Abstract
Circulating CD8+ CD28- T cells were found to be expanded more in patients with ankylosing spondylitis than in an age-matched healthy population (41.2 ± 17.7% versus 18.6 ± 7.6%). The level of CD8+CD28- T cells was dependent on the disease status, but was independent of age. Most of the CD8+ CD28- T cells produced perforin after stimulation in vitro, in contrast to their CD8+CD28+ counterparts. From the clinical perspective, the percentage of the cytotoxic CD8+ CD28- T cells reflected a more severe course of disease, as it correlated with distinct movement restrictions, as well as the metrology score summarizing cervical rotation (in sitting position), chin-to-jugulum distance, thoracic Schober, chest expansion, and fingers-to-floor distance (P = 0.032).
Keywords: ankylosing spondylitis, CD8+ T suppressor cells, CD28 molecule, cytotoxicity, interferon-γ
Introduction
Ankylosing spondylitis (AS) is a seronegative spondyloarthropathy that is frequently associated with human leucocyte antigen (HLA)-B27; 90% of AS patients test HLA-B27 positive. HLA-B27 physiologically binds antigenic peptides and thus presents them to CD8+ T lymphocytes. Single HLA-B27 restricted cytotoxic T lymphocytes (CTLs) have been described in AS patients with specificity for arthritogenic bacteria, viral peptides or autoantigens such as peptides derived from the HLA-B27 molecule itself [1,2,3]. CTLs have been reported to maintain the inflammatory process even after the eradication of the microbial pathogen by antimicrobial immune responses [4].
In vivo, MHC-class I restricted CD8+ T cells are clonally expanded and use various αβ T cell-receptors [5,6]. Some of these CD8+ T cell clones represent even more than 50% of all CD8+T cells in single AS patients [7,8,9]. This oligoclonal population which recognizes specific MHC-class I / antigen complexes is characterized by a distinct CD28-CD57+ CD11ahigh phenotype [10]. The presence of this oligoclonal CD28- population in a larger cohort of AS patients has not been examined before. It has been suggested, therefore, that the amount of CD8+ CTLs in a peripheral blood sample can be estimated by determining the percentage of CD8+ CD28- T cells. Furthermore, identification of CD8+ CTLs by the lack of CD28 has provided a helpful tool to investigate this lymphocyte subset in healthy subjects and patients with various diseases [11]. In the healthy population, CD8+ CD28-T cells are not present at birth, and the percentage of CD8+ CD28-T cells increases with age [12,13,14]. This increase of CD8+ CD28-cells, together with reduced circulating naive CD8+ T cells correlates with immunodeficiency during aging [15,16]. As CD8+ CD28-T cells have shortened telomeres, they may have reached a state of replicative senescence [17]. The question as to whether such accelerated senescence is a consequence of viral infections remains open. There may be a role for Epstein–Barr virus (EBV) in the pathogenesis of AS, as different HLA-B27 subtypes are able to present the same EBV peptide [1]. Hyper-responsiveness to EBV has also been observed in AS patients [18]. Irrespective of EBV, CD8+ CD28- T cells are markedly expanded in cytomegalovirus (CMV) seropositive healthy individuals and seropositive patients with rheumatoid arthritis [19,20].
The aim of this study was to quantify the CD8+ CD28-compartment in a cohort of AS patients in comparison with a healthy control group, and to correlate the level of these cells with clinical parameters of disease progression and seropositivity to EBV and CMV.
Materials and methods
Patient population
Patients with definite AS (as defined by the modified New York criteria [21]) were recruited from the Gasteiner Heil-stollen Hospital (Bad Gastein-Böckstein, Austria). A total of 95 patients with definite AS (13.7% female) and 53 healthy volunteers (45.3% female) were enrolled in the study (for patients' characteristics see Supplementary Table 1). In the controls, acute or chronic inflammatory diseases were excluded by physical examination and detailed history. Patients and controls did not differ in age (49.1 ± 11.4 versus 48.0 ± 14.0 years, respectively). Forty-five AS patients did not take any medication, the others took nonsteroidal anti-inflammatory drugs (n = 47), corticosteroids (n = 8), sulfasalazine (n = 2) or azathioprin (n = 1). Patients with multiple drug intakes were considered in all groups separately.
