Abstract
Background
Early-life respiratory tract infections could affect airway obstruction and increase asthma risk in later life. However, results from previous studies are inconsistent.
Objective
We examined the associations of early life respiratory tract infections with lung function and asthma in school-aged children.
Methods
This study among 5,197 children born between April 2002 and January 2006 was embedded in a population-based prospective cohort study. Information on physician-attended upper and lower respiratory tract infections until age 6 years (categorized into ≤3 and >3-6 years) was obtained by annual questionnaires. Spirometry measures and physician-diagnosed asthma were assessed at age 10 years.
Results
Upper respiratory tract infections were not associated with adverse respiratory outcomes. Compared with children without lower respiratory tract infections ≤3 years, children with lower respiratory tract infections ≤3 years had a lower forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC and forced expiratory flow at 75% of FVC (FEF75) (Z-score (95% CI): ranging from -0.22 (-0.31, -0.12) to -0.12 (-0.21, -0.03)), and an increased risk of asthma (odds ratio (95% CI): 1.79 (1.19, 2.59)). Children with lower respiratory tract infections >3-6 years had an increased risk of asthma (3.53 (2.37, 5.17)) only. Results were not mediated by antibiotic or paracetamol use, and not modified by inhalant allergic sensitization. Cross-lagged modeling showed that results were not bidirectional and independent of preschool wheezing patterns.
Conclusion
Early-life lower respiratory tract infections ≤3 years are most consistently associated with lower lung function and increased risk of asthma in school-aged children.
Keywords: Respiratory infection, asthma, clinical epidemiology
Introduction
It has been hypothesized that respiratory tract infections in early life influence the risk of lower lung function1–4 and asthma5–10 in later childhood and adulthood. Respiratory tract infections could lead to higher airway sensitization leading to airway obstruction and hyperreactivity11, and subsequently asthma. Respiratory tract infections might lead to persistent respiratory morbidity since both the immune and respiratory system are under development in early life. In observational studies, findings on the association of respiratory tract infections with lung function or asthma have been inconclusive1–10. Differences in results might be explained by power issues, ascertainment of respiratory tract infections, use of different lung function measurements, definitions of asthma, age of the outcome, and use of covariates. Most studies that examined associations of respiratory tract infections with asthma focused on children at high risk for atopy5–7 9 10, which leads to limited external validity. Additionally, child’s antibiotic12–17 and paracetamol17–19 use are suggested to be associated with increased risks of childhood asthma, and their effect on the associations of respiratory tract infections with lung function and asthma is unclear. Antibiotic or paracetamol use might have an effect on the risk of lower lung function and asthma, by influencing the microbiome or glutathione levels, respectively. However, any found association could also be the result of confounding by indication or reverse causality. Additionally, parents might change smoking behavior as a result of respiratory infections, which could subsequently influence the risk of asthma. Therefore, second-hand smoke exposure might act as a mediator in this association. Associations of respiratory tract infections with asthma seem different between subgroups of children with or without allergic sensitization, which suggests a modifying effect of allergy6 7. Last, it is unclear whether early respiratory tract infections lead to lower lung function and asthma or that lower lung function and asthma, represented by wheezing at preschool age, lead to vulnerability for respiratory tract infections.
Therefore, we examined in a population-based prospective cohort study the association of early-life respiratory tract infections with lung function and asthma in school-aged children, taking bidirectional associations and preschool wheezing into account. Next, we examined if these associations are explained by children’s paracetamol and antibiotic use or modified by current inhalant allergic sensitization status, and if findings were specific for respiratory tract infections. This study adds to the current literature by examining the association of respiratory tract infections with lung function and asthma on a population-based level, using detailed and longitudinally measured information on both upper and lower respiratory tract infections, and respiratory outcomes measures, applying a unique approach to identify bidirectional associations, and taking the effect of infections in general into account. With this, we aim to provide new evidence for a possible direct effect of respiratory tract infections on lung function and development.
Methods
Design
This study was embedded in the Generation R Study, a population-based prospective cohort study from early fetal life onwards in Rotterdam, The Netherlands. Women with a delivery data between April 2002 and January 2006 living in Rotterdam were eligible for participation in the study, as described previously20. The study has been approved by the Medical Ethical Committee of the Erasmus MC, University Medical Centre Rotterdam, Rotterdam, The Netherlands (MEC-2012-165). Written informed consent was obtained from parents or legal representatives of all participants. A total of 5,197 children were included for the current analyses (Supplementary Figure S1).
Early life respiratory tract infections
Information on parental reported physician-attended upper and lower respiratory tract infections was obtained by questionnaires at the ages of 6 months and 1, 2, 3, 4 and 6 years. Response rates ranged from 71 to 80%. Questions about physician-attended upper respiratory tract infections included ear and throat infections, false croup and whooping cough, and about lower respiratory tract infections bronchitis, bronchiolitis and pneumonia in the past 6 or 12 months (no or yes, not physician-attended; yes, physician-attended). We combined each individual respiratory tract infection into groups of upper and lower respiratory tract infections. For the cross-lagged analyses, respiratory tract infections were first analyzed per year, and to improve readability subsequently combined into early (≤3 years: at least 1 infection in this period vs. no infections or late infections only) and late (3-6 years: at least 1 infection in this period vs. no infections or early infections only) upper and lower respiratory tract infections. The cut-off point of 3 years was chosen to obtain sufficient power, based on end of highest velocity of maturation of lungs and the immune system until this age21–23, and for consistency with categorization of wheezing patterns.
School-age lung function and asthma
At age 10 years, Forced Expiratory Volume in 1 second (FEV1), Forced Vital Capacity (FVC), FEV1/FVC and Forced Expiratory Flow after expiring 75% of FVC (FEF75) were measured by spirometry (MasterScreen-Pneumo, Jaeger Toennies (Viasys) CareFusion Netherlands). All curves were scored by two trained researchers according to the ERS/ATS guidelines24 and when necessary discussed with a senior researcher. Reproducible curves were converted into sex-, age-, height- and ethnicity-adjusted z-scores25. Current asthma was defined as 1) ever diagnosis of physician-diagnosed asthma with 2) either wheezing or any asthma medication use in the past 12 months. Questions on asthma and wheezing were adapted from the International Study on Asthma and Allergy in Childhood (ISAAC), and information on medication use was obtained at the research center. Wheezing was reported by annual parental questionnaires from birth to age 4 years, and at age 6 years. We combined preschool wheezing patterns into early (≤3 years) and late (>3-6 years) wheezing, similar to groups of respiratory tract infections.
