Abstract
Objective
Platelet inhibition is a major strategy to prevent acute ischemic cardiovascular and cerebrovascular events, which may, however, be associated with an increased bleeding risk. The (hem)immunoreceptor tyrosine activation motif–bearing platelet receptors, glycoprotein VI (GPVI) and C-type lectin-like receptor 2 (CLEC-2), might be promising antithrombotic targets because they can be depleted from circulating platelets by antibody treatment, leading to sustained antithrombotic protection, but only moderately increased bleeding times in mice.
Approach and Results
We investigated whether both (hem)immunoreceptor tyrosine activation motif–bearing receptors can be targeted simultaneously and what the in vivo consequences of such a combined therapeutic GPVI/CLEC-2 deficiency are. We demonstrate that isolated targeting of either GPVI or CLEC-2 in vivo does not affect expression or function of the respective other receptor. Moreover, simultaneous treatment with both antibodies resulted in the sustained loss of both GPVI and CLEC-2, while leaving other activation pathways intact. However, GPVI/CLEC-2–depleted mice displayed a dramatic hemostatic defect and profound impairment of arterial thrombus formation. Furthermore, a strongly diminished hemostatic response could also be reproduced in mice genetically lacking GPVI and CLEC-2.
Conclusions
These results demonstrate that GPVI and CLEC-2 can be simultaneously downregulated in platelets in vivo and reveal an unexpected functional redundancy of the 2 receptors in hemostasis and thrombosis. These findings may have important implications of the potential use of anti-GPVI and anti–CLEC-2–based agents in the prevention of thrombotic diseases.
Keywords: CLEC-2, GPVI, hemostasis, platelets, thrombosis
At sites of vessel wall injury components of the extracellular matrix, most importantly, collagens are exposed to the flowing blood that triggers sudden platelet activation and platelet plug formation, followed by coagulant activity and the formation of fibrin-containing thrombi that occlude the site of injury. These events are crucial to prevent posttraumatic blood loss, but they are also a major pathomechanism in arterial thrombosis.1,2 Glycoprotein VI (GPVI) is the central platelet activating collagen receptor and is noncovalently associated with the FcRγ-chain that carries an immunoreceptor tyrosine activation motif (ITAM). Binding of GPVI to exposed subendothelial collagens finally results in platelet activation and subsequent thrombus growth.3 Patients4 and mice5–8 lacking GPVI display defective platelet responses to collagen, but only mild bleeding tendencies make this receptor a potential target for effective and safe antithrombotic therapy.9 We have previously shown that in vivo treatment of mice with anti-GPVI antibodies leads to downregulation of the receptor from the surface of circulating platelets by internalization and ectodomain shedding involving multiple proteases, resulting in a GPVI knockout-like phenotype and long-term antithrombotic protection but only very moderate effects on normal hemostasis.10,11 A comparable antibody-mediated GPVI depletion has also been observed in platelets of autoimmune patients, who had developed anti-GPVI antibodies,4 or in human platelets circulating in nonobese diabetic/severe combined immunodeficiency mice.12
Another receptor that mediates strong platelet activation is CLEC-2, a C-type lectin-like type II transmembrane receptor, that was identified as the receptor for the platelet activating snake venom, rhodocytin.13 Interestingly, CLEC-2 is a so-called hemITAM receptor containing only a single cytoplasmic YXXL motif that uses a similar signaling pathway as the GPVI/FcRγ-chain complex.14 On CLEC-2 engagement, hemITAM phosphorylation of CLEC-2 is mediated by the tyrosine kinase, Syk, which is essential for signaling and downstream phosphorylation of effector proteins, including PLCγ2.15 A developmental role for CLEC-2, which is the receptor for the lymphatic endothelial cell–expressed protein podoplanin, has been described as the constitutive CLEC-2 knockout led to embryonic/neonatal lethality in mice caused by blood-lymphatic misconnection and severe edema.16–18 However, how platelets mediate vessel separation is, at present, unclear and still controversially discussed.18–20
Principally, CLEC-2 might become a target for antithrombotic agents, but the lethality of CLEC-2 knockout mice has made studies on the function of the receptor in hemostasis and thrombosis difficult.17,21 We have demonstrated that CLEC-2 can also be downregulated in platelets by in vivo administration of a monoclonal anti–CLEC-2 antibody (INU1). Such CLEC-2–depleted mice display reduced thrombus stability and are protected from vessel occlusion in thrombosis models but show only moderately increased bleeding times.22 Shortly later, 2 studies reported partially conflicting results on the role of CLEC-2 in hemostasis and thrombosis using chimeric mice lacking CLEC-2 in the hematopoietic system (Clec2−/−), suggesting a significant or no involvement of the receptor in these processes.17,21
