Abstract
Background
C-reactive protein (CRP) pharmacodynamic (PD) models have the potential to provide adjunctive methods for predicting the individual exposure-response to antimicrobial therapy. We investigated CRP PD linked to a vancomycin PK model using routinely collected data from non-critical care adults in secondary care.
Methods
Patients receiving intermittent intravenous vancomycin therapy in secondary care were identified. A two-compartment vancomycin PK model was linked to a previously described PD model describing CRP response. PK and PD parameters were estimated using a Non-Parametric Adaptive Grid technique. Exposure-response relationships were explored with vancomycin area-under-the-curve (AUC) and the index, AUC:EC50, fitted to CRP data using a sigmoidal Emax model.
Results
Twenty-nine individuals were included. Median age was 62 (21-97) years. Fifteen (52%) patients were microbiology confirmed. PK and PD models were adequately fitted (r2 0.83 and 0.82 respectively). There was a wide variation observed in individual Bayesian posterior EC50 estimates (6.95-48.55mg/L), with mean (SD) AUC:EC50 of 31.46 (29.22). AUC:EC50 was fitted to terminal CRP with AUC:EC50 >19 associated with lower CRP value at 96-120 hours of therapy (100mg/L vs. 44mg/L; p<0.01).
Conclusion
The use of AUC:EC50 has the potential to provide in-vivo organism and host response data as an adjunct for in-vitro MIC data, which is currently used as the gold standard PD index for vancomycin therapy. This index can be estimated using routinely collected clinical data. Future work must investigate the role of AUC:EC50 in a prospective cohort and explore linkage with direct patient outcomes.
Keywords: Vancomycin, Pharmacokinetics, Phamacodynamics, Individualisation
Search terms: Inflammatory marker, glycopeptide, dosing, C-reactive protein
Introduction
Vancomycin is used for surgical prophylaxis and for treatment of established infection (1). Vancomycin is often administered empirically if the prevalence of methicillin resistant Staphylococcus aureus (MRSA) infection is high. The pharmacokinetics (PK) of vancomycin are highly variable in adult populations. The attainment of pharmacodynamic (PD) targets such as the area-under-the-concentration-time curve (AUC) to the minimum inhibitory concentration (MIC) ratio is associated with improved clinical outcomes (2). Vancomycin has a narrow therapeutic index. The use of therapeutic drug monitoring (TDM) and a range of individualised dosage strategies are required to ensure safe and effective treatment (3–6).
Vancomycin displays concentration-dependent antibacterial activity. The AUC:MIC is the PD index that best links drug exposure with the antibacterial effect. Values >400 are associated with improved clinical outcomes. Higher targets may be required in severe deep infections, such as MRSA infective endocarditis (1, 7–13). The use of AUC:MIC to guide individualised dosing requires isolation of the invading pathogen. Dosage optimisation is a recurring problem in cases where the invading pathogen is not available (14, 15). In these cases, simple measurements of Cmin, clinical judgement, physiological parameters, and biochemical markers such as C-Reactive Protein (CRP) are used to assess the therapeutic response (16). CRP is used extensively for infection diagnosis and management in clinical practice (17–19). To date, there has been little attempt to use it as a biomarker to estimate the PD of antimicrobial agents.
Recent studies in paediatric populations have used biomarkers (e.g. CRP and galactomannan) to individualise antimicrobial therapy by enabling the estimation of the AUC:EC50 (20, 21). The EC50 is the estimated concentration of a drug (mg/L) that is required to induce half maximal antimicrobial effect. The EC50 provides an in-vivo estimate of drug response (20, 21) and integrates the different aspects that govern exposure response relationships (e.g. site of infection, immune status, bacterial load, and in vitro susceptibility).
Within this study we aimed to explore whether it is possible to estimate the relationship between drug exposure and time-course of CRP using routine CRP and vancomycin TDM data from non-critical care adult patients.
Materials and Methods
Study design and characteristics
Data from non-critically ill patients receiving vancomycin therapy for the treatment of infections were identified from two audit cycles of vancomycin therapy that occurred in Imperial College Healthcare NHS Trust. The focus of the audit was to review the current standard of vancomycin use within the hospital compared to the local antimicrobial policy. The methodology used for patient identification and data collection was identical in both audits. All patients had undergone routine therapeutic drug monitoring (TDM) following local hospital guidelines (which remained stable throughout the two audit periods that spanned 15 months from April 2016 – June 2017) for treatment of confirmed or suspected infections with vancomycin.
Guidelines recommend measuring trough plasma vancomycin concentrations and targeting 10-15 mg/L, or 15-20 mg/L for more severe or deep-seated infections. Patient characteristics, biochemistry, microbiology data, and treatment history was extracted from electronic health records. CRP data (routinely collected by the patient’s clinical team as part of the infection management and clinical care) and estimated glomerular filtration rate (GRF; calculated using Modification of Diet in Renal Disease [MDRD] formula) were retrieved from patient electronic records.
