Abstract
Background
This is an update of a review published in 2012. A related review "Inhaled versus systemic corticosteroids for preventing bronchopulmonary dysplasia in ventilated very low birth weight preterm neonates" has been updated as well. Bronchopulmonary dysplasia (BPD) is a serious and common problem among very low birth weight infants, despite the use of antenatal steroids and postnatal surfactant therapy to decrease the incidence and severity of respiratory distress syndrome. Due to their anti‐inflammatory properties, corticosteroids have been widely used to treat or prevent BPD. However, the use of systemic steroids has been associated with serious short‐ and long‐term adverse effects. Administration of corticosteroids topically through the respiratory tract may result in beneficial effects on the pulmonary system with fewer undesirable systemic side effects.
Objectives
To compare the effectiveness of inhaled versus systemic corticosteroids administered to ventilator‐dependent preterm neonates with birth weight ≤ 1500 g or gestational age ≤ 32 weeks after 7 days of life on the incidence of death or BPD at 36 weeks' postmenstrual age.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 1), MEDLINE via PubMed (1966 to 23 February 2017), Embase (1980 to 23 February 2017), and CINAHL (1982 to 23 February 2017). We also searched clinical trials registers, conference proceedings and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing inhaled versus systemic corticosteroid therapy (irrespective of dose and duration) starting after the first week of life in ventilator‐dependent very low birth weight infants.
Data collection and analysis
We used standard methodological procedures expected by the Cochrane Collaboration.
Main results
We included three trials that involved a total of 431 participants which compared inhaled versus systemic corticosteroids to treat BPD. No new trials were included for the 2017 update.
Although one study randomised infants at < 72 hours (N = 292), treatment started when infants were aged > 15 days. In this larger study, deaths were included from the point of randomisation and before treatment started. Two studies (N = 139) randomised and started treatment at 12 to 21 days.
Two trials reported non‐significant differences between groups for the primary outcome: incidence of death or BPD at 36 weeks' postmenstrual age among all randomised infants. Estimates for the largest trial were Relative risk (RR) 1.04 (95% Confidence interval (CI) 0.86 to 1.26), Risk difference (RD) 0.03 (95% CI ‐0.09 to 0.15); (moderate‐quality evidence). Estimates for the other trial reporting the primary outcome were RR 0.94 (95% CI 0.83 to 1.05), RD ‐0.06 (95% CI ‐0.17 to 0.05); (low‐quality evidence).
Secondary outcomes that included data from all three trials showed no significant differences in the duration of mechanical ventilation or supplemental oxygen, length of hospital stay, or the incidence of hyperglycaemia, hypertension, necrotising enterocolitis, gastrointestinal bleed, retinopathy of prematurity or culture‐proven sepsis moderate‐ to low‐quality evidence).
In a subset of 75 surviving infants who were enrolled from the United Kingdom and Ireland, there were no significant differences in developmental outcomes at seven years of age between groups (moderate‐quality evidence). One study received grant support and the industry provided aerochambers and metered dose inhalers of budesonide and placebo for the same study. No conflict of interest was identified.
Authors' conclusions
We found no evidence that inhaled corticosteroids confer net advantages over systemic corticosteroids in the management of ventilator‐dependent preterm infants. There was no evidence of difference in effectiveness or adverse event profiles for inhaled versus systemic steroids.
A better delivery system guaranteeing selective delivery of inhaled steroids to the alveoli might result in beneficial clinical effects without increasing adverse events.
To resolve this issue, studies are needed to identify the risk/benefit ratio of different delivery techniques and dosing schedules for administration of these medications. The long‐term effects of inhaled steroids, with particular attention to neurodevelopmental outcomes, should be addressed in future studies.
Plain language summary
Inhaled versus systemic corticosteroids for the treatment of bronchopulmonary dysplasia in ventilated very low birth weight preterm infants
Review question
To compare the effectiveness of inhaled versus systemic corticosteroids administered to ventilator‐dependent preterm neonates with birth weight ≤ 1500 g or gestational age ≤ 32 weeks after 7 days of life on the incidence of chronic lung disease at 36 weeks' corrected postmenstrual age.
Background
Preterm babies (babies born before term, 40 weeks pregnancy) often need breathing (ventilator) support. Babies who need invasive (placing a breathing tube in the wind pipe) mechanical breathing support for a prolonged period often develop bronchopulmonary dysplasia (defined as requirement for supplemental oxygen at 36 weeks' postmenstrual age). It is thought that inflammation in the lungs may be part of the cause. Corticosteroid drugs reduce inflammation and swelling in the lungs, but can have serious side effects. Corticosteroid use has been associated with cerebral palsy (motor problem) and developmental delay. Inhaling steroids, so that the drug reaches the lungs directly, has been tried as a way to limit adverse effects.
Search date
23 February 2017.
Study characteristics
All three included trials were randomised, but the blinding of intervention and outcome measurement varied. Data from two trials (enrolling 139 infants) were combined as they enrolled infants between 12 and 21 days of age, but data from one trial (enrolling 292 infants) were reported separately because researchers randomised infants aged less than 72 hours. The timing when the outcomes were measured varied among studies so it was not appropriate to combine some results. In one study all deaths that occurred were reported from the time babies were randomised not from when treatment started, hence there was a greater number of babies who died in that study.
One study received grant support and the industry provided Aerochambers and metered dose inhalers of budesonide and placebo for the same study. No conflict of interest was identified.
Key results
Evidence from two studies in 370 infants, who were randomised between 12 and 21 days of age and who contributed data to the primary outcome of this review, showed that inhaled steroids administered after 7 days of age compared with systemic steroids did not decrease the incidence of death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age. Evidence from the single study in which infants were randomised at less than 72 hours of age did not show difference the incidence of death or BPD.
Evidence from three studies in 431 infants contributing to secondary outcomes showed that inhaled steroids administered after seven days of age compared with systemic steroids did not significantly alter the incidence of BPD at 36 weeks' postmenstrual age, hyperglycaemia, hypertension, duration of ventilation, duration of oxygen supplementation, length of hospital stay, intraventricular haemorrhage grade III‐IV, periventricular leukomalacia, necrotising enterocolitis, gastrointestinal bleed, retinopathy of prematurity stage > 3, culture‐proven sepsis or the incidence of adverse effects.
Adverse event profiles did not differ for inhaled versus systemic steroids but some potential complications of steroid treatment have not been reported. More research is needed to show whether any form of routine use of steroids results in overall health improvements for babies at risk of bronchopulmonary dysplasia.
Quality of the evidence
Evidence quality (according to GRADE criteria) was moderate to low.
Summary of findings
Background
Description of the condition
Bronchopulmonary dysplasia (BPD) is a serious and common problem among very low birth weight infants despite the use of antenatal steroids (Roberts 2017) and postnatal surfactant therapy (Soll 1998; Bahadue 2012) to decrease the incidence and severity of respiratory distress syndrome. The incidence of BPD varies between 23% and 26% (Lee 2000; Lemons 2001) in very low birth weight infants (< 1500 g) and has an inverse relationship to both gestational age and birth weight (Lee 2000; Sinkin 1990).
Description of the intervention
Several randomised controlled trials (Avery 1985; CDTG 1991; Cummings 1989; Harkavy 1989; Kazzi 1990; Ohlsson 1992) and systematic reviews (Bhuta 1998; Doyle 2014; Doyle 2014a; Halliday 1999; Shah 2001) have demonstrated that among infants with BPD, treatment with systemic corticosteroids facilitates extubation and improves respiratory system compliance. Marked heterogeneity regarding the dose and duration of dexamethasone administration has been noted among trials. However, corticosteroids appear to have little effect on the duration of supplemental oxygen, duration of hospitalisation or mortality (Avery 1985; CDTG 1991; Harkavy 1989; Kazzi 1990; Ohlsson 1992). There are concerns regarding the short‐ and long‐term side effects of systemic steroids in this population. These include hyperglycaemia, hypertension, hypertrophic cardiomyopathy, gastrointestinal haemorrhage and perforation, enhanced catabolism and growth failure, nephrocalcinosis, poor bone mineralization and susceptibility to infection (Ng 1993; Stark 2001).
The potential effects on brain growth and neurodevelopment are most alarming. Animal models (rat and rhesus monkey) at a similar stage of ontogeny to the human fetus have shown that steroids permanently affect brain cell division, differentiation and myelination, as well as the ontogeny of cerebral cortical development (Johnson 1979; Weichsel 1977). These effects are long‐lasting and associated with decreased head circumference and neuromotor abnormalities. Several follow‐up studies of postnatal systemic corticosteroid therapy in preterm infants have shown higher incidence of neurodevelopmental abnormalities in surviving dexamethasone‐treated infants (O'Shea 1999; Shinwell 2000; Yeh 1998).
Theoretically, the use of inhaled corticosteroids may allow for beneficial effects on the pulmonary system without concomitant high systemic concentrations and less risk of adverse effects. Results from a large multicentre study of early use of inhaled steroids concluded that among extremely preterm infants, BPD incidence was lower among those who received early inhaled budesonide than among those who received placebo, but the advantage may have been gained at the expense of increased mortality (Bassler 2015). Results from this study have been incorporated in a Cochrane Review (Shah 2017) and a meta‐analysis (Shinwell 2016). Shinwell 2016 concluded that "Very preterm infants appear to benefit from inhaled corticosteroids with reduced risk for BPD and no effect on death, other morbidities, or adverse events. Data on long‐term respiratory, growth, and developmental outcomes are eagerly awaited". Shah 2017 summarised their findings as: "There is increasing evidence from the trials reviewed that early administration of inhaled steroids to very low birth weight neonates is effective in reducing the incidence of death or CLD at 36 weeks' postmenstrual age among either all randomised infants or among survivors. Even though there is statistical significance, the clinical relevance is of question as the upper CI limit for the outcome of death or BPD at 36 weeks' postmenstrual age is infinity. The long‐term follow‐up results of the Bassler 2015 study may affect the conclusions of this review. Further studies are needed to identify the risk/benefit ratio of different delivery techniques and dosing schedules for the administration of these medications. Studies need to address both the short‐ and long‐term benefits and adverse effects of inhaled steroids with particular attention to neurodevelopmental outcome".
It is noteworthy that a Cochrane Review by Onland 2017b concluded: "Despite the fact that some studies reported a modulating effect of treatment regimens in favour of higher‐dosage regimens on the incidence of BPD and neurodevelopmental impairment, recommendations on the optimal type of corticosteroid, the optimal dosage, or the optimal timing of initiation for the prevention of BPD in preterm infants cannot be made based on current level of evidence. A well‐designed large RCT is urgently needed to establish the optimal systemic postnatal corticosteroid dosage regimen".
Apart from studies included in this review, we are not aware of any other direct comparisons of early use of inhaled versus systemic corticosteroids.
How the intervention might work
It is thought that inflammation in the lungs may be part of the cause of BPD (Nelin 2017). As part of a randomised, placebo‐controlled trial of early inhaled beclomethasone therapy, Gupta 2000 measured interleukin‐8 (IL‐8) and interleukin‐1 receptor antagonist (IL‐1ra) concentrations in tracheal aspirates as markers of pulmonary inflammation. Beclomethasone‐treated infants with moderately elevated baseline IL‐8 levels received less subsequent systemic glucocorticoid therapy and had a lower incidence of BPD than non treated infants. Gupta 2000 and co‐authors concluded that early‐inhaled beclomethasone therapy was associated with a reduction in pulmonary inflammation after one week of therapy. Corticosteroid drugs when given orally or intravenously reduces this inflammation in the lungs (Nelin 2017). Corticosteroid use has been associated with cerebral palsy and developmental delay (AAP & CPS 2002; Nelin 2017). In a retrospective study of infants born at < 29 weeks PMA and assessed at 18 to 21 months corrected age it was found that exposure to inhaled steroids was not associated with increased odds of death or neurodevelopmental impairment (Kelly 2017). However, in the same study systemic steroids use before 4 weeks of age was associated with significantly worse outcomes (Kelly 2017). It is important to minimize exposure to the potentially harmful effects of corticosteroids, particularly on the developing brain (Doyle 2017).
Why it is important to do this review
Cochrane Reviews have addressed the use of systemic or inhaled corticosteroids in the prevention or treatment of BPD or chronic lung disease. These include reviews of the early use (< 8 days) of systemic postnatal corticosteroids to prevent chronic lung disease (Doyle 2014) and the late use (> 7 days) of systemic postnatal corticosteroids for chronic lung disease (Doyle 2014a).
Other Cochrane Reviews address the use of inhaled corticosteroids in the prevention or treatment of chronic lung disease. Shah 2017 reviewed the effects of early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates and Onland 2017a reviewed the late use (≥ 7 days) of inhaled corticosteroids to reduce bronchopulmonary dysplasia in preterm infants.
Cochrane Reviews have also compared systemic and inhaled corticosteroids. Shah 2012 compared the use of inhaled versus systemic corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates, and the use of inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants (Shah 2012a).
The use of corticosteroids for other indications in neonates including intravenous dexamethasone to facilitate extubation (Davis 2001), corticosteroids for the treatment of hypotension (Ibrahim 2011) and corticosteroids for the treatment of meconium aspiration syndrome (Ward 2003) have been assessed in Cochrane Reviews.
In statements released by the European Association of Perinatal Medicine (Halliday 2001a), American Academy of Pediatrics (Watterberg 2010) and the Canadian Pediatric Society (Jefferies 2012), routine use of systemic dexamethasone for the prevention or treatment of BPD is not recommended. These recommendations were based on concerns regarding short and long‐term complications, especially cerebral palsy.
Thus, alternatives to systemic corticosteroids that may have fewer adverse consequences need to be investigated. Administration of corticosteroids topically through the respiratory tract might result in beneficial effects on the pulmonary system with fewer undesirable systemic side effects.