Here, we investigated whether the simultaneous targeting and thus downregulation of GPVI and CLEC-2, which are the only (hem)ITAM-coupled receptors in mouse platelets,23 is possible and what the functional consequences of such a treatment are. We showed that both receptors can be specifically downregulated simultaneously. Remarkably, loss of both (hem)ITAM receptors resulted in severely defective hemostasis and arterial thrombus formation, revealing partially redundant functions of GPVI and CLEC-2 in vivo.
Results
Independent and Simultaneous Downregulation of GPVI and CLEC-2 In Vivo
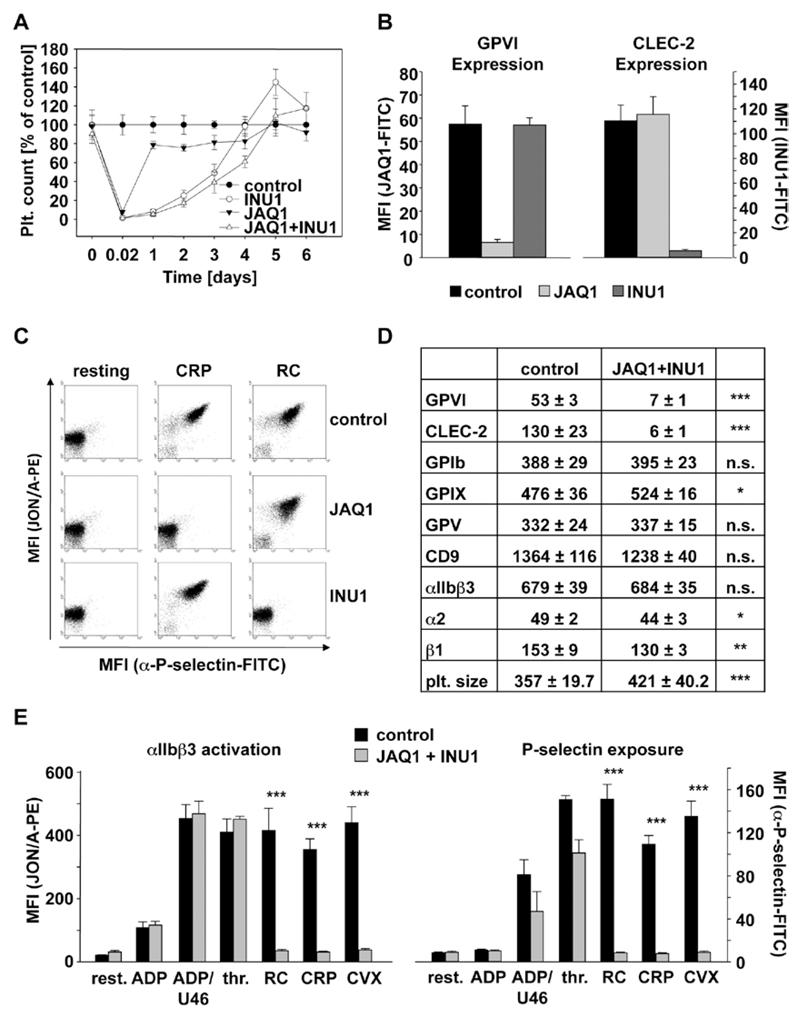
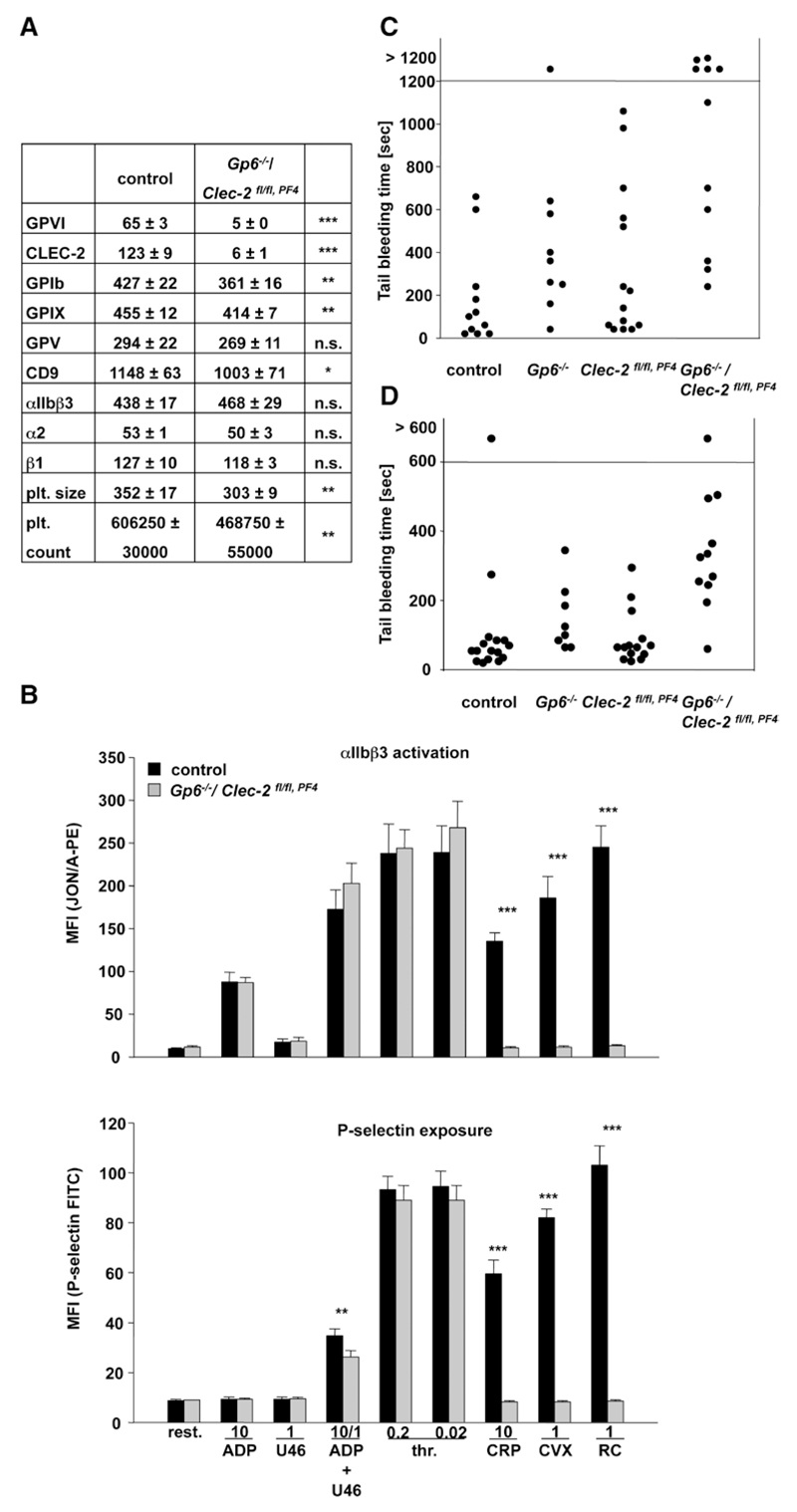
Mice were injected intravenously with the anti-GPVI antibody JAQ1 (100 µg), the anti–CLEC-2 antibody INU1 (200 µg), or both antibodies in combination. Although JAQ1 treatment induced a rapidly reversible thrombocytopenia, a more sustained thrombocytopenia was observed in mice treated with INU1 or JAQ1/INU1, with recovery to normal platelet counts on day 5 to 6 (Figure 1A). JAQ1 treatment induced the complete loss of GPVI but had no effect on CLEC-2 surface expression levels. Similarly, INU1 treatment induced the complete loss of CLEC-2 from the platelet surface but had no effect on GPVI expression (Figure 1B). Moreover, collagen-related peptide–induced GPVI signaling in CLEC-2-depleted platelets was not affected and also, vice versa, CLEC-2 signaling induced by rhodocytin was unaltered in GPVI-depleted platelets (Figure 1C). Platelets from mice treated with JAQ1 and INU1 specifically lacked GPVI and CLEC-2, whereas expression of other surface proteins was not or only slightly (GPIX, integrin α2β1) altered (Figure 1D). Slightly increased size of double-deficient platelets was observed on days 5 to 7, which is in agreement with general observations made after antibody-induced thrombocytopenia (Figure 1D). Double-deficient platelets were specifically refractory to the GPVI and CLEC-2 agonists, collagen-related peptide, convulxin, and rhodocytin, respectively (Figure 1E, integrin activation, left; P-selectin exposure, right). Only slightly decreased P-selectin exposure after thrombin stimulation was observed at early (Figure 1E) but not later time points (not shown), in line with previous observations made in JAQ1-treated mice.24 Similarly, double-deficient platelets showed absent aggregation responses to GPVI- or CLEC-2 specific agonists, whereas the cells normally aggregated in response to other agonists (Figure I in the online-only Data Supplement). These data clearly show that targeting of 1 (hem)ITAM bearing receptor specifically downregulates its expression and activity on the platelet surface but does not influence the expression and signaling-induced pathway of the other respective (hem) ITAM bearing receptor. Moreover, it is possible via simultaneous injection of both antibodies, JAQ1 and INU1, to completely shut off ITAM signaling in mouse platelets without affecting signaling by G protein–coupled receptors.
Figure 1.
Analysis of mice deficient in Glycoprotein VI (GPVI) and C-type lectin-like receptor 2 (CLEC-2) on antibody injection. A, Mice were intravenously injected with 100 µg JAQ1 and 200 µg INU1 in sterile PBS, and platelet counts were determined on a FACSCalibur at the indicated time points post injection. Results are mean±SD in % of control animals (n=5 mice per group, representative for 2 individual experiments). B and D, Flow cytometric analysis of surface protein expression 5 days post injection with the indicated antibodies. Platelets were stained for 15 minutes at room temperature with the indicated fluorophore-labeled antibodies and directly analyzed. Platelet count in number of platelets/µL. Platelet size is given as mean forward scatter (FSC) and was determined by FSC characteristics. Results are mean fluorescence intensities (MFI)±SD (n=5, representative of at least 3 independent measurements). *P<0.05; **P<0.01; ***P<0.001. C and E, Flow cytometric analysis of integrin αIIbβ3 activation (JON/A-PE) and degranulation-dependent P-selectin exposure on platelets on day 5 post injection. Washed blood was incubated with the indicated agonists for 15 minutes and analyzed on a FACSCalibur. Results are mean±SD (n=5 mice per group, representative of 3 independent experiments). ***P<0.001. ADP: 10 µmol/L; U46619: 3 µmol/L; thrombin: 0.01 U/mL; rhodocytin (RC): 1 µg/mL; collagen-related peptide (CRP): 10 µg/mL; convulxin (CVX): 1 µg/mL. All experiments were performed on day 5 to 6 after antibody injection. FITC indicates fluorescein isothiocyanate.
