Abstract
Background
This is an updated version of the original Cochrane review published in Issue 12, 2012. That review considered both fibromyalgia and neuropathic pain, but the efficacy of amitriptyline for neuropathic pain is now dealt with in a separate review.
Amitriptyline is a tricyclic antidepressant that is widely used to treat fibromyalgia, and is recommended in many guidelines. It is usually used at doses below those at which the drugs act as antidepressants.
Objectives
To assess the analgesic efficacy of amitriptyline for relief of fibromyalgia, and the adverse events associated with its use in clinical trials.
Search methods
We searched CENTRAL, MEDLINE, and EMBASE to March 2015, together with reference lists of retrieved papers, previous systematic reviews and other reviews, and two clinical trial registries. We also used our own hand searched database for older studies.
Selection criteria
We included randomised, double‐blind studies of at least four weeks' duration comparing amitriptyline with placebo or another active treatment in fibromyalgia.
Data collection and analysis
We extracted efficacy and adverse event data, and two study authors examined issues of study quality independently. We performed analysis using three tiers of evidence. First tier evidence derived from data meeting current best standards and subject to minimal risk of bias (outcome equivalent to substantial pain intensity reduction, intention‐to‐treat analysis without imputation for dropouts; at least 200 participants in the comparison, 8 to 12 weeks duration, parallel design), second tier from data that failed to meet one or more of these criteria and were considered at some risk of bias but with adequate numbers in the comparison, and third tier from data involving small numbers of participants that were considered very likely to be biased or used outcomes of limited clinical utility, or both.
For efficacy, we calculated the number needed to treat to benefit (NNT), and for harm we calculated the number needed to treat to harm (NNH) for adverse events and withdrawals. We used a fixed‐effect model for meta‐analysis.
Main results
We included seven studies from the earlier review and two new studies (nine studies, 649 participants) of 6 to 24 weeks' duration, enrolling between 22 and 208 participants; none had 50 or more participants in each treatment arm. Two studies used a cross‐over design. The daily dose of amitriptyline was 25 mg to 50 mg, and some studies had an initial titration period.
There was no first or second tier evidence for amitriptyline in the treatment of fibromyalgia. Using third tier evidence the risk ratio (RR) for at least 50% pain relief, or equivalent, with amitriptyline compared with placebo was 3.0 (95% confidence interval (CI) 1.7 to 4.9), with an NNT) of 4.1 (2.9 to 6.7) (very low quality evidence). There were no consistent differences between amitriptyline and placebo or other active comparators for relief of symptoms such as fatigue, poor sleep, quality of life, or tender points.
More participants experienced at least one adverse event with amitriptyline (78%) than with placebo (47%). The RR was 1.5 (1.3 to 1.8) and the NNH was 3.3 (2.5 to 4.9). Adverse event and all‐cause withdrawals were not different, but lack of efficacy withdrawals were more common with placebo (12% versus 5%; RR 0.42 (0.19 to 0.95)) (very low quality evidence).
Authors' conclusions
Amitriptyline has been a first‐line treatment for fibromyalgia for many years. The fact that there is no supportive unbiased evidence for a beneficial effect is disappointing, but has to be balanced against years of successful treatment in many patients with fibromyalgia. There is no good evidence of a lack of effect; rather our concern should be of overestimation of treatment effect. Amitriptyline will be one option in the treatment of fibromyalgia, while recognising that only a minority of patients will achieve satisfactory pain relief.
It is unlikely that any large randomised trials of amitriptyline will be conducted in fibromyalgia to establish efficacy statistically, or measure the size of the effect.
Plain language summary
Amitriptyline for fibromyalgia in adults
Our understanding of fibromyalgia (a condition of persistent, widespread pain and tenderness, sleep problems, and fatigue) is poor. Common pain relieving medicines such as paracetamol and ibuprofen are not usually considered effective in fibromyalgia. Medicines that are sometimes used to treat epilepsy or depression can be very effective in some people with fibromyalgia, as they are in some other forms of chronic pain where there may be nerve damage (neuropathic pain).
Amitriptyline is an antidepressant, and antidepressants are recommended for treating fibromyalgia. Although amitriptyline is commonly used to treat fibromyalgia, a review in 2012 found no good quality evidence to support its use. Most studies were small, old, and used methods or reported results that we now recognise as making benefits seem better than they are.
This review is an update of the 2012 review, which considered both fibromyalgia and neuropathic pain conditions. Neuropathic pain is now considered in a separate review. Here we examine how well amitriptyline worked in treating fibromyalgia, using a definition of what worked that involved both a high level of pain relief and the ability to take the tablets over a longer time without side effects being intolerable.
In March 2015 we performed searches to look for new studies, and found only two additional small studies to include. Neither provided any good quality evidence for benefit or harm. There were still no studies that could provide an answer that was trustworthy or reliable, because most were relatively old, and used methods or reported results that we now recognise as making benefits seem better than they are. This is disappointing, but we can still make useful comments about the drug.
Amitriptyline probably does provide good levels of pain relief for some people with fibromyalgia, although we cannot be certain of this. Our best guess is that amitriptyline provides good pain relief in about 1 in 4 (25%) more people than does placebo. About 1 in 3 (31%) more people than with placebo report having one or more adverse events, which are usually not serious but may be troublesome and interfere with taking the treatment. We cannot trust either figure based on the information available.
The most important message is that amitriptyline probably does give really good pain relief to some patients with fibromyalgia, but only a minority of them; amitriptyline will not work for most people.
Summary of findings
for the main comparison.
| Amitriptyline compared with placebo for fibromyalgia | ||||||
|
Patient or population: adults with fibromyalgia Settings: community Intervention: amitriptyline 25 to 50 mg daily Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | NNT or NNH and/or relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| At least 50% reduction in pain or equivalent (substantial) | 360 in 1000 | 110 in 1000 | RR 2.9 (1.7 to 4.9) NNT 4.1 (2.9 to 6.7) |
4 studies, 275 participants | Very low | Small number of studies and participants |
| At least 30% reduction in pain or equivalent (moderate) | no data | |||||
| Adverse event withdrawals | 80 in 1000 | 90 in 1000 | RR 1.03 (0.49 to 2.2) NNTp not calculated |
4 studies, 298 participants | Very low | Small number of studies and participants |
| Serious adverse events | none reported | |||||
| Death | none reported | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
This is an update of an earlier review of amitriptyline for neuropathic pain and fibromyalgia originally published in the Cochrane Library in 2012 (Moore 2012a). The efficacy of amitriptyline for neuropathic pain conditions is now dealt with in a separate review (Moore 2015).
In the update we have used a template for reviews of drugs used to relieve fibromyalgia. The aim is for all reviews to use the same methods, based on current criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Appendix 1).
Description of the condition
Fibromyalgia has been defined as widespread pain that lasts for longer than three months, with pain on palpation at 11 or more of 18 specified tender points (Wolfe 1990). It is frequently associated with other symptoms such as poor sleep, fatigue, and depression (Wolfe 2014). More recently, a definition of fibromyalgia has been proposed based on symptom severity and the presence of widespread pain, and which does not require palpation of tender points for diagnosis (Wolfe 2010). While some rheumatologists have thought of fibromyalgia as a specific pain disorder, other investigators have characterised it as a bodily distress syndrome or a physical symptom disorder, or somatoform disorder (Wolfe 2014). It is a heterogeneous condition in which there is abnormal processing of the sensation of pain. The cause, or causes, are not well understood, but it has features in common with neuropathic pain, including changes in the central nervous system (CNS). Moreover, people with neuropathic pain and people with fibromyalgia experience similar sensory phenomena (Koroschetz 2011).
Many people with fibromyalgia are significantly disabled, and experience moderate or severe pain for many years. Chronic painful conditions comprised five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life, employment, and increased health costs (Moore 2014a).
Fibromyalgia is common. Numerous studies have investigated prevalence in different settings and countries. The Queiroz 2013 review gives a global mean prevalence of 2.7% (range 0.4% to 9.3%), and a mean in the Americas of 3.1%, in Europe of 2.5%, and in Asia of 1.7%. Fibromyalgia is more common in women, with a female to male ratio of 3:1 (4.2%:1.4%). The change in diagnostic criteria does not appear to have significantly affected estimates of prevalence (Wolfe 2013). Estimates of prevalence in specific populations vary greatly, but have been reported to be as high as 9% in female textile workers in Turkey and 10% in metalworkers in Brazil (59% in those with repetitive strain injury; Queiroz 2013).
Fibromyalgia pain is known to be difficult to treat effectively, with only a minority of individuals experiencing a clinically relevant benefit from any one intervention. A multidisciplinary approach is now advocated, with pharmacological interventions being combined with physical or cognitive interventions, or both. Conventional analgesics are usually not effective. Treatment is often by so‐called unconventional analgesics, such as antidepressants like duloxetine and amitriptyline (Lunn 2014; Moore 2012a; Sultan 2008), or antiepileptics like gabapentin or pregabalin (Moore 2009; Moore 2014b; Wiffen 2013). The proportion of people who achieve worthwhile pain relief (typically at least a 50% reduction in pain intensity; Moore 2013a) is small, generally only 5% to 15% more than with placebo, with numbers needed to treat to benefit (NNT) usually between 6 and 20 (Moore 2013b; Wiffen 2013). This is confirmed by individual patient level analyses for duloxetine and pregabalin (Moore 2014c; Straube 2010); and is somewhat less effective than with the same drugs in neuropathic pain (Kalso 2013).
Those who do experience good levels of pain relief, however, also benefit from substantial reductions in other symptoms such as fatigue, function, sleep, depression, anxiety, and ability to work, with significant improvement in quality of life (Moore 2010b; Moore 2014a; Straube 2011). Fibromyalgia is not particularly different from other chronic pain in that only a small proportion of trial participants have a good response to treatment (Moore 2013b).
Description of the intervention
Amitriptyline is a tricyclic antidepressant. It is not licensed in the UK for treating fibromyalgia, but is commonly used for this indication, and it is commonly used for treating fibromyalgia around the world, irrespective of licensed indications. It is available as tablets (10, 25, 50 mg) and oral solutions, and is usually taken at night time to reduce any sedative effects during the day. There were over 11 million prescriptions for amitriptyline in England in 2013, mainly for 10 mg and 25 mg tablets (PCA 2014); some of these prescriptions would be for relief of depression or neuropathic pain. The main side effects are due to its anticholinergic activity, and include dry mouth, weight gain, and drowsiness.
How the intervention might work
The mechanism of action of amitriptyline in the treatment of fibromyalgia remains uncertain, although it is known to inhibit both serotonin and noradrenalin reuptake. The mechanism is likely to differ from that in depression since analgesia with antidepressants is often achieved at lower dosage than the onset of any antidepressant effect; adverse events associated with amitriptyline often wane after two or three weeks, when the benefits of the drug become apparent. In addition, there is little correlation between the effect of antidepressants on mood and pain, and antidepressants produce analgesia in patients with and without depression (Onghena 1992).
Why it is important to do this review
Amitriptyline is an established pharmacological intervention for fibromyalgia. The earlier review found some evidence of pain relief with amitriptyline compared with placebo for fibromyalgia, at the expense of increased adverse events, but this was based on small numbers of participants in studies that were susceptible to bias.
