Abstract
Background
Pressure ulcers, also known as pressure injuries and bed sores, are localised areas of injury to the skin or underlying tissues, or both. Dressings made from a variety of materials, including foam, are used to treat pressure ulcers. An evidence‐based overview of dressings for pressure ulcers is needed to enable informed decision‐making on dressing use. This review is part of a suite of Cochrane Reviews investigating the use of dressings in the treatment of pressure ulcers. Each review will focus on a particular dressing type.
Objectives
To assess the clinical and cost effectiveness of foam wound dressings for healing pressure ulcers in people with an existing pressure ulcer in any care setting.
Search methods
In February 2017 we searched: the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations); Ovid Embase; EBSCO CINAHL Plus and the NHS Economic Evaluation Database (NHS EED). We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
Published or unpublished randomised controlled trials (RCTs) and cluster‐RCTs, that compared the clinical and cost effectiveness of foam wound dressings for healing pressure ulcers (Category/Stage II or above).
Data collection and analysis
Two review authors independently performed study selection, risk of bias and data extraction. A third reviewer resolved discrepancies between the review authors.
Main results
We included nine trials with a total of 483 participants, all of whom were adults (59 years or older) with an existing pressure ulcer Category/Stage II or above. All trials had two arms, which compared foam dressings with other dressings for treating pressure ulcers.
The certainty of evidence ranged from low to very low due to various combinations of selection, performance, attrition, detection and reporting bias, and imprecision due to small sample sizes and wide confidence intervals. We had very little confidence in the estimate of effect of included studies. Where a foam dressing was compared with another foam dressing, we established that the true effect was likely to be substantially less than the study's estimated effect.
We present data for four comparisons.
One trial compared a silicone foam dressing with another (hydropolymer) foam dressing (38 participants), with an eight‐week (short‐term) follow‐up. It was uncertain whether alternate types of foam dressing affected the incidence of healed pressure ulcers (RR 0.89, 95% CI 0.45 to 1.75) or adverse events (RR 0.37, 95% CI 0.04 to 3.25), as the certainty of evidence was very low, downgraded for serious limitations in study design and very serious imprecision.
Four trials with a median sample size of 20 participants (230 participants), compared foam dressings with hydrocolloid dressings for eight weeks or less (short‐term). It was uncertain whether foam dressings affected the probability of healing in comparison to hydrocolloid dressings over a short follow‐up period in three trials (RR 0.85, 95% CI 0.54 to 1.34), very low‐certainty evidence, downgraded for very serious study limitations and serious imprecision. It was uncertain if there was a difference in risk of adverse events between groups (RR 0.88, 95% CI 0.37 to 2.11), very low‐certainty evidence, downgraded for serious study limitations and very serious imprecision. Reduction in ulcer size, patient satisfaction/acceptability, pain and cost effectiveness data were also reported but we assessed the evidence as being of very low certainty.
One trial (34 participants), compared foam and hydrogel dressings over an eight‐week (short‐term) follow‐up. It was uncertain if the foam dressing affected the probability of healing (RR 1.00, 95% CI 0.78 to 1.28), time to complete healing (MD 5.67 days 95% CI ‐4.03 to 15.37), adverse events (RR 0.33, 95% CI 0.01 to 7.65) or reduction in ulcer size (MD 0.30 cm2 per day, 95% CI ‐0.15 to 0.75), as the certainty of the evidence was very low, downgraded for serious study limitations and very serious imprecision.
The remaining three trials (181 participants) compared foam with basic wound contact dressings. Follow‐up times ranged from short‐term (8 weeks or less) to medium‐term (8 to 24 weeks). It was uncertain whether foam dressings affected the probability of healing compared with basic wound contact dressings, in the short term (RR 1.33, 95% CI 0.62 to 2.88) or medium term (RR 1.17, 95% CI 0.79 to 1.72), or affected time to complete healing in the medium term (MD ‐35.80 days, 95% CI ‐56.77 to ‐14.83), or adverse events in the medium term (RR 0.58, 95% CI 0.33 to 1.05). This was due to the very low‐certainty evidence, downgraded for serious to very serious study limitations and imprecision. Reduction in ulcer size, patient satisfaction/acceptability, pain and cost effectiveness data were also reported but again, we assessed the evidence as being of very low certainty.
None of the included trials reported quality of life or pressure ulcer recurrence.
Authors' conclusions
It is uncertain whether foam dressings are more clinically effective, more acceptable to users, or more cost effective compared to alternative dressings in treating pressure ulcers. It was difficult to make accurate comparisons between foam dressings and other dressings due to the lack of data on reduction of wound size, complete wound healing, treatment costs, or insufficient time‐frames. Quality of life and patient (or carer) acceptability/satisfaction associated with foam dressings were not systematically measured in any of the included studies. We assessed the certainty of the evidence in the included trials as low to very low. Clinicians need to carefully consider the lack of robust evidence in relation to the clinical and cost‐effectiveness of foam dressings for treating pressure ulcers when making treatment decisions, particularly when considering the wound management properties that may be offered by each dressing type and the care context.
Plain language summary
Foam dressings for treating pressure ulcers
What is the aim of this review?
The aim of this review was to find out whether foam dressings (designed to absorb fluid from wounds whilst keeping them moist) have any advantages or disadvantages in healing pressure ulcers compared with other dressings (such as silicone foam dressings, hydrocolloid, hydrogel or basic wound dressings). Researchers from Cochrane collected and analysed all relevant studies (randomised controlled trials) to answer this question and found nine relevant studies.
Key messages
There is no clear evidence from any of the studies included in this review that foam dressings are more effective at healing pressure ulcers than other types of dressings; or that foam dressings are more cost effective than other dressings. This is due in part to the low quality of the studies, many of which had small numbers of participants and did not provide accurate details of their methods.
What was studied in the review?
Pressure ulcers (pressure injuries or bed sores) are wounds that develop on bony parts of the body such as the heels, hips and lower back. Sitting or lying in the same position for long periods can cause damage to the skin and underlying tissue. People at risk of developing pressure ulcers include those with limited physical mobility such as people with spinal cord injuries, older people, or those ill in hospital.
Pressure ulcer treatment is a significant burden to patients, their carer(s) and healthcare systems worldwide. Treatments for pressure ulcers include dressings, antibiotics and antiseptics, and pressure‐relieving mattresses or cushions. There are many wound dressings available to treat pressure ulcers, which vary in cost and may have differing degrees of effectiveness.
Foam dressings are designed to absorb fluid (exudate) that comes from some pressure ulcer wounds, and to maintain a moist environment. We wanted to find out how foam dressings affected pressure ulcer healing and recurrence rates. We also wanted to find out whether foam dressings had an impact on participants’ quality of life and satisfaction with treatment, and whether there were any side effects such as infection or pain. We also evaluated the cost of foam dressings compared to other treatments.
What are the main results of the review?
We found nine studies published between 1994 and 2016 involving a total of 483 participants with pressure ulcers at Category/Stage II or above (open wounds). Seven of the nine trials had more female participants than male. On average people in these studies were 59 years or older. The studies compared foam dressings with other types of dressings, however, there was no clear evidence to indicate foam dressings were more effective at healing pressure ulcers than other types of dressings, or more cost effective. Evidence regarding reduction in ulcer size, patient satisfaction and pain is very uncertain. None of the studies reported on participants’ quality of life or pressure ulcer recurrence. The majority of studies found the dressings evaluated were no better or worse than others on the market. So, while foam dressings can be safely used for the treatment for pressure ulcers, their effect on wound healing is not supported by scientific evidence.
Generally, the studies we found did not have many participants and the results were often inconclusive. Overall the evidence that exists is of very low quality.
How up to date is this review?
We searched for studies that had been published up to February 2017.
Summary of findings
Background
Description of the condition
Pressure ulcers, also known as pressure injuries, decubitus ulcers and bed sores, are a localised injury to the skin, underlying tissue, or both, usually occurring over a bony prominence, as a result of pressure, or pressure in combination with shear stress from restrictive bedding ‐ where unaligned body weight is pushing one part of the body such as bone or muscle in one direction, and another part of the body, usually skin, in the opposite direction (NPUAP/EPUAP/PPPIA 2014). The development of a pressure ulcer is a serious complication resulting in pain, decreased quality of life and significant expenditure of both time and money for the healthcare industry (VanGilder 2009). Pressure ulcers are an internationally recognised patient safety problem, estimated to affect 2.5 million people annually (House 2011).
The main factors associated with the development of pressure ulcers are exposure of the skin to excessive pressure, and a reduced tolerance of the skin to pressure. Pressure is exerted on the skin, soft tissue, muscle, and bone by the weight of an individual or a device applied against the surface of their skin. Tissue tolerance is the ability of the skin and its supporting structures to tolerate the effects of pressure by distributing it (cushioning) and by the transfer of pressure loads from the skin surface to the skeleton (NPUAP/EPUAP/PPPIA 2014). Tissues are capable of withstanding enormous pressures briefly, but prolonged exposure to pressure initiates a series of events that lead potentially to necrosis and ulceration.
Factors that increase pressure on the skin include impairments in mobility, activity or sensory perception, because the pressure is not relieved by movement or changes to body position. Internal risk factors for the development of pressure ulcers include advancing age, poor nutrition, poor perfusion and oxygenation, whereas, external risk factors include increased moisture, shear and friction. Shear forces and friction aggravate the effects of pressure upon tissue and are important components of the mechanism of injury. A combination of pressure, shear forces, and friction causes microcirculatory occlusion (blockage), resulting in ischaemia and tissue anoxia (lack of oxygen) and stimulation of inflammatory processes, which may lead to cell death, ulceration, and tissue necrosis. Irreversible tissue damage may occur in vulnerable people after as little as 30 minutes of uninterrupted pressure (Kirman 2008). In addition, excessive contact of the skin to fluids impairs its barrier function, causes maceration and an increased risk of the development of a pressure ulcer.
A number of systems for describing the degree of tissue damage exist, but pressure ulcers are generally categorised as Category/Stage I, II, III, and IV according to the depth of tissue damage; Category/Stage I pressure ulcers are the least severe and are often difficult to detect and Category/Stage IV are the most severe with complete tissue destruction (Moore 2005), as illustrated in Table 5 (NPUAP/EPUAP/PPPIA 2014). The majority of pressure ulcers occur on the sacrum (base of the spine) or heel, but they also occur frequently over the elbow, hip ‐ including the ischium, shoulder, spinous processes on vertebrae, ankle, toe, head or face (Lahmann 2006; Shanin 2008; Vanderwee 2007).
1. International NPUAP/EPUAP/PPPIA Pressure Ulcer Classification System (2014).
| Category/Stage | Definition |
| Quoted directly fromNPUAP/EPUAP/PPPIA 2014 | |
| Category/Stage I: Nonblanchable Erythema |
Intact skin with non‐blanchable redness of a localized area usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its colour may differ from the surrounding area. The area may be painful, firm, soft, warmer or cooler as compared to adjacent tissue. Category/Stage I may be difficult to detect in individuals with dark skin tones. May indicate “at risk” individuals (a heralding sign of risk). |
| Category/Stage II: Partial Thickness Skin Loss |
Partial thickness loss of dermis presenting as a shallow open ulcer with a red pink wound bed, without slough. May also present as an intact or open/ruptured serum filled blister. Presents as a shiny or dry shallow ulcer without slough or bruising.* This Category/Stage should not be used to describe skin tears, tape burns, perineal dermatitis, maceration or excoriation. *Bruising indicates suspected deep tissue injury. |
| Category/Stage III: Full Thickness Skin Loss |
Full thickness tissue loss. Subcutaneous fat may be visible but bone, tendon or muscle are not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunnelling. The depth of a Category/Stage III pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and Category/Stage III ulcers can be shallow. In contrast, areas of significant adiposity can develop extremely deep Category/Stage III pressure ulcers. Bone/tendon is not visible or directly palpable. |
| Category/Stage IV: Full Thickness Tissue Loss |
Full thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present on some parts of the wound bed. Often include undermining and tunnelling. The depth of a Category/Stage IV pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and these ulcers can be shallow. Category/Stage IV ulcers can extend into muscle and/or supporting structures (e.g., fascia, tendon or joint capsule) making osteomyelitis possible. Exposed bone/tendon is visible or directly palpable. |
| Unstageable: Depth Unknown | Full thickness tissue loss in which the base of the ulcer is covered by slough (yellow, tan, grey, green or brown) and/or eschar (tan, brown or black) in the wound bed. Until enough slough and/or eschar is removed to expose the base of the wound, the true depth, and therefore Category/Stage, cannot be determined. Stable (dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as ‘the body’s natural (biological) cover’ and should not be removed. |
| Suspected Deep Tissue Injury: Depth Unknown | Purple or maroon localized area of discoloured intact skin or blood‐filled blister due to damage of underlying soft tissue from pressure and/or shear. The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer or cooler as compared to adjacent tissue. Deep tissue injury may be difficult to detect in individuals with dark skin tones. Evolution may include a thin blister over a dark wound bed. The wound may further evolve and become covered by thin eschar. Evolution may be rapid exposing additional layers of tissue even with optimal treatment. |
Prevalence of pressure ulcers
The prevalence of pressure ulcers is dependent upon patient factors and treatment settings (Vanderwee 2007; VanGilder 2009). A study undertaken in European acute care settings found an overall prevalence of 18.1% or 10.5% if Category/Stage I pressure ulcers were excluded with individual countries reporting prevalence rates between 8.3% and 23% (Vanderwee 2007). A more recent survey of the USA estimated a per‐annum pressure ulcer prevalence of 12% to 13% in acute care settings and 29% to 32% in longer‐term acute care settings (VanGilder 2009). It should be noted that this survey excluded Category/Stage I pressure ulcers from prevalence calculations due to the substantial inaccuracies associated with their assessment (VanGilder 2009). Within Australia, pressure ulcer point prevalence studies conducted by the Victorian Government in 136 metropolitan and rural health service sites between 2003 and 2006 resulted in a decrease in the prevalence of people with pressure ulcers (categories/stages I to IV) from 26.5% to 17.6%. However the proportion of people with pressure ulcers acquired in hospital did not change (67.6% in 2003 versus 67.7% in 2006 (QSB 2006). These international studies of prevalence illustrate the extent of the burden of pressure ulcer, however variability in prevalence in similar settings suggests pressure ulcers are amenable to intervention, with substantial potential for improvement in patient and financial outcomes.
Economic burden of pressure ulcers
Internationally, there has been substantial investment over recent decades in monitoring, preventing and treating pressure ulcers in an attempt to reduce their incidence and associated costs. As a result there is increasing evidence of the economic burden of pressure ulcers. Graves 2014 applied a probabilistic model to estimate the direct health cost of pressure ulcers in hospital and residential care settings in Australia for 2010 to 2011. They reported a mean number of pressure ulcer cases of 345,768 in public and private hospitals, at a mean cost of USD 1.64 billion In long‐term and respite residential aged care settings, they reported 10,397 cases of pressure ulcer at a mean cost of USD 13.9 million for a combined total of USD 1.65 billion. Another Australian cost‐of‐illness study (Nguyen 2015) used a prevalence approach and simulation methods to estimate the costs of pressure ulcers using 2012 to 2013 public hospital data. Based on a total number of 121,645 reported pressure ulcers cases, and 524,661 bed days lost, they estimated the cost as AUD 983 million per annum, or 1.9% of all public hospital expenditure. Opportunity costs were also estimated adding AUD 820 million per annum to the overall cost of pressure ulcers of AUD 1.8 billion. In 2011, Dealey 2012 and colleagues used a bottom‐up methodology to estimate the approximate total cost of pressure ulcers in the UK as GBP 3.36 billion annually with an expected average cost of healing a Category/Stage III or IV ulcer of between GBP 9000 and GBP 14,000. In the USA, total costs for treatment of pressure ulcers reported in 2014 were estimated at USD 9.1 to USD 11.6 billion annually, with 2.5 million people affected and approximately 60,000 deaths resulting from pressure ulcers (AHRQ 2014). The main costs incurred for the treatment and management of pressure ulcers are due to prolonged hospitalisation and the extent of nursing care required. Although the independent effects of a pressure ulcer on length of hospital stay are likely to vary between studies, authors of a report from the USA identified that the average length of acute hospital stay for adults with a pressure ulcer (Category/Stage not identified) was longer for younger age groups, and ranged from 14.1 days for people aged between 18 and 44 years, 12.4 days for people aged 65 to 84 years and 10.2 days for people aged 85 years and older (Russo 2003). In comparison, the average length of stay for all hospitalisations in 2003 was 4.6 days. In addition to the increased time spent in hospital, the discomfort and pain experienced, the burden upon the person with the pressure ulcer ‐ and the cost to the health services ‐ are compounded by the increased risk of mortality, altered body image and reduced quality of life, together with the potential cost associated with financial penalties for this largely preventable condition (VQC 2004), such as those imposed by the Queensland Government for severe pressure ulcers (Miles 2013). In spite of the level of investment in prevention and monitoring of pressure ulcers, many people continue to develop them. This is the case particularly in acute and long‐term care settings where people may present with a several risk factors such as decreased mobility, impaired perfusion, poor nutrition, and fluctuating health status (Dealey 2012). Pressure ulcer treatment strategies are often costly and complex.
Description of the intervention
Treatment of a pressure ulcer is primarily two‐fold and involves the relief of pressure allied with wound management. Other general strategies include patient education, pain management, optimising circulation/perfusion, optimising nutrition and the treatment of clinical infection (NPUAP/EPUAP/PPPIA 2014). Wound management may involve surgical or chemical debridement (removal of dead tissue) and dressings to protect the wound and possibly promote healing. Dressings can be divided into four main categories, namely, basic wound dressings, advanced wound dressings, anti‐microbial dressings and specialist dressings. Classification of a dressing depends on its purpose and the key material used in its composition. Key attributes of a dressing have been described (BNF 2016), and include: the ability of the dressing to absorb and contain exudate without leakage or strike‐through (saturation); lack of particulate contaminants left in the wound by the dressing; thermal insulation; permeability to water but not to bacteria; avoidance of wound trauma on dressing removal; frequency with which the dressing needs to be changed; provision of pain relief; and comfort.
Foam dressings, the properties of which are described below, are the focus of this review. As foam dressings are likely to be evaluated against one of the many wound dressings available, we have provided a description of potential comparators, categorised according to the British National Formulary structure, and listed by their generic names and manufacturers (BNF 2016). Dressing names, manufactures and distributors may vary between countries.
Basic wound contact dressings
Low‐adherence dressings and wound contact materials: these usually consist of cotton pads that are placed directly in contact with the wound and are designed to prevent minimal adherence to the wound bed and so present less risk of trauma to the wound as it is removed for subsequent and ongoing treatment. The addition of paraffin and similar substances is to prevent the dressing from sticking to the wound.
Absorbent dressings: these dressings are applied directly to the wound and maybe used as secondary absorbent layers in the management of heavily exuding wounds.
Advanced wound dressings
Foam dressings: these dressings normally contain hydrophilic (water absorbant) polyurethane foam designed to absorb wound exudate while maintaining a moist wound surface. There are a variety of versions including those with additional absorbent materials such as viscose and acrylate fibres, or particles of superabsorbent polyacrylate, while others are silicone‐coated for atraumatic removal.
Alginate dressings: these dressings are highly absorbent fabrics/yarns that come in the form of calcium‐alginate or calcium‐sodium‐alginate and can be combined with collagen. The alginate forms a gel when in contact with the wound surface which can be lifted off at dressing removal, or rinsed away with sterile saline. Bonding to a secondary viscose pad increases absorbency.
Hydrogel dressings: these dressings consist of cross‐linked insoluble polymers consisting of starch or carboxymethylcellulose, and up to 96% water. They are designed to absorb wound exudate or to rehydrate a wound depending on the wound moisture levels. They are supplied in either flat sheets, amorphous hydrogel or as beads.
Hydrocolloid dressings: these occlusive dressings are usually composed of a hydrocolloid matrix bonded to vapour‐permeable film or foam backing. This matrix forms a gel that provides a moist environment when in contact with the wound surface. Fibrous alternatives resembling alginates have also been developed. These are more absorbant than standard hydrocolloid dressings but are not occlusive.
Films, permeable film and membrane dressings: these dressings are permeable to water vapour and oxygen, but not to water or micro‐organisms.
Capillary‐action dressings: these dressings consist of an absorbent core of hydrophilic fibres held between two low‐adherent contact layers.
Odour‐absorbent dressings: these dressings contain charcoal and are used to absorb wound odour, often in conjunction with a secondary dressing to improve absorbency.
Antimicrobial dressings
Honey‐impregnated dressings: these dressings contain medical‐grade honey which is thought to have antimicrobial and anti‐inflammatory properties and can be used for acute or chronic wounds.
Iodine‐impregnated dressings: these dressings release free iodine, which is thought to act as a wound antiseptic when exposed to wound exudate.
Silver‐impregnated dressings: these dressings are used to treat infected wounds, as silver ions are thought to have antimicrobial properties. Silver versions of most dressing types are available (e.g. silver foam, silver hydrocolloid).
Other antimicrobial dressings: these dressings are composed of a gauze or low adherent dressing impregnated with an ointment thought to have antimicrobial properties.
Specialist dressings
Protease‐modulating matrix dressings: these dressings are designed to alter the activity of proteolytic enzymes in chronic wounds and are thought to promote natural debridement.