The aim of this review was to examine the effectiveness of inhaled versus systemic corticosteroids administered to ventilator‐dependent very low birth weight neonates of 1500 g or less after the first week of life, for the treatment of evolving BPD. This is an update of our review last published in 2012 (Shah 2012a).
Objectives
The primary objective was to compare the effectiveness of inhaled versus systemic corticosteroids administered to ventilator‐dependent preterm neonates with birth weight ≤ 1500 g or gestational age ≤ 32 weeks after 7 days of life on the incidence of death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age.
Secondary objectives
To compare the effectiveness of inhaled versus systemic corticosteroids on other indicators of BPD, the incidence of adverse events, and long‐term neurodevelopmental outcome.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised clinical trials.
Types of participants
Ventilator‐dependent preterm infants with birth weight ≤ 1500 g or gestational age ≤ 32 weeks and postnatal age of more than 7 days of age.
Types of interventions
Inhaled corticosteroids compared to systemic corticosteroids irrespective of the type, dose and duration of therapy as long as the treatment started before 7 days of age.
Types of outcome measures
For the following two comparisons:
1. Inhaled versus systemic steroids among infants ‐ outcomes including deaths (infants randomised at < 72 hours of age)
2. Inhaled versus systemic steroids among infants ‐ outcomes including deaths (infants randomised between 12 and 21 days of age)
Primary outcomes
Death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age.
Secondary outcomes
Death or BPD at 28 days of age
Death at 36 week's postmenstrual age
Death at 28 days of age
For the following comparison:
3. Inhaled versus systemic steroids among infants ‐ (infants randomised at < 72 hours of age or between 12 and 21 days of age)
Secondary outcomes
BPD at 36 weeks' postmenstrual age (requirement for supplemental oxygen at 36 weeks' postmenstrual age)
BPD at 28 days of age (requirement for supplemental oxygen at 28 days of age)
Need for ventilation amongst survivors at 36 weeks' postmenstrual age
Duration of mechanical ventilation among survivors (days)
Duration of supplemental oxygen among survivors (days)
Length of hospital stay among survivors (days)
Intraventricular haemorrhage grade III‐IV (defined as per Papile 1978)
Periventricular leukomalacia (defined as cysts in the periventricular area on ultrasound or CT scan)
Hyperglycaemia (defined as blood glucose > 10 mmol/L) during the course of intervention
Hypertension (defined as systolic or diastolic blood pressure > 2 standard deviations (SD) above the mean for infant's gestational and postnatal age (Zubrow 1995)) during the course of intervention
Necrotising enterocolitis (Bell's stage II and III) (Bell 1978)
Gastrointestinal bleed (defined as presence of bloody nasogastric or orogastric aspirate)
Retinopathy of prematurity ≥ stage 3 (ICROP 1984)
Culture‐proven sepsis
Suppression of the hypothalamic‐pituitary‐adrenal axis assessed by metyrapone or Adrenocorticotropic hormone (ACTH) stimulation test
Patent ductus arteriosus defined by presence of clinical symptoms and signs or demonstration by echocardiography
Hypertrophic cardiomyopathy defined as thickening of the intraventricular septum or of the left ventricular wall on echocardiography; sepsis defined by the presence of clinical symptoms and signs of infection and a positive culture from a normally sterile site
Pneumonia based on clinical and radiological signs and a positive endotracheal tube aspirate culture
Growth (weight, length/height and head circumference) at 36 weeks' postmenstrual age; cataracts (defined by presence of opacities in the lens)
Hypertrophy of the tongue
Nephrocalcinosis (defined by the presence of echo densities in the medulla of the kidney on ultrasound) (Saarela 1999)
Long‐term neurodevelopmental outcome (in surviving infants)
Neurodevelopmental impairment was defined as presence of cerebral palsy or mental impairment (Bayley scales of infant development, Mental Developmental Index < 70) or legal blindness (< 20/200 visual acuity) or deafness (aided or < 60 dB on audiometric testing) assessed at 18 to 24 months.
The following outcomes were reported at 7 years of age: these post‐hoc analyses were based on available data from a subsample of theHalliday 2001 study
British Ability Scales, Second Edition (provides a global measure of cognitive functioning (the general conceptual ability (GCA) score, with a standardised mean of 100 and SD of 15)
Activities, social, and school competency scales of the Child Behaviour Checklist (CBCL) for children 4 to 18 years of age
Strengths and Difficulties Questionnaire (SDQ) from which overall behavioural, emotional, conduct, hyperactivity, and peer problem scores are derived
Cerebral palsy
Severe disability defined as GCA score of < 55, no independent walking, inability to dress or feed oneself, requirement for continuous home oxygen therapy, behavioural disturbance requiring constant supervision, no useful vision, or no useful hearing
Moderate disability was defined as a GCA score of 55 to 69, restricted mobility, admission to an ICU and ventilation within the past year, secondary referral for specialised help with behaviour, ability to see gross movement only or hearing loss not corrected with aid
Death or moderate/severe disability
Systolic blood pressure > 95th percentile
Diastolic blood pressure > 95th percentile
Ever diagnosed as asthmatic by 7 years of age
Severe disability defined as GCA score of < 55, no independent walking, inability to dress or feed oneself, requirement for continuous home oxygen therapy, behavioural disturbance requiring constant supervision, no useful vision, or no useful hearing
Moderate disability was defined as a GCA score of 55 to 69, restricted mobility, admission to an ICU and ventilation within the past year, secondary referral for specialised help with behaviour, ability to see gross movement only or hearing loss not corrected with aid
Death or moderate/severe disability
Systolic blood pressure > 95th percentile
Diastolic blood pressure > 95th percentile
Ever diagnosed as asthmatic by 7 years of age
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of Cochrane and Cochrane Neonatal for the 2017 update (see the Cochrane Neonatal search strategy for specialized register).
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 1) in The Cochrane Library; MEDLINE via PubMed (1 January 2011 to 23 February 2017); Embase (1 January 2011 to 23 February 2017); and CINAHL (1 January 2011 to 23 February 2017) using the following search terms: (bronchopulmonary dysplasia OR lung diseases OR chronic lung disease OR BPD OR CLD) AND ((anti‐inflammatory agents OR steroid* OR dexamethasone OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate OR corticosteroid* OR betamethasone OR hydrocortisone) AND (inhalation OR aerosol OR inhale*)), plus database‐specific limiters for RCTs and neonates. See Appendix 1 for previous search methodologies and Appendix 2 for the full search strategies for each database searched for the 2017 update.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry Platform (WHO ICTRP); and the ISRCTN Registry). We searched Abstracts2View for abstracts from the Pediatric Academic Societies Annual Meetings from 2010 to 2016.
Searching other resources
We searched the reference lists of identified trials.
Data collection and analysis
We used the methods of the Cochrane Neonatal Review Group for data collection and analysis.
Selection of studies
Three review authors (SS, AO, VS) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search.
We retrieved full‐text study reports and three review authors (SS, AO, VS) independently screened the reports and identified studies for inclusion, and noted and recorded reasons for exclusion of ineligible studies. We resolved disagreement through discussion or, if required, we consulted a fourth review author (HH). We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies. We did not impose any language restrictions
1.
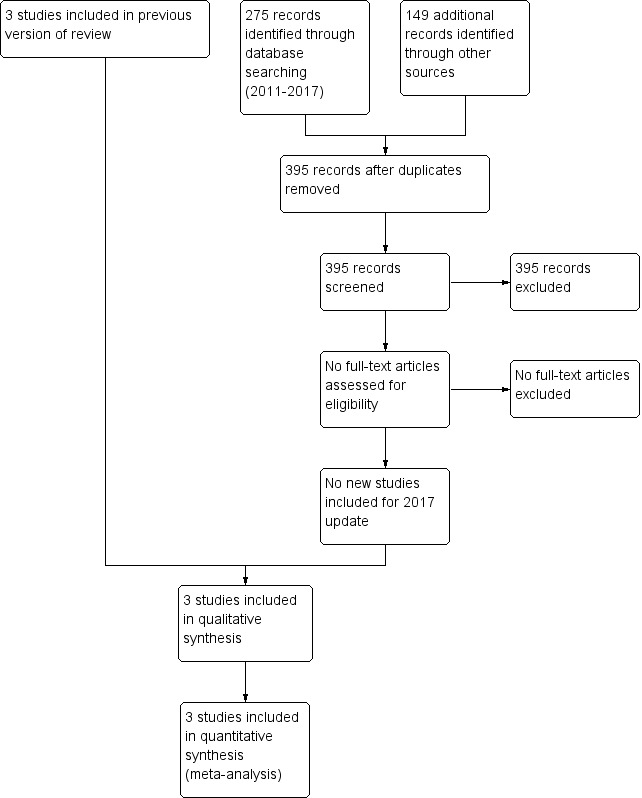
Study flow diagram: review update
Data extraction and management
For each trial, information was sought regarding the method of randomisation, blinding and reporting of outcomes of all infants enrolled in the trial. Data from primary investigators were obtained for unpublished trials or when published data were incomplete. Retrieved articles were assessed and data extracted independently by four review authors (SS, VS, AO, HH). This update was performed by two review authors (VS, AO). Discrepancies were resolved by discussion and consensus.
For each study, data were entered into RevMan by one review author and checked for accuracy by a second reviewer author. We resolved discrepancies through discussion.
We attempted to contact authors of original reports to provide further details when information in published reports was unclear.
Assessment of risk of bias in included studies
Three review authors (VS, SS, AO) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains:
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
Disagreements were resolved by discussion or by consulting a third review author. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses using Review Manager software (Review Manager 2014). Dichotomous data were analysed using relative risk (RR), risk difference (RD) and the number needed to benefit (NNTB) or number needed to harm (NNTH). The 95% confidence interval (CI) was reported for all estimates.
We analysed continuous data using weighted mean difference (WMD) or the standardised mean difference (SMD) to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
For clinical outcomes, such as episodes of sepsis, we analysed data as the proportion of neonates having one or more episodes.
Dealing with missing data
Levels of attrition were noted for the included studies. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was explored by conducting sensitivity analyses.
All outcome analyses were on an intention‐to‐treat basis i.e. we included all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined heterogeneity among trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. If noted, we planned to explore the possible causes of statistical heterogeneity using prespecified subgroup analysis (e.g. differences in study quality, participants, intervention regimens, or outcome assessments). Heterogeneity tests, including the I² statistic, were performed to assess the appropriateness of pooling data. We used the following criteria to describe heterogeneity: < 25% no heterogeneity, ≥ 25% to 49% low heterogeneity, ≥ 50% to 74% moderate heterogeneity and ≥ 75% high heterogeneity.
Assessment of reporting biases
We planned to assess possible publication bias and other biases by inspecting the symmetry or asymmetry of funnel plots had there been at least 10 trials included in an analysis.
For included trials that were recently performed (and therefore prospectively registered), we explored possible selective reporting of study outcomes by comparing the primary and secondary outcomes in the reports with the primary and secondary outcomes proposed at trial registration, using the web sites www.clinicaltrials.gov and www.controlled‐trials.com. If such discrepancies were found, we planned to contact the primary investigators to obtain missing outcome data on outcomes pre‐specified at trial registration.
Data synthesis
Meta‐analysis was conducted using Review Manager software (Review Manager 2014). We used the Mantel‐Haenszel method for estimates of typical relative risk and risk difference. We analysed continuous measures using the inverse variance method. We used the fixed‐effect model for all meta‐analyses.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) primary outcomes: death or BPD at 36 weeks' postmenstrual age for infants randomised at < 72 hours of age and for infants randomised between 12 and 21 days of age. For secondary outcomes we included infants randomised at < 72 hours or between 12 and 21 days; BPD at 36 weeks' postmenstrual age; hyperglycaemia; hypertension. For infants randomised at < 72 hours of age we included the following outcomes at 7 years of age: general conceptual ability (GCA); moderate/severe disability; death or moderate/severe disability; systolic blood pressure > 95th percentile; diastolic blood pressure > 95th percentile; and ever diagnosed as asthmatic.
Three authors (VS, SS, AO) independently assessed the quality of the evidence for each of these outcomes. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used GRADEpro GDT to create ‘Summary of findings’ tables to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
Groups were analysed based on all randomised and survivors only.
Sensitivity analysis
We planned to conduct sensitivity analyses for situations where this might affect the interpretation of significant results (e.g. where there is risk of bias associated with the quality of some of the included trials or missing outcome data). However, it was determined this was unnecessary for this review.
Results
Description of studies
Three trials were identified and included in the previous review. The updated searches of databases and trials registers in 2017 identified a total of 427 records. After removal of duplicates, we assessed 395 records by title and abstract and excluded all 395 records. The study flow from the searches is illustrated in Figure 1. One study received grant support and the industry provided Aerochambers and metered dose inhalers of budesonide and placebo for the same study. No conflict of interest was identified.
Results of the search
Five trials comparing inhaled versus systemic corticosteroids in treatment of BPD were identified. Two trials (Dimitriou 1997; Nicholl 2002) were excluded as both included non ventilator‐dependent patients and the groups of ventilated infants could not be identified separately. No new studies were included for this update.
Included studies
Three trials fulfilled the inclusion criteria and were included in the previous update of the review (Shah 2012a): Halliday 2001; Suchomski 2002 and Rozycki 2003. Although Rozycki 2003 enrolled preterm infants with birth weights between 650 g and 2000 g, on review of the published data, 93% of the enrolled infants had birth weights < 1000 g with postmenstrual age ranging from 23 to 31 weeks. Therefore, data from this trial were included in this review. Details of each trial are given in Characteristics of included studies.