It was decided to split reviews combining neuropathic pain conditions with fibromyalgia into separate reviews, so an update was performed at the same time, to capture any new studies.
Like the earlier Cochrane review, this update assessed evidence in ways that make both statistical and clinical sense, and used developing criteria for what constitutes reliable evidence in chronic pain (Appendix 1; Moore 2010a). It followed standards set out in the PaPaS Author and Referee Guidance for pain studies of the Cochrane Pain, Palliative and Supportive Care Group (PaPaS 2012).
Objectives
To assess the analgesic efficacy of amitriptyline for relief of fibromyalgia, and the adverse events associated with its use in clinical trials.
Methods
Criteria for considering studies for this review
Types of studies
We included studies if they were randomised controlled trials (RCTs) with double‐blind assessment of treatment, and outcomes reported ideally after eight weeks of treatment or longer for the highest level of evidence, but accepted studies lasting four to eight weeks as a lower level. We required full journal publication, with the exception of extended abstracts of otherwise unpublished clinical trials. We did not include short abstracts (usually meeting reports), studies that were non‐randomised, studies of experimental pain, case reports, or clinical observations. We accepted cross‐over studies only if there was clear reporting of the first phase only. We did not include studies with fewer than 10 participants in any treatment arm, or studies of topical administration.
Types of participants
We included adult participants with fibromyalgia diagnosed using the 1990 or 2010 criteria (Wolfe 1990; Wolfe 2010), aged 18 years and above, and with initial pain of at least moderate intensity
Types of interventions
Amitriptyline in any dose, by any route other than topical, administered for the relief of fibromyalgia pain, and compared to placebo or any active comparator.
Types of outcome measures
Studies needed to report pain assessment as either the primary or secondary outcome.
We anticipated that studies would use a variety of outcome measures, with most using standard subjective scales (numerical rating scale (NRS) or visual analogue scale (VAS)) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as:
at least 30% pain relief over baseline (moderate);
at least 50% pain relief over baseline (substantial);
much or very much improved on Patient Global Impression of Change (PGIC) (moderate);
very much improved on PGIC (substantial).
These outcomes were used in the earlier version of this review, but are different from many other earlier reviews, concentrating on dichotomous outcomes where pain responses are not normally distributed.
We have included a 'Summary of findings' table as set out in the author guide (PaPaS 2012), including outcomes of at least 30% and at least 50% pain intensity reduction, withdrawals due to adverse events, serious adverse events, and death, although there were no data for some of these outcomes. We used the GRADE approach to assess the quality of evidence related to each of the key outcomes listed in Types of outcome measures (Schünemann 2011), as appropriate.
Primary outcomes
Participant‐reported pain relief of 30% or greater.
Participant‐reported pain relief of 50% or greater.
PGIC much or very much improved.
PGIC very much improved.
Secondary outcomes
Any pain‐related outcome indicating some improvement.
Withdrawals due to lack of efficacy, adverse events, and for any cause
Participants experiencing any adverse event.
Participants experiencing any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or may require an intervention to prevent one of the above characteristics or consequences.
Specific adverse events, particularly somnolence, dizziness, and dry mouth.
Any disability‐related or mental health‐related outcome.
Search methods for identification of studies
Electronic searches
We searched the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL) (via The Cochrane Library 2012, Issue 9 for the earlier review and via CRSO from 2012 to 26 March 2015 for this update);
MEDLINE (via Ovid) (from inception to September 2012 for the original review, and from 2012 to 26 March 2015 for this update);
EMBASE (via Ovid) (from inception to September 2012 for the original review, and from 2012 to 26 March 2015 for this update);
Oxford Pain Relief database (Jadad 1996a) for the earlier review. This database is no longer being updated.
See Appendix 2 for the MEDLINE search strategy, Appendix 3 for the EMBASE search strategy, and Appendix 4 for the CENTRAL search strategy.
There was no language restriction.
Searching other resources
We reviewed the bibliographies of all identified RCTs and review articles, and searched clinical trial databases (ClinicalTrials.gov (ClinicalTrials.gov) and WHO ICTRP (apps.who.int/trialsearch/) to identify additional published or unpublished data. We did not contact investigators or study sponsors.
Data collection and analysis
Selection of studies
We determined eligibility by reading the abstract of each study identified by the search. Studies that clearly did not satisfy inclusion criteria were eliminated, and we obtained full copies of the remaining studies. Two review authors read these studies independently and reached agreement by discussion. We did not anonymise the studies in any way before assessment.
Data extraction and management
Two review authors independently extracted data using a standard form and checked for agreement before entry into RevMan (RevMan 2014) or any other analysis method. We included information about the pain condition and number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals and adverse events (participants experiencing any adverse event, or serious adverse event).
Assessment of risk of bias in included studies
We used the Oxford Quality Score (Jadad 1996b) as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum.
Two review authors independently assessed the risk of bias for each study, using the criteria outlined in the 'Risk of bias' tool in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process such as random number table or computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (for example, odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (for example, telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (for example, open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, for example, identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies that were not double‐blind.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (less than 10% of participants did not complete the study or used ‘baseline observation carried forward’ analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
Size (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably because the conduct of small studies is more likely to be less rigorous, allowing critical criteria to be compromised (Dechartres 2013; Kjaergard 2001; Nuesch 2010). Studies were considered to be at low risk of bias if they had 200 participants or more, at unclear risk if they had 50 to 200 participants, and at high risk if they had fewer than 50 participants.
Measures of treatment effect
We calculated numbers needed to treat to benefit (NNTs) as the reciprocal of the absolute risk reduction (ARR) (McQuay 1998). For unwanted effects, the NNT becomes the number needed to treat to harm (NNH) and is calculated in the same manner. We used dichotomous data to calculate risk ratio (RR) with 95% confidence intervals (CI) using a fixed‐effect model unless significant statistical heterogeneity was found (see below). Continuous data were not used in analyses.
Unit of analysis issues
The unit of analysis was the individual participant. For cross‐over studies we planned to use the first period data only, or any useable results if first period data were not available. The control treatment arm would be split between active treatment arms in a single study if the active treatment arms were not combined for analysis.
Dealing with missing data
We used intention‐to‐treat (ITT) analysis where the ITT population consisted of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. Missing participants were assigned zero improvement wherever possible.
Assessment of heterogeneity
We assessed statistical heterogeneity visually (L'Abbé 1987) and with the use of the I² statistic (Higgins 2003). When I² was greater than 50%, we considered the reasons.
Assessment of reporting biases
The aim of this review is to use dichotomous data of known utility and of value to patients (Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). The review did not depend on what authors of the original studies chose to report or not, though clearly difficulties arose with studies failing to report any dichotomous results. We extracted and used continuous data, which probably poorly reflect efficacy and utility, if useful for illustrative purposes only.
We undertook no assessment of publication bias due to the quality of the data identified, although we had planned to use a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean an NNT of 10 or higher) (Moore 2008).
Data synthesis
We undertook meta‐analysis using a fixed‐effect model. A random‐effects model for meta‐analysis would have been used if there was significant clinical heterogeneity and it was considered appropriate to combine studies.
We determined that we would analyse data for each painful condition in three tiers, according to outcome and freedom from known sources of bias.
The first tier used data meeting current best standards, where studies reported the outcome of at least 50% pain intensity reduction over baseline (or its equivalent), without the use of last observation carried forward analysis (LOCF) or other imputation method other than baseline observation carried forward (BOCF) for dropouts, reported an ITT analysis, lasted eight or more weeks, had a parallel‐group design, and had at least 200 participants (preferably at least 400) in the comparison (Moore 2010a; Moore 2012b). We planned to report these top‐tier results first.
The second tier used data from at least 200 participants, but where one or more of the above conditions was not met (for example, reporting at least 30% pain intensity reduction, using LOCF or a completer analysis, or lasting four to eight weeks).
The third tier of evidence used data from fewer than 200 participants, or where there were expected to be significant problems because, for example, of very short duration studies of less than four weeks, where there was major heterogeneity between studies, or where there were shortcomings in allocation concealment, attrition, or incomplete outcome data. For this third tier of evidence, no data synthesis is reasonable, and may be misleading, but an indication of beneficial effects might be possible.
Subgroup analysis and investigation of heterogeneity
We did not plan subgroup analyses since experience of previous reviews indicated that there would be too few data for any meaningful subgroup analysis.
Sensitivity analysis
We planned no sensitivity analysis because the evidence base was known to be too small to allow reliable analysis. We did examine details of dose escalation schedules in the unlikely situation that this could provide some basis for a sensitivity analysis.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, and Characteristics of studies awaiting classification.
Results of the search
New searches from January 2012 to 26 March 2015 identified 37 potentially relevant studies in CENTRAL, 107 in MEDLINE, and 259 in EMBASE. Of these, two were obtained and read in full to determine inclusion status.
Three studies still await classification because of translation requirements. Ataoğlu 1997 is a Turkish study comparing amitriptyline with paroxetine in 68 participants for six weeks. Jang 2010 is a Chinese study comparing amitriptyline with acupuncture plus cupping and with the combined treatments over four weeks, involving 186 participants in three treatment arms. The details of NCT00381199 are not clear, but it involved a comparison of amitriptyline 10 mg to 25 mg daily with nabilone 0.5 mg to 1 mg daily over a period of about three weeks in 32 participants.
Included studies
In this update we included two new studies (101 participants; Braz 2013; de Zanette 2014) and seven studies (548 participants; Carette 1986; Carette 1994; Carette 1995; Ginsberg 1996; Goldenberg 1986; Goldenberg 1996; Hannonen 1998) from the previous review that fulfilled the inclusion criteria; altogether there were nine included studies with 649 participants (Figure 1).
1.
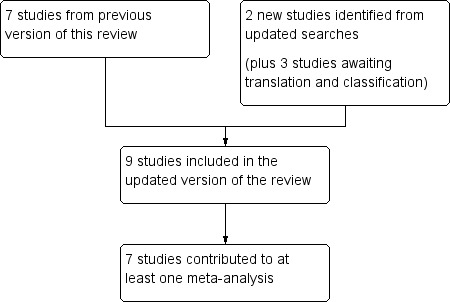
Flow diagram.
Two studies used a cross‐over design (Carette 1995; Goldenberg 1996) and the remainder used a parallel group design. All the studies except de Zanette 2014 included a placebo control, six included an active comparator (Braz 2013; Carette 1994; de Zanette 2014; Goldenberg 1986; Goldenberg 1996; Hannonen 1998), and three additionally included a treatment arm using a combination of amitriptyline and the active comparator being tested (de Zanette 2014; Goldenberg 1986; Goldenberg 1996). Two hundred and seventy participants took amitriptyline, 209 took placebo, 195 took various active comparators, and 55 took combinations. The active comparators were:
Panax ginseng extract (100 mg daily, 27% of ginsenosides);
cyclobenzaprine 30 mg daily;
melatonin 10 mg daily;
naproxen 2 x 500 mg daily;
fluoxetine 20 mg daily;
moclobemide 450 mg daily.
