Abstract
Salmonella enterica is an important intracellular bacterial pathogen of humans and animals. It replicates within host-cell vacuoles by delivering virulence (effector) proteins through a vacuolar membrane pore made by the Salmonella pathogenicity island 2 (SPI-2) type III secretion system (T3SS). T3SS assembly follows vacuole acidification, but when bacteria are grown at low pH, effector secretion is negligible. We found that effector secretion was activated at low pH from mutant strains lacking a complex of SPI-2–encoded proteins SsaM, SpiC, and SsaL. Exposure of wild-type bacteria to pH 7.2 after growth at pH 5.0 caused dissociation and degradation of SsaM/SpiC/SsaL complexes and effector secretion. In infected cells, loss of the pH 7.2 signal through acidification of host-cell cytosol prevented complex degradation and effector translocation. Thus, intravacuolar Salmonella senses host cytosolic pH, resulting in the degradation of regulatory complex proteins and effector translocation.
Type III secretion systems (T3SSs) are assembled in the cell envelope of many Gram-negative bacterial pathogens. They secrete three classes of proteins: subunits of a surface-exposed needle-like structure; translocon proteins, which form a pore in host membranes; and effectors, which pass through the needle channel and translocon pore into the host cell, where they manipulate cellular processes and promote bacterial virulence (1). Translocon pore assembly must precede effector translocation, and it is thought that upon assembly of the pore, bacteria sense host cell signals to activate effector secretion. The physiological signals and mechanisms underlying transition from translocon to effector secretion are not understood.
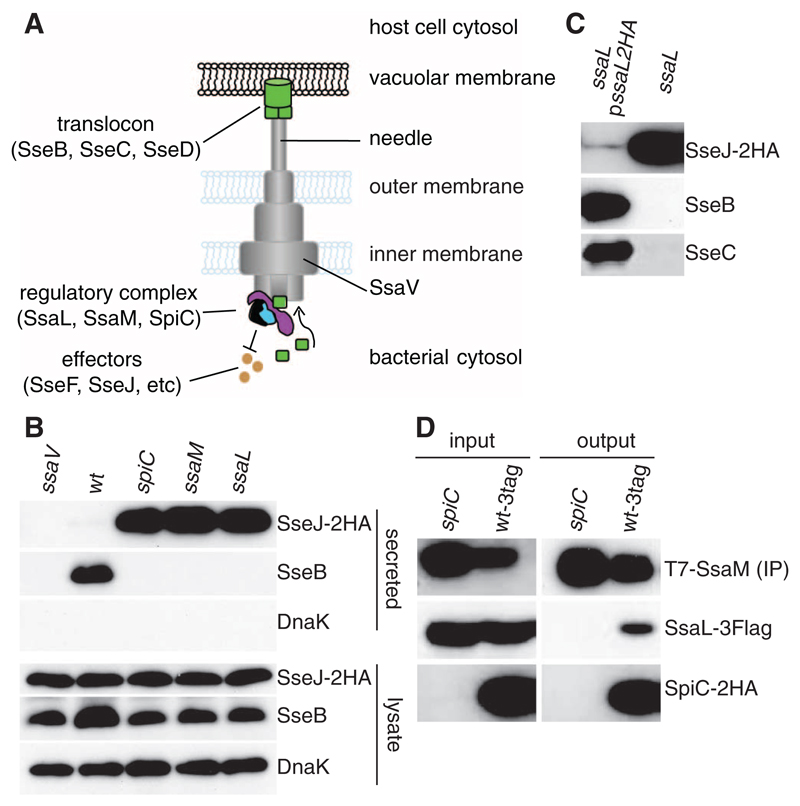
Assembly of the Salmonella pathogenicity island 2 (SPI-2) T3SS in vivo occurs after acidification of Salmonella-containing vacuoles (SCVs) to pH ~5.0 (Fig. 1A) (2, 3). Bacteria grown in vitro at pH 5.0 secrete translocon proteins but negligible levels of effectors (4). SPI-2–encoded SsaL is a member of the YopN/InvE/MxiC/SepL family of regulatory proteins found throughout T3SSs of animal pathogens (5) and is required for secretion of the translocon proteins SseB and SseD (6). To study regulation of the translocon-to-effector switch, we examined protein secretion from a Salmonella enterica serovar Typhimurium (S. Typhimurium) ssaL mutant after growth at pH 5.0 in vitro (7). This strain displayed enhanced secretion of chromosome-expressed double hemagglutinin (2HA)–tagged SPI-2 T3SS effector SseJ (Fig. 1B) and chromosome or plasmid-expressed SseF (fig. S1) and did not secrete translocon proteins SseB or SseC (Fig. 1, B and C). The secretion pattern for SseJ-2HA and translocon proteins was restored to wild type by introduction of a plasmid expressing SsaL-2HA (Fig. 1C). There was no detectable secretion of SseB or effectors by an ssaV mutant strain, which has a nonfunctional SPI-2 T3SS (Fig. 1B). The ssaL mutant phenotype is very similar to that of strains lacking SPI-2–encoded SpiC or SsaM (Fig. 1B) (4). Thus SsaL, SsaM, and SpiC are all required for secretion of translocon proteins and suppress the secretion of effectors under conditions that simulate the vacuolar environment (Fig. 1A).
