Abstract
PURPOSE
We tested the hypothesis that recombinant human VEGF-A165b and the serine arginine protein kinase (SRPK) inhibitor, SRPIN340, which controls splicing of the VEGF-A pre-mRNA, prevent neovascularisation in a rodent model of retinopathy of prematurity (ROP).
METHODS
In the 50/10 oxygen-induced retinopathy (50/10 OIR) model that exposes newborn rats to repeated cycles of 24 h of 50% oxygen alternating with 24 h of 10% oxygen, pups received intraocular injections of SRPIN340, vehicle, VEGF165b, anti-VEGF antibody or saline. Wholemounts of retinas were prepared for isolectin immunohistochemistry, and pre-retinal or intravitreal neovascularisation (PRNV) determined by clock hour analysis.
RESULTS
The anti-VEGF antibody (p<0.04), rhVEGF165b (p<0.001) and SRPIN340 (p<0.05) significantly reduced PRNV compared with control eyes. SRPIN340 reduced the expression of pro-angiogenic VEGF165 without affecting VEGF165b expression.
CONCLUSIONS
These results suggest that splicing regulation through selective downregulation of pro-angiogenic VEGF isoforms (via SRPK1 inhibition) or competitive inhibition of VEGF signalling by rhVEGF165b has the potential to be an effective alternative to potential cyto– and neuro- toxic anti-VEGF agents in the treatment of pathological neovascularisation in the eye.
Introduction
Retinopathy of Prematurity (ROP) is a potentially blinding disease that alters the normal development of retinal blood vessels in premature infants leading to retinal neovascularisation (NV)1, 2. During the initial phase the hyperoxic post-natal environment3 reduces growth factor production, induces vasoattenuation leading to a impaired retinal vascular development4, 5. Subsequently vasoproliferation and pre-retinal NV (PRNV) results, which can progress to retinal NV. Retinal NV predisposes the infant to intravitreal hemorrhages, retinal detachment, and subsequent visual loss7. The development and severity of ROP is a multifactorial process. Factors including hypoxic/hyperoxic tissue8, hypercarbia, metabolic acidosis9, temporal development and gene expression combine with clinical care to impact ROP pathogenesis10. During the second phase of ROP, vascular endothelial growth factor-A (VEGF-A, hereafter referred to as VEGF) a key regulator of angiogenesis11, is upregulated12, stimulating NV in ROP13. Not surprisingly VEGF has been identified as an attractive target for the development of novel therapeutics targeting pathological ocular angiogenesis, but anti-VEGF agents such as ranibizumab or bevacizumab, are likely to prevent the endogenous survival effects of VEGF-A, and VEGF blockade has been shown to induce retinal neurodegeneration14, 15.
The human VEGF gene is organized into eight exons and seven introns16 spanning a coding region of approximately 14 kilobases17. VEGF is alternatively spliced to form two families of isoforms that differ in structure and function. VEGFxxx isoforms are noted for their pro-angiogenic activity18-21 and VEGFxxxb isoforms are conversely noted for their anti-angiogenic activities22, 23. A key difference between these families is an altered terminal six amino-acid sequence of Ser-Leu-Thr-Arg-Lys-Asp (SLTRKD; Exon8b), compared with Cys-Arg-Lys-Pro-Arg-Arg (CDKPRR; Exon8a) in the VEGFxxx family24. It has been suggested that the differences in these terminal six amino acids and thus protein tertiary structure, are key to determining the anti-angiogenic activity of VEGFxxxb isoforms, preventing robust VEGFR-2 phosphorylation, recruitment of co-receptor neuropilin-1 (NP-1) and downstream signalling25, 26. The auxillary splicing factor, serine-rich splicing factor-1 (SRSF1) interacts with an exonic sequence enhancer (ESE) upstream of the VEGF exon 8a proximal splice site promoting VEGFxxx splicing27-29. SRSF1 is phosphorylated by SRPK1 in the cytoplasm enabling its nuclear translocation30-32 and once in the nucleus, SRSF1 can bind pre-mRNA and affect alternative splicing. SRPK1 has been identified as a target to prevent SRSF1 phosphorylation, pro-angiogenic VEGF upregulation and angiogenesis in vivo27-29. The SRPK1 small molecule inhibitor, SRPIN34033 has been shown to inhibit splicing to VEGF165 but not VEGF165b in retinal pigmented epithelial cells, and promote a modest, but significant reduction, in ocular neovascularisation in a murine oxygen induced retinopathy model (OIR)28.
Knowledge gained from models of retinal diseases have yielded much of what we know about physiological and pathological blood vessel growth in the retina10. There are currently two widely accepted OIR models for the study of ROP, the murine 75% hyperoxia model developed by Lois Smith34 and the alternating 50/10% oxygen rat model developed by John Penn35. Both models consistently reproduce retinal NV but the 50/10 model in rat is regarded as a more clinically robust model of human ROP10, 36, 37. Like infants suffering from zone II ROP, pups exposed to the 50/10 OIR develop peripheral retinal avascularity, arterial tortuosity and pre-retinal NV37-40. The neovascularisation occurs as budding at the ends of the vessels in zone II, similar to that seen in humans, rather than diffuse widespread proliferative angiogenesis throughout the intermediate region of the retina. Furthermore, increased VEGF expression in ROP in the 50/10 OIR model38, 41, which is mechanistically linked to the progression of PRNV, is also increased at mRNA level during human ROP42. During this study we investigated the splicing of VEGF during OIR and tested the specific hypothesis that both rhVEGF165b and SRPIN340 would have anti-angiogenic effects in the rat OIR model of human ROP. Such a strategy could have the potential clinical benefit of limiting PRNV without the potential adverse long-term effects of ranibizumab.
Materials and Methods
Isolation of primary cells and cell culture
Isolations were performed under cell culture hoods in class II facilities using aseptic technique, sterile instruments and autoclaved solutions. Umbilical cords for HUVEC isolation were obtained with patient consent from St. Michaels Hospital, Bristol, UK. Cords were stored in 1x phosphate buffered saline (PBS) and 1% PenStrep (Invitrogen). HUVEC were isolated by collagenase treatment of human umbilical veins as described43. HUVEC were cultured in EBM-2 supplemented with EGM-2 BulletKit (Lonza). Primary human RPE isolations were performed on human donor globes obtained within 24 hours post-mortem from the Bristol Eye bank (Bristol Eye Hospital). All human tissues were obtained with ethical approval from the Bristol Research Ethics Committee in accordance with the declaration of Helsinki as revised in 2008. Retinas with choroid-RPE sheets were removed to a petri dish, finely chopped and digested in 5ml Dulbecco’s Modified Eagle Medium (DMEM):F12(1:1)+GlutaMax (Gibco) supplemented with 0.3mg/ml collagenase for 15 minutes at 37°C. Digested choroid-RPE sheets were added to 30ml media (DMEM:F12+GlutaMax) supplemented with 10% fetal bovine serum (FBS), 0.5% PenStrep (Invitrogen) and spun at 1500 rpm (251g) for 10 minutes to pellet cells. Supernatent was aspirated off, pellet resuspended in 4ml media supplemented with 25% FBS (Gibco) and transferred to a T25 flask (Greiner). Cells were grown in cell culture flasks (Greiner) and split at 80% confluence.
Migration Assay
A series of chemoattractant solutions were made in triplicate with 1nM VEGF165 and varying concentrations of VEGF165b (0, 0.1, 0.2, 0.3, 0.5 and 1nM). Anti-VEGF165b antibody (clone 56/1, R&D) was added at 0.12ug/ml to 0.3nM VEGF165b. Full growth media and serum free media, were used as positive and negative controls, respectively. Chemoattractant solutions were added to 24-well plates (500μl/well) and HUVECs, previously serum starved for 15-16 hours, were seeded at 100,000 cells/insert in serum free media. Cells were left to migrate for 6 hours, washed and fixed in 4% paraformaldehyde for 15 minutes. Inserts were stained for Hoescht and nuclei counted at using a 40x objective.
