Abstract
Traditionally, low back-related leg pain (LBLP) is diagnosed clinically as referred leg pain or sciatica (nerve root involvement). However, within the spectrum of LBLP we hypothesised that there may be other, unrecognised patient subgroups. This study aimed to identify clusters of LBLP patients using latent class analysis (LCA) and describe their clinical course.
The study population were 609 LBLP primary care consulters. Variables from clinical assessment were included in the LCA. Characteristics of the statistically identified clusters were compared and their clinical course over one year was described.
A five cluster solution was optimal. Cluster 1 (n=104) had mild leg pain severity and was considered to represent a referred leg pain group with no clinical signs suggesting nerve root involvement (sciatica). Cluster 2 (n=122), cluster 3 (n=188) and cluster 4 (n=69) had mild, moderate and severe pain and disability respectively and response to clinical assessment items suggested categories of mild, moderate and severe sciatica. Cluster 5 (n=126) had high pain and disability, longer pain duration, more comorbidities and was difficult to map to a clinical diagnosis.
Most improvement for pain and disability was seen in the first four months for all clusters. At 12 months the proportion of patients reporting recovery ranged from 27% for cluster 5 to 45% for cluster 2 (mild sciatica).
This is the first study that empirically shows the variability in profile and clinical course of patients with LBLP including sciatica. More homogenous groups were identified which could be considered in future clinical and research settings.
Keywords: sciatica, low back-related leg pain, latent class analysis, primary care, clinical course
Background
Trials evaluating treatments for low back pain (LBP), show at best moderate effect sizes [11]. The heterogeneity of LBP patients within studies is one explanation for these results. This has stimulated research aiming to identify more homogeneous, clinically relevant subgroups of LBP patients, with the hope that these subgroups might respond more favourably to interventions or management approaches matched to the subgroup’s characteristics or presenting symptoms [12, 36].
One of the most common subgroup of LBP is back pain radiating to the leg, which represents about two thirds of back pain patients, in both primary and secondary care settings [16, 19]. Patients with low back-related leg pain (LBLP) suffer more severe pain and disability, take longer to recover and lose more time from work [14, 27, 41], compared to those with pain in the lower back alone.
When patients present with LBLP, once serious pathology (such as tumours, cauda equina compression, fracture, inflammatory causes) is ruled out, the differential diagnosis is between leg pain that is due to spinal nerve root involvement (commonly called sciatica) or to non-specific pain in the leg thought to be referred from structures in the back (e.g. disc/muscle/joint) but not involving the nerve root.
This is a rather broad brush categorisation however and currently there is a gap in the evidence regarding whether individual items from the clinical assessment, can be used to identify hitherto unrecognised subgroups of patients with LBLP who have distinct presentations of symptoms and characteristics. Early identification and differentiation of subgroups of LBLP may provide more help when informing patients about prognosis, tailoring treatment plans to match profiles and guiding the need for referrals to specialist services in a timely fashion.
The objective of this study was to use items from clinical assessment to identify new subgroups in an unselected primary care population consulting with LBLP. Statistical modelling, such as latent class analysis provides a method of classifying patients and may lead to the identification of clusters of patients with similar characteristics, over and above the binary diagnostic categories of sciatica or referred leg pain. Clusters identified in this way were compared for baseline demographic, pain, physical function, psychosocial and work features, risk of persistent disability and findings from magnetic resonance imaging (MRI) of the lumbar spine. Key characteristics reflecting pain, disability, psychological status and perceived recovery were compared at 4 and 12 months and the clinical course, in terms of monthly pain and disability scores over 12 months for the individual clusters, was described and compared to that of the clinically defined groups of LBLP patients with and without a diagnosis of sciatica.
Methods
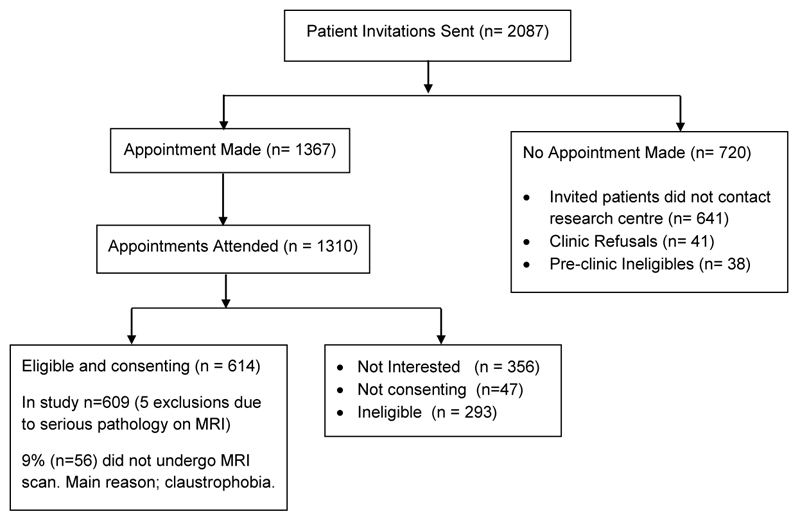
This study used data from primary care consulters with LBLP taking part in the ATLAS (Assessment and Treatment of Leg pain Associated with the Spine) multicentre prospective observational cohort study (see Fig.1 for ATLAS study flow diagram). Details of the protocol and baseline data results have been published [20, 21]. A brief overview of the ATLAS study methods is given here. Adults aged 18 years and over with LBLP of any duration and severity, who consulted their family doctor (general practitioner) were invited to take part in the ATLAS study. Patients were not eligible if they were receiving treatment, at the time of the study, for their back and leg pain. Leg pain was defined as any pain or unpleasant/abnormal sensation such as pins and needles or numbness, spreading from the back beyond the gluteal fold into the leg. Potentially eligible patients were sent a letter including information about the study, an invitation to attend a research clinic, and baseline questionnaires to complete.
Fig. 1.
ATLAS study flow diagram (adapted from Konstantinou et al. 2015 Fig. 1 p3 [21])
All patients attending the ATLAS research clinic who gave written consent underwent a standardised clinical assessment by one of seven musculoskeletal physiotherapists who documented at the end of the assessment (i) a clinical diagnosis of either sciatica or referred leg pain and (ii) confidence (0-100%) in their diagnosis. Patients received treatment according to need, with the majority of patients receiving physiotherapy intervention and a small number being referred to specialist spinal services for an opinion and consideration of further treatment options such as injections and/or surgery (see [20] for more details). Within two weeks of their assessment (providing there were no contraindications to the procedure) patients had a lumbar spine MRI scan, as part of the study. A senior consultant musculoskeletal radiologist provided a clinical report indicating presence or absence of nerve root compression, blind to any clinical information about the patient other than that the patient had LBLP.
Self-report measures were collected with questionnaires at baseline, 4 months and 12 months. Monthly measures for leg and back pain intensity and disability were collected over 12 months, using brief postal questionnaires.
Variables included in the latent class modelling
There is no restriction in latent class (LC) modelling on the number of variables or measurement level to model the clusters [44]. Twelve variables were a priori chosen from the larger set of available self-report and clinical assessment findings. Variable selection was based on (i) expert consensus from a Delphi study on items from clinical assessment considered most important for distinguishing sciatica from referred leg pain in LBLP patients [22], and (ii) clinical features of sciatica identified in a systematic review of LBLP classification systems [38].