Clinical case histories of all patients treated with vancomycin in the time period evaluated were reviewed. Patients were only included if treatment was commenced for suspected or confirmed Gram-positive pathogen(s), for which vancomycin was an appropriate agent. Patients receiving concomitant therapy with other antibacterial agents with an overlapping antimicrobial spectrum were excluded. Patients with a positive culture that was not susceptible to vancomycin (i.e. Gram-negative bacteria, anaerobes, or fungi) were excluded from the analysis. Patients without TDM data, or on renal replacement therapy were also excluded from the analysis. Statistical analysis was performed using SPSS 22.0 (IBM, NY, USA).
Vancomycin bioanalysis
Vancomycin concentrations were measured using a commercially available MULTIAGENT assay implemented on an Architect analyser (Abbott diagnostics, CA, USA). The lower limit of quantification was 1.1 mg/L. The linear range of the assay was 1.1 to 100mg/mL.
Population pharmacokinetic model
All PK and PD modelling was performed using Pmetrics and ADAPT 5 (22, 23). A two-compartment PK model with time-delimited zero-order intravenous input and first order elimination was used. The structural equations took the form:
| 1) |
| 2) |
X(1) and X(2) represent the amount of vancomycin (mg) in the central (c) and peripheral (p) compartments. R(1) is the rate of infusion of vancomycin into the central compartment (mg/h). V is the volume of the central compartment (L), from which there is clearance of drug (SCL; L/h). The two compartments are connected by first order rate constants Kcp and Kpc (h-1).
The fit of the model to the data was assessed in the following ways: (i) log-likelihood values, (ii) assessment of coefficients of determination (r2) from a linear regression of the observed-predicted data, (iii) use of the Akaike Information Criterion (AIC) (24).
Pharmacokinetic-pharmacodynamic modelling
A two-step approach to fitting PK and PD data was used. The Bayesian posterior estimates for each individual were obtained using the two compartment PK model described in equations 1) and 2). Posterior Bayesian estimated values (V, Cl, Kcp, and Kpc) for individual patients were fixed as covariates within a PK-PD model made up of equations 1), 2), and 3) to describe the exposure response dynamics of CRP. This approach was implemented given the observation of vancomycin PK variability within our population and to avoid bias in parameter estimates in the PK-PD model. The PD model chosen for use in this study was selected based on previous published work investigating the exposure response dynamics of CRP during infection (20, 25).
| 3) |
KCRPp is the maximum rate of CRP production (mg· h/L). POPmax is the maximum value of CRP (mg/L). Within our hospital a normal CRP is defined as <10 mg/L. In the literature, following acute-phase stimulus CRP can be observed to rise to greater than 500 mg/L (26). Therefore, this was used as an upper limit for the search space used in fitting the model to the data. KCRPi is the rate of maximal CRP inhibition (mg· h/L), H is the slope function for CRP inhibition, and EC50 is the concentration of vancomycin (mg/L) that produces half maximal effect on CRP reduction.
Exposure-response
The Bayesian posterior estimates for each patient were used to calculate the average AUC (i.e. total vancomycin AUC for the treatment course divided by the number of treatment days). Posterior estimates for individual patient’s EC50 were also obtained to calculate AUC:EC50. This index was then fitted to patient CRP data 96-120 hours after commencing vancomycin therapy where Gram-positive infection was microbiologically confirmed. This used an Emax sigmoidal model to identify trends in the data and describe the relationship between CRP and AUC:EC50. The findings from evaluating exposure-response in individuals with microbiology confirmed infections were then compared to individuals with no microbiology who were being treated empirically but had a high suspicion of Gram-positive infection.
Results
Subject selection & characteristics
A total of 105 non-critically ill patients receiving vancomycin were identified as potential study subjects. Twenty nine (37%) patients were eligible for consideration of inclusion in the PK-PD analysis. Of the 76 patients excluded, 20/76 (26%) were on renal replacement therapy, 16/76 (21%) had no TDM data, 8/76 (11%) had other missing data, with the remaining 22/76 (29%) dosed for less than 72 hours or treated for Gram-negative infections / non-infectious syndromes. Vancomycin therapy was used empirically in 48/105 (46%) of patients. All patients had Gram-negative and anaerobic antimicrobial cover administered at the clinician’s discretion.
