Abstract
Episodic memory is the capacity to recall an event in time and place (What? Where? When?). Impaired episodic memory is a debilitating cognitive symptom in schizophrenia but is poorly controlled by currently available antipsychotic drugs. Consistent with glutamatergic abnormality in schizophrenia, the NDMA receptor antagonist, phencyclidine (PCP), induces persistent ‘schizophrenia-like’ symptoms including memory deficits in humans and rodents and is widely used as an animal model of the disorder. However, in contrast to humans, PCP and PCP withdrawal-induced memory deficits in rodents are reversed by antipsychotic drugs such as clozapine. One possible explanation is that the memory tasks used in animal studies do not simultaneously test the What? Where? When? components that characterize episodic memory in human tasks. We investigated whether subchronic PCP withdrawal disrupts memory in rats in a task that requires simultaneous integration of memory for object, place and context. Rats learn to discriminate objects under specific spatial and contextual conditions analogous to the What? Where? When? components of human episodic memory. We found that PCP withdrawal impaired performance on this task and that the atypical antipsychotic drug clozapine did not reverse this impairment. However the acetylcholinesterase inhibitor (AChEI) donepezil, which has been shown to improve episodic memory in humans did reverse the effect of PCP. This suggests that PCP withdrawal disruption of object–place–context recognition in rats may prove to be a useful model to investigate episodic memory impairment in schizophrenia and supports the suggestion that AChEIs could prove to be a useful pharmacological strategy to specifically treat episodic memory problems in schizophrenia.
Keywords: Antipsychotic, donepezil, episodic memory, object recognition, phencyclidine (PCP), schizophrenia
Introduction
In addition to symptoms such as hallucinations and delusions, patients with schizophrenia suffer from profound impairments in episodic memory (Al-Uzri et al. 2006; Gold & Weinberger, 1995). These cognitive impairments are of particular significance for the disease as they are more closely associated with poor outcome in patients than other symptoms, such as hallucinations or delusions (Berenbaum et al. 2008; Green et al. 2004). However, unlike other core symptoms episodic memory impairment is not routinely improved by antipsychotic treatment, nor is it currently treatable using any other pharmacological strategy (Gopal & Variend, 2005; Meltzer & McGurk, 1999). One impediment to the identification and evaluation of potential new drug treatments is the lack of reliable animal models specifically designed to simulate episodic memory impairment in schizophrenia, as identified by recent research consortia, e.g. Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) (Sarter, 2006; Young et al. 2009).
Episodic memory is the capacity to recall an event in time and place, i.e. ‘What? Where? When?’ (Tulving, 1983). It was previously thought that without language animals could not show episodic memory (Mitchell, 1993), but it is now clear that with appropriate methodology many animals, including rodents, can encode events with specific What? Where? When? components (Clayton & Dickinson, 1998; Eacott & Norman, 2004; Kart-teke et al. 2006).
There is considerable evidence that N-methyl-d-aspartate receptors (NMDARs) are important for memory formation and that NMDAR function is abnormal in schizophrenia (Jentsch & Roth, 1999). NMDA antagonists [such as ketamine and phencyclidine (PCP)] exacerbate psychosis in schizophrenia patients, and in healthy volunteers induce not only symptoms such as delusions, but also episodic memory impairments (Hetem et al. 2000; Honey et al. 2005; Lahti et al. 1995). PCP and PCP withdrawal-induced learning and memory deficits in rodents are consequently widely used to attempt to model the abnormalities in memory and other cognitive functions in schizophrenia (Jentsch & Roth, 1999; Wong & Van Tol, 2003). The behavioural effects of PCP in humans have been shown to persist for several weeks after drug discontinuation, which is why withdrawal from repeated PCP administration is widely used as a pharmacological animal model relevant to schizophrenia (Enomoto et al. 2007; Jentsch & Roth, 1999; Seillier & Giuffrida, 2009). In PCP-withdrawal models animals are free from the considerable sedative effects of acute PCP that may confound interpretation of impairments in memory tasks. In addition, withdrawal models have an advantage in that they circumvent the ‘receptor tautology’ confound in pharmacological translational studies, namely, that reversal of drug-induced effects can simply reflect a pharmacological interaction and may not necessarily predict clinical efficacy (e.g. Young et al. 2009). For these reasons our investigations began with the PCP-withdrawal model.
At the behavioural level, tasks currently used to assess episodic memory such as novel object recognition following PCP withdrawal may be limited for two reasons. First, PCP withdrawal induces object recognition deficits in rodents that are reversed by antipsychotic drugs (Grayson et al. 2007; Hashimoto et al. 2005). However, it is clear that antipsychotic drugs are ineffective at reversing memory impairments in patients (Goldberg et al. 1993) representing what has been termed a ‘false positive’ (Young et al. 2009). Second, there is evidence that the brain circuitry that underpins performance in tasks that assess memory for What? When? may be different from that involved in the ability to remember What? Where? When? (Eacott & Norman, 2004; Langston & Wood, 2009). In the present study we investigated whether PCP withdrawal would disrupt object–place–context (OPC) recognition when the task includes memory for What? Where? When?, as has been shown for human episodic memory. Having established that PCP withdrawal disrupted performance on the task, we investigated whether the antipsychotic drug clozapine affected performance at a dose that reverses PCP-withdrawal effects in standard object recognition models. Finally, we investigated whether the acetylcholinesterase inhibitor (AChEI) donepezil which has been shown to improve episodic memory in humans (Grön et al. 2005) affects performance.