Supplementary Table 1.
Characteristics of patients in the study
| Patient value | Normal range | |
| Duration of AS since | 9.9 ± 9.3 | |
| diagnosis (years) | ||
| Time since onset of | 16.6 ± 12.2 | |
| symptoms (years) | ||
| ESR (mm/hour) | 31.4 ± 20.3 | <15 |
| Leucocytes (G/I) | 9.08 ± 1.92 | 3.8–10.5 |
| Erythrocytes (T/I) | 4.88 ± 0.59 | 3.9–5.7 |
| Platelets (G/I) | 344 ± 99 | 140–345 |
| HAQ-S score (0–3) | 0.89 ± 0.51 | |
| BASFI (0–10) | 4.67 ± 2.05 | |
| BASMI (0–10) | 5.04 ± 2.20 |
AS, ankylosing spondylitis; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; ESR, erythrocyte sedimentation rate; HAQ-S = Health Assessment Questionnaire; G/l, giga/liter; T/l, tara/liter.
Immunostaining and flow cytometry
Surface staining of peripheral blood mononuclear cells was performed using FITC-conjugated anti-CD8, phycoerythrin-conjugated anti-CD28 and peridinin-chlorophyll-protein-conjugated anti-CD3 or anti-CD8 monoclonal antibodies (Becton Dickinson, San Diego, CA). For intracellular detection of perforin or interferon-γ (IFN-γ), stimulated cells were stained for their surface antigens, CD28 and CD8, and with FITC-conjugated anti-perforin, anti-IFN-γ or control immunoglobulin, respectively.
Serological assays
Investigators assessing EBV- and CMV-seropositivity were blinded to the sera of 30 AS patients of different ages, with variable percentages of CD8+CD28- cells. Anti-EBV and anti-CMV IgG antibodies were determined according to the manufacturer's instructions using ELISA kits from Aventis Behring (Vienna, Austria).
Statistical analysis
The Mann–Whitney test, the two-sided Pearson test and regression analysis by ANOVA were performed using the SPSS program, version 10.0 (Chicago, IL, USA). Bonferroni adjustment was performed in case of multiple testing.
Results
Prevalence of CD8+ CD28- T cells in AS patients and controls
Peripheral blood from 95 AS patients and 53 healthy controls was analyzed by flow cytometry for the presence of CD28- cells in the CD3+ CD8+compartment. The percentage of CD3+ CD8+ T cells lacking the co-stimulatory marker CD28 was significantly increased in all AS patients compared to their age-matched healthy controls (Fig. 1). In AS patients, the mean of the percentage of CD8+ CD28- T cells was 41.1 ± 17.7%, compared to 18.6 ± 7.6% in the healthy controls (P < 0.0001). This increase of CD8+ CD28- T cells was paralleled by a decrease of the CD8+ CD28+ subset. When the level of 30% CD8+CD28- T cells was selected as a cut-off value, 68.4% of the AS patients, but only 9.4% of the healthy controls had higher levels than the cut-off value.
Figure 1.

Accumulation of CD8+ CD28- cells in peripheral blood mononuclear cells of 95 patients with ankylosing spondylitis (AS) (●) and 53 age-matched healthy controls (○). Cells were stained with fluorescence-marked antibodies directed against CD3, CD8 and CD28 and flow cytometry was used to count CD3+, CD8+, CD28+ and CD28-cells. The Mann–Whitney test was used to determine the statistical difference between patients and the healthy control group. *P < 0.001.
The finding of elevated levels of CD8+ CD28- T cells was independent of the patients' age (Fig. 2a). Regression lines were calculated as y = 0.2x + 30.6 for AS patients (R2 = 0.019) and y = 17.1 for the healthy controls (R2 = 0.003). In the ANOVA regression model AS disease, but not age, was an independent parameter for increased levels of CD8+ CD28- T cells. In receiver operating curves (ROC), the areas under the curve were calculated to be 0.861 for AS disease and 0.545 for age (Fig. 2b).
