Abstract
The transition from fish to tetrapod was arguably the most radical series of adaptive shifts in vertebrate evolutionary history. Data are accumulating rapidly for most aspects of these events1–5, but the life histories of the earliest tetrapods remain completely unknown, leaving a major gap in our understanding of these organisms as living animals. Symptomatic of this problem is the unspoken assumption that the largest known Devonian tetrapod fossils represent adult individuals. Here we present the first life history data for a Devonian tetrapod, from the Acanthostega mass-death deposit of Stensiö Bjerg, East Greenland6,7. Using propagation phase contrast synchrotron microtomography (PPC-SRµCT)8 to visualize the histology of humeri (upper arm bones) and infer their growth histories, we show that even the largest individuals from this deposit are juveniles. A long early juvenile stage with unossified limb bones, during which the individual grew to almost final size, was followed by a slow-growing late juvenile stage with ossified limbs which lasted at least six years in some individuals. The late onset of limb ossification suggests that the juveniles were exclusively aquatic, and the predominance of juveniles in the sample suggests segregated distributions of juveniles and adults at least at certain times. The absolute size at which limb ossification began differs greatly between individuals, suggesting the possibility of sexual dimorphism, adaptive strategies or competition-related size variation.
The life cycle of the earliest tetrapods, and its role in the transition from water to land, has long been a matter of speculation. For example, it has been suggested that the earliest tetrapods bred in ephemeral pools, and that the need for the larvae to locomote overland or through extremely shallow water when relocating from these drying ponds to more permanent water bodies provided selective pressure towards the evolution of terrestriality9. However, the fossil record of Devonian tetrapods, dominated by rare and incomplete specimens that frequently come from poorly constrained localities such as scree slopes10, has until now yielded virtually no life history data.
The only known Devonian tetrapod locality with good potential for revealing life history information is the Acanthostega mass-death deposit in the Britta Dal Formation (Upper Devonian, Famennian) on Stensiö Bjerg, East Greenland10. This locality, comprising a small in-situ micaceous silty sandstone body and immediately associated scree10, has yielded more than 200 skeletal elements. 14 skulls, six of them associated to partially articulated skeletons, were complete enough to measure11, and several more can be identified as individuals - such that there must have been at least 20 animals represented, although almost certainly more were present. Other vertebrates are represented only by a few isolated bones7. The Acanthostega individuals in this deposit evidently died together, probably during drought following a sheet-flood event6; they thus represent a single time-point sample from a population of this stem tetrapod.
Acanthostega humeri from the mass-death deposit show varying degrees of ossification, representing a possible partial ontogenetic series11,12. Using the non-destructive imaging technique PPC-SRµCT8, performed at beamline ID19 of the European Synchrotron Radiation Facility (ESRF) (see Methods for more details), we have undertaken histological investigations of the four humeri collected from the locality (MGUH and NHMD – Natural History Museum of Denmark – MGUH 29019, MGUH 29020, NHMD 74756, UMZC – University Museum of Zoology Cambridge – T.1295)13 (Fig. 1a; Extended Data Fig. 1), recovering data that illuminate the life history of Acanthostega. These are all the humeri of Acanthostega known so far. The humerus MGUH 29019 comes from an articulated specimen. The other humeri are isolated bones. The humeri fall into distinct size classes, large (NHMD 74756, MGUH 29020) and small (MGUH 29019, UMZC T.1295) (Fig. 1a; Extended Data Fig. 1). Confirming previous observations12, we find no correlation between size and degree of ossification: the specimens NHMD 74756 and UMZC T.1295 are weakly ossified whereas the specimens MGUH 29019 and MGUH 29020 are strongly ossified (Fig. 1a; Extended Data Fig. 1).
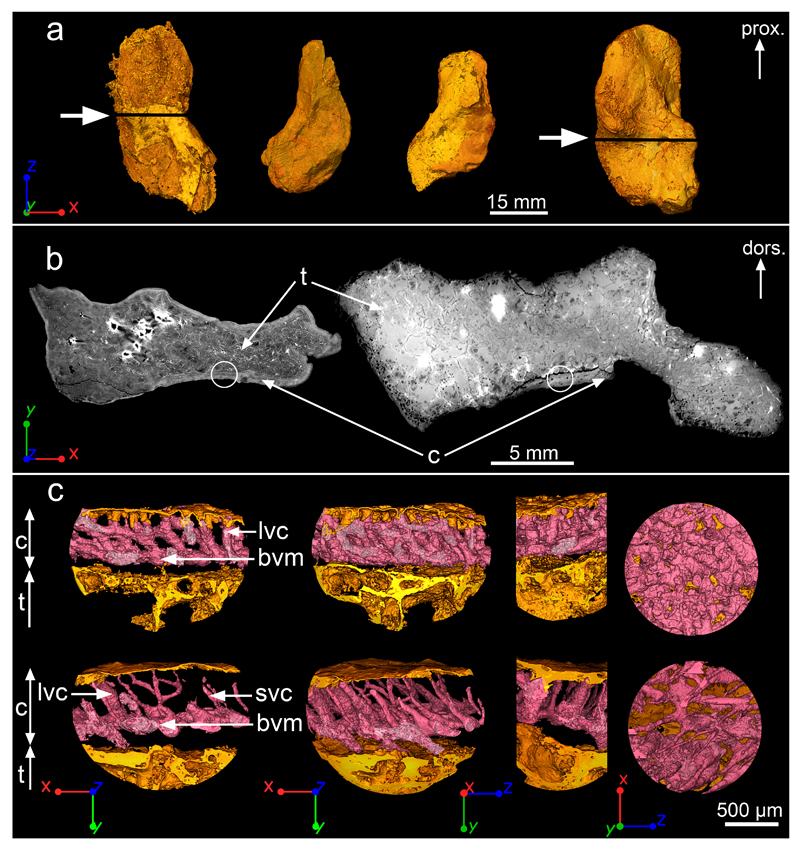
Figure 1. Midshaft bone microanatomy and histology of Acanthostega’s humerus.