Episodic memory was assessed using the OPC recognition paradigm developed by Eacott & Norman (2004). This task requires a rat to recollect the location (‘where’) of a specific object (‘what’) depending on the context in which it was encountered (‘when’) (Eacott & Norman, 2004).
Materials and methods
Animals
Twenty (expt 1) or 40 (expts 2 and 3) adult male Lister Hooded rats (Biomedical Services Unit, University of Nottingham Medical School, UK; 150–200 g on arrival, 300–350 g at the start of behavioural testing) were used. Animals received 1–2 min daily handling beginning the day after arrival at the unit and ending the day before the experiment. Animals were exposed to the test room 1 d before habituation. Animals for all experiments were kept in a temperature- (21±2 °C) and humidity- (40–60%) controlled environment on a 12-h light/dark cycle (lights on 07:00 hours). Food (standard animal chow, USA) and water were available ad libitium. Experiments were performed in accordance with the UK Animals (Scientific Procedures) Acts, 1986 and approved by a local ethical review committee (University of Nottingham) and the Home Office of the United Kingdom (project licence approval number: PPL 40/2715).
Drug administration
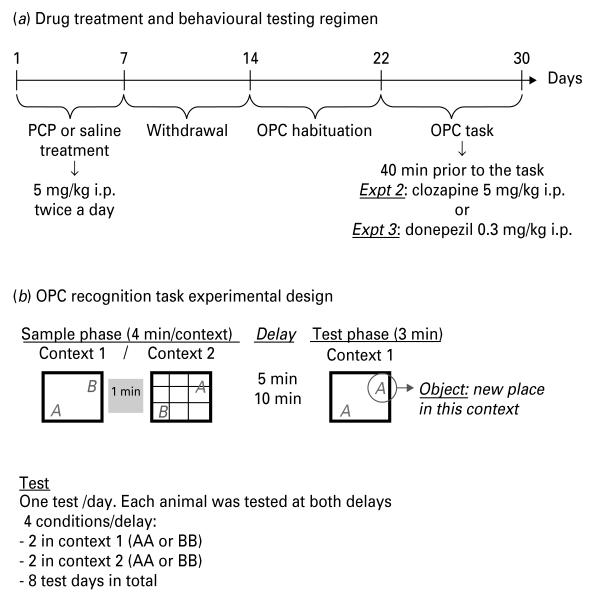
Figure 1 indicates the drug and behavioural testing regimens for expts 1–3.
Fig. 1.
(a) Shows the drug treatment and behavioural testing protocol. (b) Shows the experimental design of the object–place–context (OPC) recognition task. Rats explored two contexts with two different objects in alternating locations. After a 5- or 10-min delay, they were tested in one of the contexts (first one encountered) with two similar objects. Only one object (‘what’) was located in a new place (‘where’) depending on the context (‘which–when’).
Subchronic PCP treatment (expts 1–3)
PCP hydrochloride was obtained from Sigma-Aldrich (UK) and dissolved in saline (0.9% w/v NaCl). PCP (5 mg/kg.ml in saline) was injected intraperitoneally (i.p.) twice daily (08:00 and 18:00 hours) for 7 d followed by a 7-d drug-free period. The control group received the same treatment regimen with sodium chloride 0.9% w/v (1 ml/kg i.p.). The dose of PCP and treatment schedule was based on Jentsch et al. (1997).
Clozapine (expt 2)
Clozapine was obtained from Sigma-Aldrich (UK). Clozapine was dissolved in a minimum volume of acetic acid, pH adjusted to 5.5 with 1 m sodium hydroxide (NaOH) and saline (0.9% w/v NaCl) was added to adjust the volume. Rats received 5 mg/kg i.p. clozapine or saline (0.9% w/v NaCl) (1 ml/kg i.p., n=20) 40 min prior the task for 8 consecutive days. This regimen has previously been shown to reverse object recognition deficits in rats treated with NMDAR antagonists PCP and MK-801 (Grayson et al. 2007; Karasawa et al. 2008). One PCP-treated rat died after 2 d treatment for unknown reasons, leaving n=19 for the final experiment.
Donepezil (expt 3)
Donepezil was obtained from The National Institute of Mental Health (NIMH; Bethesda, USA). Donepezil was dissolved in saline (0.9% w/v NaCl). Rats received 0.3 mg/kg i.p. donepezil or saline (0.9% w/v NaCl) (1 ml/kg i.p., n=20) 40 min prior the task during 8 d. This regimen has been shown to improve memory in other models in rodents (Prickaerts et al. 2005).