Figure 2.
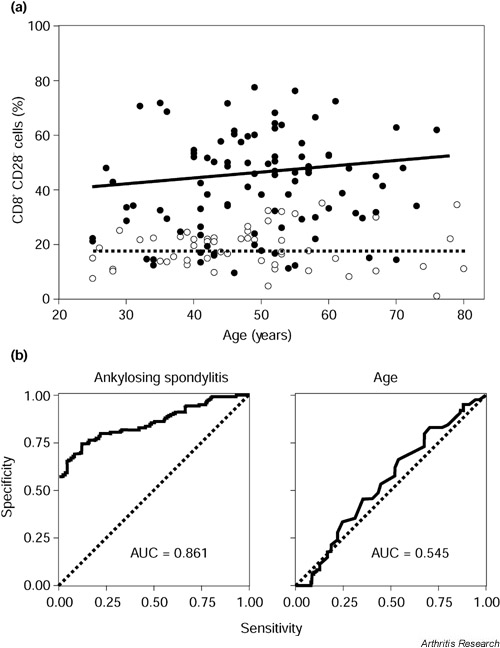
Age dependence of the percentages of CD8+ CD28- T cells in the peripheral blood. (a) The levels of CD8+ CD28- T cells are shown in correlation with age. Regression lines were calculated for patients with ankylosing spondylitis (AS) (●, unbroken line) and healthy controls (○, dashed line). (b) A regression model was applied to determine the possible effects of AS disease and age on the level of peripheral CD8+ CD28- T cells. Receiver operating curves (ROC) and the area under the curve (AUC) were determined for both independent parameters.
Perforin and interferon-γ production by CD8+ CD28- T cells
For functional characterization of CD8+ CD28- T cells in AS patients, flow cytometric single cell analysis was used, and the intracellular accumulation of perforin and IFN-γ were compared between CD28+ T cells and CD28-CD8+ T cells. Production of perforin was more frequent in CD28- CD8+ T cells (83.9 ± 17.3% versus 1.6 ± 1.2% stained with IgG control antibodies) than in their CD28+ counterparts (21.3 ± 12.0% versus 3.8 ± 1.8% stained with IgG control antibodies) (P < 0.001) (Fig. 3a). Production of IFN-γ was more frequent in CD28- CD8+ T cells (61.4 ± 10.1% versus 0.35 ± 0.0% stained with IgG control antibodies) than in their CD28+ counterparts (30.9 ± 3.2% versus 0.6 ± 0.2% stained with IgG control antibodies) (P < 0.001) (Fig. 3b).
Figure 3.

Intracellular production of perforin and interferon-γ in CD8+ CD28- and CD28+ T cells. Peripheral blood mononuclear cells of patients with ankylosing spondylitis were stimulated with phorbol 12-myristate 13-acetate and ionomycin in the presence of brefeldin A. Cells were stained with fluorescence-marked monoclonal antibodies (mAbs) directed against CD8, CD28 and either perforin or interferon-γ and counted by flow cytometry. Whiskers boxblots show the results of 5 independent experiments for perforin (a) and 6 experiments for interferon-γ (b) with 50 % of cases within the boxes and 80 % between the endpoints of the whiskers (lines). The Mann–Whitney test was used to determine the statistical difference between the percentage of perforin-positive or interferon-γ-positive CD28+ and CD28- cells out of the CD8+ T cell compartment (each with P < 0.001).
The dependencies of CD8+ CD28- T cells on clinical variables
Using the two-sided Pearson test and Bonferroni adjustment because of multiple testing, a trend of a correlation was obtained between the percentage of CD8+ CD28- T cells and cervical rotation (in sitting position) (P = 0.165) (see Supplementary Table 2). Based on these results and clinical experience, a metrology index was calculated summarizing cervical rotation (in sitting position), chin-to-jugulum distance, thoracic Schober, chest expansion, and fingers-to-floor distance. There was a significant correlation of this index with the percentage of CD8+ CD28- T cells, but not for the scores for the Health Assessment Questionnaire (HAQ-S), Bath Ankylosing Spondylitis Functional Index (BASFI) or Bath Ankylosing Spondylitis Metrology Index (BASMI) (P = 0.032 for the metrology index, after Bonferroni adjustment for multiple testing, Fig. 4) [22,23,24]. No correlation was found between the percentage of CD8+ CD28- T cells and the erythrocyte sedimentation rate, blood counts, disease duration or time since onset of symptoms.
