Abstract
Although oncogenic activation of nuclear factor (NF)-κB has been identified in various tumors, the NF-κB-activating kinases (inhibitor of NF-κB kinases, IKK) responsible for this are elusive. In this study, we determined the role of IKKα and IKKβ in KRAS-mutant lung adenocarcinomas induced by the carcinogen urethane and by respiratory epithelial expression of oncogenic KRASG12D. Using NF-κB reporter mice and conditional deletions of IKKα and IKKβ, we identified two distinct early and late activation phases of NF-κB during chemical and genetic lung adenocarcinoma development, which were characterized by nuclear translocation of RelB, IκBβ, and IKKα in tumor-initiated cells. IKKα was a cardinal tumor promoter in chemical and genetic KRAS-mutant lung adenocarcinoma, and respiratory epithelial IKKα-deficient mice were markedly protected from the disease. IKKα specifically cooperated with mutant KRAS for tumor induction in a cell-autonomous fashion, providing mutant cells with a survival advantage in vitro and in vivo. IKKα was highly expressed in human lung adenocarcinoma, and a heat shock protein 90 inhibitor that blocks IKK function delivered superior effects against KRAS-mutant lung adenocarcinoma compared with a specific IKKβ inhibitor. These results demonstrate an actionable requirement for IKKα in KRAS-mutant lung adenocarcinoma, marking the kinase as a therapeutic target against this disease.
Keywords: NF-κB, CHUK, urethane, bioluminescence, non-oncogene addiction
Introduction
Tumors harboring mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) are notoriously resistant to current treatments (1). Lung adenocarcinoma (LADC), the number one cancer killer worldwide (2), harbors KRAS mutations in up to 30-40% of the cases diagnosed in Europe and North America (3). A cardinal mechanism of KRAS mutation-associated drug resistance appears to be the oncogene’s addiction to transcriptional programs that facilitate sustained tumor-initiated cell survival, such as nuclear factor (NF)-κB (4). To this end, mutant KRAS was recently shown to interact with NF-κB-activating kinases [inhibitor of NF-κB (IκB) kinases, IKKs] to promote cancer cell survival, stemness, and drug resistance (5, 6).
NF-κB is activated via the canonical (involving IκBα, IKKβ, and RelA/P50) and non-canonical (comprising IκBβ, IKKα, and RelB/P52) pathways (7). We and others previously documented NF-κB activation in murine and human LADC (8–10). However, the IKKs responsible for this remain elusive, and most studies focused on IKKβ, IKKε, and TANK-binding kinase 1 (TBK1; 11–14). IKKα participates in both canonical and non-canonical NF-κB pathways, and co-operates with IKKβ for tumor cell growth in vitro (11, 15), but its role in LADC development in vivo is uncharted.
We deployed NF-κB reporter and conditional IKKα and IKKβ-deleted mice to decipher the timing of NF-κB activation and the mutual impact of IKKα and IKKβ on LADC development. In mouse models of tobacco carcinogen- and oncogenic KRAS G12D-triggered LADC, IKKα was cardinal for disease initiation and progression. Moreover, IKKα selectively fostered cellular proliferation in the context of mutant KRAS, and was also highly expressed in human LADC. Importantly, dual IKKα / IKKβ inhibition yielded promising results against KRAS-driven LADC, lending hope for translational applications of our findings.
Materials and Methods
Additional Methods are described in the Online Supplement.
Mice
C57BL/6J (#000664), FVB/NJ (#001800), B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (mT/mG; #007676; 16), B6.129S4-Krastm4Tyj/J (LSL.KRASG12D; #008179; 17), NOD.CB17-Prkdc<scid>/J (NOD/SCID; #001303), and FVB.129S6(B6)-Gt(ROSA)26Sortm1(Luc)Kael/J (LSL.R26.Luc; #005125; 18) mice were from Jackson Laboratories (Bar Harbor, ME). NF-κB reporter mice (NGL; NF-κB.GFP.Luciferase), B6.B4B6-Chuk<tm1Mpa>/Cgn (Chukf/f), and B6.B4B6-Ikbkb<tm2.1Mpa>/Cgn (Ikbkbf/f), B6;CBA-Tg(Scgb1a1-cre)1Vart/Flmg (Scgb1a1.Cre), and Tg(Sftpc-cre)1Blh (Sftpc.Cre) mice have been described (8, 19–21). Mice were bred >F9 to the C57BL/6 and/or FVB backgrounds at the University of Patras Center for Animal Models of Disease. The number of mice used for these studies (n = 542) is detailed in Supplementary Table S1.
Reagents
Urethane (CAS#51-79-6) and 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay were from Sigma (St. Louis, MO), adenoviruses from the Vector Development Lab of the Baylor College of Medicine (Houston, TX), D-luciferin from Gold Biotechnology (St. Louis, MO), HEK293T cells from ATCC (Wesel, Germany), and Lewis lung carcinoma (LLC) and A549 lung adenocarcinoma cells from the NCI Tumor Repository (Frederick, MD). Primers and antibodies are listed in Supplementary Tables S2 and S3. Lentiviral shRNA pools (Santa Cruz, Dallas, TX) are described in Supplementary Table S4.
Mouse models of LADC
Chemical-induced LADC was induced in FVB and C57BL/6 mice, respectively, by a single or by ten consecutive weekly intraperitoneal exposures to 1 g/Kg urethane (8, 22–24). KRASG12D-driven LADC was induced via intratracheal injections of 5 x 108 plaque-forming units (PFU) adenovirus type 5 encoding CRE recombinase (Ad-Cre) to LSL.KRASG12D mice on the C57BL/6 background (9, 17). NOD/SCID and C57BL/6 mice were anesthetized by isoflurane and received 2 x 106 HEK293T and 0.5 x 106 tumor cells into the rear flank and vertical tumor diameters (δ) were measured and mice were imaged for bioluminescent detection of cell mass weekly thereafter. Cell spot volume (V) was calculated as V = π×(δ1×δ2×δ3)/6, and mice were killed after six weeks. Flank tumors were harvested and fixed with 4% paraformaldehyde or processed for immunoblotting.
Drug treatments
LSL.KrasG12D;LSL.R26.Luc mice received 5 x 108 PFU intratracheal Ad-Cre followed by daily intraperitoneal injections of 100μL saline or 0.5 mg/Kg TPCA-1 or 17-DMAG in 100 μL saline at days14-28 or 84-112 post-Ad-Cre. Mice were imaged for bioluminescent detection of LADC burden at 0, 14, 28, 84, and 112 days post-Ad-Cre. Mice were sacrificed and lungs were harvested at 112 days post-Ad-Cre.