Halliday 2001 enrolled infants born at < 30 weeks gestation, postnatal age < 72 hours, needing mechanical ventilation and fractional inspired oxygen concentration (FiO₂) > 0.30. Infants of 30 and 31 weeks gestation could also be included if they needed FiO₂ > 0.50. Infants with lethal congenital anomalies, severe intraventricular haemorrhage (grade 3 or 4), or proven systemic infection before entry were excluded from the trial. The trial was designed to evaluate the effectiveness of early (< 72 hours) or delayed (> 15 days) administration of systemic dexamethasone or inhaled budesonide. Infants were randomly allocated to one of four treatment groups in a factorial design: early (< 72 hours) dexamethasone, early budesonide, delayed selective (> 15 days) dexamethasone and delayed selective budesonide. Only the delayed budesonide and delayed dexamethasone groups are included in this review. Halliday 2001 randomised 142 babies to delayed selective budesonide and 150 to delayed selective dexamethasone groups. Budesonide was administered by metered dose inhaler and a spacing chamber at 400 µg/kg twice daily for 12 days. Dexamethasone was given intravenously (IV) or orally in a tapering course beginning with 0.5 mg/kg/day in two divided doses for three days reducing by half every three days for a total of 12 days of therapy. Delayed selective treatment was started if infants needed mechanical ventilation and more than 30% oxygen for > 15 days. Of 142 infants randomised to the delayed selective budesonide group, 33 received a full course, 21 received a partial course and 88 babies did not receive budesonide. Of 150 infants randomised to the delayed selective dexamethasone group, 35 received a complete course, 25 received a partial course and 90 infants did not receive dexamethasone. An intention‐to‐treat analysis was performed. The primary outcome was death or oxygen dependency at 36 weeks. Secondary outcome measures included death or major cerebral abnormality, duration of oxygen treatment, and complications of preterm birth. Long‐term outcomes at 7 years of age were assessed in a sample from the UK and Ireland by assessors blinded to the treatment assignments.
Suchomski 2002 compared inhaled beclomethasone, either 400 or 800 µg/d, to intravenous dexamethasone in preterm infants dependent on conventional mechanical ventilation and supplemental oxygen at two weeks of age. The study included 78 preterm infants with birth weight ≤ 1500 g, gestational age ≤ 30 weeks and ventilatory dependence at 12 to 21 days of age with rate > 15/min and FiO₂ > 0.30 with a persistence of these ventilator settings for a minimum of 72 hours. Infants on high frequency ventilation were ineligible for inclusion in the study. Infants were excluded from the study if they had major congenital malformations, culture‐proven sepsis, hypertension or hyperglycaemia needing treatment, or persistent patent ductus arteriosus. Infants were randomly assigned to one of the three treatment groups: inhaled beclomethasone at 400 µg/d or 800 µg/d, or intravenous dexamethasone. Inhaled beclomethasone was continued until extubation. Post‐extubation the same dose was continued for another 48 hours. After that, the dose was halved every other day for six days, after which the steroids were stopped. Based on our inclusion criteria (to include all studies regardless of dosage of inhaled steroids), and because there was no significant difference in the effects of the two different doses, the two inhaled beclomethasone groups in Suchomski 2002 were combined to form one group in the present review. Intravenous dexamethasone was given as a 42 day tapering course starting with 0.5 mg/kg/day in two divided doses (Avery 1985). Cross‐over from either of the inhaled beclomethasone groups to intravenous dexamethasone was allowed if after four to five days of inhaled beclomethasone, the infant's ventilator and oxygen support had not decreased and the attending neonatologist felt that the infant could benefit from intravenous dexamethasone. It was reported that 18 infants from the inhaled steroid group crossed over to systemic dexamethasone. An intention‐to‐treat analysis was performed by the investigators. Outcome measures included adverse effects including sepsis, hypertension and hyperglycaemia; short‐term ventilatory requirements, duration of mechanical ventilation, duration of supplemental oxygen, length of stay in the hospital and need for respiratory support at 28 days or 36 weeks' postmenstrual age. Deaths at 36 weeks' postmenstrual age were not reported. For infants completing a 10 day course of either inhaled or intravenous steroids, an adrenocorticotropic hormone (ACTH) stimulation test was done two weeks after completion of the steroid course.
Rozycki 2003 enrolled 61 preterm infants with birth weights between 650 g and 2000 g who at 14 days of age were at significant risk of developing moderate to severe BPD (defined as the need for mechanical ventilation and oxygen) with x‐ray changes beyond 28 days of life. Infants with culture‐proven sepsis and who were receiving FiO₂ of ≥ 0.30 were eligible if they had a ventilatory index (10,000/ventilator rate x peak pressure x pCO₂) of < 0.8. Infants without previous sepsis were eligible if the ventilatory index was < 0.51. Infants meeting these criteria had a 75% risk of developing moderate‐severe BPD. Infants with the following were excluded: pre‐existing hyperglycaemia with blood glucose > 200 mg/dL for > 24 hours, hypertension with systolic pressures > 70 to 90 mm Hg, depending on birth weight, surgery within previous seven days, active bacterial infection unless repeat blood, urine or cerebrospinal fluid cultures were sterile after 72 hours of antibiotics, thrombocytopenia with platelet count < 100,000, any gastrointestinal bleeding within the previous seven days, significant weaning from ventilator support in the previous three days and previous exposure to postnatal steroids. Eligibility was determined at 14 days of age but the study could be delayed up to six days while awaiting resolution of infections. Infants were randomised to the following four groups: Group A: aerosol placebo‐systemic dexamethasone; Group B: high beclomethasone‐systemic placebo; Group C: medium beclomethasone‐systemic placebo; and Group D: low beclomethasone‐systemic placebo. Those receiving aerosol steroids who remained ventilator‐dependent after seven days were switched to standard 42‐day tapering doses of systemic dexamethasone. The primary outcome variable was extubation within the first seven days of the study. Secondary outcome measures included: changes in ventilatory settings and oxygen delivery over the first seven days, the incidence of hypertension, hyperglycaemia, infection and growth.
Excluded studies
We excluded two trials (Dimitriou 1997; Nicholl 2002). Both trials included non ventilator‐dependent participants and the groups of ventilated infants could not be identified separately. See Characteristics of excluded studies.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
Our searches did not find any ongoing studies.
Risk of bias in included studies
The risk of bias in the included trials are illustrated in Figure 2 and Figure 3.
2.
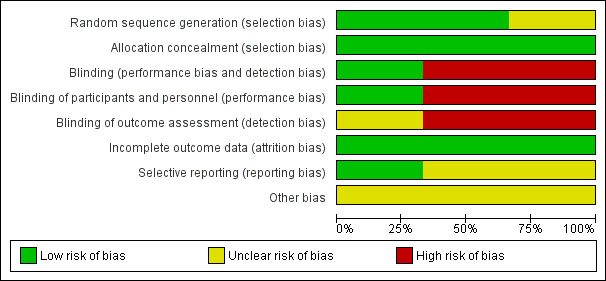
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
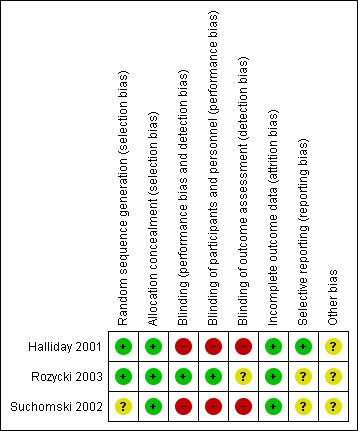
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
The risk of bias is taken into account in the 'Summary of findings' tables for the primary outcome and important secondary outcomes (Table 1; Table 2; Table 3; Table 4). Reasons for downgrading the quality of evidence is explained in the comments columns in 'Summary of findings' tables.
Summary of findings for the main comparison. Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours of age).
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours of age) | ||||||
|
Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 1.04 (95% CI 0.86 to 1.26) | 292 (1) | ⊕⊕⊕⊝ moderate | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 35/150 infants randomised to systemic steroids received full course while 33/142 infants randomised to inhaled steroids received full course. Results were presented in intention to treat analyses including deaths occurring after 72 hours of age. We downgraded the quality of the evidence by one step.
Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis.
Directness of the evidence: The study was conducted in the target population of newborn infants. Precision: Precison for the point estimate was acceptable Presence of publication bias: N/A. |
|
| 580 per 1000 | 606 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; BPD: Bronchopulmonary dysplasia; N/A: Not applicable | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Inhaled steroids compared with systemic steroids for BPD (infants randomised between 12 and 21 days of age).
| Inhaled steroids compared with systemic steroids for BPD (infants randomised between 12 and 21 days of age) | ||||||
|
Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 0.94 (95% CI 0.83 to 1.05) | 78 (1) | ⊕⊕⊝⊝ low | Bias: The risk of bias for this single study was high. There was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by one level.
Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis.
Directness of the evidence: The study was conducted in the target population of newborn infants. Precision: The precision for the point estimate was low as the sample size was small Presence of publication bias: N/A. |
|
| 963 per 1000 | 902 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; BPD: Bronchopulmonary dysplasia; N/A: Not applicable | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 3. Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours or between 12 and 21 days of age).
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours or between 12 and 21 days of age) | ||||||
|
Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| BPD at 36 weeks' postmenstrual age | High risk population | RR 1.08 (95% CI 0.88 to 1.32) | 429 (3) | ⊕⊕⊝⊝ low | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessment was unclear. In Suchomski 2002 there was no blinding of the intervention or outcomes measurements. We downgraded the quality of the evidence by two levels.
Heterogeneity/consistency: Heterogeneity was low (I² = 39%).
Directness of the evidence: The studies were conducted in the target population of newborn infants. Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. |
|
| 422 per 1000 | 485 per 1000 (394 to 776) | |||||
| Hyperglycaemia | High risk population | RR 0.86 (95% CI 0.61 to 1.22) | 429 (3) | ⊕⊕⊝⊝ low | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two levels. Heterogeneity/consistency: There was no heterogeneity (I² = 8%). Directness of the evidence: The studies were conducted in the target population of newborn infants. Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. |
|
| 255 per 1000 | 177 per 1000 (0 to 282) | |||||
| Hypertension | High risk population | RR (RR 0.86, 95% CI 0.73 to 1.01) | 429 (3) | ⊕⊕⊝⊝ low | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two steps. Heterogeneity/consistency: There was no heterogeneity (I² = 0%). Directness of the evidence: The studies were conducted in the target population of newborn infants. Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. |
|
| 604 per 1000 | 430 per 1000 (130 to 627) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; BPD: Bronchopulmonary dysplasia; N/A: Not applicable | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 4. Inhaled steroids compared with systemic steroids for BPD ‐ long‐term outcomes at 7 years of age (infants randomised at < 72 hours of age).
| Inhaled steroids compared with systemic steroids for BPD ‐ long‐term outcomes at 7 years of age (infants randomised at < 72 hours of age) | ||||||
|
Patient or population: Neonates with developing BPD Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
|
General conceptual ability (GCA) score at 7 years The test has a standardisation mean of 100 and SD of 15 |
The mean GCA score in the control group was 90.2 | The mean GCA score in the intervention groups was 3.4 units lower | MD ‐3.40 (95% CI ‐12.38 to 5.58) | 74 (1) | ⊕⊕⊕⊝ moderate | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Directness of the evidence: The study was conducted in the target population of newborn infants. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Moderate/severe disability at 7 years | 135 per 1000 | 189 per 1000 | RR 1.40 (95% CI 0.49 to 4.01) | 74 (1) | ⊕⊕⊕⊝ moderate | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Directness of the evidence: The study was conducted in the target population of newborn infants. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Death or moderate/severe disability at 7 years | 418 per 1000 | 423 per 1000 | RR 1.01 (95% CI 0.65 to 1.58) | 107 (1) | ⊕⊕⊕⊝ moderate | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Directness of the evidence: The study was conducted in the target population of newborn infants. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Systolic blood pressure > 95th percentile at 7 years | 353 per 1000 | 194 per 1000 | RR 0.55 (95% CI 0.25 to 1.23) | 70 (1) | ⊕⊕⊕⊝ moderate | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Directness of the evidence: The study was conducted in the target population of newborn infants. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Diastolic blood pressure > 95th percentile at 7 years | 121 per 1000 | 167 per 1000 | RR (1.38, 95% CI 0.43 to 4.45) | 69 (1) | ⊕⊕⊕⊝ moderate | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Directness of the evidence: The study was conducted in the target population of newborn infants. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Ever diagnosed as asthmatic by 7 years | 528 per 1000 | 459 per 1000 | RR 0.87 (95% CI 0.55 to 1.39) | 73 (1) | ⊕⊕⊕⊝ moderate | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Directness of the evidence: The study was conducted in the target population of newborn infants. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; BPD: Bronchopulmonary dysplasia; NICU: Neonatal intensive care unit; N/A: Not applicable | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
There were elements of risk of bias in the three included studies. For details see the information below.
Allocation
In the study by Halliday 2001 after identifying an eligible infant, the clinician telephoned the randomisation centre to enrol the infant and to determine the treatment group (low risk of bias).
Suchomski 2002 was a prospective randomised controlled trial. Three sets of 27 cards were assembled followed by placement of one card each into one of 81 opaque envelopes. As infants were enrolled, a card was sequentially pulled and the infant assigned to the appropriate study group (low risk of bias).
Rozycki 2003 was a prospective randomised double‐blind controlled trial. The infants were randomised using a random table number and only the pharmacy was aware of the individual group assignment (low risk of bias).
Blinding
Halliday 2001 was a multi‐centre RCT involving 47 centres. The interventions and outcome measures were not blinded in all the centres (high risk of bias). However, in 11 centres the trial was conducted double blind. In these centres, placebo metered dose inhalers and intravenous saline were used to mask treatment allocation. Comparisons were made for the primary outcome variables between the centres observing double blind strategy and the other centres. The long‐term assessments at 7 years of age were performed by assessors blinded to the group assignments (low risk of bias).
