Abstract
Individual differences in exploratory behaviour have been shown to be consistent across contexts and suggested to be part of behavioural syndromes in a diversity of species, including fish. Exploration has also been shown to be a key factor in understanding complex ecological processes such as sexual selection and cooperation. Another important question in ecology is why animals breed in colonies. Exploration syndromes, by affecting prospecting behaviour, dispersal and public information use may also contribute to our understanding of coloniality. This study aims at investigating whether an exploration syndrome exists in a colonial fish species, Neolamprologus caudopunctatus. Individuals of this species were subjected to two consecutive tests, a novel environment and a novel object test. Results show that more explorative individuals in a novel environment are also less neophobic in the presence of a novel object, suggesting that the tendency to engage with novelty per se is a consistent trait and part of an exploration syndrome. These results are discussed in light of the contribution of an exploration syndrome to explain colony formation in animals.
Keywords: temperament, personality, novel environment, novel object, consistency
1. Introduction
The study of individual variation in behaviour is gaining increasing interest. Variation in behaviour had been considered for many years as noise around an adaptive mean (Dall et al., 2004). However, many studies in a wide range of taxa, from insects to mammals (reviewed in Sih et al., 2004), suggest that when behaviours are correlated across contexts, particular combinations of trait values are less or more likely to be encountered. Some trait combinations exist with higher frequency (or lower frequency) than others, which could represent ecological constraints, pleiotropic effects of some underlying mechanism, selection for trait covariance, or any combination of the above. In humans, consistent individual differences are referred to as personality types, while in non-human animals terms such as coping styles (Koolhaas et al., 1999), behavioural syndromes (Bell, 2007) or temperaments (Réale et al., 2007) are used. We will use the term behavioural syndrome henceforth when referring to suites of correlated behaviours expressed either within a given behavioural context (e.g., exploration) or between different contexts (e.g., exploration and aggression, Sih et al., 2004). Behavioural types will refer to individuals with a particular combination of behaviour X and Y. A distinction is usually made between two behavioural types: bold (or proactive, active coping or hawk) and shy (reactive, passive coping, or dove) types. Bold individuals are generally characterised by (1) showing higher feeding motivation after transfer to a new environment (Øverli et al., 2007; Martins et al., 2011), (2) being more aggressive during dyadic interactions (Øverli et al., 2004) and (3) being less neophobic, i.e., more readily approaching a novel object and exhibiting higher levels of exploratory behaviour in a novel environment (Frost et al., 2007). Exploration is the tendency to engage with novelty per se (such as in a new environment or with a new object), and is accepted as one of the main components of behavioural syndromes in animals (Mainwaring et al., 2011; Quinn et al., 2011).
Behavioural syndromes, including exploration syndromes, have been shown to play a key role in understanding ecological and evolutionary processes such as sexual selection and mate choice (Schuett et al., 2010, 2011), animal cooperation (Lotem et al., 1999; Bergmüller et al., 2010; Witsenburg et al., 2010) and animal dispersal (Cote et al., 2010, 2011; Quinn et al., 2011). Another important question in evolutionary biology and ecology is why individuals breed in colonies (i.e., densely aggregated territories that contain no resources other than nest sites (Danchin & Wagner, 1997). Behavioural syndromes have been suggested to be part of the underlying mechanisms influencing colony formation and dynamics by mediating individual differences in exploration propensity (including prospecting breeding territories), aggression and public information use (Kurvers et al., 2010). Bold-type individuals may be more explorative (possibly an advantage to find the most appropriate breeding sites) and more aggressive towards predators, while shy-type individuals may be more vigilant, which may enhance the use of public information to select successful breeding sites (Kurvers et al., 2010). It is probably the combination of the different behavioural types that provides the advantage to colonial behaviour. Colony formation has been suggest to be a by-product of individual choices about the presence of conspecific, the prior reproductive success of conspecifics or characteristics of potential partners (habitat and sexual selection) (Danchin & Wagner, 1997). As behavioural syndromes can influence such individual choices it is likely that they play an important role in colony formation.
Coloniality is taxonomically widespread and has been predominately studied in birds, the group from which most hypotheses have been developed. However, our study fish species appears to be a useful model species for testing hypotheses about breeding aggregations (Demus, 2010; Fischer, 2010). Neolamprologus caudopunctatus is a monogamous, colonial cichlid that commonly breeds in dense aggregations in Lake Tanganyika. This species also forms colonies in aquariums, allowing the investigation of the underlying behavioural mechanisms (such as behavioural syndromes) of colony formation under controlled conditions. However, behavioural syndromes have never been reported in this colonial fish species.
