Abstract
Background
Non‐prescription (over‐the‐counter, or OTC) analgesics (painkillers) are used frequently. They are available in various brands, package sizes, formulations, and dose. They can be used for a range of different types of pain, but this overview reports on how well they work for acute pain (pain of short duration, usually with rapid onset). Thirty‐nine Cochrane reviews of randomised trials have examined the analgesic efficacy of individual drug interventions in acute postoperative pain.
Objectives
To examine published Cochrane reviews for information about the efficacy of pain medicines available without prescription using data from acute postoperative pain.
Methods
We identified OTC analgesics available in the UK, Australia, Canada, and the USA by examining online pharmacy websites. We also included some analgesics (diclofenac potassium, dexketoprofen, dipyrone) of importance in parts of the world, but not currently available in these jurisdictions.
We identified systematic reviews by searching the Cochrane Database of Systematic Reviews (CDSR) on The Cochrane Library through a simple search strategy. All reviews were overseen by a single review group, had a standard title, and had as their primary outcome numbers of participants with at least 50% pain relief over four to six hours compared with placebo. From individual reviews we extracted the number needed to treat for an additional beneficial outcome (NNT) for this outcome for each drug/dose combination, and also calculated the success rate to achieve at least 50% of maximum pain relief. We also examined the number of participants experiencing any adverse event, and whether the incidence was different from placebo.
Main results
We found information on 21 different OTC analgesic drugs, doses, and formulations, using information from 10 Cochrane reviews, supplemented by information from one non‐Cochrane review with additional information on ibuprofen formulations (high quality evidence). The lowest (best) NNT values were for combinations of ibuprofen plus paracetamol, with NNT values below 2. Analgesics with values close to 2 included fast acting formulations of ibuprofen 200 mg and 400 mg, ibuprofen 200 mg plus caffeine 100 mg, and diclofenac potassium 50 mg. Combinations of ibuprofen plus paracetamol had success rates of almost 70%, with dipyrone 500 mg, fast acting ibuprofen formulations 200 mg and 400 mg, ibuprofen 200 mg plus caffeine 100 mg, and diclofenac potassium 50 mg having success rates above 50%. Paracetamol and aspirin at various doses had NNT values of 3 or above, and success rates of 11% to 43%. We found no information on many of the commonly available low dose codeine combinations.
The proportion of participants experiencing an adverse event were generally not different from placebo, except for aspirin 1000 mg and (barely) ibuprofen 200 mg plus caffeine 100 mg. For ibuprofen plus paracetamol, adverse event rates were lower than with placebo.
Authors' conclusions
There is a body of reliable evidence about the efficacy of some of the most commonly available drugs and doses widely available without prescription. The postoperative pain model is predominantly pain after third molar extraction, which is used as the industry model for everyday pain. The proportion of people with acute pain who get good pain relief with any of them ranges from around 70% at best to less than 20% at worst; low doses of some drugs in fast acting formulations were among the best. Adverse events were generally no different from placebo. Consumers can make an informed choice based on this knowledge, together with availability and price. Headache and migraine were not included in this overview.
Plain language summary
Oral painkillers available without prescription for acute pain
Acute pain is often felt soon after injury, and is of short duration. Most people who have surgery have moderate or severe pain afterwards. Painkillers (analgesics) are tested in people with pain, often following the removal of wisdom teeth. Study participants have to have at least moderate pain levels and the pain is usually treated with painkillers taken by mouth. This overview is useful mainly for acute pain lasting only a few days or weeks, and not for chronic pain lasting for many months. For this overview we have not included information from reviews on migraine, tension headache, or period pain.
In May 2015 we looked on pharmacy websites for the range of painkillers available in the UK that could be taken by mouth, and available without a doctor's prescription. We also looked at websites in Australia, Canada, and the USA. We then looked for Cochrane reviews reporting about how well these painkillers worked, and any side effects. We used high quality evidence from 10 Cochrane reviews supplemented with information from one non‐Cochrane analysis.
The outcome we used for successful treatment was that of people with moderate or severe pain having at least 50% of the maximum possible pain relief, over a period of about six hours. This is an outcome that people with acute and chronic pain, and headache, think is useful to them.
Combinations of ibuprofen plus paracetamol worked in 7 out of 10 (70%) people, and fast acting ibuprofen formulations 200 mg and 400 mg, ibuprofen 200 mg plus caffeine 100 mg, and diclofenac potassium 50 mg worked in over 5 out of 10 (50%) people. Dipyrone 500 mg, which is available OTC in many parts of the world, also worked in about 5 out of 10 people. Paracetamol plus aspirin at various doses worked in 1 out of 10 (11%) to 4 out of 10 (43%) people. An important finding was that low doses of some medicines in fast acting formulations were among the best. We could find no information on many of the commonly available combinations containing low doses of codeine. Taking painkillers with food may reduce how well they work.
There were fewer side effects for people taking ibuprofen plus paracetamol than those taking placebo (a pretend treatment). The results for side effects may be different if the painkillers are taken for more than a few days.
Background
Description of the condition
Acute pain is experienced from time to time by almost everyone and is usually defined as short‐term pain of less than 12 weeks' duration. It may be due to tissue damage or nerve injury, or both, as a result of injury (eg sprains and strains, falls), surgery, or temporary or intermittent 'malfunction' of a body system (eg dysmenorrhoea (period pain), constipation), or to some form of headache (eg tension headache). It is frequently a manifestation of inflammation and sometimes swelling, especially in joints and muscles. By definition, it is not expected to continue indefinitely, even if not treated, but it can have a significant impact on ability to function normally.
Studies to determine the efficacy of analgesics in acute painful conditions are most commonly carried out in people who are experiencing postoperative pain. The methods have been standardised over many years and the study design has proved to be robust (McQuay 2012). Trials have to be randomised and double‐blind. Typically, in the first few hours or days after an operation, people develop pain that is moderate to severe in intensity, and will then be given the test analgesic (the intervention) or a placebo. Pain is measured using standard pain intensity scales immediately before the intervention and then afterwards using pain intensity and pain relief scales, usually over the following six hours. Pain relief of half the maximum possible pain relief or better (at least 50% pain relief) has become the standard outcome to measure successful treatment. For people given rescue medication it is usual for no additional pain measurements to be made, and for all subsequent measures to be recorded as initial pain intensity or baseline (zero) pain relief (baseline observation carried forward). This process ensures that analgesia from the rescue medication is not wrongly ascribed to the test intervention.
Non‐prescription analgesics for migraine headache will be covered in a different Cochrane overview.
Description of the interventions
The aim is to try to assess the relative efficacy of drugs such as aspirin, paracetamol (acetaminophen), ibuprofen, diclofenac, naproxen, and other drugs both alone, and in combinations with each other, with weak opioids such as codeine, or with caffeine.
There is a bewildering variety of analgesics available without prescription in various parts of the world. They include paracetamol, nonsteroidal anti‐inflammatory drugs (NSAIDs) (such as aspirin and ibuprofen), and opioids (usually codeine), as well as numerous 'complementary therapies' and 'herbal' products. They may be available in different formulations, such as standard tablets, fast acting tablets, effervescent powders, or liquids, and are frequently combined with each other, or with other products such as caffeine. Packaging and branding can make it difficult to identify similar products or to compare them.
Many of these analgesics can be bought from open shelves in pharmacies, supermarkets, and convenience stores, without any consultation with a doctor or checking by a pharmacist, while some are not displayed on open shelves and require some form of authorisation from a pharmacist before they can be sold. Regulations regarding the availability of these products vary between countries.
There are a number of websites that provide information on analgesics available without prescription. In the UK, this is the Proprietary Association of Great Britain (www.pagb.co.uk). For Europe, a searchable database is available from the European Self‐Medication Industry (www.aesgp.be), and for countries outside Europe there is the website of the World Self‐Medication Industry (www.wsmi.org).
How the intervention might work
NSAIDs reversibly inhibit the enzyme cyclo‐oxygenase (prostaglandin endoperoxide synthase or COX), now recognised to consist of two isoforms, COX‐1 and COX‐2, mediating production of prostaglandins and thromboxane A2 (FitzGerald 2001). Prostaglandins mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in inflammatory and nociceptive (pain) processes. However, relatively little is known about the mechanism of action of this class of compounds aside from their ability to inhibit cyclo‐oxygenase‐dependent prostanoid formation (Hawkey 1999). Aspirin is a special case, in that it irreversibly blocks COX‐1. It also has good antipyretic (fever reducing) properties.
Despite similarities to NSAIDs, the mode of action of paracetamol has been uncertain, but it is now generally accepted that it inhibits COX‐1 and COX‐2 through metabolism by the peroxidase function of these isoenzymes (Graham 2013). Paracetamol has previously been shown to have no significant effects on COX‐1 or COX‐2 (Schwab 2003), but has subsequently been considered as a selective COX‐2 inhibitor (Hinz 2008). Paracetamol metabolism is considerably influenced by genetic make‐up, with large intersubject and ethnic differences in susceptibility to toxicity and efficacy (Zhao 2011).
Codeine is an opioid that is metabolised in the liver to the active compounds morphine and morphine‐6‐glucuronide, but is subject to significant genetic influence (Crews 2014). Opioids bind to specific receptors in the central nervous system (CNS), causing reduced pain perception and reaction to pain, and increased pain tolerance. Binding to receptors elsewhere in the body (primarily the gastrointestinal tract) may cause nausea, vomiting, and constipation, and binding to receptors in the CNS may cause adverse events such as drowsiness and respiratory depression. In an effort to reduce the amount of opioid required for pain relief, and so reduce problematic adverse events, opioids are commonly combined with non‐opioid analgesics, such as paracetamol.