The included studies individually involved between 22 and 208 participants, and only two involved over 100 participants (Carette 1994; Hannonen 1998). The vast majority of participants were female (626 women and 33 men) with three studies enrolling only women (Braz 2013; de Zanette 2014; Hannonen 1998). Study duration ranged from 6 to 24 weeks.
Excluded studies
We excluded 15 studies (Ҫapaci 2002; Fors 2002; Hampf 1989; Heymann 2001; Isomeri 1993; Jaeschke 1991; Kempenaers 1994; McQuay 1992; McQuay 1993; Özerbil 2006; Pilowsky 1982; Pilowsky 1990; Scudds 1989; Zitman 1990; Zitman 1991). Reasons for exclusion of studies were: not being convincingly double‐blind, not demonstrating that participants had initial pain of at least moderate intensity, having fewer than 10 participants in a treatment arm, or not having a clear diagnosis of the painful condition. Details are in the Characteristics of excluded studies table.
Risk of bias in included studies
Risk of bias is shown in Figure 2 as a summary and in Figure 3 for each included study.
2.
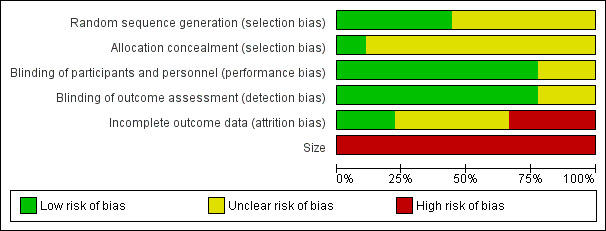
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
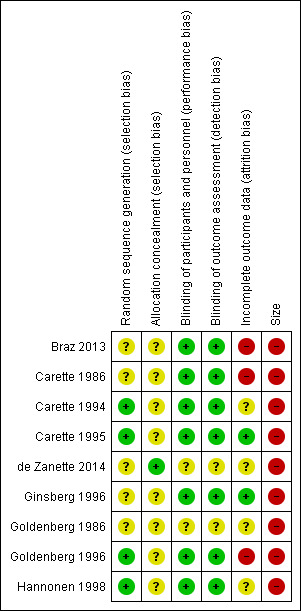
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Quality scores were good using the Oxford Quality Score; one study scored 3/5 points, three scored 4/5, and five scored 5/5.
Allocation
All studies were randomised, but only four adequately described the method used to generate the random sequence, and only one adequately described the method used to conceal the allocation of the random sequence.
Blinding
Seven studies adequately described the methods used to maintain blinding.
Incomplete outcome data
Two studies had a cross‐over design, and data on all randomised participants were not available for all outcomes (Carette 1986; Goldenberg 1996), while another study reported results only for participants who completed the study (Braz 2013). Only two studies convincingly reported on all participants (Carette 1995; Ginsberg 1996).
Selective reporting
The outcomes specified in the methods of most of these studies were not those sought for the review, so selective reporting bias was not an issue.
Other potential sources of bias
None of the studies included over 50 participants per treatment arm, and all were judged at high risk of bias for this domain.
Effects of interventions
See: Table 1
Results from individual studies are in Appendix 5 (efficacy) and Appendix 6 (adverse events and withdrawals).
Efficacy
No study met the criteria for first‐ or second‐tier evidence.
Participants with substantial pain relief
There was some third‐tier evidence that amitriptyline at 25 mg or 50 mg daily was better than placebo from four studies (Carette 1986; Carette 1994; Carette 1995; Ginsberg 1996).
The proportion of participants with substantial pain relief with amitriptyline was 36% (56/157, range 22% to 58%)
The proportion of participants with substantial pain relief with placebo was 11% (13/118, range 0% to 19%)
The RR for amitriptyline compared with placebo was 2.9 (1.7 to 4.9) (Figure 4), and the NNT was 4.1 (2.9 to 6.7).
4.
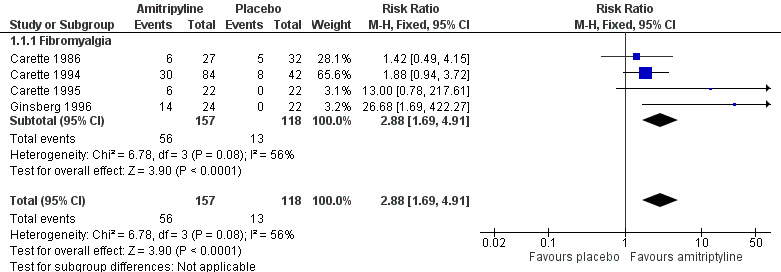
Forest plot of comparison: 1 Amitriptyline versus placebo, outcome: 1.1 Third‐tier efficacy.
These statistics should be interpreted with caution, in particular because of the small number of participants and events. The L'Abbe plot and I2 statistic estimate show no greater variation than would be expected in this number of small studies (Figure 5). Three of the four placebo‐controlled studies that did not report dichotomous outcomes also provided some support of greater analgesic effects from amitriptyline than placebo (Goldenberg 1986; Goldenberg 1996; Hannonen 1998).
5.
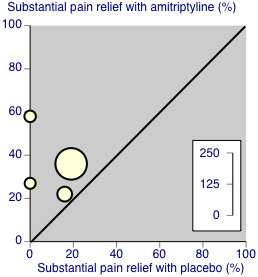
Third‐tier evidence: substantial pain relief
Amitriptyline was probably no better than cyclobenzaprine (Carette 1994), fluoxetine (Goldenberg 1996), moclobemide (Hannonen 1998) or Panax ginseng (Braz 2013) in individual small studies of limited ability to discriminate. One study claimed a benefit of melatonin over amitriptyline (de Zanette 2014).
Any disability‐related or mental health‐related outcome
All the included studies provided some information about the effects of amitriptyline on symptoms such as fatigue, sleep, tender points and quality of life (Appendix 5). These were reported as group means, but it was not always clear whether all participants were included in the analysis or what imputation method was used for missing data. Most studies reported no significant difference between treatment groups at the end of treatment for most measures, although occasionally a single measure was significantly different. There was no discernable pattern to this, and we would expect occasional significant results by chance in such a data set.
Adverse events
Participants experiencing at least one adverse event
This outcome was reported by four studies with placebo treatment arms, with 318 participants in the comparison (Carette 1986; Carette 1994; Ginsberg 1996; Hannonen 1998). At least one adverse event was experienced by 137/177 (77%) of participants taking amitriptyline, and 66/141 (47%) taking placebo. The risk ratio was 1.5 (1.3 to 1.8) (Analysis 1.2), and the number needed to treat to harm was 3.3 (2.5 to 4.9).
1.2. Analysis.
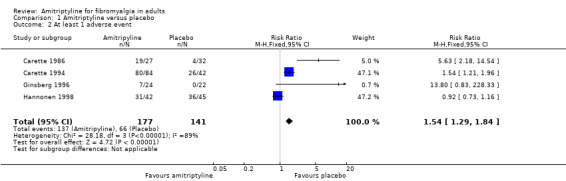
Comparison 1 Amitriptyline versus placebo, Outcome 2 At least 1 adverse event.
Serious adverse events
No studies reported any serious adverse events.
Individual adverse events
There were insufficient data for analysis of individual events. The most common events reported were dry mouth and drowsiness, somnolence or fatigue. Other events included dizziness, headache, nightmares, behavioural change, neuropsychiatric symptoms, weight gain, dyspepsia, and diarrhoea.
Withdrawals
All‐cause withdrawals were reported by seven placebo‐controlled studies (418 participants, Braz 2013; Carette 1986; Carette 1994; Carette 1995; Ginsberg 1996; Goldenberg 1986; Hannonen 1998). Overall, 38/228 (17%) withdrew for any cause with amitriptyline and 41/190 (22%) with placebo. The risk ratio was 0.77 (0.53 to 1.1) (Analysis 1.3); the number needed to treat to harm (NNH) was not calculated.
1.3. Analysis.
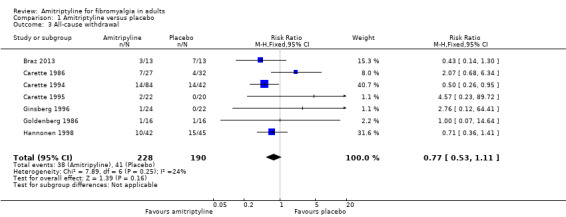
Comparison 1 Amitriptyline versus placebo, Outcome 3 All‐cause withdrawal.
Adverse event withdrawals were reported by four studies (298 participants, Braz 2013; Carette 1986; Carette 1994; Hannonen 1998). Overall, 14/166 (8%) withdrew because of adverse events with amitriptyline and 12/132 (9%) with placebo. The risk ratio was 1.03 (0.49 to 2.2) (Analysis 1.4); the NNH was not calculated.
1.4. Analysis.
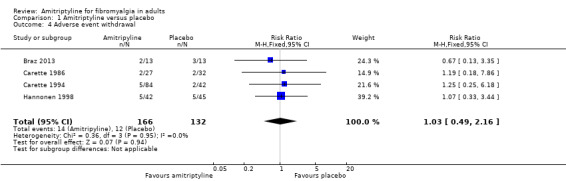
Comparison 1 Amitriptyline versus placebo, Outcome 4 Adverse event withdrawal.
Lack of efficacy withdrawals were reported by three studies (272 participants, Carette 1986; Carette 1994; Hannonen 1998). Overall, 8/153 (5%) withdrew because of lack of efficacy with amitriptyline and 14/119 (12%) with placebo. The risk ratio was 0.42 (0.19 to 0.95) (Analysis 1.5); the number needed to treat to prevent (NNTp) was 14 (7.2 to 980).
1.5. Analysis.
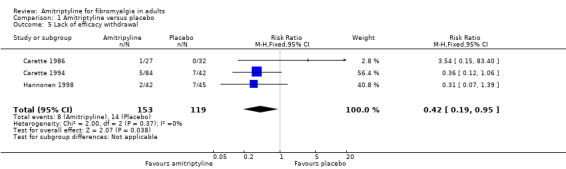
Comparison 1 Amitriptyline versus placebo, Outcome 5 Lack of efficacy withdrawal.
Discussion
Because amitriptyline is a crucially important drug in treating various forms of chronic pain, including fibromyalgia, and because experience from previous reviews was that most studies would be older, be small, and have methodological deficiencies according to present standards of evidence, we felt it appropriate to accept lower standards than those currently demanded for part of our analyses. It is important to recognise that the lower‐level evidence is likely to be subject to various positive biases, and that these lower levels of evidence cannot be used to make cross‐drug comparisons of efficacy with other drugs.
The most important finding of this review was that there were no studies that met current standards of evidence for chronic pain that minimise all known biases (Moore 2010a; Moore 2012b). All the studies accepted for third‐tier evidence contained features of design, conduct, or reporting that are known to be associated with bias in favour of the active treatment. Particular problems were reporting of outcomes of less than 50% pain intensity reduction or undefined 'improvement', having relatively short duration (although two thirds of the studies had treatment periods of eight weeks or more), and studies being small, in circumstances where small studies in chronic pain are known to be associated with over‐estimation of treatment effect (Dechartres 2013; Kjaergard 2001; Nüesch 2010), beyond the large random variation that occurs with small pain studies (Moore 1998). That means that the third‐tier efficacy results reported here offer only the best judgement possible on evidence that is not wholly trustworthy.
