Abstract
Background
Bronchopulmonary dysplasia remains a major problem in neonatal intensive care units. Persistent inflammation in the lungs is the most likely underlying pathogenesis. Corticosteroids have been used to prevent or treat bronchopulmonary dysplasia because of their potent anti‐inflammatory effects.
Objectives
To examine the relative benefits and adverse effects of systemic postnatal corticosteroids commenced within the first seven days of life for preterm infants at risk of developing bronchopulmonary dysplasia.
Search methods
For the 2017 update, we used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1); MEDLINE via PubMed (January 2013 to 21 February 2017); Embase (January 2013 to 21 February 2017); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (January 2013 to 21 February 2017). We also searched clinical trials databases, conference proceedings, and reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi‐randomised trials.
Selection criteria
For this review, we selected RCTs examining systemic postnatal corticosteroid treatment within the first seven days of life (early) in high‐risk preterm infants. Most studies evaluated the use of dexamethasone, but we also included studies that assessed hydrocortisone, even when used primarily for management of hypotension.
Data collection and analysis
We used the GRADE approach to assess the quality of evidence.
We extracted and analysed data regarding clinical outcomes that included mortality, bronchopulmonary dysplasia, death or bronchopulmonary dysplasia, failure to extubate, complications during primary hospitalisation, and long‐term health outcomes.
Main results
We included 32 RCTs enrolling a total of 4395 participants. The overall risk of bias of included studies was probably low, as all were RCTs, and most trials used rigorous methods. Investigators reported significant benefits for the following outcomes overall: lower rates of failure to extubate, decreased risks of bronchopulmonary dysplasia both at 28 days of life and at 36 weeks' postmenstrual age, death or bronchopulmonary dysplasia at 28 days of life and at 36 weeks' postmenstrual age, patent ductus arteriosus, and retinopathy of prematurity (ROP), including severe ROP. Researchers found no significant differences in rates of neonatal or subsequent mortality; they noted that gastrointestinal bleeding and intestinal perforation were important adverse effects, and that risks of hyperglycaemia, hypertension, hypertrophic cardiomyopathy, and growth failure were increased. The 13 trials that reported late outcomes described several adverse neurological effects at follow‐up examination, including cerebral palsy. However, study authors indicated that major neurosensory disability was not significantly increased, either overall in the eight studies for which this outcome could be determined, or in the two individual studies in which rates of cerebral palsy or abnormal neurological examination were significantly increased. Moreover, data show that rates of the combined outcomes of death or cerebral palsy, or of death or major neurosensory disability, were not significantly increased. Two‐thirds of studies used dexamethasone (n = 21). Subgroup analyses by type of corticosteroid revealed that most of the beneficial and harmful effects of treatment were attributable to dexamethasone. However, as with dexamethasone, hydrocortisone was associated with reduced rates of patent ductus arteriosus, mortality, and the combined outcome of mortality or chronic lung disease, but with increased occurrence of intestinal perforation. Results showed that hydrocortisone was not associated with obvious longer‐term problems.
Use of the GRADE approach revealed that the quality of evidence was high for the major outcomes considered, but review authors downgraded quality one level for several outcomes (mortality at latest age, bronchopulmonary dysplasia at 36 weeks, and death or bronchopulmonary dysplasia at 36 weeks) because of weak evidence of publication bias or moderate heterogeneity (death or cerebral palsy).
Authors' conclusions
Benefits of early postnatal corticosteroid treatment (≤ 7 days), particularly dexamethasone, may not outweigh adverse effects associated with this treatment. Although early corticosteroid treatment facilitates extubation and reduces risk of bronchopulmonary dysplasia and patent ductus arteriosus, it causes short‐term adverse effects including gastrointestinal bleeding, intestinal perforation, hyperglycaemia, hypertension, hypertrophic cardiomyopathy, and growth failure. Long‐term follow‐up studies report increased risk of abnormal findings on neurological examination and increased risk of cerebral palsy. However, the methodological quality of studies examining long‐term outcomes is limited in some cases: Surviving children have been assessed predominantly before school age; no study has been sufficiently powered to detect important adverse long‐term neurosensory outcomes; and no study has been designed with survival free of adverse long‐term neurodevelopmental disability as the primary outcome. There is a compelling need for long‐term follow‐up and reporting of late outcomes, especially neurological and developmental outcomes, among surviving infants who participated in all randomised trials of early postnatal corticosteroid treatment. Hydrocortisone reduced rates of patent ductus arteriosus, of mortality, and of the combined outcome of mortality or bronchopulmonary dysplasia, without causing any obvious long‐term harm. However, gastrointestinal perforation was more frequent in the hydrocortisone group. Longer‐term follow‐up into late childhood is vital for assessment of important effects or other effects that cannot be assessed in early childhood, such as effects of early hydrocortisone treatment on higher‐order neurological functions, including cognitive function, academic performance, behaviour, mental health, and motor function. Further randomised controlled trials of early hydrocortisone should include longer‐term survival free of neurodevelopmental disability as the main outcome.
Plain language summary
Early (up to seven days) systemic postnatal corticosteroids for preventing bronchopulmonary dysplasia in preterm infants
Review objective: To determine the relative benefits and harms of treatment with drugs that suppress inflammation, called corticosteroids, given to babies born too early during the first week after birth to prevent lung injury, known as bronchopulmonary dysplasia (sometimes also called chronic lung disease).
Background: Corticosteroids can reduce lung inflammation in newborn babies with bronchopulmonary dysplasia but may produce major adverse effects. Bronchopulmonary dysplasia is a major problem for newborn babies in neonatal intensive care units. Persistent inflammation of the lungs is the most likely cause. Corticosteroid drugs have been used to prevent or treat bronchopulmonary dysplasia through their strong anti‐inflammatory effects.
Study characteristics: We reviewed all clinical trials in preterm babies in which corticosteroids had been given as a medication during the first week after birth, and from which data on the rate of bronchopulmonary dysplasia later in the newborn period were available.
Key results: This review of trials revealed that the benefits of giving systemic corticosteroids to infants starting up to seven days after birth may not outweigh the known adverse effects. However, a particular corticosteroid called hydrocortisone shows promise in improving short‐term outcomes without adversely affecting long‐term neurodevelopment. Beneficial effects of systemic corticosteroids overall included shorter time on the ventilator and less bronchopulmonary dysplasia, but adverse effects included higher blood pressure, bleeding from the stomach or bowel, perforation of the bowel, excessive glucose in the bloodstream, and increased risk of cerebral palsy at follow‐up, particularly in those treated with dexamethasone ‐ another type of corticosteroid. Early use of corticosteroids, especially dexamethasone, to treat or prevent bronchopulmonary dysplasia should be curtailed until additional research has been performed.
Quality of evidence: Overall, the quality of evidence supporting our conclusions was high.
Summary of findings
Summary of findings for the main comparison. Early systemic postnatal corticosteroids compared with placebo or no treatment for preventing bronchopulmonary dysplasia in preterm infants.
| Early systemic postnatal corticosteroids compared with placebo or no treatment for preventing bronchopulmonary dysplasia in preterm infants | ||||||
| Patient or population: preventing bronchopulmonary dysplasia in preterm infants Setting: neonatal intensive care units Intervention: early systemic postnatal corticosteroids Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with early systemic postnatal corticosteroids | |||||
| Mortality at 36 weeks | Study population | RR 1.01 (0.89 to 1.14) | 3733 (20 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 211 per 1000 | 213 per 1000 (188 to 241) | |||||
| Mortality at latest reported age | Study population | RR 0.95 (0.85 to 1.06) | 4373 (31 RCTs) | ⊕⊕⊕⊝ MODERATEa | ||
| 232 per 1000 | 221 per 1000 (197 to 246) | |||||
| BPD (36 weeks) | Study population | RR 0.79 (0.72 to 0.87) | 3929 (24 RCTs) | ⊕⊕⊕⊝ MODERATEb | ||
| 322 per 1000 | 254 per 1000 (232 to 280) | |||||
| Death or BPD at 36 weeks | Study population | RR 0.88 (0.83 to 0.93) | 3960 (25 RCTs) | ⊕⊕⊕⊝ MODERATEb | ||
| 531 per 1000 | 467 per 1000 (441 to 494) | |||||
| Gastrointestinal perforation | Study population | RR 1.72 (1.29 to 2.30) | 3040 (16 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 43 per 1000 | 74 per 1000 (56 to 99) | |||||
| Cerebral palsy | Study population | RR 1.42 (1.06 to 1.91) | 1973 (13 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 74 per 1000 | 106 per 1000 (79 to 142) | |||||
| Death or cerebral palsy | Study population | RR 1.03 (0.91 to 1.16) | 1973 (13 RCTs) | ⊕⊕⊕⊝ MODERATEc | ||
| 335 per 1000 | 345 per 1000 (305 to 389) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious study limitations owing to weak evidence for publication bias, particularly for studies of hydrocortisone.
bDowngraded one level for serious study limitations owing to weak evidence for publication bias, for both dexamethasone and hydrocortisone studies.
cDowngraded one level for serious study limitations owing to moderate heterogeneity, particularly for studies of dexamethasone.
Background
Description of the condition
Advances in neonatal care, including use of antenatal corticosteroids and surfactant therapy, have improved the outcomes of preterm infants with respiratory distress syndrome, but risk of chronic lung disease or bronchopulmonary dysplasia (BPD) has been only modestly reduced (Egberts 1997). The terms 'chronic lung disease' and 'bronchopulmonary dysplasia' are often used interchangeably; for the purposes of this review, we have decided to use 'bronchopulmonary dysplasia' to describe the condition of infants with oxygen dependency at 28 days of life or at 36 weeks' postmenstrual age. More infants with BPD are now cared for in neonatal intensive care units (NICUs), and management of their condition is both time‐consuming and costly. Bronchopulmonary dysplasia refers to injury and maldevelopment of the lung that follows preterm birth and is a major problem in NICUs. Persistent inflammation in the lungs is the most likely underlying pathogenesis.
Description of the intervention
Postnatal corticosteroid treatment has been shown to have some beneficial acute effects on lung function in infants with established BPD, especially among those who are ventilator‐dependent (CDTG 1991; Mammel 1983). However, clinicians have been concerned that the benefits of corticosteroids might not outweigh associated adverse effects, which include hypertension, hyperglycaemia, intestinal perforation, and extreme catabolism (Anonymous 1991; Ng 1993).
Corticosteroids have been used to try to prevent BPD by treating at‐risk preterm infants, starting within the first four days of life. They are given parenterally or enterally. It is not clear whether early use of corticosteroids provides long‐term benefits, nor is it clear whether adverse neurological outcomes observed in animal studies do not apply to the immature human newborn infant.
How the intervention might work
Corticosteroids might prevent BPD through their potent anti‐inflammatory effects.
Why it is important to do this review
Multiple systematic reviews have examined the use of postnatal corticosteroids in infants with or at risk of BPD (Arias‐Camison 1999; Bhuta 1998; Doyle 2000; Doyle 2010a; Doyle 2010b; Doyle 2010c; Doyle 2014a; Doyle 2014b; Halliday 1997; Halliday 1999; Tarnow‐Mordi 1999). Other systematic reviews have explored early versus late use of inhaled corticosteroids and comparisons of systemic versus inhaled steroids for prevention or treatment of BPD (Onland 2017; Shah 2007b; Shah 2012a; Shah 2012b; Shah 2017).
Two existing Cochrane reviews have reviewed separately trials in which postnatal corticosteroids were started before 8 days of birth or after the first 7 days following birth (Doyle 2014a; Doyle 2014b). This review examines the outcomes of trials in which preterm infants were treated with corticosteroids up to seven days after birth. It is an update of previous Cochrane reviews and includes long‐term outcome data from 13 trials (Doyle 2014a; Halliday 2000; Halliday 2010).
Objectives
To examine the relative benefits and adverse effects of systemic postnatal corticosteroids commenced within the first seven days of life for preterm infants at risk of developing bronchopulmonary dysplasia.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify randomised controlled trials (RCTs) of systemic postnatal corticosteroid therapy in preterm infants at risk of developing BPD, who were enrolled within the first seven days of life (early postnatal corticosteroids). We included trials that provided hydrocortisone in the first days of life, even if it had been used primarily to treat or prevent hypotension.
Types of participants
Preterm infants at risk of developing BPD, including those who are ventilator‐dependent.
Types of interventions
Intravenous or oral corticosteroids versus control (placebo or no treatment). We did not include in this review trials of inhaled corticosteroids.
Types of outcome measures
Primary outcomes
Mortality
Bronchopulmonary dysplasia (at 28 days of life, at 36 weeks' postmenstrual age, and at 36 weeks' postmenstrual age in survivors)
Death or bronchopulmonary dysplasia (at 28 days of life and at 36 weeks' postmenstrual age)
Long‐term outcomes (including blindness, deafness, cerebral palsy, and major neurosensory disability)
Secondary outcomes
Failure to extubate
Late rescue with corticosteroids (in all infants and in survivors)
Need for home oxygen therapy
Complications during primary hospitalisation (including infection, hyperglycaemia, hypertension, pulmonary air leak, patent ductus arteriosus, severe intraventricular haemorrhage, periventricular leukomalacia, necrotising enterocolitis, gastrointestinal bleeding, intestinal perforation, and severe retinopathy of prematurity)
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library; MEDLINE via PubMed (January 2013 to 21 February 2017); Embase (January 2013 to 21 February 2017); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (January 2013 to 21 February 2017), using the following search terms: (adrenal cortex hormones OR dexamethasone OR betamethasone OR hydrocortisone OR steroid OR corticosteroid), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for full search strategies for each database). We did not apply language restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization International Trial Registry and Platform (www.whoint/ictrp/search/en/); and the ISRCTN Registry).
See Appendix 2 for search strategies used previously.
Searching other resources
We also searched the reference lists of all published trials to identify trials overlooked during the electronic literature search.
Data collection and analysis
We used methods of the Cochrane Neonatal Group for data collection and analysis.
Selection of studies
We included all randomised and quasi‐randomised controlled trials that fulfilled the selection criteria described in the previous section. Two review authors (LWD and JC) independently reviewed results of the updated search and selected studies for inclusion. We resolved disagreements by discussion.
Data extraction and management
For each trial, we sought information regarding methods of randomisation, blinding, stratification, and reporting of outcomes for all infants enrolled, and whether the trial used a single‐centre or multi‐centre setting. Information on trial participants included birth weight, gestational age, severity of respiratory distress syndrome, need for mechanical ventilation and surfactant, and sex. We analysed information on clinical outcomes for mortality, survival without BPD, BPD defined at 28 days of life and at 36 weeks' postmenstrual age, failure to extubate, pneumothorax, infection, hyperglycaemia, hypertension, severe retinopathy of prematurity, patent ductus arteriosus, severe intraventricular haemorrhage, periventricular leukomalacia, necrotising enterocolitis, gastrointestinal bleeding, intestinal perforation, and need for late corticosteroid treatment, as well as long‐term outcomes such as developmental delay, blindness, deafness, cerebral palsy, and major neurosensory disability.
For each study, one review author entered final data into Review Manager (RevMan) 5 software (RevMan 2014); a second review author checked the data for accuracy. We resolved discrepancies through discussion or by consultation with a third assessor.
We attempted to contact authors of the original reports to request further details when information regarding any of the above was unclear.
Assessment of risk of bias in included studies
Two review authors (LWD, JC) independently assessed risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2011).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We used standard methods of the Cochrane Neonatal Group when analysing data.
We performed statistical analyses using RevMan 5. We analysed dichotomous data using risk ratio (RR), risk difference (RD), and the number needed to treat for an additional beneficial (NNTB) or harmful outcome (NNTH). We reported 95% confidence intervals (CIs) for all estimates.
We included no continuous outcomes in this review. If included, we planned to analyse continuous data using the mean difference (MD) or the standardised mean difference (SMD) to combine trials that measure the same outcome using different methods.
Unit of analysis issues
For clinical outcomes such as episodes of sepsis, we analysed the data as proportions of neonates having one or more episodes.
Dealing with missing data
For included studies, we noted levels of attrition. If we had concerns regarding the impact of including studies with high levels of missing data in the overall assessment of treatment effect, we planned to explore this concern via sensitivity analysis.
We conducted all outcome analyses on an intention‐to‐treat basis, that is, we included in the analyses all participants randomised to each group. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting forest plots and by quantifying the impact of heterogeneity using the I² statistic. If noted, we planned to explore possible causes of statistical heterogeneity by conducting prespecified subgroup analyses (e.g. differences in study quality, participants, intervention regimens, or outcome assessments).
Assessment of reporting biases
We assessed possible publication bias and other biases by examining symmetry/asymmetry of funnel plots.
For included trials that were recently performed (and therefore were prospectively registered), we explored possible selective reporting of study outcomes by comparing primary and secondary outcomes described in the reports against primary and secondary outcomes proposed at trial registration, using the websites www.clinicaltrials.gov and www.controlled‐trials.com. If we found such discrepancies, we planned to contact the primary investigators to request missing outcome data on outcomes prespecified at trial registration.
Data synthesis
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook, to assess the quality of evidence for the following (clinically relevant) outcomes: mortality, BPD, death or BPD, intestinal perforation, and cerebral palsy (Schünemann 2013).
Two review authors independently assessed the quality of evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEproGDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of evidence (GRADEpro GDT).
Through the GRADE approach, we assessed the quality of a body of evidence by assigning one of four grades.
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
When we judged meta‐analysis to be appropriate, we carried out the analysis using RevMan 5, as supplied by Cochrane. We used the Mantel‐Haenszel method to obtain estimates of typical risk ratio and risk difference. We included no continuous outcomes in this review. We planned to use the inverse variance method to analyse continuous measures, if included.
We used the fixed‐effect model for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses by type of corticosteroid used (dexamethasone or hydrocortisone) when we identified sufficient numbers of trials to make such subgroup analyses meaningful.
Sensitivity analysis
We planned to perform sensitivity analyses for situations that might affect interpretation of significant results (e.g. when risk of bias is associated with the quality of some included trials, when we note missing outcome data). We thought no such analyses were necessary for this review.
Results
Description of studies
Results of the search
Thirty‐two studies qualified for inclusion in this review (Figure 1). Most of these trials enrolled low birth weight infants with respiratory distress syndrome who were receiving mechanical ventilation.
1.
Study flow diagram: review update.
Included studies
See Characteristics of included studies.
The corticosteroid that was usually administered was dexamethasone, and the most common treatment regimen consisted of 0.50 mg/kg/d for three days followed by 0.25 mg/kg/d for three days, then 0.12 mg/kg/d for three days followed by 0.05 mg/kg/d for three days. However, trialists described considerable variation in treatment regimens, including short courses of one to two days and longer courses of up to four weeks. Eleven studies used hydrocortisone (Baden 1972; Batton 2012; Baud 2016; Biswas 2003; Bonsante 2007; Efird 2005; Hochwald 2014; Ng 2006; Peltoniemi 2005; Watterberg 1999; Watterberg 2004). In some cases, when low (almost physiological) doses were used, the indication was management of hypotension (see under Description of studies).
Anttila 2005 was a multi‐centre, double‐blind, placebo‐controlled trial of infants with birth weight of 500 grams to 999 grams, gestation less than 32 weeks, and respiratory failure by four hours of age. Investigators randomised 109 infants to receive four doses of dexamethasone (0.25 mg/kg at 12‐hour intervals) or saline placebo.
Baden 1972 included 44 infants with respiratory distress syndrome, mild hypoxia and hypercapnia, and a chest radiograph compatible with respiratory distress syndrome. Researchers randomised infants to receive hydrocortisone 15 mg/kg on admission and 12 hours later intravenously, or placebo. Birth weights ranged from 800 grams to 2805 grams, and gestational ages from 26 to 36 weeks.
Batton 2012 was a pilot study of infants at 23 to 26 weeks' gestation with low blood pressure in the first 24 hours of life. Investigators compared dopamine and hydrocortisone versus placebo using a factorial design. The dose of hydrocortisone was 1 mg/kg loading, then 0.5 mg/kg 12‐hourly for six doses (total dose, 3.5 mg/kg). The trial was stopped early because of slow recruitment after only 10 infants were enrolled.
Baud 2016 was a multi‐centre double‐blind randomised controlled trial of 523 infants at 24 to 27 weeks’ gestational age who were recruited from 21 French centres with NICU facilities in the first 24 hours after birth between 25 May 2008 and 31 January 2014. The treatment group received hydrocortisone hemisuccinate 1 mg/kg/d divided into two doses for seven days, then 0.5 mg/kg/d once per day for three days (total dose, 8.5 mg/kg). Control infants were given an equivalent volume of 5% glucose placebo. The trial was halted early because of lack of funding.
Biswas 2003 was a multi‐centre randomised trial of 253 infants of less than 30 weeks' gestational age. Investigators mechanically ventilated infants and entered them into the study within nine hours of birth. They gave all infants surfactant during the first 24 hours of life. Trialists randomised those in the treatment group (n = 125) to receive an infusion of hydrocortisone 1 mg/kg/d and tri‐iodothyronine (T3) 6 µg/kg/d for five days, then hydrocortisone 0.5 mg/kg/d and T3 3 µg/kg/d for two days. The placebo group (n = 128) received an equal volume of 5% dextrose.
Bonsante 2007 enrolled a total of 50 infants of birth weight less than 1250 grams or at 24 to 30 weeks' gestation who were less than 48 hours old and were ventilator‐dependent after surfactant treatment. Exclusion criteria were cardiopulmonary malformations, perinatal asphyxia, death within 12 hours after recruitment, or use of steroids for any reason within 12 days after birth. Researchers excluded no infants for these latter two reasons. They stratified infants by birth weight (not specified), gestational age (not specified), and antenatal steroid exposure, then randomly allocated infants to a 12‐day course of hydrocortisone (1.0 mg/kg for nine days, then 0.5 mg/kg/d for three days) (n = 25) or an equivalent volume of 0.9% saline placebo (n = 25). Study authors based the sample size calculation on the results of Watterberg 1999, resulting in an estimate of 138 infants to be recruited. The study was stopped early when 50 infants had been enrolled because of reports from other trials of spontaneous intestinal perforation with early hydrocortisone treatment.
Efird 2005 was a randomised controlled trial of hydrocortisone to prevent hypotension in infants of birth weight less than 1000 grams and gestation of 24 to 28 weeks. Trialists randomised 34 infants to receive 1 mg/kg of intravenous hydrocortisone 12‐hourly for two days, followed by 0.3 mg/kg 12‐hourly for three days, or a normal saline placebo.
Garland 1999 reported a prospective, multi‐centre, randomised trial comparing a three‐day course of dexamethasone therapy, beginning at 24 to 48 hours of life, versus placebo. Researchers enrolled 241 preterm infants (dexamethasone n = 118, placebo n = 123) who weighed between 500 grams and 1500 grams, had received surfactant therapy, and were at significant risk for BPD or death, using a predictive model at 24 hours. Trial authors gave dexamethasone to infants in a three‐day tapering course at 12‐hour intervals. The first two doses were 0.4 mg/kg, the third and fourth doses were 0.2 mg/kg, and the fifth and sixth doses were 0.1 mg/kg and 0.05 mg/kg, respectively. They gave a similar volume of normal saline to placebo‐treated infants at similar time intervals.
Halac 1990 was a randomised trial undertaken to determine if prenatal corticosteroid therapy would reduce the incidence of necrotising enterocolitis. Investigators randomised women to prenatal betamethasone or placebo when they were admitted in preterm labour and were expected to deliver within 24 hours. They then randomised infants of mothers who had received placebo to postnatal dexamethasone or placebo; we included in this review only infants who were randomised to postnatal therapy. Study infants weighed less than 1501 grams at birth or were born at less than 34 weeks' gestation and had evidence of "birth asphyxia" (one‐minute Apgar score < 5, prolonged resuscitation, and metabolic acidosis (bicarbonate < 15 mmol/L within one hour of birth)). Trialists assigned study groups via a table of random numbers. The treatment group (n = 130) received 2 mg/kg/d of dexamethasone phosphate intravenously for seven days; the control group (n = 118) received an equal volume of 10% dextrose. The major endpoint of this study was necrotising enterocolitis.
Hochwald 2014 reported a single‐centre randomised trial conducted to determine the effects of hydrocortisone on vasopressor dosing in hypotensive infants at < 31 weeks' gestation or with birth weight < 1251 grams during the first 48 hours after birth. Researchers randomly allocated 22 infants to hydrocortisone 2 mg/kg for one dose and 1 mg/kg for three doses, six hours apart, then 0.5 mg/kg for four doses, six hours apart (total dose, 7 mg/kg), or an equal volume of saline placebo.
Kopelman 1999 was a prospective blinded randomised controlled trial of 70 infants who required mechanical ventilation at less than 28 weeks' gestation. Thirty‐seven infants received dexamethasone 0.20 mg/kg at delivery, and 33 infants received placebo consisting of an equal volume of saline.
Lauterbach 2006 presented a single‐centre randomised trial to determine the effects of two active drugs on occurrence of BPD at 36 weeks. The two active drugs were nebulised pentoxifylline diluted in distilled water and intravenous dexamethasone. Infants weighing < 1251 grams at birth who were receiving supplemental oxygen on the fourth day after birth were eligible if they did not have a grade 3 or 4 intraventricular haemorrhage. Study authors randomly allocated a total of 150 infants to nebulised pentoxifylline every six hours for three days, intravenous dexamethasone 0.25 mg/kg/12‐hourly for three days, or nebulised saline placebo every six hours for three days. Study drugs could be repeated every seven days if the infant was still ventilator‐ or oxygen‐dependent and a diagnosis of BPD had not been established. Trialists did not report the number of repeat doses for any group. Researchers entered only data from the dexamethasone group and the control group into the meta‐analysis.