While there may be other components in some non‐prescription analgesics, these are usually present infrequently, and at doses too low to have any major impact. An exception is caffeine, which adds to analgesic effects in acute pain, in conjunction with conventional analgesics, and at caffeine doses of 100 mg or above (Derry 2012).
Why it is important to do this overview
This review is intended to provide information to consumers about non‐prescription oral analgesics for treating acute (of short duration, usually with rapid onset) pain conditions such as toothache or strains and sprains. Many products are available, but there is little information about their relative efficacy.
Scanning the pharmacy online databases demonstrates how many drugs, formulations, and combinations are available to treat mild headache, joint and muscle pain, dental, back, and period pain. The amount of high quality information about the efficacy of these analgesics is limited. Although Cochrane reviews are intended for use by consumers as well as healthcare providers and commissioners, there is to date no overview that directly addresses consumer issues relating to acute pain treatments that can be obtained without a prescription, and their effectiveness. The consumer is faced with a variety of different pain relieving medicines, at different amounts per tablet, sometimes alone, and sometimes with other ingredients; this makes choosing a product rather difficult for the consumer.
A broad range of analgesics is available without prescription in many parts of the world. This review is intended to cover most of the less costly analgesics available almost anywhere in the world. Licensing of analgesics available without prescription in the UK is broadly similar to that in the USA, Australia, and much of Europe. It was also our aim to provide sufficient information for individuals to work out for themselves what the efficacy may be for specific over‐the‐counter (OTC) products not included by name in this review.
Use of non‐prescription analgesics
OTC analgesics are used frequently, though the reasons for their use are not often described. For example, in Norway, one large population survey found that the prevalence of using OTC analgesics at least once per week in the last month was 47% (Dale 2015). Prevalence of paracetamol use was almost 40%, compared to 19% for NSAIDs and 8% for aspirin; more women used OTC analgesics. A lower figure of 25% over three months was found in Germany (Freytag 2014), with paracetamol and aspirin predominating. There was a much higher prevalence of 76% over one month in the USA (Paulose‐Ram 2003), again with use higher in women.
A systematic review found the prevalence of non‐prescription analgesic use in individual studies examining older people varied between 4% and 87%, with most reporting between 20% and 60% (Jerez‐Roig 2014). Studies report that 5% to 94% of adolescents use OTC analgesics (Shehnaz 2014).
Objectives
To examine published Cochrane reviews for information about the efficacy of pain medicines available without prescription using data from acute postoperative pain.
Methods
Criteria for considering reviews for inclusion
All Cochrane reviews of randomised controlled trials (RCTs) of single dose oral analgesics for acute postoperative pain in adults (aged 15 years or over).
We included reviews providing information on any drug, dose, or formulation matching the content of analgesic products available without prescription in the UK, where the usual recommended dose involves taking two tablets of most products. A list of available medication was drawn up from the Internet sites of Boots (www.boots.com/en/Pharmacy‐Health/Health‐shop/Pain‐relief/) and Lloyds Pharmacy (www.lloydspharmacy.com/en/medicines‐treatments/pain‐relief/) on 21 May 2015 (Appendix 1). We limited the overview to medication available in the UK because it is almost impossible to know with certainty what is available in other parts of the world. However, for completeness, we examined online pharmacy websites in the USA (Walgreens, CVS), Canada (Shoppers Drug Mart), and Australia (Guardian, Terry White Chemists) to check on any major drugs or combinations not available in the UK, and for alternatively named products sold in those countries. We planned specifically to include dipyrone (metamizole), which, while not available in any of the countries listed, is available without prescription in many parts of the world.
Medicines covered in the review are applicable to all parts of the world, and will include the most commonly used, although there are likely to be many other products, doses, and combinations available in different countries. Four examples highlight the difficulties in trying to produce an overview for OTC analgesics. Diclofenac potassium was available as an OTC product in the UK until early 2015, when market authorisation was withdrawn because of concerns about potential cardiovascular events. But it is still available in a number of other countries (though not Australia, Canada, or the USA), and so we have included it in the overview for completeness. Dipyrone is similarly available in many parts of the world, but not in Australia, Canada, the UK, or USA. Again, combinations of ibuprofen plus caffeine are available in some parts of South America, and we have included it here because caffeine is known to increase analgesic efficacy (Derry 2014a), and the amount required to provide greater efficacy can be derived from a cup of coffee or other drinks, or as tablets, and so is available for possible use by consumers. We also became aware that dexketoprofen is available as an OTC analgesic in Estonia, Italy, Lithuania, and Spain.
We have included Cochrane reviews of randomised controlled trials (RCTs) carried out to high methodological standards, using validated methods and outcomes of interest to patients. Each medicine was compared with placebo to allow indirect comparison. We anticipated that included reviews would be of medication used in acute postoperative pain, where study participants have established pain of moderate to severe intensity before treatment. In most circumstances it is considered appropriate to extrapolate these results to acute pain generally. The outcome had to be of direct relevance to patients, namely no worse than mild pain, or at least 50% of maximum pain relief (McQuay 2012; Moore 2013a).
We expected that the bulk of studies contributing information would have been performed in pain following third molar extractions, as demonstrated in a Cochrane overview (Moore 2015a). For this review, we have not included information on reviews on migraine, tension headache, or period pain.
Search methods for identification of reviews
We searched theCochrane Database of Systematic Reviews Issue 4 on The Cochrane Library for relevant reviews. See Appendix 2 for the search strategy. A series of Cochrane reviews have been conducted by the same team, covering analgesics identified in the British National Formulary.
Data collection and analysis
Two review authors (RAM, SD) independently carried out searches, selected reviews for inclusion, carried out assessment of methodological quality, and extracted data. We resolved any disagreements by discussion, involving a third review author if necessary.
Selection of reviews
Included reviews assessed RCTs evaluating the effects of a single oral dose of analgesic given for relief of moderate to severe postoperative pain in adults, compared to placebo, and included:
a clearly defined clinical question;
details of inclusion and exclusion criteria;
details of databases searched and relevant search strategies;
patient‐reported pain relief; and
summary results for at least one desired outcome.
Data extraction and management
We extracted data from the included reviews using a standard data extraction form. We used original study reports only if specific data were missing.
We collected information on the following.
Number of included studies and participants.
Drug, dose, and formulation (if formulation was an issue).
50% or greater maximum pain relief over four to six hours (participant‐reported): this outcome encapsulates both degree of pain relief and duration of the effect, and is a dichotomous (yes/no) measure of success over a defined period following drug ingestion.
Success and failure rates, where success (as a percentage of the maximum possible) was calculated from the drug‐specific effect and maximum possible effect (Moore 2013b).
Participants experiencing one or more adverse events.
Assessment of methodological quality of included reviews
All included reviews were carried out according to a standard protocol that satisfied the criteria specified in the 'assessment of multiple systematic reviews' (AMSTAR) measurement tool for rigorous methodological quality (Shea 2007).
Each review was required to:
provide an a priori design;
carry out duplicate study selection and data extraction;
carry out a comprehensive literature search;
include published and unpublished studies irrespective of language of publication;
provide a list of studies (included and excluded);
assess and document the scientific quality of the included studies;
use the scientific quality of the included studies appropriately in formulating conclusions;
use appropriate methods to combine the findings of studies; and
state conflicts of interests.
Data synthesis
We used information on the selected efficacy outcomes to draw up comparisons of analgesic efficacy, using indirect comparison of different drugs from almost identical clinical trial conditions, with placebo as a common comparator (Glenny 2005; Song 2003). We required a minimum of 200 participants in any comparison of an intervention with placebo (Moore 1998).
We have expressed comparative results at recommended doses as:
participants achieving at least 50% maximum pain relief, as a percentage, and as number needed to treat for an additional beneficial outcome (NNT) for at least 50% maximum pain relief over four to six hours, compared with placebo;
success and failure rates;
participants experiencing at least one adverse event.
We planned to list marketed products for which no good quality evidence is available from Cochrane reviews, and to attempt to produce estimates of their efficacy based on a model that predicts the analgesic efficacy of combination products (Moore 2012).
Results
A similar range of OTC analgesics was available in the UK, Australia, Canada, and the USA (Appendix 1). We added diclofenac, dipyrone, and ibuprofen plus caffeine to the list of reviews to look for.
We examined 39 separate Cochrane reviews investigating 41 analgesics or analgesic combinations given as single oral doses in acute postoperative pain conditions (Aceclofenac 2009; Acemetacin 2009; Aspirin 2012; Celecoxib 2013; Codeine 2010; Dexibuprofen 2009; Diclofenac 2015; Diflunisal 2010; Dihydrocodeine 2000; Dipyrone 2010; Etodolac 2009; Etoricoxib 2014; Fenbufen 2009; Fenoprofen 2011; Flurbiprofen 2009; Gabapentin 2010; Ibuprofen 2009; Ibuprofen + caffeine 2015; Ibuprofen + codeine 2015; Ibuprofen + oxycodone 2013; Ibuprofen + paracetamol 2013; Indometacin 2004; Ketoprofen and dexketoprofen 2009; Lornoxicam 2009; Lumiracoxib 2010; Mefenamic acid 2011; Meloxicam 2009; Nabumetone 2009; Naproxen 2009; Nefopam 2009; Paracetamol 2008; Paracetamol ± dextropropoxyphene 1999; Paracetamol + codeine 2009; Paracetamol ± oxycodone 2009; Piroxicam 2000; Rofecoxib 2009; Sulindac 2009; Tenoxicam 2009; Tiaprofenic acid 2009). Results for ibuprofen formulations were supplemented with data from a non‐Cochrane review but based on Cochrane reviews (Moore 2014a).