Behavioural testing
Behavioural testing was performed using an adaptation of a previously published method (see Eacott & Norman, 2004 for full details).
Apparatus
All testing was carried out in two different clear Perspex chambers (30×30×30 cm). A clear Perspex lid was placed on top of each chamber to prevent escape but had holes ~1 cm to allow the circulation of air. A camera was fixed to the lid to record each trial. One chamber (context 1) had a white plastic floor and the walls were covered in black and white squares (3 cm2). The second chamber (context 2) had a black plastic floor and four natural wood walls. Both chambers had crosses marked at the two locations where objects would be presented. These locations were in the lower right and upper left corners 5 cm from the walls of the chamber. The chambers remained in the same position throughout the experiment. The room was lit by a single, centrally placed overhead fluorescent light (650 lx measured from the base of the experimental chamber, 640 lx measured from the centre of the room). Objects were chosen to fulfil the criteria of being easily cleaned and not easily gnawed by the rats. They were of a similar size and shape with different textures (e.g. glass, sand-blasted glass bottles), or colours (e.g. black and white), and sufficiently heavy to prevent the rats from pushing them over. Copies of the objects were used during test trials to eliminate the use of odour cues. Objects and contexts were the same for all experiments.
Habituation
Each rat received one habituation session a day for 8 d. A habituation session consisted of placement of the rat in the behavioural chamber for 10 min with an object at the centre of the arena. Four of these habituation sessions were carried out in context 1 and four habituation sessions in context 2, the order of which was counterbalanced. The objects used during habituation were different from those used during the rest of the experiment but were of a similar size and weight.
OPC recognition task
Trials consisted of two sample phases and a test phase separated by a delay. For all phases the rat started from the same point in the arena. Different copies of objects were used in each trial to prevent the use of odour cues.
Sample phase 1
The first sample phase was carried out in context 1 with object A in the lower left corner and object B in the upper right corner. Rats were allowed to explore freely for 4 min. Rats were then placed in their holding cage for 1 min while the arena was cleaned using 70% v/v ethanol.
Sample phase 2
The second sample phase was carried out in context 2 with the location of the object switched (B now in the lower left corner and A in the upper right corner). Rats were allowed to explore freely for 4 min, after which they were placed in their holding cage for a delay period (5 or 10 min) before the test phase.
Test phase
The test phase was carried out in context 1 with two copies of the same object placed in the lower left and upper right corners (either two copies of A or two copies of B). Rats were allowed to explore freely for 3 min. Each object, location and context were familiar to the rat; however, one of the objects had never been seen before in this specific location in this specific context with two different time delays used as described below.
Delays of 5 and 10 min were used, being selected on the basis of a pilot study in our laboratory replicating Eacott & Norman’s (2004) experiment at seven different delays. For each delay each rat underwent four different trials, one per day, comprising sample 1, sample 2 and test as described above according to the following schedule. Two trials used context 1 and two trials used context 2 in the test phase. There were thus four possible combinations: context 1 or 2 with two objects A or two objects B (context 1 AA, context 1 BB context 2 AA, context 2 BB). Animals were tested in sample phase 1 context only as pilot studies in our laboratory showed a potential confound of recency vs. primacy (better memory for the last event compared to the first one; Barker et al. 2007; Dere et al. 2005a). (See Fig. 1 for more details on experimental design). The location of the objects during the sample phase was counterbalanced between delays and animals. In all experiments rats began the task on day 8 after the last PCP injection.
Data analysis
Time spent exploring both objects during test was scored from a video recording of the test phase. The video was independently scored twice and the Pearson correlation coefficient between two independent ratings of object exploration was r=0.8. A discrimination index (DI) was calculated as
A score of 0 indicates no discrimination between novel and familiar object. Note that the novel object in this procedure is the one that is in a new location for a specific context as the rat is already familiar with the object, location and context.
For the sample phase a split-plot ANOVA was used with exploration time (in seconds) as dependent variable with treatment and experimental day as factors. For the test phase one-way ANOVA for each delay was performed with DI as dependent variable and treatment as factor followed by planned post-hoc t tests where appropriate. Treatment levels were (expt 1: PCP–saline, saline–saline; expt 2: PCP–saline, saline–saline, saline–clozapine, PCP–clozapine; expt 3: PCP–saline, saline–saline; saline–donepezil, PCP–donepezil; In all experiments one-sample t test for individual treatment groups was used to determine if the DI was significantly greater than zero as reported in Eacott & Norman (2004). All statistics were conducted using SPSS software, version 16.1 (SPSS Inc., USA).