In Suchomski 2002 blinding of the intervention was not performed (high risk of bias). Blinding of outcome measurement was not ensured (high risk of bias). Cross‐over from inhaled steroid groups to intravenous dexamethasone was allowed at the discretion of attending neonatologist.
In Rozycki 2003 blinding of intervention was performed (low risk of bias). Blinding of outcome measurement was unclear (unclear risk of bias).
Incomplete outcome data
There was complete follow up of all randomised infants in all three studies (low risk of bias in all three studies).
Selective reporting
There was no selective reporting in the trial by Halliday 2001 (low risk of bias ). The protocols for the other trials were not available, so we can not judge if there were any deviations or not.
Other potential sources of bias
We are not aware of any other sources of bias in the included trials (unclear risk).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Halliday 2001 randomised infants at < 72 hours of age; Suchomski 2002 randomised infants at 12 to 21 days; and Rozycki 2003 randomised after 14 days of age. All trials reported outcomes from the age of randomisation. Although infants received steroids after the first two weeks of life in all trials, the time period over which outcomes were measured differed among studies. Data from all three trials were combined for meta‐analyses of secondary outcomes (Halliday 2001; Suchomski 2002; Rozycki 2003).
For outcomes that included death we performed separate analyses for Halliday 2001 which reported on deaths from randomisation at < 72 hours and we combined results from Suchomski 2002 and Rozycki 2003.
1. Inhaled versus systemic steroids among infants ‐ outcomes including deaths (infants randomised at < 72 hours of age)
Because only Halliday 2001 was included in these analyses, tests for heterogeneity were not applicable.
Primary outcome
Death or BPD at 36 weeks' postmenstrual age
There was no statistically significant difference between the groups for the combined outcome of death or BPD at 36 weeks' postmenstrual age (RR 1.04, 95% CI 0.86 to 1.26; RD 0.03, 95% CI ‐0.09 to 0.14; 1 study, N = 292; Analysis 1.1; moderate‐quality evidence).
1.1. Analysis.
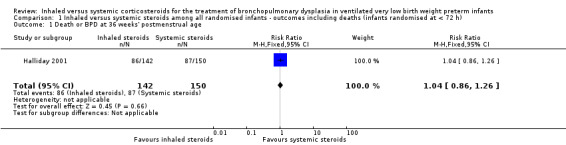
Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 1 Death or BPD at 36 weeks' postmenstrual age.
Secondary outcomes
Death or BPD at 28 days of age
There was no statistically significant difference between the groups for the combined outcome of death or BPD at 28 days of age (RR 1.00, 95% CI 0.90 to 1.12; RD 0.00, 95% CI ‐0.09 to 0.09; 1 study, N = 292; Analysis 1.2).
1.2. Analysis.
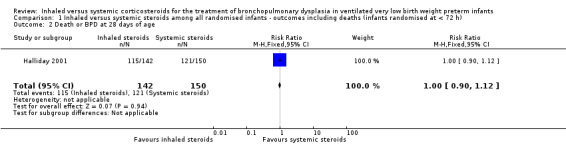
Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 2 Death or BPD at 28 days of age.
Death at 36 weeks' postmenstrual age
There was no statistically significant effect on death at 36 weeks' postmenstrual age between groups (RR 0.96, 95% CI 0.62 to 1.49; RD ‐0.01, 95% CI ‐0.10 to 0.09; 1study, N = 292; Analysis 1.3).
1.3. Analysis.
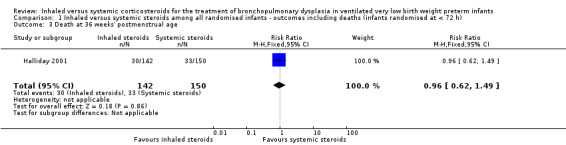
Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 3 Death at 36 weeks' postmenstrual age.
Death at 28 days of age
No statistically significant effect on mortality by 28 days was noted between the groups (RR 0.85, 95% CI 0.52 to 1.37; RD ‐0.03, 95% CI ‐0.12 to 0.06; 1 study, N = 292; Analysis 1.4).
1.4. Analysis.
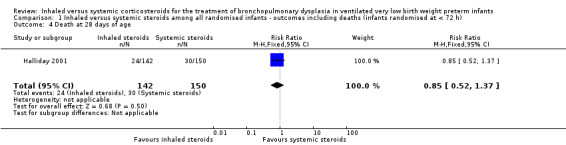
Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 4 Death at 28 days of age.
2. Inhaled versus systemic steroids among infants ‐ outcomes including deaths (infants randomised between 12 and 21 days of age)
Because only Suchomski 2002 was included in these analyses, tests for heterogeneity were not applicable. Rozycki 2003 did not report on deaths at 36 weeks' PMA or at 28 days of age.
Primary outcome
Death or BPD at 36 weeks' postmenstrual age
There was no statistically significant difference between groups for the combined outcome of death or BPD at 36 weeks' postmenstrual age (RR 0.94, 95% CI 0.83 to 1.05; RD ‐0.06, 95% CI ‐0.17 to 0.05; 1 study, N = 78; Analysis 2.1; low‐quality evidence).
2.1. Analysis.
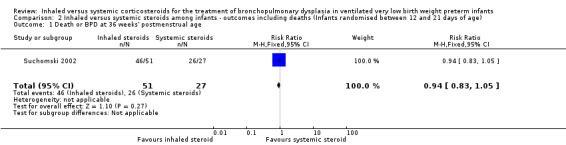
Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 1 Death or BPD at 36 weeks' postmenstrual age.
Secondary outcomes
Death at 36 weeks' postmenstrual age
There was no statistically significant effect on death at 36 weeks' postmenstrual age between groups (RR 2.69, 95% CI 0.13 to 54.15; RD 0.04, 95% CI ‐0.04 to 0.12; 1 study, N = 78; Analysis 2.2).
2.2. Analysis.
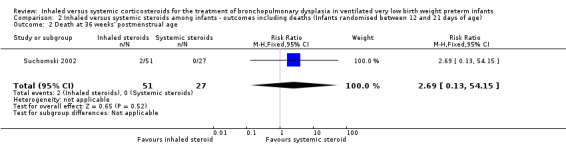
Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 2 Death at 36 weeks' postmenstrual age.
Death at 28 days of age
No statistically significant effect on mortality by 28 days was noted between groups (RR 2.69, 95% CI 0.13 to 54.15; RD 0.04, 95% CI ‐0.04 to 0.12; 1 study, N = 78; Analysis 2.3).
2.3. Analysis.
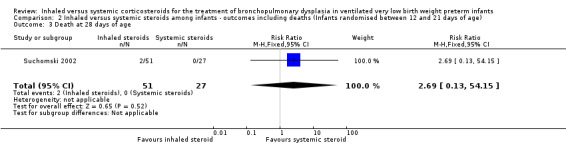
Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 3 Death at 28 days of age.
3. Inhaled versus systemic steroids among infants ‐ secondary outcomes (infants randomised at < 72 hours of age or between 12 and 21 days of age)
Secondary outcomes
BPD at 36 weeks' postmenstrual age
There was no statistically significant difference in the incidence of BPD at 36 weeks' postmenstrual age in the inhaled steroid compared to systemic steroid group (typical RR 1.08, 95% CI 0.88 to 1.32; typical RD 0.03, 95% CI ‐0.06 to 0.12; 3 studies, N = 429; Analysis 3.1; low‐quality evidence). There was low heterogeneity for both RR (39%) and RD (28%).
3.1. Analysis.
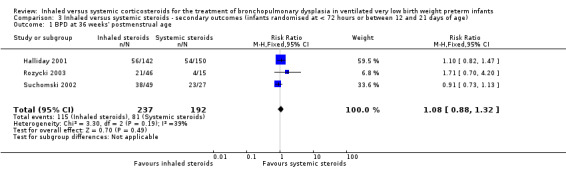
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 1 BPD at 36 weeks' postmenstrual age.
BPD at 28 days of age
There was no statistically significant difference in the incidence of BPD at 28 days between groups (typical RR 1.04, 95% CI 0.91 to 1.18; typical RD 0.03, 95% CI ‐0.06 to 0.12; 2 studies, N = 368; Analysis 3.2). There was low heterogeneity for both RR (46%) and RD (0 %).
3.2. Analysis.
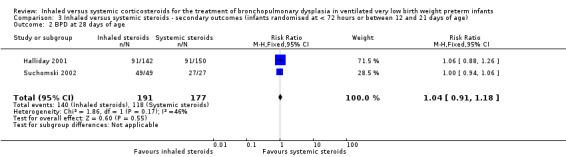
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 2 BPD at 28 days of age.
Need for ventilation amongst survivors at 36 weeks' postmenstrual age
There was no statistically significant difference for this outcome between groups (RR 1.10, 95% CI 0.30 to 4.06; RD 0.01, 95% CI ‐0.14 to 0.16; 1 study, N = 76; Analysis 3.3).
3.3. Analysis.
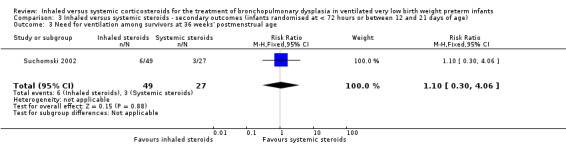
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 3 Need for ventilation among survivors at 36 weeks' postmenstrual age.
Duration of mechanical ventilation among survivors (days)
The duration of mechanical ventilation was not statistically significantly different between groups (typical WMD ‐ 0.3 days, 95% CI ‐5.2 to 4.6; 2 studies, N = 368; Analysis 3.4). There was no heterogeneity for WMD (0%).
3.4. Analysis.
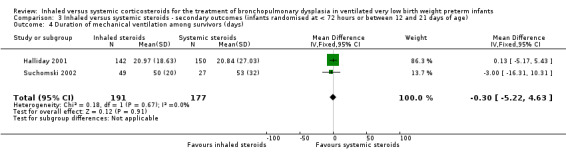
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 4 Duration of mechanical ventilation among survivors (days).
Duration of supplemental oxygen among survivors (days)
The duration of supplemental oxygen was not statistically significantly different between groups (typical WMD ‐4.91 days, 95% CI ‐21 to 11; 2 studies, N = 368; Analysis 3.5).
3.5. Analysis.
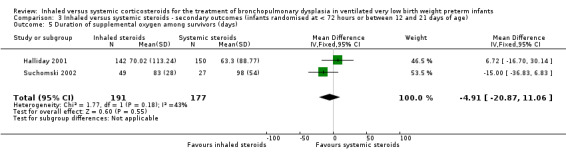
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 5 Duration of supplemental oxygen among survivors (days).
Length of hospital stay among survivors (days)
There was no statistically significant difference in the length of hospital stay among survivors between groups (MD ‐13, 95% CI ‐33 to 7; 1 study, N = 76; Analysis 3.6). Test for heterogeneity not applicable.
3.6. Analysis.
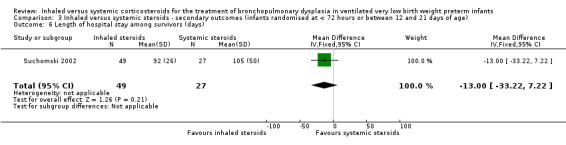
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 6 Length of hospital stay among survivors (days).
Intraventricular haemorrhage grade III‐IV
There was no statistically significant difference in length of hospital stay among survivors between groups (RR 0.90, 95% CI 0.33 to 2.40; RD ‐0.03, 95% CI ‐0.28 to 0.23; 1 study, N = 61; Analysis 3.7). Test for heterogeneity not applicable.
3.7. Analysis.
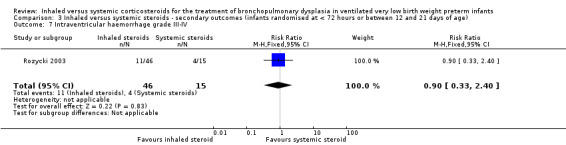
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 7 Intraventricular haemorrhage grade III‐IV.
Periventricular leukomalacia
There was no statistically significant difference in the incidence of periventricular leukomalacia between groups (typical RR 0.85, 95% CI 0.34 to 2.13; typical RD ‐0.02, 95% CI ‐0.15 to 0.10; 2 studies, N = 137; Analysis 3.8). There was no heterogeneity for either RR (0%) or RD (0%).
3.8. Analysis.
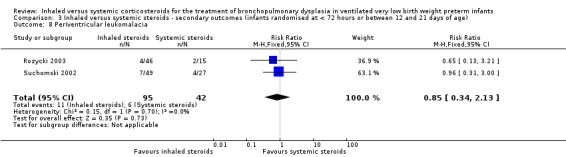
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 8 Periventricular leukomalacia.
Hyperglycaemia
There was no statistically significant difference in the incidence of hyperglycaemia between groups (typical RR 0.86, 95% CI 0.61 to 1.22; typical RD ‐0.03, ‐0.11 to 0.05; 3 studies, N = 429; Analysis 3.9; low‐quality evidence). There was no heterogeneity for either RR (8%) or RD (0 %).
3.9. Analysis.
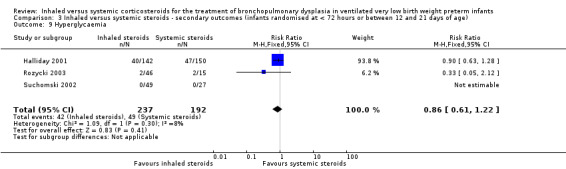
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 9 Hyperglycaemia.
Hypertension
There was no statistically significant difference in the incidence of hypertension between groups (typical RR 0.86, 95% CI 0.73 to 1.01; typical RD ‐0.08, 95% Ci ‐0.17 to 0.00; 3 studies, N = 429; Analysis 3.10; low‐quality evidence). There was no heterogeneity for either RR (0%) or RD (0%).