The diversity of dressings available to clinicians (including variation within each type listed above) makes evidence‐based decision making difficult when determining the optimum treatment regimen for a particular person (Gillespie 2012). Some dressings are formulated with an 'active' ingredient such as silver that is promoted as a dressing treatment option to reduce infection and possibly to promote healing. With increasingly sophisticated technology being applied to wound care, practitioners need to know how effective these, often expensive, dressings are compared with more traditional and usually less costly dressings. However, far from providing critical evaluation of dressing types for clinical use, studies have shown wide variation in practice and wound care knowledge (Reddy 2008; Maylor 1997; Pieper 1995), and the number of economic evaluations of wound dressings available is limited (NICE 2017).
How the intervention might work
The principle of moist wound healing directs contemporary wound care. This is optimised through the application of occlusive or semi‐occlusive dressings and preparation of the wound bed (NPUAP/EPUAP/PPPIA 2014). Animal experiments performed 50 years ago suggested that acute wounds healed more quickly when their surface was kept moist, rather than being left to dry and scab (Winter 1962; Winter 1963a; Winter 1963b). A moist environment is thought to provide optimal conditions for the cells involved in the healing process, as well as allowing autolytic debridement (removal of dead tissue by natural processes), which is thought to be an important component of the healing pathway (Cardinal 2009). The desire to maintain a moist wound environment is an important factor in the choice of wound dressing. Wound dressings vary in their level of absorbency so that a dry wound may be treated with an occlusive dressing to maintain a moist environment to promote healing. Alternatively a wet wound may be treated with a more absorbant dressing (such as a foam dressing) to draw excess moisture away from the area of injury and avoid skin damage.
Why it is important to do this review
Pressure ulcers are a relatively common yet complex type of wound that are a significant source of suffering for patients and their loved ones and an economic burden to healthcare systems (Reddy 2008). They are an internationally recognised patient safety problem and serve as a clinical indicator for the standard of care provided. As a result, significant investment has been made in strategies aimed at pressure ulcer prevention. However, pressure ulcers remain a prevalent condition in many care settings. Dressings are widely used as a treatment strategy for pressure ulcers, and understanding the existing evidence base and potential uncertainty around clinical efficacy and cost‐effectiveness of different dressing types is important for effective decision making.
Internationally accepted guidelines recommend that dressings that keep the wound moist should be used, based upon level C evidence that is "supported by indirect evidence (e.g., studies in healthy humans, humans with other types of chronic wounds, animal models) and/or expert opinion" (NPUAP/EPUAP/PPPIA 2014). The same guidelines suggest that foam dressings be used to treat pressure ulcers in various scenarios, mainly for the treatment of exuding Category/Stage II and shallow Category/Stage III pressure ulcers, however these recommendations are based on limited evidence (NPUAP/EPUAP/PPPIA 2014).
Two notable systematic reviews of treatments for pressure ulcers have included trials of dressings (Reddy 2008; Smith 2013). Reddy 2008 reported that "No single dressing was consistently superior to other dressings in the trials of pressure ulcers we examined" (p. 2659). This finding was consistent with earlier systematic reviews by Chaby 2007 and Hamilton 2008, which found no evidence that one particular dressing type was more clinically effective or cost effective than another. More recently a review by Smith 2013 included dressing interventions but did not specifically identify foam dressings. We conclude that up‐to‐date and transparent information on evidence for the use of dressings to treat pressure ulcers and cost effectiveness is required.
This review is part of a suite of Cochrane Reviews investigating the use of dressings in the treatment of pressure ulcers. Each review will focus on a particular dressing type and then be summarised in an overview of reviews that will draw together all existing Cochrane Review evidence regarding the use of dressings to treat pressure ulcers.
Objectives
To assess the clinical and cost effectiveness of foam wound dressings for healing pressure ulcers in people with an existing pressure ulcer in any care setting.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and cluster‐RCTs irrespective of publication status or language. We excluded non‐randomised, clinical controlled trials and cross‐over trials.
The critical review of health economic evidence included, where possible, comparative full and partial economic evaluations conducted within the framework of eligible RCTs and cluster‐RCTs (i.e. cost‐effectiveness analyses, cost‐utility analyses, cost‐benefit analyses and cost analyses that included a dressing intervention and a relevant comparator), as well as RCTs reporting more limited information, such as estimates of resource use or costs associated with dressings and a comparator. We only considered health economics studies conducted alongside effectiveness studies that were included in the clinical effectiveness component of the review.
Types of participants
We included studies that recruited people of any age (no upper age limit was set) with a diagnosis of pressure ulcer of Category/Stage II or above in any care setting using the NPUAP/EPUAP/PPPIA 2014. We also used alternative pressure ulcer classification systems, such as the Stirling (Reid 1994) and Torrence classification systems (Harker 2000), as well as earlier versions published by the NPUAP (NPUAP 1989), on the condition that the definitions of these alternative and previous versions closely matched the contemporary International NPUAP/EPUAP/PPPIA Pressure Ulcer Classification System Criteria (NPUAP/EPUAP/PPPIA 2014). See Table 6 'Comparison of pressure ulcer classification systems'. We excluded studies involving participants with Category/Stage I ulcers because although 'at‐risk' signs and symptoms of potential pressure ulcer such as non‐blanchable redness, pain, hardness or softness, heat or coolness are present, the skin remains intact (NPUAP/EPUAP/PPPIA 2014). A posteriori uncertainty about what constituted a Category/Stage I and II pressure ulcer in alternative pressure ulcer classification systems required changes to original protocol. These are outlined in Differences between protocol and review.
2. Comparison of pressure ulcer classification systems.
| NPUAP/EPUAP/PPPIA Classification System (2014, 2009) | NPUAP (1989) | The UK Consensus (Stirling) Classification of Pressure Sore Severity (1994) | The Torrence Classification System (1983) | ||||
| Category/Stage | Definition | Category/Stage | Definition | Category/Stage | Definition | Category/Stage | Definition |
| Quoted directly fromNPUAP/EPUAP/PPPIA 2014 | Quoted directly from NPUAP 1989 | Quoted directly fromReid 1994 | Quoted directly fromHarker 2000 | ||||
| Category/Stage I: Nonblanchable Erythema | Intact skin with non‐blanchable redness of a localized area usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its colour may differ from the surrounding area. The area may be painful, firm, soft, warmer or cooler as compared to adjacent tissue. Category/Stage I may be difficult to detect in individuals with dark skin tones. May indicate “at risk” individuals (a heralding sign of risk). | Stage I | Non‐blanchable erythema of intact skin: the heralding lesion of skin ulceration. Identification of Stage I pressure ulcers may be difficult in patients with darkly pigmented skin. | Stage 1 | Discoloration of intact skin (light finger pressure applied to the site does not alter the discolouration) 1.1 Non‐blanchable erythema with increased local heat 1.2 Blue/purple/black discolouration |
Stage 1 | Blanching hyperaemia: Reactive hyperaemia is a temporary dilation of the capillaries which bring oxygen to the area and remove accumulated carbon dioxide and other waste products. It causes a distinct erythema after pressure is released. Light finger pressure is said to cause blanching of this erythema, indicating that the microcirculation is intact. |
| Category/Stage II: Partial Thickness Skin Loss | Partial thickness loss of dermis presenting as a shallow open ulcer with a red pink wound bed, without slough. May also present as an intact or open/ruptured serum filled blister. Presents as a shiny or dry shallow ulcer without slough or bruising.* This Category/Stage should not be used to describe skin tears, tape burns, perineal dermatitis, maceration or excoriation. *Bruising indicates suspected deep tissue injury. | Stage II | Partial thickness skin loss involving epidermis and/or dermis. The ulcer is superficial and presents clinically as an abrasion, blister or shallow crater. | Stage 2 | Partial thickness skin loss or damage involving epidermis and/or dermis 2.1 Blister 2.2 Abrasion 2.3 Shallow ulcer, without undermining of adjacent tissue 2.4 Any of these with underlying blue/purple/black discolouration or induration. |
Stage 2 | Non‐blanching hyperaemia: the erythema remains when light pressure is applied indicating a degree of microcirculatory disruption and inflammation. Oedema distorts and thickens all tissues compressed between the bone and the support surface. Superficial damage may present as swelling, induration, blistering or epidermal ulceration, which might expose the dermis. |
| Category/Stage III: Full Thickness Skin Loss | Full thickness tissue loss. Subcutaneous fat may be visible but bone, tendon or muscle are not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunnelling. The depth of a Category/Stage III pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and Category/Stage III ulcers can be shallow. In contrast, areas of significant adiposity can develop extremely deep Category/Stage III pressure ulcers. Bone/tendon is not visible or directly palpable. | Stage III | Full thickness skin loss involving damage or necrosis of subcutaneous tissue that may extend down to, but not through, underlying fascia. The ulcer presents clinically as a deep crater with or without undermining of adjacent tissue. | Stage 3 | Full‐thickness skin loss involving damage or necrosis of subcutaneous tissues but not extending to underlying bone, tendon or joint capsule 3.1 Crater, without undermining of adjacent tissue 3.2 Crater, with undermining of adjacent tissue 3.3 Sinus, the full extent of which is not certain 3.4 Full‐thickness skin loss but wound bed covered with necrotic tissue (hard or leathery black/brown tissue or softer yellow/cream/grey slough) which masks the true extent of tissue damage. Until debrided it is not possible to observe whether damage extends into the muscle or involves damage to bone or supporting structures. |
Stage 3 | Ulceration progresses through the dermis to the junction with subcutaneous tissue. The ulcer edges are distinct but it is surrounded by erythema and induration. At this stage the damage is still reversible. |
| Category/Stage IV: Full Thickness Tissue Loss | Full thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present on some parts of the wound bed. Often include undermining and tunnelling. The depth of a Category/Stage IV pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and these ulcers can be shallow. Category/Stage IV ulcers can extend into muscle and/or supporting structures (e.g., fascia, tendon or joint capsule) making osteomyelitis possible. Exposed bone/tendon is visible or directly palpable. The depth of a Category/Stage IV pressure ulcer varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and these ulcers can be shallow. Category/Stage IV ulcers can extend into muscle and/or supporting structures (e.g., fascia, tendon or joint capsule) making osteomyelitis possible. Exposed bone/tendon is visible or directly palpable. | Stage IV | Full thickness skin loss with extensive destruction, tissue necrosis or damage to muscle, bone, or supporting structures (for example, tendon or joint capsule). Note: undermining and sinus tracts may also be associated with Stage IV pressure ulcers. | Stage 4 | Full‐thickness skin loss with extensive destruction and tissue necrosis extending to underlying bone, tendon or joint capsule 4.1 Visible exposure of bone, tendon or capsule 4.2 Sinus assesses as extending to bone, tendon or capsule. |
Stage 4 | Ulceration extends into the subcutaneous fat. Small‐vessel thrombosis and infection compound fat necrosis. Underlying muscle is swollen and inflamed, and undergoes pathological changes. The relative avascular deep fascia temporarily impedes downward progress of the damage but promotes lateral extension, causing undermining of the skins. Epidermal thickening creates a distinct ulcer margin but inflammation, fibrosis and retraction distort the deeper areas of the sore. |
| Unstageable: Depth Unknown | Full thickness tissue loss in which the base of the ulcer is covered by slough (yellow, tan, grey, green or brown) and/or eschar (tan, brown or black) in the wound bed. Until enough slough and/or eschar is removed to expose the base of the wound, the true depth, and therefore Category/Stage, cannot be determined. Stable (dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as ‘the body’s natural (biological) cover’ and should not be removed. | Unstageable | When eschar is present, accurate staging of the pressure ulcer is not possible until the eschar has sloughed or the wound has been debrided. | Stage 5 | Infective necrosis penetrates the deep fascia, and muscle destruction progresses rapidly. The wound spreads along the fascial planes and bursae, and may even reach the joints and body cavities. Osteomyelitis can easily develop. Multiple pressure ulcers may join, resulting in massive areas of tissue destruction. | ||
| Suspected Deep Tissue Injury: Depth Unknown | Purple or maroon localized area of discoloured intact skin or blood‐filled blister due to damage of underlying soft tissue from pressure and/or shear. The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer or cooler as compared to adjacent tissue. Deep tissue injury may be difficult to detect in individuals with dark skin tones. Evolution may include a thin blister over a dark wound bed. The wound may further evolve and become covered by thin eschar. Evolution may be rapid exposing additional layers of tissue even with optimal treatment. | ||||||
Types of interventions
The primary intervention under investigation is the use of any foam wound dressing for treating Category/Stage II pressure ulcers or above. We included any trial in which the presence or absence of a foam dressing was the only systematic difference between treatment groups. We anticipated that comparisons would include the following:
different types of foam dressings compared with each other;
foam dressings compared with other dressings or active treatments, or both, and;
foam dressings compared with no dressing treatment.
Types of outcome measures
For clarity we present data for short‐term follow‐up (8 weeks or less); medium follow‐up (24 weeks or less) and long‐term follow‐up (more than 24 weeks). This change is noted in Differences between protocol and review.
Primary outcomes
Incidence of healed pressure ulcers (proportion of participants in whom a pressure ulcer healed)
Time to complete healing
Adverse events per participant (such as wound or systematic infection, or both, or increase in ulcer size and severity)
Secondary outcomes
Reduction in ulcer size
Quality of life (measured using any validated tool)
Patient satisfaction/acceptability measured using any validated tool
Pressure ulcer recurrence (Category/Stage II or above)
Pain (associated with a pressure ulcer or dressing removal, or both, measured by any validated tool)
Economic outcomes
Cost (including but not limited to: costs of dressings; costs of related nursing or other health practitioner time or consultations; treatment costs per participant per pressure ulcer; costs to treat adverse events, infections or complications associated with the pressure ulcer; duration or costs of hospital stay for pressure ulcer wound healing, adverse events and complications; indirect costs to society associated with pressure ulcer such as lost productivity)
Utility scores representing health‐related quality of life
Incremental cost per event such as per additional pressure ulcer healed; incremental cost per life year gained; incremental cost per quality‐adjusted life year (QALY); net health or monetary benefit)
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials and economic studies:
the Cochrane Wounds Specialised Register (searched 27 February 2017);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library (searched 27 February 2017);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 27 February 2017);
Ovid Embase (1974 to 27 February 2017);
EBSCO CINAHL Plus (1937 to 27 February 2017);
the NHS Economic Evaluation Database (NHS EED) in the Cochrane Library (searched 27 February 2017).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, NHS EED, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL Plus searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2017). To identify economic studies, we combined Ovid MEDLINE, Ovid Embase, and EBSCO CINAHL Plus searches with filters developed by the Centre for Reviews and Dissemination (CRD 2017). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
ClinicalTrials.gov (searched 3 March 2017);
World Health Organization (WHO) International Clinical Trials Registry Platform (searched 3 March 2017);
EU Clinical Trials Register (searched 3 March 2017).
Search strategies for clinical trials registries can be found in Appendix 1.
Searching other resources
Searching reference lists of included trials and relevant reviews
We aimed to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses and Health Technology Assessment reports.
Contacts
We attempted to contact authors of papers and abstracts that were identified as having omissions of reported data, to request further information about their trials. However given that eight of the nine studies were published nine to 23 years ago, we had limited success making contact with authors, or where contact was made, authors were unable to access original data.
Adverse effects
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
We carried out data collection and analysis using Review Manager 5 (RevMan 5) (RevMan 2014) according to methods pre‐specified in a published protocol (Walker 2014).
Selection of studies
Two review authors independently assessed the titles and abstracts of all citations retrieved by the search for relevance against the inclusion criteria. After this initial assessment, we retrieved full‐text versions of all studies considered to be potentially eligible. The same two authors then independently assessed the full papers for eligibility and disagreement between review authors was resolved through discussion and, when required, via input by a third independent review author (Higgins 2011a). When the eligibility of a study was unclear, we attempted to contact study authors to request clarification. We recorded all the reasons for exclusion of studies we obtained as full copies, and completed a PRISMA flow chart to summarise this process (Liberati 2009). We also attempted to obtain all relevant publications when studies had reported more than once.
Data extraction and management
We extracted and summarised details from eligible studies using a pre‐designed data extraction sheet. Two review authors extracted data independently and then performed a cross‐check for accuracy and agreement. Any disagreements were resolved though discussion and arbitration by a third review author when necessary. Where studies were reported multiple times, we obtained all publications to ensure that we extracted the maximum amount of relevant data and included the study once in the review. When we included a study with more than two intervention arms, we extracted data only from the intervention and control groups as per the eligibility criteria. If there were any data missing from the papers, we attempted to contact study authors to retrieve the missing information.
Where possible, we extracted the following data from those trial arms relevant to the review:
country of origin;
type/Category/Stage of pressure ulcer;
location of pressure ulcer;
unit of investigation (per participant) ‐ single injury versus multiple injuries per participant;
care setting;
eligibility criteria and key baseline participant data;
number of participants randomised to each trial arm;
details of the dressing treatment/regimen received by each group;
details of any co‐interventions;
primary and secondary outcome(s) with definitions;
outcome data for primary and secondary outcomes (by group);
duration of follow‐up;
number of withdrawals (by group); and,
source of funding.
We extracted the following data from economic studies relevant to the review:
estimates of specific items of resource use per participant;
estimates of unit costs (extracted separately to resource use);
price year and currency;
decision making jurisdiction;
analytic perspective;
both a point estimate and a measure of uncertainty (e.g. standard error or confidence interval) for measures of incremental resource use, costs and cost effectiveness, if reported; and
details of any sensitivity analyses undertaken, and any information regarding the impact of varying assumptions on the magnitude and direction of results.
Assessment of risk of bias in included studies
Two review authors independently assessed included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2011b). The tool addresses six specific domains (refer to Appendix 2), namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues that may potentially bias the study (Higgins 2011b). We linked Cochrane 'Risk of bias' ratings to the GRADE assessment using an adaptation by Guyatt 2011 to define the four 'Risk of bias' ratings (Westby 2017):
very high ‐ two or more key domains with a high risk of bias or a single domain with very high levels of uncertainty (e.g. very high degree of differential missing data);
high ‐ high risk of bias for any one domain or we judged the risk of bias to be ’almost high’ across more than one domain;
low ‐ low risk of bias for each of the key domains;
unclear ‐ insufficient information for at least one key domain (with the other domains being at low risk of bias).
As we only included RCTs and cluster‐RCTs in this review, our GRADE ratings started at 'high' (according to the GRADE quality rating system of high, moderate, low, very low). However we downgraded studies according to five factors: 1) limitations in the design and implementation suggesting the high likelihood of bias; 2) indirectness of evidence (indirect population, intervention, control, outcomes); 3) unexplained heterogeneity or inconsistency of results; 4) imprecision of results; 5) high probability of bias (Schünemann 2011a). Explanations for our GRADE assessment decisions are presented in the footnotes to the 'Summary of findings' tables.
We completed a 'Risk of bias' table for each included study, and conducted a separate assessment for each outcome. We have presented 'Risk of bias' assessment using two 'Risk of bias' summary figures: one that provides a summary of bias for each item across all studies and another that provides a cross‐tabulation of each trial for all risk of bias items. For economic evaluations, we used the Consolidate Health Economic Evaluation Reporting Standards (CHEERS) checklist to assess the methodological quality of full and partial economic evaluations (Husereau 2013).
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratio (RR) with 95% confidence intervals (CI). For continuous outcomes, we used the mean difference (MD) with 95% CIs for trials that used the same assessment scale. When trials used different assessment scales, we planned to use the standardised mean difference (SMD) with 95% CIs. Time‐to‐event data (e.g. time‐to‐healing) were intended to be reported as hazard ratio (HR) when possible, in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If studies reporting time‐to‐event data (e.g. time to healing) did not report a hazard ratio then, when feasible, we planned to estimate this using other reported outcomes, such as numbers of events via an appropriate statistical method (Tierney 2007).
Review of economic evaluations
We planned to present a tabulated analysis of the identified economic data in accordance with advice outlined in the CHEERS checklist (Husereau 2013). However, limited data made it impractical to do so. Instead we have presented a narrative description of the economic data.
For any included studies, given the likely lack of direct comparability in resource use and cost data between different healthcare contexts and settings, we did not intend to pool economic outcomes. Rather we planned to incorporate a discussion of key drivers and impact of assumptions on the cost‐effectiveness of foam dressings, scenarios that are likely to lead to the most and least cost‐effective use of foam dressings, as well as guidance on future research that might be required to assess the economic value of foam dressings as an intervention for pressure ulcer treatment.
Costs
We planned to report resource utilisation and unit costs separately, along with the currency and price year in each original study. We would then convert these costs to current values by employing a web‐based conversion tool that applies implicit price deflators for gross domestic product (GDP) of that currency and then converts into the currency most frequently observed in the articles reviewed using GDP Purchasing Power Parities (Shemilt 2011). This would allow readers of the review to make meaningful comparisons between costs in studies that may have been conducted in different countries and at different times. However, given that only three studies reported costs for different components of pressure ulcer treatment, across different comparisons, we did not consider it appropriate to convert costs to a common currency and year.
The main costs were likely to be those associated with the development of pressure ulcers and their treatment (e.g. dressings), nursing time for dressing changes, specialist and other practitioner costs as measured by time or number of visits, potential cost‐savings from a reduced length of stay in hospital, and costs stemming from differing rates of adverse events and complications (including procedures initiated due to the failure of wounds to heal, such as amputation). We planned to identify the key cost drivers from the studies included to enable users of the review to gain a clear understanding of the nature of resource use associated with foam dressing for pressure ulcer treatment.
