Abstract
Anemia is frequently encountered in patients with inflammatory bowel disease (IBD), decreasing the quality of life and significantly worsening the prognosis of the disease. The pathogenesis of anemia in IBD is multifactorial and results mainly from intestinal blood loss in inflamed mucosa and impaired dietary iron absorption. Multiple studies have proposed the use of the polyphenolic compound curcumin to counteract IBD pathogenesis since it has significant preventive and therapeutic properties as an anti-inflammatory agent and very low toxicity, even at high dosages. However, curcumin has been shown to possess properties consistent with those of an iron-chelator, such as the ability to modulate proteins of iron metabolism and decrease spleen and liver iron content. Thus, this property may further contribute to the development and severity of anemia of inflammation and iron deficiency in IBD. Herein, we evaluate the effects of curcumin on systemic iron balance in the dextran sodium sulfate (DSS) model of colitis in C57Bl/6 and BALB/c mouse strains that were fed an iron-sufficient diet. In these conditions, curcumin supplementation caused mild anemia, lowered iron stores, worsened colitis and significantly decreased overall survival, independent of the mouse strain. These findings suggest that curcumin usage as an anti-inflammatory supplement should be accompanied by monitoring of erythroid parameters to avoid exacerbation of iron deficiency anemia in IBD.
Introduction
For patients with inflammatory bowel disease (IBD), anemia is one of the major causes of hospitalization [1, 2] and has a debilitating effect on the quality of life (QoL) [3, 4], increasing disease morbidity and tightly associating the disease with mortality [5]. IBD is an inflammatory disease consisting of a group of gastrointestinal tract disorders, namely ulcerative colitis and Crohn's disease, which are characterized by blood loss from the intestinal mucosa and reduced iron absorption. Up to two-thirds of patients with IBD develop anemia, with the most common types being iron-deficiency anemia and anemia of chronic disease, which often overlap [2, 6, 7].
IBD pathophysiology includes the activation of inflammatory cytokines such as TNF-α [8, 9]. Therefore, many studies on IBD therapies have focused on anti-inflammatory treatments or natural compounds such as curcumin that have anti-inflammatory properties to mitigate the disease [10, 11].
Curcumin, the yellow pigment obtained from the rhizome of Curcuma longa (turmeric), is commonly used as a spice and food-coloring agent [12]. Curcumin features complex and multifactorial mechanisms of action that have demonstrated a variety of therapeutic properties, including those described as anti-oxidant, anti-infection, anti-tumor, and anti-inflammatory [13]. Moreover, curcumin use has no major side effects and has low toxicity at high dosages (up to 8 g/day) [14]. Anti-cancer activities of curcumin are mediated by a variety of biological pathways in mutagenesis, oncogene expression, cell cycle regulation, apoptosis, tumorigenesis, and metastasis. Additionally, the effects of curcumin as an anti-inflammatory agent have been previously associated with the regulation of different inflammatory cytokines (extracellular matrix metalloproteinase inducer (EMMPRIN); matrix metalloproteinase-9 (MMP-9); IL-1β; and mitogen-activated protein kinase (MAPK)) [15–17]. Recently, Kong et al. demonstrated curcumin’s mechanism of action in macrophages during the inflammasome response [18]. Furthermore, different studies have highlighted curcumin’s properties to inhibit the toll-like receptor 4/myeloid differential factor 88 (TLR4/MyD88) pathway via the repression of TLR4 homodimerization and the subsequent decrease of MyD88 expression [19, 20]. TLR4 has a critical role in the inflammatory response inducing nuclear factor-kappa B (NF-κB) expression via protein adaptor MyD88 stimulation.
Curcumin has also been shown to have anticoagulant and antiplatelet activities [21], which may sustain or prolong active bleeding [22] and has been proposed to have the properties of an iron chelator [23–25]. Consistent with its iron chelating properties, curcumin has been reported to reduce spleen and liver iron stores in mice [24, 26]. Moreover, curcumin has been also shown to affect hepcidin expression [24], the main regulator of iron homeostasis [27]. Hepcidin, encoded by the HAMP gene, controls the levels of intestinal iron absorption and plays a major role in regulating iron release from macrophages. These cells are responsible for iron recycling and, in inflammatory settings, will accumulate iron at high hepcidin levels [28].
The potentially detrimental effects of curcumin on iron homeostasis in the inflammatory context could exacerbate anemia and iron deficiency; however, this aspect has been often ignored in studies of gastrointestinal disorders and IBD mouse models, which exhibit marginal or depleted iron stores. Herein, we investigated the effects of curcumin on the dextran sodium sulfate (DSS)-induced colitis mouse model in the context of an iron-sufficient diet.
Materials and methods
Ethical statements
All procedures were performed in accordance with the Canadian Council of Animal Care guidelines after approval by the Institutional Animal Care Committee of the Centre de recherche du Centre Hospitalier de l'Université de Montréal (CRCHUM).
Experimental animals
Female C57Bl/6 and BALB/c mice aged 8 weeks old were purchased from Charles River Laboratories (Wilmington, CA, USA). Animals were kept at the CRCHUM animal facility in temperature (22°C), humidity (65%) and lighting-controlled (12:12 light–dark cycle, light on at 07:00) rooms and had free access to chow and water. They were housed in specific pathogen free (SPF) conditions in cages layered with bedding material at 3–4 mice per cage. Nesting material as environmental enrichment was added to each cage and was changed every two weeks. A total number of 8–10 of mice were used in each group for statistical power/significance calculation. Minimum of three independent replications of each experiments were done.
Animal treatments, diets, and induction of colitis
Two weeks before DSS treatment, 8 weeks old female C57BL/6 or BALB/c mice received ad libitum a control diet containing 50 mg/kg of iron in the form of iron sulfate (Teklad TD.120515; Envigo, IN, United States) with or without 2% (wt/wt) curcumin supplementation (Teklad TD.140182). Colitis was induced by the administration of DSS (molecular weight 40000; TdB Consultancy, Uppsala, Sweden) at 1–2% w/v in drinking water for 5 days, followed by 7 days of rest for 3–4 cycles of DSS [29]. All mice were observed daily for general health conditions, adequacy of food, water, and where weighed and monitored for signs of inflammation. Animals treated with DSS showed severe signs of illness, associated with colitis symptoms such as blood in stool and body weight loss. A body weight loss exceeding 20% of total body weight in combination with hunched posture and/or lack of activity, was defined as a humane endpoint. At the end of the experimental period, mice were anaesthetized with 75 mg/kg of pentobarbital sodium via intraperitoneal injection and were sacrificed by cervical dislocation. No animal deaths without euthanasia were recorded during the duration of the studies.
Blood and tissue samples collection
Blood samples were collected by orbital puncture under terminal anesthesia using capillary tubes. Samples were collected in BD Microtainer blood collection tubes containing EDTA anticoagulant (BD Diagnostics, Franklin Lake, NJ, USA) to measure erythroid parameters, and in blood collection tubes with separation gel for serum samples (Vet lab supplies Ltd, Pulborough, UK). For histological analysis, tissue samples were placed in histology cassettes and submerged in glass jar containing 10% neutral buffered formalin. For protein extraction, tissue samples were immediately snap frozen through submersion in liquid nitrogen and stored at -80°C. For RNA extraction, tissue samples were collected in RNA later-containing microcentrifuge tubes (Qiagen, Mississauga, ON, Canada). Finally, for tissue iron concentration measurements, samples were collected in ice-shilled microcentrifuge tubes and stored at -20°C.