3.10. Analysis.
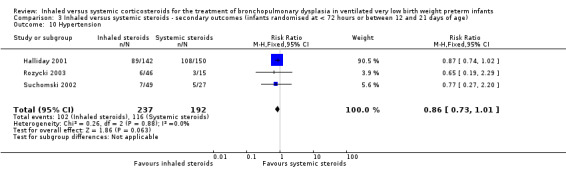
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 10 Hypertension.
Necrotising enterocolitis
There was no statistically significant difference in the incidence of necrotising enterocolitis between groups (typical RR 0.96, 95% CI 0.50 to 1.85; typical RD ‐0.00, 95% CI ‐0.06 to 0.06; 2 studies, N = 368; Analysis 3.11). There was no heterogeneity for either RR (0%) or RD (0%).
3.11. Analysis.
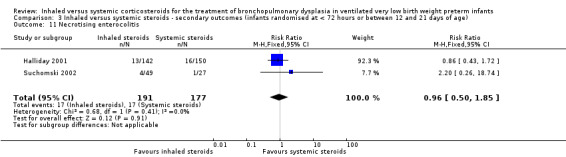
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 11 Necrotising enterocolitis.
Gastrointestinal bleed
There was no statistically significant difference in the incidence of gastrointestinal bleed between groups (typical RR 0.89, 95% CI 0.41 to 1.93; typical RD ‐0.01, 95% CI ‐0.06 to 0.04; 2 studies, N = 368; Analysis 3.12). As there were no outcomes in either group in one trial, test for heterogeneity for RR was not applicable. There was no heterogeneity for RD (0%).
3.12. Analysis.
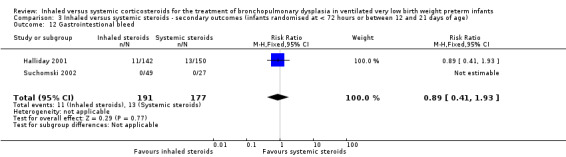
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 12 Gastrointestional bleed.
Retinopathy of prematurity ≥ stage 3
There was no statistically significant difference in the incidence of retinopathy of prematurity between groups (typical RR 1.32, 95% CI 0.77 to 2.25; typical RD 0.04, 95% CI ‐0.03 to 0.11; 3 studies, N = 363; Analysis 3.13). There was no heterogeneity for either RR (0%) or RD (0%).
3.13. Analysis.
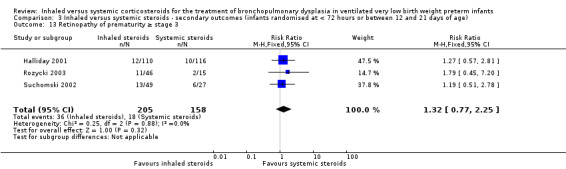
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 13 Retinopathy of prematurity ≥ stage 3.
Culture‐proven sepsis
There was no statistically significant difference in the incidence of culture‐proven sepsis between groups (typical RR 1.07, 95% CI 0.79 to 1.45; RD 0.02, 95% CI ‐0.07 to 0.12; 2 studies, N = 368; Analysis 3.14). There was no heterogeneity for either RR (0%) or RD (0%).
3.14. Analysis.
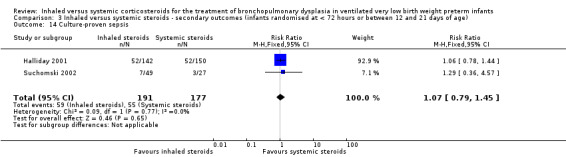
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 14 Culture‐proven sepsis.
Other outcomes
Adrenocorticotropic hormone (ACTH) stimulation test
Suchomski 2002 reported that the ACTH test was completed for 24 infants. The baseline cortisol levels before the ACTH stimulation test for the 800 µg/d inhaled group (3 ± 2.3 µg/dL; N = 7) and the intravenous group (1.6 ± 1.3 µg/dL; N = 10) were statistically significantly lower than for the 400 µg/d inhaled group (7.3 ± 4.2 µg/dL; N = 7). However, the response to ACTH (i.e. relative rise in cortisol level) was similar in all three groups: 12 ± 5.7 µg/dL in the 400 µg/d inhaled group, 15.6 ± 8.5 µg/dL in the 800 µg/d inhaled group and 10.7 ± 4.6 µg/dL in the intravenous group, P = 0.408. Post ACTH stimulation cortisol levels were 18.4 ± 8.0 µg/dL in the 800 µg/d inhaled group, 19.3 ± 5.9 µg/dL in the 400 µg/d inhaled group and 12.3 ± 5.7 µg/dL in the intravenous group, P = 0.048.
No relevant data for the following outcomes were available for analysis: measurement of pulmonary functions, growth at 36 week PMA, nephrocalcinosis, hypertrophy of tongue, cataract, pneumonia or hypertrophic cardiomyopathy.
4. Inhaled versus systemic steroids among children at 7 years of age (infants randomised at < 72 hours of age)
A subset of infants enrolled in the OSECT study (Halliday 2001) were followed to a median age of 7 years. The study followed 127 (84%) of 152 survivors from the United Kingdom and Ireland. Of these, 75 children belonged to the late budesonide and late dexamethasone groups; 38 children belonged to the late budesonide group; and 37 children to the late dexamethasone group.
Tests for heterogeneity were not applicable to any of these analyses because only one study was included in each analysis.
There were no statistically significant differences between the early inhaled and the early systemic corticosteroid groups for the following outcomes in the Halliday 2001 study which reported on 75 children.
General conceptual ability score at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (MD ‐3.40, 95% CI ‐12.38 to 5.58; 1 study, N = 74; Analysis 4.1; moderate‐quality evidence).
4.1. Analysis.
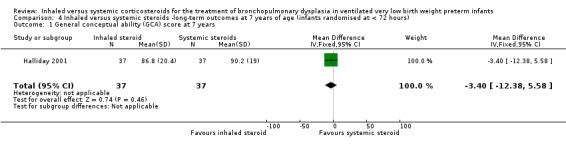
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 1 General conceptual ability (GCA) score at 7 years.
Child Behaviour Checklist at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (MD 0.20, 95% CI ‐4.75 to 5.15; 1 study, N = 74; Analysis 4.2; moderate‐quality evidence).
4.2. Analysis.
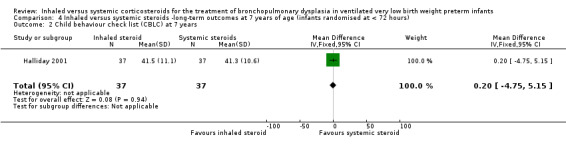
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 2 Child behaviour check list (CBLC) at 7 years.
Strengths and Difficulties Questionnaire at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids, (MD 1.00, 95% CI ‐2.19 to 4.19; 1 study, N = 74; Analysis 4.3; moderate‐quality evidence).
4.3. Analysis.
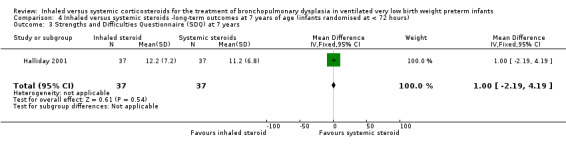
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years.
Cerebral palsy at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (RR 0.97, 95% CI 0.35 to 2.72; RD ‐0.01, 95% CI ‐0.18 to 0.17; 1 study, N = 69; Analysis 4.4; moderate‐quality evidence).
4.4. Analysis.
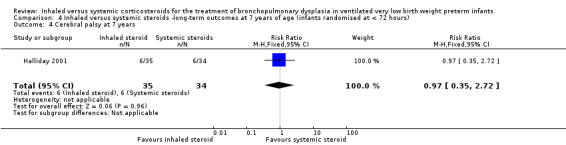
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 4 Cerebral palsy at 7 years.
Moderate/severe disability at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (RR 1.40, 95% CI 0.49 to 4.01; RD 0.05, 95% CI ‐0.11 to 0.22; 1 study, N = 74; Analysis 4.5; moderate‐quality evidence).
4.5. Analysis.
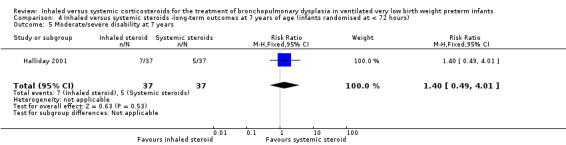
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 5 Moderate/severe disability at 7 years.
Death or moderate/severe disability at 7 years
There was no significant difference between the group who received inhaled steroids versus the group who received systemic steroids (RR 1.01, 95% CI 0.65 to 1.58; RD 0.00, 95% CI ‐0.18 to 0.19; 1 study, N = 107; Analysis 4.6; moderate‐quality evidence).
4.6. Analysis.
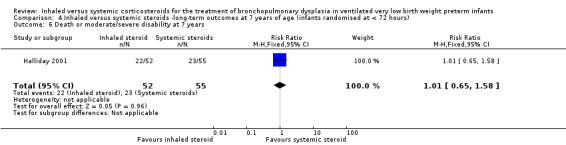
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 6 Death or moderate/severe disability at 7 years.
Systolic blood pressure > 95th percentile at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (RR 0.55, 95% CI 0.25 to 1.23; RD ‐0.16, 95% CI ‐0.36 to 0.05; 1 study, N = 70; Analysis 4.7; moderate‐quality evidence).
4.7. Analysis.
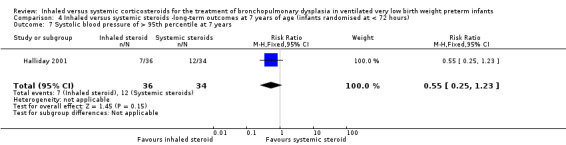
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 7 Systolic blood pressure of > 95th percentile at 7 years.
Diastolic blood pressure > 95th percentile at 7 years
There was no significant difference between the groups of infants who received inhaled or systemic steroids (RR 1.38, 95% CI 0.43 to 4.45; RD 0.05, 95% CI ‐0.12 to 0.21; 1 study, N = 69; Analysis 4.8; moderate‐quality evidence).
4.8. Analysis.
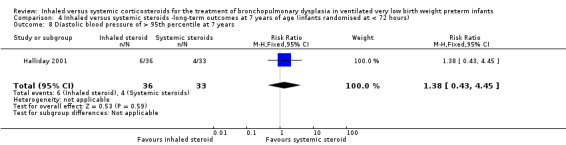
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 8 Diastolic blood pressure of > 95th percentile at 7 years.
Ever diagnosed with asthma by 7 years
There was no significant difference between the groups who received inhaled or systemic steroids (RR 0.87, 95% CI 0.55 to 1.39; RD ‐0.07, 95% CI ‐0.30 to 0.16; 1 study, N = 73; Analysis 4.9; moderate‐quality evidence).
4.9. Analysis.
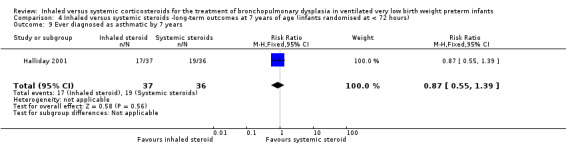
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 9 Ever diagnosed as asthmatic by 7 years.
Discussion
Summary of main results
We included three studies that involved a total of 431 infants.
Evidence from two studies in 370 infants contributing data to the primary outcome of this review showed that inhaled steroids administered after 7 days of age compared with systemic steroids did not decrease the incidence of death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age.
Evidence from three studies in 431 infants contributing to secondary outcomes showed that inhaled steroids administered after seven days of age compared with systemic steroids did not significantly alter the incidence of BPD at 36 weeks' postmenstrual age, hyperglycaemia, hypertension, duration of ventilation, duration of oxygen supplementation, length of hospital stay, intraventricular haemorrhage grade III‐IV, periventricular leukomalacia, necrotising enterocolitis, gastrointestinal bleed, retinopathy of prematurity stage > 3, culture‐proven sepsis or the incidence of adverse effects.
One study received grant support and the industry provided Aerochambers and metered dose inhalers of budesonide and placebo for the same study. No conflict of interest was identified.
Overall completeness and applicability of evidence
For the primary outcome death or BPD at 36 weeks' postmenstrual age data from Halliday 2001 were reported separately as they randomised infants < 72 hours of age. Suchomski 2002 reported on the primary outcome but enrolled infants between 12 to 21 days of age. Rozycki 2003 did not report on the primary outcome. Data from three trials (Suchomski 2002; Rozycki 2003) were combined for secondary outcomes as treatment started in infants > 7 days old. The period of measurement of outcomes varied between Halliday 2001 and Suchomski 2002 making combination of results inappropriate. This may possibly explain differences in the incidence of adverse events such as mortality. Halliday 2001 counted deaths from < 72 hours onwards and Suchomski 2002 counted deaths from 12 to 21 days onwards. This means that in Halliday 2001, all deaths < 72 hours were attributed to the randomised treatment policy, whereas in Suchomski 2002, only deaths from 12 to 21 days were so attributed. Looking at the control event rate we see what we would expect ‐ a much higher death rate in Halliday 2001 than in Suchomski 2002 (death at 36 weeks' was 33/150 in Halliday 2001 and 0/27 in Suchomski 2002). Similar explanations could be provided for the other outcomes of interest. Due to these concerns, aggregation of data from the two trials was not performed when outcomes included deaths.
Quality of the evidence
According to GRADE assessment of the quality of the evidence for the outcome of death or BPD at 36 weeks' postmenstrual age (primary outcome) for infants randomised at < 72 hours of age was moderate (Table 1). The quality of the evidence according to GRADE for the outcome of death or BPD at 36 weeks' postmenstrual age (primary outcome) for infants randomised between 12 to 21 days of age was low (Table 2).