The goal of this study is to investigate whether individuals of a colonial fish species show consistent individual differences in exploratory behaviour across contexts. Exploratory behaviour represents one of the best studied and most common behavioural syndromes trait (e.g., Sih et al., 2004; Réale et al., 2007) and, therefore, was selected to investigate behavioural syndromes in N. caudopunctatus. Individuals of N. caudopunctatus were subjected to two consecutive exploration tests: spatial and novel object tests. We show that individual differences in exploration are highly correlated across contexts. This suggests that the tendency to engage with novelty per se is a consistent trait and provides preliminary evidence of an exploration syndrome in N. caudopunctatus.
2. Material and methods
2.1. Experimental animals, housing and feeding
Forty-three experimental animals (20 females and 23 males, sexually mature) were used with a starting standard body length (mass) of 5.37±0.10 cm (4.10 ± 0.33 g) and 5.77 ± 0.38 cm (4.92 ± 0.39 g) for females and males, respectively. Males and females were kept in separate tanks (home tanks, 130 × 65 × 50 cm, length × width × height). Each individual was tagged with a visible implant elastomer (VIE, Northwest Marine Technologies http://www.nmt.us/products/vie/vie.shtml) that allowed a unique code based on body location (3 positions above the anal fin and 3 positions under the dorsal fin) and colour (green, blue, black, brown, pink, violet). The water temperature and salinity were kept at 25.5–26.5°C and 450–550 μS, respectively, by regular controls (Russell RL060C Portable Conductivity/Temperature Meter, Thermo Electron). The tanks included 4 cm substrate on the base of the tank (light coloured aquarium gravel/sand), a heater and an air powered sponge filter. In addition, halves of clay flower pots were provided for shelter.
Fish were fed once a day until apparent satiation with commercial flake food for tropical aquarium fishes and three times per week with frozen food (Artemia sp., Cyclops sp., red mosquito larvae, and Daphnia sp.). A 14-h light:10-h dark photoperiod was maintained with daybreak set at 9:30 h.
2.2. Experimental procedures
2.2.1. Novel environment test
The novel environment test consisted of transferring fish into a new tank where they were kept in isolation for a period of 24 h. Experimental facilities allowed testing 8 fish (netted from the home tank in a random order) simultaneously. Fish were kept visually isolated from each other by black-painted wooden walls. No front walls were used so that the behavioural observations could be carried out by direct observation. Each tank (39.5 × 25 × 25.5 cm, length × width × height, filled with water up to 17 cm) was illuminated by a neon tube light (18 watt, Cool White, Osram). The water temperature was kept at 25.5–26.5°C by a heater and the water salinity at 550-650 μS by regular controls. The test tanks contained a halved clay flower pot, a heater, an air stone and 2 cm of sand on the base of the tank. Flower pots, heaters and air stones were kept in identical positions throughout the tanks. Flower pots were located in one corner of the tank opposite to the location of the heater and air stone.
This test comprised five observation periods each of 15 min duration: 0 h (i.e., immediately after transfer into the novel environment), 1 h 40 min, 3 h 40 min, 6 h and 24 h after transfer. The following behavioural parameters were measured: time spent swimming (s), time spent in the flower pot (s), and the number of dorsal fin spreads (defined as a sudden elevation of the dorsal fin). Fin spreads have been previously been shown to be a sensitive indicator of behavioural responses to environmental changes (Vainikka et al., 2005; Ylönen et al., 2007).
Except for the 0-h observation period, all the other periods started after the researchers stood motionless for 15 min in front of the test tank to minimize the influence of human presence on the measured parameters.