Health state utility scores
We planned to examine information on the change in health‐related quality of life reported by the included trials via utilities measured by a multi‐attribute utility instrument (MAUI) or other approaches (such as the time trade‐off, standard gamble).
Unit of analysis issues
In most of the studies included in our review, the participant was the unit of analysis, taking into account the level at which randomisation occurred. For parallel‐group designs, we analysed a single measure for each outcome for each person participating, thereby avoiding ’unit‐of‐analysis’ errors that can result in a false positive conclusion that the intervention had an effect (Deeks 2011). For cluster‐RCTs (e.g. where outcome data were presented for multiple ulcers per participant) we had planned to adjust sample size based on methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011); that is, where possible, use an estimate of intra‐cluster correlation coefficient (ICC) derived from the trial, or calculate the design effect using the formula: DE = 1 (M‐1) x ICC (Deeks 2011). However, in the one study with clustered data, we did not do this due to the small amount of data, the age of the study and resulting lack of detail about the data. Instead we matched the number of observations with the number of 'units' (i.e. participants) randomised, and reflected the lack of independence in the study via the risk of bias and GRADE assessment.
Dealing with missing data
We considered it likely that studies included in our review would have missing data, which would increase the possibility of bias. Where there was evidence of missing data, we attempted to contact study authors to request the missing information. In cases where this approach was unsuccessful, we assumed that missing data were due to loss of follow‐up (missing at random) and analysed the available information. If we considered that data were not missing at random, we planned to either impute missing data, acknowledging that these were imputed with uncertainty or to use statistical models to allow for missing data by making assumptions about their relationship with the available data (Deeks 2011), or adopt both process (we did not use these options in the review). We considered intention‐to‐treat (ITT) analysis (keeping participants in the intervention groups to which they were randomised, regardless of the intervention they actually received) where some randomised participants were excluded from the analysis. Where we assessed ITT analysis as inappropriate (in cases of unintended/adverse events), we considered available case analysis (Deeks 2011). We planned to perform sensitivity analyses to assess how robust the results were to reasonable changes in the assumptions that we made. We have addressed the impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
Based on previous reviews of the literature, there was an expectation that included studies would have considerable heterogeneity due to clinical variation (differences in participants, interventions and outcomes), and methodological diversity related to design and risk of bias difference (Deeks 2011), which resulted in statistical heterogeneity (Higgins 2003). Therefore, we attempted to identify potential sources of clinical, methodological and statistical heterogeneity prior to meta‐analysis. We analysed studies of each intervention and presented data separately. If studies were sufficiently homogeneous, we pooled data using meta‐analysis with RevMan 5 (RevMan 2014). We used the Chi2 test to quantify our assessment of statistical heterogeneity, with significance being set at P value less than 0.10 and the I2 measure. We did not pool studies with high returned values ‐ classed as when I2 exceeded 75% (Deeks 2011). Where there were sufficiently similar studies to consider pooling, we used a fixed‐effect model to quantify an estimate of low to moderate levels of heterogeneity (I2 0% to 50%). We planned to use a random‐effects model in the absence of clinical heterogeneity and in the presence of statistical heterogeneity (I2 > 50%), However this was not possible due to the high degree of clinical variation.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Sterne 2011). Publication bias is one example of a number of possible 'small study effects', such as a tendency to over‐estimate the effect of interventions in smaller RCTs. We planned to explore reporting bias using funnel plots. A funnel plot is a simple scatter plot that enables a visual assessment of intervention effect estimates from individual RCTs against some measure of each trial's size or precision (Sterne 2011). We had planned to present funnel plots if at least 10 studies were available for the meta‐analysis, however this situation did not arise.
Data synthesis
We described included studies in a structured narrative summary based upon comparators.
We entered quantitative data into RevMan 5 (RevMan 2014), and analysed the data using the RevMan 5 analysis software. For dichotomous outcomes, we calculated RR plus 95% CI. For continuous outcomes, we intended to calculate SMD and MD plus 95% CI. For time‐to‐event outcomes we planned to calculate pooled HR with 95% CI. The decision to pool data in a meta‐analysis was dependent upon the availability of outcome data and assessment of between‐trial heterogeneity. We explored the robustness of meta‐analyses using appropriate meta‐analytical models ‐ such as fixed‐effect or random‐effects models, based on the level of heterogeneity as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
'Summary of findings' tables
We have presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011b). The 'Summary of findings' tables also include an overall grading of the body of evidence related to each of the main outcomes using the GRADE approach (Schünemann 2011a). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. As this review is part of a suite of Cochrane Reviews investigating the use of a wide range of dressing types for the treatment of pressure ulcers, reviewed studies here include those from a select range of advanced wound dressing types. Based on the characteristics of included studies, the 'Summary of Findings' tables have been organised according to the following comparisons with each table comprising results from several individual studies:
different types of foam dressings compared with each other;
foam dressings compared with hydrocolloid dressings;
foam dressings compared with hydrogel dressings;
foam dressings compared with basic wound contact dressings.
We have presented data on the following outcomes:
incidence of healed pressure ulcers (proportion of participants in whom a pressure ulcer healed);
time to complete healing;
adverse events per patient (such as wound or systematic infection, or both, or increase in ulcer size and severity;
quality of life.
Subgroup analysis and investigation of heterogeneity
We had planned, if data allowed, to undertake the following subgroup analysis: type of setting (community, hospital, inpatient, outpatient) however this was not possible and we have not presented any subgroup analyses.
Sensitivity analysis
When possible we planned to perform sensitivity analysis to explore the influence of risk of bias on clinical, methodological and statistical heterogeneity (Deeks 2011). As a result of this process, we planned to exclude those studies assessed as having high risk of bias from meta analysis and consider the effects of those studies at unclear risk or low risk of bias. We considered studies as having overall low risk of bias if they had low risk of bias in all key domains, namely adequate generation of the randomisation sequence, adequate allocation concealment and blinding of outcome assessor for the estimates of treatment effect. We did not conduct this analysis.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies
Results of the search
The search generated 1352 records (registers were checked on 27 February 2017). In total, we excluded 1326 studies and assessed 26 as full text for eligibility. See Figure 1. Of these, we included nine studies and excluded 16, as per our a priori objectives reported in the protocol for this review (Walker 2014).
1.
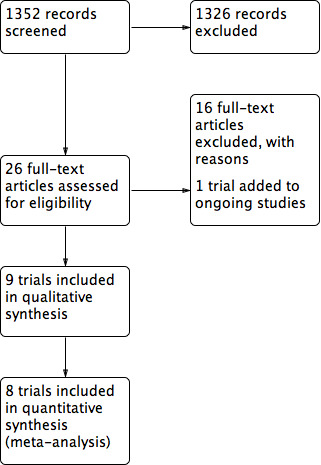
Figure 1: Study flow diagram
We only identified one trial as a relevant ongoing study (ISRCTN57842461); however results from that study had not been published at the time of this review. Refer to Characteristics of ongoing studies for more details about this trial. We located no new studies by searching reference lists, as any relevant studies had been identified in the electronic searching.
Included studies
Study design and setting
Nine studies met the inclusion criteria for this review (refer to Characteristics of included studies), although only eight were suitable for meta‐analyses (Bale 1997; Banks 1994a; Meaume 2003; Payne 2009; Seeley 1999; Sopata 2002; Souliotis 2016; Thomas 1997). One study (Bale 1998) used multiple subgroup analyses for which results may have been misleading (Deeks 2011). Therefore we did not include Bale 1998 in the meta‐analyses but considered it important for the narrative description. Apart from Bale 1998, the included studies were randomised controlled trials (RCTs) with two arms, for a total of 483 participants. Health settings comprised community, aged and palliative‐care facilities. Six included studies used an intention‐to‐treat (ITT) approach (Polit 2010), where there was limited or no participant loss following randomisation (Bale 1998; Meaume 2003; Payne 2009; Seeley 1999; Sopata 2002; Thomas 1997). The remaining studies (Banks 1994a; Souliotis 2016), used a per‐protocol approach, which potentially contributed to bias in their studies (Polit 2010). We made attempts to contact study authors to request additional information about missing data but no further information was received.
Participants
Participants from included trials were recruited from:
five centres (not specified) in the UK (Bale 1997);
the community in the UK (Bale 1998; Thomas 1997), and Greece (Souliotis 2016);
aged care facilities in Belgium, France and Italy (Meaume 2003);
a palliative care unit in Poland (Sopata 2002);
a combination of community, aged care and palliative settings in the UK and USA (Banks 1994a; Payne 2009; Seeley 1999).
The mean age of participants in eight trials was ≥ 73 years (Bale 1997; Bale 1998; Banks 1994a; Meaume 2003; Payne 2009; Seeley 1999; Souliotis 2016; Thomas 1997). However the mean age of participants was 59 years in Sopata 2002.
All included trials apart from Payne 2009 and Souliotis 2016 had more female participants than male (Bale 1997; Bale 1998; Banks 1994a; Meaume 2003; Seeley 1999; Sopata 2002; Thomas 1997).
The most commonly reported locations for pressure ulcer were the sacrum (Bale 1997; Banks 1994a; Meaume 2003; Payne 2009; Seeley 1999; Souliotis 2016; Thomas 1997), hips and buttocks (Payne 2009; Souliotis 2016; Thomas 1997), heel and ankle (Meaume 2003; Seeley 1999; Souliotis 2016; Thomas 1997). Location of pressure ulcer was not reported by Bale 1998 or Sopata 2002.
Interventions
We considered all types of dressing that were manufactured using foam as 'foam dressings'. Within the included studies these consisted of hydrocellular foam (Bale 1998; Seeley 1999); hydropolymer foam (Thomas 1997; Meaume 2003); polyurethane foam (Bale 1997; Banks 1994a; Payne 2009; Sopata 2002); silicone foam (Meaume 2003); as well as foam dressings with anti‐microbial (silver and silver‐sulfadiazine), and analgesic (ibuprofen) properties (Souliotis 2016). See Summary of outcomes, Table 7.
3. Summary of outcomes.
| Study | Intervention | Comparator | Follow‐up (weeks) | Incidence of healed PU |
Time to complete healing |
Adverse events |
Reduction in ulcer size |
Quality of life | Patient satisfaction | PU recurrence | Pain | Economic |
| Bale 1997 | Polyurethane foam (n = 29) | Hydrocolloid (n = 31) | 4 | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Bale 1998 | Hydrocellular foam (n = 17) | Hydrocolloid (n = 15) | 8 | ✗ | ✗ | ✓ Data not separated by wound type | ✓ Data not separated by wound type | ✗ | ✓ | ✗ | ✗ | ✓ |
| Banks 1994a | Polyurethane foam (n = 26) | Knitted viscous secured with a vapour‐permeable film dressing (n = 24) | 12 | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Meaume 2003 | Silicone polyurethane foam (n = 18) | Hydropolymer foam (n = 20) | 8 | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Payne 2009 | Polyurethane foam (n = 20) | Saline‐soaked gauze (n = 16) | 4 | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ |
| Seeley 1999 | Hydrocellular foam (n = 20) | Hydrocolloid (n = 19) | 8 | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ |
| Sopata 2002 | Polyurethane foam (n = 17) | Hydrogel (n = 17) | 8 | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Souliotis 2016 | Foam dressings, foam with silver, silver‐sulfadiazine and ibuprofen (n = 47) | Plain and saline‐soaked gauze (n = 48) | Until complete healing (less than 24 weeks) | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
| Thomas 1997 | Hydropolymer (n = 50) | Hydrocolloid (n = 49) | 6 | ✓ | ✗ | ✓ | ✓ Data not separated by wound type | ✗ | ✗ | ✗ | ✓ | ✗ |
We considered foam dressings as a single group where possible. Four studies compared a foam dressing with a hydrocolloid dressing (Bale 1997; Bale 1998; Seeley 1999; Thomas 1997), three compared foam dressing(s) with basic wound contact dressing (Banks 1994a; Payne 2009; Souliotis 2016), one compared a foam dressing with a hydrogel dressing (Sopata 2002) and one study compared two different types of foam dressing (Meaume 2003).
Outcomes
A summary of reported outcomes relevant to the review is reported in Table 7.
The primary outcome, incidence of healed pressure ulcer was the most frequently reported (Bale 1997; Banks 1994a; Meaume 2003; Payne 2009; Seeley 1999; Sopata 2002; Thomas 1997), followed by adverse events (Bale 1997; Meaume 2003; Seeley 1999; Sopata 2002; Souliotis 2016; Thomas 1997), and time to complete healing (Sopata 2002; Souliotis 2016). For secondary outcomes five trials reported reduction in ulcer size (Bale 1998Meaume 2003; Payne 2009; Sopata 2002; Thomas 1997), two reported patient satisfaction (Bale 1998; Banks 1994a), and pain (Banks 1994a; Seeley 1999). None of the included studies reported outcomes for quality of life or pressure ulcer recurrence. Economic outcomes were reported in three trials (Bale 1998; Payne 2009; Souliotis 2016).
Excluded studies
In total we excluded 16 studies from the review for the following reasons (refer to Characteristics of excluded studies).
Where there was uncertainty about the classification system used in studies, following contact or attempted contact with the study authors, we deemed this a potential source of bias and did not consider their inclusion. Four studies did not report the classification system used to assess pressure ulcers (Banks 1994b; Banks 1994c; Banks 1997; Reynolds 2004) and we were unable to access the original data to clarify the classification used (Banks 1994b; Banks 1994c; Banks 1997), or contact the study author (Reynolds 2004).
Four studies were not RCTs or cluster‐RCTs (Ashby 2012; Diehm 2005; Oleske 1986; Parish 2008).
Two studies did not report subgroup analyses for participants with pressure ulcers in study arms comprising mixed dressings (Münter 2006; Palao i Domenech 2008), and we were unable to access original data (Münter 2006), or contact the study authors (Palao i Domenech 2008).
One study did not investigate or report a priori objectives identified in the protocol for this review; that is, wound exudate was the primary interest of the study and not the effectiveness of the foam dressing in treating pressure ulcers (Piatkowski 2012).
One pilot study was an RCT; however dressing choice in the control group was based upon health professional and participant choice (of which foam dressings were one option) rather than randomisation (Ashby 2012).
One study manuscript was incomplete and we could not access it (Avanzi 2000).
One study compared an intervention dressing comprising hydrogel and foam layers with a hydrocolloid dressing. We excluded the study as the hydrogel layer was closest to the skin, and the foam was an outer layer that provided cushioning (Brown‐Etris 1996).
One study compared two foams for the treatment of pressure ulcers, however their application occurred as a component of negative pressure wound therapy following surgical debridement rather than as a wound dressing (Wagstaff 2014).
One study included participants with neuropathic foot ulcers, not pressure ulcers (Zimny 2003).
Risk of bias in included studies
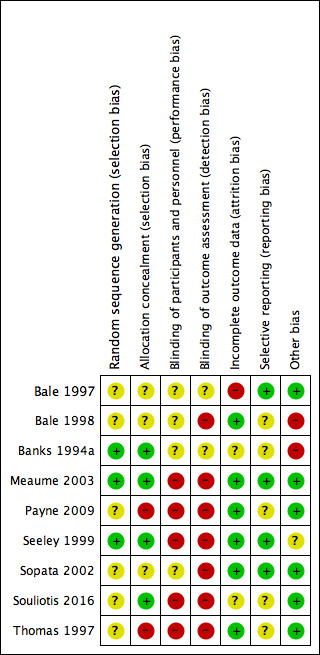
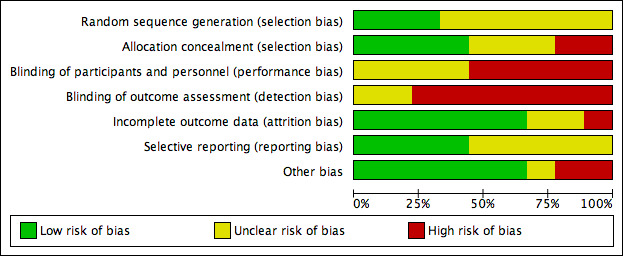
Risk of bias was an important consideration when assessing the quality of evidence reported in trials evaluated for this review as reported in the 'Risk of bias' summary (Figure 2) and 'Risk of bias' graph (Figure 3). We have outlined our 'Risk of bias' judgements in the Characteristics of included studies. Eight of the nine included studies were at high risk of bias for one or more domains. Overall, the quality of reporting was limited due to lack of clarity and detail as outlined below.
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.
Allocation
We assessed three trials as being low risk of bias for random sequence generation (Banks 1994a; Meaume 2003; Seeley 1999), with appropriate use of computer‐generated randomisation lists. The remaining six studies did not provide enough information about the generation of a randomising sequence (Bale 1997; Bale 1998; Payne 2009; Sopata 2002; Souliotis 2016; Thomas 1997). We judged only Banks 1994a, Meaume 2003, Seeley 1999 and Souliotis 2016 to have a low risk of bias for allocation concealment. The remaining trials we assessed as having unclear risk of bias (Bale 1997; Bale 1998; Sopata 2002) or high risk of bias (Payne 2009; Thomas 1997).
Blinding
While it is difficult to blind participants and personnel in studies where there was a physical evidence of treatment allocation, there was no indication of blind‐to‐intervention assessment. As such, we did not assess any trials as being at low risk of bias for blinding of participants, personnel or outcome assessment of reported outcomes relevant to this review. We assessed five trials as having a high risk of bias for blinding of personnel (Meaume 2003; Payne 2009; Seeley 1999; Souliotis 2016; Thomas 1997), and seven trials as being high risk of bias for blinding of outcome assessment (Bale 1998; Meaume 2003; Payne 2009; Seeley 1999; Sopata 2002; Souliotis 2016; Thomas 1997). The bias aspect of the remaining studies we considered to be unclear (Bale 1997; Banks 1994a).
Incomplete outcome data
We assessed six studies as being at low risk of attrition bias (Bale 1998; Meaume 2003; Payne 2009; Seeley 1999; Sopata 2002; Thomas 1997) as they used an ITT approach (Polit 2010) where there was no participant loss following randomisation or missing data were unlikely to be related to the true outcome (Higgins 2011b). We judged other studies to have an unclear risk of bias, as they used a per‐protocol approach, which potentially contributed to bias (Souliotis 2016), or reported incomplete outcome data with insufficient descriptions for follow‐up and comparator data (Banks 1994a). We assessed one trial (Bale 1997) as being at high risk of bias for incomplete outcome data, with a significant loss of 67% of participants from the study. The study did not report the number of participants who required a dressing change due to discomfort and provided little detail regarding time to complete wound healing.
Selective reporting
We judged four trials to be at low risk of bias for selective reporting (Bale 1997; Meaume 2003; Seeley 1999; Sopata 2002). The remaining studies we considered to be at unclear risk of reporting bias.
Other potential sources of bias
Other potential sources of bias included: acknowledgment that dressing wearing time was not a true reflection of the average (unclear risk of bias) (Seeley 1999); and inequality of wound sizes between groups (high risk of bias) (Bale 1998; Banks 1994a). Indeed Bale 1998 undertook subgroup analyses in a subset of the trial population, hence results may be misleading as they were not based on randomised comparisons (Deeks 2011).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Hydropolymer foam dressing compared with silicone foam dressing for treating pressure ulcers.
| Hydropolymer foam dressing compared with silicone foam dressing for treating pressure ulcers | ||||||
| Patient or population: people of any age with an existing pressure ulcer of Category/Stage II or above Setting: any care setting Intervention: silicone foam dressing Comparison: hydropolymer foam dressing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with hydropolymer foam dressing | Risk with silicone foam dressings | |||||
| Incidence of healed pressure ulcers, short‐term follow‐up (8 weeks or less) | 500 per 1000 | 445 per 1000 (225 to 875) | RR 0.89 (0.45 to 1.75) | 38 (1 RCT) | ⊕⊝⊝⊝ very low1 | |
| Time to complete healing | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison | |
| Adverse events, short‐term follow‐up (8 weeks or less) | 150 per 1000 | 56 per 1000 (6 to 488) | RR 0.37 (0.04 to 3.25) | 38 (1 RCT) | ⊕⊝⊝⊝ very low2 | |
| Quality of life | Not estimable | Not estimable | n/a | n/a | Outcome not measured or reported for this comparison | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Majority of evidence at high risk of bias due to limitations in design and implementation due to lack of blinding (downgraded once); very serious imprecision of results due to small sample size and wide confidence intervals (0.45 to 1.75) (downgraded twice). 2Majority of evidence at high risk of bias due to limitations in design and implementation (downgraded once); very serious imprecision of results due to low number of events and wide confidence intervals (0.04 to 3.25) (downgraded twice).