Supplementary Table 2.
Percentages of CD8+ CD28- T cells in patients with ankylosing spondylitis, classified according to the grade of their movement restriction
| CD8+ CD28- T cells in patients (%) | |||
| Minor | Mean | Major | |
| Cervical rotation (sitting) | 34.2 ± 17.3 | 45.0 ± 18.1 | 44.1 ± 16.3 |
| Cervical rotation (lying) | 36.9 ± 18.1 | 48.8 ± 15.3 | 44.7 ± 15.6 |
| Tragus to wall | 44.1 ± 17.2 | 41.8 ± 18.0 | 44.4 ± 16.5 |
| Chin to jugulum | 34.7 ± 18.2 | 43.5 ± 17.5 | 45.1 ± 16.3 |
| Head to wall | 39.8 ± 16.8 | 44.6 ± 18.6 | 40.5 ± 18.6 |
| Chest expansion | 35.5 ± 15.0 | 43.7 ± 18.9 | 43.2 ± 17.6 |
| Thoracic Schober | 39.3 ± 16.6 | 41.4 ± 18.7 | 42.8 ± 17.9 |
| Modified Schober | 41.0 ± 18.3 | 45.2 ± 15.3 | 43.7 ± 17.7 |
| Lumbar side flexion | 39.3 ± 16.3 | 45.5 ± 18.1 | 44.6 ± 16.9 |
| Fingers to floor | 36.8 ± 16.4 | 42.8 ± 18.8 | 43.9 ± 17.8 |
| Intermalleolar distance | 39.8 ± 17.4 | 44.5 ± 16.9 | 45.2 ± 17.2 |
Patients were grouped in those with minor restrictions (head-to-wall distance <5, chin-to jugulum distance <3, cervical rotation in sitting position >70, thoracic Schober >32, chest expansion >6 and fingers-to-floor distance <20), those with mean restrictions, and those with major restrictions (head-to-wall distance >20, chin-to-jugulum distance >6, cervical rotation in sitting position <15, thoracic Schober <30.5, chest expansion <2 and fingers-to-floor distance >50). Correlations between the level of CD8+ CD28- T cells and the extent of movement restrictions were not significant (using Bonferroni adjustment after Pearson test).
Figure 4.
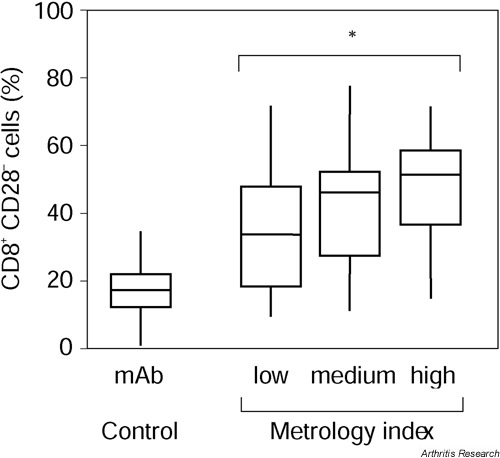
Disease status of ankylosing spondylitis (AS) and percentages of CD8+ CD28- T cells. AS patients were subdivided into groups with low, medium and high grades of movement restriction by a metrology index which includes cervical rotation, chin-to-jugulum distance, thoracic Schober, chest expansion, and the fingers-to-floor distance. Whiskers boxblots show 50% of cases within the boxes and 80% between the endpoints of the whiskers (lines). The two-sided Pearson test (with subsequent Bonferroni adjustment due to multiple testing) was used to assess statistical significance. *P = 0.032.
