Abstract
Background
Asthma is a chronic inflammatory disease that affects the airways and is common in both adults and children. It is characterised by symptoms including wheeze, shortness of breath, chest tightness, and cough. People with asthma may be helped to manage their condition through shared decision‐making (SDM). SDM involves at least two participants (the medical practitioner and the patient) and mutual sharing of information, including the patient's values and preferences, to build consensus about favoured treatment that culminates in an agreed action. Effective self‐management is particularly important for people with asthma, and SDM may improve clinical outcomes and quality of life by educating patients and empowering them to be actively involved in their own health.
Objectives
To assess benefits and potential harms of shared decision‐making for adults and children with asthma.
Search methods
We searched the Cochrane Airways Trials Register, which contains studies identified in several sources including CENTRAL, MEDLINE, and Embase. We also searched clinical trials registries and checked the reference lists of included studies. We conducted the most recent searches on 29 November 2016.
Selection criteria
We included studies of individual or cluster parallel randomised controlled design conducted to compare an SDM intervention for adults and children with asthma versus a control intervention. We included studies available as full‐text reports, those published as abstracts only, and unpublished data, and we placed no restrictions on place, date, or language of publication. We included interventions targeting healthcare professionals or patients, their families or care‐givers, or both. We included studies that compared the intervention versus usual care or a minimal control intervention, and those that compared an SDM intervention against another active intervention. We excluded studies of interventions that involved multiple components other than the SDM intervention unless the control group also received these interventions.
Data collection and analysis
Two review authors independently screened searches, extracted data from included studies, and assessed risk of bias. Primary outcomes were asthma‐related quality of life, patient/parent satisfaction, and medication adherence. Secondary outcomes included exacerbations of asthma, asthma control, acceptability/feasibility from the perspective of healthcare professionals, and all adverse events. We graded and presented evidence in a 'Summary of findings' table.
We were unable to pool any of the extracted outcome data owing to clinical and methodological heterogeneity but presented findings in forest plots when possible. We narratively described skewed data.
Main results
We included four studies that compared SDM versus control and included a total of 1342 participants. Three studies recruited children with asthma and their care‐givers, and one recruited adults with asthma. Three studies took place in the United States, and one in the Netherlands. Trial duration was between 6 and 24 months. One trial delivered the SDM intervention to the medical practitioner, and three trials delivered the SDM intervention directly to the participant. Two paediatric studies involved use of an online portal, followed by face‐to‐face consultations. One study delivered an SDM intervention or a clinical decision‐making intervention through a mixture of face‐to‐face consultations and telephone calls. The final study randomised paediatric general practice physicians to receive a seminar programme promoting application of SDM principles. All trials were open‐label, although one study, which delivered the intervention to physicians, stated that participants were unaware of their physicians' involvement in the trial. We had concerns about selection and attrition bias and selective reporting, and we noted that one study substantially under‐recruited participants. The four included studies used different approaches to measure fidelity/intervention adherence and to report study findings.
One study involving adults with poorly controlled asthma reported improved quality of life (QOL) for the SDM group compared with the control group, using the Asthma Quality of Life Questionnaire (AQLQ) for assessment (mean difference (MD) 1.90, 95% confidence interval (CI) 1.24 to 2.91), but two other trials did not identify a benefit. Patient/parent satisfaction with the performance of paediatricians was greater in the SDM group in one trial involving children. Medication adherence was better in the SDM group in two studies ‐ one involving adults and one involving children (all medication adherence: MD 0.21, 95% CI 0.11 to 0.31; mean number of controlled medication prescriptions over 26 weeks: 1.1 in the SDM group (n = 26) and 0.7 in the control group (n = 27)). In one study, asthma‐related visit rates were lower in the SDM group than in the usual care group (1.0/y vs 1.4/y; P = 0.016), but two other studies did not report a difference in exacerbations nor in prescriptions for short courses of oral steroids. Finally, one study described better odds of reporting no asthma problems in the SDM group than in the usual care group (odds ratio (OR) 1.90, 95% CI 1.26 to 2.87), although two other studies reporting asthma control did not identify a benefit with SDM. We found no information about acceptability of the intervention to the healthcare professional and no information on adverse events. Overall, our confidence in study results ranged from very low to moderate, and we downgraded outcomes owing to risk of bias, imprecision, and indirectness.
Authors' conclusions
Substantial differences between the four included randomised controlled trials (RCTs) indicate that we cannot provide meaningful overall conclusions. Individual studies demonstrated some benefits of SDM over control, in terms of quality of life; patient and parent satisfaction; adherence to prescribed medication; reduction in asthma‐related healthcare visits; and improved asthma control. Our confidence in the findings of these individual studies ranges from moderate to very low, and it is important to note that studies did not measure or report adverse events.
Future trials should be adequately powered and of sufficient duration to detect differences in patient‐important outcomes such as exacerbations and hospitalisations. Use of core asthma outcomes and validated scales when possible would facilitate future meta‐analysis. Studies conducted in lower‐income settings and including an economic evaluation would be of interest. Investigators should systematically record adverse events, even if none are anticipated. Studies identified to date have not included adolescents; future trials should consider their inclusion. Measuring and reporting of intervention fidelity is also recommended.
Plain language summary
Can shared decision‐making between the patient and the healthcare professional help people with asthma?
Background to the question
Asthma is a long‐term disease that is common in adults and children. People with asthma often wheeze, cough, and have difficulty breathing. Shared decision‐making means fully involving individuals with asthma in decisions about their care. It usually involves the patient and his or her doctor or nurse, and key features include sharing information to help individuals with asthma make the best decisions for themselves. By including individuals with asthma in the decision‐making process, it is hoped that their asthma will be better controlled and will cause them fewer problems.
Review question
We wanted to review the evidence on shared decision‐making for people with asthma compared with standard asthma care, or a different way of making healthcare decisions. We wanted to know if shared decision‐making has an effect on quality of life, asthma attacks, patient satisfaction with care, asthma control, sticking to medication plans, and unwanted effects.
Study characteristics
We reviewed the evidence up to November 2016. We found four studies, including 1342 people, that attempted to answer this question. All participants had asthma; participants in three studies were children and those in one study were adults. Three studies took place in the United States and one in the Netherlands; studies lasted from six months to two years. Different studies used different methods of shared decision‐making, including face‐to‐face discussions, telephone calls, and online messages.
Key results
Because these studies were conducted in different ways, we were unable to combine their findings. We found evidence from individual studies indicating that shared decision‐making may improve quality of life and asthma control and may reduce healthcare visits for asthma. Shared decision‐making may also help people to take their asthma inhaler(s) more regularly owing to better understanding of why they need to do that. Going through this process may make people feel more satisfied with their care, as they may feel empowered about making choices. However, all of these findings were reported by different studies, and some studies showed benefit of shared decision‐making, while others did not. It is important to mention that none of these studies looked into whether shared decision‐making causes unwanted side effects. All four studies measured how well the shared decision‐making intervention had been delivered or received but did this in different ways.
Quality of the evidence
We were not very confident in the quality of the evidence presented in this review. We were concerned about the small number of studies and about differences in the way included studies were designed. Also, participants knew which group they were in (i.e. shared decision‐making or standard care), and this may have affected how they answered questions about their asthma during the trial.
Take‐home message
Some evidence suggests that shared decision‐making might help people with asthma, but we are not sure whether it is helpful. In the future, larger studies that include adolescents while looking out for side effects, harms, and benefits should prove useful in answering this question.
Summary of findings
Summary of findings for the main comparison. Shared decision‐making compared with usual care for people with asthma.
| Shared decision‐making compared with usual care for people with asthma | |||||||
| Patient or population: adults and children with asthma Setting: primary care/outpatient clinics Intervention: shared decision‐making Comparison: usual care | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Risk with usual care | Risk with shared decision‐making | ||||||
| Asthma‐related quality of life (follow‐up: 6 to 24 months) |
AQLQ responders | 556 per 1000 | 704 per 1000 (608 to 784) | OR 1.90 (1.24 to 2.91) | 371 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | Participants achieving > 0.5‐point improvement (MCID for this scale) |
| ITG‐ASF daytime symptom scale | Mean ITG‐ASF daytime symptom score was 12 | MD 4 higher (3.54 lower to 11.54 higher) | ‐ | 53 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | Higher score = Better quality of life The same study also reported mean night‐time symptom scale and functional limitation scale (see Analysis 1.2). |
|
| Mini‐AQLQ | Mini‐AQLQ score was 5.5 | MD 0.4 higher (0.18 higher to 0.62 higher) | ‐ | 371 (1 RCT) | ⊕⊕⊝⊝ LOWa,c,d | Higher score = Better quality of life. MCID 0.5 | |
| Parent/patient satisfaction | Presentation on forest plot not possible; summarised narratively in text and Table 3 | ‐ | ‐ | ‐ | |||
| Medication adherence (follow‐up: 12 to 24 months) |
ICS only | The ICS adherence was 0.59 | MD 0.22 higher (0.11 higher to 0.33 higher) | ‐ | 371 (1 RCT) | ⊕⊕⊕⊝ MODERATEe | Adherence calculated using continuous medication acquisition (CMA) from pharmacy data. Maximum score 1. The same study reported all‐medication adherence (see Analysis 1.4). |
| Exacerbations of asthma (follow‐up: 6 months) |
Requiring ED visit | 222 per 1,000 | 77 per 1,000 (14 to 314) | OR 0.29 (0.05 to 1.60) | 53 (1 RCT) | ⊕⊕⊝⊝ LOWf | The same study reported exacerbations requiring hospital admission, "specialist visits", and GP visits (see Analysis 1.5). |
| Asthma control (follow‐up: 12 to 24 months) |
Asthma well controlled; ATAQ = 0 | No control group risk presented | Not estimable | OR 1.90 (1.26 to 2.87) | 371 (1 RCT) |
⊕⊕⊕⊝ MODERATEa | Lower score = Better asthma control A different small study reported asthma control on ACT and ACQ (see Analysis 1.6). |
| Adverse events (all) | Included trials did not measure or report any adverse events. | ‐ | ‐ | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; AQLQ: asthma quality of life questionnaire; ATAQ: Asthma Therapy Assessment Questionnaire CI: confidence interval; ED: emergency department; GP: general practitioner; ICS: inhaled corticosteroid; ITG‐ASF: Integrated Therapeutics Group ‐ Child Asthma Short Form; MCID: mean clinically important difference; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||||
aRisk of performance and detected bias. Downgraded once.
bOne study. Confidence intervals include possible harm and benefit of intervention. Downgraded once.
cOnly quality of life subscales reported. Downgraded once for indirectness.
dAlthough the mean difference for this scale lies below the MCID, the responder analysis suggests that significantly more people achieved the MCID change with the intervention. No downgrade.
eAdherence calculated using continuous medication acquisition from pharmacy data. This is a proxy measure and may overestimate true adherence. Downgraded once.
fOne study. Confidence intervals very wide and include possible harm and benefit of intervention. Downgraded twice.
Background
Description of the condition
Asthma is a chronic disease that affects the airways. It is usually characterised by chronic inflammation of the airways, which causes wheeze, shortness of breath, chest tightness, cough, and variable airflow limitation (GINA 2016). Symptoms vary significantly in nature, frequency, and severity, both within and between individuals with a diagnosis of asthma. Day‐to‐day symptoms vary according to the presence of external stimuli (e.g. exercise, allergens), and people with asthma can experience flare‐ups or 'exacerbations', which are associated with significant morbidity and mortality worldwide (GINA 2016; Global Asthma Network 2014; NRAD 2014). Long‐term goals of asthma management include maintaining control of symptoms and minimising risk of exacerbations, airflow limitation, and treatment side effects (GINA 2016). Educating adults and children to self‐manage their asthma is widely recognised as integral to achieving these goals (Gibson 2002; Guevara 2003).
Description of the intervention
Shared decision‐making (SDM) should involve at least two participants (the medical practitioner and the patient) and is defined as mutual sharing of information to build consensus about preferred treatment that culminates in an agreed action (Charles 1997). Decisions about management of long‐term conditions are based on a multitude of factors, including relative efficacy and safety of treatments, costs, and palatability. Shared decision‐making provides a way of balancing these factors by considering the values and preferences of the patient and the opinions of healthcare providers. Légaré describes the three essential elements of SDM as follows (Légaré 2013).
Recognizing and acknowledging that a decision is required.
Knowing and understanding the best available evidence.
Incorporating the patient's values and preferences into all decisions.
For asthma, management guidelines increasingly acknowledge the role of "the patient and healthcare provider partnership" for a shared‐care approach (GINA 2016). Interventions provided to encourage patient‐centred care in clinical consultations across a range of conditions generally put the onus on the healthcare provider; some seek to offer a pathway for patients or parents to better engage in their asthma care; and others suggest a combination of these approaches (Dwamena 2012; Fiks 2015; Wilson 2010). Thus different approaches may have different aims and outcomes. Interventions aimed at changing healthcare provider behaviour might include open communications, efforts to identify and address patient and family concerns about asthma and its treatment, discussion of treatment preferences and barriers to implementation, shared development of treatment goals, and encouragement of active self‐assessment and self‐management (NHLBI/NAEPP 2007).
How the intervention might work
The potential benefit of SDM is dependent on the willingness and ability of both sides to interact, and this ability might depend on factors such as "ethnicity, literacy, understanding of health concepts (health literacy), numeracy, beliefs about asthma and medications, desire for autonomy, and the health care system" (GINA 2016). As such, SDM will not necessarily be equally acceptable to all patients or care‐givers and may not be applied in the same way across healthcare contexts. Benefits of SDM may be seen for individuals and more widely for health services and society as enhanced uptake of evidence‐based options and reduction in overuse of options that confer minimal benefit, thus reducing practice and geographic variations in care and avoiding unnecessary expenditures (Coulter 2011; Légaré 2014).
Preferences for an active, collaborative, or passive role in decision‐making vary among populations, but patient roles are often passive, and many patients report that they wish to be more involved (Caress 2005; Sleath 2011). Patient preferences for involvement in decision‐making are related to education level, perceptions of the healthcare provider, financial barriers to receiving appropriate care, and psychosocial factors, but preferences have not been strongly associated with demography or asthma severity (Adams 2001; Caress 2005). Nonetheless, evidence regarding how best to achieve SDM in practice is sparse, especially in paediatric asthma with regards to the child‐parent relationship and adapted emphasis on SDM as the child matures (Rivera‐Spoljaric 2014).
