Abstract
The sub-classification of immunology into innate and adaptive immunity is challenged by innate-like T lymphocytes that use innate receptors to respond rapidly to stress despite expressing T cell antigen receptors, a hallmark of adaptive immunity. Explaining how such cells straddle innate and adaptive immunity, the current study shows that antigen receptor signaling, whose conventional role is to facilitate clonal T cell activation, is critical for innate-like T cell development but then is rapidly attenuated, thus accommodating the cells’ innate responsiveness. These findings permitted the identification de novo of an innate-like T cell subset, and argue that T cell hypo-responsiveness, a state traditionally implicated in tolerance, may be fundamental to T cells entering the innate compartment and thereby providing lymphoid stress surveillance.
In the innate phase of an immune response, myelomonocytic cells such as dendritic cells (DC) are rapidly activated by microbe-associated molecules that engage the cells’ pattern recognition receptors (PRRs), particularly Toll like receptors. As a consequence, DC take up microbial materials and/or infected cells and migrate to lymphoid tissues where they present antigen to T lymphocytes, thus initiating the adaptive phase of the response. For the next several days, such lymphocytes clonally expand, differentiate, and then migrate to the affected tissues where they provide antigen-specific effector functions and commonly contribute to antigen-specific memory. Thus the biologies of myelomonocytic cells and lymphocytes largely segregate with innate and adaptive immunity, respectively1.
While extremely powerful, this frame of reference is incomplete. In particular, there are T lymphocytes that by definition express T cell antigen receptors (TCRs), but which respond rapidly to infection or tissue dysregulation in synchrony with the innate response. This responsiveness permits such cells to contribute to lymphoid stress-surveillance with implications for tumour immunology, allergy, and inflammation2,3. Moreover the functional potentials of these cells are developmentally pre-programmed rather than requiring the time-consuming step of differentiation de novo in the periphery2,4,5. Such ‘innate-like’ T lymphocytes are functionally diverse and have attracted much recent attention based on their significant contributions to host protection, and on their widespread implication in immunopathologies2. However, the means by which these T cells can straddle the distinct biologies of innate and adaptive immunity are unresolved. In particular, engagement of the antigen receptor is a fundamental checkpoint over the safety of conventional lymphocyte activation, without which cells of inappropriate specificities could be activated by cytokines and/or stress-antigens alone. And yet the response modes of innate-like T cells suggest that they lack this key checkpoint.
To investigate this, the current study began with a prototypic subset of innate-like γδ T cells which rapidly produces interleukin (IL)-17A solely in response to the pro-inflammatory cytokines IL-1 and IL-236. These cells are particularly abundant in human and murine neonates and provide immune-protection against bacteria and fungi, while also contributing to immunopathologies such as rheumatoid arthritis, psoriasis and experimental allergic encephalomyelitis7-12. Such cells well illustrate the uncertainties surrounding the role of the TCR in the biology of innate-like T lymphocytes. Thus, murine IL-17A-producing γδ T cells, which do not express CD27 and are therefore widely referred to as γδ27− cells5, are considered to emerge by default from thymic progenitors that receive only weak TCR signals during development, by comparison to strong signals received by γδ27+ cells4,13. And yet, γδ27− cells and their thymic progenitors are developmentally pre-programmed and constitutively display markers (TCRhi, CD127hi, IL-1Rhi, CD62Llo, CCR6+, CD44hi) associated with TCR activation5,12,14. Thus it seemed appropriate to use these cells to re-assess the contribution of TCR signaling to the development and biology of innate-like T cells. Far from emerging by default, the developmental maturation of γδ27− cells was found to selectively depend on strong, TCR-associated signaling that then revised the cells’ TCR responsiveness. Such properties were likewise shown by other, diverse subsets of innate-like T cells, suggesting that the developmental suppression of conventional TCR checkpoint-control may be a general means by which lymphocytes acquire rapid responsiveness to innate stimuli.
RESULTS
Innate-like γδ T cells selectively depend on Zap70
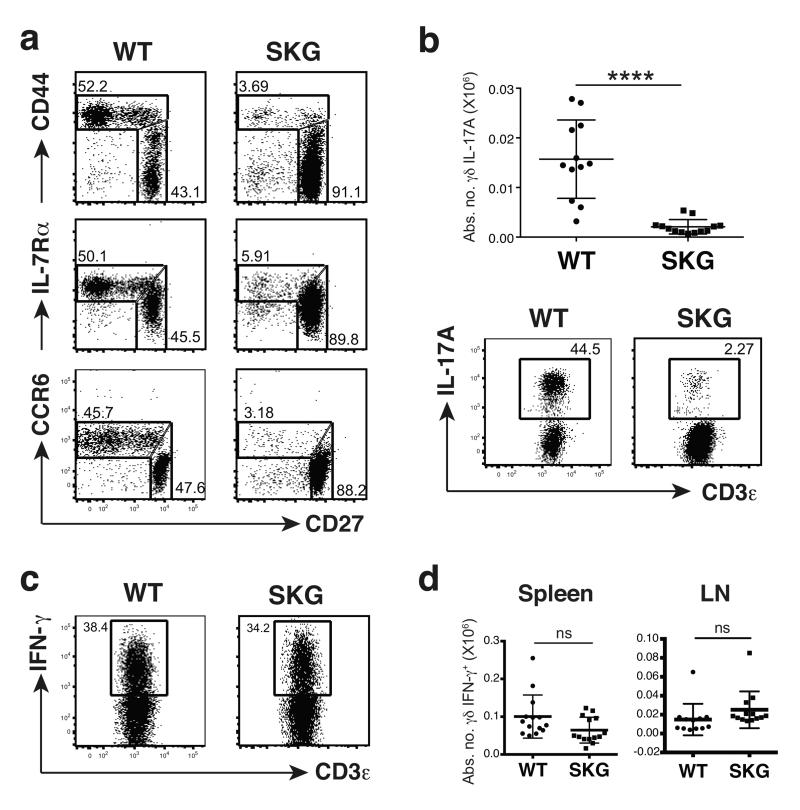
Experimental ablation of the TCR was not a practical approach to addressing its role in innate-like γδ T cell development, since it would most likely affect all T cells and it would specifically remove the capacity to accurately phenotype γδ T cell subsets. Therefore, the role of TCR signaling was examined using the SKG mouse that carries a mutation reducing by ~90% the function of the kinase domain of the TCR-proximal signaling kinase, Zap7015. γδ T cell numbers in SKG versus wild type (WT) mice were comparable in the thymus, slightly decreased in the spleen, and slightly increased in the lymph nodes (LNs) (Supplementary Fig. 1a). However, by contrast to this mild phenotype, the γδ subset profile was dramatically altered. Specifically, SKG LNs were severely depleted of γδ27− cells that could rapidly produce IL-17A upon polyclonal stimulation with PMA + ionomycin (Fig. 1a,b). Likewise, γδ T cells expressing the CCR6 chemokine receptor (a marker of γδ27− cells14) were markedly depleted from LNs and from the dermis, a site ordinarily rich in γδ27− cells10 (Fig. 1a; Suplementary Fig. 1b). Conversely, there was no such marked loss of γδ27+ cells, nor overt impairment in the capacity to produce interferon-γ (IFN-γ upon stimulation with PMA + ionomycin (Fig. 1a,c,d). Rather, the absolute numbers of such cells in SKG versus WT mice were slightly decreased in the spleens and slightly increased in the LNs, paralleling total γδ T cell numbers (Fig. 1d; Supplementary Fig. 1a). Thus, a striking dependence on Zap70 is shown by innate-like γδ27− cells but not by the bulk of systemic γδ T cells, thus providing a novel criterion by which to subdivide the peripheral γδ T cell compartment.