Severely Defective Hemostasis and Arterial Thrombus Formation in GPVI/CLEC-2 Double-Depleted Mice
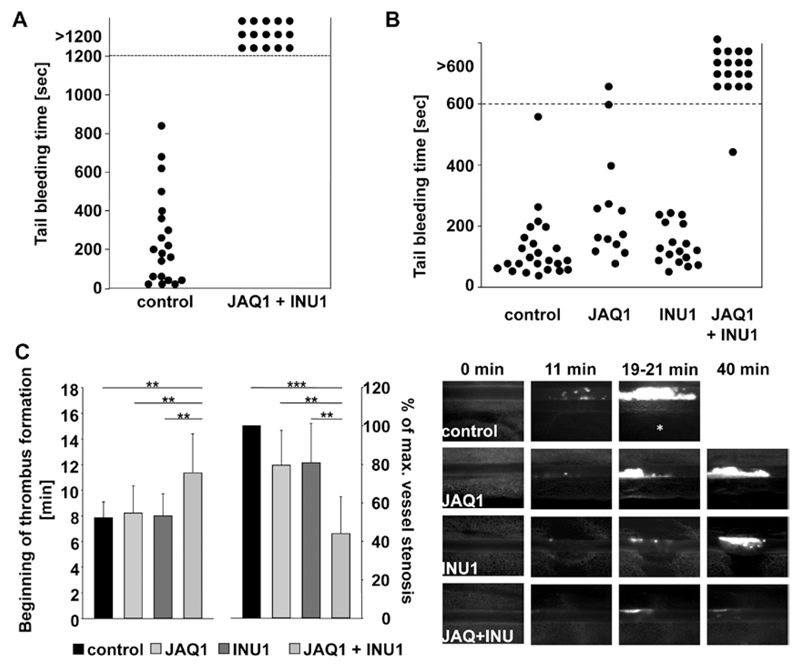
We have previously shown that JAQ1-treated mice display very mild prolonged bleeding times and also INU1-treated mice show only moderately increased, but generally more, variable tail bleeding times in the filter paper model,10,22 and this was confirmed in the current study (Figure II in the online-only Data Supplement). Remarkably, however, the depletion of both (hem)ITAM-bearing receptors led to a virtually complete loss of hemostatic activity as evident by the lack of cessation of tail bleeding (Figure 2A). The same observation was made when the wound of the tail tip was immersed in 37°C prewarmed saline. Here, single-deficient mice displayed normal hemostatic function, whereas double-deficient mice displayed again a strong bleeding phenotype (Figure 2B). Importantly, however, we did not observe any signs of spontaneous bleeding in any of these animals. These data suggest that GPVI and CLEC-2 may have at least partially redundant roles in hemostasis but that their simultaneous loss does not induce spontaneous hemorrhage.
Figure 2.
Determination of hemostatic function and pathological thrombus formation in Glycoprotein VI (GPVI)/C-type lectin-like receptor 2 (CLEC-2)–depleted mice. A, A 1-mm segment of the tail tip was cut, and bleeding was determined to have ceased when no blood drop was observed on the filter paper. Each symbol represents 1 individual. B, A 1-mm segment of the tail tip was cut, and the tail tip was immersed in saline. Each symbol represents 1 individual. Differences of bleeding times between wild-type (WT), single GPVI-depleted, and single CLEC-2–depleted mice are nonsignificant. C, Mesenteric arterioles were treated with 20% FeCl3, and adhesion and thrombus formation of fluorescently labeled platelets were monitored by in vivo fluorescence microscopy. Statistical evaluation of the time to appearance of a first thrombus (left) and percentage of maximal vessel stenosis (right) are depicted, n≥10. At most 2 arterioles of each mouse were analyzed. **P<0.01; ***P<0.001. Vessel stenosis was determined by measuring maximal thrombus size divided by vessel diameter using the Metamorph software (Visitron). Representative images are shown. White asterisk indicates occluded vessel. All experiments were performed on day 5 to 6 after antibody injection.
The effect of single- and double-receptor depletion on pathological thrombus formation was studied by intravital fluorescence microscopy of ferric chloride–injured mesenteric arterioles.7,22 In control mice, small aggregate formation was observed at 7.8±1.2 minutes after injury (Figure 2C, left), with complete vessel occlusion occurring at 16.4±2.2 minutes (not shown, Video I in the online-only Data Supplement). GPVI and CLEC-2 single-depleted mice showed similar kinetics of small aggregate formation, whereas in most cases the vessels did not occlude (GPVI-depleted: 8/12, Video II in the online-only Data Supplement; CLEC-2-depleted: 7/10, Video III in the online-only Data Supplement, see also references).7,22 Remarkably, onset of small aggregate formation was significantly delayed in GPVI/CLEC-2–depleted mice (Figure 2C, left), and the maximal vessel stenosis reached within the 40-minute observation period was strongly reduced compared with all other groups (Figure 2C, right). As a consequence, blood flow was maintained in all vessels (12/12, Video IV in the online-only Data Supplement). Representative images from the experiment are shown (Figure 2C). These data demonstrate that the lack of both GPVI and CLEC-2 results in almost completely abolished thrombus formation, suggesting partially redundant functions of the 2 receptors in vivo and that their simultaneous targeting provides profound antithrombotic protection, but also severely impairs normal hemostasis
Defective Hemostasis in CLEC-2–Depleted Gp6−/− Mice
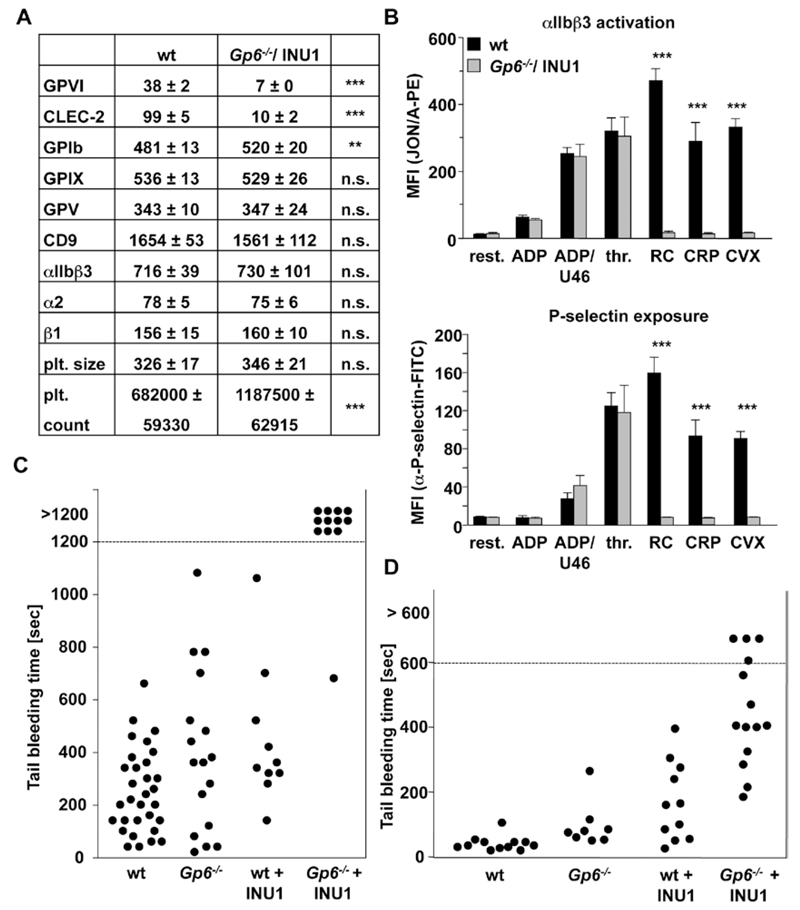
The severe hemostatic defect in JAQ1/INU1-treated wild-type mice indicated that the therapeutic depletion of either receptor may induce bleeding in individuals, genetically deficient in or expressing very low levels of the respective other receptor. To test this hypothesis directly, we studied platelet function in newly generated Gp6−/− mice (Figure III in the online-only Data Supplement) on day 5 after vehicle or INU1 treatment. As expected, Gp6−/− platelets were refractory to GPVI specific agonists as measured by flow cytometry and aggregometry, whereas responses to other agonists were normal (not shown). In contrast, platelets from Gp6−/−/INU1-treated mice lacked GPVI and CLEC-2 (Figure 3A) and were unresponsive toward collagen-related peptide, convulxin, and rhodocytin, whereas all other tested activation pathways were unaffected (Figure 3B). Slightly increased GPIb expression levels were noted, which may be explained by the slightly increased platelet size. Similar to double-depleted mice (Figure 2A and 2B), CLEC-2–depleted Gp6−/− mice showed a severe hemostatic defect in both bleeding time assays (filter paper: Figure 3C; saline: Figure 3D), mirroring the JAQ1/INU1 antibody-induced double deficiency.