Cellular Assays
Mouse primary lung adenocarcinoma cells and airway epithelial cells were derived from the lungs of urethane (single dose 1 g/Kg) or saline-treated FVB or C57BL/6 mice by simple tumor or large airway dissection or epithelial stripping, respectively, and 5-month or 5-day culture, respectively, as described elsewhere (25). These cell lines were named XYLA# with X signifying the mouse strain (F, FVB; C, C57BL/6), Y the carcinogen used (U, urethane), LA lung adenocarcinoma, and # their serial number by derivation date. Cells were cultured at 37°C in 5% CO2-95% air using DMEM supplemented with 10% FBS, 2mM L-glutamine, 1 mM pyruvate, 100 U/ml penicillin, and 100mg/ml streptomycin. Cells were tested biannually for identity (by the short tandem repeat method) and for Mycoplasma Spp. (by PCR). For experiments, frozen cells were reconstituted and were passaged 2-5 times for less than two weeks. In vitro cancer cell proliferation was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For this, 2 x 104 cells/well were plated onto 96-well plates. Daily thereafter, 15 μL of 5 mM MTT working solution in PBS was added to wells to be measured that day. The plate was incubated for 4 h at 37 °C in a 5% CO2 humidified incubator followed by addition of 100 μL acidified isopropanol per well for sediment solubilization and absorbance measurement at 492 nm on a MR-96A photometer (Mindray, Shenzhen, China). For soft agar colony formation assay, 7.5 x 103 cells were plated in 60 mm culture vessels in semi-solid 0.7% agarose in full culture medium and were incubated for 30 days at 37 °C in a 5% CO2 humidified incubator. 2 mL fresh culture medium was added to each vessel biweekly. After incubation, 500 μL MTT working solution was added to each vessel and plates were dried, inverted, photographed, and colonies were counted, as described elsewhere (25).
Human samples
Matched tumor and normal lung tissue RNA and sections of 23 and 35, respectively, previously reported patients with LADC from Institution 3 were used for microarray and immunohistochemistry for IKKα and IKKβ (26). Human studies were approved a priori by the ethics committee of the University of Lübeck, Germany (approval # AZ 12-220) and were conducted according to the Declaration of Helsinki. Written informed consent was obtained from all patients. IKK score was 0, 1, 2, or 3 for no, cytoplasmic only, cytoplasmic and nuclear, and nuclear only immunoreactivity, respectively (modified from 11).
Statistics
Sample size (n; always biological) was determined using G*power (http://www.gpower.hhu.de/), assuming α = 0.05, β = 0.05, and d = 1.5. Data were acquired by two blinded readers, reevaluated if >20% deviant (no data were excluded), examined for normality by Kolmogorov-Smirnof test, and presented as median (interquartile range) or mean ± SEM. Differences in frequencies were examined by Fischer’s exact/χ2 tests, in means of normally distributed variables by t-test or one-way ANOVA/Bonferroni post-tests, and in medians of non-normally distributed variables by Mann-Whitney test or Kruskal-Wallis/Dunn’s post-tests. Survival and flank tumor volume were examined by Kaplan-Meier estimates/log-rank tests and two-way ANOVA/Bonferroni post-tests. Probability (P) is two-tailed; P < 0.05 was considered significant. Statistics and plots were done on Prism v5.0 (GraphPad, La Jolla, CA).
Study approval
All animal experiments were approved a priori by the Veterinary Administration of Western Greece according to a full and detailed protocol (approval # 276134/14873/2). Male and female mice were sex-, weight (20-25 g)-, and age (6-12 week)-matched. Human studies were approved a priori by the ethics committee of the University of Lübeck, Germany (approval # AZ 12-220).
Results
NF-κB is activated in KRAS-mutant LADC
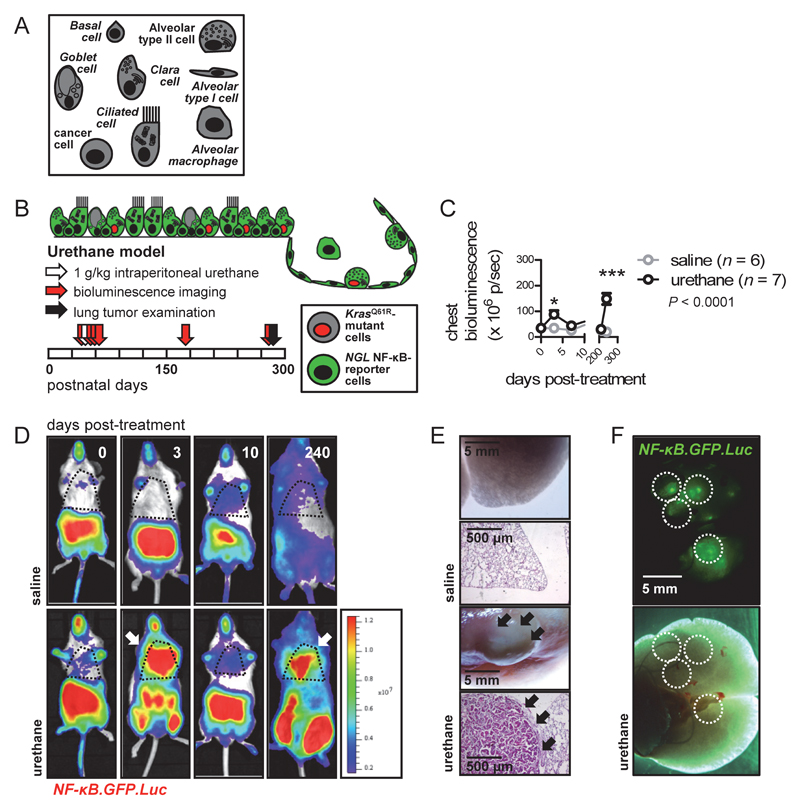
To map pulmonary NF-κB activity during KRAS-driven neoplasia, NF-κB reporter mice (NGL) on the carcinogen-susceptible FVB background expressing NF-κB-driven Photinus Pyralis luciferase (LUC) in-frame with EGFP (8, 23) received a single intraperitoneal injection of saline or the tobacco carcinogen urethane (1g/Kg) and were serially imaged for bioluminescence. Urethane causes respiratory epithelial KrasG12V/Q61R mutations and progressive inflammation, hyperplasias, adenomas, and adenocarcinomas in FVB mice (22–25) that in this experiment also expressed the NGL reporter (Figs. 1A, B). In addition to the baseline signals of these mice, markedly increased light emission from the chest was exclusively detected in urethane-treated mice at early and late time-points corresponding to carcinogen-induced inflammation and LADC, respectively (8). Enhanced NF-κB activation indicated by the EGFP reporter emanated exclusively from LADC (Figs. 1C-F). In a second approach, NGL mice were intercrossed with mice carrying a conditional loxP-STOP-loxP.KRASG12D allele (LSL.KRASG12D; 17), and NGL and NGL;LSL.KRASG12D offspring (all C57BL/6 background) received intratracheal Ad-Cre and were longitudinally imaged. In LSL.KRASG12D mice, progressive inflammation, hyperplasia, adenomas, and adenocarcinomas carrying the KRAS mutation are inflicted by Ad-Cre (9, 17). To titrate Ad-Cre, mT/mG CRE-reporters that switch from membranous Tomato (mT) to EGFP (mG) fluorescence upon CRE-recombination (16), received 0, 5 x 107, 5 x 108, or 5 x 109 PFU intratracheal Ad-Luc or Ad-Cre and were killed upon subsidence of transient Ad-mediated transgene expression at two weeks post-injection (27). The low, intermediate, and high Ad-Cre titers, respectively, caused infrequent, stochastic, and ubiquitous respiratory epithelial recombination (Supplementary Figs. S1A, B). We selected 5 x 108 PFU Ad-Cre to stochastically induce recombination into the respiratory epithelium of NGL and NGL;LSL.KRASG12D mice (Supplementary Fig. S1C). Similar to the urethane model, two phases of enhanced chest light emission by NGL;LSL.KRASG12D but not NGL mice were observed, coinciding with early inflammation and late LADC development (8, 22–24, 28). Again, NF-κB-dependent EGFP expression was confined to LADC (Supplementary Figs. S1D-S1G). These data demonstrate biphasic pulmonary NF-κB activation during KRAS-driven LADC development.