Lin 1999 was a randomised trial with a sequential design involving infants weighing 500 grams to 1999 grams. Investigators stratified infants by birth weight into three groups: 500 grams to 999 grams, 1000 grams to 1500 grams, and 1501 grams to 1999 grams. Within each group, equal numbers of dexamethasone‐treated or control cards were placed into envelopes for random selection of the first infant of each pair. The next infant of the appropriate birth weight stratum was enrolled for the match. A pharmacist opened the envelope, and investigators administered dexamethasone or saline placebo blind. Entry criteria included the presence of severe radiographic respiratory distress syndrome, the need for assisted ventilation within six hours of birth, and receipt of one dose of surfactant. Treated infants were given dexamethasone starting within 12 hours of birth at 0.25 mg/kg/dose 12‐hourly for seven days, 0.12 mg/kg/dose 12‐hourly for seven days, 0.05 mg/kg/dose 12‐hourly for seven days, and 0.02 mg/kg/dose 12‐hourly for seven days, resulting in a total of four weeks of treatment. Trialists presented results for 20 treated and 20 control infants.
Mukhopadhyay 1998 reported a randomised trial that included untreated controls. Study authors did not describe the method of randomisation used. Treated infants received dexamethasone 0.5 mg/kg/dose 12‐hourly for three days, beginning within six hours of birth. Researchers included 19 infants at less than 34 weeks' gestation and weighing less than 2000 grams who could be provided with mechanical ventilation. These infants had severe respiratory distress syndrome but were not given surfactant.
Ng 2006 was a double‐blind randomised controlled trial of a "stress dose" of hydrocortisone for treatment of refractory hypotension. Investigators randomised 48 infants of birth weight less than 1500 grams to receive hydrocortisone 1 mg/kg eight‐hourly for five days, or an equivalent volume of isotonic saline.
Peltoniemi 2005 enrolled a total of 51 infants weighing less than 1251 grams at birth or born at less than 31 weeks' gestation, who were under 36 hours old and were ventilator‐dependent. Investigators conducted this trial at three collaborating centres in Finland. They stratified infants by centre and by birth weight (501 grams to 749 grams, 750 grams to 999 grams, and 1000 grams to 1250 grams) and randomly allocated them to a 10‐day tapering course of hydrocortisone (2 mg/kg/d for two days, 1.5 mg/kg/d for two days, 0.75 mg/kg/d for six days) (n = 25) or an equivalent volume of 0.9% saline placebo (n = 26). Researchers based the sample size calculation on detecting an increase in survival without BPD from 50% to 70% and required inclusion of 160 participants per study arm (alpha and beta error 0.05 and 0.20, respectively). This study was stopped early at 51 infants because two of the hydrocortisone‐treated infants had intestinal perforation and other RCTs of early hydrocortisone had reported the same complication. Trialists followed up with children at two years and at five to seven years of age. Long‐term outcomes included in the meta‐analysis pertain to the five‐ to seven‐year follow‐up study only.
Rastogi 1996 recruited 70 infants with birth weight of 700 grams to 1500 grams who had severe respiratory distress syndrome (assisted ventilation with at least 40% oxygen and/or 7 cmH2O mean airway pressure, alveolar/arterial (a/A) partial pressure of oxygen (PO2) ratio ≤ 0.24) and had been treated with surfactant before entry. Infants were less than 12 hours old, and trialists excluded them if they had major malformations, chromosome abnormalities, five‐minute Apgar scores < 3, or severe infection. The intervention group received dexamethasone intravenously every 12 hours according to the following schedule: 0.50 mg/kg/d on days one to three, 0.30 mg/kg/d on days four to six, 0.20 mg/kg/d on days seven to nine, and finally 0.10 mg/kg/d on days 10 to 12. The control group received a saline placebo intravenously.
Romagnoli 1999 was a randomised trial that used numbered, sealed envelopes involving 25 dexamethasone‐treated infants and 25 untreated controls. Entry criteria were birth weight < 1251 grams, gestational age < 33 weeks, ventilator‐ and oxygen‐dependent at 72 hours, and high risk of BPD based on a local scoring system that predicted 90% risk. Treated infants were given dexamethasone beginning on the fourth day at a dose of 0.5 mg/kg/d for three days, 0.25 mg/kg/d for three days, and 0.125 mg/kg/d for one day.
Sanders 1994 enrolled 40 infants at less than 30 weeks' gestation who had respiratory distress syndrome diagnosed by clinical and radiographic signs, required mechanical ventilation at 12 to 18 hours of age, and had received at least one dose of surfactant. Exclusion criteria at entry included a strong suspicion of sepsis or pneumonia, congenital heart disease, chromosome abnormalities, and receipt of an exchange transfusion. Trialists randomised infants to receive dexamethasone 0.50 mg/kg at between 12 and 18 hours of age and a second dose 12 hours later, or a saline placebo. They administered both treatments intravenously.
Shinwell 1996 reported a multi‐centre trial that randomised 248 infants of birth weight 500 grams to 2000 grams if they had clinical and radiographic evidence of respiratory distress syndrome, required mechanical ventilation with more than 40% oxygen, were less than 12 hours old, and had no contraindications to corticosteroid treatment, such as a bleeding tendency, hypertension, hyperglycaemia, or active infection. Investigators excluded infants with lethal congenital malformations. The intervention group received dexamethasone 0.25 mg/kg intravenously every 12 hours for a total of six doses. The control group received intravenous saline.
Sinkin 2000 was a multi‐centre randomised double‐blind trial that included 384 infants of less than 30 weeks' gestation with respiratory distress syndrome. A total of 189 infants received dexamethasone 0.50 mg/kg at 12 to 18 hours of age and a second dose 12 hours later, and 195 infants received an equal volume of saline placebo.
Soll 1999 described a multi‐centre randomised double‐blind controlled trial that compared dexamethasone given at 12 hours of age versus selective late dexamethasone therapy in premature infants weighing 501 grams to 1000 grams (early dexamethasone n = 272, late selective therapy n = 270). Infants required assisted ventilation, had received surfactant therapy, were physiologically stable, had no obvious life‐threatening congenital anomaly, had blood cultures obtained, and had started antibiotic therapy. Trialists randomly assigned infants to early dexamethasone therapy or saline placebo. They administered intravenous dexamethasone for 12 days according to the following schedule: 0.5 mg/kg/d for three days, 0.25 mg/kg/d for three days, 0.1 mg/kg/d for three days, and 0.05 mg/kg/d for three days. Infants in either group could receive late postnatal corticosteroids beginning on day 14 if they needed assisted ventilation with supplemental oxygen greater than 30%.
Stark 2001 was a randomised multi‐centre controlled trial conducted to compare a tapering course of stress‐dose corticosteroid started on the first day versus placebo. Infants with birth weight 501 grams to 1000 grams needing mechanical ventilation before 12 hours of age were eligible for the study. Infants with birth weight over 750 grams also needed to have received surfactant and required an oxygen concentration of 30% or greater. The initial dose of dexamethasone was 0.15 mg/kg/d for three days, then tapered over seven days. After enrolling 220 infants (sample size was 1200), the trial was halted because of an excess of intestinal perforations in the dexamethasone‐treated group. Researchers randomised 111 infants to receive dexamethasone and 109 to receive placebo.
Subhedar 1997 reported a randomised trial that enrolled infants into one of four treatment groups using a factorial design. Investigators compared both inhaled nitric oxide (iNO) and early dexamethasone separately versus controls. They randomised 42 infants: 10 to receive iNO alone, 11 dexamethasone alone, 10 both treatments, and 11 neither treatment. Researchers compared 21 infants receiving dexamethasone versus 21 controls. Infants were eligible for entry into the trial at 96 hours of age if they met the following criteria: gestational age less than 32 weeks, mechanical ventilation from birth, had received surfactant therapy, and were thought to be at high risk of developing BPD based on a scoring system (Ryan 1996). Exclusion criteria were major congenital anomaly, structural cardiac defect, significant ductus shunting, culture‐positive sepsis, intraventricular haemorrhage with parenchymal involvement, pulmonary or gastrointestinal haemorrhage, disordered coagulation, and platelet count < 50,000. Infants received dexamethasone intravenously at 12‐hourly intervals for six days: 0.50 mg/kg/dose for six doses and 0.25 mg/kg/dose for a further six doses. Control infants did not receive a placebo.
Suske 1996 randomised 26 infants with gestational age of 24 to 34 weeks who had respiratory distress syndrome and had been treated with surfactant. Trialists excluded infants with known septicaemia during the first week of life, haemodynamically relevant cardiac anomalies except for patent ductus arteriosus, or malformations of the lung or central nervous system (CNS). They performed randomisation by drawing lots before the age of two hours. The intervention group received dexamethasone 0.50 mg/kg intravenously in two divided doses for five days, and controls received no placebo.
Tapia 1998 was a multi‐centre double‐blind placebo‐controlled trial of 109 preterm infants with respiratory distress syndrome and birth weight between 700 grams and 1600 grams who were treated with mechanical ventilation and surfactant. Researchers randomised 55 infants to receive dexamethasone 0.50 mg/kg/d for three days, followed by 0.25 mg/kg/d for three days, followed by 0.12 mg/kg/d for three days, then 0.06 mg/kg/d for three days. A total of 54 control infants received an equal volume of saline.
Vento 2004 enrolled 20 neonates with birth weight less than 1251 grams and gestation less than 33 weeks who were oxygen‐ and ventilator‐dependent on the fourth day of life and randomised them to receive dexamethasone 0.50 mg/kg/d for three days, 0.25 mg/kg/d for three days, and 0.125 mg/kg/d for one day (total dose 2.375 mg/kg), or no corticosteroid treatment.
Wang 1996 reported a randomised trial of a 21‐day course of dexamethasone or saline placebo given in a double‐blind fashion. Study authors did not state the method of randomisation used. Entry criteria were birth weight 1000 grams to 1999 grams, appropriate for gestational age, clinical and radiological severe respiratory distress syndrome, mechanical ventilation, and age less than 12 hours. Surfactant was not given, as it was not commercially available in Taiwan at the time of the study. Treated infants were given dexamethasone 0.25 mg/kg/dose 12‐hourly for seven days, 0.125 mg/kg/dose 12‐hourly for seven days, and 0.05 mg/kg/dose 12‐hourly for seven days, for a total course of 21 days. The first dose of dexamethasone was given during the first 12 hours of life. Participants included 34 infants in the dexamethasone group and 29 in the placebo control group.
Watterberg 1999 described a randomised double‐masked placebo‐controlled pilot study conducted to compare early treatment with low‐dose hydrocortisone (1.0 mg/kg/d for nine days, then 0.5 mg/kg/d for three days), begun before 48 hours of age, versus placebo. Researchers enrolled at two centres 40 infants weighing between 500 grams and 999 grams who were mechanically ventilated: 20 hydrocortisone‐treated infants and 20 placebo controls.
Watterberg 2004 was a multi‐centre masked randomised trial of hydrocortisone to prevent early adrenal insufficiency. Investigators randomised 360 infants with birth weight of 500 grams to 999 grams who were mechanically ventilated to receive hydrocortisone 1 mg/kg/d for 12 days, then 0.5 mg/kg/d for three days, or saline placebo. They enrolled infants at between 12 and 48 hours of life. The trial was stopped because of an increase in spontaneous gastrointestinal perforation in the hydrocortisone group.
Yeh 1990 enrolled 57 infants whose birth weight was < 2000 grams and who had severe respiratory distress syndrome diagnosed on the basis of a chest radiograph and the need for mechanical ventilation within four hours after birth. Absence of infection was also required for inclusion. Trialists randomly assigned infants to receive dexamethasone 0.50 mg/kg/dose 12‐hourly from days one to three, then 0.25 mg/kg/dose 12‐hourly from days four to six, then 0.12 mg/kg/dose 12‐hourly from days seven to nine, and finally 0.05 mg/kg/dose 12‐hourly from days 10 to 12. Researchers administered all doses intravenously and gave a saline solution to infants in the placebo group.
Yeh 1997 reported a multi‐centre randomised double‐blind clinical trial of 262 preterm infants (< 2000 grams) who had respiratory distress syndrome and required mechanical ventilation from shortly after birth. The treated group received dexamethasone 0.25 mg/kg/dose 12‐hourly intravenously from day one to day seven; 0.12 mg/kg/dose 12‐hourly intravenously from day 8 to day 14; 0.05 mg/kg/dose 12‐hourly intravenously from day 15 to day 21; and 0.02 mg/kg/dose 12‐hourly intravenously from day 22 to day 28. Control infants received a saline placebo.
Excluded studies
We excluded 25 studies. See Characteristics of excluded studies.
We excluded studies for several reasons. In one study, the primary outcome was the need for an epinephrine infusion 12 hours after treatment (Gaissmaier 1999). Study authors reported no long‐term outcomes. Another study was not an RCT (Tsukahara 1999). This study included 26 study infants and 12 historical controls.
We included studies of late postnatal corticosteroids in the review titled "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants." We excluded these studies from this review and included the following studies: Ariagno 1987; Avery 1985; Brozanski 1995; CDTG 1991; Cummings 1989; Doyle 2006; Durand 1995; Harkavy 1989; Kari 1993; Kazzi 1990; Kothadia 1999; Kovacs 1998; Noble‐Jamieson 1989; Ohlsson 1992; Papile 1998; Parikh 2013; Romagnoli 1997; Scott 1997; Vento 2004; Vincer 1998; and Walther 2003.
Investigators in one trial randomised 120 very low birth weight infants to both hydrocortisone and caffeine as active treatments, compared with treatment described in "standard guidelines", which presumably meant no hydrocortisone or caffeine (Dobryansky 2012). Major outcomes reported were BPD and BPD combined with death. As caffeine alone reduces BPD, the independent effect of hydrocortisone cannot be determined (Schmidt 2006).
Although researchers in another trial randomly allocated 29 very low birth weight infants to dexamethasone or placebo before six hours of age, they reported none of the outcomes that are applicable to this review (Yaseen 1999). Outcomes reported comprised only changes in mean values over the first five days for oxygenation, blood pressure and serum creatinine, and urea and glucose ‐ not rates of BPD, hypertension, or hypoglycaemia, for example.
We found no studies that are currently awaiting further assessment.
Risk of bias in included studies
The overall risk of bias was low for most studies. All were randomised controlled trials, although the method of random allocation was not always clear. Allocation concealment applied to most studies. Blinding of investigators and others was achieved most often through the use of placebo, usually saline solution. Follow‐up reporting for short‐term outcomes was mostly complete but was more variable for long‐term outcomes beyond discharge and later into childhood. Most studies reported primary outcomes as specified in their methods.
Anttila 2005 carried out randomisation in the pharmacy of the co‐ordinating centre using coded vials, with blinding of study investigators. Open‐label dexamethasone was allowed when deemed necessary by the attending neonatologist, but its use was discouraged. Trialists performed intention‐to‐treat analysis and reported no follow‐up component.
Baden 1972 performed randomisation by using vials and a table of random numbers. Clinical personnel were not aware of the content of any vial. Study authors reported outcomes for all enrolled infants. Follow‐up consisted of the following: One paediatrician and one psychologist saw survivors at 12 months of age, corrected for prematurity. A neurologist saw all children with abnormal neurological signs. Observers were blinded to treatment group allocation. The follow‐up rate of survivors was 93% (25/27). Study authors did not specify criteria for the diagnosis of cerebral palsy, nor did they provide specific criteria for blindness or deafness (children were tested by free‐field pure‐tone audiometry). Psychological assessment consisted of the Griffiths Scales. Study authors did not report major neurosensory disability (Fitzhardinge 1974).
Batton 2012 did not state the method of randomisation used. Trialists administered an identical placebo and reported no follow‐up component.
Baud 2016 generated the randomisation sequence electronically using nQuery. After enrolment, researchers assigned treatment through a secure study website after verifying eligibility and consent status. They electronically randomised all infants before they reached 24 completed hours after birth and reported short‐term outcomes for all but two participants who were randomised. They followed up on 93% of survivors at 22 months' corrected age, although only 75% were given the full neurodevelopmental assessment battery. Investigators maintained double‐blinding through all aspects of the study.
Biswas 2003 conducted randomisation as performed by the Perinatal Trials Unit in Oxford, with stratification for centre and gender, and the study pharmacist held the code. Controls received an equal infusion rate of 5% dextrose. One pharmacy made the syringes and transported them to individual study centres. Trialists reported short‐term outcomes for all enrolled infants. Study authors reported no follow‐up component.
Bonsante 2007 conducted centralised randomisation using a computer‐generated random number sequence. Researchers stratified infants into six risk groups to ensure a homogeneous number of infants with regard to birth weight, gestation, and antenatal corticosteroid administration. They prepared drugs each day in the pharmacy, and the care team, parents, and personnel collecting data had no knowledge of the random assignment at any time. Study authors reported results of follow‐up at two years of age (follow‐up component) in conjunction with data from another study but did not describe clinical criteria for various outcomes (Peltoniemi 2009). Study authors reported follow‐up data for 92% (33/36) of survivors up to hospital discharge.
Efird 2005 performed randomisation by opening sequentially numbered, opaque envelopes containing preassigned treatment designations. Investigators randomised infants of multiple gestations as separate participants and blinded clinicians to treatment identity. If hypotension persisted, the randomisation assignment could be unblinded and hydrocortisone administered if the infant had been assigned to the placebo group. Study authors reported no follow‐up component.
Garland 1999 randomised infants at each centre within each of four strata on the basis of birth weight (≤ 1000 grams, > 1000 grams) and a/A ratio before surfactant (≤ 0.15, > 0.15). Study pharmacists at each centre maintained randomisation codes. Investigators, caregivers, and parents were blinded to treatment allocation. The first interim analysis (n = 75) showed increased risk of gastrointestinal perforation in the dexamethasone group. After adjustment for severity of illness, the difference was not sufficiently statistically significant to stop enrolment. However, to ensure participant safety, the Data Monitoring Committee recommended that the dexamethasone dose should be reduced. Investigators changed the dosing schedule to four doses of 0.25 mg/kg/dose every 12 hours, begun at 24 to 48 hours, followed by doses of 0.125 mg/kg and 0.05 mg/kg at the next two 12‐hour periods, respectively. After the first interim analysis, all enrolled infants received ranitidine therapy during the first three days of the study. It appears that study authors reported outcome measures for all 241 infants enrolled in the study and included no follow‐up component.
Halac 1990 used a table of random numbers for randomisation, along with placebo blinding. Study authors stated that they had excluded from the study deaths before 10 days of age; they reported a total of five early deaths from sepsis, but it was not clear how often this occurred in each group. Apart from these infants, investigators provided outcome data for all remaining enrolled infants. They reported limited follow‐up to six months of age but provided no follow‐up results (apart from a statement that "growth and development were not hampered in any of these patients").
Hochwald 2014 did not state methods used for random sequence generation, allocation concealment, blinding of personnel and families, and blinding of outcomes, apart from use of a placebo, and reported no follow‐up component.
Kopelman 1999 performed randomisation in the pharmacy after stratifying infants for treatment with antenatal corticosteroids. The blinded clinical team provided care. Study authors provided outcome data for all enrolled infants and reported no follow‐up component.
Lauterbach 2006 used a computer‐generated random number table for randomisation. Investigators allocated infants to groups by opening numbered containers on the fourth day of life. They provided no placebo for the dexamethasone arm and hence reported no blinding of dexamethasone treatment. Study authors reported no follow‐up component.
Lin 1999 performed randomisation by opening sealed envelopes in the pharmacy. This study used a sequential analysis design and paired 12 infants successfully. Study authors reported outcome measures for all 40 enrolled infants, including those who remained unpaired. They described no follow‐up component.
Mukhopadhyay 1998 did not state the method of randomisation used. Investigators were able to provide ventilation for only 28 of 43 eligible infants and subsequently excluded eight infants owing to non‐availability of blood gases due to a technical fault, and excluded one baby because of congenital heart block. This left 19 infants included in the study: 10 received intravenous dexamethasone, and nine received no drug treatment. Study authors did not mention placebo. They reported outcome measures for these 19 infants and described no follow‐up component.
Ng 2006 performed randomisation by using computer‐generated random numbers and opening sequentially numbered, sealed, opaque envelopes in the pharmacy. They assigned infants in blocks of six, and once an envelope was opened, an infant would be irrevocably entered into the trial. To ensure effective blinding of medications, both types of trial drug were colourless and odourless and were filled to the same volume before they were sent to the ward. Study authors reported no follow‐up component.
In the Peltoniemi 2005 study, non‐clinical staff achieved randomisation centrally, independent of the chief investigators, using random variation in block sizes of two to eight, separately for each centre. Study authors did not specify the method used for randomisation. Researchers had syringes prepared and labelled identically in the pharmacy department of the centre, thereby concealing allocation from study site investigators and caregivers of the infant. Open‐label corticosteroids were discouraged after randomisation but were not prohibited; some infants may have received both a second course of their initially allocated study drug and open‐label corticosteroids. No one apart from the pharmacist at study sites had access to the treatment codes. Study authors reported short‐term outcomes for all enrolled infants. Follow‐up consisted of the following: Investigators assessed surviving children at 24 months of age, corrected for prematurity, and at five to seven years of age, when it was not stated that age was corrected for prematurity. Paediatricians, paediatric neurologists, speech therapists, and psychologists at individual study sites were blinded to treatment group allocation. At two years, children were considered to have a major neurosensory impairment if they had cerebral palsy, blindness (inability to see any objects, with the exception of light), deafness (failure to pass an evoked otoacoustic emission test during the neonatal period and no response in brainstem auditory evoked potentials), or developmental delay (defined as a Mental Developmental Index (MDI) on the Bayley Scales of Infant Development < 70 (< ‐2 standard deviations (SDs)) or a developmental quotient < 70 on the Griffiths Cognitive Scales). Researchers assessed cognitive development of children at five to seven years of age by using the Wechsler Presechool and Primary Scale of Intelligence ‐ Revised (WPPSI‐R). They diagnosed minor neurological dysfunction on the basis of the number of dysfunctional domains. Speech assessment included the Reynell Developmental Language Scale III (RDLS III). Study authors did not provide the criteria for blindness or deafness and reported the follow‐up rate of survivors at two years (98%; 45/46) and at five to seven years of age (80%; 37/46) (Peltoniemi 2009; Peltoniemi 2016).
Rastogi 1996 performed randomisation in the pharmacy, using a random number list after stratifying infants for birth weight into three groups: 700 grams to 999 grams, 1000 grams to 1249 grams, and 1250 grams to 1500 grams. The clinical team and other study personnel were blinded to assignments until the study was completed, and they recorded all outcome variables for all infants. Study authors reported no follow‐up component.
Romagnoli 1999 achieved randomisation through random number allocation by opening numbered, sealed envelopes. Trialists excluded infants with prenatal infections, congenital malformations, and evidence of sepsis at randomisation. They did not mention placebo and reported outcome measures for all 50 enrolled infants. Follow‐up consisted of the following: One paediatrician and one neurologist saw survivors at 34 to 42 months of age, corrected for prematurity, and observers were blinded to treatment group allocation. The follow‐up rate of survivors was 100% (45/45). The neurologist diagnosed cerebral palsy, but study authors did not specify the criteria used for this, nor for the diagnosis of blindness or deafness. Psychological assessment included the Stanford‐Binet 3rd Revision, and intellectual impairment comprised an IQ < 70. Major neurosensory impairment consisted of either blindness or deafness (Romagnoli 2002).
Sanders 1994 randomised participants in the pharmacy after opening sealed envelopes. Trialists dispensed dexamethasone or placebo via labelled syringes. Clinical personnel were not aware of assignment of the intervention. Study authors reported outcomes for all 40 enrolled infants. Follow‐up consisted of the following: A paediatrician, a neurologist, and a psychologist saw survivors at mean ages of 64 (SD 8) months (dexamethasone) and 61 (SD 4) months (controls), not corrected for prematurity, with observers blinded to treatment group allocation. Researchers sought additional data from parents and teachers. The follow‐up rate of survivors was 100% (31/31). The criterion for the diagnosis of cerebral palsy was a fixed motor deficit diagnosed by the neurologist. Blindness comprised visual acuity < 6/60 in the better eye, and study authors defined deafness as the need for a hearing aid. Psychological assessment was based on the Wechsler Scales (Wechsler Intelligence Scale for Children (WISC) and WPPSI‐R) ‐ intellectual impairment comprised a full‐scale IQ < 70. Study authors did not specify major neurosensory disability and planned further follow‐up at 15 years of age (Sinkin 2002 (personal communication follow‐up to Sanders 1994)).
Shinwell 1996 supplied each participating unit with numbered sets of syringes containing dexamethasone or physiological saline. Syringes containing dexamethasone were not distinguishable from those containing saline. Trialists numbered syringe sets according to a random number list and stratified randomisation by centre and by two birth weight groups: 500 grams to 1000 grams, and 1001 grams to 2000 grams. No investigators knew the drug assignment until after the three‐month observation period of the last enrolled infant. Study authors reported outcomes for 248 of 255 enrolled infants. The seven infants subsequently excluded from analysis included three with major congenital abnormalities (two with myotonic dystrophy and one with cyanotic congenital heart disease), three with errors in drug administration, and one randomised after the age of 12 hours. Follow‐up consisted of the following: Survivors were seen at a mean age of 53 (SD 18; range 24 to 71) months, presumably not corrected for prematurity. Multiple paediatricians saw these children in multiple follow‐up clinics, with observers blinded to treatment group allocation. The follow‐up rate of survivors was 83% (159/190). Trialists did not specify criteria for the diagnosis of cerebral palsy, but neurologists made the diagnosis in all cases. Study authors did not specify criteria for blindness but defined deafness as the need for hearing aids. Study personnel performed no formal psychological assessments, and multiple assessors assigned the judgement of developmental delay. Major neurosensory disability comprised any of non‐ambulant cerebral palsy, global retardation (not specified), blindness, or deafness. Researchers planned further follow‐up at school age (Shinwell 2002).
Sinkin 2000 performed randomisation with stratification by centre, using a set of sealed envelopes in the pharmacy. It appears that study authors provided outcome data for all enrolled infants. Follow‐up consisted of the following (Sinkin 2002 (personal communication follow‐up to Sinkin 2000)): Researchers obtained data from one of the four original centres in the study, from follow‐up clinic appointments, and from questionnaires completed by parents and paediatricians. A paediatrician, a neurologist, and a psychologist saw survivors at approximately 12 months of age, corrected for prematurity, with observers blinded to treatment group allocation. The follow‐up rate of survivors was 13% (41/311) at 36 weeks' postmenstrual age overall but was confined to one of four individual study centres, within which the follow‐up rate was 100% (41/41). The criterion for the diagnosis of cerebral palsy was a fixed motor deficit diagnosed by the neurologist. Blindness comprised visual acuity < 6/60 in the better eye, and study authors defined deafness as the need for a hearing aid. Psychological assessment included the Bayley Scales of Infant Development. Investigators did not specify major neurosensory disability.
