Abstract
Replicable risk factors for ALS include increasing age, family history and being male. The male: female ratio has been reported as being between 1 and 3. We tested the hypothesis that the sex ratio changes with age in a population register covering the south-east of England. The sex ratio before and after the age of 51 years was compared using a Z-test for proportions. Kendall’s tau was used to assess the relationship between age group and sex ratio using incidence and prevalence data. Publicly available data from Italian and Irish population registers were compared with results. There was a significant difference in the proportion of females with ALS between those in the younger group (30.11%) and those in the older group (43.66%) (p = 0.013). The adjusted male: female ratio dropped from 2.5 in the younger group to 1.4 in the older group using prevalence data (Kendall’s tau = –0.73, p = 0.039). Similar ratios were found in the Italian but not the Irish registry. We concluded that sex ratios in ALS may change with age. Over-representation of younger patients in clinic registers may explain the variation in sex ratios between studies. Menopause may also play a role.
Keywords: Amyotrophic lateral sclerosis, epidemiology, testosterone, oestrogen, menopause
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease primarily affecting motor neurons, which leads to progressive weakness, usually culminating in respiratory failure and death within three to five years (1). The most widely accepted risk factors are increasing age, family history of ALS, and being male (2,3). The reported male to female ratio varies widely, but in some more recent population studies approaches 1.1 (4–7).
Conversely, in many clinic based series, a ratio of 2 or higher is observed (4,8). Clinic based registers have patients with a young mean age at onset, typically around 56 years, because younger individuals are more motivated to seek the specialist help they provide (9,10). Population registers are representative of the true disease population structure and have patients with a higher mean age at onset, more typically 70 + years (8–10). These findings are consistent with the observation that older patients with ALS are more likely to be female and therefore that the ratio changes as the age structure of the study population changes. We therefore tested the hypothesis that there is a change in sex ratio with age in population registries of ALS. We used a population register, the South East England ALS (SEALS) register, and examined the sex ratio across different age groups.
Methods
SEALS register
The catchment area of the SEALS register includes south-east London boroughs Lambeth, Southwark, Lewisham, Bexley, Greenwich and Bromley, and Brighton and Hove, East Sussex and Kent, covering a total population of approximately 3,219,366. Further details including case ascertainment are available elsewhere (10). Only patients with a confirmed diagnosis of ALS made by two neurologists after exclusion of other conditions were included in the final register. The incidence in this area has previously been established as 1.06 per 100,000 population (10). Data for the present study consisted of patients diagnosed between 1 January 1990 and 1 February 2006, which includes patients added to the register retrospectively (from 1990 to 1997) and prospectively (1997 to 2006). The demographics of the two parts of the register are not significantly different. A total of 472 patients were included in the study. This research was approved by the ethics committees of the relevant institutions.
Sex and age
We used the menopause as an arbitrary cut-off for defining young and old age groups because before this, hormonal differences between the sexes are more pronounced. The numbers of cases of ALS observed in males and females were calculated pre-menopause (15–49 years of age) and post-menopause (>55 years of age) with menopause operationally defined as occurring at 51 years of age, the mean age of menopause in the United Kingdom (13). This excluded 31 patients (11 female) who were peri-menopausal (50–54 years of age). The prevalence rates in females were compared using the Z-test for proportions. Age was further categorized by decade as shown in Table II. The incidence was calculated for each age group within each sex to allow comparisons of male: female ratios at different ages, thereby controlling for the underlying population structure in making the comparisons. The population figures for the catchment area were commissioned from the UK Office of National Statistics using data from the 2001 census (14). Incidence data from a 10-year period (1995–2005) were used for this analysis as data capture was considered complete from 1995 onwards. For comparison, publicly available data from an Italian registry (15) of the Lombardy region and from an Irish registry (7) were also analysed. A comparison of rates pre- and post-menopause, using prevalence rather than incidence, was also performed for completeness. Confidence intervals were calculated for the male: female ratios using Graph pad, an online calculator (http://graphpad.com/quickcalcs/ErrorProp1.cfm). Intervals could not be calculated if the CI of the denominator included 0 so these are missing if this was the case. A Z-test was used to compare proportions between two groups. The Kendall’s tau-b correlation coefficient, which is a non-parametric test based on ranks, was used to test for a relationship between sex ratio and age categorized by decade, since a plot (Figure 1) clearly shows they are non-linearly related. Data analysis was carried out using SPSS version 15 (SPSS), Microsoft Excel and Programs for Epidemiologists (PEPI, freely available from http://www.brixtonhealth.com).