Figure 1. NF-κB activation in urethane-induced lung adenocarcinoma.
(A-F) NGL mice were backcrossed > F9 to the carcinogen- susceptible FVB strain and received single intraperitoneal injections of saline (n = 6) or the tobacco-contained carcinogen urethane (1 g/Kg; n = 7) and were imaged longitudinally for bioluminescence. (A) Legend to respiratory epithelial cells used in schematics throughout. (B) Schematic of experimental time-course (boxes = months) and topology of NF-κB-reporter (green cytosol) versus KrasQ61R-mutant (red nucleus) cells in this model. (C) Data summary of chest bioluminescence shown as mean (points), SEM (bars), and two-way ANOVA P value. * and ***: P < 0.05 and P < 0.001, respectively, for urethane- compared with saline-treated mice by Bonferroni post-tests. (D) Representative merged bioluminescent/photographic images with pseudocolor scale showing increased chest light emission of urethane-treated mice at early and late time- points after carcinogen injection (arrows). (E) Representative images of gross lungs and hematoxylin/eosin-stained lung sections of saline- and urethane-treated mice at eight months post-injection showing lung adenocarcinomas in the latter (arrows). (F) Light- optic and green fluorescent lung images of representative urethane-treated mouse at eight months post-injection showing NF-κB-driven GFP expression in lung adenocarcinomas (dashed lines).
KRAS-mutant LADC displays both canonical and non-canonical NF-κB activity
To investigate the NF-κB pathway at play during KRAS-mutant inflammation, hyperproliferation, and LADC formation, the immunoreactivity of nuclear and cytoplasmic protein extracts of whole lungs of urethane-treated FVB mice and of Ad-Cre-treated LSL.KRAS G12D mice for NF-κB subunits, kinases, and inhibitors were probed longitudinally (Figs. 2A-D). In the urethane model, marked RelB and RelA immunoreactivity was detected in nuclear extracts and enhanced IκBα, IκBβ, IKKα, IKKβ, and TBK1 immunoreactivity in cytoplasmic extracts of the neoplastic stage. Some immunoreactivity was also present in early stages but their expression peaked in tumor-bearing lungs, while no IKKε signal was evident at any time-point. In the LSL.KRASG12D model, enhanced nuclear RelB and P52 and modest RelA immunoreactivity was detected in nuclear extracts of tumor bearing lungs, together with some cytoplasmic immunoreactivity for IκBβ, IKKα, and TBK1 (120 days). In addition, some RelA, RelB, P52, IκBα, IκBβ, and TBK1 immunoreactivity was evident in same-day-treated lungs (0 days), some RelA, RelB, P52, IKKα, and TBK1 immunoreactivity in inflammatory and proliferative lungs (30 and 60 days), and no IKKβ and IKKε signal at any stage. IKK expression patterns were corroborated using immunofluorescent detection of IKKα/IKKβ on lung sections of urethane-treated FVB and Ad-Cre-treated LSL.KRASG12D mice at 240 and 120 days post-treatment, respectively. In both models, IKKα was expressed by a significant proportion of LADC cells, while minimal IKKβ expression was detectable (Figs. 2E, F). To further characterize NF-κB activity of LADC, LADC cells were derived from the lungs of saline and urethane-treated FVB mice, according to established methods (Supplementary Figs. S2A,B; 25, 29). LADC cells exhibited enhanced nuclear RelB (but not RelA) localization and activity compared with saline- and urethane-treated lungs (Supplementary Figs. S2C,D). Taken together, these results indicate co-activation of the canonical and non-canonical NF-κB pathways in LADC.
Figure 2. Increased NF-κB activity and enhanced IKKα expression of KRAS- driven lung adenocarcinoma.
(A, C, E) FVB mice (n = 14) received 1 g/Kg intraperitoneal urethane and were sacrificed after the indicated time intervals. (B, D, F) Mice carrying a conditional loxP-STOP-loxP.KRASG12D allele (LSL.KRASG12D; C57BL/6 strain; n = 12) received 5 x 108 intratracheal PFU adenovirus encoding CRE (Ad-Cre) and were sacrificed after the indicated time intervals. (A, B) Schematic representations of intensity and time-course of inflammation, hyperplasia, and tumorigenesis in the two models (8, 20-22, 25). (C, D) Immunoblots of whole lung nuclear and cytoplasmic extracts for NF-κB pathway components. (C) Note the increased expression of RelA, RelB, IκBβ, IKKα, IKKβ, and TBK1 at late time-points post-urethane, when lung adenocarcinomas have developed. (D) Note the increased expression of RelB, P52, and TBK1 at four months post-Ad-Cre, when lung adenocarcinomas have developed. (E) FVB mice (n = 5) received 1 g/Kg intraperitoneal urethane and were sacrificed after eight months for fluorescent detection of IKKα and IKKβ immunoreactivity on cryosections of lungs with bronchi (b) and alveoli (a) and lung tumors (dashed lines). (F) LSL.KRASG12D mice (C57BL/6 strain; n = 5) received 5 x 108 intratracheal PFU Ad-Cre and were sacrificed after four months for fluorescent detection of IKKα and IKKβ immunoreactivity on cryosections of lungs with bronchi (b) and alveoli (a) and lung tumors (dashed lines). Note the increased immunoreactivity of lung adenocarcinomas for IKKα (arrows). Rel, v-rel avian reticuloendotheliosis viral oncogene homolog; IκB, inhibitor of NF-κB; IKK, inhibitor of NF-κB kinase; TBK, TANK-binding kinase.