Soll 1999 performed randomisation in hospital pharmacies after opening opaque, sealed envelopes supplied by the Vermont Oxford Neonatal Network. The study was stopped before sample size goals were met owing to concern regarding adverse effects in the early corticosteroid therapy group. It appears that outcome measures were reported for most of the 542 enrolled infants. Study authors reported no follow‐up component.
Stark 2001 performed random allocation in hospital pharmacies using a random number scheme. This study used a factorial design so that infants were also randomised to routine ventilator management or to a strategy of minimal ventilator support aimed at reducing mechanical lung injury. After 220 infants were enrolled from a sample size estimate of 1200, the trial was halted. It appears that study authors have reported outcome measures for all 220 participants enrolled in the trial. Follow‐up consisted of the following: Trained developmental observers blinded to treatment group allocation saw survivors at 18 to 22 months of age, corrected for prematurity. The follow‐up rate of survivors was 88% (144/164). Criteria for the diagnosis of cerebral palsy included non‐progressive abnormalities of tone in at least one limb and abnormal control of movement and posture. Study authors defined blindness as no useful vision in either eye, and deafness as disability with bilateral hearing amplification. Psychological assessment included the MDI and the Psychomotor Developmental Index (PDI) of the Bayley Scales of Infant Development‐II (Bayley 1993). Major neurosensory disability comprised any of moderate or severe cerebral palsy (sitting independently with support or worse), blindness, deafness, or an MDI or PDI < ‐2 SD (Stark 2014).
Subhedar 1997 performed block randomisation by using computer‐generated random numbers and sealed envelopes. Researchers used no placebo and provided no evidence of blinding of clinicians. Study authors reported outcome measures for all enrolled infants. Follow‐up consisted of the following (Subhedar 2002 (personal communication follow‐up to Subhedar 1997)): One developmental paediatrician who was blinded to treatment group allocation saw survivors at 30 months of age, corrected for prematurity. The follow‐up rate of survivors was 95% (21/22). Study authors specified criteria for the diagnosis of cerebral palsy but not for deafness; an ophthalmologist diagnosed blindness. Psychological assessment included the MDI and the PDI of the Bayley Scales of Infant Development. Major neurosensory disability comprised any of cerebral palsy, an MDI or PDI < 71, blindness, or deafness.
Suske 1996 performed randomisation by drawing lots; lot numbers corresponded to numbers on non‐transparent envelopes. A neutral, uninvolved person drew random lots and envelopes. This was considered a pilot trial conducted before a multi‐centre study was begun, and researchers planned that the trial would be stopped if they found a statistically significant difference between groups. A total of 41 infants met the inclusion criteria. Owing to lack of co‐operation and co‐ordination at the beginning of the study, investigators did not randomise nine infants. They excluded four infants after randomisation because they showed definite signs of septicaemia. Study authors reported results for 26 of the 28 remaining infants and described no follow‐up component.
Tapia 1998 achieved random assignment at each centre using ampoules of dexamethasone and saline prepared in the hospital pharmacy at one of the centres. Researchers reported outcomes for 109 of 113 enrolled infants. They excluded two infants from the dexamethasone group ‐ one because of congenital cystic adenomatoid malformation, and another because of early sepsis. Investigators also excluded two patients from the placebo group ‐ one because of early sepsis, and the other because of transfer to another hospital at two weeks of age. Study authors did not provide further data on outcomes and reported no follow‐up component.
Vento 2004 did not state the method of randomisation used. Whether clinicians caring for infants were blinded to treatment allocation remains unclear. Control infants did not receive a placebo, and study authors reported no follow‐up component.
Wang 1996 reported that random allocation was double‐blind but did not describe the exact method used. Study authors reported outcome measures for all 63 infants enrolled in the study and reported no follow‐up component.
Watterberg 1999 randomised Infants at each centre by using a constant block design with four participants per block to minimise imbalance over time. Investigators used separate randomisation tables for infants exposed to antenatal corticosteroids. Hospital pharmacies prepared hydrocortisone doses and the placebo of normal saline. Study authors reported outcome measures for all of the 40 infants enrolled in the trial. Follow‐up consisted of the following (Watterberg 2002 (personal communication follow‐up to Watterberg 1999): A neonatologist and a physiotherapist saw survivors at a regular follow‐up clinic for one of the two study sites at a mean age of 11 (SD 2) months, corrected for prematurity, with observers blinded to treatment group allocation. The follow‐up rate of survivors was 53% (18/34) for the study overall, but 86% (18/21) for the study centre with follow‐up data. Researchers specified criteria for the diagnosis of cerebral palsy, which comprised abnormal tone and movement. An ophthalmologist diagnosed blindness, and investigators screened participants for deafness in early infancy and at follow‐up. They performed no formal psychological testing and did not define major neurosensory disability.
Watterberg 2004 performed randomisation centrally, stratifying infants for birth weight (500 grams to 749 grams, and 750 grams to 999 grams) and by centre, using permuted block sizes of six within each stratum. Only pharmacists at individual sites who prepared the drug were aware of group assignment. All other personnel were masked. Trialists randomised twins together to the same study arm. They reported mortality for all enrolled infants but described other short‐term outcomes for all but three infants who were withdrawn from the study. Follow‐up consisted of the following: Assessors at individual study sites who were blinded to treatment group allocation assessed surviving children at 18 to 22 months of age, corrected for prematurity. They considered children to have a neurodevelopmental (neurosensory) impairment if they had cerebral palsy (criteria included abnormalities of tone, movement, and posture), functional blindness (inability to complete the Bayley Scales of Infant Development ‐ Second Edition (BSID‐II) because of visual impairment), functional deafness (inability to complete BSID‐II because of hearing impairment), developmental delay (defined as MDI on the BSID‐II < 70 (< ‐2 SD), or motor delay (defined as a Psychomotor Developmental Index on the BSID‐II < 70 (< ‐2 SD)) (Bayley 1993). The follow‐up rate of survivors at 18 to 22 months was 86% (252/294), or 87% (252/291) when three children whose families had withdrawn consent were excluded (Watterberg 2007).
Yeh 1990 performed randomisation in the pharmacy using balanced blocks of 10. Personnel working in the pharmacy labelled vials, and clinical staff were unaware of assignment. Trialists included 60 infants in the study and subsequently withdrew three: one because of death from Haemophilus influenzae septicaemia six hours after enrolment, and two because of an error in measurement of birth weight (581 grams and 2200 grams). Study authors did not report outcomes for these three infants and described no follow‐up component.
Yeh 1997 completed randomisation in the central pharmacy using an assignment list. Investigators calculated sample size on the basis of an expected 50% reduction in the incidence of BPD with early dexamethasone, allowing a 5% chance of a type I error, and a 10% chance of a type II error. Study authors reported short‐term outcome data for all 262 enrolled infants and described the study as double‐blind. Follow‐up consisted of the following: In 1998, researchers reported that one neurologist and one psychologist saw survivors at a mean age of 25 months, corrected for prematurity, with observers blinded to treatment group allocation (Yeh 1998). The follow‐up rate of survivors was 81% (133/164). Study authors did not specify criteria for the diagnosis of cerebral palsy, blindness, or deafness. Psychological assessment included the MDI and the PDI of the Bayley Scales of Infant Development. Major neurosensory disability comprised severe motor dysfunction (child non‐ambulant) or an MDI or PDI < ‐2 SD. In 2004, investigators in the Yeh trial reported that trial personnel reassessed survivors at seven to nine years of age (Yeh 2004). The follow‐up rate of survivors was 92% (146/159). Assessors were blind to treatment allocation. A paediatric neurologist evaluated children for cerebral palsy, assessing motor skills using the Movement ABC, and IQ using the WISC‐III. Trial personnel formally evaluated vision and hearing. Major neurological disability comprised any of cerebral palsy, vision worse than 20/60, deafness requiring hearing aids, or an IQ < 5th centile. Whether age was corrected for prematurity remained unclear. We used data for cerebral palsy at eight years in the meta‐analysis, as the diagnosis of cerebral palsy is more certain at eight years than at two years of age, and because the follow‐up rate was higher when participants were eight years of age. Trialists measured blood pressure, height, weight, and head circumference at eight years of age but did not report these as standardised scores (SD or Z‐scores), to enable pooling of data for meta‐analysis.
Effects of interventions
See: Table 1
Results of meta‐analysis
Meta‐analysis of these 32 studies of early postnatal corticosteroid treatment yielded the following results.
Mortality
No evidence suggests that early postnatal corticosteroid treatment reduced mortality at 28 days of life (typical risk ratio (RR) 1.02, 95% confidence interval (CI) 0.88 to 1.19, 19 studies, 2950 infants; Analysis 1.1), at 36 weeks' gestational age (OR 1.01, 95% CI 0.89 to 1.14, 20 studies, 3733 infants; Analysis 1.2), before discharge (typical RR 0.95, 95% CI 0.85 to 1.07, 30 studies, 4273 infants; Analysis 1.3), or at the latest age possible to determine the outcome (typical RR 0.95, 95% CI 0.85 to 1.06, 31 studies, 4373 infants; Analysis 1.4). We found weak evidence of publication bias for mortality at latest age, particularly for studies examining treatment with hydrocortisone (Figure 2).
1.1. Analysis.
Comparison 1 Mortality, Outcome 1 Neonatal mortality (up to 28 days).
1.2. Analysis.
Comparison 1 Mortality, Outcome 2 Mortality at 36 weeks.
1.3. Analysis.
Comparison 1 Mortality, Outcome 3 Mortality to hospital discharge.
1.4. Analysis.
Comparison 1 Mortality, Outcome 4 Mortality at latest reported age.
2.
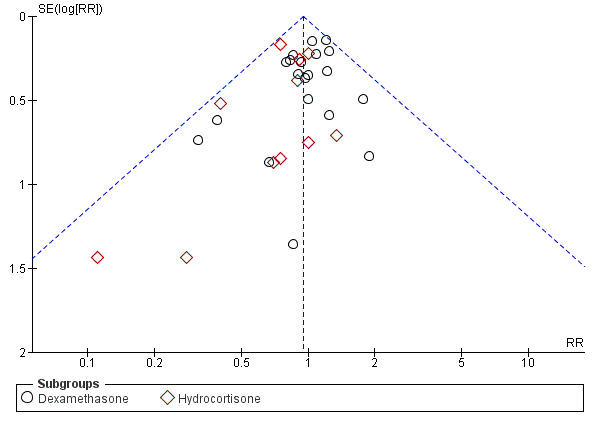
Funnel plot of comparison: 1 Mortality, outcome: 1.4 Mortality at latest reported age.
Bronchopulmonary dysplasia
Early corticosteroids reduced the incidence of BPD, defined as needing oxygen supplementation at 28 days of life (typical RR 0.87, 95% CI 0.81 to 0.93; typical RD (RD) ‐0.07, 95% CI ‐0.10 to ‐0.03; 17 studies, 2874 infants; Analysis 2.1) or at 36 weeks' postmenstrual age (typical RR 0.79, 95% CI 0.72 to 0.87; typical RD ‐0.07, 95% CI ‐0.09 to ‐0.04; 24 studies, 3929 infants; Analysis 2.2). We found weak evidence of publication bias for oxygen supplementation at 36 weeks (Figure 3) and a reduction in BPD at 36 weeks' postmenstrual age among survivors (typical RR 0.81, 95% CI 0.74 to 0.88; typical RD ‐0.08, 95% CI ‐0.11 to ‐0.05; 21 studies, 2970 infants; Analysis 2.3). Early corticosteroids reduced the need for later corticosteroid treatment overall (typical RR 0.75, 95% CI 0.68 to 0.82; typical RD ‐0.11, 95% CI ‐0.15 to ‐0.07; 14 studies, 2483 infants; Analysis 2.4) and among survivors (typical RR 0.77, 95% CI 0.67 to 0.89; typical RD ‐0.11, 95% CI ‐0.77 to ‐0.05; seven studies, 895 infants; Analysis 2.5). Results of analysis showed no significant reduction in the proportion of survivors discharged home on oxygen, although fewer studies were able to determine this outcome (typical RR 0.72, 95% CI 0.51 to 1.03, six studies, 691 infants; Analysis 2.6).
2.1. Analysis.
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 1 BPD (28 days of life).
2.2. Analysis.
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 2 BPD (36 weeks' postmenstrual age).
3.
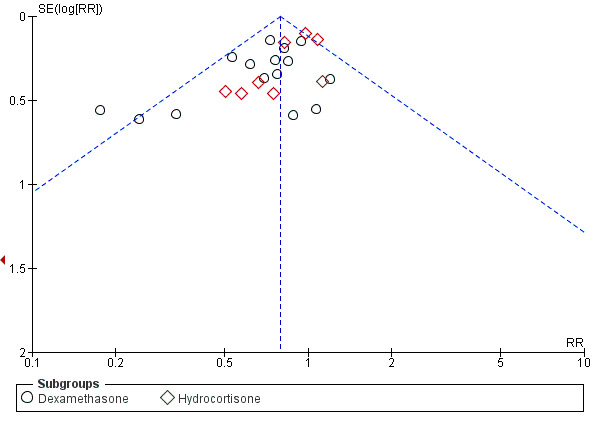
Funnel plot of comparison: 2 Bronchopulmonary dysplasia (BPD), outcome: 2.2 BPD (36 weeks' postmenstrual age).
2.3. Analysis.
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 3 BPD at 36 weeks' postmenstrual age in survivors.
2.4. Analysis.
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 4 Late rescue with corticosteroids.
2.5. Analysis.
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 5 Survivors who had late rescue with corticosteroids.
2.6. Analysis.
Comparison 2 Bronchopulmonary dysplasia (BPD), Outcome 6 Survivors discharged home on oxygen.
Death or bronchopulmonary dysplasia
Early corticosteroids reduced the incidence of death or BPD, defined as needing oxygen supplementation at 28 days of life (typical RR 0.92, 95% CI 0.88 to 0.96; typical RD ‐0.06, 95% CI ‐0.09 to ‐0.03; 15 studies, 2546 infants; Analysis 3.1) or at 36 weeks' postmenstrual age (typical RR 0.88, 95% CI 0.83 to 0.93; typical RD ‐0.06, 95% CI ‐0.09 to ‐0.03; 25 studies, 3960 infants; Analysis 3.2). We found weak evidence of publication bias for mortality or BPD at 36 weeks (Figure 4).
3.1. Analysis.
Comparison 3 Death or bronchopulmonary dysplasia (BPD), Outcome 1 Death or BPD at 28 days of life.
3.2. Analysis.
Comparison 3 Death or bronchopulmonary dysplasia (BPD), Outcome 2 Death or BPD at 36 weeks' postmenstrual age.
4.
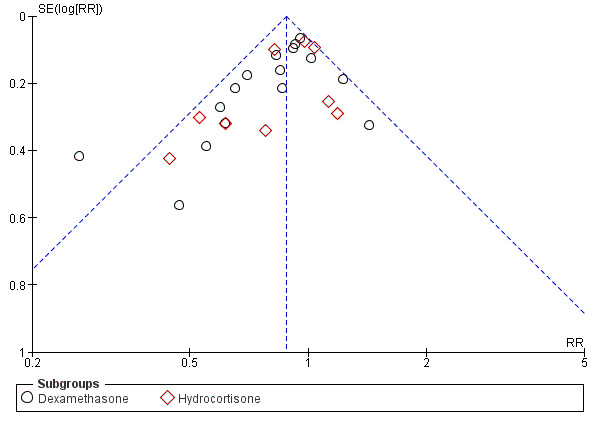
Funnel plot of comparison: 3 Death or bronchopulmonary dysplasia (BPD), outcome: 3.2 Death or BPD at 36 weeks' postmenstrual age.
Failure to extubate
Early corticosteroids reduced rates of failure to extubate at three days (typical RR 0.85, 95% CI 0.75 to 0.95; typical RD ‐0.09, 95% CI ‐0.16 to ‐0.03; four studies, 887 infants; Analysis 4.1), seven days (typical RR 0.76, 95% CI 0.68 to 0.85; typical RD ‐0.12, 95% CI ‐ 0.17 to ‐0.07; eight studies, 1448 infants; Analysis 4.2), 14 days (typical RR 0.77, 95% CI 0.62 to 0.97; typical RD ‐0.10, 95% CI ‐0.19 to ‐0.02; four studies, 443 infants; Analysis 4.3), and 28 days of life (typical RR 0.84, 95% CI 0.72 to 0.98; typical RD ‐0.07, 95% CI ‐0.13 to ‐0.01; seven studies, 902 infants; Analysis 4.4).
4.1. Analysis.
Comparison 4 Failure to extubate, Outcome 1 Failure to extubate by third day.
4.2. Analysis.
Comparison 4 Failure to extubate, Outcome 2 Failure to extubate by seventh day.
4.3. Analysis.
Comparison 4 Failure to extubate, Outcome 3 Failure to extubate by 14th day.
4.4. Analysis.
Comparison 4 Failure to extubate, Outcome 4 Failure to extubate by 28th day.
Complications during primary hospitalisation
Metabolic complications
Early corticosteroids increased risks of hyperglycaemia (typical RR 1.33, 95% CI 1.20 to 1.47; typical RD 0.11, 95% CI 0.07 to 0.15; 13 studies, 2167 infants; Analysis 5.2) and hypertension (typical RR 1.85, 95% CI 1.54 to 2.22; typical RD 0.10, 95% CI 0.07 to 0.13; 11 studies, 1993 infants; Analysis 5.3).
5.2. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 2 Hyperglycaemia.
5.3. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 3 Hypertension.
Gastrointestinal complications
Early corticosteroids increased risks of gastrointestinal bleeding (typical RR 1.86, 95% CI 1.35 to 2.55; typical RD 0.05, 95% CI 0.03 to 0.08; 12 studies, 1816 infants; Analysis 5.14) and gastrointestinal perforation (typical RR 1.72, 95% CI 1.29 to 2.30; typical RD 0.03, 95% CI 0.02 to 0.05; 16 studies, 3040 infants; Analysis 5.15), but we found no evidence of an effect on the incidence of necrotising enterocolitis (typical RR 0.90, 95% CI 0.74 to 1.11; 25 studies, 4050 infants; Analysis 5.13).
5.14. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 14 Gastrointestinal bleeding.
5.15. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 15 Gastrointestinal perforation.
5.13. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 13 Necrotising enterocolitis (NEC).
Other effects
Early corticosteroids increased risks of hypertrophic cardiomyopathy (RR 4.33, 95% CI 1.40 to 13.4; RD 0.40, 95% CI 0.17 to 0.63; one study, 50 infants; Analysis 5.4) and growth failure (RR 6.67, 95% CI 2.27 to 19.6; RD 0.68, 95% CI 0.48 to 0.88; one study, 50 infants; Analysis 5.5) in one study in which these were reported. Early corticosteroids reduced the risk of patent ductus arteriosus (typical RR 0.78, 95% CI 0.72 to 0.85; typical RD ‐0.09, 95% CI ‐0.12 to ‐0.06; 24 studies, 4013 infants; Analysis 5.7). Results showed no significant effects on infection (typical RR 1.05, 95% CI 0.96 to 1.15; 25 studies, 4101 infants; Analysis 5.1), pulmonary air leaks (typical RR 0.91, 95% CI 0.74 to 1.13; 16 studies, 3225 infants; Analysis 5.6), severe intraventricular haemorrhage (typical RR 0.96, 95% CI 0.83 to 1.11; 26 studies, 4103 infants; Analysis 5.8), periventricular leukomalacia (typical RR 1.07, 95% CI 0.78 to 1.46; 15 studies, 2807 infants; Analysis 5.10), or pulmonary haemorrhage (typical RR 1.16, 95% CI 0.87 to 1.54; 10 studies, 1820 infants; Analysis 5.16). Early corticosteroids reduced any retinopathy of prematurity (typical RR 0.88, 95% CI 0.80 to 0.97; nine studies, 1345 infants; Analysis 5.17) and both severe retinopathy of prematurity (typical RR 0.81, 95% CI 0.66 to 0.98; RD ‐0.03, 95% CI ‐0.05 to ‐0.00; 14 studies, 2577 infants; Analysis 5.18) and severe retinopathy of prematurity among survivors (typical RR 0.77, 95% CI 0.64 to 0.94; RD ‐0.05, 95% CI ‐0.09 to ‐0.01; 12 studies, 1575 infants; Analysis 5.19).
5.4. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 4 Hypertrophic cardiomyopathy.
5.5. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 5 Growth failure.
5.7. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 7 Patent ductus arteriosus (PDA).
5.1. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 1 Infection.
5.6. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 6 Pulmonary air leak.
5.8. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 8 Severe IVH.
5.10. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 10 Periventricular leukomalacia (PVL).
5.16. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 16 Pulmonary haemorrhage.
5.17. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 17 Any retinopathy of prematurity (ROP).
5.18. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 18 Severe ROP.
5.19. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 19 Severe ROP in survivors.
Follow‐up data
Follow‐up studies are few compared with the total number of studies: Of 32 studies, 13 provided follow‐up data.
Developmental delay
Corticosteroids increased developmental delay in one study of dexamethasone, but criteria for the diagnosis were not explicit (RR 1.68, 95% CI 1.08 to 2.61; RD 0.14, 95% CI 0.03 to 0.24; one study, 248 infants; Analysis 6.5). Results showed no significant differences when developmental delay was determined by formal developmental assessments (Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4).
6.5. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 5 Developmental delay (criteria not specified).
6.1. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 1 Bayley Mental Developmental Index (MDI) <‐2 SD.
6.2. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 2 Bayley MDI <‐2 SD in tested survivors.
6.3. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 3 Bayley Psychomotor Developmental Index (PDI) <‐2 SD.
6.4. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 4 Bayley PDI <‐2 SD in tested survivors.
Cerebral palsy
Corticosteroids increased cerebral palsy (typical RR 1.42, 95% CI 1.06 to 1.91; typical RD 0.02, 95% CI 0.00 to 0.05; 13 studies, 1973 infants; Analysis 6.11), but results showed little difference in the combined outcome, death or cerebral palsy (typical RR 1.03, 95% CI 0.91 to 1.16; 13 studies, 1973 infants; Analysis 6.13). We noted moderate heterogeneity (I² = 52%) for the combined outcome, death or cerebral palsy, among the dexamethasone studies and little evidence of publication bias for the outcome of cerebral palsy (Figure 5).
6.11. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 11 Cerebral palsy.
6.13. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 13 Death or cerebral palsy.
5.
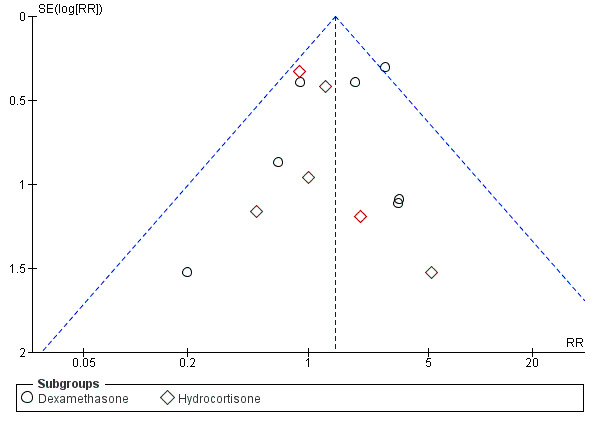
Funnel plot of comparison: 6 Long‐term follow‐up, outcome: 6.11 Cerebral palsy.
Major neurosensory disability
Results showed few effects on major neurosensory disability (typical RR 1.09, 95% CI 0.89 to 1.33; eight studies, 1754 infants; Analysis 6.15) and on the combined outcome, death or major neurosensory disability (typical RR 0.97, 95% CI 0.88 to 1.08; eight studies, 1754 infants; Analysis 6.17).
6.15. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 15 Major neurosensory disability (variable criteria ‐ see individual studies).
6.17. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 17 Death or major neurosensory disability (variable criteria).
Abnormal neurological examination
Results showed a significant increase in the rate of abnormal neurological examination findings (typical RR 1.81, 95% CI 1.33 to 2.47; typical RD 0.10, 95% CI 0.05 to 0.15; five studies, 829 infants; Analysis 6.19) and in the combined outcome, death or abnormal neurological examination (typical RR 1.23, 95% CI 1.06 to 1.42; typical RD 0.10, 95% CI 0.03 to 0.16; five studies, 829 infants; Analysis 6.21). Although criteria for this diagnosis were vague and varied between studies, the size of the difference in this outcome in trials for which data were available was similar to the size of the difference in cerebral palsy in the corresponding study. Yeh 1997 provided data for cerebral palsy obtained at age eight to nine years, whereas investigators obtained abnormal examination data from earlier in childhood ‐ at two years of age.
6.19. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 19 Abnormal neurological exam (variable criteria ‐ see individual studies).
6.21. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 21 Death or abnormal neurological exam (variable criteria).
Other long‐term outcomes
Results showed no significant effects on other long‐term outcomes of blindness, deafness, formal psychometric testing, abnormal electroencephalogram (EEG), behaviour problems, or rehospitalisation in infancy.
Subgroup analysis by type of corticosteroid used
Mortality
Data show little difference in the effects of dexamethasone or hydrocortisone on mortality at 28 days of life (typical RR dexamethasone 1.06, 95% CI 0.90 to 1.24; 16 studies, 2603 infants; hydrocortisone 0.78, 95% CI 0.50 to 1.23; three studies, 347 infants; P value for interaction = 0.22; Analysis 1.1), or of dexamethasone on mortality at 36 weeks' postmenstrual age (typical RR 1.08, 95% CI 0.94 to 1.25; 14 studies, 2487 infants; Analysis 1.2), before discharge (typical RR 1.03, 95% CI 0.90 to 1.18; 19 studies, 2840 infants; Analysis 1.3), or at the latest age possible to determine the outcome (typical RR 1.02, 95% CI 0.90 to 1.16; 20 studies, 2940 infants; Analysis 1.4). However, hydrocortisone reduced mortality to discharge (typical RR hydrocortisone 0.80, 95% CI 0.65 to 0.98; 11 studies, 1433 infants; P value for interaction = 0.04; Analysis 1.3) and at the latest age possible to determine the outcome (typical RR hydrocortisone 0.80, 95% CI 0.65 to 0.99; 11 studies, 1433 infants; P value for interaction = 0.05; Analysis 1.4), and had a borderline effect on mortality at 36 weeks (typical (RR 0.83, 95% CI 0.65 to 1.06; six studies, 1246 infants; P value for interaction = 0.07; Analysis 1.2).