We checked the contents of these reviews against the list of available OTC analgesics in Appendix 1, and included in this review only the relevant reviews (Aspirin 2012; Diclofenac 2015; Dipyrone 2010; Ibuprofen 2009; Ibuprofen + caffeine 2015; Ibuprofen + codeine 2015; Ibuprofen + paracetamol 2013; Ketoprofen and dexketoprofen 2009; Naproxen 2009; Paracetamol 2008).
We found information on 21 different OTC analgesic drugs, doses, and formulations, using information from 10 Cochrane reviews, supplemented by information from one non‐Cochrane review with additional information on ibuprofen formulations (Moore 2014a, high quality evidence). The included reviews provided information on several doses of aspirin, dexketoprofen (as the trometamol salt), diclofenac potassium, dipyrone, ibuprofen in various formulations, naproxen, and paracetamol, and the combinations of ibuprofen plus caffeine and ibuprofen plus paracetamol. No or insufficient information was available on combinations of analgesics with low doses of codeine, or a range of the other combinations identified in Appendix 1.
The amount of information available for included interventions varied greatly, from a minimum of two studies with about 200 participants to over 50 studies and about 5000 participants (Appendix 3).
Description of included reviews
Included reviews each had the same structure and organisation, and used identical methods based on criteria established by extensive analysis and validation, using individual participant data. They all used the same criteria and typically these were as follows.
Adults with established pain of at least moderate intensity (Collins 1997).
Single dose oral administration of analgesic or placebo (with additional analgesia available, typically after 60 to 120 minutes).
Randomised, double‐blind studies.
Pain assessed by participants using standard pain intensity and pain relief scales.
Study duration of four hours or more.
Searching included electronic searches, plus databases created by hand searching the older literature, now part of the Cochrane Central Register of Controlled Trials (CENTRAL). Searching also included different retail names for drugs.
No language restriction on included papers.
Assessment of study quality according to established criteria and minimum criteria for inclusion.
Methodological quality of included reviews
All the reviews:
had an a priori design;
performed duplicate study selection and data extraction;
had a comprehensive literature search;
used published and any unpublished studies included irrespective of language of publication, though not all reviews contacted companies or researchers for unpublished trial data;
provided a list of included and excluded studies;
provided characteristics of included studies;
assessed and documented the scientific quality of the included studies;
used the scientific quality of the included studies appropriately in formulating conclusions, because only studies with minimal risk of bias were included (a particular issue was trial size, but conclusions were not drawn from inadequate data sets, based on previously established criteria (Moore 1998));
used appropriate methods to combine findings of studies and importantly provided analyses according to drug dose; and
had universal conflict of interest statements.
The reviews all used validated methods for conversion of mean to dichotomous data (Moore 1996; Moore 1997a; Moore 1997b), providing the number and proportion of participants with the clinically relevant outcome of at least 50% maximum pain relief. Remedication is common within a few hours with placebo, therefore the method of imputing data after withdrawal is potentially of importance to the measurement of treatment effect. In the case of the primary outcome of the reviews, that of NNT for at least 50% maximum pain relief compared with placebo over four to six hours, the imputation method had been shown not to make any appreciable difference (Moore 2005), although use of last observation carried forward tended to overestimate treatment effect compared with baseline observation carried forward over longer periods (Moore 2005).
Effect of interventions
The effects of the analgesics are reported according to dose in milligrams, not as the number of tablets required to obtain that dose. This is because the dose per tablet can be quite variable (Appendix 1).
1 Number needed to treat for an additional beneficial outcome (NNT)
NNT is used widely in medicine to report the magnitude of the effect of an intervention. In this case it describes the number of participants in studies who need to be treated with an analgesic for one more person to have good pain relief than if the same number had been treated with placebo. Lower numbers are clearly better, and the ideal number is an NNT of 1, which would occur if every participant had good pain relief with an analgesic and none with placebo. Summary of results A and Figure 1 shows the NNTs for OTC drugs where a minimum of two studies with 200 participants was available. Full details are in Appendix 3.
1.
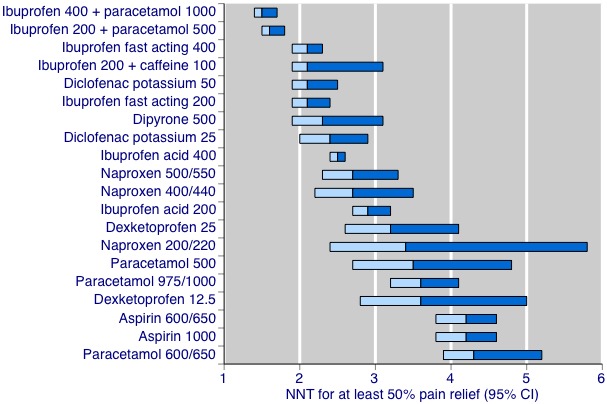
Number needed to treat for an additional beneficial outcome (NNT for at least 50% maximum pain relief over four to six hours compared with placebo. The bars show the 95% confidence interval (CI), and the colour change is the point estimate.
The lowest (best) NNT values were for combinations of ibuprofen plus paracetamol, with NNT values below 2. Analgesics with values close to 2 included fast acting formulations of ibuprofen 200 mg and 400 mg, ibuprofen 200 mg plus caffeine 100 mg, and diclofenac potassium 50 mg. By contrast, paracetamol plus aspirin at various doses had NNT values of 3 or above.
Summary of results A
| Drug | Dose (mg) | NNT (95% confidence interval) |
| Aspirin | 500 | Not better than placebo |
| Aspirin | 600/650 | 4.2 (3.8 to 4.6) |
| Aspirin | 1000 | 4.2 (3.8 to 4.6) |
| Dexketoprofen | 12.5 | 3.6 (2.8 to 5.0) |
| Dexketoprofen | 25 | 3.2 (2.6 to 4.1) |
| Diclofenac potassium | 25 | 2.4 (2.0 to 2.9) |
| Diclofenac potassium | 50 | 2.1 (1.9 to 2.5) |
| Dipyrone | 500 | 2.3 (1.9 to 3.1) |
| Ibuprofen acid | 200 | 2.9 (2.7 to 3.2) |
| Ibuprofen acid | 400 | 2.5 (2.4 to 2.6) |
| Ibuprofen fast acting | 200 | 2.1 (1.9 to 2.4) |
| Ibuprofen fast acting | 400 | 2.1 (1.9 to 2.3) |
| Ibuprofen + caffeine | 200+100 | 2.1 (1.9 to 3.1) |
| Ibuprofen + paracetamol | 200+500 | 1.6 (1.5 to 1.8) |
| Ibuprofen + paracetamol | 400+1000 | 1.5 (1.4 to 1.7) |
| Naproxen | 200/220 | 3.4 (2.4 to 5.8) |
| Naproxen | 400/440 | 2.7 (2.2 to 3.5) |
| Naproxen | 500/550 | 2.7 (2.3 to 3.3) |
| Paracetamol | 500 | 3.5 (2.7 to 4.8) |
| Paracetamol | 600/650 | 4.6 (3.9 to 5.5) |
| Paracetamol | 975/1000 | 3.6 (3.2 to 4.1) |
2 Success rate
Success rate is a different way of describing different degrees of effectiveness between drugs. It is calculated by taking the proportion of participants who get good pain relief with analgesic minus the proportion who get good pain relief with placebo, and expressing this as a percentage of the maximum possible success rate for the analgesic, namely 100 minus the response rate with placebo. It has been used to explore high failure rates with analgesics, especially in more complex chronic conditions (Moore 2013b).
Summary of results B and Figure 2 show the success rates for OTC drugs where a minimum of two studies with 200 participants was available. Full details are in Appendix 4. Results expressed in this way show the same general order as with NNT. Again combinations of ibuprofen plus paracetamol have the highest success rates, at almost 70%, with dipyrone 500 mg, fast acting ibuprofen formulations 200 mg and 400 mg, ibuprofen 200 mg plus caffeine 100 mg, and diclofenac potassium 50 mg having success rates above 50%. Again, doses of aspirin plus paracetamol tended to have the lowest success rates.
2.
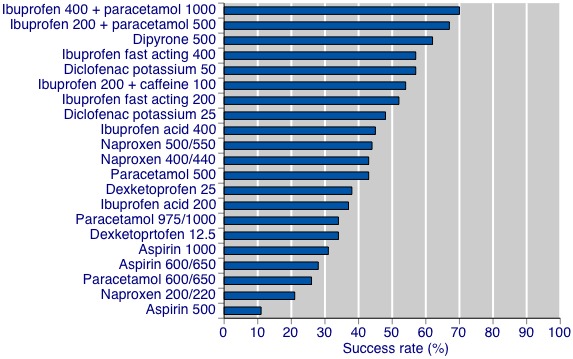
Success rate ‐ percentage achieving at least 50% maximum pain relief.
Figure 3 shows the relationship between success rate and NNT for these drugs.
3.