Erythroid parameters and serum iron
Red blood cell, hemoglobin, hematocrit, and mean corpuscular volume levels were measured with an automated cell counter calibrated for murine samples (ABC vet counter; ABX Hématologie, Montpellier, France). Serum iron was measured by a colorimetric assay with the Kodak Ektachem DT60 system (Johnson & Johnson, Ortho-Clinical Diagnostics, Mississauga, ON, Canada).
Histology
Histological scoring was assessed on colon samples from each mouse. The samples were fixed in 10% neutral buffered formalin (Chapter Chemicals, Montreal, QC, Canada), cut, fixed and stained with hematoxylin and eosin. All histological evaluations were assessed in a blinded fashion. Histological scoring was calculated as follows: presence of occasional inflammatory cells in the lamina propria (assigned a value of 0); increased numbers of inflammatory cells in the lamina propria (value of 1); confluence of inflammatory cells, extending into the submucosa (value of 2); and transmural extension of the infiltrate (value of 3). For tissue damage score: no mucosal damage (value of 0); lymphoepithelial lesions (value of 1); surface mucosal erosion or focal ulceration (value of 2); and extensive mucosal damage and extension into a deeper structure (value of 3) [30].
Disease activity index
The disease activity index was scored accordingly to previous studies [31, 32]. The index consists of the sum of all scores attributed to weight loss (0, none; 1, 1%–5%; 2, 5%–10%; 3, 10%–20%; 4, >20%), stool consistency (0, normal; 2, soft; 4, diarrhea) and fecal blood (0, negative; 2, blood in the stool; 4, gross bleeding), divided by 3.
SDS-PAGE and western blot analysis
Protein concentrations were measured using a Pierce BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Same concentrations of protein from colon or liver extracts were loaded to perform electrophoresis. More precisely, equivalent amounts of proteins were boiled in loading buffer containing 4% SDS, 20% glycerol, and bromophenol blue for 5 minutes. Proteins were resolved on 10% SDS-PAGE gels and then were transferred onto nitrocellulose membranes (GE Healthcare; Amersham Biosciences, Baie d’Urfé, QC, Canada). The membranes were blocked with 5% non-fat dry milk solution and incubated with antibodies against lipocalin 2 (R&D Systems, Minneapolis, MN, USA), MyD88 (Cell Signaling, Danvers, MA, USA) and β-actin (Abcam, Cambridge, MA, USA). To detect the formation of immunocomplexes, peroxidase-conjugated anti-goat IgG (Santa Cruz, Dallas, TX, USA) and anti-mouse IgG (R&D Systems, Minneapolis, MN, USA) were used as secondary antibodies. Staining intensity was developed with an Amersham enhanced chemiluminescence system (GE Healthcare, Amersham Biosciences, Baie d’Urfé, QC, Canada).
Quantitative RT-PCR
Total RNA from tissue samples was isolated by phenol chloroform using the TRIzol reagent (Invitrogen, Burlington, ON, Canada) as recommended by the manufacturer, and reverse transcription was performed with an Omniscript RT-PCR system (Qiagen, Mississauga, ON, Canada). The mRNA levels of selected genes were measured by real-time PCR with a Rotor-Gene 3000 real-time DNA detection system (Montreal Biotech, Kirkland, QC, Canada) and QuantiTect SYBR Green I PCR kits (Qiagen, Mississauga, ON, Canada) as previously described [33]. Expression levels were normalized to the housekeeping gene β-actin. The following primers were used: Hamp (F) CCTATCTCCATCAACAGATG; Hamp (R) AACAGATACCACACTGGGAA; β-actin (F) TGTTACCAACTGGGACGACA; β-actin (R) GGTGTTGAAGGTCTCAAA.
Measurement of iron in the liver and the spleen
Non-heme iron concentrations were assessed by acid digestion of liver and spleen tissue samples [34], followed by measurement by colorimetry using the ferrozine reagent and measuring absorption at 560 nm [35].
Statistical analysis
All statistics were calculated using Prism GraphPad (GraphPad, San Diego, CA) with a pre-specified significant P-value of 0.05. Student’s t-test (two-tailed) was used for comparisons between two groups, and multiple comparisons were evaluated by one-way analysis of variance (ANOVA), followed by the Bonferroni multiple comparison test.
Results
Curcumin supplementation of an iron-sufficient diet causes mild anemia in a DSS-induced colitis mouse model
We investigated the potential contribution of curcumin in the systemic iron balance in an IBD setting using the DSS-induced colitis mouse model. To evaluate such effects, colitis was induced by administration of DSS in C57Bl/6 mice with or without 2% curcumin supplementation of an iron-sufficient diet (50 mg/kg chow). Repeated cycles of DSS administration in the drinking water of mice were intercalated with resting periods of 7 days with untreated water to model the chronic pattern of IBD [36, 37].
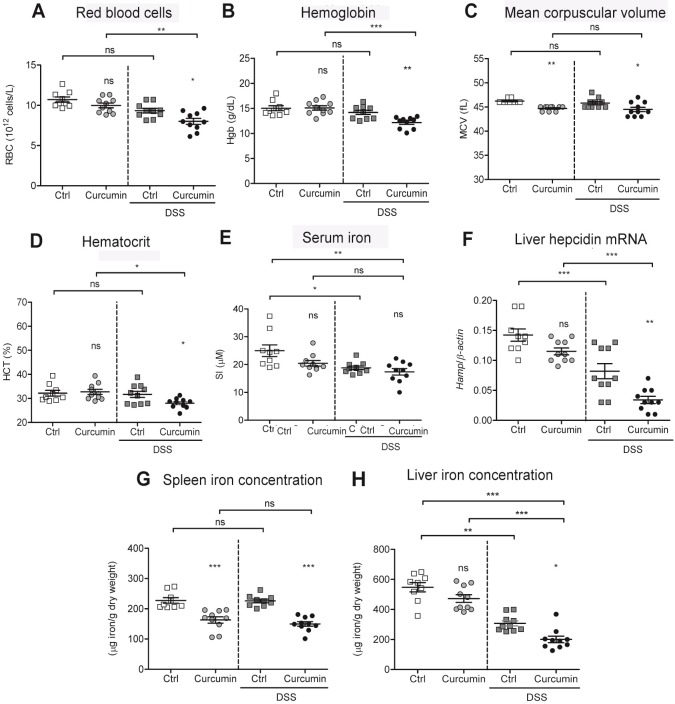
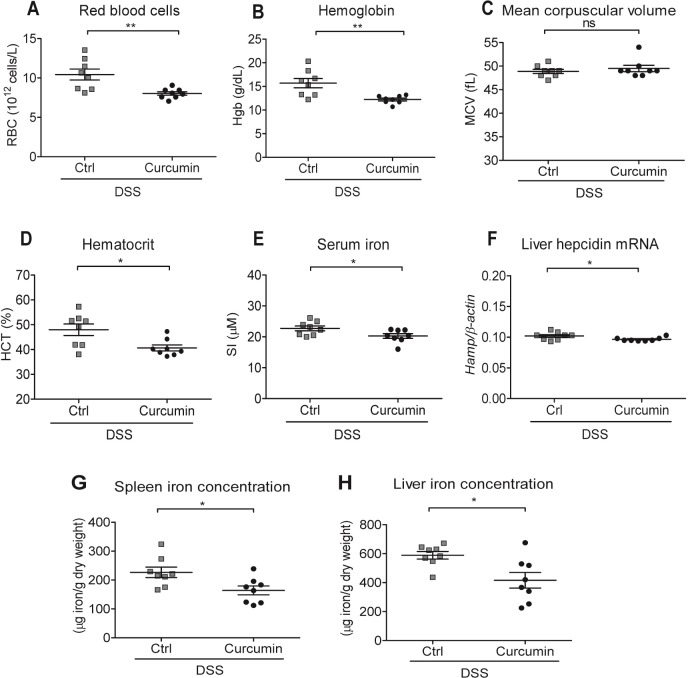
We found that erythroid parameters including red blood cells, hemoglobin, mean corpuscular volume and hematocrit values were significantly lower in DSS-treated mice on the curcumin-supplemented diet (Curcumin-DSS), compared with mice treated with DSS without curcumin supplementation (Ctrl-DSS) (Fig 1A–1D). In control conditions with non-colitic mice (Ctrl and Curcumin, without DSS), only the mean corpuscular volume was lowered by curcumin supplementation, with all other erythroid parameters remaining similar in both groups. The lowest values for serum iron levels were found in the Curcumin-DSS group, with levels significantly lower compared to non-colitic Ctrl mice (Fig 1E).