a, 3D models of humeri shown in ventral view, based on synchrotron data. From left to right: NHMD 74756 (voxel size: 14.95μm), UMZC T.1295 (voxel size: 12.62μm), MGUH 29019 (voxel size: 12.62μm) and MGUH 29020 (bottom, voxel size: 20.24μm). The white arrows indicate the location of the virtual thin sections in b. b, Humeral microanatomy of NHMD 74756 (left) and MGUH 29020 (right) showing an extended trabecular cavity (t) surrounded by a thin layer of compact cortical bone (c). The transverse virtual thin sections are 80μm thick. The white circles indicate the location of the high-resolution scans modelled in c. c, 3D models (voxel size: 0.638μm) of the cortical bone microstructure of NHMD 74756 (top) and MGUH 29020 (bottom) showing a dense oblique mesh of large (lvc) and small (svc) vascular canals (in pink) connected to a horizontal basal vascular mesh (bvm). From left to right: transverse 3D thin section (250μm thick), complete 3D model in transverse orientation, 3D model in longitudinal orientation, tangential section showing the inner view of the 3D vascular mesh. Abbreviations: dors., dorsal; prox., proximal.
All humeri exhibit an extensive spongiosa surrounded by a thin compact cortex (Fig. 1a,b). This arrangement resembles that of the humerus of the lobe-finned fish Eusthenopteron14, a less crownward member of the tetrapod stem group15. Remnants of calcified cartilage in the metaphyseal region (close to the articular extremities; Extended Data Fig. 2c) show that the spongiosa formed by endochondral ossification as in extant tetrapods16 and Eusthenopteron14,17. Tubular structures at the base of the epiphyses (Extended Data Fig. 2a,b) resemble the marrow processes in the growth plate of the humerus of Eusthenopteron14.
The midshaft cortex of all Acanthostega humeri contains a dense arrangement of radial vascular canals (Fig. 1c) similar to that of juvenile Eusthenopteron14. The radial canals connect to a basal mesh of surface-parallel canals (Fig. 1c). In the largest specimen, MGUH 29020, the radial canals vary in diameter between different parts of the scanned area (Fig. 1c), probably reflecting local blood-supply needs. Although the cortex shows evidence of patchy basal erosion in three of the humeri, all appear to retain areas of primary internal cortical surface (Fig. 2). Clusters of large aligned globular cell lacunae between the endosteal bone and the cortex (Fig. 2b,d and Extended Data Fig. 3) can be identified as chondrocyte lacunae, by comparison with juvenile Eusthenopteron in which similar lacunae lie between the cortical bone and unresorbed remnants of calcified cartilage14. These numerous alignments of chondrocyte lacunae at midshaft (Fig. 2 and Extended Data Fig. 3) mark the perichondral surface of the original cartilaginous humerus. Midshaft being the location of the origin of limb bone growth, this implies that the cartilaginous rod was very large, relative to the observed final size of the bone, and that cortical bone growth conversely made only a modest contribution to the final size. In other words, the Acanthostega individuals grew almost to full observed size before their humeri began to ossify.
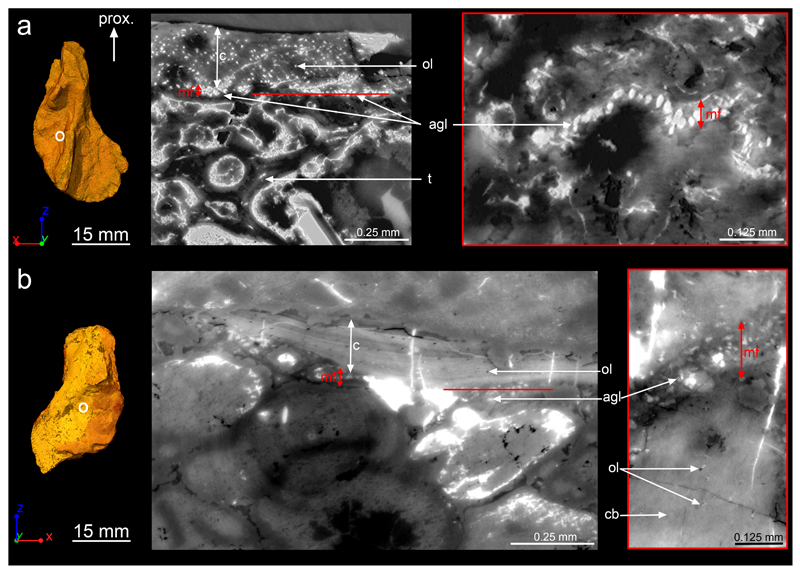
Figure 2. Humeral bone development.
a, 3D model of the humerus of NHMD 74756 in dorsal view. The white circle indicates the midshaft location where the virtual thin sections were made in b. b, Humeral cortical histology (voxel size: 0.638μm; thickness: 10μm) showing the complete bone deposit of periosteal bone (pb) from the mineralisation front (mf) to the surface of the humerus. The aligned globular cell lacunae (agl) are identified as remnants of chondrocyte lacunae – which are much larger than osteocyte lacunae (ol), and typically closely aligned in rows. Trabeculae (t) are numerous in the medullary cavity (mc) and covered with endosteal bone (eb). From left to right: longitudinal virtual thin section; transverse virtual thin section showing the location of the next section in red; tangential virtual thin section. c, 3D model of the humerus of MGUH 29020 in dorsal view showing the high-resolution scanned location. d, Humeral cortical histology (voxel size: 0.638μm; thickness: 10μm) showing the complete record of cortical bone deposition. In addition, remnants of calcified cartilage (cc) within the trabeculae explain their endochondral origin. From left to right: longitudinal virtual thin section in the trabecular region; transverse virtual thin section showing the location of the next section in red; oblique virtual thin section. Abbreviation: prox., proximal.
