Abstract
The object of this study was to evaluate the potential of a recently developed preactivated thiolated pectin derivative as mucoadhesive excipient in drug delivery to the gastric cavity. Pectin (Pec) was chemically modified with L-cysteine (Cys). The free thiol groups of resulting thiomer were activated with 2-mercaptonicotinic acid (MNA) in order to improve stability and reactivity of attached thiol groups over a broad pH range. Multiunit dosage form properties of the resulting conjugate (Pec-Cys-MNA) were compared to unmodified pectin and the intermediate thiolated using rosuvastatin calcium as a model drug in loaded minitablets. Obtained results were compared with unmodified pectin and the intermediate thiolated pectin. Approximately half of attached thiol groups (507 μmol/g polymer) have been preactivated. Minitablets were evaluated regarding mucoadhesive properties, hardness, disintegration behavior, swelling characteristics and release of rosuvastatin calcium. Mediated by covalent bonds between the polymer and cysteine-rich subdomains in mucus, total work of adhesion increased more than 5-fold. The modification had no impact on hardness of compressed tablets but implementation of the aromatic ligand went along with reduction in hydrophilic properties. Disintegration time was prolonged more than 2-fold while water uptake capacity increased. Weight gain for Pec-Cys-MNA was at least 16-fold. Further, a sustained release of rosuvastatin calcium over 36 hours was determined. Neither biodegradability nor CaCo-2 cell viability was affected. The study shows that Pec-Cys-MNA is a promising excipient for the development of mucoadhesive gastric dosage form.
Keywords: mucoadhesive drug delivery system, preactivated thiomers, swellable polymer, rosuvastatin, gastric
Introduction
In the 1980s mucoadhesive polymers were pioneered as potential excipients in order to prolong the residence time of drug delivery system on all kind of mucosal membranes like gastrointestinal, nasal, pulmonary, ocular, buccal, rectal and vaginal mucosa. The increased contact time of delivery system with mucus results in an increased drug concentration gradient on the absorption membrane and therefore in improved drug uptake (Bernkop-Schnurch, 2005). However, in particularly in case of oral delivery mucoadhesive polymers could so far not convince as “super glue”. This is likely caused by rapid mucus turn over and strong peristaltic moves pushing even adhering delivery systems forward. Since application in dry form is not possible the effect of adhesion by hydration cannot be utilized. Despite all these challenges there is encouraging data for gastric and intestinal mucoadhesive delivery systems available (Prinderre et al., 2011). For instance, Akiyama et al. showed higher plasma levels of riboflavin and furosemide having their absorption window in the proximal segments of the GI-tract, in rats as well as in volunteers after administration in an adhesive microsphere formulation compared to application of same amount of drug in non-adhesive microspheres. The area under the plasma-concentration curve of furosemide was found to be 1.8 fold higher when administered in mucoadhesive dosage form (Akiyama et al., 1998). These results are of even higher value since they were achieved with non-covalent binding mucoadhesive polymers exhibiting much lower mucoadhesive properties than covalently binding polymers such as thiolated polymers (thiomers) forming disulfide bonds with cysteine-rich subdomains of the mucus gel layer (Bernkop-Schnurch et al., 1999a; Leitner et al., 2003c; Perez-Vilar and Mabolo, 2007).
So far, thiomers could not be applied for gastric mucoadhesive delivery systems as their thiol groups are not sufficiently reactive at low pH. Recently, a novel generation of thiomers – namely preactivated thiomers – was established(Iqbal et al., 2012). These polymers are characterized by bearing a thiol group, which is activated by the attachment of pyridyl substructures via disulfide bond formation, resulting in higher activities of polymeric thiol groups over a broader pH range (Iqbal et al., 2012). According to these developments it can be regarded as logical next step to utilize preactivated thiomers for mucoadhesive gastric drug delivery systems. The aim of this study was to evaluate the potential of a promising candidate out of this new class of modified polymers in regards for usage in mucoadhesive systems for drug delivery in the gastric cavity. As a hydrophilic polymeric backbone the biodegradable fiber pectin was chosen and to activate attached thiol groups 2-mercaptonicotinic acid was utilized. The synthesized excipient was examined in terms of mucoadhesive properties, hardness of compressed minitablets, disintegration time, swelling capacity and release of incorporated rosuvastatin calcium. Further, biodegradability and cytotoxicity of the excipient was examined. Since pH in the stomach fluctuates over a wide range investigations were carried out in acidic and neutral environments. As model drug rosuvastatin calcium, a HMG-CoA reductase inhibitor which is used in treatment of dyslipidemia, was employed and incorporated into mini tablets, to generate a multi-unit dosage form. Like other statins, the bioavailability of rosuvastatin is low. The absolute oral bioavailability of the sparingly soluble drug is only about only 20 % (Martin et al., 2003a; Martin et al., 2003b). By now, several attempts have been made to improve bioavailability of different statins like fluvastatin, simvastatin and atorvastatin calcium in gastro retentive systems (Hussain et al., 2012). Besides enhanced uptake, modified release formulations of HMG-CoA reductase inhibitors may lead to reduced incidence of serious side effects like rhabdomyolysis. Therefore, rosuvastatin is a promising candidate for improved bioavailability by gastroretentive delivery systems (Garg and Gupta, 2008).
2. Materials and Methods
2.1. Materials
Lemon pectin (degree of esterification approximately 75%) was obtained from Herbafood Ingredients GmbH, Weder, Germany and 2-mercaptonicotinic acid 98% (MNA) from ABCR GmbH & Co KG, Karlsruhe, Germany. L-Cysteine hydrochloride, 5,5′-dithiobis(2-nitrobenzoic acid) (Ellman’s reagent), hydrogen peroxide, dialysis tubes (MWCO 12 kDa), glutathione (GSH), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC), porcine gastric mucin, pectinase from Aspergillus aculeatus, resazurin, phosphoric acid 85% and acetonitrile HPLC grade were purchased from Sigma Aldrich, Austria. Rosuvastatin calcium was obtained from CHEMOS GmbH, Germany. Water HPLC gradient grade was purchased from Fisher Chemical, United Kingdom. All other reagents used were of analytical grade.