Semi-quantitative: reverse transcriptase (RT)-PCR for VEGF in human cells
Conventional PCR was used to detect VEGF165 and VEGF165b mRNA. Five-ten percent of the cDNA was added to a reaction mixture containing: 2x PCR Master Mix (Promega), primers (1μM each) complementary to exon 7b (5′-GGC AGC TTG AGT TAA ACG AAC-3′) and the 3’UTR of exon 8b (5′-ATG GAT CCG TAT CAG TCT TTC CTG G-3’) and DNase/RNase free water. All samples were run in parallel with negative controls (water and cDNA without reverse transcriptase (-RT)) and positive controls (VEGF165 in a plasmid expression vector (pcDNA) and VEGF165b pcDNA). The reaction mixture was thermo-cycled (PCR Express, Thermo Electron Coorporation, Basingstoke) 30-35 times, denaturing at 95°C for 60 seconds, annealing at 55°C for 60 seconds and extending at 72°C for 60 seconds. PCR products were separated on 2.5% agarose gels containing 0.5μg/ml ethidium bromide (BioRad) and visualized under an ultraviolet transilluminator (BioRad).
Equal cDNA loading was determined by PCR with GAPDH primers (Forward: 5′-CAC CCA CTC CTC CAC CTT TGA C-3’; Reverse: 5′-GTC CAC CAC CCT GTT GCT GTA G-3’). Primers result in one amplicon at ~112bp after thermo cycling 30 times, denaturing at 94°C for 45 seconds, annealing at 65°C for 45 seconds and extending at 72°C for 60 seconds.
PanVEGF and VEGFxxxb enzyme-linked immunosorbent assay (ELISA)
One μg/ml pan-VEGF capture antibody (Duoset VEGF ELISA DY-293; R&D systems) was incubated overnight at room temperature. The plates were blocked (Superblock; Thermo Scientific) and serial dilutions of recombinant human (rh)VEGF165 or rhVEGF165b standards (ranging from 4ng/ml to 16.25pg/ml) were added, incubated alongside sample lysates, typically diluted 1:10. The plate was incubated for one hour at 37oC with shaking, washed and incubated with 100μl/well of either biotinylated goat anti-human VEGF (0.1μg/ml; R & D systems) or mouse anti-human VEGF165b (0.25μg/ml) for one further hour at 37oC. After washing, 100μl/well of Horseradish Peroxidase (HRP)-conjugated streptavidin (1:200; R&D Systems) was added and plates were left at room temperature for 20 minutes.
The plates were washed and colour change induced with substrate A and B (DY-999; R&D Systems) for 20mins under light protection. The reaction was stopped by addition of 100μl/well of 1M H2SO4 and the absorbance was read immediately in an ELISA plate reader (Dynex Technologies Opsys MR system plate reader) at 450nm with a control reading at 570nm. Revelation Quicklink 4.25 software was also used to calculate a standard curve from mean absorbance values of standards enabling the estimation of VEGF concentration for each sample.
Western Blotting
Subconfluent cells were lysed in RIPA lysis buffer supplemented with Protease Inhibitor cocktail (Sigma). Thirty micrograms of total protein was resuspended in sample buffer, heated at 95°C for 5 min, and subjected to sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Subsequently, proteins were electrotransferred for 2 hours at 4°C to polyvinylidene fluoride (PVDF) membranes. The membranes were then exposed to primary antibodies (VEGF A20, 1:1000 2.5% milk (sc-152); anti-VEGFxxxb, (clone 56/1) 1:1000 5% BSA), washed in Tris-buffered saline/0.3% Tween 20, (TBS-T) and incubated with a secondary peroxidase-conjugated antibody at a 1:10,000 dilution. Signals were detected by enhanced chemoluminescence (ECL) substrate.
50/10 Oxygen Induced Retinopathy (OIR) model
Female Sprague dawley (SD) rats and their newborn litters were placed in the oxygen chamber within 4 hours of birth, or raised in normoxia. Combined litters of between 14-17 pups were exposed to alternating 24-hour cycles of hyperoxia (50% O2) and hypoxia (10% O2). Pups received intraocular injection of (i) 25ng SRPIN340 or (ii) vehicle (saline+0.05%DMSO) at p12, or (iii) 25ng VEGF165b, (iv) 1μg anti-VEGF (G6-31) or (v) saline at p14. In a previous study of OIR mouse IgG (control for G6-31) was compared to saline injection and showed no significant difference (data not shown). At p14 pups were removed from the chamber and returned to normoxia until p20. Contralateral eyes were injected with saline controls. On day 14, 17 or 20 pups were culled, unfixed retinas were taken for protein extraction and fixed retinas were dissected, stained, and flatmounted. The 50/10 OIR model develops vascular tortuosity at p12 and PRNV at p18. Moreover, the peripheral retina remains avascular at p20. Survival rates were in excess of 90% for hyperoxia/hypoxia exposed neonatal rats and there was no obvious maternal oxygen toxicity. Rats reared in room air were used as untreated normal controls. All animals were treated according to the institutional guidelines regarding animal experimentation and the ARVO regulations for the use of animals in research.
Intraocular Injection
SD rat pups were anaesthetized with an intraperitoneal injection of a mixture of 50mg/kg ketamine and 0.5mg/kg medetomidine at time points ranging from P5-P8 for normal vasculature studies and P12-P14 during OIR. Intraocular injections were administered using a 35gauge needle (P5-P8; 1μl injection volume) or 33 gauge needle (P12-P14; 2.5μl injection volume), each injection was sustained for a duration of one minute to minimise loss of solution. Injections resulting in intravitreal haemorrhaging or clouding of the lens were not included within the study.
Quantification of pathology and statistical analysis
Flatmounted isolectin stained eyes were imaged and images merged to obtain whole retinal pictures. Clock hour analysis was performed on coded samples under the microscope and PRNV scored by two masked observers. ImageJ was used to measure the total retinal area, vascular area and PRNV area of masked images. Tortuosity was calculated using Image J where the length of the vessel and the length of the line of best fit of the vessel path was calculated and the two values expressed as a ratio. Statistical analysis was performed using GraphPad Prism software. Means are expressed ± standard error of the mean. Clock hours were analysed using a two-sample Mann-Whitney rank sum test (two-sided) as the data was integral. Other analyses used students paired t-test and one-way ANOVA with bonferroni post hoc unless otherwise stated.
Results
The 50/10 OIR insult induced PRNV and increased pro-angiogenic VEGF expression
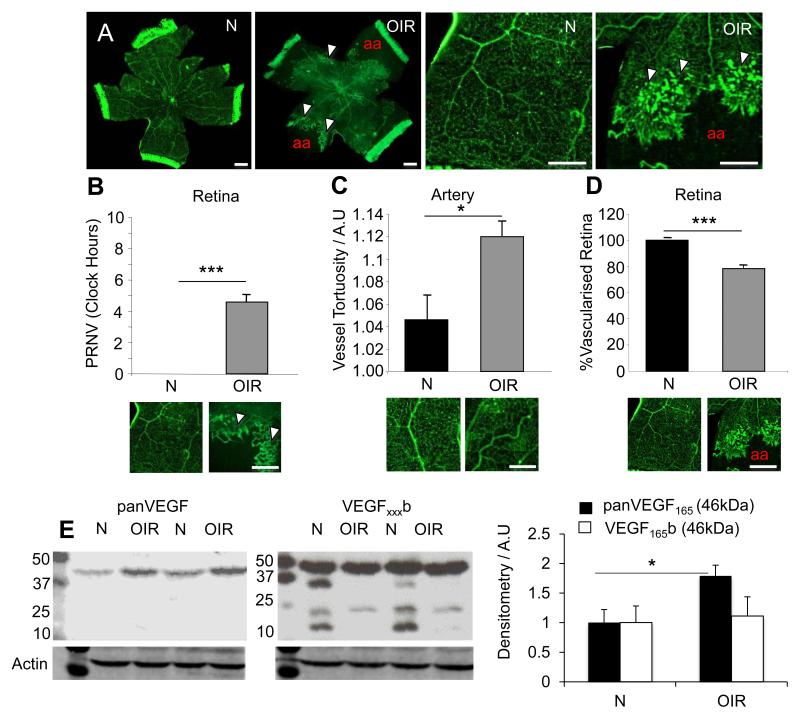
Compared with normoxia raised age-matched controls 50/10 OIR retinae stained with isolectin showed the presence of PRNV simulating a characteristic of ROP pathology (Figure 1A-B). PRNV developed from ends of post-capillary venules prior to the vascular/avascular boundary and are characterised as intensely stained swellings often merged together to produce a brush border of pathological angiogenesis. Retinae also showed increased tortuosity (p<0.05, figure 1C) of retinal arteries, and a significant reduction in the area of vascularised retina (p<0.001) (Figure 1D).