Fig. 1. SsaL is required for translocon protein secretion, suppresses effector secretion, and interacts with SsaM and SpiC.
(A) Model of the SPI-2 T3SS spanning the inner and outer membranes of the bacterial cell and connected to a translocon pore formed in the vacuolar membrane. Translocon proteins must be secreted before effectors can be translocated. SsaV is thought to be located in the inner membrane and is essential for the function of the secretion system. (B) Wild-type (wt), ssaV, spiC, ssaM, or ssaL deletion mutant strains expressing 2HA-tagged SseJ from chromosome were grown in minimal medium pH 5.0, and secreted and bacterial-associated (lysate) proteins were examined by means of immunoblotting to detect the HA epitope, SseB, and DnaK. (C) A plasmid expressing SsaL-2HA was introduced into the ssaL deletion mutant, and secreted levels of SseJ-2HA, SseB, and SseC were compared with the ssaL mutant by means of immunoblotting. (D) Interaction between SpiC-2HA, T7-SsaM, and SsaL-3Flag. In the wt-3tag strain, spiC, ssaM, and ssaL are replaced with versions expressing epitope-tagged proteins. This and an isogenic strain lacking SpiC (spiC) were grown in minimal medium pH 5.0, and whole-cell lysates were immunoprecipitated with an antibody to T7. The presence of the three proteins was detected in input samples (input) and after immunoprecipitation (output) by means of immunoblotting.
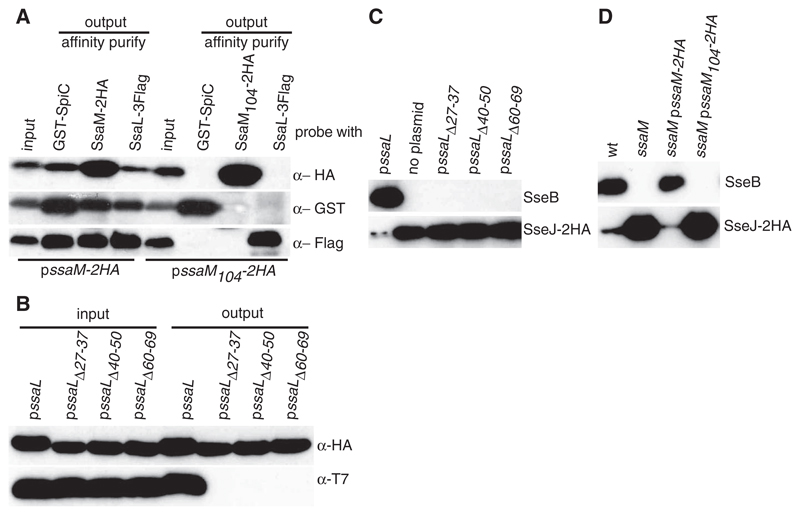
SpiC and SsaM form a complex (4). To determine whether SsaL interacts with the SpiC/SsaM complex when produced at physiological levels, we constructed a bacterial strain in which chromosomal copies of spiC, ssaM, and ssaL were replaced with versions expressing epitope-tagged proteins (SpiC-2HA, T7-SsaM, and SsaL-3Flag). This strain (wt-3tag) was indistinguishable from the wild-type strain in terms of intracellular replication and SPI-2 T3SS effector-dependent tubule formation in infected cells, indicating a functional T3SS (fig. S2). The wt-3tag strain and an isogenic mutant lacking endogenous or epitope-tagged SpiC (spiC) were grown at pH 5.0, and whole-cell lysates were immunoprecipitated with an antibody to T7. SsaL-3Flag was coprecipitated from the wt-3tag strain but not from the spiC mutant (Fig. 1D). Co-immunoprecipitation and pull-down experiments also showed that the C-terminal 18 amino acids of SsaM were required for interaction with SpiC (4) and SsaL (Fig. 2A). Thus, SsaL, SsaM, and SpiC form a complex when bacteria are grown at pH 5.0 (Fig. 1A).