2.2.2. Novel object test
The novel object test was performed 30 min after the 24-h observation period of the novel environment test and lasted 20 min. Immediately after the 24-h observation period of the novel environment test, 4 blood worms were provided to all fish as a way to determine whether food intake was already recovered. All fish consumed the food immediately, and the novel object test began immediately after. The novel object consisted of a red Lego brick (3 × 3 × 2 cm, length × width × height) that was dropped suddenly in the middle of the tank. To make sure that the Lego brick would sink in the water, four little iron pieces were put inside the brick and fixed by a rubber band. A transparent fishing line was tied up around the brick to make it easy to drop it from a distance, reducing visual contact between the fish and researcher. The fishing line with the novel object was placed on top of the tank since the start of the novel environment test and was kept hidden until the start of the novel object test. The front glass walls of the test tanks were divided into three equal vertical zones, which were marked with a text marker outside the tank. The middle zone was named ‘hot zone’, where the novel object was dropped. The other zones were named ‘safe zones’, where the heater and the flower pot were located.
During the 20-min observation period the following parameters were measured: time spent in the hot zone and inside the flower pot (s), number of fin spreads and number of times the fish touched the object.
2.3. Statistical analyses
Statistical analyses were performed using SPSS 16.0 for Windows. Differences in behavioural responses between males and females exposed to the novel environment and object tests were investigated using an independent t -test (Levene’s test for equality of variances confirmed homogeneity of variance). In addition, a mixed model repeated measures analysis was used to examine temporal trends in exploratory behaviour. Relationships between variables were investigated using Spearman rank tests. Data collected during the novel environment were analysed using the first 15 min of observation or the average of the five observation periods. To test for the correlation between the spatial and the novel object exploration contexts, individual traits that best represented each of these behavioural contexts were collapsed into first principal component scores for each axis of interest using Principal Components Analysis (PCA). The correlation matrix was used to check multicollinearity, i.e., to identify variables that did not correlate with any other variable, or correlate very highly (r = 0.9) with one or more other variables. Kaiser–Meyer–Olkin (KMO) test for sample adequacy was greater than 0.5 and the Bartlett’s test of sphericity was significant for all tests. Across-context correlations between these scores were then calculated using Spearman’s rank correlation test. Statistical significance was set a p <0.05.
3. Results
3.1. Behavioural responses within each test
In both tests, males and females did not differ in behavioural responses (Table 1, p > 0.05). Temporal trends in exploratory behaviour in a novel environment are depicted in Figure 1. While the time spent in flower pots did not differ between the observation periods, the number of fin spreads and time spent swimming were significantly reduced at 6 h after transfer to the new environment as compared to the first 15 min of observation (fin spreads, F4,156 = 0.001; pairwise comparison between 15 min and 6 h: p = 0.015) and 1 h 40 min after transfer (time spent swimming, F4,164 = 0.002; pairwise comparison between 1 h 40 min and 6 h: p = 0.006). Time spent swimming was significantly higher at 24 h as compared to 6 h after transfer into the novel environment (p = 0.024).
Table 1.
Means and standard deviations of the different behaviours measured in females and males during the novel environment and the novel object test (N = 43).
No differences were found between females and males (p >0.05).
| Test/behaviour | Females | Males |
|---|---|---|
| Novel environment test | ||
| Time spent swimming (s) | 145.46 ± 115.79 | 139.40 ± 95.48 |
| Time spent in flower pot (s) | 439.10 ± 289.66 | 332.64 ± 258.47 |
| No. of fin spreads | 4.40 ± 5.60 | 5.53 ± 5.83 |
| Novel object test | ||
| Time spent in flower pot (s) | 811.78 ± 396.53 | 747.34 ± 403.24 |
| Time spent in hot zone (s) | 127.84 ± 160.07 | 116.91 ± 141.59 |
| No. of touched objects | 1.21 ± 1.90 | 1.39 ± 1.85 |
| No. of fin spreads | 2.73 ± 3.89 | 5.91 ± 8.10 |
Figure 1.
Temporal trends in exploratory behaviour while in a new environment in males and females of N. caudopunctus. Different letters between different time points indicate statistical significance over time for both males and females at p <0.05.
In the new environment test, individuals spending more time swimming were also the individuals that spent less time in the flower pot (rs =−0.84, p <0.001) and exhibited a higher number of fin spreads throughout the 24-h observation period (Figure 2).
Figure 2.
Relationship between behavioural responses to a novel environment in N. caudopunctus during the first 15 min of observation (open circles and dashed line) and during the 24 h of observation (average of 5 observation points, filled circles and solid line). (A) Relationship between time spent swimming and time spent in flower pot; (B) relationship between time spent swimming and No. of fin spreads.