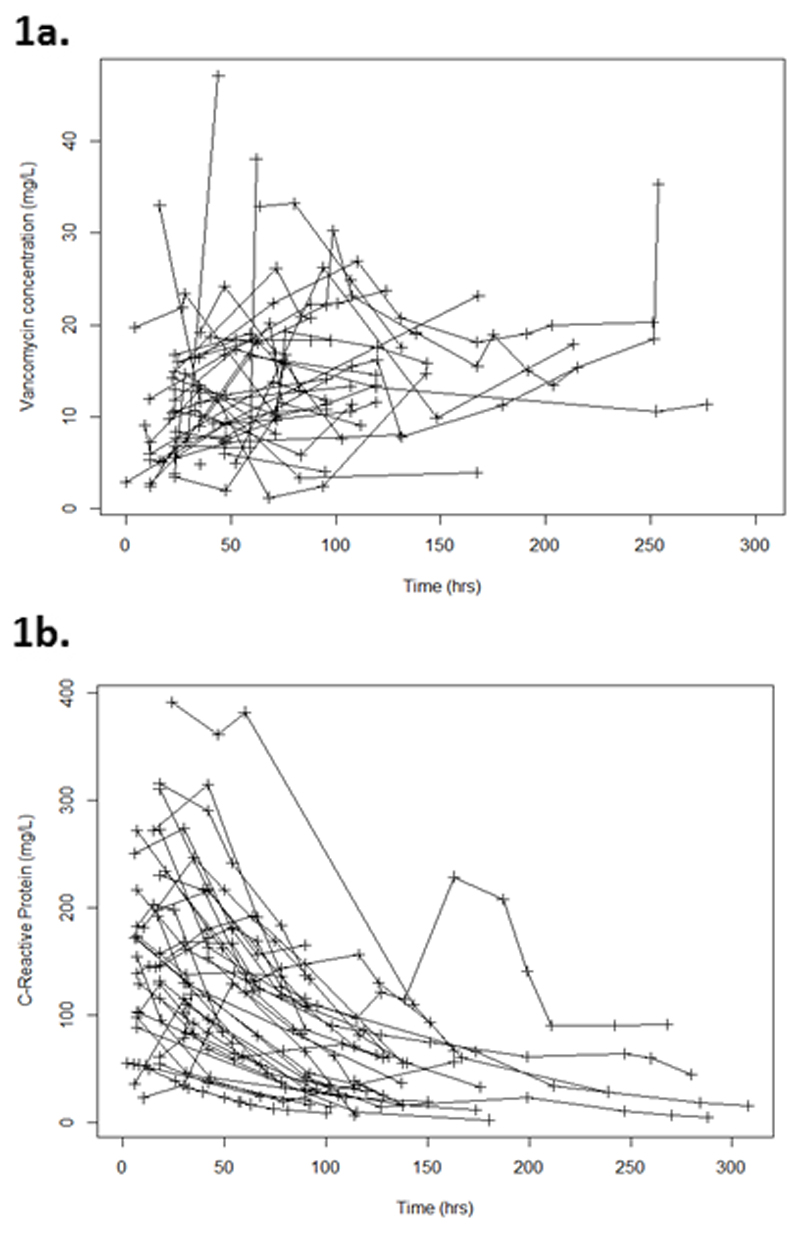
For the 29 subjects included in the PK-PD analysis (Table 1), median (range) age was 62 (21-97) years. The majority were female (18/29; 62%) and 15/29 (52%) had microbiology confirmed Gram-positive infection. The mean (SD; range) number of doses of vancomycin received were 10 (4; 4-22), with a mean dose (range) of 1000 mg (500-2000 mg) per day. Each subject had a mean (SD) of 5 (3) TDM samples taken during the time period under investigation. Mean (SD) GFR for the cohort was 82 (37) ml/min/1.73m2 and initial mean (SD) CRP on commencement of vancomycin therapy was 154 (110) mg/L. Patients had a mean (range) of 5 (2-13) CRP measurements during the time period that they were receiving vancomycin therapy. Concentration-time profiles of the raw-data for vancomycin TDM and CRP used for modelling are shown in Figure 1.
Table 1.
Summary of patient characteristics included in the pharmacokinetic-pharmacodynamic model
| Parameter | Value (%) | |
|---|---|---|
|
| ||
| Demographics | ||
| Age (range) | 62 (21-97) | |
| Female | 18 (62) | |
| Infection | ||
| Blood Stream Infection | 7 (24) | |
| Pneumonia | 2 (7) | |
| Skin and soft tissue | 10 (34) | |
| CNS infection | 1 (3) | |
| Intra-abdominal infection | 2 (7) | |
| Joint (inc. prosthetic) | 1 (3) | |
| Line sepsis | 1 (3) | |
| Urinary tract infection | 2 (7) | |
| Other | 3 (10) | |
| Organism | ||
| Empirical therapy (no growth) | 14 (49) | |
| Staphylococcus aureus | 7 (24) | |
| Coagulase negative Staphylococcus | 4 (14) | |
| Other Gram-positive | 4 (14) | |
|
| ||
Figure 1. Concentration time plots for (a) recorded vancomycin concentrations and (b) C-Reactive protein results.