Lack of association with serological EBV- and CMV-seropositivity
Of the AS patients tested, 96.7% were positive for EBV-IgG and 60% were positive for CMV-IgG. Levels of CD8+ CD28- T cells were independent of seropositivity of CMV and EBV. Levels of CD8+ CD28-T cells did not differ between patients who were positive or negative for CMV-IgG (39.4 ± 23.1% and 38.6 ± 21.1%, respectively). The CMV-IgG negative AS patients showed levels of CD8+ CD28- T cells ranging up to 77.32%. The only patient who was seronegative for EBV was seropositive for CMV, and had 60.0% CD28- T cells in the CD8+compartment. Assuming a cut-off level of 30% between normal and pathological percentages of CD8+ CD28- T cells, we did not find a difference in either CMV-specific or EBV-specific IgG titers between patients with low and high levels of CD8+ CD28- T cells (see Supplementary Fig. 1).
Supplementary Figure 1.
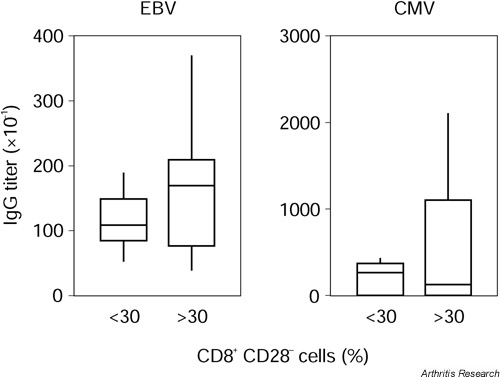
Epstein–Barr virus (EBV)-IgG and cytomegalovirus (CMV)-IgG titers in patients with ankylosing spondylitis (AS). AS patients were grouped according to the carrier status of circulating CD8+ CD28- T cells. Carrier status was defined as expressing >30% CD8+ CD28- T cells. IgG titers were determined by ELISA. No differences were seen.
Discussion
Circulating CD8+ CD28- T cells (characterized by a lack of the CD28 co-stimulatory molecule on the cell surface and production of perforin) are increased not only in various infectious diseases but also in patients with autoimmune diseases like AS. The fact that the accumulation of CD8+CD28- T cells correlated with anatomical restrictions of the joints routinely measured in AS patients further underlines the pathogenic relevance in AS. This metrology index, as well as the established BASMI score, quantify anatomical restrictions of joints, whereas the HAQ-S and the BASFI scores include more complex restrictions of daily life activities. We believe that a specific metrology index might be the best tool to describe disease status as an integral function of disease duration and activity. This could explain why the percentage of CD8+ CD28- T cells in AS patients reflects the anatomical restrictions and status of disease, but not functional and life quality restrictions. In our study, the levels of CD8+ CD28- T cells were not correlated with the age of the subjects, whereas others had observed an accumulation of CD8+ CD28-T cells in the elderly, including centenarians [12,13,14]. Clonal populations known to be part of the CD28- compartment have been discussed earlier as a T cell equivalent of benign monoclonal gammopathy [25]. Our results can be explained by the fact that 83% of our controls were younger than 60 years and all of them were preselected for the absence of acute or chronic inflammatory disease.
In view of the different functions of CD8+ CD28- T cells, it is difficult to propose a specific role for these cells in AS. CD8+CD28- T cells have been reported to be effector cells, producing perforin, granzyme B, tumour necrosis factor-α and IFN-γ [10,26,27,28,29], although others have reported only low levels of tumour necrosis factor-α and IFN-γ in unselected CD8+ T cells from AS patients [30]. Our data confirms a possible role of IFN-γ in the pathogenesis of AS. The CD8+ CD28- T cells contain clonally expanded CTLs specific for human CMV or with unknown antigen-specificity [31], and act as suppressor cells on antigen-presenting cells, inhibiting their ability to elicit T helper cell activation and proliferation [32]. The peripheral increment of CTLs may develop as a consequence of an oligoclonal outgrowth of CD8+ CD28- T cells during the course of the disease [33], or by CD8+ CD28+ T cells preferentially migrating to peripheral sites [14].