Figure 3.
Analysis of Glycoprotein VI (Gp6−/−)/C-type lectin-like receptor 2 (CLEC-2)–depleted mice. A, Flow cytometric analysis of surface protein expression of Gp6−/− platelets 5 days post injection with the anti–CLEC-2 antibody INU1. Platelets were stained for 15 minutes at room temperature with the indicated fluorophore-labeled antibodies and directly analyzed. Platelet count in number of platelets/µL. Platelet size is given as mean forward scatter (FSC) and was determined by FSC characteristics. Results are mean fluorescence intensities (MFI)±SD (n=5, representative of at least 3 independent measurements). **P<0.01; ***P<0.001. B, Flow cytometric analysis of degranulation-dependent P-selectin exposure and integrin αIIbβ3 activation on platelets. Washed blood was incubated with the indicated agonists for 15 minutes at room temperature and analyzed on a FACSCalibur. Results are mean±SD (n=5 mice per group, representative of 3 individual experiments). ***P<0.001. ADP: 10 µmol/L; U46619: 3 µmol/L; thrombin (thr): 0.01 U/mL; rhodocytin (RC): 1 µg/mL; collagen-related peptide (CRP): 10 µg/mL; convulxin (CVX): 1 µg/mL. C, A 1-mm segment of the tail tip was cut, and bleeding was determined to have ceased when no blood drop was observed on the filter paper. Each symbol represents 1 individual. Differences of bleeding times among control, Gp6−/− mice, and CLEC-2–-depleted mice are nonsignificant. D, An 1-mm segment of the tail tip was cut, and the tail tip was immersed in saline. Each symbol represents 1 individual. All experiments were performed on day 5 to 6 after antibody injection. Bleeding time of Gp6−/−/CLEC-2–depleted mice is significantly prolonged compared with control and Gp6−/− mice ***P<0.001, and to CLEC-2–depleted mice **P<0.01. Bleeding time of CLEC-2–depleted mice is prolonged compared with control **P<0.01. WT indicates wild-type mice.
Defective Hemostasis in Mice Genetically Deficient in Platelet GPVI and CLEC-2
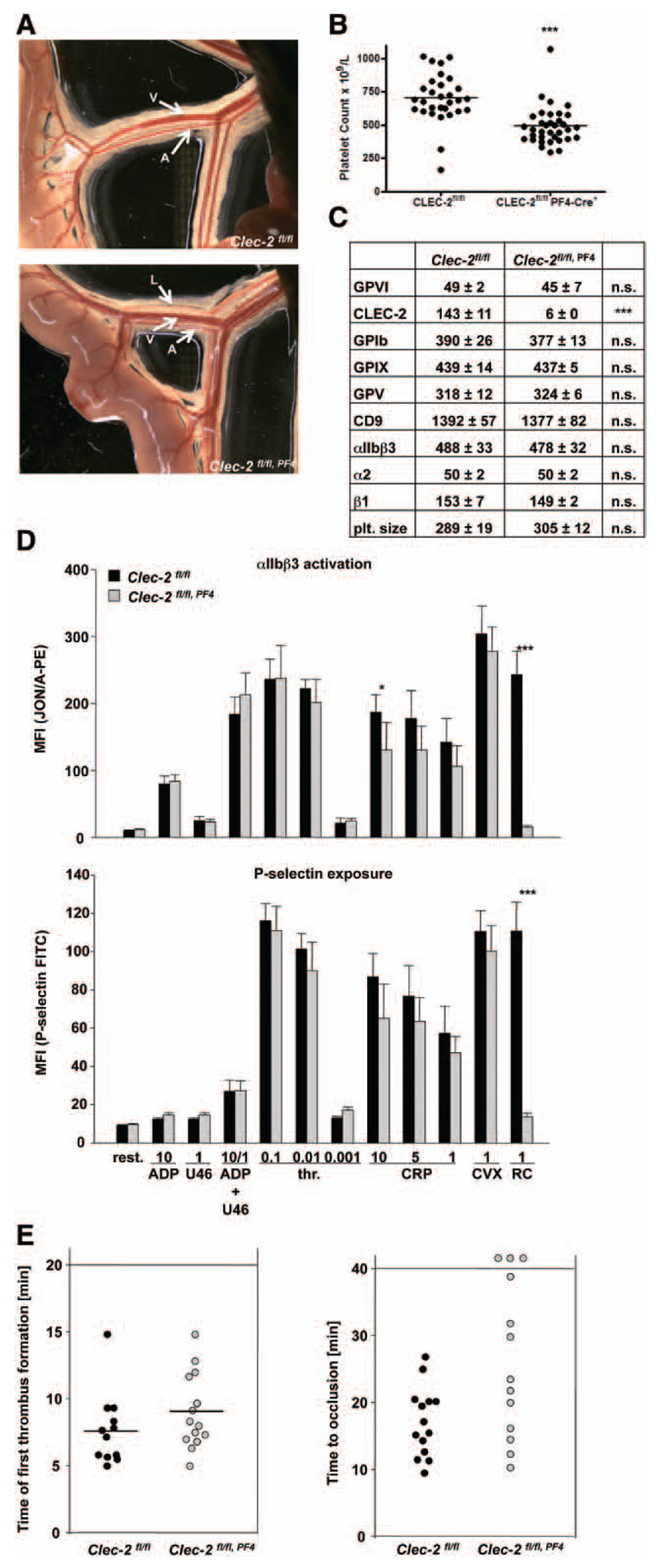
To test the possibility that side effects of the antibody treatment contributed to the observed bleeding phenotype in receptor-depleted animals, we generated mice genetically deficient in both receptors in platelets. Because mice constitutively lacking CLEC-2 die perinatally,17,21 Clec-2fl/fl, Pf4-Cre mice specifically lacking CLEC-2 in megakaryocytes and platelets were used for analysis.18 However, as previously described, these mice are not healthy in that they display a pronounced defect in blood–lymph separation (Figure 4A)16–18,20 and other vascular defects, which may influence the hemostatic system as indicated by a reduction in platelet count to ≈70% of control (Figure 4B). Platelets of Clec-2fl/fl, Pf4-Cre mice lacked CLEC-2, whereas all other tested surface receptors were normally expressed (Figure 4C). Consequently, rhodocytin-induced platelet activation was abolished in the mutant cells, whereas responses to other agonists were fully intact (Figure 4D, integrin activation and P-selection exposure and Figure IV in the online-only Data Supplement: aggregometry). We have previously shown that CLEC-2 single-depleted mice display normal small aggregate formation in FeCl3-injured mesenteric arterioles but were, in most cases, unable to fully occlude the vessels.22 Similarly, Clec-2fl/fl, Pf4-Cre mice showed only slightly, but not significantly, delayed first appearance of small thrombi (Figure 4E, left), and vessel occlusion was in most of the animals delayed or absent (Figure 4E, right and Videos V and VI in the online-only Data Supplement). These results indicated that antibody-induced and genetic loss of platelet CLEC-2 provides comparable protection from occlusive thrombus formation.