While it is possible that amitriptyline is effective in some patients with fibromyalgia, the evidence we have cannot rule out the possibility that amitriptyline is no better than placebo for this condition. This rather bleak conclusion should be tempered by many years of clinical experience indicating that amitriptyline can give really good pain relief to some patients with fibromyalgia, but only a minority of them; amitriptyline will not work for most people.
Summary of main results
There is limited evidence based on small numbers of small studies that amitriptyline may provide good pain relief in fibromyalgia. Our best estimate is that for every four people treated, one will experience a good level of pain relief (equivalent to at least 50% pain reduction) who would not have done with placebo (very low quality evidence; Table 1). Given the caveats above, this is probably an overestimation of treatment effect, but the consistency of effect within these four studies does provide some confidence that amitriptyline benefits are real, at least for some patients.
The effect of amitriptyline on other fibromyalgia symptoms, such as fatigue, quality of sleep, and tender points is less clear. Mean data indicate that all treatment groups show an overall improvement during the study, but there were no consistent findings of significant differences between amitriptyline and placebo or other comparators. This differs from results of individual patient level analyses linking improvements in pain with changes in other outcomes; these demonstrate that pain reduction is closely linked with improvements in sleep, depression, quality of life, and ability to work (Moore 2010c; Straube 2011). The small number of studies and participants and the use of mean data for these outcomes may have limited the ability to demonstrate a significant difference.
Overall completeness and applicability of evidence
It is likely that all of the completed clinical trials have been found, but those we found and included had deficiencies because the design or reporting included features known to be associated with potential bias towards the active treatment over placebo. For example, two had a cross‐over design, all were small, and fewer than half reported efficacy outcomes based on individual participants obtaining a high degree of pain relief.
This limits considerably the applicability of the evidence. Although amitriptyline is widely used as the mainstay of treatment of fibromyalgia, there is no unbiased evidence on which to base clinical practice beyond extensive clinical experience, and no evidence for comparison with other potential treatments of fibromyalgia.
There are also significant limits in what the review can say about appropriate doses of amitriptyline. Studies used daily doses of 25 mg to 50 mg, with titration in some.
Quality of the evidence
All studies had to be randomised and double‐blind to be included, and all had to have participants with at least moderate pain relief to ensure that studies were sensitive. No single study fulfilled all the qualities of reliability now used in chronic pain. It is disappointing that the more recent studies were not of higher reporting quality.
Potential biases in the review process
We used an extensive search strategy, which was based on previous Cochrane reviews and on other reviews with different strategies, and included a comprehensive manual journal search (Jadad 1996a). It is unlikely that relevant high‐quality large studies of amitriptyline in fibromyalgia have been overlooked, especially because amitriptyline is the mainstay of treatment.
Agreements and disagreements with other studies or reviews
Our earlier review looked at both neuropathic pain and fibromyalgia, although different conditions were analysed separately for efficacy. The new studies did not contribute to efficacy data and did not change the conclusions for the analyses of withdrawals to which they did contribute.
A review of amitriptyline, duloxetine, and milnacipran in fibromyalgia included seven of the nine studies in this review, and three studies that we excluded because they had no baseline pain requirement or baseline pain data (Heymann 2001; Scudds 1989), or had fewer than 10 participants in each treatment arm (Kempenaers 1994). The authors acknowledged the poor quality of the studies, reporting an NNT of 3.5 (2.7 to 5.0) for at least 30% pain relief compared with placebo, and small or moderate effects on fatigue, quality of life, and sleep, using mean data. These results are consistent with those found in this review. Corresponding NNTs for duloxetine and milnacipran were 8 and 11, with similar small to moderate effects on other symptoms. The higher (worse) NNTs may in part be due to inclusion of larger studies of better quality for these two drugs. There were no significant differences in overall withdrawal rates between each drug and placebo, or between the three drugs, but adverse event withdrawals were not specifically reported (Hausser 2011).
An earlier review of amitriptyline for fibromyalgia again included seven of the nine studies in this review, and three studies that we excluded because they had no baseline pain requirement or baseline pain data (Fors 2002; Heymann 2001; Scudds 1989). The authors chose not to pool data, but reported a therapeutic response for amitriptyline 25 mg, but not 50 mg, compared with placebo for pain, sleep, fatigue, and global impression. Neither dose had an effect on tender points. No clear statement was made about adverse events because of inconsistent reporting, although there was no difference between amitriptyline and placebo for adverse event withdrawals (Nishishinya 2008).
Amitriptyline had similar effects on the intensity of care in a large cohort of US patients treated with a variety of antidepressant and antiepileptic drugs (Kim 2015).
Authors' conclusions
Implications for practice.
Amitriptyline has been a first‐line treatment for fibromyalgia for many years. The fact that there is no supportive unbiased evidence for substantial pain relief has to be balanced against decades of successful treatment in many tens of thousands of patients with fibromyalgia. There is no reliable evidence of a lack of effect: rather our concern should be of overestimation of treatment effect.
For clinicians
Amitriptyline will continue to be used as part of the treatment of fibromyalgia, but we should be cognisant of the fact that only a small number of patients will achieve satisfactory pain relief.
For policy makers
Amitriptyline will continue to be used as part of the treatment of fibromyalgia, but a range of drugs will be needed to provide good pain relief for a population of people with fibromyalgia.
For Funders
Amitriptyline will continue to be used as part of the treatment of fibromyalgia, but a range of drugs will be needed to provide good pain relief for a population of people with fibromyalgia.
Implications for research.
General
There is no convincing evidence about effectiveness of the most commonly used first line therapy for fibromyalgia.
It is unlikely that any large randomised trials of amitriptyline will be conducted in fibromyalgia to establish efficacy. Such trials are expensive. The bigger implication is for research in clinical practice, to determine whether there is a sequence of using drugs that will provide overall better clinical effectiveness (Moore 2010d). Another area for research, though extremely difficult, is to identify characteristics that predict which patients are likely to benefit from amitriptyline.
Design
This review highlights the design weaknesses of older trials in fibromyalgia. It is notable that probably the only treatment in fibromyalgia that reaches first tier level of evidence is duloxetine, and then because of a post‐hoc individual‐patient‐level analysis to change LOCF to BOCF, and use a common defined outcome (Moore 2014c).
Measurement (endpoints)
There are no lessons here about endpoints. We know that individuals with high levels of pain relief obtain benefit in a range of other symptoms, like sleep, depression, quality of life, and function.
Comparison between active treatments
This is not possible given the present state of knowledge, with generally inadequate trials and reporting for older therapies. More recently introduced therapies for fibromyalgia ‐ duloxetine, milnacipran, and pregabalin ‐ have been licensed in the USA and elsewhere based on large, high quality, clinical trials. While issues around imputation methods in those trials remains, we can be confident that they work well in a small proportion of people with fibromyalgia.
What's new
| Date | Event | Description |
|---|---|---|
| 28 May 2019 | Amended | Contact details updated. |
| 11 October 2017 | Review declared as stable | No new studies likely to change the conclusions are expected. |
History
Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 31 July 2015 | Review declared as stable | This review will be assessed for further updating in 2020. |
| 31 March 2015 | New citation required but conclusions have not changed | Previous review split into two new reviews, with separate reviews dealing with neuropathic pain and with fibromyalgia. Title changed from Amitriptyline for neuropathic pain and fibromyalgia in adults to Amitriptyline for fibromyalgia in adults New studies did not provide data that changed conclusions |
| 26 March 2015 | New search has been performed | New searches run. Two new included studies identified, with 101 participants (Braz 2013; de Zanette 2014) |
| 24 September 2010 | Amended | Contact details updated. |
Acknowledgements
Support for this review came from the Oxford Pain Relief Trust.
The protocol for this review was written with funding support from the NHS Cochrane Collaboration Programme Grant Scheme (UK) and European Union Biomed 2 Grant no. BMH4 CT95 0172 (UK). We are grateful to the peer reviewers for some very useful comments relating to that protocol, and the earlier review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Methodological considerations for chronic pain
There have been several recent changes in how the efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria for what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, avoiding problems from the random play of chance. Newer trials also tend to be of longer duration, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for the inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. To summarise some of the recent insights that must be considered in this new review:
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011a; Moore 2011b), back pain (Moore 2010d), and arthritis (Moore 2010c), as well as in fibromyalgia (Straube 2010); in all cases average results usually describe the experience of almost no‐one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
As a consequence, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In arthritis, trials of less than 12 weeks duration, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010c); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
The proportion of patients with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis to 30% in fibromyalgia (Moore 2009; Moore 2010c; Moore 2013b; Moore 2014b; Straube 2010; Sultan 2008). A Cochrane review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so.
Individual patient analyses indicate that patients who get good pain relief (moderate or better) have major benefits in many other outcomes, affecting quality of life in a significant way (Moore 2010b; Moore 2014a).
Imputation methods such as last observation carried forward (LOCF), used when participants withdraw from clinical trials, can overstate drug efficacy especially when adverse event withdrawals with drug are greater than those with placebo (Moore 2012b).
Appendix 2. MEDLINE (via OVID) search strategy
exp PAIN/
exp PERIPHERAL NERVOUS SYSTEM DISEASES/
exp SOMATOSENSORY DISORDERS/
FIBROMYALGIA/ or exp MYOFASCIAL PAIN SYNDROMES/ or POLYMYALGIA RHEUMATICA/
((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp.
(fibromyalgi* or fibrosti* or FM or FMS).mp.
((neur* or nerv*) adj6 (compress* or damag*)).mp.
1 or 2 or 3 or 4 or 5 or 6 or 7
Amitriptyline/
(am?tr?pt?lin* or amitriptyliini).mp.
9 or 10
8 and 11
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/13‐20
exp animals/ not humans.sh.
21 not 22
23 and 12
Appendix 3. EMBASE (via OVID) search strategy
exp chronic pain/
exp peripheral neuropathy/
exp somatosensory disorder/
fibromyalgia/ or exp myofascial pain/ or rheumatic polymyalgia/
((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp.
(fibromyalgi* or fibrosti* or FM or FMS).mp.
((neur* or nerv*) adj6 (compress* or damag*)).mp.
1 or 2 or 3 or 4 or 5 or 6 or 7
amitriptyline/
(am?tr?pt?lin* or amitriptyliini or Tryptomer or Elavil or Tryptizol or Laroxyl or Sarotex or Lentizol or Endep).mp.
9 or 10
8 and 11
random*.ti,ab.
factorial*.ti,ab.
(crossover* or cross over* or cross‐over*).ti,ab.
placebo*.ti,ab.
(doubl* adj blind*).ti,ab.
assign*.ti,ab.
allocat*.ti,ab.
RANDOMIZED CONTROLLED TRIAL.sh.
DOUBLE‐BLIND PROCEDURE.sh.
CROSSOVER PROCEDURE.sh.