Covariates
Information on maternal characteristics included educational level, body mass index at intake, parity, smoking during pregnancy, psychiatric symptoms, pet keeping, and history of asthma and atopy, and were obtained from multiple questionnaires during pregnancy. Mode of delivery and child’s sex, gestational age at birth, and birth weight were obtained from midwife and hospital records at birth. Information on country of birth, breastfeeding, day care attendance, environmental tobacco smoke exposure, antibiotic and paracetamol use were obtained by multiple questionnaires at age 6 months to 2 years. Ethnicity was based on country of birth of both parents. Inhalant allergic sensitization at the age of 10 years was measured by skin prick test using the ‘scanned area method’26.
Other infections
Information on infections other than respiratory tract infections were collected by similar questionnaires as respiratory tract infections and included physician-diagnosed gastro-enteritis and urinary tract infection (no or yes, not physician-attended; yes physician-attended). Similarly to respiratory tract infections, other infections were combined into early (≤3 years) and late (3-6 years) infections.
Statistical analysis
First, we examined associations of respiratory tract infections with lung function measures and asthma using linear and logistic regression models, respectively. Analyses were adjusted for potential confounders, which were selected from literature8 9 27–29, if they were related to respiratory tract infections and the outcomes of interest, or if the effect estimate of the unadjusted analyses changed ≥10% when we additionally adjusted for a confounder. Missing data of covariates were imputed by the multiple imputation method using chained equations to select the most likely value for a missing response. Ten new datasets were created by imputation. Additionally, to take correlations between respiratory tract infections into account, both any and individual upper respiratory tract infections were adjusted for any preceding upper respiratory tract infections, and any and individual lower respiratory tract infections for any preceding lower respiratory tract infections. We did not apply multiple testing due to strong correlations between the different infections and lung function measures, since that could potentially lead to false negative findings. Second, we examined if associations were mediated by children’s antibiotic or paracetamol use or environmental tobacco smoke exposure by additionally adjusting for these variables, or were modified by inhalant allergic sensitization by testing their interactive effects. Statistical analyses were performed with the Statistical Package of Social Sciences version 21.0 for Windows (SPSS Inc). Third, we applied a cross-lagged model using Mplus version 7.11 for Windows (Muthén & Muthén). With a cross-lagged model, bidirectional associations between the exposure, respiratory tract infections until age 5 years, and the outcome, wheezing until age 5 years, and lung function or asthma at age 10 years, can be studied within the same model. In this model, logistic or linear regression models are used to study the associations of early respiratory tract infections with early and late wheezing and lung function or asthma, of late respiratory tract infections with late wheezing and lung function or asthma, and of early wheezing with late respiratory tract infections, while taking associations over time between respiratory tract infections, and wheezing and lung function or asthma into account30 31. These models enable us to disentangle the direction of the observed associations, such as whether respiratory tract infections influence the risk of lower lung function and asthma, or vice versa. Last, sensitivity analyses were performed to examine the associations of gastro-enteritis and urinary tract infection with lung function and asthma. These sensitivity analyses could prove insight to whether any associations of respiratory tract infections with lung function and asthma are due to a specific respiratory tract infection effect, or due to a general infection status. All measures of association are presented with their 95% Confidence Intervals (95% CI).
Results
Subject characteristics
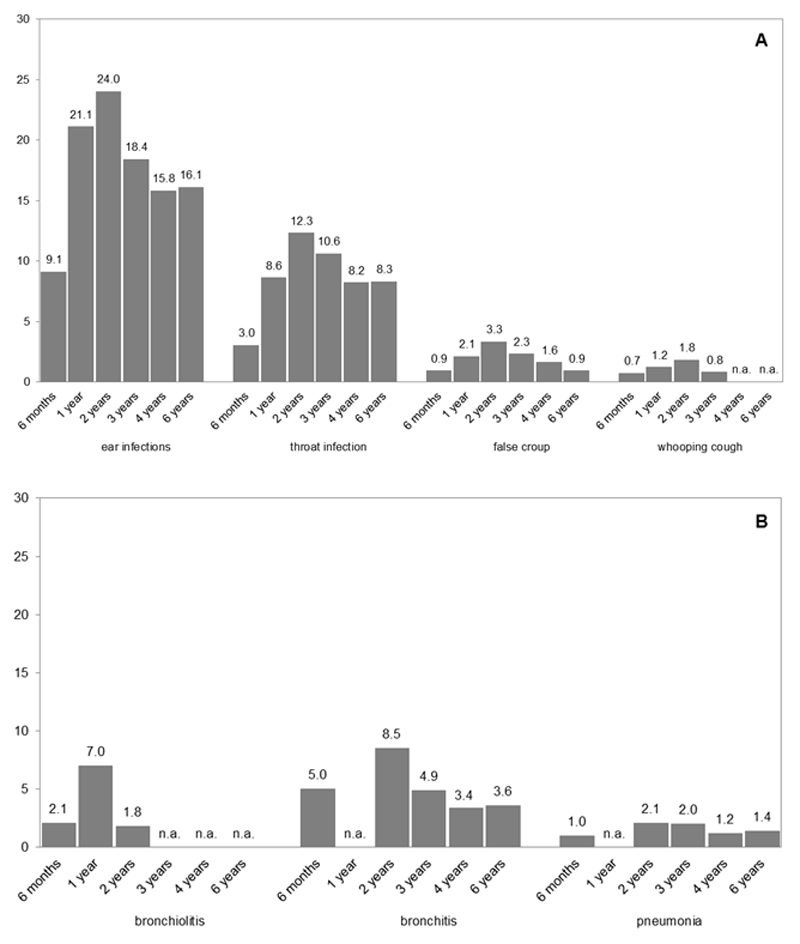
Maternal and child characteristics are shown in Table 1. The highest prevalence of upper and lower respiratory tract infections was at the age of 2 years (24.0% and 8.5%, Figure 1A and B, respectively). At age 10 years, mean FEV1 (SD) was 2.02 L (0.30), FVC 2.33 L (0.36), FEV1/FCV 86.6% (5.7) and FEF75 1.14 L/s (0.35) (Table 1). Current asthma was present in 5.5% of the children. Current asthma was defined as ever asthma (9.4%), combined with wheezing (4.4) or medication use (17.4%) in the past 12 months. Those not included in the analysis had, among others, mothers who were lower educated and had a higher prevalence of smoking during pregnancy. These children were more often born younger, had a lower birth weight, and a lower prevalence of breastfeeding (Supplementary Table S1).