Researchers have highlighted organisational factors that may serve as a barrier to feelings of satisfaction among patients or families regarding the role they play in their asthma care, especially quality and duration of consultations, which vary substantially across healthcare contexts (Caress 2005). A narrative synthesis of the fast‐growing trend toward patient involvement in medicine has identified that the preparedness of service systems can enable successful SDM, alongside empowerment, patient education, communication for involvement, and staff training (Snyder 2016). It is possible that engaging in SDM may cause unintended harms, for example, by allowing a patient to choose an option without proper discussion of harms and benefits, so it is important that staff are appropriately trained, and that decision aids are used correctly (Coulter 2011).
Why it is important to do this review
Shared decision‐making (SDM) may improve clinical outcomes and quality of life by educating and empowering patients to be actively involved in their own health (Butz 2007; Wilson 2010). These interventions may be particularly beneficial in people with asthma, as self‐management behaviours are important for, and make SDM particularly relevant to, the population with asthma (Gibson 2002; Guevara 2003). The US Institute of Medicine has prioritised SDM, and Asthma UK has identified methods to "empower and enable people to take control of their own asthma" as a research priority (Asthma UK 2011; Institute of Medicine 2009).
A recent Cochrane review found 43 studies that tested effects of interventions to encourage patient‐centred care in clinical consultations, and found mixed results in terms of patient satisfaction, health behaviour, and health status (Dwamena 2012). Review authors suggested that complex interventions with condition‐specific materials aimed at both providers and patients might be promising, but acknowledge that evidence was limited at the time. Similarly, Légaré focused on interventions aimed at improving uptake of SDM by healthcare professionals across medical disciplines with a primary focus on how well this is adopted in practice (Légaré 2014). Review of available evidence for SDM in asthma will allow us to conduct wider searches of the asthma literature to find additional studies and to focus on important condition‐specific outcomes. Attention to clinical outcomes is particularly important, given the possible tension between SDM and adherence to clinical guidelines. Growth of SDM research means it is likely that new evidence will have been published since the time existing reviews were prepared.
Objectives
To assess benefits and potential harms of shared decision‐making for adults and children with asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that used individual or cluster randomisation. We planned to exclude cross‐over trials; however, we will include the first phase of cross‐over trials in future versions of the review. We did not identify any relevant cross‐over studies. We excluded non‐randomised studies because they would restrict our ability to imply causation of intervention effects and are more likely to be subject to selection biases and confounders. However, we summarised narratively any non‐randomised evidence identified by our searches and contrasted this summary with results presented in our discussion. We planned to include studies reported as full‐text articles, those published as abstracts only, and unpublished data.
Types of participants
We included studies of adults and children with a diagnosis of asthma, confirmed by a medical practitioner or by spirometry according to guidelines (e.g. GINA 2016). We excluded studies that included participants with other long‐term conditions, in particular, chronic obstructive pulmonary disease (COPD), unless researchers presented separate results for those with asthma. We also excluded studies looking at shared decision‐making (SDM) in asthma specifically for people with cognitive impairments, as these interventions are likely to have a different focus. If a study included a subset of eligible participants (e.g. a mixed population that includes participants with other health conditions), we included it only if we could analyse separately disaggregated data for eligible participants.
Types of interventions
We included studies that assessed SDM interventions for people with asthma. We included interventions aimed at healthcare professionals (specialists, general practitioners, nurses, pharmacists, etc.), patients and their families or care‐givers, or both. We included studies that compared the intervention against usual care or a minimal control intervention and those compared an SDM intervention versus another active intervention, such as clinical decision‐making. We excluded studies of interventions that involved multiple components other than the SDM intervention unless the control group also received these components.
Types of outcome measures
Primary outcomes
Asthma‐related quality of life (on a validated scale e.g. Asthma Quality of Life Questionnaire (AQLQ))
Patient/parent satisfaction
Medication adherence
Secondary outcomes
Exacerbations of asthma (leading to a course of oral corticosteroid (OCS) treatment or an unscheduled visit to a healthcare professional)
Asthma control (e.g. Asthma Control Questionnaire (ACQ))
Acceptability/feasibility from the perspective of healthcare professionals
Adverse events (all)
Reporting one or more of the outcomes listed here was not a criterion for inclusion of studies in this review.
Trial authors and editorial teams chose primary outcomes by consensus as those most likely to be relevant to the intervention under investigation and most important to patients and their families/care‐givers.
We prioritised extraction of any validated measures of patient/parent satisfaction, medication adherence, asthma control, and acceptability/feasibility but did not predefine accepted measures in advance, so as not to restrict analyses unnecessarily. If study authors used non‐validated measures, or used a mixture of validated and non‐validated measures across studies, we planned to assess which were sufficiently similar for pooling to make sense.
We planned to extract and analyse data from both parent and child perspectives as provided by paediatric studies.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
Weekly searches of MEDLINE Ovid SP 1946 to date.
Weekly searches of Embase Ovid SP 1974 to date.
Monthly searches of PsycINFO Ovid SP.
Monthly searches of the Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO).
Monthly searches of the Allied and Complementary Medicine database (AMED EBSCO).
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register were identified through search strategies based on the scope of Cochrane Airways. We have presented in Appendix 1 details of these strategies, as well as a list of handsearched conference proceedings.See Appendix 2 for search terms used to identify studies for this review. We based our search terms for 'shared decision‐making' on those used in a Cochrane Review by Légaré (Légaré 2014).
We also conducted a search of ClinicalTrials.gov (http://ClinicalTrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; http://who.int/ictrp/en/). We searched all databases from their inception to the present, and we imposed no restriction on language of publication. We conducted the most recent searches on 29 November 2016.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references.
On 15 November 2016, we searched for errata or retractions from included studies published in full text on PubMed (http://ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (KK and RN) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text study reports/publications for all studies in the 'retrieve' category. Two review authors (KK and PM) independently screened full‐text articles and identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion; if required, we consulted a third person. We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram (Figure 1) and Characteristics of excluded studies tables (Moher 2009).
1.
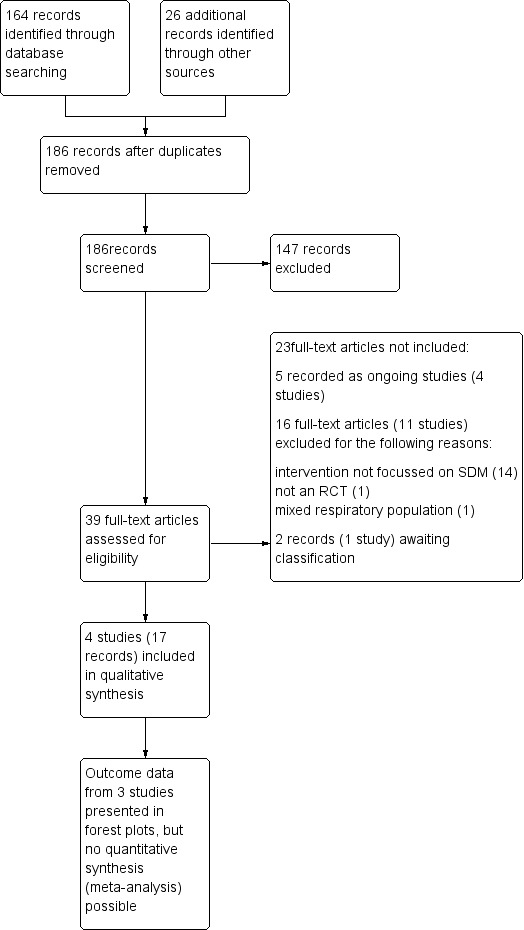
Study flow diagram.
Data extraction and management
We used a data collection form piloted on one included study to record study characteristics and outcome data. One review author (KK) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, and dates of the study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (KK and RN) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if a study reported outcome data that were not useable in an analysis. We resolved disagreements by reaching consensus or by involving a third person. One review author (KK) transferred data into the Review Manager (RevMan) file (RevMan 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review versus data provided in the study reports.
Assessment of risk of bias in included studies
Two review authors (KK and RN) independently assessed risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with a third person. We assessed the risk of bias of each included study according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for each study that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and reported deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios and continuous data as mean differences. Had we been able to combine data presented on different scales, we planned to use standardised mean differences. We entered data presented as a scale with a consistent direction of effect.
We planned to undertake meta‐analyses only when this was meaningful (i.e. if treatments, participants, and the underlying clinical question were similar enough for pooling to make sense).
We narratively described skewed data reported as medians and interquartile ranges.
When a single study reported multiple trial arms, we planned to include only the relevant arms. If we had combined two comparisons (e.g. two types of SDM vs usual care) in the same meta‐analysis, we planned to halve the control group to avoid double counting.
If both change from baseline and endpoint scores were available for continuous data, we planned to use change from baseline unless most studies reported endpoint scores. If a study reported outcomes at multiple time points, we used the end‐of‐study measurement.
If both an analysis that included only participants who completed the trial and an analysis that imputed data for participants who were randomly assigned but did not provide endpoint data (e.g. last observation carried forward) were available, we planned to use the latter.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of people admitted with one or more exacerbation, rather than number of exacerbations per person). We planned to meta‐analyse data from cluster RCTs only if available data had been adjusted (or could be adjusted) to account for clustering.
Dealing with missing data
We planned to contact investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when we identify a study as an abstract only). However, we identified full‐text reports of all included studies.
Assessment of heterogeneity
We planned to use the I² statistic to measure heterogeneity among the studies in each analysis. If we had identified substantial heterogeneity, we planned to report this and to explore possible causes by conducting prespecified subgroup analyses.
Assessment of reporting biases
We were not able to pool more than 10 studies, so we could not create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We planned to use a random‐effects model and to perform a sensitivity analysis using a fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table using the outcomes listed in this review. We used the five Grading of Recommendations Assessment, Development and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to meta‐analyses for prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we used the GRADEpro Guideline Development Tool (GRADEpro GDT). We used footnotes to justify all decisions to downgrade or upgrade the quality of the evidence, and we made comments to aid the reader's understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analysesa for primary outcomes.
Age of the asthma population (children < 12 years of age, 12 to 18 years of age, adults > 18 years of age).
Focus of the intervention (i.e. population randomised to the intervention: healthcare providers vs patients/parents).
Duration/extensiveness of intervention (e.g. one‐off or simple intervention vs ongoing SDM sessions).
aChildren, adolescents, and adults may have quite different needs and preferences with respect to SDM, so interventions may have different focuses and effects across age groups. We expected study effects to vary regarding focus and extent of the intervention, and we tried to assess this in the other two subgroup analyses. However, a subgroup analysis can look at only one of these effect modifiers at a time and does not imply causation; therefore, we planned to interpret the results cautiously. We presented these and other possible effect modifiers in Table 2.
1. Summary of study characteristics.
| Study ID | Country | Population | Age (years) | Design | Intervention | Aimed at | Control |
| Clark 1998 | USA | 74 physicians; 637 children | 1 to 12 | Cluster RCT | SDM seminars | HCPs | Usual care |
| Fiks 2015 | USA | 60 families | 6 to 12 | Individual RCT | SDM portal | HCPs and patients/parents | Usual care + decision support |
| van Bragt 2015 | Holland | 33 children | 6 to 12 | Cluster RCT | SDM online tool | HCPs and patients/parents | Enhanced usual care |
| Wilson 2010 | USA | 612 adults | 18 to 65 | Individual RCT | SDM structured sessions | HCPs | 1. Guideline‐led decision‐making 2. Usual care |
HCP: healthcare provider; RCT: randomised controlled trial; SDM: shared decision‐making.
We planned to use the formal test for subgroup interactions provided in RevMan (RevMan 2014).
Sensitivity analysis
We planned to perform the following sensitivity analyses by removing the following from the primary analyses.
Unpublished data.
Studies at high risk in any selection bias domain.
Results
Description of studies
Results of the search
We identified 152 records in main database searches (including a search of clinicaltrials.gov), 21 from the WHO trials portal, four from reference lists of included studies, and one through author correspondence. We found that four were duplicates, and we screened the remaining 174 records. We excluded 137 records that did not meet review inclusion criteria by looking at titles and abstracts, and we obtained full texts for the 37 remaining records. After reviewing full texts, we deemed that 21 records were ineligible for inclusion in the review: 16 because they did not meet the inclusion criteria and five because they were ongoing studies (related to four studies: Federman 2015; Hoskins 2013; NCT02516449; Tapp 2011). We collated the 16 excluded records into 11 unique studies, which we have described under Excluded studies. We collated the other 17 records into four unique studies and included them in the review (Figure 1).
We conducted a further search on 27 June 2017 before preparation of this publication. One study investigating the use of decision aids may meet the inclusion criteria for this review, and we will fully assess this trial for inclusion when we update the review (Studies awaiting classification).
Included studies
Four studies, including a total of 1342 participants, met the inclusion criteria for this review (Clark 1998; Fiks 2015; van Bragt 2015; Wilson 2010). We have presented a summary of study characteristics in Table 2. We have provided more information about each study's design, setting, inclusion criteria, population and intervention, and risk of bias assessments in the Characteristics of included studies tables.
Study design and setting
Wilson individually randomised 612 adults with asthma across five US clinical Kaiser Permanante (KP; a large not‐for‐profit integrated managed care consortium) sites (Wilson 2010). The three remaining studies involved children and their families. Clark cluster‐randomised 74 US general practice paediatricians, with 637 children enrolled under their care, in Michigan and New York State (Clark 1998). Fiks individually randomised 60 families of children with asthma across three primary care practices in Philadelphia (Fiks 2015). Finally, van Bragt randomised five outpatient clinics in the Netherlands, enrolling a total of 33 children with asthma (van Bragt 2015).
Population characteristics
Forty‐three per cent (266/612) of participants in the only adult study were male, and investigators reported a mean age of 45.1 to 46.9 years across the three intervention arms (Wilson 2010). Approximately 60% of participants were Caucasian, 15% Africian American, and 10% Asian, with the remaining participants from Hispanic, Pacific Islander, and American Indian ethnic groups. Approximately 70% of participants reported a household income greater than $40,000 per year, and more than 95% had completed at least high school level education. Eighty‐four per cent of participants were reported to have poorly or very poorly controlled asthma at baseline, with forced expiratory volume in one second (FEV1) < 80% predicted in 70% of participants. Approximately 16% were current smokers.