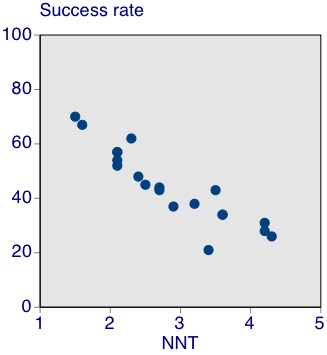
Relationship between success rate and number needed to treat for an additional beneficial outcome (NNT
Summary of results B
| Drug | Dose (mg) | Success rate (%) |
| Aspirin | 500 | 11 |
| Aspirin | 600/650 | 28 |
| Aspirin | 1000 | 31 |
| Dexketoprofen | 12.5 | 34 |
| Dexketoprofen | 25 | 38 |
| Diclofenac potassium | 25 | 48 |
| Diclofenac potassium | 50 | 57 |
| Dipyrone | 500 | 62 |
| Ibuprofen acid | 200 | 37 |
| Ibuprofen acid | 400 | 45 |
| Ibuprofen fast acting | 200 | 52 |
| Ibuprofen fast acting | 400 | 57 |
| Ibuprofen + caffeine | 200+100 | 54 |
| Ibuprofen + paracetamol | 200+500 | 67 |
| Ibuprofen + paracetamol | 400+1000 | 70 |
| Naproxen | 200/220 | 21 |
| Naproxen | 400/440 | 43 |
| Naproxen | 500/550 | 44 |
| Paracetamol | 500 | 43 |
| Paracetamol | 600/650 | 26 |
| Paracetamol | 975/1000 | 34 |
3 Adverse events
The reviews reported the number of participants who experienced at least one adverse event during the course of the studies. This could be any adverse event, such as headache, nausea, or dizziness. Full details are in Appendix 5. For the most part there was no difference in adverse event rates between analgesics and placebo in these studies involving single doses.
For two analgesics, aspirin 1000 mg and ibuprofen 200 mg plus caffeine 100 mg, adverse event rates were statistically significantly higher with analgesic than placebo, although barely so for ibuprofen and caffeine. For aspirin 1000 mg the number needed for one participant to be harmed more than with placebo was 7.5.
For both doses of ibuprofen plus paracetamol in combination, the adverse event rate was lower with analgesic than placebo, with a number needed to treat to prevent one more adverse event happening with analgesic than with placebo of about 5.
Serious adverse events were rare. In total there were four reported for ibuprofen, four for placebo, and one for naproxen.
4 Non‐prescription analgesics with no or insufficient data
We found no data for a range of OTC analgesics, particularly those combining a low dose of codeine plus aspirin or paracetamol, and combinations that included caffeine. We conducted some searches of PubMed to find any other non‐Cochrane review or randomised trials with low dose codeine combinations, but found none. Within a review of ibuprofen plus codeine, we found information on one trial of an OTC combination product (Ibuprofen + codeine 2015), but concluded that the amount of information was too small to make the estimate of efficacy reliable.
5 Predicting analgesic efficacy where there were no direct data
It became clear after looking at the range of combination products available, principally those with low doses of codeine, that this part of the review could not be completed as there were no reliable data to use.
Discussion
This overview of non‐prescription (OTC) oral analgesics for acute pain builds on evidence from a large number of clinical studies of oral analgesics for acute postoperative pain, a common test of analgesic efficacy that has been in use since the 1950s (McQuay 2012). It has proved a reliable indicator of whether drugs can act as analgesics, and in systematic review, how well they work. Two other overviews have looked in detail at all available data on efficacy (Moore 2015a) and adverse events (Moore 2015b).
New information continues to inform on analgesic effectiveness. For example, in the recent past we have learned much about the importance of drug formulation on efficacy, and recognise that speedy absorption into the body not only provides more rapid pain relief, but better overall pain relief and longer lasting pain relief (Moore 2014a; Moore 2015c). Taking analgesics with food can delay absorption and is likely to reduce the effectiveness of drugs for acute pain (Moore 2015d); the studies in the included reviews were probably all conducted in fasting participants, reducing the relevance of food for this overview. However, the advice is often given to take analgesics with food, with the ostensible aim of reducing adverse effects in the gastrointestinal tract, and this advice needs urgent re‐evaluation. Finally, relative analgesic efficacy determined in this acute postoperative pain model generally holds up in other circumstances (Moore 2015e).
This overview is directed specifically at acute painful conditions, and not at migraine, tension headache, dysmenorrhoea, chronic musculoskeletal conditions, or other chronic painful conditions such as neuropathic pain or fibromyalgia. For those conditions different drugs might be used, or the same drugs at different doses, or using different formulations. Increasingly, overviews are being produced for some of these, such as antiepileptic drugs for neuropathic pain and fibromyalgia (Wiffen 2013), or sumatriptan (all routes of administration) for migraine Derry 2014b).
Summary of main results
A wide range of analgesic efficacy exists for OTC products for acute pain, whether expressed as NNT or success rate. Taking success rate as the more easily understood method of reporting results, success ranged from below 20% to about 70%. Better‐performing analgesics included combinations of ibuprofen plus paracetamol, dipyrone 500 mg, fast acting ibuprofen formulations 200 mg and 400 mg, ibuprofen 200 mg plus caffeine 100 mg, and diclofenac potassium 50 mg. Aspirin and paracetamol at various doses had the lowest success rates.
It was clear that combination products and fast acting formulations performed particularly well, and that it might easily be possible to obtain greater analgesic effects with lower doses by varying formulation and combination. For example, the ibuprofen plus paracetamol combination that performed best used a formulation designed for rapid dissolution, and resulted in very good analgesia with only ibuprofen 200 mg with paracetamol 500 mg. This also produced long lasting analgesia (Ibuprofen + paracetamol 2013), so that fewer doses need to be taken, resulting in a lower exposure to the drugs. This is also the case with fast acting formulations (Moore 2014a), and the combination of ibuprofen plus caffeine. The combination of ibuprofen plus caffeine need not be in a single tablet, and it might well be that individuals could achieve very good analgesic results by taking a fast acting 200 mg tablet of ibuprofen with a drink containing caffeine, like a mug of strong coffee or a caffeinated beverage.
Some formulations sold without prescription are described as being slow release (Appendix 1). There is no reason to expect that these will be of any value for acute pain where rapid pain relief is needed.
For the most part, the OTC analgesics in single dose studies resulted in no more participants experiencing an adverse event than with placebo. Serious adverse events were rare, and not necessarily related to the drug taken.
The available data do not cover every available dose or combination product available in four large Western countries, and there will be many other products available worldwide. Even so, there is a reasonable amount of evidence to help consumers choose therapies that can help them. Some are available from open shelves, while some have to be asked for at a pharmacy counter; regulations vary. We did not consider the cost of analgesics to consumers.
Overall completeness and applicability of evidence
For many of the analgesic doses and combinations available OTC, there was simply no evidence. Combination products with low doses of codeine represent the major gap in the evidence, but probably represent a large part of OTC analgesic sales.
Moreover, the evidence we have is rather general, and there is no way of knowing whether a product with ibuprofen 200 mg in it is any different in the 30 different versions that we found (Appendix 1), other than whether the formulation is a standard acid or fast acting. There may well be differences in the actual formulation process, in the way the drug dissolves in the stomach, or the way it is absorbed and acts.
We know of no registry of studies or source of study results we could use to determine whether any one of these different products is better than any other, except that fast acting tends to be better than standard formulations. We just cannot know, for any product, how much analgesia is produced by it: all we have are the average values from all trials ever done, and the expectation that the average will apply to a particular product. The exception is for ibuprofen plus paracetamol combination, where all the studies used the same formulation (Appendix 1).
The postoperative pain model used in this overview is predominantly (80%) pain after third molar extraction, which is used as the industry model for everyday pain (Derry 2011). That makes the results relevant to many pain conditions for people who use non‐prescription analgesics. The ideal would be to have results also available for other common pain conditions where non‐prescription analgesics are used, but where the efficacy may be different. The most common pain conditions are dental caries, tension‐type headache, and migraine (Vos 2012). There were few data for tension‐type headache (Moore 2014b), while there were Cochrane reviews for some OTC analgesics in migraine (Derry 2013; Kirthi 2013; Rabbie 2013). A review of dysmenorrhoea did not give efficacy results according to drug, but suggested that the NNT for providing moderate or excellent pain relief for NSAIDs was around 3.2, with success rates up to 43% (Marjoribanks 2015); NSAIDs were better than paracetamol, mirroring results in this overview in acute pain.
The results do not apply to treatment of chronic musculoskeletal conditions, such as osteoarthritis.
Quality of the evidence
The quality of the evidence was good, using standard reviews examining standard clinical trials designed to measure the analgesic efficacy of drugs in sensitive assays in acute painful conditions (McQuay 2012). The overview process further removed any results likely to be the object of potential publication bias, so that only reliable results remained. This leaves a very large body of efficacy results presented by dose and formulation.
These results report a clinically useful level of pain relief over a sensible period, and with the common comparator of placebo. Though indirect comparisons are often criticised, this is one circumstance where indirect comparison can be justified because of the clinical homogeneity of trials and outcomes, and because data like these have been tested and indirect comparison found to be a reasonable approach (Song 2003).
Potential biases in the overview process
No obvious biases in the overview process existed, for the reasons given above.
Small data sets are clearly more variable than larger ones, as would be expected (Moore 1998). However, with few exceptions placebo response rates were within limited ranges, typically between 5% and 20%.