Fig. 1. SKG mice are severely depleted of IL-17A-producing γδ T cells.
Flow cytometry of γδ T cells (gated on TCRδ+CD3+) from LNs of wild type (WT) or SKG animals: (a) cells stained for CD27, CD44, IL-7Rα or CCR6; plots are representative of at least 3 independent experiments (n ≥ 8 per group); the difference in MFI for CD27 in the different panels reflects the fact that the data were collected from different independent experiments but were internally controlled (b) cells stained intracellularly for IL-17A after stimulation with PMA + ionomycin; plots are representative of 4 independent experiments (n = 12 per group); error bars in cell enumeration are ± SD. (c) Intracellular staining for IFN-γ of sorted TCRδ+CD3+CD27+ cells from pooled LNs and spleens after PMA + ionomycin stimulation; plots representative of 2 independent experiments (n = 7 per group). (d) Absolute numbers of IFN-γ-producing γδ T cells from spleen and LNs after stimulation with PMA + ionomycin; error bars ± SD; 4 independent experiments (n ≥12 per group). NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 (Student’s t-test)
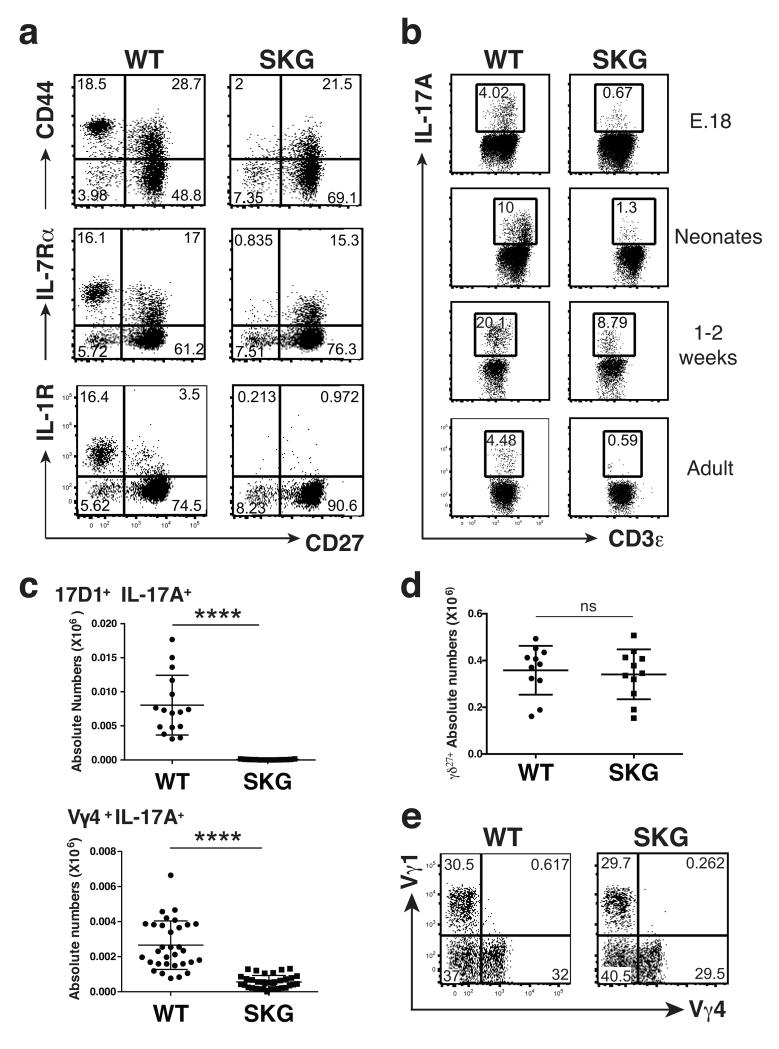
The selective loss of IL-17A producing γδ cells was developmental in that it was overt in the thymus (Fig. 2a,b; Supplementary Fig. 1c). Any possibility that this loss was attributable to reduced mature, peripheral γδ27− cells recirculating through the thymus was excluded by the marked reduction in IL-17A-producing γδ27− cells in fetal and in neonatal mice (Fig. 2b). Furthermore, these highly significant decreases applied both to Vγ6+ IL-17A-producing thymocytes, that develop primarily in the fetus, display very limited TCR diversity, and that principally populate the uterine and tongue epithelia16-18, and to Vγ4+ IL-17A-producing thymocytes that are more diverse than Vγ6+ cells, and that primarily populate the peritoneum, spleen, LNs, dermis and intestinal lamina propria5,10 (Fig. 2c). By contrast, SKG and WT mice showed comparable numbers and subset composition of γδ27+ thymocytes (Fig. 2d,e). In sum, a substantial defect in Zap70, a major TCR-proximal kinase, selectively attenuated the developmental maturation of γδ T cells competent to produce IL-17A rapidly upon infection and/or stress.
Fig. 2. The loss of IL-17A-producing γδ T cells in SKG animals is developmental.