Figure 1. The 50/10 OIR insult induces pathologies reminiscent of human ROP.
Litters of 12-14 Sprague Dawley pups were raised with their dams in either normoxia or under conditions of fluctuating oxygen levels between 50% and 10% every 24 hours, for the first 14 days of life (N = Normoxia raised. OIR = 50/10 OIR raised; White arrowheads indicate PRNV; aa – avascular area). A) Examples of whole flatmounted retinas from p20 rats were fixed and stained for isolectin-B4. Exposure to the 50/10 OIR conditions induced: B) PRNV (p<0.001, unpaired t-test), C) increased arterial tortuosity (P<0.05, unpaired t-test) and D) Significantly decreased retinal vascular area compared with normoxic raised pups (p<0.001, unpaired t-test). In addition protein was extracted from P20 rats raised either in normoxia (N) or in altered oxygen levels between 50-10% (OIR) and subjected to immunoblotting using panVEGF, VEGFxxxb, or β-actin antibodies. E) OIR increased total VEGF expression compared to normoxic controls (left), however it reduced the number of VEGFxxxb splice isoforms detected (right). Scale bar = 1mm.
Protein extracted from retinae of normoxia raised (N) and 50/10 OIR raised (OIR) pups was assessed for VEGF expression. Total VEGF (A20; Santa Cruz) showed a clear upregulation in OIR samples compared to normoxic samples (Figure 1E; left panel). However, assessment of the VEGFxxxb isoforms (56/1; R&D) showed a reduction in the expression of certain isoforms. In addition to previously identified bands at 46kDa (MW of VEGF dimer = 46kDa), bands were observed at 23kDa, the expected size of glycosylated VEGF165b monomers, and protein from retinas of rats exposed to OIR showed decreased expression of this product. In addition other bands were observed in normoxic retinal protein, at approximately 33kDa (VEGF121b), and 16kDa (VEGF121b monomer) - these bands were absent from OIR retinal protein (Figure 1E; right hand blot). Densitometry analysis revealed a significant upregulation of pro-angiogenic VEGF165 but not VEGF165b (46kDa band; p<0.05, students t-test).
Recombinant human VEGF165b blocks VEGF165 mediated cell migration and reduces PRNV comparable to anti-VEGF treatment, following OIR insult
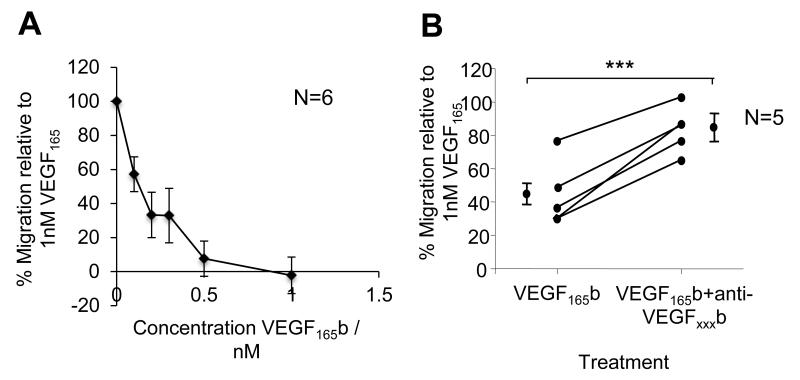
Endothelial cell migration is a critical component of angiogenesis and it has been reported that anti-angiogenic rhVEGF165b can be used to prevent VEGF-mediated cell migration. Here we have used pro-angiogenic and pro-migratory rhVEGF165 (1nM) as a chemoattractant to promote HUVEC migration. Recombinant human VEGF165b >dose-dependently inhibited VEGF165–mediated HUVEC migration, achieving significance (p<0.01, one-way ANOVA with Dunnetts post hoc) at 0.2, 0.3, 0.5 and 1nM VEGF165b concentrations (Figure 2A). When VEGF165 and VEGF165b were in combined to reduce migration by 56% compared to VEGF165 alone, pre-incubation with anti-VEGFxxxb specific antibody (56/1; R&D Systems) abolished the inhibition on cell migration observed with rhVEGF165b treatment, returning VEGF165 mediated migration to 83.1±7.61% of VEGF165 treatment alone (p<0.001, unpaired t-test; Figure 2B).
Figure 2. Recombinant human VEGF165b blocks VEGF165 mediated cell migration.
A) HUVECs were left to migrate towards chemoattractant solutions of 1nM VEGF165 + varying concentrations of VEGF165b. Increasing concentrations of VEGF165b inhibited the pro-migratory effect of VEGF165, as seen by a reduction of % migration. B) Pre-incubation with 0.12ng/μl of a VEGF165b blocking antibody 4 hours previously successfully rescued the inhibitory action of VEGF165b over the pro-migratory VEGF165, as seen by an increase of % migration relative to VEGF165 alone (***, p<0.001, unpaired t-test).
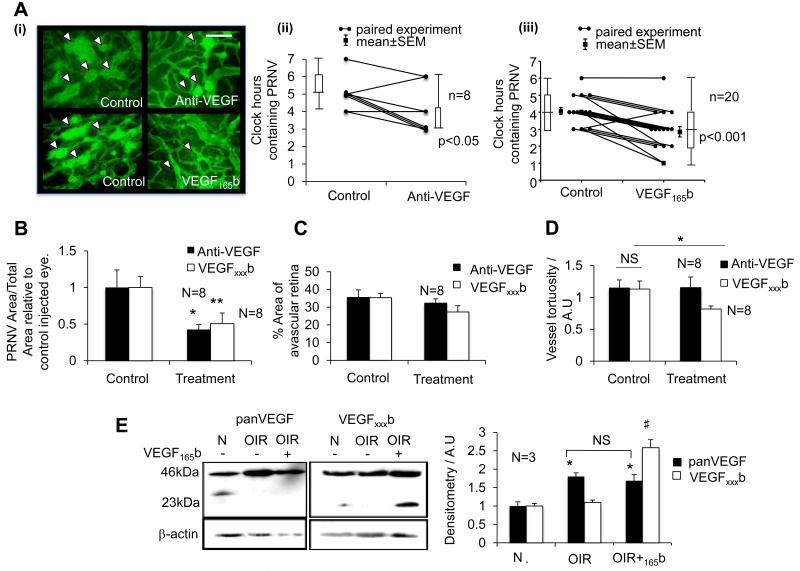
When tested in a mouse model of OIR, rhVEGF165b has been shown to be anti-angiogenic to hypoxia-driven angiogenesis in the eye44. Here we have tested rhVEGF165b in a rat model of OIR, a model that better reflects human ROP36. Intraocular injection of 25ng rhVEGF165b, (Figure 3A), reduced PRNV in the eye following 50/10 OIR insult and was equivalent to anti-VEGF (G6-31; Roche) treatment. Retinas from eyes treated with rhVEGF165b showed a significant reduction in the number of clock hours showing PRNV, 2.8±0.31, compared with control eyes in the same pup, 4.1±0.21 (p<0.001, Mann Whitney rank sum test; n=20, power = 99.8%. Figure 3A). Treatment with G6-31 also demonstrated a significant reduction in clock hours possessing PRNV, 4±0.63, compared to 5.33±0.33 in controls, respectively (p<0.05; Mann Whitney rank sums test). Further analysis revealed the total area of PRNV relative to total retinal area, was decreased in retinas from rhVEGF165b (49.4±14.6%; p<0.01) and G6-31 (57.5±6.6%; p<0.05) treated eyes relative to their respective control eyes. A positive correlation (rs=0.71, Spearmans Rank correlation coefficient) between PRNV area and number of clock hours showing PRNV was observed (data not shown). In this model we found rhVEGF165b had a consistently inhibitory effect on the development of pathological PRNV, similar to non-isoform specific pan VEGF inhibition (Figure 3B). We also compared the effects of rhVEGF165b and G6-31 treatment on vessel tortuosity and avascular area. Although neither of the treatments affected the area of the avascular retina (p>0.05; Figure 3C), rhVEGF165b significantly reduced arterial tortuosity (p<0.05, unpaired t-test; Figure 3D).