Fig. 2. Phenotypes of SsaL and SsaM variants that block ternary complex formation.
(A) The SsaL/SpiC/SsaM complex requires the C-terminal 18 amino acids of SsaM. A strain expressing SsaL-3Flag, glutathione S-transferase (GST)–SpiC, and either SsaM-2HA or a nonfunctional version lacking its C-terminal 18 amino acids (SsaM104-2HA) (4) were grown in minimal medium pH 5.0, and whole lysates were used for GST pull-down (GST-SpiC) or immunoprecipitation (SsaL-3Flag, SsaM-2HA, and SsaM104-2HA). (B) Plasmids encoding T7-SsaM and 2HA-tagged SsaL or mutant variants were introduced into an ssaL deletion mutant. Whole bacterial lysates were immunoprecipitated with antibody to HA. SsaL-2HA and T7-SsaM were detected in input samples (input) and after immunoprecipitation (output) by means of immunoblotting. (C) The ssaL deletion strain expressing SseB and SseJ-2HA, and SsaL or mutant variants from a plasmid, were grown in minimal medium pH 5.0 for 5 hours. Secreted fractions were analyzed by means of immunoblotting for SseB and SseJ-2HA. (D) The wild-type strain, an ssaM mutant, and the mutant with or without a plasmid expressing SsaM-2HA or SsaM104-2HA were grown at pH 5.0 and analyzed as in (C).
To determine whether an intact complex is necessary to suppress effector secretion at pH 5.0, we constructed three small deletion mutants in an N-terminal region of SsaL corresponding to the chaperone-binding domain of YopN (fig. S3), which is necessary for its interaction with SycN and YscB (8). These mutants, and a truncated SsaM protein lacking its 18 C-terminal amino acids, all prevented formation of the ternary complex (Fig. 2, A and B) and caused the same phenotype as individual null mutants: no detectable translocon secretion and greatly enhanced effector secretion at pH 5.0 (Fig. 2, C and D). Thus, an intact ternary complex is necessary to promote translocon secretion and to suppress effector secretion at pH 5.0.
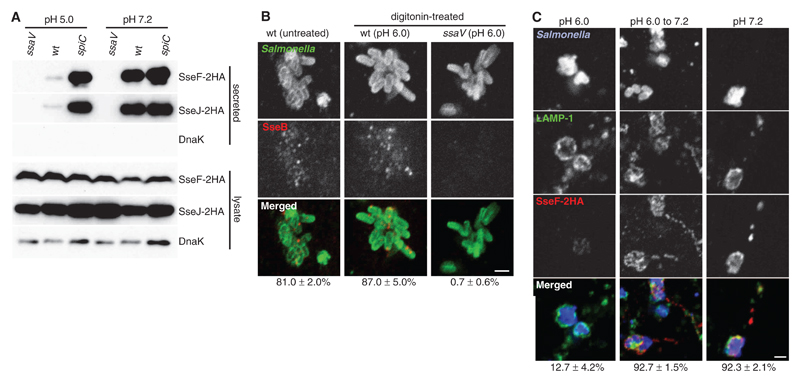
The ssaM, spiC, and ssaL mutant phenotypes raised the possibility that their corresponding proteins might regulate the secretion switch in infected cells once the translocon pore has been assembled. The translocon spans the membrane separating the vacuole lumen (pH ~5.0) (9) from the cytosol (pH 7.2). We tested whether a shift in ambient pH from 5.0 to 7.2 enhanced SPI-2 effector secretion by using wild-type bacteria in vitro. Bacterial strains expressing either SseJ-2HA or SseF-2HA were grown at pH 5.0 for 4 hours in order to activate the SPI-2 T3SS and then exposed to pH 7.2 or 5.0. Secretion of these and other effectors was enhanced by an order of magnitude when wild-type bacteria were subjected to pH shift (Fig. 3A and fig. S4A). The overall amounts of each secreted protein were similar to those secreted by the spiC mutant strain at pH 5.0 or after shift to pH 7.2, and there was no detectable secretion by the ssaV mutant strain (Fig. 3A). In contrast, intrabacterial and secreted levels of SseB were reduced by approximately 50% after pH shift (fig. S4A). Inhibition of protein synthesis showed that the pH shift enhanced the secretion of preformed effectors, suggesting that it acted on the T3SS apparatus or associated proteins (fig. S4B). Enhanced secretion of SseF-2HA was detected at pH 6.4 and reached maximal levels at pH 6.8 (fig. S4C). Thus, wild-type bacteria respond to an extracellular pH shift from 5.0 to 7.2 by reducing SseB levels and triggering effector secretion.