(a) Flow cytometry of neonatal WT and SKG γδ thymocytes; representative of 2 independent experiments (n ≥ 6 per group). (b) Intracellular staining as in Fig. 1b of γδ thymocytes isolated at times indicated and stimulated with PMA + ionomycin; plots representative of 2 to 4 independent experiments per time point (n ≥ 8 per group); the difference in MFI for CD3 in the different panels reflects the fact that the data were collected from different independent experiments but were internally controlled. (c) Absolute numbers of IL-17A-producing γδ thymocytes from neonates stained with antibodies for 17D1 (that detects Vγ6+ cells when used simultaneously with anti-TCRδ, upper panel) or Vγ4 (lower panel). Error bars are ± SD; at least 3 independent experiments (n ≥ 15 per group). (d) Absolute numbers of TCRδ+CD3+CD27+ adult thymocytes; error bars ± SD; 4 independent experiments (n = 11 per group). (e) Flow cytometry of Vγ chain usage by TCRδ+CD27+ adult thymocytes; plots representative of 3 independent experiments (n = 7 per group). NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 (Student’s t-test)
Hypo-responsive TCR signaling in innate-like T cells
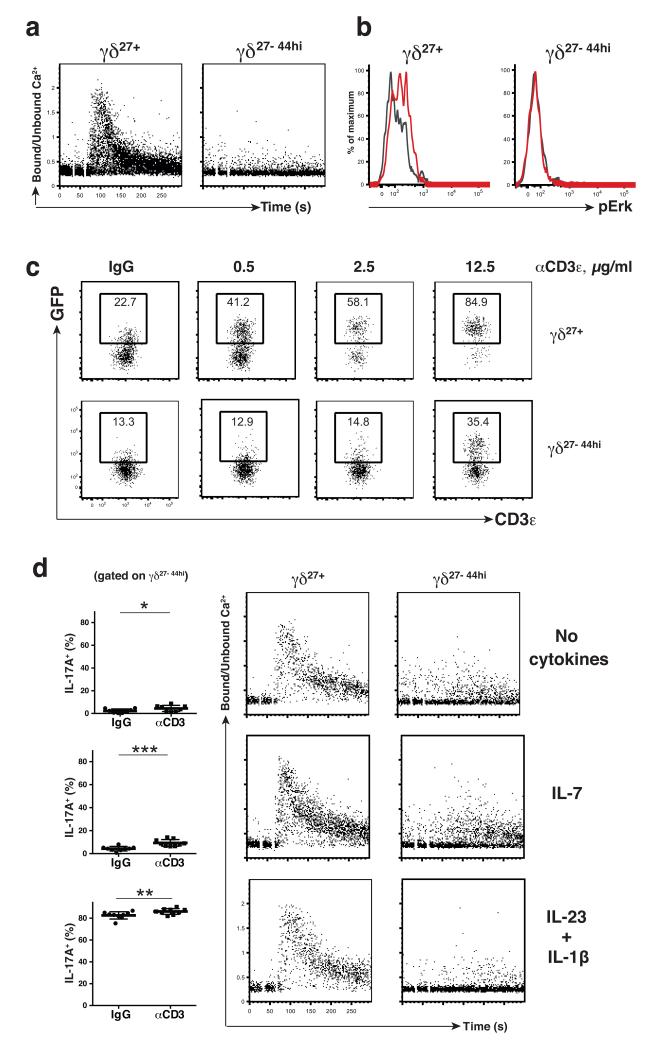
Whereas B cells experiencing strong antigen receptor signaling during development enter a quasi-anergic state in which the antigen receptor becomes markedly hypo-responsive to ligand engagement, no such analogous state was found for developing T cells19. However, considering that only conventional T cells have been examined in this regard, it remained possible that an analogous quasi-anergic state applied to innate-like γδ27− cells, based on their strong developmental dependence on Zap70. A signature response of T cells to antigen receptor engagement is the rapid and transient increase in cytosolic calcium concentration reflecting release from intracellular stores and import via plasma-membrane channels20. Following antigen receptor cross-linking, this conventional T cell pattern was displayed by peripheral γδ27+ cells from WT and SKG mice (Fig. 3a; Supplementary Fig. 2a) and by γδ27+ WT thymocytes (Supplementary Fig. 2b), but it was not displayed by either peripheral or thymic γδ27− cells from WT mice (Fig. 3a; Supplementary Fig. 2b), even over extended time frames (data not shown). (Note that the γδ27− cells were gated on γδ27−CD44hi cells since this subset includes the majority of γδ27− cells that produce IL-17A12). In addition to adult γδ27−CD44hi thymocytes, neonatal Vγ6+γδ27− thymocytes displayed quantitatively impaired, qualitatively distinct calcium fluxes, supporting the hypothesis that the TCR response is markedly revised early in the cells’ development (Supplementary Fig. 2c).
Fig. 3. γδ27− cells are hypo-responsive to TCR stimulation.
(a) WT LN cells were stained for surface markers to identify the indicated subsets, and assayed over a 5 minute timeframe for intracellular Ca2+ mobilization upon TCR stimulation with biotinylated anti-CD3ε (10 μg/ml) followed by streptavidin cross-linking (10 μg/ml); data representative of at least 6 independent experiments (n ≥14). (b) WT LN cells were stimulated in vitro with soluble anti-CD3ε (10 μg/ml) for 5min and phosphorylation of Erk1/2 assessed by flow cytometry in indicated subsets (colored line depicts stimulated cells; grey line depicts cells treated with isotype control antibody); data representative of at least 3 independent experiments (n = 6). (c) LN cells from Nur77.GFP reporter mice were cultured overnight with increasing amounts of plate-coated anti-CD3ε (0.5; 2.5 and 12.5 μg/ml). GFP expression was assessed by flow cytometry in indicated subsets; data representative of 5 independent experiments (n = 16). (d) WT LN cells were cultured for 16h with no cytokines, IL-7 (20 ng/ml) or IL-1β (10 ng/ml) + IL-23 (50 ng/ml). Cells represented in the left panels were cultured for an additional 5 hours with coated anti-CD3ε (10 μg/ml) or IgG-control (10 μg/ml) in the presence of Brefeldin A and IL-17A production assessed by intracellular flow cytometry in the γδ CD27−CD44hi subset. Dataset comparison was performed using a paired t test. Cells represented in the two rightmost panels were cultured an additional 2h under the specified conditions and the indicated subsets (defined by cell surface staining) were assessed for intracellular calcium mobilization following anti-CD3ε as in Fig.3a; data representative of at least 4 independent experiments (n ≥ 8). NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 (Student’s t-test)
A downstream consequence of TCR engagement is transient phosphorylation of Erk. However, whereas phospho-Erk was readily detected in γδ27+ LN cells at 5 minutes after TCR cross-linking, essentially none was detected in γδ27− cells (Fig. 3b). Also downstream of T and B cell antigen receptor signaling is calcineurin-dependent transcription of the Nur77 gene21. Thus, Nur77.GFP reporter mice have been employed to identify cells experiencing antigen receptor signaling19,22. When peripheral γδ27+ cells were cultured overnight they displayed a low background of GFP expression, whereas TCR-agonist stimulation induced a clear, dose-dependent increase in signal, with up to ~85% of cells becoming GFP+ (Fig. 3c). Conversely, only the highest concentration of antibody induced GFP expression above background in γδ27− cells, and then only in a minority of cells (Fig. 3c). In sum, innate-like γδ27− cells and their thymic progenitors could be contrasted with most systemic γδ T cells and thymocytes by multiple metrics of hypo-responsiveness to TCR engagement. The cells were not completely anergic, but responded only to very high levels of antigen receptor engagement.
Consistent with the cells’ hypo-responsiveness, TCR agonist antibody evoked IL-17A production in <5% of peripheral γδ27− cells, relative to the background of cells treated with control antibodies (Fig. 3d), and there was little appreciable calcium flux relative to γδ27+ cells treated in parallel. To further investigate whether the cells were intrinsically hypo-responsive or simply lacked the provision of additional signals, several co-stimulants were employed together with anti-CD3. None showed any significant impact with the exception of pre-exposure to IL-7 which slightly increased the fraction of γδ27− cells producing IL-17A to between 5% and 10% (Fig. 3d), consistent with IL-7 being a selective growth factor for γδ27− cells12. However, cells stimulated with anti-CD3 + IL-7 still displayed very little calcium flux (Fig. 3d). By contrast, IL-1β and IL-23 induced IL-17A production, often by >80% of cells, whereas this was not the case for γδ27+ cells in response to cytokines and/or TCR engagement (Supplementary Fig. 2d). Although TCR stimulation often (but not always) fractionally increased the amount of IL-17A produced by IL-23 - IL-1β-stimulated γδ27− cells (Fig. 3d; Supplementary Fig. 2e), there was still minimal calcium flux (Fig. 3d), and the high amounts of IL-17A induced by IL-1β and IL-23 were completely resistant to Cyclosporin A (Supplementary Fig. 2e). Thus, the profound response of γδ27− cells to innate stimuli occurs in the context of sustained antigen receptor hypo-responsiveness.
This hypo-responsiveness is acquired following a developmental period when there is an acute and selective dependence on antigen receptor signaling components that most likely induced the high expression levels of IL-7R and of receptors for innate stimuli (such as IL-1β) expressed by γδ27− cells (see Fig. 1a). Consistent with this, the expression of IL-1R and IL-7R by developing γδ27− thymocytes increased between embryionic day (E)18 and E19 from about 15% to >45% together with a coincident increase in the Mean Fluorescence Intensity (Supplementary Fig. 3a). In sum, for innate-like, IL-17-producing γδ T cells the most essential role of the TCR is during development, rather than directing peripheral responses to challenge.