Two variables were on a continuous scale (0-10) (leg pain intensity; back pain intensity). The remaining variables were binary (yes/no): subjective sensory changes in the lower limb; below knee pain; leg pain worse than back pain; leg pain on cough/sneeze/strain; leg pain on forward or backward spinal bend; positive neural tension test (straight leg raise or slump or femoral nerve stretch); myotomal (strength) deficit, reflex deficit, sensory deficit. (See Supplementary digital content file for description of variables).
Latent class model development
LC modelling aims to identify unobserved heterogeneity in a population and to find meaningful groups that are similar in their responses to measured variables [28] with minimal within group variation and maximum between-group variation [18]. LC models were fitted consecutively starting with a two cluster solution. The optimal number of clusters was determined by a combination of the following:
-
(i)
Goodness of fit statistics: Bayesian Information Criterion (BIC) (a model with lower BIC is preferred) and the bootstrapped parametric likelihood ratio test (LRT) which assesses if the addition of a cluster significantly improves the model fit [31].
-
(ii)
Uncertainty of classification measures: entropy measuring the distinction between classes, (0 to 1 where number closer to 1 is optimal) [5], and average posterior probabilities [4] where values should exceed 0.7, indicating clear separation for individuals allocated to that cluster.
-
(iii)
At least 5% of the sample in each cluster [45].
-
(iv)
Face validity of the clusters in terms of their clinical interpretability.
When assigning a “descriptive label” to the clusters, the following was taken into consideration:
-
(i)
Probabilities of a positive response (range 0 to 1) to the categorical clinical assessment items entered in the LC modelling. A probability of 1 means that all patients in that cluster responded “yes” to that item e.g. all had ‘pain below the knee’. Probabilities closer to 0.5 reflect more ambiguity in distinguishing clusters [13].
-
(ii)
Average back and leg pain intensity of patients within the cluster.
-
(iii)
Proportion of patients within the cluster with a clinical diagnosis (made by the assessing physiotherapist at the end of assessment) of referred leg pain or sciatica.
Cluster characteristics
Baseline characteristics were compared across the identified clusters. These included age, gender, socioeconomic status, body mass index (BMI) (height and weight measured in clinic), currently smoking, time off work (only for those at work), pain duration, pain trajectory over the previous year dichotomised as either “mild” or “moderate/severe” based on seven available responses ranging from first ever episode to severe pain all the time [7], presence of widespread pain derived from the shaded body manikin [23] and defined as pain present above and below the waist, in the right and left-hand sides of the body and in the axial skeleton [43], neuropathic (self-report) pain score (S-LANSS questionnaire [2] scored from 0-24, with values ≥12 indicating possible neuropathic pain), Sciatica Bothersomeness Index (SBI) (scored from 0-24, based on self-reported ratings (0-6) of bothersomeness of (i) leg pain (ii) numbness or tingling in the leg, foot or groin (iii) weakness in the leg/foot, and (iv) back or leg pain while sitting giving a composite score from 0-24 higher scores indicating worse symptoms) [32], pain self-efficacy (scored from 0 to 60, higher scores representing greater pain self-efficacy beliefs) [30], anxiety and depression using the Hospital Anxiety and Depression Scale (HADS)[46] scored from 0 to 21 with a score of ≥ 11 indicative of probable depression/anxiety, risk of poor outcome in terms of back pain related disability using the STarT Back Tool [15] with cut off scores to predict low, medium or high risk, number of comorbidities (from a list of five conditions: chest problems, heart problems, hypertension, diabetes, circulation problems in legs), sleep disturbances (self-report) due to LBLP, general health (Short Form Health Questionnaire)[42] ranked as either good/very good/excellent or fair/poor. A single value for health status index was calculated from the EQ-5D-3L [10] between zero and one, with values closer to one indicating better quality of health. Also compared among clusters was the proportion of patients with MRI evidence of nerve root compression and the proportion of patients where clinicians had high confidence in their diagnosis of either referred leg pain or sciatica (dichotomised to at least 80% confident in diagnosis (yes/no), at this cut-off the inter-rater reliability is high [39]).
Clinical course
The clinical course of the identified clusters was examined over a 12 month period for leg pain, LBP, and back and leg pain related disability. Leg and back pain intensity were measured using the mean of three 0 to 10 numerical rating scales (NRS) for current pain and least and usual pain over the previous 2 weeks [9]. Disability was measured using the sciatica version of the Roland Morris Disability Questionnaire (RMDQ) with total score ranging from 0 to 23, higher values representing greater disability [32, 35]. At 4 and 12 months, self-report characteristics to reflect pain (SBI, pain self-efficacy, possible neuropathic pain), psychological status (HADS, 12 month only), and health status (EQ-5D-3L), were compared for the identified clusters.
The proportion of patients referred to secondary care for spinal specialist opinion within the clusters, were described and global perceived recovery from baseline was compared across the clusters with recovery defined as ‘completely recovered’ or ‘much better’ [3].
LC modelling was performed in Mplus version 5 (Muthen and Muthen Los Angeles, CA). Graphs of clinical course were performed in Stata 14 (StataCorp. College Station Texas). All other analyses were performed in SPSS version 21. Each characteristic was compared across the number of identified clusters using ANOVA for continuous variables (Kruskall Wallis test when normality and homogeneity of variance assumptions were not met) and Pearson’s Chi squared test (Fisher’s exact test used for cell frequencies <5) for categorical variables. Analyses were two tailed and considered statistically significant if p<0.05.
Results
At baseline, data were available for 609 LBLP consulters (63% female, mean (SD) age 50 (13.9) years). Forty three percent (n=251) of patients had leg pain for less than 6 weeks, 36% (n=212) had leg pain for greater than three months. Based on clinical assessment, clinicians diagnosed 74% (n=452) of the patients as having sciatica. On neurological examination, 54% (n=327) of patients had either myotomal, reflex or sensory deficit of the lower limb. Monthly questionnaire response rates for pain and disability scores (Table 1) ranged from 46% (282/609) to 75% (450/609). Overall response rates to 4 and 12-month questionnaires were 66% and 74% respectively. Response rates for individual clusters were similar to the overall average across the five clusters.
Table 1. Monthly response rates to questionnaires.
| Month | No. of participants | Follow-up response rate compared to baseline |
|---|---|---|
| 0 | 609 | 100.0% |
| 1 | 455 | 74.7% |
| 2 | 410 | 67.3% |
| 3 | 396 | 65.0% |
| 4 | 402 | 66.0% |
| 5 | 282 | 46.3% |
| 6 | 325 | 53.4% |
| 7 | 300 | 49.3% |
| 8 | 308 | 50.6% |
| 9 | 286 | 47.0% |
| 10 | 287 | 47.1% |
| 11 | 287 | 47.1% |
| 12 | 450 | 73.9% |
Bolded row represent full questionnaires, the rest are short monthly questionnaires on pain severity and disability (RMDQ)
Model development
A five cluster LC solution was optimal (see Table 2 for indices of fit data) because the BIC was lowest and compared to two, three and four cluster solution, the entropy was highest (0.74). Entropy improved in the six cluster solution (0.79) but the BIC was higher. The bootstrapped LRT p-value remained significant for all cluster solutions suggesting the model fit improved every time a cluster was added to the model. With seven clusters the sample size of the smallest cluster was below 4%. There was a high probability of individuals in the five cluster solution being classified in their allocated group, with all average probabilities > 0.80.