The role of prior EBV- or CMV-infection in AS and peripheral accumulation of CD8+ CD28- T cells is still unclear. Clonally expanded CD8+ T cells reactive against these viruses are frequently present in chronic inflammatory lesions [34]. In rheumatoid arthritis patients, CMV-seropositivity has been directly correlated with increased levels of CD8+ CD28- T cells [20]. It may be argued, however, that patients with more severe rheumatoid arthritis disease and higher levels of CD8+CD28- T cells have undergone more consistent immunosuppressive therapy and thus have an iatrogenic risk for CMV and EBV infections. Most of our AS patients, however, cannot be considered to be immunosuppressed, except for the fact that their immune system is disturbed by the underlying disease. As a considerable number of AS patients tested seronegative for CMV, but showed an accumulation of CD8+ CD28- T cells in the peripheral blood, the two phenomena appear to be independent of each other in AS patients.
Conclusions
Cytotoxic CD28- T cells accumulate in the circulating CD8+ T cell population of AS patients and are associated with more severe anatomical joint restrictions. In AS patients, occurrence of CD8+ CD28- T cells is independent of prior CMV infection.
Abbreviations
AS = ankylosing spondylitis; BASFI = Bath Ankylosing Spondylitis Functional Index; BASMI = Bath Ankylosing Spondylitis Metrology Index; CMV = cytomegalovirus; CTL = cytotoxic T lymphocyte; EBV = Epstein Barr virus; HAQ-S = Health Assessment Questionnaire; HLA = human leucocyte antigen; IFN-γ = interferon-γ.
Supplementary material
Materials and methods
Patient population
Patients underwent routine evaluation by experienced rheumatologists. Clinical assessment included time since onset of symptoms, duration of disease, measurements of typical ankylosing spondylitis (AS)-specific parameters and scores including the Health Assessment Questionnaires (HAQ-S, n = 55), the Bath Ankylosing Spondylitis Metrology Index (BASMI, n = 55) and the Bath Ankylosing Spondylitis Functional Index (BASFI, n = 75) (Supplementary Table 1). All patients were screened for blood counts and erythrocyte sedimentation rate, and after informed consent, 15 ml of peripheral blood was obtained for flow cytometric studies and serology.
Immunostaining and flow cytometry
Peripheral blood mononuclear cells were isolated by Ficoll density gradient centrifugation. Surface staining of peripheral blood mononuclear cells was performed using FITC-conjugated anti-CD8, phycoerythrin-conjugated anti-CD28 and peridinin chlorophyll protein-conjugated anti-CD3 or anti-CD8 monoclonal antibodies, respectively (Becton Dickinson, San Diego, CA). Cells were simultaneously stained for 30 minutes at 4°C and, after washing with phosphate-buffered saline, fixed with 4% paraformaldehyde in phosphate-buffered saline for 60 minutes. For intracellular detection of perforin or IFN-γ, cells were stimulated with 25 ng/ml phorbol 12-myristate 13-acetate and 1 μg/ml ionomycin in the presence of 10 μg/ml brefeldin A for four hours (Sigma, St. Louis, MO). Cells were then stained for their surface antigens CD28 and CD8 and, after permeabilization, with FITC-conjugated anti-perforin, anti-IFN-γ or control immunoglobulin, respectively. Fixed cells were analyzed on a FACS-Calibur flow cytometer (Becton Dickinson). Data were analyzed using WinMDI software (Joseph Trotter, Scripps Research Institute, La Jolla, CA).
Statistical analysis
The Mann–Whitney test was used to compare the percentages of CD8+CD28- T cells between AS patients and healthy controls, the percentages of perforin or IFN-γ positive T cells between the CD8+ CD28+ and the CD8+ CD28- compartments, and the EBV- and CMV- IgG titers of AS patients with less than and more than 30% of CD8+ CD28- T cells. The Pearson test (two-sided) was used to examine the correlations between clinical measurements or scores and the percentage of CD8+ CD28- T cells. Regression analysis by ANOVA testing and ROC curves were used to evaluate the dependence of the level of CD8+ CD28- T cells on AS disease and age. Bonferroni adjustment was performed in case of multiple testing. Statistical analysis was performed using the SPSS program, version 10.0 (Chicago, IL, USA).