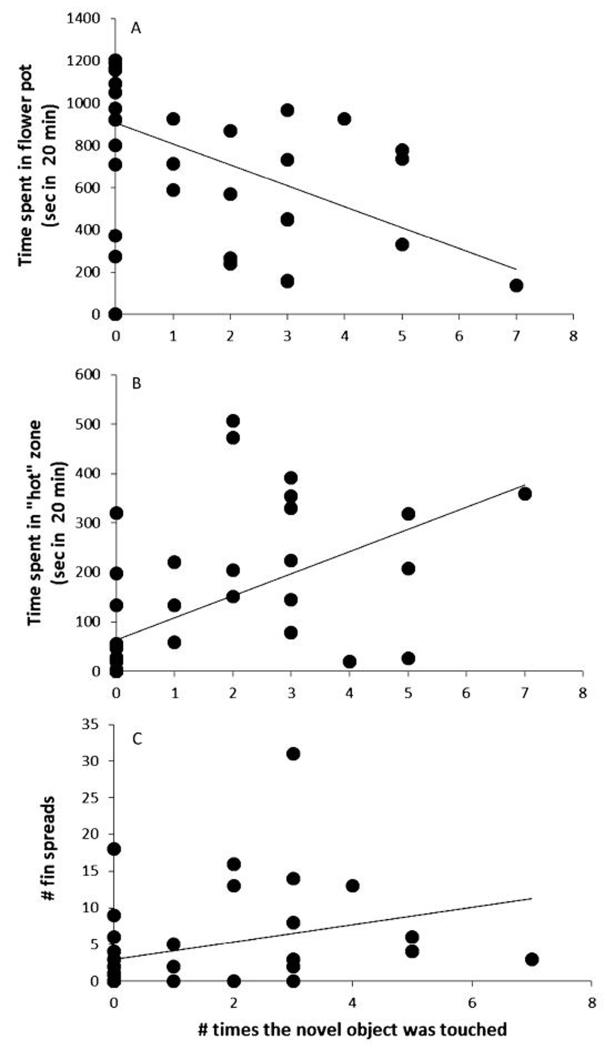
In the novel object test, less neophobic individuals were characterised by touching the object several times (up to 7 times), spending less time hiding in the flower pot and spending more time in the ‘hot zone’. They also exhibited a higher number of fin spreads during the presence of the novel object (Figure 3).
Figure 3.
Relationship between behavioural responses to a novel object in N. caudopunctatus (A, No. of times the novel object was touched and time spent in flower pot; B, No. of times the novel object was touched and time spent in hot zone; C, No. of times the novel object was touched and No. of fin spreads).
3.2. Correlation of exploratory behaviour across contexts: spatial and novel object
Figure 4 depicts the relationship between temporal changes in the time spent swimming after transfer into a novel environment and the PC1 for exploration of a novel object. Individuals that explored a novel object faster were those that (in all observation intervals except at time 0, rs = 0.282, p = 0.07) spent significantly more time swimming in the novel environment (1 h 40 min: rs = 0.685, p <0.001; 3 h 40 min: rs = 0.44, p = 0.004; 6 h: rs = 0.649, p <0.001 and 24 h: rs = 0.617, p <0.001). PCA was used to collapse the individual traits that best represented the novel environment and the novel object contexts. The PCA loadings of within-context behavioural variables used to generate a principal component score (PC1) to assess cross-context correlations are shown in Table 2. The PC1 for the exploration of a novel environment and a novel object were significantly correlated when considering both the 15 min of observation during the novel environment and the averaged five observation intervals (first 15 min: rs = 0.35, p < 0.022; overall 24 h: rs = 0.70, p < 0.001, Figure 5), suggesting that individuals that spent more time exploring a novel environment were also less neophobic.
Figure 4.
Relationship between time spent swimming over time while in a new environment and exploration of a novel object in N. caudopunctatus.
Table 2.
PCA loadings of within-context behavioural variables used to generate a principal component scores (PC1) to assess cross-context correlations in exploration of a novel environment and a novel object in N. caudopunctatus.
| Behavioural context | Behaviours within each context |
Loadings for PC1 (component matrix) |
% Variation explained |
|---|---|---|---|
| Exploration of a novel environment (first 15 min) |
Time swimming | 0.894 | 72.7 |
| Time in pot | −0.848 | ||
| No. of fin spreads | 0.815 | ||
| Exploration of a novel environment (overall 24 h) |
Time swimming | 0.913 | 72.1 |
| Time in pot | −0.891 | ||
| No. of fin spreads | 0.732 | ||
| Exploration of a novel object |
Time in hot zone | 0.888 | 62.4 |
| Time in flower pot | −0.809 | ||
| No. of times object was touched | 0.740 | ||
| No. of fin spreads | 0.710 |
Figure 5.