1a Legend: Concentration time plot for recorded vancomycin concentrations
1b Legend: Concentration time plot for C-Reactive Protein results
Pharmacokinetic – pharmacodynamic model
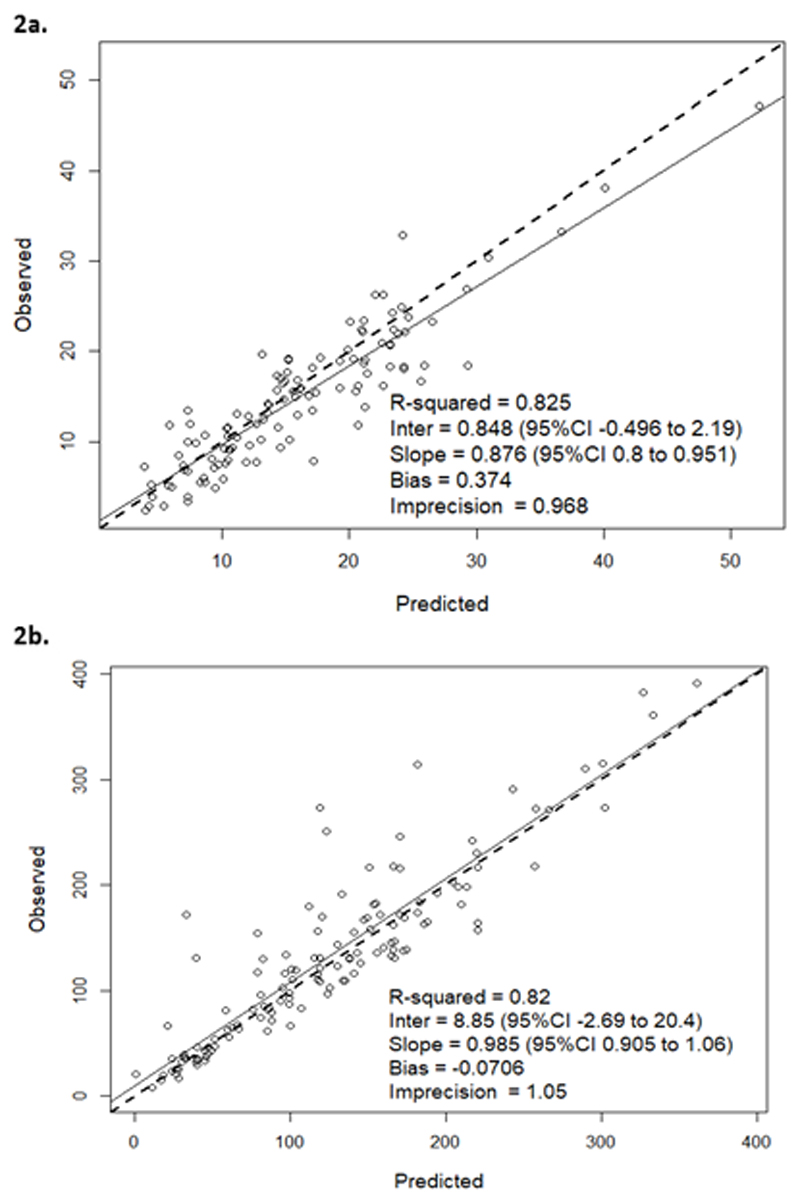
A two-compartment model was found to be the optimal model for the data. The final vancomycin model had an individual posterior observation versus predicted plot r2 of 0.83 with a bias of 0.37 and imprecision of 0.97 (Figure 2a). The CRP PD model was fitted with an individual posterior observation versus predicted plot r2 of 0.82, a bias of -0.07, and imprecision of 1.05 (Figure 2b). As only individual posterior estimates were required for this study, covariate modelling was not performed. A summary of the final population PK-PD parameter estimates are outlined in Table 2. Population estimates of vancomycin PK were similar to previously reported observations in the literature (12, 27). There was a substantial variability in the individual Bayesian posterior estimates for EC50 values estimated, with mean (SD; range) of 23.40 (13.55; 6.95-48.55). Mean (SD; range) AUC:EC50 was 31.46 (29.22; 7.30-128.41).
Figure 2. Individual posterior observed versus predicted plots for the vancomycin pharmacokinetic (PK) and CRP pharmacodynamic (PD) components from the PK-PD model.
2a Legend: Posterior observed vs. predicted plots for vancomycin PK model
2b Legend: Posterior observed vs. predicted plots for CRP PD model
Table 2.
Population estimates of the pharmacokinetic – pharmacodynamic parameters for a model linking CRP to vancomycin concentrations in a population of non-critical care patients in secondary care.