Acknowledgments
Acknowledgements
The study was supported by the Verein zur Förderung der Hämatologie, Onkologie und Immunologie (Innsbruck, Austria) and by the Gasteiner Heilstollen GesmbH (Badgastein, Austria). We want to thank Ms. Rajam Csordas (PhD, Oxon) for her help in preparing the manuscript.
References
- Brooks JM, Murray RJ, Thomas WA, Kurilla MG, Rickinson AB. Different HLA-B27 subtypes present the same immunodominant Epstein–Barr virus peptide. J Exp Med. 1993;178:879–887. doi: 10.1084/jem.178.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann E, Yu DTY, Meyer zum Büschenfelde KH, Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993;342:646–650. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- Fiorillo MT, Maragno M, Butler R, Dupuis ML, Sorrentino R. CD8(+) T-cell autoreactivity to an HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J Clin Invest. 2000;106:47–53. doi: 10.1172/JCI9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann E, Fleischer B, Meyer zum Büschenfelde KH. Bacteria-specific cytotoxic CD8+ T cells: a missing link in the pathogenesis of the HLA-B27-associated spondylarthropathies. Ann Medicine. 1994;26:365–369. doi: 10.3109/07853899409148352. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC-class I restricted CD8+ CD28- T cells. Intl Immunol. 1998;10:775–783. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- Pennesi G, Liu Z, Ciubotariu R, Jiang S, Colovai A, Cortesini R, Suciu-Foca N, Harris P. TCR repertoire of suppressor CD8+ CD28- T cell populations. Human Immunol. 1999;60:291–304. doi: 10.1016/s0198-8859(98)00134-7. [DOI] [PubMed] [Google Scholar]
- Posnett DN, Gottlieb A, Bussel JB, Friedman SM, Chiorazzi N, Li Y, Szabo P, Farid NR, Robinson MA. T cell antigen receptors in autoimmunity. J Immunol. 1988;141:1963–1969. [PubMed] [Google Scholar]
- Grunewald J, Jeddi-Tehrani M, Dersimonian H, Andersson R, Wigzell H. A persistent expansion in the peripheral blood of a normal adult male: a new clinical entity? Clin Exp Immunol. 1992;89:279–284. doi: 10.1111/j.1365-2249.1992.tb06945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JE, Ricalton NS, Meyer AC, West SG, Kaplan H, Berendt C, Kotzin BL. Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol. 1995;154:3538–3547. [PubMed] [Google Scholar]
- Azuma M, Phillips JH, Lanier LL. CD28- T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- Speiser DE, Valmori D, Rimoldi D, Pittet ML, Lienard D, Cerundolo V, Mac Donald HR, Cerottini J-C, Romero P. CD28 – negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur J Immunol. 1999;29:1990–1999. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovivi R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+ CD28- T-cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino J, Martinez-Gonzalez MA, Rubio M, Inoges S, Sanchez-Ibarrola A, Subira ML. Progressive decrease of CD8high+ CD28+ CD57- cells with ageing. Clin Exp Immunol. 1998;112:48–51. doi: 10.1046/j.1365-2249.1998.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nociari MM, Telford W, Russo C. Postthymic development of CD28- CD8+ T cells subset: Age-associated expansion and shift from memory to naive phenotype. J Immunol. 1999;162:3327–3335. [PubMed] [Google Scholar]
- Bandres E, Merino J, Vazquez B, Inoges S, Moreno C, Subira ML, Sanchez-Ibarrola A. The increase of IFN-gamma production through aging correlates with the expanded CD8(+high) CD28(-) CD57(+) subpopulation. Clin Immunol. 2000;96:230–235. doi: 10.1006/clim.2000.4894. [DOI] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, Casti A, Franceschi C, Passeri M, Sansoni P. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28- CD8+ T cells imply a replicative history that is distinct from their CD28+ CD8+ counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- Winrow VR, Perry JD. Hyper-responsiveness to EBV in ankylosing spondylitis. Ann Rheum Dis. 1987;46:493–494. doi: 10.1136/ard.46.6.493-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratama JW, Naipal AM, Oosterveer MA, Stijnen T, Kluin-Nele-mans HC, Ginsel LA, den Ottolander GJ, Hekker AC, D'Amaro J, van der Giessen M, Tanke HJ. Effects of herpes virus carrier status on peripheral T lymphocyte subsets. Blood. 1987;70:516–523. [PubMed] [Google Scholar]