Summary of findings 2. Foam dressings compared with hydrocolloid dressings for treating pressure ulcers.
| Foam dressings compared with hydrocolloid dressings for treating pressure ulcers | |||||||
|
Patient or population: people of any age with an existing pressure ulcer of Category/Stage II or above Settings: any care setting Intervention: hydrocellular, hydropolymer and polyurethane foam dressings Comparison: hydrocolloid dressing | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Heterogeneity | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with hydrocolloid dressing | Risk with foam dressing | ||||||
| Incidence of healing, short‐term follow‐up (8 weeks or less) | 293 per 1000 | 249 per 1000 ( 158 to 393 ) | RR 0.85 (0.54 to 1.34) |
Chi2 = 2.12, df = 2, (P = 0.35), I2 = 6% | 198 (3 RCTs) | ⊕⊝⊝⊝ very low1 | |
| Time to complete healing | Outcome not measured or reported for this comparison | Outcome not measured or reported for this comparison | n/a | ‐ | n/a | Outcome not measured or reported for this comparison | |
| Adverse events, short‐term follow‐up (8 weeks or less) | 91 per 1000 | 81 per 1000 (34 to 192) |
RR 0.88 (0.37 to 2.11) |
Chi2 = 0.82, df = 2, (P = 0.66), I2 = 0.0% | 198 (3 RCTs) |
⊕⊝⊝⊝ very low2 | A fourth RCT reported adverse events. However these data were not separated by wound type. |
| Quality of life | Outcome not measured or reported for this comparison | Outcome not measured or reported for this comparison | n/a | ‐ | n/a | Outcome not measured or reported for this comparison | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||||||
1Majority of evidence at very high risk of bias due to limitations in design and implementation due to lack of blinding and allocation concealment (downgraded twice); serious imprecision of results due to small sample size and wide confidence intervals (0.54 to 1.34) (downgraded once). 2Majority of evidence at high risk of bias due to limitations in design and implementation due to lack of blinding and allocation concealment (downgraded once); very serious imprecision of results due small sample size and wide confidence intervals (0.37 to 2.11) (downgraded twice).
Summary of findings 3. Foam dressing compared with hydrogel dressing for treating pressure ulcers.
| Foam dressing compared with hydrogel dressing for treating pressure ulcers | ||||||
|
Patient or population: people of any age with an existing pressure ulcer of Category/Stage II or above Settings: any care setting Intervention: polyurethane foam dressing Comparison: hydrogel dressing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with hydrogel dressing | Risk with foam dressing | |||||
| Incidence of healing, short‐term follow‐up (8 weeks or less) | 882 per 1000 | 882 per 1000 (159 to 1129) | RR 1.00 (0.78 to 1.28) | 34 (1 RCT) | ⊕⊝⊝⊝ very low1 | |
| Time to complete healing | Treatment time reported in days reported. The medium time was 20.10 days (for 20 wounds) | Treatment time reported in days reported. The medium time was 5.67 days more days (4.03 to 15.37 days more, for 18 wounds) | n/a | 34 (1 RCT) | ⊕⊝⊝⊝ very low2 |
|
|
Adverse events, short‐term follow‐up (8 weeks or less) |
59 per 1000 | 20 per 1000 (1 to 450) | RR 0.33 (0.01 to 7.65) | 34 (1 RCT) | ⊕⊝⊝⊝ very low3 | |
| Quality of life | Outcome not measured or reported for this comparison | Outcome not measured or reported for this comparison | n/a | n/a | Outcome not measured or reported for this comparison | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Majority of evidence at high risk of bias due to limitations in design and implementation due to lack of blinding (downgraded once); very serious imprecision of results due to small sample size and wide confidence intervals (0.78 to 1.28) (downgraded twice). 2Majority of evidence at high risk of bias due to limitations in design and implementation due to lack of blinding (downgraded once); very serious imprecision of results due to small sample size, wide confidence intervals (‐4.03 to ‐15.37) (downgraded twice).
3Majority of evidence at high risk of bias due to limitations in design and implementation due to lack of blinding (downgraded once); very serious imprecision of results due small sample size and wide confidence intervals (0.0.1 to 7.65) (downgraded twice).
Summary of findings 4. Foam dressings compared with basic contact dressings for treating pressure ulcers.
| Foam dressings compared with basic contact dressings for treating pressure ulcers | |||||||
|
Patient or population: people of any age with an existing pressure ulcer Category/Stage II or above Settings: any care setting Intervention: polyurethane, silver and ibuprofen‐releasing foam dressings Comparison: basic contact dressings (gauze, saline‐soaked gauze, low‐adherence dressing secured by a vapour‐permeable film) | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Subgroup differences | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with basic contact dressings | Risk with foam dressing | ||||||
| Incidence of healing, short‐term follow‐up (8 weeks of less) | 375 per 1000 | 500 per 1000 (233 to 1080) | RR 1.33 (0.62 to 2.88) | Chi2 = 0.09, df = 1, (P = 0.77), I2 = 0.0% | 36 (1 RCT) | ⊕⊝⊝⊝ very low1 | |
| Incidence of healing, medium‐term follow‐up (8 to 24 weeks) | 625 per 1000 | 731 per 1000 (494 to 1075) | RR 1.17 (0.79 to 1.72) | 50 (1 RCT) | ⊕⊝⊝⊝ very low2 | ||
| Time to complete healing (days) medium‐term follow‐up (8 to 24 weeks) | The mean time to complete healing (days) was 121.4 days | The mean time to complete healing with foam dressing was 35.80 days less (56.77 to 14.83 less) | 95 (1 RCT) | ⊕⊝⊝⊝ very low3 |
|||
| Adverse events, medium‐term follow‐up (8 to 24 weeks) | 438 per 1000 | 254 per 1000 (145 to 460) | RR 0.58 (0.33 to 1.05) | 95 (1 RCT) | ⊕⊕⊝⊝ low4 | ||
| Quality of life | Outcome not measured or reported for this comparison | Outcome not measured or reported for this comparison | n/a | n/a | Outcome not measured or reported for this comparison | ||
| Incremental cost per event, short‐term follow‐up (8 weeks or less) | Per patient cost USD 781 | Per patient cost USD 315 | n/a | 36 (1 RCT) | ⊕⊝⊝⊝ very low5 | Cost difference between intervention and comparator dressings = USD 466. Treatment cost data for intervention and comparator dressings, other materials and nurse time based on national standard costs in the USA in mid‐2007 and hourly wages for nurses based on 2006 rates |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||||||
1Majority of evidence at high risk of bias due to limitations in design and implementation due to lack of blinding (downgraded once); very serious imprecision of results due to small sample size, wide confidence intervals and incomplete reporting (0.62 to 2.88) (downgraded twice) 2Majority of evidence at high risk of bias due to limitations in design and implementation due to lack of blinding (downgraded once); very serious imprecision of results due to small sample size, wide confidence intervals and incomplete reporting (0.79 to 1.72) (downgraded twice) 3Majority of evidence at high risk of bias due to limitations in design and implementation due to lack of blinding (downgraded twice); very serious imprecision of results due to small sample size, wide confidence intervals and incomplete reporting (‐56.77 to ‐14.835) (downgraded once). 4Majority of evidence at high risk of bias due to limitations in design and implementation due to lack of blinding (downgraded once); very serious imprecision of results due to small sample size, wide confidence levels and incomplete reporting (0.33 to 1.05) (downgraded once).
We have organised findings by comparison and a priori outcome measures as outlined above (Types of outcome measures).
The nine trials included 483 participants. The trials were small (median sample size = 20), and while there was some clinical and methodological heterogeneity, we undertook meta‐analysis where there was similarity between dressings (intervention foam versus comparator hydrocolloid), follow‐up periods and category/stages of pressure ulcer subgroups. Where there was no similarity, we summarised studies narratively.
Comparison 1: hydropolymer foam dressing compared with silicone foam dressing (1 trial; 38 participants)
Only one trial compared a foam dressing (hydropolymer foam) with another foam dressing (silicone foam) (Meaume 2003) with 8 weeks of follow‐up.
Primary outcomes
Incidence of healed pressure ulcers (short‐term follow‐up, 8 weeks or less)
It is uncertain whether alternative types of foam dressing affected the incidence of healed pressure ulcers over a short‐term follow‐up period: RR 0.89 (95% CI 0.45 to 1.75) (Analysis 1.1). The certainty of evidence was very low due to high risk of bias, downgraded once due to serious limitations in design and implementation (lack of blinding) and twice for very serious imprecision of results due small sample size and wide confidence intervals. See Table 1.
1.1. Analysis.
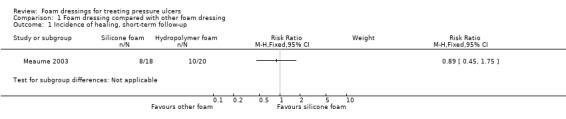
Comparison 1 Foam dressing compared with other foam dressing, Outcome 1 Incidence of healing, short‐term follow‐up.
Meaume 2003 did not report our primary outcome: time to complete healing
Adverse events (short‐term follow‐up, 8 weeks or less)
It is uncertain whether alternative types of foam dressing affected the risk of adverse events in people with pressure ulcers: RR 0.37 (95% CI 0.04 to 3.25) (Analysis 1.2). The certainty of evidence was very low due to high risk of bias, downgraded once due to serious limitations in design and implementation (lack of blinding) and twice for very serious imprecision of results due to low number of events and wide confidence intervals. See Table 1.
1.2. Analysis.
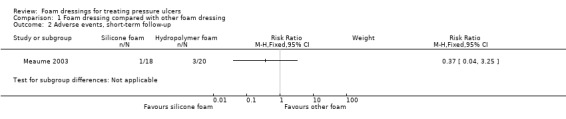
Comparison 1 Foam dressing compared with other foam dressing, Outcome 2 Adverse events, short‐term follow‐up.
Secondary outcomes
Reduction in ulcer size (short‐term follow‐up, 8 weeks or less)
Reduction of wound size was measured in cm2 from tracings of the each participant's wound at baseline and final assessment (Meaume 2003). Wounds dressed with the silicone foam dressing had a mean reduction in wound area of 3.1 cm2 compared with 3.3 cm2 in the hydropolymer foam dressing. No standard deviation or standard error data were reported and so could not be analysed further. We assessed the evidence as very low certainty due to high risk of bias, downgraded once due to imitations in design and implementation (lack of blinding) and twice for very serious imprecision of results due to small sample size and lack of reporting.
Meaume 2003 did not report the secondary outcomes quality of life, patient satisfaction/acceptability or pressure ulcer recurrence and pain, or the economic outcomes, cost, utility scores representing health‐related quality of life and incremental cost per event.
Comparison 2: foam* dressings compared with hydrocolloid dressings (4 trials; 230 participants)
*Hydrocellular, hydropolymer and polyurethane foam dressings
This comparison included four trials with 230 participants (Bale 1997; Bale 1998; Seeley 1999; Thomas 1997). All the studies had short‐term follow‐up (up to 8 weeks). It should be noted that Bale 1997 and Bale 1998 were both conducted in community settings that were not specifically described, and used the same dressings: hydrocolloid versus foam (described as a polyurethane foam dressing in Bale 1997 and a hydrocellular dressing in Bale 1998). However, reported sample sizes between the studies were different as was the focus. Bale 1997 focused on "ease of application, removal, adhesion, conformability, absorbency and wear time", whereas Bale 1998 compared the costs of dressing and "dressing durability, time to competed healing, ease of wound cleansing and dressing removal."
Primary outcomes
Incidence of healed pressure ulcers (short‐term follow‐up, 8 weeks or less)
Only three trials reported incidence of healed pressure ulcers in this comparison (Bale 1997; Seeley 1999; Thomas 1997), while Bale 1998 primarily reported costs associated with the dressings. Follow‐up times ranged from four weeks (Bale 1997), six weeks (Thomas 1997) and eight weeks (Seeley 1999). It is uncertain whether foam dressings affected the incidence of healed pressure ulcers compared with hydrocolloid dressings over a short‐term period: RR 0.85 (95% CI 0.54 to 1.34) (Analysis 2.1). We assessed this as very low‐certainty evidence, downgraded twice due to very serious limitations in design and implementation (lack of blinding and allocation concealment) and once for very serious imprecision of results due small sample size and wide confidence intervals. See Table 2.
2.1. Analysis.
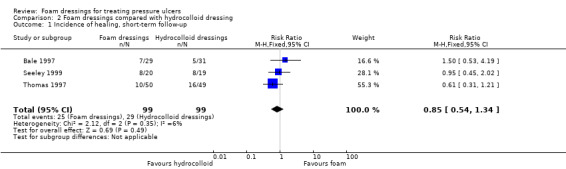
Comparison 2 Foam dressings compared with hydrocolloid dressing, Outcome 1 Incidence of healing, short‐term follow‐up.
None of the trials included in this comparison reported time to complete healing (Bale 1997; Seeley 1999; Thomas 1997)
Adverse events (short‐term follow‐up, 8 weeks of less)
Three studies reported dressing‐related adverse events (Bale 1997; Seeley 1999; Thomas 1997). It is uncertain whether foam dressings affected the risk of adverse events compared with hydrocolloid dressings RR 0.88 (95% CI 0.37 to 2.11) (Analysis 2.2). The certainty of evidence was very low due to high risk of bias, downgraded once due to limitations in design and implementation (lack of blinding and allocation concealment) and twice for very serious imprecision of results due small sample size and wide confidence intervals. See Table 2.
2.2. Analysis.
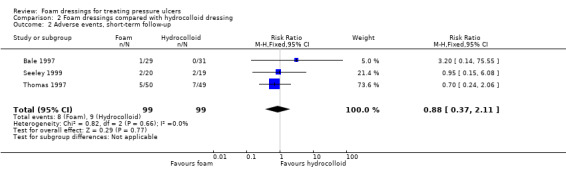
Comparison 2 Foam dressings compared with hydrocolloid dressing, Outcome 2 Adverse events, short‐term follow‐up.
Bale 1998 reported adverse events. However these data were not separated by wound type. It is uncertain whether foam dressings affected the risk of adverse events compared with hydrocolloid dressings because we assessed the quality of the evidence as being very low due to high risk of bias, and downgraded twice due to limitations in design and implementation (uncertain blinding and allocation concealment) and once for imprecision of results due to small sample size and incomplete reporting.
Secondary outcomes
Reduction in ulcer size (short‐term follow‐up, 8 weeks or less)
Two studies (Bale 1998; Thomas 1997) (n = 131) reported on reduction in ulcer size. However data were not separated by wound type in both studies preventing further analysis. It is uncertain whether foam dressings led to reduction in ulcer size compared to hydrocolloid dressings because the quality of the evidence was very low due to high risk of bias, downgraded twice due to limitations in design and implementation (lack of blinding and allocation concealment) and once for imprecision of results due to small sample size and lack of reporting.
Bale 1997 and Seeley 1999 did not report reduction in ulcer size.
None of the trials included in this comparison reported our secondary outcome, quality of life (Bale 1997; Bale 1998; Seeley 1999; Thomas 1997)
Patient satisfaction/acceptability (short‐term follow‐up, 8 weeks or less)
Bale 1998 reported patient satisfaction based on the comfort of the foam and hydrocolloid dressings. However these data were not separated by wound type. It is uncertain whether foam dressings led to patient satisfaction/acceptability because we assessed the certainty of the evidence as being very low due to high risk of bias, and downgraded twice due to limitations in design and implementation (uncertain blinding and allocation concealment) and once for imprecision of results due to small sample size and incomplete reporting.
Bale 1997; Seeley 1999 and Thomas 1997 did not report patient satisfaction/acceptability.
None of the four trials reported secondary outcome pressure ulcer recurrence.
Pain (short‐term follow‐up, 8 weeks or less)
Seeley 1999 used a 4‐point rating scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) to assess wound pain. It is uncertain if the foam dressing affected wound pain (mean 0.15, SD 0.8, n = 20) compared with the hydrocolloid dressing (MD ‐0.32, 95% CI ‐0.86 to 0.22) (Analysis 2.3). Thomas 1997 recorded pain and discomfort associated with the dressing (comfortable or otherwise and reported P = 0.023) however did not report any further details. It is uncertain whether the foam dressings led to pain compared with the hydrocolloid dressing because the certainty of the evidence was very low for both studies due to high risk of bias, downgraded twice due to limitations in design and implementation (lack of blinding), and once for imprecision of results.
2.3. Analysis.
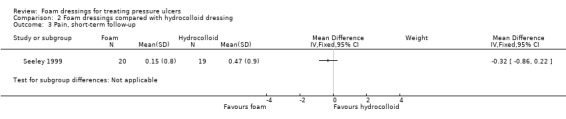
Comparison 2 Foam dressings compared with hydrocolloid dressing, Outcome 3 Pain, short‐term follow‐up.
Economic outcomes
Costs and incremental cost per event (short‐term follow‐up, 8 weeks or less)
Bale 1998 compared the material costs for foam and hydrocolloid dressing changes, which included costs of dressing and saline. Costs were reported as GBP using a 1994 cost year. The total cost of treatment was GBP 844 (mean GBP 50 per participant, n = 17) for using the foam dressing compared to GBP 1142 (mean GBP 76 per participant, n = 15) for the hydrocolloid dressing. However, these costs related to an already small participant subgroup (n = 32) and the authors did not report the statistical significance of the difference. In addition, they did not include the costs of nursing time for the dressing change or management of complex wounds and participants in the hydrocellular group were more likely to have a less severe stage of pressure ulcer at enrolment, representing a significant limitation of the study.
Although Bale 1998 reported healing rates in addition to materials costs for dressing changes, the study authors did not draw conclusions regarding the cost‐effectiveness of foam dressings for the management of pressure ulcers, which is appropriate given this analysis was based on a small subgroup sample without tests of statistical significance and with only partial costs included. This study had very low‐certainty evidence due to high risk of bias, downgraded twice due to limitations in design and implementation (lack of blinding and allocation concealment), and once for imprecision of results due to small sample size and lack of reporting.
The other studies in this comparison (Bale 1997; Seeley 1999; Thomas 1997) did not report economic outcomes.
Comparison 3: polyurethane foam dressing compared with hydrogel dressing (1 trial; 34 participants)
Sopata 2002 compared a foam dressing with a hydrogel dressing, with a short‐term follow‐up period of eight weeks. One participant in the foam dressing group had two pressure ulcers and one or more participants in the hydrogel dressing group had more than one wound, which we could not specify through communication with the study author. We allocated one wound to each participant in the analysis. See Table 3.
Primary outcomes
Incidence of healed pressure ulcers (short‐term follow‐up, 8 weeks or less)
It is uncertain whether treatment with a foam dressing affected the incidence of healed pressure ulcers compared with a hydrogel dressing: RR 1.00 (95% CI 0.78 to 1.28) (Analysis 3.1). We assessed the evidence as very low certainty due to high risk of bias, downgraded once due to limitations in design and implementation (lack of blinding) and twice for very serious imprecision of results due small sample size and wide confidence intervals. See Table 3.
3.1. Analysis.
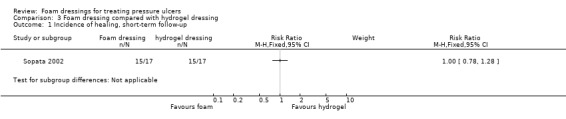
Comparison 3 Foam dressing compared with hydrogel dressing, Outcome 1 Incidence of healing, short‐term follow‐up.
Time to complete healing (short‐term follow‐up, 8 weeks or less)
This study (n = 34) reported treatment times in days (mean ± SD). Compared to the hydrogel dressings, foam dressings were associated with an increased number of treatment days MD 5.67 days, (95% CI ‐4.03 to 15.37) (Analysis 3.2), although this increase was not statistically significant. This was very low certainty evidence due to high risk of bias, downgraded once due to limitations in design and implementation (lack of blinding) and twice for serious imprecision of results due small sample size and wide confidence intervals. See Table 3.
3.2. Analysis.
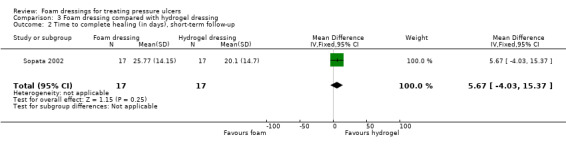
Comparison 3 Foam dressing compared with hydrogel dressing, Outcome 2 Time to complete healing (in days), short‐term follow‐up.
Adverse events (short‐term follow‐up, 8 weeks or less)
One adverse event was reported in the hydrogel dressing group (1/17) where the Category/Stage II pressure ulcer increased in size. It is uncertain whether use of a foam dressing affected the incidence of adverse events compared with a hydrogel dressing: RR 0.33 (95% CI 0.01 to 7.65) (Analysis 3.3). This was very low‐certainty evidence due to high risk of bias, downgraded once due to limitations in design and implementation (lack of blinding) and twice for very serious imprecision of results due small sample size and wide confidence intervals. See Table 3.
3.3. Analysis.
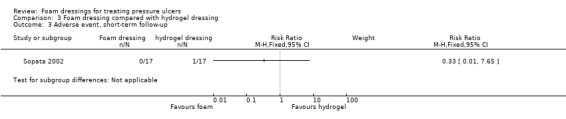
Comparison 3 Foam dressing compared with hydrogel dressing, Outcome 3 Adverse event, short‐term follow‐up.
Secondary outcomes
Reduction in ulcer size per day (short‐term follow‐up, 8 weeks or less)
Sopata 2002 reported reduction of ulcer size for healed pressure ulcers only (n = 30). The mean difference was 0.30 cm2 per day (95% CI ‐0.15 to 0.75) (Analysis 3.4). It is uncertain whether treatment with foam or hydrogel dressings had any impact on the reduction of pressure ulcer size. This trial did not report total overall reduction in ulcer size nor its categorised treatment effect; rather it reported the duration of treatment time by day. While Sopata 2002 compared wound healing rates with Banks 1994a, no supporting data were presented. We assessed the evidence as very low certainty due to high risk of bias, downgraded once due to limitations in design and implementation (lack of blinding) and twice for very serious imprecision of results due to small sample size, wide confidence interval and incomplete reporting.