Relationship between two component behaviours (PC1), exploration in a novel environment (considering the first 15 min observations (open circles and dashed line) and the mean of the 5 observation points collected over 24 h (filled circles and solid line)) and exploration of a novel object in N. caudopunctatus.
4. Discussion
This study provides evidence for the existence of an exploration syndrome in N. caudopunctatus. The individual differences in exploratory behaviour in a new environment persisted when individuals were exposed to a novel object. This suggests that the tendency of individuals to engage in exploration is consistent over time and across contexts in N. caudopunctatus, a requisite for a behavioural trait to be considered part of a behavioural syndrome. Exploration is considered one of the main traits of behavioural syndromes and it has been studied in a diversity of animal species (reviewed in Conrad et al., 2011) including fish.
In this study it was not possible to quantify the distance fish covered or the proportion of the tank substrate area that was explored as no video recording was done. Such information would allow us to better validate the use of swimming activity as a measure of exploration in this species as fish could have spent a considerable amount of time swimming but exploring very little. Although we cannot exclude this possibility our visual observations indicate that swimming activity measured in this study corresponded to an active swimming covering all areas of the tank and not restricted to specific areas.
The approach in this study to investigate exploration syndromes in N. caudopunctatus was to subject each individual first to a novel environment and after to a novel object test. This approach could raise the question whether the behavioural responses observed in the novel object test were simply a carry-over effect of the novel environment test instead of a direct measure of an exploration syndrome. Individuals were subjected to the novel object after they were already recovered from netting and transfer into a novel environment. Therefore, we waited 24 h (and in the meanwhile assessed the exploration behaviour in a novel environment) before adding the new object. Starting immediately with a novel object would only be possible if the novel object would have been introduced in the tank where the groups were being held. As this study aimed at looking at individual responses we decided to undertake the novel object test in individually housed fish and, therefore, a period of acclimatization prior to the novel object test would always be needed. Since there is no knowledge on behavioural syndromes in this species we decided to test individuals without the influence of conspecifics, although further studies should assess the influence of social context on exploration syndromes. In such a design exposing half of the groups, first to a novel object and the other half to a novel environment could minimize any artefacts of the experimental design.
Individual differences in exploratory behaviour in both tests did not differ between females and males. Similar results have been reported in other fish species exposed to novelty tests (Archard & Braithwaite, 2011). One could have expected differences in exploratory behaviour between females and males due to differences in life-history trajectories. The fact that in some fish species, including N. caudopunctatus, females and males exhibit similar levels of exploratory behaviour may reflect similar investments in parental care. Although very little is known about the breeding strategies of N. caudopunctatus there is evidence that this species is monogamous and that both females and males engage in defensive behaviour (aggression) towards neighbours to protect their territories (Ochi & Yanagisawa, 1999; Fischer, 2010).
In addition to the classical behaviours used to describe exploration syndromes such as swimming activity and proximity to a novel object, this study showed for the first time that the number of fin spreads is worthy of further attention. In both the novel environment and the novel object test, the individuals of the bold type exhibited a higher number of fin spreads. Fin spreads have been related to antipredator responses as shown in Ylönen et al. (2007) and Vainikka et al. (2005). However, the extent to which such behaviour reflects appraisal mechanisms (i.e., the way environmental stimuli are evaluated or perceived), remains to be further investigated. Previous studies support the hypothesis that appraisal of stimuli differs between behavioural types (Geerse et al., 2006; Timmermans et al., 2009; Tong, 2010; in fish see, e.g., Martins et al., 2011). Studies in sheep, for instance, showed that the position of the ears is a sign of appraisal and emotional reactivity (Reefmann et al., 2009).