Table II.
Age- and sex-adjusted incidence according to menopause status.
| Incidence per 100,000 population years (95% CI) |
|||
|---|---|---|---|
| Male | Female | Male:female ratio (95% CI) | |
| Pre-menopause (15–49) | 0.68 (0.53–0.86) | 0.17 (0.10–0.27) | 3.98 (3.73–4.30) |
| Post-menopause (>55) | 3.64 (3.28–4.03) | 2.23 (1.95–2.54) | 1.63 (1.61–1.65) |
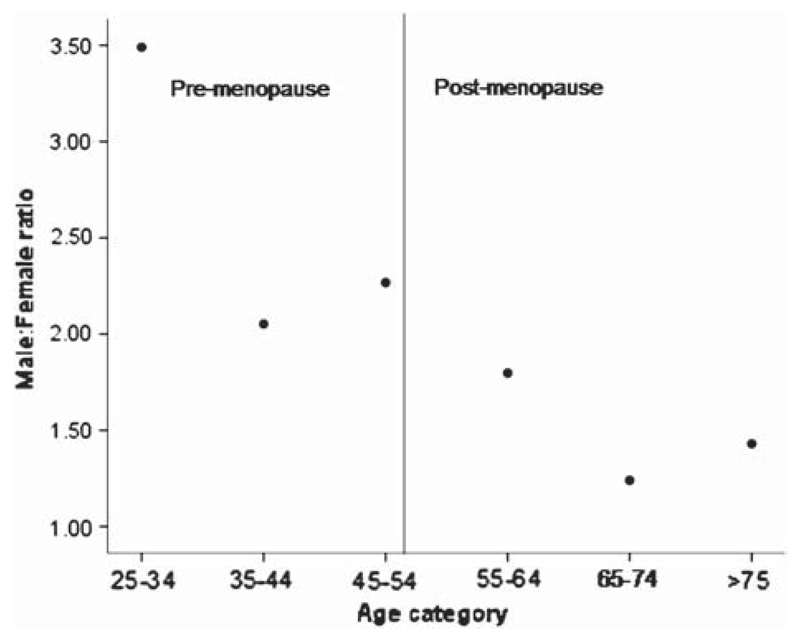
Figure 1.
Relationship between age group and sex ratio (incidence). This figure shows a plot of the age- and sex-adjusted sex ratio by age group. To the right of the vertical line are older age groups (operationally defined in this context as being post menopausal). The male: female ratio is clearly lower in the younger groups.
Results
Of the 472 patients in the register, nine had inaccurate or missing birth dates and were excluded from further study. Two hundred and seventy-six of the remaining 463 patients were male, making a crude male: female ratio of 1.5, not taking into account the underlying population structure. The proportion of women increased from 30.1% pre-menopause to 43.7% post-menopause (Table I). The difference in proportions was significant (Z = 2.24, p = 0.013). Between 1 January 1995 and 31 December 2005, 380 cases with complete date of birth information were registered (226 males). The age- and sex-adjusted male: female ratio based on mean incidence data from this period from the SEALS registry showed a marked drop from > 2.5 to < 1.5 at the menopause (Tables II, III and V). There was a relationship between age group by decage and male: female ratio (Kendall’s tau-b correlation coefficient = −0.73, p = 0.039) (see Figure 1 and 2). Using data from the Lombardy registry (16), a similar pattern was observed, with the ratio dropping from about 2.5 pre-menopause to about 1.7 post-menopause (Kendall’s tau-b correlation coefficient −0.8, p = 0.05) (see Table IV). This pattern was not seen in the Irish data where large male: female ratios were seen in those less than 44 years of age but also in those over 75 years of age (see Table IV). Using prevalence data, the age- and sex-adjusted male: female ratio changed from 2.5 before to about 1.4 after menopause (Table V). There was a relationship between age and the male: female ratio (Kendall’s tau-b correlation coefficient = –0.73, p = 0.03).