Table 2. Statistical indices of fit of the latent cluster models of LBLP patients (n=609).
| Number of clusters | BIC | Bootstrapped parametric LRT p value |
Entropy | Smallest sample size a (%) |
|---|---|---|---|---|
| 2 | 12101.838 | <0.001 | 0.714 | 281 (46.3) |
| 3 | 12005.723 | <0.001 | 0.738 | 147 (24.1) |
| 4 | 11951.353 | <0.001 | 0.728 | 121 (19.9) |
| 5 | 11941.422 | <0.001 | 0.742 | 69 (11.3) |
| 6 | 11974.379 | <0.001 | 0.791 | 51 (8.4) |
| 7 | 12002.221 | <0.001 | 0.802 | 24 (3.9) |
BIC Bayesian Information Criteria; LRT likelihood ratio test.
The number (proportion) of patients in the smallest class; at least 5% of sample should be in each class. The bold text indicates the model selected as having the optimal number of clusters
Description of clusters
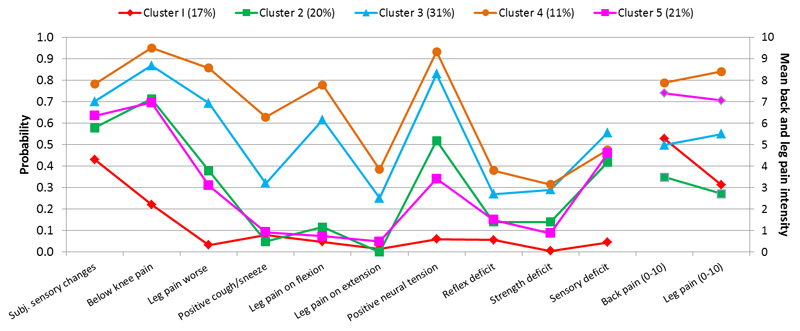
The five clusters’ response probabilities to individual clinical assessment items and their corresponding back and leg pain intensity is displayed in Fig. 2.
Fig. 2.
Five cluster latent class analysis solution. Item response probabilities of categorical variables (left vertical axis) and baseline mean leg and back pain intensity (right vertical axis).
Patients in cluster 1 (n=104, 17%) had moderate LBP (mean 5.3, standard deviation (SD)1.7), low intensity leg pain (mean 3.1, SD 1.4) and moderate probability of subjective sensory changes (0.43). All other clinical items had very low probability of being positive (≤0.22). 81% were given a “referred pain” diagnosis by the assessing physiotherapists. On the basis of these characteristics, we assigned the label “referred leg pain” to this cluster.
Patients in cluster 2 (n= 122, 20%) had low intensity back (mean 3.4, SD 1.4) and leg pain (mean 2.6, SD 1.2), high probability of below knee pain (0.7) and moderate probability of subjective sensory changes (0.57), objective sensory deficits (0.42) and positive neural tension (0.52). 81% were given a “sciatica” clinical diagnosis by the assessing physiotherapists. Based on these characteristics, we assigned the label “mild sciatica” to this cluster.
Patients in cluster 3 (n=188, 31%) had moderate leg and back pain with slightly higher leg pain (mean 5.5, SD 1.3) than back pain (mean 5.0, SD 1.5) intensity. They had very high probability of below knee pain (0.86) and positive neural tension (0.83) and low probability of reflex or myotome deficit (<0.3) but higher probability of sensory deficit (0.56). 93% were given a “sciatica” clinical diagnosis by the assessing physiotherapist. Based on these characteristics, we assigned the label “moderate sciatica” to this cluster.
Patients in cluster 4 (n=69, 11%) had high intensity back pain (mean 8.0, SD 1.3) and leg pain (mean 8.5, SD 1.1) and high probability of most clinical assessment items being positive, especially leg pain worse than back pain (0.86), below knee pain (0.95) and neural tension (0.9). They had the highest probability among all the clusters of neurological deficits (0.38, 0.32, 0.48, for reflex, myotome and sensory deficit respectively) and positive cough/sneeze (0.63). 100% were given a “sciatica” clinical diagnosis by the assessing physiotherapists. Based on these characteristics, we assigned the label “severe sciatica” to this cluster.
Patients in cluster 5 (n=126, 21%) had high intensity back pain (mean 7.5, SD 1.4) and leg pain (mean 7.2 SD 1.4) and high probability (0.7) of pain below the knee. They were not likely to have positive neural tension (0.34) or leg pain worse than back pain (0.31) and likely to have subjective sensory changes (0.63) and objective sensory deficit (0.46), compared to other clusters. They had a very similar response to clinical assessment as cluster 2 but with much higher pain severity. 71% were given a “sciatica” clinical diagnosis by the physiotherapist. Based on these characteristics, we assigned the label “atypical sciatica” to this cluster.
Cluster characteristics
The clusters did not differ significantly in age, gender or BMI (Table 3). There was a greater proportion of smokers in clusters 4 (severe sciatica) and 5 (atypical sciatica) and these two clusters had more patients categorised as manual workers.
Table 3. Baseline characteristics of the five clusters of low back- related leg pain patients.
| Socio-demographics Denominator a |
Cluster 1 Referred leg pain n=104 |
Cluster 2 Mild sciatica n=122 |
Cluster 3 Moderate sciatica n=188 |
Cluster 4 Severe sciatica n=69 |
Cluster 5 Atypical sciatica n=126 |
p value Δ |
|---|---|---|---|---|---|---|
| Age (years) mean (SD) | 47.2 (13.8) | 50.4 (13.3) | 50.9 (14.4) | 49.2 (12.7) | 51.9 (14.1) | 0.111 |
| Age category 65+ | 13 (12.5) | 17 (13.9) | 33 (17.6) | 7 (10.1) | 22 (17.5) | 0.238 |
| Gender, Female | 76 (73.1) | 72 (59.0) | 113 (60.1) | 42 (60.9) | 80 (63.5) | 0.187 |
| Current smoker | 27 (26.0) | 29 (23.8) | 52 (27.7) | 30 (43.5) | 56 (44.4) | <0.001 |
| BMI (607) category: | 31 (29.8) | 49 (40.5) | 78 (41.5) | 36 (52.2) | 54 (43.2) | 0.056 |
| Obese/Morbidly obese | ||||||
| Socioeconomic status: Manual | 41 (39.4) | 43 (36.1) | 85 (46.4) | 36 (55.4) | 78 (63.9) | <0.001 |
| occupation (593) | ||||||
| Self-certified time off work (363) | 25 (35.7) | 20 (25.6) | 42 (35.0) | 11 (29.7) | 8 (13.8) | 0.032 |
| or current sick note (365) | 22 (31.4) | 16 (20.3) | 34 (28.3) | 14 (37.8) | 14 (16.2) | 0.279 |
| Back pain duration (607) >6 wks | 64 (61.5) | 72 (59.0) | 117 (62.6) | 47 (68.1) | 89 (71.2) | 0.279 |
| Leg pain duration (583) > 6wks | 50 (50.5) | 52 (45.2) | 105 (57.7) | 38 (57.6) | 87 (71.9) | 0.001 |
| > 3 months | 31 (31.3) | 24 (20.9) | 69 (37.9) | 20 (30.3) | 68 (56.2) | <0.001 |