For secondary outcomes we included for infants randomised at < 72 hours or between 12 and 21 days: BPD at 36 weeks' postmenstrual age, hyperglycaemia, and hypertension. The quality of the evidence according to GRADE was low (Table 3). For infants randomised at < 72 hours of age we included in GRADE assessments the following outcomes at seven years of age: general conceptual ability; moderate/severe disability; death or moderate/severe disability; systolic blood pressure > 95th percentile; diastolic blood pressure > 95th percentile and ever diagnosed with asthma. According to GRADE the quality of the evidence was moderate for these outcomes (Table 4).
Potential biases in the review process
We are not aware of any potential biases in our review process. One author (HH) is the first author of an included trial (Halliday 2001). That study was assessed by the other three authors (AO, SS, VS).
Agreements and disagreements with other studies or reviews
We found no evidence that inhaled steroids confer any net advantages over systemic steroids in the management of ventilator‐dependent preterm infants. Systemic steroids given late (after 1 week) suggest that late therapy may reduce neonatal mortality without significantly increasing the risk of adverse long‐term neurodevelopmental outcomes (Doyle 2014a; Shah 2001). However, systemic steroids, especially dexamethasone given early (< 7 days of age), are associated with an increase in abnormal neurological exam findings and cerebral palsy (Doyle 2014). Further studies with particular attention on long‐term neurodevelopmental outcomes need to be performed before delayed steroids, either inhaled or systemic, can be confirmed as safe for treatment of evolving BPD in preterm infants. Follow‐up studies are extremely important. A subset of surviving infants from the OSECT study (Halliday 2001) have been followed to seven years of age and the results are included in this updated review.
A major concern with studies of inhaled steroid therapy is uncertainty regarding drug delivery and deposition of steroids in the oropharynx and peripheral airways. Numerous factors affect drug delivery and deposition including numbers of particles in the respirable range, delivery technique (use of a metered dose inhaler with or without a spacer), nebuliser (jet or ultrasonic) and the presence or absence of an endotracheal tube. Previous reports have shown that the amount of aerosol delivery varies from 0.4% to 14% based on the technique used (Arnon 1992; Grigg 1992; O'Callaghan 1992). The delayed onset of activity (Dimitriou 1997) and a similar risk profile of inhaled steroids (Shah 2017) suggests that effects may be secondary to systemic absorption.
Inhaled steroids are believed to be less effective compared to systemic steroids. The drug type, dosage and delivery methods may be inadequate. More refinements in the inhalational drug delivery system guaranteeing selective delivery in the alveoli and smaller airways may improve the clinical efficacy and decrease the side effect profile of inhaled steroids.
In the present review, there is no evidence of difference in effectiveness or side effect profiles for inhaled versus systemic steroids. A better delivery system or higher dose of inhaled steroids may result in equivalence of effectiveness for the two modes of administration, but may also show evidence of side effects. To resolve this issue, studies are needed to identify the risk/benefit ratio of different delivery techniques and dosing schedules for the administration of these medications.
Authors' conclusions
Implications for practice.
This updated review of three trials of moderate to low quality found no evidence that inhaled corticosteroids confer net advantages over systemic corticosteroids in the management of ventilator‐dependent preterm infants. The lack of evidence leads to the conclusion that inhaled steroids cannot be recommended as part of standard practice.
Implications for research.
To date, only three studies reporting on 431 infants have been published. The quality of the evidence based on GRADE was moderate to low. Larger high quality studies are needed to identify the effectiveness of inhaled steroids versus systemic steroids. The risk/benefit ratio of different delivery techniques and dosing schedules for the administration of steroids needs to be assessed. Studies are needed to address the long‐term effects of inhaled steroids, with particular attention to neurodevelopmental outcome. We are not aware of any ongoing trials.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 7 November 2017 | Amended | Corrected typo and republished the October 2017 update. |
| 6 March 2017 | New search has been performed | The review was updated in March, 2017. Literature searches on 23 February 2017 did not identify any new trials. Summary of findings tables were added. |
| 6 March 2017 | New citation required but conclusions have not changed | No changes to conclusions. |
| 26 March 2012 | New search has been performed | This review updates the existing review "Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants" published in the Cochrane Database of Systematic Reviews Shah 2012a. |
| 26 March 2012 | New citation required but conclusions have not changed | Updated search in June 2011 found no new trials. No changes to conclusions. |
| 26 June 2008 | Amended | Converted to new review format. |
| 3 August 2007 | New search has been performed | This updates the review "Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants" published in The Cochrane Library Issue 2, 2003 (Shah 2003). For this update, one additional trial was identified and included in this review. The conclusions of the review did not change. |
Acknowledgements
We thank Dr HL Halliday and Dr Chris Patterson for providing additional data for the infants included in the OSECT trial.
Appendices
Appendix 1. Previous search methodology
For previous versions of the review, randomised controlled trials comparing inhaled versus systemic corticosteroid therapy in preterm infants were identified from MEDLINE (1966 to 2011) using MeSH headings: infant‐newborn, chronic lung disease, bronchopulmonary dysplasia, anti‐inflammatory agents, steroids; dexamethasone, administration, inhalation; aerosols, budesonide, beclomethasone dipropionate, flunisolide and fluticasone propionate.
Other databases were searched including: Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 6, 2011), EMBASE (1980 to 2011), CINAHL (1982 to 2011), reference lists of published trials and abstracts published in Pediatric Research or electronically on the Pediatric Academic Societies web site (1990 to 2011). No language restrictions were applied.
For the 2012 update, we searched Clinicaltrials.gov, Controlled‐trials.com and Web of Science, which were not searched for previous reviews.
We used the following search strategies for the 2012 updated searches:
PubMed
((bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)) AND ((infant, newborn[MeSH] OR newborn OR neon* OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) AND (("2007"[PDat] : "3000"[PDat]))
CINAHL
(bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate) ) and ( ( infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND ( randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) 2007 ‐ Present
Cochrane Central Register of Controlled Trials
(bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate) and (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW), from 2007 to 2011
EMBASE
1 ((bronchopulmonary dysplasia or lung diseases or chronic lung disease) and (anti‐inflammatory agents or steroids or dexamethasone or inhalation or aerosols or budesonide or beclomethasone dipropionate or flunisolide or fluticasone propionate)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1849)
2 (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (603948)
3 (human not animal).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (11849457)
4 (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1256505)
5 1 and 2 and 3 and 4 (336)
6 limit 5 to yr="2007 ‐Current" (76)
Clinicaltrials.gov
(infant OR newborn) AND (bronchopulmonary dysplasia OR lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)
Controlled‐trials.com
(infant OR newborn) AND (bronchopulmonary dysplasia OR lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)
Appendix 2. Standard search methodology for 2017 update
PubMed
((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
EMBASE
(infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL
(infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library
(infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 3. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomisation, and the blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as low, high, or unclear risk. Two review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the table Characteristics of included studies. We evaluated the following issues and entered the findings into the risk of bias table:
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
a. Low risk (any truly random process e.g. random number table; computer random number generator);
b. High risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number);
c. Unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
a. Low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
b. High risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
c. Unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
a. Low risk, high risk or unclear risk for participants;
b. Low risk, high risk or unclear risk for personnel;
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
a. Low risk for outcome assessors.
b. High risk for outcome assessors.
c. Unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
a. Low risk (< 20% missing data);
b. High risk (≥ 20% missing data);
c. Unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
a. Low risk (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
b. High risk (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
c. Unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
a. Low risk;
b. High risk;
c. Unclear risk
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or BPD at 36 weeks' postmenstrual age | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.26] |
| 2 Death or BPD at 28 days of age | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 3 Death at 36 weeks' postmenstrual age | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.49] |
| 4 Death at 28 days of age | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.37] |
Comparison 2. Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or BPD at 36 weeks' postmenstrual age | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.05] |
| 2 Death at 36 weeks' postmenstrual age | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| 3 Death at 28 days of age | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
Comparison 3. Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BPD at 36 weeks' postmenstrual age | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| 2 BPD at 28 days of age | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3 Need for ventilation among survivors at 36 weeks' postmenstrual age | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.30, 4.06] |
| 4 Duration of mechanical ventilation among survivors (days) | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐5.22, 4.63] |
| 5 Duration of supplemental oxygen among survivors (days) | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐4.91 [‐20.87, 11.06] |
| 6 Length of hospital stay among survivors (days) | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐33.22, 7.22] |
| 7 Intraventricular haemorrhage grade III‐IV | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.33, 2.40] |
| 8 Periventricular leukomalacia | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.34, 2.13] |
| 9 Hyperglycaemia | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.22] |
| 10 Hypertension | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 11 Necrotising enterocolitis | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.50, 1.85] |
| 12 Gastrointestional bleed | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 13 Retinopathy of prematurity ≥ stage 3 | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.77, 2.25] |
| 14 Culture‐proven sepsis | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.45] |
Comparison 4. Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 General conceptual ability (GCA) score at 7 years | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐12.38, 5.58] |
| 2 Child behaviour check list (CBLC) at 7 years | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐4.75, 5.15] |
| 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.19, 4.19] |
| 4 Cerebral palsy at 7 years | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.35, 2.72] |
| 5 Moderate/severe disability at 7 years | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.49, 4.01] |
| 6 Death or moderate/severe disability at 7 years | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.58] |
| 7 Systolic blood pressure of > 95th percentile at 7 years | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.23] |
| 8 Diastolic blood pressure of > 95th percentile at 7 years | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.43, 4.45] |
| 9 Ever diagnosed as asthmatic by 7 years | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.39] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Halliday 2001.
| Methods | Multicentre, randomised open study. 1. Blinding of randomisation: Yes. 2. Blinding of intervention: Not in all centres. 11 centres ‐ Yes; 36 centres ‐ No 3. Blinding of outcome measurement: No 4. Complete follow up: Yes Study period: February 1994 to December 1998. Study location: 47 neonatal intensive care units worldwide (UK, Ireland, Canada, Switzerland, Norway, Greece, Portugal, Sweden, Slovenia, Poland, Israel, Singapore, UAE) |
|
| Participants | 570 infants from 47 neonatal intensive care units worldwide (UK, Ireland, Canada, Switzerland, Norway, Greece, Portugal, Sweden, Slovenia, Poland, Israel, Singapore, UAE) were enrolled. Inclusion criteria: Gestational age < 30 weeks, postnatal age < 72 hours and need for mechanical ventilation and inspired FiO₂ > 30%. Delayed selective treatment was started if infants needed mechanical ventilation and > 30% FiO₂ for > 15 days. Infants of 30 ‐ 31 weeks GA could also be included if they needed > 50% FiO₂. Exclusion criteria: congenital lethal anomalies, severe intraventricular haemorrhage (grade 3 or 4) and proven systemic infection before entry. A strong suspicion of infection, uncontrolled hypertension and hyperglycaemia were considered to be indications to postpone trial entry until they resolved, provided that this occurred within 72 hours of birth. The trial had factorial design and a similar number of infants was allocated to each group. Group 1 received early (< 72 hours) dexamethasone (N = 135); group 2 received delayed (> 15 days) dexamethasone (N = 150); group 3 received early budesonide (N = 143); Group 4 received delayed selective budesonide (N = 142). Demographic data: values presented as mean (SD) or as appropriate Group 1: Early (< 72 hours) dexamethasone (N = 135) Data not included in this systematic review Group 2: Delayed (> 15 days) dexamethasone (N = 150) Gestational age: 27.1 weeks (1.9) Birth weight: 1007 g (283) Sex (f/m): 71/79 Antenatal steroids: N = 82 (55%) Surfactant treatment: N = 138 (92%) Clinical risk index for babies score: median 7, range 1 to 16 Group 3: Early budesonide (N = 143) Data not included in this systematic review Group 4: Delayed selective budesonide group N = 142 Gestational age: 27 weeks (2) Birth weight: 994 g (279) Sex (f/m): 64/78 Antenatal steroids: N = 89 (63%) Surfactant treatment: N = 132 (93%) Clinical risk index for babies score: median = 6, range 1 to 18 |
|
| Interventions | 1. Dexamethasone was administered IV or orally in initial dose of 0.5 mg/kg/day in 2 divided doses for 3 days, followed by 0.25 mg/kg/day in 2 divided doses for 3 days, then 0.10 mg/kg/day for 3 days, and finally 0.05 mg/kg/day in 2 divided doses for 3 days for a total of 12 days of treatment. 2. Budesonide was administered using a metered dose inhaler (200 µg/puff; Pulmicort, Astra Draco, Lund, Sweden) connected to spacing device (Aerochamber MV 15; Trudell Medical, Canada). The aero chamber was a rigid, clear plastic cylinder, 11 by 4.1 cm with an approximate capacity of 145 mL. After endotracheal suctioning, the metered dose inhaler was shaken and inserted into the spacing chamber. The spacer was then filled with 100% oxygen and the infant's FiO₂ was increased by 20%. The aero chamber was connected into the ventilatory circuit and manual inflations were given through the chamber using an inflatable bag. Budesonide was administered as soon as chest wall movements were established. A 500 to 1000 g infant was given 2 puffs twice daily and 1000 to 1500 g infant was given 3 puffs twice daily. The puffs were given one at a time, activating metered dose inhaler at end expiration and allowing 10 breaths after each activation. After each administration, the chamber was removed from the ventilator circuit and the infant was reconnected to the ventilator at the previous settings. The duration of budesonide treatment was up to 12 days provided the infant remained intubated. If the infant was extubated before 12 days budesonide was discontinued |
|
| Outcomes | 1. Primary outcome measure was death or oxygen dependency at 36 weeks' postmenstrual age. 2. Secondary outcome measures included death or major cerebral abnormality on ultrasound nearest to 6 weeks' postnatal age, death or oxygen dependency at 28 days and expected date of delivery, duration > 40% FiO₂, duration of any supplemental oxygen, duration of assisted ventilation by endotracheal tube and duration of hospital stay. 3. Complications such as pneumothorax, other pulmonary air leaks, necrotising enterocolitis, acquired pneumonia, patent ductus arteriosus requiring treatment, pulmonary haemorrhage requiring increased ventilation, seizures treated with anticonvulsants, recurrent apnoea needing treatment, retinopathy of prematurity at 36 weeks' postmenstrual age, gastric haemorrhage and GI perforation were noted. All neonates were monitored daily for blood pressure and blood glucose. Also, withdrawals from the intervention because of hypertension, hyperglycaemia, sepsis, gastric bleeding, or intestinal perforation were noted. An intention‐to‐treat analysis was performed |
|
| Notes | The study was performed double blind in 11 centres, and in these centres placebo metered dose inhalers and intravenous saline were used to mask treatment allocation. The study design was open rather than double‐blind because some clinicians wanted to prescribe broad spectrum antibiotics or H₂ blockers such as cimetidine or ranitidine to infants receiving dexamethasone. However, in 11 centres, the trial was conducted double‐blind, and in these centres placebo metered dose inhalers and intravenous saline were used to mask treatment allocation This study was supported by a grant from Action Research, United Kingdom. Trudell Medical, London Ontario, Canada supplied Aerochambers, and Astra, Draco, Lund, Sweden supplied the metered dose inhalers (MDIs) of budesonide and placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Multicentre, randomised open study. Random number sequence generation performed by the trial statistician, independent of researchers |
| Allocation concealment (selection bias) | Low risk | Once an infant had full filled entry criteria, the supervising clinician telephoned the randomisation centre in Belfast to enrol an infant and determine the treatment group |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not in all centres. 11 centres ‐ Yes; 36 centres ‐ No |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not in all centres. 11 centres ‐ Yes; 36 centres ‐ No |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not in all centres. 11 centres ‐ Yes; 36 centres ‐ No |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported on all enrolled infants |
| Selective reporting (reporting bias) | Low risk | There was no selective reporting according to the first author (HH) |
| Other bias | Unclear risk | Appears free of other bias |
Rozycki 2003.