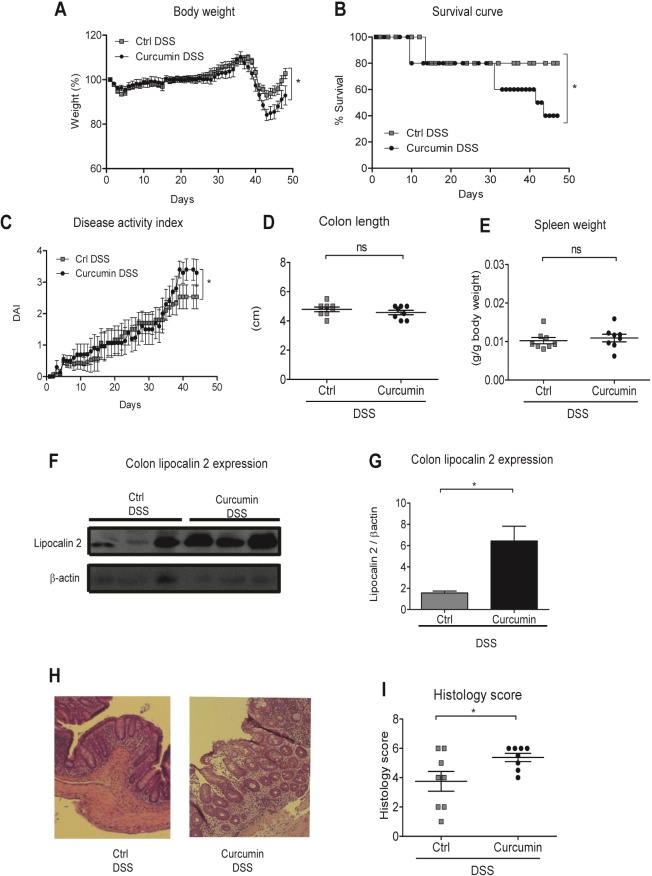
Fig 1. Curcumin supplementation of an iron-sufficient diet causes mild anemia in a DSS-mouse model.
C57BL/6 mice were fed an iron-sufficient diet (50 mg/kg chow; Ctrl) or an iron-sufficient diet supplemented with 2% curcumin (Curcumin). For dextran sodium sulfate (DSS) treatment, mice were fed an iron-sufficient diet with or without curcumin, starting 2 weeks before administration of DSS. (A-D) Erythroid parameters: red blood cells, hemoglobin, mean corpuscular volume, and hematocrit. (E) Serum iron levels. (F) Liver hepcidin (Hamp) mRNA expression against housekeeping β-actin mRNA. (G-H) Iron content in spleen (G) and liver (H). Results are representative of a minimum of three independent experiments; n = 9–10 mice per group. Statistical analysis was performed with one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, and ns = not significant between Curcumin to Ctrl groups and when indicated between non-DSS and DSS groups.
Next, we measured liver hepcidin mRNA expression (Hamp), the major regulator of iron homeostasis [27]. As shown in Fig 1F, Curcumin-DSS mice had the lowest hepcidin expression of all groups, and the values were also significantly lower when compared to Ctrl-DSS mice that did not received curcumin supplementation.
We then analyzed the iron concentration in the spleen and liver and found that splenic iron concentrations in mice fed with 2% curcumin with or without DSS treatment were significantly lower compared with their respective controls (Ctrl and Ctrl-DSS; Fig 1G). In regards to liver concentration, both groups treated with DSS had lower liver iron content compared to non-DSS mice, indicating that DSS treatment lowers liver iron concentrations (Fig 1H). The lowest liver iron content was found in the Curcumin-DSS group when compared to all other treatment groups.
Taken together, these findings indicate that mice fed an iron-sufficient diet supplemented with curcumin develop mild anemia accompanied by marked lower iron levels in the spleen and liver in the DSS-mouse model. In contrast, mice treated with DSS alone do not develop anemia despite a modest lowering of serum and liver iron concentrations.