Bronchopulmonary dysplasia
Most of the benefit of early corticosteroids in reducing the incidence of BPD was provided by dexamethasone, with little effect of hydrocortisone, regardless of the definition of BPD: needing oxygen supplementation at 28 days of life (typical RR dexamethasone 0.85, 95% CI 0.79 to 0.92; typical RD ‐0.07, 95% CI ‐0.11 to ‐0.04; 16 studies, 2621 infants; hydrocortisone 1.00, 95% CI 0.85 to 1.18; one study, 253 infants; Analysis 2.1), or needing oxygen at 36 weeks' postmenstrual age (typical RR dexamethasone 0.71, 95% CI 0.62 to 0.81; typical RD ‐0.08, 95% CI ‐0.12 to ‐0.05; 16 studies, 2584 infants; hydrocortisone 0.91, 95% CI 0.80 to 1.05; eight studies, 1345 infants; Analysis 2.2). Benefit in reducing the need for late rescue with postnatal corticosteroids also was largely confined to the dexamethasone group (typical RR dexamethasone 0.72, 95% CI 0.65 to 0.80; typical RD ‐0.14, 95% CI ‐0.18 to ‐0.10; 10 studies, 1974 infants; hydrocortisone 1.01, 95% CI 0.73 to 1.40; four studies, 509 infants; Analysis 2.4). Strong evidence showed subgroup differences for some of these outcomes, with low P values for Analysis 2.2, Analysis 2.3, and Analysis 2.4. We noted substantial or moderate heterogeneity for some of these comparisons (2.1, 2.3, and 2.4), suggesting that results should be interpreted with caution.
Death or bronchopulmonary dysplasia
Most of the benefit of early corticosteroids in reducing the incidence of the combined outcome of death or bronchopulmonary dysplasia at 28 days of life was provided by dexamethasone, with little effect of hydrocortisone (typical RR dexamethasone 0.91, 95% CI 0.86 to 0.96; typical RD ‐0.07, 95% CI ‐0.10 to ‐0.03; 14 studies, 2293 infants; hydrocortisone 1.00, 95% CI 0.90 to 1.12; one study, 253 infants; Analysis 3.1), but both agents reduced the combined outcome of death or bronchopulmonary dysplasia at 36 weeks' postmenstrual age (typical RR dexamethasone 0.87, 95% CI 0.80 to 0.94; typical RD ‐0.07, 95% CI ‐0.10 to ‐0.03; 16 studies, 2581 infants; hydrocortisone 0.90, 95% CI 0.82 to 0.99; typical RD ‐0.06, 95% CI ‐0.11 to ‐0.01; nine studies, 1379 infants; Analysis 3.2). Heterogeneity was substantial for one of these comparisons (Analysis 3.1) and was moderate for the other (Analysis 3.2), suggesting the need for caution when results are interpreted.
Complications during primary hospitalisation
Some of the short‐term complications observed with corticosteroids were related more to dexamethasone than to hydrocortisone, including hyperglycaemia (typical RR dexamethasone 1.35, 95% CI 1.21 to 1.49; typical RD 0.11, 95% CI 0.08 to 0.15; 12 studies, 2117 infants; hydrocortisone 0.92, 95% CI 0.50 to 1.67; one study, 50 infants; Analysis 5.2), hypertension (typical RR dexamethasone 1.84, 95% CI 1.53 to 2.21; typical RD 0.10, 95% CI 0.07 to 0.13; 10 studies, 1943 infants; hydrocortisone RR 3.00, 95% CI 0.33 to 26.92; one study, 50 infants; Analysis 5.3), and gastrointestinal haemorrhage (typical RR dexamethasone 1.87, 95% CI 1.35 to 2.58; typical RD 0.05, 95% CI 0.03 to 0.08; 10 studies, 1725 infants; hydrocortisone 1.53, 95% CI 0.27 to 8.74; two studies, 91 infants; Analysis 5.14). However, both types of corticosteroid were associated with greater gastrointestinal perforation (typical RR dexamethasone 1.73, 95% CI 1.20 to 2.51; typical RD 0.03, 95% CI 0.01 to 0.05; nine studies, 1936 infants; hydrocortisone 1.70, 95% CI 1.07 to 2.70; typical RD 0.03, 95% CI 0.00 to 0.06; seven studies, 1104 infants; Analysis 5.15) and lower rates of patent ductus arteriosus (typical RR dexamethasone 0.76, 95% CI 0.69 to 0.84; typical RD ‐0.10, 95% CI ‐0.13 to ‐0.06; 17 studies, 2706 infants; hydrocortisone 0.82, 95% CI 0.71 to 0,95; typical RD ‐0.07, 95% CI ‐0.12 to ‐0.02; seven studies, 1307 infants; Analysis 5.7). Only dexamethasone was associated with reductions in the rates of any retinopathy of prematurity (typical RR dexamethasone 0.84, 95% CI 0.72 to 0.99; eight studies, 1042 infants; hydrocortisone 0.93, 95% CI 0.84 to 1.04; one study, 303 infants; Analysis 5.17), severe retinopathy of prematurity (typical RR dexamethasone 0.77, 95% CI 0.60 to 0.99; eight studies, 1507 infants; hydrocortisone 0.87, 95% CI 0.63 to 1.21; six studies, 1070 infants; Analysis 5.18), and severe retinopathy of prematurity among survivors (typical RR dexamethasone 0.75, 95% CI 0.59 to 0.95; 10 studies, 1238 infants; hydrocortisone 0.83, 95% CI 0.60 to 1.17; two studies, 337 infants; Analysis 5.19).
Follow‐up data
Cerebral palsy and the combined outcome of death or cerebral palsy were more common with dexamethasone than with hydrocortisone (cerebral palsy: typical RR dexamethasone 1.75, 95% CI 1.20 to 2.55; typical RD 0.05, 95% CI 0.01 to 0.09; seven studies, 921 infants; typical RR hydrocortisone 1.05, 95% CI 0.66 to 1.66; six studies, 1052 infants; Analysis 6.11; death or cerebral palsy: typical RR dexamethasone 1.17, 95% CI 1.00 to 1.37; typical RD 0.07, 95% CI 0.00 to 0.13; seven studies, 921 infants; typical RR hydrocortisone 0.86, 95% CI 0.71 to 1.05; typical RD ‐0.04, 95% CI ‐0.09 to 0.01; six studies, 1052 infants; Analysis 6.13).
We noted that some subgroup analyses included few studies and small sample sizes for the hydrocortisone subgroup; hence the power to detect beneficial or harmful effects of hydrocortisone was limited under these circumstances.
Results of individual trials
Anttila 2005: The primary outcome was survival without BPD, intraventricular haemorrhage (grade 3 or 4), or periventricular leukomalacia, and although this tended to be greater in the dexamethasone group, differences compared with controls were not statistically significant. The risk ratio for death or BPD at 36 weeks' postmenstrual age was 0.78 (95% CI 0.54 to 1.13) overall, and 0.61 (95% CI 0.33 to 1.11) in the subgroup with birth weight 750 grams to 999 grams. We noted no detectable trends in mortality, severe intraventricular haemorrhage, or periventricular leukomalacia. Rates of patent ductus arteriosus, retinopathy of prematurity, or sepsis did not differ between groups. Mean arterial blood pressures were increased in the dexamethasone group during the first week (P = 0.015), and the dexamethasone group tended to need more insulin therapy (49% vs 39%; P = 0.25).
Baden 1972: Results of this study showed no significant effects on blood gases, pH, oxygen requirement, need for assisted ventilation, or survival. Data indicate no significant differences in rates of cerebral palsy or deafness among survivors, in mean scores on Griffiths Scales, or in the combined rate of death or cerebral palsy (Fitzhardinge 1974).
Batton 2012: Data show minimal effects on rates of death during primary hospitalisation, BPD (undefined), intraventricular haemorrhage, periventricular leukomalacia, or necrotising enterocolitis.
Baud 2016: Death or BPD at 36 weeks' gestational age occurred in 40% (102/255) of the hydrocortisone group compared with 49% (130/266) of the placebo group (OR 0.82, 95% CI 0.67 to 0.99). Rates of any neurodevelopmental impairment (NDI) among assessed survivors were similar in the two groups (hydrocortisone 27% (53/194); placebo 30% (55/185)), as were rates of moderate to severe impairment (hydrocortisone 7% (14/194); placebo 11% (21/185)). The combined outcome of death or moderate severe impairment in all randomised infants was lower in the hydrocortisone group than in the control group (hydrocortisone 24% (62/255); placebo 33% (88/266); OR 0.73, 95% CI 0.56 to 0.97).
Biswas 2003: Results showed no significant effects of infusion of hydrocortisone and T3 on the primary endpoint of death or failure to extubate by seven days, or death or oxygen dependency at 14 days. Patent ductus arteriosus was significantly reduced in the treatment group (41/125 vs 60/128; RR 0.70, 95% CI 0.51 to 0.96), but data show no other significant differences in secondary outcomes.
Bonsante 2007: Oxygen‐free survival was significantly greater in the hydrocortisone group than in the control group (64% vs 32%; P = 0.023). The effect of hydrocortisone was particularly evident in the subgroup not exposed to prenatal corticosteroids. Four infants in the hydrocortisone group died compared with 10 in the control group (16% vs 40%; P = 0.05). Duration of ventilation, patent ductus arteriosus, severe retinopathy of prematurity, severe intraventricular haemorrhage, and periventricular leukomalacia were not different between groups.
Efird 2005: Vasopressor was used less in the hydrocortisone‐treated group, significantly so on the second day of life. Results showed no significant differences in cortisol levels between groups at any time point, and no significant differences in mortality, duration of ventilation, BPD (oxygen at 36 weeks' postmenstrual age), nosocomial infections, necrotising enterocolitis, spontaneous intestinal perforations, or intraventricular haemorrhage. No infants were treated or removed from the study as a result of hypertension. Data show no differences in the rate of glucose intolerance between groups, but two infants in the hydrocortisone group received insulin for five days.
Garland 1999: Early dexamethasone‐treated infants were more likely than placebo‐treated controls to survive without BPD (83/118 vs 71/123; P = 0.03). They also were less likely to develop BPD if they survived to 28 days of life (16/99 vs 27/98; P = 0.042). Mortality rates were not significantly different. Subsequent dexamethasone therapy was used less often among early dexamethasone‐treated infants who survived (68/99 vs 81/98; P = 0.01). Intestinal perforation was more common, but not significantly so, among dexamethasone‐treated infants (12/118 vs 7/122; P = 0.20); during the first week of life, the difference was significant (9/118 vs 1/122; P = 0.009). Infants in the dexamethasone group also spent less time receiving oxygen (median days 43 vs 50; P = 0.04). Any grade of intraventricular haemorrhage (36% vs 52%; P = 0.02) and of patent ductus arteriosus ligation (14% vs 28%; P = 0.01) was also less common in the dexamethasone group. Hypertension (P<0.001) and treatment with insulin (P=0.007) ocurred more often for dexamethasone‐treated infants than controls.
Halac 1990: Investigators reported no substantial or statistically significant effects of dexamethasone on neonatal mortality, mortality to hospital discharge, necrotising enterocolitis, sepsis, patent ductus arteriosus, or severe intraventricular haemorrhage.
Hochwald 2014: Data show no statistically significant effects of hydrocortisone on mortality to hospital discharge, BPD, death or BPD, necrotising enterocolitis, or sepsis.
Kopelman 1999: Intermittent mandatory ventilation (IMV) rate and ventilation index improved more rapidly in the dexamethasone‐treated group. Mean blood pressure was higher in the dexamethasone group after the first day. Patent ductus arteriosus was less common in dexamethasone‐treated infants (13/37 vs 19/33; P = 0.08), and fewer received indomethacin (8/37 vs 15/33; P = 0.03). At the study hospital, where early extubation was practised, more dexamethasone‐treated infants were extubated during the first week (10/22 vs 2/16, P < 0.03). Results show no difference in intraventricular haemorrhage and no occurrence of adverse effects.
Lin 1999: For the endpoint of BPD at 28 days of life, statistical significance favouring dexamethasone was reached when analysis of 12 consecutive pairs in which one infant had BPD and the other did not showed that 10 pairs favoured dexamethasone and two pairs favoured control. Data presented for 40 infants (20 in each group) show a lower incidence of BPD at 28 days of life in the dexamethasone group (n = 4) than in the control group (n = 11; P < 0.05). Duration of oxygen therapy was also shorter in the dexamethasone group, at 7 ± 6 days versus 13 ± 12 days (P < 0.05). Among survivors, 12 of 15 in the dexamethasone group were extubated compared with 9 of 16 in the control group at the end of the study. Infants in the treated group had transient hyperglycaemia and hypertension, but data showed no differences between groups for mortality, incidence of sepsis, or intraventricular haemorrhage.
Mukhopadhyay 1998: Oxygen requirement was lower in the treated group than in the control group on days three, four, and five, although differences were not statistically significant. Mean duration of ventilation was shorter in the dexamethasone group (87 ± 42 hours) than in the control group (120 ± 46 hours; P value not given). Study authors reported one case of culture‐positive sepsis in the dexamethasone group, and two in the control group. None of the infants developed BPD (definition not given). Four infants in the dexamethasone group developed a pneumothorax versus three in the control group. Survival was 60% in the treated group and 55% in the control group.
Ng 2006: Nineteen infants (79%) in the hydrocortisone group were weaned from vasopressor support within 72 hours compared with eight controls (33%) (P < 0.001). Cumulative doses of dopamine and dobutamine after randomisation were significantly lower in the hydrocortisone group. Duration of ventilation, duration of oxygen, and incidence of BPD (oxygen at 36 weeks' postmenstrual age) were not significantly different between groups. Results showed no differences between groups for highest serum glucose, culture‐proven sepsis, necrotising enterocolitis, intestinal perforation, duration of hospitalisation, and mortality. However, significantly more hydrocortisone‐treated infants had glycosuria (P = 0.029).
Peltoniemi 2005: Hydrocortisone‐treated infants did not show a significant increase in survival without BPD (64% vs 54% placebo) or a significant decrease in BPD among survivors (odds ratio (OR) 0.53, 95% CI 0.17 to 1.71). However, the study enrolled only 16% of its intended sample size. Two infants in the hydrocortisone group and three in the placebo group died. During the first week of life, infants in the hydrocortisone group needed lower mean airway pressures than infants in the placebo group (P = 0.03). Patent ductus arteriosus (36% vs 73%; P = 0.01) and duration of oxygen therapy (34 vs 62 days; P = 0.02) occurred less often in the hydrocortisone group, but intraventricular haemorrhage, cystic periventricular leukomalacia, retinopathy of prematurity, sepsis, necrotising enterocolitis, gastrointestinal haemorrhage, open corticosteroid treatment, and duration of intubation and of hospitalisation were not different between groups. Risk of gastrointestinal perforation was increased in the hydrocortisone group (16% vs 0%; P = 0.05). Data showed no differences in the rate of hyperglycaemia requiring insulin nor in blood pressures (diastolic and systolic). At six‐year follow‐up, data showed no substantial differences between groups in rates of cerebral palsy, blindness, deafness, or intellectual impairment (IQ < 69; steroid group 8% (2/25) vs placebo group 4% (1/26)).
Rastogi 1996: Ventilator variables at 5 to 14 days were significantly improved among infants who received dexamethasone compared with infants who received placebo. The effect seemed to be more marked in infants weighing less than 1250 grams at birth. Significantly more infants could be extubated by 14 days in the dexamethasone group (26/32 vs 14/32; P = 0.004). Dexamethasone therapy reduced the incidence of BPD at 28 days of life (OR 0.10, 95% CI 0.03 to 0.30) and eliminated BPD at 36 weeks' postmenstrual age. Dexamethasone‐treated infants were more likely to show weight loss at 14 days (12.9% vs 3.7%; P = 0.01) and higher blood pressure from days 3 to 10. However, data showed no differences in time to regain birth weight, hypertension (one infant in each group), or incidence of intraventricular haemorrhage.
Romagnoli 1999: The incidence of BPD at 28 days of life and at 36 weeks' postmenstrual age was significantly lower in the dexamethasone group than in the control group (P < 0.001). Infants in the dexamethasone group remained intubated and required oxygen therapy for a shorter period than those in the control group (P < 0.001). Hyperglycaemia, hypertension, growth failure, and hypertrophy of the left ventricle were transient side effects of early corticosteroid administration. Data show no significant differences in rates of cerebral palsy, blindness, deafness, intellectual impairment, or mean IQ, or in the combined rate of death or cerebral palsy (Romagnoli 2002).
Sanders 1994: The dexamethasone group required less ventilatory support (mean airway, peak inspiratory and end‐expiratory pressures, and IMV) and supplemental oxygen after study day 4 (all P < 0.05). Improved tidal volume in the dexamethasone group, as assessed by pulmonary function testing of infants who remained intubated, was seen on study day 7 (P = 0.02). The dexamethasone group required a shorter time in hospital (median 95 days vs 106 days; P = 0.01). Survival in the dexamethasone group was 89% versus 67% in the placebo group (P = 0.08). Survival without BPD was 68% in the dexamethasone group versus 43% in the placebo group (P = 0.14). Mean blood pressure was elevated on study days 4 to 7. Data show no differences in rates of hyperglycaemia, incidence or severity of intraventricular haemorrhage, or days to regain birth weight, and no significant differences in rates of cerebral palsy, blindness, deafness, or intellectual impairment, nor in the combined rate of death or cerebral palsy (Sinkin 2002 (personal communication follow‐up to Sanders 1994)).
Shinwell 1996: Results showed no differences in any outcome variable, except for a reduction in the need for mechanical ventilation at three days in dexamethasone‐treated infants (54/122, 44% vs 71/106, 67%; P = 0.001). Gastrointestinal haemorrhage, hypertension, and hyperglycaemia were more common among treated infants, but no life‐threatening complications, such as gastrointestinal perforation, were encountered. Follow‐up of survivors at two to six years showed no significant differences in rates of blindness, deafness, or major neurosensory disability, nor in the combined rate of death or major neurosensory disability. However, data showed significant increases in rates of abnormal neurological examination findings, developmental delay, and cerebral palsy, and a significant increase in the combined rate of death or cerebral palsy (Shinwell 2002).
Sinkin 2000: Results showed no differences between dexamethasone and placebo groups, respectively, for the primary outcomes of survival (79% vs 83%), survival without oxygen at 36 weeks' postmenstrual age (both 59%), and survival without oxygen at 36 weeks' postmenstrual age without late corticosteroids (46% vs 44%). We noted no significant differences between groups for median time in oxygen (50 vs 56 days), ventilation (20 vs 27 days), time to regain birth weight (15.5 vs 15.0 days), nor length of stay (88 vs 89 days). Infants given early dexamethasone were less likely to receive late corticosteroids for BPD during their hospital stay (25% vs 35%; P = 0.042). We noted no clinically significant side effects in the dexamethasone group, although transient elevations in blood glucose and blood pressure with return to baseline were evident by study day 10. Among infants who died (40 vs 33), data show no differences in median days on oxygen, ventilation, or length of hospital stay. However, among survivors (149 vs 162), we observed the following: median days on oxygen 37 vs 45, ventilation 14 vs 19 days, and length of stay 79 vs 81 days, for dexamethasone versus placebo groups, respectively. Data show no significant differences in rates of cerebral palsy, in the combined rate of death or cerebral palsy, or in mean Bayley scores (Sinkin 2002 (personal communication follow‐up to Sinkin 2000).
Soll 1999: Results showed no differences in the primary outcome of BPD or death at 36 weeks' postmenstrual age (early therapy 135/272 vs 143/267; RR 0.93, 95% CI 0.79 to 1.09). Infants who received early corticosteroid therapy were less likely to need late treatment (114/270 vs 164/267; RR 0.69, 95% CI 0.58 to 0.81). They also had a lower risk of patent ductus arteriosus (92/272 vs 117/269; RR 0.78, 95% 0.63 to 0.96) and were less likely to receive indomethacin therapy (132/273 vs 176/269; RR 0.74, 95% CI 0.64 to 0.86). However, infants who received early corticosteroid therapy were more likely to have complications such as hyperglycaemia (200/271 vs 151/263; RR 1.29, 95% CI 1.13 to 1.46) and to require insulin therapy (168/271 vs 102/267; RR 1.62, 95% CI 1.36 to 1.94). Data show trends towards increased gastrointestinal haemorrhage (33/271 vs 21/267; RR 2.55, 95% CI 0.92 to 2.61) and gastrointestinal perforation (31/271 vs 20/267; RR 1.53, 95% CI 0.89 to 2.61). Infants who had cranial ultrasound scans showed a trend towards an increase in periventricular leukomalacia in the early corticosteroid group (18/252 vs 8/250; RR 2.23, 95% CI 0.99 to 5.04). Infants who received early corticosteroid therapy received fewer days of supplemental oxygen but experienced poorer weight gain.
Stark 2001: Corticosteroid‐treated infants had a lower incidence of the primary outcome, death or BPD at 36 weeks' postmenstrual age (63% vs 69%; P < 0.05). Fewer infants in the corticosteroid group had pulmonary interstitial emphysema (9% vs 23%; P < 0.05), required oxygen at 28 days of life (78% vs 94%; P < 0.05), or received subsequent corticosteroid treatment (34% vs 51%; P < 0.05). Rates of severe intraventricular haemorrhage, periventricular leukomalacia, retinopathy of prematurity, and nosocomial infection were similar. Hypertension and hyperglycaemia were more frequent in the corticosteroid group (27% vs 4% and 23% vs 12%, respectively; both with P < 0.05). During the first 14 days, 14/111 (13%) infants in the corticosteroid group and 3/109 (3%) in the placebo group had spontaneous gastrointestinal perforation without necrotising enterocolitis (P < 0.05). Spontaneous perforation was also associated with indomethacin treatment (P = 0.005), as was an interaction between indomethacin and corticosteroid therapy (P = 0.04). Data showed no significant differences in rates of cerebral palsy, developmental delay, or major neurosensory disability, in the combined rate of death or cerebral palsy, or in the combined rate of death or major neurosensory disability (Stark 2001).
Subhedar 1997: Results showed no differences in the combined incidence of BPD and/or death before discharge from hospital between infants treated with dexamethasone and control infants (RR 0.95, 95% CI 0.79 to ‐1.18) or between those treated with inhaled nitric oxide and controls (RR 1.05, 95% CI 0.84 to 1.25). Data showed no significant differences in rates of cerebral palsy, blindness, deafness, developmental delay, the combined rate of death or cerebral palsy, or the combined rate of death or major neurosensory disability (Subhedar 2002 (personal communication follow‐up to Subhedar 1997)).
Suske 1996: Infants in the dexamethasone group were extubated earlier (6.6 days vs 14.2 days; P < 0.02) and required less time in supplemental oxygen (4.2 days vs 12.5 days; P < 0.02). Pulmonary complications tended to be fewer in the dexamethasone group (1/14 vs 4/12), and retinopathy of prematurity tended to occur less frequently (2/14 vs 6/12; P < 0.05).
Tapia 1998: Results showed no significant differences in mortality and/or BPD between groups. The number of infants requiring oxygen at 36 weeks' postmenstrual age was significantly reduced in the dexamethasone group (8% vs 33%; P < 0.05). Stepwise logistic regression analysis with oxygen dependency at 36 weeks as the dependent variable, and birth weight, gestational age, gender, prenatal corticosteroids, and study treatment as the independent variables, showed that study treatment was the only variable significantly associated with oxygen dependency at 36 weeks. Data showed no differences between groups in the number of days of mechanical ventilation and oxygen treatment, and no differences in the incidence of major morbidity and possible complications related to corticosteroid administration, except for a lower incidence of necrotising enterocolitis in the dexamethasone group.
Vento 2004: Seven dexamethasone‐treated infants and two control infants were extubated during the study period of seven days. Data showed no differences between groups for respiratory distress syndrome, patent ductus arteriosus, or severe intraventricular haemorrhage (grade 3 or 4). Dexamethasone‐treated infants had lower absolute cell count and proportion of polymorphs in tracheal aspirate fluid compared with control infants as early as day 1 of treatment. They also had significantly higher dynamic compliance values compared with control infants (P < 0.01) as early as day 2 of treatment. Inspired oxygen concentrations were significantly lower on day 2 (0.24 vs 0.31; P < 0.05), and mean airway pressure on day 5 (4.8 vs 7.2 cmH2O; P < 0.05).
Wang 1996: Dexamethasone treatment decreased fractional inspired oxygen concentration, arterial carbon dioxide tension, and mean airway pressure, and facilitated successful weaning from mechanical ventilation. Surfactant protein (SP)‐A concentrations in tracheal aspirates were increased at days 7 and 14, and SP‐D concentrations were increased from days 3 to 14 in the dexamethasone‐treated group, compared with the control group.
Watterberg 1999: In this study, more infants treated with hydrocortisone survived without supplemental oxygen at 36 weeks' postmenstrual age (12/20 vs 7/20; P = 0.023). Hydrocortisone treatment was also associated with a reduction in duration of oxygen > 40% (7 vs 28 days; P = 0.06), duration of oxygen > 25% (48 vs 69 days; P = 0.02), and duration of mechanical ventilation (25 vs 32 days; P = 0.03). Data show no differences in rates of death, sepsis, patent ductus arteriosus, necrotising enterocolitis, gastrointestinal perforation, intraventricular haemorrhage, or retinopathy of prematurity, and no significant differences in rates of cerebral palsy, blindness, or deafness, or in the combined rate of death or cerebral palsy (Watterberg 2002 (personal communication follow‐up to Watterberg 1999)).
