI) | 1.11 [0.67, 1.84] |
| 2 Progression | 2 | 212 | Peto Odds Ratio (95% CI) | 0.16 [0.09, 0.29] |
| 3 Response | 2 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.23] |
| 4 Vertebral compression | 2 | 188 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.59] |
| 5 Acute leukemia | 3 | 262 | Peto Odds Ratio (95% CI) | 3.20 [0.55, 18.72] |
1.1. Analysis.
Comparison 1 Outcome, Outcome 1 Mortality.
1.2. Analysis.
Comparison 1 Outcome, Outcome 2 Progression.
1.3. Analysis.
Comparison 1 Outcome, Outcome 3 Response.
1.4. Analysis.
Comparison 1 Outcome, Outcome 4 Vertebral compression.
1.5. Analysis.
Comparison 1 Outcome, Outcome 5 Acute leukemia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hjorth 1993.
| Methods | RCT ITT‐Yes Jadad=2 Power analysis not described Duration of follow‐up: 5 yr | |
| Participants | Stage I MM (Asymptonic) Durie and Salmon Early treatment: Enrolled 25, analyzed 25; Deferred treatment: Enrolled 25, analyzed 25 | |
| Interventions | Melphalan (MPH): 0.25mg/kg prednisone (P): 2 mg/kg 4 days, 6 week intervals | |
| Outcomes | Mortality Progression Response Vertebral compression Renal insufficiency Leukemia | |
| Notes | Not double‐blind Withdrawls and dropouts described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Riccardi 1994.
| Methods | RCT ITT‐No Jadad=3 Power analysis not described Duration of follow‐up: 3 yr | |
| Participants | Stage I MM Durie and Salmon Early treatment: Enrolled 38, analyzed 34; Deferred treatment: Enrolled 40, analyzed 40 | |
| Interventions | MPH: 0.21mg/kg 4 days, P: 0.5 mg/kg 10 days, 6 week intervals | |
| Outcomes | Mortality Progression Response Leukemia | |
| Notes | Not double‐blind Withdrawls and dropouts described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Riccardi 2000.
| Methods | RCT ITT‐No Jadad=3 Power analysis not described Duration of follow‐up: 6 yr | |
| Participants | Stage I MM Durie and Salmon Early treatment: Enrolled 75, analyzed 72; Deferred treatment: Enrolled 70, analyzed 66 (all patients evaluable for response) | |
| Interventions | MPH: 0.21mg/kg 4 days, P: 0.5 mg/kg 10 days, 6 week intervals | |
| Outcomes | Mortality Progression Response Osteolysis Hypercalcemia Vertebral compression Renal insufficiency Leukemia | |
| Notes | Not double‐blind Withdrawls and dropouts described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Contributions of authors
BD oversaw and participated in all phases of the project, coordinated the group activity, and maintained contact with the Cochrane Collaboration. YH wrote the draft of the review and edited it. YH searched and extracted data. KW provided statistical expertise. AG hand‐searched and extracted data. KW and AG helped with drafting and provided assistance with statistics, data analysis and data presentation. OC and JR helped with drafting.
Sources of support
Internal sources
H Lee Moffitt Cancer Center and Research Institute (This work was performed as part of the fulfilment for MPH degree for YH), USA.
External sources
No sources of support supplied
Declarations of interest
Ben Djulbegovic is serves on the editorial board of the Cochrane Haematology Malignancy Group.