Results
Expt 1: effect of subchronic PCP withdrawal on OPC recognition memory
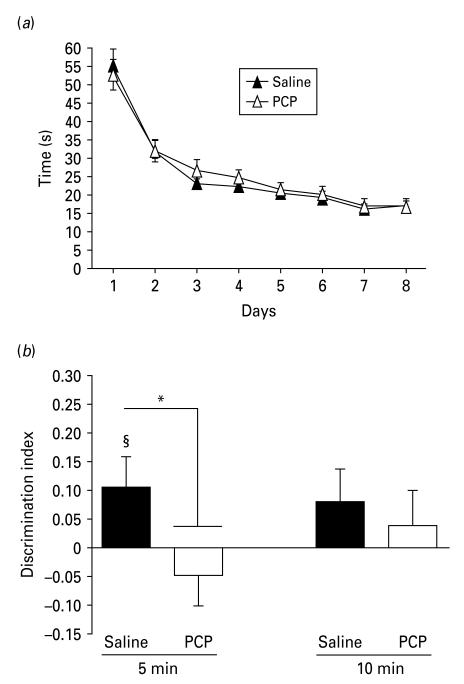
During the sample phase (Fig. 2a), there was a significant decrease in time spent exploring the objects over the 8 d of procedure (ANOVA effect of day: F7,126=46.16, p < 0.01, but not treatment).
Fig. 2.
Phencyclidine (PCP) disrupts object–place–context memory in rats. (a) Object exploration (time in seconds) decreased similarly in both groups during the sample phase and did not differ between groups. (b) Mean discrimination index (DI). At 5-min delay, only the saline group was significantly greater than zero (§ p < 0.05) and was significantly different from the PCP group (* p < 0.05). Error bars represent ± s.e.m.
During the test phase (Fig. 2b), at the 5-min delay there was a significant effect of treatment on the DI (F1,19=8.01, p < 0.01). The DI of saline-treated rats was significantly greater than 0 at the 5-min but not 10-min delay (t9=2.62, p < 0.05).
Expt 2: effect of clozapine on PCP-withdrawal disruption of OPC recognition
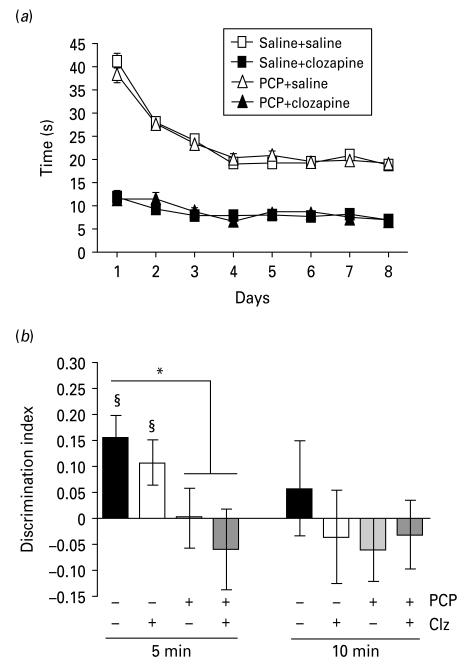
As found in expt 1, total time spent exploring the objects during the sample phase decreased over days in saline–saline and PCP–saline groups (Fig. 3a). Both clozapine-treated groups showed markedly reduced exploration of the objects during this phase (ANOVA significant effect of day: F7,245=57.53, p < 0.01, and treatment: F3,35=307.28, p < 0.01).
Fig. 3.
Clozapine does not reverse phencyclidine (PCP)-induced object–place–context memory deficit in rats. (a) During the sample phase, clozapine-treated rats showed reduced exploration compared to saline (p < 0.01). (b) Mean discrimination index. At 5-min delay, both saline–saline and saline–clozapine (Clz) groups showed performance significantly greater than zero (§ p < 0.05). * Indicates p < 0.05 significant difference from saline–saline group at 5-min delay. Errors bars represent ± s.e.m.
During the test phase, at the 5-min delay (Fig. 3b), there was a significant effect of treatment on the DI (F3,35=3.08, p < 0.05). DI in the PCP–saline group was significantly lower than the saline–saline group (t17=2.21, p < 0.05) but not significantly different from the PCP–clozapine group (p>0.05). DI was significantly greater than zero in the saline–saline (t9=3.82, p < 0.01) and saline–clozapine (t9=2.33, p < 0.05) groups, suggesting that clozapine did not impair the capacity of rats to perform the task despite reduced object exploration in the sample phase. At the 10-min delay none of the experimental groups recognized the correct new triad of object, place and context.
Expt 3: effect of donepezil on PCP-withdrawal disruption of OPC recognition
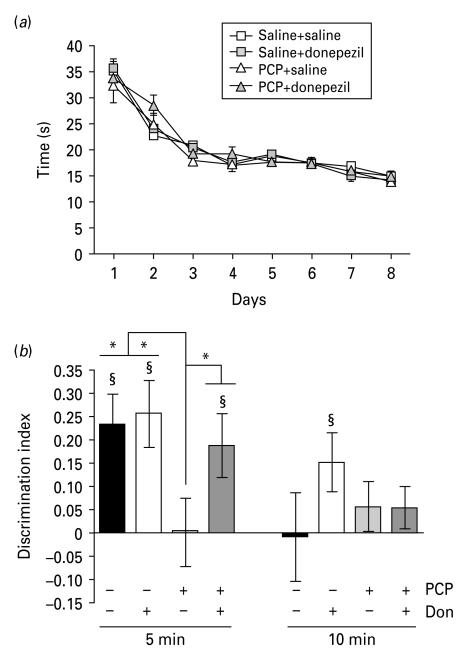
During the sample phase (Fig. 4a) rats showed decreased object exploration over days (ANOVA effect of day: F7,252=83.24, p < 0.01) but no effect of treatment.