Watterberg 2004: Results showed no differences in primary outcomes between groups (hydrocortisone vs placebo): survival without BPD (35% vs 34%), death before 36 weeks' postmenstrual age (15% vs 16%), and death before discharge (16% vs 17%). In a subgroup of infants exposed to chorioamnionitis, the hydrocortisone‐treated group had significantly improved survival without BPD (38% vs 24%; P = 0.005) and lower mortality at 36 weeks' postmenstrual age (10% vs 18%; P = 0.02) and before discharge (12% vs 21%; P = 0.02). During treatment, rates of hyponatraemia, hypernatraemia, hyperkalaemia, hyperglycaemia, hypertension, and gastrointestinal bleeding were similar between groups. Seventy‐four infants (41%) in the hydrocortisone group and 62 (34%) in the placebo group were treated with insulin (P = 0.19). Serum sodium and mean arterial blood pressure were significantly higher in hydrocortisone‐treated infants (P < 0.001 and P = 0.022, respectively). Other outcomes included no differences in weight gain or head circumference, durations of oxygen and ventilation, pulmonary air leaks, pulmonary haemorrhage, patent ductus arteriosus, sepsis, intraventricular haemorrhage, periventricular leukomalacia, retinopathy of prematurity and necrotising enterocolitis. However, hydrocortisone‐treated infants were less likely to receive open‐label corticosteroids during the treatment period (18% vs 28%; P = 0.02) and were more likely to have a spontaneous gastrointestinal perforation (9% vs 2%; P = 0.01). Follow‐up data reveal no significant differences in rates of cerebral palsy, major neurological disability, developmental delay, or rehospitalisation, or in combined rates of death or cerebral palsy, or death or major neurological disability (Watterberg 2007).
Yeh 1990: Infants in the dexamethasone group had significantly higher pulmonary compliance, tidal volume, and minute ventilation, and required lower mean airway pressure for ventilation than infants in the placebo group. The endotracheal tube was successfully removed from more infants in the dexamethasone group (16/28 vs 8/29; P < 0.025) at two weeks of age. Nineteen infants (65%) in the placebo group and 11 (39%) in the dexamethasone group (P < 0.05) had lung injury characterised by the following: surviving infants with BPD; infants who died of intractable respiratory failure and had evidence of BPD at autopsy; and infants who died of intractable respiratory failure with clinical evidence of BPD. Dexamethasone therapy was associated with a temporary increase in blood pressure and plasma glucose concentration and delayed somatic growth.
Yeh 1997: Infants in the dexamethasone group had a significantly lower incidence of BPD than those in the placebo group, judged at 28 postnatal days (21/132 vs 40/130, P < 0.05) or at 36 weeks' postmenstrual age (20/132 vs 37/130, P < 0.05). More infants in the dexamethasone group were extubated during the study. Data showed no difference in mortality between groups (39/130 vs 44/132); however, a higher proportion of infants in the dexamethasone group died during the late study period, probably as the result of infection. Results showed no differences between groups in duration of oxygen therapy and hospitalisation. Significantly more infants in the dexamethasone group had bacteraemia or clinical sepsis (44/132 vs 27/130; P < 0.05). Other immediate but transient side effects observed in the dexamethasone group were hyperglycaemia, hypertension, cardiac hypertrophy, hyperparathyroidism, and delay in growth rate. At 25 months of age, data revealed no significant differences in rates of blindness, developmental delay, or major neurosensory disability, or in the combined rate of death or cerebral palsy, or the combined rate of death or major neurosensory disability. However, we noted significant increases in rates of abnormal neurological examination and cerebral palsy among survivors (Yeh 1998). The follow‐up rate of survivors at eight years was 92% (146/159). Although rates of cerebral palsy were not significantly higher in the dexamethasone group, overall motor performance on the Movement ABC was worse than in controls. IQ and other cognitive performance were significantly worse in the dexamethasone group. Overall, survivors in the dexamethasone group had greater major neurological disability.
Discussion
Corticosteroids are potent drugs that may improve lung function in infants with bronchopulmonary dysplasia (BPD) through several different mechanisms. It has been suggested that corticosteroids might have a role to play in prevention of BPD by suppressing the inflammatory response in the lungs of infants at risk (Groneck 1995). It has been shown that infants who develop BPD have low cortisol levels following adrenocorticotrophic hormone (ACTH) stimulation during the first week of life (Watterberg 1999). To be effective in preventing BPD, corticosteroids may have to be given within the first few days of life.
This review has demonstrated that early corticosteroid treatment facilitates weaning from the ventilator. Additional advantages include increased survival without BPD at 28 days of life and at 36 weeks' postmenstrual age, reduced risks of BPD at 28 days of life and at 36 weeks' postmenstrual age, and reduced risks of late treatment with corticosteroids, patent ductus arteriosus, and retinopathy of prematurity. On the other hand, risks of gastrointestinal bleeding, intestinal perforation, hyperglycaemia, hypertension, hypertrophic cardiomyopathy, and growth failure may be increased.
Potential hazards of corticosteroid treatment for the neonate include growth restriction, protein breakdown, cardiac hypertrophy, and possible adverse effects on development of the central nervous system and lungs (Gibson 1993; Gramsbergen 1998; Tschanz 1995; van Goudoever 1994; Weichsel 1977; Werner 1992). One study showed a significant decline in the growth of head circumference with early corticosteroid treatment (Papile 1996). Long‐term follow‐up results showed that early corticosteroid treatment is associated with a significant increase in risks of developmental delay and cerebral palsy, but has no significant effects on the combined outcome of death or cerebral palsy, except in the subgroup of infants treated with dexamethasone. One study in which the rate of cerebral palsy was significantly higher at two years of age used a four‐week tapering course of dexamethasone, so is similar in duration to the six‐week tapering course of late corticosteroids reported by Kothaia and included in the systematic review of late corticosteroids (Doyle 2014a; Kothadia 1999; Yeh 1997). However, in Yeh 1997, the numbers of surviving children with cerebral palsy declined between two and eight to nine years of age, and the difference became statistically non‐significant. In the 2002 follow‐up study to the 1996 Shinwell study, adverse long‐term neurological outcomes were reported in children treated with only a three‐day course of early dexamethasone starting within 12 hours of birth (Shinwell 1996; Shinwell 2002). This finding is of extreme importance and concern, as data show about a three‐fold increased risk of cerebral palsy among survivors, including children with spastic diplegia, spastic quadriplegia, and hemiplegia. Why dexamethasone given early for a short course should have such devastating effects remains unknown. Certainly some infants would have been treated with repeat courses of dexamethasone, but this would have been more likely among control infants. Periventricular leukomalacia is an obvious cause of cerebral palsy, but studies have shown no excess of this disorder in corticosteroid‐treated infants compared with controls. Despite an increase in the diagnosis of cerebral palsy, it is important to note that this does not necessarily translate into major functional disability for the children concerned.
This systematic review found that early (≤ 7 days) systemic postnatal corticosteroids for preventing BPD in preterm infants, in the regimens used, have had significant short‐term and long‐term effects ‐ both beneficial and harmful. A significant problem in interpreting late follow‐up data is that only 13 of 32 trials of early postnatal corticosteroids have reported follow‐up results; therefore, the possibility of follow‐up bias and publication bias must be considered. Potential limitations of the study with a significant increase in the rate of cerebral palsy are that only 84% of surviving infants were examined, and children were assessed during early childhood (Shinwell 1996). It is important to remember that cerebral palsy had been diagnosed before the children were five years of age in most cases; diagnosing cerebral palsy with certainty before five years of age is problematic (Stanley 1982). In another study, in which the rate of cerebral palsy was significantly worse at two years of age, with 81% follow‐up, the difference became non‐significant at eight to nine years ‐ an age when the diagnosis of cerebral palsy is more certain, and when the follow‐up rate was much better (92%), illustrating the importance of age of assessment and high follow‐up rates (Yeh 1997). No study was designed primarily to test effects of postnatal corticosteroids on adverse long‐term neurosensory outcomes, and all studies were underpowered to detect clinically important differences in long‐term neurosensory outcomes.
Subgroup analyses by type of corticosteroid revealed that most beneficial and harmful effects were attributable to dexamethasone, and that hydrocortisone had little effect in most analyses, but the power to detect beneficial or harmful effects of hydrocortisone was low for most comparisons. However, the addition of data from the latest randomised controlled trial of early hydrocortisone reported by Baud and colleagues, which is also the largest trial, has revealed some advantages for early hydrocortisone in improving rates of mortality, extubation failure, and patent ductus arteriosus (Baud 2016).
In an observational study of infants born after antenatal corticosteroid therapy, an excess of periventricular leukomalacia was evident among those whose mothers had received dexamethasone rather than betamethasone (Baud 1999). Most studies of postnatal corticosteroids used dexamethasone in high doses of 0.5 to 1.0 mg/kg/d. Other corticosteroids or lower doses of dexamethasone may prove to be safer, and emerging evidence supports the use of hydrocortisone as prophylaxis for bronchopulmonary dysplasia. Further studies are needed to compare lower doses of corticosteroids, other corticosteroids, and alternative routes of administration (e.g. inhalation) (see Cochrane Review ‐ Shah 2017).
Quality of the evidence
Review authors assessed the quality of evidence for seven major outcomes (Table 1). We assessed the evidence as high quality for mortality at 36 weeks, gastrointestinal perforation, and cerebral palsy. We downgraded several outcomes (mortality at latest age, BPD at 36 weeks, and death or BPD at 36 weeks) by one level to moderate quality because of weak evidence for publication bias, or because of moderate heterogeneity (death or cerebral palsy).
Authors' conclusions
Implications for practice.
Benefits of early postnatal corticosteroids in preterm infants at risk of developing bronchopulmonary dysplasia may not outweigh real or potential adverse effects. Early postnatal corticosteroid treatment resulted in short‐term benefits, including earlier extubation and decreased risks of bronchopulmonary dysplasia and of 'death or bronchopulmonary dysplasia' at 28 days of life and at 36 weeks' postmenstrual age, but was also associated with significant short‐term and long‐term adverse effects. Adverse effects included short‐term risk of gastrointestinal bleeding, intestinal perforation, hyperglycaemia, and hypertension, as well as long‐term risks of abnormal neurological examination findings and cerebral palsy. However, the methodological quality of studies determining long‐term outcomes was limited in some cases; children were assessed predominantly before school age, and no study was sufficiently powered to detect important adverse long‐term neurosensory outcomes. Therefore, given the risks of potential short‐term and long‐term adverse effects versus potential short‐term benefits, it appears appropriate to curtail early corticosteroid treatment for prevention of bronchopulmonary dysplasia.
Implications for research.
Through this systematic review, we have identified a compelling need for long‐term follow‐up and reporting of late outcomes, especially neurological and developmental outcomes, among surviving infants who participated in all randomised trials of early postnatal corticosteroid treatment. These follow‐up studies should include tests of gross motor function, cognitive functioning, hearing, and visual acuity.
Future studies are needed to identify accurately infants who are at greatest risk of developing bronchopulmonary dysplasia. Future placebo‐controlled trials of postnatal corticosteroids in preterm infants should include long‐term neurological follow‐up. Studies comparing different types, doses, and durations of corticosteroid treatment, and examining effects of inhaled corticosteroids, are urgently needed.
Short‐term and longer‐term effects of early hydrocortisone to prevent bronchopulmonary dysplasia require further evaluation.
What's new
| Date | Event | Description |
|---|---|---|
| 23 January 2018 | Amended | Additional data was incorrectly presented in table 1.3.2. It has been removed. The text of the review remains unchanged, as it did not reflect this error. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 10 July 2017 | New citation required and conclusions have changed | Made changes to conclusions regarding hydrocortisone during first week of life |
| 2 July 2017 | New search has been performed | Updated searches 21 February 2017; updated text and data in May 2017, and again in July 2017. Added data from new studies (Baud 2016; Hochwald 2014). Also added data from 2 arms of a 3‐arm study (Lauterbach 2006), which was not included in earlier reviews |
| 8 January 2014 | New citation required but conclusions have not changed | Added data from a pilot study of hydrocortisone for blood pressure support (Batton 2012). Made minor changes to discussion of another study (Stark 2001) with full publication of follow‐up data (2013) |
| 7 September 2013 | New search has been performed | Updated searches 22 August 2013 |
| 5 November 2009 | Amended | Edited reference citation (Peltoniemi 2005) |
| 10 November 2008 | New citation required but conclusions have not changed | Made substantive updates |
| 10 September 2008 | New search has been performed | This review updates the existing review, "Early postnatal (< 96 hours) corticosteroids for preventing chronic lung disease in preterm infants," which was published in the Cochrane Library (2003, Issue 1). This update includes data from a total of 28 trials, 12 of which provided long‐term follow‐up data. |
| 10 April 2008 | Amended | Converted to new review format |
| 11 November 2002 | New citation required and conclusions have changed | Made substantive amendments |
| 11 November 2002 | New search has been performed | This review updates the existing review, "Early postnatal (< 96 hours) corticosteroids for preventing chronic lung disease in preterm infants," which was published in the Cochrane Library (2001, Issue 1). Included in this update are additional long‐term neurodevelopmental follow‐up data from 7 trials: Data for Baden 1972 and Romagnoli 1999 were published in full reports; data for Subhedar 1997 were published as a letter to the editor; data for Stark 2001 were obtained from a presented and published abstract; and data for Sanders 1994, Sinkin 2000, and Watterberg 1999 were provided by trial investigators. Also included 2 trials reporting short‐term outcome data: Halac 1990 and Biswas 2003 Although early steroid treatment facilitates extubation and reduces risk of chronic lung disease, long‐term follow‐up studies indicate potentially increased risk of adverse neurosensory outcomes. Furthermore, short‐term complications such as gastrointestinal bleeding, intestinal perforation, hyperglycaemia, hypertension, hypertrophic cardiomyopathy, and growth failure are increased by early steroid treatment. |
Acknowledgements
None noted.
Appendices
Appendix 1. Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
The Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Previous search methods
For previous versions of this review, we sought randomised controlled trials of postnatal corticosteroid therapy from the Cochrane Central Register of Controlled Trials (CENTRAL; 2013, Issue 8), MEDLINE (1966 to August 2013), handsearching of paediatric and perinatal journals, and examination of previous review articles and information received from practising neonatologists. We searched MEDLINE using the terms: adrenal cortex hormones or dexamethasone or betamethasone or hydrocortisone or steroids or corticosteroids, limits randomised controlled trials, human, all infant: birth to 23 months. We contacted the authors of all studies, when possible, to confirm details of reported follow‐up studies, or to obtain any information about long‐term follow‐up when none had been reported.
Appendix 3. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomisation, and of blinding and reporting of all outcomes for all infants enrolled in the trial. We assessed each criterion as having low, high, or unclear risk. Two review authors separately assessed each study. We resolved disagreements by discussion. We added this information to the table 'Characteristics of included studies'. We evaluated the following issues and entered findings into the 'Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
a. low risk (any truly random process e.g. random number table; computer random number generator);
b. high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
c. unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
a. low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
b. high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
c. unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We categorised methods as:
a. low risk, high risk, or unclear risk for participants; and
b. low risk, high risk, or unclear risk for personnel;
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. We assessed blinding separately for different outcomes or classes of outcomes. We categorised the methods as:
a. low risk for outcome assessors;
b. high risk for outcome assessors; or
c. unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with total randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When trial authors reported or supplied sufficient information, we re‐included missing data in the analyses. We categorised methods as:
a. low risk (< 20% missing data);
b. high risk (≥ 20% missing data); or
c. unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
a. low risk (when it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
b. high risk (when not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
c. unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
a. low risk;
b. high risk; or
c. unclear risk
If needed, we explored the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal mortality (up to 28 days) | 19 | 2950 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.19] |
| 1.1 Dexamethasone | 16 | 2603 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.24] |
| 1.2 Hydrocortisone | 3 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.50, 1.23] |
| 2 Mortality at 36 weeks | 20 | 3733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.14] |
| 2.1 Dexamethasone | 14 | 2487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.25] |
| 2.2 Hydrocortisone | 6 | 1246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.65, 1.06] |
| 3 Mortality to hospital discharge | 30 | 4273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.07] |
| 3.1 Dexamethasone | 19 | 2840 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.18] |
| 3.2 Hydrocortisone | 11 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.98] |
| 4 Mortality at latest reported age | 31 | 4373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.06] |
| 4.1 Dexamethasone | 20 | 2940 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.16] |
| 4.2 Hydrocortisone | 11 | 1433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.99] |
Comparison 2. Bronchopulmonary dysplasia (BPD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BPD (28 days of life) | 17 | 2874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.93] |
| 1.1 Dexamethasone | 16 | 2621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.79, 0.92] |
| 1.2 Hydrocortisone | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| 2 BPD (36 weeks' postmenstrual age) | 24 | 3929 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.72, 0.87] |
| 2.1 Dexamethasone | 16 | 2584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.62, 0.81] |
| 2.2 Hydrocortisone | 8 | 1345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.05] |
| 3 BPD at 36 weeks' postmenstrual age in survivors | 21 | 2970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.88] |
| 3.1 Dexamethasone | 14 | 1917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.64, 0.83] |
| 3.2 Hydrocortisone | 7 | 1053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 4 Late rescue with corticosteroids | 14 | 2483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.82] |
| 4.1 Dexamethasone | 10 | 1974 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.80] |
| 4.2 Hydrocortisone | 4 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.40] |
| 5 Survivors who had late rescue with corticosteroids | 7 | 895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.89] |
| 5.1 Dexamethasone | 6 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.91] |
| 5.2 Hydrocortisone | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.24, 0.98] |
| 6 Survivors discharged home on oxygen | 6 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.51, 1.03] |
| 6.1 Dexamethasone | 3 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.48, 1.26] |
| 6.2 Hydrocortisone | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.40, 1.11] |
Comparison 3. Death or bronchopulmonary dysplasia (BPD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or BPD at 28 days of life | 15 | 2546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.88, 0.96] |
| 1.1 Dexamethasone | 14 | 2293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.86, 0.96] |
| 1.2 Hydrocortisone | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 2 Death or BPD at 36 weeks' postmenstrual age | 25 | 3960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.83, 0.93] |
| 2.1 Dexamethasone | 16 | 2581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.94] |
| 2.2 Hydrocortisone | 9 | 1379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.82, 0.99] |
Comparison 4. Failure to extubate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to extubate by third day | 4 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.95] |
| 1.1 Dexamethasone | 3 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.86] |
| 1.2 Hydrocortisone | 1 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.14] |
| 2 Failure to extubate by seventh day | 8 | 1448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.68, 0.85] |
| 2.1 Dexamethasone | 6 | 703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.61, 0.84] |
| 2.2 Hydrocortisone | 2 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.69, 0.94] |
| 3 Failure to extubate by 14th day | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.62, 0.97] |
| 4 Failure to extubate by 28th day | 7 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 0.98] |
Comparison 5. Complications during primary hospitalisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection | 25 | 4101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.15] |
| 1.1 Dexamethasone | 18 | 2821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.15] |
| 1.2 Hydrocortisone | 7 | 1280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.25] |
| 2 Hyperglycaemia | 13 | 2167 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.20, 1.47] |
| 2.1 Dexamethasone | 12 | 2117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.21, 1.49] |
| 2.2 Hydrocortisone | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.50, 1.67] |
| 3 Hypertension | 11 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.54, 2.22] |
| 3.1 Dexamethasone | 10 | 1943 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.53, 2.21] |
| 3.2 Hydrocortisone | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 26.92] |
| 4 Hypertrophic cardiomyopathy | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.33 [1.40, 13.37] |
| 5 Growth failure | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [2.27, 19.62] |
| 6 Pulmonary air leak | 16 | 3225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 6.1 Dexamethasone | 12 | 2041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.08] |
| 6.2 Hydrocortisone | 4 | 1184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.75, 1.65] |
| 7 Patent ductus arteriosus (PDA) | 24 | 4013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.72, 0.85] |
| 7.1 Dexamethasone | 17 | 2706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.69, 0.84] |
| 7.2 Hydrocortisone | 7 | 1307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.71, 0.95] |
| 8 Severe IVH | 26 | 4103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.11] |
| 8.1 Dexamethasone | 17 | 2736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.14] |
| 8.2 Hydrocortisone | 9 | 1367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 9 Severe intraventricular haemorrhage (IVH) in infants examined | 7 | 1909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 10 Periventricular leukomalacia (PVL) | 15 | 2807 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.78, 1.46] |
| 10.1 Dexamethasone | 8 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.84, 1.81] |
| 10.2 Hydrocortisone | 7 | 1293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.46, 1.40] |
| 11 PVL in infants with cranial ultrasound scans | 7 | 1841 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.60] |
| 12 PVL in survivors seen at follow‐up | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.60, 2.48] |
| 13 Necrotising enterocolitis (NEC) | 25 | 4050 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 13.1 Dexamethasone | 15 | 2661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
| 13.2 Hydrocortisone | 10 | 1389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.37] |
| 14 Gastrointestinal bleeding | 12 | 1816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.35, 2.55] |
| 14.1 Dexamethasone | 10 | 1725 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.35, 2.58] |
| 14.2 Hydrocortisone | 2 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.27, 8.74] |
| 15 Gastrointestinal perforation | 16 | 3040 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.02, 0.05] |
| 15.1 Dexamethasone | 9 | 1936 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.05] |
| 15.2 Hydrocortisone | 7 | 1104 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.06] |
| 16 Pulmonary haemorrhage | 10 | 1820 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.54] |
| 16.1 Dexamethasone | 7 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.45] |
| 16.2 Hydrocortisone | 3 | 1134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.92, 2.03] |
| 17 Any retinopathy of prematurity (ROP) | 9 | 1345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.97] |
| 17.1 Dexamethasone | 8 | 1042 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 0.99] |
| 17.2 Hydrocortisone | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.04] |
| 18 Severe ROP | 14 | 2577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.98] |
| 18.1 Dexamethasone | 8 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.99] |
| 18.2 Hydrocortisone | 6 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
| 19 Severe ROP in survivors | 12 | 1575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.94] |
| 19.1 Dexamethasone | 10 | 1238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.95] |
| 19.2 Hydrocortisone | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.60, 1.17] |
5.9. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 9 Severe intraventricular haemorrhage (IVH) in infants examined.
5.11. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 11 PVL in infants with cranial ultrasound scans.
5.12. Analysis.
Comparison 5 Complications during primary hospitalisation, Outcome 12 PVL in survivors seen at follow‐up.
Comparison 6. Long‐term follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bayley Mental Developmental Index (MDI) <‐2 SD | 3 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.78, 1.29] |
| 2 Bayley MDI <‐2 SD in tested survivors | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.25] |
| 3 Bayley Psychomotor Developmental Index (PDI) <‐2 SD | 3 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.85, 1.60] |
| 4 Bayley PDI <‐2 SD in tested survivors | 3 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.87, 1.57] |
| 5 Developmental delay (criteria not specified) | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.08, 2.61] |
| 6 Developmental delay (criteria not specified) in tested survivors | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.30, 2.88] |
| 7 Blindness | 8 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.74, 5.50] |
| 8 Blindness in survivors assessed | 8 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.80, 5.86] |
| 9 Deafness | 8 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.39, 3.37] |
| 10 Deafness in survivors assessed | 8 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.29] |
| 11 Cerebral palsy | 13 | 1973 | Risk Ratio (IV, Fixed, 95% CI) | 1.42 [1.06, 1.91] |
| 11.1 Dexamethasone | 7 | 921 | Risk Ratio (IV, Fixed, 95% CI) | 1.75 [1.20, 2.55] |
| 11.2 Hydrocortisone | 6 | 1052 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.66, 1.66] |
| 12 Death before follow‐up in trials assessing cerebral palsy | 13 | 1973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.05] |
| 12.1 Dexamethasone | 7 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.21] |
| 12.2 Hydrocortisone | 6 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| 13 Death or cerebral palsy | 13 | 1973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.16] |
| 13.1 Dexamethasone | 7 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.00, 1.37] |
| 13.2 Hydrocortisone | 6 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.05] |
| 14 Cerebral palsy in survivors assessed | 13 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.11, 1.90] |
| 14.1 Dexamethasone | 7 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.29, 2.57] |
| 14.2 Hydrocortisone | 6 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.58] |
| 15 Major neurosensory disability (variable criteria ‐ see individual studies) | 7 | 1703 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.33] |
| 15.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.03, 1.83] |
| 15.2 Hydrocortisone | 3 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.64, 1.14] |
| 16 Death before follow‐up in trials assessing major neurosensory disability (variable criteria) | 6 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.17] |
| 16.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.25] |
| 16.2 Hydrocortisone | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.58, 1.28] |
| 17 Death or major neurosensory disability (variable criteria) | 7 | 1703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.08] |
| 17.1 Dexamethasone | 4 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.99, 1.30] |
| 17.2 Hydrocortisone | 3 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| 18 Major neurosensory disability in survivors examined (variable criteria) | 8 | 1178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.28] |
| 18.1 Dexamethasone | 4 | 469 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.05, 1.77] |
| 18.2 Hydrocortisone | 4 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.10] |
| 19 Abnormal neurological exam (variable criteria ‐ see individual studies) | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.33, 2.47] |
| 20 Death before follow‐up in trials assessing abnormal neurological exam (variable criteria) | 6 | 1350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.07] |
| 20.1 Dexamethasone | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.21] |
| 20.2 Hydrocortisone | 1 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.54, 1.04] |
| 21 Death or abnormal neurological exam (variable criteria) | 5 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.06, 1.42] |
| 22 Abnormal neurological exam in tested survivors (variable criteria) | 5 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.41, 2.52] |
| 23 Intellectual impairment (IQ < 70) | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.57, 3.31] |
| 24 Intellectual impairment (IQ < 70) in survivors assessed | 2 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.47, 2.65] |
| 25 "Major neurosensory impairment" ‐ blindness or deafness | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.16, 2.25] |
| 26 "Major neurosensory impairment" ‐ blindness or deafness ‐ in survivors assessed | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.16, 2.12] |
| 27 Behaviour abnormalities | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.16, 2.25] |
| 28 Behaviour abnormalities in 3‐year‐old survivors assessed | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.16, 2.22] |
| 29 Abnormal EEG | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.66, 2.33] |
| 30 Abnormal EEG in tested survivors | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.61, 2.08] |
| 31 Rehospitalisation in infancy | 3 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| 32 Rehospitalisation in infancy in survivors | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.07] |
6.6. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 6 Developmental delay (criteria not specified) in tested survivors.