2.2. Synthesis of pectin-cysteine-mercaptonicotinic acid
2.2.1. Synthesis of pectin-cysteine conjugate
Thiolated pectin (Pec) was synthesized by covalent attachment of L-cysteine hydrochloride via amid bond formation according to a method published previously (Majzoob et al., 2006)). Amide bond formation was mediated by EDAC (Kunkel et al., 1981). Briefly, 1.5 g of pectin were dissolved in demineralized water and carboxylic acid moieties were activated by addition of 1.5 g of EDAC. After 1 h of incubation 3 g of L-cysteine hydrochloride (Cys) were added. The pH of all components was adjusted to 4.5 using 5 M NaOH. The mixture was stirred for 5 hours. Product was purified via dialysis and lyophilized for 2 days under reduced pressure.
2.2.2. Synthesis of pectin-cysteine-2-mercaptonicotinic acid
The thiomer was preactivated according to a method described previously(Iqbal et al., 2012). First, the dimer of 2-mercaptonicotinic acid was gained via oxidative coupling of the monomer using H2O2. The product (2,2′-dithiodinicotinic acid) was freeze-dried for 2 days under reduced pressure. The aromatic ligand was attached covalently by disulfide bond formation. 200 mg of Pec-Cys were dissolved in 50 mL of demineralized water under stirring. Next, 50 mg of MNA dimer were added and pH adjusted to 8 with 1 M NaOH. The mixture was stirred for 6 h at room temperature (Dünnhaupt et al., 2012; Iqbal et al., 2012);(Whitesides et al., 1977)). To separate the conjugate from unbound MNA dimer, the reaction solution was dialyzed for 7 days using Spectra/Por® 3 membrane (MWCO: 12 kDa) in 5 L of demineralized water under stirring in the dark at 10 °C. The resulting product was freeze-dried for 2 days under reduced pressure.
2.3. Quantification of conjugated L-cysteine hydrochloride
The amount of bound L-cysteine hydrochloride was determined spectrophotometrically using Ellman’s reagent (5,5′-dithio-bis(2-nitrobenzoic acid)) according to a method described previously (Bernkop-Schnurch et al., 1999c). To ensure that there is no unbound cysteine in the sample, a TNBS test has been carried out to quantify unbound amino groups allowing direct correlation to unbound cysteine (Goodwin and Choi, 1970)).
2.4. Determination of conjugated 2-mercaptonicotinic acid
The amount of immobilized 2-mercaptonicotinic acid was determined spectrophotometrically according a method described previously by our group (Iqbal et al., 2012)). To determine the amount of bound MNA, the ligand was released by adding GSH. The absorbance of liberated MNA was measured at 354 nm. To ensure that there is no unbound MNA in the sample, absorption measurements at 354 nm have been carried out prior to reduction with GSH.
2.5. Manufacturing of minitablets
For the manufacturing of the minitablets 30 mg of lyophilisates were compressed at 10 kN for 25 s yielding 2 mm thick flat-faced discs of 5 mm diameter (Paul Weber, Remshalden-Grünbach, Germany). The hardness of resulting test discs was determined using a Schleuniger 2-E/205 tablet-hardness tester (Dr. K. Schleuniger and Co., Switzerland).
2.6. In vitro mucoadhesion studies
To evaluate the mucoadhesive capacity, tensile studies were performed as previously described by our research group (Kast and Bernkop-Schnurch, 2001). Briefly, freshly excised porcine stomach was cleaned and cut into pieces. The tissue was fixed on a glass base with cyanoacrylate adhesive and placed in beakers with 0.1 M phosphate buffer pH 6.8 and 0.1 M HCl, respectively. The beaker was placed on a balance. The polymer discs were glued to stainless steel basis using the same adhesive. The basis with the test disc were arranged above the mucosa. The disc was attached to the tissue by applying mild force. After an incubation time of 20 min at room temperature the mucosa was pulled down at a rate of 0.1 mm/s by lowering the balance. Data points were collected every second by a computer software (Sarta Collect software; Satorius AG). The total work of adhesion (TWA) is represented by the area under the force/distance curve and the maximum detachment force (MDF) were determined (Leitner et al., 2003a).
2.7. Disintegration behavior
Disintegration time of test discs was evaluated with disintegration apparatus in accordance with the European Pharmacopoeia. The oscillating frequency was adjusted to 0.5 s−1 (Leitner et al., 2003b). The test was carried out at two different pH values: 0.1 M HCl and 0.1 M phosphate buffer pH 6.8. The temperature was set to 37 ± 1 °C.
2.8. Water uptake capacity
Swelling/erosion characteristics of the different pectin conjugates and control Pec were evaluated by determination of weight change. Test discs were fixed on pin needles and incubated in 0.1 M HCl and phosphate buffer 0.1 M pH 6.8. At predetermined time points the surface water was daped away with paper tissue and the amount of water uptake was determined gravimetrically. Water uptake was calculated according to the following equation with W0 = initial weight and Wt = weight of the test disk at the time t (Perera et al., 2010b).