Most studies in the individual reviews will have been sponsored or conducted by manufacturers. This is not likely to be a source of any bias, since specific analyses have been conducted on some of the larger data sets to demonstrate that no industry bias exists in like‐for‐like comparisons (Barden 2006).
Agreements and disagreements with other studies or reviews
The only other overviews of this type known to exist for acute pain studies are non‐Cochrane overviews in dental pain (Barden 2004; Derry 2011), and a review of OTC analgesics based on Cochrane reviews (Moore 2013c). The general methods used were similar and there were no major differences. Two other overviews have looked in detail at all available data on efficacy (Moore 2015a) and adverse events (Moore 2015b).
Authors' conclusions
Implications for practice.
For people with acute pain
The major implication for people with acute pain is the knowledge that there is a body of reliable evidence about the efficacy of some of the most commonly available drugs and doses that are available without prescription. The proportion of people with acute pain who get good pain relief with any of them ranges from about 70% at best to less than 20% at worst. Low doses in fast acting formulations can provide good analgesia in many people. Adverse events are generally no different from placebo. Consumers can make an informed choice based on this knowledge, together with availability, and price.
Advice is often given to take analgesics with food, with the ostensible aim of reducing adverse effects in the gastrointestinal tract, and this advice needs urgent re‐evaluation.
For clinicians
There is also a clear message that simple drug combinations and fast acting formulations deliver good analgesia in many people with acute pain, and at relatively low doses. Clinicians can use this knowledge to provide good evidence‐based advice to people who want to self medicate. There are no drugs available with prescription that are more effective.
Advice is often given to take analgesics with food, with the ostensible aim of reducing adverse effects in the gastrointestinal tract, and this advice needs urgent re‐evaluation.
For policy makers
Simple drug combinations and fast acting formulations can deliver good analgesia in many people with acute pain at relatively low doses. This can help provide potentially useful public health messages about maximising pain relief while minimising population exposure to analgesics.
Advice is often given to take analgesics with food, with the ostensible aim of reducing adverse effects in the gastrointestinal tract, and this advice needs urgent re‐evaluation.
For funders
The knowledge that no better analgesics are available with a prescription than without a prescription may have implications for prescribing policy and the health economy.
Implications for research.
General
It appears to be possible to provide useful evidence‐based information directed at consumers concerning commonly used drugs available without prescription. That this is based on an in‐depth understanding of clinical trials and systematic reviews, consistently done to high quality levels, and using a simple message about outcomes of value to people with pain, provides an exemplar for how more can be done to inform consumers.
The lack of any information on the efficacy of low dose codeine combination therapies is a major gap in knowledge. While the doses of codeine may be small in individual doses, this possibly represents substantial population consumption, and we need to know that there is some benefit in terms of analgesic efficacy in individuals as a balance to possible harm to the community.
Design
Perhaps the most important design issue relates to the outcome. The outcome used is one of value to people with pain, and results can be expressed in a simple and understandable way.
Measurement (endpoints)
Pain measurement is not an issue.
What's new
| Date | Event | Description |
|---|---|---|
| 19 February 2020 | Amended | Clarification added to Declarations of interest. |
| 11 October 2017 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 10, 2013 Review first published: Issue 11, 2015
| Date | Event | Description |
|---|---|---|
| 28 May 2019 | Amended | Contact details updated. |
| 6 November 2015 | Amended | PLS stated that the overview included nine Cochrane reviews. This has been corrected to read 10 Cochrane reviews. |
Notes
No updates of the included reviews are expected in the next 5 years, and no new data are likely to be available that change the conclusions for at least 10 years. This overview has now been stabilised, and will be reassessed for updating in 2027. If appropriate, we will update the overview earlier if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The Oxford Pain Relief Trust provided institutional support for this work.
We wish to acknowledge the significant contribution made by Christopher Derry in searching for information about OTC analgesics (drug content and availability) in the early planning of this review.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. List of available non‐prescription analgesics in the UK in May 2015, from Boots and Lloyds Pharmacy websites
| Drug and dose (mg) | UK OTC product | Comments |
| Aspirin only products | ||
| Aspirin 300 | Disprin Direct | ‐ |
| Disprin soluble | ‐ | |
| Boots Aspirin Tablets | ‐ | |
| Boots Aspirin Dispersible Tablets | ‐ | |
| Lloyds Pharmacy Aspirin Tablets | ‐ | |
| Lloyds Pharmacy Dispersible Aspirin Tablets | ‐ | |
| Aspro Clear Regular Strength | ‐ | |
| Disprin Original Tablets | ‐ | |
| Aspirin 325 | Aspirin Regular Strength Tablets | ‐ |
| Aspro Tablets | ‐ | |
| Bayer Aspirin Coated Tablets | ‐ | |
| Bayer aspirin Tablets | ‐ | |
| CVS Regular Strength Aspirin | ‐ | |
| CVS Enteric Aspirin Regular Strength | ‐ | |
| Aspirin 500 | Aspro Clear Extra Strength | ‐ |
| Disprin Max | ‐ | |
| Bayer Extra Strength Coated Caplets | ‐ | |
| Combination products containing aspirin | ||
| Aspirin 500 + codeine 8 | Codis | ‐ |
| Boots Aspirin & Codeine Tablets | ‐ | |
| Aspirin 300 + paracetamol 200 + caffeine 45 | Anadin Extra | ‐ |
| Anadin Extra Soluble | ‐ | |
| Anadin Extra Triple Action Tablets | ‐ | |
| Boots Aspirin Extra | ‐ | |
| Lloyds Pharmacy Extra Power Pain Reliever Caplets | ‐ | |
| Aspirin 250 + paracetamol 250 + caffeine 65 | Excedrin Extra Strength | ‐ |
| Aspirin 500 + caffeine 50 | Beechams Powders | ‐ |
| Aspirin 500 + caffeine 32.5 | Bayer Back & Body Caplets | ‐ |
| CVS Extra Strength Back & Body Pain Caplets | ‐ | |
| Aspirin 325 + caffeine 15 | Anadin Original | ‐ |
| Diclofenac only products (not now available in the UK) | ||
| Diclofenac potassium 12.5 | Boots joint pain relief 12.5 mg tablets | Fast acting |
| Voltarol joint pain 12.5 mg tablets | Fast acting | |
| Diclofenac potassium 25 | Voltarol pain‐eze extra strength 25 mg tablets | Fast acting |
| First resort double action pain relief tablets | Fast acting | |
| Ibuprofen only products | ||
| Ibuprofen 200 | Nurofen Tablets | ‐ |
| Nurofen Caplets | ‐ | |
| Value Health Ibuprofen | ‐ | |
| Boots Ibuprofen Caplet | ‐ | |
| Anadin Joint Pain | ‐ | |
| Lloyds Pharmacy Ibuprofen Caplets | ‐ | |
| Life Brand ibuprofen Tablets | ‐ | |
| Advil Ibuprofen Tablets | ‐ | |
| Gold Cross Ibuprofen | ‐ | |
| Guardian Ibuprofen | ‐ | |
| Herron Blue Ibuprofen Tapsules | ‐ | |
| Value Choice Ibuprofen | ‐ | |
| CVS Dye‐Free Ibuprofen Tablets | ‐ | |
| Nurofen Meltlet Lemon | Probably fast acting | |
| Nurofen Migraine Pain (as lysine) | Fast acting | |
| Nurofen Tension Headache (as lysine) | Fast acting | |
| Nurofen Express Liquid Capsule | Fast acting | |
| Nurofen Express Period Pain | Fast acting | |
| Feminax Express | Fast acting | |
| Nurofen Liquid capsules | Fast acting | |
| Boots Ibuprofen Liquid Capsules | Fast acting | |
| Boots Rapid Ibuprofen Lysine | Fast acting | |
| Nurofen Express Caplet (as sodium salt) | Fast acting | |
| Anadin Ultra Ibuprofen Liquid capsules | Fast acting | |
| Life Brand Ibuprofen Liquid Capsules | Fast acting | |
| Nurofen Zavance (Capsules and Tablets) | Fast acting | |
| Advil Liqui‐Gels | Fast acting | |
| Advil Migraine solubilised ibuprofen | Fast acting | |
| Boots Ibuprofen Long Lasting | Slow release | |
| Lloyds Pharmacy Ibuprofen Long Lasting Capsules | Slow release | |
| Ibuprofen 300 | Nurofen Back Pain Sustained Release Capsules | Slow release |
| Ibuprofen 400 | Boots Ibuprofen Caplets | ‐ |
| Nurofen Maximum Strength Migraine | ‐ | |
| Nurofen Express Caplet | ‐ | |
| Lloyds Pharmacy Ibuprofen Caplets | ‐ | |
| Advil Extra Strength Caplets | ‐ | |
| Advil Muscle & Joint Extra Strength Tablets | ‐ | |
| Life Brand Extra Strength Ibuprofen Muscle & Joint | ‐ | |
| Nurofen Express Liquid Capsule | Fast acting | |
| Combination products containing ibuprofen | ||
| Ibuprofen 200 + codeine 12.8 | Nurofen Plus | ‐ |
| Solpadeine Migraine | ‐ | |
| Boots Ibuprofen and Codeine | ‐ | |
| Ibuprofen 200 + paracetamol 500 | Nuromol | ‐ |