Fig. 4.
Donepezil reverses phencyclidine (PCP)-induced object–place–context memory deficit in rats. (a) Exploration decreased over days during sample phase but there was no difference between drug groups. (b) Mean discrimination index (DI). At 5-min delay, only the PCP–saline group DI was not significantly greater than zero (§ p < 0.05) and there was a significant difference between scores from the PCP–saline group with drug groups (* p < 0.05). At 10-min delay, the saline–donepezil-treated rats’ DI was significantly greater than zero (§ p < 0.05). Errors bars represent ± s.e.m.
During the test phase (Fig. 4b), at the 5-min delay there was an effect of treatment (F3,36=2.81, p=0.05). The DI was lower in the PCP–saline group compared to the saline–saline (t18=2.43, p < 0.05), PCP–donepezil (t18=2.21, p < 0.05) and saline–donepezil (t18=2.50, p < 0.05) groups. At the 5-min delay the DI was significantly greater than zero in saline–saline (t9=3.71, p < 0.01), saline–donepezil (t9=3.57, p < 0.001) and PCP–donepezil (t9=2.74, p < 0.05) groups. At the 10-min delay DI was significantly greater than zero in saline–donepezil (t9=2.45, p < 0.05) but not saline–saline (t9=0.083, p>0.05) groups, suggesting that the saline–donepezil group successfully performed the task while the saline–saline control group did not.
Discussion
These data first indicate that rats can perform a recognition memory task that requires simultaneous memory for What? Where? When? in a robust and replicable manner. In controls, reduced memory was seen at 10-min delay, confirming the delay dependence of the task (Eacott & Norman, 2004). PCP-treated rats showed impaired memory at both 5- and 10-min delays. Clozapine, an atypical antipsychotic reduced exploratory behaviour but despite this did not affect memory performance either when given alone or to PCP-treated rats. In contrast, donepezil, an AChEI, improved OPC recognition performance in control rats and furthermore restored the PCP-withdrawal-induced memory deficit. These findings show first that memory simultaneously measuring the What? Where? When? triad is sensitive to disruption by withdrawal from a NMDAR antagonist. Second, in contrast to previous studies using PCP disruption in learning and memory tasks which do not require object–place and context components, clozapine did not reverse these deficits. Third, these data suggest that it is possible to pharmacologically distinguish between memory for What? Where? When? (OPC recognition) and ‘what’ (one-trial object recognition) which supports the theoretical position of separate memory systems.
Fourth, these data show that PCP-withdrawal memory deficit is sensitive to the effects of donepezil both confirming that memory in this model can be improved by drugs which enhance central cholinergic function, and the potential of this class of drugs to ameliorate episodic memory deficits in schizophrenia.
PCP withdrawal disrupts episodic memory incorporating the What? Where? When? triad
Many previous studies have demonstrated the ability of rodents to discriminate between objects using either spatial or non-spatial information in a time-dependent manner (Dere et al. 2005a, b; Good, 2007; Kart-Teke et al. 2006). However, in contrast to other paradigms, in the present OPC recognition task, rats have to discriminate objects in terms of context (‘when’=in which context was it seen) as well as in time (‘how long ago was it seen?), thereby using contextual information to differentiate events that have occurred in the past (Smith, 2008). It is unlikely that rats have solved the task on the basis of simple familiarity as objects, places and contexts are equally familiar and it is only the combination of object–context–location that is novel in the task (Eacott & Norman, 2004). These results demonstrate a replicable deficit in OPC recognition memory after withdrawal from a subchronic PCP regimen in rats (5 mg/kg twice daily during 7 d). These data support prior demonstrations of memory impairment following PCP treatment in rodents, and extend these to include memory that requires the encoding of What? Where? When? characteristics (Javitt, 2007; Jentsch et al. 1997). These data are consistent with human studies showing that acute non-competitive NMDAR antagonists disrupt episodic memory performance (Hetem et al. 2000; Honey et al. 2005) and more specifically for the PCP-withdrawal model that these deficits are persistent after drug discontinuation (Malhotra et al. 1996).
As this model involves withdrawal from PCP rats are not tested under the acute influence of the drug, thus it is unlikely that results are mediated by extraneous effects of PCP on general performance in the rats. There were no significant differences in total object exploration between PCP and control rats during the sample phases confirming that withdrawal from PCP had no effect on general exploration pattern.