| >12 months | 15 (15.2) | 10 (8.7) | 24 (13.2) | 3 (4.5) | 29 (24.0) | <0.001 |
| Back pain intensity, mean (SD) | 5.3 (1.7) | 3.4 (1.4) | 5.0 (1.5) | 8.0 (1.3) | 7.5 (1.4) | <0.001 |
| Leg pain intensity, mean (SD) | 3.1 (1.4) | 2.6 (1.2) | 5.5 (1.3) | 8.5 (1.1) | 7.2 (1.4) | <0.001 |
| RMDQ disability score (0-23) | 11.5 (5.6) | 8.6 (5.0) | 12.8 (4.7) | 16.7 (5.1) | 15.1 (5.5) | <0.001 |
| mean (SD) (607) | ||||||
| Sciatica Bothersomeness Index | 11.1 (4.9) | 10.0 (4.4) | 14.7 (4.0) | 19.8 (3.5) | 17.2 (4.4) | <0.001 |
| (0-24)mean (SD) (582) | ||||||
| S-LANSS, possible neuropathic | 37 (35.6) | 44 (36.4) | 100 (53.2) | 45 (66.2) | 67 (53.6) | <0.001 |
| pain (≥12) (606) | ||||||
| STarT Back subgroup (589) | <0.001 | |||||
| Low risk | 17 (17.0) | 44 (37.0) | 16 (13.4) | 0 (0.0) | 5 (4.0) | |
| Medium risk | 52 (52.0) | 59 (49.6) | 105 (58.0) | 20 (30.8) | 40 (32.3) | |
| High risk | 31 (31.0) | 16 (8.8) | 60 (33.1) | 45 (69.2) | 79 (63.7) | |
| Widespread pain b (592) | 50 (49.9) | 48 (40.7) | 72 (38.9) | 15 (22.4) | 65 (54.2) | <0.001 |
| HADS Anxiety (607)subscale | ||||||
| Probable c | 32 (31.1) | 20 (16.4) | 34 (18.2) | 33 (47.8) | 52 (41.3) | <0.001 |
| HADS Depression subscale | ||||||
| Probable c | 12 (11.5) | 9 (7.4) | 21 (11.2) | 26 (37.7) | 30 (23.8) | <0.001 |
| Pain self-efficacy score (0-60), | 37.6 (12.4) | 42.9 (12.5) | 34.7 (12.3) | 22.5 (15.6) | 28.4 (14.3) | <0.001 |
| mean (SD) (593) | ||||||
| EQ—5D-3L summary index (590) | 0.54 (0.3) | 0.66 (0.2) | 0.48 (0.3) | 0.13 (0.3) | 0.29 (0.3) | <0.001 |
| Co-morbidities | ||||||
| Two or more other health | 16 (15.4) | 15 (12.3) | 21 (11.2) | 5 (7.2) | 23 (18.3) | 0.139 |
| problems | ||||||
| General Health (608) | ||||||
| Fair/poor | 38 (36.5) | 31 (25.5) | 59 (31.4) | 32 (47.1) | 62 (49.2) | <0.001 |
| Sleep Disturbance (yes) d | 69 (66.3) | 73 (59.8) | 133 (70.7) | 61 (88.4) | 92 (73.0) | 0.001 |
| Clinical diagnosis sciatica | 20 (19.2) | 99 (81.1) | 175 (93.1) | 69 (100.0) | 89 (70.6) | <0.001 |
| Clinician confidence in diagnosis | 72 (69.2) | 75 (61.4) | 156 (83.0) | 63 (91.3) | 70 (55.6) | <0.001 |
| ≥80% | ||||||
| MRI (554) | ||||||
| Clear or possible nerve root | 25 (26.3) | 56 (50.5) | 106 (63.1) | 57 (89.1) | 53 (45.7) | <0.001 |
| compression | ||||||
| Disc prolapse | 17 (68.0) | 47 (83.9) | 84 (79.2) | 49 (86.0) | 33 (62.3) | |
| Stenosis | 6 (24.0) | 7 (12.5) | 19 (17.9) | 7 (12.3) | 16 (30.2) | |
| Other e | 2 (8.0) | 2 (3.6) | 3 (2.8) | 1 (1.8) | 4 (7.5) | |
SD, standard deviation; BMI, body mass index; RMDQ, Roland Morris Disability Questionnaire; s-LANSS, self-report Leeds Assessment of Neuropathic Symptoms and Signs; HADS, Hospital Anxiety and Depression Scale; EQ-5D-3L EuroQoL; MRI, magnetic resonance imaging
All figures are frequencies (percentages) unless otherwise stated as mean (SD).
Significance p-value (α=0.05) for the difference between patients in the five latent clusters on ANOVA for continuous variables (Kruskill Wallis for variables BMI, HADS(depression) and EQ-5D) and Chi squared test for categorical variables (Fisher’s exact test for variable socioeconomic cluster and general health).
Denominator varies for some participants due to missing data or non-applicable cases All figures are frequencies (percentages) unless otherwise stated as mean (SD)
Widespread pain derived from the shaded body manikin (defined as pain present above and below the waist in the right and left hand sides of the body and in the axial skeleton).
Score of ≥ 11 indicative of probable depression/anxiety
Question on back and/or leg pain associated sleep disturbance was asked during the clinical assessment.
Other MRI diagnoses (n= 11) included spondylolisthesis, epidural lipomatosis, synovial cyst, osteophyte
In ascending order of severity for pain (back and leg pain intensity and S-LANSS neuropathic pain score), sciatica bothersomeness index, and disability (RMDQ) scores, was cluster 2 (mild sciatica), cluster 1 (referred leg pain), cluster 3 (moderate sciatica), cluster 5 (atypical sciatica) and cluster 4 (severe sciatica). In cluster 5, 24% of patients had leg pain for over one year compared to 13% or less for the other four clusters. The proportions of patients with moderate/severe pain over the last year was lowest in cluster 1 (30%) and highest in cluster 5 (71%).
The STarT Back tool grouped 69% and 64% of patients in clusters 4 and 5 respectively, as being at high risk of poor prognosis in terms of disability. Clusters 1 and 3 had approximately one third of patients categorised as high risk and only 13% of patients in cluster 2 were at high risk. Anxiety and depression cases were highest for cluster 4, followed by cluster 5. Cluster 2 had the lowest proportion of patients categorised as anxious or depressed. Cluster 1 (referred leg pain) had higher anxiety levels than clusters 2 and 3. Pain self-efficacy was lowest for cluster 4 and highest for cluster 2. A higher proportion in cluster 5 reported poorer general health, more widespread pain and two or more other health problems. EQ5D summary index was considerably lower for cluster 4 (mean 0.13 SD 0.3) and cluster 5 (mean 0.29, SD 0.3), indicating poorer quality of health.
Clinicians had high confidence (≥80%) in their diagnosis for 90% of patients in cluster 4, whereas in cluster 5, just over half (51%) of the group were diagnosed by clinicians with high confidence. Concordant MRI findings of nerve root compression were highest in cluster 4 (89%) and lowest in cluster 1 (26%). Clusters 2 and 5 had similar proportion of patients with nerve root compression on MRI (51% and 46% respectively). Cluster characteristics are summarised in Table 3.