| Ibuprofen 200 + phenylephrine 5 | Nurofen Sinus Pain PE | ‐ |
| Ibuprofen 100 + caffeine 100 | Not in UK | ‐ |
| Ibuprofen 200 + caffeine 100 | Not in UK | ‐ |
| Naproxen only products | ||
| Naproxen 250 | Boots Period Pain Relief | Probably slow release |
| Feminax Ultra 9 Tablets | Probably slow release | |
| Naproxen 220 | Life Brand Naproxen Sodium Tablets | ‐ |
| Aleve Liquid Gels Naproxen Capsules | ‐ | |
| Aleve Naproxen Tablets | ‐ | |
| Aleve All Day Strong Tablets | ‐ | |
| Aleve Liquid Gels | ‐ | |
| Aleve Tablets and Caplets | ‐ | |
| CVS All Day Pain Relief Caplets | ‐ | |
| CVS All Day Pain Relief Tablets | ‐ | |
| Paracetamol only products | ||
| Paracetamol 650 | Tylenol Arthritis Pain | ‐ |
| Life Brand Muscle Aches & Body Pain Acetaminophen Extended Release Tablets | ‐ | |
| CVS 8 Hour Acetaminophen Extended Release | ‐ | |
| Paracetamol 500 | Paracetamol 500 mg Tablets or Caplets | ‐ |
| Panadol Actifast | ‐ | |
| Panadol Advance | ‐ | |
| Panadol Rapid Soluble | ‐ | |
| Panadol Rapid Caplets | ‐ | |
| Value Health Paracetamol Tablets | ‐ | |
| Anadin Paracetamol | ‐ | |
| Lloyds Pharmacy Paracetamol Caplets | ‐ | |
| Lloyds Pharmacy Paracetamol Capsules | ‐ | |
| Life Brand Extra Strength Acetaminophen | ‐ | |
| Tylenol Extra Strength Acetaminophen Tablets | ‐ | |
| Guardian Paracetamol | ‐ | |
| Herron Gold Paracetamol | ‐ | |
| Value Choice Paracetamol | ‐ | |
| CVS Extra Strength Pain Relief 500 mg Caplets | ‐ | |
| Paracetamol 325 | Life Brand Regular Strength Caplets | ‐ |
| Tylenol Regular Strength | ‐ | |
| Combination products containing paracetamol | ||
| Paracetamol 500 + diphenhydramine HCl 25 | Panadol night pain | ‐ |
| Paracetamol 500 + caffeine 65 | Boots Paracetamol Extra | ‐ |
| Panadol Extra Advance | ‐ | |
| CVS Tension Headache Coated caplet | ‐ | |
| Excedrin Tension Headache | ‐ | |
| Paracetamol 500 + caffeine 60 + prilamine maleate 15 | Maximum Strength Midol | ‐ |
| Paracetamol 500 + pseudoephedrine HCl 60 | Boots Decongestant with Pain Relief | ‐ |
| Paracetamol 500 + codeine 8 | Boots Paracetamol and Codeine | ‐ |
| Paracodol | ‐ | |
| Lloyds Pharmacy Co‐Codamol | ‐ | |
| Lloyds Pharmacy Co‐Codamol Effervescent Tablets | ‐ | |
| Codapane | ‐ | |
| Migraleve Yellow | ‐ | |
| Paracetamol 500 + codeine 8 + buclizine HCl 6.25 | Migraleve Pink | ‐ |
| Paracetamol 500 + codeine 8 + caffeine 30 | Solpadeine Plus | ‐ |
| Lloyds Pharmacy Paracetamol Codeine Extra Effervescent Tablets | ‐ | |
| Paracetamol 500 + codeine 12.8 | Panadol Ultra | ‐ |
| Solpadeine Max | ‐ | |
| Paracetamol 500 + dihydrocodeine tartrate 7.5 | Paramol | ‐ |
| Aspirin 300 + paracetamol 200 + caffeine 45 | Anadin Extra | ‐ |
| Anadin Extra Soluble | ‐ | |
| Anadin Extra Triple Action Tablets | ‐ | |
| Boots Aspirin Extra | ‐ | |
| Lloyds Pharmacy Extra Power Pain Reliever Caplets | ‐ | |
HCl: hydrochloride; OTC: over‐the‐counter.
Appendix 2. Search strategy for Cochrane reviews
(postoperative):ti,ab,kw or (post NEXT operative):ti,ab,kw
(pain):ti,ab,kw or (painful):ti,ab,kw or (analgesi*):ti,ab,kw
(1 AND 2) in Cochrane Database of Systematic Reviews
Appendix 3. Results for the efficacy outcome of NNT for at least 50% of maximum pain relief over four to six hours
| Drug | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Risk ratio (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| Aspirin | 500 | 2 | 213 | 45/135 | 20/78 | 34 | 26 | 1.3 (0.8 to 2.0) | Not calculated |
| Aspirin | 600/650 | 60 | 4965 | 983/2496 | 379/2469 | 39 | 15 | 2.5 (2.3 to 2.8) | 4.2 (3.8 to 4.6) |
| Aspirin | 1000 | 6 | 618 | 138/340 | 40/278 | 41 | 14 | 2.7 (2.0 to 3.7) | 4.2 (3.8 to 4.6) |
| Dexketoprofen | 12.5 | 5 | 452 | 104/230 | 38/222 | 45 | 17 | 2.7 (2.0 to 3.7) | 3.6 (2.8 to 5.0) |
| Dexketoprofen | 25 | 6 | 523 | 129/225 | 38/248 | 47 | 15 | 3.3 (2.4 to 4.5) | 3.2 (2.6 to 4.1) |
| Diclofenac potassium | 25 | 4 | 502 | 140/248 | 37/274 | 56 | 15 | 3.9 (2.8 to 5.3) | 2.4 (2.0 to 2.9) |
| Diclofenac potassium | 50 | 7 | 757 | 253/398 | 60/359 | 64 | 17 | 3.7 (2.9 to 4.7) | 2.1 (1.9 to 2.5) |
| Dipyrone | 500 | 5 | 288 | 106/143 | 45/145 | 74 | 31 | 2.4 (1.8 to 3.1) | 2.3 (1.9 to 3.1) |
| Ibuprofen acid | 200 | 18 | 2103 | 448/1094 | 67/1009 | 41 | 7 | 6.5 (5.1 to 8.2) | 2.9 (2.7 to 3.2) |
| Ibuprofen acid | 400 | 51 | 5604 | 1596/3070 | 289/2543 | 52 | 12 | 4.6 (4.0 to 5.1) | 2.5 (2.4 to 2.6) |
| Ibuprofen fast acting | 200 | 7 | 828 | 270/478 | 34/350 | 57 | 10 | 5.7 (4.2 to 7.9) | 2.1 (1.9 to 2.4) |
| Ibuprofen fast acting | 400 | 13 | 1364 | 427/658 | 85/466 | 65 | 18 | 3.9 (3.2 to 4.7) | 2.1 (1.9 to 2.3) |
| Ibuprofen + caffeine | 200+100 | 4 | 334 | 103/174 | 16/160 | 59 | 10 | 5.5 (3.5 to 8.7) | 2.1 (1.9 to 3.1) |
| Ibuprofen + paracetamol | 200+500 | 3 | 508 | 240/349 | 10/159 | 69 | 6 | 10 (5.7 to 19) | 1.6 (1.5 to 1.8) |
| Ibuprofen + paracetamol | 400+1000 | 3 | 543 | 278/384 | 10/159 | 72 | 6 | 11 (6.2 to 20) | 1.5 (1.4 to 1.7) |
| Naproxen | 200/220 | 2 | 202 | 54/120 | 16/82 | 45 | 30 | 2.9 (1.6 to 5.1) | 3.4 (2.4 to 5.8) |
| Naproxen | 400/440 | 3 | 334 | 103/210 | 14/124 | 49 | 11 | 4.8 (2.8 to 8.4) | 2.7 (2.2 to 3.5) |
| Naproxen | 500/550 | 9 | 784 | 200/394 | 59/390 | 52 | 15 | 3.4 (2.6 to 4.4) | 2.7 (2.3 to 3.3) |
| Paracetamol | 500 | 6 | 561 | 176/290 | 86/271 | 61 | 32 | 1.9 (1.6 to 2.3) | 3.5 (2.7 to 4.8) |
| Paracetamol | 600/650 | 19 | 1886 | 358/954 | 145/932 | 38 | 16 | 2.4 (2.0 to 2.8) | 4.6 (3.9 to 5.5) |
| Paracetamol | 975/1000 | 28 | 3232 | 876/1906 | 241/1329 | 46 | 18 | 2.7 (2.4 to 3.0) | 3.6 (3.2 to 4.1) |
| CI: confidence interval; NNT: number needed to treat for an additional beneficial outcome. Note: NNT not calculated when result not significantly different from placebo. | |||||||||
Appendix 4. Success and failure rates for at least 50% maximum pain relief
| Drug | Dose (mg) | Percent with outcome | Success rate (%) | Failure rate (%) | |
| Active | Placebo | ||||
| Aspirin | 500 | 34 | 26 | 11 | 89 |
| Aspirin | 600/650 | 39 | 15 | 28 | 72 |
| Aspirin | 1000 | 41 | 14 | 31 | 69 |
| Dexketoprofen | 12.5 | 45 | 17 | 34 | 66 |
| Dexketoprofen | 25 | 47 | 15 | 38 | 62 |
| Diclofenac potassium | 25 | 56 | 15 | 48 | 52 |
| Diclofenac potassium | 50 | 64 | 17 | 57 | 43 |
| Dipyrone | 500 | 74 | 31 | 62 | 38 |
| Ibuprofen acid | 200 | 41 | 7 | 37 | 63 |
| Ibuprofen acid | 400 | 52 | 12 | 45 | 55 |
| Ibuprofen fast acting | 200 | 57 | 10 | 52 | 48 |
| Ibuprofen fast acting | 400 | 65 | 18 | 57 | 43 |
| Ibuprofen + caffeine | 200+100 | 59 | 10 | 54 | 46 |
| Ibuprofen + paracetamol | 200+500 | 69 | 6 | 67 | 33 |
| Ibuprofen + paracetamol | 400+1000 | 72 | 6 | 70 | 30 |
| Naproxen | 200/220 | 45 | 30 | 21 | 79 |
| Naproxen | 400/440 | 49 | 11 | 43 | 57 |
| Naproxen | 500/550 | 52 | 15 | 44 | 56 |
| Paracetamol | 500 | 61 | 32 | 43 | 57 |
| Paracetamol | 600/650 | 38 | 16 | 26 | 74 |
| Paracetamol | 975/1000 | 46 | 18 | 34 | 66 |
Appendix 5. Results for participants with at least one adverse event
| Drug | Dose (mg) | Number of | Percent with outcome | Risk ratio (95% CI) | NNH (95% CI) | NNTp (95% CI) | ||
| Studies | Participants | Active | Placebo | |||||
| Aspirin | 500 | 3 | 319 | 7 | 6 | 0.9 (0.4 to 1.9) | ‐ | ‐ |
| Aspirin | 600/650 | 46 | 3633 | 11 | 9.5 | 1.2 (1.0 to 1.4) | ‐ | ‐ |
| Aspirin | 1000 | 4 | 404 | 26 | 12 | 1.6 (1.1 to 2.3) | 7.5 (4.8 to 17) | ‐ |
| Dexketoprofen | 12.5 | 3 | 258 | 9 | 14 | 0.6 (0.4 to 1.3) | ‐ | ‐ |
| Dexketoprofen | 25 | 5 | 413 | 20 | 13 | 1.5 (0.9 to 2.3) | ‐ | ‐ |
| Diclofenac potassium | 25 and 50 | 7 | 1090 | 8 | 46 | 1.0 (0.7 to 1.6) | ‐ | ‐ |
| Dipyrone | 500 | No data | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ibuprofen acid | 200 | 14 | 1808 | 19 | 19 | 1.2 (0.7 to 2.1) | ‐ | ‐ |
| Ibuprofen acid | 400 | 40 | 4867 | 17 | 16 | 0.9 (0.7 to 1.02) | ‐ | ‐ |
| Ibuprofen fast acting | 200 | No data | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ibuprofen fast acting | 400 | No data | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Ibuprofen + caffeine | 200+100 | 4 | 336 | 11 | 6 | 2.2 (1.03 to 4.9) | 19 (8.9 to ‐220) | ‐ |
| Ibuprofen + paracetamol | 200+500 | 3 | 508 | 30 | 48 | 0.7 (0.6 to 0.9) | ‐ | 5.4 (3.6 to 11) |
| Ibuprofen + paracetamol | 400+1000 | 3 | 543 | 29 | 48 | 0.6 (0.5 to 0.8) | ‐ | 5.1 (3.5 to 9.5) |
| Naproxen | 200/220 | No data | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Naproxen | 400/440 | 3 | 334 | 22 | 17 | 1.3 (0.8 to 2.2) | ‐ | ‐ |
| Naproxen | 500/550 | 9 | 784 | 27 | 29 | 1.0 (0.7 to 1.2) | ‐ | ‐ |
| Paracetamol | 500 | 3 | 319 | 7 | 6 | 0.9 (0.4 to 1.9) | ‐ | ‐ |
| Paracetamol | 600/650 | 13 | 1522 | 16 | 14 | 1.2 (0.9 to 1.5) | ‐ | ‐ |
| Paracetamol | 975/1000 | 19 | 2342 | 18 | 16 | 1.1 (0.9 to 1.3) | ‐ | ‐ |
| NNH: number needed to treat for an additional harmful outcome; NNTp: number needed to treat to prevent an adverse event occurring. Note: NNH and NNTp not calculated when result not significantly different from placebo | ||||||||
Contributions of authors