Fig. 3. Effect of pH on effector secretion and translocation.
(A) Bacterial strains were grown in minimal medium pH 5.0 for 4 hours, then exposed to pH 5.0 or 7.2 for 90 min. Secreted and bacteria-associated (lysate) 2HA-tagged effectors and DnaK were examined by means of immunoblotting. (B) HeLa cells were infected with wild-type or ssaV mutant Salmonella for 3.5 hours in order to allow expression of the SPI-2 T3SS, then some samples were permeabilized with digitonin and exposed to pHe 6.0. Cells were fixed 2.5 hours later and immunolabeled in order to detect Salmonella and secreted SseB. (C) HeLa cells were infected for 3.5 hours with wild-type Salmonella expressing SseF-2HA, then permeabilized with digitonin and exposed to pH 6.0 or 7.2 for a further 2.5 hours. In one sample, pHe was changed from 6.0 to 7.2, 1 hour before fixation. Fixed cells were immunolabeled to detect Salmonella, LAMP-1, and SseF-2HA. In (B) and (C), values below the images represent the percentage of cells in which secreted SseB or translocated SseF-2HA was detected, ±SE of three experiments (n > 100 cells per experiment). Scale bars, 2 µm.
To determine whether host cytosolic pH (pHcyt) acted as a signal for the secretion switch, digitonin was used to selectively permeabilize plasma membranes of infected epithelial cells exposed to external media at different pH (pHe). After digitonin permeabilization, pHcyt was reduced by pHe 6.0, but SCV luminal pH remained within the normal range (fig. S5). Secreted SseB was detected at similar levels in untreated and permeabilized cells at pHe 6.0 (Fig. 3B). Translocation of SseF-2HA was detected in over 90% of infected permeabilized cells at pHe 7.2 (Fig. 3C) but in less than 13% of infected cells at pHe 6.0 (Fig. 3C). This inhibition was reversed if pHe was subsequently raised to 7.2 (Fig. 3C). Thus, we have observed a pH-dependent, reversible inhibition of effector translocation, which suggests that near-neutral host cytosolic pH is necessary for effector delivery across the vacuolar membrane.