6.7. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 7 Blindness.
6.8. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 8 Blindness in survivors assessed.
6.9. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 9 Deafness.
6.10. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 10 Deafness in survivors assessed.
6.12. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 12 Death before follow‐up in trials assessing cerebral palsy.
6.14. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 14 Cerebral palsy in survivors assessed.
6.16. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 16 Death before follow‐up in trials assessing major neurosensory disability (variable criteria).
6.18. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 18 Major neurosensory disability in survivors examined (variable criteria).
6.20. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 20 Death before follow‐up in trials assessing abnormal neurological exam (variable criteria).
6.22. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 22 Abnormal neurological exam in tested survivors (variable criteria).
6.23. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 23 Intellectual impairment (IQ < 70).
6.24. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 24 Intellectual impairment (IQ < 70) in survivors assessed.
6.25. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 25 "Major neurosensory impairment" ‐ blindness or deafness.
6.26. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 26 "Major neurosensory impairment" ‐ blindness or deafness ‐ in survivors assessed.
6.27. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 27 Behaviour abnormalities.
6.28. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 28 Behaviour abnormalities in 3‐year‐old survivors assessed.
6.29. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 29 Abnormal EEG.
6.30. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 30 Abnormal EEG in tested survivors.
6.31. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 31 Rehospitalisation in infancy.
6.32. Analysis.
Comparison 6 Long‐term follow‐up, Outcome 32 Rehospitalisation in infancy in survivors.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anttila 2005.
| Methods | Multi‐centre double‐blind placebo‐controlled randomised trial | |
| Participants | 109 infants with birth weight 500 grams to 999 grams, gestation < 32 weeks, need for mechanical ventilation and supplemental oxygen by 4 hours of age. Stratified by weight (500 grams to 749 grams vs 750 grams to 999 grams) Exclusions: life‐threatening congenital anomalies or known chromosomal anomaly | |
| Interventions | 4 doses of dexamethasone 0.25 mg/kg each at 12‐hourly intervals or normal saline as placebo. First dose was given before 6 hours. Open‐label dexamethasone was allowed when deemed necessary by attending physician, but its use was discouraged. | |
| Outcomes | Survival to 36 weeks without IVH (grade III to IV), PVL (echodensities after first week or periventricular cysts on ultrasound), or BPD (oxygen at 36 weeks); growth, duration of assisted ventilation and oxygen, late corticosteroid treatment, infection, hyperglycaemia, hypertension, ROP, PDA, GI bleeding and perforation, NEC | |
| Notes | This paper also reported a meta‐analysis of early short vs early prolonged dexamethasone treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation by coded vials prepared in the pharmacy at each centre |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified primary and secondary outcomes reported |
Baden 1972.
| Methods | Double‐blind placebo‐controlled randomised trial | |
| Participants | 44 preterm infants < 24 hours old with respiratory distress confirmed both clinically and radiologically | |
| Interventions | Hydrocortisone 25 mg/kg on admission and 12 hours later intravenously Control group given placebo | |
| Outcomes | Death, FiO2, cortisol levels, and blood gases | |
| Notes | The oldest study, carried out in 1972. Used hydrocortisone in a very short course of treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation via random numbers and sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Batton 2012.
| Methods | Multi‐centre randomised placebo‐controlled trial | |
| Participants | Infants at 23 to 26 completed weeks' gestation with study‐defined low blood pressure | |
| Interventions | Hydrocortisone 1 mg/kg loading, then 0.5 mg/kg at 12‐hourly intervals for 6 doses | |
| Outcomes | Short‐term outcomes during primary hospitalisation of death, BPD (not defined), IVH grade III or IV, PVL, and NEC requiring surgery | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Enrolled infants were randomised from a prespecified sequence, allocated by centre, and received treatment from an investigational pharmacist. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome of the study was to determine the feasibility of a randomised trial of blood pressure management, rather than effects on bronchopulmonary dysplasia. |
Baud 2016.
| Methods | Multi‐centre double‐blind randomised controlled trial | |
| Participants | 523 inborn infants at 24 to 27 weeks’ gestational age in the first 24 hours after birth recruited from 21 French centres with NICU facilities between 25 May 2008 and 31 January 2014. Exclusions: rupture of membranes at < 22 weeks’ gestation; birth weight < third centile according to French sex‐customised curves; severe perinatal asphyxia (Apgar score = 0–3 for longer than 5 minutes, cord blood pH < 7·00, or both) and expected to die shortly after birth; congenital malformations (birth defects or major structural abnormalities detectable prenatally); known chromosomal aberrations |
|
| Interventions | Hydrocortisone hemisuccinate 1 mg/kg/d divided into 2 doses for 7 days, then 0.5 mg/kg/d once per day for 3 days (total dose 8.5 mg/kg) Control infants were given an equivalent volume of 5% glucose placebo. Open‐label corticosteroids were not allowed during first 10 days of treatment. |
|
| Outcomes | Short‐term primary outcome: survival free of bronchopulmonary dysplasia (BPD) at 36 weeks’ postmenstrual age. BPD was diagnosed at 36 weeks (± 3 days) without additional testing if an infant required mechanical ventilation, non‐invasive ventilation with continuous positive airway pressure, or 30% or more supplemental oxygen concentration. BPD was diagnosed in infants requiring only 22% to 29% oxygen if the oxygen requirement was confirmed by a standardised oxygen‐reduction test, which was completed by neonatologists masked to treatment groups. Secondary outcomes: bronchopulmonary dysplasia at 36 weeks’ postmenstrual age; death; surgical ligation of patent ductus arteriosus; air leaks; pulmonary haemorrhage; insulin requirement; late‐onset sepsis (positive blood culture or symptomatic pneumonia); necrotising enterocolitis, gastrointestinal perforation; grade 3 or 4 IVH; cystic PVL; death before discharge; severe retinopathy of prematurity (requiring laser treatment or surgery) Longer term: Children were assessed at approximately 22 months’ corrected age. Children underwent a French‐based developmental assessment that was standardised in the mid‐1990s, and a standardised neurodevelopmental assessment based on the Amiel‐Tison and Denver scales. Neurodevelopmental impairment (NDI) was defined as any disability on the standardised neurodevelopmental assessment, cerebral palsy, blindness, deafness, or a formal developmental assessment score < ‐1 SD (< 85). |
|
| Notes | Study was stopped early because of lack of funding, rather than because any predetermined threshold had been reached, at approximately 2/3 of projected sample size of 786. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned (1:1) via a secure study website Strata for 24 to 25 weeks and 26 to 27 weeks |
| Allocation concealment (selection bias) | Low risk | Remote electronic allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding maintained by identical placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to knowledge of treatment group at both primary hospitalisation phase and 22‐month follow‐up phase |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low risk for short‐term outcomes, as all but 2 randomised participants have short‐term outcomes reported. However, moderate risk at follow‐up phase because although 93% (379/406) of long‐term survivors were assessed at 22 months’ corrected age, only 75% (304/406) had full neurological and developmental assessment. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported |
Biswas 2003.
| Methods | Multi‐centre placebo‐controlled randomised trial | |
| Participants | 253 infants < 30 weeks' gestation, within 9 hours of birth at entry; all mechanically ventilated | |
| Interventions | Hydrocortisone 1 mg/kg/d as continuous infusion for 5 days, then 0.5 mg/kg/d for 2 days. Also given tri‐iodothyronine 6 µg/kg/d for 5 days, halving to 3 µg/kg/d for 2 days Controls given equal volume infusion of 5% dextrose | |
| Outcomes | Primary outcome was death or ventilator dependence at 7 days, or death or oxygen dependence at 14 days. Secondary outcomes included duration of ventilation, oxygen dependence, and hospitalisation; oxygen dependency at 36 weeks; IVH, PVL, PDA, and NEC | |
| Notes | Hydrocortisone combined with T3 infusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by Oxford Perinatal Trials Unit |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Bonsante 2007.
| Methods | Two‐centre randomised double‐blind placebo‐controlled trial | |
| Participants | 70 infants with birth weight < 1000 grams or < 28 weeks' gestation, ventilator‐dependent after 7 days of age, and considered to be a candidate for corticosteroids Exclusions: major anomaly likely to affect long‐term neurological outcome | |
| Interventions | Active treatment – total dose of hydrocortisone 10.5 mg/kg over 10 days Placebo group ‐ equal volume of 0.9% saline | |
| Outcomes | Primary outcomes: survival free of disability at 2 years of age, mortality up to 2 years of age, and neurological outcome after discharge Secondary outcomes: rate of BPD, death or BPD, failure to extubate, other complications during primary hospital stay including GI perforation, severe IVH (grade 3 or 4) and cystic PVL, long‐term neurosensory impairment (blindness, deafness, developmental delay assessed by MDI on Bayley Scales, cerebral palsy), and disabilities (severe ‐ any of severe cerebral palsy (not likely to walk), blindness, or severe developmental delay (MDI < 55), moderate‐moderate cerebral palsy (not walking at 2 years but likely to do so), deafness, moderate developmental delay (MDI 55 to < 70), mild‐mild cerebral palsy (walking at 2 years), or mild developmental delay (MDI 70 to < 85) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation centrally |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment blind: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up reporting: yes for outcomes during primary hospital stay ‐ 98% of surviving infants traced to 2 years of age |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Efird 2005.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | 34 infants of gestation > 23 weeks and < 29 weeks, and birth weight > 500 grams and < 1000 grams enrolled by 2 hours of age Exclusions: major malformations, chromosomal abnormalities, congenital heart disease | |
| Interventions | Hydrocortisone intravenously at dose of 1 mg/kg every 12 hours for 2 days, followed by 0.3 mg/kg every 12 hours for 3 days Control infants received an equivalent volume of normal saline as placebo | |
| Outcomes | Blood pressure, urine output, hyperglycaemia, mortality, durations of mechanical ventilation and hospital stay, BPD (oxygen at 36 weeks), infection, NEC, intestinal perforation, PDA, IVH, PVL, cortisol levels | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation via sequentially numbered, preassigned treatment designations in sealed, opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Garland 1999.
| Methods | Multi‐centre placebo‐controlled randomised trial | |
| Participants | 241 infants weighing between 500 grams and 1500 grams, received surfactant, at significant risk for BPD or death using a model to predict at 24 hours | |
| Interventions | 3‐Day course of dexamethasone beginning at 24 to 48 hours. First 2 doses were 0.4 mg/kg, third and fourth doses 0.2 mg/kg, and fifth and sixth doses 0.1 mg/kg and 0.05 mg/kg, respectively. Dexamethasone dose reduced slightly after first interim analysis (see Notes) Similar volume of normal saline was given to control infants | |
| Outcomes | Primary outcomes were survival without BPD defined as oxygen therapy at 36 weeks to maintain SaO2 above 91% and mortality. Secondary outcomes included duration of ventilation and supplemental oxygen, respiratory support at 28 days of life, length of stay for survivors, use of subsequent dexamethasone therapy, and usual complications of prematurity. | |
| Notes | At first interim analysis (n = 75), increased risk of GI perforation was noted in the dexamethasone group. Data Monitoring Committee recommended reducing the dexamethasone dose to 4 doses of 0.25 mg/kg/dose every 12 hours begun at 24 to 48 hours, followed by doses of 0.125 mg/kg and 0.05 mg/kg at the next two 12‐hour periods, respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by study pharmacists at each centre |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Halac 1990.
| Methods | Placebo‐controlled randomised trial | |
| Participants | 248 infants, birth weight ≤ 1500 grams, gestation < 34 weeks, with evidence of "birth asphyxia" (1‐minute Apgar score < 5, prolonged resuscitation, and metabolic acidosis (HCO3 < 15 mmol/L within 1 hour of birth)) | |
| Interventions | 7‐Day course of dexamethasone 1 mg/kg 12‐hourly beginning on first day of life | |
| Outcomes | Neonatal mortality, mortality to discharge, NEC, PDA, sepsis, severe IVH | |
| Notes | Possible exclusion of 5 deaths after randomisation, but not clear which group they came from | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation via list of random numbers |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Primary prespecified outcome of NEC was reported, as were a large number of other outcomes. |
Hochwald 2014.
| Methods | Placebo‐controlled randomised trial | |
| Participants | 22 infants, gestational age ≤ 30 weeks or birth weight ≤ 1250 grams, and < 48 hours after birth, with an arterial catheter in place, invasive mean blood pressure < gestational age on 3 consecutive measurements 10 minutes apart, and after treatment with 1 or 2 boluses of 10 mL of 0.9% saline. Excluded if blood loss, hydrops, or major cardiac lesions | |
| Interventions | Hydrocortisone 7 mg/kg total over 48 hours, or equal volume of 0.9% saline placebo | |
| Outcomes | Mortality (presumably to discharge), NEC, BPD, positive blood culture, insulin treatment | |
| Notes | Major outcome was to determine whether hydrocortisone reduced vasopressor doses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Short‐term outcomes reported for all participants |
| Selective reporting (reporting bias) | Unclear risk | Only short‐term outcomes reported, but major outcome of effects on vasopressor doses not reported |
Kopelman 1999.
| Methods | Two‐centre randomised placebo‐controlled trial | |
| Participants | 70 infants < 28 weeks' gestation requiring intermittent mandatory ventilation and arterial catheterisation | |
| Interventions | Dexamethasone 0.2 mg/kg within 2 hours of delivery Control infants given an equal volume of saline | |
| Outcomes | Ventilation Index (VI), IMV rate, mean blood pressure, incidence of PDA, need for indomethacin, number extubated during first week, usual complications of RDS | |
| Notes | After an interim analysis showed that the incidence of IVH was much lower than expected, enrolment was stopped and analysis was limited to a comparison of ventilator settings, blood pressure, and pressor use during first 7 days. Outcome of successful extubation was available at only 1 hospital, where 38 infants were enrolled. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in the hospital pharmacy stratified by use of antenatal corticosteroids; exact method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, but others reported too |
Lauterbach 2006.
| Methods | Three‐armed randomised controlled trial: (1) nebulised pentoxifylline, (2) intravenous dexamethasone, (3) nebulised water placebo | |
| Participants | 150 infants < 1500 grams birth weight who needed oxygen on fourth day of life, regardless of the need for assisted ventilation. Major malformations and grade 3 or 4 IVH led to exclusions. | |
| Interventions | Dexamethasone 0.25 mg/kg/dose every 12 hours for 3 days | |
| Outcomes | Primary endpoint BPD (oxygen dependency at 36 weeks). Secondary endpoints included PDA, IVH and PVL, | |
| Notes | All prespecified outcomes reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not clearly stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind treatment groups for comparison of dexamethasone vs nebulised water placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unable to blind treatment groups for comparison of dexamethasone vs nebulised water placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Short‐term outcomes reported for all participants |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Lin 1999.
| Methods | Placebo‐controlled randomised trial | |
| Participants | 40 infants of 500 grams to 1999 grams with severe RDS, needing IPPV within 6 hours of birth | |
| Interventions | Dexamethasone 0.25 mg/kg 12‐hourly from 1 to 7 days, 0.12 mg/kg 12‐hourly from 8 to 14 days, 0.05 mg/kg 12‐hourly from 15 to 21 days, 0.02 mg/kg 12‐hourly from 22 to 28 days Saline placebo was given to controls. | |
| Outcomes | Mortality at 28 days; discharge, failure to extubate (during study), death or BPD (36 weeks), BPD (28 days and 36 weeks), infection (clinical), severe IVH, plasma glucose, mean blood pressure on days 2, 5, 7, and 16; weight at 2 weeks | |
| Notes | Sequential analysis for 12 pairs. Data given for 40 infants as randomised into the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in a paired sequential trial. Assignment determined by pharmacist and groups stratified by birth weight: 500 grams to 999 grams, 1000 grams to 1500 grams, and 1501 grams to 1999 grams. Allocation by drawing lots |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Mukhopadhyay 1998.
| Methods | Single‐centre randomised controlled trial | |
| Participants | 19 infants < 34 weeks and < 2000 grams who could be provided with ventilation. Clinical and radiographic evidence of RDS; IPPV with oxygen > 30% | |
| Interventions | Dexamethasone 0.5 mg/kg/dose 12‐hourly for 3 days starting within 6 hours of birth Control group did not receive any drug | |
| Outcomes | Changes in oxygen requirements, mean duration of ventilation, culture‐positive sepsis, PDA, BPD (not defined), pneumothorax, mortality | |
| Notes | Infants were entered into the trial only if a ventilator was available. Surfactant was not given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment: not sure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Ng 2006.
| Methods | Double‐blind randomised controlled trial | |
| Participants | 48 infants of gestation < 32 weeks and birth weight < 1500 grams who had systemic hypotension despite treatment with volume expanders and dopamine within the first 7 days of life Infants also had to have an indwelling arterial catheter for continuous BP monitoring. Exclusions: major or lethal congenital or chromosomal abnormalities, congenital heart defects, previous postnatal systemic or inhaled corticosteroids, proven infection, NEC | |
| Interventions | Hydrocortisone 1 mg/kg every 8 hours for 5 days Control infants received isotonic saline as placebo for 5 days. | |
| Outcomes | BP, use of vasopressors, duration of ventilation, oxygen and hospital stay, PIE, pulmonary haemorrhage, pneumothorax, hyperglycaemia, glycosuria, IVH (grade III or IV), PVL, NEC, GI perforation, sepsis, ROP (> stage II), mortality | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation in blocks of 6 by computer‐generated random numbers and opening numbered, sealed, opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Primary outcome was blood pressure, which was reported. |
Peltoniemi 2005.
| Methods | Multi‐centre double‐blind randomised controlled trial | |
| Participants | 51 infants with birth weight 501 grams to 1250 grams, gestation 23 to 30 weeks, needing mechanical ventilation before the age of 24 hours. The subgroup 1000 grams to 1250 grams had to need supplemental oxygen and mechanical ventilation > 24 hours despite surfactant. Exclusions: lethal malformations, suspected chromosomal abnormalities |
|
| Interventions | Hydrocortisone 2.0 mg/kg/d intravenously 8‐hourly for 2 days, 1.5 mg/kg/d 8‐hourly for 2 days, 0.75 mg/kg/d 12‐hourly for 6 days Control infants received isotonic saline as placebo. First dose was given before 36 hours. Use of open‐label corticosteroids was discouraged. | |
| Outcomes | Survival without BPD (oxygen at 36 weeks), IVH (grades III or IV), cystic PVL, durations of ventilation, oxygen and hospital stay, sepsis, hyperglycaemia, hypertension, PDA, GI bleeding, GI perforation, NEC, ROP, and cortisol levels Long‐term outcomes: At 2 years ‐ neurosensory impairments (blindness, deafness, developmental delay assessed by MDI on Bayley Scales, cerebral palsy) and disabilities (severe ‐ any of severe cerebral palsy (not likely to walk), blindness, or severe developmental delay (MDI < 55, moderate‐moderate cerebral palsy (not walking at 2 years but likely to do so), deafness, moderate developmental delay (MDI 55 to < 70), mild‐mild cerebral palsy (walking at 2 years), or mild developmental delay (MDI 70 to < 85). Follow‐up rate was 87% (40/46). At 6 years ‐ IQ (Wechsler Preschool and Primary Scale of Intelligence ‐ Revised) and language (Reynell Developmental Language Scale III) were assessed, as were diagnoses of cerebral palsy, blindness, and deafness. Follow‐up rate was 80% (37 of the 46 survivors). |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation at each centre via identical coded syringes. Exact method of randomisation not stated. Stratified by birth weight (501 grams to 750 grams vs 750 grams to 999 grams vs 1000 grams to 1250 grams) |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes (for primary hospital outcomes). Follow‐up rates at 2 and 6 years listed above |
| Selective reporting (reporting bias) | Low risk | Primary outcome was reported as specified. |
Rastogi 1996.
| Methods | Double‐blind randomised controlled trial | |
| Participants | 70 preterm infants < 12 hours old, weighing 700 grams to 1500 grams with respiratory distress syndrome (RDS) confirmed clinically and radiologically; infants needed mechanical ventilation > 30% O2 and/or MAP 7 cmH2O a/A < 0.25 after surfactant treatment. Exclusions: major malformations, chromosome abnormalities, severe infection, Apgar < 3 at 5 minutes | |
| Interventions | Intravenous dexamethasone 0.5 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, 0.15 mg/kg/d for 3 days, 0.05 mg/kg/d for 3 days Control group given saline placebo | |
| Outcomes | FiO2, MAP, BPD (28 days and CXR), severe BPD (36 weeks), duration O2, infections, deaths, pneumothorax, pulmonary haemorrhage, PDA, IVH, NEC, hyperglycaemia, insulin use, hypertension, ROP | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation: via a pharmacy list; stratified for birth weight |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Romagnoli 1999.
| Methods | Randomised non‐blinded controlled trial | |
| Participants | 50 infants < 1251 grams or < 33 weeks, oxygen‐dependent at 72 hours, and at high risk of BPD according to a scoring system predicting 90% risk of BPD | |
| Interventions | Dexamethasone 0.5 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, and 0.125 mg/kg/d for 1 day Control group: no mention of placebo | |
| Outcomes | Survival to 28 days, survival to discharge, PDA, IVH (grades 3 and 4), PVL, sepsis, NEC, ROP (stages III and above), requiring ventilation at 28 days, BPD at 28 days and 36 weeks, hyperglycaemia, hypertension, needed late corticosteroids, growth failure, left ventricular hypertrophy | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation via random numbers, concealed in numbered sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurements: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Sanders 1994.
| Methods | Randomised double‐blind controlled trial | |
| Participants | 40 infants < 30 weeks' gestation and 12 to 18 hours old with RDS, both clinical and radiological. Infants were treated with mechanical ventilation and surfactant Exclusions: sepsis, congenital heart disease, chromosome abnormalities, need for exchange transfusion | |
| Interventions | Dexamethasone 0.5 mg/kg twice, 12 hours apart Control group given saline placebo | |
| Outcomes | MAP, FiO2, mortality, extubation < 7 days, pulmonary function tests, duration IPPV, O2, hospital, mortality, BPD (36 weeks O2), late corticosteroids | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in the pharmacy via sealed envelopes. Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported, but definitions vague |
Shinwell 1996.
| Methods | Multi‐centre double‐blind randomised controlled trial | |
| Participants | 248 preterm infants with birth weight 500 grams to 2000 grams, 1 to 3 days old, requiring mechanical ventilation with more than 40% oxygen Exclusions: active bleeding, hypertension, hyperglycaemia, active infection, lethal congenital anomalies | |
| Interventions | Intravenous dexamethasone 0.25 mg/kg every 12 hours 6 times Controls given saline placebo | |
| Outcomes | Mortality, survival with no O2, mechanical ventilation at 3 and 7 days, BPD, duration in hospital, IVH, PVL, pneumothorax, PIE, PDA, sepsis, hypertension, hyperglycaemia | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation, stratified by centre and birth weight, from random numbers list in the pharmacy |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes for short‐term; 84% for long‐term |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Sinkin 2000.
| Methods | Multi‐centre randomised double‐blind trial | |
| Participants | 384 infants < 30 weeks' gestation with RDS by clinical and radiographic signs, needing IPPV at 12 to 18 hours of age; had received at least 1 dose of surfactant | |
| Interventions | Dexamethasone 0.5 mg/kg at 12 to 18 hours of age, second dose 12 hours later Control group given an equal volume of placebo | |
| Outcomes | Primary outcomes were survival, survival without oxygen at 28 days or 36 weeks, and survival without oxygen at 28 days or 36 weeks and without late corticosteroids Length of time in oxygen, on ventilation, to regain birth weight, and in hospital. Hyperglycaemia, hypertension, IVH, PDA, sepsis, NEC, isolated GI perforation, ROP, air leak, discharged home on oxygen | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in the pharmacy via labelled syringes. Stratification by centre. Exact method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Soll 1999.
| Methods | Multi‐centre randomised double‐blind trial | |
| Participants | 542 infants weighing 501 grams to 1000 grams who required assisted ventilation < 12 hours, had received surfactant by 12 hours, were physiologically stable, and had no life‐threatening congenital anomalies | |
| Interventions | Dexamethasone 0.5 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, 0.10 mg/kg/d for 3 days, and 0.05 mg/kg/d for 3 days. Control infants received a similar volume of normal saline. Infants in either group could receive late postnatal corticosteroids beginning on day 14 if they were on assisted ventilation with supplemental oxygen > 30%. | |
| Outcomes | Primary outcome was BPD or death at 36 weeks' adjusted age. Secondary outcome measures included clinical status at 14 days and 28 days, duration of assisted ventilation, supplemental oxygen and hospital stay, treatment with late postnatal corticosteroids, proven sepsis, hypertension and hyperglycaemia requiring therapy, weight at 36 weeks, usual complications of prematurity | |
| Notes | Published as an extended abstract and presented at a clinical meeting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in hospital pharmacies by opening opaque, sealed envelopes. Precise method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Stark 2001.
| Methods | Multi‐centre randomised double‐blind trial | |
| Participants | 220 infants with birth weight 501 grams to 1000 grams, mechanically ventilated < 12 hours. Infants > 750 grams also needed to receive surfactant and have FiO2 > 0.29. | |
| Interventions | Dexamethasone 0.15 mg/kg/d for 3 days, then tapered over 7 days Saline placebo | |
| Outcomes | Death or BPD, oxygen at 28 days, PIE, late corticosteroid treatment, hypertension, hyperglycaemia, GI perforation | |
| Notes | Factorial design; infants also randomised to routine ventilator management or a strategy of minimal ventilator support to reduce mechanical lung injury. After enrolling 220 infants (sample size estimate was 1200), the trial was halted owing to unanticipated adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation via numbers generated by a random, permuted block algorithm, stratified by birth weight |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Subhedar 1997.