YR and LT performed an initial search for medicines available without prescription, supplemented with additional searches by RAM.
RAM and SD extracted data from Cochrane reviews relating to drug efficacy.
RAM and PW checked data extraction and assessment.
TM provided input and insight from a retail pharmacy perspective.
RAM produced the initial draft, and all authors contributed to developing the manuscript.
RAM will be responsible for updates.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
Institutional support
External sources
No sources of support supplied
Declarations of interest
RAM has no conflicts relating to this review or any similar product.
PJW has no conflicts relating to this review or any similar product.
SD has no conflicts relating to this review or any similar product.
TM has no conflicts relating to this review or any similar product.
YR has no conflicts relating to this review or any similar product.
LT has no conflicts relating to this review or any similar product.
We are funded by the NIHR for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain but this review is not supported by that funding.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Stable (no update expected for reasons given in 'What's new')
References
References to included reviews
Aspirin 2012
- Derry S, Moore RA. Single dose oral aspirin for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD002067.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diclofenac 2015
- Moore RA, Derry S, Wiffen PJ. Single dose oral diclofenac for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004768.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dipyrone 2010
- Edwards J, Meseguer F, Faura C, Moore RA, McQuay HJ, Derry S. Single dose dipyrone for acute postoperative pain. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD003227.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibuprofen + caffeine 2015
- Derry S, Wiffen PJ, Moore RA. Single dose oral ibuprofen plus caffeine for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD011509.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibuprofen + codeine 2015
- Derry S, Karlin S, Moore RA. Single dose oral ibuprofen plus codeine for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD010107.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibuprofen + paracetamol 2013
- Derry CJ, Derry S, Moore RA. Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010210.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibuprofen 2009
- Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral ibuprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001548.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ketoprofen and dexketoprofen 2009
- Barden J, Derry S, McQuay HJ, Moore RA. Single dose oral ketoprofen and dexketoprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007355.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naproxen 2009
- Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral naproxen and naproxen sodium for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004234.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paracetamol 2008
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004602.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to excluded reviews
Aceclofenac 2009
- Moore RA, Derry S, McQuay HJ. Single dose oral aceclofenac for postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007588.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Acemetacin 2009
- Moore RA, Derry S, McQuay HJ. Single dose oral acemetacin for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007589.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Celecoxib 2013
- Derry S, Moore RA. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD004233.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Codeine 2010
- Derry S, Moore RA, McQuay HJ. Single dose oral codeine, as a single agent, for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD008099.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dexibuprofen 2009
- Moore RA, Derry S, McQuay HJ. Single dose oral dexibuprofen [S(+)‐ibuprofen] for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007550.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diflunisal 2010
- Wasey JO, Derry S, Moore RA, McQuay HJ. Single dose oral diflunisal for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD007440.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dihydrocodeine 2000
- Moore RA, Rees J, Derry S, McQuay HJ. Single dose oral dihydrocodeine for acute postoperative pain. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002760] [DOI] [PMC free article] [PubMed] [Google Scholar]
Etodolac 2009
- Tirunagari SK, Derry S, Moore RA, McQuay HJ. Single dose oral etodolac for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007357.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Etoricoxib 2014
- Clarke R, Derry S, Moore RA. Single dose oral etoricoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD004309.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fenbufen 2009
- Moore RA, Derry S, McQuay HJ. Single dose oral fenbufen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007547.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fenoprofen 2011
- Traa MX, Derry S, Moore RA. Single dose oral fenoprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD007556.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flurbiprofen 2009
- Sultan A, McQuay HJ, Moore RA, Derry S. Single dose oral flurbiprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007358.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabapentin 2010
- Straube S, Derry S, Moore RA, Wiffen PJ, McQuay HJ. Single dose oral gabapentin for established acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD008183.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibuprofen + oxycodone 2013
- Derry S, Derry CJ, Moore RA. Single dose oral ibuprofen plus oxycodone for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010289.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Indometacin 2004
- Moore RA, Derry S, Mason L, McQuay HJ, Rees J. Single dose oral indometacin for the treatment of acute postoperative pain. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004308.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lornoxicam 2009
- Hall PE, Derry S, Moore RA, McQuay HJ. Single dose oral lornoxicam for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007441.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumiracoxib 2010
- Roy YM, Derry S, Moore RA. Single dose oral lumiracoxib for postoperative pain in adults. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD006865.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mefenamic acid 2011
- Moll R, Derry S, Moore RA, McQuay HJ. Single dose oral mefenamic acid for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007553.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meloxicam 2009
- Moore RA, Derry S, McQuay HJ. Single dose oral meloxicam for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007552.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabumetone 2009
- Moore RA, Derry S, Moore M, McQuay HJ. Single dose oral nabumetone for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007548.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nefopam 2009
- Kakkar M, Derry S, Moore RA, McQuay HJ. Single dose oral nefopam for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007442.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paracetamol + codeine 2009
- Toms L, Derry S, Moore RA, McQuay HJ. Single dose oral paracetamol (acetaminophen) with codeine for postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001547.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paracetamol ± dextropropoxyphene 1999
- Moore RA, Collins S, Rees J, Derry S, McQuay HJ. Single dose oral dextropropoxyphene, alone and with paracetamol (acetaminophen), for postoperative pain. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001440] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paracetamol ± oxycodone 2009
- Gaskell H, Derry S, Moore RA, McQuay HJ. Single dose oral oxycodone and oxycodone plus paracetamol (acetaminophen) for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002763.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piroxicam 2000
- Moore RA, Rees J, Loke Y, Derry S, McQuay HJ. Single dose oral piroxicam for acute postoperative pain. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002762] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rofecoxib 2009
- Bulley S, Derry S, Moore RA, McQuay HJ. Single dose oral rofecoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004604.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sulindac 2009
- Moore RA, Derry S, McQuay HJ. Single dose oral sulindac for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007540.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tenoxicam 2009