Table I.
Observed cases according to menopause status.
| Male cases (%) | Female cases (%) | Total | |
|---|---|---|---|
| Pre-menopause (15–49 years) | 65 (69.99) | 28 (30.11) | 93 |
| Post-menopause (>55 years) | 191 (56.44) | 148 (43.66) | 339 |
Table III.
Age and sex adjusted incidence.
| Incidence (per 100,000 population) (95% CI) |
|||
|---|---|---|---|
| Age group | Male | Female | Male:female ratio (95% CI) |
| 25–34 | 0.37 (0.01–2.25) | 0.10 (0.07–0.15) | 3.7** |
| 35–44 | 0.58 (0.01–2.22) | 0.28 (0.21–0.35) | 2.07** |
| 45–54 | 2.13 (0.53–5.00) | 0.94 (0.11–3.39) | 2.26 (0.48–17.06) |
| 55–64 | 4.65 (1.75–8.99) | 2.58 (0.65–6.07) | 1.80 (0.48–17.86) |
| 65–74 | 3.83 (1.32–9.46) | 3.08 (0.75–7.08) | 1.24 (0.09–10.7) |
| >75 | 1.62 (0.25–7.57) | 1.13 (0.14–4.29) | 1.43 (0–4.06) |
**CI of denominator includes 0.
Table V.
Period prevalence in each age group and sex.
| Age group | Male prevalence (per 100,000 population) (95% CI) | Female prevalence (per 100,000 population) (95% CI) | Male:female ratio (95% CI) |
|---|---|---|---|
| 15–24 | 0 | 1.41 (0.29 to 4.13) | – |
| 25–34 | 4.45 (2.30 to 7.77) | 1.78 (0.58 to 4.15) | 2.5 (0.89–19.66) |
| 35–44 | 7.81 (4.83 to 11.94) | 3.27 (1.50 to 6.21) | 2.4 (1.13–7.08) |
| 45–54 | 29.59 (22.84 to 37.72) | 12.27 (8.16 to 17.74) | 2.4 (1.58–4.00) |
| 55–64 | 56.22 (45.59 to 68.58) | 33.51 (25.64 to 43.05) | 1.67 (1.23–2.34) |
| 65–74 | 47.33 (36.37 to 60.55) | 37.95 (28.89 to 48.95) | 1.24 (0.87–1.80) |
| 75+ | 17.62 (10.44 to 27.85) | 12.26 (7.68 to 18.56) | 1.4 (0.71–2.77) |
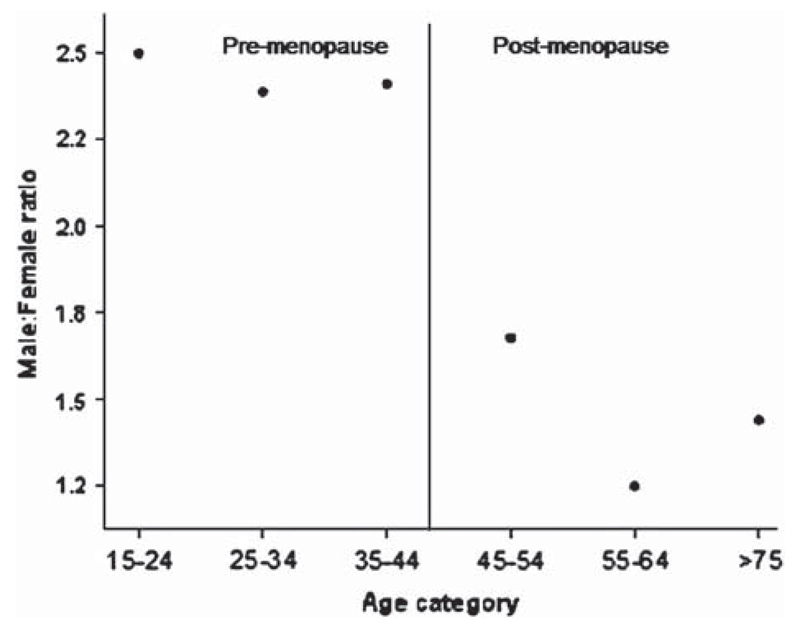
The male:female ratio drops after age 55 years.