2.9. Release studies
In vitro release of rosuvastatin calcium was investigated. Mixing with the different excipients was achieved by dissolving 40 mg of rosuvastatin calcium in aqueous solutions containing 120 mg of polymer. After freeze-drying, tablets of 40 mg were compressed as described above. Studies were performed with an ERWEKA DT 700 dissolution apparatus with paddles according to the European Pharmacopoeia. Temperature was set to 37 ± 0.5° C and speed to 100 rpm. Investigations were made with and without pH change of release medium. Studies without pH change were carried out using 0.1 M HCl (900 mL/vessel). For studies with change in pH the 0.1 M HCl was replaced by phosphate buffer pH 6.8 0.1 M after 2 hours simulating the transition from stomach to intestine corresponding to the European Pharmacopoeia. Samples of 1 mL were withdrawn at predetermined time points and volume was replaced with fresh release medium. The concentration of rosuvastatin calcium was determined via HPLC (Hitachi EliteLaChrom HPLC-System). A CN-RP column (250 ×4.6 mm, 5 μm) (Machery-Nagel, Germany) was used as stationary phase. The mobile phase consisted of acetonitrile:water pH 3.5 (adjusted with phosphoric acid) 40:60 v/v. Flow rate was set to 1.0 mL/min. A diode array detector was used to measure absorption of the eluate at λ = 242 nm (Kaila et al., 2010). Retention time for rosuvastatin calcium was 7.2 min. Rosuvastatin calcium concentration was calculated from a linear calibration from 3-50 μg/mL. The experiment was carried out in six replicates.
2.10. Viscosity and degradability
Solutions (10 mg/mL) of Pec, Pec-Cys and Pec-Cys-MNA in 0.1 M acetate buffer pH 4.5 were incubated at 37 °C overnight in a thermal mixer. Thereafter, dynamic viscosity was measured at a shear rate of 50 s−1 at 37 °C using a plate-plate viscometer (RotoVisco RT20, Haake GmbH, Karlsruhe, Germany). The enzymatic degradability was determined by the addition of pectinase to the polymer solutions (final concentration of ≥ 3.8 units/mL of pectinase). After an incubation time of 90 min, the dynamic viscosity was measured.
2.11. Cytotoxicity studies – resazurin assay
The resazurin assay is based on the reducing environment of viable cells. The blue, non-fluorescent resazurin is reduced to red and fluorescent resorufin in the environment of metabolic active cells (O’Brien et al., 2000). The colorimetric and fluorescence changes were measured at 540 nm with background subtraction at 590 nm with a Tecan infinite, M200 spectrophotometer, Grödig, Austria (Jennings et al., 2007). In detail, the assay was performed on Caco-2 cells which were cultured in 24-well plates for 14 days at 37 °C in a 5% CO2 environment. The minimum essential medium (MEM) with FCS was replaced every second day. 500 μL of the prepared test solutions (5 mg/mL, Pec, Pec-Cys, Pec-Cys-MNA in MEM without FCS and phenol red) were added. As positive control MEM without FCS and phenol red was added whereas Triton X® 100 in a 4% (w/w) solution in MEM was used as negative control. Each sample was prepared 4-times, incubation time was 3 h. Subsequently, cell were washed twice with phosphate buffered saline. To each well 250 μL of a 2.2 μM resazurin solution were added. After 3 h the fluorescence was measured as described above.
2.12. Statistical data analysis
Statistical data analysis was performed (GraphPad Prism 5) using one way ANOVA with P<0.05 as the minimal level of significance followed by Bonferroni’s Multiple Comparison Test with p<0.05 as minimal level of significance.
3. Results
3.1. Characterization of pectin-cysteine-mercaptonicotinic acid
3.1.1. Characterization of pectin-cysteine conjugate
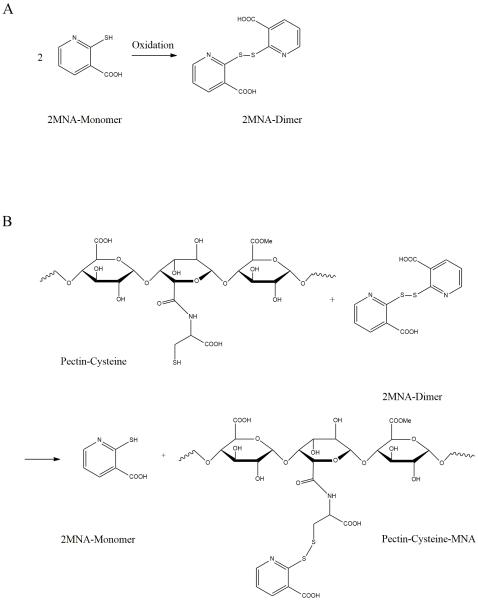
A schematic diagram of a thiolated substructure of the formed pectin derivative are given in Figure 1. Ellman’s test showed that 507 ± 35 μmol (0.06 g) L-cysteine hydrochloride were bound per gram pectin. The control sample prepared in the same manner omitting EDAC showed a negligible number of remaining traces of L-cysteine. For all experiments the fibrous lyophilized polymer were used.
Figure 1.
Synthesis of Pec-Cys-MNA
Dimerization of 2-mercaptonicotinic acid using hydrogen peroxide as oxidizer (A). The resulting dimer was added to Pec-Cys to preactivate the SH-Group (B).
3.1.2. Characterization of 2,2′-dithiodinicotinic acid
The 2-2-dithionicotinic acid was generated by addition of a H2O2 solution to a solution of the MNA monomer (Figure 1A). During the reaction, color changed from yellow to colorless. Further, UV spectra (UVmini1240, Shimadzu Co., Japan) were measured during reaction time. Thereby, the yellow MNA solution showed two at 278 nm and 353 nm. Oxidized product showed one absorption peak at 257 nm.
3.1.3. Characterization of pectin-cysteine-2-mercaptonicotinic acid
Pec-Cys-MNA formation was achieved by disulfide exchange as illustrated in Figure 1B. As the 2-mercaptonicotinic acid monomer was released during reaction the solution turned from colorless to light yellow. Approximately 50 % of free thiol groups adhering to pectin were modified: 263 ± 13 μmol (0.041 g) of MNA per gram Pec-Cys-MNA were detected.