The Clark study reported that 60% (44/74) of included paediatricians were male, as were 70% (471/637) of enrolled children (Clark 1998). Researchers provided data on an average of 10 children per paediatrician (range 1 to 33). Seven per cent of enrolled children were younger than two years of age, 59% were between two and seven years, and 34% were 8 to 12 years old. Fifteen per cent of enrolled children were Latino/Hispanic, and 15% were Africian American. Study authors provided no details about the ethnicity of the remaining 70%. Approximately 20% of participating families reported a household income less than $20,000 per year, and 16% were below the poverty level of $15,000 annual household income. Almost 90% of parents had at least a high school level education. Investigators did not report baseline asthma severity.
Fiks did not report the gender of the 60 paediatric participants in this trial (Fiks 2015). Children had a mean age of 8.3 years, 47% were black/Africian American, and 42% were white, with the remainder described as Asian, Hispanic, or other. Seventy‐one per cent of parents had at least some college level education, and 75% were in paid employment. Data show that baseline asthma severity was mild in 53% of children, moderate in 42%, and severe in 5%.
Finally, 62% (18/29) of the children included in the last study were male, and their mean age was approximately 8.5 years (van Bragt 2015). Ninety‐seven per cent of children were Caucisian. Eighty‐seven per cent of families in the intervention arm were reported to be from a high socioeconomic group, as were 64% in the control group. Mean FEV1% predicted was > 100% in both groups at baseline. Data indicate that asthma was uncontrolled (ACQ score ≥ 1) at baseline in 3/15 (20%) in the intervention group and in 6/14 (43%) in the control group.
Inclusion and exclusion criteria
Wilson specifically recruited adults whose asthma was not well controlled and were therefore likely to have inadequate adherence to their asthma regimen (Wilson 2010). Eligible patients were between 18 and 70 years of age. Poorly controlled asthma was evident in medical records by overuse of reliever medication or a recent emergency department (ED) visit or hospitalisation for asthma. Participants were excluded if they had intermittent asthma or a primary diagnosis of COPD, or were using regular OCSs. Participants were also excluded if they were already enrolled in an asthma management programme.
Clark enrolled children aged 1 to 12 years through participating paediatric general practitioners (Clark 1998). Eligible children must have had physician‐diagnosed asthma and no other chronic disorders with pulmonary complications, and must have had at least one emergency medical visit for asthma during the past year.
Fiks recruited children aged 6 to 12 years with persistent asthma and an English‐speaking parent or guardian who had consistent access to a computer and the Internet (Fiks 2015). Children were excluded if their asthma was not a primary or current health concern for their parent or guardian, or if they were not taking a "controller medication".
van Bragt recruited children aged 6 to 12 years with physician‐diagnosed asthma who had used asthma medication (bronchodilators and/or inhaled corticosteroids (ICSs)) for at least six weeks over the preceding year (van Bragt 2015). Children were excluded if they had comorbid conditions that would significantly impact their health‐related quality of life, were not receiving mainstream education, or had insufficient Dutch language skills.
Interventions and comparisons
Wilson 2010
Group 1. Shared decision‐making (SDM)
Participants received two face‐to‐face sessions and three phone calls over nine months. Sessions involved eliciting the patient's asthma history, classifying the level of control, and providing asthma education. In the SDM model, this was followed by negotiation of a treatment plan that took into account the participant's goals and preferences. Researchers shared with participants a full list of appropriate guideline‐based treatment options for all levels of asthma severity before arriving at a treatment plan that best accommodated the participant's and the care manager's goals.
Investigators provided a written asthma management and action plan at the end of the first session and adapted it as required in subsequent sessions.
Group 2. Clinical decision‐making (CDM)
As above for SDM, but instead of a negotiated treatment plan, the care manager prescribed an appropriate regimen based on the patient's level of asthma control and explained this decision to the patient.
Group 3. Usual care
Usual care at KP is based on a guideline‐based stepped‐care approach to pharmacotherapy with the goal of long‐term asthma control.
Intervention fidelity
Sixteen nurses, respiratory therapists, pharmacists, nurse practitioners, and physician assistants were recruited to deliver the intervention. Most were already trained asthma care managers. Researchers scored audiotapes of both sessions for 10% of participants against a checklist to ensure fidelity to the study protocol. They also asked participants to report their perceived role in the treatment decision after session one. The SDM model was based on "four key defining features described by Charles and colleagues" (Charles 1997; Charles 1999).
Clark 1998
Group 1. Interactive seminar programme
General practice paediatricians in this group received two interactive face‐to‐face seminars, each lasting approximately 2.5 hours, over a two‐ to three‐week period. Seminars were based on the theory of self‐regulation, "guiding physicians to examine their own behaviour and to identify ways that they could develop a better partnership with their patients". This included a focus on deriving information for making therapeutic decisions, creating a supportive atmosphere, reinforcing self‐management, giving a view of the long‐term therapeutic plan, and building patients’ confidence in controlling symptoms and using medicines. Seminars included brief lectures from respected asthma specialists, a video example, case studies, and a self‐assessment protocol for physicians.
Group 2. Control
General practice paediatricians in this group continued their usual asthma care practices.
Intervention fidelity
Physicians were asked to rate their own performance through a survey. Questions were related not only to prescribing practices but also to procedures such as encouraging self‐management, providing patient teaching, and exhibiting supportive communication and behaviour. Investigators collected similar data from patients and their parents and correlated this information with physicians' reports, noting a good level of agreement. The trial did not include an explicit assessment of intervention fidelity and did not attempt to record or observe physicians interacting with patients and parents.
Fiks 2015
Group 1. MyAsthma shared decision‐making portal
Participants in this group used "MyAsthma", a shared decision‐making portal linked to their electronic health record. Clinicians and families had developed MyAsthma with the aim of promoting SDM. The main features of this online portal included eliciting parents' concerns and asthma treatment goals; tracking symptoms and side effects; providing educational content; and granting access to participants' individual asthma care plans. Families were prompted to complete a monthly survey, the results of which were used to provide guideline‐based decision support for parents and clinicians.
Group 2. Control
Participants in this group did not have access to the MyAsthma portal, but their clinician had access to the decision support system designed to promote guideline‐based asthma care.
Intervention fidelity
Study staff provided "brief training" to families randomised to receive the MyAsthma intervention and sent monthly emails to remind them to complete portal surveys, on which subsequent decision support was based. Acceptibility of the intervention was recorded through surveys at baseline, at three months, and at six months; these surveys included questions about satisfaction with asthma care. The proportion of participants completing the monthly portal survey was used as a measure of feasibility.
van Bragt 2015
Group 1. PELICAN online tool
Children in this group used a self‐administered online health‐related quality of life instrument, specifically developed for children aged 6 to 11 years. Children were invited to respond to a series of questions using a 5‐point Likert scale and to choose from a list of specific asthma problems the ones that may bother them in their daily life. Children completed the PELICAN tool before each study visit, and investigators used their answers to guide asthma management, based on SDM between child, parent, and nurse. After the first session, researchers produced a written action plan that would be reviewed at subsequent sessions.
Group 2. Enhanced usual care
Children in this group were assessed every three months. Specific issues addressed included symptoms, medication use, and exposure to asthma triggers, according to the guidelines of the Dutch College of General Practitoners. Consultations provided by the child's usual general practitioner or nurse typically lasted 10 minutes.
Intervention fidelity
Study authors did not describe the procedure used to train children to use the online tool. Nurses delivering the face‐to‐face shared decision‐making consultation were trained in the process during a two‐hour meeting before the study began and were monitored for a fixed number of "feedback/observation moments". Telephone support was provided for specific questions.
Outcomes
Clark 1998: physician survey (items related to using clinical practice methods/medicines, encouraging self‐management, and providing patient teaching and communications); parent interview form (questions related to symptom status of the child, medicines prescribed, and use of healthcare services for asthma (ED visits, hospitalisations, physician office visits);as well as parents’ observations and opinions of physicians’ teaching and communication behaviours and other aspects of the clinician–patient interaction). Data were collected from physicians at baseline, at five months ("mid‐point"), and at one year after the mid‐point. Investigators tracked patient visits over 22 months and collected data from patients on average two months after their visit.
Fiks 2015: feasibility (assessed as percentage of participants in the intervention group completing the monthly portal survey); acceptability of asthma care (measured at six months on an 11‐point Likert scale); clinical outcomes (numbers of asthma ED visits, hospitalisations, and specialist and general practitioner visits over the six‐month study); number of prescriptions assessed through electronic health records; number of days of missed school (child) or work (parent) over past month; Parent Patient Activation Measure (tool that can be used to assess the knowledge, skills, and confidence needed to manage a child's health care; regarded as a measure of satisfaction (higher score = higher activation)); Integrated Therapeutics Group ‐ Child Asthma Short Form (ITG‐ASF); and asthma control test. Families completed surveys at enrolment and at three and six months.
van Bragt 2015: primary outcome: quality of life (Pediatric Asthma Quality of Life Questionnaire (PAQLQ)); secondary outcomes: asthma control (ACQ); symptoms and medication via a diary; cost‐effectiveness; caregiver quality of life (Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ)); process outcomes. Investigators collected data at three, six, and nine months after the baseline assessment.
Wilson 2010: primary outcomes: adherence to controller medications; better asthma‐related quality of life; and improved healthcare utilisation; secondary outcomes: short‐acting beta‐agonist (SABA) use; lung function; and asthma control. Investigators collected data at 12 and 24 months post randomisation.
Excluded studies
We excluded 11 studies after viewing full texts: Nine studies tested an intervention that was not focused on improving shared decision‐making (Ford 1996; Gorelick 2006; Moffat 2008; NCT00170248; NCT00214669; Smith 2008; Sockrider 2001; Tapp 2014; Tieffenberg 2000). One was not an RCT (NCT01522144). Another study recruited a mixed respiratory population (Early 2015). In addition, we have listed four relevant studies as ongoing (Federman 2015; Hoskins 2013; NCT02516449; Tapp 2011).
Risk of bias in included studies
We have provided a summary of our risk of bias judgements in Figure 2.
2.
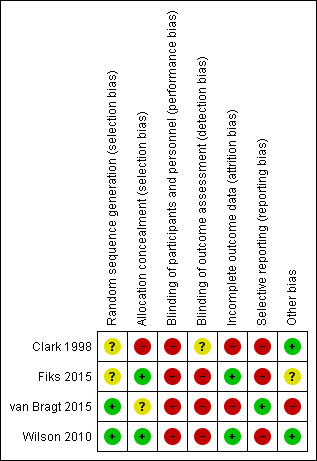
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered one study to be at low risk of bias because trial authors described computerised methods of generating the random sequence and concealing allocation (Wilson 2010). Another study used minimisation software to generate the random sequence but did not describe allocation concealment, so we rated risks of bias as low and unclear, respectively (van Bragt 2015). We rated another study as having unclear and low risks of bias because it did not describe random sequence generation but used sealed envelopes to conceal allocation (Fiks 2015). We rated the remaining study as having unclear risk of bias for random sequence generation and high risk of bias for allocation concealment because the method of selecting participants for inclusion was not well concealed, and this may have introduced a selection bias (Clark 1998).
Blinding
We considered three studies to be at high risk of bias for both blinding domains because patients, physicians, or both were aware of group allocation, and this may have affected how they behaved and responded during and after the intervention (Fiks 2015; van Bragt 2015; Wilson 2010). The other included study blinded patients and parents to physicians' involvement in the study, so outcomes measured by patients and parents would be at low risk of detection bias, but outcomes rated by physicians would be at higher risk (Clark 1998). We assessed separately the likelihood that each outcome would be subject to performance and detection biases when GRADE ratings were applied.
Incomplete outcome data
Two studies were at low risk of attrition bias because a similar and low proportion of participants from either group could not be included in the final analyses (Fiks 2015; Wilson 2010). We considered the other two studies to be at high risk of attrition bias because overall dropout was high and numbers randomised and completed in each group were not reported fully, or because all dropouts came from the control group (Clark 1998; van Bragt 2015).
Selective reporting
We rated one study as having low risk of reporting bias because it was prospectively registered and researchers reported all specified outcomes as planned (van Bragt 2015). We rated three studies as having high risk of reporting bias; two were prospectively registered and the full report did not include data for all planned outcomes or time points, and one reported some outcomes narratively or in a way that meant data could not be pooled in a meta‐analysis (Clark 1998; Fiks 2015; Wilson 2010).
Other potential sources of bias
We did not note any additional sources of bias in two studies (Clark 1998; Wilson 2010). In another study, study authors noted: "The study population was a convenience sample based largely on clinician recommendation and was not designed to be representative of all children with asthma in the care network", but it is unclear whether this introduces bias (Fiks 2015). We rated another study as having high risk of bias because the 33 children recruited were significantly fewer than the 170 planned, potentially leading to underpowered analyses. In addition, groups were not balanced at baseline for asthma control or for socioeconomic status (van Bragt 2015).
Effects of interventions
See: Table 1
We did not consider interventions, comparisons, or outcomes reported in the included studies to be sufficiently similar for pooling to make sense. We present a narrative description of the outcomes of interest for each included study, structured according to our prespecified primary and secondary outcomes. When possible, we present findings from individual studies on forest plots to provide a visual representation of the effect estimate.
Primary outcomes
Asthma‐related quality of life
Three studies reported asthma quality of life.
Fiks reported three subscales of the ITG‐ASF (higher score = poorer quality of life) as change from baseline for 53 participants but did not report a measure of variance (Fiks 2015). We back‐calculated standard deviations (SDs) from reported P values for differences between arms. Confidence intervals include no differences for each of the subscales. We presented results in Analysis 1.2. (very low‐quality evidence).
1.2. Analysis.
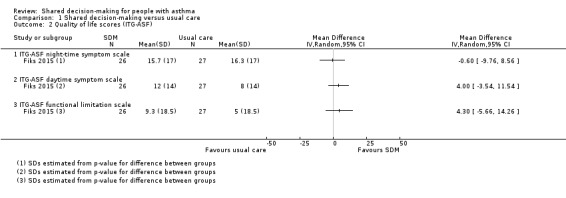
Comparison 1 Shared decision‐making versus usual care, Outcome 2 Quality of life scores (ITG‐ASF).
Wilson (a three‐arm trial) also reported on the endpoint quality of life, using the symptoms domain of the mini‐AQLQ (Wilson 2010). We have presented SDM versus usual care comparisons in Analysis 1.3. We back‐calculated SDs from the P value given for the difference (P = 0.0003). Although the mean difference falls below the minimal clinically important difference (MCID) of 0.5 for this scale, responder analysis demonstrates that significantly more people experienced an improvement of at least 0.5 units (odds ratio (OR) 1.90, 95% confidence interval (CI) 1.24 to 2.91; participants = 371; studies = 1; Analysis 1.1). We have moderate confidence in these results.