Figure 2.
Relationship between age group and sex ratio (prevalence). This figure shows a plot of the age- and sex-adjusted sex ratio by age group. To the right of the vertical line are the older age groups (operationally defined as post menopausal as with figure 1). The male: female ratio is clearly lower in the older groups.
Table IV.
Comparison of sex ratios in different population registries.
| Age group SEALS | M:F ratio | Age group Lombardy | M:F ratio | Age group Ireland | M:F ratio |
|---|---|---|---|---|---|
| 35–44* | 2.5 | <45 | 2.5 | 35–44 | 2.25 |
| 45–54 | 2.27 | 45–54 | 2.45 | 45–54 | 1.17 |
| 55–64 | 1.8 | 55–64 | 1.77 | 55–64 | 1.54 |
| 65–74 | 1.24 | 65–74 | 1.13 | 65–74 | 1.14 |
| >75 | 1.43 | >75 | 1.76 | >75 | 2.62 |
The male:female (M:F) ratio drops in the Italian and English registers (but not the Irish) after age 55 years.
No incident cases were recorded in one or other category below age 35 years, so a meaningful ratio cannot be constructed.
Discussion
We have found that in the SEALS population-based ALS register, there is an increase in the proportion of females with ALS in older age groups. The change is seen both in simple proportions of incident and prevalent cases and in data adjusted for the underlying population structure thereby controlling for the relative increase in the proportion of women alive in the elderly population. We also examined the ratio change by decade and observed a step change between the 45–54 years and 55–64 years age groups, with those younger than 54 years of age at onset having a ratio > 2 and those older having a ratio < 2. Such findings would be consistent with the hypothesis that the menopause marks the point at which the sex ratio changes. If the menopause, and presumably associated hormonal changes, were the explanation for a sex ratio change in ALS, this effect should be seen in other population based registers. We therefore examined the ratio in the Lombardy and Irish registers. We found that the Lombardy register gave a very similar picture to the SEALS register, but the Irish register did not show the same change at menopause. The Irish data suggest that an increased risk for females occurs about a decade before the menopause. There is no evidence that the menopause occurs earlier in Ireland, and the ascertainment rates for all three registers are likely to be similar as they produce comparable incidence and prevalence rates. Thus, it seems probable that a risk factor that occurs in a similar age range to the menopause is mediating the change in risk for females, rather than hormonal changes related to menopause itself. For example, if pregnancy were protective and women in Ireland completed families at an earlier age than in Italy or England, this would result in the observed patterns at least until the over 75 years age group. Although we could find no evidence that Irish mothers completed their families earlier, Irish families tend to be larger than English and Italian families, with a mean number of children per woman of 3.5 for older age groups, dropping to 2.2 for those now aged between 40 and 44 years. The equivalent figures for England and Italy are 2.2 and 2.4, dropping to 1.7 and 1.6, respectively.