Curcumin supplementation aggravates colitis in the DSS-mouse model
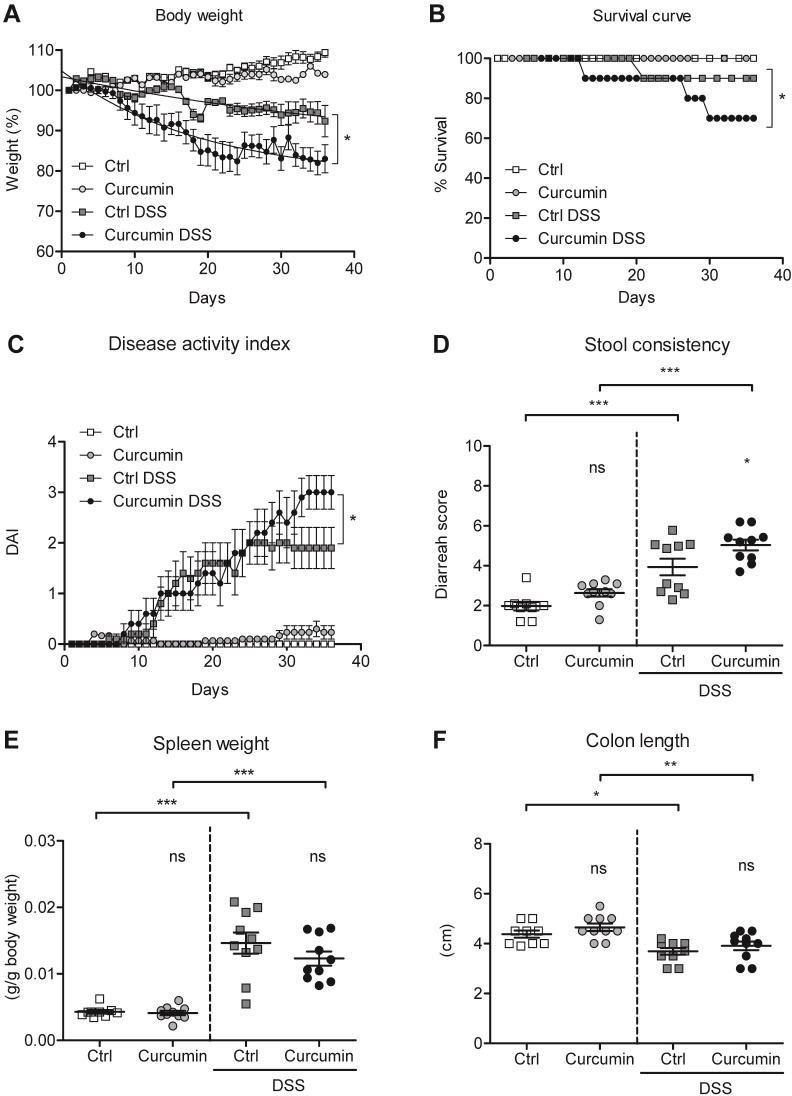
We evaluated the effect of curcumin supplementation on colitis severity. Curcumin supplementation in DSS-treated mice contributed to a greater weight loss compared to Ctrl-DSS mice (Fig 2A). This result was in line with survival (Fig 2B), where both Ctrl-DSS and Curcumin-DSS groups demonstrated mortality. However, only Curcumin-DSS mice showed a steady decrease in survival reaching 60% compared to 90% in the Ctrl-DSS group at the end of the experiment.
Fig 2. Curcumin supplementation of an iron-sufficient diet aggravates colitis in a DSS-mouse model.
C57BL/6 mice were fed an iron-sufficient diet (Ctrl) or iron-sufficient diet supplemented with curcumin (Curcumin). For dextran sodium sulfate (DSS) treatment, mice were fed an iron-sufficient diet with or without curcumin, starting 2 weeks before administration of DSS. (A) Body weight. (B) Survival. (C) Disease activity index (DAI). (D) Stool consistency. (E) Spleen weight. (F) Colon length. Results are representative of a minimum of three independent experiments; n = 9–10 mice per group. Statistical analysis was performed with one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, and ns = not significant between Curcumin to Ctrl groups and when indicated between non-DSS and DSS groups.
The disease activity index, consisting of the sum of the scores attributed to body weight loss, stool consistency and observance of blood in feces divided by three, was measured according to previous studies [31, 32]. We found that around 30 days after the initiation of the treatment Curcumin-DSS mice start having an increased disease activity index compared to Ctrl-DSS mice (Fig 2C). The stool consistency score, highest for diarrhea, reflected the same trend and showed a significant increase in Curcumin-DSS mice compared to Ctrl-DSS (Fig 2D).
Spleen weight was significantly higher in both groups of mice treated with DSS (Ctrl-DSS and Curcumin-DSS) than non-colitic mice (Fig 2E), with no significant differences found between Ctrl-DSS and Curcumin-DSS. We additionally analyzed the colon length as a marker of disease severity and found that it was significantly shorter in DSS-treated mice (Ctrl-DSS and Curcumin-DSS) than in non-colitic mice, but curcumin treatment did not did not appear to enhance the shortening of the colon (Fig 2F).
Overall, these results show that, in our experimental setting, curcumin supplementation decreases survival and is associated with higher scores of disease activity in DSS-induced colitis.
Curcumin supplementation in DSS-treated mice enhances inflammation in the colon
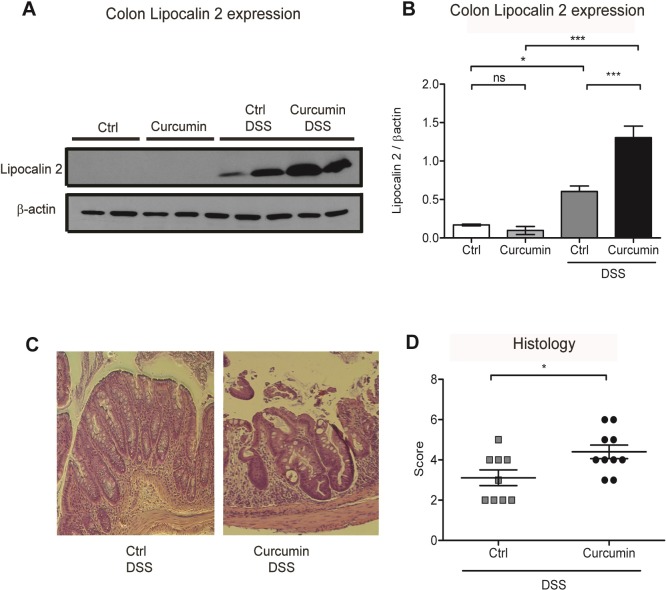
We assessed protein levels of lipocalin 2 in the colon as it is highly expressed in response to injury and inflammation and is used as a biomarker of intestinal inflammation [38]. Our results showed that curcumin supplementation of DSS-treated mice was associated with enhanced lipocalin 2 expression (Fig 3A–3B) compared to Ctrl-DSS mice, indicating heightened inflammation.
Fig 3. Curcumin supplementation in DSS-treated mice enhances inflammation in the colon.
(A) Representative western blot of colon protein extracts probed with antibodies against lipocalin 2 and β-actin. Each lane represents an individual mouse. (B) Graphic depicting densitometric quantification of western blots from three independent experiments. Data are presented as mean ± SEM. (C) Representative hematoxylin and eosin staining of mouse colon histological sections. (D) Graphic depicting quantification of colonic histology scores. Statistical analysis was performed by two-tailed Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001; ns = not significant; n = 8 mice per group.
We then assessed the severity of DSS-induced colitis using histology scoring (see materials and methods). We found that cellular infiltration and tissue damage followed by epithelial destruction were more severe in mice supplemented with curcumin (Curcumin-DSS) compared to mice treated with DSS without curcumin supplementation (Ctrl-DSS; Fig 3C and 3D).