- Moore OA, McIntyre M, Moore RA, Derry S, McQuay HJ. Single dose oral tenoxicam for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007591.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiaprofenic acid 2009
- Moore RA, Derry S, Moore M, McQuay HJ. Single dose oral tiaprofenic acid for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007542.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Barden 2004
- Barden J, Edwards JE, McQuay HJ, Moore AR. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain 2004;107(1‐2):86‐90. [DOI: 10.1016/j.pain.2003.09.021] [DOI] [PubMed] [Google Scholar]
Barden 2006
- Barden J, Derry S, McQuay HJ, Moore RA. Bias from industry trial funding? A framework, a suggested approach, and a negative result. Pain 2006;121(3):207‐18. [DOI: ] [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72:95‐7. [DOI] [PubMed] [Google Scholar]
Crews 2014
- Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clinical Pharmacology and Therapeutics 2014;95(4):376‐82. [DOI: 10.1038/clpt.2013.254] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dale 2015
- Dale O, Borchgrevink PC, Fredheim OM, Mahic M, Romundstad P, Skurtveit S. Prevalence of use of non‐prescription analgesics in the Norwegian HUNT3 population: impact of gender, age, exercise and prescription of opioids. BMC Public Health 2015;15(1):461. [DOI: 10.1186/s12889-015-1774-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2011
- Derry S, Wiffen PJ, Moore RA. Relative efficacy of oral analgesics after third molar extraction ‐ a 2011 update. British Dental Journal 2011;211(9):419‐20. [DOI: 10.1038/sj.bdj.2011.905] [DOI] [PubMed] [Google Scholar]
Derry 2012
- Derry CJ, Derry S, Moore RA. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009281.pub2] [DOI] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Moore RA. Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008040.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2014a
- Derry CJ, Derry S, Moore RA. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD009281.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2014b
- Derry CJ, Derry S, Moore RA. Sumatriptan (all routes of administration) for acute migraine attacks in adults ‐ overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD009108.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
FitzGerald 2001
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase‐2. New England Journal of Medicine 2001;345(6):433‐42. [PUBMED: 11496855] [DOI] [PubMed] [Google Scholar]
Freytag 2014
- Freytag A, Quinzler R, Freitag M, Bickel H, Fuchs A, Hansen H, et al. Use and potential risks of over‐the‐counter analgesics [Gebrauch und potenzielle Risiken durch nicht verschreibungspflichtige Schmerzmittel]. Schmerz 2014;28(2):175‐82. [DOI: 10.1007/s00482-014-1415-5] [DOI] [PubMed] [Google Scholar]
Glenny 2005
- Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D'Amico R, et al. International Stroke Trial Collaborative Group. Indirect comparisons of competing interventions. Health Technology Assessment 2005;9(26):1‐134, iii‐iv. [DOI] [PubMed] [Google Scholar]
Graham 2013
- Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Immunopharmacology 2013;21(3):201‐32. [DOI: 10.1007/s10787-013-0172-x] [DOI] [PubMed] [Google Scholar]
Hawkey 1999
- Hawkey CJ. Cox‐2 inhibitors. Lancet 1999;353(9149):307‐14. [DOI] [PubMed] [Google Scholar]
Hinz 2008
- Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase‐2 inhibitor in man. FASEB Journal 2008;22(2):383‐90. [DOI: 10.1096/fj.07-8506com] [DOI] [PubMed] [Google Scholar]
Jerez‐Roig 2014
- Jerez‐Roig J, Medeiros LF, Silva VA, Bezerra CL, Cavalcante LA, Piuvezam G, et al. Prevalence of self‐medication and associated factors in an elderly population: a systematic review. Drugs and Ageing 2014;31(12):883‐96. [DOI: 10.1007/s40266-014-0217-x] [DOI] [PubMed] [Google Scholar]
Kirthi 2013
- Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008041.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marjoribanks 2015
- Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti‐inflammatory drugs for dysmenorrhoea. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD001751.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2012
- McQuay HJ, Derry S, Eccleston C, Wiffen PJ, Moore RA. Evidence for analgesic effect in acute pain ‐ 50 years on. Pain 2012;153(7):1364‐7. [DOI: 10.1016/j.pain.2012.01.024] [DOI] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66:229‐37. [DOI: 10.1016/0304-3959(96)03032-1] [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 1997;69:127‐30. [DOI: 10.1016/S0304-3959(96)03251-4] [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain 1997;69:311‐15. [DOI: 10.1016/S0304-3959(96)03306-4] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer M, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2005
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta‐analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116(3):322‐31. [DOI: 10.1016/j.pain.2005.05.001] [DOI] [PubMed] [Google Scholar]
Moore 2012
- Moore RA, Derry S, McQuay HJ, Wiffen PJ. A conservative method of testing whether combination analgesics produce additive or synergistic effects using evidence from acute pain and migraine. European Journal of Pain 2012;16(4):585‐91. [DOI: 10.1016/j.ejpain.2011.08.009] [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore RA, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI: 10.1136/bmj.f2690] [DOI] [PubMed] [Google Scholar]
Moore 2013c
- Moore RA, Derry S. Efficacy of OTC analgesics. International Journal of Clinical Practice 2013;178:21‐5. [DOI: 10.1111/ijcp.12054] [DOI] [PubMed] [Google Scholar]
Moore 2014a
- Moore RA, Derry S, Straube S, Ireson‐Paine J, Wiffen PJ. Faster, higher, stronger? Evidence for formulation and efficacy for ibuprofen in acute pain. Pain 2014;155(1):14‐21. [DOI: 10.1016/j.pain.2013.08.013] [DOI] [PubMed] [Google Scholar]
Moore 2014b
- Moore RA, Derry S, Wiffen PJ, Straube S, Bendtsen L. Evidence for efficacy of acute treatment of episodic tension‐type headache: methodological critique of randomised trials for oral treatments. Pain 2014;155(11):2220‐8. [10.1016/j.pain.2014.08.009] [DOI] [PubMed] [Google Scholar]
Moore 2015a
- Moore RA, Derry S, McQuay HJ, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD008659.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015b
- Moore RA, Derry S, Aldington D, Wiffen PJ. Adverse events associated with single dose oral analgesics for acute postoperative pain in adults ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011407.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015c
- Moore RA, Derry S, Straube S, Ireson‐Paine J, Wiffen PJ. Validating speed of onset as a key component of good analgesic response in acute pain. European Journal of Pain 2015;19(2):187‐92. [DOI: 10.1002/ejp.536] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015d
- Moore RA, Derry S, Wiffen PJ, Staube S. Effects of food on pharmacokinetics of immediate release oral formulations of aspirin, dipyrone, paracetamol, and NSAIDs ‐ systematic review. British Journal of Clinical Pharmacology 2015;80(3):381‐8. [DOI: 10.1111/bcp.12628; PUBMED: 25784216] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015e
- Moore RA, Derry S, Wiffen PJ, Straube S, Aldington DJ. Overview review: comparative efficacy of oral ibuprofen and paracetamol (acetaminophen) across acute and chronic pain conditions. European Journal of Pain 2015;19(9):1213‐23. [DOI: 10.1002/ejp.649; PUBMED: 25530283] [DOI] [PubMed] [Google Scholar]
Paulose‐Ram 2003
- Paulose‐Ram R, Hirsch R, Dillon C, Losonczy K, Cooper M, Ostchega Y. Prescription and non‐prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III). Pharmacoepidemiology and Drug Safety 2003;12(4):315‐26. [DOI] [PubMed] [Google Scholar]
Rabbie 2013
- Rabbie R, Derry S, Moore RA. Ibuprofen with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008039.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwab 2003
- Schwab JM, Schluesener HJ, Laufer S. COX‐3: just another COX or the solitary elusive target of paracetamol?. Lancet 2003;361:981‐2. [DOI: 10.1016/S0140-6736(03)12841-3] [DOI] [PubMed] [Google Scholar]
Shea 2007
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:10. [DOI: 10.1186/1471-2288-7-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shehnaz 2014
- Shehnaz SI, Agarwal AK, Khan N. A systematic review of self‐medication practices among adolescents. Journal of Adolescent Health 2014;55(4):467‐83. [DOI: 10.1016/j.jadohealth.2014.07.001] [DOI] [PubMed] [Google Scholar]
Song 2003
- Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. BMJ 2003;326(7387):472. [DOI: 10.1136/bmj.326.7387.472] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2013
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice AS, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010567.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2011
- Zhao L, Pickering G. Paracetamol metabolism and related genetic differences. Drug Metabolism Reviews 2011;43(1):41‐52. [DOI: 10.3109/03602532.2010.527984] [DOI] [PubMed] [Google Scholar]