TCR attenuation during DETC development
To investigate whether the biology of γδ27− cells might describe other innate-like T cells, analysis was made of murine Dendritic Epidermal T Cells (DETC) that compose a quasi-monoclonal T cell subset expressing a Vγ5Vδ1 TCR, and that make substantial contributions to the maintenance of epithelial integrity and to cutaneous atopic responses2,3,23-25. By several criteria, DETC and γδ27− cells are very different. Thus, activated DETC produce IFN-γ, IL-13, and several chemokines and growth factors, but rarely if ever produce IL-17A3,26. However, DETC resemble γδ27− cells in displaying innate-like biology by which they can rapidly respond in vivo to the up-regulation on keratinocytes of high levels of self-encoded stress-responsive ligands for the innate receptor, NKG2D3,25.
Normal development of Vγ5Vδ1+ DETC progenitors between E15 and E17 depends on TCR signaling components activated when the cells engage medullary thymic epithelial cells (mTEC) expressing Skint1, the founding member of a B7-like/Butyrophilin-like gene family27,28. Skint1 imposes in DETC progenitors a gene regulatory network suppressing Rorc and Il17a and simultaneously up-regulating CD45RB; the expression of genes associated with mature DETC function, e.g. Tbx21 and Ifng; and epidermal–homing potential13,29. Of note, these effects of Skint1 could be phenocopied by TCR agonists27, and DETC also failed to mature in mice deficient in Syk, an antigen receptor-proximal kinase substituting for ZAP70 in fetal DETC progenitors30. However, whether or not DETC progenitors developmentally acquire antigen receptor hypo-responsiveness had not been examined.
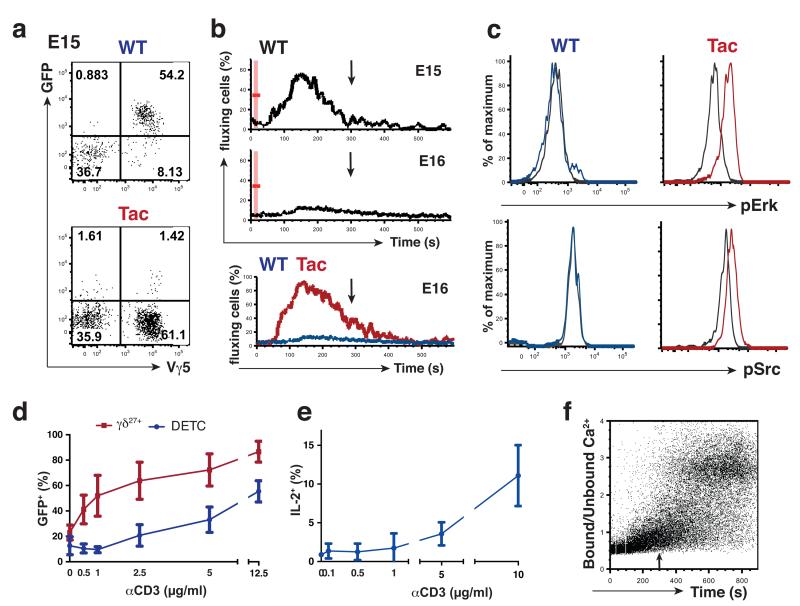
The majority of Vγ5+ DETC progenitors sampled at E15 from Nur77.GFP mice were GFP+, whereas this was not true for Vγ5+ thymocytes in Nur77.GFP mice bred onto an FVB.Tac background that carries a hypomorphic mutation in Skint127,28 (Fig. 4a). Thus, these data support the conclusion that TCR signaling is initiated by engagement of Skint1-expressing mTEC. However, whereas E15 Vγ5Vδ1+ progenitors showed conventional calcium flux in response to TCR cross-linking, this was starkly attenuated within one day (Fig. 4b), by which time the cells also failed to phosphorylate Erk or Src proteins (Fig. 4c). Conversely, Vγ5Vδ1+ progenitors from FVB.Tac mice showed conventional calcium fluxes and Erk and Src phosphorylation (Fig. 4b,c) upon TCR cross-linking, thus establishing a causal link between Skint1-initiated TCR-signaling and hypo-responsiveness. Consistent with TCR-induced hypo-responsiveness, >25% of Vγ5Vδ1+ progenitors had by E16 upregulated inhibitory receptors of the Ly49 family whereas none was induced in Vγ5Vδ1+ progenitors from FVB.Tac mice (Supplementary Fig. 3b). In sum, whereas DETC and γδ27− cells are phenotypically and functionally distinct cell types, they share a developmental dependence on discrete TCR signaling modalities and a consequent attenuation of antigen receptor responsiveness.
Fig. 4. Atypical TCR responses of DETC and their progenitors.
(a) E15 fetal thymocytes of Nur77.GFP mice bred onto FVB.Tac backgrounds were analysed by flow cytometry with littermate embryos heterozygous for Tac mutation used as a WT control. Plots are gated on CD45+TCRδ+ thymocytes; data are representative of 3 independent experiments (n = 8 per group). (b) Fetal thymocytes (gated on CD45+TCRδ+Vγ5+) isolated from FVB.WT or Tac embryos at indicated gestational ages and assayed for intracellular Ca2+ mobilization as in Fig. 3a. Arrow indicates 5 min after beginning of cell acquisition; data are representative of at least 3 independent experiments with pooled fetal thymic lobes. (c) E16 fetal thymocytes from FVB.WT and Tac embryos were stimulated in vitro with soluble anti-CD3ε (10 μg/ml). Phosphorylation of Erk1/2 and Src was assessed by flow cytometry 2 min after stimulation; data are representative of 3 independent experiments with pooled fetal thymic lobes. Histograms are gated on CD45+Vγ5+TCRδ+ cells; grey line – unstimulated, colored line – stimulated. (d) Epidermal and LN T cells isolated from Nur77.GFP reporter mice were cultured overnight with increasing amounts of coated anti-CD3ε (0; 0.5; 1; 2.5; 5 and 12.5 μg/mL) and GFP expression assessed in TCRδ+Vγ5+ epidermal cells by comparison with CD3+TCRδ+CD27+ LN cells; graph is a summary of 5 independent experiments. (e) Epidermal T cells were isolated from WT animals and cultured as in (d). Brefeldin A (1 μg/ml) was added for the last 10 hours and IL-2 production analyzed by intracellular cytometry in TCRδ+ Vγ5+ epidermal cells; data are representative of 3 independent experiments (n ≥ 6). (f) Analysis of intracellular Ca2+ mobilization in WT Vγ5+ TCRδ+ DETC was performed as in Fig. 3a. Arrow indicates 5min after beginning of cell acquisition; data are representative of 8 out of 13 independent experiments.