Respiratory epithelial IKKα promotes KRAS-driven LADC
We next functionally assessed the role of IKKα and IKKβ in LADC development, utilizing conditional IKKα and IKKβ gene-deleted mice (Chukf/f and Ikbkbf/f) that feature loxP-flanked alleles excised upon CRE expression (19). In a first line of experiments, mT/mG CRE-reporter (control), Chukf/f, and Ikbkbf/f mice on the urethane-resistant C57BL/6 background (8) received 5 x 109 PFU intratracheal Ad-Cre (a titer causing recombination in ~75% of the respiratory epithelium within two weeks; Supplementary Figs. S1A, B), and were started two weeks thereafter on ten weekly doses of 1g/kg intraperitoneal urethane, a regimen that reproducibly induces LADC in C57BL/6 mice (23, 29). In this multi-hit model, stochastic KRAS mutations, inflammation, apoptosis, and regeneration were repeatedly inflicted across IKK-deleted and non-deleted respiratory epithelium (Fig. 3A). Interestingly, Ikbkbf/f mice displayed decreased survival during repeated urethane exposures, suggesting a role for IKKβ in epithelial repair (Fig. 3B). However, at six months post-urethane start, IKKα-deleted mice had markedly decreased LADC incidence, multiplicity, and burden per lung compared with controls, while IKKβ-deleted mice displayed only minor reductions in tumor multiplicity but not burden (Figs. 3C-G). These experiments were replicated on Chukf/f and Ikbkbf/f mice back-crossed >F9 to the single-hit FVB model that recapitulates the mutation spectrum of human LADC and allows separate insights into the effects of IKK deletion on tumor initiation and progression via observations on LADC number and size after six months (8, 22–24). For this, WT control, Chukf/f, and Ikbkbf/f mice (all FVB) received 5 x 109 PFU intratracheal Ad-Cre, followed by a single intraperitoneal exposure to 1g/kg urethane (Supplementary Fig. S3A). All genotypes comparably survived single-hit urethane (Supplementary Fig. S3B). Again, Chukf/f mice developed fewer and smaller LADC compared with controls, indicating marked tumor-initiating and promoting effects of IKKα, but Ikbkbf/f mice displayed tumor incidence, number, size, and load closely resembling WT littermates, suggesting that the minor tumor-promoting effects of IKKβ require repetitive carcinogen challenge to become evident (Supplementary Figs S3C-G). Chukf/f and Ikbkbf/f mice were also intercrossed with Scgb1a1.Cre (20) and Sftpc.Cre (21) CRE-drivers (all C57BL/6) and their offspring received ten consecutive weekly intraperitoneal injections of 1 g/Kg urethane starting at six weeks of age (Supplementary Fig. S4A). Interestingly, both Scgb1a1.Cre and Sftpc.Cre-driven IKKα-deletion was equally protective against LADC, while IKKβ-deletion had no effect (hence pooled Scgb1a1.Cre and Sftpc.Cre data are presented; Supplementary Figs. S4B-E). To solidify the link between IKKα and mutant KRAS and to discriminate between cell-autonomous and paracrine IKKα effects, Chukf/f and Ikbkbf/f mice were intercrossed with LSL.KRASG12D mice (all C57BL/6) and their offspring received 5 x 108 PFU intratracheal Ad-Cre, a model where KRASG12D-expression and IKK-deletion coincide (Fig. 4A). Lung morphometry (30) at four months post-Ad-Cre showed that IKKα-deleted mice had markedly decreased LADC burden compared with controls, while IKKβ-deleted mice displayed an intermediate phenotype (Figs. 4B-E). Collectively these findings show that IKKα promotes KRAS-mutant LADC in a cell-autonomous fashion, independent from and more pronounced than IKKβ.
Figure 3. Adenoviral-mediated IKKα deletion from the respiratory epithelium protects C57BL/6 mice from multi-hit urethane-induced lung adenocarcinoma.
Conditional CRE-reporter (mT/mG) and IKKα (Chukf/f) or IKKβ (Ikbkbf/f) gene-deleted mice (C57BL/6 background) received 5 x 109 PFU intratracheal Ad-Cre followed by ten consecutive weekly intraperitoneal urethane injections (1 g/Kg) commenced two weeks post-Ad-Cre and were killed six months later. (A) Schematic of experimental time-course (boxes = months) and topology of IKK-deleted (pink cytosol) versus KrasQ61R-mutant (red nucleus) cells in this model. (B) Kaplan-Meier plot of survival with log-rank P value. ns and *: P > 0.05 and P < 0.05, respectively, for the indicated comparisons by log-rank test. (C) Frequency distribution of lung tumors with n and χ2 P value. ns and **: P > 0.05 and P < 0.01, respectively, for the indicated comparisons by Fischer’s exact test. (D-F) Data summary of lung tumor number, mean diameter, and total volume (burden) per lung with raw data points (dots), Tukey’s whiskers (boxes: interquartile range; bars: 50% extreme quartiles), and Kruskal-Wallis ANOVA P values. ns, *, **, and ***: P > 0.05, P < 0.05, P < 0.01, and P < 0.001, respectively, for the indicated comparisons by Dunn’s post-tests. (G) Representative images of gross lungs. Arrows denote lung tumors.
Figure 4. IKKα deletion ameliorates respiratory epithelial oncogenicity of mutant KRASG12D.
Conditional IKKα (Chukf/f) or IKKβ (Ikbkbf/f) gene-deleted mice were intercrossed with mice carrying a loxP-STOP-loxP.KRASG12D conditional allele (LSL.KRASG12D; all C57BL/6) and their offspring received 5 x 108 PFU intratracheal Ad- Cre and was killed four months later. (A) Schematic of experimental time-course (boxes= months) and topology of IKK-deleted (pink cytosol) versus KRASG12D-mutant (red nucleus) cells in this model, where IKK-deletion and oncogenic KRASG12D expression uniquely coincide in the same cells. (B) Frequency distribution of lung tumorigenesis with n and χ2 P values. ns, *, **, and ***: P > 0.05, P < 0.05, P < 0.01, and P < 0.001, respectively, for the indicated comparisons by Fischer’s exact test. (C, D) Data summary of relative lung tumor fraction and total lung tumor volume (burden) per lung with raw data points (dots), Tukey’s whiskers (boxes: interquartile range; bars: 50% extreme quartiles), and Kruskal-Wallis ANOVA P values. ns, *, **, and ***: P > 0.05, P < 0.05, P < 0.01, and P < 0.001, respectively, for the indicated comparisons by Dunn’s post-tests. (E) Representative hematoxylin and eosin-stained lung tissue sections. Arrows denote lung tumors.