1.3. Analysis.
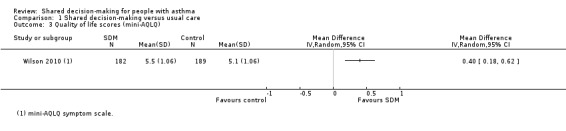
Comparison 1 Shared decision‐making versus usual care, Outcome 3 Quality of life scores (mini‐AQLQ).
1.1. Analysis.
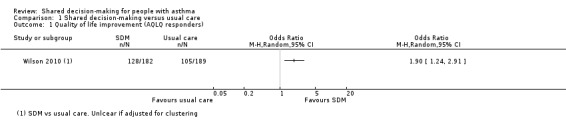
Comparison 1 Shared decision‐making versus usual care, Outcome 1 Quality of life improvement (AQLQ responders).
Of note, Wilson reported that the mean difference in mini‐AQLQ symptom score for SMD versus CDM was 0.1 and described this finding as non‐significant. A responder analysis for this comparison revealed that the number of people in the CDM group with improvement greater than 0.5 units was 110/180. If this is used as the control group, the effect is smaller and the lower confidence interval shows no difference (OR 1.51, 95% CI 0.97 to 2.34; data not presented).
van Bragt reported child and parent scores on the AQLQ as medians and interquartile ratios (IQRs) (van Bragt 2015). We noted baseline imbalances, and although investigators stated in the methods section that they would adjust for this, it is unclear whether this was done, as data were not normally distributed. Scores were slightly higher in the SDM group than in the control group, and the number of participants was small (6.78 vs 6.5 children (n = 29); 6.96 vs 6.85 parents (n = 25); IQRs between 0.31 and 0.96).
Patient/parent satisfaction
Clark reported parental views on the "demeanour and communications skills of the paediatrician", adjusted for clustering, using a number of different measures, but these investigators presented results without a measure of variance, so we have not presented them graphically (Clark 1998). Study authors followed up a total of 472 parents of enrolled children for this outcome. Parents in the intervention group were significantly more likely to report that the paediatrician was reassuring and encouraging; described as a goal that the child could be fully active; looked into how the family managed asthma on a day‐to‐day basis; and gave parents information to relieve their specific worries and concerns about asthma (Table 3).
2. "Parents’ Views of Pediatricians’ Performance"; adapted from Clark 1998.
| Was/did the clinician: | SDM | Control |
P value (GEEa) |
| Reassuring and encouragingb | 4.63 | 4.42 | 0.006 |
| Look into how family managed day to dayb | 3.98 | 3.69 | 0.02 |
| Describe how child should be fully activec | 71.% | 59% | 0.007 |
| Describe at least 1 of 3 goals: child should sleep through the night; have no symptoms when active; be fully activec | 75% | 64% | 0.07 |
| Give information to relieve specific worriesb | 4.1 | 3.9 | 0.007 |
| Enable family to know how to make asthma management decisionsb | 4.3 | 4.2 | 0.07 |
aGEE method to assess "Time2" (follow‐up) scores with baseline scores and group assignment as covariates in regression models. bA Likert‐type response scale was used, where 1 = strongly disagree and 6 = strongly agree. cQuestion asked at "Time2" (follow‐up) only.
NB: A total of 472 parents were followed up; numbers in each group are not given.
Fiks reported the number of parents who completed the portal survey for each of the six months of the study and considered this to be a measure of acceptability of the intervention (Fiks 2015). It should be noted that parents of children in the control group did not have access to the portal, and therefore this outcome was measured only in the SDM group. Of the 30 families randomised to the intervention group, 17 (57%) completed the survey five or more times, which was defined as frequent use, and 77% completed the survey more than once. It was also noted that parents of children with more severe asthma were more likely to be frequent users of the portal (75% vs 47% with mild persistent asthma). Twenty‐two out of 24 parents reported that the MyAsthma intervention made it easier to care for their child with asthma, and 10 of 24 parents reported that the portal made it easier to communicate with their child’s healthcare providers. Six parents reported that the portal increased their awareness of the importance of asthma management.
This same study reported "parental activation" using the Parent Patient Activation Measure. This tool assesses the knowledge, skills, and confidence needed to manage a child's health care and could be regarded as a measure of satisfaction (higher score = higher activation). Data showed no significant differences between study arms; change scores were reported as 2.3 and 2.4 in SDM and control groups, respectively (P = 0.9).
Medication adherence
Fiks reported the mean number of "controller" medication prescriptions over 26 weeks as 1.1 in the SDM group (n = 26) and 0.7 in the control group (n = 27) (Fiks 2015).
Wilson reported medication adherence for all medications and for inhaled corticosteroid (ICS) alone as continuous medication acquisition (CMA) (Wilson 2010). This is calculated as the total days’ supply acquired in a given year divided by 365 days. Results suggest that SDM increases CMA when compared with usual care (Analysis 1.4; all medication: mean difference (MD) 0.21, 95% CI 0.11 to 0.31; ICS alone: MD 0.22, 95% CI 0.11 to 0.33; participants = 371; moderate‐quality evidence). Our confidence in this finding was reduced by the potentially indirect nature of using CMA to measure adherence. The CMA mean difference between SDM and CDM in the Wilson study was 0.029 for all medication and 0.017 for ICS alone; these mean differences are smaller than those for SDM versus usual care but are also reported as statistically significant (Wilson 2010). Of note, trialists also collected CMA data at two years and reported that between‐group differences were no longer significant.
1.4. Analysis.
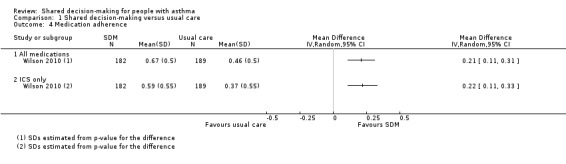
Comparison 1 Shared decision‐making versus usual care, Outcome 4 Medication adherence.
CMA findings are supported by an additional metric of the beclomethasone dipropionate (BDP) equivalent of canisters acquired, which shows an effect in favour of SDM at one year and at two years, although a smaller difference after two years (data not shown).
Secondary outcomes
Exacerbations of asthma (leading to a course of oral corticosteroids or unscheduled visit to a healthcare professional)
Clark reported mean numbers of ED visits and hospitalisations per child and showed no clear between‐group differences (mean number of ED visits: SDM = 0.65, usual care = 0.67; hospitalisations: SDM = 0.081, usual care = 0.076; both P values were adjusted for clustering and were reported as non‐significant) (Clark 1998).
Fiks reported the mean number of oral corticosteroid (OCS) prescriptions over 26 weeks, without variance, as 0.4 in the SDM group (n = 26) and 1 in the control group (n = 27) (Fiks 2015). This study also reported the numbers of children with exacerbations requiring hospital admission, an ED visit, a specialist visit, and a general practitioner visit. We have presented these data in Analysis 1.5; all four point estimates favour shared decision‐making, but confidence intervals are wide, and our confidence in these findings is low. Finally, study authors reported the change in the number of asthma exacerbations, captured by the "Asthma Control Tool" (a validated instrument in children), as ‐3.3 in the SDM group and ‐1.3 in the control group (25‐point scale; P = 0.02).
1.5. Analysis.
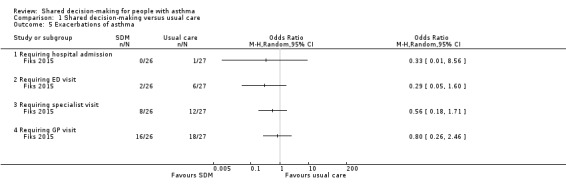
Comparison 1 Shared decision‐making versus usual care, Outcome 5 Exacerbations of asthma.
Wilson reported rates of asthma‐related visits in this three‐arm study (Wilson 2010). During year 1, both SDM and CDM groups had significantly lower visit rates (1.0/y and 1.1/y) than the usual care group (1.4/y; P = 0.0161 and 0.0147, respectively).
Asthma control
Fiks reported change in "asthma symptoms while at best" on the "Asthma Control Tool" as ‐2.8 in the SDM group and ‐0.6 in the control group (P = 0.10), with a lower score indicating less severe symptoms (Fiks 2015).
van Bragt assessed asthma control using the Asthma Control Questionnaire (ACQ) and the Asthma Control Test (ACT) and presented results as medians and IQRs. Baseline imbalances were notable (ACQ in favour of intervention and ACT in favour of control), and, as data were not normally distributed, it is unclear whether scores were adjusted accordingly (van Bragt 2015). This same trial dichotomised participants into well controlled and not well controlled (well controlled seen as < 1 on the ACQ and > 22 on the ACT). Study authors detected no between‐group differences, but confidence intervals were wide and the number classified as 'well controlled' at baseline was unbalanced (Analysis 1.6; low‐quality evidence).
1.6. Analysis.
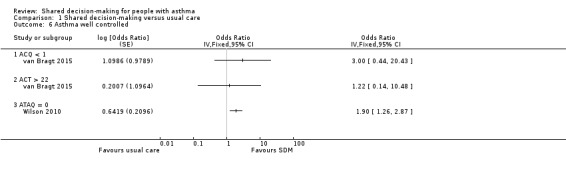
Comparison 1 Shared decision‐making versus usual care, Outcome 6 Asthma well controlled.
Wilson reported change from baseline on the Asthma Therapy Assessment Questionnaire (ATAQ) but did not give any measure of variance (Wilson 2010). Changes were as follows: ‐0.8 in the SDM group, ‐0.54 in the CDM group, and ‐0.46 in the usual care group, with lower scores indicating better control. This same study used the ATAQ to report the number of people with 'no asthma problems' (ATAQ score = 0). We have presented SDM versus usual care in Analysis 1.6 (moderate‐quality evidence); the odds ratio for the SDM versus CDM comparison shows a smaller but still significant effect in favour of SDM: 1.6 (1.1 to 2.4, P = 0.0239).
Acceptability/feasibility from the perspective of healthcare professionals
We did not find any data about this.
Adverse events (all)
None of the included studies measured or reported adverse events.
Subgroup and sensitivity analyses
It was not possible to conduct any of the planned subgroup analyses (age; who the intervention was aimed at; extensiveness of intervention), as we did not perform any meta‐analyses. We have presented a summary of study characteristics in Table 2.
Similarly, it was not possible to test the robustness of study results by performing sensitivity analyses while excluding unpublished data and studies at high risk of selection bias.
Discussion
Summary of main results
This review includes four studies of shared decision‐making (SDM), allocating a total of 1342 participants to either SDM interventions or control. Study design, populations, interventions, comparisons, and outcomes are substantially different between the four studies. Three studies recruited children with asthma and their care‐givers (Clark 1998; Fiks 2015; van Bragt 2015). One study recruited adults (Wilson 2010). Asthma severity ranged from mild to severe. Three studies took place in the United States (Clark 1998; Fiks 2015; Wilson 2010). One was conducted in the Netherlands (van Bragt 2015). Trial duration was between six and 24 months, and outcomes were measured at a range of time points from six months to two years.
All studies were conducted in a primary care or outpatient setting, and the intervention was delivered in various ways, either to participants directly or to healthcare professionals. Two studies in children used an online portal to elicit key asthma management concerns and goals; this was followed by face‐to‐face discussions with a healthcare professional based on shared decision principles (Fiks 2015; van Bragt 2015). Clark provided seminars aimed at developing skills in SDM among paediatric general practitioners, who in turn enrolled their patients into the study (Clark 1998). Wilson provided to participants a mixture of face‐to‐face discussions and telephone calls with personnel trained in SDM or in clinical decision‐making (CDM) (Wilson 2010). The duration and content of interventions varied, but SDM was a key component of the intervention provided in all included studies. Owing to the nature of the intervention, it was not possible to blind participants or trial personnel to group allocation. Review authors considered the impact of the lack of blinding on an outcome‐specific basis when assigning GRADE ratings.
Meta‐analysis of results was not possible owing to the small number of heterogenous trials included. Three studies used different tools to assess asthma‐related quality of life and reported inconsistent results. Fiks conducted a study in children that compared an SDM online portal versus guideline‐based care presented in subscales of the Integrated Therapeutics Group ‐ Child Asthma Short Form (ITG‐ASF) and did not demonstrate between‐group differences, although confidence intervals were wide (Fiks 2015). Similarly, van Bragt conducted a study in children using an online tool and found little difference between SDM and control groups (van Bragt 2015). Wilson completed a study in adults involving face‐to‐face and telephone consultations and identified benefit of SDM over usual care, using the mini‐Asthma Quality of Life Questionnaire (AQLQ) symptom scale (Wilson 2010). This benefit was confirmed by a responder analysis.
Two studies reported patient/parent satisfaction, or proxy measures. In a cluster‐randomised trial in which SDM training was provided to physicians, Clark reported that parents of children in the intervention group were significantly more likely to report satisfaction with the paediatrician (Clark 1998). Fiks reported "parental activation" using the Parent Patient Activation Measure but noted no significant differences between study arms (Fiks 2015).
Two studies reported medication adherence. Fiks indicated that the mean number of controller medication prescriptions over 26 weeks was greater in the SDM group (Fiks 2015). Wilson reported medication adherence for all medications and for inhaled corticosteroids (ICSs) alone as continuous medication acquisition (CMA) (Wilson 2010). Results suggest that SDM increases CMA when compared with usual care, but that differences are lessened over time.
Of our secondary outcomes, study authors reported only exacerbations and asthma control. Three studies reported exacerbations.Mean numbers of emergency department (ED) visits and hospitalisations per child reported by Clark show no clear between‐group differences (Clark 1998). Fiks indicated that the mean number of oral corticosteroid (OCS) prescriptions over 26 weeks was reduced in the SDM group compared with the control group (Fiks 2015). This study also reported the number of children with exacerbations requiring an unscheduled visit or hospital admission; point estimates favoured SDM, but confidence intervals were wide. Wilson reported rates of asthma‐related visits and indicated that the SDM group had significantly lower visit rates than the usual care group (Wilson 2010). Three studies reported asthma control. Changes in "asthma symptoms while at best" on the "Asthma Control Tool" as reported by Fiks were noted to be lower in the SDM group than in the control group (Fiks 2015). van Bragt assessed asthma control using the Asthma Control Questionnaire (ACQ) and the Asthma Control Test (ACT) and dichotomised participants into two groups: well controlled and not well controlled (van Bragt 2015). Researchers reported no between‐group differences, but confidence intervals were wide. One study used the Asthma Therapy Assessment Questionnaire (ATAQ) to report the number of people with 'no asthma problems' (ATAQ score = 0) and described benefit of SDM over control (Wilson 2010).