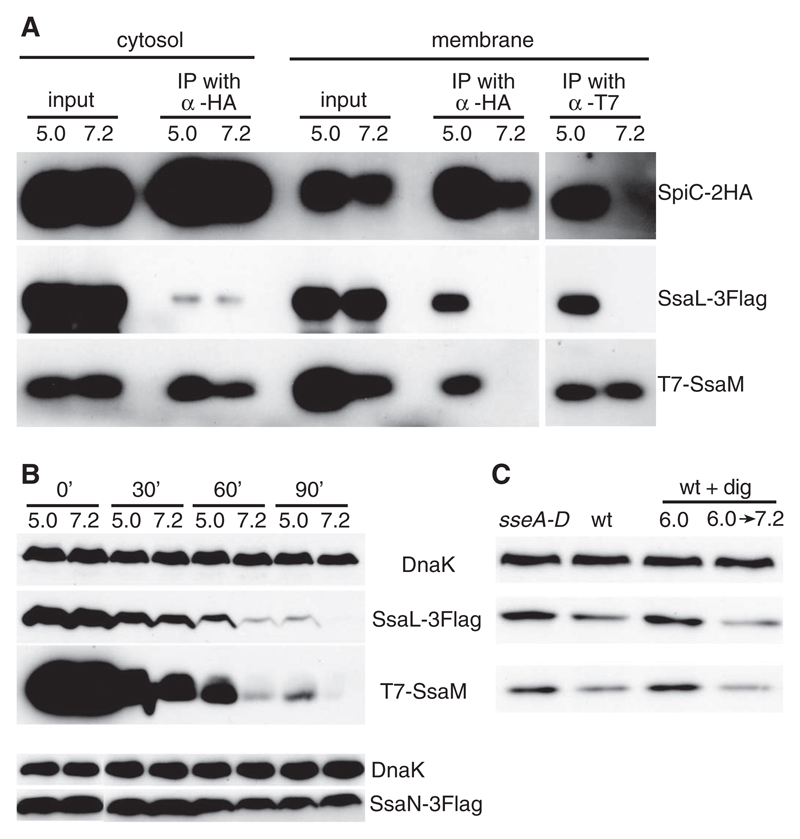
We next investigated SsaL/SsaM/SpiC complex integrity in bacteria after pH shift. The wt-3tag strain was grown at pH 5.0 for 4 hours and then exposed to pH 7.2 for 1 hour. Because substrate recognition in T3SSs involves proteins associated with the secretion machinery in the bacterial inner membrane (1), bacterial cells were lysed and separated into membrane and cytosolic fractions. Membrane-associated SpiC-2HA was immunoprecipitated and immunoblots probed so as to detect SsaL-3Flag and T7-SsaM. As expected, both proteins were co-precipitated at pH 5.0, but neither was detected when SpiC-2HA was precipitated from membranes of cells shifted to pH 7.2 (Fig. 4A). When T7-SsaM was immunoprecipitated from membranes recovered from bacteria at pH 5.0, both SsaL-3Flag and SpiC-2HA were co-precipitated, but not after shift of bacteria to pH 7.2 (Fig. 4A). Thus, an increase in extracellular pH leads to dissociation of the membrane-bound SsaL/SsaM/SpiC complex.
Fig. 4. Effect of pH on the SsaL/SsaM/SpiC complex.
(A) The wt-3tag strain was grown in minimal medium at pH 5.0 for 4 hours, then exposed to pH 5.0 or 7.2 for 1 hour, then membrane-associated and cytosolic SpiC-2HA and membrane-associated T7-SsaM were immunoprecipitated (IP). Proteins were detected in input samples (input) and after immunoprecipitation (output) by means of immunoblotting. (B) The wt-3tag strain and a strain in which the chromosomal copy of ssaN is replaced with a functional version carrying a 3Flag epitope (bottom) were subjected to pH shift in the presence of tetracycline. Samples were removed at various times, and lysates were analyzed by means of immunoblot. (C) HeLa cells were infected with the wt-3tag strain (wt) or an isogenic translocon mutant (sseA-D). At 3.5 hours after invasion, some wt-infected cells were treated with digitonin (wt + dig), and exposed to pHe 6.0 for 2.5 hours (6.0) or pHe 6.0 for 1.5 hours, then changed to pHe 7.2 for another 1 hour (6.0 → 7.2). Cells were lysed at 6 hours after invasion and analyzed by means of immunoblotting.
We were unable to detect secretion or translocation of SsaM, SpiC (4), or SsaL (fig. S6). To study the stability of SsaL and SsaM after pH shift, the wt-3tag strain grown at pH 5.0 was exposed to either pH 5.0 or pH 7.2 in the presence of tetracycline in order to inhibit further protein synthesis, and protein levels were examined. Whereas the levels of DnaK and a functional 3Flag-tagged SsaN (an essential component of the SPI-2 T3SS) were relatively stable over this period at either pH, levels of both SsaL-3Flag and T7-SsaM declined. However, the rate of decline was much greater in bacteria after exposure to pH 7.2 as compared with that after exposure to pH 5.0 (Fig. 4B). To determine whether intrabacterial levels of SsaL and SsaM were affected after translocon pore assembly in vivo, HeLa cells were infected for 6 hours with the wt-3tag strain or by a mutant version lacking all three translocon proteins. The levels of SsaL-3Flag and T7-SsaM in intracellular wt-3tag bacteria were consistently lower (by 36.7 ± 1.3% and 33.7 ± 3.6%, respectively) as compared with their levels in the strain that could not assemble a translocon pore (Fig. 4C) but had an otherwise functional T3SS (fig. S7). Furthermore, loss of SsaL-3Flag and T7-SsaM was prevented by reducing pHcyt and induced after a subsequent increase in pHcyt (causing a loss of 57.3 ± 0.6% and 60.2 ± 1.9%, respectively) (Fig. 4C). Thus, in infected cells, loss of SsaL and SsaM requires near-neutral pHcyt and formation of the translocon. The translocon pore provides a means by which intravacuolar bacteria could sense pHcyt, leading to dissociation and degradation of regulatory complex components, triggering effector delivery (fig. S8).