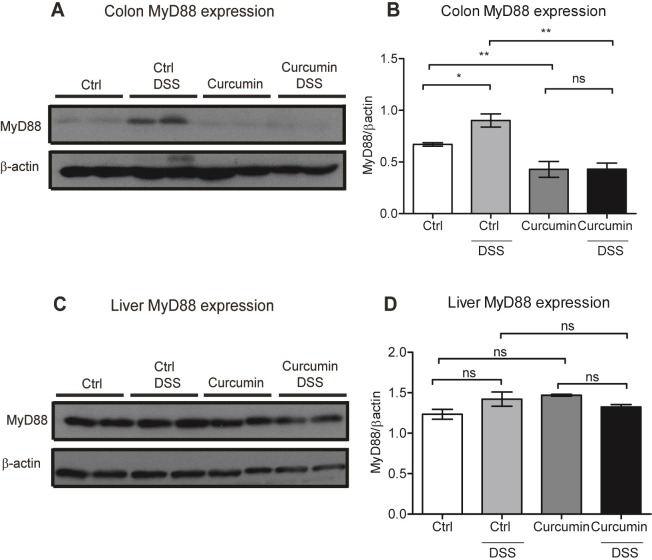
We also analyzed curcumin modulation of MyD88 protein expression. MyD88 is the major adaptor protein essential for the inflammatory cytokine activation upon stimulation of almost all the TLRs except for TLR3 [39]. As shown in Fig 4A and 4B, DSS treatment resulted in MyD88 activation (Ctrl vs. Ctrl-DSS groups). Curcumin supplementation significantly decreased MyD88 expression in colonic tissues in both Curcumin and Curcumin-DSS groups compared to Ctrl and Ctrl-DSS mice. In contrast to the colon, no significant modulation of MyD88 was observed in the liver between the four mouse groups (Fig 4C and 4D).
Fig 4. Curcumin decreases MyD88 protein expression in the colon but not in the liver.
(A-B) Colonic MyD88 protein expression in C57BL/6 mice treated with or without dextran sodium sulfate (DSS) and fed an iron-sufficient diet (Ctrl) or iron-sufficient diet supplemented with curcumin (Curcumin). (A) Representative western blot of colon protein extracts probed with antibodies against MyD88 and β-actin. Each lane represents an individual mouse. (B) Graphic depicting densitometric quantification of western blots from three independent experiments. (C-D) Hepatic MyD88 protein expression. (C) Representative western blot of liver protein extracts probed with antibodies against MyD88 and β-actin. (D) Graphic depicting densitometric quantification of western blots from three independent experiments. Statistical analysis was performed with one-way ANOVA: data in B and D are presented as mean ± SEM. Statistical analysis was performed by two-tailed Student’s t-test. *P < 0.05, **P < 0.01; ns = not significant; n = 8 mice per group.
These findings show that when mice are fed an iron-sufficient diet, curcumin enhances inflammation and aggravates colitis induced by DSS. In addition, curcumin treatment suppresses MyD88 protein expression in the colon but not in the liver and is independent of DSS induction of colitis.
Curcumin supplementation of an iron-sufficient diet induces mild anemia independent of mouse strain
To investigate whether the aggravation of colitis in mice by curcumin is dependent on the mouse strain, we assessed the DSS-induced colitis mouse model on BALB/c mice using the same iron-sufficient diets with or without curcumin supplementation at 2% (w/w).
We found that BALB/c mice showed, as previously reported [40], higher resistance to the DSS treatment, recovering more readily from the successive DSS-treatment cycles. However, around day 35, body weight loss and survival decreased in Curcumin-DSS mice compared to Ctrl-DSS mice (Fig 5A and 5B). Disease activity index was higher in Curcumin-DSS mice compared to Ctrl-DSS (Fig 5C), whereas no significant differences were found in the colon length and spleen weight (Fig 5D and 5E). Lipocalin 2 expression and histology scoring in the distal colon were higher in mice supplemented with curcumin (Curcumin-DSS) than in Ctrl-DSS mice (Fig 5F–5I), indicating more severe inflammatory cell infiltration and tissue damage in Curcumin-DSS mice.
Fig 5. Curcumin supplementation of an iron-sufficient diet in BALB/C mice exacerbates DSS-induced colitis.
BALB/c mice were fed an iron-sufficient diet (50 mg/kg chow; Ctrl) or iron-sufficient diet supplemented with 2% curcumin (Curcumin) starting at 2 weeks before administration of dextran sodium sulfate (DSS). (A) Body weight. (B) Survival. (C) Disease activity index (DAI). (D) Colon length. (E) Spleen weight. (F-G) Colonic lipocalin 2 expression. (F) Representative western blot of colonic lipocalin 2 and β-actin expression. Each lane represents an individual mouse. (G) Graphic depicting densitometric quantification of western blots from three independent experiments. Data are presented as mean ± SEM. (H) Representative hematoxylin and eosin staining of mouse colon histological section. (I) Graphic depicting quantification of colonic histology scores. Results are representative of a minimum of three independent experiments; n = 8 mice per group. Statistical analysis was performed by two-tailed Student’s t-test. *P < 0.05; ns = not significant.
Regarding erythroid parameters, we found that Curcumin-DSS mice had significantly lower red blood cells numbers as well as lower hemoglobin and hematocrit values, while mean corpuscular volume remained unaffected when compared to Ctrl-DSS mice (Fig 6A–6D). Significant differences were also found in serum iron levels and liver hepcidin expression, with lower values found in Curcumin-DSS than in Ctrl-DSS mice (Fig 6E and 6F). Finally, spleen and liver iron concentrations were significantly lower in Curcumin-DSS mice compared to Ctrl-DSS mice (Fig 6G and 6H).
Fig 6. Curcumin supplementation of an iron-sufficient diet induces mild anemia independent of mouse strain.
BALB/c mice were fed an iron-sufficient diet (50 mg/kg chow; Ctrl) or iron-sufficient diet supplemented with 2% curcumin (Curcumin) starting at 2 weeks before administration of dextran sodium sulfate (DSS). (A-D) Erythroid parameters: red blood cells, hemoglobin, mean corpuscular volume and hematocrit. (E) Serum iron levels. (F) Liver hepcidin (Hamp) mRNA expression against housekeeping β-actin mRNA. (G-H) Iron content of spleen or liver. Statistical analysis was performed by two-tailed Student’s t-test. *P < 0.05, **P < 0.01; ns = not significant; n = 8 mice per group.
These results show that curcumin in an iron-sufficient diet causes mild anemia in a mouse model of IBD, reduces iron stores in the spleen, worsens colitis and decreases survival even in a DSS-resistant mouse strain.
Discussion
In this study, we aimed to investigate the potential of curcumin’s chelating activity to affect body iron stores and anemia development in a murine model of IBD. We found that curcumin supplementation in DSS-treated mice led to a decrease of several erythroid parameters, including the number of red blood cells, hemoglobin, mean corpuscular volume, and hematocrit, indicating the development of mild anemia. We also showed that Curcumin-DSS mice developed splenomegaly, which is indicative of extramedullary erythropoiesis in mice responding to iron-deficiency anemia [41]. These changes were accompanied by a reduction in liver hepcidin mRNA levels, further indicating that mice become anemic since hepcidin levels are inhibited by anemia [42]. Hepcidin levels decreased despite the presence of inflammation, which has the opposite effect of anemia and activates hepcidin expression, confirming previous studies showing that erythropoietic drive can inhibit hepcidin activation through the inflammatory pathway [43]. Our results are in agreement with previous work reporting that curcumin supplementation decreases hepcidin levels both in mice [26] and humans [44]. This regulation has been associated with the inhibition of phosphorylation of the signal transducer and activator of transcription 3 (STAT3) [44, 45] and TNFα activation [46]. MyD88 has also been implicated as an important factor for hepcidin regulation as MyD88-deficient mice are unable to appropriately upregulate hepcidin expression when iron-challenged and develop iron-loading in the liver [47]. However, we show that curcumin does not affect MyD88 levels in the liver; hence, MyD88 would not be expected to further interfere with hepatic hepcidin expression in our experimental settings. The fact that MyD88 expression in the liver was not affected and contrasted with its downregulation in colonic tissue, indicates that curcumin regulation of MyD88 expression could be more relevant locally, within the intestine.