3.2. In vitro mucoadhesion studies
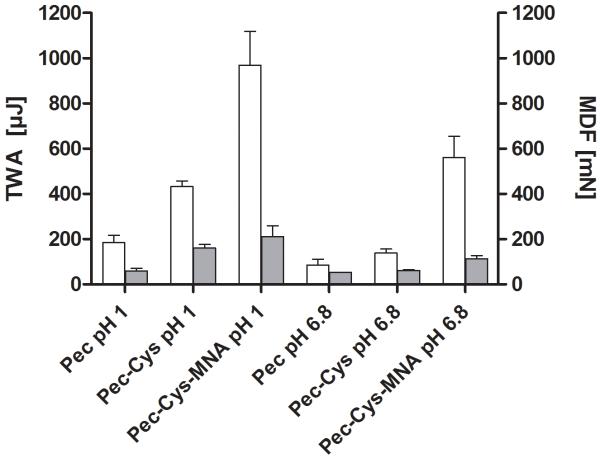
To test mucoadhesive properties tensile studies on porcine mucosa were performed.The results are given in Figure 2 including the maximum detachment force (MDF) and the total work of adhesion (TWA). The improvement ratio for total work of adhesion of Pec-Cys-MNA in comparison to Pec was 5.2-fold at pH 6.8 and 6.5-fold under acidic conditions. After incubation time of 20 minutes, cohesiveness of all tablets was still provided. Swelling process has started but no erosion was observed.
Figure 2.
Diagram shows mucoadhesive properties of Pec, Pec-Cys and Pec-Cys-MNA. White bars display the mean detachment force (MDF), grey bars the total work of adhesion (TWA) at 37° C. Indicated values are means ± SD, n = 5. TWA-means differ from each other significantly: Pec pH 1 from Pec-Cys-MNA pH 1, Pec-Cys 1 from Pec-Cys-MNA pH, Pec pH 6.8 from Pec-Cys-MNA pH 6.8 and Pec-Cys pH 6.8 from, Pec-Cys-MNA pH 6.8. MDF-means differ from each other significantly: Pec pH 1 from Pec-Cys pH 1, Pec pH 1 from Pec-Cys-MNA pH 1 and Pec pH 6.8 from Pec-Cys-MNA (Bonferroni’s Multiple Comparison Test, p< 0.05).
3.3. Hardness of test tablets
The results of hardness test are shown in Table 1. No significant difference between the modified pectin compared to the native pectin could be observed.
Table 1.
Hardness of test tablets. Indicate values are mean ± S.D. of 10 tablets.
| Polymers | Hardness [N] |
|---|---|
| Pectin | 85 ± 11 |
| Pectin-Cysteine | 94 ± 16 |
| Pectin-Cysteine-MNA | 86 ± 7 |
3.4. Disintegration studies
The endpoint of the experiment was reached after tablets were fully soaked with no firm core remaining. Results are shown in Figure 3. For pectin-tablets bulking was observed but dissolving of the tablet occurred early. Well-defined shape was lost after 30min, even though there was still a dry core visible. The disintegration time was prolonged for Pec-Cys 1.9-fold (pH 6.8) and 2.4-fold (pH 1.2), for Pec-Cys-MNA the improvement ratio was 2.3 at pH 6.8 and 2.6 at pH 1.2. Until the endpoint of the test was reached a well-defined shape and a clear borderline between the swelling tablets and the surrounding medium could be observed.
Figure 3.
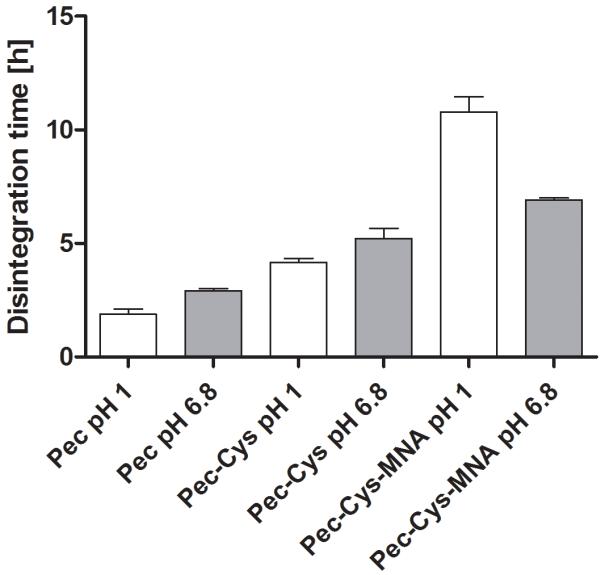
Histogram shows time until disintegration of all three polymers. The time was determined at two different pH values. Indicated values are means ± SD, n= 6. Means differ from each other significantly (Bonferroni’s Multiple Comparison Test, p < 0.05).
3.5. Water uptake capacity
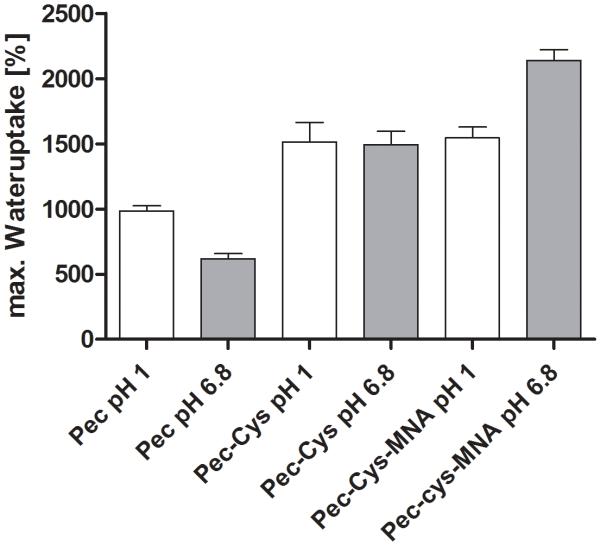
Water uptake capacity increased 1.5-fold under acidic conditions for both pectin-derivatives, Pec-Cys and Pec-Cys-MNA, compared to the unmodified pectin. In the phosphate buffer pH 6.8 the water uptake raised 2.4-times for Pec-Cys and 3.5-times for Pec-Cys-MNA. The maximum water uptake for all tested polymers can be seen in figure 4. After 4.5 h of incubation in aqueous environment pectin was completely moistened and turned into a shapeless, jelly mass. Further weighing was not feasible. In contrasts tablets comprising the modified polymer showed a greater cohesiveness and no erosion could be observed for Pec-Cys and Pec-Cys-MNA. After 4.5 hours no further significant weight-change was observed for Pec-Cys and Pec-Cys-MNA. Weight of pectin-tablets increased 11-fold under acidic conditions whereas at pH 6.8 a 7-fold gain was observed. Pec-Cys and Pec-Cys-MNA tablets experienced a 16-fold weight gain under acidic conditions. In the pH 6.8 buffer solution the weight increased for Pec-Cys and for Pec-Cys-MNA 16-fold and 22-fold, respectively.