Overall completeness and applicability of evidence
Only four studies met the inclusion criteria for this review, thus the body of evidence available from randomised controlled trials (RCTs) is limited at this time. Substantial differences in study design, populations, interventions, comparisons, and outcomes prevent overall conclusions. Although we identified several randomised trials in asthma that included an element of SDM, we considered this to be only one element of a broader intervention and thus excluded these studies (see Characteristics of excluded studies). This may have resulted in loss of useful information, but we judged it would not have been possible to confidently ascribe any clinical benefit to SDM in the context of a much broader intervention. The small number of trials identified also meant that no subgroup analysis could be performed as planned on the basis of content, intensiveness, or duration of the intervention; these are all likely to be important effect modifiers.
Whether or not the intervention was delivered with a high level of fidelity is also an important consideration when outcomes of SDM interventions are assessed. All four studies attempted to capture fidelity or intervention adherence using different approaches. Investigators in two studies reported observing or recording trial staff to ensure that the intervention was delivered as planned (van Bragt 2015; Wilson 2010). Investigators in another trial asked physicians, who were the primary recipients of the intervention, to rate their own performance, which was reported as having a high level of correlation with their patients' reports (Clark 1998). However, this trial report did not describe attempting to observe or record physicians while interacting with patients. Families recruited in another study received "brief training" by study staff on use of the online portal and recorded acceptability through surveys that included questions about satisfaction with asthma care (Fiks 2015). The proportion of participants completing the monthly portal survey was used as a measure of feasibility, and trialists reported that 77% of parents completed the survey at least twice, out of a possible six times. Fifty‐seven per cent completed the survey five or more times.
Although adverse events might not be anticipated in trials of SDM, none of the included studies set out to systematically measure and report this outcome; this is another limitation of the evidence presented. Another important gap is the fact that none of the included studies focused on adolescents. Adolescents are at higher risk of poor asthma outcomes, including death, when compared with younger children (Akinbami 2002; Akinbami 2006). Asthma management during adolescence may require particularly high levels of trust and good communication between care providers and patients; therefore SDM interventions have the potential for substantial impact (de Benedictis 2007).
Three out of the four included studies were conducted in the United States, and the fourth in another high‐income setting (the Netherlands). This may limit applicability of findings to other healthcare systems facing greater resource constraints and with different cultural approaches to the relationship between healthcare professionals and patients. Cost‐effectiveness is also not addressed in this review nor in the included studies. Evidence suggests that SDM interventions may not be cost‐neutral, so studies including an economic evaluation would be a useful addition to the evidence base (Veroff 2013).
We also noted that baseline asthma severity and control varied between studies (e.g. most participants in the Wilson study had poorly controlled asthma, whereas mean ACQ score in the van Bragt trial was < 1, suggesting overall good asthma control; Fiks reported that a large majority of participants had mild or moderate asthma) (Fiks 2015; van Bragt 2015; Wilson 2010). A possible direction for future research would be to investigate whether people with more or less severe asthma benefit more or less from SDM than those given usual care. The limited number of studies in this review means that we cannot currently comment on this. A further consideration is that those who agree to participate in SDM trials and those who adhere to the trial protocol once recruited may differ substantially from those not recruited. This may limit generalisability of findings from such trials to the wider asthma population.
Finally, choice of control group and treatment setting may have an impact on whether an SDM intervention leads to improvement in asthma outcomes. Usual care practices vary widely between settings; some may include elements of SDM routinely, which would likely limit differences seen between intervention and control groups. A thorough description of routine practices is important for an understanding of local applicability of findings from individual trials.
Quality of the evidence
We were not able to apply GRADE to all outcomes as planned because we had no pooled data for some analyses, including patient/parent satisfaction; acceptability from the perspective of the healthcare professional; and adverse events. When we were able to make a judgement, our confidence ranged from very low to moderate. We downgraded subjective outcomes (quality of life and asthma control) owing to inherent risk of bias introduced by unblinded trials, although it is difficult to conceive a trial of SDM in which effective participant and personnel blinding would be possible. We did not consider the open‐label design of trials to pose such a threat to outcomes such as medication adherence and exacerbations.
We had concerns about indirectness in trials that reported subscale scores from a quality of life questionnaire, rather than total scores, and we downgraded evidence for this reason. We also downgraded medication adherence evidence, as we judged continuous medication acquisition to be a proxy measure of adherence that may overestimate true adherence. We noted that imprecision was a problem for several outcomes, including quality of life, exacerbations, and asthma control, with confidence intervals including the possibility of both harm and benefit from the intervention.
We did not detect statistical heterogeneity because we did not pool studies owing to differences in study design, outcomes reported, or both (i.e. high clinical heterogeneity); therefore we ran no tests for heterogeneity. We have reported findings narratively when relevant. We did not suspect publication bias but did not include sufficient studies to produce a funnel plot.
Potential biases in the review process
We carried out the review according to methods provided in the published protocol and detailed deviations from the protocol in the Differences between protocol and review section (Kew 2016). As planned, two review authors independently screened search results and resolved discrepancies by discussion. We did not restrict the search by date or by language. At least two review authors extracted all study characteristics and numerical data and resolved discrepancies through discussion. The same was true for risk of bias ratings and GRADE ratings, for which a third person was consulted as required to resolve disagreements. Two additional review authors joined the team to complete the update (RN and KA). Insufficient data prevented completion of planned meta‐analyses and subgroup and sensitivity analyses.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review that synthesises evidence from RCTs on SDM in asthma. Several other systematic reviews have explored the association between SDM and health outcomes and behaviours across a range of medical conditions. A consistent theme across these reviews, in keeping with the present review, is the difficulty of meaningfully combining evidence from the wide range of trials taking place in this field.
A recent review, including 39 studies, most of which were observational, found that although affective‐cognitive outcomes may be favourable if participants perceive that SDM has occurred, evidence linking empirical measures of SDM to health and behavioural outcomes is lacking (Shay 2015). Joosten and colleagues identified 11 RCTs of SDM involving adults across various medical conditions (Joosten 2008). Although these review authors concluded that SDM may be beneficial, especially in the context of chronic illness, they noted that evidence from RCTs regarding impact on health outcomes is lacking.
A 2015 review of SDM in paediatrics identified 61 studies, most of which were observational in design, and focused on satisfaction, decisional conflict, and knowledge, rather than health outcomes. Only 15 studies could be meta‐analysed, and review authors concluded that SDM interventions in paediatrics remain poorly defined, but limited available evidence suggests that SDM may reduce decisional conflict,and improve parent knowledge (Wyatt 2015). Durand and colleagues in their systematic review specifically addressed whether SDM interventions can reduce health inequalities (Durand 2014). Review authors concluded following a narrative synthesis of evidence that SDM interventions may be more beneficial for those from disadvantaged groups, but confidence in their findings was reduced by between‐study heterogeneity.
Légaré and colleagues synthesised evidence related to effectiveness of interventions aimed at patients or healthcare professionals to improve SDM (Légaré 2014). They identified 39 studies, 38 of which were RCTs. Despite the large number of studies included, review authors were not able to conclude whether interventions to improve adoption of SDM are effective, although they suggest that targeting both patients and healthcare professionals is likely to be more effective than targeting just one or the other.
Reviews investigating the role of SDM in other specific conditions have demonstrated benefit; for example, one trial addressed SDM in the context of antibiotic prescribing for acute respiratory illness, and another investigated SDM for people with dementia (Coxeter 2015; Daly 2016).
Authors' conclusions
Implications for practice.
We have presented findings from four heterogeneous studies of shared decision‐making in asthma. Substantial differences between studies mean that we cannot form overall conclusions. Individual studies have demonstrated some benefits of shared decision‐making over control, including quality of life; patient and parent satisfaction; adherence to prescribed medication; reduction in asthma‐related visits/exacerbations; and improved asthma control (Clark 1998; Wilson 2010). Our confidence in these findings from individual studies ranges from moderate to very low, primarily owing to concerns about performance and detection bias, indirectness, and imprecision. It is important to note that studies did not measure or report adverse events, so no information on harmful effects of shared decision‐making is available.
Implications for research.
At this time, the body of evidence from randomised controlled trials of shared decision‐making is limited. Future trials should be adequately powered and of sufficient duration to detect differences in patient‐important outcomes such as exacerbations and hospitalisations. We recommend use of core asthma outcomes and validated scales when possible and urge that the study population should be clearly characterised. Three of the four studies identified were conducted in the United States, and the fourth in the Netherlands; future studies conducted in lower‐income settings would be of interest. Adverse events should be systematically recorded, even if none are anticipated. Adolescents have not been represented in the studies identified to date and should be considered for future trials. Economic evaluations of future interventions could be considered, and trialists should seek to explicitly measure and report intervention fidelity.
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways. We are grateful for advice and editorial expertise provided by the Cochrane Airways staff.
Sally Spencer was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| CENTRAL (the Cochrane Library) | Monthly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Condition search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
17. exp Aspergillosis, Allergic Bronchopulmonary/
18. lung diseases, fungal/
19. aspergillosis/
20. 18 and 19
21. (bronchopulmonar$ adj3 aspergillosis).mp.
22. 17 or 20 or 21
23. 16 or 22
24. Lung Diseases, Obstructive/
25. exp Pulmonary Disease, Chronic Obstructive/
26. emphysema$.mp.
27. (chronic$ adj3 bronchiti$).mp.
28. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
29. COPD.mp.
30. COAD.mp.
31. COBD.mp.
32. AECB.mp.
33. or/24‐32
34. exp Bronchiectasis/
35. bronchiect$.mp.
36. bronchoect$.mp.
37. kartagener$.mp.
38. (ciliary adj3 dyskinesia).mp.
39. (bronchial$ adj3 dilat$).mp.
40. or/34‐39
41. exp Sleep Apnea Syndromes/
42. (sleep$ adj3 (apnea$ or apnoea$)).mp.
43. (hypopnoea$ or hypopnoea$).mp.
44. OSA.mp.
45. SHS.mp.
46. OSAHS.mp.
47. or/41‐46
48. Lung Diseases, Interstitial/
49. Pulmonary Fibrosis/
50. Sarcoidosis, Pulmonary/
51. (interstitial$ adj3 (lung$ or disease$ or pneumon$)).mp.
52. ((pulmonary$ or lung$ or alveoli$) adj3 (fibros$ or fibrot$)).mp.
53. ((pulmonary$ or lung$) adj3 (sarcoid$ or granulom$)).mp.
54. or/48‐53
55. 23 or 33 or 40 or 47 or 54
Filter to identify randomised controlled trials (RCTs)
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 shared* NEAR decision*:ti,ab
#6 sharing* NEAR decision*:ti,ab
#7 informed* NEAR decision*:ti,ab
#8 informed* NEAR choice*:ti,ab
#9 decision* NEAR aid*:ti,ab
#10 ((share* or sharing* or informed*) AND (decision* or deciding* or choice*)):ti
#11 MeSH DESCRIPTOR Decision Making
#12 MeSH DESCRIPTOR Decision Support Techniques
#13 MeSH DESCRIPTOR Decision Support Systems, Clinical
#14 MeSH DESCRIPTOR Choice Behavior
#15 decision* NEAR making*:ti,ab
#16 decision* NEAR support*:ti,ab
#17 choice* NEAR behavio?r*:ti,ab
#18 ((decision* or choice*) AND (making* or support* or behavior* or behaviour*)):ti
#19 MeSH DESCRIPTOR Patient Participation
#20 patient* NEAR participation*:ti,ab
#21 consumer* NEAR participation*:ti,ab
#22 patient* NEAR involvement*:ti,ab
#23 consumer* NEAR involvement*:ti,ab
#24 ((patient* or consumer*) AND (involvement* or involving* or participation* or participating*)):ti
#25 MeSH DESCRIPTOR Professional‐Patient Relations
#26 MeSH DESCRIPTOR Physician‐Patient Relations
#27 MeSH DESCRIPTOR Patient‐Centered Care
#28 ((patient* or person* or client* or consumer*) NEAR (centred or centered or focused or oriented)):ti,ab
#29 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28
#30 #4 AND #29
(Note: In search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, asthma.)
Data and analyses
Comparison 1. Shared decision‐making versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life improvement (AQLQ responders) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Quality of life scores (ITG‐ASF) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 ITG‐ASF night‐time symptom scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 ITG‐ASF daytime symptom scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 ITG‐ASF functional limitation scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life scores (mini‐AQLQ) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Medication adherence | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 All medications | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 ICS only | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Exacerbations of asthma | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Requiring hospital admission | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Requiring ED visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Requiring specialist visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Requiring GP visit | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Asthma well controlled | 2 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 6.1 ACQ < 1 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 ACT > 22 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 ATAQ = 0 | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Clark 1998.