3.4. Analysis.
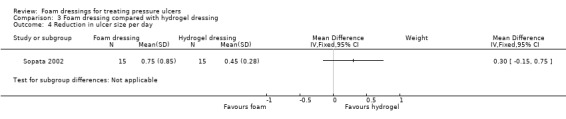
Comparison 3 Foam dressing compared with hydrogel dressing, Outcome 4 Reduction in ulcer size per day.
Sopata 2002 did not report our secondary outcomes, quality of life, patient satisfaction/acceptability, pressure ulcer recurrence and pain, or economic outcomes, cost, utility scores representing health‐related quality of life and incremental cost per event.
Comparison 4: foam dressings* compared with basic wound contact dressings** (3 trials; 181 participants)
*Polyurethane, silver and ibuprofen‐releasing foam dressing
** Gauze, saline‐soaked gauze, low‐adherence dressing secured by a vapour‐permeable film
Three trials (Banks 1994a; Payne 2009; Souliotis 2016) compared foam dressings with basic wound contact dressings (plain or saline‐soaked gauze and knitted multi‐filament yarns secured with a vapour‐permeable film). Follow‐up times ranged from short‐term (4 weeks for Payne 2009) and medium term (12 weeks for Banks 1994a and just over 17 weeks for Souliotis 2016). See Table 4.
Primary outcomes
Incidence of healed pressure ulcers (short‐term follow‐up, 8 weeks or less)
Using data from one study (Payne 2009) (n = 36) it is uncertain if there is a difference in the incidence of healed pressure ulcers over a short‐term follow‐up period: RR 1.33 (95% CI 0.62 to 2.88) (Analysis 4.1). We assessed the evidence as very low certainty due to high risk of bias, downgraded once because of limitations in design and implementation (lack of blinding) and twice for serious imprecision of results due small sample size, wide confidence intervals and incomplete reporting. See Table 4.
4.1. Analysis.
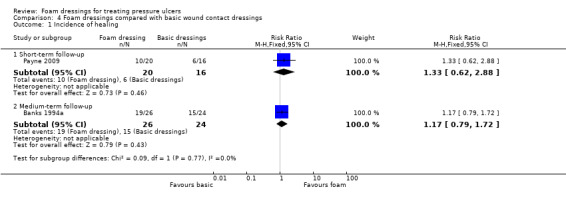
Comparison 4 Foam dressings compared with basic wound contact dressings, Outcome 1 Incidence of healing.
Incidence of healed pressure ulcers (medium‐term follow‐up, 8 to 24 weeks)
Using data from Banks 1994a (n = 50), it is uncertain whether foam dressings impact on the incidence of healed pressure ulcers compared with the control dressing consisting of a layer of knitted viscous multifilament yarns: RR 1.17 (95% CI 0.79 to 1.72) (Analysis 4.1). We also assessed this evidence as very low certainty due to high risk of bias, downgraded once due to limitations in design and implementation (lack of blinding) and twice for serious imprecision of results due small sample size, wide confidence intervals and incomplete reporting. See Table 4.
Souliotis 2016 did not report incidence of healed pressure ulcers.
Time to complete healing (medium‐term follow‐up, 8 to 24 weeks)
Souliotis 2016 (n = 95) reported all participants achieving complete wound healing within 24 weeks (17.3 weeks). This study reported time to complete healing in days (mean ± SD). Compared to basic contact dressings, we observed that in this single study, foam dressings were associated with a decreased time to complete healing MD ‐35.8 days, (95% CI ‐56.77 to ‐14.83) (Analysis 4.2). However this was very low certainty evidence due to high risk of bias, downgraded once due to limitations in design and implementation (lack of blinding) and twice for serious imprecision of results due small sample size, wide confidence intervals and incomplete reporting. See Table 4.
4.2. Analysis.
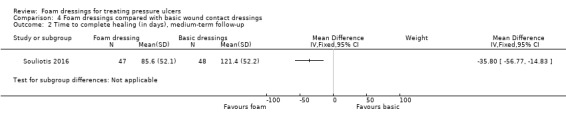
Comparison 4 Foam dressings compared with basic wound contact dressings, Outcome 2 Time to complete healing (in days), medium‐term follow‐up.
Banks 1994a and Payne 2009 did not report our primary outcome, time to complete healing.
Adverse events (medium‐term follow‐up, 8 to 24 weeks)
Souliotis 2016 reported 12 adverse events‐related wound infections in the foam dressings group (n = 48), compared with 21 in the basic wound contact dressing group: RR 0.58 (95% CI 0.33 to 1.05) Analysis 4.3. Hence more adverse events related to wound infection occurred in the basic wound contact dressing group, compared to the foam dressing group. This was low‐certainty evidence, due to high risk of bias, downgraded once due to limitations in design and implementation (lack of blinding) and twice for serious imprecision of results due small sample size, wide confidence intervals and incomplete reporting. See Table 4.
4.3. Analysis.
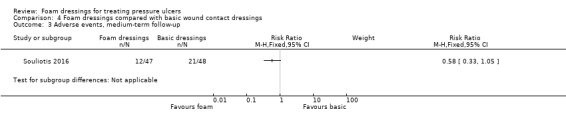
Comparison 4 Foam dressings compared with basic wound contact dressings, Outcome 3 Adverse events, medium‐term follow‐up.
Banks 1994a and Payne 2009 did not report the primary outcome, adverse events.
Secondary outcomes
Reduction in ulcer size (short‐term follow‐up, 8 weeks or less)
Payne 2009 (n = 36) documented the size of participants' ulcers in cm2 as a baseline in the polyurethane foam and saline‐soaked gauze dressing groups, however did not report the final assessment of wound size to enable comparison. It is uncertain whether foam dressings led to a reduction in ulcer size compared with basic wound contact dressings. This was very low‐certainty evidence due to high risk of bias, downgraded twice due to limitations in design and implementation (lack of blinding) and once for serious imprecision of results due small sample size and incomplete reporting.
Banks 1994a and Souliotis 2016 did not report the secondary outcome, reduction in ulcer size.
Patient satisfaction/acceptability (medium‐term follow‐up, 8 to 24 weeks)
Banks 1994a (n = 50) used a patient acceptance questionnaire to record dressing comfort using a scale from 0 = poor to 10 = very comfortable. While they reported mean scores, they did not provide any other information, such as standard deviation or variance data, from which we could make a meaningful interpretation. It is uncertain whether foam dressings affected patient satisfaction/acceptability compared with basic wound contact dressings. We assessed the evidence as very low certainty due to high risk of bias, downgraded twice due to limitations in design and implementation (lack of blinding) and once for imprecision of results due small sample size and incomplete reporting.
Payne 2009 and Souliotis 2016 did not report our secondary outcome, patient satisfaction/acceptability.
Pain (medium‐term follow‐up, 8 to 24 weeks)
Banks 1994a also used a patient acceptance questionnaire to record pain on dressing removal using a scale from 0 = painful to 10 = painless. While they reported mean scores, they did not provide any other information, such as standard deviation or variance data, from which we could make a meaningful interpretation. It is uncertain whether foam dressings affected pain compared with basic wound contact dressings. This was very low‐certainty evidence due to high risk of bias, downgraded twice due to limitations in design and implementation (lack of blinding) and once for imprecision of results due small sample size and incomplete reporting.
Payne 2009, Souliotis 2016 and Thomas 1997 did not report our secondary outcome, pain.
Banks 1994a; Payne 2009; Souliotis 2016 did not report our secondary outcome, quality of life.
Economic outcomes
Cost and incremental cost per event (short‐term follow‐up, up to 8 weeks)
Payne 2009 (n = 36) analysed treatment costs (dressings, other materials, and nurse time) until ulcer healing or 28 days, whichever occurred first. They reported costs as USD using a cost year of 2006/7. The polyurethane foam dressing was less costly per participant (USD 315) than saline‐soaked gauze (USD 781), representing a mean saving of USD 466 per participant in the foam group (P = 0.055). The study authors reported the foam dressing to be dominant; that is, less costly and more effective in terms of number of participants healed by 28 days and ulcer‐free days per participant. They concluded that the foam dressing was cost effective compared to saline‐soaked gauze for the treatment of Category/Stage II pressure ulcers. However, the study was not powered to detect differences in time to healing nor sensitivity analyses undertaken for participants who withdrew before their wounds had healed or before the treatment period. Due to a lack of data, additional analysis was not possible and we are uncertain about the relative impact of basic wound contact dressings on economic outcomes compared with foam dressings. We assessed this evidence as very low certainty due to high risk of bias, downgraded twice due to limitations in design and implementation (lack of blinding) and once for serious imprecision of results due small sample size.
Costs and incremental cost per event (medium‐term follow‐up, 8 to 24 weeks)
Souliotis 2016 reported total and per‐participant treatment costs in the home setting until healing (including dressings, labour and materials). The cost year was not stated. Treatment costs over the study period (to ulcer healing) indicated foam dressings were less costly overall (EUR 63,543 for 47 participants) and per participant (EUR 1351) than plain gauze overall (EUR 186,638 for 48 participants) or per participant (EUR 3888). However, they did not report the statistical significance of this difference. Therefore, although the study authors also reported a shorter average healing time for the foam dressing than the gauze dressing group, it is not possible to draw strong conclusions around cost effectiveness. A paucity of data prevented further analysis and we are uncertain about the relative impact of foam dressings on economic outcomes compared with basic wound contact dressings. This was very low‐certainty evidence due to high risk of bias, downgraded once due to limitations in design and implementation (lack of blinding) and twice for serious imprecision of results due to small sample size and incomplete reporting.
Banks 1994a did not report economic outcomes.
Discussion
Summary of main results
This review of nine trials with 483 participants includes all the currently available RCT evidence evaluating foam dressings to treat pressure ulcers. The primary outcomes for this review were incidence of healed pressure ulcers, time to complete healing and adverse events per participant. Secondary outcomes included reduction in ulcer size, quality of life, patient satisfaction/acceptability, pressure ulcer recurrence, and pain. None of the included trials reported quality of life or pressure ulcer recurrence. We also sought economic outcomes, such as cost, utility scores and incremental costs.
We assessed trials according to the following comparisons: 1) different types of foam dressings compared with each other (1 trial, 38 participants); 2) foam dressings compared with hydrocolloid dressings (4 trials; 230 participants); 3) foam dressings compared with hydrogel dressings (1 trial; 34 participants) and; 4) foam dressings compared with basic wound contact dressings (3 trials; 181 participants).
We judged GRADE assessments as being of low to very low certainty due to serious risk of bias related to lack of blinding and allocation concealment, and imprecision due to small samples or lack of data, or both. Overall, the majority of evidence for all of the included trials was at high risk of bias due to limitations in design and implementation (related to lack of blinding or allocation concealment, or both) and serious imprecision of results (related to all or a combination of small sample size, wide confidence intervals and lack of reporting). Hence we can draw no firm conclusions about clinical advantages, cost effectiveness or patient satisfaction/acceptability between the different types of foam dressings or foam dressing compared with other dressings.
More specifically, we found uncertain evidence about whether foam dressings presented any substantial clinical advantages when compared with other dressings in terms of impact on incidence of pressure ulcers, increasing the time to healing of pressure ulcer, preventing adverse events associated with pressure ulcers, or reducing the size of pressure ulcers. There was also limited available evidence on which to draw conclusions about the comparative impacts of foam dressings for pressure ulcers on quality of life, pain, and satisfaction and acceptability for participants. Available cost evaluations also provided low‐certainty evidence due to missing data and absence of cost‐benefit analyses that would benefit decision makers.
Overall completeness and applicability of evidence
Overall, there were significant weaknesses in the completeness and approachability of evidence reported in the included studies. The trials had small samples (median sample size = 20), and there was no evidence of replication of studies or progression to larger trials, hence comparisons were limited.
There was an overlap of investigators in the teams of four trials (Bale 1997; Bale 1998; Banks 1994a; Thomas 1997). These trials are dated by 20 or more years, hence we were unable to contact the study authors with requests for additional information. Where we were able to contact study authors, they no longer had access to data or could not recall details of individual trials (Bale 1997; Bale 1998; Banks 1994a; Sopata 2002; Thomas 1997). Apart from an included trial published in 2016, the date of publication for the remaining eight trials (1994 to 2009), may also explain the absence of a standardised approach (such as CONSORT (Schulz 2010)), to report methods and results. Consequently, there was a high degree of variability between studies in terms of dressings used, follow‐up periods, interventions and outcomes. Similarly there was methodological diversity due to: selection bias related to the generation of randomisation sequences (Bale 1998; Payne 2009; Sopata 2002; Souliotis 2016;Thomas 1997); allocation concealment (Bale 1997; Bale 1998; Payne 2009; Sopata 2002; Thomas 1997); lack of blinding of participants and personnel (Meaume 2003; Payne 2009; Seeley 1999; Sopata 2002; Souliotis 2016); outcome assessment (Bale 1998; Meaume 2003; Payne 2009; Seeley 1999; Sopata 2002; Souliotis 2016; Thomas 1997); and attrition bias (Bale 1997). While we acknowledge that it is difficult to blind participants and personnel in studies where there is a physical evidence of treatment allocation, none of the eight included studies demonstrated blind‐to‐intervention assessment.
Six trials did not report a funding source (Banks 1994a; Meaume 2003; Seeley 1999; Sopata 2002; Souliotis 2016; Thomas 1997). However the authors of three trials, acknowledged industry sponsorship (Bale 1997; Bale 1998; Payne 2009). Sponsorship was also disclosed in half of the excluded trials (Banks 1994b; Banks 1994c; Münter 2006; Palao i Domenech 2008; Parish 2008; Piatkowski 2012; Reynolds 2004; Wagstaff 2014).
Quality of the evidence
The body of evidence from this review cannot provide robust conclusions regarding the objectives. Hence its downgrade to low or very low‐certainty evidence related to risk of bias and imprecision.
Risk of bias was evident due to small study samples, unblinded outcome assessment, and occasional selective reporting. None of the included studies actively tried to avoid performance bias, although this may be a defendable action due to the difficulty of allocation concealment inherent in wound studies. All of the studies in the review failed to report time to complete healing, quality of life, pressure ulcer recurrence, and economic outcomes utility score representing health‐related quality of life, or incremental costs per event. These are important outcomes that could provide essential information for health policy makers to ensure cost‐effective, patient‐focused care.
Included trials were small and underpowered with wide confidence intervals indicating imprecision in the point estimates, leading to very little confidence in the estimate of effect. Most included trials had relatively short to medium follow‐up times (mean 8 weeks), which led to imprecise results. RCTs need to be adequately powered for treatment effects to be detected. Sample size calculations help estimate the number of participants required. Trials should also have an appropriate follow‐up period to enable important outcome measures (such as wound healing) to occur.
Potential biases in the review process
The review considered the evidence that it was possible to obtain and included studies that were not published in English‐language journals. It is possible that there may be unpublished data that we have not been able to access, as well a potential for publication bias; however, this is very unlikely given the range of findings from this review. There were deviations from the protocol based on a posteriori conditions related to alternative pressure ulcer classification systems, namely the Stirling (Reid 1994) and Torrence classification systems (Harker 2000), and earlier versions published by the NPUAP (NPUAP 1989), that deviated form the contemporary International NPUAP/EPUAP/PPPIA Pressure Ulcer Classification System Criteria (NPUAP/EPUAP/PPPIA 2014). We accepted these alternative classification systems on the condition that the definitions of stage/grade closely matched the contemporary International NPUAP/EPUAP/PPPIA Pressure Ulcer Classification System Criteria (NPUAP/EPUAP/PPPIA 2014). See Table 6 for a comparison of pressure ulcer classification systems. We also included studies that recruited participants with Category/Stage II pressure ulcers or above alongside people with other types of chronic wounds, such as venous and arterial leg ulcers or diabetic foot ulcers, if the results for people with relevant pressure ulcers were presented separately (or this data were available from study authors). Similarly when a study included both Category/Stage I and more advanced pressure ulcers, we included it in the review only if data for Category/Stage II and above were reported separately, or if the data were available on request from study authors. We also included studies where pressure ulcers from Category/Stage II and above were reported collectively. It was not possible to evaluate the wider possibility of publication bias as there was variability of reporting between the included studies, and there were challenges in contacting or sourcing additional information from authors due to age of the studies. As a result of this heterogeneity, we were only able to combine studies for comparison based on their shared outcomes.
Agreements and disagreements with other studies or reviews
No other reviews have presented data on foam dressings as they are presented here; however the findings of this review concur with the conclusion of the large review by Reddy 2008, that looked at several treatments for pressure ulcers and stated that, “No single dressing was consistently superior to other dressings in the trials of pressure ulcers we examined” (p. 2659). The recent National Institute of Health and Clinical Effectiveness (NICE) Pressue Ulcer Guidelines state that “a dressing for adults that promotes a warm, moist wound healing environment to treat Grade 2, 3 and 4 pressure ulcers” should be considered (NICE 2014). The guidelines further state that gauze dressings should not be offered to treat a pressure ulcer in adults. We included all studies examined in the NICE review and a further two studies not mentioned in the NICE guidelines (Bale 1997; Banks 1994a).
Authors' conclusions
Implications for practice.
A comprehensive review of current evidence found no indication of differential effects of foam dressings compared with alternative wound treatments on the outcomes that matter for pressure ulcers (including healing), or cost‐effectiveness. We assessed all of the review trials (Bale 1997; Bale 1998; Banks 1994a; Meaume 2003; Payne 2009; Seeley 1999; Sopata 2002; Souliotis 2016Thomas 1997) as having low‐ to very low‐quality evidence due to risk of bias stemming from unblinded outcome assessment, and occasional selective reporting; inconsistent reporting and; imprecision of results from small and underpowered trials, with relatively short follow‐up times (mean 8 weeks).
Health clinicians may therefore elect to consider other characteristics of wound dressings for the treatment of pressure ulcers such as cost, symptom management properties (such as exudate) and context when choosing a suitable dressing.
Implications for research.
There is an urgent need to evaluate the clinical and cost‐effectiveness of foam dressings to treat pressure ulcers. Currently there is no evidence of a difference in ulcer healing between pressure ulcers dressed with foam dressings and those treated with the other dressings that have been evaluated. In terms of dressing choice, any investment in future research must maximise its value in terms of clinical and cost‐effectiveness to decision makers. Given the large number of dressing options, the design of future trials should be driven by high priority questions from patients and other decision makers. It is also important for researchers to ensure that the outcomes that are collected in research studies are those that matter to patients, carers and health professionals and that the follow‐up times for trials are long enough to capture these. Where trials are conducted, good practice guidelines must be followed for their design, implementation and reporting. Further reviews are being conducted to synthesise evidence regarding the effect of other dressings on the treatment of pressure ulcers. It would then be useful to conduct further evidence synthesis (overviews of reviews, network meta‐analyses or both) to aid decision making about the choice of dressings for pressure ulcers across all dressing options.
Acknowledgements
The authors are grateful to peer reviewers Julie Bruce (editor), Susanne Hempel, Paulo Alves, Louise O'Connor and Janet Wale for their comments on the review; and to Suzanne Hartley, Gill Worthy and Durhane Wong‐Reiger for their feedback on the protocol. Thanks are also due to copy editors Elizabeth Royle and Denise Mitchell. The authors would also like to thank Samantha Keogh for her assistance in developing the protocol for this review.
The NHMRC has provided funding for this review from its Centre of Research Excellence Scheme, which funds one or more of the authors.