Figure 4.
Maximum water uptake of unmodified pectin, Pec-Cys and Pec-Cys-MNA. Indicated values are means ± SD, n = 4. Means of max. wateruptake differ from each other significantly: Pec pH 1 from Pec-Cys pH 1, Pec pH 1 from Pec-Cys-MNA pH 1, Pec pH 6.8 from Pec-Cys pH 6.8 and from Pec-Cys-MNA pH 6.8, Pec-Cys pH 6.8 from Pec-Cys-MNA pH 6.8 (Bonferroni’s Multiple Comparison Test, p< 0.05).
3.6. Release studies
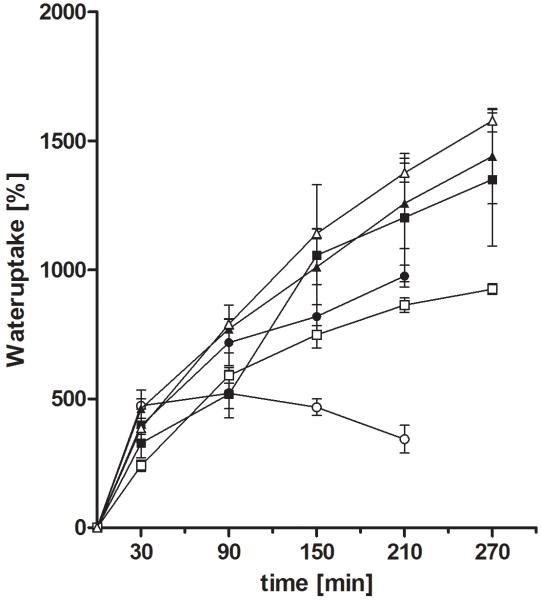
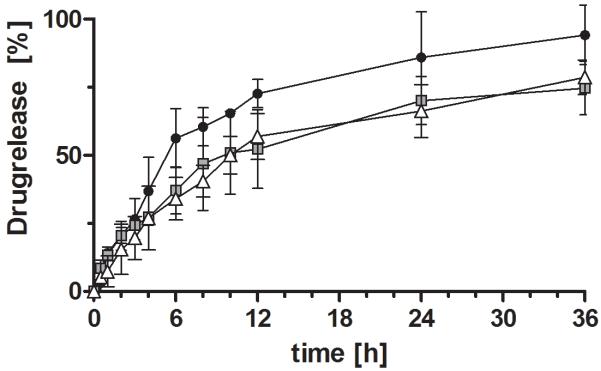
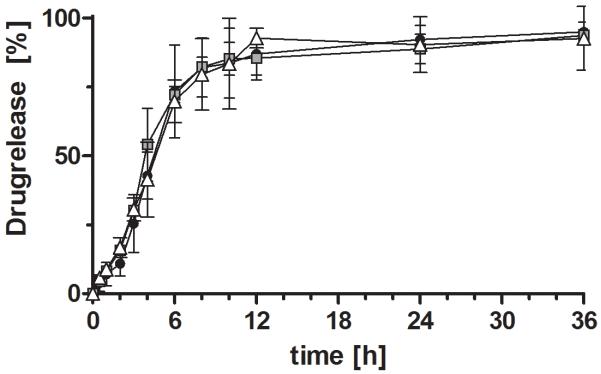
For all formulations a sustained release could be observed (Figure 5 and 6). Under acidic conditions a less sustained but almost complete (95%) release was observed for the pectin-based formulation. Formulations based on Pec-Cys and Pec-Cys-MNA had released 50 % after 10 hours. In the pH-change setup of the experiment, release profiles of different formulations did not differ. After pH change to 6.8, release occurred more rapidly, 50% of rosuvastatin calcium were released after 4 hours.
Figure 5.
Release profiles of rosuvastatin calcium from tablets based on unmodified pectin (-●-), Pec-Cys ( ) and Pec-Cys-MNA (-△-) over 36 hours. Experiments were carried out in 0.1 M HCl. Indicated values are means (n=3 ± SD).
) and Pec-Cys-MNA (-△-) over 36 hours. Experiments were carried out in 0.1 M HCl. Indicated values are means (n=3 ± SD).
Figure 6.
Release profiles of rosuvastatin calcium from tablets based on unmodified pectin (-●-), Pec-Cys ( ) and Pec-Cys-MNA (-△-) over 36 hours. First two hours were carried out, than medium was changed to 0.1 M phosphate buffer pH 6.8. Indicated values are means (n=3 ± SD).
) and Pec-Cys-MNA (-△-) over 36 hours. First two hours were carried out, than medium was changed to 0.1 M phosphate buffer pH 6.8. Indicated values are means (n=3 ± SD).
3.7. Viscosity and degradability
The dynamic viscosity of modified polymers in comparison to the native pectin increased 1250-fold in case of Pec-Cys and 3480-fold in case of the Pec-Cys-MNA (1% m/v solutions, pH 4.5). Due to the addition of the pectin splitting enzyme pectinase the dynamic viscosity decreased in case of all three polymers. After the reaction time of 90 minutes the dynamic viscosity for all three tested polymers equaled. Table 2 provides a synopsis of results.