Figure 4.
Analysis of megakaryocyte/platelet-specific C-type lectin-like receptor 2 (CLEC-2)–deficient mice. A, Representative images of the intestine are shown. L indicates lymphatic vessel; A, arteriole; and V, vein. B, Platelet count was determined by flow cytometric analysis. ***P<0.001. C, Flow cytometric analysis of surface protein expression. Platelets were stained for 15 minutes at room temperature with the indicated fluorophore-labeled antibodies and directly analyzed. Platelet size is given as mean forward scatter (FSC) and was determined by FSC characteristics. Results are mean fluorescence intensities (MFI)±SD (n=4, representative of at least 3 independent measurements). ***P<0.001. D, Flow cytometric analysis of degranulation-dependent P-selectin exposure and integrin αIIbβ3 activation on platelets. Washed blood was incubated with the indicated agonists for 15 minutes at room temperature and analyzed on a FACSCalibur. Results are mean±SD (n=5 mice per group, representative of 3 individual experiments). *P<0.05; ***P<0.001. ADP [µmol/L]; U46619: [µmol/L); thrombin (thr): [U/mL]; rhodocytin (RC): [µg/mL]; collagen-related peptide (CRP): [µg/mL]; convulxin (CVX): [µg/mL]. E, Mesenteric arterioles were treated with 20% FeCl3 and adhesion, and thrombus formation of fluorescently labeled platelets were monitored by in vivo fluorescence microscopy. Evaluation of the time to appearance of a first thrombus (left) and time to vessel occlusion (right) are depicted. n≥10. Time of first appearance of thrombi is nonsignificant. Time to occlusion is *P<0.05. GPVI indicates Glycoprotein VI.
We intercrossed Gp6−/− and Clec-2fl/fl mice and thereafter mated Gp6−/−/Clec-2fl/fl females with Gp6−/−/Clec-2fl/fl, Pf4-Cre males to obtain double-deficient animals. These breedings only yielded small litters (2–6 mice) and <35% Gp6−/−/Clec-2fl/fl, Pf4-Cre mice, indicating increased embryonic or perinatal lethality. The surviving Gp6−/−/Clec-2fl/fl, Pf4-Cre animals displayed dramatically altered vascular structure and blood-filled lymphatics in the intestine, and this phenotype was clearly more pronounced than in CLEC-2 single-deficient mice (Figure V in the online-only Data Supplement). Flow cytometric analysis confirmed the absence of both receptors in the platelets of Gp6−/−/Clec-2fl/fl, Pf4-Cre mice, which was associated with some minor changes in the expression pattern of other surface receptors and a moderately reduced platelet count, similar to that observed in CLEC-2 single-deficient mice (Figure 5A). As expected, the platelets of these mice showed a complete loss of (hem)ITAM signaling as revealed by measurement of αIIbβ3 activation and P-selection expression with GPVI or CLEC-2 specific agonists, while leaving activation of these pathways by ADP, thromboxane, and thrombin receptors intact (Figure 5B). In addition, we studied GPIb function in GPVI- and CLEC-2–deficient platelets by 2 different assays, namely platelet spreading on a vWF-coated matrix25 and platelet adhesion on vWF under flow conditions.26 In both cases, there was no significant difference as compared with control, indicating intact GPIb function in the mutant platelets (data not shown).
Figure 5.
Analysis of Glycoprotein VI (GPVI) and C-type lectin-like receptor 2 (CLEC-2) double-mutant mice. A, Flow cytometric analysis of surface protein expression of Gp6−/−/Clec-2fl/fl, Pf4-Cre platelets. Platelets were stained for 15 minutes at room temperature with the indicated fluorophore-labeled antibodies and directly analyzed. Platelet count in number of platelets/µL. Platelet size is given as mean forward scatter (FSC) and was determined by FSC characteristics. Results are mean fluorescence intensities (MFI)±SD (n=5, representative of at least 3 independent measurements). *P<0.05; **P<0.01; ***P<0.001. B, Flow cytometric analysis of degranulation-dependent P-selectin exposure and integrin αIIbβ3 activation on platelets. Washed blood was incubated with the indicated agonists for 15 minutes at room temperature and analyzed on a FACSCalibur. Results are mean±SD (n=4 mice per group, representative of 3 individual experiments). **P<0.01; ***P<0.001. ADP [µmol/L]; U46619: [µmol/L]; thrombin (thr): [U/mL]; rhodocytin (RC): [µg/mL]; collagen-related peptide (CRP): [µg/mL]; convulxin (CVX): [µg/mL]. C, A 1-mm segment of the tail tip was cut, and bleeding was determined to have ceased when no blood drop was observed on the filter paper. Each symbol represents 1 individual. Fisher test: Gp6−/−/Clec-2fl/fl, Pf4-Cre vs control: 0.0351; Gp6−/−/Clec-2fl/fl, Pf4-Cre vs Clec-2fl/fl, Pf4-Cre: 0.0087. Other conditions nonsignificant. D, An 1-mm segment of the tail tip was cut, and the tail tip was immersed in saline. Each symbol represents 1 individual. Bleeding time of Gp6−/−/Clec-2fl/fl, Pf4-Cre mice is prolonged compared with control and Clec-2fl/fl, Pf4-Cre*, P<0.05.
To test the effect of genetic GPVI/CLEC-2 double deficiency on hemostasis, tail bleeding times in mutant and control mice were assessed by the filter paper model (Figure 5C) and the saline model (Figure 5D). Gp6−/−/Clec-2fl/fl, Pf4-Cre mice showed in both models markedly prolonged bleeding times as compared with control or single-deficient mice confirming that GPVI and CLEC-2 have unexpected redundant roles in normal hemostasis. The bleeding time prolongation was less pronounced than in double-depleted mice, which may at least partially be explained by the vascular alterations and the reduced general state of health in these animals.
Attempts to study thrombus formation by intravital microscopy in FeCl3-injured mesenteric arterioles as shown for GPVI/CLEC-2–depleted mice (which displayed no vessel separation defect [Figure VI in the online-only Data Supplement]) failed for Gp6−/−/Clec-2fl/fl, Pf4-Cre mice because of a dramatically altered vascular structure and blood-filled lymphatics in the intestine of these animals (Figure V in the online-only Data Supplement).
Discussion
In this study, we have shown that the 2 major (hem)ITAM receptors, GPVI and CLEC-2, can be simultaneously depleted with high specificity in circulating platelets in vivo and that their combined loss results in a severe hemostatic defect and virtually abolished thrombus formation in mice. These findings reveal for the first time that GPVI and CLEC-2 have partially redundant functions in normal hemostasis and pathological thrombus formation and that their simultaneous targeting may be an effective, but not necessarily safe antithrombotic approach.