IKKα selectively fosters KRAS-mutant cell prevalence in vitro and in vivo
We next stably transfected HEK293T benign human embryonic kidney cells with vectors encoding control random sequence (pC), RFP (pRFP), EGFP (peGFP), wild-type (peGFP.KrasWT) or mutant (peGFP.KrasG12C) murine Kras in-frame with EGFP, and murine IKKα (pChuk) or IKKβ (pIkbkb) in various combinations. After transgene expression was validated, RFP-expressing control cells and EGFP-expressing intervention cells co-transfected with various combinations of peGFP.KrasWT/ peGFP.KrasG12C and pChuk/pIkbkb were mixed at equal ratios and co-cultured for one week, followed by quantification by fluorescent microscopy and flow cytometry (Supplementary Figs. S5A, B). Of note, as opposed to successful pIkbkb co-expression with peGFP.KrasWT, pIkbkb co-expression with peGFP.KrasG12C was repeatedly impossible (n = 5), indicating mutual repulsion of mutant KrasG12C and IKKβ, similar to what was previously observed with other RAS/IκB-like GTPases called κB-RAS (Supplementary Fig. S5B; 31). Despite this, IKKβ provided a proliferative advantage to HEK293T cells expressing KrasWT, whereas KrasG12C-expressing HEK293T cells proliferated more efficiently upon IKKα overexpression (Supplementary Figs. S5C, D). Subsequently, HEK293T cells were stably transfected with pCAG.Luc followed by various combinations of pC, peGFP.KrasWT, peGFP.KrasG12C, pChuk, and/or pIkbkb, were validated, and two million cells were injected at different dorsal skin spots of NOD/SCID mice followed by serial spot volume assessment and bioluminescence imaging. Again, IKKβ boosted in vivo growth of HEK293T cells expressing KrasWT, while KrasG12C-expressing HEK293T cells were rendered more tumorigenic upon IKKα overexpression (Figs. 5A-C). Interestingly, none of eight NOD/SCID mice bearing subcutaneous KrasWT cells developed pulmonary lesions, while five of eight mice with subcutaneous KrasG12C cells developed lung metastases (P = 0.0256, χ2 test; Fig. 5D). We next examined HEK293T spots that had grown into tumors for NF-κB pathway component immunoreactivity. By immunoblotting, we observed nuclear localization of IKKα but not IKKβ in control tumors expressing KrasWT that was further enhanced by co-expression of IKKβ. KrasG12C tumors showed both IKKα and IKKβ nuclear immunoreactivity, while KrasG12C-IKKα-expressing tumors had enhanced IKKα and diminished IKKβ nuclear signals, and KrasG12C-IKKβ-expressing tumors displayed loss of both nuclear signals (Fig. 5E). The nuclear localization of IKKα in KrasG12C-IKKα co-expressing tumors was also evident on tissue sections by immunofluorescence (Fig. 5F). In addition to KRAS-IKK co-expression in benign cells, we stably expressed shRNAs specifically targeting IKKα and IKKβ transcripts (Chuk and Ikbkb respectively) in different lung adenocarcinoma cell lines [LLC, Lewis lung adenocarcinoma cells; and primary lung adenocarcinoma cells derived from urethane-induced lung tumors of FVB (FULA) and C57BL/6 (CULA) mice] bearing wild-type KrasWT (CULA cells), KrasG12C (LLC cells), KrasQ61R (FULA1 and FULA3 cells), or silenced KrasQ61R (FULA3 cells stably expressing shKras) (25, 29). Interestingly, IKKα silencing resulted in decreased clonogenic capacity in vitro and decreased tumor growth in vivo specifically of KRAS-mutant tumor cells (Supplementary Figs. 6A-6C). Moreover, this effect was not obvious in vitro, in line with recent observations on the in vivo-restricted effects of the oncogene (32). Collectively, these results indicated selective addiction of mutant KRAS to IKKα during carcinogenesis, possibly via nuclear IKKα functions reported elsewhere (33, 34).
Figure 5. IKKα selectively promotes the growth of KRAS-mutant cells in vivo.
HEK293T cells were stably transfected with a constitutive luciferase reporter (pCAG.Luc), followed by plasmids encoding control random sequence (pC), wild-type (peGFP.KrasWT) or mutant (peGFP.KrasG12C) murine Kras in frame with eGFP, and murine IKKα (pChuk) or IKKβ (pIkbkb) in various combinations. Two million cells were injected at different spots of the skin of NOD/SCID mice (n = 16) followed by serial spotvolume assessment and bioluminescent imaging of spot cell mass. Mice were killed aftersix weeks for assessment of primary spots and lungs for tumorigenicity of the injected cells. (A) Schematic of in vivo competition studies between bioluminescent cells expressing combinations of peGFP.KrasWT, peGFP.KrasG12C, pChuk, and pIkbkb and representative bioluminescent images. (B, C) Data summary of spot bioluminescence (B) and volume (C) of pC (grey), pChuk (red), and pIkbkb (blue) -expressing cells shown as mean (points), SEM (bars), and two-way ANOVA P values. ns, *, and ***: P > 0.05, P < 0.05, and P < 0.001, respectively, for comparisons of the indicated color-matched data-points to pC-expressing cells at the same time-point by Bonferroni post-tests. (D) Representative lung images of mice carrying peGFP.KrasWT (top) and peGFP.KrasG12C (bottom) tumors showing lung metastases of luminescent cells in the latter. (E) Immunoblots of tumor nuclear and cytoplasmic extracts for NF-κB pathway components. (F) IKKα and IKKβ immunoreactivity of flank tumor cryosections showing increased immunoreactivity of peGFP.KrasG12C/pChuk tumors for IKKα (arrows). Rel, v-rel avian reticuloendotheliosis viral oncogene homolog; IKK, inhibitor of NF-κB kinase.
Combined targeting of IKKα/IKKβ is effective against LADC
We subsequently evaluated the therapeutic potential of our findings using cellular and animal systems tailored to non-invasively monitor tumor growth and NF-κB activity. For this, three KRAS-mutant LADC cell lines (mouse primary LADC, KrasQ61R; murine Lewis lung carcinoma, KrasG12C; A549 human LADC, KRASG12S) were stably transfected with constitutive (pCAG.Luc) and NF-κB-dependent (pNGL) LUC reporters, inducibility of the NF-κB reporter was validated, and cells were treated with increasing concentrations of the selective IKKβ inhibitor TPCA-1 {2-[(aminocarbonyl)amino]-5-[4-fluorophenyl]-3-thiophenecarboxamide; 35) or the heat shock protein 90 (HSP90) inhibitor 17-DMAG (alvespimycin; 17-dimethylaminoethylamino-17-demethoxygeldanamycin; 36, 37) that blocks, among other targets, IKKα and IKKβ function; bioluminescence imaging of live pCAG.Luc cells after 48-hour treatments was used to determine cell killing and of pNGL cells after 4-hour treatments NF-κB inhibition. Intriguingly, 17-DMAG displayed superior efficacy in halting cell proliferation and NF-κB activity in all three cell lines compared with TPCA-1, as evident by 4-5-fold lower 50% inhibitory concentrations of pCAG.Luc activity (mean ± SD: 28 ± 12 μM for 17-DMAG and 114 ± 30 for TPCA-1) and 200-1000-fold lower 50% inhibitory concentrations of pNGL activity (mean ± SD: 0.133 ± 0.068 μM for 17-DMAG and 62 ± 30 for TPCA-1) (Supplementary Figs. S7A-S7E). Based on these results and the data from NGL mice with KRASG12D tumors (Supplementary Fig. S1), we designed an in vivo study where mice with KRASG12D-mutant LADCs received low doses of either drug tailored to inhibit NF-κB activity rather than cell proliferation in both preventive and curative modes. To enable repetitive non-invasive bioluminescent quantification of tumor burden in vivo, mice harboring a conditional loxP-STOP-loxP.R26.Luc allele (LSL.R26.Luc; 18) were intercrossed with LSL.KRASG12D mice (17; all C57BL/6), yielding a model where CRE-recombination leads to simultaneous KRASG12D and LUC expression (Fig. 6A; 38). LSL.KRASG12D;LSL.R26.Luc mice (n = 30) received 5 x 108 intratracheal PFU Ad-Cre and were allocated to drug treatments during the two distinct phases of NF-κB activation identified from LSL.KRASG12D;NGL mice (Supplementary Fig. S1): between days 14-28 post-Ad-Cre (prevention trial) or between days 84-112 post-Ad-Cre (regression trial; Fig. 6B). Treatments consisted of 100 μL daily intraperitoneal saline, TPCA-1, or 17-DMAG, both at 0.5 mg/Kg in 100 μL saline, equivalent by body volume extrapolation to maximal in vivo concentrations of 1.79 μM for TPCA-1 and of 0.77 μM for 17-DMAG, far inferior to cytotoxic concentrations (Supplementary Fig. S7). Bioluminescent detection of developing LADC revealed that TPCA-1 had no effect, while 17-DMAG prevention and regression regimens efficiently blocked tumor development compared with controls (Figs. 6C, D). Collectively, these results indicate that 17-DMAG exerts beneficial effects against KRAS-mutant LADCs in vitro and in vivo, even at low doses tailored to inhibit NF-κB. On the contrary, a specific IKKβ inhibitor failed to show any effect, further supporting a druggable addiction of IKKα with mutant KRAS in LADC.