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Pressure Ulcer EXPLODE ALL AND INREGISTER 2 (pressure next (ulcer* or sore* or injur*)) AND INREGISTER 3 (decubitus next (ulcer* or sore*)) AND INREGISTER 4 ((bed next sore*) or bedsore) AND INREGISTER 5 #1 OR #2 OR #3 OR #4 6 MESH DESCRIPTOR Bandages EXPLODE ALL AND INREGISTER 7 MESH DESCRIPTOR Polyurethanes EXPLODE ALL AND INREGISTER 8 MESH DESCRIPTOR Silicones EXPLODE ALL AND INREGISTER 9 foam* AND INREGISTER 10 polyurethane* AND INREGISTER 11 silicone* AND INREGISTER 12 hydrocellular or hydropolymer* AND INREGISTER 13 ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex AND INREGISTER 14 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 15 #5 AND #14
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Pressure Ulcer] explode all trees #2 (pressure next (ulcer* or sore* or injur*)):ti,ab,kw #3 (decubitus next (ulcer* or sore*)):ti,ab,kw #4 ((bed next sore*) or bedsore):ti,ab,kw #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Bandages] explode all trees #7 MeSH descriptor: [Polyurethanes] explode all trees #8 MeSH descriptor: [Silicones] explode all trees #9 (foam*):ti,ab,kw #10 (polyurethane*):ti,kw,ab #11 (silicone*):ti,kw,ab #12 (hydrocellular or hydropolymer*):ti,kw,ab #13 (ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex):ti,kw,ab #14 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 #15 #5 and #14
The NHS Economic Evaluation Database (NHS EED)
#1 MeSH descriptor: [Pressure Ulcer] explode all trees #2 (pressure next (ulcer* or sore* or injur*)):ti,ab,kw #3 (decubitus next (ulcer* or sore*)):ti,ab,kw #4 ((bed next sore*) or bedsore):ti,ab,kw #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Bandages] explode all trees #7 MeSH descriptor: [Polyurethanes] explode all trees #8 MeSH descriptor: [Silicones] explode all trees #9 (foam*):ti,ab,kw #10 (polyurethane*):ti,kw,ab #11 (silicone*):ti,kw,ab #12 (hydrocellular or hydropolymer*):ti,kw,ab #13 (ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex):ti,kw,ab #14 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 #15 #5 and #14
Ovid MEDLINE
1 exp Pressure Ulcer/ 2 (pressure adj (ulcer* or sore* or injur*)).ab,kw,ti. 3 (decubitus adj (ulcer* or sore*)).ab,kw,ti. 4 (bed next sore* or bedsore).ab,kw,ti. 5 or/1‐4 6 exp Bandages/ 7 Polyurethanes/ 8 exp Silicones/ 9 foam*.ab,kw,ti. 10 polyurethane*.ab,kw,ti. 11 silicone*.ab,kw,ti. 12 (hydrocellular or hydropolymer*).ab,kw,ti. 13 (ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex).ab,kw,ti. 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 5 and 14 16 randomized controlled trial.pt. 17 controlled clinical trial.pt. 18 randomi?ed.ab. 19 placebo.ab. 20 clinical trials as topic.sh. 21 randomly.ab. 22 trial.ti. 23 or/16‐22 24 exp animals/ not humans.sh. 25 23 not 24 26 15 and 25
Search for economic studies in Ovid MEDLINE:
1 exp Pressure Ulcer/ 2 (pressure adj (ulcer* or sore* or injur*)).ab,kw,ti. 3 (decubitus adj (ulcer* or sore*)).ab,kw,ti. 4 (bed next sore* or bedsore).ab,kw,ti. 5 or/1‐4 6 exp Bandages/ 7 Polyurethanes/ 8 exp Silicones/ 9 foam*.ab,kw,ti. 10 polyurethane*.ab,kw,ti. 11 silicone*.ab,kw,ti. 12 (hydrocellular or hydropolymer*).ab,kw,ti. 13 (ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex).ab,kw,ti. 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 5 and 14 16 economics/ 17 exp "costs and cost analysis"/ 18 economics, dental/ 19 exp "economics, hospital"/ 20 economics, medical/ 21 economics, nursing/ 22 economics, pharmaceutical/ 23 (economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic*).ti,ab. 24 (expenditure* not energy).ti,ab. 25 value for money.ti,ab. 26 budget*.ti,ab. 27 or/16‐26 28 ((energy or oxygen) adj cost).ti,ab. 29 (metabolic adj cost).ti,ab. 30 ((energy or oxygen) adj expenditure).ti,ab. 31 or/28‐30 32 27 not 31 33 letter.pt. 34 editorial.pt. 35 historical article.pt. 36 or/33‐35 37 32 not 36 38 exp Animals/ not humans/ 39 37 not 38 40 15 and 39
Ovid Embase
1 exp decubitus/ 2 (pressure adj (ulcer* or sore* or injur*)).ti,kw,ab. 3 (decubitus adj (ulcer* or sore*)).ti,kw,ab. 4 (bed next sore* or bedsore).ti,kw,ab. 5 or/1‐4 6 exp foam dressing/ 7 exp polyurethan/ 8 exp silicone derivative/ 9 foam*.ti,kw,ab. 10 polyurethan*.ti,kw,ab. 11 silicone*.ti,kw,ab. 12 (hydrocellular or hydropolymer*).ab,kw,ti. 13 (ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex).ti,kw,ab. 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 5 and 14 16 Randomized controlled trials/ 17 Single‐Blind Method/ 18 Double‐Blind Method/ 19 Crossover Procedure/ 20 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab. 21 (doubl* adj blind*).ti,ab. 22 (singl* adj blind*).ti,ab. 23 or/16‐22 24 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 25 human/ or human cell/ 26 and/24‐25 27 24 not 26 28 23 not 27 29 15 and 28
Search for economic studies in Ovid Embase:
1 exp decubitus/ 2 (pressure adj (ulcer* or sore* or injur*)).ti,kw,ab. 3 (decubitus adj (ulcer* or sore*)).ti,kw,ab. 4 (bed next sore* or bedsore).ti,kw,ab. 5 or/1‐4 6 exp foam dressing/ 7 exp polyurethan/ 8 exp silicone derivative/ 9 foam*.ti,kw,ab. 10 polyurethan*.ti,kw,ab. 11 silicone*.ti,kw,ab. 12 (hydrocellular or hydropolymer*).ab,kw,ti. 13 (ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex).ti,kw,ab. 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 5 and 14 16 health‐economics/ 17 exp economic‐evaluation/ 18 exp health‐care‐cost/ 19 exp pharmacoeconomics/ 20 or/16‐19 21 (econom* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic*).ti,ab. 22 (expenditure* not energy).ti,ab. 23 (value adj2 money).ti,ab. 24 budget*.ti,ab. 25 or/21‐24 26 20 or 25 27 letter.pt. 28 editorial.pt. 29 note.pt. 30 or/27‐29 31 26 not 30 32 (metabolic adj cost).ti,ab. 33 ((energy or oxygen) adj cost).ti,ab. 34 ((energy or oxygen) adj expenditure).ti,ab. 35 or/32‐34 36 31 not 35 37 exp animal/ 38 exp animal‐experiment/ 39 nonhuman/ 40 (rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. 41 or/37‐40 42 exp human/ 43 exp human‐experiment/ 44 or/42‐43 45 41 not (41 and 44) 46 36 not 45 47 15 and 46
EBSCO CINAHL Plus
S29 S15 AND S28 S28 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 S27 TI allocat* random* or AB allocat* random* S26 MH "Quantitative Studies" S25 TI placebo* or AB placebo* S24 MH "Placebos" S23 TI random* allocat* or AB random* allocat* S22 MH "Random Assignment" S21 TI randomi?ed control* trial* or AB randomi?ed control* trial* S20 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S19 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S18 TI clinic* N1 trial* or AB clinic* N1 trial* S17 PT Clinical trial S16 MH "Clinical Trials+" S15 S5 AND S14 S14 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S13 TI ( ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex ) OR AB ( ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex ) S12 TI ( hydrocellular or hydropolymer* ) OR AB ( hydrocellular or hydropolymer* ) S11 TI silicone* OR AB silicone* S10 TI polyurethan* OR AB polyurethan* S9 TI foam* OR AB foam* S8 (MH "Silicones+") S7 (MH "Polyurethanes") S6 (MH "Foam Dressings") S5 S1 OR S2 OR S3 OR S4 S4 TI decubitus or AB decubitus S3 TI ( bed sore* or bedsore* ) or AB ( bed sore* or bedsore* ) S2 TI ( pressure ulcer* or pressure sore* ) or AB ( pressure ulcer* or pressure sore* ) S1 (MH "Pressure Ulcer+")
Search for economic studies in EBSCO CINAHL Plus:
S38 S15 AND S37 S37 S33 NOT S36 S36 S19 NOT (S19 AND S35) S35 MH "Human" S34 MH "Animal Studies" S33 S28 NOT S32 S32 S29 or S30 or S31 S31 PT commentary S30 PT letter S29 PT editorial S28 S26 OR S27 S27 TI (cost or costs or economic* or pharmacoeconomic* or price* or pricing*) OR AB (cost or costs or economic* or pharmacoeconomic* or price* or pricing*) S26 S22 OR S25 S25 S23 OR S24 S24 MH "Health Resource Utilization" S23 MH "Health Resource Allocation" S22 S16 NOT S21 S21 S17 OR S18 or S19 OR S20 S20 MH "Business+" S19 MH "Financing, Organized+" S18 MH "Financial Support+" S17 MH "Financial Management+" S16 MH "Economics+" S15 S5 AND S14 S14 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S13 TI ( ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex ) OR AB ( ActivHeal or Allevyn or Advazorb or Biatain or Copa or LyoFoam or PermaFoam or PolyMem or Suprasorb or Tegaderm or Tielle or Transorbent or Trufoam or UrgoCell or Cutimed or Kendall or Askina or Kerraboot or Cavi‐care or Mepilex ) S12 TI ( hydrocellular or hydropolymer* ) OR AB ( hydrocellular or hydropolymer* ) S11 TI silicone* OR AB silicone* S10 TI polyurethan* OR AB polyurethan* S9 TI foam* OR AB foam* S8 (MH "Silicones+") S7 (MH "Polyurethanes") S6 (MH "Foam Dressings") S5 S1 OR S2 OR S3 OR S4 S4 TI decubitus or AB decubitus S3 TI ( bed sore* or bedsore* ) or AB ( bed sore* or bedsore* ) S2 TI ( pressure ulcer* or pressure sore* ) or AB ( pressure ulcer* or pressure sore* ) S1 (MH "Pressure Ulcer+")
ClinicalTrials.gov
Foam dressing OR bandage AND pressure ulcer treatment Foam dressing OR bandage AND bed sore treatment Foam dressing OR bandage AND decubitis ulcer treatment
World Health Organization International Clinical Trials Registry Platform
Foam dressing OR bandage AND pressure ulcer treatment Foam dressing OR bandages OR polyurethanes OR silicones AND pressure ulcer OR pressure injury OR bed sore OR decubitis ulcer AND treatment Foam dressing OR bandage AND pressure ulcer OR pressure injury OR bed sore OR decubitis ulcer AND treatment
EU Clinical Trials Register
Foam dressing OR bandage AND pressure ulcer OR pressure injury OR bed sore OR decubitis ulcer AND treatment
Appendix 2. Assessment of risk of bias criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence‐generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open, random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Either of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study's pre‐specified primary outcomes were reported.
One or more primary outcomes were reported using measurements, analysis methods or subsets of the data (e.g. subscales) that had not been pre‐specified.
One or more reported primary outcomes had not been pre‐specified (unless clear justification for their reporting was provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis.
The study report failed to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Foam dressing compared with other foam dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of healing, short‐term follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Adverse events, short‐term follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 2. Foam dressings compared with hydrocolloid dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of healing, short‐term follow‐up | 3 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.54, 1.34] |
| 2 Adverse events, short‐term follow‐up | 3 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.37, 2.11] |
| 3 Pain, short‐term follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 3. Foam dressing compared with hydrogel dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of healing, short‐term follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Time to complete healing (in days), short‐term follow‐up | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 5.67 [‐4.03, 15.37] |
| 3 Adverse event, short‐term follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Reduction in ulcer size per day | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 4. Foam dressings compared with basic wound contact dressings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of healing | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short‐term follow‐up | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.62, 2.88] |
| 1.2 Medium‐term follow‐up | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.79, 1.72] |
| 2 Time to complete healing (in days), medium‐term follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Adverse events, medium‐term follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bale 1997.
| Methods | A multicentre, prospective RCT with 61 participants Conducted in the UK Follow‐up: 30 d Participants recruited from 5 centres. Centres not specifically described |
|
| Participants |
Inclusion criteria: aged ≥ 18 years, not pregnant, able to understand and consent to the trial, no history of poor compliance or previous involvement in the study, Stage II‐III PUs (using the Stirling classification) the largest wound diameter ≤ 11 cm with no sign of infection (identified as absence of bleeding, friable granulation tissue, offensive odour and pus secretion) Exclusion criteria: NR In the polyurethane foam dressing group at baseline (n = 29):
In the hydrocolloid dressing group at baseline (n = 31):
The study received local research ethics committee approval at each centre and informed consent was obtained from all participants |
|
| Interventions |
Group A: polyurethane foam dressing (Allevyn Adhesive) until the wound healed, or for a maximum of 30 d (n = 29) hydrocolloid dressing (Granuflex) until the wound healed, or for a maximum of 30 d (n = 31) |
|
| Outcomes |
Primary outcomes: these outcomes are recorded as per the review in relation to primary and secondary Incidence of healed PUs (referred to as "healed wounds") Adverse events per participant Secondary outcomes: NR Economic outcomes: NR |
|
| Notes | Missing data/exclusions: of total number of participants recruited (n = 61); "Sixty patients were included in the statistical analysis. One patient was excluded who died shortly after the first dressing application (this was not dressing related)." (p. 464) High rate of participant dropout: 18 in the polyurethane foam group, 22 in the hydrocolloid group Wounds healed in 12 participants: 7 in the polyurethane foam dressing group, 5 in the hydrocolloid dressing group Adverse events: "There was only one dressing‐related adverse incident, where a patient treated with the polyurethane foam dressing developed a localised skin rash" (p. 464). Mention of "Damage to the surrounding skin, although rare, was reported in both groups…" (p. 466) Stirling classification system similar to NPUAP/EPUAP/PPPIA classification Study funded by Smith & Nephew, manufacturer of Allevyn Adhesive |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "Patients allocated to one of two treatment groups sequentially for each centre, using an open randomisation list." Comment: unclear how random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quotation: "Patients allocated to one of two treatment groups sequentially for each centre, using an open randomisation list." Comment: not clear how allocation to group was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No direct quotation addressing this aspect Comment: unclear whether participants and personnel were blinded to treatment group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No direct quotation addressing this aspect Comment: unclear, blinding of outcome assessment is not specifically stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotations: "One patient was excluded who died shortly after the first dressing application (this was not dressing related)" “Forty of the 61 patients enrolled in the study were withdrawn. The main reasons for this were that the patients died or were discharged before their wounds healed." Comment: 67% of participants were withdrawn from the study |
| Selective reporting (reporting bias) | Low risk | No direct quotation addressing this aspect Comment: some data not reported, (e.g. the number of participants who required a dressing change due to discomfort) |
| Other bias | Low risk | No direct quotation addressing this aspect. Comment: none noted |
Bale 1998.
| Methods | A single‐centre, prospective, parallel‐group RCT with 100 participants Conducted in the UK Follow‐up: 8 weeks Participants recruited from the community over 10 month period from 26 April 1993 with the last follow‐up visit completed on 13 June 1994. No other specific details provided | |
| Participants |
Inclusion criteria: judgement by investigator if treatment with study dressings was appropriate, leg ulcers of any aetiology except those with venous ulceration who were able to tolerate compression therapy, and those with Stage II and III sore (classified using an early version of the NPUAP), or other granulating wounds with moderate‐high levels of exudate Exclusion criteria: pregnant or lactating women, people with pressure sores classified Stage I or Stage IV (classified using an early version of the NPUAP), wound expected to heal within 1 week or wound with sloughy or necrotic tissue or grossly infected wound (although these people could be included in the study after the wound had been debrided or the infection had been resolved) If a participant presented with more than one suitable wound, data on the largest wound was collected during the study period In the hydrocellular foam dressing group at baseline (n = 50) from combined data of wound types, PUs, leg ulcers and other
In the hydrocolloid dressing group at baseline (n = 46) from combined data of wound types, PUs, leg ulcers and other
Ethcial approval reported as being obtained, and written consent from participants obtained |
|
| Interventions | Group A: hydrocellular dressing (Allevyn) until the wound healed, or for a maximum of 8 weeks (n = 50, of which PU n = 17) Group B: hydrocolloid dressing (Granuflex) until the wound healed, or for a maximum of 8 weeks (n = 46, of which PU n = 15) | |
| Outcomes |
Primary outcomes Adverse events per participant (data not separated by wound type) Secondary outcomes Reduction in ulcer size (data not separated by wound type) Patient satisfaction/acceptability (data not separated by wound type) Economic outcomes Cost and incremental cost per event (total costs of study materials and cost effectiveness per participant) |
|
| Notes | Missing data/exclusions: "Four patients have been excluded from the efficacy, performance and economic analysis." 21/49 (43%) of participants in Group B (hydrocolloid dressing) were withdrawn from the study before completion compared to 10/51 (20%) of participants in Group A (hydrocellular foam dressing). However, it is unclear what proportion of those participants with PUs were withdrawn from the study Wounds treated in Group A (hydrocellular foam dressing) were significantly larger than those treated in Group B (hydrocolloid dressing) Study funded by Smith & Nephew, manufacturer of Allevyn |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "...subjects were randomised in blocks of four to Group A, to receive the hydrocellular dressing (Allevyn) or to Group B, to receive the hydrocolloid dressing (Granuflex)." Comment: no indication of how randomisation was achieved |
| Allocation concealment (selection bias) | Unclear risk | Quotation: "...subjects were randomised in blocks of four to Group A, to receive the hydrocellular dressing (Allevyn) or to Group B, to receive the hydrocolloid dressing (Granuflex)." Comment: no indication if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotation: "All patients in the trial were treated by research nurses from the Wound Healing Research Unit in accordance with the study protocol." Comment: unclear if participants and personnel were blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quotation: "It must be noted that one of the problems of collecting subjective data when it is not a blind assessment is the introduction of unconscious bias." Comment: suggests that those assessing the wounds were not blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotation: "Four patients have been excluded from the efficacy, performance and economic analysis. One patient in Group A (hydrocellular foam dressing) had a wound which was too large. In Group B (hydrocolloid dressing), two patients were withdrawn from the study within seven days and a third died during the study and the case report form was not recovered. All other patients recruited (including later withdrawals) have been incorporated in all statistical analyses; 43% of patients were withdrawn after the initial; seven days in Group B compared to 20% in Group A. Thus the difference was statistically significant ( p = 0.012, x 2 = 6.32, df = 1)." Comment: it appears that all 32 participating in the PU subgroup were included in the analysis. Only 4 participants were excluded for the larger combined group as described above |
| Selective reporting (reporting bias) | Unclear risk | No direct quotation addressing this aspect Comment: limited information on which to judge |
| Other bias | High risk | Comment: Subgroup analyses undertaken in only part of the trial population Quotation: “Wounds treated in Group A when the study commenced were significantly larger than those treated in Group B (median 4.7cm2 (p=0.037)." Comment: dissimilarity of study groups may be a source of bias, although group A (hydrocellular foam dressing) were reported as having better outcomes at 56 days than the hydrocolloid dressing |
Banks 1994a.
| Methods | A randomised controlled comparative study trial with 50 participants in 2 centres Conducted in the UK Follow‐up: 12 weeks Participants recruited from a hospital and the community over a 10‐month period in 1992. Centres not specifically described |
|
| Participants |
Inclusion criteria: people with Grade 2 or 3 pressure sores (using the Torrance 5‐stage classification system) Exclusion criteria: people who were terminally ill or unavailable for the full 12‐week trial period, or if the sores were necrotic, infected or over 7 cm in any direction Limited baseline participant data reported Combined data reported for age (years): 34, 68% > 75 Combined data reported for gender: female (n = 32, 64%), male (n = 18, 36%) In the polyurethane foam dressing group at baseline (n = 26)
In the Control dressing at baseline (n = 24)
Location of PU reported as percentages for the combined group (n = 100): sacral (n = 53, 52.9%), buttocks (n = 32, 32.4%), trochanter (n = 6, 5.9%), foot (n = 6, 5.9%), heels (n = 3, 2.9%) Ethical approval was granted for the study and informed consent obtained from participants, next‐of‐kin of legal guardian |
|
| Interventions |
Group A: polyurethane foam dressing (Lyofoam A) until wound healed, or for a maximum of 12 weeks (n = 26) Group B: control dressing (NA) consisting of a layer of knitted viscous multifilament yarns, which allows exudate through its open structure. This was placed in contact with the wound surface and secured with a vapour‐permeable film dressing (Tegaderm) until the wound healed, or for a maximum of 12 weeks (n = 24) |
|
| Outcomes |
Primary outcomes Incidence of healed PUs (i.e., duration of pressure sore healing time in weeks, and categorised as "completely healed, improved") Secondary outcomes Patient satisfaction (comfort of dressing) Pain associated with removal of dressing Economic outcomes: NR |
|
| Notes | Randomisation resulted in an inequality of initial sore size between the 2 dressing groups Duration of pressure sites was not known for 14 participants (28%) Torrence 5‐stage pressure sore grading system similar to NPUAP/EPUAP/PPPIA classification Missing data/exclusions for 12 withdrawn participants Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotation: "Randomisation was provided by an independent statistician. Each patient was allocated via a sealed envelope containing one of two treatment codes indicating the trial or the control." Comment: evidence of appropriate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quotation: "Randomisation was provided by an independent statistician. Each patient was allocated via a sealed envelope containing one of two treatment codes indicating the trial or the control." Comment: evidence of appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotation: “The weekly patient questionnaires recorded dressing comfort and pain on its removal of dressings on a scale of 0‐10.” Quotation: “The weekly nurse questionnaires recorded ease of application and removal of dressings on a scale of 0‐10.” Comment: unclear if participants and personnel were blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotation: “The initial wound assessment and recruitment to the trial was undertaken by the trial coordinator who also visited the patients on a weekly basis throughout the trial period.” Comment: unclear if those assessing the wounds were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quotation: “Following up the patients each week for 12 weeks proved difficult, particularly when patients were transferred to other wards or discharged”; “In the control group it is not possible to identify which aspect of the dressing benefited healing” Comment: follow‐up and comparator data potentially incomplete |
| Selective reporting (reporting bias) | Unclear risk | No direct quotation addressing this aspect Comment: limited information on which to judge |
| Other bias | High risk | Quotation: “The patient population may have influenced wound site, and evaluation of the patient characteristics show a large elderly population.”; “In the control group it is not possible to identify which aspect of the dressing benefited healing” Quotation: “Randomisation did result in an inequality of initial sore size between the two dressing groups. This could have had some influence on outcome and future studies may provide more accurate data if the initial wound size, and possibly wound site, were specified.” Comment: dissimilarity of study groups may be a source of bias |
Meaume 2003.