Our finding that curcumin modulates iron status is in line with its iron chelating properties that have been demonstrated both in vitro [25] and in vivo [23, 24]. In long-term experiments in mice, curcumin supplementation has been shown to significantly lower liver and spleen iron levels [26]. Others have reported that the usage of a high dosage of curcumin in a low iron diet modulates erythroid and iron parameters, exacerbating iron deficiency symptoms [24]. Our present work strengthens and adds to these previous studies by demonstrating that the effect of curcumin in iron metabolism is of importance in the context of chronic intestinal inflammation.
Under our experimental conditions, Curcumin-DSS mice exhibited more severe symptoms of intestinal inflammation. This contradicts previous reports on curcumin’s anti-inflammatory effects in colitis, which had been linked to the attenuation of the TLR4/MyD88/NF-κB inflammatory pathway by inhibiting TLR4 homodimerization [19] and decreasing MyD88 expression [48]. Although the reasons for these differences are not clear at present, it is worth mentioning that MyD88 deficiency in mice seems to increase susceptibility to DSS-induced colitis. Araki et al. reported that MyD88 is crucial in intestinal homeostasis by playing a protective role against the development of colitis since DSS induced a more severe colitis in MyD88-/- mice [49]. In their experiments, they observed that mice lacking MyD88 in the colon had a subsequent higher intestinal permeability, causing more severe infiltration of bacterial products from the lumen [49]. Similarly, our data show that MyD88 downregulation by curcumin in DSS-treated mice resulted in an aggravation of the inflammatory responses in our experimental conditions. In fact, DSS-curcumin mice showed more severe colonic tissue damage compared to DSS-treated mice that were not supplemented with curcumin. While the precise mechanism of action remains to be investigated, the susceptibility of MyD88-deficient mice to colitis has been linked to gut microbiota composition, which is altered in IBD [50]. Furthermore, MyD88-/- mice, in contrast with wild-type mice, are unable to respond to treatment with probiotic bacteria in the context of DSS-induced colitis, further highlighting the link between MyD88 and the gut microbiota [51]. Overall, the implications of these studies are that bacterial components may play both detrimental [50] and protective roles, at least partially, in a MyD88-dependent manner [49, 51].
Regarding colitis severity, our study is in contrast with previous reports revealing a protective effect of curcumin in DSS-induced acute [11, 52] and chronic [53] colitis in mice. Such differences may be due to the amount of iron found in standard rodent chow, which tends to have excess iron ranging from 350 mg/kg up to 900 mg iron/kg diet in some related studies [53]. This strikingly contrasts with our iron-sufficient diet that contained 50 mg/kg, which is more in accordance to mouse iron requirements [54]. Excess dietary iron may compensate for the iron chelating effect of curcumin [55] and presumably avoids the development of iron deficiency and anemia in this model. In addition to iron, other components of the rodent diet, such as fermentable fibers, may have a role in altering the effects of curcumin in DSS-induced colitis. Indeed, recent studies have shown that the presence of fermentable fibers in the diet can ameliorate low-grade inflammation while exacerbating disease severity in response to acute colitis [56].
Previous studies have reported that the efficacy of dietary curcumin in trinitrobenzene sulfonic acid (TNBS)-induced colitis, another rodent model for IBD, may vary depending on the mouse strain [10]. Furthermore, mouse strain has also been shown to influence the severity of DSS-induced colitis [40]. We tested BALB/c mice since they are known to be substantially more resistant to DSS acute colitis in comparison to C57BL/6 mice [40]. We found that curcumin aggravated colitis and induced an iron-deficiency anemia phenotype in BALB/c mice as well, indicating that the detrimental effect of curcumin in the context of an iron-sufficient diet is independent of mouse strain.
In conclusion, we found that long-term curcumin administration in mice has potentially adverse effects in a DSS-induced model of ulcerative colitis, lowering iron stores and leading to the development of anemia. While beneficial effects of curcumin as an anti-inflammatory agent have been documented in animal models as well as in patients with mild to moderate ulcerative colitis [57, 58], the iron chelating properties of curcumin should be considered. This is particularly pertinent in situations of iron-deficiency, a condition that is found in up to 78% of Crohn’s disease patients with active inflammation [59]. Our study highlights the potential risks of curcumin, which is commonly taken as an over-the-counter supplement without monitoring erythroid and iron status parameters.
Supporting information
(PDF)
(A-B) Unaltered representative western blot of colon protein extracts of C57BL/6 mice probed with antibodies against lipocalin 2 (A) and (B) β-actin. Each lane represents an individual mouse. (C-D) Unaltered representative western blot of colon protein extracts of C57BL/6 mice probed with antibodies against (C) MyD88 and (D) β-actin. Each lane represents an individual mouse. (E-F) Unaltered representative western blot of liver protein extracts of C57BL/6 mice probed with antibodies against (E) MyD88 and (F) β-actin. Each lane represents an individual mouse. (G-H) Unaltered representative western blot of colon protein extracts of BALB/c mice probed with antibodies against (G) lipocalin 2 and (H) β-actin. Each lane represents an individual mouse.