Both activatory receptors have been proposed as possible pharmacological targets for antithrombotic therapy because they can easily be immunodepleted from circulating platelets in vivo, resulting in a knockout-like phenotype for the respective receptor for a prolonged period of time.10,22 Such a targeted downregulation of GPVI or CLEC-2 provides profound antithrombotic protection in different models of thrombosis, while having only (very) moderate effects on normal hemostasis.7,10,17,21,22 In this study, we could confirm these previous findings and show that the antibody-induced loss of either GPVI or CLEC-2 does not affect expression or function of the respective other receptor. This seems to be different from the previously described phenomenon in human platelets in which cross-inhibition between GPVI and another ITAM-bearing receptor, FcγRIIa, which is only present on human but not mouse platelets, was observed.23 Gardiner et al27 showed that FcγRIIa-ligation resulted in metalloproteinase-mediated ectodomain shedding of GPVI in vitro, demonstrating that signaling by 1 ITAM-bearing receptor not only influences its own expression and signaling but also causes effects on the other ITAM-bearing receptor. This suggests that this transinhibition effect of FcγRIIa and GPVI in human platelets in vitro occurs through a mechanism that is not operating in the regulation of CLEC-2 and GPVI in mouse platelets in vivo. Recently, a role for FcγRIIa as a functional conduit for αIIbβ3-mediated outside-in signaling and thus in controlling thrombosis was described in human platelets.28
The antibody-induced downregulation of GPVI occurs through 2 different pathways, namely internalization/degradation and more importantly metalloproteinase-dependent ectodomain shedding.11,29 Both processes require signaling through the FcRγ-chain ITAM29 and can occur in circulating platelets and presumably also in megakaryocytes.10 This GPVI immunodepletion seems to be very specific because GPVI-depleted and Gp6−/− mice display virtually identical defects in different thrombosis models and a comparable minor prolongation of tail bleeding times.7 In contrast, much less is known about the mechanisms underlying the antibody-induced loss of CLEC-2 in platelets, which is associated with a prolonged phase of marked thrombocytopenia.22 It is currently not clear whether the induced loss of CLEC-2 can occur in circulating platelets in the periphery or also in megakaryocytes and whether it is mediated by ectodomain shedding, internalization or another, yet undefined mechanism. However, very similar to the loss of GPVI, CLEC-2 depletion is a surprisingly specific process leaving G protein–coupled receptor signaling pathways largely intact (Figure 1B and 1C and data not shown).22 We have previously shown that CLEC-2–depleted mice display variable tail bleeding times when assessed in the filter paper model, which was also confirmed here (Figure IIB in the online-only Data Supplement).22 In contrast to this, we have also reported that radiation chimeric mice lacking CLEC-2 in the hematopoietic system have unaltered tail bleeding times compared with wild-type controls when assessed using a version of the tail bleeding assay, which monitors the time to cessation of bleeding without the use of filter paper.21 Interestingly, we also found no prolongation of tail bleeding times in CLEC-2–depleted mice using a third version of this assay, namely monitoring bleeding in saline (Figure 2B), suggesting that the mechanisms contributing to hemostasis in the various bleeding time models may be partially different and that CLEC-2 depletion very well mirrors genetic loss of CLEC-2 in platelets. This is further corroborated by the observation that Clec-2fl/fl, Pf4-Cre mice show a very similar thrombus formation defect as CLEC-2–depleted mice as revealed by intravital microscopy using the ferric chloride injury model (Figure 4E and Videos V and VI in the online-only Data Supplement).22 We tested whether the thrombus formation defect in CLEC-2–deficient mice could be attributable to a defect in fibrinogen binding. However, we found no differences between CLEC-2 depleted and control platelets when assessing spreading on a fibrinogen-coated surface, adhesion under flow on a fibrinogen matrix (rate: 1000/s; data not shown),30 or aggregation in response to ADP, thromboxane, and thrombin receptor activation. Together, the data indicate that the targeted depletion of GPVI or CLEC-2 in circulating platelets can be induced in a highly specific manner and reproduces the phenotypes observed in mice with genetic deletions of these receptors.
The simultaneous injection of both antibodies, JAQ1 and INU1, resulted in a highly selective and complete loss of GPVI and CLEC-2 in platelets, respectively, while leaving other activation pathways largely intact. This demonstrates for the first time that it is possible to completely delete (hem)ITAM receptor function in circulating platelets in vivo. Further, the normal platelet count in these animals demonstrates that neither receptor is required for the steady level of platelet production. We found that the combined loss of GPVI and CLEC-2 resulted in markedly impaired hemostasis and a severe thrombus formation defect that by far exceeded that seen in GPVI- or CLEC-2 single-depleted animals (Figure 2). Possible off-target effects of the antibody treatment likely do not explain this pronounced defect because it was fully reproduced in CLEC-2–depleted Gp6−/− mice and in Gp6−/−/Clec-2fl/fl, Pf4-Cre mice, although to a somewhat lesser extent in the latter which most likely could be attributable to the mixture of their blood and lymph (Figures 3C, 3D, 5C, and 5D) and other vascular defects that may account for their increased embryonic/perinatal lethality and reduced general state of health at the adult stage. This assumption is also corroborated because neither the coagulation system (activated partial thromboplastin time and prothrombin time, Figure VII in the online-only Data Supplement) was impaired nor relevant cytokine levels were released in double-depleted mice (Figure VIII in the online-only Data Supplement), further excluding off-target effects of antibody treatment. Together, these findings demonstrate that GPVI and CLEC-2 have partially redundant functions in hemostasis and occlusive thrombus formation, but the exact underlying mechanisms remain to be determined. For normal hemostasis, however, classic (hem)ITAM signaling downstream of the 2 receptors does not seem to be essential because mice lacking Syk, a crucial proximal molecule in this signaling pathway, did not show such a bleeding defect.31 Similarly, it has been reported that the Syk inhibitor, PRT060318, did not affect hemostasis in mice.32 Together, these findings point to functions of GPVI, CLEC-2, or both receptors in hemostasis and possibly also thrombosis independent of their classic signal transduction capacity. On the basis of this assumption, one may speculate that adhesive functions of these receptors and their ability to bind and activate putative counter receptors in platelets might account for this unexpected activity. However, currently no intravascular ligands for GPVI and CLEC-2 are known, but based on our results we postulate that they may exist.
Taken together, we have demonstrated that antibody-mediated independent and simultaneous downregulation of the platelet activating proteins, GPVI and CLEC-2, is possible and revealed unexpected redundant functions of these 2 receptors in arteriolar thrombus formation but also, and more importantly, in normal hemostasis in mice. Although data obtained in mice cannot be directly extrapolated to the human system (which is further complicated by the possible role of a third ITAM receptor, FcγRIIa, which is absent in the mouse genome), these results indicate that anti-GPVI or anti–CLEC-2 treatment might bear the risk of uncontrolled bleeding in patients exhibiting defects in the respective other (hem)ITAM signaling pathway. Supporting data come from a very recent study that has appeared during revision of this article showing that the ITAM receptors, GPVI and CLEC-2, are critical for vascular integrity in inflammatory processes.33 Our results may have important implications for the development of anti-GPVI and anti–CLEC-2–based antithrombotic therapeutics.
Materials and Methods
Mice
Male NMRI and C57BL/6JRj mice 3-6 weeks of age were obtained from Harlan (Borchen, Germany) or Janvier (Le Genest St. Isle, France). Animal studies were approved by the local authorities (Bezirksregierung Unterfranken). Mice were intravenously injected with 100 µg anti-GPVI (JAQ1) and/or with 200 µg anti-CLEC-2 (INU1) antibody. Gp6-/- mice were generated as described in the supplement. Clec-2fl/fl 1 and Pf4-Cre2 mice were described earlier.
Reagents and Antibodies
The anesthetic drugs medetomidine (Pfizer), midazolam (Roche), fentanyl (Janssen-Cilag), and the antagonists atipamezol (Pfizer), flumazenil (Delta Select) and naloxon (Delta Select) were used according to the regulation of the local authorities. High-molecular-weight heparin (Ratiopharm), Apyrase Grade III, human fibrinogen, ADP (Sigma-Aldrich), prostacycline (PGI2, Calbiochem), U-46619 (Enzo Life Sciences), thrombin (Roche), collagen (Kollagenreagens Horm; Nycomed), convulxin (Axxora), were purchased. Collagen-related peptide (CRP) was generated as previously described.3 Rhodocytin was isolated as described.4 JON/A-PE antibody against the activated form of integrin αIIbβ3 was from Emfret Analytics. All other antibodies were generated and modified in our laboratory as previously described.5, 6
Determination of Platelet Count, Size, Surface Protein Expression and Platelet Activation
To measure platelet size and surface protein expression, heparinized blood was diluted 1:20 and stained for 15 minutes with saturating amounts of fluorophore-conjugated antibodies and immediately analyzed on a FACSCalibur (Becton Dickinson, Heidelberg, Germany). For platelet activation, samples were activated with agonists at the indicated concentrations, stained with fluorophore-conjugated monoclonal antibodies at saturating concentrations for 15 minutes at 37°C and directly analyzed.