| Methods | A multi‐centre, open, RCT with 38 participants Conducted in 3 European countries Follow‐up: 8 weeks Participants recruited from 3 nursing homes in Belgium, France and Italy. No other specific details provided |
|
| Participants |
Inclusion criteria: participants ≥ 65 years, Stage II PU as per EPUAP which had not improved in the preceding 4 weeks, a Modified Norton Scale Score ≥ 11, red/yellow wound according to the Red‐Yellow‐Black System Exclusion criteria: underlying disease that may interfere with the treatment of the PU, food and/or fluid intake score of ≤ 2 on the Modified Norton Scale, allergic/hypersensitivity to any material in the dressings, a wound larger than 11 cm x 11 cm, a wound with black necrotic tissue or clinical signs of local infection at baseline In the silicone foam dressing group at baseline (n = 18):
In the hydropolymer foam dressing group at baseline (n = 20):
Study performed in accordance with ethical principals outlined in the Declaration of Helsinki and informed, written consent obtained from all participants |
|
| Interventions |
Group A: silicone foam dressing (Mepilex Border by Molnlycke) until the wound healed, or for a maximum of 8 weeks (n = 18) Group B: hydropolymer foam dressing (Tielle by Johnson & Johnson) until the wound healed, or for a maximum of 8 weeks (n = 20) |
|
| Outcomes |
Primary outcomes Incidence of healed PUs Adverse events Secondary outcomes Reduction in ulcer size Economic outcomes: NR |
|
| Notes | Only 1 PU per participant Adverse events per participant
Funding source: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotation: "Participants were randomly assigned to one of the two treatment options by a predetermined computer‐generate randomisation list stratified by study centre, and the block size was unknown to the investigators. Each centre received numbered, sealed envelopes to be opened in consecutive order.” Comment: evidence of appropriate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quotation: "Participants were randomly assigned to one of the two treatment options by a predetermined computer‐generate randomisation list stratified by study centre, and the block size was unknown to the investigators. Each centre received numbered, sealed envelopes to be opened in consecutive order.” Comment: evidence of appropriate randomisation method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotation: “The study was not blinded because dressing differences make blinding difficult to achieve.” Comment: participants and personnel were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quotation: “The study was not blinded because dressing differences make blinding difficult to achieve.” Comment: outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No direct quotation addressing this aspect Comment: data appear to be presented for all participants |
| Selective reporting (reporting bias) | Low risk | Quotation: “The results of this exploratory study indicate that the soft silicone dressing is superior to dressings using traditional adhesive technologies. These findings need to be confirmed in other controlled studies” Comment: acknowledged to be an exploratory study. Data appear to be presented for all participants. |
| Other bias | Low risk | Quotation: “Patients were excluded from this study if they suffered from an underlying disease that, according to the investigator, might possibly interfere with the treatment of the pressure ulcer” Comment: limited information on which to judge |
Payne 2009.
| Methods | A multicentre prospective, RCT with 36 participants Conducted in the USA Follow‐up: 4 weeks Participants recruited from 3 hospital inpatient wards, 1 hospital‐based outpatients' clinic, 1 long‐term residential care centre and a community‐based wound clinic in the USA between November 2005 and March 2007 |
|
| Participants |
Inclusion criteria: participants had to be ≥ 18 years of age, either gender, not pregnant or using contraception, and have a Stage II PU as per the NPUAP classification system with slight to moderate levels of exudate. If the participant had more than one eligible wound, the largest was selected for the study. Exclusion criteria: people with a history of poor compliance, presence of clinical infection in the wounds, presence of Stage I, III or IV PU, previous participation in the study In the polyurethane foam dressing group at baseline (n = 20):
In the saline‐soaked gauze dressing group at baseline (n = 16):
Ethics approval was obtained from each of the 5 participating centres and participants, their legal representative, guardian or care‐giver gave informed written consent |
|
| Interventions |
Group A: self‐adhesive polyurethane foam dressing (Allevyn Thin, Smith & Nephew) until the wound healed, or for a maximum of 4 weeks (n = 20) Group B: saline‐soaked gauze until the wound healed, or for a maximum of 4 weeks (n = 16) |
|
| Outcomes |
Primary outcomes Incidence of healed PUs Reduction in ulcer size Secondary outcomes: NR Economic outcomes Cost and incremental cost per event (cost of materials per participant, per ulcer healed and per ulcer‐free day) |
|
| Notes | Missing data/exclusions: 2 participants were excluded from the costing analyses Reason for withdrawals: "Nine patients were withdrawn from the study ‐ six in the foam group (three died, one developed a wound infection, one developed an abscess unrelated to the study wound, and one became ineligible for other reasons) and three in the gauze group (two died and one asked to be discharged form hospital)." (p. 53) Wound preparation (cleaning and drying the wound) was not standardised and each participant was treated according to the normal practice of each study centre Study authors acknowledged a measure of participant‐assessed quality of life should have been included in the study to ensure that results did not impact on patient quality of life Study funded by Smith & Nephew Authors Posnett, Sharma and Hartwell were employees of Smith & Nephew at the time of the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "Consenting patients were assigned a sequential patient number at each study centre. A randomisation schedule determined treatment allocation to either self‐adhesive polyurethane foam dressing…or saline‐soaked gauze." Comment: not clear how randomisation schedule was devised or implemented |
| Allocation concealment (selection bias) | High risk | Quotation: "Consenting patients were assigned a sequential patient number at each study cent er. A randomisation schedule determined treatment allocation to either self‐adhesive polyurethane foam dressing…or saline‐soaked gauze." Comment: not clear how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotation: “Study participants were not blinded.” Quotation: “In both study groups, dressing change frequency was determined at the discretion of the clinical investigator.” Comment: participants and personnel were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quotation: “In both study groups, dressing change frequency was determined at the discretion of the clinical investigator.” Quotation: “Details of wound healing and dressing changes at each assessment were recorded by the study investigator directly to a case report from (CRF) Comment: outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotation: “Two patients in the foam group had no information recorded on dressing changes between weekly assessment. These patients were included in the full data analysis set, but because it is likely these patients had additional dressing changes that were not recorded, including costs for these patients, may have led to an underestimation of average treatment costs for patients in the foam group. For this reason these patients were excluded from the costing analysis.” Comment: adequate evidence to assume low risk judgement |
| Selective reporting (reporting bias) | Unclear risk | No direct quotation addressing this aspect Comment: limited information on which to judge |
| Other bias | Low risk | Quotations: “There was no evidence of a difference in the time to wound closure between the two treatment groups (P=0.817).”; “The study was not powered to detect a difference in time to healing and although a difference favouring the foam group was observed, this difference was not statistically significant at the 5% level” Comment: limited information on which to judge |
Seeley 1999.
| Methods | A multi‐centre, prospective, randomised, stratified, parallel‐group study with 40 participants Conducted in the USA Follow‐up: 8 weeks Participants recruited over 17 months from “several” long‐term care facilities and from outpatients from diabetic foot and wound centre. No other details provided |
|
| Participants |
Inclusion criteria: either sex, > 18 years with ≥ 1 Stage II or III PU (as per an early version of the NPUAP classification system known as the Agency for Health Care Policy and Research system), were recruited sequentially into the study. If the participant had more than one PU, the largest ulcer that met the inclusion criteria was selected as the study ulcer. Exclusion criteria: If the ulcer was smaller than 1 cm2 or larger then 50 cm2 or if the ulcer was considered to be clinically infected, people with uncontrolled diabetes or a known history of poor compliance with medical treatment In the hydrocellular foam dressing group at baseline (n = 20):
In the hydrocolloid dressing group at baseline (n = 19):
Ethics approval was obtained and participant, or their authorised representative gave informed written consent |
|
| Interventions |
Group A: hydrocellular dressing (Allevyn Hydorcellular, Smith & Nephew) until the wound healed, the participant was withdrawn, or for a maximum of 8 weeks (n = 20) Group B: hydrocolloid dressing (Duoderm CGF Border Dressing, ConvaTec) until the wound healed, the participant was withdrawn, or for a maximum of 8 weeks (n = 19) |
|
| Outcomes |
Primary outcomes Incidence of healed PUs Adverse events per participant Secondary outcomes Pain associated with a PU or dressing removal, or both Economic outcomes: NR |
|
| Notes | Only 1 PU per participant Missing data/exclusions ‐ "Forty patients were recruited into the study. One patient in the hydrocolloid group died shortly after enrolment and was excluded from the statistical analysis. The death was not related to the study dressing." (p. 41) Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotation: "The randomisation was stratified into three groups (small, medium, large) according to the initial ulcer size (1 to 10 cm2, 10.1 to 20 cm2, and 20.1 to 50 cm2, respectively). Within each strata, patients were randomised to the hydrocellular or hydrocolloid dressing using a computer generated list." Comment: evidence of appropriate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quotation: "The frequency of dressing changes was dictated by the individual wound's condition and was left to the judgement of the clinical investigator." Quotation: "The time needed to change each of the dressings at the weekly assessments was recorded in minutes. The dressing wear times were calculated from data recorded in the dressing diaries." Comment: study group allocation not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotation: “The frequency of dressing changes was dictated by the individual wound’s condition and was left to the judgement of the clinical investigator.” Quotation: “The time needed to change each of the dressings at the weekly assessments was recorded in minutes. The dressing wear times were calculated from data recorded in the dressing diaries.” Comment: participants and personnel were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quotation: "The frequency of dressing changes was dictated by the individual wound's condition and was left to the judgement of the clinical investigator." Comment: outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotation: "Forty patients were recruited into the study. One patient in the hydrocolloid group shortly after enrolment and was excluded from the statistical analysis. This death was not related to the study dressing." Comment: adequate evidence to assume low risk judgement |
| Selective reporting (reporting bias) | Low risk | Quotation: "The study ulcer was evaluated, traced and photographed at baseline (week 0) and thereafter at weeks 1 to 8." Quotation: "The mean percentage reductions in ulcer area from the patient's initial weeks to final week were 50% and 52% for the hydrocellular and hydrocolloid groups, respectively (P=0.31). " Comment: although the study ulcer was accessed and measured each week, results for ulcer area reported as a mean percentage rather than in cm2 |
| Other bias | Unclear risk | Quotation: “It therefore can be argued that the wear time generated in this study is not a true reflection of the maximum average wear time for either dressing” Comment: limited information on which to judge |
Sopata 2002.
| Methods | A single‐centre prospective, RCT with 34 participants with advanced cancer Conducted in Poland Follow‐up: 8 weeks Participants recruited over 3 years from January 1996‐January 1999 in a palliative care department |
|
| Participants |
Inclusion criteria: advanced cancer and a life expectancy of > 8 weeks, Grade II or III PUs (using the Torrance 5‐stage classification system) Exclusion criteria: poor general condition, with low levels of haemoglobin and albumin and use of drugs such as corticosteroids that could affect wound healing In the polyurethane foam dressing group at baseline (n = 17, with 18 ulcers):
In the hydrogel dressing group at baseline (n = 17, with 20 ulcers):
|
|
| Interventions |
Group A: polyurethane dressing (Lyofoam) until the wound healed, or for a maximum of 8 weeks (n = 17, with 18 ulcers) Group B: hydrogel dressing (Aquagel) until the wound healed, or for a maximum of 8 weeks (n = 17, with 20 ulcers) |
|
| Outcomes |
Primary outcomes Note: primary outcomes reported in this study were different to a priori criteria reported in the review protocol Incidence of healed PUs Time to complete healing (in days) Adverse events per participant Secondary outcomes Reduction in ulcer size Economic outcomes: NR |
|
| Notes | A primary outcome of this review was to measure the incidence of healed PUs with respect to the unit of analysis being the proportion of participants in whom a PU healed. There is limited information to extrapolate this information from the analysis presented Ethical approval for the study and informed consent for participants NR Funding source NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "Patients were randomly allocated, using a computer number system, to treatment with either Lyofoam or Aquagel." Comment: not clear how randomisation schedule was devised or implemented |
| Allocation concealment (selection bias) | Unclear risk | Quotation: "Patients were randomly allocated, using a computer number system, to treatment with either Lyofoam or Aquagel." Comment: not clear if allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotation: “All patients were treated by the main researcher (M. Sopata) or by one of two departmental nurses.” Comment: not clear if some participants and personnel were blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quotation: “All patients were treated by the main researcher (M. Sopata) or by one of two departmental nurses.” Comment: outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotation: “In the Lyofoam group, six (100%) Grade II and nine (75%) Grade II ulcers healed. Three Grade III ulcers improved although two of these belonged to patients who died before the end of the study and one did not heal. In the Aqucel group, six (100%) Grade II and nine 64%) Grade III ulcers healed. Four patients had four wounds that improved (29%) but, again, these wounds belonged to three patients who does before the end of the study and one did not heal. The treatment failed in one ulcer.” Comment: ITT analysis assumed suggesting complete reporting of outcome data |
| Selective reporting (reporting bias) | Low risk | No direct quotation addressing this aspect Comment: outcome measures reported in methods section were reported in the results section |
| Other bias | Low risk | No direct quotation addressing this aspect Comment: none noted |
Souliotis 2016.
| Methods | A RCT of 100 people with full thickness PUs treated at home Greece Participants recruited from the community and treated in their homes Follow‐up until complete healing |
|
| Participants |
Inclusion criteria: Stage III or IV PU using the EPUAP classification system) Exclusion criteria:
In the moist wound healing group (n = 47)
In the plain gauze group (n = 48)
Participants were fully informed about the aim of the study and that participation was optional and that they could drop out of the study. All participants signed an informed consent form, and in those cases where they were unable to do so, a designated person signed for them. Participants’ personal data were codified to ensure anonymity and confidentiality. |
|
| Interventions |
Group A: moist wound dressings (foam dressing with anti‐microbial and analgesic variations) until the wound healed, with no time limit (participants with pressure sores, n = 47) Group B: plain gauze (including gauze soaked in saline) until the wound healed, with no time limit (participants with pressure sores, n = 48) |
|
| Outcomes |
Primary outcomes Time to complete healing (in days) Adverse events per participant (local wound infection) Secondary outcomes: NR Economic outcomes Cost and incremental cost per event (cost of clinical materials per participant, daily wages and cost of healthcare professionals per home visit) |
|
| Notes | Only 1 ulcer per person Data collection and ulcer measurements took place once a month until complete healing Costs of dressings and materials informed by the (Greek) Committee for Health Supplies and the average purchase prices paid by public hospitals. Costs per home visit came from official sources regarding public servants’ monthly wages and labour costs Funding source NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: "The allocation of the subjects in the group of moist wound dressings and the gauze group was randomised by using sealed opaque envelopes." Comment: not clear how randomisation schedule was devised |
| Allocation concealment (selection bias) | Low risk | Quotation: "The allocation of the subjects in the group of moist wound dressings and the gauze group was randomised by using sealed opaque envelopes." Comment: evidence of appropriate randomisation method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No direct quote addressing this aspect Comment: participants and personnel not blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quotation: “Home treatment was performed by healthcare professionals according to the patients’ needs and the ulcer treatment protocols applied by each healthcare service. For the ulcer surface measuring, sterile transparent graded films were used.” Comment: outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quotation: "One patient of the group under treatment with plain gauze and two from the group under treatment with moist wound healing dressings had to be withdrawn from the study. One patient from each group died during the course of the study. The data of the patients who did not complete the study were not included in the data analysis." Comment: a per‐protocol approach which potentially contributed to bias |
| Selective reporting (reporting bias) | Unclear risk | No direct quotation addressing this aspect Comment: limited information on which to judge |
| Other bias | Low risk | No direct quotation addressing this aspect Comment: none noted |
Thomas 1997.
| Methods | A 2‐centred, open, randomised, controlled, comparative study with 199 participants (PUs n = 99, leg ulcers n = 100) Place of study NR. Assumed to be UK (Wales) where authors are located Follow‐up: 6 weeks Participants recruited from the community. No other details provided |
|
| Participants |
Inclusion criteria: Grade 2 or 3 PUs (using the Stirling Classification), had an ulcer less then 10 mm deep and a maximum diameter of 8 cm (to allow a single dressing to cover the entire ulcer) Exclusion criteria: participants < 16 years, were known to have a history or poor compliance with medical treatment, had insulin‐dependent diabetes, were considered unlikely to survive the period of the study, had previously demonstrated adverse reactions to one of the dressings being tested, had wounds that were clinically infected In the hydropolymer foam dressing group at baseline (n = 50):
In the hydrocolloid dressing group at baseline (n = 49):
Ethics committee approval obtained. Participants provided written informed consent prior to randomisation and collection of demographic data |
|
| Interventions |
Group A: hydropolymer foam dressing (Tielle) until the wound healed, or for a maximum of 6 weeks (participants with PUs, n = 50) Group B: hydrocolloid dressing (Granuflex) until the wound healed, or for a maximum of 6 weeks (participants with PUs, n = 49) |
|
| Outcomes |
Primary outcomes Incidence of healed PUs (categorised as "totally healed, improved, not healed, unchanged") Adverse events per participant (categorised as ulcer "deteriorated") Secondary outcomes Reduction in ulcer size (data not separated by wound type) Economic outcomes: NR |
|
| Notes | Only one ulcer per person Participants with PU were cared for using appropriate pressure‐relieving devices Adverse events linked to dressings (most frequently related to the adhesive nature of the dressings causing trauma) Funding source NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotation: “...patients who…gave informed consent in writing were allocated to the two treatment groups on a randomised basis, using a system of sealed envelopes.” Comment: method of randomisation not described. Not clear if envelopes were sequentially numbered to ensure random sequence was maintained |
| Allocation concealment (selection bias) | High risk | Quotation: “An open, randomised, controlled, two‐centred, comparative study…” Comment: an open study. Group allocation not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotation: “An open, randomised, controlled, two‐centred, comparative study…” Comment: an open study. Participants and personnel not blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quotation: “An open, randomised, controlled, two‐centred, comparative study…” Quotation: “To ensure accurate data collection, all dressing changes were undertaken by dedicated research nurses…” Comment: outcome assessment not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotation: "Details of all adverse events were documented. If a patient was withdrawn from the study, the reason was recorded and a decision made as to whether this was the result of an adverse reaction related to the use of the dressing or a non‐dressing related event." Comment: data reported for all participants randomised |
| Selective reporting (reporting bias) | Unclear risk | No direct quotation addressing this aspect Comment: limited information on which to judge |
| Other bias | Low risk | No direct quotation addressing this aspect Comment: none noted |
ITT: intention‐to‐treat; NR: not reported; PU: pressure ulcer; RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ashby 2012 | Although the study was an RCT, it had a mixed‐dressing control arm with foam dressings being 1 of 3 dressing options based on health professional and participant choice rather than randomisation |
| Avanzi 2000 | Paper incomplete and review authors unable to access it |
| Banks 1994b | PU classification system not stated. Review authors unable to access original data |
| Banks 1994c | PU classification system not stated. Review authors unable to access original data |
| Banks 1997 | PU classification system not stated. Review authors unable to access original data |
| Brown‐Etris 1996 | The intervention dressing (Transorbant) has dry hydrogel and foam layers. We excluded this study as the hydrogel layer was closest to the skin, and the foam was an outer layer that provided cushioning |
| Diehm 2005 | Not a RCT or cluster‐RCT |
| Münter 2006 | No subgroup analysis of participants with PUs. Review authors unable to access original data |
| Oleske 1986 | Not a RCT or cluster‐RCT |
| Palao i Domenech 2008 | No subgroup analysis of participants with PUs. Unable to contact study authors |
| Parish 2008 | Not a RCT or cluster‐RCT |
| Piatkowski 2012 | Administered foam dressing to participants on both trial arms |
| Reynolds 2004 | PU classification system not stated. Unable to contact study author |
| Romanelli 2009 | Subanalysis of larger study by Palao i Domenech 2008. Does not include participants with PUs |
| Wagstaff 2014 | While this trial compared two foams for the treatment of PUs, their application occurred as a component of negative pressure wound therapy following surgical debridement rather than as a wound dressing |
| Zimny 2003 | Participants had neuropathic foot ulcers, not PUs. |
PU: pressure ulcer; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ISRCTN57842461.
| Trial name or title | A multi‐centre, randomised, clinical trial comparing adhesive polyurethane foam dressing and adhesive hydrocolloid dressing in patients with grade II pressure ulcers in primary care and nursing homes |
| Methods | RCT |
| Participants | Planning to recruit 820 participants from primary health care and home care centres |
| Interventions | Adhesive polyurethane foam |
| Outcomes | Percentage of wounds healed after 8 weeks |
| Starting date | ISRCTN record shows starting date of 30 September 2012 and end date of 30 September 2015 |
| Contact information | M Guillén‐Solà: mguillen@ibsalut.caib.es |
| Notes | Country: Spain |
RCT: randomised controlled trial
Differences between protocol and review
Alternative pressure ulcer classification systems
The main difference between the protocol and the review is the description for assessing a Category/Stage I and II pressure ulcer.