Clinical course
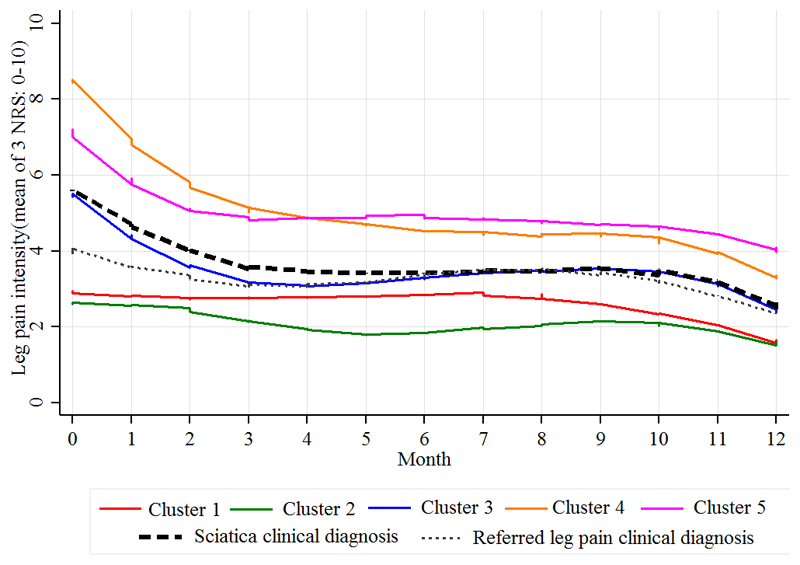
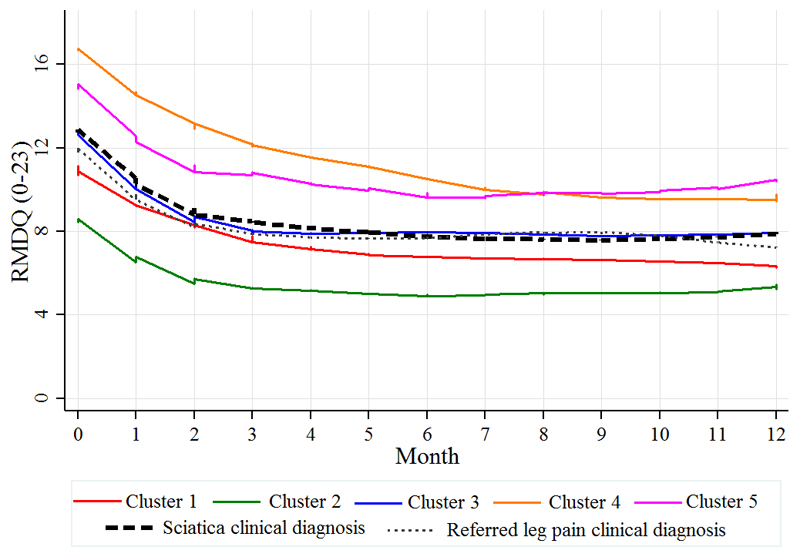
Disability, leg pain and LBP scores improved over time for all clusters. Similar to the baseline pattern, the order of severity of monthly leg pain (Fig. 3) and disability scores (Fig. 4) remained almost the same across the five clusters with clusters 4 and 5 remaining with the highest pain and disability scores at 12 months.
Fig. 3.
Clinical course over 12 months of monthly leg pain intensity scores (0-10) for the five clusters and the clinically diagnosed groups (referred leg pain and sciatica), calculated from the mean of 3 numeric rating scores (NRS) for current and least and usual leg pain over the previous two weeks.
Fig. 4.
Clinical course over 12 months of disability for the five clusters and the clinically diagnosed groups (referred leg pain and sciatica), measured by the monthly mean Roland Morris Disability Questionnaire (RMDQ) score.
The most reduction in pain and disability for all clusters was seen in the first 4 months, after which the values remained relatively stable. Cluster 2 (mild sciatica) presented with the mildest pain at baseline and remained relatively unchanged over the year.
When patients are classified to two groups according to the clinical diagnosis of either referred leg pain (n= 157) or sciatica (n=452), their clinical course was very similar for leg pain (Fig. 3), disability (Fig. 4) and back pain (Fig. 5).
Fig. 5.
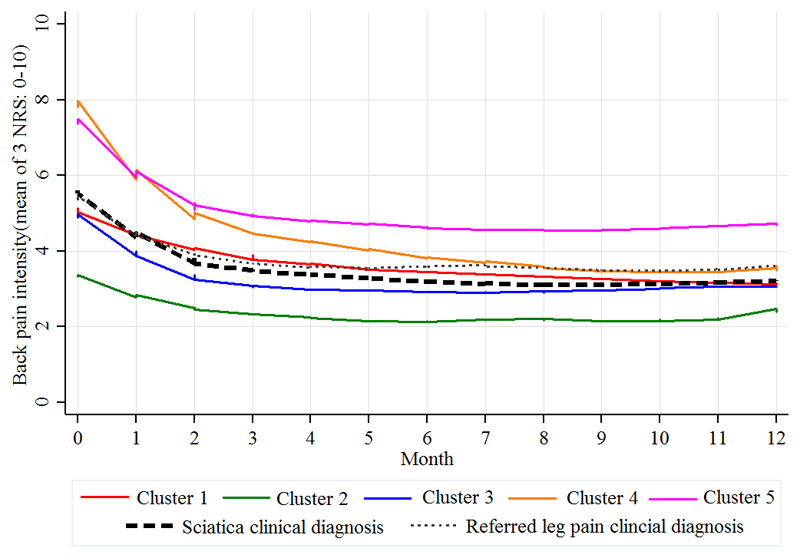
Clinical course over 12 months of monthly back pain intensity scores (0-10) for the five clusters and the clinically diagnosed groups (referred leg pain and sciatica), calculated from the mean of 3 numeric rating scores (NRS) for current and least and usual back pain over the previous two weeks.
Among the whole cohort, 70 patients were referred to specialist spinal services. Patients in the moderate, severe and atypical sciatica clusters accounted for the majority of these onward referrals. On all key characteristics (Table 4), scores improved across all domains measured at 4 and 12 months. Cluster 5 showed least improvement and had the lowest proportion of patients reporting recovery (completely recovered or much better) at 4 months (19%) and 12 months (27%). At 12 months, overall recovery proportions in the other four clusters ranged from 37% in cluster 1 to 45% in cluster 2.