Tail Bleeding Time
Filter paper
Mice were anesthetized and a 1-mm segment of the tail tip was removed with a scalpel. Tail bleeding was monitored by gently absorbing blood with filter paper at 20-second intervals, without making contact with the wound site. When no blood was observed on the paper, bleeding was determined to have ceased. Otherwise, experiments were stopped after 20 minutes.
Saline
Mice were anesthetized and 1 mm of the tail tip was cut off. Immediately, tails were immersed in 0.9% isotonic saline at 37°C. The time until stop of bleeding (no blood flow for longer than 1 minute) was determined. Otherwise, experiments were stopped after 10 minutes.
Intravital Microscopy of Thrombus Formation in FeCl3-Injured Mesenteric Arterioles
Mice (15-18 g or 4-5 weeks old) were anesthetized, and the mesentery was exteriorized through a midline abdominal incision. Arterioles were visualized with a Zeiss Axiovert 200 inverted microscope (x10) equipped with a fluorescent lamp source, and a CoolSNAP-EZ camera (Visitron). Digital images were recorded and analyzed off-line using a Metavue software. Injury was induced by topical application of a 3-mm2 filter paper saturated with FeCl3 (20%). Adhesion and aggregation of fluorescently labeled platelets (i.v. injection of Dylight-488 conjugated anti-GPIX Ig derivative beforehand) in arterioles were monitored for 40 minutes or until complete occlusion occurred (blood flow stopped for longer than 1 minute).7
Statistics
Results from at least 3 experiments per group are presented as mean ± SD. Differences between two groups were assessed by Welch’s test, whereas differences between more than two groups were analyzed by one-way analysis of variance (ANOVA) with Dunnett’s T3 as post-hoc test using SPSS Statistics 20. The Fischer’s exact test was applied to assess variance in occurrence of occlusion. P-values <0.05 were considered statistically significant.
Supplementary Material
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.112.300672/-/DC1.
Significance.
Platelet inhibition is a major strategy to prevent acute ischemic cardiovascular and cerebrovascular events, which may, however, be associated with an increased bleeding risk. The receptors, Glycoprotein VI (GPVI) and C-type lectin-like receptor 2 (CLEC-2), which are the only (hem) immunoreceptor tyrosine activation motif–bearing receptors in mouse platelets, might be promising antithrombotic targets because they can be depleted from circulating platelets by antibody treatment, leading to sustained antithrombotic protection, but only moderately increased bleeding times in mice. Here, we found that combined loss of GPVI and CLEC-2, and thus (hem) immunoreceptor tyrosine activation motif signaling, resulted in markedly impaired hemostasis and a severe thrombus formation defect that by far exceeded that seen in GPVI- or CLEC-2 single-depleted animals. These results indicate that anti-GPVI or anti–CLEC-2 treatment might bear the risk of uncontrolled bleeding in patients exhibiting defects in the respective other (hem) immunoreceptor tyrosine activation motif signaling pathway. Our results may have important implications for the development of anti-GPVI and anti–CLEC-2–based antithrombotic therapeutics.
Acknowledgments
We thank Steffi Hartmann, Birgit Midloch, and Jens Antons for excellent technical assistance and Prof Johannes Eble for kindly providing purified rhodocytin. We also thank Prof Robert K. Andrews for providing botrocetin and Dr Karin Sauer for measuring activated partial thromboplastin time and prothrombin time.
Sources of Funding
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 688 to B. Nieswandt), the Rudolf Virchow Center, and the Wellcome Trust (088410).
Footnotes
Disclosures
None.
Contributor Information
Markus Bender, Chair of Vascular Medicine, University Hospital Würzburg and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany.
Frauke May, Chair of Vascular Medicine, University Hospital Würzburg and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany.
Viola Lorenz, Chair of Vascular Medicine, University Hospital Würzburg and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany.
Ina Thielmann, Chair of Vascular Medicine, University Hospital Würzburg and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany.
Ina Hagedorn, Chair of Vascular Medicine, University Hospital Würzburg and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany.
Brenda A. Finney, Centre for Cardiovascular Sciences, Institute for Biomedical Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom
Timo Vögtle, Chair of Vascular Medicine, University Hospital Würzburg and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany.
Katharina Remer, Chair of Vascular Medicine, University Hospital Würzburg and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany.
Attila Braun, Chair of Vascular Medicine, University Hospital Würzburg and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany.
Michael Bösl, Chair of Vascular Medicine, University Hospital Würzburg and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany.
Steve P. Watson, Centre for Cardiovascular Sciences, Institute for Biomedical Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom
Bernhard Nieswandt, Chair of Vascular Medicine, University Hospital Würzburg and Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Würzburg, Germany.
References
- 1.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP. Arterial thrombosis–insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 3.Nieswandt B, Pleines I, Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J Thromb Haemost. 2011;9(suppl 1):92–104. doi: 10.1111/j.1538-7836.2011.04361.x. [DOI] [PubMed] [Google Scholar]
- 4.Arthur JF, Dunkley S, Andrews RK. Platelet glycoprotein VI-related clinical defects. Br J Haematol. 2007;139:363–372. doi: 10.1111/j.1365-2141.2007.06799.x. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, Marchese P, Reininger A, Ruggeri ZM, Ware J. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 2003;102:1701–1707. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- 6.Lockyer S, Okuyama K, Begum S, Le S, Sun B, Watanabe T, Matsumoto Y, Yoshitake M, Kambayashi J, Tandon NN. GPVI-deficient mice lack collagen responses and are protected against experimentally induced pulmonary thromboembolism. Thromb Res. 2006;118:371–380. doi: 10.1016/j.thromres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Bender M, Hagedorn I, Nieswandt B. Genetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl(3) -induced thrombosis. J Thromb Haemost. 2011;9:1423–1426. doi: 10.1111/j.1538-7836.2011.04328.x. [DOI] [PubMed] [Google Scholar]
- 8.Nieswandt B, Bergmeier W, Schulte V, Rackebrandt K, Gessner JE, Zirngibl H. Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRgamma chain. J Biol Chem. 2000;275:23998–24002. doi: 10.1074/jbc.M003803200. [DOI] [PubMed] [Google Scholar]
- 9.Dütting S, Bender M, Nieswandt B. Platelet GPVI: a target for antithrombotic therapy?! Trends Pharmacol Sci. 2012;33:583–590. doi: 10.1016/j.tips.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Nieswandt B, Schulte V, Bergmeier W, Mokhtari-Nejad R, Rackebrandt K, Cazenave JP, Ohlmann P, Gachet C, Zirngibl H. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med. 2001;193:459–469. doi: 10.1084/jem.193.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender M, Hofmann S, Stegner D, Chalaris A, Bösl M, Braun A, Scheller J, Rose-John S, Nieswandt B. Differentially regulated GPVI ectodomain shedding by multiple platelet-expressed proteinases. Blood. 2010;116:3347–3355. doi: 10.1182/blood-2010-06-289108. [DOI] [PubMed] [Google Scholar]