The observation that there is a significant correlation between age and male: female ratio with a decline of male predominance, and a ratio nearly equal over 65 years of age has been made by others (11,12). Our study, together with data from Italian and Irish registries, suggest that the biological explanation is likely to be some external factor changing ALS risk in females in the fifth or sixth decades of life. It also suggests that as populations age and population registers develop in their ability to capture incident cases, the sex ratio in ALS will appear to decrease towards 1 because the proportion of older patients will increase. Furthermore, we would expect that clinic based registers will always show a higher male: female ratio than population registers because the study population tends to be younger (1).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Beghi E, Logroscino G, Chiò A, Hardiman O, Mitchell D, Swingler R, et al. The epidemiology of ALS and the role of population based registries. Biochim Biophys Acta. 2006;1762:1150–7. doi: 10.1016/j.bbadis.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Armon C. An evidence based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyotrophic lateral sclerosis. Neuroepidemiology. 2003;22:217–28. doi: 10.1159/000070562. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell JD. Amyotrophic lateral sclerosis: toxins and environment. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000 Sep;1(4):235–50. doi: 10.1080/14660820050515061. [DOI] [PubMed] [Google Scholar]
- 4.Roman GC. Neuroepidemiology of amyotrophic lateral sclerosis: clues to aetiology and pathogenesis. J Neurol Neurosurg Psychiatry. 1996;61:131–7. doi: 10.1136/jnnp.61.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O. Incidence and prevalence of ALS in Ireland, 1995–1997: a population based study. Neurology. 1999;52:504–9. doi: 10.1212/wnl.52.3.504. [DOI] [PubMed] [Google Scholar]
- 6.Piemonte and Valle d’Aosta Register for Amyotrophic Lateral Sclerosis (PARALS) Incidence of ALS in Italy: evidence for a uniform frequency in Western countries. Neurology. 2001;56:239–44. doi: 10.1212/wnl.56.2.239. [DOI] [PubMed] [Google Scholar]
- 7.O’Toole O, Traynor BJ, Brennan P, Sheehan C, Frost E, Corr B, et al. Epidemiology and clinical features of amyotrophic lateral sclerosis in Ireland between 1995 and 2004. J Neurol Neurosurg Psychiatry. 2008;79:30–2. doi: 10.1136/jnnp.2007.117788. [DOI] [PubMed] [Google Scholar]
- 8.Logroscino G, Traynor BJ, Hardiman O, Chio’ A, Couratier P, Mitchell JD, et al. Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry. 2008;79:6–11. doi: 10.1136/jnnp.2006.104828. [DOI] [PubMed] [Google Scholar]
- 9.Forbes RB, Colville S, Swingler RJ, Scottish ALS/MND Register The epidemiology of amyotrophic lateral sclerosis (ALS/MND) in people aged 80 years or over. Age Ageing. 2004;33:131–4. doi: 10.1093/ageing/afh013. [DOI] [PubMed] [Google Scholar]
- 10.Abhinav K, Stanton B, Johnston C, Hardstaff J, Orrell RW, Howard R, et al. Amyotrophic lateral sclerosis in south-east England: a population based study (South-East England register for Amyotrophic Lateral Sclerosis (SEALS) registry) Neuroepidemiol. 2007;29:44–8. doi: 10.1159/000108917. [DOI] [PubMed] [Google Scholar]
- 11.Eisen A. Clinical Neurophysiology of the Motor Neurone Diseases. USA: Elsevier; 2004. pp. 1–3. [Google Scholar]
- 12.Chancellor AM, Hendry A, Caird FI, Warlow CP, Weir AI. Motor neuron disease: a disease of old age. Scott Med J. 1993;38:178–82. doi: 10.1177/003693309303800606. [DOI] [PubMed] [Google Scholar]
- 13.Drife J, Magowan B. Clinical Obstetrics and Gynaecology. Edinburgh: Saunders; 2004. p. 233. [Google Scholar]
- 14.Office of National Statistics. Commissioned table C0381, received 18/07/07. London: ONS; 2007. [accessed 24/04/2008]. http://www.statistics.gov.uk/census2001/downloads/com_tab_finder.xls [Google Scholar]
- 15.Beghi E, Millul A, Micheli A, Vitelli E, Logroscino G, SLALOM Group Incidence of ALS in Lombardy, Italy. Neurology. 2007;68:141–5. doi: 10.1212/01.wnl.0000250339.14392.bb. [DOI] [PubMed] [Google Scholar]