| Methods | Randomised controlled trial ‐ factorial design | |
| Participants | 42 preterm infants, entry at 96 hours if gestation < 32 weeks, mechanical ventilation from birth, surfactant treatment, and high risk of developing BPD based on score (Ryan 1996) Exclusion criteria: major congenital anomaly, structural cardiac defect, significant ductus shunting, culture‐positive sepsis, IVH with parenchymal involvement, pulmonary or GI haemorrhage, abnormal coagulation, thrombocytopenia (platelets < 50,000) | |
| Interventions | Intravenous dexamethasone at 12‐hourly intervals for 6 days; 0.5 mg/kg/dose for 6 doses and 0.25 mg/kg/dose for a further 6 doses. Inhaled NO 5 to 20 ppm for 72 hours Control groups were not given placebo | |
| Outcomes | Mortality, BPD at 28 days and > 36 weeks with abnormal chest radiograph Duration of ventilation, time to extubation, duration of hospitalisation, maximum grade of IVH, pulmonary haemorrhage, pneumothorax, severe PDA, NEC, ROP (stage 3 or 4) Complications including ileal perforation, upper GI haemorrhage, hyperglycaemia, hypertension, septicaemia | |
| Notes | Note factorial design, which means that half of treated infants and half of control infants also received 72 hours of inhaled NO | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation by computer‐generated random numbers and sealed envelopes. Factorial design provided 4 groups: early dexamethasone, inhaled NO, both drugs together, and neither drug. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurements: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes reported |
Suske 1996.
| Methods | Randomised controlled trial | |
| Participants | 26 preterm infants < 2 hours old, with birth weight < 1500 grams if FiO2 > 0.50, or > 1500 grams birth weight with FiO2 > 0.70 Exclusions: known sepsis, cardiac anomalies, malformations of lung or CNS |
|
| Interventions | Intravenous dexamethasone 0.5 mg/kg/d for 5 days Controls were not given placebo. | |
| Outcomes | Blood gases, ventilator settings, mortality IVH, BPD (O2 28 days), NEC, late sepsis, PDA, ROP, air leak, duration in hospital | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation via sealed envelopes. Randomisation achieved by drawing lots |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Tapia 1998.
| Methods | Multi‐centre double‐blind placebo‐controlled randomised trial | |
| Participants | 113 (4 exclusions for congenital abnormality, early sepsis, and failure to obtain follow‐up data) infants with birth weight between 700 and 1600 grams, clinical and radiological diagnosis of RDS, needing mechanical ventilation, and < 36 hours of age Exclusion criteria: life‐threatening congenital malformation or chromosome abnormality, strong suspicion of infection at birth (maternal chorioamnionitis) or early sepsis (positive blood culture in the first 36 hours of life) | |
| Interventions | Intravenous dexamethasone 0.5 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, 0.12 mg/kg/d for 3 days, and 0.06 mg/kg/d for 3 days Placebo group received an equivalent volume of saline solution. | |
| Outcomes | Primary outcomes were death before hospital discharge, BPD (oxygen need at 28 days and x‐ray changes), death or BPD, and oxygen need at 36 weeks. Other outcomes included time on ventilator, time in over 40% oxygen, and time in oxygen. Major morbidity and complications included pneumothorax, PIE, PDA, pulmonary haemorrhage, pneumonia, sepsis, NEC, ROP, hypertension, hyperglycaemia, and IVH (grades I to II and III to IV). | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation via ampoules of dexamethasone and saline prepared in the hospital pharmacy. Exact method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: almost (109/113) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Vento 2004.
| Methods | Randomised controlled trial | |
| Participants | 20 infants with birth weight < 1251 grams and gestation < 33 weeks who were oxygen‐ and ventilator‐dependent on fourth day of life and were at high risk of BPD by study authors' own scoring system Exclusions: none stated | |
| Interventions | Intravenous dexamethasone 0.5 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, and 0.125 mg/kg/d for 1 day (total dose 2.375 mg/kg) Control group received no corticosteroid treatment. | |
| Outcomes | Tracheal aspirates for cell counts, pulmonary mechanics, PDA, IVH (grades III and IV), extubation during study period | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation but method not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment: uncertain |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of intervention: uncertain |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: uncertain |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Wang 1996.
| Methods | Double‐blind randomised controlled trial | |
| Participants | 63 infants with birth weight from 1000 grams to 1999 grams, AGA, clinical and radiographic RDS, IPPV (0 to 12, age after birth) | |
| Interventions | Dexamethasone 0.25 mg/kg 12‐hourly from 1 to 7 days, 0.125 mg/kg 12‐hourly from 8 to 14 days, 0.05 mg/kg 12‐hourly from 15 to 21 days. First dose administered at < 12 hours Controls received saline placebo. | |
| Outcomes | Oxygen requirements; PCO2; MAP; SP‐A and SP‐D in tracheal aspirate; failure to extubate by third day, 7th day, 14th day, and 28th day; mortality before discharge; sepsis; BPD at 28 days | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in a double‐blind fashion; method not stated |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Watterberg 1999.
| Methods | Two‐centre double‐blind randomised controlled trial | |
| Participants | 40 infants weighing between 500 grams and 999 grams who were AGA and needed mechanical ventilation < 48 hours of age Exclusion criteria: maternal diabetes, congenital sepsis, SGA | |
| Interventions | Hydrocortisone 1.0 mg/kg/d every 12 hours for 9 days, 0.5 mg/kg/d for 3 days Control infants were given an equal volume of normal saline. | |
| Outcomes | Primary outcome was survival without supplemental oxygen at 36 weeks' post conception. Secondary outcomes among survivors: BPD at 36 weeks, duration of mechanical ventilation, > 40% oxygen, > 25% oxygen, hospital stay, weight and head circumference at 36 weeks | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation at each centre by constant block design with 4 participants per block to minimise bias over time. Separate randomisation tables were used for infants exposed to antenatal corticosteroids. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Watterberg 2004.
| Methods | Multi‐centre double‐blind randomised controlled trial | |
| Participants | 360 infants of 500 grams to 999 grams birth weight, needing mechanical ventilation, aged 12 to 48 hours Exclusions: major congenital anomaly, congenital sepsis, postnatal corticosteroids, triplet or higher‐order gestation | |
| Interventions | Hydrocortisone 1 mg/kg/d 12‐hourly for 12 days, then 0.5 mg/kg/d for 3 days Control group infants received an equal volume of normal saline placebo. | |
| Outcomes | Survival without BPD (oxygen at 36 weeks), physiological BPD, death before 36 weeks, death before discharge, BPD in survivors, durations of mechanical ventilation and oxygen, hospital stay, weight and OFC at 36 weeks, PDA, infection, NEC, GI perforation, major IVH (grade 3 or 4), cystic PVL, ROP, and open‐label corticosteroid therapy Longer‐term outcomes included neurosensory impairments (any of cerebral palsy, blindness, deafness, or developmental or motor delay, as assessed by Bayley Scales (MDI or PDI, respectively)). | |
| Notes | Sample size estimate was 712, but the study was stopped early because of increased incidence of apparently spontaneous GI perforation in the hydrocortisone group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation, stratified by centre and birth weight (500 grams to 749 grams vs 750 grams to 999 grams), via a permuted block scheme with blocks of 6 in each stratum. Randomisation lists in each pharmacy in a sealed envelope |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Yeh 1990.
| Methods | Double‐blind randomised controlled trial | |
| Participants | 57 preterm infants weighing between 700 grams and 1999 grams, < 13 hours old, with severe RDS both clinically and radiologically. They needed mechanical ventilation < 4 hours and were excluded if they had infection. | |
| Interventions | Intravenous dexamethasone 0.50 mg/kg/d for 3 days, 0.25 mg/kg/d for 3 days, 0.12 mg/kg/d for 3 days, 0.05 mg/kg/d for 3 days Control infants were given saline placebo. | |
| Outcomes | MAP, FiO2, pulmonary function tests, BP, glucose, mortality, BPD, duration O2, hospital, weight loss, sepsis, PDA, IVH (> grade I), ROP | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in blocks of 10 via a pharmacy list. Exact method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurements: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: almost |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Yeh 1997.
| Methods | Multi‐centre double‐blind randomised controlled trial | |
| Participants | 262 infants of birth weight < 2000 grams with RDS and requiring mechanical ventilation after birth | |
| Interventions | Dexamethasone 0.25 mg/kg/dose every 12 hours intravenously on days 1 to 7; 0.12 mg/kg/dose every 12 hours intravenously from days 8 to 14; 0.05 mg/kg/dose every 12 hours intravenously from days 15 to 21; and 0.02 mg/kg/dose every 12 hours intravenously from days 22 to 28 Control infants were given saline placebo. | |
| Outcomes | BPD judged at 28 days or at 36 weeks Extubation during the study, mortality, bacteraemia or clinical sepsis, and side effects of hyperglycaemia, hypertension, cardiac hypertrophy, hyperparathyroidism, and growth failure | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation via central pharmacy random number list; exact method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Allocation concealment: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: almost for short‐term; 81% for long‐term |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
ACTH: adrenocorticotrophic hormone. ABA: appropriate for gestational age. BP: blood pressure. BPD: bronchopulmonary dysplasia. CLD: chronic lung disease. CNS: central nervous system. CXR: chest x‐ray. FiO2: fraction of inspired oxygen. GI: gastrointestinal. HCO3: bicarbonate. IMV: intermittent mandatory ventilation. IPPV: intermittent positive‐pressure ventilation. IVH: intraventricular haemorrhage. MAP: mean airway pressure. MDI: Mental Developmental Index. NDI: neurodevelopmental impairment. NEC: necrotising enterocolitis. NO: nitric oxide. NRN: Neonatal Research Network. O2: oxygen. OFC: occipito‐frontal circumference. PDA: patent ductus arteriosus. PDI: Psychomotor Developmental Index. PIE: pulmonary interstitial emphysema. ppm: parts per million. PVL: periventricular leukomalacia. RDS: respiratory distress syndrome. ROP: retinopathy of prematurity. SaO2: oxygen saturation. SGA: small for gestational age. SP‐A: surfactant protein‐A. SP‐D: surfactant protein‐D. T3: triiodothyronine. VI: Ventilation Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ariagno 1987 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Avery 1985 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Brozanski 1995 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| CDTG 1991 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Cummings 1989 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Dobryansky 2012 | 20 VLBW infants were randomised to both hydrocortisone and caffeine as active treatments, compared with "standard guidelines", which presumably meant no hydrocortisone or caffeine. Major outcomes reported included BPD and BPD combined with death. As caffeine reduces BPD (Schmidt 2006), the independent effect of hydrocortisone cannot be determined. |
| Doyle 2006 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Durand 1995 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Gaissmaier 1999 | Primary outcome was need for an epinephrine infusion 12 hours after treatment. No long‐term outcomes reported |
| Gross 2005 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Harkavy 1989 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Kari 1993 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Kazzi 1990 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Kothadia 1999 | Study of late postnatal corticosteroids included in the review "'Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Kovacs 1998 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Noble‐Jamieson 1989 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Ohlsson 1992 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Papile 1998 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Parikh 2013 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Romagnoli 1997 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Salas 2014 | Recruited term infants only for a study of early hydrocortisone to treat hypotension |
| Scott 1997 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Smolkin 2014 | Before after study only ‐ not an RCT |
| Tsukahara 1999 | Not an RCT; 26 study infants and 12 historical controls |
| Vincer 1998 | Study of late postnatal corticosteroids included in the review "'Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Walther 2003 | Study of late postnatal corticosteroids included in the review "Late (> 7 days) systemic postnatal corticosteroids for bronchopulmonary dysplasia in preterm infants" (Doyle 2017) |
| Yaseen 1999 | Study of early dexamethasone, but no outcomes relevant to this review were reported |
BPD: bronchopulmonary dysplasia. RCT: randomised controlled trial. VLBW: very low birth weight.
Differences between protocol and review
We added the methods and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol nor in the last version of this review. For the 2017 update, we changed the title to "Early (< 8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants."
Contributions of authors
Lex Doyle collated data concerning long‐term neurosensory outcomes; he assisted Henry Halliday and Richard Ehrenkranz in identifying all studies, synthesising the data, and writing earlier versions of this review. Richard Ehrenkranz assisted Henry Halliday in identifying all studies, synthesising the data, and writing earlier versions of this review. Henry Halliday identified all studies, synthesised the data, and wrote earlier versions of this review. Jeanie Cheong assisted Lex Doyle in identifying all studies in the most recent literature search, synthesising the data, and writing the current version of this review.
Sources of support
Internal sources
Action Research UK Grant to study effects of postnatal steroids, UK.
Action Research UK Grant to study long‐term follow‐up, UK.
External sources
National Health and Medical Research Council, Australia.
Declarations of interest
Lex Doyle was Chief Investigator in the DART study, a randomised controlled trial of low‐dose, short‐course dexamethasone in ventilator‐dependent infants that was funded by the National Health and Medical Research Council of Australia.
Henry Halliday (HLH) is a retired neonatologist. He is joint Editor‐in‐Chief of the journal Neonatology and sits on many Data Monitoring and Trial Steering Committees for various neonatal/perinatal trials. He has received support in the past for co‐ordinating the OSECT study (2000), for which AstraZeneca (Sweden) supplied metered‐dose inhalers of budesonide and placebo. HLH also acts as a consultant for Chiesi Farmiceutici (Italy), a company that sells two neonatal drugs ‐ Curosurf (a surfactant to treat respiratory distress syndrome) and Peyona (a caffeine preparation to treat apnoea of prematurity).
Edited (no change to conclusions)
References
References to studies included in this review
Anttila 2005 {published data only}
- Anttila E, Peltonemi O, Haumont D, Herting E, ter Horst H, Heinonen K, et al. Early neonatal dexamethasone treatment for prevention of bronchopulmonary dysplasia. Randomised trial and meta‐analysis evaluating the duration of dexamethasone therapy. European Journal of Pediatrics 2005;164(8):472‐81. [DOI: 10.1007/s00431-005-1645-8; PUBMED: 15864643] [DOI] [PubMed] [Google Scholar]
Baden 1972 {published data only}
- Baden M, Bauer CR, Cole E, Klein G, Taeusch HW, Stern L. A controlled trial of hydrocortisone therapy in infants with respiratory distress syndrome. Pediatrics 1972;50(4):526‐34. [PUBMED: 4561296] [PubMed] [Google Scholar]
- Fitzhardinge PM, Eisen A, Lejtenyi C, Metrakos K, Ramsay M. Sequelae of early steroid administration to the newborn infant. Pediatrics 1974;53(6):877‐83. [PUBMED: 4598934] [PubMed] [Google Scholar]
Batton 2012 {published data only}
- Batton BJ, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Feasibility study of early blood pressure management in extremely preterm infants. Journal of Pediatrics 2012;161(1):65‐9. [DOI: 10.1016/j.jpeds.2012.01.014; PUBMED: 22336574] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baud 2016 {published data only}
- Baud O, Maury L, Lebail F, Ramful D, Moussawi F, Nicaise C, et al. PREMILOC Trial Study Group. Effect of early low‐dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double‐blind, placebo‐controlled, multicentre, randomised trial. Lancet 2016;387(10030):1827‐36. [DOI: 10.1016/S0140-6736(16)00202-6; PUBMED: 26916176] [DOI] [PubMed] [Google Scholar]
- Baud O, Trousson C, Biran V, Leroy E, Mohamed D, Alberti C, PREMILOC Trial Group. Association between early low‐dose hydrocortisone therapy in extremely preterm neonates and neurodevelopmental outcomes at 2 years of age. JAMA 2017;317(13):1329‐37. [DOI: 10.1001/jama.2017.2692; PUBMED: 28384828] [DOI] [PubMed] [Google Scholar]
Biswas 2003 {published data only}
- Biswas S. Personal communication. email 2002.
- Biswas S, Buffery J, Enoch H, Bland M, Markiewicz M, Walters D. Pulmonary effects of triiodothyronine (T3) and hydrocortisone (HC) supplementation in preterm infants less than 30 weeks gestation: results of the THORN trial ‐ thyroid hormone replacement in neonates. Pediatric Research 2003;53(1):48‐56. [DOI: 10.1203/00006450-200301000-00011; PUBMED: 12508081] [DOI] [PubMed] [Google Scholar]
Bonsante 2007 {published data only}
- Bonsante F, Latorre G, Lacobelli S, Forziati V, Laforgia N, Esposito L, et al. Early low‐dose hydrocortisone in very preterm infants: a randomized placebo‐controlled trial. Neonatology 2007;91(4):217‐21. [DOI: 10.1159/000098168; PUBMED: 17568152] [DOI] [PubMed] [Google Scholar]
Efird 2005 {published data only}
- Efird MM, Heerens AT, Gordon PV, Bose CL, Young DA. A randomized‐controlled trial of prophylactic hydrocortisone supplementation for the prevention of hypotension in extremely low birth weight infants. Journal of Perinatology 2005;25(2):119‐24. [DOI: 10.1038/sj.jp.7211193; PUBMED: 15329742] [DOI] [PubMed] [Google Scholar]
Garland 1999 {published data only}
- Garland JS, Alex CP, Pauly TH, Whitehead VL, Brand J, Winston JF, et al. A three‐day course of dexamethasone therapy to prevent chronic lung disease in ventilated neonates: a randomized trial. Pediatrics 1999;104(1 Pt 1):91‐9. [PUBMED: 10390266] [DOI] [PubMed] [Google Scholar]
Halac 1990 {published data only}
- Halac E, Halac J, Begue EF, Casañas JM, Indiveri DR, Petit JF, et al. Prenatal and postnatal corticosteroid therapy to prevent neonatal necrotizing enterocolitis: a controlled trial. Journal of Pediatrics 1990;117(1 Pt 1):132‐8. [PUBMED: 2196355] [DOI] [PubMed] [Google Scholar]
Hochwald 2014 {published data only}
- Hochwald O, Palegra G, Osiovich O. Adding hydrocortisone as 1st line of inotropic treatment for hypotension in very low birth weight infants. Indian Journal of Pediatrics 2014;81(8):808‐10. [DOI: 10.1007/s12098-013-1151-3; PUBMED: 23904065] [DOI] [PubMed] [Google Scholar]
Kopelman 1999 {published data only}
- Kopelman AE, Moise AA, Holbert D, Hegemier SE. A single very early dexamethasone dose improves respiratory and cardiovascular adaptation in preterm infants. Journal of Pediatrics 1999;135(3):345‐50. [PUBMED: 10484801] [DOI] [PubMed] [Google Scholar]
Lauterbach 2006 {published data only}
- Lauterbach R, Szymura‐Oleksiak J, Pawlik D, Warchol J, Lisowska‐Miszczyk I, Rytlewski K. Nebulized pentoxifylline for prevention of bronchopulmonary dysplasia in very low birth weight infants: a pilot clinical study. Journal of Maternal‐Fetal & Neonatal Medicine 2006;19(7):433‐8. [DOI: 10.1080/14767050600736754; PUBMED: 16923699] [DOI] [PubMed] [Google Scholar]
Lin 1999 {published data only}
- Lin YJ, Yeh TF, Hsieh WS, Chi YC, Lin HC, Lin CH. Prevention of chronic lung disease in preterm infants by early postnatal dexamethasone therapy. Pediatric Pulmonology 1999;27(1):21‐6. [PUBMED: 10023787] [DOI] [PubMed] [Google Scholar]
Mukhopadhyay 1998 {published data only}
- Mukhopadhyay K, Kumar P, Narang A. Role of early postnatal dexamethasone in respiratory distress syndrome. Indian Pediatrics 1998;35(2):117‐22. [PUBMED: 9707853] [PubMed] [Google Scholar]
Ng 2006 {published data only}
- Ng PC, Lee CH, Bnur FL, Chan IH, Lee AW, Wong E, et al. A double‐blind randomized controlled study of a stress dose of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics 2006;117(2):367‐75. [DOI: 10.1542/peds.2005-0869; PUBMED: 16452355] [DOI] [PubMed] [Google Scholar]
Peltoniemi 2005 {published data only}
- Peltoniemi O, Kari A, Heinonen K, Saarela T, Nikolajev K, Andersson S, et al. Pretreatment cortisol values may predict responses to hydrocortisone administration for the prevention of bronchopulmonary dysplasia in high‐risk infants. Journal of Pediatrics 2005;146(5):632‐7. [DOI: 10.1016/j.jpeds.2004.12.040; PUBMED: 15870666] [DOI] [PubMed] [Google Scholar]
- Peltoniemi OM, Lano A, Puosi R, Yliherva A, Bonsante F, Kari MA, et al. Neonatal Hydrocortisone Working Group. Trial of early neonatal hydrocortisone: two‐year follow‐up. Neonatology 2009;95(3):240‐7. [DOI: 10.1159/000164150; PUBMED: 18931525] [DOI] [PubMed] [Google Scholar]
- Peltoniemi OM, Lano A, Yliherva A, Kari MA, Hallman M, Neonatal Hydrocortisone Working Group. Randomised trial of early neonatal hydrocortisone demonstrates potential undesired effects on neurodevelopment at preschool age. Acta Paediatrica 2016;105(2):159‐64. [DOI: 10.1111/apa.13074; PUBMED: 26058477] [DOI] [PubMed] [Google Scholar]
Rastogi 1996 {published data only}
- Morales P, Rastogi A, Bez ML, Akintorin SM, Pyati S, Andes SM, et al. Effect of dexamethasone therapy on the neonatal ductus arteriosus. Pediatric Cardiology 1998;19(3):225‐9. [DOI: 10.1007/s002469900290; PUBMED: 9568218] [DOI] [PubMed] [Google Scholar]
- Rastogi A, Akintorin SM, Bez ML, Morales P, Pildes PS. A controlled trial of dexamethasone to prevent bronchopulmonary dysplasia in surfactant‐treated infants. Pediatrics 1996;98(2 Pt 1):204‐10. [PUBMED: 8692619] [PubMed] [Google Scholar]
Romagnoli 1999 {published data only}
- Romagnoli C, Zecca E, Luciano R, Torrioli G, Tortorolo G. Controlled trial of early dexamethasone treatment for the prevention of chronic lung disease in preterm infants: a 3‐year follow‐up. Pediatrics 2002;109(6):e85. [PUBMED: 12042579] [DOI] [PubMed] [Google Scholar]
- Romagnoli C, Zecca E, Vento G, Carolis MP, Papacci P, Tortorolo G. Early postnatal dexamethasone for the prevention of chronic lung disease in high‐risk preterm infants. Intensive Care Medicine 1999;25(7):717‐21. [PUBMED: 10470576] [DOI] [PubMed] [Google Scholar]
- Romagnoli C, Zecca E, Vento G, Maggio L, Papacci P, Tortorolo G. Effect on growth of two different dexamethasone courses for preterm infants at risk of chronic lung disease. A randomized controlled trial. Pharmacology 1999;59(5):266‐74. [DOI: ; PUBMED: 10529659] [DOI] [PubMed] [Google Scholar]
Sanders 1994 {published data only}
- Sanders RJ, Cox C, Phelps DL, Sinkin RA. Two doses of early intravenous dexamethasone for the prevention of bronchopulmonary dysplasia in babies with respiratory distress syndrome. Pediatric Research 1994;36(1 Pt 1):122‐8. [DOI: 10.1203/00006450-199407001-00022; PUBMED: 7936832] [DOI] [PubMed] [Google Scholar]
- Sinkin RA. Personal communication. email 2002.
Shinwell 1996 {published data only}
- Shinwell ES. Early dexamethasone therapy is associated with increased incidence of cerebral palsy. Hot Topics' 99 in Neonatology 1999:240‐54.
- Shinwell ES. Personal communication. email 2002.
- Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D, et al. Early postnatal dexamethasone treatment and incidence of cerebral palsy. Archives of Disease in Childhood. Fetal and Neonatal Edition 2000;83(3):F177‐81. [PUBMED: 11040164] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinwell ES, Karplus M, Zmora E, Reich D, Rothschild A, Blazer S, et al. Failure of early postnatal dexamethasone to prevent chronic lung disease in infants with respiratory distress syndrome. Archives of Disease in Childhood. Fetal and Neonatal Edition 1996;74(1):F33‐7. [PUBMED: 8653433] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinkin 2000 {published data only}
- D'Angio CT, Maniscalco WM, Ryan RM, Avissar NE, Basavegowda K, Sinkin RA. Vascular endothelial growth factor in pulmonary lavage fluid from premature infants: effects of age and postnatal dexamethasone. Biology of the Neonate 1999;76(5):266‐73. [DOI: ; PUBMED: 10516393] [DOI] [PubMed] [Google Scholar]
- Sinkin RA. Personal communication. email 2002.
- Sinkin RA, Dweck HS, Horgan MJ, Gallaher KJ, Cox C, Maniscalco WM, et al. Early dexamethasone ‐ attempting to prevent chronic lung disease. Pediatrics 2000;105(3 Pt 1):542‐8. [PUBMED: 10699107] [DOI] [PubMed] [Google Scholar]
Soll 1999 {published data only}
- Soll RF, Vermont Oxford Network Steroid Study Group. Early postnatal dexamethasone therapy for the prevention of chronic lung disease. Pediatric Research 1999;45:226A. [DOI] [PubMed] [Google Scholar]
- Vermont Oxford Network Steroid Study Group. Early postnatal dexamethasone therapy for the prevention of chronic lung disease. Pediatrics 2001;108(3):741‐8. [PUBMED: 11533345] [DOI] [PubMed] [Google Scholar]
Stark 2001 {published data only}
- Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, et al. National Institute of Child Health and Human Development Neonatal Research Network. Adverse effects of early dexamethasone in extremely‐low‐birth‐weight infants. New England Journal of Medicine 2001;344(2):95‐101. [DOI: 10.1056/NEJM200101113440203; PUBMED: 11150359] [DOI] [PubMed] [Google Scholar]
- Stark AR, Carlo WA, Vohr BR, Papile L, Saha S, Bauer CR, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Death or neurodevelopmental impairment at 18 to 22 months corrected age in a randomized trial of early dexamethasone to prevent death or chronic lung disease in extremely low birth weight infants. Journal of Pediatrics 2014; Vol. 164, issue 1:34‐9 e2. [DOI: 10.1016/j.jpeds.2013.07.027; PUBMED: 23992673] [DOI] [PMC free article] [PubMed]
Subhedar 1997 {published data only}
- Subhedar NV. Personal communication. email 2002.