Table 4. Key characteristics for five clusters at 4 and 12 months.
| Cluster 1 Referred leg pain n=104 |
Cluster 2 Mild sciatica n=122 |
Cluster 3 Moderate sciatica n=188 |
Cluster 4 Severe sciatica n=69 |
Cluster 5 Atypical sciatica n=126 |
||
|---|---|---|---|---|---|---|
| HADS anxiety probable n (%) | Baseline (n=609) |
32 (31.1) | 20 (16.4) | 34 (18.2) | 33 (47.8) | 52 (41.3) |
| 12 months (n=365) |
9 (15.0) | 8 (10.3) | 14 (11.7) | 7 (20.0) | 17 (23.6) | |
| HADs depression probable) n (%) | Baseline (n=609) |
12 (11.5) | 9 (7.4) | 21 (11.2) | 26 (37.7) | 30 (23.8) |
| 12 months (n=373) |
3 (4.8) | 2 (2.5) | 6 (5.0) | 5 (13.5) | 14 (19.2) | |
| EQ-5D-3L summary index (0-1) mean (SD) | Baseline (n=590) 4 months (n=341) 12 months (n=357) |
0.54 (0.30) | 0.66 (0.20) | 0.48 (0.30) | 0.13 (0.30) | 0.29 (0.30) |
| 0.73 (0.24) | 0.76 (0.21) | 0.65 (0.29) | 0.49 (0.40) | 0.44 (0.37) | ||
| 0.72 (0.23) | 0.77 (0.21) | 0.68 (0.28) | 0.62 (0.32) | 0.53 (0.38) | ||
| Sciatica bothersomeness index (SBI) (0-24) mean (SD) |
Baseline (n=582) |
11.1 (4.9) | 10.0 (4.4) | 14.7 (4.0) | 19.8 (3.5) | 17.2 (4.4) |
| 4 months a (n= 236) |
8.5 (5.2) | 8.5 (4.9) | 10.2 (5.4) | 14.5 (7.0) | 14.3 (5.7) | |
| 12 months a (n=187) |
9.6 (5.4) | 8.2 (4.7) | 10.9 (5.7) | 13.3 (5.6) | 14.1 (6.1) | |
| Pain self-efficacy (PSEQ) 0-60, mean (SD) | Baseline n=593 |
37.6 (12.4) | 42.9 (12.5) | 34.7 (12.3) | 22.5 (15.6) | 28.4 (14.3) |
| 4 months n=378 |
48.4 (10.7) | 49.1 (12.7) | 42.9 (14.3) | 37.8 (18.4) | 35.8 (17.5) | |
| 12 months n=364 |
48.6 (10.1) | 50.0 (11.6) | 44.1 (14.1) | 41.7 (17.7) | 38.4 (17.1) | |
| S-LANSS Neuropathic pain score (≥12) n (%) | Baseline n= 606 |
37 (35.6) | 44 (36.4) | 100 (53.2) | 45 (66.2) | 67 (53.6) |
| 4 months n=376 |
9 (15.3) | 12 (15.6) | 32 (25.4) | 15 (41.7) | 26 (33.3) | |
| 12 months n=348 |
8 (13.8) | 10 (13.7) | 31 (27.0) | 10 (28.6) | 20 (29.9) | |
| Global perceived recovery b (completely recovered, much better) n (%) | 4 months n= 394 |
19 (31) | 37 (46) | 55 (42) | 12 (32) | 16 (19) |
| 12 months n= 444 |
28 (38) | 42 (45) | 88 (40) | 19 (41) | 22 (27) |
SD, standard deviation; HADS, hospital anxiety and depression scale; EQ-5D-3L, EuroQoL; S-LANSS, self-report Leeds Assessment of Neuropathic Symptoms and Signs
SBI questionnaire only answered by patients whose pain from the back had spread down their legs in the last 2 weeks
compared to 4 (12) months ago, how do you think your back and/or leg pain has changed?
Discussion
This study is the first to use LC modelling to identify potentially clinically relevant clusters of primary care consulters with symptoms of low back and leg pain. Clusters were identified based on response to clinical assessment items used to guide diagnosis in LBLP patients. One cluster represents a referred leg pain group. Three clusters represent varying severity of sciatica (mild, moderate and severe). The fifth cluster (atypical sciatica) is more difficult to define, with similar responses to clinical assessment items as the mild sciatica cluster but with much higher pain intensity. The work gives a novel insight into the clinical spectrum of LBLP, not previously highlighted in the literature.
The main items that distinguished between the four “sciatica” clusters were severity of back and leg pain, whether leg pain was worse than back pain, location of the leg pain (below the knee), and presence of neural tension. Neurological examination tests did not add much information to distinguishing the sciatica clusters, neither did leg pain on lumbar extension. The probability of having leg pain on forward bending was higher for patients in clusters 3 and 4 which could be explained by similarity to mechanics of performing a straight leg raise.
Two clusters (‘severe sciatica’ and ‘atypical sciatica) had considerably greater severity in terms of pain, disability, risk of poor outcome, work impact and psychological and health-related characteristics. Mean disability levels for the ‘severe sciatica’ cluster 4, measured by RMDQ, was 16.7, comparable to secondary care sciatica populations in clinical trials involving surgery (16.4) [33]. By comparison, the ‘mild sciatica’ cluster had the lowest level of disability (8.6) comparable to other primary care LBP cohorts with and without leg pain (8.8 [16], (8.7) [24]). In cluster 5, ‘atypical sciatica’, although 70% of patients had a clinical diagnosis of sciatica, clinicians had low confidence in their diagnosis (<80% confidence) in almost half of the patients and MRI findings confirmed nerve root compression in 46% of patients. Arguably, labelling this cluster as “sciatica” may be unrepresentative of the signs and symptoms of the condition, and during discussions with clinicians, this was the most difficult group to “label”. Only 27% of patients in cluster 5 reported recovery at 12 months, considerably lower than the other four clusters. This perhaps reflects their more complex presentation with more patients in this cluster having longer pain duration, more comorbidities and a higher proportion with widespread pain.
The observed differences in cluster characteristics at baseline persisted over time. All clusters showed improved pain and disability scores over 12 months, with most improvement seen within the first three to four months following baseline assessment. LBP trajectory studies confirm this early improvement for most patients and show findings similar to our cohort that the majority of LBP patients remain in some level of pain at 12 months [1, 6]. When patients were classified according to clinical diagnosis of either referred leg pain or sciatica, their clinical course over 12 months was very similar for leg pain, disability and back pain. The latent class modelling gave richer information about the whole LBLP cohort as opposed to considering the group as with or without a clinical diagnosis of sciatica. Cluster 1 (labelled ‘referred leg pain’) and the group of patients with the clinical diagnosis of referred leg pain, consist of mostly the same patients, hence their clinical course is similar. Cluster 3 (moderate sciatica) mirrored the clinical course of patients with a clinical diagnosis of sciatica. Clusters 2, 4, and 5 however revealed the existing variability in terms of characteristics and clinical course in patients with sciatica, and provide more detailed information and insight compared to the information provided by the overall average for this group.
Probably the most extensively investigated LBP and leg pain classification system is the Quebec Task Force Classification (QTFC) system ([37]) which categorises back and leg pain patients based on pain location and presence of neurological deficit. Patients with leg pain and signs of nerve root involvement were most severely affected in terms of pain, disability and work ability [17], improve more than other LBLP categories over time, but have poorer outcomes measured by absolute disability scores [19]. This is similar to the clinical course of our ‘severe sciatica’ cluster.
Previous work using longitudinal LCA and pain trajectories identified four LBP clusters (with and without leg pain): persistent mild, recovering, fluctuating, severe chronic [8]. The severe chronic pain cluster, with the greatest numbers with leg pain (89%), scored worse on disability scores, psychological distress and work absence, suggesting it might reflect patients from both our “severe sciatica” and “atypical sciatica” cluster. In acute LBP, similar trajectories were identified but “pain below the knee” was not associated with membership of any of the clusters [6].
Strengths and limitations
The key strengths of our study include using a statistical approach to develop clusters based on patient data, with clinical judgement to aid cluster interpretation. The sample represents a true primary care population presenting initially to their GP, with variable symptom severity and duration. The modelling and description of the clusters was based on a comprehensive dataset of clinical assessment, self-report and imaging items, and longitudinal data.