Table 1.
Characteristics of children and their mothers
| n = 5,197 |
|
|---|---|
| Maternal characteristics | |
| Education, higher (%) | 51.6 (2,685) |
| Body mass index at intake (kg/m2) | 24.6 (4.25) |
| Parity, nullipara (%) | 56.6 (2,942) |
| Smoking during pregnancy, yes (%) | 14.0 (729) |
| Psychiatric symptoms during pregnancy (GSI)1 | 0.17 (0.06,0.38) |
| Pet keeping, yes (%) | 38.1 (1,982) |
| History of asthma or atopy, yes (%) | 37.2 (1,936) |
| Mode of delivery, caesarian section (%) | 13.6 (709) |
| Children’s characteristics | |
| Female sex (%) | 50.5 (2,626) |
| Gestational age at birth (weeks)1 | 40.0 (39.1, 41.0) |
| Preterm birth <37 weeks (%) | 4.3 (223) |
| Birth weight (grams) | 3,444 (550) |
| Breastfeeding never (%) | 8.1 (419) |
| Day care attendance 1st year, yes (%) | 58.2 (3,025) |
| Environmental tobacco smoke exposure first 2 years, yes (%) | 19.3 (1,005) |
| Use of antibiotics 1st year, yes (%) | 63.0 (3,278) |
| Use of paracetamol 1st year, yes (%) | 87.8 (4,569) |
| Inhalant allergic sensitization at age 10 years, yes (%) | 31.8 (1.172) |
| FEV1 (L) | 2.02 (0.30) |
| FEV1 (Z-score) | 0.17 (0.97) |
| FVC (L) | 2.33 (0.36) |
| FVC (Z-score) | 0.21 (0.93) |
| FEV/FEV (%) | 86.6 (5.7) |
| FEV1/FVC (Z-score) | -0.11 (0.95) |
| FEF75 (L/s) | 1.14 (0.35) |
| FEF75 (Z-score) | 0.00 (0.91) |
| Current asthma, yes (%) | 5.5 (252) |
| Early wheezing, yes (%) | 59.5 (1,828) |
| Late wheezing, yes (%) | 20.0 (690) |
Values are means (SD), valid percentages (absolute numbers) or 1medians (25-75% range) based on imputed data. Global Severity Index (GSI). Data on Forced Vital Capacity (FVC) (available n=4,672), Forced Expiratory Flow in 1 second (FEV1) (n=4,672), FEV1/FVC ratio (n=4,672), Forced Expiratory Flow when 75% of the FVC is exhaled (FEF75) (n=4,672), current asthma (n=4,556) and early (n=3,074) and late (n=3,443) wheezing was not imputed.
Figure 1.
Prevalence of upper (A) and lower (B) respiratory tract infections.
Values represent % of specific upper and lower respiratory tract infections per age, and were not imputed. Not available – no data on this specific infection at this timepoint (n.a.)
Respiratory tract infections and lung function or asthma
Results from models on crude and adjusted complete cases associations of upper and lower respiratory tract infections with lung function and asthma are shown in Supplementary Table S2 and S3, respectively. No major differences in the magnitude or direction of the effect estimates were observed between analyses with imputed missing data and complete cases only. Results of imputed datasets using per year analyses showed that upper respiratory tract infections were not consistently associated with lung function measures or asthma (Table 2 and Supplementary Table S4). Lower respiratory tract infections at ages 6 months, 1, 2, 3 and 6 years were associated with lower FEV1, FVC, FEV1/FVC or FEF75, with Z-score differences (95% CI) ranging from -0.19 (-0.32, -0.05) to -0.34 (-0.50, -0.18) (Table 2). Lower respiratory tract infections at ages 2 to 6 years were associated with an increased risk of current asthma at 10 years, with odds ratios (95% CI) ranging from 4.19 (2.65, 6.64) to 13.45 (7.22, 25.05). Associations of individual upper and lower respiratory tract infections with lung function and current asthma are given in Supplementary Table S4 and S5.
Table 2.