Figure 6. Dual blockade of IKKα and IKKβ is effective against KRASG12D-driven lung adenocarcinoma in vivo.
Mice harboring a conditional loxP-STOP-loxP.R26.Luc allele constitutively expressed in the ROSA locus (LSL.R26.Luc) were intercrossed with conditional mice carrying a loxP-STOP-loxP.KRASG12D allele (LSL.KRASG12D; all C57BL/6). Double transgenic LSL.KRASG12D;LSL.R26.Luc mice (n = 30) received 5 x 108 intratracheal PFU adenovirus encoding CRE (Ad-Cre) and were allocated to daily intraperitoneal treatment with the selective IKKβ inhibitor TPCA-1 or the dual IKKα/IKKβ inhibitor 17-DMAG (both at 0.5 mg/Kg in 100 μL saline; approximately equivalent to 1-2 μM by body volume extrapolation) before (prevention trial; days 14-28 post-Ad-Cre) or after (regression trial; days 84-112 post-Ad-Cre) lung adenocarcinoma establishment. Thereafter mice were imaged longitudinally for bioluminescence. (A) Topology of luminescent R26.Luc (green cytosol) versus KRASG12D-expressing (red nucleus) cells in this model. (B) Schematic of experimental time-course (boxes = weeks). (C) Data summary of chest bioluminescence shown as mean (points), SEM (bars), and two-way ANOVA P value. ** and ***: P < 0.01 and P < 0.001, respectively, for comparisons of the indicated data-points to saline-treated mice at the same time-point by Bonferroni post-tests. (D) Representative merged bioluminescent/photographic images with pseudocolor scale showing decreased chest (dashed lines) light emission of 17-DMAG-treated mice at 112 days post-Ad-Cre.
IKKα in human LADC
The relevance of our findings with human LADC was subsequently addressed. For this, tumor and adjacent normal-appearing lung tissues of 23 patients with LADC were analyzed for CHUK and IKBKB expression by microarray and of another 35 patients from the same series for IKKα and IKKβ by immune-labelling (26). CHUK mRNA was overrepresented in normal-appearing and LADC tissue compared with IKBKB mRNA, while the levels of both were not different between normal-appearing and tumor tissue (Fig. 7A). However, using a modified NF-κB scoring system that examines staining intensity and localization (10), IKKα protein was significantly overexpressed in LADC compared with both normal-appearing tissues and with IKKβ (Figs. 7B, C), suggesting its possible involvement in the pathogenesis of human LADC.
Figure 7. IKKα in human lung adenocarcinoma.
(A) Data summary of normalized CHUK and IKBKB expression in tumor and adjacent normal-appearing lung tissues of 23 patients with lung cancer (43) by microarray. Data are shown as mean (columns), SEM (bars), raw data points (dots), and two-way repeated measures ANOVA P value. ns and ***: P > 0.05 and P < 0.001 for the indicated comparisons by Bonferroni post-tests. (B) Data summary of IKKα and IKKβ immunoreactivity of tumor and adjacent normal- appearing lung tissues of 35 patients with lung cancer (43) by immunohistochemistry. Data are shown as mean (columns), SEM (bars), raw data points (dots), and two-way repeated measures ANOVA P value. ns, **, and ***: P > 0.05, P < 0.01, and P < 0.001 for the indicated comparisons by Bonferroni post-tests. (C) Representative IKKα- and IKKβ-immunostained lung and tumor tissue sections showing IKKα-immunoreactive cells (arrows). (D) Schematic of proposed role of IKKα in KRAS-mutant lung adenocarcinoma. Endogenous IKKα activity sporadically prevails over IKKβ signalling across different cell types of the respiratory epithelium of smokers. Upon chemical induction of stochastic KRAS mutations across the respiratory field, pre-existing IKKα activity fosters the survival of KRAS-mutant cells and is therefore addicted to the oncogene, while IKKβ signaling promotes the survival and maintenance of non-mutated cells and IKKβ-dependent cells that suffer KRAS-mutations are destined to death. This opposing addiction of IKKα and IKKβ to mutant and wild-type KRAS, respectively, leads over time to the appearance of KRAS-mutant lung adenocarcinomas with enhanced IKKα activity. (E) Summary of in vivo IKK deletion/targeting experiments shown as mean percentile reduction of lung tumor burden by IKKα and IKKβ-targeted intervention (lines), SEM (bars), raw data (each dot represents one arm of an experiment), and paired Student’s t-test P value.
Discussion
We report an actionable requirement for IKKα in KRAS-mutant LADC. Using chemical and transgenic delivery of KRAS mutations to the respiratory tract in combination with NF-κB reporter and conditional IKK-deleted mice we map the patterns of NF-κB activation in the lungs and identify the critical role of IKKα. We show that IKKα drives LADC through cell-autonomous effects that are specifically exerted in the cellular context of mutant KRAS. These findings have implications for human disease, since IKKα is overexpressed in human LADC and oncogenic KRAS-IKKα addiction was annihilated by treatment with 17-DMAG.