| Population parameters | Value | |
|---|---|---|
|
| ||
| Pharmacokinetic parameters | ||
| Clearance (CL, L/hr) | mean (SD) | 3.77(2.23) |
| Volume (central, L) | mean (SD) | 25.89 (12.08) |
| Kcp (hr-1) | mean (SD) | 3.32 (3.81) |
| Kpc (hr-1) | mean (SD) | 2.59 (3.17) |
| Pharmacodynamic parameters | ||
| KCRPp (mg∙h/L) | mean (SD) | 0.07 (0.07) |
| POPmax (mg/L) | mean (SD) | 494.24 (242.46) |
| H | mean (SD) | 11.14 (8.18) |
| KCRPi (mg∙h/L) | mean (SD) | 0.11 (0.07) |
| EC50 (mg/L) | mean (SD) | 23.40 (13.55) |
| Initial condition of CRP (mg) | mean (SD) | 154 (110) |
| Glomerular filtration rate (ml/min/1.73m2) | mean (SD) | 82 (37) |
|
| ||
Exposure response
Individual cases were then assessed with Bayesian posterior estimates of individual vancomycin AUC:EC50 fitted to an sigmoid Emax model for Gram-positive confirmed patients and the relationships to CRP values at 96-120 hours post initiation of vancomycin therapy assessed (Figure 3). This was repeated for those individuals treated empirically. In the microbiology confirmed cohort, one individual was excluded due to being taken back to surgery during the first 120 hours of therapy. Two individuals from the empirically treated group were also excluded as one developed active pancreatitis during the therapy and one was taken back to theatre for further surgery.
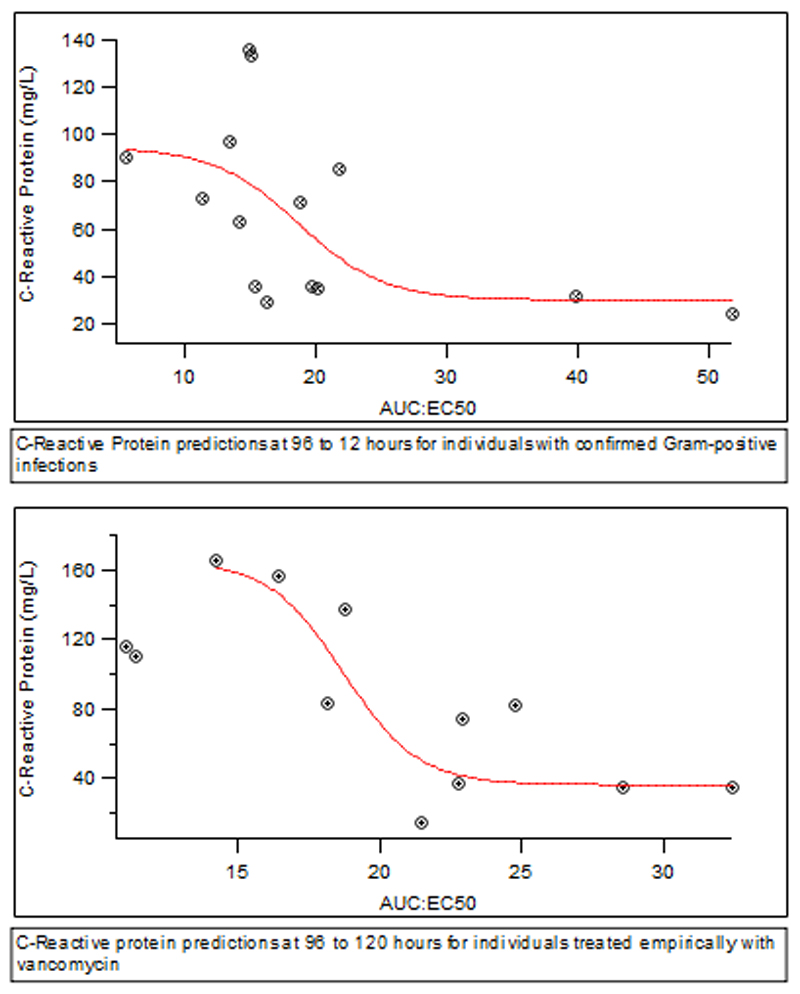
Figure 3. Exploration of dose – response relationship using vancomycin AUC:EC50 against C-reactive protein.
Assuming a mean MIC of 1 mg/L for Staphylococcus aureus and other associated organisms treated within this study (only 4/14 microbiology specimens had individual MIC data available), the optimal target vancomycin AUC would be >400 based on previously published clinical outcome data (1, 7–13). Using AUC:EC50 as a surrogate, values greater than 19 (given a vancomycin AUC of 400 and median EC50 of 21 mg/L) would therefore be expected to correlate to this. Thus, AUC:EC50 values could potentially be expected to demonstrate a better CRP response to therapy above this. In the Gram-positive confirmed cohort, 5/14 (36%) individuals had AUC:EC50 >19. There was an association with lower CRP values at 96-120 hours with mean (SD) CRP of 42 (24) mg/L in those with AUC:EC50 >19 vs. 81 (38) mg/L for those with AUC:EC50 <19 (p=0.06). For those individuals treated empirically, 6/12 (50%) had AUC:EC50 >19. Once again, an association was observed toward lower CRP at 96-120 hours. The mean (SD) for those with AUC:EC50 >19 was 46 (26) vs. 128 (31) (p<0.01).