Associations of any upper and lower respiratory tract infections with lung function and asthma at age 10 years
| FEV1 Z-score (95% CI) n = 4,672 | FVC Z-score (95% CI) n = 4,672 | FEV1/FVC Z-score (95% CI) n = 4,672 | FEF75 Z-score (95% CI) n = 4,672 | Current asthma OR (95% CI) n = 4,556 | ||
|---|---|---|---|---|---|---|
| Any URTI | ||||||
| Age 6 months | n = 2,930 | -0.02 (-0.13, 0.10) | -0.04 (-0.15, 0.07) | 0.02 (-0.09, 0.14) | 0.00 (-0.11, 0.11) | 1.46 (0.93, 2.28) |
| Age 1 year | n = 3,601 | -0.03 (-0.12, 0.07) | -0.02 (-0.11, 0.07) | -0.02 (-0.11, 0.08) | -0.03 (-0.12, 0.05) | 1.42 (0.96, 2.12) |
| Age 2 years | n = 3,768 | 0.06 (-0.03, 0.16) | 0.04 (-0.05, 0.12) | 0.04 (-0.05, 0.13) | 0.05 (-0.04, 0.14) | 1.56 (1.04, 2.34)* |
| Age 3 years | n = 3,645 | 0.03 (-0.08, 0.13) | 0.06 (-0.04, 0.16) | -0.07 (-0.18, 0.03) | -0.05 (-0.15, 0.05) | 1.04 (0.65, 1.66) |
| Age 4 years | n = 3,658 | 0.01 (-0.11, 0.14) | -0.02 (-0.13, 0.10) | 0.06 (-0.06, 0.18) | 0.07 (-0.05, 0.18) | 0.84 (0.48, 1.46) |
| Age 6 years | n = 4,681 | 0.03 (-0.09, 0.16) | 0.02 (-0.10, 0.13) | 0.03 (-0.09, 0.15) | 0.03 (-0.09, 0.15) | 1.01 (0.57, 1.79) |
| Any LRTI | ||||||
| Age 6 months | n = 2,996 | -0.21 (-0.35, -0.07)** | -0.12 (-0.26, 0.01) | -0.12 (-0.26, 0.02) | -0.11 (-0.24, 0.02) | 1.66 (0.97, 2.82) |
| Age 1 year | n = 3,623 | -0.34 (-0.50, -0.18)** | -0.25 (-0.40, -0.10)** | -0.14 (-0.29, 0.02) | -0.25 (-0.40, -0.10)** | 1.27 (0.86, 2.87) |
| Age 2 years | n = 3,828 | -0.19 (-0.32, -0.05)** | -0.03 (-0.16, 0.10) | -0.25 (-0.38, -0.12)** | -0.28 (-0.40, -0.15)** | 4.19 (2.65, 6.64)** |
| Age 3 years | n = 3,678 | -0.21 (-0.41, -0.01)* | -0.07 (-0.26, 0.11) | -0.22 (-0.41, -0.03)* | -0.16 (-0.34, 0.03) | 4.15 (2.33, 7.42)** |
| Age 4 years | n = 3,649 | -0.08 (-0.32, 0.16) | 0.03 (-0.20, 0.25) | -0.17 (-0.41, 0.07) | -0.13 (-0.35, 0.10) | 7.80 (4.13, 14.71)** |
| Age 6 years | n = 4,649 | 0.08 (-0.15, 0.30) | 0.23 (0.02, 0.45)* | -0.27 (-0.49, -0.05)* | -0.15 (-0.36, 0.07) | 13.45 (7.22, 25.05)** |
Values are Z-scores or odds ratios (OR) with 95% confidence interval (95% CI), derived from linear or binomial logistic regression models. * p-value <0.05, ** p-value <0.01. Models are adjusted for maternal education, body mass index, parity, smoking during pregnancy, psychiatric symptoms during pregnancy, pet keeping, history of asthma or atopy, mode of delivery, and child’s sex, gestational age at birth, birth weight corrected for gestational age at birth, breastfeeding and day care attendance. Additionally, upper respiratory tract infections were adjusted for any preceding upper respiratory tract infections, and lower respiratory tract infections for any preceding lower respiratory tract infections. Upper respiratory tract infections (URTI), lower respiratory tract infections (LRTI), Forced Expiratory Flow in 1 second (FEV1), Forced Vital Capacity (FVC), Forced Expiratory Flow when 75% of the FVC is exhaled (FEF75).
After adjustment for antibiotic and paracetamol use, and environmental tobacco smoke exposure, the strength and direction of the effect estimates did not materially change (data not shown). We observed no consistent interactive effect of upper and lower respiratory tract infections and inhalant allergic sensitization for the associations with lung function and asthma, (p-values>0.05).
Cross-lagged modelling
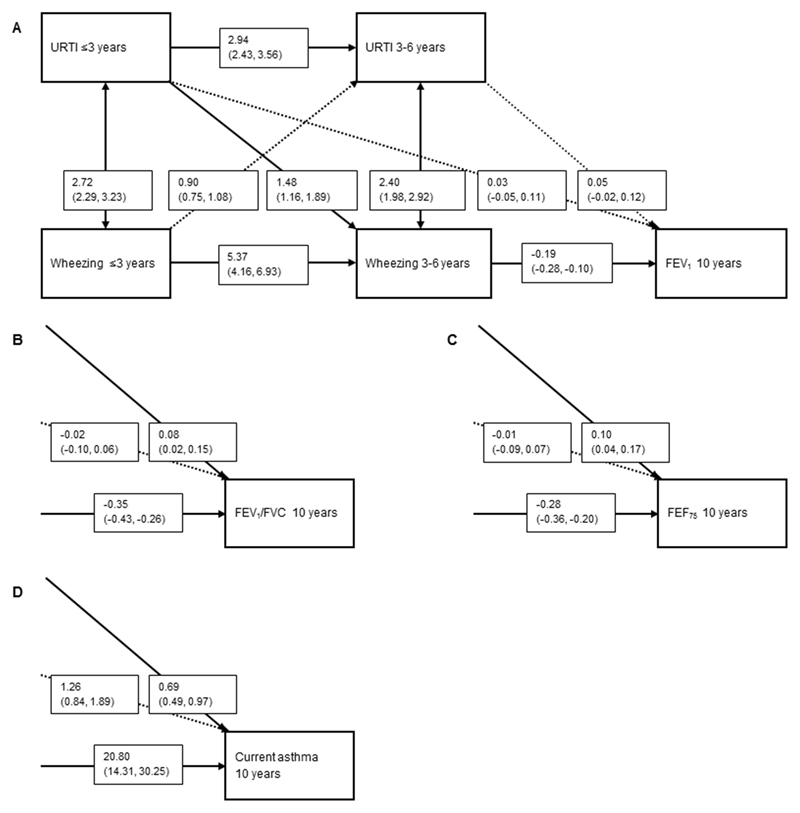
Cross-lagged modelling showed no association of upper respiratory tract infections ≤3 years with lung function measures (Figure 2A, B and C) or asthma (Figure 2D). Compared with no upper respiratory tract infections >3-6 years, upper respiratory tract infections >3-6 years were associated with a higher FEV1/FVC and FEF75 (0.08 (0.02, 0.15) and 0.10 (0.04, 0.17), respectively, but not FEV1. Upper respiratory tract infections >3-6 years were associated with a decreased risk of current asthma (0.69 (0.49, 0.97)) (Figure 2D).