Table 2.
Viscosity of pectin and derivatives before and after addition of pectinase. Indicated values are mean ± SD, n = 3.
| Dynamic viscosity [Pas] | ||
|---|---|---|
|
| ||
| polymer | 1% solution | 1% solution with pectinase |
| Pec | 0.144 ± 0.014 | 0.005 ± 0.004 |
| Pec-Cys | 179.767 ± 17.954 | 0.007 ± 0.002 |
| Pec-Cys-MNA | 499.600 ± 49.201 | 0.008 ± 0.007 |
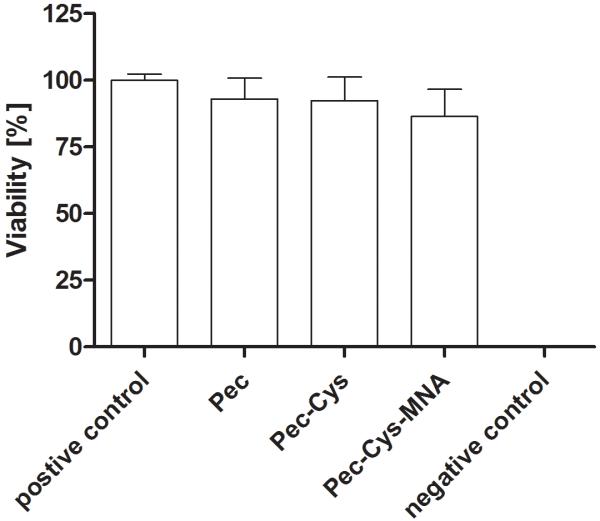
3.8. Cytotoxicity studies – resazurin assay
The resazurin fluorometric cell viability assay is a simple, safe and reliable way to investigate cytotoxicity based on measuring metabolic activity of vibrant cells (Perrot et al., 2003). There were no significant differences for all tested polymers during the incubation period of 3 hours.
4. Discussion
Within this study, a preactivated thiomer was characterized in order to evaluate its potential as mucoadhesive drug delivery system. Therefore, pectin was thiolated by covalent attachment of cysteine via disulfide bond formation. Thiol groups were further modified by disulfide bond formation with MNA in order to activate sulfhydryl groups immobilized on the polymer and to gain a higher activity over a broader pH range.
The formulation is meant to improve bioavailability of the model drug rosuvastatin calcium, whose oral bioavailability is approximately 20%, by prolongation of contact time with the gastric mucosa. Gastroretention can be prolonged by mucoadhesive properties of the excipient and can be supported by the size of the tablet after water uptake in the stomach (Garg and Sharma, 2003). The chosen polymeric backbone, pectin, shows mucoadhesive properties per se. Due to hydroxyl groups pectin is able to form hydrogen bonds with other functional groups such as hydroxyl, carboxyl or amide groups. Thereby interaction between the polymer and gastric mucus is possible. Smart et al. found an adhesiveness of pectin when investigated with mucus gel employing the Wilhemy plate method (Smart et al., 1984). In addition, mucoadhesive properties of the polymer on gastrointestinal mucosa could be shown (Liu et al., 2003; Thirawong et al., 2008). This study showed that the mucoadhesion is improved by the implementation of free thiol groups and further activation of these thiol groups with an aromatic ligand. Former studies revealed that thiol groups are able to form covalent bonds with cysteine-rich subdomains of the mucus gel layer by disulfide exchange reactions or oxidative coupling resulting in enhanced mucoadhesion (Bernkop-Schnurch et al., 1999b; Leitner et al., 2003c; Perez-Vilar and Mabolo, 2007). Unfortunately, reactivity of thiol groups strongly depends on pH values of the environment. In aqueous solutions, crosslinking between attached thiol groups takes place easily especially at pH values above 5 being closer to pka value of attached thiol groups. Inter- and intramolecular disulfide formation results in a decrease of mucoadhesion. Furthermore, at pH values below 5, reactivity against thiol bearing mucus components is decreased as well as concentration of thiol anion –S−, being required as reactive form, is very low (Bernkop-Schnurch et al., 2004). To overcome this restrained reactivity of thiomers, more recently, so-called preactivated thiolated polymers have been introduced. Formation of inter- and intramolecular disulfide bonds should be limited and further reactivity against other thiols should be increased. Covalent chromatography provides strong evidence that proteins can be linked to thiol group bearing resins efficiently when activated with pyridyl substructures (Carlsson et al., 1978; Iqbal et al., 2012; Norris and Brocklehurst, 1976). Hence, preactivated thiol groups should be able to form disulfide bonds with thiol bearing subdomains of mucin by disulfide exchange reaction whereat the aromatic thiol moiety is released, explaining improved mucoadhesion (Carlsson et al., 1978; Norris and Brocklehurst, 1976). Results of tensile studies are in line with the hypothesis about protecting and activating thiol groups by implementing an aromatic leaving group. The improvement of TWA of Pec-Cys-MNA was outstanding for both conditions, pH 1.2 and pH 6.8. Total values for test series in acidic conditions were considerable higher than in phosphate buffer pH 6.8.
Besides enhanced mucoadhesion, coupling MNA to the thiol groups of the synthesized polymer changed other characteristics of the polysaccharide like disintegration time and water uptake. Water uptake increased considerable from Pec to Pec-Cys to Pec-Cys-MNA. Swelling of polymeric delivery systems is of advantage for several reasons. The higher volume of dosage form may lead to prolongation of retention time in stomach (Garg and Sharma, 2003). Further, swelling is important for mucoadhesion, employing simple adhesion by hydration effect and swelling leads to chain relaxation making interpenetration of polymer chains and mucus gel layer easier(Quintanar-Guerrero et al., 2001; Smart, 2005). Next, swelling of the dosage form is also important for drug release as swelling together with erosion and diffusion are the most important mechanisms for controlled release (Siepmann and Peppas, 2001). Diffusion depends on the water content of the formulation, as the drug has to be dissolved and chain relaxation opens ways out for the drug. After the change of pH in the release medium release profiles of the three investigated formulations are similar.