(TIF)
Acknowledgments
We thank Jacqueline Chung for editing the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by grants from the Canadian Network for Research and Innovation in Machining Technology, Natural Sciences and Engineering Research Council of Canada (412326-2011 awarded to Dr Manuela M. Santos), Canadian Institutes of Health Research (CIHR, grants no. PJT – 159775 and MOP123246) and the Natural Sciences and Engineering Research Council of Canada (NSERC, grant no. RGPIN-2018-06442) to MMS.MC is the recipient of a postdoctoral fellowship from MITACS (Accelerate fellowship, # IT07618) and MSM received a Ph.D. scholarship from the Institute du Cancer de Montréal (ICM, bourse Canderel).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24(2):109–16. Epub 2011/12/14. 10.1097/MEG.0b013e32834f3140 . [DOI] [PubMed] [Google Scholar]
- 2.Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther. 2006;24(11–12):1507–23. 10.1111/j.1365-2036.2006.03146.x [DOI] [PubMed] [Google Scholar]
- 3.Pizzi LT, Weston CM, Goldfarb NI, Moretti D, Cobb N, Howell JB, et al. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(1):47–52. Epub 2005/12/24. . [DOI] [PubMed] [Google Scholar]
- 4.Wells CW, Lewis S, Barton JR, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis. 2006;12(2):123–30. Epub 2006/01/25. 10.1097/01.MIB.0000196646.64615.db . [DOI] [PubMed] [Google Scholar]
- 5.Cucino C, Sonnenberg A. Cause of death in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2001;7(3):250–5. Epub 2001/08/23. . [DOI] [PubMed] [Google Scholar]
- 6.Patel D, Trivedi C, Khan N. Management of Anemia in Patients with Inflammatory Bowel Disease (IBD). Curr Treat Options Gastroenterol. 2018. Epub 2018/02/07. 10.1007/s11938-018-0174-2 . [DOI] [PubMed] [Google Scholar]
- 7.Murawska N, Fabisiak A, Fichna J. Anemia of Chronic Disease and Iron Deficiency Anemia in Inflammatory Bowel Diseases: Pathophysiology, Diagnosis, and Treatment. Inflamm Bowel Dis. 2016;22(5):1198–208. Epub 2016/01/29. 10.1097/MIB.0000000000000648 . [DOI] [PubMed] [Google Scholar]
- 8.MacDonald TT, Biancheri P, Sarra M, Monteleone G. What's the next best cytokine target in IBD? Inflamm Bowel Dis. 2012;18(11):2180–9. Epub 2012/04/18. 10.1002/ibd.22967 . [DOI] [PubMed] [Google Scholar]
- 9.Neurath MF. Cytokines in inflammatory bowel disease. Nature reviews Immunology. 2014;14(5):329–42. Epub 2014/04/23. 10.1038/nri3661 . [DOI] [PubMed] [Google Scholar]
- 10.Billerey-Larmonier C, Uno JK, Larmonier N, Midura AJ, Timmermann B, Ghishan FK, et al. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis. 2008;14(6):780–93. Epub 2008/01/18. 10.1002/ibd.20348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deguchi Y, Andoh A, Inatomi O, Yagi Y, Bamba S, Araki Y, et al. Curcumin prevents the development of dextran sulfate Sodium (DSS)-induced experimental colitis. Dig Dis Sci. 2007;52(11):2993–8. Epub 2007/04/13. 10.1007/s10620-006-9138-9 . [DOI] [PubMed] [Google Scholar]
- 12.Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin—from molecule to biological function. Angew Chem Int Ed Engl. 2012;51(22):5308–32. Epub 2012/05/09. 10.1002/anie.201107724 . [DOI] [PubMed] [Google Scholar]
- 13.Shishodia S. Molecular mechanisms of curcumin action: gene expression. Biofactors. 2013;39(1):37–55. Epub 2012/09/22. 10.1002/biof.1041 . [DOI] [PubMed] [Google Scholar]
- 14.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–900. Epub 2001/11/20. . [PubMed] [Google Scholar]
- 15.Cao J, Han Z, Tian L, Chen K, Fan Y, Ye B, et al. Curcumin inhibits EMMPRIN and MMP-9 expression through AMPK-MAPK and PKC signaling in PMA induced macrophages. J Transl Med. 2014;12:266 Epub 2014/09/23. 10.1186/s12967-014-0266-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Zhao Y, Hu J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS One. 2013;8(6):e67078 Epub 2013/07/05. 10.1371/journal.pone.0067078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min KJ, Um HJ, Cho KH, Kwon TK. Curcumin inhibits oxLDL-induced CD36 expression and foam cell formation through the inhibition of p38 MAPK phosphorylation. Food Chem Toxicol. 2013;58:77–85. Epub 2013/04/23. 10.1016/j.fct.2013.04.008 . [DOI] [PubMed] [Google Scholar]
- 18.Kong F, Ye B, Cao J, Cai X, Lin L, Huang S, et al. Curcumin Represses NLRP3 Inflammasome Activation via TLR4/MyD88/NF-kappaB and P2X7R Signaling in PMA-Induced Macrophages. Front Pharmacol. 2016;7:369 Epub 2016/10/26. 10.3389/fphar.2016.00369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youn HS, Saitoh SI, Miyake K, Hwang DH. Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem Pharmacol. 2006;72(1):62–9. Epub 2006/05/09. 10.1016/j.bcp.2006.03.022 . [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Zhang T, Wang X, Wei X, Chen Y, Guo L, et al. Curcumin Modulates Macrophage Polarization Through the Inhibition of the Toll-Like Receptor 4 Expression and its Signaling Pathways. Cell Physiol Biochem. 2015;36(2):631–41. Epub 2015/05/23. 10.1159/000430126 . [DOI] [PubMed] [Google Scholar]
- 21.Keihanian F, Saeidinia A, Bagheri RK, Johnston TP, Sahebkar A. Curcumin, hemostasis, thrombosis, and coagulation. J Cell Physiol. 2018;233(6):4497–511. Epub 2017/10/21. 10.1002/jcp.26249 . [DOI] [PubMed] [Google Scholar]
- 22.Kim DC, Ku SK, Bae JS. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012;45(4):221–6. Epub 2012/04/26. . [DOI] [PubMed] [Google Scholar]
- 23.Jiao Y, Wilkinson Jt, Christine Pietsch E, Buss JL, Wang W, Planalp R, et al. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 2006;40(7):1152–60. Epub 2006/03/21. 10.1016/j.freeradbiomed.2005.11.003 . [DOI] [PubMed] [Google Scholar]
- 24.Jiao Y, Wilkinson Jt, Di X, Wang W, Hatcher H, Kock ND, et al. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2009;113(2):462–9. Epub 2008/09/26. 10.1182/blood-2008-05-155952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernabe-Pineda M, Ramirez-Silva MT, Romero-Romo MA, Gonzalez-Vergara E, Rojas-Hernandez A. Spectrophotometric and electrochemical determination of the formation constants of the complexes Curcumin-Fe(III)-water and Curcumin-Fe(II)-water. Spectrochim Acta A Mol Biomol Spectrosc. 2004;60(5):1105–13. Epub 2004/04/16. 10.1016/S1386-1425(03)00344-5 . [DOI] [PubMed] [Google Scholar]
- 26.Chin D, Huebbe P, Frank J, Rimbach G, Pallauf K. Curcumin may impair iron status when fed to mice for six months. Redox Biology. 2014;2:563–9. 10.1016/j.redox.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta 2012;1823(9):1434–43. 10.1016/j.bbamcr.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2009;1790(7):682–93. 10.1016/j.bbagen.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 29.Constante M, Fragoso G, Calvé A, Samba-Mondonga M, Santos MM. Dietary heme induces gut dysbiosis, aggravates colitis, and potentiates the development of adenomas in mice. Frontiers in Microbiology. 2017;8(1809). 10.3389/fmicb.2017.01809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, Davidson LA, et al. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68(10):3985–91. Epub 2008/05/17. 10.1158/0008-5472.CAN-07-6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falcone EL, Abusleme L, Swamydas M, Lionakis MS, Ding L, Hsu AP, et al. Colitis susceptibility in p47(phox-/-) mice is mediated by the microbiome. Microbiome. 2016;4:13 Epub 2016/04/06. 10.1186/s40168-016-0159-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69(2):238–49. Epub 1993/08/01. . [PubMed] [Google Scholar]