The findings are novel and important on various counts. First, NF-κB activity of KRAS-mutant LADC is charted in living mice and is shown to be activated early after KRAS mutation induction and late in established LADC. This pattern is in line with observations from smokers at risk for LADC that feature airway epithelial NF-κB activation (39) and from patients with established LADC that display oncogenic NF-κB activation (10). The results are consistent with the hypothesis that NF-κB activation occurs together with field mutagenesis in the respiratory tract, persists in mutated cells, and reappears during clinical manifestation of late disease (40), bearing implications for NF-κB-based therapy and prevention (Fig. 7D).
Second, non-canonical together with canonical NF-κB pathway components are shown to be activated in KRAS-driven LADC. Canonical NF-κB signaling is long known to be important in human and experimental LADC (8–10), but activity of the alternative pathway has not been described. This finding is in accord with our previous observations of enhanced RelB activity of tumor cells in human LADC (10) and suggests important roles for alternative NF-κB signaling in KRAS-driven LADC.
Importantly, IKKα is identified as the critical kinase for oncogenic NF-κB activation of KRAS-mutant LADC. IKKα deletion provided beneficial effects in four different mouse models of combined KRAS-driven carcinogenesis and IKK depletion from the respiratory epithelium. In addition, 17-DMAG protected mice from KRASG12D-driven LADC when given early (preventive treatment) or late (regression trial), while the IKKβ blocker TPCA-1 did not. Although 17-DMAG likely suppresses a spectrum of targets broader than IKKα and IKKβ (41), inclusively targeting IKKα utilizing even this non-specific approach provided superior overall effects in reducing tumor burden compared with IKKβ-specific inhibition (Fig. 7E). We were the first to identify that indirect IKKβ blockade via overexpression of dominant negative IκBα protects mice from urethane-induced LADC (8), a finding thereafter recapitulated in KRASG12D-mutant (9), and tobacco-smoke-induced (14) LADC. Urethane-triggered LADCs were recently genomically characterized and shown to harbor KrasQ61R/KrasG12V mutations (22), similar to human LADC (42). Based on these findings and results from other tumor types, research and drug discovery focused on IKKβ yielding proteasome and IKKβ inhibitors (35, 43). However, these provide poor outcomes in human LADC (44) and cause resistance or paradoxical tumor promotion in animal LADC models via myeloid NF-κB inhibition, secondary mutation development, and/or enhanced neutrophil-provided IL-1β (23, 45, 46). In addition, recent evidence indicates that IKKβ might not be the only kinase responsible for oncogenic NF-κB activation of KRAS-mutant LADC (47). To this end, TBK1 emerged as a KRAS addiction partner, and was found to mediate EGFR-inhibitor resistance (5, 6), while IKKε promoted tumorigenesis together with TBK1 (13). Only one study addressed the role of IKKα depletion together with IKKβ in lung cancer cells in vitro and found both kinases to be important (11). Our results identify for the first time the pivotal role of IKKα in de novo development of KRAS-mutant LADC in vivo and position the kinase as a marked therapeutic target.
Using in vitro and in vivo competition studies, we determine that IKKα selectively fosters the survival of KRAS-mutant cells and is therefore addicted to the oncogene, while IKKβ promotes the survival and maintenance of non-mutated cells. We hypothesize that in a stochastically KRAS-mutated respiratory field, this opposing addiction of IKKα and IKKβ to mutant and wild-type KRAS, respectively, would lead over time via clonal selection to KRAS-mutant LADCs with enhanced IKKα activity (Fig. 7D). This cell-autonomous model is supported by the results from KRASG12D mice (where IKKα was selectively deleted in KRAS-mutant cells) and from HEK293T cells (where IKK/KRAS combinations functioned similarly in vitro and in vivo), notwithstanding the possibility for autocrine IKKα-triggered cytokine networks identified elsewhere (48,49). To this end, IKKα localized to the nucleus of our murine LADCs, a phenomenon that could enhance gene transcription or repress oncogenes (33, 34). Nuclear IKKα was also present in human LADC, which displayed enhanced nuclear IKKα immunoreactivity. The proposed IKKα function to site-independently foster KRAS-mutant cells also emanates from tissue-restricted IKK-deletion studies where IKKα was critical in both airway and alveolar cells, a result of importance given the cellular and histologic diversity of human LADC (50).
Finally, a feasible approach for translation of the findings is explored. Treatment with 17-DMAG was tailored to target NF-κB activation of KRAS-mutant LADC in vitro and was translated to a preclinical study, where it was well-tolerated and effective against LADC in vivo, both preventively and therapeutically. The efficacy of 17-DMAG and the inefficacy of TPCA-1 strengthen the proposed link between mutant KRAS and IKKα and open up new avenues for therapy/prevention of KRAS-mutant LADC (1). In summary, we report a requirement for IKKα in KRAS-driven LADC, implicate IKKα as a KRAS non-oncogene addiction partner, and show that targeting IKKα may confer beneficial effects against a currently untreatable disease that is the number one cancer killer in the world.
Supplementary Material
Precis.
Findings report a novel requirement for IKKα in mutant KRAS lung tumor formation with potential therapeutic applications.
Acknowledgements
The authors thank the University of Patras Center for Animal Models of Disease and Advanced Light Microscopy Cores for experimental support.
Financial Support: This work was supported by European Research Council 2010 Starting Independent Investigator and 2015 Proof of Concept Grants (260524 and 679345, respectively, awarded to G.T. Stathopoulos), as well as a Research Award by the Hellenic Thoracic Society (awarded to M. Vreka).