A limitation of our study is that although the five cluster solution was based on optimal statistical fit of the data and clinical interpretability of the clusters, they may not reflect the precise clustering of LBLP patients among primary care consulters. Replication of these clusters in other LBLP populations is needed to explore their external validity. Available longitudinal data gave insight to the clinical course of patients within the five clusters but there was missing data at each time point owing to non-response to monthly questionnaires or to individual items within the questionnaire. Age and gender characteristics for non-participants (invited patients who did not attend the research clinics or were not interested in participation) were similar to those who participated. As we do not have data on other variables, participation bias is possible if participants differed from non-participants on certain characteristics.
When considering the clinical course of the five clusters, generalizability to primary care may be influenced by nature of patients’ involvement in the study. Receiving a lumbar spine MRI scan with subsequent feedback from clinicians in relation to findings and having timely access to appropriate management may have positively influenced patient outcomes. Despite this process, the proportion of patients reporting recovery (completely recovered or much better) was no higher than 45% for all clusters, and cluster 5 (“atypical sciatica”) was considerably lower at 12 months with only 27% of patients reporting recovery.
Clinical implications
This work gives detailed insight into the complexity of LBLP and shows that information on initial presentation can help classify patients into distinct clusters.
Even within a specific condition/presentation such as sciatica, variation is overlooked if only ‘average’ population measures are considered. Heterogeneous study populations in clinical research can potentially confound outcomes [40] and recent clinical practice guidelines for LBP treatments conclude there is insufficient evidence to better match treatment for presentations of leg pain/sciatica [34]. The clusters identified in this work may represent groups likely to need a different management approach.
Currently management of sciatica is a stepped care approach in those not deteriorating or with signs suggestive of sinister pathology, starting with non-invasive treatments and progressing to more invasive treatment options [26]. Timing and when to move to the next step is not clear, particularly in those with higher pain levels [25] and it still remains unknown which patient will benefit from what intervention at which point (e.g. conservative management/surgery /injection) [29]. Two of the clusters represent patients that could preferentially respond to this stepped approach (cluster 2 and 3). Cluster 4 patients may benefit from a more intensive initial approach earlier in their management e.g. specialist opinion regarding more invasive options (surgery/ injections). Patients in cluster 1 and cluster 5 may be more suitable for pain management options that include psychosocial interventions.
Levels of depression and anxiety were highest in the clusters with most severe symptoms, which is unsurprising and levels remained highest in cluster 5 at 12 months. Mechanisms driving the high pain and anxiety are potentially different between the two groups and management should reflect this. The atypical sciatica cluster resembles profiles of patients with persistent/ widespread pain, whereas cluster 4 have a clear diagnosis and in the clinical setting are more likely to be considered for treatment options such as injections and/or surgery.
These clusters could be more homogenous groups that represent uniquely different responders to specific interventions. The next step is to consider optimum management pathways for these clusters and formally test whether different management options improve outcomes.
Conclusion
This work shows the variation in profile and clinical course of patients that present with a seemingly similar condition of LBLP. This is more informative than describing simple averages among a more heterogenous population. We recommend these clusters and their potentially differential treatment responses should be considered in current clinical settings and when designing future studies in the treatment of LBLP including sciatica.
Supplementary Material
Acknowledgements
This research is supported by National Institute for Health Research (NIHR) funding awards to Siobhan Stynes NIHR/CNO Clinical Doctoral Research Fellowship (CDRF-2010-055) and Kika Konstantinou HEFCE/NIHR Senior Clinical Lectureship. Elaine Hay is an NIHR Senior Investigator. The authors are solely responsible for content of the manuscript. The funders did not participate in study design, data collection, data analysis, interpretation of the data or preparation for the manuscript.
Ethical approval for the ATLAS study was granted by the South Birmingham Research Ethics committee, reference number 10/H1207/82.
Footnotes
Conflict of interest statement
There are no conflicts of interest associated with this study.
References
- [1].Axen I, Leboeuf-Yde C. Trajectories of low back pain. Best Pract Res Clin Rheumatol. 2013;27:601–12. doi: 10.1016/j.berh.2013.10.004. [DOI] [PubMed] [Google Scholar]
- [2].Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6(3):149–58. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- [3].Beurskens AJ, de Vet HC, Koke AJ. Responsiveness of functional status in low back pain: a comparison of different instruments. Pain. 1996;65(1):71–76. doi: 10.1016/0304-3959(95)00149-2. [DOI] [PubMed] [Google Scholar]
- [4].Clark DB, Jones BL, Wood DS, Cornelius JR. Substance use disorder trajectory classes: diachronic integration of onset age, severity, and course. Addict Behav. 2006;31(6):995–1009. doi: 10.1016/j.addbeh.2006.03.016. [DOI] [PubMed] [Google Scholar]
- [5].Collins L, Lanza S. Latent class and latent transition analysis: With applications in the social, behavioural, and health sciences. New York: John Wiley & Sons; 2010. [Google Scholar]
- [6].Costa Lda C, Koes BW, Pransky G, Borkan J, Maher CG, Smeets RJ. Primary care research priorities in low back pain: an update. Spine. 2013;38(2):148–156. doi: 10.1097/BRS.0b013e318267a92f. [DOI] [PubMed] [Google Scholar]
- [7].Downie AS, Hancock MJ, Rzewuska M, Williams CM, Lin CW, Maher CG. Trajectories of acute low back pain: a latent class growth analysis. Pain. 2016;157(1):225–234. doi: 10.1097/j.pain.0000000000000351. [DOI] [PubMed] [Google Scholar]
- [8].Dunn KM, Croft PR. The importance of symptom duration in determining prognosis. Pain. 2006;121(1–2):126–132. doi: 10.1016/j.pain.2005.12.012. [DOI] [PubMed] [Google Scholar]
- [9].Dunn KM, Jordan KP, Croft PR. Recall of medication use, self-care activities and pain intensity: a comparison of daily diaries and self-report questionnaires among low back pain patients. Primary Health Care Research & Development. 2010;11(01):93–102. [Google Scholar]
- [10].EuroQuol, Group. EuroQol--a new facility for the measurement of health-related quality of life. Health policy (Amsterdam, Netherlands) 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- [11].Foster NE, Hill JC, O'Sullivan P, Hancock M. Stratified models of care. Best Pract Res Clin Rheumatol. 2013;27(5):649–661. doi: 10.1016/j.berh.2013.10.005. [DOI] [PubMed] [Google Scholar]
- [12].Fritz JM, Lindsay W, Matheson JW, Brennan GP, Hunter SJ, Moffit SD, Swalberg A, Rodriquez B. Is there a subgroup of patients with low back pain likely to benefit from mechanical traction? Results of a randomized clinical trial and subgrouping analysis. Spine. 2007;32(26):E793–800. doi: 10.1097/BRS.0b013e31815d001a. [DOI] [PubMed] [Google Scholar]