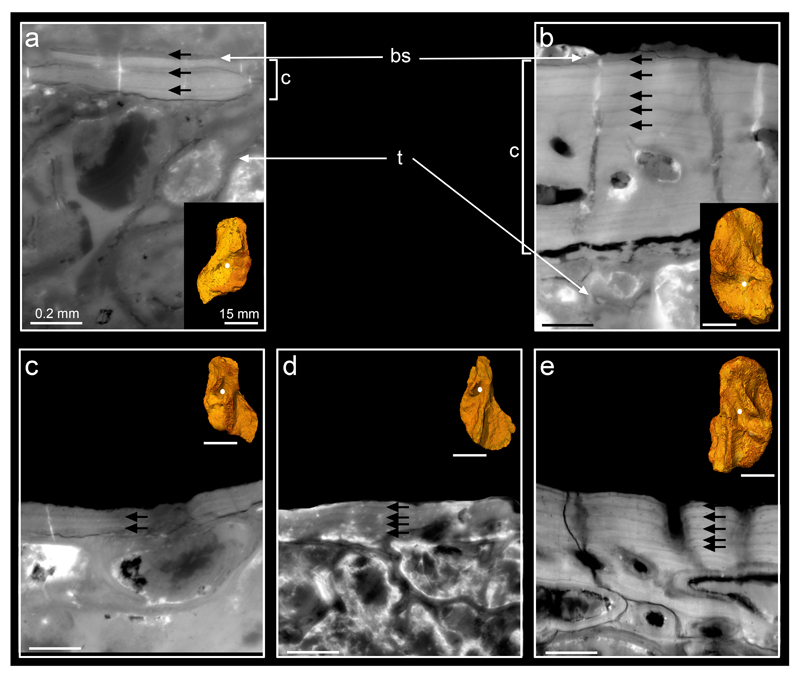
The presence of lines of arrested growth (LAGs) in the cortical bone permits us to infer how many years were occupied by the deposition of this tissue, on the assumption that the deposit between two LAGs represents an annual cycle as in most extant tetrapods18,19. Observations in homologous regions (Extended Data Fig. 4) of the four humeri reveal a maximal amount of six LAGs in MGUH 29020 (Fig. 3b,d and Extended Data Fig. 5b,e), four in NHMD 74756 (Fig. 3c,e) and UMZC T.1295 (Extended Data Fig. 5d), and three in MGUH 29019 (Extended Data Fig. 5a,c). All observations were made in areas at least partly covered with matrix and thus unlikely to have been affected by external erosion. These LAG patterns are regular and show no tightening (Extended Data Table 1), i.e. no deceleration of the growth rate (Extended Data Fig. 6), as would be expected at sexual maturity in adult tetrapods19–21. This suggests that the four specimens of Acanthostega were still juveniles when they died, assuming that their humeri had begun to ossify before the onset of sexual maturity (as they do in all known tetrapods22–24 and in Eusthenopteron14: See Extended Data Supplementary Information). The juvenile stage must therefore have lasted at least six years in Acanthostega. Indeed, it probably lasted a good deal longer, because the cartilaginous humerus grew to almost full size before cortical bone deposition, and thus the recording of annual growth increments, even began. Acanthostega is not the only member of the tetrapod stem group to show a late ossification onset. Juvenile Eusthenopteron exhibits a large spongiosa and a cortex with no internal resorption, showing that the original cartilaginous rod was approximately 2/3 of adult spongiosa size and presumably formed over several years14. How this relates to final adult size in Acanthostega is difficult to say but the slow growth rate of the juvenile Acanthostega suggests that final adult size may not have been much greater than the largest individuals recorded from the mass-death deposit.
Figure 3. Bone skeletochronology.
a, 3D models of the humeri of MGUH 29020 (left) and NHMD 74756 (right) in dorsal and ventral views, showing the locations where the high-resolution scans were done. b-e, Virtual thin sections (voxel size: 0.638μm; thickness: 30μm) revealing lines of arrested growth (yellow and white arrows) resulting from the cyclical growth of the cortical deposit (c). b, Longitudinal section (voxel size: 0.638μm; thickness: 30μm). c, Transverse section (voxel size: 0.638μm; thickness: 30μm). d, Transverse section (voxel size: 1.12μm; thickness: 80μm). e, Transverse section (voxel size: 0.638μm; thickness: 30μm). The annual bone growth rate (Extended Table 1) was measured in each specimen in regions of cortical bone not distorted by taphonomic or biological factors (such as muscle insertions) and exhibiting regular LAG patterns labelled with white arrows. Abbreviation: prox., proximal.
The complete lack of correlation between size and degree of ossification could reflect some form of individual variation, such as the competition-related size variation observed in certain extant tetrapods25, adaptive strategies or sexual dimorphism26. Under these interpretations, some individuals (represented by MGUH 29019 and UMZC T.1295) began to ossify their humeri – and presumably approach sexual maturity – at a much smaller size than the others (represented by MGUH 29020 and NHMD 74756). Unfortunately the very small sample size does not allow us to determine whether the apparently discrete size classes reflect a real bimodal size distribution, or whether they are simply the outcome of randomly sampling a continuous size variation. However, the observed combination of sizes and ossification states categorically invalidates the construction of an ontogenetic sequence from smallest to largest humerus.
The synchrotron virtual histological data from the humeri shed new light on several aspects of the palaeobiology and life history of Acanthostega. It had a prolonged juvenile stage, no less than six years (as shown by the LAGs) but more probably at least a decade, given that it grew almost to full recorded size before the onset of cortical bone ossification. This aligns it with a range of sarcopterygian fishes and tetrapods including Neoceratodus (15-20 years27), Eusthenopteron (adulthood at 11 years14), Discosauriscus (10 years20) and Andrias (larval period of 4-5 years and 10 years to adulthood28), suggesting that a long juvenile stage could be primitive for tetrapods. The late onset of ossification in Acanthostega implies that the early juvenile stage was aquatic, as a cartilaginous humerus would be ill-suited for terrestrial locomotion; this also agrees with the presence of aquatic adaptations such as a large caudal fin and well-developed gill skeleton in Acanthostega1,29, and contradicts the hypothesis of juvenile terrestriality9 at least for this particular tetrapod.
The fact that all four humeri appear to belong to juvenile individuals suggests that the mass-death assemblage is dominated by, and may in fact consist exclusively of, juveniles. The assemblage does not include a subset of distinctively larger individuals. The specimens MGUH 29019 and MGUH 29020 are the most fully ossified humeri12 of the assemblage. MGUH 29019, the smallest humerus, is associated with a 12 cm long skull; MGUH 29020, the largest humerus, is an isolated find from the scree but appears to represent one of the largest individuals in the assemblage (pers. obs. JAC and PEA).