Pooling of all cases, both microbiologically confirmed and empirical, followed by assessment of AUC:EC50 demonstrated a significant association with AUC:EC50 >19 and prediction of CRP at 96-120 hours. Individuals with AUC:EC50 >19 had mean (SD) CRP of 44 (24) mg/L vs. 100 (41) mg/L in those with AUC:EC50 <19 (p<0.01). Mean (SD) estimated AUC:MIC’s for the cohort were then compared using MIC breakpoint estimates used in clinical practice (28). Using an estimated MIC of 1 mg/L, CRP response at 96-120 hours was compared between individuals with AUC:MIC greater or less than 400. There was no difference between groups with AUC:MIC <400 obtaining a mean (SD) CRP of 65.7 (32) mg/L vs. 80.1 (49) mg/L in those with AUC:MIC >400 (p=0.45).
Discussion
The use of vancomycin in non-critically ill adults is always challenging. There is considerable PK variability. Many patients are culture negative meaning an MIC is not available to guide individualised therapy. Therefore, clinicians are forced to use TDM targets derived from a population of patients and MICs at the breakpoint in an attempt to optimise therapy for the individual patient. These approaches are not necessarily optimal for the individual being treated and fail to consider patient-level factors that determine a response to antimicrobial therapy. In an attempt to individually estimate the clinical response to an infection and subsequent antimicrobial therapy physicians commonly use non-specific markers such as CRP. To date there has been very little linkage using this inflammatory marker, and other similar routinely collected biomarkers of infection, to PK-PD parameters (20, 21).
The use of AUC:EC50 offers a novel measure to asses individual patient’s response to therapy. The EC50 value is a measure of the in-vivo potency of a drug taking into account both the host factors (such as immune response and comorbid status) as well as organisms factors (such as resistance to the therapy being delivered and bacterial load). When linked to the exposure of the drug in question (i.e. AUC), this allows integration of factors that affect the ultimate exposure-response relationships. The use of MIC alone only provides information on the in vitro potency of the drug for its microbiological target. Thus, the AUC:EC50 may augment this, acting as a more inclusive estimate of pharmacodynamics. This may be of benefit when MIC data is not available, which is a common scenario, especially outside of the critical care setting (14, 15).
Within this study we have demonstrated a potential of AUC:EC50 estimates obtained through analysis of routinely available data to be able to predict greater response of CRP during therapy. The breakpoint was estimated based on current non-individualised AUC:MIC estimates that would routinely be considered during empirical therapy in clinical practice (i.e. target AUC:MIC of >400). On comparison to estimated AUC:MIC for individual cases within this study, using published MIC breakpoints (28), AUC:MIC >400 did not correlate with lower CRP at 96-120 hours. This may support some of the potential benefits of using EC50 to provide more individualised assessment of response to therapy.
However, this study also highlights several challenges that individualised therapy using measures such as the AUC:EC50 face in the future in adult populations. In a previous study performed by Ramos-Martin et al, AUC:EC50 values in neonates predicted the likelihood of the normalisation of CRP (defined as <10mg/L) for infants receiving teicoplanin therapy for the treatment of coagulase negative staphylococcus line infections (20). In our study population, very few subjects CRP returned to <10mg/L on cessation of vancomycin therapy. This is, in part, likely to be due to local antimicrobial stewardship policies for adults in the non-critical care setting that means patients are regularly reviewed and therapy is de-escalated before patient’s biochemical markers have returned to normal limits (usually within 72-120 hours) (29). It also reflects the co-morbid state of adult patient populations in this setting. Therefore, we chose a time point of 96-120 hours given that most individuals will be treated for at least this period of time with vancomycin.
Given that both AUC and EC50 can be estimated with minimal vancomycin and CRP data it is possible that future studies could incorporate consideration of AUC:EC50 estimation into medication reviews. This may act as a tool to help inform the likelihood of success of continued empirical therapy when no organism has been identified, providing an individualised estimate of treatment success. A further observation from this study was that a number of individuals appeared to have vancomycin concentrations below recommended targets during therapy. On review of mean dose received by individuals within the study, the observed mean of 1000 mg/24 hours may have been lower than is often recommended. However, this is a common problem observed with vancomycin therapy in similar populations and highlights some of the challenges with conventional approaches to dosing and TDM using trough concentrations (12, 30, 31).
With the development of continuous monitoring of biomarkers and antimicrobials, and translation into closed-loop control systems, the AUC:EC50 may also provide a source for dynamic individualisation of therapy given that changes in the individuals physiological state over will also be considered alongside organism response (32–33). Further work is required to explore newer, more specific clinical biomarkers (such as procalcitonin and CD64) that have the ability to improve population PD models for delivering individualised therapy (18). The model described within this study serves as a framework from which PK-PD models for these biomarkers can be developed and explored.