Figure 2.
Direction of associations of upper respiratory tract infections (URTI) with wheezing and FEV1 (A), FEV1/FVC (B) FEF75 (C) and current asthma (D) at age 10 years.
Values are odds ratios (OR) or change in Z-scores with their corresponding 95% confidence interval (95% CI) derived from binomial logistic or linear regression models, respectively, using cross-lagged modeling which takes bidirectional associations into account. Models are adjusted for maternal education, body mass index, parity, smoking during pregnancy, psychiatric symptoms during pregnancy, pet keeping, history of asthma or atopy, mode of delivery, and child’s sex, gestational age at birth, birth weight corrected for gestational age at birth, breastfeeding and day care attendance. Arrows indicate the direction of the associations and if they are significant (bold) or non-significant (dashed). Forced Expiratory Flow in 1 second (FEV1), Forced Vital Capacity (FVC), Forced Expiratory Flow when 75% of the FVC is exhaled. For Figure 2B, C and D only the right lower quadrant of the figure is presented. All other directions and effect estimates of the associations were approximately the same as presented in Figure 2A.
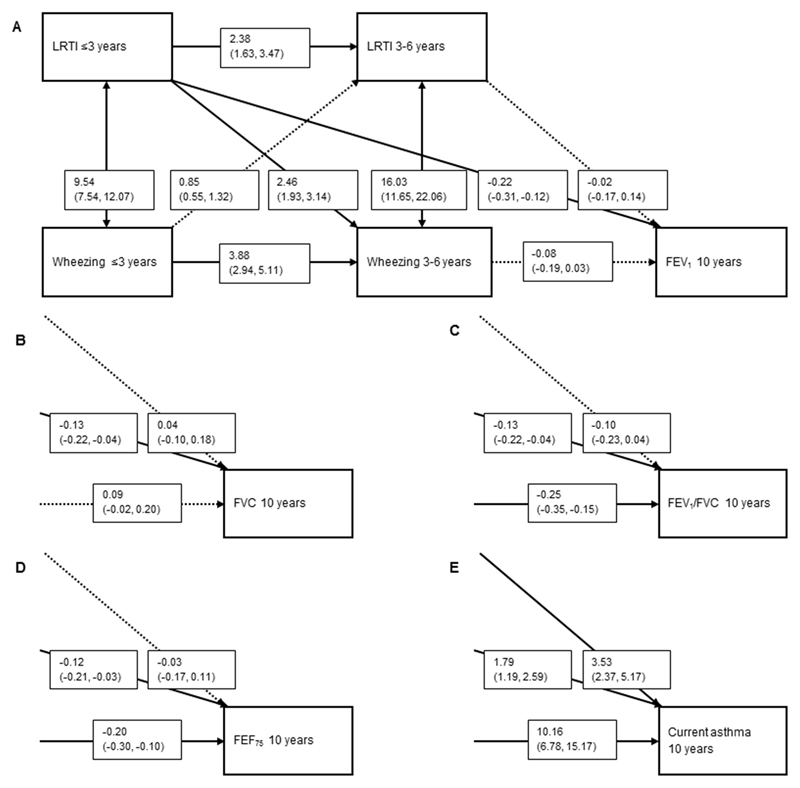
Lower respiratory tract infections ≤3 years were associated with lower FEV1, FVC, FEV1/FVC and FEF75 (range -0.12 (-0.21, -0.03) to -0.22 (-0.31, -0.12) (Figure 3A, B, C and D). Lower respiratory tract infections ≤3 and >3-6 years were also associated with an increased risk of current asthma (1.79 (1.19, 2.59) and 3.53 (2.37, 5.17), respectively) (Figure 3E). Lower respiratory tract infections >3-6 years were not associated with any lung function measurements. The directions from preschool wheezing patterns to later upper and lower respiratory tract infections were not significant.
Figure 3.
Direction of associations of lower respiratory tract infections (LRTI) with wheezing patterns and FEV1 (A), FVC (B), FEV1/FVC (C), FEF75 (D) and current asthma (E) at age 10 years.
Values are odds ratios (OR) or Z-scores with their corresponding 95% confidence interval (95% CI) derived from binomial logistic or linear regression models, respectively, using cross-lagged modeling which takes bidirectional associations into account. Models are also adjusted for maternal education, body mass index, parity, smoking during pregnancy, psychiatric symptoms during pregnancy, pet keeping, history of asthma or atopy, mode of delivery, and child’s sex, gestational age at birth, birth weight corrected for gestational age at birth, breastfeeding and day care attendance. Arrows indicate the direction of the associations and if they are significant (bold) or non-significant (dashed). Forced Expiratory Flow in 1 second (FEV1), Forced Vital Capacity (FVC), Forced Expiratory Flow when 75% of the FVC is exhaled. For Figure 3B, C, D and E, only the right lower quadrant of the figure is presented. All other directions and effect estimates of the associations were approximately the same as presented in Figure 3A.
Other infections
Cross-lagged modelling showed that gastro-enteritis was not associated with lung function measures (data not shown). Gastro-enteritis ≤3 years, but not >3-6 years, was associated with an increased risk of current asthma (1.68 (1.13, 2.47)) (Supplementary Figure S2A). Urinary tract infections ≤3 year were associated with a higher FEV1 (0.18 (0.00, 0.35), but not with current asthma (Supplementary Figure S2B).
Discussion
We observed in large population-based prospective cohort study that lower respiratory tract infections ≤3 years were most strongly and consistently associated with lower lung function and an increased risk of current asthma at school-age. These associations were not mediated by use of antibiotics or paracetamol and environmental tobacco smoke exposure, and not modified by inhalant allergic sensitization. Additionally, we observed that these associations were not bidirectional, independent of preschool wheezing patterns, and not due to a general infection status. Upper respiratory tract infections at any age were not associated with lower lung function, and only upper respiratory tract infections at the age of 2 years were associated with an increased risk of asthma.