The palaeoenvironmental data from the locality provide a context for these observations. It forms part of a large ephemeral fluvial system in an otherwise arid tropical landscape6, extending northwards for more than 200 km from an unpreserved source water body that must have been large and permanent as it housed large lobe-finned fishes like Eusthenodon and Holoptychius7. The Acanthostega individuals appear to have been flushed out into this fluvial system during a flood event, after which the ensuing drought concentrated them in a shrinking pool and eventually killed them6. The almost complete absence of other taxa in the death assemblage suggests that it is not a concentrate of a whole fauna (like the near-contemporary mass-death deposit from Canowindra, Australia30) but rather a reflection of schooling behaviour in Acanthostega. We can thus tentatively conclude that Acanthostega had a long aquatic juvenile stage characterised, at least at certain times, by the formation of schools that included few or no adults.
Whereas the unique palaeoenvironmental and population-related data provided by the Acanthostega mass-death deposit are dependent on the context of that particular locality, the type of life history information provided by the humeri is dependent only on the preservation of the bone itself and can potentially be matched in a wide range of stem tetrapods. Even a single limb bone can, in principle, provide decisive answers to questions about the length of the juvenile stage and the onset of limb ossification, which in turn help to constrain palaeobiological hypotheses. We are undertaking a systematic PPC-SRµCT survey of stem tetrapod limb histology with this aim in mind. For now, Acanthostega provides a first glimpse of the actual life history of a Devonian tetrapod.
Online Methods Section
Sampling protocol
All the humeri of Acanthostega known so far from the Upper Devonian locality of Stensiö Bjerg (East Greenland) were brought together from museum collections to perform the current study (MGUH and NHMD – Natural History Museum of Denmark – MGUH 29019, MGUH 29020, NHMD 74756, UMZC – University Museum of Zoology Cambridge – T.1295). They were all imaged both at low and high resolutions at the beamline ID19 of the European Synchrotron Radiation Facility (see experimental parameters below).
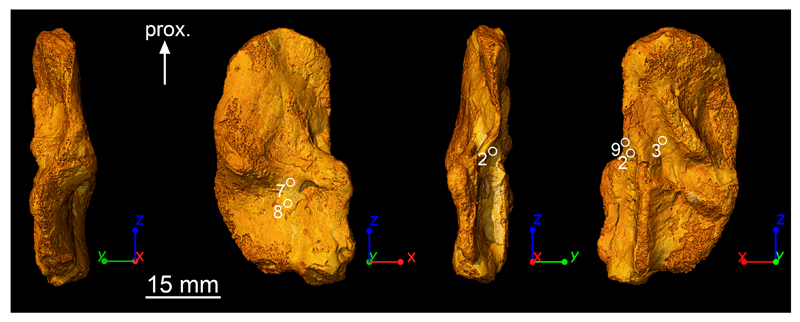
In the diaphyseal region, nine homologous regions were investigated at high resolution in the four humeri but only regions 2, 3, 7, 8 and 9 could provide quantifiable information regarding growth patterns (Extended Data Fig. 4, 6; Extended Data Table 1). Areas of muscle insertions31 were avoided as much as possible. Most of the regions (9, 2, 3) are non-muscle attachment areas. Regions 7 and 8 are located between two regions of muscle insertions but their LAG patterns remain undisturbed (Fig. 3e and Extended Data Fig. 5a). Only the LAG pattern of region 7 of MGUH 29020 (Extended Data Fig. 5b) presents an inner cortex which is highly vascularised. In this case, measurements were done in the most external part of the cortex exhibiting a regular untouched LAG pattern.
In the epiphyseal region, one scan was performed at high resolution in the specimen MGUH 29020.
Imaging experiments
X-ray imaging was done using propagation phase-contrast synchrotron microtomography (PPC-SRμCT) at the beamline ID19 of the European Synchrotron Radiation Facility (ESRF, France). A multiscale approach permitted to perform scans with voxel sizes varying from 20.24 to 0.638 μm with average energies ranging from 60 to 123 keV
1). Low-resolution experiments
The scan of MGUH 29020, at 20.24μm voxel-size, was done with a monochromatic beam, using a double Si111 Bragg monochromator and a FreLON 2k14 CCD detector32 mounted on the lenses coupling optics. The optical system was associated to a Gadox scintillator of 20μm thickness. The distance between the sample and detector was 950mm. The scans were performed at 60keV.
NHMD 74756 was imaged with a voxel size of 14.95μm with a monochromatic beam, using a double Si111 Bragg monochromator and a 2k14 CCD detector31. The optical system was associated to a Gadox scintillator of 10μm thickness. The sample was positioned at 900mm from the detector. The scans were performed at 60keV. 5000 projections were taken over 360° in half-acquisition mode. The time of exposure was of 0.25s.
In order to obtain a voxel size of 12.62μm while scanning the humeri MGUH 29019, MGUH 29020 and UMZC T.1295, the experiment was done using pink beam with a Frelon 2k14 CCD detector31 mounted on an optical system associated to a 1000μm-thick LuAG scintillator. The gap of the wiggler was opened to 50mm. The beam was filtered with 2mm of aluminium and 9mm of copper. The resulting average energy was of 123keV. The samples were placed at a distance of 13m from the detector in order to obtain an enhanced propagation phase-contrast. 4998 projections were taken over 360° in half-acquisition mode. The time of exposure was of 0.15s.
2). High-resolution experiments
The epiphysis of MGUH 29020 was imaged with a voxel size of 1.12μm. The experiment was performed in monochromatic conditions with a FReLoN 2K1432, an objective 10x, N.A. 0.3, coupled with a 2.5x eyepiece and a 10μm-thick GGG scintillator mounted on the microscope optics in binning conditions. The multilayer was set to the energy of 52keV. The distance of propagation was of 150mm.
The high-resolution scans at midshaft were done with a voxel size of 0.638μm using pink beam. A Frelon 2k14 CCD detector32 associated to a microscope with a 25μm-thick GGG scintillator permitted to obtain the submicron voxel size. The gap of the U32u undulator was of 11.5mm. The beam was filtered with 2mm of aluminium, 0.1mm of copper and 0.1mm of tungsten. The average energy was probably of 55keV. 22 transfocator 2D CRL lenses, made of beryllium, and with a radius of curvature of 0.5 mm, were used to reduce the actual divergence of the beam at this energy, thereby increasing the flux going through the samples. The fossils were at a distance of propagation of 150mm. 6000 projections were taken over 360° in half-acquisition mode. The time of exposure was of 1s.