Figure 3. Recombinant human VEGF165b significantly reduces PRNV and vessel tortuosity in the rat 50/10 OIR model.
Litters of 12-14 Sprague Dawley pups were raised with their dams under conditions of fluctuating oxygen levels between 50% and 10% every 24 hours, for the first 14 days of life. At P14 pups received IO injections of either anti-VEGF antibody, G6-31 (1μg) or rhVEGF165b (25ng) in the ipsilateral eye and control injections in the contralateral eye. A) (i) Examples of control and treated microvasculature shown (White arrowheads indicate PRNV) (ii) G6-31 (p<0.05; n=8) and (iii) rhVEGF165b (p<0.001; n=20) significantly reduced the number of clock hours showing PRNV (Mann-Whitney U test) and B) PRNV area relative to contralateral controls (Paired t-test). C) Neither G6-31 nor rhVEGF165b were capable of significantly reducing retinal avascular area (P>0.05; paired t-test) however D) rhVEGF165b significantly reduced arterial vessel tortuosity. E) Immunoblot for pan-VEGF and VEGF165b showing expression of VEGF dimers and monomers in uninjected and expression in rhVEGF165b injected eyes six days after injection. Densitometry shows a signficant upregulation of pan-VEGF but not VEGF165b during OIR (*), and significantly increased VEGF165b after rhVEGF165b injection six days earlier (#). (*, #=p<0.05. NS=not significant).
VEGF165b expression was assessed in protein extracted from unfixed retinas of P20 OIR pups and saline injected eyes were compared to VEGF165b injected eyes. Recombinant human VEGF165b was detected in retinal protein from VEGF165b injected eyes as a monomer at 23kDa even six days after its injection, suggesting its stability in the eye (Figure 3E).
SRPK1 inhibition modulates pro-angiogenic VEGF expression and reduces PRNV
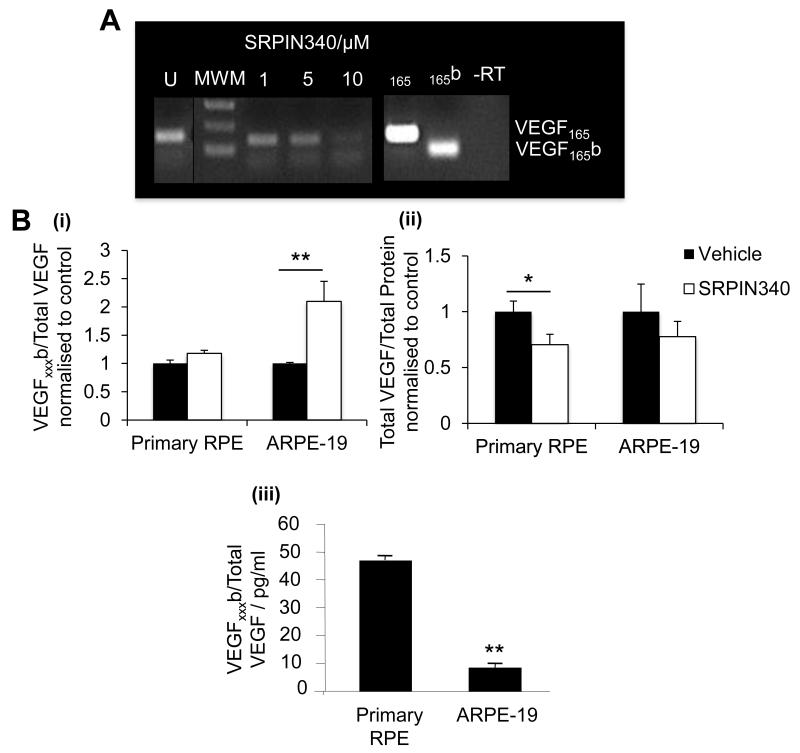
The SRPK selective inhibitor, SRPIN340 dose-dependently and selectively reduced VEGF165 expression, achieving maximal inhibition at 10μM (Figure 4A). Furthermore, treatment with 10μM SRPIN340 altered VEGF protein expression in ARPE-19 and primary RPE cells. SRPIN340 significantly increased the expression of VEGFxxxb protein isoforms relative to total VEGF in ARPE-19 cells (figure 4Bi), which express low endogenous VEGFxxxb levels (figure 4Biii), and significantly reduced pro-angiogenic VEGF expression in primary RPE cells (figure 4Bii), which had significantly higher endogenous VEGFxxxb expression (Figure 4Biii).
Figure 4. SRPK1 inhibition modulates pro-angiogenic VEGF expression in vitro.
A) Primary RPE cells were treated with varying concentration of SRPIN340 (1, 5, 10μM) for 24 hours. RT-PCR was performed with primers spanning VEGF exon 7b and 8b. SRPIN340 switched the expression of VEGF isoforms to favour VEGF165b dose-dependently from 1-10μM achieving significance at 5μM (p<0.05) and 10μM (p<0.01) (One-way ANOVA, Dunnetts post hoc). B) VEGF protein levels in primary RPE and ARPE-19 were assessed by ELISA, (i) SRPIN340 significantly increased the expression of VEGFxxxb/Total VEGF in ARPE-19 cells (p<0.01), and (ii) significantly reduced pro-angiogenic VEGF in primary RPE cells (p<0.05; students t-test). (iii) VEGFxxxb expression was greater in primary RPE cells compared to ARPE-19 (p<0.01),
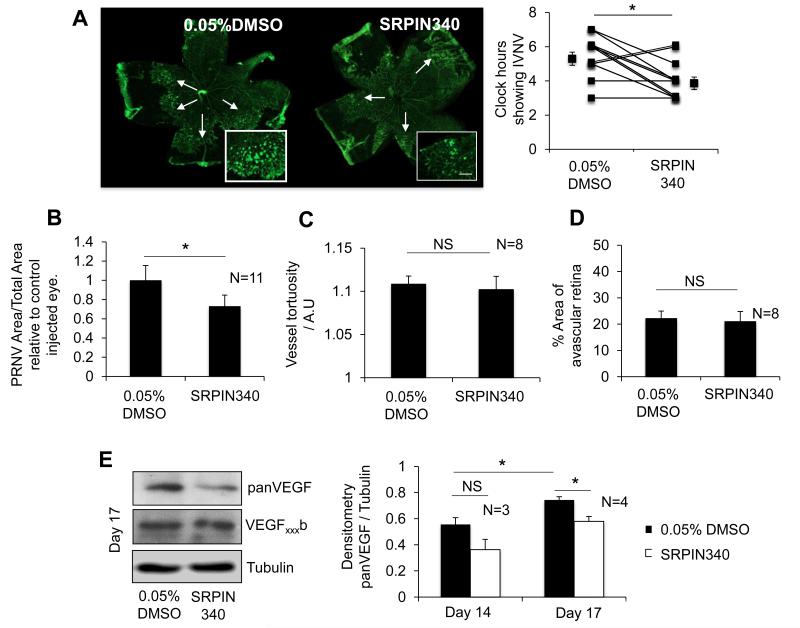
SRPIN340 was subsequently tested in the 50/10 OIR model. SRPIN340 was injected two days earlier than rhVEGF165b to allow the splicing factor to take effect prior to the VEGF surge experienced on day 1445, 46. A total of 25ng SRPIN340 was injected intraocularly in a volume of 2.5μl; saline controls were supplemented with 0.05% DMSO and injected at the same volume. Retinas from eyes treated with SRPIN340 showed a significant reduction of clock hours possessing PRNV compared with control eyes in the same pup (p<0.05, paired t-test; Figure 5A). This was confirmed by PRNV area analysis showing a 33.3±15.7% reduction in PRNV area compared with control eyes (Figure 5B). In addition we assessed the effect of SRPIN340 administration on avascular area (Figure 5C) and arterial tortuosity (Figure 5D). SRPIN340 had no effect on either vascularity or tortuosity (p<0.05; students t-test). Furthermore retinal protein was assessed for VEGF expression. VEGF expression significantly increased between P14 and P17, SRPIN340 injected eyes expressed significantly less VEGF compared with control although VEGFxxxb isoform expression was unchanged (Figure 5E). A single dose of SRPIN340, was capable of significantly reducing pro-angiogenic VEGF mediated induction of PRNV, albeit modestly.