Comparison with previous studies
Previous prospective cohort studies observed inconclusive associations of respiratory tract infections with lung function or asthma, both in children and in adulthood1–10. Some studies that used viral sampling found that specific viral triggers, such as respiratory syncytial virus6–9 or rhinovirus6 9 were associated with an 2.1 to 3.9-fold increased risk of wheeze or 2.6 to 9.8-fold increased risk of asthma in children aged 5 to 10 years. However, other studies showed that not a specific viral trigger, but the number of infections was associated with an increased risk of asthma, current wheeze or bronchial hyperreactivity at age 7 years5 10. Two previous studies among children age 56 and 10 years7 showed that associations of specific lower respiratory tract infections with asthma and wheeze were only present in children who were sensitized before the age of 2 years. Others showed no modifying effect of sensitization for the associations of respiratory syncytial virus, rhinovirus infections or both with the risk of asthma at age 6 years9. Some differences could be explained by differences in the size of study groups4–7 9. Also, most studies examined children at high risk for atopy, which reduces generalizability4–7 9 10. Most studies were focused on respiratory tract infections in either the first year or the first 3 years of life4–7 9 10 32. Our findings are consistent with previous findings, and now show that results are applicable to a general population, are not mediated by use of antibiotics or paracetamol and environmental tobacco smoke exposure, and not modified by inhalant allergic sensitization. Our sensitivity analysis for non-respiratory tract infections showed an association of gastro-enteritis ≤3 years with asthma only. Since urinary tract infections at any age were not associated with any adverse respiratory outcome, the effect of gastro-enteritis might be specific, and we speculate that this could be mediated by the intestinal microbiome 33. Another possibility however is that infections in general could have an effect on the immune system, and thereby influence the risk of asthma.
When we took possible bidirectional associations into account, cross-lagged modeling showed that lower respiratory tract infections ≤3 years were associated with lower lung function and current asthma, and lower respiratory tract infections at 3-6 years only with current asthma. By using cross-lagged models, we confirmed the direction of the association of respiratory tract infections with lung function and asthma, and not vice versa. These findings are supported by a randomized clinical trial, which observed that vaccination for respiratory syncytial virus infections led to a reduction of the number of wheezing days in the first year of life, suggesting causality from viral infection to wheeze34.
We observed that lower respiratory tract infections, specifically bronchitis, bronchiolitis and pneumonia, were associated with lower lung function and an increased risk of asthma. Previous studies suggested that childhood respiratory tract infections influence lung function at school age and in adulthood1–3 35 36. In adults up to 70 years of age, early life bronchitis3 36 and pneumonia1–3 36 were associated with a lower FEV1, FVC and, in lesser extent, a lower FEV1/FVC. Together with the present finding, these findings suggest that exposure to respiratory tract infections in early life might affect respiratory health during the life course.
Potential mechanisms
Lung development starts in utero, and continues during childhood21 37, similar to the development of the immune system22 37 38. In early life, the developing respiratory and immune system could be affected by lower respiratory tract infections leading to persistent adverse adaptations and subsequently lower lung function and an increased risk of asthma. Non-specific immune responses mediated by epithelial cells and phagocytes, and adaptive immune responses mediated by T cells, could contribute to airway inflammation in response to viral triggers38. Early airway infections could lead to changes in endothelial cell physiology, such as increased vascular permeability and hence, bronchial wall edema39 40. Because of the immaturity of the respiratory and immune systems at a young age, these processes with potential persistent consequences are more likely to occur when lower respiratory tract infections occur early in life. Our findings are in line with this hypothesis because we observed that mainly lower respiratory tract infections ≤3 years were associated with lower lung function and increased risk of asthma. The effect of upper respiratory tract infections on the lungs seems to be less distinct, most probably because upper respiratory tract infections do not affect the lungs directly.
The immune system is also important for the resolution of airway inflammation after infections and a less developed immune system could potentially lead to persistent changes of airway physiology and function after infections39. To date, the immunological pathways responsible for the persistence of structural and functional abnormalities after respiratory tract infections have not yet been identified. The immune response to infections and the risk of developing lower lung function and asthma could both be dependent on common factors, such as the microbiome41 or (epi)genetic factors39, and need to be examined in future studies.
Strengths and limitations
This study was embedded in a population-based, prospective cohort study with a large number of participants, and detailed and longitudinally measured information on both upper and lower respiratory tract infections, and respiratory outcomes measures. Furthermore, we applied a unique method to identify bidirectional associations using cross-lagged modeling. Since the study was not limited to a high-risk population, the findings are applicable to the general population. However, some limitations apply to our study. First, selection bias towards a more affluent and healthy population might have been present. Also, we used multiple imputation to reduce potential bias due to missing data in covariates. By using this method we assumed that data in covariates was missing at random. However, it might be possible that some data was missing not at random, which may have led to bias 42. Second, information on respiratory tract infections was parental based information, collected with questionnaires, which might have led to recall or misclassification bias. If so, we expect this possible misclassification to be random because information on infections was collected before the outcome was known, and would most probably have led to an underestimation of our observed effect estimates. Our study lacked viral or bacteriological sampling at the time of symptoms of respiratory tract infections, although it is suggested that not the specific microbial trigger but respiratory tract infections in general are important for the risk of asthma5. Third, information on asthma and wheezing was obtained by questionnaires. Although these questionnaires were adapted from ISAAC43, which was validated in various age groups and is considered as a reliable measure in epidemiological studies, misclassification due to self-report might still have been present. Prevalences of early and late wheezing in our study slightly differ from previous birth cohort studies44 due to the definition used for optimal cross-lagged modeling. Fourth, we did not have lung function measurements before the occurrence of respiratory tract infections. Therefore, we cannot distinguish whether the lower lung function is a result of the respiratory tract infections only, or whether it was already present before the infections occurred45. It has been suggested however, that lower respiratory tract infections impair lung function independent of lung function at younger age46. We partly addressed this issue by our statistical effort using cross-lagged modeling. Fifth, inhalant allergic sensitization was measured at the age of 10 years. Early measurements or longitudinal patterns of allergic sensitization might be needed to better examine the intermediating role of allergy. Last, even though we corrected for numerous confounders, residual confounding due to unmeasured confounders, for example genetic susceptibility47 or maternal infections during pregnancy48, could have affected our results.