Several other limitations with the use of this model within our population were identified during the study. Given the nature of how our data were collected, PK estimates were made using sparse data, which may have influenced our estimates of vancomycin PK parameters. In future work, use of a PK model, built with informative vancomycin PK data may be of benefit in improving the precision of PK-PD estimates. Secondly, a large number of individuals identified as receiving vancomycin therapy in were excluded from the analysis as they lacked TDM data, were receiving renal replacement therapy, or received vancomycin inappropriately (in Gram-negative infections). Therefore, the small and highly selected sample of individuals included means that generalizability of our findings is difficult. This also means that certain aspects may have been underpowered to demonstrate significance statistically. We now plan to investigate whether AUC:EC50 estimates derived from a cohort of patients undergoing rich vancomycin PK-PD analysis can enhance the predictive ability of AUC:EC50 in this scenario. This includes exploration of whether more intensive CRP monitoring can improve the accuracy of EC50 estimates within the PD model. Finally, as we were only able to use estimated organism MIC for the majority of clinical isolates within this study, future work will also look to ensure that individual MIC data is available to enable a comparison of the predictive power of AUC:MIC versus AUC:EC50.
Conclusion
In conclusion, we have demonstrated that it is possible to link antimicrobial PK and PD with direct markers of treatment response, such as CRP, using routinely collected patient data in adult, non-critical care settings. Future work must ensure that local and national antimicrobial policies are considered when investigating and setting novel PK-PD targets within specific cohorts to ensure that these can translate into clinical practice. With larger, prospective, rich data PK-PD models the possibility of truly individualised, precision antimicrobial therapy may become a possibility.
Acknowledgements
The authors would like to acknowledge the National Institute of Health Research Imperial Biomedical Research Centre and the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infection and Antimicrobial Resistance at Imperial College London in partnership with Public Health England and the NIHR Imperial Patient Safety Translational Research Centre. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the UK Department of Health.
Funding
This report is independent research funded by the National Institute for Health Research Invention for Innovation (i4i) Scheme, Enhanced, Personalized and Integrated Care for Infection Management at Point of Care (EPIC IMPOC), II-LA-0214-20008.
Footnotes
Contribution statement
All authors contributed significantly towards the planning and undertaking of this study. TMR drafted the initial draft of the manuscript with all authors significantly contributing to the development and finalisation of the final iteration for submission.
Competing interests
The authors have no competing interests to declare.
References
- 1.Vandecasteele SJ, De Vriese AS, Tacconelli E. The pharmacokinetics and pharmacodynamics of vancomycin in clinical practice: Evidence and uncertainties. J Antimicrob Chemother. 2013;68:743–748. doi: 10.1093/jac/dks495. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh N, Chavada R, Maley M, van Hal SJ. Impact of source of infection and vancomycin AUC0-24/MICBMD targets on treatment failure in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2014;20:O1098–O1105. doi: 10.1111/1469-0691.12695. [DOI] [PubMed] [Google Scholar]
- 3.Vance-Bryan K, Guay DRP, Gilliland SS, Rodvold KA, Rotschafer JC. Effect of obesity on vancomycin pharmacokinetic parameters as determined by using a Bayesian forecasting technique. Antimicrob Agents Chemother. 1993;37:436–440. doi: 10.1128/aac.37.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinks Aa. The application of population pharmacokinetic modeling to individualized antibiotic therapy. Int J Antimicrob Agents. 2002;19 doi: 10.1016/s0924-8579(02)00023-7. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen EI, Cars O, Friberg LE. Pharmacokinetic/Pharmacodynamic (PK/PD) Indices of Antibiotics Predicted by a Semimechanistic PKPD Model: a Step toward Model-Based Dose Optimization. Antimicrob Agents Chemother. 2011;55:4619–4630. doi: 10.1128/AAC.00182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jelliffe RW, Schumitzky A, Leary R, Botnen A, Gandhi A, Maire P, Barbaut X, Bleyzac N. Optimizing individualized dosage regimens of potentially toxic drugs. In: Krishna R, editor. Applications of Pharmacokinetic Principles in Drug Development. Springer; Boston, MA: 2004. [Google Scholar]
- 7.Morrill HJ, Caffrey AR, Noh E, LaPlante KL. Vancomycin dosing considerations in a real-world cohort of obese and extremely obese patients. Pharmacotherapy. 2015;35:869–875. doi: 10.1002/phar.1625. [DOI] [PubMed] [Google Scholar]
- 8.Medellı SE, Romano-moreno S, Barcia E. Pharmacokinetics of vancomycin and dosing recommendations for trauma patients. 2015:1–9. doi: 10.1093/jac/dkv372. [DOI] [PubMed] [Google Scholar]