Figure 5. SRPIN340 significantly reduces PRNV and reduces pro-angiogenic VEGF in vivo.
On day 12 of the OIR protocol the rats were briefly removed from the chamber and given a 2.5μl IO injection of 25ng SRPIN340 and vehicle (Saline +0.05% DMSO) in the contralateral eye. A) SRPIN340 treatment significantly reduced PRNV clock hours compared to control eyes (p<0.05, students paired t-test), example flat mounts shown (White arrows indicate PRNV, scale bar = 500μm) and B) PRNV area relative to total retinal area (p<0.05, students paired t-test). C) Retinas treated with SRPIN340 showed no significant change in vessel tortuosity, or the % of vascularized retina, compared to control eyes also subjected to the OIR paradigm. E) Protein extracted from the retinae of these pups showed a reduction in VEGF in SRPIN340 eyes compared with control injected, however no change was observed in VEGFxxxb expression.
Total VEGF blockade retards normal vasculature development
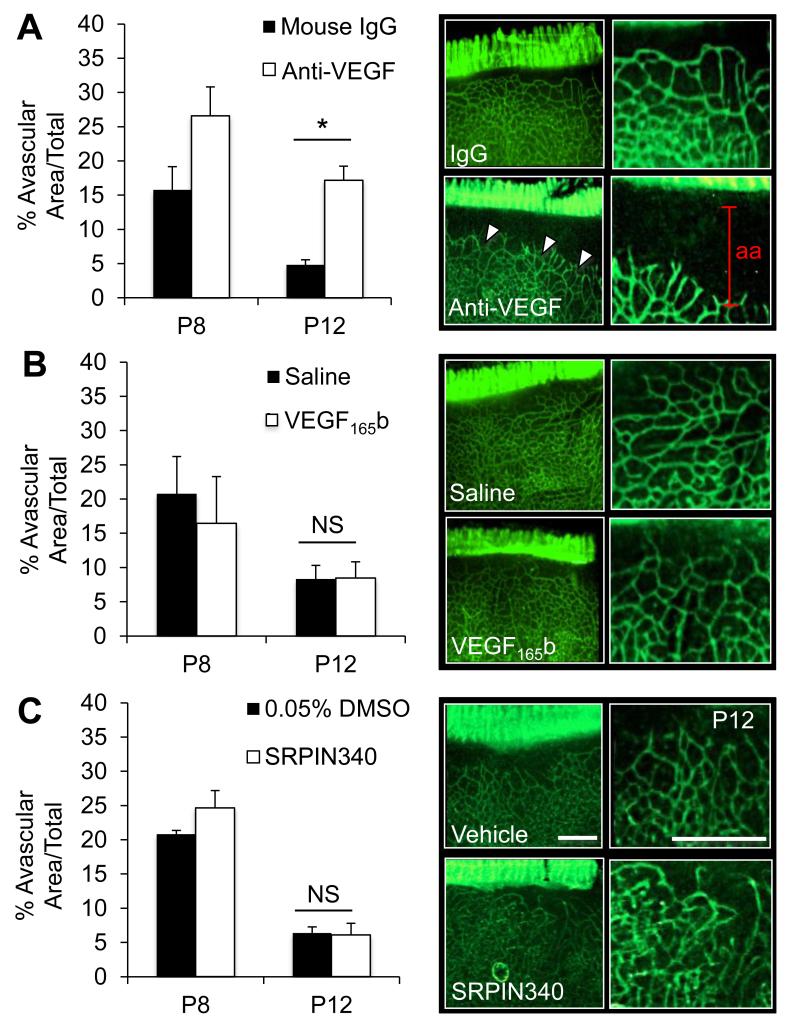
To determine whether anti-VEGF, rhVEGF165b or SRPIN340 affect the development of the normal retinal vasculature, developing eyes were injected at P5 (1μl injection volume, concentration as before) and control eyes injected with mouse IgG, saline or saline supplemented with 0.05% DMSO, respectively. Pups were culled at P8 and P12, retinae excised stained, flatmounted and avascular area assessed. Anti-VEGF treatment retarded growth of the vasculature leading to a significant increase in avascular area at P12 (p<0.05, paired t-test). Conversely, both 25ng rhVEGF165b and SRPIN340 failed to affect the developing retina at either time point. These results were reproduced when treatments were injected at P8 and pups culled at P12 (Suppl. Figure 1).
Discussion
We have demonstrated selective upregulation of pro-angiogenic but not anti-angiogenic VEGF whilst using Penn’s 50/10 OIR model to stimulate neovascular growth by cyclically fluctuating oxygen levels35. The 50/10 OIR rat model is widely accepted as a clinically relevant model of ROP36 resulting in previously described pre-retinal neovascularisation (PRNV), increased vessel width and tortuosity, as well an avascular retinal periphery35, 37, 38. We confirmed increased VEGF expression at P20, namely the VEGF164 isoform41, coinciding with maximal PRNV47. VEGFxxxb expression has not been previously investigated in this model, although it has been suggested that VEGF165b decreases during mouse OIR48. It is likely that the secondary anti-mouse IgG antibody used to detect the VEGFxxxb antibody (56/1; R&D) will also bind to endogenous mouse IgG which also runs at ~46kDa. For this reason the use of rat tissue can be more illuminating for detection of VEGF165b by mouse antibodies that do not cross-react with rat IgG. Studies in the human fetal eye indicate that VEGF165b was not notable until vascular development neared completion, while VEGF165 was very prominent as the vessels developed49, suggesting both a developmental regulation of VEGF isoform expression and an ability of VEGFxxxb to ‘switch off’ or prevent VEGFxxx activity. In physiological angiogenesis, for example during follicular development50, and wound healing51, pro-angiogenic VEGF isoforms are selectively upregulated. Alterations in the splicing of VEGF creating an ‘angiogenic switch’ have also been observed in pathological situations where angiogenesis promotes disease progression. Pro-angiogenic isoform expression is selectively upregulated in numerous human cancers23, 24, 52 and pathological eye disease such as age-related macular degeneration53. Conversely VEGF165b is upregulated in systemic sclerosis, a disease characterized by a lack of angiogenesis54. We confirmed that the rhVEGF165b used in this study can inhibit VEGF165 induced cell migration, essential to angiogenesis55. Previous reports have shown VEGF165b overexpression56 and rhVEGF165b treatment inhibited VEGF165 induced HUVEC and HMREC migration57, 58, but this study also showed rhVEGF165b could be inhibited by pre-incubation with a VEGFxxxb specific antibody (Figure 2B). These are important controls to show the activity of the rhVEGF165b, as C′-terminal exopeptidase activity, or incomplete synthesis of the protein could lead to a truncated form of the protein, such as VEGF159, which has been shown to have no anti-angiogenic activity, and some angiogenic activity25. This is also the first evidence to show that VEGF165b antibodies can be neutralizing, in the same way that bevacizumab is neutralizing to all VEGF isoforms59. The ability of the anti-VEGF165b antibody to block the anti-migratory properties of VEGF165b suggests that the C′-terminal region of VEGF165b may be involved in receptor binding, or at least binding to this region interferes with receptor interaction of the VEGFxxx isoforms.