- [13].Green DJ, Jordan KP, Protheroe J, van der Windt D. Development of hand phenotypes and changes in hand pain and problems over time in older people. Pain. 2016;157(3):569–576. doi: 10.1097/j.pain.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grotle M, Brox JI, Veierod MB, Glomsrod B, Lonn JH, Vollestad NK. Clinical course and prognostic factors in acute low back pain: Patients consulting primary care for the first time. Spine. 2005;3098:976–82. doi: 10.1097/01.brs.0000158972.34102.6f. [DOI] [PubMed] [Google Scholar]
- [15].Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, Hay EM. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59(5):632–41. doi: 10.1002/art.23563. [DOI] [PubMed] [Google Scholar]
- [16].Hill JC, Konstantinou K, Egbewale BE, Dunn KM, Lewis M, van der Windt D. Clinical outcomes among low back pain consulters with referred leg pain in primary care. Spine. 2011;36(25):2168–2175. doi: 10.1097/BRS.0b013e31820712bb. [DOI] [PubMed] [Google Scholar]
- [17].Kongsted A, Kent P, Albert H, Jensen TS, Manniche C. Patients with low back pain differ from those who also have leg pain or signs of nerve root involvement - a cross-sectional study. BMC Musculoskelet Disord. 2012;13:236. doi: 10.1186/1471-2474-13-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kongsted A, Kent P, Hestbaek L, Vach W. Patients with low back pain had distinct clinical course patterns that were typically neither complete recovery nor constant pain. A latent class analysis of longitudinal data. Spine J. 2015;15(5):885–94. doi: 10.1016/j.spinee.2015.02.012. [DOI] [PubMed] [Google Scholar]
- [19].Kongsted A, Kent P, Jensen TS, Albert H, Manniche C. Prognostic implications of the Quebec Task Force classification of back-related leg pain: an analysis of longitudinal routine clinical data. BMC Musculoskelet Disord. 2013;14:171. doi: 10.1186/1471-2474-14-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Konstantinou K, Beardmore R, Dunn KM, Lewis M, Hider SL, Sanders T, Jowett S, Somerville S, Stynes S, van der Windt DA, Vogel S, et al. Clinical course, characteristics and prognostic indicators in patients presenting with back and leg pain in primary care. The ATLAS study protocol. BMC Musculoskelet Disord. 2012;13:4. doi: 10.1186/1471-2474-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Konstantinou K, Dunn KM, Ogollah R, Vogel S, Hay EM, team Asr Characteristics of patients with low back and leg pain seeking treatment in primary care: baseline results from the ATLAS cohort study. BMC Musculoskelet Disord. 2015;16(1) doi: 10.1186/s12891-015-0787-8. 332-015-0787-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Konstantinou K, Hider SL, Vogel S, Beardmore R, Somerville S. Development of an assessment schedule for patients with low back-associated leg pain in primary care: a Delphi consensus study. Eur Spine J. 2012;21(7):1241–1249. doi: 10.1007/s00586-011-2057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lacey RJ, Lewis M, Jordan K, Jinks C, Sim J. Interrater reliability of scoring of pain drawings in a self-report health survey. Spine. 2005;30(16):E455–458. doi: 10.1097/01.brs.0000174274.38485.ee. [DOI] [PubMed] [Google Scholar]
- [24].Lamb SE, Lall R, Hansen Z, Castelnuovo E, Withers EJ, Nichols V, Griffiths F, Potter R, Szczepura A, Underwood M. A multicentred randomised controlled trial of a primary care-based cognitive behavioural programme for low back pain. The Back Skills Training (BeST) trial. Health Technol Assess. 2010;14(41):1–253. doi: 10.3310/hta14410. iii-iv. [DOI] [PubMed] [Google Scholar]
- [25].Lee J, Gupta S, Price C, Baranowski AP, British Pain S Low back and radicular pain: a pathway for care developed by the British Pain Society. Br J Anaesth. 2013;111(1):112–120. doi: 10.1093/bja/aet172. [DOI] [PubMed] [Google Scholar]
- [26].Lewis R, Williams N, Matar HE, Din N, Fitzsimmons D, Phillips C, Jones M, Sutton A, Burton K, Nafees S, Hendry M, et al. The clinical effectiveness and cost-effectiveness of management strategies for sciatica: systematic review and economic model. Health Technol Assess. 2011;15(39):1–578. doi: 10.3310/hta15390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miranda H, Viikari-Juntura E, Martikainen R, Takala EP, Riihimaki H. Individual factors, occupational loading, and physical exercise as predictors of sciatic pain. Spine. 2002;27(10):1102–1109. doi: 10.1097/00007632-200205150-00017. [DOI] [PubMed] [Google Scholar]
- [28].Muthen B. Latent variable analysis: Growth mixture modelling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newbury Park, Ca: Sage; 2004. [Google Scholar]
- [29].NICE. Low Back Pain and Sciatica in Over 16s: Assessment and management. London: 2016. [PubMed] [Google Scholar]
- [30].Nicholas MK. The pain self-efficacy questionnaire: Taking pain into account. Eur J Pain. 2007;11(2):153–63. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- [31].Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling. 2007;14(4):535–569. [Google Scholar]
- [32].Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine. 1995;20(17):1899–908. doi: 10.1097/00007632-199509000-00011. [DOI] [PubMed] [Google Scholar]
- [33].Peul WC, van Houwelingen HC, van den Hout WB, Brand R, Eekhof JA, Tans JT, Thomeer RT, Koes BW, Leiden-The Hague Spine Intervention Prognostic Study Group Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356(22):2245–2256. doi: 10.1056/NEJMoa064039. [DOI] [PubMed] [Google Scholar]
- [34].Qaseem A, Wilt TJ, McLean RM, Forciea M, Clinical Guidelines Committee of the American College of Physicians Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the american college of physicians. Ann Int Med. 2017;166(7):514–530. doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- [35].Roland M, Morris R. A Study of the Natural History of Back Pain: Part I: Development of a Reliable and Sensitive Measure of Disability in Low-Back Pain. Spine. 1983;8(2):141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- [36].Schafer A, Hall T, Briffa K. Classification of low back-related leg pain-A proposed patho-mechanism-based approach. Man Ther. 2009;14(2):222–231. doi: 10.1016/j.math.2007.10.003. [DOI] [PubMed] [Google Scholar]
- [37].Spitzer W, LeBlanc FE, Dupuis M. Scientific approach to the assessment and management of activity related spinal disorders. A monograph for clinicians. Report of the Quebec Task Force on Spinal Disorders. Spine. 1987;12:S1–S59. [PubMed] [Google Scholar]
- [38].Stynes S, Konstantinou K, Dunn KM. Classification of patients with low back-related leg pain: a systematic review. BMC Musculoskelet Disord. 2016;17:226. doi: 10.1186/s12891-016-1074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stynes S, Konstantinou K, Dunn KM, Lewis M, Hay EM. Reliability among clinicians diagnosing low back-related leg pain. Eur Spine J. 2016;25(9):2734–2740. doi: 10.1007/s00586-015-4359-2. [DOI] [PubMed] [Google Scholar]
- [40].Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- [41].Tubach F, Beauté J, Leclerc A. Natural history and prognostic indicators of sciatica. J Clin Epidemiol. 2004;57(2):174–179. doi: 10.1016/S0895-4356(03)00257-9. [DOI] [PubMed] [Google Scholar]
- [42].Ware JE., Jr SF-36 health survey update. Spine. 2000;25(24):3130. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- [43].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam A, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- [44].Wurpts IC, Geiser C. Is adding more indicators to a latent class analysis beneficial or detrimental? Results of a Monte-Carlo study. Front Psychol. 2014;5:920. doi: 10.3389/fpsyg.2014.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yang C-C. Evaluating latent class analysis models in qualitative phenotype identification. Computational Statistics & Data Analysis. 2006;50(4):1090–1104. [Google Scholar]
- [46].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.