Atypical TCR responsiveness of DETC
Like peripheral γδ27− cells, mature DETC did not recover conventional TCR responsiveness even when the cells were cultured with any of several cytokines and putative co-stimulators, including those for the NKG2D and JAML receptors, respectively (data not shown)31. Thus, when compared with γδ27+ cells from Nur77.GFP mice (the same comparison as was employed in Fig. 3c, above), DETC incubated with low doses of TCR agonist antibody showed no acquisition of GFP expression above the background of overnight culture, and even at very high doses they did not display the levels of GFP expression achieved by systemic γδ27+ cells (Fig. 4d). A strikingly parallel dose-response to TCR agonist antibodies was evident for IL-2 production by DETC (Fig. 4e). Thus, like peripheral γδ27− cells, mature DETC were not fully anergic, but showed a substantially higher threshold for response through the TCR than did most systemic γδ T cells. The revision of TCR signaling in DETC relative to conventional T cells was both quantitative and qualitative, as shown by calcium flux assays. Thus, whereas this was completely attenuated over the first 5 minutes post-stimulation (arrow in Fig. 4f), during which time other T cell responses had peaked and returned to baseline (for example, see Figs. 3a; 4b), this was followed, in eight out of thirteen experiments, by an unusual, delayed but sustained response commencing ≥6 minutes post-stimulation (Fig. 4f). This highly atypical, revised response mode might explain how the DETC TCR can be utilized for constitutive immune surveillance of keratinocytes, sustaining CD3ζ phosphorylation but without provoking cytokine production or cell activation26. However, in five of thirteen experiments, the sustained response was less evident, with attenuation being the overt evident phenotype. In sum, whereas TCR signaling in mature DETC shows some recovery from the attenuation imposed during DETC development, its properties remain distinctive relative to those of most systemic T cells.
A novel innate-like T cell subset
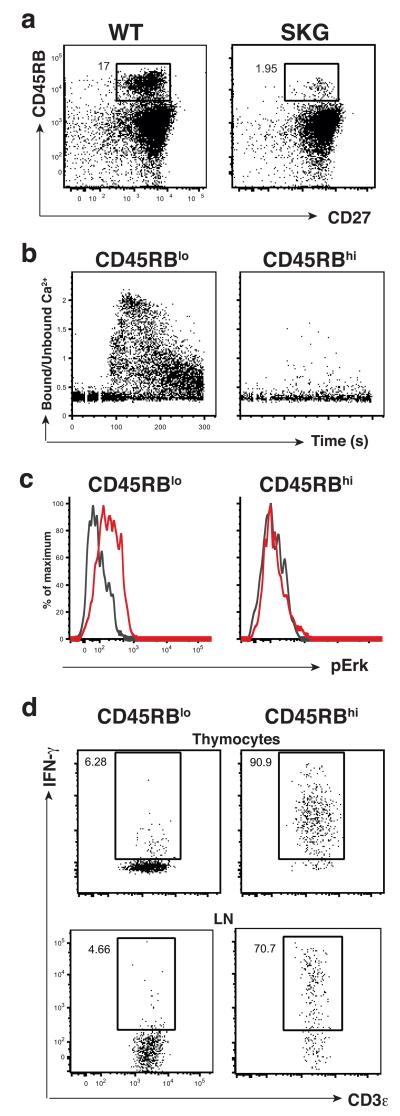
The striking commonalities between two otherwise dissimilar innate-like T cell subsets suggested that most, possibly all, innate-like T cells might depend for their development on strong TCR signals that are then revised. Were this to be the case, these criteria might be used to identify novel innate-like T cell subsets. To test this, adult mice were examined for additional thymocyte subsets expressing markers indicative of developmental TCR signaling. One such marker is high expression of CD45RB13,27 which identified a subset of CD27+CD45RBhi thymocytes that was ~90% depleted in SKG mice (Fig. 5a). A large fraction of these cells readily produced IFN-γ upon stimulation with PMA + ionomycin (Supplementary Fig. 4a), and consistent with their loss, absolute numbers of IFN-γ-producing thymocytes were slightly reduced in post-natal SKG mice (Supplementary Fig. 4b). However, by comparison to the bulk of γδ27+ cells (which are CD45RBlo), these CD27+CD45RBhi cells failed to flux calcium or phosphorylate Erk upon TCR cross-linking (Fig. 5b,c). Thus, a candidate innate-like T cell subset was identified de novo within the bulk of CD27+ systemic γδ T cells.
Fig. 5. CD27+CD45RBhi γδ cells are innate-like T cells.
(a) Flow cytometry analysis of TCRδ+ γδ thymocytes isolated from adult WT and SKG mice stained for CD27 and CD45RB; data representative of 5 independent experiments (n ≥ 14 for each group). (b) Analysis of intracellular Ca2+ mobilization in WT TCRδ+ adult thymocytes performed as in Fig. 3a; data are representative of 3 independent experiments (n = 11). (c) WT LN cells stimulated in vitro with soluble anti-CD3ε (10 μg/ml) for 5 min at which time point Erk1/2 phosphorylation was assessed by flow cytometry in TCRδ+CD3+ subsets, as indicated; (colored line depicts stimulated cells; grey line depicts cells treated with isotype control antibody); data representative of at least 2 independent experiments (n ≥ 4). (d) WT thymocytes or LN cells were cultured for 21h with IL-12 (100 ng/mL) + IL-18 (100 ng/mL) with (thymocytes) or without (LN) IL-15 (100 ng/mL). Brefeldin A (10 μg/ml) was added for the final 5 hours and IFNγ production was assessed by intracellular cytometry in TCRδ+CD3+ subsets, as indicated; data representative of 3 independent experiments (thymocytes; n = 9 per condition) or 2 (LN; n = 6 per condition).
To test whether these cells were truly innate-like, CD27+CD45RBhi thymocytes and LN cells were cultured with several combinations of cytokines (Supplementary Table 1), among which there was a striking response to IL-12 plus IL-18, with or without IL-15. This provoked IFN-γ production by >80% of CD27+CD45RBhi thymocytes and >70% of CD27+CD45RBhi LN cells, but did not have any substantive effect on the bulk of γδ27+ cells, emphasizing the distinction between these T cell subtypes (Fig. 5d; Supplementary Table 1). IL-18 is a member of the IL-1 family. Hence, the induction of IFN—γ but not IL-17A in CD27+CD45RBhi cells by IL-18 + IL-12 seemed complementary to the induction of IL-17A by IL1β + IL-23 in >80% of γδ27− cells (see above).
In sum, different γδ T cell subsets with distinct cellular phenotypes and effector functions share rapid, bulk responsiveness to combinations of IL-1-family and STAT-activating cytokines. However, whereas the differential response of γδ27− cells by comparison to CD27+CD45RBhi cells to IL-1 might be explained by the high amounts of IL-1R expressed by γδ27− cells (Supplementary Fig. 4c), such reasoning could not explain the differential response of CD27+CD45RBhi cells to IL-18R, since although this was highly expressed by CD27+CD45RBhi cells, it was expressed even more strongly by γδ27− cells that responded only poorly to IL-18 (Supplementary Fig. 4c, d). Most likely the respective responses of innate-like T cell subsets to cytokines reflects both their expression of the appropriate receptors and their developmentally pre-programmed effector differentiation5.
In seeking shared properties of the two systemic innate-like γδ T cell subsets that might relate to their common hypo-responsiveness to TCR-stimulation, neither subset was found to express LAG-3 (Supplementary Fig. 4c), or CR-TAM, or CTLA-4 (not shown), all of which have been associated with reduced TCR-responsiveness in αβ T cells32-34. Likewise, neither subset expressed high levels of Egr2 or Egr313 or diacylglycerol kinase α (Dgka) and Dgkz and Cblb which are Egr-target genes associated with anergy35, and which were, by contrast, expressed more highly by the bulk of γδ27+ cells (Supplementary Fig. 4e and data not shown). In sum, the TCR hypo-responsiveness of the two innate-like γδ T cell subsets was not obviously maintained by signaling components thus far implicated in NFAT-mediated anergy36.