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
12 and 23
Appendix 4. CENTRAL search strategy
MeSH descriptor Pain explode all trees
MeSH descriptor Peripheral Nervous System Diseases explode all trees
MeSH descriptor Somatosensory Disorders explode all trees
MeSH descriptor Fibromyalgia, this term only
MeSH descriptor Myofascial Pain Syndromes explode all trees
MeSH descriptor Polymyalgia Rheumatica explode all trees
((pain* or discomfort*) and (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)):ti,ab,kw
(fibromyalgi* or fibrosti* or FM or FMS):ti,ab,kw
((neur* or nerv*) and (compress* or damag*)):ti,ab,kw
(1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9)
MeSH descriptor Amitriptyline
(am?tr?pt?lin* or amitriptyliini or Tryptomer or Elavil or Tryptizol or Laroxyl or Sarotex or Lentizol or Endep).ti,ab,kw
11 or 12
10 and 13
Limit 14 to CENTRAL
Appendix 5. Summary of outcomes in individual studies: efficacy
| Study |
Treatment (taken at night, unless stated) |
Pain outcome | Other efficacy outcome |
| Braz 2013 | Amitriptyline 25 mg = 13 P. ginseng extract = 12 Placebo = 13 | VAS PI reduced in all groups compared with baseline, but no statistically significant difference between groups | No significant differences between groups on any of the measures: fatigue, sleep, QoL |
| Carette 1986 | Amitriptyline 50 mg = 27 Placebo = 32 Dose titration: Week 1 ‐ 10 mg/d Weeks 2 to 4 ‐ 25 mg/d Weeks 5 to 9 ‐ 50 mg/d | Global impression of change ‐ moderate or marked at 9 weeks
Amitriptyline = 17/27 Placebo = 10/32 Global impression of change ‐ marked Amitriptyline = 6/27 Placebo = 5/32 |
No difference in tender points, but improved sleep with amitriptyline |
| Carette 1994 | Amitriptyline 25 mg = 84 Cyclobenzaprine 30 mg = 82 Placebo = 42 Dose titration: Amitriptyline week 1 ‐ 10 mg/d Weeks 2 to 12 ‐ 25 mg/d Weeks 13 to 24 ‐ 50 mg/d Cyclobenzaprine week 1 ‐ 10 mg/d Weeks 2 to 12 ‐ 20 mg/d Weeks 13 to 24 ‐ 30 mg/d | Significant improvers (50% improvement in pain, sleep, fatigue, global, myalgic score, 4 of 6):
Amitriptyline = 30/84
Cyclobenzaprine = 27/82 Placebo = 8/42 No difference for change in mean pain score (from graph): Amitriptyline dropped 67 to 48 mm Placebo dropped 69 to 54 mm |
No significant end of trial difference for sleep, fatigue, global, tender points |
| Carette 1995 | Amitriptyline 25 mg = 22 Placebo = 20 Cross‐over |
Significant improvers (50% improvement in pain, sleep, fatigue, global, myalgic score, 4 of 6) Amitriptyline = 6/22 Placebo = 0/22 VAS pain at 8 weeks (mean ± SD) Amitriptyline = 5.1 ± 3.2 Placebo = 7.1 ± 2.1 |
Significant difference for sleep, patient global, fatigue, but not tender points |
| de Zanette 2014 | Amitriptyline 25 mg daily = 21 Melatonin 10 mg daily = 21 Amitriptyline 25 mg + melatonin 10 mg daily = 21 |
Mean PI in last 24 h during last week of treatment (100 mm VAS) vs before treatment Amitriptyline 63 to 50 = 13 Melatonin 65 to 48 = 17 Amitriptyline + melatonin 69 to 49 = 20 | No significant difference observed between groups in the numbers of analgesic used in last week of treatment, sleep quality and number of tender points |
| Ginsberg 1996 | Amitriptyline 25 mg = 24 Placebo = 22 |
Responder (at least 50% improvement pain and or global) at 8 weeks:
Amitriptyline = 14/ 24 Placebo = 0/22 VAS pain (mean ± SD) Amitriptyline baseline 3.8 ± 2.4 Amitriptyline end 7.0 ± 1.3 Placebo baseline 7.0 ± 1.4 Placebo end 5.0 ± 2.1 |
Major changes in patient global, tender point count and score, sleep, fatigue, and stiffness |
| Goldenberg 1986 | Balanced assignment quoted, but actual numbers in each group not given Therefore we assume:
Amitriptyline 25 mg = 16 Placebo = 16 (Also included naproxen 2 x 500 mg and amitriptyline + naproxen treatment arms) |
VAS pain at 6 weeks (mean, from graph):
Amitriptyline = 5.4 Placebo = about 7.4 Significant difference only at 4 weeks, not at 6 weeks. No dispersion given |
End of trial ‐ significant benefit for amitriptyline versus placebo for fatigue, sleep, and patient global assessment, but not tender points |
| Goldenberg 1996 | Amitriptyline 25 mg = 21
Fluoxetine 20 mg = 22
Amitriptyline + Fluoxetine = 19 Placebo = 19 Cross‐over |
VAS pain at 6 weeks (mean ± SD)
Amitriptyline = 64 ± 28
Fluoxetine = 58 ± 26
Amitriptyline + Fluoxetine = 43 ± 29 Placebo = 82 ± 17 |
Apparent significant results, probably amitriptyline versus placebo, for pain, FIQ, sleep, and global, but not fatigue or tender points Generally effect amitriptyline + fluoxetine > fluoxetine ≥ amitriptyline > placebo % change before/after calculated for each patient gave similar pattern to group means ‐ numbers given for > 25% improvement in FIQ only: Amitriptyline = 5/21, Fluoxetine = 7/22, Amitriptyline + Fluoxetine = 12/19, Placebo = 1/19 |
| Hannonen 1998 | Amitriptyline 25 mg = 42
Moclobemide 450 mg (am and pm) = 43 Placebo = 45 Titration to max 37.5 mg A, 600 mg M |
VAS (mean ± SD)
Amitriptyline baseline 6.0 ± 2.1
Amitriptyline end 4.5 ± 2.8
Moclobemide baseline 5.7 ± 2.1
Moclobemide end 4.5 ± 2.7 Placebo baseline 5.7 ± 2.3 Placebo end 5.2 ± 2.7 Number of responders not given, but some response in 74% (amitriptyline) versus 49% (placebo), 54% (moclobemide) |
General health, sleep fatigue tender points, and clinician severity all improved with amitriptyline and placebo, but no obvious between group difference, except perhaps sleep |
AE: adverse effect; d: day; FIQ: Fibromyalgia Impact Questionnaire; QoL: quality of life; SD: standard deviation; VAS: visual analogue scale
Appendix 6. Summary of outcomes in individual studies: adverse events and withdrawals
| Study |
Treatment (taken at night, unless stated) |
Adverse events | Withdrawals |
| Braz 2013 | Amitriptyline 25 mg = 13 P. ginseng extract = 12 Placebo = 13 | Not reported | All cause: Amitriptyline 3/13 P. ginseng 4/12 Placebo 7/13 AE: Amitriptyline 2/13 P. ginseng 3/12 Placebo 3/13 No specific LoE withdrawals |
| Carette 1986 | Amitriptyline 50 mg = 27 Placebo = 32 Dose titration: Week 1 ‐ 10 mg/d Weeks 2 to 4 ‐ 25 mg/d Weeks 5 to 9 ‐ 50 mg/d | Patients with ≥ 1 AE:
Amitriptyline = 19/27 Placebo = 4/32 "minor side effects" ‐ mostly drowsiness and xerostomia |
Total: Amitriptyline = 7/27, Placebo = 4/32 LoE: Amitriptyline = 1/27 Placebo = 0/32 AE: Amitriptyline = 2/27 Placebo = 2/32 Other: Amitriptyline = 4/27 Placebo = 2/32 |
| Carette 1994 | Amitriptyline 25 mg = 84 Cyclobenzaprine 30 mg = 82 Placebo = 42 Dose titration: Amitriptyline week 1 ‐ 10 mg/d Weeks 2 to 12 ‐ 25 mg/d Weeks 13 to 24 ‐ 50 mg/d Cyclobenzaprine week 1 ‐ 10 mg/d Weeks 2 to 12 ‐ 20 mg/d Weeks 13 to 24 ‐ 30 mg/d | Patients with ≥ 1 AE: Amitriptyline = 80/84 Cyclobenzaprine = 80/82 Placebo = 26/42 Most common ‐ dry mouth, somnolence, dizziness, weight gain |
Total: Amitriptyline = 14/84, Cyclobenzaprine = 24/82, Placebo = 14/42 LoE: Amitriptyline = 5/84, Cyclobenzaprine = 6/82, Placebo = 7/42 AE: Amitriptyline = 5/84, Cyclobenzaprine = 11/82, Placebo = 2/42 Other: Amitriptyline = 4/84, Cyclobenzaprine = 7/82, Placebo = 5/42 |
| Carette 1995 | Amitriptyline 25 mg = 22 Placebo = 20 Cross‐over |
Not reported | 2 withdrawals after first period A (not drug‐related) |
| de Zanette 2014 | Amitriptyline 25 mg daily = 21 Melatonin 10 mg daily = 21 Amitriptyline 25 mg + melatonin 10 mg daily = 21 |
Minor
Amitriptyline 8/21 (nausea, dizziness, weight gain, dry mouth, headache)
Melatonin 5/21 (no details) Amitriptyline + melatonin not reported Major Amitriptyline 5/21 (dizziness, nightmares, drowsiness, headache, behavioural change, worsening pain) Melatonin 6/21 (no details) Amitriptyline + melatonin not reported |
AE: Amitriptyline 2/21 Melatonin 2/21 Amitriptyline + melatonin 2/21 No other withdrawals reported |
| Ginsberg 1996 | Amitriptyline 25 mg = 24 Placebo = 22 |
Amitriptyline = 7/24 (3 dry mouth, 2 digestive symptoms, 1 vertigo, 2 neuro‐psychic symptoms) Placebo = 0/22 |
1 in amitriptyline due to AE |
| Goldenberg 1986 | Balanced assignment quoted, but actual numbers in each group not given. Therefore we assume:
Amitriptyline 25 mg = 16 Placebo = 16 (Also included naproxen 2 x 500 mg, and amitriptyline + naproxen treatment arms) |
8 patients (across groups) complained of side effects but did not discontinue medication (dry mouth, dyspepsia, diarrhoea) | Amitriptyline = 1 (lost to follow‐up)
N = 1 (lost to follow‐up)
Amitriptyline + naproxen = 1 (AE ‐ somnolence) Placebo = 1 (AE ‐ epigastric distress) |
| Goldenberg 1996 | Amitriptyline 25 mg = 21
Fluoxetine 20 mg = 22
Amitriptyline + Fluoxetine = 19 Placebo = 19 Cross‐over |
Not reported | 12/31 did not complete
Amitriptyline = 1 (other)
Fluoxetine = 4 (1 AE, 3 LoE)
Amitriptyline + Fluoxetine = 5 (3 AE, 2 other) Placebo = 1 (AE) Washout after Amitriptyline + Fluoxetine = 1 (LoE) |
| Hannonen 1998 | Amitriptyline 25 mg = 42
Moclobemide 450 mg (am and pm) = 43 Placebo = 45 Titration to max 37.5 mg amitriptyline, 600 mg moclobemide |
Patients with ≥ 1 AE:
Amitriptyline = 31/42 (dry mouth, fatigue)
Moclobemide = 33/43 (headache, difficulty falling asleep) Placebo = 36/45 (fatigue, headache) |
Withdrawals:
Amitriptyline = 10/42 (2 LoE, 5 AE, 3 other)
Moclobemide = 13/43 (4 LoE, 6 AE, 3 other) Placebo = 15/45 (7 LoE, 5 AE, 3 other) |
AE: adverse effect; LoE: lack of efficacy; SAE: serious adverse effect
Data and analyses
Comparison 1. Amitriptyline versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Third‐tier efficacy | 4 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.69, 4.91] |
| 1.1 Fibromyalgia | 4 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.69, 4.91] |
| 2 At least 1 adverse event | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.29, 1.84] |
| 3 All‐cause withdrawal | 7 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 4 Adverse event withdrawal | 4 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.16] |
| 5 Lack of efficacy withdrawal | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.95] |
1.1. Analysis.
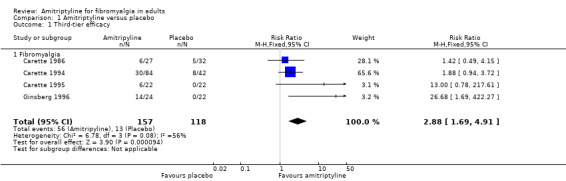
Comparison 1 Amitriptyline versus placebo, Outcome 1 Third‐tier efficacy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Braz 2013.