Image reconstruction
The data was reconstructed using a single distance phase retrieval approach8 based on a modified version of the algorithm of Paganin et al.33, applying an unsharp mask on the radiographs after the phase retrieval to compensate the partial loss of the high frequencies due to the original algorithm. This is based on a relative chemical homogeneity assumption. In-house filters were used to enhance the contrast between the microstructures and reduce the noise induced by metallic infillings.
Segmentation
The data were segmented using the software VGStudio MAX version 2.2 (Volume Graphics Inc., Germany).
Extended Data
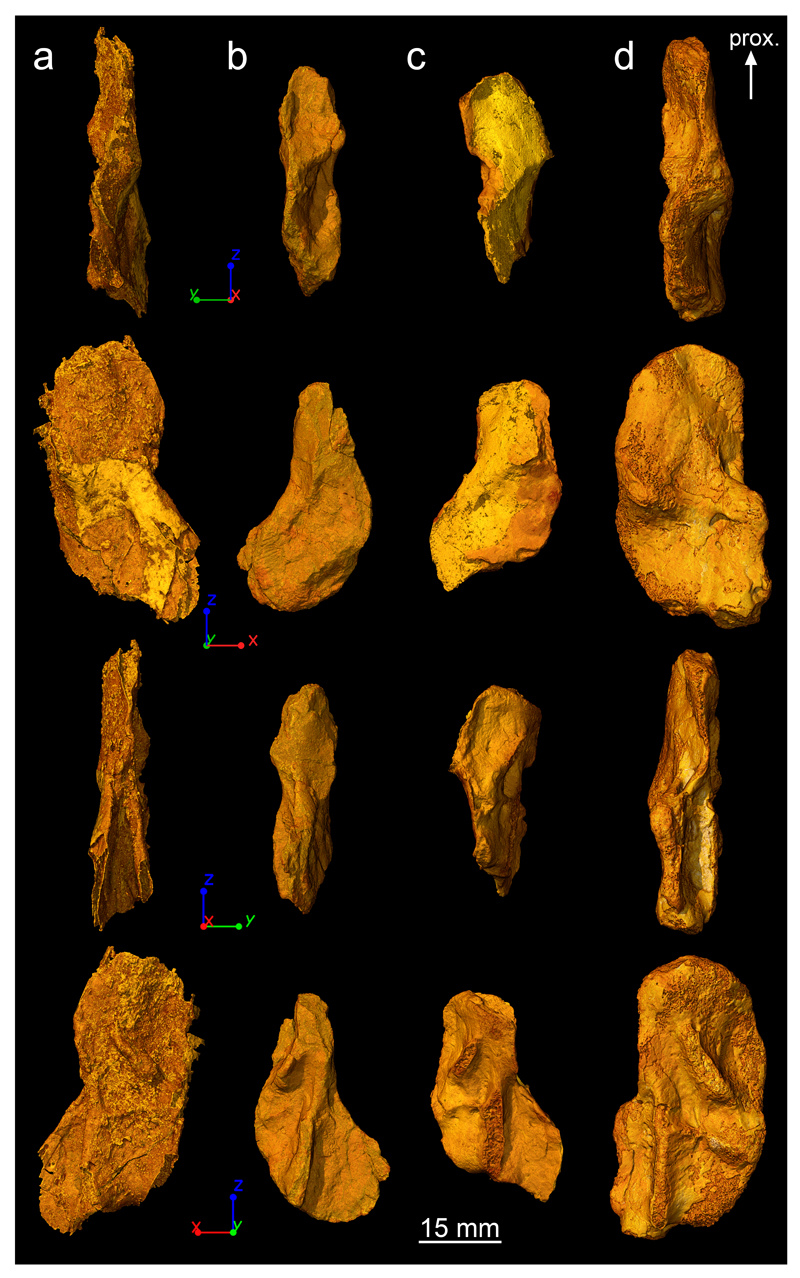
Extended Data Figure 1. 3D models of Acanthostega’s humeri based on synchrotron microtomography data.
a,NHMD 74756. b, UMZC T.1295. c, MGUH 29019. d, MGUH 29020. From top to bottom: preaxial view, ventral view, postaxial view, dorsal view. They are all oriented with their proximal epiphysis towards the top. Abbreviation: prox., proximal.
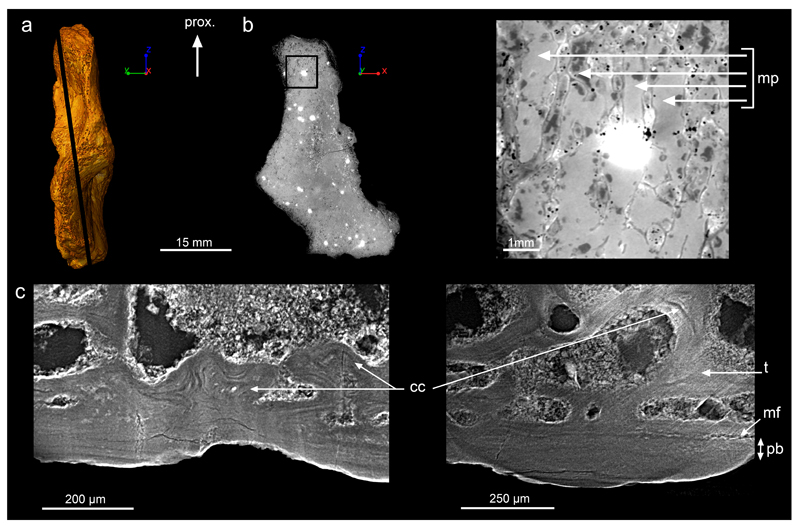
Extended Data Figure 2. Epiphyseal microanatomy and histology of Acanthostega’s humerus (MGUH 29020).
a, 3D model in preaxial view, based on synchrotron microtomography data. It is oriented with the proximal extremity (epiphysis6) towards the top. The black line indicates the virtual thin section illustrated in b. b, Longitudinal virtual thin section (thickness: 50μm, voxel size: 1.12μm, same scale bar and orientation as in a) showing the location where the detailed image on the right comes from. The latter shows the marrow processes (mp) formed in the growth plate by endochondral ossification. c, High-resolution virtual thin section (thickness: 50μm, voxel size: 1.12μm) from the epiphyseal region showing obvious Liesegang’s rings as remnants of calcified cartilage6 (cc), formed during endochondral ossification. These remnants are entrapped in the trabeculae (t), at the vicinity of the ossification notch6, where the thickness of the periosteal bone (pb) – between the mineralisation front (mf) and the surface – drastically reduces. The bone is oriented with its surface towards the bottom. From left to right: longitudinal thin section, transverse section. Abbreviation: prox., proximal.