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Stephen AG, Esposito D, Bagni RK, McCormick F. Dragging Ras Back in the Ring. Cancer Cell. 2014;25:272–281. doi: 10.1016/j.ccr.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. [DOI] [PubMed] [Google Scholar]
- 5.Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seguin L, Kato S, Franovic A, Camargo MF, Lesperance J, Elliott KC, et al. An integrin β₃-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16:457–468. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death-a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stathopoulos GT, Sherrill TP, Cheng DS, Scoggins RM, Han W, Polosukhin VV, et al. Epithelial NF-kappaB activation promotes urethane-induced lung carcinogenesis. Proc Natl Acad Sci U S A. 2007;104:18514–18519. doi: 10.1073/pnas.0705316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–107. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giopanou I, Lilis I, Papaleonidopoulos V, Marazioti A, Spella M, Vreka M, et al. Comprehensive Evaluation of Nuclear Factor-κB Expression Patterns in Non-Small Cell Lung Cancer. PLoS One. 2015;10:e0132527. doi: 10.1371/journal.pone.0132527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassères DS, Ebbs A, Cogswell PC, Baldwin AS. IKK is a therapeutic target in KRAS-Induced lung cancer with disrupted p53 activity. Genes Cancer. 2014;5:41–55. doi: 10.18632/genesandcancer.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bivona TG, Hieronymus H, Parker J, Chang K, Taron M, Rosell R, et al. FAS and NF-κB signaling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471:523–526. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, et al. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–354. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nottingham LK, Yan CH, Yang X, Si H, Coupar J, Bian Y, et al. Aberrant IKKalpha and IKKbeta cooperatively activate NF-kappaB and induce EGFR/AP1 signaling to promote survival and migration of head and neck cancer. Oncogene. 2014;33:1135–1147. doi: 10.1038/onc.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 17.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safran M, Kim WY, Kung AL, Horner JW, DePinho RA, Kaelin WG., Jr Mouse reporter strain for noninvasive bioluminescent imaging of cells that have undergone Cre-mediated recombination. Mol Imaging. 2003;2:297–302. doi: 10.1162/15353500200303154. [DOI] [PubMed] [Google Scholar]
- 19.Luedde T, Heinrichsdorff J, de Lorenzi R, De Vos R, Roskams T, Pasparakis M. IKK1 and IKK2 cooperate to maintain bile duct integrity in the liver. Proc Natl Acad Sci U S A. 2008;105:9733–9738. doi: 10.1073/pnas.0800198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oikonomou N, Mouratis MA, Tzouvelekis A, Kaffe E, Valavanis C, Vilaras G, et al. Pulmonary autotoxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47:566–574. doi: 10.1165/rcmb.2012-0004OC. [DOI] [PubMed] [Google Scholar]
- 21.Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 22.Westcott PM, Halliwill KD, To MD, Rashid M, Rust AG, Keane TM, et al. The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. Nature. 2015;517:489–492. doi: 10.1038/nature13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karabela SP, Psallidas I, Sherrill TP, Kairi CA, Zaynagetdinov R, Cheng DS, et al. Opposing effects of bortezomib-induced nuclear factor-κB inhibition on chemical lung carcinogenesis. Carcinogenesis. 2012;33:859–867. doi: 10.1093/carcin/bgs024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karabela SP, Kairi CA, Magkouta S, Psallidas I, Moschos C, Stathopoulos I, et al. Neutralization of tumor necrosis factor bioactivity ameliorates urethane-induced pulmonary oncogenesis in mice. Neoplasia. 2011;13:1143–1151. doi: 10.1593/neo.111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agalioti T, Giannou AD, Krontira AC, Kanellakis NI, Kati D, Vreka M, et al. Mutant KRAS promotes malignant pleural effusion formation. Nat Commun. 2017;8:15205. doi: 10.1038/ncomms15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marwitz S, Depner S, Dvornikov D, Merkle R, Szczygieł M, Müller-Decker K, et al. Downregulation of the TGFβ Pseudoreceptor BAMBI in Non-Small Cell Lung Cancer Enhances TGFβ Signaling and Invasion. Cancer Res. 2016;76:3785–3801. doi: 10.1158/0008-5472.CAN-15-1326. [DOI] [PubMed] [Google Scholar]
- 27.Stathopoulos GT, Sherrill TP, Han W, Sadikot RT, Yull FE, Blackwell TS, et al. Host nuclear factor-kappaB activation potentiates lung cancer metastasis. Mol Cancer Res. 2008;6:364–671. doi: 10.1158/1541-7786.MCR-07-0309. [DOI] [PubMed] [Google Scholar]
- 28.Nikitin AY, Alcaraz A, Anver MR, Bronson RT, Cardiff RD, Dixon D, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64:2307–2316. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]
- 29.Giopanou I, Lilis I, Papaleonidopoulos V, Agalioti T, Kanellakis NI, Spiropoulou N, et al. Tumor-derived osteopontin isoforms cooperate with TRP53 and CCL2 to promote lung metastasis. Oncoimmunology. 2017;6:e1256528. doi: 10.1080/2162402X.2016.1256528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsia CC, Hyde DM, Ochs M, Weibel ER, ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Vallee S, Wu J, Vu D, Sondek J, Ghosh G. Inhibition of NF-kappaB activity by IkappaBbeta in association with kappaB-Ras. Mol Cell Biol. 2004;24:3048–3056. doi: 10.1128/MCB.24.7.3048-3056.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X, et al. Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor. Cell. 2018;172:578–589. doi: 10.1016/j.cell.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 35.Birrell MA, Hardaker E, Wong S, McCluskie K, Catley M, De Alba J, et al. Ikappa-B kinase-2 inhibitor blocks inflammation in human airway smooth muscle and a rat model of asthma. Am J Respir Crit Care Med. 2005;172:962–971. doi: 10.1164/rccm.200412-1647OC. [DOI] [PubMed] [Google Scholar]
- 36.Rastelli G, Tian ZQ, Wang Z, Myles D, Liu Y. Structure-based design of 7-carbamate analogs of geldanamycin. Bioorg Med Chem Lett. 2005;15:5016–5021. doi: 10.1016/j.bmcl.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Hertlein E, Wagner AJ, Jones J, Lin TS, Maddocks KJ, Towns WH 3rd, et al. 17-DMAG targets the nuclear factor-kappaB family of proteins to induce apoptosis in chronic lymphocytic leukemia: clinical implications of HSP90 inhibition. Blood. 2010;116:45–53. doi: 10.1182/blood-2010-01-263756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Y, Yeddula N, Leblanc M, Ke E, Zhang Y, Oldfield E, et al. Reduced cell proliferation by IKK2 depletion in a mouse lung-cancer model. Nat Cell Biol. 2012;14:257–265. doi: 10.1038/ncb2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Stefano A, Caramori G, Oates T, Capelli A, Lusuardi M, Gnemmi I, et al. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J. 2002;20:556–563. doi: 10.1183/09031936.02.00272002. [DOI] [PubMed] [Google Scholar]
- 40.Franklin WA, Gazdar AF, Haney J, Wistuba II, La Rosa FG, Kennedy T, et al. Widely dispersed p53 mutation in respiratory epithelium. A novel mechanism for field carcinogenesis. J Clin Invest. 1997;100:2133–2137. doi: 10.1172/JCI119748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl Bancroft C, Sausville E, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res. 2001;7:1419–1428. [PubMed] [Google Scholar]
- 44.Lilenbaum R, Wang X, Gu L, Kirshner J, Lerro K, Vokes E. Randomized phase II trial of docetaxel plus cetuximab or docetaxel plus bortezomib in patients with advanced non-small-cell lung cancer and a performance status of 2: CALGB 30402. J Clin Oncol. 2009;27:4487–4491. doi: 10.1200/JCO.2009.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLoed AG, Sherrill TP, Cheng DS, Han W, Saxon JA, Gleaves LA, et al. Neutrophil-Derived IL-1β Impairs the Efficacy of NF-κB Inhibitors against Lung Cancer. Cell Rep. 2016;16:120–132. doi: 10.1016/j.celrep.2016.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xue W, Meylan E, Oliver TG, Feldser DM, Winslow MM, Bronson R, et al. Response and resistance to NF-κB inhibitors in mouse models of lung adenocarcinoma. Cancer Discov. 2011;1:236–47. doi: 10.1158/2159-8290.CD-11-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Waes C. Targeting NF-κB in mouse models of lung adenocarcinoma. Cancer Discov. 2011;1:200–202. doi: 10.1158/2159-8290.CD-11-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, et al. KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S, et al. An NF-κB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519–1528. doi: 10.1172/JCI59743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.