- Hooper M, Kallas EG, Coffin D, Campbell D, Evans TG, Looney RJ. Cytomegalovirus seropositivity is associated with the expansion of CD4+ CD28- and CD8+ CD28- T cells in rheumatoid arthritis. J Rheumatol. 1999;26:1452–1457. [PubMed] [Google Scholar]
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- Abbott CA, Helliwell PS, Chamberlain MA. Functional assessment in ankylosing spondylitis: evaluation of a new self-administered questionnaire and correlation with anthropometric variables. Br J Rheumatol. 1994;33:1060–1066. doi: 10.1093/rheumatology/33.11.1060. [DOI] [PubMed] [Google Scholar]
- Jenkinson TR, Mallorie PA, Whitelock HC, Gail Kennedy L, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol. 1994;21:1694–1698. [PubMed] [Google Scholar]
- Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, Jenkinson T. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21:2281–2285. [PubMed] [Google Scholar]
- Posnett DN, Sinha R, Kabak S, Russo C. Clonal population of T cells in normal elderly humans: the T cell equivalent to "benign monoclonal gammopathy". J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthou C, Legros-Maida S, Soulié A, Wargnier A, Guillet J, Rabian C, Gluckman E, Sasportes M. Cord blood T lymphocytes lack constitutive perforin expression in contrast to adult peripheral blood T lymphocytes. Blood. 1995;85:1540–1546. [PubMed] [Google Scholar]
- Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnett DN, Edinger JW, Manavalan JS, Irwin C, Marodon G. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+ CD28- cytotoxic effector clones. Int Immunol. 1999;11:229–241. doi: 10.1093/intimm/11.2.229. [DOI] [PubMed] [Google Scholar]
- Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8+ T cells is induced by IL-2 receptor γ chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12:1005–1013. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- Rudwaleit M, Siegert S, Yin Z, Eick J, Thiel A, Radbruch A, Sieper J, Braun J. Low T cell production of TNFα and IFNγ in ankylosing spondylitis: its relation to HLA-B27 and influence of the TNF-308 gene polymorphism. Ann Rheum Dis. 2001;60:36–42. doi: 10.1136/ard.60.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weekes MP, Wills MR, Mynard K, Hicks R, Sissons JG, Carmichael AJ. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28- CD8+ T-cell population. Immunology. 1999;98:443–449. doi: 10.1046/j.1365-2567.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu Z, Jiang S, Cortesini R, Lederman S, Suciu-Foca N. T suppressor lymphocytes inhibit NF-κB-mediated transcription of CD86 gene in APC. J Immunol. 1999;163:6386–6392. [PubMed] [Google Scholar]
- Duchmann R, Lambert C, May E, Hohler T, Marker-Hermann E. CD4+ and CD8+ clonal T cell expansions indicate a role of antigens in ankylosing spondylitis; a study in HLA-B27+ monozygotic twins. Clin Exp Immunol. 2001;123:315–322. doi: 10.1046/j.1365-2249.2001.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotet E, Peyrat MA, Saulquin X, Retiere C, Couedel C, Davodeau F, Dulphy N, Toubert A, Bignon JD, Lim A, Vie H, Hallet MM, Liblau R, Weber M, Berthelot JM, Houssaint E, Bonneville M. Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur J Immunol. 1999;29:973–985. doi: 10.1002/(SICI)1521-4141(199903)29:03<973::AID-IMMU973>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]