To evaluate the importance of VEGF in this model a neutralizing antibody to all VEGF isoforms, G6-31 (the mouse monoclonal antibody that was a precursor of bevacizumab), was tested and compared to rhVEGF165b in rat. Human VEGF165b protein activity has been demonstrated when over-expressed in the mammary gland of transgenic mice60, and in the rabbit cornea and rat mesentery23. Previous reports have suggested that anti-VEGF therapy demonstrates a sustained and significant decrease in PRNV in the 50/10 OIR model41. A single intra-ocular injection of 1μg G6-31 or 25ng rhVEGF165b on day 14 significantly reduced the prevalence of PRNV on day 20 by clock hour analysis and by PRNV area analysis, previously described61, 62(Figure 3A-B). Of particular interest was that, SRPIN340, a highly selective SRPK inhibitor33, significantly reduced PRNV compared to 0.05% DMSO controls when given as a single IVT injection on day 12 (p<0.05). This demonstrates that selective downregulation of pro-angiogenic VEGF isoforms (Figure 4), but not anti-angiogenic isoforms is capable of reducing PRNV (Figure 5). Being upstream of VEGF, selective inhibition of SRPK1 and therefore the activity of SRSF1, may result in altered alternative splicing of other genes and thus introduce potential non-specific effects, although initial toxicology tests showed gram quantities of SRPIN340 administered to animals resulted in no ill effect33. Other downstream targets of SRPIN340 are currently being investigated and it is possible that other angiogenic genes, similarly altered during OIR, may also be regulated by SRPK1. Modulation of SRPK1 activity may therefore have the potential benefit of resulting in a coordinated regulation of alternative splicing during disease progression.
Other studies have investigated whether anti-VEGF treatment affects other characteristics associated with ROP, micro-vessel density and vessel tortuosity, and the latter has been linked to a poor prognosis in patients suffering from ROP63. In this study we observed that neither G6-31 nor rhVEGF165b was capable of increasing normal retinal vascularisation (Figure 3C), but we did note a significant reduction in vessel tortuosity for rhVEGF165b (p<0.05; students t-test; Figure 3D). SRPIN340 failed to affect either avascular area or vessel tortuosity (Figure 5C-D). Neutralising antibodies have previously been shown to decrease vessel tortuosity in this model38, but we observed no effect with G6-31 treatment. It would be interesting to determine the effect of a VEGF165 specific neutralising antibody in this model, but such an antibody has not yet become available.
PRNV has clearly been identified as the most damaging pathology of VEGF mediated ROP progression; the abnormal growth of vessels increases in line with the increase in severity of ROP64 and can lead to the formation of fibrous tissue and retinal detachment65. Current treatments for ROP include cryotherapy and peripheral diode laser photocoagulation. Studies have shown laser therapy to be the superior of the two66, 67, however laser therapy poses severe risks including intra-ocular hemorrhage and cataract formation68, 69. With the identification of VEGF as a critical factor in the progression of ROP70-72, off-label use of anti-VEGF inhibitors has been reported. Lee and colleagues reported regression of disease and a more rapid development of the peripheral retinal vascular bed after IVT bevacizumab injection combined with laser photocoagulation73. The BEAT-ROP trial tested anti-VEGF (intravitreal bevacizumab) therapy in premature babies suffering from ROP. This prospective randomized and controlled multicenter trial for zone I and zone II severe human ROP showed a significant benefit for zone I disease suggesting an impressive benefit with anti-VEGF therapy74. Other studies have suggested treating the BEAT-ROP trial results with caution stating concerns over the safety (there was a non significant increase in deaths in the bevacizumab group, and the trial was not large enough to demonstrate safety), data interpretation (time to endpoint was dependent on time to recurrence which was greater for bevacizumab, and therefore recurrence may have been outside the endpoint, even though it occurred), post-hoc determination of outcomes, and alteration of the primary endpoint75, as well as highlighting the failing of the trial to examine longer term ocular and systemic side effects76.
Previous debates concerning VEGF blockade77 which can be damaging to cells and tissues78, have shown that perturbation of normal VEGF can prevent normal retinal function79. Administration of anti-VEGF therapies in age related macular degeneration was shown during the Seven-up study to result in retinal atrophy in almost all (98%) of patients followed over the 7-8 year period (Bhisitkul RB et al. IOVS 2012;53:ARVO E-Abstract 3679). Moreover, while VEGF-A has been shown for some time to be neuroprotective for retinal cells15, it has recently been shown that pan VEGF-A blockade exacerbated retinal ganglion cell death in animal models of glaucoma14. We tested G6-31, rhVEGF165b and SRPIN340 during development and observed retarded growth of the retinal vasculature following anti-VEGF treatment but not by a single dose of 25ng rhVEGF165b or SRPIN340 (Figure 6; Suppl. Figure 1). Although VEGFxxxb isoforms are largely considered as being anti-angiogenic22, 23, we have previously shown that rhVEGF165b is also cytoprotective for both endothelial cells and epithelial cells including retinal pigmented epithelial cells58. Furthermore, we have recently demonstrated that VEGF165b is neuroprotective for sensory neurons including retinal ganglion cells in vivo81.
Figure 6. SRPIN340 and rhVEGF165b do not influence normal retinal development.
Eighteen P5 SD rat pups were injected IO (1μl) with either (A) 1μg G6-31, (B) 10ng rhVEGF165b or (C) 10ng SRPIN340 in one eye and 1μg Mouse IgG, saline or 0.05% DMSO respectively in the contralateral control injected eye. Pups were culled at P8 (n=3 each group) and at P12 (n=3). Flatmounted retinae were stained for isolectin IB4 imaged and avascular area quantified using image J. Both rhVEGF165b and SRPIN340 did not affect normal retinal vascularisation whereas anti-VEGF G6-31 (Roche) significantly reduced vascularisation compared to control eyes at P12 (P<0.05, students paired t-test).
Conclusion
Here we have shown that cytoprotective rhVEGF165b administration is capable of reducing pathological PRNV in rats without the need for total VEGF blockade. Unlike anti-VEGF therapy this treatment reduced arterial tortuosity and maintained the development of the normal retinal vasculature. Like anti-VEGF, rhVEGF165b failed to promote peripheral vascularisation during OIR, although some reports suggest VEGF may not be the key mediator of this process. The SRPK1 inhibitor, SRPIN340, mechanistically demonstrated that selectively reducing pro-angiogenic VEGF isoforms is sufficient to significantly reduce PRNV in this model. More potent SRPK inhibitors or targeting other factors involved in the splicing of VEGF may therefore be worth exploring as novel strategies for identifying potential ROP therapeutics. Recombinant human VEGF165b could be an alternative, potentially less damaging therapy to laser photocoagulation, or even anti-VEGF IVT injections in premature babies suffering from ROP. It will be important to determine whether rhVEGF165b treatment leads to long-term complications, toxicities and systemic side effects, which have been associated with anti-VEGF therapy.
Supplementary Material
Acknowledgements
We would like to thank Professor John S. Penn (Vanderbilt University) and Dr Xiaolin Gu (Alcon) for key advice and information on setting up the 50/10 OIR model and PRNV analysis. This work has been supported by Fight for Sight, Skin Cancer Research Foundation, the British Heart Foundation, Richard Bright VEGF Research Trust, The British Microcirculation Society and NERC. SJH is funded by the MRC G10002073.