| Methods |
Study design: parallel, open‐label, cluster RCT Length of observation: 22 months Setting: 74 general practices in Michigan and New York, USA |
|
| Participants |
Population: 74 physicians were randomised and 69 completed the trial. It is not clear how many were randomised to each group, but the study states that 637 children were recruited in total, and outcome data were available for 472. Baseline characteristics Baseline data were reported for the whole population rather than for each group. 60% of physicians and 70% of children were male. Physician and child ages were reported in brackets rather than as a mean per group. 30% of families were Latino/Hispanic (15%) or African American (15%). Inclusion criteria:Physician criteria: primary specialty of general paediatrics; licensed no earlier than 1960; providing direct patient care; if board‐specialised, certified only in paediatrics; willing to take part in the interactive seminar if randomised to the treatment group. Child criteria: 1 to 12 years of age; diagnosis of asthma made by a physician; no other chronic disorders with pulmonary complications; at least 1 emergency medical visit for asthma in the previous year. An emergency visit was a hospitalisation, emergency department (ED) visit, or physician office visit on an emergency basis defined as administration of epinephrine subcutaneously or bronchodilators by aerosol. Exclusion criteria: none in addition to inclusion criteria |
|
| Interventions |
Intervention: shared decision‐making seminars for clinicians Interactive seminar based on self‐regulation theory to guide physicians in NAEPP care and to engage in interactive conversations with patients to derive information for making therapeutic decisions, create a supportive atmosphere, reinforce self‐management, give a view of the long‐term therapeutic plan, and build patients’ confidence in controlling symptoms and using medicines. Materials included brief lectures from respected asthma specialists; a video depicting effective clinician teaching and communications behaviour; case studies presenting troublesome clinical problems; a protocol by which physicians could assess their own behaviour regarding patient communications; and review of messages to communicate and materials to use when teaching patients. Resources: The seminar comprised 2 face‐to‐face group meetings, each lasting 2 ½ hours, held over a 2‐ to 3‐week period. Control: usual care Physicians in the control group were randomly assigned a date corresponding to 1 of the 3 seminar time points, to determine when follow‐up interviews of their patients should begin. |
|
| Outcomes | Physician survey (items related to using clinical practice methods/medicines, encouraging self‐management, and providing patient teaching and communications). Analysis of data illustrated close correlation between physician and parent descriptions of behaviour. Questions on the parent interview form related to symptom status of the child, medicines prescribed, use of healthcare services for asthma (ED visits, hospitalisations, physician office visits), parents’ observations and opinions of physicians’ teaching and communications behaviours, other aspects of the clinician–patient interaction | |
| Notes |
Trial registration: not reported Funding: supported by MD/Family Partnership ‐ Education in Asthma Management grant number HL‐44976 from the Lung Division of the National Heart, Lung, and Blood Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized, controlled study design" but no description of how this was done |
| Allocation concealment (selection bias) | High risk | "Names of patients meeting criteria were selected by the investigators at random from the roster provided by physicians", which may have introduced recruitment bias within practices, even if practices were themselves randomised adequately to groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patients and their parents were blind to physicians’ involvement in the intervention." "A potential source of bias in the study was that physicians would give positive reports of their behavior to be consistent with good clinical and communications practices. To guard against such bias, data were collected from parents of patients regarding physician behavior as a means of corroborating physician reports." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Patients and parents were blind so outcomes measured by them can be considered low risk of bias. Outcomes measured or self‐assessed by the physicians taking part in the study are at high risk of detection bias." "A potential source of bias in the study was that physicians would give positive reports of their behavior to be consistent with good clinical and communications practices. To guard against such bias, data were collected from parents of patients regarding physician behavior as a means of corroborating physician reports." Patients and parents were blinded to their physician's participation in the intervention. Depends who is reporting the outcome, and to whom. Will be assessed separately when GRADE is applied |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Data were collected from physicians at baseline, and 69 (93%) provided follow‐up data 5 months after the program. Data were also collected from 637 of their patients at baseline, and in a 22‐month window after the intervention, 472 (74%) of this number provided follow‐up data." Unclear how many were randomised to each group and whether dropout was balanced, but nonetheless quite high attrition overall |
| Selective reporting (reporting bias) | High risk | Study does not report methods fully, for example, number of people assigned to each group and participant flow. In terms of data, uncertainty regarding the number of participants per group means that data are difficult to analyse reliably in meta‐analyses. Some data relevant to this review are presented narratively. We did not identify a study protocol or trial registration |
| Other bias | Low risk | None noted |
Fiks 2015.
| Methods |
Study design: parallel, individually randomised, open‐label RCT Length of observation: 6 months Setting: 3 primary care practices (1 urban, 2 suburban) in Philadephia, USA |
|
| Participants |
Population: 60 families were randomised to the online portal for SDM (30) or to the control group (30). Baseline characteristics Mean age was 8.3 years (SD 1.9) in the intervention group and 8.2 years (SD 1.9) in the control group. 43% of the intervention group and 40% of the control group were white. In the intervention group, 60% had mild asthma, 37% moderate, and 3% severe. In the control group, 47% had mild asthma, 47% moderate, and 6% severe. Inclusion criteria: Eligible participants were children aged 6 to 12 years with persistent asthma who received care at a study site, along with their parent or legal guardian. We enrolled English‐speaking parents/guardians who served as the primary member of their household involved in communicating with the doctor’s office and had consistent computer and Internet access. Exclusion criteria: At clinicians’ discretion, parents of children whose asthma was not a primary or current health concern were excluded, as were those not currently taking a controller medication. |
|
| Interventions |
Intervention: shared decision‐making portal MyAsthma, developed with input from families and clinicians with the goal of fostering ongoing SDM, provided decision support to both clinicians and parents. The clinician interface appeared in the electronic health record (EHR), and the parent interface appeared within MyChart, the EHR vendor’s patient portal. Features include identification of parents’ concerns and goals for asthma treatment; monthly symptom tracking, drug side effects, goal progress; educational content; and asthma care plan. Parents were encouraged via email to complete monthly portal surveys. Answers informed guideline‐based decision support for parents and clinicians, directing them to speak to one another if asthma was not well controlled, or if side effects occurred, or to continue current therapy. Control: usual care + decision support Families in the control group did not have access to the portal; however, clinicians caring for control group children had access to a clinician‐focused decision support system proven effective in fostering guideline‐based care. |
|
| Outcomes | Families completed surveys at enrolment and at 3 and 6 months. Feasibility assessed as % of participants in intervention group completing the monthly portal survey. Acceptability of asthma care measured at 6 months on 11‐point Likert scale. Clinical outcomes were numbers of asthma ED visits, hospitalisations, and specialist and GP visits over the 6‐month study (parental report validated when possible by chart review); number of prescriptions assessed through EHR; and number of days of missed school (child) or work (parent) over past month. Parent Patient Activation Measure. Integrated Therapeutics Group ‐ Child Asthma Short Form (ITG‐ASF) as quality of life measure. ACT as control measure | |
| Notes |
Trial registration: NCT01715389 Funding: supported by the Chair’s Initiative Grant and the William Wikoff Smith Endowed Chair in Pediatric Genomics from Children’s Hospital of Philadelphia, and by award number K23HD059919 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A randomization sequence was generated by the study coordinator (SLM). Randomization was stratified by practice and by whether the child had mild or moderate versus severe persistent asthma." |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes were used to ensure blinding of study staff to treatment condition before enrolment and randomization." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'Parents either had access to the portal or not so it was not possible to blind them to treatment allocation. This knowledge may have affected clinician and parent behaviour during the study and potentially biased outcomes." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were generally parent rated, which would introduce high risk of detection bias. Resource use outcomes and prescription refills would be less subject to detection biases. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 families in the intervention group (13.3%) and 3 in the control group (10%) could not be reached via phone or email. These families were not included in the analysis, but dropout was judged to be low and balanced enough that outcomes are unlikely to have been biased. |
| Selective reporting (reporting bias) | High risk | Some outcomes listed in the protocol that were of interest to this review were not fully reported in the paper or on clinicaltrials.gov (e.g. satisfaction with asthma care between groups, total scores on the ITG‐ASF and ACT). |
| Other bias | Unclear risk | Study authors noted: "The study population was a convenience sample based largely on clinician recommendation and was not designed to be representative of all children with asthma in the care network." This does not necessarily introduce bias. |
van Bragt 2015.
| Methods |
Study design: parallel, cluster‐randomised, single‐blind RCT Acronym: PELICAN Length of observation: 9 months Setting: 5 outpatient clinics in Holland |
|
| Participants |
Population: 33 children were randomised within the 5 clusters to the intervention group (15) or the control group (18) Baseline characteristics 66.7% of the intervention group and 57.1% of the control group were male. Mean age was 8.4 years (SD 1.7) in the intervention group and 8.7 years (SD 1.7) in the control group. 93.3% of the intervention group and 100% of the control group were white. In the intervention group, mean FEV1 was 111%; 80% were on ICS; mean PAQLQ was 6.35 (1.17); and ACQ 0.5 (0.6). In the control group, mean FEV1 was 101%; 57% were on ICS; mean PAQLQ was 6.02 (0.89); and ACQ 0.8 (1.4). Inclusion criteria: Children had physician‐diagnosed asthma, were 6 to 12 years of age, and used asthma medication (i.e. bronchodilators and/or inhaled corticosteroids) for at least 6 weeks during the previous year. Exclusion criteria: comorbid conditions that significantly influence health‐related quality of life, not able to attend a regular school class (as an indicator of normal intelligence), and insufficient skills in speaking and/or reading the Dutch language |
|
| Interventions |
Intervention: shared decision‐making online tool Nurse‐led patient‐centred care via an online tool. First, children completed the PAQLQ and selected 1 to 3 personal asthma problems, which were forwarded to the nurse. Then at the consultation, the nurse discussed with the child and parent which problem to prioritise, discussed details of the problem and chose a treatment goal through shared decision‐making, formulated a SMART goal (specific, measurable, acceptable, realistic, and time‐bound), brainstormed solutions together and documented an action plan, discussed results at the next visit, and repeated if necessary. Nurses were trained in the process during a 2‐hour meeting before the study. Control: enhanced usual care Besides usual care, the intervention group also received recommendations based on the Pelican outcome by a practice nurse. Described as enhanced usual care as seen more regularly than would be the case in practice. |
|
| Outcomes | Primary: quality of life (PAQLQ). Secondary: asthma control (ACQ), symptoms and medication via a diary, cost‐effectiveness, caregiver quality of life (PACQLQ), process outcomes | |
| Notes |
Trial registration: NCT01109745 Funding: Dutch Lung Foundation (previously Dutch Asthma Foundation), NutsOhra foundation, and a grant from the Nijmegen Centre of Evidence‐Based Practice (RadboudUMC grant) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "assigned children…in a 1:1 ratio using minimization software (Minim) that forced a balance between study arms for age (6–8 vs. 9–11 years old) and asthma control (ACQ score <1 vs greater than or equal to 1)" |
| Allocation concealment (selection bias) | Unclear risk | Not described but states that individual practices managed allocation to groups, which may not have adequately controlled for selection biases |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Children, parents, and nurses were aware of treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "This was a single‐blinded study. The analyses presented in this manuscript were based on blinded data. The study code was broken after the analyses were concluded." Study does not specify who was blinded. Outcome assessment and several outcomes were patient‐rated, which would introduce high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Protocol states: "The primary analysis is an intention‐to‐treat analysis, however both explanatory and intention‐to‐treat analyses will be performed." "A total of 33 children started with the study, 15 in the intervention group and 18 in the usual care group. One child was lost to follow‐up during the study and three children had too many missing data of the primary outcome, leaving 29 children for the analysis." All dropouts were from the usual care group. |
| Selective reporting (reporting bias) | Low risk | Outcomes relative to the review that were defined in the trial registration were reported but could not be included in meta‐analyses owing to non‐parametric methods. |
| Other bias | High risk | The 33 children recruited were significantly fewer than the 170 planned, which (1) meant the study was underpowered and (2) may reflect the feasibility of the intervention. "112 general practices was invited to participate of which 28 practices did not respond and 73 other practices refused participation for reasons such as lack of time, participation in other research projects, too few pediatric asthma patients or no affinity. Of the 11 practices that decided on participation, two practices were withdrawn due to lack of sufficient participants." |
Wilson 2010.
| Methods |
Study design: parallel, individually randomised, open‐label RCT Acronym: BOAT Length of observation: 52 weeks and 104 weeks Setting: 5 clinical Kaiser Permanante (a not‐for‐profit health plan) sites in the USA |
|
| Participants |
Population: 612 adults were randomised to a shared decision‐making intervention (204), clinical decision‐making (204), or a usual care group (204). Baseline characteristics 43.6% of the SDM group was male, 44.1% of the CDM group, and 42.6% of the usual care group. Mean age was 45.7 (SD 13.3) in the SDM group, 46.9 (SD 12.1) in the CDM group, and 45.1 (SD 12.4) in the usual care group. Most participants were white (62.8% SDM, 60.8% CDM, 62.3% usual care). Most participants' asthma symptoms were poorly or very poorly controlled (85.8% SDM, 82.9 CDM, 83.2 usual care). Other characteristics presented included education level, family income, smoking, controller medication use, recent hospitalisation, symptom frequency, and categories of FEV1 % predicted. Inclusion criteria: patients whose asthma was not well controlled, and whose adherence to their asthma regimen was likely to be inadequate. KP members, aged 18 to 70 years, with evidence suggestive of poorly controlled asthma, were identified at 5 clinical sites using computerised records of overuse of rescue medications (a controller/[controller + rescue medication] ratio < 0.5 and at least 3 beta‐agonist dispensings in the past year) or a recent asthma‐related ED visit or hospitalisation. Exclusion criteria: intermittent asthma (brief exacerbations or symptoms less than once/week), primary diagnosis of chronic obstructive pulmonary disease or emphysema, insufficient pulmonary function reversibility (for ex‐/current smokers and those without regular controller use), regular use of oral corticosteroids, current asthma care management |
|
| Interventions |
Intervention: shared decision‐making Sessions followed the same structure as clinical decision‐making but with the following added: description of SDM approach, identification and summary of patient goals and preferences, discussion of options and relative merits in terms of patients' goals and preferences, and negotiation of a treatment decision. Five sessions; 2 face‐to‐face and 3 over the phone at 3, 6, and 9 months. Intervention delivered to participants by 16 nurses, respiratory therapists, pharmacists, nurse practitioners, and physicians' assistants, most of whom were already asthma care managers. Specific training in shared decision‐making was provided. Control 1: clinical decision‐making Sessions included the following: building rapport, schedule for sessions, symptom/medication/triggers assessed, asthma understanding assessed and improved, spirometry reviewed, asthma severity and control determined using GINA, adherence problems addressed, new regimen recommended based on guidelines, prescription, action plan, inhaler technique instruction and asthma diary given, follow‐up appointment set. Five sessions; 2 face‐to‐face and 3 over the phone at 3, 6, and 9 months. Intervention delivered to participants by 16 nurses, respiratory therapists, pharmacists, nurse practitioners, and physicians' assistants, most of whom were already asthma care managers. Specific training in clinical decision‐making was provided. Control 2: usual care Usual care based on a stepped‐care approach to pharmacotherapy with the aim of long‐term asthma control, as recommended by the National Asthma Education Prevention Program’s Expert Panel Report 2. At some sites, clinicians had the option to refer patients to an asthma care management program similar to but less structured than the clinician decision‐making intervention. |
|
| Outcomes | Primary: adherence to controller medications, better asthma‐related quality of life, lower health care utilisation for acute symptoms than among patients who received usual care (no asthma care management). Secondary: short‐acting beta‐agonist (SABA) use, lung function, asthma control | |
| Notes |
Trial registration: NCT00149526; NCT00217945 Funding: supported by National Institutes of Health grants R01 HL69358 and R18 HL67092 Notes: Adherence was measured using a continuous medication acquisition (CMA) index for each year, calculated as the total days’ supply acquired in a given year divided by 365 days (30–32). The index represents the proportion of the prescribed medication supply acquired by the patient during each 365‐day period, and may potentially overestimate, but not underestimate, actual use. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐based adaptive randomization algorithm was used to ensure concealment from randomization staff and better‐than‐chance balance among the three groups on age (18–34, 35–50, and 51–70 yr), sex, race/ethnicity, hospitalisation in the prior two years (yes/no), and frequency of asthma controller use in the past week (none,1–3, ≥4 d)." |
| Allocation concealment (selection bias) | Low risk | "computer‐based adaptive randomization algorithm was used to ensure concealment from randomization staff" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study investigators and participants could not be kept blind to treatment allocation owing to the nature of the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Most outcomes would be subject to some form of detection bias by knowledge of treatment allocation, particularly self‐rated outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar proportions of participants in each group were followed up at 12 months (89.2% in the SDM group, 88.2% in the CDM group, and 92.6% in the usual care group). Attendance was similar in SDM and CDM groups for all time points except 9 months, where fewer people in the CDM group (59.3%) than the SDM group (75.5%) attended. It is assumed that attendance at the session resulted in gathering of appropriate outcome data at this time point. |
| Selective reporting (reporting bias) | High risk | Several outcomes are not reported fully for year 2 (including adherence and asthma control), and only results for the symptom subscale are given for the quality of life measure, rather than the total score. |
| Other bias | Low risk | None noted |
ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; CDM: clinician decision‐making; CMA: continuous medication acquisition; ED: emergency department; EHR: electronic health record; FEV1: forced expiratory volume in one second; GINA: Global Initiative for Asthma; GP: general practitioner; ICS: inhaled corticosteroid; ITG‐AST: Integrated Therapeutics Group ‐ Child Asthma Short Form; NAEPP: National Asthma Education and Prevention Program; PACQLQ: Pediatric Asthma Caregiver's Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; RCT: randomised controlled trial; SD: standard deviation; SDM: shared decision‐making; SMART: specific, measurable, acceptable, realistic, and time‐bound (goal).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Early 2015 | Population does not match the inclusion criteria: mixed respiratory population; only 17% had asthma and results are not given separately |
| Ford 1996 | Intervention does not match the inclusion criteria: Focus is asthma education, self‐management, and empowerment, rather than shared decision‐making. |
| Gorelick 2006 | Intervention does not match the inclusion criteria: case management/discharge planning from emergency department. Emphasis is not on shared decision‐making. |
| Moffat 2008 | Intervention does not match the inclusion criteria: Main emphasis is on communication skills. Not enough information about the intervention to include confidently (only abstracts, no full publication identified) |
| NCT00170248 | Intervention does not match the inclusion criteria: Focus is on supporting physicians' decisions, not on sharing decisions with patients |
| NCT00214669 | Intervention does not match inclusion criteria; broad intervention in which shared decision‐making was not the primary focus |
| NCT01522144 | Not an RCT: single group assignment |
| Smith 2008 | Intervention does not match the inclusion criteria: patient‐centred education following ED visit, not decision‐making |
| Sockrider 2001 | Intervention does not match the inclusion criteria: video to educate asthma families to follow an action plan. Some emphasis on communication but not strictly on shared decision‐making with a clinician |
| Tapp 2014 | Intervention does not match the inclusion criteria: testing different methods of disseminating a shared decision‐making intervention, rather than assessing whether it works |
| Tieffenberg 2000 | Intervention does not match the inclusion criteria: child‐centred care and empowerment to self‐manage asthma, not shared decision‐making |
ED: emergency department; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Gagné 2017.