Finally, other T lymphocyte subsets were examined for the newly-established joint criterion of innate-like cells; namely, developmental dependence on TCR signaling followed by its subsequent revision. The bulk of TCRαβ+ and TCRγδ+ iNKT cells were present in normal numbers in SKG mice and displayed conventional TCR responsiveness in Nur77.GFP mice (Supplementary Fig. 5a,b), showing them to be distinct from the innate-like T cells described here. Conversely, the intestine includes TCRαβ+ and TCRγδ+ T cells that are developmentally dependent on Syk kinase30 and reportedly agonist-selected37,38, and these cells showed stark TCR hypo-responsiveness, consistent with their being developmentally anergised38 (Supplementary Fig. 5c). Thus, the distinct biologies of different T cell subsets can be segregated according to the developmental dependence on discrete TCR signaling modalities and the impact of this on the cells’ peripheral TCR responsiveness.
Discussion
It should not be possible for a lymphocyte to straddle innate and adaptive immunity, since lymphocyte activation requires antigen receptor-mediated signaling that thereby prevents the expansion in the adaptive response of cells with inappropriate specificities. While co-stimulatory ligands and cytokines collaborate with antigen to activate T cells, this is distinct from the response of innate-like lymphocytes to innate signals alone, as was first described for DETC responding to NKG2D ligand upregulation25, and as described for γδ27− cells responding to IL-1β + IL-236. Such responses profoundly expand lymphocyte biology by permitting T cell participation in the early phases of immune responses that are classically attributed to myeloid cells. This study shows how this can be achieved.
During innate-like T cell development, a requisite activation of TCR signaling components markedly revises the antigen receptor response mode, removing it as the primary checkpoint for peripheral responsiveness. Thus freed, the cells can respond rapidly to innate signals. These dual criteria of developmental dependence on TCR signaling components and the consequent revision of TCR responsiveness proved capable of identifying a new innate-like T cell subset that responds selectively to IL-12 + IL-18.
By revising their TCR response mode, innate-like T cells resemble Innate Lymphoid Cells (ILC)39 that by definition lack constraint by antigen receptors. Nonetheless, two factors distinguish these cell types. First, although the residual TCR responsiveness of innate-like T cells is highly atypical relative to other T cells, the cells are not fully anergic and do not obviously display the molecular signature of anergy induced by NFAT activation in the absence of AP-1 mobilization36. Thus, these cells may employ their TCRs in novel ways, e.g. the steady-state TCR-mediated engagement of epithelial cells by IEL26, or innate-like T cell responses to very high amounts of antigen in the context of particular cytokines12. By such means, the TCR may powerfully expand the innate response repertoire which in invertebrates can be amplified by massive expansion of PRRs40. The diverse specificities of γδ T cells seem highly suited to expanding innate immune recognition41. Second, by contrast to ILC, the pre-requisite of developmental TCR signaling may provide a means to quality control lymphocytes entering the innate compartment.
Importantly, the current study shows that the developmental imposition of hypo-responsiveness does not solely lead to T cell tolerance, but appears critical to the construction of lymphoid compartments that will respond rapidly to tissue dysregulation, reflected by epithelial stress-ligands, such as Rae-1, and/or IL-1 cytokines3,42, the latter in combination with particular STAT-activating cytokines43. Thus, IL-17A production by innate-like T cells is induced by IL-1β + IL-23, whereas IFN-γ production is provoked by IL-18 (another IL-1 family member) plus IL-12. Echoing this, IL-12 + IL-18 provokes IFN-γ production by NK cells44. Such potent, stress-responsive lymphocyte compartments inevitably incur risks, and IL-17A-producing γδ T cells have been widely implicated in inflammatory diseases41.
This study emphasizes subdivision in the γδ lineage. Contrary to common perspective, many γδ T cells are not strictly innate-like since they show no responses to the cytokine and stress-ligand combinations tested, but instead display ‘textbook’ TCR-dependent response modes. This is consistent with the adaptive contributions of murine, bovine, and human γδ cells to infection, vaccination, and nominal antigen challenge41. Indeed, the rarity of some biochemically-validated γδ TCR reactivities, e.g. the endothelial protein C receptor (EPCR), is consistent with a polyclonal, adaptive repertoire45. Likewise, most γδ thymocytes and their mature progeny display a phenotype (CD127loIL-1RloCD62L+CD44lo) of naive T cells5,12, and some γδ cells responding in an antigen-specific fashion can show peripheral differentiation to IL-17A production, in contrast to the developmental pre-programming of innate-like γδ27− cells46. Although the downstream consequences of antigen activation in γδ T cells may differ in fine detail from those of conventional αβ T cell activation, including an acquired capacity to respond to innate stimuli46, the cells’ primary peripheral responses were adaptive because they were driven by the specificities of their TCRs. Moreover, there is growing evidence that innate-like and ‘adaptive’ γδ T cells develop in waves from distinct progenitors that might respond differently to developmental TCR signaling41,47. Such intrinsic differences in cellular responses to TCR signaling should qualify ‘strength-of-signal models’ of αβ:γδ lineage commitment that are mostly based on varying the quality of the ligand48.
The innate-like IFN-γ-producing CD27+CD45RBhi compartment identified here probably comprises γδ27+ cells that engage thymic ligands4,13. It was previously considered that this diverted thymocytes from a default potential to produce IL-17A4,13. This study extends that view, predicting that the functional maturation of any such ‘default cells’ would still require strong, Zap70-dependent TCR signaling. Consistent with this, Vγ5Vδ1+ progenitors that cannot mature as IFN-γ-producing DETC in Skint1-mutant FVB.Tac mice display IL-17A-producing potential, but few emerge into the periphery13. Moreover, the few γδ27− thymocytes with IL-17A-producing potential detected in SKG mice displayed markedly lower TCR levels than their WT counterparts, suggesting abortive maturation.
By proposing key criteria for innate-like T lymphocytes, this study provokes a reassessment of other T cell subsets. First, TCRαβ+ TH17 cells show largely normal TCR responsiveness49, re-emphasizing their fundamental biological distinction from γδ27− cells. It would likewise seem inappropriate to classify TCRαβ+ and TCRγδ+ iNKT cells as innate-like, since they are present in approximately normal numbers in SKG mice and display conventional TCR responsiveness. Indeed, whereas these cells can make pleiotropic cytokine responses notoriously quickly and without requirement for extensive clonal expansion, their activation depends primarily on TCR engagement. Furthermore, the small percentage of iNKT cells that responds purely to innate signals displays hypo-responsive TCR signaling50, and innate responsiveness could be experimentally established in bulk NKT cells by TCR ablation51. This association of innate-like responsiveness with revision of the TCR response mode is clearly consistent with this study’s findings.
Although this study emphasizes the developmental origin of innate-like T cells, this response state might also be acquired in the periphery, for example following chronic antigen exposure, as alluded to for NKT cells and for some γδ27+ T cells. Likewise, human intestinal T cells in celiac disease acquired with time the capacity to respond to IL-15 and NKG2D ligands alone52. Based on this study, such cells would be predicted to have revised their TCR response mode, which casts a new light on this common immunopathology. Finally, the revised TCR responsiveness of innate-like T cells, many of which are tissue-associated, might justify re-assessment of the use calcineurin inhibitors as broad immunosuppressants.