References
- 1.Ashton N, Ward B, Serpell G. Role Of Oxygen In The Genesis Of Retrolental Fibroplasia - A Preliminary Report. British Journal of Ophthalmology. 1953;37 doi: 10.1136/bjo.37.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 3.Patz A, Fine SL, Finkelstein D, Yassur Y. Diseases of the macula: the diagnosis and management of choroidal neovascularization. Transactions. Section on Opthalmology. American Academy of Ophthalmology and Otolaryngology. 1977;83:468–475. [PubMed] [Google Scholar]
- 4.Chanling T, Stone J. Retinopathy Of Prematurity: Origins In The Architecture Of The Retina. Progress in Retinal Research. 1993;12:155–178. [Google Scholar]
- 5.Hardy P, Dumont I, Bhattacharya M, et al. Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: a basis for ischemic retinopathy. Cardiovascular Research. 2000;47:489–509. doi: 10.1016/s0008-6363(00)00084-5. [DOI] [PubMed] [Google Scholar]
- 6.Penn JS, Thum LA. The rat as an animal model for retinopathy of prematurity. Progress in clinical and biological research. 1989;314:623–642. [PubMed] [Google Scholar]
- 7.Foos RY. Chronic retinopathy of prematurity. Ophthalmology. 1985;92:563–574. doi: 10.1016/s0161-6420(85)34007-1. [DOI] [PubMed] [Google Scholar]
- 8.Katzman G, Satish M, Krishnan V. Hypoxemia and retinopathy of prematurity. Pediatrics. 1987;80:972–972. [PubMed] [Google Scholar]
- 9.Biglan AW, Brown DR, Reynolds JD, Milley JR. Risk-factors associated with retrolental fibroplasia. Ophthalmology. 1984;91:1504–1511. doi: 10.1016/s0161-6420(84)34104-5. [DOI] [PubMed] [Google Scholar]
- 10.Barnett JM, Yanni SE, Penn JS. The development of the rat model of retinopathy of prematurity. Documenta Ophthalmologica. 2010;120:3–12. doi: 10.1007/s10633-009-9180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. Journal of Genetics. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozaki H, Yu AY, Della N, et al. Hypoxia inducible factor-1 alpha is increased in ischemic retina: Temporal and spatial correlation with VEGF expression. Investigative Ophthalmology and Visual Science. 1999;40:182–189. [PubMed] [Google Scholar]
- 13.Good WV, Gendron RL. Retinopathy of prematurity: gone today, here tomorrow? Clinical and Experimental Ophthalmology. 2005;33:339–340. doi: 10.1111/j.1442-9071.2005.01057.x. [DOI] [PubMed] [Google Scholar]
- 14.Foxton RH, Finkelstein A, Vijay S, et al. VEGF-A Is Necessary and Sufficient for Retinal Neuroprotection in Models of Experimental Glaucoma. The American journal of pathology. 2013;182:1379–1390. doi: 10.1016/j.ajpath.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishijima K, Ng Y-S, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. American Journal of Pathology. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature Medicine. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 17.Vincenti V, Cassano C, Rocchi M, Persico G. Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation. 1996;93:1493–1495. doi: 10.1161/01.cir.93.8.1493. [DOI] [PubMed] [Google Scholar]
- 18.Jingjing L, Xue Y, Agarwal N, Roque RS. Human Muller cells express VEGF183, a novel spliced variant of vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1999;40:752–759. [PubMed] [Google Scholar]
- 19.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth-factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 20.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. Faseb Journal. 1999;13:9–22. [PubMed] [Google Scholar]
- 21.Tischer E, Gospodarowicz D, Mitchell R, et al. Vascular endothelial growth-factor - a new member of the platelet-derived growth-factor gene family. Biochemical and Biophysical Research Communications. 1989;165:1198–1206. doi: 10.1016/0006-291x(89)92729-0. [DOI] [PubMed] [Google Scholar]
- 22.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolard J, Wang WY, Bevan HS, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 24.Bates DO, Cui TG, Doughty JM, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 25.Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cellular and Molecular Life Sciences. 2006;63:601–615. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawamura H, Li XJ, Goishi K, et al. Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood. 2008;112:3638–3649. doi: 10.1182/blood-2007-12-125856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin EM, Oltean S, Hua J, et al. WT1 Mutants Reveal SRPK1 to Be a Downstream Angiogenesis Target by Altering VEGF Splicing. Cancer Cell. 2011;20:768–780. doi: 10.1016/j.ccr.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowak DG, Amin EM, Rennel ES, et al. Regulation of Vascular Endothelial Growth Factor (VEGF) Splicing from Pro-angiogenic to Anti-angiogenic Isoforms A novel therapeutic strategy for angiogenesis. Journal of Biological Chemistry. 2010;285:5532–5540. doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowak DG, Woolard J, Amin EM, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. Journal of Cell Science. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aubol BE, Chakrabarti S, Ngo J, et al. Processive phosphorylation of alternative splicing factor/splicing factor 2. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12601–12606. doi: 10.1073/pnas.1635129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kataoka N, Bachorik JL, Dreyfuss G. Transportin-SR, a nuclear import receptor for SR proteins. Journal of Cell Biology. 1999;145 doi: 10.1083/jcb.145.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngo JCK, Chakrabarti S, Ding JH, et al. Interplay between SRPK and Clk/Sty kinases in phosphorylation of the splicing factor ASF/SF2 is regulated by a docking motif in ASF/SF2. Molecular Cell. 2005;20:77–89. doi: 10.1016/j.molcel.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Fukuhara T, Hosoya T, Shimizu S, et al. Utilization of host SR protein kinases and RNA-splicing machinery during viral replication. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11329–11333. doi: 10.1073/pnas.0604616103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith LEH, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Investigative Ophthalmology and Visual Science. 1994;35:101–111. [PubMed] [Google Scholar]
- 35.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatric Research. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham S, Fleck BW, Elton RA, McIntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–1465. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 37.Liu K, Akula JD, Falk C, Hansen RM, Fulton AB. The retinal vasculature and function of the neural retina in a rat model of retinopathy of prematurity. Investigative Ophthalmology and Visual Science. 2006;47 doi: 10.1167/iovs.06-0016. [DOI] [PubMed] [Google Scholar]
- 38.Hartnett ME, Martiniuk D, Byfield G, Geisen P, Zeng G, Bautch VL. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: Relevance to plus disease. Investigative Ophthalmology and Visual Science. 2008;49 doi: 10.1167/iovs.08-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartnett ME, Martiniuk DJ, Saito Y, Geisen P, Peterson LJ, McColm JR. Triamcinolone reduces neovascularization, capillary density and IGF-1 receptor phosphorylation in a model of oxygen-induced retinopathy. Investigative Ophthalmology and Visual Science. 2006;47 doi: 10.1167/iovs.06-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Investigative Ophthalmology and Visual Science. 1993;34 [PubMed] [Google Scholar]
- 41.Geisen P, Peterson LJ, Martiniuk D, Uppal A, Saito Y, Hartnett ME. Neutralizing antibody to VEGF reduces intravitreous neovascularization and may not interfere with ongoing intraretinal vascularization in a rat model of retinopathy of prematurity. Molecular Vision. 2008;14:345–357. [PMC free article] [PubMed] [Google Scholar]
- 42.Young TL, Anthony DC, Pierce E, Foley E, Smith LE. Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J AAPOS. 1997;1 doi: 10.1016/s1091-8531(97)90008-2. [DOI] [PubMed] [Google Scholar]
- 43.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins - identification by morphologic and immunological criteria. Journal of Clinical Investigation. 1973;52 doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF(165)b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Molecular Vision. 2006;12:626–632. [PubMed] [Google Scholar]