The published protocol for the section titled 'Types of participants' described the following inclusion and exclusion criteria:
"People of any age with an existing pressure ulcer of Category/Stage II or above in any care settings. Studies including people with only Category/Stage I pressure ulcers are excluded, as, although ’at‐risk’ signs and symptoms of potential pressure ulcer such as non‐blanchable redness, pain, hardness or softness, heat or coolness are present, the skin remains intact AWMA 2012."
A posteriori uncertainty about what constituted a Category/Stage I and II pressure ulcer in alternative pressure ulcer classification systems led to the current review being changed. This was because the initial working definition was based upon the then Australian Wound Management Association and Pan Pacific Partners 'Pan Pacific Clinical Practice Guideline for the Prevention and Management of Pressure Injury'. At the time of writing the protocol it was a draft document for consultation. The accepted system of grading now more closely resembles other internationally accepted pressure ulcer grading systems.
In this review, as reported in 'Types of participants', we accepted study authors' alternative pressure ulcer classification systems, such as the Stirling (Reid 1994) and Torrence classification systems (Harker 2000), as well as earlier versions published by the NPUAP (NPUAP 1989), on the condition that the definitions of these alternative and previous versions closely matched the contemporary International NPUAP/EPUAP/PPPIA Pressure Ulcer Classification System Criteria (NPUAP/EPUAP/PPPIA 2014). See Table 6 'Comparisons of pressure ulcer classification systems'.
We also included studies that recruited participants with Category/Stage II pressure ulcers or above alongside with people with other types of chronic wounds, such as venous and arterial leg ulcers or diabetic foot ulcers, if the results for people with relevant pressure ulcers were presented separately (or this data were available from study authors). Similarly when a study included both Category/Stage I and more advanced pressure ulcers, we included it in the review only if data for Category/Stage II and above were reported separately or if the data were available on request from study authors. We also included studies where pressure ulcers from Category/Stage II and above were reported collectively.
Follow‐up periods
The included studies presented analyses for primary and secondary outcomes based on different time points. For clarity and consistency, we have presented the pooled analyses relative to short‐term follow‐up (8 weeks or less); medium follow‐up (24 weeks or less) and long‐term follow‐up (more than 24 weeks).
Contributions of authors
Rachel Walker: designed and coordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; made an intellectual contribution to the review; advised on the review; performed economic analysis and is guarantor of the review.
Brigid Gillespie: designed the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; checked the quality of the statistical analysis; contributed to writing or editing the review; made an intellectual contribution to the review and advised on the review.
Lukman Thalib: analysed or interpreted data; undertook quality assessment; performed statistical analysis; checked the quality of the statistical analysis; contributed to writing or editing the review; made an intellectual contribution to the review and advised on the review.
Niall Higgins: extracted data; checked the quality of data extraction; analysed or interpreted data; undertook quality assessment; contributed to writing or editing the review and made an intellectual contribution to the review.
Jennifer Whitty: analysed or interpreted data; contributed to writing or editing the review; made an intellectual contribution to the review; advised on the review and performed economic analysis.
Contributions of editorial base
Nicky Cullum (Coordinating Editor): advised on content, edited the protocol and approved the final protocol prior to submission.
Joan Webster (Editor): advised on content, edited the review and approved the final review prior to submission.
Gill Rizzello (Managing Editor) and Jo Dumville (Deputy Coordinating Editor): coordinated the editorial process and advised on methodology, interpretation and content.
Zipporah Iheozor‐Ejiofor (Methodologist): advised on methodology.
Reetu Child and Naomi Shaw (Information Specialists): designed the search strategy and edited the search methods sections.
Ursula Gonthier (Editorial Assistant): edited the reference sections of the review and the Plain Language Summary.
Sources of support
Internal sources
-
National Health and Medical Research Council, Australia.
The NHMRC has provided funding for this review from its former Centre of Research Excellence in Nursing Scheme, which funds one or more of the authors.
-
Griffith University, Australia.
Griffith University’s former NHMRC Centre for Research Excellence in Nursing has provided in‐kind support (time and overheads).
External sources
-
The National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Rachel Walker: none known
Brigid M Gillespie: none known
Lukman Thalib: none known
Niall S Higgins: none known
Jennifer Whitty: none known.
New
References
References to studies included in this review
Bale 1997 {published data only}
- Bale S, Squires D, Varnon T, Walker A, Benbrow M, Harding KG. A comparison of two dressings in pressure sore management. Journal of Wound Care 1997;6(10):463‐6. [PUBMED: 9455271] [DOI] [PubMed] [Google Scholar]
Bale 1998 {published data only}
- Bale S, Hagelstein S, Banks V, Harding KG. Costs of dressings in the community. Journal of Wound Care 1998;7(7):327‐30. [PUBMED: 9791356] [DOI] [PubMed] [Google Scholar]
Banks 1994a {published data only}
- Banks V, Harding KG. Superficial pressure sores: comparing two regimes. Journal of Wound Care 1994;3(1):8‐10. [ISSN: 0969‐0700] [DOI] [PubMed] [Google Scholar]
Meaume 2003 {published data only}
- Meaume S, Looverbosch D, Heyman H, Romanelli M, Ciangherotti A, Charpin S. A study to compare a new self‐adherent soft silicone dressing with a self‐adherent polymer dressing in Stage II pressure ulcers. Ostomy Wound Management 2003;49(9):44‐51. [PUBMED: 14581709] [PubMed] [Google Scholar]
Payne 2009 {published data only}
- Payne WG, Posnett J, Alvarez O, Brown‐Etris M, Jameson G, Wolcott R, et al. A prospective, randomized clinical trial to assess the cost‐effectiveness of a modern foam dressing versus a traditional saline gauze dressing in the treatment of Stage II pressure ulcers. Ostomy Wound Management 2009;55(2):50‐5. [PUBMED: 19246785] [PubMed] [Google Scholar]
Seeley 1999 {published data only}
- Seeley J, Jensen JL, Hutcherson J. A randomized clinical study comparing a hydrocellular dressing to a hydrocolloid dressing in the management of pressure ulcers. Ostomy Wound Management 1999;45(6):39‐44, 46‐7. [PUBMED: 10655861] [PubMed] [Google Scholar]
Sopata 2002 {published data only}
- Sopata M, Lucak J, Ciupinska M. Effect of bacteriological status on pressure ulcer healing in patients with advanced cancer. Journal of Wound Care 2002;11(3):107‐10. [PUBMED: 11933727] [DOI] [PubMed] [Google Scholar]
Souliotis 2016 {published data only}
- Souliotis K, Kalemikerakis I, Saridi M, Papageorgiou M, Kalokerinou A. A cost and clinical effectiveness analysis among moist wound healing dressings versus traditional methods in home care patients with pressure ulcers. Wound Repair and Regeneration 2016;24:596‐601. [PUBMED: 27037729] [DOI] [PubMed] [Google Scholar]
Thomas 1997 {published data only}
- Thomas S, Banks V, Bale S, Fear‐Price M, Hagelstein S, Harding KG, et al. A comparison of two dressings in the management of chronic wounds. Journal of Wound Care 1997;6(8):383‐6. [PUBMED: 9341430] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ashby 2012 {published data only}
- Ashby RL, Dumville JC, Soars MO, McGinnis E, Stubbs N, Torgerson DJ, et al. A pilot randomised controlled trial of negative pressure wound therapy to treat Grade III/IV pressure ulcers. Trials 2012;13(119):16. [ISRCTN69032034; PUBMED: 22839453] [DOI] [PMC free article] [PubMed] [Google Scholar]
Avanzi 2000 {unpublished data only}
- Avanzi A, Martinelli M, Accardi S, Giraudi C, Peroli P. Adhesive hydrocellular dressing vs hydrocolloid dressing in the treatment of 2nd and 3rd degree PUS: a prospective, controlled randomised comparative multi‐centre clinical evaluation. www.akademie‐zwm.ch/uploads/tx_scpublications/Allevynheel_ProfBodi_Firenze2001.pdf (accessed 10 August 2016).
Banks 1994b {published data only}
- Banks V, Bale S, Harding K. The use of two dressings for moderately exuding pressure sores. Journal of Wound Care 1994;3(3):132‐4. [DOI] [PubMed] [Google Scholar]
Banks 1994c {published data only}
- Banks V, Bale SE, Harding KG. Comparing two dressings for exuding pressure sores in community patients. Journal of Wound Care 1994;3(4):175‐8. [DOI] [PubMed] [Google Scholar]
Banks 1997 {published data only}
- Banks V, Bale S, Harding K, Harding EF. Evaluation of a new polyurethane foam dressing. Journal of Wound Care 1997;6(6):266‐8. [PUBMED: 9274262 ] [DOI] [PubMed] [Google Scholar]
Brown‐Etris 1996 {published data only}
- Brown‐Etris M, Fowler E, Papen J, Stanfield J, Harris A, Tintle T, et al. Comparison and evaluation of the performance characteristics, usability and effectiveness on wound healing of Transorbent versus Duoderm CGF. Fifth European Conference on Advances in Wound Management; 1995 November; Harrogate. London: Macmillan Magazines Ltd, 1996:151‐4. [ISBN: 0333680820]
Diehm 2005 {published data only}
- Diehm C, Lawall H. Evaluation of Tielle hydropolymer dressings in the management of chronic exuding wounds in primary care. International Wound Journal 2005;2(1):26‐35. [PUBMED: 16722852 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Münter 2006 {published data only}
- Münter KC, Beele H, Russell L, Crespi A, Gröchenig E, Basse P, et al. Effect of a sustained silver‐releasing dressing on ulcers with delayed healing: the CONTORP study. Journal of Wound Care 2006;15(5):199‐206. [PUBMED: 16711173] [DOI] [PubMed] [Google Scholar]
Oleske 1986 {published data only}
- Oleske DM, Smith XS, White P, Pottage J, Donovan MI. A randomized controlled trial of two dressing methods for the treatment of low‐grade pressure ulcers. Journal of Enterostomal Therapy 1986;13(3):90‐8. [PUBMED: 3519718] [DOI] [PubMed] [Google Scholar]
Palao i Domenech 2008 {published data only}
- Palao i Domenech R, Romanelli M, Tsiftsis DD, Slonková V, Jortikka A, Johannesen N, et al. Effect of an ibuprofen‐releasing foam dressing on wound pain: a real‐life RCT. Journal of Wound Care 2008;17(8):342, 344‐8. [PUBMED: 18754195] [DOI] [PubMed] [Google Scholar]
Parish 2008 {published data only}
- Parish LC, Dryjski M, Cadden S, Versiva XC Pressure Ulcer Study Group. Prospective clinical study of new adhesive gelling foam dressing in pressure ulcers. International Wound Journal 2008;5(1):60‐7. [DOI: 10.1111/j.1742-481X.2007.00428.x.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piatkowski 2012 {published data only}
- Piatkowski A, Ulrich D, Seidel D, Abel M, Pallua N, Andriessen A. Randomized, controlled pilot to compare collagen and foam in stagnating pressure ulcers. Journal of Wound Care 2012;21(10):505‐11. [PUBMED: 23103485 ] [DOI] [PubMed] [Google Scholar]
Reynolds 2004 {published data only}
- Reynolds T, Russell L, Deeth M, Jones H, Birchall L. A randomised controlled trial comparing Drawtex with standard dressings for exuding wounds. Journal of Wound Care 2004;14(2):71‐4. [PUBMED: 14999992 ] [DOI] [PubMed] [Google Scholar]
Romanelli 2009 {published data only}
- Romanelli M, Dini V, Polignano R, Bonadeo P, Maggio G. Ibuprofen slow‐release foam dressing reduces wound pain in painful exuding wounds: preliminary findings from an international real‐life study. Journal of Dermatological Treatment 2009;20(1):19‐26. [DOI: 10.1080/09546630802178232.] [DOI] [PubMed] [Google Scholar]
Wagstaff 2014 {published data only}
- Wagstaff MJ, Driver S, Coghlan P, Greenwood JE. A randomized, controlled trial of negative pressure wound therapy of pressure ulcers via a novel polyurethane foam. Wound Repair and Regeneration 2014;22(2):205‐11. [DOI: 10.1111/wrr.12146] [DOI] [PubMed] [Google Scholar]
Zimny 2003 {published data only}
- Zimny S, Schatz H, Pfohl U. The effects of applied felted foam on wound healing and healing times in the therapy of neuropathic diabetic foot ulcers. Diabetic Medicine 2003;20(8):622‐5. [PUBMED: 12873288] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN57842461 {published data only}
- ISRCTN57842461. To compare the polyurethane foam dressing (hydrocellular) and the hydrocolloid dressing in patients with pressure ulcers (stage II) in primary care. www.isrctn.com/ISRCTN5784246 (accessed 11 August 11 2016). [DOI: 10.1186/ISRCTN57842461] [DOI]
Additional references
AHRQ 2014
- US Agency for Healthcare Research and Quality (AHRQ). Are we ready for this change? Preventing pressure ulcers in hospitals: a toolkit for improving quality of care. www.ahrq.gov/professionals/systems/hospital/pressureulcertoolkit/putool1.html (accessed 26 October 2016).
BNF 2016
- British Medical Association, British Royal Pharmaceutical Society of Great Britain. Wound management products and elasticated garments. www.medicinescomplete.com/mc/bnf/current/PHP101071‐wound‐management‐products‐and‐elasticated‐garments.htm (accessed 10 August 2016).
Cardinal 2009
- Cardinal M, Eisenbud DE, Armstrong DG, Zelen C, Driver V, Attinger C, et al. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair and Regeneration 2009;17(3):306‐11. [PUBMED: 19660037] [DOI] [PubMed] [Google Scholar]
Chaby 2007
- Chaby G, Senet P, Vaneau M, Martel P, Guillaume JC, Meaume S, et al. Dressings for acute and chronic wounds: a systematic review. Archives of Dermatology 2007;143:1297‐304. [DOI] [PubMed] [Google Scholar]
CRD 2017
- Centre for Reviews and Dissemination (CRD). Search strategies. www.crd.york.ac.uk/crdweb/searchstrategies.asp (accessed 21 September 2017).
Dealey 2012
- Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. Journal of Wound Care 2012;21(6):261‐2, 264, 266. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Gillespie 2012
- Gillespie BM, Chaboyer W, Niuewenhoven P, Rickard CM. Drivers and barriers of surgical wound management in a large healthcare organisation: results of an environmental scan. Wound Practice and Research 2012;20(2):90‐202. [Google Scholar]
Graves 2014
- Graves N, Birrell F, Whitby M. Modelling the direct health care costs of chronic wounds in Australia. Wound Practice & Research: Journal of the Australian Wound Management Association 2014;22(2):20‐4, 26‐33. [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64:407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Hamilton 2008
- Hamilton C. Speculating to accumulate: reducing the cost of wound care by appropriate dressing selection. Journal of Wound Care 2008;17(8):359‐63. [DOI] [PubMed] [Google Scholar]
Harker 2000
- Harker J. Pressure ulcer classification: the Torrance system. Journal of Wound Care 2000;9(6):275‐7. [PUBMED: 11933341] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
House 2011
- House S, Giles T, Whitcomb J. Benchmarking to the international pressure ulcer prevalence survey. Journal of Wound, Ostomy and Continence Nursing 2011;38(3):254‐9. [DOI] [PubMed] [Google Scholar]
Husereau 2013
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) ‐ explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value in Health 2013;16:231‐50. [DOI] [PubMed] [Google Scholar]
Kirman 2008
- Kirman CN, Geibel J. Pressure ulcers and wound care treatment and management. emedicine.medscape.com/article/190115‐treatment (accessed 10 August 2016).
Lahmann 2006
- Lahmann N, Halfens R, Dassen T. Pressure ulcers in German nursing homes and acute care hospitals: prevalence, frequency and ulcer characteristics. Ostomy Wound Management 2006;52(2):20‐33. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maylor 1997
- Maylor ME. Knowledge base and use in the management of pressure sores. Journal of Wound Care 1997;6(5):244‐7. [DOI] [PubMed] [Google Scholar]
Miles 2013
- Miles SJ, Fulbrook P, Nowicki T, Franks C. Decreasing pressure injury prevalence in an Australian general hospital: a 10‐year review. Wound Practice & Research: Journal of the Australian Wound Management Association 2013;21:148‐56. [Google Scholar]
Moore 2005
- Moore Z. Pressure ulcer grading. Nursing Standard 2005;19:56‐64. [DOI] [PubMed] [Google Scholar]
Nguyen 2015
- Nguyen K‐H, Chaboyer W, Whitty JA. Pressure injury in Australian public hospitals: a cost‐of‐illness study. Australian Health Review 2015;39(3):329‐36. [PUBMED: 25725696 ] [DOI] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence (NICE). Pressure ulcers: prevention and management. Clinical guideline [CG179]. April 2014. www.nice.org.uk/guidance/cg179 (accessed 9 October 2017). [PubMed]
NICE 2017
- National Institute for Health and Care Excellence (NICE). Surgical site infections: prevention and treatment. Clinical guideline [CG74]. February 2017. www.nice.org.uk/guidance/cg74 (accessed 9 October 2017).
NPUAP 1989
- National Pressure UIcer Advisory Panel (NPUAP). Pressure ulcers prevalence, cost and risk assessment: consensus development conference statement. Decubitus 1989;2(2):24‐8. [PUBMED: 2665781] [PubMed] [Google Scholar]
NPUAP/EPUAP/PPPIA 2014
- National Pressure Ulcer Advisory Panel (NPUAP), European Pressure Ulcer Advisory Panel (EPUAP) and Pan Pacific Pressure Injury Alliance (PPPIA). Prevention and treatment of pressure ulcers: quick reference guide October 2014. www.npuap.org/wp‐content/uploads/2014/08/Updated‐10‐16‐14‐Quick‐Reference‐Guide‐DIGITAL‐NPUAP‐EPUAP‐PPPIA‐16Oct2014.pdf (accessed 18 May 2016).
Pieper 1995
- Pieper B, Mott M. Nurses' knowledge of pressure ulcer prevention, staging, and description. Advances in Wound Care 1995;8(3):34, 38, 40 passim. [PubMed] [Google Scholar]
Polit 2010
- Polit DF, Gillespie BM. Intention‐to‐treat in randomized controlled trials: recommendations for a total trial strategy. Research in Nursing & Health 2010;33:355‐68. [PUBMED: 20645423] [DOI] [PubMed] [Google Scholar]
QSB 2006
- Quality, Safety Branch (QSB). PUPPS 3 ‐ Pressure ulcer point prevalence survey. Statewide report 2006. www2.health.vic.gov.au/about/publications/researchandreports/pressure‐ulcer‐prevalence‐survey (accessed 9 October 2017).
Reddy 2008
- Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA 2008;300(22):2647‐62. [DOI] [PubMed] [Google Scholar]
Reid 1994
- Reid J, Morison M. Towards a consensus: classification of pressure sores. Journal of Wound Care 1994;3(3):157‐60. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russo 2003
- Russo CA, Elixhauser A. Hospitalizations related to pressure sores, 2003. www.hcup‐us.ahrq.gov/reports/statbriefs/sb3.pdf (accessed 18 May 2016). [PubMed]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e1000251. [PUBMED: 20352064] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting Results and Drawing Conculsions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shanin 2008
- Shanin E, Dassen T, Halfens R. Pressure ulcer prevalence and incidence in intensive care patients: a literature review. Nursing in Critical Care 2008;13(2):71‐9. [DOI] [PubMed] [Google Scholar]
Shemilt 2011
- Shemilt I, Mugford M, Byford S, Drummond M, Eisenstein E, Knapp M, et al. Chapter 15: Incorporating economics evidence. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
SIGN 2017
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/search‐filters.html (accessed 9 October 2017).
Smith 2013
- Smith ME, Totten A, Hickman DH, Fu R, Wasson N, Rahman B, et al. Pressure ulcer treatment strategies: a systematic comparative effectiveness review. Annals of Internal Medicine 2013;159(1):39‐50. [PUBMED: 23817703 ] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;7(8):16. [PUBMED: 17555582 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanderwee 2007
- Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. Journal of Evaluation in Clinical Practice 2007;13:227‐35. [DOI] [PubMed] [Google Scholar]
VanGilder 2009
- VanGilder C, Amlung S, Harrison P, Meyer S. Results of the 2008–2009 International Pressure Ulcer Prevalence ™ Survey and a 3‐year, acute care, unit‐specific analysis. Ostomy Wound Management 2009;55:39‐45. [PubMed] [Google Scholar]
VQC 2004
- Victorian Quality Commission (VQC). State‐wide pressure ulcer point prevalence survey 2 report 2004. health.vic.gov.au/pu_basics/resources/vcq_pupps_2.pdf (accessed 18 May 2016).
Westby 2017
- Westby MJ, Dumville JC, Soares MO, Stubbs N, Norman G. Dressings and topical agents for treating pressure ulcers. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD011947.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winter 1962
- Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293‐4. [DOI] [PubMed] [Google Scholar]
Winter 1963a
- Winter GD, Scales JT. Effect of air drying and dressings on the surface of a wound. Nature 1963;197:91‐2. [DOI] [PubMed] [Google Scholar]
Winter 1963b
- Winter GD. Effect of air exposure and occlusion on experimental human skin wounds. Nature 1963;200:378‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Walker 2014
- Walker RM, Keogh SJ, Higgins NS, Whitty JA, Thalib L, Gillespie BM, et al. Foam dressings for treating pressure ulcers. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD011332] [DOI] [PMC free article] [PubMed] [Google Scholar]