Exploration syndromes may be particularly relevant in species that breed in colonies such as the N. caudopunctatus because individual differences in exploratory behaviour may influence key factors underlying colony formation and dynamics such as prospecting behaviour (i.e., exploratory activity of individuals aimed at collecting information about potential future breeding sites, Calabuig et al., 2010), dispersal and settlement decisions (Hénaux et al., 2007; Cote et al., 2010, 2011; Quinn et al., 2011) and public information use (Kurvers et al., 2010; Wagner & Danchin, 2010). Studies on public information use (i.e., all forms of social and non-social information that are accessible to others, Wagner & Danchin, 2010) for instance highlight a differential use of environmental cues by individuals with divergent behavioural syndromes (Kurvers et al., 2010; Ruiz-Gomez et al., 2011). Kurvers et al. (2010) showed that the way public information is used is dependent on an individual’s behavioural type. These authors show that bold individuals use less public information provided by conspecifics compared to shy individuals.
In summary, we found evidence that individual differences in exploratory behaviour are consistent across different contexts. Individuals that were more likely to explore a novel environment were also more likely to explore a novel object. This suggests that an exploration syndrome is present in the colonial fish species N. caudopunctatus. Further studies are needed to understand the contribution of exploration syndromes to colonial breeding.
Acknowledgements
C.I.M. was supported by a grant provided by the Foundation for Science and Technology, Portugal (SFRH/BPD/42015/2007). This study was funded by the Austrian Science Fund (FWF) grants P20401-B17 and P17468-B06.
References
- Archard GA, Braithwaite VA. Increased exposure to predators increases both exploration and activity level in Brachyrhaphis episcope. J. Fish Biol. 2011;78:593–601. doi: 10.1111/j.1095-8649.2010.02880.x. [DOI] [PubMed] [Google Scholar]
- Bell AM. Animal personalities. Nature. 2007;447:539–540. doi: 10.1038/447539a. [DOI] [PubMed] [Google Scholar]
- Bergmüller R, Schürch R, Hamilton IM. Evolutionary causes and consequences of consistent individual variation in cooperative behaviour. Phil. Trans. Roy. Soc. B. 2010;365:2751–2764. doi: 10.1098/rstb.2010.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabuig G, Ortego J, Aparicio JM, Cordero PJ. Intercolony movements and prospecting behaviour in the colonial lesser kestrel. Anim. Behav. 2010;79:811–817. [Google Scholar]
- Conrad JL, Weinersmith KL, Brodin T, Saltz JB, Sih A. Behavioural syndromes in fishes: a review with implications for ecology and fisheries management. J. Fish Biol. 2011;78:395–435. doi: 10.1111/j.1095-8649.2010.02874.x. [DOI] [PubMed] [Google Scholar]
- Cote J, Fogarty S, Brodin T, Weinersmith K, Sih A. Personality-dependent dispersal in the invasive mosquitofish: group composition matters. Proc. R. Soc. Lond. B Biol. 2011;278:1670–1678. doi: 10.1098/rspb.2010.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A. Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis) Proc. Roy. Soc. Lond. B: Biol. 2010;277:1571–1579. doi: 10.1098/rspb.2009.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall SRX, Houston AI, McNamara JM. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 2004;7:734–739. [Google Scholar]
- Danchin E, Wagner RH. The evolution of coloniality: the emergence of new perspectives. Trends Ecol. Evol. Biol. 1997;12:842–847. doi: 10.1016/s0169-5347(97)01124-5. [DOI] [PubMed] [Google Scholar]
- Demus P. Colony formation and mate choice in Neolamprologus caudopunctatus. University of Vienna; Vienna: 2010. p. 52. Diploma thesis. [Google Scholar]
- Fischer S. Breeding aggregation and anti-predator defense in a monogamous cichlid. University of Vienna; Vienna: 2010. p. 42. Diploma thesis. [Google Scholar]
- Frost AJ, Winrow-Giffen A, Ashley PJ, Sneddon LU. Plasticity in animal personality traits: does prior experience alter the degree of boldness? Proc. Roy. Soc. Lond. B: Biol. 2007;274:333–339. doi: 10.1098/rspb.2006.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerse G-J, van Gurp LCA, Wiegant VM, Stam R. Individual reactivity to the open-field predicts the expression of stress-induced behavioural and somatic pain sensitisation. Behav. Brain Res. 2006;174:112–118. doi: 10.1016/j.bbr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Hénaux V, Bregnballe T, Lebreton J-D. Dispersal and recruitment during population growth in a colonial bird, the great cormorant Phalacrocorax carbo sinensis. J. Avian Biol. 2007;38:44–57. [Google Scholar]