Online methods
Mice
Balb/c and FVB/NHsd (FVB.WT) were purchased from Charles River and Harlan laboratories, respectively. FVB/NTac (FVB.Tac) mice carrying a mutation in Skint1 gene were from Taconic farms. Nur77.GFP reporter mice on a C57/BL6 background22 were provided by Prof K. Hogquist (University of Minnesota, USA). Zap70SKG (SKG) mice on a Balb/c background15 were provided by S. Sakaguchi (University of Osaka, Japan). The Skint1 mutation was crossed onto the Nur77.GFP reporter by intercrossing the reporter with FVB.Tac and breeding the F1 to FVB.Tac. All adult mice used were more than 5 weeks old. For timed pregnancies mice were mated overnight and the day a vaginal plug was observed was considered as E0. All animal experiments were undertaken in full compliance with UK Home Office regulations under a project licence to A. Hayday.
Cell isolation
Fetal, newborn and adult thymi (from 5 to 8 week old mice), lymph nodes (axillary, inguinal and brachial) and spleen were homogenized in PBS 2% FCS by mechanical disaggregation and filtered through a 70 μm or 40 μm filter (BD Bioscience). Splenocyte suspensions were incubated with a red blood cell lysing buffer (Sigma). Epidermal T cells were isolated as previously described27. In some experiments (IL-2 production and calcium mobilization) Trypsin-GNK was replaced by TriplE (Invitrogen), in which case the tissue was incubated for 4 hours instead of 2 hours to separate epidermis from dermis. Dermal T cells were prepared as follows: epidermis was removed after 2 hours of incubation with T-GNK as described above. Dermis was minced and further digested with 85 μg/ml Liberase TM (Roche), 0.25 mg/ml Hyaluronidase (Sigma), 0.2 mg/ml DNAse (Roche), 10 mM Hepes (Sigma) and 1mM Sodium Pyruvate (Gibco), for 2 hours at 37°C. Viable dermal and epidermal lymphocytes were isolated using a 40/80% Percoll gradient (GE Life Sciences). To isolate intestinal intraepithelial lymphocytes (IEL), small intestines were excised, flushed, opened longitudinally and Peyer’s patches removed. Intestines were cut into 0.5 cm pieces and incubated for 20 min in RPMI (Gibco) supplemented with 100 U/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco) and 10% FCS (Sigma) (RPMI 10%) containing 1mM DTT (Sigma). Gut pieces were then vortexed and the resulting cell suspension filtered through a 70 μm strainer. IEL were isolated from the 80/40% interface following 80/40/20% Percoll gradient centrifugation. To isolate liver lymphocytes, mice were perfused with PBS and the livers were removed and homogenized in PBS 2% FCS by mechanical disaggregation and filtered through a 70 μm filter. Most hepatocytes were removed by allowing them to settle at the bottom of the tube. The resulting supernatant was subjected to 35/70% Percoll gradient centrifugation and iNKT cells isolated from the interface.
Cell culture and media
For anti-CD3ε stimulation of DETC and LN cells from Nur77.GFP-reporter mice, cells were cultured overnight in IMDM (Invitrogen) supplemented with 50 μM 2-mercaptoethanol (Gibco), 100 U/mL penicillin (Gibco), 100 μg/mL streptomycin (Gibco), and 10% FCS (Sigma) on plates coated with control IgG or the indicated amount of anti-CD3ε (145-2C11, LEAF Purified, eBioscience). To assess IL-2 production, Brefeldin A (1 μg/ml, Sigma) was added to the culture for the last 10h. IEL from Nur77.GFP-reporter mice were cultured in RPMI 10% for 4 hours on plates coated with control IgG or the indicated amount of anti-CD3ε. For flow cytometric assays of cytokine production, cells were cultured in RPMI 10%, and stimulated with 25 ng/ml PMA and 1μg/ml ionomycin in the presence of 10 μg/ml Brefeldin A (all from Sigma). To assess the effect of cytokines on γδ responsiveness to anti-CD3ε antibodies, LN cells were cultured for 16 hours in complete RPMI with or without IL-7 (20 ng/ml; R&D) or IL-1β (10 ng/ml; Peprotech) + IL-23 (50 ng/ml; eBioscience). Cells were cultured for an additional 2h and assayed for intracellular calcium mobilization upon TCR stimulation (as described below) or transferred onto plates coated with anti-CD3ε or control IgG (10 μg/ml) and cultured for an additional 5 hours in presence of Brefeldin A (10 μg/ml). IL-17A production was assessed by intracellular cytometry. For inhibitor experiments, LN cells were cultured in RPMI 10% for 21h in the presence or absence of IL-1β (10 ng/ml) + IL-23 (50 ng/ml) on plates coated with IgG-control or anti-CD3ε (10 μg/ml), and in the presence of Cyclosporin A (80nM; Sigma) or DMSO control. Brefeldin A (10 μg/ml) was added for the final 5 hours and IL-17A production assessed by intracellular cytometry. To assess IFN-γ production following innate stimulation of the CD45RBhi subset, thymocytes or LN cells were incubated in RPMI 10% with IL-1β (10ng/mL); IL-3 (100U/mL; R&D); IL-6 (20ng/mL; Peprotech); IL-12 (100ng/mL; R&D); IL15 (100ng/mL; Immunotool); IL-18 (100ng/mL; R&D); IL-23 (50ng/mL), in different combinations. Brefeldin A (10 μg/ml) was added for the final 5 hours and IFN-γ or IL-17A production assessed by intracellular cytometry.
Flow cytometry
Flow cytometry was performed essentially as described12,13. Antibodies against TCRδ (GL3), Vγ1 (2.11), Vγ4 (UC3-10A6), Vγ5 (536), Vδ6.3 (8F4H7B7), CD3 (145-2C11), CD27 (LG.7F9; LG3A10), CD44 (IM7), CD45 (30-F11), CD45RB (C363.16A), Rat IgM (HIS40), CD127 (IL-7R; SB/199), IL-1R (JAMA-147), CD218a (IL-18Ra; P3TUNYA), CCR-6 (29-2L17), CD223 (LAG-3; C9B7W), IL-17A (TC11-18H10.1), IFN-γ (XMG1.2), IL-2R (JES6-5H4) coupled to appropriate fluorochromes were from eBioscience, BioLegend or BD-Pharmingen. 17D1 rat IgM antibody recognising Vγ5Vδ1 and Vγ6Vδ1 53 was produced in-house, with hybridoma supernatant used as a primary staining reagent followed by staining with anti rat IgM. iNKT cells were identified using R-PE labelled mouse CD1d αGalCer tetramers (ProImmune) following the manufacturers’ instructions. Intracellular phosphoproteins were detected as described 54 using anti-phospho-Erk1/2 and anti-phospho-Src antibodies from Cell Signalling. For assays of intracellular Ca2+ mobilisation, cells were loaded with Indo-1AM (Invitrogen) (1 μM in IMDM or RPMI with no FCS) for 30 min at 37°C. Thereafter, cells were stained for surface markers and kept on ice. Prior to stimulation cell aliquots were allowed to equilibrate to 37°C for 5 min, and then analysed by flow cytometry. After acquiring the background level of intracellular Ca2+ for 30 seconds, cells were stimulated with 10 μg/ml of biotinylated anti-CD3ε antibody (145-2C11, eBioscience), and then crosslinked by addition of streptavidin. Samples were acquired with LSRII or LSR-Fortessa cytometers (BD) and analysed using FlowJo software (TreeStar). Cell sorting was performed on a MoFlo sorter (Beckman-Coulter) or a BD Aria at the flow cytometry facilities of the LRI Cancer Research UK or the Peter Gorer Department of Immunobiology, KCL, London.