There are a number of possible underlying mechanisms that could mediate PCP-withdrawal-induced deficit in episodic-like memory. Subchronic PCP reduces the number of parvalbumin-immunoreactive (parvalbumin-IR) neurons in the CA2/3 subregion of the hippocampus measured 6 wk after treatment and reduces prefrontal cortical dopaminergic activity (Abdul-Monim et al. 2007; Jentsch et al. 1997). Given that previous studies have demonstrated a role of both the hippocampus (Eichenbaum, 2003) and frontal cortical networks in the temporal discrimination of events and episodic memory (Barker et al. 2007; Lee & Kesner, 2003) these are possible mechanisms that could be explored in future studies. It will be of interest in future studies to investigate whether other regimens of PCP administration produce similar OPC memory impairment, e.g. repeated low doses (Beraki et al. 2009).
Clozapine does not reverse PCP-induced deficits in OPC recognition memory
Clozapine (5 mg/kg) did not restore the PCP withdrawal-induced episodic memory deficit in OPC recognition. The dose used produced significant sedation which is reflected in reduced exploration times during the sample phase of the task reproducing the known sedative effects in patients and in rodents (Kumra et al. 2008; Wiley, 2008). However, no disruption in DI in clozapine-treated rats suggests that in this task a degree of sedation does not confound memory performance. It is possible that clozapine may have an influence at a higher dose than that tested here. This is unlikely, as at this dose clozapine reverses PCP- and MK-801- (a non-competitive NMDAR antagonist) induced deficits in one-trial object recognition (Grayson et al. 2007; Hashimoto et al. 2005; Karasawa et al. 2008). These studies used one-trial object recognition tasks addressing ‘what’ (which object is new?), that only refer to a previous event, and are considered as working-memory tasks (Ennaceur & Delacour, 1988). This is in contrast to OPC recognition in the present study which requires a comparison between events. The possibility that these tasks may be dissociable is suggested by the finding that hippocampal lesion does not impair one-trial object recognition but does impair OPC recognition and by the finding that fornix lesion impairs OPC but not object–place tasks (Dere et al. 2007; Eacott & Norman, 2004, Langston & Wood, 2009). In the present study clozapine did not improve the PCP deficit in OPC recognition. The present findings confirm that this reversal does not extend to episodic memory defined as memory for What? Where? When? While the present findings suggest a reproduction of the lack of clinical effectiveness of antipsychotics such as clozapine to treat episodic memory deficits seen in schizophrenia, further studies using a variety of doses and antipsychotics including putatively antipsychotic mGluR2/3 agonists, are warranted before such a conclusion could be definitively drawn (Harvey & Keefe, 2001; Patil et al. 2007; Riutort et al. 2003).
PCP deficit in OPC recognition is reversed by donepezil
Donepezil (0.3 mg/kg) increased performance in saline-treated rats and reversed the PCP-induced deficit in the episodic memory task of OPC recognition. Donepezil is known to have a beneficial effect on learning and memory in Alzheimer’s disease (Bullock & Dengiz, 2005; Tsuno, 2009). In schizophrenia, anomalies in the cholinergic pathway have been reported such as lower numbers of muscarinic (Crook et al. 2001) and nicotinic (Freedman et al. 1995) receptors in the prefrontal cortex and hippocampus (Raedler et al. 2006). Few studies have investigated the cognitive effect of donepezil in rodent models relevant to schizophrenia. Studies in mice have shown donepezil reversal of MK-801 and PCP-induced deficits in spatial reversal learning, contextual and cued memory, and one-trial object recognition (Csernansky et al. 2005; Kunitachi et al. 2009). In middle-aged mice, Béracochéa (2007) demonstrated that donepezil given alone improves contextual memory (Béracochéa et al. 2007). Furthermore, human studies have also revealed episodic memory improvement after donepezil administration (Grön et al. 2005). Our results are consistent with these findings as in the present study at the 10-min delay donepezil-treated rats showed clear object discrimination where controls did not, suggesting that it enhances basal memory performance. AChEIs have been tested in initial clinical trials in schizophrenia with some studies suggesting a beneficial effect in general cognitive impairment but others demonstrating minimal benefits (Buchanan et al. 2003; Chung et al. 2009; Friedman et al. 2002; Howard et al. 2002; Keefe et al. 2007; Lee et al. 2007; Risch et al. 2007; Tuğal et al. 2004). However, the studies performed to date have used general cognitive assessment batteries typically measuring executive function and attention and do not include specific tests of episodic memory. While large-scale double-blind studies are required to fully assess the therapeutic potential of AChEIs, our study suggests that episodic memory impairment, in particular, might benefit from the effects of AChEIs in schizophrenia.
Acknowledgements
The authors are grateful to the University of Nottingham, Institute of Neuroscience IDTC programme for studentship support for R.L.
Footnotes
Statement of Interest None.