| Methods | Parallel randomised controlled trial |
| Participants | Convenience sample of participants 18 to 65 years, with diagnosis of mild to severe asthma, and prescribed inhaled corticosteroids, alone or in combination with long‐acting β2‐agonists |
| Interventions | Asthma eduction plus decision aid vs asthma education alone |
| Outcomes | Knowledge of asthma; decisional conflict; appropriate use of asthma pharmacotherapy; asthma control |
| Notes | Funding: Principal investigator and co‐investigator received a grant from the Allergy, Genes and Environment Network for funding of the research (reference number for the project: 11CKT2). Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Publication: peer‐reviewed journal article |
Characteristics of ongoing studies [ordered by study ID]
Federman 2015.
| Trial name or title | Rationale and design of a comparative effectiveness trial of home‐ and clinic‐based self‐management support coaching for older adults with asthma |
| Methods | Pragmatic randomised controlled trial with 3 arms |
| Participants | 425 adults with asthma aged ≥ 60, based in New York |
| Interventions | 1. Intervention delivered in primary care 2. Intervention delivered at home 3. Usual care "In the intervention, care coaches use a novel screening tool to identify the specific barriers to asthma control and self‐management they experience. Once identified, the coach and patient choose from a menu of actions to address it. The intervention emphasizes efficiency, flexibility, shared decision making and goal setting, communication strategies appropriate for individuals with limited cognition and literacy skills, and ongoing reinforcement and support. Additionally, we introduced asthma‐specific enhancements to the electronic health records of all participating clinical practices, including an asthma severity assessment, clinical decision support, and a patient‐tailored asthma action plan." |
| Outcomes | Patients will be followed for 12 months and interviewed at baseline, and at 3, 6, and 12 months; data on emergency department visits and hospitalisations will be obtained through the New York State Statewide Planning and Research Cooperative System. |
| Starting date | Unclear |
| Contact information | Alex D Federman ‐ Division of General Internal Medicine, Icahn School of Medicine at Mount SInai, New York |
| Notes |
Hoskins 2013.
| Trial name or title | Goal‐setting intervention in patients with active asthma |
| Methods | Two‐armed, single‐blind, multi‐centre, cluster‐randomised controlled feasibility trial |
| Participants | Planned recruitment: 80 Primary care patients with active asthma from at least 8 practices across 2 health boards in Scotland (10 patients per practice, resulting in ˜40 in each arm) |
| Interventions | "Patients in the intervention arm will be asked to complete a novel goal‐setting tool immediately prior to an asthma review consultation. This will be used to underpin a focused discussion about their goals during the asthma review. A tailored management plan will then be negotiated to facilitate achieving their prioritised goals. Patients in the control arm will receive a usual care guideline‐based review of asthma." |
| Outcomes | "Data on quality of life, asthma control and patient confidence will be collected from both arms at baseline and 3 and 6 months post‐intervention. Data on health services resource use will be collected from all patient records 6 months pre‐ and post‐intervention. Semi‐structured interviews will be carried out with healthcare staff and a purposive sample of patients to elicit their views and experiences of the trial. The outcomes of interest in this feasibility trial are the ability to recruit patients and healthcare staff, the optimal method of delivering the intervention within routine clinical practice, and acceptability and perceived utility of the intervention among patients and staff." |
| Starting date | Overall trial start date: 01/09/2012 Overall trial end date: 30/11/2013 |
| Contact information | Dr Gaylor Hoskins ‐ Nursing Midwifery and Allied Health Professions (NMAHP) Research Unit, Iris Murdoch Building, University of Stirling |
| Notes |
NCT02516449.
| Trial name or title | Assessment of shared decision‐making aids in asthma |
| Methods | Randomised, parallel, double‐blind study (investigators and outcome assessors blinded) |
| Participants | Planned enrolment: 51 Men or women, aged 18 to 65 years, with current diagnosis of mild to severe asthma (details of asthma eligibility given on clinicaltrials.gov) People with COPD or recent asthma education (last 6 months) excluded |
| Interventions | Patient decision aid that participants read and fill before being provided education on asthma. The decision aid is a 12‐page A3 booklet entitled "Should I take asthma inhaled controller medication to optimize asthma control?" Control group received no intervention. |
| Outcomes | Primary outcomes: asthma knowledge measured by QCALF score and decisional conflict measured by DCS score (both as change from baseline to 2 months) Secondary outcomes: adherence to treatment, measured by questionnaire, and asthma control, measured by ACSS score (both as change from baseline to 2 months) |
| Starting date | March 2013 ‐ Study authors confirmed that study was undergoing amendments at the time of writing of this review. |
| Contact information | Louis‐Philippe Boulet, MD, Centre de Recherche de l'Institut Universitaire de Cardiologie et de Pneumologie de Quebec |
| Notes |
Tapp 2011.
| Trial name or title | Comparative effectiveness of asthma interventions within a practice‐based research network |
| Methods | Unclear if randomised. A centralised database will be created with the goal of facilitating comparative effectiveness research on asthma outcomes specifically for this study. Patient and community level analysis will include results from patient surveys, focus groups, and asthma patient density mapping. Community variables such as income and housing density will be mapped for comparison. |
| Participants | This study will include 95 practices, 171 schools, and more than 30,000 asthmatic patients. |
| Interventions |
|
| Outcomes | Hospitalisations and emergency department visits; improved adherence to medication; improved quality of life; reduced school absenteeism; improved self‐efficacy; improved school performance |
| Starting date | Unclear |
| Contact information | Lisa.Hebert@carolinashealthcare.org ‐ Carolinas Physicians Network, Carolinas HealthCare System, Charlotte, NC |
| Notes |
ACSS: Asthma Control Scoring System; COPD: chronic obstructive pulmonary disease; DCS: Decisional Conflict Scale; EMR: electronic medical record; QCALF: self‐administered French scale assessing four domains of asthma knowledge.
Differences between protocol and review
In the Dealing with missing data section, we changed the wording after "Where this was not possible, and we considered that the missing data may introduce serious bias" from "we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis" to "we explored the impact in the Grading of Recommendations Assessment, Development and Evaluation (GRADE) rating for that outcome."
Rebecca Normansell joined the review author team at the review stage. She extracted data and assessed studies for risk of bias, instead of PM, as had been planned. This was a more practical approach, as KK and RN are based in the same office.
We had planned to exclude cross‐over trials owing to the likelihood of carry‐over of effects, but for future updates, we will include the first phase of a cross‐over trial. We did not identify any relevant cross‐over trials during our searches.
Contributions of authors
KK wrote the Background and Methods sections of this review with support from PM.
For the full review, KK, PM, and RN screened search results and selected studies for inclusion. KK and RN finalised the included studies, extracted data, and assessed risk of bias in the included studies. KK conducted the analyses and wrote up the results, with input from RN. RN and KK assessed the quality of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. RN, PM, and KA wrote the Discussion section, with input from KK. All review authors contributed to interpretation of findings and assisted in preparing the manuscript for submission.
Sources of support
Internal sources
-
Kayleigh M Kew, UK.
Supported by St George's, University of London
External sources
-
National Institute for Health Research (NIHR), UK.
Evidence to guide care in adults and children with asthma, 13/89/14
This project was supported by the NIHR, via Cochrane Infrastructure, Cochrane Programme Grant, or Cochrane Incentive funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS, or the Department of Health
Declarations of interest
KK is funded to prepare Cochrane reviews by a Programme Grant awarded by the NIHR to the Cochrane Airways Group.
PM has reported no conflicts.
KA is a consultant respiratory paediatrician with respiratory interest in the NHS. He has no alternative sources of funding.
RN is a qualified general practitioner and the deputy Co‐ordinating Editor of Cochrane Airways. She is funded by an NIHR grant to Cochrane Airways.
New
References
References to studies included in this review
Clark 1998 {published data only}
- Clark NM, Gong M, Schork MA, Evans D, Roloff D, Hurwitz M, et al. Impact of education for physicians on patient outcomes. Pediatrics 1998;101(5):831‐6. [CENTRAL: 688433; CRS: 4900100000023539; PUBMED: 9565410] [DOI] [PubMed] [Google Scholar]
- Clark NM, Gong M, Schork MA, Kaciroti N, Evans D, Roloff D, et al. Long‐term effects of asthma education for physicians on patient satisfaction and use of health services. European Respiratory Journal 2000;16(1):15‐21. [CENTRAL: 316303; CRS: 4900100000009731; 4900100000009731; PUBMED: 10933079] [DOI] [PubMed] [Google Scholar]
- Frasca MA. Participation in an interactive seminar improved paediatricians' patient teaching and communication skills: commentary. Evidence‐Based Medicine 1999;4(1):31. [CENTRAL: 310793; CRS: 4900100000009226] [Google Scholar]
Fiks 2015 {published data only}
- Fiks AG, Mayne SL, Karavite DJ, Suh A, O'Hara R, Localio AR, et al. Parent‐reported outcomes of a shared decision‐making portal in asthma: a practice‐based RCT. Pediatrics 2015;135(4):e965‐73. [CENTRAL: 1066974; CRS: 4900126000027102; EMBASE: 2015934354; PUBMED: 25755233] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01715389. Evaluation of a shared decision making portal for pediatric asthma. clinicaltrials.gov/show/NCT01715389 (first received 10 October 2012). [CRS: 4900132000030028]
van Bragt 2015 {published data only}
- NCT01109745. Effectiveness of the pelican Instrument in medical care (PELICANII). clinicaltrials.gov/show/NCT01109745 (first received 22 April 2010).
- Bragt S, Bemt L, Kievits R, Merkus P, Weel C, Schermer T. PELICAN: a randomized controlled trial in Dutch General Practices to assess the effectiveness of individualised self‐management for paediatric asthma [Abstract]. 7th International Primary Care Respiratory Group (IPCRG) World Conference; 2014 May 21‐24; Athens. 2014:OR‐009. [CENTRAL: 994138; CRS: 4900126000016465]
- Bragt S, Bemt L, Cretier R, Weel C, Merkus P, Schermer T. PELICAN: content evaluation of patient‐centered care for children with asthma based on an online tool. Pediatric Pulmonology 2016;51(10):993‐1003. [CENTRAL: 1152866; CRS: 4900132000019804; PUBMED: 27128738] [DOI] [PubMed] [Google Scholar]
- Bragt S, Bemt L, Kievits R, Merkus P, Weel C, Schermer T. PELICAN: a cluster‐randomized controlled trial in Dutch general practices to assess a self‐management support intervention based on individual goals for children with asthma. Journal of Asthma 2015;52(2):211‐9. [CENTRAL: 1077131; CRS: 4900132000004118; EMBASE: 2015136467; PUBMED: 25166455] [DOI] [PubMed] [Google Scholar]
- Bragt S, Bemt L, Thoonen B, Weel C, Merkus P, Schermer T. PELICAN: a quality of life instrument for childhood asthma: study protocol of two randomized controlled trials in primary and specialized care in the Netherlands. BMC Pediatrics 2012;12(137):137. [CENTRAL: 834422; CRS: 4900100000061225; EMBASE: 2012715722; PUBMED: 22935133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2010 {published data only}
- Does shared decision‐making improve asthma outcomes?. https://clinicaltrials.gov/ct2/show/NCT00149526 (accessed 9 September 2016).