| Methods | R, DB, AC, PC, parallel groups, duration 12 weeks Medication taken once daily at 6 pm Assessment at baseline and 1, 3, 6, 9, 12 weeks |
|
| Participants | Inclusion: women with fibromyalgia (ACR 1990), age 21 to 60 years, normal laboratory tests Exclusion: untreated inflammatory or endocrine disease; neurological, renal, infectious or bone disease; glaucoma, urinary retention, cardiovascular abnormalities; use of tricyclics within 3 months, any contraindication to study medication N = 38, mean age 43 years, all F Mean duration of symptoms > 33 months (least in placebo group, mean baseline pain 9/10 (5.7 to 9.6) |
|
| Interventions | Amitriptyline 25 mg daily, n = 13 Panax ginseng extract (100 mg daily, 27% of ginsenosides), n = 12 Placebo, n = 13 Analgesics, opioids, anti‐inflammatory drugs all stopped for ≥ 3 weeks before start of study |
|
| Outcomes | Mean pain intensity AE withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generation of random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "identical form as capsules .... in sealed black bottles" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "identical form as capsules .... in sealed black bottles" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Results for completers only |
| Size | High risk | < 50 participants per treatment arm |
Carette 1986.
| Methods | Multicentre, R, DB, PC, parallel groups, duration 9 weeks Medication taken as single dose at bedtime. Initial daily dose of amitriptyline 10 mg, increased to 25 mg after 1 week, and to 50 mg after 4 weeks. Dose reduction allowed if not tolerated Pain, sleep, overall change in disease assessed at baseline, week 5 and week 9 |
|
| Participants | Inclusion: primary fibrositis (Smythe's criteria) Exclusion: evidence of traumatic, neurologic, muscular, infectious, osseous, endocrine, or other rheumatic conditions. History of glaucoma, urinary retention, cardiovascular abnormalities. Use of amitriptyline within previous year N = 70 enrolled, 57 completed, mean age 41 years, M 5/F 54 Mean duration of symptoms ˜85 months (significantly longer in placebo group), mean baseline pain ˜6/10 |
|
| Interventions | Amitriptyline 50 mg/day, n = 27 Placebo, n = 32 All NSAIDs, antidepressants and hypnotic medication stopped ≥ 3 weeks before start of study Paracetamol permitted throughout study |
|
| Outcomes | Patient global impression of change Mean pain intensity Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ stated to be "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules "were identical" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Capsules "were identical" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis |
| Size | High risk | Fewer than 50 participants/treatment arm |
Carette 1994.
| Methods | Multicentre, R, DB (DD), PC and AC, parallel groups, treatment period 24 weeks Amitriptyline taken as single dose at bedtime; initial daily dose 10 mg, increased to 25 mg after 1 week, and to 50 mg after 12 weeks. Cyclobenzaprine initial daily dose 10 mg at bedtime, increased to 20 mg at bedtime after 1 week, and to 10 mg in the morning +20 mg at bedtime after 12 weeks. Dose reduction permitted if not tolerated Pain, fatigue, sleep, fibromyalgia symptoms assessed at baseline, and each month |
|
| Participants | Inclusion: fibromyalgia (ACR 1990), age ≥ 18 years, ≥ 4/10 for pain and/or global assessment of fibromyalgia symptoms Exclusion: evidence of inflammatory rheumatic disease, untreated endocrine, neurologic, infectious, or osseous disorder. Glaucoma, urinary retention, cardiovascular abnormalities. Previous treatment with study drugs N = 208, mean age 45 years, M 13/F 195 Median duration of symptoms 5 years, baseline pain ≥ 66/100 |
|
| Interventions | Amitriptyline 50 mg/day, n = 84 Cyclobenzaprine 30 mg/day, n = 82 Placebo, n = 42 All NSAIDs, hypnotics, and antidepressants discontinued ≥ 3 weeks before start of study Paracetamol permitted throughout study |
|
| Outcomes | Responder (at least 4/6 from ≥ 50% improvement in pain, sleep, fatigue, patient global assessment, physician global assessment, and increase of 1 kg in total myalgic score) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "generated using a table of random numbers .... assigned in blocks of 5" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy method described. ''Either amitriptyline 25mg or an identical appearing inert cyclobenzaprine placebo or active cyclobenzaprine and inert amitriptyline placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy method described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Responder analysis, but unclear whether withdrawal = non responder or LOCF |
| Size | High risk | Fewer than 50 participants in placebo treatment arm |
Carette 1995.
| Methods | Single centre, R, DB, PC, cross‐over study. 2 x 8‐week treatment periods with no washout. Medication taken as single dose, 1 hour before bedtime Pain, fibromyalgia, sleep, and fatigue assessed at baseline and end of each treatment period |
|
| Participants | Inclusion: fibromyalgia (ACR), age ≥ 18 years, baseline pain and/or global assessment of fibromyalgia ≥ 4/10 Excluded: evidence of neurologic, muscular, infectious, endocrine, osseous, or other rheumatological diseases, history of glaucoma, urinary retention, cardiovascular disease, sleep apnoea N = 22, mean age 44 years, M 1/F 21 Mean (SD) duration of fibromyalgia 83 (± 75) months, mean baseline pain 7/10 |
|
| Interventions | Amitriptyline 25 mg/d (reduced to 10 mg/day if not tolerated), n = 22 Placebo, n = 20 Washout before start of study: 2 weeks for NSAIDs and hypnotics, minimum 4 weeks for antidepressants Paracetamol permitted throughout study |
|
| Outcomes | Responder (at least 4/6 from ≥ 50% improvement in pain, sleep, fatigue, patient global assessment, physician global assessment, and increase of 1 kg in total myalgic score) Mean pain intensity Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "generated using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "identically appearing placebo tablet" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "identically appearing placebo tablet" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in responder analysis. Unclear how missing data were handled for mean data |
| Size | High risk | Fewer than 50 participants/treatment arm |
de Zanette 2014.
| Methods | R, DB (DD), AC, parallel groups, duration 6 weeks Medication taken as single dose at bedtime Assessment at baseline and end of treatment |
|
| Participants | Inclusion: fibromyalgia (ACR), refractory to current treatment and PI ≥ 50/100 Exclusion: inflammatory rheumatic disease or other painful conditions that might confound assessment; history of substance abuse, neurologic or oncologic disease, ischaemic heart disease, kidney or hepatic insufficiency N = 63, mean age 48 years, mean baseline pain 66/100 |
|
| Interventions | Amitriptyline 25 mg daily, n = 21 Melatonin 10 mg daily, n = 21 Amitriptyline 25 mg + melatonin 10 mg daily, n = 21 Current analgesics continued unchanged (paracetamol, ibuprofen, codeine, tramadol) Rescue medication: paracetamol (maximum 4 x 750 mg daily) and ibuprofen (maximum 4 x 20 mg daily) |
|
| Outcomes | Pain intensity Fibromyalgia Impact Questionnaire Use of additional analgesics in final week |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | "Before the recruitment phase, envelopes containing the protocol materials were prepared. Each envelope was sealed and numbered sequentially" "Two investigators who were not involved in patient evaluations were responsible for the blinding and randomization procedures" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Placebo and active treatment [capsules] had the same size, color, smell and flavor" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Placebo and active treatment [capsules] had the same size, color, smell and flavor" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method for mean data unclear. No dichotomous data |
| Size | High risk | < 50 participants per treatment arm |
Ginsberg 1996.
| Methods | Single centre, R, DB, PC, parallel groups, 8‐week treatment period Medication taken as single dose, 1 hour before bedtime Pain, fibromyalgia, sleep, and fatigue assessed at baseline and end of weeks 4 and 8 |
|
| Participants | Inclusion: fibromyalgia (ACR) Exclusion: glaucoma, urinary retention, cardiovascular problems, epilepsy, treatment with amitriptyline within 6 months N = 46, mean age 46 years, M 8/F 38 Duration of fibromyalgia 0.3 to 20 years, mean baseline pain 7/10 |
|
| Interventions | Amitriptyline 25 mg/day n = 24 (sustained‐release formulation) Placebo, n = 22 Not permitted during study: vitamin D/magnesium, muscle relaxants, analgesics/anti‐inflammatory except paracetamol, antidepressants, hypnotics, tranquillisers Paracetamol permitted throughout study for severe pain |
|
| Outcomes | Responder (at least 3/4 from ≥ 50% improvement in patient global, physician global, pain, and ≥ 25% reduction in tender point score) Mean pain intensity Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported ‐ stated as "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was "identical to the amitriptyline capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo was "identical to the amitriptyline capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in responder analysis. Unclear how missing data were handled for mean data |
| Size | High risk | Fewer than 50 participants/treatment arm |
Goldenberg 1986.
| Methods | R, DB (DD), AC, and PC, parallel groups, 6‐week treatment period Amitriptyline taken as single dose at night, naproxen as divided dose morning and night ‐ implication is DD Assessments at baseline, 2, 4, and 6 weeks for patient global fibromyalgia symptoms, pain or stiffness, fatigue, sleep |
|
| Participants | Inclusion: fibromyalgia (not ACR, but probably equivalent), baseline pain and/or fibromyalgia symptoms ≥ 4/10 Excluded: peptic ulcer disease or cardiac arrhythmias N = 62, mean age 44 years, M 3/F 59 Duration of chronic pain 0.3 to 20 years |
|
| Interventions | Amitriptyline 25 mg/day, n = assume 16 Naproxen 2 x 500 mg/day, n = assume 15 Amitriptyline 25 mg + naproxen 2 x 500 mg/day, n = assume 15 Placebo, n = assume 16 All analgesics, anti‐inflammatory medications, antidepressants, sleeping medication and CNS‐active medications stopped ≥ 72 h before start Paracetamol (2 x 650 mg every 4 hours) allowed for severe pain throughout study |
|
| Outcomes | Mean pain intensity Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported ‐ stated to be "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated to be 'blinded'. Describes double‐dummy design but not stated to be matching |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated to be 'blinded'. Describes double‐dummy design but not stated to be matching |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. No responder data |
| Size | High risk | Fewer than 50 participants/treatment arm |
Goldenberg 1996.