References
- Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. Journal of Psychopharmacology. 2007;21:198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- Al-Uzri MM, Reveley MA, Owen L, Bruce J, et al. Measuring memory impairment in community-based patients with schizophrenia. Case-control study. British Journal of Psychiatry. 2006;189:132–136. doi: 10.1192/bjp.bp.105.013631. [DOI] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. Journal of Neuroscience. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béracochéa D, Philippin J, Meunier S, Morain P, et al. Improvement of episodic contextual memory by S 18986 in middle-aged mice: comparison with donepezil. Psychopharmacology. 2007;193:63–73. doi: 10.1007/s00213-007-0765-4. [DOI] [PubMed] [Google Scholar]
- Beraki S, Diaz-Heijtz R, Tai F, Ogren SO. Effects of repeated treatment of phencyclidine on cognition and gene expression in C57BL/6 mice. International Journal of Neuropsychopharmacology. 2009;12:243–255. doi: 10.1017/S1461145708009152. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Kerns JG, Vernon LL, Gomez JJ. Cognitive correlates of schizophrenia signs and symptoms: III. Hallucinations and delusions. Psychiatry Research. 2008;159:163–166. doi: 10.1016/j.psychres.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Summerfelt A, Tek C, Gold J. An open-labeled trial of adjunctive donepezil for cognitive impairments in patients with schizophrenia. Schizophrenia Research. 2003;59:29–33. doi: 10.1016/s0920-9964(01)00387-5. [DOI] [PubMed] [Google Scholar]
- Bullock R, Dengiz A. Cognitive performance in patients with Alzheimer’s disease receiving cholinesterase inhibitors for up to 5 years. International Journal of Clinical Practice. 2005;59:817–822. doi: 10.1111/j.1368-5031.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- Chung YC, Lee CR, Park TW, Yang KH, et al. Effect of donepezil added to atypical antipsychotics on cognition in patients with schizophrenia: an open-label trial. World Journal of Biological Psychiatry. 2009;10:156–162. doi: 10.1080/15622970701432551. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. American Journal of Psychiatry. 2001;158:918–925. doi: 10.1176/appi.ajp.158.6.918. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Martin M, Shah R, Bertchume A, et al. Cholinesterase inhibitors ameliorate behavioral deficits induced by MK-801 in mice. Neuropsychopharmacology. 2005;30:2135–2143. doi: 10.1038/sj.npp.1300761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. Episodic-like memory in mice: simultaneous assessment of object, place and temporal order memory. Brain Research Protocols. 2005a;16:10–19. doi: 10.1016/j.brainresprot.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. Integrated memory for objects, places, and temporal order: evidence for episodic-like memory in mice. Neurobiology of Learning and Memory. 2005b;84:214–221. doi: 10.1016/j.nlm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience and Biobehavioral Reviews. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? Journal of Neuroscience. 2004;24:1948–1953. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus, episodic memory, declarative memory, spatial memory … where does it all come together? International Congress Series. 2003;1250:235–244. [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behavioural Brain Research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Enomoto T, Noda Y, Nabeshima T. Phencyclidine and genetic animal models of schizophrenia developed in relation to the glutamate hypothesis. Methods and Findings in Experimental and Clinical Pharmacology. 2007;29:291–301. doi: 10.1358/mf.2007.29.4.1075358. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biological Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Friedman J, Adler DN, Howanitz E, Harvey PD, et al. A double blind placebo controlled trial of donepezil adjunctive treatment to risperidone for the cognitive impairment of schizophrenia. Biological Psychiatry. 2002;51:349–357. doi: 10.1016/s0006-3223(01)01342-7. [DOI] [PubMed] [Google Scholar]
- Gold JM, Weinberger DR. Cognitive deficits and the neurobiology of schizophrenia. Current Opinion in Neurobiology. 1995;5:225–230. doi: 10.1016/0959-4388(95)80030-1. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Greenberg RD, Griffin SJ, Gold JM, et al. The effect of clozapine on cognition and psychiatric symptoms in patients with schizophrenia. British Journal of Psychiatry. 1993;162:43–48. doi: 10.1192/bjp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Good MA, Hale G, Staal V. Impaired “episodic-like”; object memory in adult APPswe transgenic mice. Behavioural Neuroscience. 2007;121:443–448. doi: 10.1037/0735-7044.121.2.443. [DOI] [PubMed] [Google Scholar]
- Gopal YV, Variend H. First-episode schizophrenia: review of cognitive deficits and cognitive remediation. Advances Psychiatric Treatment. 2005;11:38–44. [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behavioural Brain Research. 2007;184:31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia Research. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Grön G, Kirstein M, Thielscher A, Riepe M, et al. Cholinergic enhancement of episodic memory in healthy young adults. Psychopharmacology. 2005;182:170–179. doi: 10.1007/s00213-005-0043-2. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Keefe RSE. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. American Journal of Psychiatry. 2001;158:176–184. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Shimizu E, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. European Journal of Pharmacology. 2005;519:114–117. doi: 10.1016/j.ejphar.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hetem LA, Danion JM, Diemunsch P, Brandt C. Effect of a subanesthetic dose of ketamine on memory and conscious awareness in healthy volunteers. Psychopharmacology. 2000;152:283–288. doi: 10.1007/s002130000511. [DOI] [PubMed] [Google Scholar]