Solubility of pectin carrying carboxyl groups is decreased in acidic environment. Under less acidic conditions carboxyl groups are deprotonated and chain relaxation may occur more easily. This may facilitate diffusion of the drug resulting in faster and higher drug release (Dwivedi et al., 2011). Unmodified, naturally occurring pectin is expected to be degraded under influence of polysaccharidases like pectinases produced by bacterial flora in the colon (Perera et al., 2010a). To assure biodegradability of the derivative it was tested using pectinase from Aspergillus aculeatus. Modification of pectin turned out to be no hindrance for enzymatic degradation. Pectin and L-cysteine are two naturally occurring substances which are known to be nontoxic and biocompatible. Conjugation of these two substances and further modification with 2-mercaptonicotinic acid showed no significant change in cell viability under tested conditions. Nevertheless, these kind of tests can only give a hint about a substances potential to cause damage or not. It is not possible to predict any long term damages, effects on other kind of tissues and the influence of concentration. Further, under given conditions, it is difficult to investigate cell toxicity over a long time period as pectin is a microbial susceptible substance. It may be contaminated during reaction time as well as storage, as conditions were non sterile.
5. Conclusion
Within this present study, a recently developed pectin derivative was evaluated in vitro for its usage as a novel excipient for mucoadhesive delivery systems on the gastric mucosa using the model drug rosuvastatin calcium. The covalent attachment of 2-mercaptonicotinic acid to thiolated pectin led to improved mucoadhesion especially in an acidic environment where mucoadhesion of non preactivated thiolated thiomers is restricted. Moreover, it could be shown that by preactivation with a pyridylic group the pH dependences of properties of thiomers are decreased. Furthermore, a considerable gain in water-binding capacity was observed. These properties combined with improved cohesiveness and sustained release for 36 hours under acidic conditions indicate a great potential of the excipient for a mucoadhesive drug delivery system, especially useful for sparingly soluble drug with a low bioavailability like the statin rosuvastatin calcium which can be released very slowly from the formulation to target side which may resulting in enhanced uptake.
Acknowledgement
The work was supported by the University of Innsbruck and the FWF (Fonds zur Förderung der wissenschaftlichen Forschung) project No.ZFP 235150.
Footnotes
Chemical compounds studied in the article
Pectin (CID 441476)
2-Mercaptonicotinic acid (CID 673681)
References
- Akiyama Y, Nagahara N, Nara E, Kitano M, Iwasa S, Yamamoto I, Azuma J, Ogawa Y. Evaluation of oral mucoadhesive microspheres in man on the basis of the pharmacokinetics of furosemide and riboflavin, compounds with limited gastrointestinal absorption sites. J Pharm Pharmacol. 1998;50:159–166. doi: 10.1111/j.2042-7158.1998.tb06171.x. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A. Thiomers: a new generation of mucoadhesive polymers. Adv Drug Deliv Rev. 2005;57:1569–1582. doi: 10.1016/j.addr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A, Krauland AH, Leitner VM, Palmberger T. Thiomers: potential excipients for non-invasive peptide delivery systems. Eur J Pharm Biopharm. 2004;58:253–263. doi: 10.1016/j.ejpb.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A, Schwarz V, Steininger S. Polymers with thiol groups: a new generation of mucoadhesive polymers? Pharm Res. 1999a;16:876–881. doi: 10.1023/a:1018830204170. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A, Schwarz V, Steininger S. Polymers with thiol groups: a new generation of mucoadhesive polymers? Pharm Res. 1999b;16:876–881. doi: 10.1023/a:1018830204170. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnurch A, Schwarz V, Steininger S. Polymers with thiol groups: A new generation of mucoadhesive polymers? Pharmaceutical Research. 1999c;16:876–881. doi: 10.1023/a:1018830204170. [DOI] [PubMed] [Google Scholar]
- Carlsson J, Drevin H, Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978;173:723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dünnhaupt S, Barthelmes J, Thurner CC, Waldner C, Sakloetsakun D, Bernkop-Schnürch A. S-protected thiolated chitosan: Synthesis and in vitro characterization. Carbohydrate Polymers. 2012;90:765–772. doi: 10.1016/j.carbpol.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi J, Dwivedi R, Ashawat MS, Singhvi I, Mehta A, Chandwani U, Mahatma OP. Solubility and dissolution rate determination of rosuvastatin calcium in different pH media using UV visible spectrophotometer. 2011 2011, Inventi:ppt/359/311. [Google Scholar]
- Garg R, Gupta G. Progress in Controlled Gastroretentive Delivery Systems. Tropical Journal of Pharmaceutical Reasearch, Benin City. 2008:1055–1066. [Google Scholar]
- Garg S, Sharma S. Gastroretentive Drug Delivery Systems. Buisness Briefing: Pharmatech; 2003. 2003. [Google Scholar]
- Goodwin JF, Choi SY. Quantification of protein solutions with trinitrobenzenesulfonic acid. Clin Chem. 1970;16:24–31. [PubMed] [Google Scholar]
- Hussain N, Al Masum A, Akhter S, Sharmin F, Reza S. Formulation and Evaluation of Gastr Retentive Floating Tablets of Simvastatin using Hydrophilic Rate Retardant. Bangladesh Pharmaceutical Journal. 2012:119–126. [Google Scholar]