- NCT00149526. Does shared decision‐making improve asthma outcomes?. https://clinicaltrials.gov/ct2/show/NCT00149526 (first received 6 September 2005). [CRS: 4900100000021091]
- NCT00217945. Does shared decision‐making improve adherence in asthma. clinicaltrials.gov/ct2/show/NCT00217945 (first received 19 September 2005).
- Wilson SR, Knowles S, Qian Y, Buist AS, Strub P, Lapidus J, et al. Does involving patients in treatment decisions reduce use of asthma rescue medication? [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:[A242]. [CENTRAL: 651696; CRS: 4900100000022587]
- Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. American Journal of Respiratory and Critical Care Medicine 2010;181(6):566‐77. [CENTRAL: 728584; CRS: 4900100000024367; EMBASE: 2010157400; PUBMED: 20019345] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Strub P, Buist AS, Verghese S, Brown N, Luna V, et al. Does involving patients in treatment decisions improve asthma controller medication adherence? [Abstract]. Proceedings of the American Thoracic Society. 2006:A469. [CENTRAL: 592304; CRS: 4900100000020891]
- Wilson SR, Strub P, Knowles SB, Buist AS, Huang Q, Nguyen M. Ethnicity, income, and education as potential modifiers of the effects of shared treatment decision‐making (SDM) between asthma care manager and patient on asthma controller medication adherence: the better outcomes of asthma treatment (BOAT) trial [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S72. [CENTRAL: 756675; CRS: 4900100000025163] [Google Scholar]
References to studies excluded from this review
Early 2015 {published data only}
- Early F, Everden AJ, O'Brien CM, Fagan PL, Fuld JP. Patient agenda setting in respiratory outpatients: a randomized controlled trial. Chronic Respiratory Disease 2015;12(4):347‐56. [CENTRAL: 1101025; CRS: 4900132000009524; EMBASE: 2015440958; PUBMED: 26272499] [DOI] [PubMed] [Google Scholar]
Ford 1996 {published data only}
- Ford ME, Edwards G, Rodriguez JL, Gibson RC, Tilley BC. An empowerment‐centered, church‐based asthma education program for African American adults. Health and Social Work 1996;21(1):70‐5. [DOI] [PubMed] [Google Scholar]
Gorelick 2006 {published data only}
- Gorelick MH, Meurer JR, Walsh‐Kelly CM, Brousseau DC, Grabowski L, Cohn J, et al. Emergency department allies: a controlled trial of two emergency department‐based follow‐up interventions to improve asthma outcomes in children. Pediatrics 2006;117(4 Suppl 2):S127‐34. [DOI] [PubMed] [Google Scholar]
Moffat 2008 {published data only}
- Cleland A, Price DB, Hall S. Implementing asthma action plans: practice nurse training in patient centered communication skills has no impact on patient outcomes. Thorax 2004;59:ii32. [Google Scholar]
- Cleland JA, Price DB, Moffat M, Hall S. Training in the community‐based doctors and nurses in patient‐centred asthma care: recognition of need and prerequisities to training [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego. 2005:[D93] [Poster: 603]. [CENTRAL: 524613; CRS: 4900100000018663]
- Moffat M, Cleland J, Clark N, Cotton, Bucknall C, Griffiths C, et al. An educational intervention for patient‐centred asthma care (PACE) modified for training practice nurses (PNs) and general practitioners (GPs) in Scotland: first stage evaluation [Abstract]. Primary Care Respiratory Journal 2008;17(2):122. [CENTRAL: 642736; CRS: 4900100000022294] [Google Scholar]
NCT00170248 {published data only}
- NCT00170248. Computer‐based decision support in managing asthma in primary care. clinicaltrials.gov/ct2/show/NCT00170248 (first received 13 September 2005).
NCT00214669 {published data only}
- Griffiths C, Bremner S, Islam K, Sohanpal R, Vidal DL, Dawson C, et al. Effect of an education programme for South Asians with asthma and their clinicians: a cluster randomised controlled trial (OEDIPUS). PLOS One 2016;11(12):e0158783. [DOI: 10.1371/journal.pone.0158783] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00214669. Can education for South Asians with asthma and their clinicians reduce unscheduled care? A randomised trial (OEDIPUS). clinicaltrials.gov/ct2/show/NCT00214669 (first received 14 September 2005).
NCT01522144 {published data only}
- NCT01522144. An electronic decision support tool to improve outpatient asthma care. https://clinicaltrials.gov/ct2/show/NCT01522144 (accessed 9 September 2016).
Smith 2008 {published data only}
- Smith S, Mitchell C, Bowler S. Standard versus patient‐centred asthma education in the emergency department: a randomised study [see comment]. European Respiratory Journal 2008;31(5):990‐7. [CENTRAL: 637591; CRS: 4900100000022025; EMBASE: 2009045444; PUBMED: 18216062] [DOI] [PubMed] [Google Scholar]
- Smith S, Mitchell C, Fleming M, Bowler S. A randomised control trial (RCT) of patient centred education in emergency department (EDS) [Abstract]. Respirology 2005;10(Suppl):A37. [CENTRAL: 518753; CRS: 4900100000018405] [Google Scholar]
Sockrider 2001 {published data only}
- Sockrider MM, Czyzewski DI, West BL, Pella JJ, Swank PR. Promoting family decision‐making using a pediatric asthma action plan. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A293. [CENTRAL: 394615; CRS: 4900100000012784] [Google Scholar]
Tapp 2014 {published data only}
- NCT02047929. Comparing types of implementation of a shared decision making intervention (ADAPT‐NC) [Comparing traditional and participatory dissemination of a shared decision making intervention]. clinicaltrials.gov/show/NCT02047929 (first received 27 January 2014). [CRS: 4900132000022864]
- Tapp H, McWilliams A, Ludden T, Kuhn L, Taylor Y, Alkhazraji T, et al. Comparing traditional and participatory dissemination of a shared decision making intervention (ADAPT‐NC): a cluster randomized trial. Implementation Science 2014;9(1):158. [CENTRAL: 1015317; CRS: 4900126000020910; PUBMED: 25359128] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tieffenberg 2000 {published data only}
- Tieffenberg JA, Wood EI, Alonso A, Tossutti MS, Vicente MF. A randomized field trial of ACINDES: a child‐centered training model for children with chronic illnesses (asthma and epilepsy). Journal of Urban Health 2000;77(2):280‐97. [CENTRAL: 297472; CRS: 4900100000008730; PUBMED: 10856009] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gagné 2017 {published data only}
- Gagné M, Légaré F, Moisan J, Boulet L. Adding a decision aid to asthma education: impact on decisional conflict and appropriate medication usage. Value in Health. 2016; Vol. 19, issue 7:A557.
- Gagné ME, Légaré F, Moisan J, Boulet LP. Impact of adding a decision aid to patient education in adults with asthma: a randomized clinical trial. PLoS One 2017;12(1):e0170055. [DOI: 10.1371/journal.pone.0170055] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Federman 2015 {published data only}
- Federman AD, Martynenko M, O'Conor R, Kannry J, Karp A, Lurio J, et al. Rationale and design of a comparative effectiveness trial of home‐ and clinic‐based self‐management support coaching for older adults with asthma. Contemporary Clinical Trials 2015;44:103‐11. [DOI] [PubMed] [Google Scholar]
Hoskins 2013 {published data only}
- Hoskins G, Abhyankar P, Taylor AD, Duncan E, Sheikh A, Pinnock H, et al. Goal‐setting intervention in patients with active asthma: protocol for a pilot cluster‐randomised controlled trial. Trials 2013;14(1):289. [CENTRAL: 870965; CRS: 4900100000088569; EMBASE: 2013573201; PUBMED: 24021033] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02516449 {published data only}
- Gagné ME, Legare F, Moisan J, Boulet L‐P. Impact of adding a decision aid to asthma education: a randomized clinical trial. Under review. [DOI] [PMC free article] [PubMed]
- NCT02516449. Assessment of shared decision making aids in asthma. clinicaltrials.gov/ct2/show/NCT02516449 (first received 2 July 2015).
Tapp 2011 {published data only}
- Tapp H, Hebert L, Dulin M. Comparative effectiveness of asthma interventions within a practice based research network. BMC Health Services Research 2011;11:188. [CENTRAL: 833357; CRS: 4900100000050044; EMBASE: 21846401; PUBMED: 21846401] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adams 2001
- Adams RJ, Smith BJ, Ruffin RE. Patient preferences for autonomy in decision making in asthma management. Thorax 2001;56(2):126‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akinbami 2002
- Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics 2002;110(2 pt 1):315‐22. [DOI] [PubMed] [Google Scholar]
Akinbami 2006
- Akinbami L, Centers for Disease Control and Prevention National Center for Health Statistics. The state of childhood asthma, United States, 1980‐2005. Advance Data 2006;12(381):1‐24. [PubMed] [Google Scholar]
Asthma UK 2011
- Asthma UK. Asthma UK’s Research Strategy: 2011–2016. asthma.org.uk/globalassets/research/research_strategy_2011‐2016.pdf (accessed 11 May 2016).
Butz 2007
- Butz AM, Walker JM, Pulsifer M, Winkelstein M. Shared decision making in school age children with asthma. Journal of Pediatric Nursing 2007;33(2):111‐6. [PMC free article] [PubMed] [Google Scholar]
Caress 2005
- Caress A‐L, Beaver K, Luker K, Campbell M, Woodcock A. Involvement in treatment decisions: what do adults with asthma want and what do they get? Results of a cross sectional survey. Thorax 2005;60(3):199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charles 1997
- Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: what does it mean? (or it takes at least two to tango). Social Science and Medicine 1997;44(5):681‐92. [DOI] [PubMed] [Google Scholar]
Charles 1999
- Charles C, Gafni A, Whelen T. Decision‐making in the physician‐patient encounter: revisiting the shared treatment decision‐making model. Social Science and Medicine 1999;49:651‐61. [DOI] [PubMed] [Google Scholar]
Coulter 2011
- Coulter A, Collins A. Making shared decision‐making a reality: no decision about me, without me. kingsfund.org.uk/sites/files/kf/Making‐shared‐decision‐making‐a‐reality‐paper‐Angela‐Coulter‐Alf‐Collins‐July‐2011_0.pdf (accessed 16 August 2016).
Coxeter 2015
- Coxeter P, Mar CB, McGregor L, Beller EM, Hoffmann TC. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD010907.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daly 2016
- Daly R, Bunn F, Goodman C. Shared decision‐making for people living with dementia in extended care settings: protocol for a systematic review. BMJ Open 2016;6(11):e012955. [DOI: 10.1136/bmjopen-2016-012955] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Benedictis 2007
- Benedictis D, Bush A. The challenge of asthma in adolescence. Pediatric Pulmonology 2007;42:683‐92. [DOI] [PubMed] [Google Scholar]
Durand 2014
- Durand MA, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, et al. Do interventions designed to support shared decision making reduce health inequalities? A systematic review and meta‐analysis. PLoS One 2014;9(4):e94670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwamena 2012
- Dwamena F, Holmes‐Rovner M, Gaulden CM, Jorgenson S, Sadigh G, Sikorskii A, et al. Interventions for providers to promote a patient‐centred approach in clinical consultations. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD003267.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2002
- Gibson PG, Powell H, Wilson A, Abramson MJ, Haywood P, Bauman A, et al. Self‐management education and regular practitioner review for adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001117] [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2016
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. http://ginasthma.org/2017‐gina‐report‐global‐strategy‐for‐asthma‐management‐and‐prevention/ (accessed 28 February 2017).
Global Asthma Network 2014
- Global Asthma Network. The Global Asthma Report 2014. http://globalasthmareport.org/index.php (accessed 3 May 2016).
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 11 May 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guevara 2003
- Guevara JP, Wolf FM, Grum CM, Clark NM. Effects of educational interventions for self management of asthma in children and adolescents: systematic review and meta‐analysis. BMJ 2003;326(7402):1308‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. http://handbook.cochrane.org/.
Institute of Medicine 2009
- Committee On Comparative Effectiveness Research Prioritization. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academy Press, 2009. [Google Scholar]
Joosten 2008
- Joosten EAG, DeFuentes‐Merillas L, Weert GH, Sensky T, Staak CPF, Jong CAJ. Systematic review of the effects of shared decision‐making on patient satisfaction, treatment adherence and health status. Psychotherapy and Psychosomatics 2008;77:219‐26. [DOI] [PubMed] [Google Scholar]
Légaré 2013
- Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Affairs 2013;32(2):276‐84. [DOI] [PubMed] [Google Scholar]
Légaré 2014
- Légaré F, Stacey D, Turcotte S, Cossi MJ, Kryworuchko J, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006732.pub3] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHLBI/NAEPP 2007
- National Heart, Lung and Blood Institute and the National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma (EPR‐3). Section 3, Component 2: education for a partnership in asthma care. https://nhlbi.nih.gov/files/docs/guidelines/05_sec3_comp2.pdf (accessed 11 May 2016).
NRAD 2014
- National Review of Asthma Deaths. Why asthma still kills: the national review of asthma deaths. https://rcplondon.ac.uk/sites/default/files/why‐asthma‐still‐kills‐full‐report.pdf (accessed 3 May 2016).
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivera‐Spoljaric 2014
- Rivera‐Spoljaric K, Halley M, Wilson SR. Shared clinician‐patient decision‐making about treatment of pediatric asthma: what do we know and how can we use it?. Current Opinions in Allergy and Clinical Immunology 2014;14(2):161‐7. [DOI] [PubMed] [Google Scholar]
Shay 2015
- Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Medical Decision Making 2015;35(1):114‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sleath 2011
- Sleath BL, Carpenter DM, Sayner R, Ayala GX, Williams D, Davis S, et al. Child and caregiver involvement and shared decision‐making during asthma pediatric visits. Journal of Asthma 2011;48(10):1022‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snyder 2016
- Snyder H, Engström J. The antecedents, forms and consequences of patient involvement: a narrative review of the literature. International Journal of Nursing Studies 2016;53:351‐78. [DOI] [PubMed] [Google Scholar]
Veroff 2013
- Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference‐sensitive conditions. Health Affairs 2013;32(2):285‐93. [DOI] [PubMed] [Google Scholar]
Wyatt 2015
- Wyatt KD, List B, Brinkman WB, Prutsky Lopez G, Asi N, et al. Shared decision making in pediatrics: a systematic review and meta‐analysis. Systematic Review 2015;51:573–83. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kew 2016
- Kew KM, Malik P. Shared decision‐making for people with asthma. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012330] [DOI] [PMC free article] [PubMed] [Google Scholar]