In conclusion, our results suggest that lower respiratory tract infections ≤3 years are most strongly and consistently associated with lower lung function and increased risk of asthma at school-age, while lower respiratory tract infections at 3-6 years were associated with asthma only. Upper respiratory tract infections were not associated with lower lung function or an increased risk of asthma, except at the age of 2 years with asthma. Cross-lagged modeling showed that observed associations were not bidirectional, independent of wheezing in early life and most likely not due to infections in general. Therefore, our findings support the hypothesis that early-life respiratory tract infections might have a direct effect on lung development and the risk of asthma. Further studies are needed to explore the possible underlying immunological and pathophysiological pathways.
Supplementary Material
What is the key question?
Are early-life respiratory tract infections associated with lower lung function and an increased risk of asthma in school-aged children?
What is the bottom line?
When taking bidirectional associations into account, early-life lower respiratory tract infections are associated with lower lung function and an increased risk of asthma, while upper respiratory tract infections are not.
Why read on?
This large prospective cohort study shows, by using cross-lagged modelling, that early-life lower respiratory tract infections might have a direct effect on lung development and the risk of asthma.
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Centre in close collaboration with the School of Law and the Faculty of Social Sciences at the Erasmus University, Rotterdam, the Municipal Health Service, Rotterdam area, and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (Star-MDC), Rotterdam. We gratefully acknowledge the contribution of children and their parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Funding and Competing Interests
The Generation R Study is made possible by financial support from the Erasmus Medical Centre, Rotterdam, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development and the Ministry of Health, Welfare and Sport. Dr Pauline W. Jansen received a grant from the Dutch Diabetes Foundation (grant number 2013.81.1664). Dr Vincent W.V. Jaddoe received grants from the Netherlands Organization for Health Research and Development (VIDI o16.136.3610) and the European Research Council (ERC-2014-CoG-648916). Dr Liesbeth Duijts received funding from the Lung Foundation Netherlands (no 3.2.12.089; 2012). The project received funding from the European Union's Horizon 2020 research and innovation programme (LIFECYCLE project, grant agreement no 733206; 2016), and from cofunded ERA-Net on Biomarkers for Nutrition and Health (ERA HDHL), Horizon 2020 (grant agreement no 696295; 2017), ZonMW The Netherlands (no 529051014; 2017), Science Foundation Ireland (no SFI/16/ERA-HDHL/3360), and the European Union (ALPHABET project). The researchers are independent from the funders. The study sponsors had no role in the study design, data analysis, interpretation of data, or writing of this report. Drs. Evelien R. van Meel, Drs. Herman T. den Dekker, Drs. Niels J. Elbert, Dr. Henriëtte A. Moll, Dr. Irwin K. Reiss and Dr. Johan C. de Jongste report no conflict of interest.
Abbreviations
- FEF75
Forced Expiratory Flow after expiring 75% of FVC
- FEV1
Forced Expiratory Volume in 1 second
- FVC
Forced Vital Capacity
- GE-itis
Gastro-enteritis
- ISAAC
International Study of Asthma and Allergy in Childhood
- LRTI
Lower respiratory tract infections
- URTI
Upper respiratory tract infection
- UTI
Urinary tract infection
Footnotes
Author Contribution
EM, HD and LD contributed to the conception and design, acquisition of data, analyses and interpretation of the data, drafted the article, revised it critically for important intellectual content, and gave final approval of the version to be published.
NE, PJ, HM, IR, JJ and VJ contributed to the conception and design, acquisition of data, revised the drafted manuscript critically for important intellectual content, and gave final approval of the version to be published.
References
- 1.Chan JY, Stern DA, Guerra S, et al. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135(4):607–16. doi: 10.1542/peds.2014-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston ID, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood on adult lung function. The New England journal of medicine. 1998;338(9):581–7. doi: 10.1056/NEJM199802263380904. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen SO, Sterne JA, Tucker JS, et al. Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax. 1998;53(7):549–53. doi: 10.1136/thx.53.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilbert TW, Singh AM, Danov Z, et al. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. The Journal of allergy and clinical immunology. 2011;128(3):532–8.e1-10. doi: 10.1016/j.jaci.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnelykke K, Vissing NH, Sevelsted A, et al. Association between respiratory infections in early life and later asthma is independent of virus type. The Journal of allergy and clinical immunology. 2015 doi: 10.1016/j.jaci.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusel MM, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. The Journal of allergy and clinical immunology. 2007;119(5):1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusel MM, Kebadze T, Johnston SL, et al. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J. 2012;39(4):876–82. doi: 10.1183/09031936.00193310. [DOI] [PubMed] [Google Scholar]
- 8.Stensballe LG, Simonsen JB, Thomsen SF, et al. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. The Journal of allergy and clinical immunology. 2009;123(1):131–37 e1. doi: 10.1016/j.jaci.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Illi S, von Mutius E, Lau S, et al. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. Bmj. 2001;322(7283):390–5. doi: 10.1136/bmj.322.7283.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Openshaw PJ, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005;18(3):541–55. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penders J, Kummeling I, Thijs C. Infant antibiotic use and wheeze and asthma risk: a systematic review and meta-analysis. Eur Respir J. 2011;38(2):295–302. doi: 10.1183/09031936.00105010. [DOI] [PubMed] [Google Scholar]
- 13.Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics. 2011;127(6):1125–38. doi: 10.1542/peds.2010-2092. [DOI] [PubMed] [Google Scholar]