| Methods | Single centre, R, DB, PC, and AC, cross‐over study. 4 x 6‐week treatment periods with 2‐week washout between periods. Amitriptyline taken as single dose at bedtime, fluoxetine as single dose in the morning Pain, fibromyalgia, sleep, and fatigue assessed at baseline and end of each treatment period |
|
| Participants | Inclusion: fibromyalgia (ACR), age 18 to 60 years, baseline pain ≥ 30/100, baseline HRS‐D ≤ 18 Exclusion: current or history of systemic disease N = 31, mean age 43 years, M 3/F 28 Duration of symptoms 24 to 240 months, mean baseline pain 67/100 |
|
| Interventions | Amitriptyline 25 mg/day, n = 21 Fluoxetine 20 mg/day, n = 22 Amitriptyline 25 mg + fluoxetine 20 mg, n = 19 Placebo, n = 19 All CNS‐active medications, NSAIDs, analgesics other than paracetamol stopped ≥ 7 days before start Paracetamol permitted |
|
| Outcomes | Mean pain intensity Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "order of treatment was generated from a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All tablets were identical in appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All tablets were identical in appearance" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | LOCF implied |
| Size | High risk | Fewer than 50 participants/treatment arm |
Hannonen 1998.
| Methods | Multicentre, R, DB, PC, and AC, parallel groups, 12‐week treatment period Amitriptyline taken 2 h before bedtime, moclobemide taken as divided dose in morning and afternoon. Initial daily dose amitriptyline 12.5 mg, increased to 25 mg at 2 weeks, and again to 37.5 mg at 6 weeks if response unsatisfactory. Initial daily dose of moclobemide 300 mg, increased to 450 mg at 2 weeks, and again to 600 mg if response unsatisfactory Pain, general health (fibromyalgia), sleep, and fatigue assessed at baseline and 2, 6, 12 weeks |
|
| Participants | Inclusion: fibromyalgia (ACR 1990), female, age 18 to 65 years, score ≥ 4/10 for at least three of pain, general health (fibromyalgia), sleep, and fatigue Exclusion: severe cardiovascular, pulmonary, hepatic, haematological or renal disease N = 130, mean age 49 years, all F Mean duration of symptoms 8 years, baseline pain ≥ 5.7/10 |
|
| Interventions | Amitriptyline 25 mg/day, n = 42 Moclobemide 450 mg/day, n = 43 Placebo, n = 45 All CNS‐active medications, NSAIDs, and analgesics (other than paracetamol) discontinued before start of study Paracetamol permitted throughout study |
|
| Outcomes | Mean pain intensity, global health Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ''The randomisation was organised centrally with sequentially numbered envelopes consisting of blocks of six''. Probably low risk |
| Allocation concealment (selection bias) | Unclear risk | Does not state that envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "placebo capsules were identical to the active drugs". Implies double‐dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "placebo capsules were identical to the active drugs". Implies double‐dummy method |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not reported. No obvious imbalance for discontinuations between groups, but > 25% withdrawals in all groups |
| Size | High risk | Fewer than 50 participants/treatment arm |
AC: active control; ACR: American College of Rheumatology; BOCF: baseline observation carried forward; CNS: central nervous system; DB: double‐blinding; DD: double dummy; ECG: electrocardiogram; HRS‐D: Hamilton Rating Scale ‐ Depression; ITT: intention‐to‐treat; LOCF: last observation carried forward; NSAIDs: non‐steroidal anti‐inflammatory drugs; PC: placebo controlled; PDN: painful diabetic neuropathy; PHN: postherpetic neuralgia; R: randomisation; W: withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fors 2002 | No initial pain requirement for inclusion, no baseline pain reported |
| Hampf 1989 | Fewer than 10 participants in amitriptyline treatment arm |
| Heymann 2001 | No initial pain requirement for inclusion, no baseline pain reported |
| Isomeri 1993 | Study not blinded |
| Jaeschke 1991 | Fewer than 10 participants/treatment group (N of 1 trials) |
| Kempenaers 1994 | Fewer than 10 participants/treatment group |
| McQuay 1992 | Predominantly neuropathic or musculoskeletal pain |
| McQuay 1993 | Neuropathic pain |
| Pilowsky 1982 | Unclear diagnosis of pain condition ("a wide range of intractable pain problems ..... without readily treatable somatic pathology") |
| Pilowsky 1990 | Study not double‐blind |
| Scudds 1989 | No initial pain requirement for inclusion, no baseline pain reported |
| Zitman 1990 | Unclear diagnosis of pain condition ("somatoform pain disorder"). Included some participants with < moderate baseline pain intensity |
| Zitman 1991 | Unclear diagnosis of pain condition ("chronic pain .....no selection on organic or psychogenic aetiology"). Included some participants with < moderate baseline pain intensity |
| Özerbil 2006 | No pain evaluation, duration of each treatment period only 2 weeks |
| Ҫapaci 2002 | Study not convincingly double‐blind, no patient evaluation of pain |
Characteristics of studies awaiting assessment [ordered by study ID]
Ataoğlu 1997.
| Methods | Randomised, 6‐week trial |
| Participants | Fibromyalgia N = 68 |
| Interventions | Amitriptyline Paroxetine |
| Outcomes | |
| Notes | Turkish (with English abstract) ‐ awaiting translation |
Jang 2010.
| Methods | Randomised, controlled trial, 4 weeks |
| Participants | Fibromyalgia syndrome N = 186 |
| Interventions | Oral amitriptyline (Western medicine), once daily Acupuncture combined with cupping and Western medicine Acupuncture combined with cupping |
| Outcomes | |
| Notes | Chinese (with English abstract) ‐ awaiting translation |
NCT00381199.
| Methods | Randomised, double‐blind, cross‐over study. Duration 43 days (possibly 2 x 3 weeks) Assessment at 1, 15, 29, 43 days |
| Participants | Fibromyalgia (ACR 1990) with self‐reported sleep disturbance. Age 18 years or over N = 32 |
| Interventions | Amitriptyline 10 to 25 mg daily Nabilone 0.5 to 1 mg daily |
| Outcomes | Pain intensity (VAS) Pain quality (McGill Pain Questionnaire) Quality of Life (Fibromyalgia Impact Questionnaire) Mood (Profile of Mood States) |
| Notes | Reported complete in May 2007 |
ACR: American College of Rheumatology; N: number of participants; VAS: visual analogue scale
Differences between protocol and review
This update considers fibromyalgia only. Neuropathic pain conditions are the subject of a separate review. It is based on a template for reviews of drugs used to relieve fibromyalgia. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Appendix 1).
We have used three‐tiers of evidence, not two, to better distinguish the strength of evidence and in line with other reviews of interventions for neuropathic pain. We assessed the data according to different neuropathic pain conditions, and planned no further subgroup analysis because the amount of data was expected to be small.
Contributions of authors
PW, RAM, and SD wrote the protocol.
For the original review, and again for this update, RAM and SD carried out searches, assessed studies for inclusion, and extracted data. RAM acted as arbitrator. All authors were involved in writing the review.
RAM will be responsible for updating the review.
Sources of support
Internal sources
Oxford Pain Relief Trust, UK.
External sources
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
SD has no conflicts relating to this review or any similar product.
PW has no conflicts relating to this review or any similar product.
RAM has no conflicts relating to this review or any similar product.
DA has no conflicts relating to this review or any similar product.
PC has received research support from industry sources at various times but none related to this review.