RNA expression analysis
LN cells from C57Bl/6 animals were FACS sorted with a combination of TCRδ, CD27, CD45RB antibodies. Total RNA was isolated with NucleoSpin RNA XS kit (Macherey-Nagel) as per manufacturer’s instructions. RNA was reverse transcribed with SuperScriptIII (Invitrogen) using random hexamers. Quantitative PCR was performed with SensiMix SYBR HI-ROX kit (Bioline) on RotorGene 3000 equipment (Corbett). Relative expression is displayed in arbitrary units normalized to Tbp (TATA-box binding protein) by ΔΔCt method. Primer sequences are available upon request.
Statistical analysis
Normal distribution was assumed a priori for all samples. Unless indicated otherwise, an unpaired non-parametric t-test with Welch correction was used for dataset comparison. In cases where data point distribution was not Gaussian, a non-parametric t-test was also applied.
Supplementary Material
Acknowledgements
We are grateful to many colleagues for help and discussions, but particularly: Marie-Laure Michel, Pierre Vantourout, Olga Sobolev, Rosie Hart, Mahima Swamy, Bruno Silva-Santos, Dan Pennington, Peter Parker, Manoj Saini and the staff of the flow cytometry and biological services units of LRI and of the Peter Gorer Dept of Immunobiology, KCL; to Shimon Sakaguchi (Osaka) for SKG mice and to Kristin Hogquist (Minnesota) for Nur77.GFP mice. The work was supported by Cancer Research UK; a Marie Curie Fellowship (LD); a UCL MBPhD programme (RdiMB); and a Wellcome Trust Programme Grant to ACH.
References
- 1.Medzhitov R, Janeway CA. Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–353. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 2.Hayday AC. gamma delta; T Cells and the Lymphoid Stress-Surveillance Response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Strid J, Sobolev O, Zafirova B, Polic B, Hayday A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science. 2011;334:1293–1297. doi: 10.1126/science.1211250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen KDC, et al. Thymic selection determines gamma delta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17–producing γδ T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton CE, et al. Interleukin-1 and IL-23 Induce Innate IL-17 Production from γδ T Cells, Amplifying Th17 Responses and Autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gamma delta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 8.Hamada SS, et al. IL-17A produced by gamma delta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petermann F, et al. Gamma delta T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism. Immunity. 2010;33:13–13. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumaria N, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. Journal of Experimental Medicine. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Y, et al. Pivotal Role of Dermal IL-17-Producing γδ T Cells in Skin Inflammation. Immunity. 2011 doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel M-L, et al. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing γδ cells. Proceedings of the National Academy of Sciences. 2012;109:17549–17554. doi: 10.1073/pnas.1204327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-γ-secreting versus interleukin-17-secreting γδ T cells. Immunity. 2011;35:59–68. doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Haas JD, et al. CCR6 and NK1. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi N, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 16.Itohara SS, et al. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 17.Shibata K, et al. Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 18.Haas JD, et al. Development of Interleukin-17-Producing γδ T Cells Is Restricted to a Functional Embryonic Wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss A, Imboden J, Shoback D, Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proceedings of the National Academy of Sciences. 1984;81:4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborne BA, et al. Identification of genes induced during apoptosis in T lymphocytes. Immunol Rev. 1994;142:301–320. doi: 10.1111/j.1600-065x.1994.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 22.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. Journal of Experimental Medicine. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jameson JJ, et al. A role for skin gamma delta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 24.Hayday A, Tigelaar R. Immunoregulation in the tissues by gamma delta T cells. Nature Publishing Group. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 25.Strid J, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 26.Chodaczek GG, Papanna VV, Zal MAM, Zal TT. Body-barrier surveillance by epidermal γδ TCRs. Nature Immunology. 2012;13:272–282. doi: 10.1038/ni.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis JM, et al. Selection of the cutaneous intraepithelial γδ+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 28.Boyden LM, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin YY, et al. Cutting edge: Intrinsic programming of thymic γδT cells for specific peripheral tissue localization. J Immunol. 2010;185:7156–7160. doi: 10.4049/jimmunol.1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallick-Wood CA, et al. Disruption of epithelial gamma delta T cell repertoires by mutation of the Syk tyrosine kinase. Proc Natl Acad Sci USA. 1996;93:9704–9709. doi: 10.1073/pnas.93.18.9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witherden DAD, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gamma delta T cell activation. Science. 2010;329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y, et al. Egr2-dependent gene expression profiling and ChIP-Seq reveal novel biologic targets in T cell anergy. Mol Immunol. 2013;55:283–291. doi: 10.1016/j.molimm.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 34.Hannier SS, Tournier MM, Bismuth GG, Triebel FF. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161:4058–4065. [PubMed] [Google Scholar]
- 35.Olenchock BA, et al. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nature Immunology. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 36.Müller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 37.Leishman AJ, et al. Precursors of functional MHC class I- or class II-restricted CD8alphaalpha(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–364. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- 38.Pobezinsky LA, et al. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spits H, Cupedo T. Innate Lymphoid Cells: Emerging Insights in Development, Lineage Relationships, and Function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 40.Rast JP, Smith LC, Loza-Coll M, Hibino T, Litman GW. Genomic Insights into the Immune System of the Sea Urchin. Science. 2006;314:952–956. doi: 10.1126/science.1134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vantourout PP, Hayday AA. Six-of-the-best: unique contributions of γδ T cells to immunology. Nature Publishing Group. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinarello CAC. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- 43.Guo L, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proceedings of the National Academy of Sciences. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaix J, et al. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willcox CRC, et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nature Immunology. 2012;13:872–879. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- 46.Zeng XX, et al. γδ T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. 2012;37:524–534. doi: 10.1016/j.immuni.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kisielow J, Tortola L, Weber J, Karjalainen K, Kopf M. Evidence for the divergence of innate and adaptive T-cell precursors before commitment to the αβ and γδ lineages. Blood. 2011;118:6591–6600. doi: 10.1182/blood-2011-05-352732. [DOI] [PubMed] [Google Scholar]
- 48.Wong GW, Zúñiga-Pflücker JC. gamma delta and alpha beta T cell lineage choice: Resolution by a stronger sense of being. Semin Immunol. 2010;22:228–236. doi: 10.1016/j.smim.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Weber KS, Miller MJ, Allen PM. Th17 cells exhibit a distinct calcium profile from Th1 and Th2 cells and have Th1-like motility and NF-AT nuclear localization. J Immunol. 2008;180:1442–1450. doi: 10.4049/jimmunol.180.3.1442. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, et al. Human invariant natural killer T cells acquire transient innate responsiveness via histone H4 acetylation induced by weak TCR stimulation. 2012;209:987–1000. doi: 10.1084/jem.20111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vahl JC, et al. NKT Cell-TCR Expression Activates Conventional T Cells in Vivo, but Is Largely Dispensable for Mature NKT Cell Biology. PLoS Biol. 2013;11:e1001589. doi: 10.1371/journal.pbio.1001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meresse B, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Mallick-Wood CA, et al. Conservation of T Cell Receptor Conformation in Epidermal. Science. 1998;279:1729. doi: 10.1126/science.279.5357.1729. [DOI] [PubMed] [Google Scholar]
- 54.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.