- Iqbal J, Shahnaz G, Dünnhaupt S, Müller C, Hintzen F, Bernkop-Schnürch A. Preactivated thiomers as mucoadhesive polymers for drug delivery. Biomaterials. 2012;33:1528–1535. doi: 10.1016/j.biomaterials.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings P, Koppelstaetter C, Aydin S, Abberger T, Wolf AM, Mayer G, Pfaller W. Cyclosporine A induces senescence in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293:F831–838. doi: 10.1152/ajprenal.00005.2007. [DOI] [PubMed] [Google Scholar]
- Kaila HO, Ambasana MA, Thakkar RS, Saravaia HT, Shah AK. A New Improved RP-HPLC Method for Assay of Rosuvastatin Calcium in Tablets. Indian J Pharm Sci. 2010;72:592–598. doi: 10.4103/0250-474X.78526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast CE, Bernkop-Schnurch A. Thiolated polymers--thiomers: development and in vitro evaluation of chitosan-thioglycolic acid conjugates. Biomaterials. 2001;22:2345–2352. doi: 10.1016/s0142-9612(00)00421-x. [DOI] [PubMed] [Google Scholar]
- Kunkel GR, Mehrabian M, Martinson HG. Contact-site cross-linking agents. Mol Cell Biochem. 1981;34:3–13. doi: 10.1007/BF02354846. [DOI] [PubMed] [Google Scholar]
- Leitner VM, Marschutz MK, Bernkop-Schnurch A. Mucoadhesive and cohesive properties of poly(acrylic acid)-cysteine conjugates with regard to their molecular mass. Eur J Pharm Sci. 2003a;18:89–96. doi: 10.1016/s0928-0987(02)00245-2. [DOI] [PubMed] [Google Scholar]
- Leitner VM, Marschutz MK, Bernkop-Schnurch A. Mucoadhesive and cohesive properties of poly(acrylic acid)-cysteine conjugates with regard to their molecular mass. Eur J Pharm Sci. 2003b;18:89–96. doi: 10.1016/s0928-0987(02)00245-2. [DOI] [PubMed] [Google Scholar]
- Leitner VM, Walker GF, Bernkop-Schnurch A. Thiolated polymers: evidence for the formation of disulphide bonds with mucus glycoproteins. Eur J Pharm Biopharm. 2003c;56:207–214. doi: 10.1016/s0939-6411(03)00061-4. [DOI] [PubMed] [Google Scholar]
- Liu L, Fishman ML, Kost J, Hicks KB. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials. 2003;24:3333–3343. doi: 10.1016/s0142-9612(03)00213-8. [DOI] [PubMed] [Google Scholar]
- Majzoob S, Atyabi F, Dorkoosh F, Kafedjiiski K, Loretz B, Bernkop-Schnurch A. Pectin-cysteine conjugate: synthesis and in-vitro evaluation of its potential for drug delivery. J Pharm Pharmacol. 2006;58:1601–1610. doi: 10.1211/jpp.58.12.0006. [DOI] [PubMed] [Google Scholar]
- Martin PD, Warwick MJ, Dane AL, Brindley C, Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003a;25:2553–2563. doi: 10.1016/s0149-2918(03)80316-8. [DOI] [PubMed] [Google Scholar]
- Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, Lenz E. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003b;25:2822–2835. doi: 10.1016/s0149-2918(03)80336-3. [DOI] [PubMed] [Google Scholar]
- Norris R, Brocklehurst K. A convenient method of preparation of high-activity urease from Canavalia ensiformis by covalent chromatography and an investigation of its thiol groups with 2,2′-dipyridyl disulphide as a thiol titrant and reactivity probe. Biochem J. 1976;159:245–257. doi: 10.1042/bj1590245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- Perera G, Barthelmes J, Bernkop-Schnurch A. Novel pectin-4-aminothiophenole conjugate microparticles for colon-specific drug delivery. J Control Release. 2010a;145:240–246. doi: 10.1016/j.jconrel.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Perera G, Hombach J, Bernkop-Schnurch A. Hydrophobic Thiolation of Pectin with 4-Aminothiophenol: Synthesis and In Vitro Characterization. Aaps Pharmscitech. 2010b;11:174–180. doi: 10.1208/s12249-009-9370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Vilar J, Mabolo R. Gel-forming mucins. Notions from in vitro studies. Histol Histopathol. 2007;22:455–464. doi: 10.14670/HH-22.455. [DOI] [PubMed] [Google Scholar]
- Perrot S, Dutertre-Catella H, Martin C, Rat P, Warnet JM. Resazurin metabolism assay is a new sensitive alternative test in isolated pig cornea. Toxicol Sci. 2003;72:122–129. doi: 10.1093/toxsci/kfg014. [DOI] [PubMed] [Google Scholar]
- Prinderre P, Sauzet C, Fuxen C. Advances in gastro retentive drug-delivery systems. Expert Opin Drug Deliv. 2011;8:1189–1203. doi: 10.1517/17425247.2011.592828. [DOI] [PubMed] [Google Scholar]
- Quintanar-Guerrero D, Villalobos-Garciá R, Alvarez-Colín E, Cornejo-Bravo JM. In vitro evaluation of the bioadhesive properties of hydrophobic polybasic gels containing N,N-dimethylaminoethyl methacrylate-co-methyl methacrylate. Biomaterials. 2001;22:957–961. doi: 10.1016/s0142-9612(00)00260-x. [DOI] [PubMed] [Google Scholar]
- Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Advanced Drug Delivery Reviews. 2001;48:139–157. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev. 2005;57:1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Smart JD, Kellaway IW, Worthington HEC. An in-vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. Journal of Pharmacy and Pharmacology. 1984;36:295–299. doi: 10.1111/j.2042-7158.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]
- Thirawong N, Kennedy RA, Sriamornsak P. Viscometric study of pectin-mucin interaction and its mucoadhesive bond strength. Carbohydrate Polymers. 2008;71:170–179. [Google Scholar]
- Whitesides GM, Lilburn JE, Szajewski RP. Rates of thiol-disulfide interchange reactions between mono- and dithiols and Ellman’s reagent. The Journal of Organic Chemistry. 1977;42:332–338. [Google Scholar]