- 33.Makui H, Soares RJ, Jiang W, Constante M, Santos MM. Contribution of Hfe expression in macrophages to the regulation of hepatic hepcidin levels and iron loading. Blood. 2005;106(6):2189–95. Epub 2005/05/26. 10.1182/blood-2005-02-0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wienk KJH, Marx JJM, Santos M, Lemmens AG, Brink EJ, Van Der Meer R, et al. Dietary ascorbic acid raises iron absorption in anaemic rats through enhancing mucosal iron uptake independent of iron solubility in the digesta. British Journal of Nutrition. 1997;77(01):123–31. 10.1079/BJN19970014 [DOI] [PubMed] [Google Scholar]
- 35.Fillebeen C, Gkouvatsos K, Fragoso G, Calve A, Garcia-Santos D, Buffler M, et al. Mice are poor heme absorbers and do not require intestinal Hmox1 for dietary heme iron assimilation. Haematologica. 2015;100(9):e334–7. 10.3324/haematol.2015.126870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702. Epub 1990/03/01. . [DOI] [PubMed] [Google Scholar]
- 37.Whittem CG, Williams AD, Williams CS. Murine Colitis Modeling using Dextran Sulfate Sodium (DSS). Journal of Visualized Experiments: JoVE. 2010;(35):1652 10.3791/1652 PubMed PMID: PMC2841571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS ONE. 2012;7(9):e44328 10.1371/journal.pone.0044328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34. 10.3109/08830185.2010.529976 [DOI] [PubMed] [Google Scholar]
- 40.Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005;288(6):G1328–G38. 10.1152/ajpgi.00467.2004 . [DOI] [PubMed] [Google Scholar]
- 41.Bennett MPP, Cudkowicz G, Bannerman RM. Hemopoietic Progenitor Cells in Marrow and Spleen of Mice with Hereditary Iron Deficiency Anemia. Blood. 1968;32(6):908–21. [PubMed] [Google Scholar]
- 42.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–44. 10.1172/JCI15686 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang H, Constante M, Layoun A, Santos MM. Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli. Blood. 2009;113(15):3593–9. Epub 2009/02/11. doi: blood-2008-08-173641 [pii] 10.1182/blood-2008-08-173641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laine F, Laviolle B, Bardou-Jacquet E, Fatih N, Jezequel C, Collet N, et al. Curcuma decreases serum hepcidin levels in healthy volunteers: a placebo-controlled, randomized, double-blind, cross-over study. Fundam Clin Pharmacol. 2017;31(5):567–73. Epub 2017/04/04. 10.1111/fcp.12288 . [DOI] [PubMed] [Google Scholar]
- 45.Fatih N, Camberlein E, Island ML, Corlu A, Abgueguen E, Detivaud L, et al. Natural and synthetic STAT3 inhibitors reduce hepcidin expression in differentiated mouse hepatocytes expressing the active phosphorylated STAT3 form. J Mol Med (Berl). 2010;88(5):477–86. Epub 2010/02/20. 10.1007/s00109-009-0588-3 . [DOI] [PubMed] [Google Scholar]
- 46.Shanmugam NK, Ellenbogen S, Trebicka E, Wang L, Mukhopadhyay S, Lacy-Hulbert A, et al. Tumor necrosis factor alpha inhibits expression of the iron regulating hormone hepcidin in murine models of innate colitis. PLoS One. 2012;7(5):e38136 Epub 2012/06/08. 10.1371/journal.pone.0038136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Layoun A, Samba-Mondonga M, Fragoso G, Calve A, Santos MM. MyD88 Adaptor Protein Is Required for Appropriate Hepcidin Induction in Response to Dietary Iron Overload in Mice. Front Physiol. 2018;9:159 Epub 2018/03/21. 10.3389/fphys.2018.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lubbad A, Oriowo MA, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. 2009;322(1–2):127–35. Epub 2008/11/13. 10.1007/s11010-008-9949-4 . [DOI] [PubMed] [Google Scholar]
- 49.Araki A, Kanai T, Ishikura T, Makita S, Uraushihara K, Iiyama R, et al. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40(1):16–23. Epub 2005/02/05. 10.1007/s00535-004-1492-9 . [DOI] [PubMed] [Google Scholar]
- 50.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9(10):599–608. 10.1038/nrgastro.2012.152 [DOI] [PubMed] [Google Scholar]
- 51.Souza DG, Senchenkova EY, Russell J, Granger DN. MyD88 Mediates the Protective Effects of Probiotics Against the Arteriolar Thrombosis and Leukocyte Recruitment Associated with Experimental Colitis. Inflammatory Bowel Diseases. 2015;21(4):888–900. 10.1097/MIB.0000000000000331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J-y, Zhong X, Kim S-J, Kim D-H, Kim HS, Lee J-S, et al. Comparative Effects of Curcumin and Tetrahydrocurcumin on Dextran Sulfate Sodium-induced Colitis and Inflammatory Signaling in Mice. Journal of Cancer Prevention. 2018;23(1):18–24. 10.15430/JCP.2018.23.1.18 PubMed PMID: PMC5886491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villegas I, Sánchez‐Fidalgo S, de la Lastra CA. Chemopreventive effect of dietary curcumin on inflammation‐induced colorectal carcinogenesis in mice. Molecular Nutrition & Food Research. 2011;55(2):259–67. 10.1002/mnfr.201000225 [DOI] [PubMed] [Google Scholar]
- 54.National Research Council (U.S.). Subcommittee on Laboratory Animal Nutrition Nutrient requirements of laboratory animals. 4th rev. ed. Washington, D.C.: National Academy of Sciences; 1995. xii, 173 p. p. [Google Scholar]
- 55.Badria FA, Ibrahim AS, Badria AF, Elmarakby AA. Curcumin Attenuates Iron Accumulation and Oxidative Stress in the Liver and Spleen of Chronic Iron-Overloaded Rats. PLOS ONE. 2015;10(7):e0134156 10.1371/journal.pone.0134156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miles JP, Zou J, Kumar M-V, Pellizzon M, Ulman E, Ricci M, et al. Supplementation of Low- and High-fat Diets with Fermentable Fiber Exacerbates Severity of DSS-induced Acute Colitis. Inflammatory Bowel Diseases. 2017;23(7):1133–43. 10.1097/MIB.0000000000001155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lang A, Salomon N, Wu JC, Kopylov U, Lahat A, Har-Noy O, et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2015;13(8):1444–9 e1. Epub 2015/03/01. 10.1016/j.cgh.2015.02.019 . [DOI] [PubMed] [Google Scholar]
- 58.Suskind DL, Wahbeh G, Burpee T, Cohen M, Christie D, Weber W. Tolerability of Curcumin in Pediatric Inflammatory Bowel Disease: A forced dose titration study. Journal of pediatric gastroenterology and nutrition. 2013;56(3):277–9. 10.1097/MPG.0b013e318276977d PubMed PMID: PMC3701433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neville A, Ellul P. Iron deficiency in Crohn's disease: Iron supplementation or disease control? Journal of Crohn's and Colitis. 2014;8(10):1333–. 10.1016/j.crohns.2014.03.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(A-B) Unaltered representative western blot of colon protein extracts of C57BL/6 mice probed with antibodies against lipocalin 2 (A) and (B) β-actin. Each lane represents an individual mouse. (C-D) Unaltered representative western blot of colon protein extracts of C57BL/6 mice probed with antibodies against (C) MyD88 and (D) β-actin. Each lane represents an individual mouse. (E-F) Unaltered representative western blot of liver protein extracts of C57BL/6 mice probed with antibodies against (E) MyD88 and (F) β-actin. Each lane represents an individual mouse. (G-H) Unaltered representative western blot of colon protein extracts of BALB/c mice probed with antibodies against (G) lipocalin 2 and (H) β-actin. Each lane represents an individual mouse.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.