| Methods | Prospective randomised controlled trial.
1. Blinding of randomisation: Yes
After parental consent, infants were stratified into two birth weight groups (650 to 1000 g and 1001 to 2000 g birth weight) and then into four dosing groups using a random table number
2. Blinding of intervention: Yes
3. Blinding of outcome assessment: Unclear
4. Complete follow up: Yes Study period: January 1992 to March 1995. Study location: Division of Neonatal‐Perinatal Medicine, Medical College of Virginia at Virginia Commonwealth University, Richmond, Virginia, USA |
|
| Participants | 61 preterm infants with birth weights between 650 g and 2000 g if at 14 days of age were at significant risk for developing moderate to severe BPD, defined as need for mechanical ventilation and oxygen, along with X‐ray changes beyond 28 days of life were enrolled.
Infants with proven sepsis and receiving FiO₂ of ≥ 0.3 and had a ventilatory index of < 0.8 were eligible while for infants without culture‐proven sepsis, the oxygen requirement was the same but the ventilatory index threshold was < 0.510. Demographic data: values presented as mean (SE) or as appropriate Group A: Dexamethasone group N = 15 Birth weight: 773 g (132) Gestational age: 26 weeks (24 to 27) Sex (m/f): 7/8 Prenatal steroids: 2/15 Inborn: 12 Prestudy sepsis: 8 Initial ventilation index: 0.252 (0.094) Group B: High dose beclomethasone group N = 16 Birth weight: 710 g (148) Gestational age: 26 weeks (23 to 29) Sex (m/f): 6/10 Prenatal steroids: 1/15 Inborn: 13 Prestudy sepsis: 10 Initial ventilation index: 0.300 (0.184) Group C: Medium dose beclomethasone group N = 15 Birth weight: 796 g (152) Gestational age: 26 weeks (24 to 30) Sex (m/f): 8/7 Prenatal steroids: 3/16 Inborn: 11 Prestudy sepsis: 7 Initial ventilation index: 0.294 (0.106) Group D: Low dose beclomethasone group N = 15 Birth weight: 760 g (124) Gestational age: 25 weeks (24 to 31) Sex (m/f): 9/6 Prenatal steroids: 2/15 Inborn: 13 Prestudy sepsis: 9 Initial ventilation index: 0.293 (0.158) |
|
| Interventions | Infants were randomised into 4 groups:
Group A: aerosol placebo‐systemic dexamethasone
Group B: high dose beclomethasone‐systemic placebo
Group C: medium dose beclomethasone‐systemic placebo
Group D: low dose beclomethasone‐systemic placebo Dexamethasone group: 42 day course of dexamethasone as described by Avery et al followed by a 7 day course of placebo to ensure that all subjects ended the study at the same time. Subjects in the aerosol steroid groups (B, C, or D) began a similar 42 day systemic dexamethasone course on day 8 if extubation was unsuccessful while on inhaled steroids. Aerosol steroid groups (limited to data from infants 650 to 1000 g) randomised to receive: High dose beclomethasone group (2.4 to 3.69 µg/kg/day) Medium dose beclomethasone group (1.0 change to 1.20 to 1.85 µg/kg/day) Low dose beclomethasone group (0.48 to 0.74 µg/kg/day) Beclomethasone dipropionate (42 µg/actuation, Vanceril, Schering‐Plough, Kenilworth, NJ) was administered using a metered dose inhaler placed between the bag and the spacer. After disconnecting the ventilator circuit, a 250 mL Laerdal resuscitation bag with oxygen reservoir connected to an oxygen source (> 90% FiO₂) was connected through a spacer to the endotracheal tube. After activating the metered dose inhaler, infants were given three manual breaths. Infants < 1001 g at birth received one dose of beclomethasone every 12 hourly while larger infants received a dose every 8 hourly. Inhaled steroids were administered for a maximum of 7 days. The medication was stopped if the infant was successfully extubated for more than 12 hours even if not all doses had been administered. |
|
| Outcomes | 1. Primary outcome measure was extubation within the first 7 days of the study 2. Secondary outcome measures included changes in ventilator settings and oxygen delivery over the first 7 days. The rates of hypertension, hyperglycaemia, infection and growth over the first 7 days were analysed. Death was not included as an outcome Long‐term outcomes were not included. Our primary outcome of death or BPD at 36 weeks' postmenstrual age was not reported on. BPD at 36 weeks' postmenstrual age and deaths during hospital stay were reported on. There were 3 deaths in the dexamethasone group (3/15) and 4 deaths in the beclomethasone groups (4/46) |
|
| Notes | Infants were ventilated with time‐cycled, pressure limited, non synchronized ventilators during this study. Ventilator management was prescribed during the first 2 weeks of the study. If the pCO₂ was < 50 mm Hg, the peak pressure was lowered until it was < 15 cm H₂O. Then, if the pCO₂ was < 50 mm Hg, the ventilator rate was lowered, FiO₂ was adjusted to maintain oxygen saturation between 88 to 92%. No subjects received bronchodilators during the study period. The use of caffeine or diuretics was left to the discretion of the attending physician No funding resources were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used. |
| Allocation concealment (selection bias) | Low risk | After parental consent, infants were stratified into two birth weight groups (650 to 1000 g and 1001 to 2000 g birth weight) and then into four dosing group using a random table number. Only the pharmacy was aware of the individual group assignment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | There was aerosol and systemic placebo in all participants in the control groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Yes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were provided on all enrolled infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot judge if there were any deviations from the protocol |
| Other bias | Unclear risk | Appears free of other bias |
Suchomski 2002.
| Methods | Prospective randomised controlled trial.
1. Blinding of randomisation: Yes
Random allocation using 3 sets of 27 assembled, opaque envelopes.
As infants were enrolled, a card was sequentially pulled and infant assigned to appropriate study group. In case of multiple gestation, all eligible siblings were assigned to same group to minimize parental anxiety. 2. Blinding of intervention: No 3. Blinding of outcome measurement: No 4. Complete follow up: Yes Study period: July 1994 to July 1996. Study location: Neonatal intensive care unit (NICU) at Children’s Hospital of Buffalo, State of New York. |
|
| Participants | 78 preterm infants ≤ 30 weeks, birth weight ≤ 1500 g and conventional ventilator dependence at 12 to 21 days of age with a rate > 15/min and FiO₂ > 0.30 with persistence of these ventilator settings for a minimum of 72 hours were enrolled in the study. Demographic data: Values presented as mean (SD) or as appropriate Inhaled beclomethasone 800 µg/d group N = 25 Gestational age: 26 weeks (1) Birth weight: 843 g (177) Sex (female/male) (number of infants): 14/11 Maternal steroids (number and percentage): 12 (48%) Age of commencement of steroids (days): 17 (3) Baseline mean airway pressure: 7.1 (1.2) Baseline FiO₂: 0.44 (0.10) Inhaled beclomethasone 400 µg/day group N = 26 Gestational age: 26 weeks (2) Birth weight: 846 g (N = 139) Sex (female/male) (number of infants): 12/14 Maternal steroids (number and percentage): 22 (84.6%) Age of commencement of steroids (days): 18 (3) Baseline mean airway pressure: 7.3 (1.9) Baseline FiO₂: 0.42 (0.13) Intravenous dexamethasone group N = 27 Gestational age: 26 weeks (2) Birth weight: 843 g (227) Sex (female/male) (number of infants): 8/19 Maternal steroids (number and percentage): 16 (59.2%) Age of commencement of steroids (days): 17 (2) Baseline mean airway pressure: 7.9 (1.6) Baseline FiO₂: 0.49 (0.13) Infants were ineligible if they were on high frequency oscillatory ventilation. Exclusion criteria: major congenital malformations, culture positive sepsis, hypertension which required medical management, persistent patent ductus arteriosus, or hyperglycaemia requiring insulin. Infants were also excluded if they received any postnatal steroid therapy (either inhaled or intravenous) before 12 days of age or before entry in the study. |
|
| Interventions | 1. The inhaled beclomethasone was delivered through a metered dose inhaler with a spacer device (Aerovent) connected in line with the ventilator at 50 µg per puff. Between each puff, the infant was ventilated with 4 or 5 manual breaths delivered at a peak pressure identical to that delivered during mechanical ventilation.
The 400 µg/d group (N = 26) received 4 puffs every 12 hours. The 800 µg/puff group (N = 25) received 4 puffs every 6 hours. Beclomethasone was continued until extubation. If the infant was successfully extubated, then the same dose was administered for 48 hours more. Thereafter, the steroid dose was halved every other day for 6 days, after which the steroid was stopped. After extubation, the inhaled beclomethasone was given using a face mask with inhaler and spacer device. 2. The intravenous dexamethasone group (N = 27) received a 42 day tapering course (Avery 1985), starting with 0.5 mg/kg/day, divided every 12 hours. Cross over from either of the inhaled beclomethasone groups to intravenous dexamethasone was allowed if, after 4 to 5 days of inhaled beclomethasone, the infant's ventilator and oxygen support had not decreased and the attending neonatologist felt that the infant might benefit from intravenous dexamethasone. 3. All data were analysed according to original group assignment. |
|
| Outcomes | 1. Primary outcome measures: hypertension or hyperglycaemia needing treatment or culture positive sepsis. 2. Other outcome variables: ventilatory settings, specifically rate, mean airway pressure (MAP), peak inspiratory pressure, positive end expiratory pressure, and supplemental oxygen requirement every 6 hours daily on all enrolled patients starting from 5 days before initiation of steroid therapy and daily thereafter, until extubation. After extubation, the supplemental oxygen was recorded daily until patient was discharged or supplemental oxygen was discontinued. 3. The occurrence of the following was also recorded: intraventricular haemorrhage, periventricular leukomalacia, necrotising enterocolitis, retinopathy of prematurity, GI bleed and death. 4. For infants who completed at least a 10 day course of either inhaled or intravenous steroids, an ACTH stimulation test was done 2 weeks after completion of steroid course. The test was completed in 24 neonates and the morning cortisol levels were noted before and one hour after intravenous bolus of 36 µg/kg synthetic ACTH (Cortrosyn, Organon, West Orange, NJ). |
|
| Notes | Sample size was determined by assuming a rate of adverse effects (as defined by sepsis or hyperglycaemia or hypertension requiring treatment) of 80% in intravenous steroid‐treated infants. The sample size was calculated to detect a 30% difference in adverse effects at 80% power and P < 0.05.
There was a statistically significant difference between the groups regarding some maternal characteristics such as maternal steroid use and need for caesarean section. No funding resources were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Random allocation using 3 sets of 27 assembled, opaque envelopes As infants were enrolled, a card was sequentially pulled and infants were assigned to appropriate study group. In case of multiple gestation, all eligible siblings were assigned to same group to minimize parental anxiety |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were reported on all enrolled infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us, so we cannot judge if there were any deviations from the protocol or not |
| Other bias | Unclear risk | Appears free of other bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dimitriou 1997 | The authors included non ventilator‐dependent infants and the age of commencement of steroids varied from 5 to 118 days of life |
| Nicholl 2002 | Non ventilator‐dependent infants were included in the study |
Differences between protocol and review
There is no published protocol. In this 2017 update the primary outcome was changed to death or bronchopulmonary dysplasia at 36 weeks' postmenstrual age. The title was changed from "Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants" to "Inhaled versus systemic corticosteroids for the treatment of bronchopulmonary dysplasia in ventilated very low birth weight preterm infants" to reflect this change. These changes were made following recommendations from the Editorial team so that different reviews on the topic of postnatal steroids would use the same primary outcome and the same nomenclature. These changes necessitated changes to the secondary outcomes that were listed in the previous update of the review.