- 45.Hartmann JS, Thompson H, Wang H, et al. Expression of vascular endothelial growth factor and pigment epithelial-derived factor in a rat model of retinopathy of prematurity. Molecular Vision. 2011;17 [PMC free article] [PubMed] [Google Scholar]
- 46.McColm J, Geisen P, Hartnett M. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: Relevance to clinical ROP. Molecular Vision. 2004;10:512–520. [PMC free article] [PubMed] [Google Scholar]
- 47.Werdich XQ, Penn JS. Specific involvement of Src family kinase activation in the pathogenesis of retinal neovascularization. Investigative Ophthalmology and Visual Science. 2006;47:5047–5056. doi: 10.1167/iovs.05-1343. [DOI] [PubMed] [Google Scholar]
- 48.Zhao M, Shi X, Liang J, et al. Expression of pro- and anti-angiogenic isoforms of VEGF in the mouse model of oxygen-induced retinopathy. Experimental Eye Research. 2011;93:921–926. doi: 10.1016/j.exer.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Baba T, McLeod DS, Edwards MM, et al. VEGF 165 b in the developing vasculatures of the fetal human eye. Developmental Dynamics. 2012;241:595–607. doi: 10.1002/dvdy.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmermann RC, Hartman T, Kavic S, et al. Vascular endothelial growth factor receptor 2-mediated angiogenesis is essential for gonadotropin-dependent follicle development. Journal of Clinical Investigation. 2003;112 doi: 10.1172/JCI18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bates DO, Jones ROP. The role of vascular endothelial growth factor in wound healing. The international journal of lower extremity wounds. 2003;2:107–120. doi: 10.1177/1534734603256626. [DOI] [PubMed] [Google Scholar]
- 52.Pritchard-Jones RO, Dunn DBA, Qiu Y, et al. Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. British Journal of Cancer. 2007;97:223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das A, Fanslow W, Cerretti D, Warren E, Talarico N, McGuire P. Angiopoietin/Tek interactions regulate MMP-9 expression and retinal neovascularization. Laboratory Investigation. 2003;83:1637–1645. doi: 10.1097/01.lab.0000097189.79233.d8. [DOI] [PubMed] [Google Scholar]
- 54.Manetti M, Guiducci S, Romano E, et al. Overexpression of VEGF165b, an Inhibitory Splice Variant of Vascular Endothelial Growth Factor, Leads to Insufficient Angiogenesis in Patients With Systemic Sclerosis. Circ Res. 2011;109:e14–26. doi: 10.1161/CIRCRESAHA.111.242057. [DOI] [PubMed] [Google Scholar]
- 55.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circulation Research. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 56.Rennel ES, Waine E, Guan H, et al. The endogenous anti-angiogenic VEGF isoform, VEGF(165)b inhibits human tumour growth in mice. British Journal of Cancer. 2008;98:1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hua J, Spee C, Kase S, et al. Recombinant Human VEGF(165)b Inhibits Experimental Choroidal Neovascularization. Investigative Ophthalmology and Visual Science. 2010;51:4282–4288. doi: 10.1167/iovs.09-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magnussen AL, Rennel ES, Hua J, et al. VEGF-A(165)b Is Cytoprotective and Antiangiogenic in the Retina. Investigative Ophthalmology and Visual Science. 2010;51:4273–4281. doi: 10.1167/iovs.09-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature Reviews Drug Discovery. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 60.Qiu Y, Bevan H, Weeraperuma S, et al. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. Faseb J. 2008;22:1104–1112. doi: 10.1096/fj.07-9718com. [DOI] [PubMed] [Google Scholar]
- 61.Penn JS, McCollum GW, Barnett JM, Werdich XQ, Koepke KA, Rajaratnam VS. Angiostatic effect of penetrating ocular injury: Role of pigment epithelium-derived factor. Investigative Ophthalmology and Visual Science. 2006;47:405–414. doi: 10.1167/iovs.05-0673. [DOI] [PubMed] [Google Scholar]
- 62.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Investigative Ophthalmology and Visual Science. 2008;49:1591–1598. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freedman SF, Kylstra JA, Capowski JJ, Realini TD, Rich C, Hunt D. Observer sensitivity to retinal vessel diameter and tortuosity in retinopathy of prematurity: a model system. J Pediatr Ophthalmol Strabismus. 1996;33:248–254. doi: 10.3928/0191-3913-19960701-10. [DOI] [PubMed] [Google Scholar]
- 64.Werdich XQ, McCollum GW, Rajaratnam VS, Penn JS. Variable oxygen and retinal VEGF levels: correlation with incidence and severity of pathology in a rat model of oxygen-induced retinopathy. Experimental Eye Research. 2004;79:623–630. doi: 10.1016/j.exer.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Chen J, Smith LEH. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 66.Connolly BP, Ng EYJ, McNamara JA, Regillo CD, Vander JF, Tasman W. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years - Part 2. Refractive outcome. Ophthalmology. 2002;109:936–941. doi: 10.1016/s0161-6420(01)01015-6. [DOI] [PubMed] [Google Scholar]
- 67.Ng EYJ, Connolly BP, McNamara JA, Regillo CD, Vander JF, Tasman W. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years - Part 1. Visual function and structural outcome. Ophthalmology. 2002;109:928–934. doi: 10.1016/s0161-6420(01)01017-x. [DOI] [PubMed] [Google Scholar]
- 68.Lambert SR, Capone A, Cingle KA, Drack AV. Cataract and phthisis bulbi after laser photoablation for threshold retinopathy of prematurity. American Journal of Ophthalmology. 2000;129:585–591. doi: 10.1016/s0002-9394(99)00475-4. [DOI] [PubMed] [Google Scholar]
- 69.O’Neil JW, Hutchinson AK, Saunders RA, Wilson ME. Acquired cataracts after argon laser photocoagulation for retinopathy of prematurity. J AAPOS. 1998;2:48–51. doi: 10.1016/s1091-8531(98)90110-0. [DOI] [PubMed] [Google Scholar]
- 70.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in-vivo by inhibition of vascular endothelial growth-factor (VEGF) using soluble vegf-receptor chimeric proteins. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato T, Kusaka S, Shimojo H, Fujikado T. Vitreous Levels of Erythropoietin and Vascular Endothelial Growth Factor in Eyes with Retinopathy of Prematurity. Ophthalmology. 2009;116:1599–1603. doi: 10.1016/j.ophtha.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 72.Smith LEH, Shen W, Perruzzi C, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nature Medicine. 1999;5:1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 73.Lee JY, Chae JB, Yang SJ, Yoon YH, Kim J-G. Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2010;248:1257–1262. doi: 10.1007/s00417-010-1375-0. [DOI] [PubMed] [Google Scholar]
- 74.Mintz-Hittner HA, Kennedy KA, Chuang AZ, Grp B-RC. Efficacy of Intravitreal Bevacizumab for Stage 3+ Retinopathy of Prematurity. New England Journal of Medicine. 2011;364:603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moshfeghi DM, Berrocal AM. Retinopathy of Prematurity in the Time of Bevacizumab: Incorporating the BEAT-ROP Results into Clinical Practice. Ophthalmology. 2011;118 doi: 10.1016/j.ophtha.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 76.Gilbert CE, Zin A, Darlow B. Bevacizumab for Retinopathy of Prematurity. New England Journal of Medicine. 2011;364 doi: 10.1056/NEJMc1103460. [DOI] [PubMed] [Google Scholar]
- 77.Raizada S, Al Kandari J, Al Sabti K. Will the BEAT-ROP Study Results Really Beal ROP? Investigative Ophthalmology and Visual Science. 2011;52:9288–9289. doi: 10.1167/iovs.11-8479. [DOI] [PubMed] [Google Scholar]
- 78.Hara A, Wada T, Furuichi K, et al. Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glornerulonephritis. Kidney International. 2006;69:1986–1995. doi: 10.1038/sj.ki.5000439. [DOI] [PubMed] [Google Scholar]
- 79.Pierce EA, Foley ED, Smith LEH. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Archives of Ophthalmology. 1996;114 doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 80.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group Seven-Year Outcomes in Ranibizumab-Treated Patients in ANCHOR, MARINA, and HORIZON: A Multicenter Cohort Study (SEVEN-UP) Ophthalmology. 2013 May 3; doi: 10.1016/j.ophtha.2013.03.046. pii: S0161-6420(13)00331-X; doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 81.Beazley Long N, Hulse RP, Hua J, Jehle T, Dersch R, Lehrling C, Bevan H, Lagreze W, Wynick D, Gittenberger de Groot A, Kehoe P, Harper SJ, Churchill AJ, Bates DO, Donaldson LF. The anti-angiogenic isoform of VEGF, VEGF165b, is neuroprotective in vivo. Am J Pathol. doi: 10.1016/j.ajpath.2013.05.031. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.