Acidic ambient pH suppresses effector secretion but is necessary for translocon protein secretion (3, 4, 10). The SsaL/SsaM/SpiC regulatory complex has a critical role in this process by acting as a “gatekeeper,” enabling translocon protein secretion while suppressing effector secretion at pH 5.0. This complex is likely to interact with the cytoplasmic region of basal body of the secretion apparatus and to respond to an unidentified pH sensor. The sensor is unlikely to be part of the translocon because the translocon deletion mutant displayed wild-type levels of effector secretion upon pH upshift (fig. S7). The sensor might be the needle subunit itself, which has been implicated in signaling the translocator to effector switch in Shigella (11) and Yop secretion by Yersinia (12). Another possibility is that translocon pore assembly changes the pH gradient within the needle channel and that the sensor is located toward the base of the secretion apparatus. Changes in pH from mildly acidic to neutral can have dramatic effects on protein folding; for example, some bacterial toxins refold after their translocation from acidic endosomes to the host-cell cytosol in a partially unfolded state (13). The SPI-2 T3SS pH sensor might thus undergo a conformational change on exposure to neutral pH and transduce a dissociation signal to the SsaL/SsaM/SpiC complex.
Supplementary Material
Footnotes
We thank J. Mota, C. Tang, and members of the Holden laboratory for comments on the manuscript. This research was supported by grants G0800148 and 074553/Z/04/Z to D.W.H. from the Medical Research Council and Wellcome Trust.
References
- 1.Galán JE, Wolf-Watz H. Nature. 2006;444:567. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 2.Rappl C, Deiwick J, Hensel M. FEMS Microbiol Lett. 2003;226:363. doi: 10.1016/S0378-1097(03)00638-4. [DOI] [PubMed] [Google Scholar]
- 3.Chakravortty D, Rohde M, Jäger L, Deiwick J, Hensel M. EMBO J. 2005;24:2043. doi: 10.1038/sj.emboj.7600676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu X-J, Liu M, Holden DW. Mol Microbiol. 2004;54:604. doi: 10.1111/j.1365-2958.2004.04297.x. [DOI] [PubMed] [Google Scholar]
- 5.Pallen MJ, Beatson SA, Bailey CM. BMC Microbiol. 2005;5:9. doi: 10.1186/1471-2180-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coombes BK, Brown NF, Valdez Y, Brumell JH, Finlay BB. J Biol Chem. 2004;279:49804. doi: 10.1074/jbc.M404299200. [DOI] [PubMed] [Google Scholar]
- 7.Materials and methods are available as supporting material on Science Online
- 8.Schubot FD, et al. J Mol Biol. 2005;346:1147. doi: 10.1016/j.jmb.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 9.Drecktrah D, Knodler LA, Howe D, Steele-Mortimer O. Traffic. 2007;8:212. doi: 10.1111/j.1600-0854.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beuzón CR, Banks G, Deiwick J, Hensel M, Holden DW. Mol Microbiol. 1999;33:806. doi: 10.1046/j.1365-2958.1999.01527.x. [DOI] [PubMed] [Google Scholar]
- 11.Kenjale R, et al. J Biol Chem. 2005;280:42929. doi: 10.1074/jbc.M508377200. [DOI] [PubMed] [Google Scholar]
- 12.Torruellas J, Jackson MW, Pennock JW, Plano GV. Mol Microbiol. 2005;57:1719. doi: 10.1111/j.1365-2958.2005.04790.x. [DOI] [PubMed] [Google Scholar]
- 13.Young JA, Collier RJ. Annu Rev Biochem. 2007;76:243. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.