Contributions of authors
Sachin Shah (SS): performance of literature search, identification of studies, abstraction of the data, analysis of data and writing of the review. Arne Ohlsson (AO): writing of the protocol, identification of studies (literature search), abstraction of data, analysis of data and editing of the review. Henry Halliday (HH): writing of protocol, identification of studies, abstraction of data, analysis of data and editing of review. Vibhuti Shah (VS): writing of the protocol, identification of studies (literature search), abstraction of data, analysis of data and editing of the review.
The searches for the 2011 update were completed by SS, AO, VS. The administrative update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Diane Haughton, and Roger Soll).
The literature searches for the 2017 update were conducted by Jennifer Spano, Information Specialist, Cochrane Neonatal.
This 2017 update was reviewed and approved by SS, AO, VS, HH.
Sources of support
Internal sources
Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
No sources of support supplied
Declarations of interest
Sachin S Shah, no conflict of interest to declare.
Arne Ohlsson, no conflict of interest to declare.
Henry L Halliday, is the author of an included trial. He did not assess the quality of his own trial.
Vibhuti S Shah, no conflict of interest to declare.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Halliday 2001 {published data only}
- Halliday HL, Patterson CC, Halahakoon CW, European Multicenter Steroid Study Group. A multicenter, randomized open study of early corticosteroid treatment (OSECT) in preterm infants with respiratory illness: comparison of early and late treatment and of dexamethasone and inhaled budesonide. Pediatrics 2001;107(2):232‐40. [DOI] [PubMed] [Google Scholar]
- Wilson TT. Long Term Neurodevelopmental and Psychosocial Outcomes Following Premature Birth: Has Postnatal Corticosteroid Treatment Been an Over‐looked Factor? [PhD thesis]. Belfast (UK): Queen’s University Belfast, 2005. [Google Scholar]
- Wilson TT, Waters L, Patterson CC, McCusker CG, Rooney NM, Marlow N, et al. Neurodevelopmental and respiratory follow‐up results at 7 years for children from the United Kingdom and Ireland enrolled in a randomized trial of early and late postnatal corticosteroid treatment, systemic and inhaled (the Open Study of Early Corticosteroid Treatment). Pediatrics 2006;117(6):2196‐205. [DOI] [PubMed] [Google Scholar]
Rozycki 2003 {published data only}
- Rozycki HJ, Byron PR, Elliott GR, Carroll T, Gutcher GR. Randomized controlled trial of three different doses of aerosol beclomethasone versus systemic dexamethasone to promote extubation in ventilated premature infants. Pediatric Pulmonology 2003;35(5):375‐83. [DOI] [PubMed] [Google Scholar]
Suchomski 2002 {published data only}
- Suchomski SJ, Cummings JJ. A randomised trial of inhaled versus intravenous steroids in ventilator dependent preterm infants. Journal of Perinatology 2002;22(3):196‐203. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dimitriou 1997 {published data only}
- Dimitriou G, Greenhough A, Giffin FJ, Kavadia V. Inhaled versus systemic steroids in chronic oxygen dependency of preterm infants. European Journal of Pediatrics 1997;156(1):51‐5. [DOI] [PubMed] [Google Scholar]
Nicholl 2002 {published data only}
- Nicholl RM, Greenough A, King M, Cheeseman P, Gamsu HR. Growth effects of systemic versus inhaled steroids in chronic lung disease. Archives of Disease in Childhood 2002;87(1):F59‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAP & CPS 2002
- American Academy of Pediatrics, Committee on Fetus and Newborn, Canadian Paediatric Society, Fetus and Newborn Committee. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 2002;109(2):330–8. [DOI] [PubMed] [Google Scholar]
Arnon 1992
- Arnon S, Grigg J, Nikander K, Silverman M. Delivery of micronized budesonide suspension by metered dose inhaler and jet nebulizer into a neonatal ventilator circuit. Pediatric Pulmonology 1992;13(3):172‐5. [DOI] [PubMed] [Google Scholar]
Avery 1985
- Avery GB, Fletcher AB, Kaplan M, Brudno DS. Controlled trial of dexamethasone in respirator‐dependent infants with bronchopulmonary dysplasia. Pediatrics 1985;75(1):106‐11. [PubMed] [Google Scholar]
Bahadue 2012
- Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD001456.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassler 2015
- Bassler D, Plavka R, Shinvell ES, Hallman M, Jarreau PH, Carnielli V, et al. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. New England Journal of Medicine 2015;573(16):1497‐506. [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotising enterocolitis: Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhuta 1998
- Bhuta T, Ohlsson A. Systematic review and meta‐analysis of early postnatal dexamethasone for prevention of chronic lung disease. Archives of Disease in Childhood 1998;79(1):26‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDTG 1991
- Collaborative Dexamethasone Trial Group. Dexamethasone therapy in neonatal chronic lung disease: an international placebo controlled trial. Pediatrics 1991;88(3):421‐7. [PubMed] [Google Scholar]
Cummings 1989
- Cummings JJ, D'Eugenio DB, Gross SJ. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. New England Journal of Medicine 1989;320(23):1505‐10. [DOI] [PubMed] [Google Scholar]
Davis 2001
- Davis PG, Henderson‐Smart DJ. Intravenous dexamethasone for extubation of newborn infants. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000308] [DOI] [PubMed] [Google Scholar]
Doyle 2014
- Doyle LW, Halliday HL, Ehrenkranz RA. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD001146.pub4] [DOI] [PubMed] [Google Scholar]
Doyle 2014a
- Doyle LW, Halliday HL, Ehrencranz RA. Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD001145.pub3] [DOI] [PubMed] [Google Scholar]
Doyle 2017
- Doyle LW, Cheong JLY. Postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Who might benefit?. Seminars in Fetal & Neonatal Medicine 2017;22(5):290‐5. [DOI: 10.1016/j.siny.2017.07.003; PUBMED: 28734731] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 23 February 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grigg 1992
- Grigg J, Arnon S, Jones T, Clarke A, Silverman M. Delivery of therapeutic aerosols to intubated babies. Archives of Disease in Childhood 1992;67(1 Spec No):25‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2000
- Gupta GK, Cole CH, Abbasi S, Demissie S, Nijnimbam C, Nielsen HC. Effects of early inhaled beclomethasone therapy on tracheal aspirate inflammatory mediators IL8 and IL‐Ira in ventilated preterm infants at risk for bronchopulmonary dysplasia. Pediatric Pulmonology 2000;30(4):275‐81. [DOI] [PubMed] [Google Scholar]
Halliday 1999
- Halliday HL. Clinical trials of postnatal steroids: inhaled and systemic. Biology of the Neonate 1999;76(Suppl 1):29‐40. [DOI] [PubMed] [Google Scholar]
Halliday 2001a
- Halliday HL. Guidelines on neonatal steroids. Prenatal and Neonatal Medicine 2001;6(6):371‐3. [Google Scholar]
Harkavy 1989
- Harkavy KL, Scanlon JW, Chowdhry PK, Grylack LJ. Dexamethasone therapy for chronic lung disease in ventilator‐and‐oxygen‐ dependent infants: a controlled trial. Journal of Pediatrics 1989;115(6):979‐83. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Cochrane Collaboration.
Ibrahim 2011
- Ibrahim H, Sinha IP, Subhedar NV. Corticosteroids for treating hypotension in preterm infants. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003662.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICROP 1984
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Archives of Ophthalmology 1984;102:1130‐4. [DOI] [PubMed] [Google Scholar]
Jefferies 2012
- Jefferies AL. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Journal of Paediatrics and Child Health 2012;17(10):573‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1979
- Johnson JW, Mitzner W, London WT, Palmer AE, Scott R. Betamethasone and the rhesus fetus: multisystemic effects. American Journal of Obstetrics and Gynecology 1979;133(6):677‐84. [DOI] [PubMed] [Google Scholar]
Kazzi 1990
- Kazzi NJ, Brans YW, Poland RL. Dexamethasone effects on the hospital course of infants with bronchopulmonary dysplasia who are dependent on artificial ventilation. Pediatrics 1990;86(5):722‐7. [PubMed] [Google Scholar]
Kelly 2017
- Kelly EN, Shah VS, Levenbach J, Vincer M, DaSilva O, Shah PS, Canadian Neonatal Network and Canadian Neonatal Follow‐Up Network Investigators. Inhaled and systemic steroid exposure and neurodevelopmental outcome of preterm neonates. Journal of Maternal‐fetal & Neonatal Medicine 2017 July 16 [Epub ahead of print]. [DOI: 10.1080/14767058.2017.1350644; PUBMED: 28714339] [DOI] [PubMed]
Lee 2000
- Lee SK, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU Network: 1996‐1997. Pediatrics 2000;106(5):1070‐9. [DOI] [PubMed] [Google Scholar]
Lemons 2001
- Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001;107(1):E1. [DOI] [PubMed] [Google Scholar]
Nelin 2017
- Nelin LD, Logan JW. The use of inhaled corticosteroids in chronically ventilated preterm infants. Seminars in Fetal & Neonatal Medicine 2017;22(5):296‐301. [DOI: 10.1016/j.siny.2017.07.005; PUBMED: 28768578] [DOI] [PubMed] [Google Scholar]
Ng 1993
- Ng PC. The effectiveness and side effects of dexamethasone in preterm infants with bronchopulmonary dysplasia. Archives of Disease in Childhood 1993;68(3 Spec No):330‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Callaghan 1992
- O'Callaghan C, Hardy J, Stammers J, Stephensen T, Hull D. Evaluation of techniques for delivery of steroids to lungs of neonates using a rabbit model. Archives of Disease in Childhood 1992;67(1 Spec No):20‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Shea 1999
- O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG 3rd, et al. Randomized placebo‐controlled trial of a 42 day tapering course of dexamethasone to reduce the duration of ventilatory dependency in very low birth weight infants: outcomes of study participants at 1‐year adjusted age. Pediatrics 1999;104(1 Pt 1):15‐21. [DOI] [PubMed] [Google Scholar]
Ohlsson 1992
- Ohlsson A, Calvert SA, Hosking M, Shennan AT. Randomized controlled trial of dexamethasone treatment in very‐low‐birth‐weight infants with ventilatory dependent chronic lung disease. Acta Paediatrica 1992;81(10):751‐6. [DOI] [PubMed] [Google Scholar]
Onland 2017a
- Onland W, Offringa M, Kaam A. Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD002311.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Onland 2017b
- Onland W, Jaegere APMC, Offringa M, Kaam A. Systemic corticosteroid regimens for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD010941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 grams. Journal of Pediatrics 1978;92(4):529‐34. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2017
- Roberts D, Brown J, Medley N, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD004454.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saarela 1999
- Saarela T, Vaarala A, Lanning P, Koivisto M. Incidence, ultrasonic patterns and resolution of nephrocalcinosis in very low birth‐weight infants. Acta Paediatrica 1999;86(6):655‐60. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (eds), GRADE Working Group. GRADE Handbook 2013. gdt.gradepro.org/app/handbook/handbook.html (accessed prior to 8 September 2017).
Shah 2001
- Shah V, Ohlsson A. Postnatal dexamethasone in the prevention of chronic lung disease. In: David TJ editor(s). Recent Advances in Paediatrics. London, England: Churchill Livingstone, 2001:77‐96. [Google Scholar]
Shah 2012
- Shah SS, Ohlsson A, Halliday H, Shah VS. Inhaled versus systemic corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD002058.pub2] [DOI] [PubMed] [Google Scholar]
Shah 2017
- Shah VS, Ohlsson A, Halliday HL, Dunn M. Early administration of inhaled corticosteroids for preventing chronic lung disease in very low birth weight preterm neonates. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD001969.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinwell 2000
- Shinwell ES, Karplus M, Reich D, Weintraub Z, Blaazer S, Bader D, et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Archives of Disease in Childhood Fetal Neonatal Edition 2000;83(3):F177‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinwell 2016
- Shinwell ES, Portnov I, Meerpohl JJ, Karen T, Bassler D. Inhaled corticosteroids for bronchopulmonary dysplasia: A meta‐analysis. Pediatrics 2016;138(6):e20162511. [DOI] [PubMed] [Google Scholar]
Sinkin 1990
- Sinkin RA, Cox C, Phelps DL. Predicting risk for bronchopulmonary dysplasia: selection criteria for clinical trials. Pediatrics 1990;86(5):728‐36. [PubMed] [Google Scholar]
Soll 1998
- Soll RF. Synthetic surfactant for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD001149] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stark 2001
- Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran EF, et al. Adverse effects of early dexamethasone treatment in extremely‐low‐birth‐weight infants. National Institute of Child Health and Human Development Neonatal Research Network. New England Journal of Medicine 2001;344(2):95‐101. [DOI] [PubMed] [Google Scholar]
Ward 2003
- Ward MC, Sinn JKH. Steroid therapy for meconium aspiration syndrome in newborn infants. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003485] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watterberg 2010
- Watterberg KL, American Academy of Pediatrics. Committee of Fetus and Newborn. Policy statement ‐ postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010;126(4):800‐8. [DOI] [PubMed] [Google Scholar]
Weichsel 1977
- Weichsel ME Jr. The therapeutic use of glucocorticoid hormones in the perinatal period: potential neurologic hazards. Annals of Neurology 1977;2(5):364‐6. [DOI] [PubMed] [Google Scholar]
Yeh 1998
- Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow up study. Pediatrics 1998;101(5):E7. [DOI] [PubMed] [Google Scholar]
Zubrow 1995
- Zubrow A, Hulman S, Kushner H, Falkner B. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Philadelphia Neonatal Blood Pressure Study Group. Journal of Perinatology 1995;15(6):470‐9. [PubMed] [Google Scholar]
References to other published versions of this review
Shah 2003b
- Shah SS, Ohlsson A, Halliday H, Shah VS. Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD002057] [DOI] [PubMed] [Google Scholar]
Shah 2012a
- Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD002057.pub3] [DOI] [PubMed] [Google Scholar]