Extended Data Figure 3. Midshaft bone histology of two humeri of Acanthostega (UMZC T.1295 and MGUH 29019).
a, 3D model of the humerus UMZC T.1295 in dorsal view and oriented with their proximal epiphyses6 towards the top. The white circle indicates the midshaft location where the transverse virtual section was made. The latter (single tomographic slice, voxel size: 0.638μm) shows the complete bone deposit of cortical bone (c) from the mineralisation front (mf) to the surface of the humerus (top). The cortical bone comprises numerous osteocyte lacunae (ol), which are much smaller than the aligned globular cell lacunae (agl) present at the location of the mineralisation front. Trabeculae (t) are numerous in the medullary cavity. The red line in the transverse virtual section indicates the location of the next tangential virtual section which details the mineralisation front. b, 3D model of the humerus MGUH 29019 in ventral view showing the high-resolution scanned location. The virtual section shows the humeral cortical histology at midshaft (single tomographic slice, voxel size: 0.638μm). As in UMZC T.1295, the cortical bone matrix (cb) is very compact, pierced with small osteocyte lacunae. At this location, its surface (top), although still embedded in the rock matrix, is not well preserved. The red line in the transverse virtual section indicates the location of the next tangential virtual section detailing the cellular structure of the mineralisation front. Abbreviation: prox., proximal.
Extended Data Figure 4. Regions where high-resolution scans were made.
Skeletochronological observations were done at submicron resolution in nine homologous regions of the four humeri of Acanthostega. The specimen MGUH 29020 is used here to illustrate the regions providing quantifiable information to calculate annual bone growth rates (Extended Data Table 1). Areas of muscle insertions were avoided when possible. Regions 2, 3 and 9 are non-muscle attachment areas. Regions 7 and 8 are located between two regions of muscle insertions but annual bone growth rates (Extended Data Table 1) were only measured in undisturbed cortical parts exhibiting regular LAG patterns.
Extended Data Figure 5. Humeral midshaft skeletochronology.
All virtual thin sections (voxel size: 0.638μm) reveal lines of arrested growth (LAGs, black arrows) resulting from the cyclical growth of the cortical deposit (c). They are oriented with the surface (bs) of the bone towards the top and medullary trabeculae (t) downwards. The locations of the thin sections are shown as white dots on the associated 3D models. All 3D models are oriented with their proximal epiphyses6 towards the top. a, Transverse virtual thin section (thickness: 30μm) showing 3 LAGs in the cortical bone of the ventral midshaft of the humerus MGUH 29019 (region 7). The inner surface of the cortical bone has been eroded. b, Longitudinal virtual thin section (thickness: 30μm) showing 5 LAGs in the cortical bone of the ventral midshaft of MGUH 29020 (region 7). The inner cortical bone is disturbed by a highly vascularised period. LAGs cannot be identified with accuracy in this region. The growth deposits between the LAGs in region 7 are similar in MGUH 29019 and MGUH 29020 (Extended Data Table 1). c, Transverse virtual thin section (thickness: 30μm) showing 2 LAGs in the cortical bone of the dorsal midshaft of the specimen MGUH 29019 (region 3). d, Longitudinal virtual thin section (thickness: 50μm) showing 4 LAGs in the cortical bone of the dorsal midshaft of UMZC T.1295 (region 3). e, Longitudinal virtual thin section (thickness: 30μm) showing 5 LAGs in the cortical bone of the dorsal midshaft of MGUH 29020 (region 3). The growth deposits between the LAGs in region 3 are similar in UMZC T.1295, MGUH 29019 and MGUH 29020 (Extended Data Table 1). Scale bars for virtual thin sections: 0.2mm. Scale bars for 3D models: 15mm.
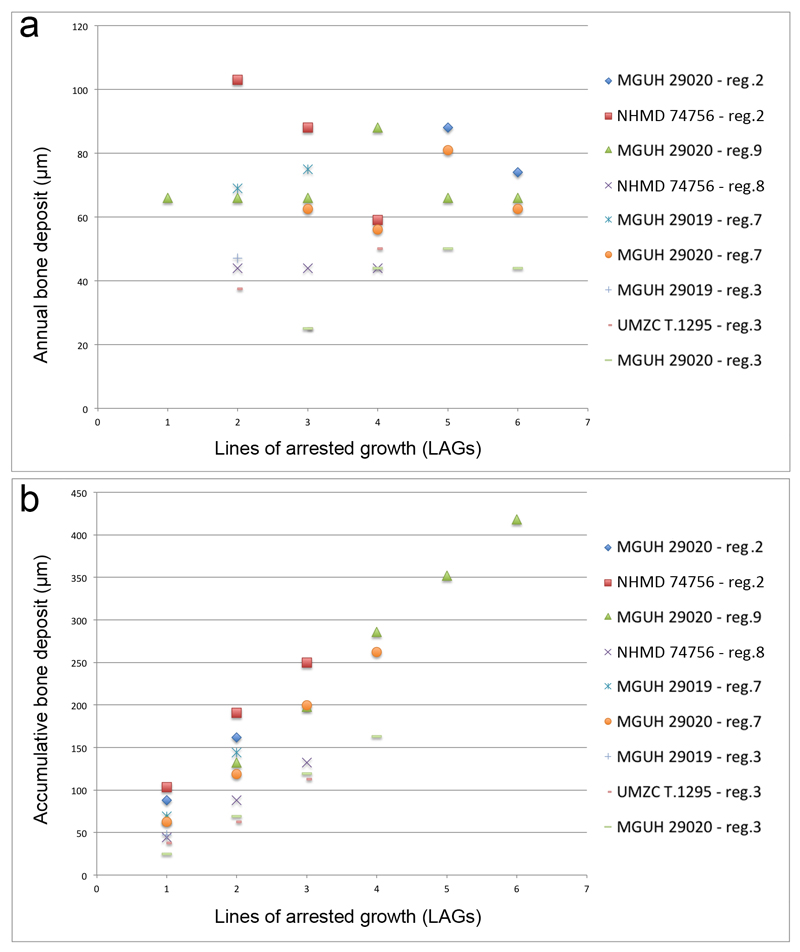
Extended Data Figure 6. Graphic visualisations of bone deposits based on the measurements provided in Extended Data Table 1.