For transparency SD, PW and RAM have received research support from charities, government, and industry sources at various times, but none relate to this review. We are funded by the NIHR for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Braz 2013 {published data only}
- Braz AS, Morais LC, Paula AP, Diniz MF, Almeida RN. Effects of Panax ginseng extract in patients with fibromyalgia: a 12‐week, randomized, double‐blind, placebo‐controlled trial. Revista Brasileira de Psiquiatria 2013;35(1):21‐8. [DOI: 10.1016/j.rbp.2013.01.004] [DOI] [PubMed] [Google Scholar]
Carette 1986 {published data only}
- Carette S, McCain GA, Bell DA, Fam AG. Evaluation of amitriptyline in primary fibrositis. A double‐blind, placebo‐controlled study. Arthritis and Rheumatism 1986;29(5):655‐9. [DOI] [PubMed] [Google Scholar]
Carette 1994 {published data only}
- Carette S, Bell MJ, Reynolds WJ, Haraoui B, McCain GA, Bykerk VP, et al. Comparison of amitriptyline, cyclobenzaprine, and placebo in the treatment of fibromyalgia. A randomized, double‐blind clinical trial. Arthritis and Rheumatism 1994;37(1):32‐40. [DOI] [PubMed] [Google Scholar]
Carette 1995 {published data only}
- Carette S, Oakson G, Guimont C, Steriade M. Sleep electroencephalography and the clinical response to amitriptyline in patients with fibromyalgia. Arthritis and Rheumatism 1995;38(9):1211‐7. [DOI] [PubMed] [Google Scholar]
de Zanette 2014 {published data only}
- Cauma W (Principle investigator). Immune‐Pineal Axis Function in Fibromyalgia. www.clinicaltrials.gov/ct2/show/NCT02041455?term=NCT02041455&rank=1 (accessed 30 Mar 15) 2014. [CTG: NCT02041455]
- Zanette SA, Vercelino R, Laste G, Rozisky JR, Schwertner A, Machado CB, et al. Melatonin analgesia is associated with improvement of the descending endogenous pain‐modulating system in fibromyalgia: a phase II, randomized, double‐dummy, controlled trial. BMC Pharmacology and Toxicology 2014;15:40. [DOI: 10.1186/2050-6511-15-40] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginsberg 1996 {published data only}
- Ginsberg F, Mancaux A, Joos E, Vanhove P, Famey J‐P. A randomized placebo‐controlled trial of sustained‐release amitriptyline in fibromyalgia. Journal of Musculoskeletal Pain 1996;4(3):37‐47. [Google Scholar]
Goldenberg 1986 {published data only}
- Goldenberg DL, Felson DT, Dinerman H. A randomized, controlled trial of amitriptyline and naproxen in the treatment of patients with fibromyalgia. Arthritis and Rheumatism 1986;29(11):1371‐7. [DOI] [PubMed] [Google Scholar]
Goldenberg 1996 {published data only}
- Goldenberg D, Mayskiy M, Mossey C, Ruthazer R, Schmid C. A randomized, double‐blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis and Rheumatism 1996;39(11):1852‐9. [DOI] [PubMed] [Google Scholar]
Hannonen 1998 {published data only}
- Hannonen P, Malminiemi K, Yli‐Kerttula U, Isomeri R, Roponen P. A randomized, double‐blind, placebo‐controlled study of moclobemide and amitriptyline in the treatment of fibromyalgia in females without psychiatric disorder. British Journal of Rheumatology 1998;37(12):1279‐86. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fors 2002 {published data only}
- Fors EA, Sexton H, Götestam KG. The effect of guided imagery and amitriptyline on daily fibromyalgia pain: a prospective, randomized, controlled trial. Journal of Psychiatric Research 2002;36(3):179‐87. [DOI] [PubMed] [Google Scholar]
Hampf 1989 {published data only}
- Hampf G, Bowsher D, Nurmikko T. Distigmine and amitriptyline in the treatment of chronic pain. Anesthesia Progress 1989;36(2):58‐62. [PMC free article] [PubMed] [Google Scholar]
Heymann 2001 {published data only}
- Heymann RE, Helfenstein M, Feldman D. A double‐blind, randomized, controlled study of amitriptyline, nortriptyline and placebo in patients with fibromyalgia. An analysis of outcome measures. Clinical and Experimental Rheumatology 2001;19(6):697‐702. [PubMed] [Google Scholar]
Isomeri 1993 {published data only}
- Isomeri R, Mikkelsson M, Latikka P, Kammonen K. Effects of amitriptyline and cardiovascular fitness training on pain in patients with primary fibromyalgia. Journal of Musculoskeletal Pain 1993;1(3/4):253‐60. [Google Scholar]
Jaeschke 1991 {published data only}
- Jaeschke R, Adachi J, Guyatt G, Keller J, Wong B. Clinical usefulness of amitriptyline in fibromyalgia: the results of 23 N‐of‐1 randomized controlled trials. Journal of Rheumatology 1991;18(3):447‐51. [PubMed] [Google Scholar]
Kempenaers 1994 {published data only}
- Kempenaers C, Simenon G, Vander Elst M, Fransolet L, Mingard P, Maertelaer V, et al. Effect of an antidiencephalon immune serum on pain and sleep in primary fibromyalgia. Neuropsychobiology 1994;30(2‐3):66‐72. [DOI] [PubMed] [Google Scholar]
McQuay 1992 {published data only}
- McQuay HJ, Carroll D, Glynn CJ. Low dose amitriptyline in the treatment of chronic pain. Anaesthesia 1992;47(8):646‐52. [DOI] [PubMed] [Google Scholar]
McQuay 1993 {published data only}
- McQuay HJ, Carroll D, Glynn CJ. Dose‐response for analgesic effect of amitriptyline in chronic pain. Anaesthesia 1993;48(3):281‐5. [DOI] [PubMed] [Google Scholar]
Özerbil 2006 {published data only}
- Özerbil O, Okudan N, Gökbel H, Levendoğlu F. Comparison of the effects of two antidepressants on exercise performance of the female patients with fibromyalgia. Clinical Rheumatology 2006;25(4):495‐7. [DOI] [PubMed] [Google Scholar]
Pilowsky 1982 {published data only}
- Pilowsky I, Hallett EC, Bassett DL, Thomas PG, Penhall RK. A controlled study of amitriptyline in the treatment of chronic pain. Pain 1982;14(2):169‐79. [DOI] [PubMed] [Google Scholar]
Pilowsky 1990 {published data only}
- Pilowsky I, Barrow CG. A controlled study of psychotherapy and amitriptyline used individually and in combination in the treatment of chronic intractable, 'psychogenic' pain. Pain 1990;40(1):3‐19. [DOI] [PubMed] [Google Scholar]
Scudds 1989 {published data only}
- Scudds RA, McCain GA, Rollman GB, Harth M. Improvements in pain responsiveness in patients with fibrositis after successful treatment with amitriptyline. Journal of Rheumatology Supplement 1989;19:98‐103. [PubMed] [Google Scholar]
Zitman 1990 {published data only}
- Zitman FG, Linssen AC, Edelbroek PM, Stijnen T. Low dose amitriptyline in chronic pain: the gain is modest. Pain 1990;42(1):35‐42. [DOI] [PubMed] [Google Scholar]
Zitman 1991 {published data only}
- Zitman FG, Linssen AC, Edelbroek PM, Kempen GM. Does addition of low‐dose flupentixol enhance the analgetic effects of low‐dose amitriptyline in somatoform pain disorder?. Pain 1991;47(1):25‐30. [DOI] [PubMed] [Google Scholar]
Ҫapaci 2002 {published data only}
- Ҫapaci K, Hepgüler S. Comparison of the effects of amitriptyline and paroxetine in the treatment of fibromyalgia syndrome. The Pain Clinic 2002;14(3):223‐8. [Google Scholar]
References to studies awaiting assessment
Ataoğlu 1997 {published data only}
- Ataoglu S, Ataoglu A, Erdogan F, Sarac J. Comparison of paroxetine, amitriptyline in the treatment of fibromyalgia. Turkish Journal of Medical Science 1997;27(6):535‐9. [Google Scholar]
Jang 2010 {published data only}
- Jang ZY, Li CD, Qiu L, Guo JH, He LN, Yue Y, et al. Combination of acupuncture, cupping and medicine for treatment of fibromyalgia syndrome: a multi‐central randomized controlled trial. Zhongguo Zhen Jiu [Chinese Acupuncture and Moxibustion] 2010;30(4):265‐9. [PubMed] [Google Scholar]
NCT00381199 {published data only}
- Ware MA (Principle Investigator). Nabilone versus amitriptyline in improving quality of sleep in patients with fibromyalgia. www.clinicaltrials.gov/ct2/show/NCT00381199?term=NCT00381199&rank=1 (accessed 30 March 2015) 2007. [CTG: NCT00381199]
Additional references
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Hausser 2011
- Häuser W, Petzke F, Üçeyler N, Sommer C. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta‐analysis. Rheumatology (Oxford) 2011;50(3):532‐43. [DOI: 10.1093/rheumatology/keq354] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore RA, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kalso 2013
- Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ 2013;347:f7339. [DOI: 10.1136/bmj.f7339] [DOI] [PubMed] [Google Scholar]
Kim 2015
- Kim SC, Landon JE, Lee YC. Patterns of health care utilization related to initiation of amitriptyline, duloxetine, gabapentin or pregabalin in fibromyalgia. Arthritis Research and Therapy 2015;17(1):18. [DOI: 10.1186/s13075-015-0530-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Koroschetz 2011
- Koroschetz J, Rehm SE, Gockel U, Brosz M, Freynhagen R, Tölle TR, et al. Fibromyalgia and neuropathic pain ‐ differences and similarities. A comparison of 3057 patients with diabetic painful neuropathy and fibromyalgia. BMC Neurology 2011;11:55. [DOI: 10.1186/1471-2377-11-55] [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
Lunn 2014
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007115.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 1998
- McQuay H, Moore R. An evidence‐based resource for pain relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐263048‐2] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978–0–931092–69–5] [Google Scholar]
Moore 2009
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007076] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010a
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010c
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. [DOI: 10.1016/j.pain.2010.02.039] [DOI] [PubMed] [Google Scholar]
Moore 2010d
- Moore RA, Derry S, McQuay HJ, Straube S, Aldington D, Wiffen P, et al. Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain 2010;149(2):173‐6. [DOI: 10.1016/j.pain.2009.08.007] [DOI] [PubMed] [Google Scholar]
Moore 2011a
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Moore 2011b
- Moore RA, Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post‐operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28(6):427‐32. [DOI: 10.1097/EJA.0b013e328343c569] [DOI] [PubMed] [Google Scholar]
Moore 2012b
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265‐8. [DOI: 10.1016/j.pain.2011.10.00] [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI: 10.1136/bmj.f2690] [DOI] [PubMed] [Google Scholar]
Moore 2014a
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. [DOI] [PubMed] [Google Scholar]
Moore 2014b
- Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD007938.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2014c
- Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions ‐ individual patient data responder analysis. European Journal of Pain 2014;18(1):67‐75. [DOI: 10.1002/j.1532-2149.2013.00341.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD008242.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishishinya 2008
- Nishishinya B, Urrútia G, Walitt B, Rodriguez A, Bonfill X, Alegre C, et al. Amitriptyline in the treatment of fibromyalgia: a systematic review of its efficacy. Rheumatology (Oxford) 2008;47(12):1741‐6. [DOI: 10.1093/rheumatology/ken317] [DOI] [PubMed] [Google Scholar]
Nuesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Onghena 1992
- Onghena P, Houdenhove B. Antidepressant‐induced analgesia in chronic non‐malignant pain: a meta‐analysis of 39 placebo‐controlled studies. Pain 1992;49:205‐20. [DOI] [PubMed] [Google Scholar]
PaPaS 2012
- PaPaS author and referee guidance. http://papas.cochrane.org/papas‐documents (accessed 9 March 2015).
PCA 2014
- Anonymous. Prescription Cost Analysis, England 2013. Available at: hscic.gov.uk/catalogue/PUB13887/pres‐cost‐anal‐eng‐2013‐rep.pdf (Accessed 9 March 2015). Health and Social Care Information Centre, 2014:100‐1. [ISBN: 978‐1‐78386‐089‐0]
Queiroz 2013
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Current Pain and Headache Reports 2013;17(8):356. [DOI: 10.1007/s11916-013-0356-5] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Straube 2010
- Straube S, Derry S, Moore RA, Paine J, McQuay HJ. Pregabalin in fibromyalgia‐‐responder analysis from individual patient data. BMC Musculoskeletal Disorders 2010;11:150. [DOI: 10.1186/1471-2474-11-150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2011
- Straube S, Moore RA, Paine J, Derry S, Phillips CJ, Hallier E, et al. Interference with work in fibromyalgia: effect of treatment with pregabalin and relation to pain response. BMC Musculoskeletal Disorders 2011;12:125. [DOI: 10.1186/1471-2474-12-125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sultan 2008
- Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology 2008;8:29. [DOI: 10.1186/1471-2377-8-29] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2013
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice ASC, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010567.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 1990
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33:160‐72. [DOI] [PubMed] [Google Scholar]
Wolfe 2010
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care and Research 2010;62(5):600‐10. [DOI: 10.1002/acr.20140] [DOI] [PubMed] [Google Scholar]
Wolfe 2013
- Wolfe F, Brähler E, Hinz A, Häuser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care and Research 2013;645(5):777‐85. [DOI: 10.1002/acr.21931] [DOI] [PubMed] [Google Scholar]
Wolfe 2014
- Wolfe F, Walitt BT, Häuser W. What is fibromyalgia, how is it diagnosed and what does it really mean?. Arthritis Care and Research 2014;66(7):969‐71. [DOI: 10.1002/acr.22207] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
McQuay 1996
- McQuay HJ, Tramer M, Nye BA, Carroll D, Wiffen PJ, Moore RA. A systematic review of antidepressants in neuropathic pain. Pain 1996;68:217‐27. [DOI] [PubMed] [Google Scholar]
Moore 2012a
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008242.pub2] [DOI] [PubMed] [Google Scholar]
Saarto 2007
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005454.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