Edited (no change to conclusions)
References
References to studies included in this review
Hjorth 1993 {published data only}
- Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rodjer S, Westin J. Initial versus deferred melphalan‐prednisone therapy for asymptomatic multiple myeloma stage I‐‐a randomized study. Myeloma Group of Western Sweden. European Journal of Haematology 1993;50(2):95‐102.. [DOI] [PubMed] [Google Scholar]
Riccardi 1994 {published data only}
- Riccardi A, Ucci G, Luoni R, Brugnatelli S, Mora O, Spanedda R, Paoli A, Barbarano L, Stasi M, Alberio F, et al. Treatment of multiple myeloma according to the extension of the disease: a prospective, randomised study comparing a less with a more aggressive cystostatic policy. Cooperative Group of Study and Treatment of Multiple Myeloma. British Journal of Cancer 1994;70(6):1203‐10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riccardi 2000 {published data only}
- Riccardi A, Mora O, Tinelli C, Valentini D, Brugnatelli S, Spanedda R, Paoli A, Barbarano L, Stasi M, Giordano M, Delfini C, Nicoletti G, Bergonzi C, Rinaldi E, Piccinini L, Ascari E. Long‐term survival of stage I multiple myeloma given chemotherapy just after diagnosis or at progression of the disease: a multicentre randomized study. Cooperative Group of Study and Treatment of Multiple Myeloma. British Journal of Cancer 2000;82(7):1254‐60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alexanian 1994
- Alexanian R, Dimopoulos M. The treatment of multiple myeloma. The New England Journal of Medicine 1994;330(7):484‐9.. [DOI] [PubMed] [Google Scholar]
Altman 2001
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gotzsche PC, Lang T. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Annals of Internal Medicine 2001;134(8):663‐94.. [DOI] [PubMed] [Google Scholar]
Anderson 1998
- Anderson KC, Noga SJ, Bensinger Wea. NCCN practice guidelines for multiple myeloma.. Oncology 1998;12:317‐51. [PubMed] [Google Scholar]
Attal 1996
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. The New England Journal of Medicine 1996;335(2):91‐7.. [DOI] [PubMed] [Google Scholar]
Castro 1997
- Castro AA, Clark AO, Atallah AN. Optimal search strategy for clinical trials in the Latin Anerican and Caribbean Health Science Literature Database (LILIACS).. Revista paulista de medicina 1997;115:1423‐1426. [DOI] [PubMed] [Google Scholar]
Clarke 1999
- Clarke M, Oxman AD. Cochrane Reviewer's Handbook 4.1 [update July 2000]. Oxford: The Cochrane Collaboration; Review Manager (Revman) 4.1. 1999. [Google Scholar]
Cooper 1986
- Cooper MR, McIntyre OR, Propert KJ, Kochwa S, Anderson K, Coleman M, Kyle RA, Prager D, Rafla S, Zimmer B. Single, sequential, and multiple alkylating agent therapy for multiple myeloma: a CALGB Study. Journal of Clinical Oncology 1986;4(9):1331‐9.. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. Bmj 1994;309(6964):1286‐91.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Djulbegovic 2001
- Djulbegovic B, Adams JR, Lyman GH, 23.Djulbegovic B, Adams JR, Lyman GH, et al. Evaluation and appraisal of randomized controlled trials in myeloma.. Ann Oncology 2001;12:1611‐1617. [DOI] [PubMed] [Google Scholar]
Djulbegovic 2002
- Djulbegovic B, Clark O, Hozo I. Database for health outcomes and quality of randomized trials in multiple myeloma. Proceeding of American Society of Clinical Oncology 2002;21:251a. [Google Scholar]
Durie 1975
- Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 1975;36(3):842‐54.. [DOI] [PubMed] [Google Scholar]
Egger 2001
- Egger M, Smith DS, Altman D. Systematic reviews in health care.. London: BMJ Books 2001;2nd ed.:87‐108. [Google Scholar]
Harley 1979
- Harley JB, Pajak TF, McIntyre OR, Kochwa S, Cooper MR, Coleman M, Cuttner J. Improved survival of increased‐risk myeloma patients on combined triple‐alkylating‐agent therapy: a study of the CALGB. Blood 1979;54(1):13‐22.. [PubMed] [Google Scholar]
Hussein 1994
- Hussein M. Multiple myeloma: an overview of diagnosis and management. Cleveland Clinic Journal of Medicine 1994;61(4):285‐98.. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12.. [DOI] [PubMed] [Google Scholar]
Jadad 1998
- Jadad A. Randomized Controlled Trials.. London: BMJ Books. 1998. [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. Bmj 2001;323(7303):42‐6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Landis 1998
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA: a Cancer Journal for Clinicians 1998;48(1):6‐29.. [DOI] [PubMed] [Google Scholar]
Myeloma 1998
- Myeloma Trialists' Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. Myeloma Trialists' Collaborative Group. Journal of Clinical Oncology 1998;16(12):3832‐42.. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34.. [DOI] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93; discussion 661‐6.. [DOI] [PubMed] [Google Scholar]
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31(1):150‐3.. [DOI] [PubMed] [Google Scholar]
Salmon 1983
- Salmon SE, Haut A, Bonnet JD, Amare M, Weick JK, Durie BG, Dixon DO. Alternating combination chemotherapy and levamisole improves survival in multiple myeloma: a Southwest Oncology Group Study. Journal of Clinical Oncology 1983;1(8):453‐61.. [DOI] [PubMed] [Google Scholar]
Shakespeare 2001
- Shakespeare TP, Gebski VJ, Veness MJ, Simes J. Improving interpretation of clinical studies by use of confidence levels, clinical significance curves, and risk‐benefit contours. Lancet 2001;357(9265):1349‐53.. [DOI] [PubMed] [Google Scholar]
Verhagen 1998
- Verhagen AP, Vet HC, Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of Clinical Epidemiology 1998;51(12):1235‐41.. [DOI] [PubMed] [Google Scholar]