- 12.Boylan B, Berndt MC, Kahn ML, Newman PJ. Activation-independent, antibody-mediated removal of GPVI from circulating human platelets: development of a novel NOD/SCID mouse model to evaluate the in vivo effectiveness of anti-human platelet agents. Blood. 2006;108:908–914. doi: 10.1182/blood-2005-07-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki-Inoue K, Fuller GL, García A, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 14.Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pöhlmann S, Suzuki-Inoue K, Ozaki Y, Watson SP, Pearce AC. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–12409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Séverin S, Pollitt AY, Navarro-Nuñez L, Nash CA, Mourão-Sá D, Eble JA, Senis YA, Watson SP. Syk-dependent phosphorylation of CLEC-2: a novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling. J Biol Chem. 2011;286:4107–4116. doi: 10.1074/jbc.M110.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertozzi CC, Schmaier AA, Mericko P, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K, Shin Y, et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285:24494–24507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finney BA, Schweighoffer E, Navarro-Núñez L, et al. CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood. 2012;119:1747–1756. doi: 10.1182/blood-2011-09-380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertozzi CC, Hess PR, Kahn ML. Platelets: covert regulators of lymphatic development. Arterioscler Thromb Vasc Biol. 2010;30:2368–2371. doi: 10.1161/ATVBAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osada M, Inoue O, Ding G, Shirai T, Ichise H, Hirayama K, Takano K, Yatomi Y, Hirashima M, Fujii H, Suzuki-Inoue K, et al. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem. 2012;287:22241–22252. doi: 10.1074/jbc.M111.329987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes CE, Navarro-Núñez L, Finney BA, Mourão-Sá D, Pollitt AY, Watson SP. CLEC-2 is not required for platelet aggregation at arteriolar shear. J Thromb Haemost. 2010;8:2328–2332. doi: 10.1111/j.1538-7836.2010.04006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May F, Hagedorn I, Pleines I, Bender M, Vögtle T, Eble J, Elvers M, Nieswandt B. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. 2009;114:3464–3472. doi: 10.1182/blood-2009-05-222273. [DOI] [PubMed] [Google Scholar]
- 23.Gardiner EE, Al-Tamimi M, Mu FT, Karunakaran D, Thom JY, Moroi M, Andrews RK, Berndt MC, Baker RI. Compromised ITAM-based platelet receptor function in a patient with immune thrombocytopenic purpura. J Thromb Haemost. 2008;6:1175–1182. doi: 10.1111/j.1538-7836.2008.03016.x. [DOI] [PubMed] [Google Scholar]
- 24.Schulte V, Reusch HP, Pozgajová M, Varga-Szabó D, Gachet C, Nieswandt B. Two-phase antithrombotic protection after anti-glycoprotein VI treatment in mice. Arterioscler Thromb Vasc Biol. 2006;26:1640–1647. doi: 10.1161/01.ATV.0000225697.98093.ed. [DOI] [PubMed] [Google Scholar]
- 25.David T, Ohlmann P, Eckly A, Moog S, Cazenave JP, Gachet C, Lanza F. Inhibition of adhesive and signaling functions of the platelet GPIb-V-IX complex by a cell penetrating GPIbalpha peptide. J Thromb Haemost. 2006;4:2645–2655. doi: 10.1111/j.1538-7836.2006.02198.x. [DOI] [PubMed] [Google Scholar]
- 26.Elvers M, Stegner D, Hagedorn I, Kleinschnitz C, Braun A, Kuijpers ME, Boesl M, Chen Q, Heemskerk JW, Stoll G, Frohman MA, et al. Impaired alpha(IIb)beta(3) integrin activation and shear-dependent thrombus formation in mice lacking phospholipase D1. Sci Signal. 2010;3:ra1. doi: 10.1126/scisignal.2000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardiner EE, Karunakaran D, Arthur JF, Mu FT, Powell MS, Baker RI, Hogarth PM, Kahn ML, Andrews RK, Berndt MC. Dual ITAM-mediated proteolytic pathways for irreversible inactivation of platelet receptors: de-ITAM-izing FcgammaRIIa. Blood. 2008;111:165–174. doi: 10.1182/blood-2007-04-086983. [DOI] [PubMed] [Google Scholar]
- 28.Zhi H, Rauova L, Hayes V, Gao C, Boylan B, Newman D, McKenzie S, Cooley B, Poncz M, Newman P. Cooperative integrin/itam signaling in platelets enhances thrombus formation in vitro and in vivo. Blood. 2012 doi: 10.1182/blood-2012-07-443325. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabie T, Varga-Szabo D, Bender M, Pozgaj R, Lanza F, Saito T, Watson SP, Nieswandt B. Diverging signaling events control the pathway of GPVI down-regulation in vivo. Blood. 2007;110:529–535. doi: 10.1182/blood-2006-11-058107. [DOI] [PubMed] [Google Scholar]
- 30.Gupta S, Braun A, Morowski M, Premsler T, Bender M, Nagy Z, Sickmann A, Hermanns HM, Bösl M, Nieswandt B. CLP36 is a negative regulator of glycoprotein VI signaling in platelets. Circ Res. 2012;111:1410–1420. doi: 10.1161/CIRCRESAHA.112.264754. [DOI] [PubMed] [Google Scholar]
- 31.Law DA, Nannizzi-Alaimo L, Ministri K, Hughes PE, Forsyth J, Turner M, Shattil SJ, Ginsberg MH, Tybulewicz VL, Phillips DR. Genetic and pharmacological analyses of Syk function in alphaIIbbeta3 signaling in platelets. Blood. 1999;93:2645–2652. [PubMed] [Google Scholar]
- 32.Andre P, Morooka T, Sim D, et al. Critical role for Syk in responses to vascular injury. Blood. 2011;118:5000–5010. doi: 10.1182/blood-2011-06-360743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulaftali Y, Hess P, Getz T, Cholka A, Stolla M, Mackman N, Owens A, Ware J, Kahn M, Bergmeier W. Platelet itam signaling is critical for vascular integrity in inflammation. [Accessed March 6, 2013];J Clin Invest. 2013 Jan 25; doi: 10.1172/JCI65154. http://www.jci.org/articles/view/65154 doi:pii: 65154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Finney B, Schweighoffer E, Navarro-Núñez L, et al. Clec-2 and syk in the megakaryocytic/platelet lineage are essential for development. Blood. 2012;119:1747–1756. doi: 10.1182/blood-2011-09-380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiedt R, Schomber T, Hao-Shen H, Skoda R. Pf4-cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 3.Knight C, Morton L, Onley D, Peachey A, Ichinohe T, Okuma M, Farndale R, Barnes M. Collagen-platelet interaction: Gly-pro-hyp is uniquely specific for platelet gp vi and mediates platelet activation by collagen. Cardiovascular research. 1999;41:450–457. doi: 10.1016/s0008-6363(98)00306-x. [DOI] [PubMed] [Google Scholar]
- 4.Bergmeier W, Bouvard D, Eble J, Mokhtari-Nejad R, Schulte V, Zirngibl H, Brakebusch C, Fässler R, Nieswandt B. Rhodocytin (aggretin) activates platelets lacking alpha(2)beta(1) integrin, glycoprotein vi, and the ligand-binding domain of glycoprotein ibalpha. The Journal of biological chemistry. 2001;276:25121–25126. doi: 10.1074/jbc.M103892200. [DOI] [PubMed] [Google Scholar]
- 5.Nieswandt B, Bergmeier W, Rackebrandt K, Gessner J, Zirngibl H. Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood. 2000;96:2520–2527. [PubMed] [Google Scholar]
- 6.Nieswandt B, Echtenacher B, Wachs F, Schröder J, Gessner J, Schmidt R, Grau G, Männel D. Acute systemic reaction and lung alterations induced by an antiplatelet integrin gpiib/iiia antibody in mice. Blood. 1999;94:684–693. [PubMed] [Google Scholar]
- 7.Grosse J, Braun A, Varga-Szabo D, et al. An ef hand mutation in stim1 causes premature platelet activation and bleeding in mice. The Journal of clinical investigation. 2007;117:3540–3550. doi: 10.1172/JCI32312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.