- 9.Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 10.Patel N, Pai MP, Rodvold Ka, Lomaestro B, Drusano GL, Lodise TP. Vancomycin: We can’t get there from here. Clin Infect Dis. 2011;52:969–974. doi: 10.1093/cid/cir078. [DOI] [PubMed] [Google Scholar]
- 11.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O’Sullivan MVN, Anderson TL, Roberts SA, Warren SJC, Gao W, Howden BP, et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013;57:1654–1663. doi: 10.1128/AAC.01485-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, Lodise TP. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;58:309–316. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casapao AM, Lodise TP, Davis SL, Claeys KC, Kullar R, Levine DP, Rybak MJ. Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob Agents Chemother. 2015;59:2978–2985. doi: 10.1128/AAC.03970-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phua J, Ngerng W, See K, Tay C, Kiong T, Lim H, Chew M, Yip H, Tan A, Khalizah H, Capistrano R, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care. 2013;17:R202. doi: 10.1186/cc12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarb P, Goossens H. European Surveillance of Antimicrobial Consumption (ESAC): Value of a point-prevalence survey of antimicrobial use across Europe. Drugs. 2011;71:745–755. doi: 10.2165/11591180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Rawson TM, Charani E, Moore LSPLSP, Hernandez B, Castro-Sánchez E, Herrero P, Georgiou P, Holmes aHAH. Mapping the decision pathways of acute infection management in secondary care among UK medical physicians: a qualitative study. BMC Med. 2016;14:208. doi: 10.1186/s12916-016-0751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markanday A. Acute Phase Reactants in Infections: Evidence-Based Review and a Guide for Clinicians. Open forum Infect Dis. 2015;2 doi: 10.1093/ofid/ofv098. ofv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobeissi ZA, Zanotti-Cavazzoni SL. Biomarkers of sepsis. Yearb Crit Care Med. 2010;2010:227–228. [Google Scholar]
- 19.Nargis W, Ahamed B, Ibrahim M. Procalcitonin versus C-reactive protein: Usefulness as biomarker of sepsis in ICU patient. Int J Crit Illn Inj Sci. 2014;4:195. doi: 10.4103/2229-5151.141356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos-Martín V, Neely MN, McGowan P, Siner S, Padmore K, Peak M, Beresford MW, Turner MA, Paulus S, Hope WW. Population pharmacokinetics and pharmacodynamics of teicoplanin in neonates: making better use of C-reactive protein to deliver individualized therapy. J Antimicrob Chemother. 2016 doi: 10.1093/jac/dkw295. dkw295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huurneman LJ, Neely M, Veringa A, Pérez FD, Ramos-Martin V, Tissing WJ, Alffenaar JWC, Hope W. Pharmacodynamics of voriconazole in children: Further steps along the path to true individualized therapy. Antimicrob Agents Chemother. 2016;60:2336–2342. doi: 10.1128/AAC.03023-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate Detection of Outliers and Subpopulations With Pmetrics, a Nonparametric and Parametric Pharmacometric Modeling and Simulation Package for R. Ther Drug Monit. 2012;34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Argenio David Z, Schumitzky Alan WX. ADAPT 5 User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software 316. 2009 https://bmsr.usc.edu/files/2013/02/ADAPT5-User-Guide.pdf.
- 24.Akaike H. A Bayesian Extension of the Minimal AIC Procedures of Autoregressive Model Fitting. Biometrika. 1979;66:237–242. [Google Scholar]
- 25.Ramos-Martín V, Johnson a, Livermore J, McEntee L, Goodwin J, Whalley S, Docobo-Pérez F, Felton TW, Zhao W, Jacqz-Aigrain E, Sharland M, et al. Pharmacodynamics of vancomycin for CoNS infection: experimental basis for optimal use of vancomycin in neonates. J Antimicrob Chemother. 2016:992–1002. doi: 10.1093/jac/dkv451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: A review of population pharmacokinetic analyses. Clin Pharmacokinet. 2012;51:1–13. doi: 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters. 2016 http://www.eucast.org/clinical_breakpoints/
- 29.ESPAUR SSTF Implementation subgroup. Start Smart - Then Focus Antimicrobial Stewardship Toolkit for English Hospitals. 2015 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/417032/Start_Smart_Then_Focus_FINAL.PDF.
- 30.Kang JS, Lee MH. Overview of therapeutic drug monitoring. Korean J Intern Med. 2009;24:1–10. doi: 10.3904/kjim.2009.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts JA, Norris R, Paterson DL, Martin JH. Therapeutic drug monitoring of antimicrobials. Br J Clin Pharmacol. 2012;73:27–36. doi: 10.1111/j.1365-2125.2011.04080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawson TM, O’Hare D, Herrero P, Sharma S, Moore LSP, de Barra E, Roberts JA, Gordon AC, Hope W, Georgiou P, Cass AEG, et al. Delivering precision antimicrobial therapy through closed-loop control systems. J Antimicrob Chemother. 2017 doi: 10.1093/jac/dkx458. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrero P, Rawson TM, Philip A, Moore LSP, Holmes AH, Georgiou P. Closed-loop Control for Precision Antimicrobial Delivery: an In Silico Proof-of-Concept. IEEE Trans Biomed Eng. 2017 doi: 10.1109/TBME.2017.2787423. http://ieeexplore.ieee.org/document/8239859/?reload=true. [DOI] [PubMed] [Google Scholar]