- 14.Lapin B, Piorkowski J, Ownby D, et al. The relationship of early-life antibiotic use with asthma in at-risk children. The Journal of allergy and clinical immunology. 2014;134(3):728–9. doi: 10.1016/j.jaci.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortqvist AK, Lundholm C, Kieler H, et al. Antibiotics in fetal and early life and subsequent childhood asthma: nationwide population based study with sibling analysis. Bmj. 2014;349:g6979. doi: 10.1136/bmj.g6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoskin-Parr L, Teyhan A, Blocker A, et al. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose-dependent relationship. Pediatr Allergy Immunol. 2013;24(8):762–71. doi: 10.1111/pai.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heintze K, Petersen KU. The case of drug causation of childhood asthma: antibiotics and paracetamol. Eur J Clin Pharmacol. 2013;69(6):1197–209. doi: 10.1007/s00228-012-1463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etminan M, Sadatsafavi M, Jafari S, et al. Acetaminophen use and the risk of asthma in children and adults: a systematic review and metaanalysis. Chest. 2009;136(5):1316–23. doi: 10.1378/chest.09-0865. [DOI] [PubMed] [Google Scholar]
- 19.Kreiner-Moller E, Sevelsted A, Vissing NH, et al. Infant acetaminophen use associates with early asthmatic symptoms independently of respiratory tract infections: the Copenhagen Prospective Study on Asthma in Childhood 2000 (COPSAC(2000)) cohort. The Journal of allergy and clinical immunology. 2012;130(6):1434–6. doi: 10.1016/j.jaci.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Jaddoe VW, van Duijn CM, Franco OH, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27(9):739–56. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 21.Dunnill MS. Postnatal growth of the lung. Thorax. 1962;17(4):329–33. [Google Scholar]
- 22.de Vries E, de Groot R, de Bruin-Versteeg S, et al. Analysing the developing lymphocyte system of neonates and infants. Eur J Pediatr. 1999;158(8):611–7. doi: 10.1007/s004310051162. [DOI] [PubMed] [Google Scholar]
- 23.Kajekar R. Environmental factors and developmental outcomes in the lung. Pharmacol Ther. 2007;114(2):129–45. doi: 10.1016/j.pharmthera.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Valk JP, Gerth van Wijk R, Hoorn E, et al. Measurement and interpretation of skin prick test results. Clin Transl Allergy. 2015;6:8. doi: 10.1186/s13601-016-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dick S, Friend A, Dynes K, et al. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open. 2014;4(11):e006554. doi: 10.1136/bmjopen-2014-006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotecha SJ, Gallacher DJ, Kotecha S. The respiratory consequences of early-term birth and delivery by caesarean sections. Paediatr Respir Rev. 2016;19:49–55. doi: 10.1016/j.prrv.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Guxens M, Sonnenschein-van der Voort AM, Tiemeier H, et al. Parental psychological distress during pregnancy and wheezing in preschool children: the Generation R Study. The Journal of allergy and clinical immunology. 2014;133(1):59–67 e1-12. doi: 10.1016/j.jaci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 30.Luijk MP, Sonnenschein-van der Voort AM, Mileva-Seitz VR, et al. Is parent-child bed-sharing a risk for wheezing and asthma in early childhood? Eur Respir J. 2015;45(3):661–9. doi: 10.1183/09031936.00041714. [DOI] [PubMed] [Google Scholar]
- 31.Hays RD, Marshall GN, Wang EY, et al. Four-year cross-lagged associations between physical and mental health in the Medical Outcomes Study. J Consult Clin Psychol. 1994;62(3):441–9. doi: 10.1037//0022-006x.62.3.441. [DOI] [PubMed] [Google Scholar]
- 32.Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed) 1982;284(6330):1665–9. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6:1085. doi: 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. The New England journal of medicine. 2013;368(19):1791–9. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 35.Dharmage SC, Erbas B, Jarvis D, et al. Do childhood respiratory infections continue to influence adult respiratory morbidity? Eur Respir J. 2009;33(2):237–44. doi: 10.1183/09031936.00062907. [DOI] [PubMed] [Google Scholar]
- 36.Barker DJ, Godfrey KM, Fall C, et al. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. Bmj. 1991;303(6804):671–5. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz J. Air pollution and children's health. Pediatrics. 2004;113(4 Suppl):1037–43. [PubMed] [Google Scholar]
- 38.Holt PG. Programming for responsiveness to environmental antigens that trigger allergic respiratory disease in adulthood is initiated during the perinatal period. Environ Health Perspect. 1998;106(Suppl 3):795–800. doi: 10.1289/ehp.98106795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folkerts G, Busse WW, Nijkamp FP, et al. Virus-induced airway hyperresponsiveness and asthma. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1708–20. doi: 10.1164/ajrccm.157.6.9707163. [DOI] [PubMed] [Google Scholar]
- 40.Shaheen SO. Changing patterns of childhood infection and the rise in allergic disease. Clin Exp Allergy. 1995;25(11):1034–7. doi: 10.1111/j.1365-2222.1995.tb03248.x. [DOI] [PubMed] [Google Scholar]
- 41.Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35. doi: 10.1186/s13223-015-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Bmj. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 44.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. The Journal of allergy and clinical immunology. 2014;133(5):1317–29. doi: 10.1016/j.jaci.2013.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drysdale SB, Wilson T, Alcazar M, et al. Lung function prior to viral lower respiratory tract infections in prematurely born infants. Thorax. 2011;66(6):468–73. doi: 10.1136/thx.2010.148023. [DOI] [PubMed] [Google Scholar]
- 46.Gray DM, Turkovic L, Willemse L, et al. Lung Function in African Infants in the Drakenstein Child Health Study: Impact of Lower Respiratory Tract Illness. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201601-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Sanchez A, Isidoro-Garcia M, Garcia-Solaesa V, et al. Genome-wide association studies (GWAS) and their importance in asthma. Allergol Immunopathol (Madr) 2015;43(6):601–8. doi: 10.1016/j.aller.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Zhu T, Zhang L, Qu Y, et al. Meta-analysis of antenatal infection and risk of asthma and eczema. Medicine (Baltimore) 2016;95(35):e4671. doi: 10.1097/MD.0000000000004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.