- Honey G, Honey R, Sharar S, Turner D, et al. Impairment of specific episodic memory processes by sub-psychotic doses of ketamine: the effects of levels of processing at encoding and of the subsequent retrieval task. Psychopharmacology. 2005;181:445–457. doi: 10.1007/s00213-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Howard AK, Thornton AE, Altman S, Honer WG. Donepezil for memory dysfunction in schizophrenia. Journal of Psychopharmacology. 2002;16:267–270. doi: 10.1177/026988110201600313. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. International Reviews of Neurobiology. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, et al. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Hashimoto K, Chaki S. d-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behavioural Brain Research. 2008;186:78–83. doi: 10.1016/j.bbr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kart-Teke E, De Souza Silva MA, Huston JP, Dere E. Wistar rats show episodic-like memory for unique experiences. Neurobiology of Learning and Memory. 2006;85:173–182. doi: 10.1016/j.nlm.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Malhotra AK, Meltzer HY, Kane JM, et al. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-bBlind, placebo-controlled trial. Neuropsychopharmacology. 2007;33:1217–1228. doi: 10.1038/sj.npp.1301499. [DOI] [PubMed] [Google Scholar]
- Kumra S, Oberstar J, Sikich L, Findling R, et al. Efficacy and tolerability of second-generation antipsychotics in children and adolescents with schizophrenia. Schizophrenia Bulletin. 2008;34:60–71. doi: 10.1093/schbul/sbm109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitachi S, Fujita Y, Ishima T, Kohno M, et al. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent subchronic administration of donepezil: role of sigma-1 receptors. Brain Research. 2009;1279:189–196. doi: 10.1016/j.brainres.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lahti A, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20:1139–1153. doi: 10.1002/hipo.20714. doi:10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. Journal of Neuroscience. 2003;23:1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BJ, Lee JG, Kim YH. A 12-week, double-blind, placebo-controlled trial of donepezil as an adjunct to haloperidol for treating cognitive impairments in patients with chronic schizophrenia. Journal of Psychopharmacology. 2007;21:421–427. doi: 10.1177/0269881106070996. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophrenia Bulletin. 1999;25:233–256. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Mitchell RW. Mental models of mirror-self-recognition: two theories. New Ideas in Psychology. 1993;11:295–325. [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nature Medicine. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Şık A, Van Der Staay FJ, De Vente J, et al. Dissociable effects of acetylcholinesterase inhibitors and phosphodiesterase type 5 inhibitors on object recognition memory: acquisition versus consolidation. Psychopharmacology. 2005;177:381–390. doi: 10.1007/s00213-004-1967-7. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Bymaster FP, Tandon R, Copolov D, et al. Towards a muscarinic hypothesis of schizophrenia. Molecular Psychiatry. 2006;12:232–246. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- Risch SC, Horner MD, McGurk SR, Palecko S, et al. Double-blind donepezil-placebo crossover augmentation study of atypical antipsychotics in chronic, stable schizophrenia: a pilot study. Schizophrenia Research. 2007;93:131–135. doi: 10.1016/j.schres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Riutort M, Cuervo C, Danion JM, Peretti CS, et al. Reduced levels of specific autobiographical memories in schizophrenia. Psychiatry Research. 2003;117:35–45. doi: 10.1016/s0165-1781(02)00317-7. [DOI] [PubMed] [Google Scholar]
- Sarter M. Preclinical research into cognition enhancers. Trends in Pharmacological Sciences. 2006;27:602–608. doi: 10.1016/j.tips.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Seillier A, Giuffrida A. Evaluation of NMDA receptor models of schizophrenia: divergences in the behavioral effects of sub-chronic PCP and MK-801. Behavioural Brain Research. 2009;204:410–415. doi: 10.1016/j.bbr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Smith DM. Handbook of Behavioral Neuroscience. Vol. 18. Elsevier; Amsterdam, London: 2008. The hippocampus, context processing and episodic memory; pp. 465–481. [Google Scholar]
- Tsuno N. Donepezil in the treatment of patients with Alzheimer’s disease. Expert Review of Neurotherapeutics. 2009;9:591–598. doi: 10.1586/ern.09.23. [DOI] [PubMed] [Google Scholar]
- Tuğal O, Yazici KM, Anil Yağcioğlu AE, Göğüş A. A double-blind, placebo controlled, cross-over trial of adjunctive donepezil for cognitive impairment in schizophrenia. International Journal of Neuropsychopharmacology. 2004;7:117–123. doi: 10.1017/S1461145703004024. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of Episodic Memory. Clarendon Press; Oxford: 1983. [Google Scholar]
- Wiley JL. Antipsychotic-induced suppression of locomotion in juvenile, adolescent and adult rats. European Journal of Pharmacology. 2008;578:216–221. doi: 10.1016/j.ejphar.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AH, Van Tol HH. Schizophrenia: from phenomenology to neurobiology. Neuroscience and Biobehavioral Reviews. 2003;27:269–306. doi: 10.1016/s0149-7634(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, et al. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacology and Therapeutics. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]