- Koolhaas JM, Korte SM, de Boer SF, van der Vegt BJ, van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Kurvers RHJM, Oers KV, Nolet BA, Jonker RM, van Wieren SE, Prins HHT, Ydenberg RC. Personality predicts the use of social information. Ecol. Lett. 2010;13:829–837. doi: 10.1111/j.1461-0248.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- Lotem A, Fishman MA, Stone L. Evolution of cooperation between individuals. Nature. 1999;400:226–227. doi: 10.1038/22247. [DOI] [PubMed] [Google Scholar]
- Mainwaring MC, Beal J, Hartley IR. Zebra finches are bolder in an asocial, rather than social, context. Behav. Process. 2011;87:171–175. doi: 10.1016/j.beproc.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Martins CIM, Silva PIM, Conceição LEC, Costas B, Höglund E, Øverli Ø, Schrama JW. Linking fearfulness and coping styles in fish. PLoS ONE. 2011;6:e28084. doi: 10.1371/journal.pone.0028084. doi:10.1371/journal.pone.0028084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi H, Yanagisawa Y. Sand-transfer behaviour outside the nest by guarding parents of the Tanganyikan cichlid Neolamprologus caudopunctatus. Ichthyol. Res. 1999;46:419–422. [Google Scholar]
- Øverli Ø, Korzan WJ, Höglund E, Winberg S, Bollig H, Watt M, Forster GL, Barton BA, Øverli E, Renner KJ, Summers CH. Stress coping style predicts aggression and social dominance in rainbow trout. Horm. Behav. 2004;45:235–241. doi: 10.1016/j.yhbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Øverli Ø, Sørensen C, Pulman KG, Pottinger TG, Korzan W, Summers CH, Nilsson GE. Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci. Biobehav. Rev. 2007;31:396–412. doi: 10.1016/j.neubiorev.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Quinn JL, Cole EF, Patrick SC, Sheldon BC. Scale and state dependence of the relationship between personality and dispersal in a great tit population. J. Anim. Ecol. 2011;80:918–928. doi: 10.1111/j.1365-2656.2011.01835.x. [DOI] [PubMed] [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- Reefmann N, Bütikofer Kaszàs F, Wechsler B, Gygax L. Ear and tail postures as indicators of emotional valence in sheep. Appl. Anim. Behav. Sci. 2009;118:199–207. [Google Scholar]
- Ruiz-Gomez MDL, Huntingford FA, Øverli Ø, Thörnqvist P-O, Höglund E. Response to environmental change in rainbow trout selected for divergent stress coping styles. Physiol. Behav. 2011;102:317–322. doi: 10.1016/j.physbeh.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Schuett W, Godin J-GJ, Dall SRX. Do female zebra finches, Taeniopygia guttata, choose their mates based on their ‘personality’? Ethology. 2011;117:908–917. [Google Scholar]
- Schuett W, Tregenza T, Dall SRX. Sexual selection and animal personality. Biol. Rev. 2010;85:217–246. doi: 10.1111/j.1469-185X.2009.00101.x. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 2004;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- Timmermans T, Mechlen IV, Nezlek JB. Individual differences in core affect reactivity. Pers. Indiv. Differ. 2009;47:510–515. [Google Scholar]
- Tong EMW. Personality influences in appraisal-emotion relationships: the role of neuroticism. J. Pers. 2010;78:393–417. doi: 10.1111/j.1467-6494.2010.00620.x. [DOI] [PubMed] [Google Scholar]
- Vainikka A, Jokelainen T, Kortet R, Ylönen H. Predation risk allocation or direct vigilance response in the predator interaction between perch (Perca fluviatilis L.) and pike (Esox lucius L.)? Ecol. Freshw. Fish. 2005;14:225–232. [Google Scholar]
- Wagner RH, Danchin E. A taxonomy of biological information. Oikos. 2010;119:203–209. [Google Scholar]
- Witsenburg F, Schürch R, Heg D. Behavioural types and ecological effects in a natural population of the cooperative cichlid Neolamprologus pulcher. Anim. Behav. 2010;80:757–767. [Google Scholar]
- Ylönen H, Kortet R, Myntti J, Vainikka A. Predator odor recognition and antipredatory response in fish: does the prey know the predator diel rhythm? Acta Oecol. 2007;31:1–7. [Google Scholar]