a, Amount of bone deposited every year – i.e. between two lines of arrested growth (LAG) – in the regions of interest (reg.) of the four studied humeri. Except from region 2 (measured in MGUH 29020 and NHMD 74756), all other regions show a relatively constant or increasing growth rate during the animal development. b, Bone deposition accumulated to form the cortex. Despite a slight variation of values due to growth allometries, the growth rate (illustrated by the slope angle) is relatively constant in all regions of all specimens, meaning that all specimens grow with the same rate.
Extended Data Table 1. Measurements of humeral cyclical growth deposits between lines of arrested growth – LAG (in μm).
Measurements are based on the LAGs labelled with white arrows in Fig. 3 and black arrows in Extended Data Fig. 5. Nine regions were investigated but only regions 2, 3, 7, 8 and 9 could provide quantifiable information (Extended Data Fig. 4). Symbol: -, there is no more LAGs as the bone stopped growing.
| Region | Specimen | Figure | LAG 0-1 | LAG 1-2 | LAG 2-3 | LAG 3-4 | LAG 4-5 | LAG 5-6 | Average deposit |
|---|---|---|---|---|---|---|---|---|---|
| 2 | MGUH 29020 | Fig. 3b | LAG pattern disturbed in a highly vascularised region | 88 | 74 | 81 | |||
| 2 | NHMD 74756 | Fig. 3c | eroded | 103 | 88 | 59 | - | - | 83 |
| 9 | MGUH 29020 | Fig. 3d | 66 | 66 | 66 | 88 | 66 | 66 | 70 |
| 8 | NHMD 74756 | Fig. 3e | eroded | 44 | 44 | 44 | - | - | 44 |
| 7 | MGUH 29019 | Extended Data Fig. 5a | eroded | 69 | 75 | - | - | 72 | |
| 7 | MGUH 29020 | Extended Data Fig. 5b | LAG pattern disturbed in a highly vascularised region | 65.5 | 56 | 81 | 62.5 | 65.5 | |
| 3 | MGUH 29019 | Extended Data Fig. 5c | eroded | 47 | - | - | - | - | 47 |
| 3 | UMZC T.1295 | Extended Data Fig. 5d | eroded | 37.5 | 25 | 50 | - | - | 37.5 |
| 3 | MGUH 29020 | Extended Data Fig. 5e | LAG pattern disturbed in a highly vascularised region | 25 | 44 | 50 | 44 | 41 | |
Supplementary Material
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Acknowledgements
Beamtime was allocated as inhouse beamtime and thanks to a proposal accepted by the ESRF (EC203, S.S.). This research was supported by an ERC grant (233111, P.E.A.) and a grant from the Vetenskapsrådet (2015-04335, S.S.). The authors thank J. Castanet, J.-S. Steyer and G. Clement (MNHN, Paris), M. Coates (University of Chicago) T. Smithson (University of Cambridge), A. R. Milner (Birkbeck University of London), H. Blom and D. Snitting (Uppsala University), I. Adameyko (KI, Stockholm), A. Soler and S. Martin (La Ferme aux Crocodiles, Pierrelatte) and R. R. Schoch (SMNS) for interesting discussions; G. Cuny and Bent E. Kramer Lindow for access to the collections housed in the Natural History Museum of Denmark and M. Lowe for access to the collections of the University Museum of Zoology Cambridge.
Footnotes
Author Contributions S.S., P.E.A. and P.T. conceived and designed the project. S.S. and P.T. performed the synchrotron experiments. The localities were excavated by J.A.C. and P.E.A. in 1987. P.T. processed and reconstructed the raw PPC-SRµCT scan data. S.S. segmented the scan data. S.S., P.E.A. and P.T. analysed the data. All authors discussed the interpretations. S.S. and P.E.A. developed the main text. S.S. made the figures and supplementary information. All authors provided a critical review of the manuscript and approved the final draft.
The authors declare no competing financial interests.
Author information The synchrotron data will be made available through the ESRF palaeontology database (http://paleo.esrf.eu).
References
- 1.Clack JA. Gaining ground. 2nd edn. Indiana Univ. Press; 2012. [Google Scholar]
- 2.Niedzwiedzki G, Szrek P, Narkiewicz K, Narkiewicz M, Ahlberg PE. Tetrapod trackways from the early Middle Devonian period of Poland. Nature. 2010;463:43–48. doi: 10.1038/nature08623. [DOI] [PubMed] [Google Scholar]
- 3.Shubin NH, Daeschler EB, Jenkins FA., Jr The pectoral fin of Tiktaalik roseae and the origin of the tetrapod limb. Nature. 2006;440:764–771. doi: 10.1038/nature04637. [DOI] [PubMed] [Google Scholar]
- 4.Friedman M, Coates MI, Anderson P. First discovery of a primitive coelacanth fin fills a major gap in the evolution of lobed fins and limbs. Evol Dev. 2007;9:329–337. doi: 10.1111/j.1525-142X.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- 5.Boisvert CA, Mark-Kurik E, Ahlberg PE. The pectoral fin of Panderichthys and the origin of digits. Nature. 2008;456:636–638. doi: 10.1038/nature07339. [DOI] [PubMed] [Google Scholar]
- 6.Astin TR, Marshall JEA, Blom H, Berry CM. The sedimentary environment of the Late Devonian East Greenland tetrapods. Geol Soc Lond. 2010;339:93–109. [Google Scholar]
- 7.Blom H, Clack JA, Ahlberg PE, Friedman M. Devonian vertebrates from East Greenland: a review of faunal composition and distribution. Geodiversitas. 2007;29:119–141. [Google Scholar]
- 8.Sanchez S, Ahlberg PE, Trinajstic K, Mirone A, Tafforeau P. Three dimensional synchrotron virtual paleohistology: a new insight into the world of fossil bone microstructures. Microsc Microanal. 2012;18:1095–1105. doi: 10.1017/S1431927612001079. [DOI] [PubMed] [Google Scholar]
- 9.Warburton FE, Denman NS. Larval competition and the origin of tetrapods. Evolution. 1961;15:566. [Google Scholar]
- 10.Blom H, Clack JA, Ahlberg PE. Localities, distribution and stratigraphical context of the Late Devonian tetrapods of East Greenland. Medd Grönl. 2005;43:4–50. [Google Scholar]
- 11.Clack JA. The dermal skull roof of Acanthostega gunnari, an early tetrapod from the Late Devonian. Trans R Soc Edinb Earth Sci. 2002;93:17–33. [Google Scholar]
- 12.Callier V, Clack JA, Ahlberg PE. Contrasting developmental trajectories in the earliest known tetrapod forelimbs. Science. 2009;324:364–367. doi: 10.1126/science.1167542. [DOI] [PubMed] [Google Scholar]
- 13.Coates MI. The Devonian tetrapod Acanthostega gunnari Jarvik: postcranial anatomy, basal tetrapod interrelationships and patterns of skeletal evolution. Trans R Soc Edinb Earth Sci. 1996;87:363–421. [Google Scholar]
- 14.Sanchez S, Tafforeau P, Ahlberg PE. The humerus of Eusthenopteron: a puzzling organization presaging the establishment of tetrapod limb bone marrow. Proc R Soc B. 2014;281:20140299. doi: 10.1098/rspb.2014.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coates MI, Ruta M, Friedman M. Ever since Owen: changing perspectives on the early evolution of tetrapods. Annu Rev Ecol Evol Syst. 2008;39:571–592. [Google Scholar]
- 16.Francillon-Vieillot H, et al. In: Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. Carter JG, editor. I. Van Nostrand Reinhold; 1990. pp. 471–530. [Google Scholar]
- 17.Laurin M, Meunier F-J, Germain D, Lemoine M. A microanatomical and histological study of the paired fin skeketon of the Devonian sarcopterygian. Eusthenopteron foordi J Paleontology. 2007;81:143–153. [Google Scholar]
- 18.Castanet J, Francillon-Vieillot H, Ricqlès A. In: de in Amphibian Biology. Heatwole H, Davies M, editors. V-Osteology. Surrey Beatty & Sons; 2003. pp. 1598–1683. [Google Scholar]
- 19.Padian K. Evolutionary physiology: A bone for all seasons. Nature. 2012;487:310–311. doi: 10.1038/nature11382. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez S, Klembara J, Castanet J, Steyer J-S. Salamander-like development in a seymouriamorph revealed by palaeohistology. Biol Lett. 2008;4:411–414. doi: 10.1098/rsbl.2008.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castanet J, Francillon-Vieillot H, Meunier F-J, de Ricqlès A. In: Bone. Hall BK, editor. Vol. 7. CRC Press; 1993. pp. 245–283. Bone Growth-B. [Google Scholar]
- 22.Fröbisch NB. Ossification patterns in the tetrapod limb–conservation and divergence from morphogenetic events. Biol Rev. 2008;83:571–600. doi: 10.1111/j.1469-185X.2008.00055.x. [DOI] [PubMed] [Google Scholar]
- 23.Witzmann F. Developmental patterns and ossification sequence in the Permo-Carboniferous temnospondyl Archegosaurus decheni (Saar-Nahe Basin, Germany) J Vertebr Paleontol. 2006;26:7–17. [Google Scholar]
- 24.Schoch RR. Skeleton Formation in the Branchiosauridae: a case study in comparing ontogenetic trajectories. J Vertebr Paleontol. 2004;24:309–319. [Google Scholar]
- 25.Peacor SD, Pfister CA. Experimental and model analyses of the effects of competition on individual size variation in wood frog (Rana sylvatica) tadpoles. J Anim Ecol. 2006;75:990–999. doi: 10.1111/j.1365-2656.2006.01119.x. [DOI] [PubMed] [Google Scholar]
- 26.Badyaev AV. Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol Evol. 2002;17:369–378. [Google Scholar]
- 27.Kind PK. Movement patterns and habitat use in the Queensland lungfish Neoceratodus forsteri (Krefft 1870) PhD Thesis thesis; University of Queensland: 2002. [Google Scholar]
- 28.Sparreboom M. Salamanders of the old world: the salamanders of Europe, Asia and Northern Africa. KNNV Publishing; 2014. [Google Scholar]
- 29.Ahlberg PE, Milner AR. The origin and early diversification of tetrapods. Nature. 1994;368:507–514. [Google Scholar]
- 30.Johanson Z. The Upper Devonian Fish Bothriolepis (Placodermi: Antiarchi) from near Canowindra, New South Wales, Australia. Records-Australian Museum. 1998;50:315–348. [Google Scholar]
- 31.Bishop PJ. The humerus of Ossinodus pueri a stem tetrapod from the Carboniferous of Gondwana, and the early evolution of the tetrapod forelimb. Alcheringa. An Australasian Journal of Palaeontology. 2014;38:209–238. [Google Scholar]
- 32.Labiche J-C, et al. The fast readout low noise camera as a versatile X-ray detector for time resolved dispersive extended X-ray absorption fine structure and diffraction studies of dynamic problems in materials science, chemistry, and catalysis. Rev Sci Instrum. 2007;78:091301–091311. doi: 10.1063/1.2783112. [DOI] [PubMed] [Google Scholar]
- 33.Paganin D, Mayo SC, Gureyev TE, Miller PR, Wilkins SW. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J Microsc. 2002;206:33–40. doi: 10.1046/j.1365-2818.2002.01010.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.