- Subhedar NV, Bennett AJ, Wardle SP, Shaw NJ. More trials on early treatment with corticosteroids are needed. BMJ 2000;320(7239):941. [PUBMED: 10742018 ] [PMC free article] [PubMed] [Google Scholar]
- Subhedar NV, Ryan SW, Shaw NJ. Open randomised controlled trial of inhaled nitric oxide and early dexamethasone in high risk preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1997;77(3):F185‐90. [PUBMED: 9462187] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suske 1996 {published data only}
- Suske G, Oestreich K, Varnholt V, Lasch P, Kachel W. Influence of early postnatal dexamethasone therapy on ventilator dependency in surfactant‐substituted preterm infants. Acta Paediatrica 1996;85(6):713‐8. [PUBMED: 8816210] [DOI] [PubMed] [Google Scholar]
Tapia 1998 {published data only}
- Tapia JL, Ramirez R, Cifuentes J, Fabres J, Hubner ME, Bancalari A, et al. The effect of early dexamethasone administration on bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome. Journal of Pediatrics 1998;132(1):48‐52. [PUBMED: 9469999] [DOI] [PubMed] [Google Scholar]
Vento 2004 {published data only}
- Vento G, Matassa PG, Zecca E, Tortorolo L, Martelli M, Carolis MP, et al. Effect of dexamethasone on tracheobronchial aspirate fluid cytology and pulmonary mechanics in preterm infants. Pharmacology 2004;71(3):113‐9. [DOI: 10.1159/000077444; PUBMED: 15161992] [DOI] [PubMed] [Google Scholar]
Wang 1996 {published data only}
- Wang JY, Yeh TF, Lin YC, Miyamura K, Holmskov U, Reid KB. Measurement of pulmonary status and surfactant protein levels during dexamethasone treatment of neonatal respiratory distress syndrome. Thorax 1996;51(9):907‐13. [PUBMED: 8984701] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Yeh TF, Lin YJ, Chen WY, Lin CH. Early postnatal dexamethasone therapy may lessen lung inflammation in premature infants with respiratory distress syndrome on mechanical ventilation. Pediatric Pulmonology 1997;23(3):193‐7. [PUBMED: 9094727] [DOI] [PubMed] [Google Scholar]
Watterberg 1999 {published data only}
- Watterberg KL. Personal communication. email 2002.
- Watterberg KL, Gerdes JS, Gifford KL, Lin HM. Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics 1999;104(6):1258‐63. [PUBMED: 10585975] [DOI] [PubMed] [Google Scholar]
Watterberg 2004 {published data only}
- Watterberg KL, Gerdes JS, Cole CH, Aucott SW, Thilo EH, Mammel MC, et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics 2004;114(6):1649‐57. [DOI: 10.1542/peds.2004-1159; PUBMED: 15574629] [DOI] [PubMed] [Google Scholar]
- Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, Couser RJ, et al. Growth and neurodevelopmental outcomes after early low‐dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 2007;120(1):40‐8. [DOI: 10.1542/peds.2006-3158; PUBMED: 17606560] [DOI] [PubMed] [Google Scholar]
Yeh 1990 {published data only}
- Yeh TF, Torre JA, Rastogi A, Anyebuno MA, Pildes RS. Early postnatal dexamethasone therapy in premature infants with severe respiratory distress syndrome: a double‐blind, controlled study. Journal of Pediatrics 1990;117(2 Pt 1):273‐82. [PUBMED: 2199642] [DOI] [PubMed] [Google Scholar]
Yeh 1997 {published data only}
- Lin YJ, Lin CH, Wu JM, Tsai WH, Yeh TF. The effects of early postnatal dexamethasone therapy on pulmonary outcome in premature infants with respiratory distress syndrome: a two‐year follow‐up study. Acta Paediatrica 2005;94(3):310‐6. [PUBMED: 16028649] [DOI] [PubMed] [Google Scholar]
- Lin YJ, Yeh TF, Lin HC, Wu JM, Lin CH, Yu CY. Effects of early postnatal dexamethasone therapy on calcium homeostasis and bone growth in preterm infants with respiratory distress syndrome. Acta Paediatrica 1998;87(10):1061‐5. [PUBMED: 9825973] [DOI] [PubMed] [Google Scholar]
- Peng CT, Lin HC, Lin YJ, Tsai CH, Yeh TF. Early dexamethasone therapy and blood cell count in preterm infants. Pediatrics 1999;104(3 Pt 1):476‐81. [PUBMED: 10469772] [DOI] [PubMed] [Google Scholar]
- Yeh TF, Lin I, Shieh W, Lin H, Chen J, Kao S. Prevention of chronic lung disease (CLD) in premature RDS infants with early and prolonged dexamethasone (D) therapy ‐ a multicenter double‐blind controlled study. Pediatric Research 1994;35(4):262A. [Google Scholar]
- Yeh TF, Lin YJ, Hsieh WS, Lin HC, Lin CH, Chen JY, et al. Early postnatal dexamethasone therapy for the prevention of chronic lung disease in preterm infants with respiratory distress syndrome: a multicenter clinical trial. Pediatrics 1997;100(4):E3. [PUBMED: 9310536] [DOI] [PubMed] [Google Scholar]
- Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow‐up study. Pediatrics 1998;101(5):E7. [PUBMED: 9565440] [DOI] [PubMed] [Google Scholar]
- Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. New England Journal of Medicine 2004;350(13):1304‐13. [DOI: 10.1056/NEJMoa032089; PUBMED: 15044641] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ariagno 1987 {unpublished data only}
- Ariagno RL, Sweeney TE, Baldwin RB, Inguillo D, Martin D. Controlled trial of dexamethasone in preterm infants at risk for bronchopulmonary dysplasia: lung function, clinical course and outcome at three years (as supplied 2000). Data on file.
- Ariagno RL, Sweeney TJ, Baldwin RB, Inguillo D, Martin D. Dexamethasone effects on lung function and risks in 3 week old ventilatory dependent preterm infants. American Reviews of Respiratory Disease 1987;135:A125. [Google Scholar]
Avery 1985 {published data only}
- Avery GB, Fletcher AB, Kaplan M, Brudno DS. Controlled trial of dexamethasone in respirator‐dependent infants with bronchopulmonary dysplasia. Pediatrics 1985;75(1):106‐11. [PUBMED: 3880879] [PubMed] [Google Scholar]
Brozanski 1995 {published data only}
- Brozanski BS, Jones JG, Gilmour CH, Balsan MJ, Vazquez RL, Israel BA, et al. Effect of pulse dexamethasone therapy on the incidence and severity of chronic lung disease in the very low birth weight infant. Journal of Pediatrics 1995;126(5 Pt 1):769‐76. [PUBMED: 7752005] [DOI] [PubMed] [Google Scholar]
- Gilmour CH, Sentipal‐Walerius JM, Jones JG, Doyle JM, Brozanski BS, Balsan MJ, et al. Pulse dexamethasone does not impair growth and body composition of very low birth weight infants. Journal of the American College of Nutrition 1995;14(5):455‐62. [PUBMED: 8522724] [DOI] [PubMed] [Google Scholar]
- Hofkosh D, Brozanski BS, Edwards DE, Williams LA, Jones JG, Cheng KP. One year outcome of infants treated with pulse dexamethasone for prevention of BPD. Pediatric Research 1995;37(4):259A. [Google Scholar]
CDTG 1991 {published data only}
- Collaborative Dexamethasone Trial Group. Dexamethasone therapy in neonatal chronic lung disease: an international placebo‐controlled trial. Pediatrics 1991;88(3):421‐7. [PUBMED: 1881718] [PubMed] [Google Scholar]
- Jones R, Wincott E, Elbourne D, Grant A. Controlled trial of dexamethasone in neonatal chronic lung disease: a 3‐year follow‐up. Pediatrics 1995;96(5 Pt 1):897‐906. [PUBMED: 7478833] [PubMed] [Google Scholar]
- Jones RA, Collaborative Dexamethasone Trial Follow‐up Group. Randomized, controlled trial of dexamethasone in neonatal chronic lung disease: 13‐ to 17‐year follow‐up study: I. Neurologic, psychological, and educational outcomes. Pediatrics 1995;116(2):370‐8. [DOI: 10.1542/peds.2004-1818; PUBMED: 16061591] [DOI] [PubMed] [Google Scholar]
- Jones RA, Collaborative Dexamethasone Trial Follow‐up Group. Randomized, controlled trial of dexamethasone in neonatal chronic lung disease: 13‐ to 17‐year follow‐up study: II. Respiratory status, growth, and blood pressure. Pediatrics 2005;116(2):379‐84. [DOI: 10.1542/peds.2004-1819; PUBMED: 16061592] [DOI] [PubMed] [Google Scholar]
Cummings 1989 {published data only}
- Cummings JJ. Personal communication. email 2002.
- Cummings JJ, D'Eugenio DB, Gross SJ. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. New England Journal of Medicine 1989;320(23):1505‐10. [DOI: 10.1056/NEJM198906083202301; PUBMED: 2657423] [DOI] [PubMed] [Google Scholar]
- Gross SJ, Anbar RD, Mettelman BB. Follow‐up at 15 years of preterm infants from a controlled trial of moderately early dexamethasone for the prevention of chronic lung disease. Pediatrics 2005;115(3):681‐7. [DOI: 10.1542/peds.2004-0956; PUBMED: 15741372] [DOI] [PubMed] [Google Scholar]
Dobryansky 2012 {published data only}
- Dobryansky D, Borysiuk O, Salabay Z, Dubrovna Y. Clinical effectiveness of early administration of caffeine and low‐dose hydrocortisone to preterm newborns with a high risk of BPD development. Archives of Disease in Childhood 2012;97:A119. [DOI: 10.1136/archdischild-2012-302724.0405] [DOI] [Google Scholar]
Doyle 2006 {published data only}
- Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB. Low‐dose dexamethasone facilitates extubation among chronically ventilator‐dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics 2006;117(1):75‐83. [PUBMED: 16396863] [DOI] [PubMed] [Google Scholar]
- Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB, DART Study Investigators. Outcome at 2 years of age of infants from the DART study: a multicenter, international, randomized, controlled trial of low‐dose dexamethasone. Pediatrics 2007;119(4):716‐21. [DOI: 10.1542/peds.2006-2806; PUBMED: 17403842 ] [DOI] [PubMed] [Google Scholar]
Durand 1995 {published data only}
- Durand M, Sardesai S, McEvoy C. Effects of early dexamethasone therapy on pulmonary mechanics and chronic lung disease in very low birth weight infants: a randomized, controlled trial. Pediatrics 1995;95(4):584‐90. [PUBMED: 7700763] [PubMed] [Google Scholar]
Gaissmaier 1999 {published data only}
- Gaissmaier RE, Pohlandt F. Single‐dose dexamethasone treatment of hypotension in preterm infants. Journal of Pediatrics 1999;134(6):701‐5. [PUBMED: 10356137] [DOI] [PubMed] [Google Scholar]
Gross 2005 {published data only}
- Gross SJ, Anbar RD, Mettelman BB. Follow‐up at 15 years of preterm infants from a controlled trial of moderately early dexamethasone for the prevention of chronic lung disease. Pediatrics 2005;115(3):681‐7. [10.1542/peds.2004‐0956; PUBMED: 15741372] [DOI] [PubMed] [Google Scholar]
Harkavy 1989 {published data only}
- Harkavy KL, Scanlon JW, Chowdhry PK, Grylack LJ. Dexamethasone therapy for chronic lung disease in ventilator‐ and oxygen‐dependent infants: a controlled trial. Journal of Pediatrics 1989;115(6):979‐83. [PUBMED: 2685220] [DOI] [PubMed] [Google Scholar]
Kari 1993 {published data only}
- Kari MA, Heinonen K, Ikonen RS, Koivisto M, Raivio KO. Dexamethasone treatment in preterm infants at risk for bronchopulmonary dysplasia. Archives of Disease in Childhood 1993;68(5 Spec No):566‐9. [PUBMED: 8323356] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari MA, Raivio KO, Venge P, Hallman M. Dexamethasone treatment of infants at risk for chronic lung disease: surfactant components and inflammatory parameters in airway specimens. Pediatric Research 1994;36(3):387‐93. [DOI: 10.1203/00006450-199409000-00020; PUBMED: 7808837] [DOI] [PubMed] [Google Scholar]
- Mieskonen S, Eronen M, Malmberg LP, Turpeinen M, Kari MA, Hallman M. Controlled trial of dexamethasone in neonatal chronic lung disease: an 8‐year follow‐up of cardiopulmonary function and growth. Acta Paediatrica 2003;92(8):896‐904. [PUBMED: 12948063] [PubMed] [Google Scholar]
Kazzi 1990 {published data only}
- Kazzi NJ, Brans YW, Poland RL. Dexamethasone effects on the hospital course of infants with bronchopulmonary dysplasia who are dependent on artificial ventilation. Pediatrics 1990;86(5):722‐7. [PUBMED: 2235226] [PubMed] [Google Scholar]
Kothadia 1999 {published data only}
- Bensky AS, Kothadia JM, Covitz W. Cardiac effects of dexamethasone in very low birth weight infants. Pediatrics 1996;97(6 Pt 1):818‐21. [PUBMED: 8657520] [PubMed] [Google Scholar]
- Goldstein DJ, Waldrep EL, VanPelt JC, O'Shea TM. Developmental outcome at 5 years following dexamethasone use for very low birth weight infants. Pediatric Research 2000;47(4):310A. [Google Scholar]
- Kothadia JM, O'Shea TM, Roberts D, Auringer ST, Weaver RG 3rd, Dillard RG. Randomized placebo‐controlled trial of a 42‐day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants. Pediatrics 1999;104(1 Pt 1):22‐7 Erratum in: Pediatrics 2004; 114(6):1746. [PUBMED: 10390255] [DOI] [PubMed] [Google Scholar]
- Nixon PA, Washburn LK, Schechter MS, O'Shea TM. Follow‐up study of a randomized controlled trial of postnatal dexamethasone therapy in very low birth weight infants: effects on pulmonary outcomes at age 8 to 11 years. Journal of Pediatrics 2007;150(4):345‐50. [DOI: 10.1016/j.jpeds.2006.12.013; PUBMED: 17382108] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea TM, Goldstein DJ, Jackson BG, Kothadia JM, Dillard RG. Randomized trial of a 42‐day tapering course of dexamethasone in very low birth weight infants: neurological, medical and functional outcome at 5 years of age. Pediatric Research 2000;47(4):319A. [Google Scholar]
- O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG 3rd, et al. Randomized placebo‐controlled trial of a 42‐day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1‐year adjusted age. Pediatrics 1999;104(1 Pt 1):15‐21. [PUBMED: 10390254] [DOI] [PubMed] [Google Scholar]
- Washburn LK, Nixon PA, O'Shea TM. Follow‐up of a randomized, placebo‐controlled trial of postnatal dexamethasone: blood pressure and anthropometric measurements at school age. Pediatrics 2006;118(4):1592‐9. [DOI: 10.1542/peds.2006-0973; PUBMED: 17015551] [DOI] [PubMed] [Google Scholar]
Kovacs 1998 {published data only}
- Kovacs L, Davis GM, Faucher D, Papageorgiou A. Efficacy of sequential early systemic and inhaled corticosteroid therapy in the prevention of chronic lung disease of prematurity. Acta Paediatrica 1998;87(7):792‐8. [PUBMED: 9722255] [DOI] [PubMed] [Google Scholar]
Noble‐Jamieson 1989 {published data only}
- Noble‐Jamieson CM, Regev R, Silverman M. Dexamethasone in neonatal chronic lung disease: pulmonary effects and intracranial complications. European Journal of Pediatrics 1989;148(4):365‐7. [PUBMED: 2651132] [DOI] [PubMed] [Google Scholar]
Ohlsson 1992 {published data only}
- Ohlsson A, Calvert SA, Hosking M, Shennan AT. Randomized controlled trial of dexamethasone treatment in very‐low‐birth‐weight infants with ventilator‐dependent chronic lung disease. Acta Paediatrica 1992;81(10):751‐6. [PUBMED: 1421877] [DOI] [PubMed] [Google Scholar]
Papile 1998 {published data only}
- Papile LA, Tyson JE, Stoll BJ, Wright LL, Donovan EF, Bauer CR, et al. A multicenter trial of two dexamethasone regimens in ventilator‐dependent premature infants. New England Journal of Medicine 1998;338(16):1112‐8. [DOI: 10.1056/NEJM199804163381604; PUBMED: 9545359] [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Temprosa M, Tyson JE, Papile LA, Wright LL, Bauer CR, et al. Dexamethasone therapy increases infection in very low birth weight infants. Pediatrics 1999;104(5):e63. [PUBMED: 10545589] [DOI] [PubMed] [Google Scholar]
Parikh 2013 {published data only}
- Parikh NA, Kennedy KA, Lasky RE, McDavid GE, Tyson JE. Pilot randomized trial of hydrocortisone in ventilator‐dependent extremely preterm infants: effects on regional brain volumes. Journal of Pediatrics 2013;162(4):685‐90. [DOI: 10.1016/j.jpeds.2012.09.054; PUBMED: 23140612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romagnoli 1997 {published data only}
- Romagnoli C, Vento G, Zecca E, Tortorolo G, Papacci P, Carolis M, et al. Dexamethasone for the prevention of chronic lung disease in preterm neonates: a prospective randomized study [II desametazone nella prevenzione della patologia polmonare cronica del neonato pretermine: studio prospettico randomizzato]. Rivista Italiana di Pediatria [Italian Journal of Pediatrics] 1997;24:283‐8. [Google Scholar]
- Romagnoli C, Zecca E, Luciano R, Torrioli G, Tortorolo G. A three year follow up of preterm infants after moderately early treatment with dexamethasone. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;87(1):F55‐8. [12091294] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli C, Zecca E, Vento G, Maggio L, Papacci P, Tortorolo G. Effect on growth of two different dexamethasone courses for preterm infants at risk of chronic lung disease. A randomized trial. Pharmacology 1999;59(5):266‐74. [PUBMED: 10529659] [DOI] [PubMed] [Google Scholar]
Salas 2014 {published data only}
- Salas G, Travaglianti M, Leone A, Couceiro C, Rodríguez S, Fariña D. Hydrocortisone for the treatment of refractory hypotension:a randomised controlled trial [Hidrocortisona para el tratamiento de hipotensión refractaria: ensayo clínico controlado y aleatorizado]. Anales de Pediatria (Barcelona, Spain : 2003) 2014;80(6):387‐93. [DOI: 10.1016/j.anpedi.2013.08.004; PUBMED: 24139558] [DOI] [PubMed] [Google Scholar]
Scott 1997 {published data only}
- Scott SM, Backstrom C, Bessman S. Effect of five days of dexamethasone therapy on ventilator dependence and adrenocorticotropic hormone‐stimulated cortisol concentrations. Journal of Perinatology 1997;17(1):24‐8. [PUBMED: 9069060] [PubMed] [Google Scholar]
Smolkin 2014 {published data only}
- Smolkin T, Ulanovsky I, Jubran H, Blazer S, Makhoul IR. Experience with oral betamethasone in extremely low birthweight infants with bronchopulmonary dysplasia. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(6):F517‐8. [DOI: 10.1136/archdischild-2014-306619; PUBMED: 25074982] [DOI] [PubMed] [Google Scholar]
Tsukahara 1999 {published data only}
- Tsukahara H, Watanabe Y, Yasutomi M, Kobata R, Tamura S, Kimura K, et al. Early (4‐7 days of age) dexamethasone therapy for prevention of chronic lung disease in preterm infants. Biology of the Neonate 1999;76(5):283‐90. [DOI: ; PUBMED: 10516395] [DOI] [PubMed] [Google Scholar]
Vincer 1998 {published data only}
- Vincer MJ, Allen AC. Double blind randomized controlled trial of 6‐day pulse of dexamethasone for very low birth weight infants (VLBW <1500 grams) who are ventilator dependent at 4 weeks of age. Pediatric Research 1998;43:201A. [Google Scholar]
Walther 2003 {published data only}
- Walther FJ, Findlay RD, Durand M. Adrenal suppression and extubation rate after moderately early low‐dose dexamethasone therapy in very preterm infants. Early Human Development 2003;74(1):37‐45. [PUBMED: 14512180] [DOI] [PubMed] [Google Scholar]
Yaseen 1999 {published data only}
- Yaseen H, Okash I, Hanif M, al‐Umran K, al‐Faraidy A. Early dexamethasone treatment in preterm infants treated with surfactant: a double blind controlled trial. Journal of Tropical Pediatrics 1999;45(5):304‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Additional references
Anonymous 1991
- [No authors listed]. Dexamethasone for neonatal chronic lung disease. Lancet 1991;338(8773):982‐3. [PUBMED: 1681347] [PubMed] [Google Scholar]
Arias‐Camison 1999
- Arias‐Camison JM, Lau J, Cole CH, Frantz ID 3rd. Meta‐analysis of dexamethasone therapy started in the first 15 days of life for prevention of chronic lung disease in premature infants. Pediatric Pulmonology 1999;28(3):167‐74. [PUBMED: 10495332] [DOI] [PubMed] [Google Scholar]
Baud 1999
- Baud O, Foix‐L'Helias L, Kaminski M, Audibert F, Jarreau PH, Papiernik E, et al. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very preterm infants. New England Journal of Medicine 1999;341(16):1190‐6. [DOI: 10.1056/NEJM199910143411604; PUBMED: 10519896] [DOI] [PubMed] [Google Scholar]
Bayley 1993
- Bayley, N. Bayley Scales of Infant Development. 2nd Edition. San Antonio: The Psychological Corporation, 1993. [Google Scholar]
Bhuta 1998
- Bhuta T, Ohlsson A. Systematic review and meta‐analysis of early postnatal dexamethasone for prevention of chronic lung disease. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(1):F26‐33. [PUBMED: 9797621] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2000
- Doyle LW, Davis PG. Postnatal corticosteroids in preterm infants: systematic review of effects on mortality and motor function. Journal of Paediatrics and Child Health 2000;36(2):101‐7. [PUBMED: 10760004] [DOI] [PubMed] [Google Scholar]
Doyle 2010a
- Doyle LW, Ehrenkranz RA, Halliday HL. Postnatal hydrocortisone for preventing or treating bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 2010;98(2):111‐7. [DOI: 10.1159/000279992; PUBMED: 20150750] [DOI] [PubMed] [Google Scholar]
Doyle 2010b
- Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment in the first week of life for preventing bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 2010;98(3):217‐24. [DOI: 10.1159/000286210; PUBMED: 20389126] [DOI] [PubMed] [Google Scholar]
Doyle 2010c
- Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment after the first week of life for bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 2010;98(4):289‐96. [DOI: 10.1159/000286212; PUBMED: 20453523] [DOI] [PubMed] [Google Scholar]
Doyle 2014b
- Doyle LW, Ehrenkranz RA, Halliday HL. Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD001145.pub3] [DOI] [PubMed] [Google Scholar]
Doyle 2017
- Doyle LW, Cheong J, Ehrenkranz RA, Halliday HL. Late (> 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD001145.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egberts 1997
- Egberts J, Brand R, Walti H, Bevilacqua G, Breart G, Gardini F. Mortality, severe respiratory distress syndrome and chronic lung disease of the newborn are reduced more after prophylactic than after therapeutic administration of the surfactant Curosurf. Pediatrics 1997;100(1):E4. [PUBMED: 9200378] [DOI] [PubMed] [Google Scholar]
Fitzhardinge 1974
- Fitzhardinge PM, Eisen A, Lejtenyi C, Metrakos K, Ramsay M. Sequelae of early steroid administration to the newborn infant. Pediatrics 1974;53(6):877‐83. [PUBMED: 4598934] [PubMed] [Google Scholar]
Gibson 1993
- Gibson AT, Pearse RG, Wales JKH. Growth retardation after dexamethasone administration: assessment by knemometry. Archives of Disease in Childhood 1993;69(5 Spec No):505‐9. [PUBMED: 8285754] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 21 February 2017. Hamilton (ON): GRADE Working Group, McMaster University (developed by Evidence Prime), 2015. Available from gradepro.org.
Gramsbergen 1998
- Gramsbergen A, Mulder EJH. The influence of betamethasone and dexamethasone on motor development in young rats. Pediatric Research 1998;44(1):105‐10. [DOI: 10.1203/00006450-199807000-00017; PUBMED: 9667379] [DOI] [PubMed] [Google Scholar]
Groneck 1995
- Groneck P, Speer CP. Inflammatory mediators and bronchopulmonary dysplasia. Archives of Disease in Childhood. Fetal and Neonatal Edition 1995;73(1):F1‐3. [PUBMED: 7552588] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halliday 1997
- Halliday HL. A review of postnatal corticosteroids for treatment and prevention of chronic lung disease in the preterm infant. Prenatal and Neonatal Medicine 1997;2:1‐12. [Google Scholar]
Halliday 1999
- Halliday HL. Clinical trials of postnatal corticosteroids: inhaled and systemic. Biology of the Neonate 1999;76(Suppl 1):29‐40. [PUBMED: 10393391] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Mammel 1983
- Mammel MC, Green TP, Johnson DE, Thompson TR. Controlled trial of dexamethasone therapy in infants with bronchopulmonary dysplasia. Lancet 1983;1(8338):1356‐8. [PUBMED: 6134136] [DOI] [PubMed] [Google Scholar]
Ng 1993
- Ng PC. The effectiveness and side effects of dexamethasone in preterm infants with bronchopulmonary dysplasia. Archives of Disease in Childhood 1993;68(3 Spec No):330‐6. [PUBMED: 8466274] [DOI] [PMC free article] [PubMed] [Google Scholar]
Onland 2017
- Onland W, Offringa M, Kaam A. Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD002311.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papile 1996
- Papile LA, Stoll B, Donovan E, Tyson I, Bauer C, Wright L, et al. Dexamethasone therapy in infants at risk for chronic lung disease (CLD): a multicenter, randomized, double‐masked trial. Pediatric Research 1996;39:236A. [Google Scholar]
Peltoniemi 2009
- Peltoniemi OM, Lano A, Puosi R, Yliherva A, Bonsante F, Kari M A, et al. Neonatal Hydrocortisone Working Group. Trial of early neonatal hydrocortisone: two‐year follow‐up. Neonatology 2009;95(3):240‐7. [DOI: 10.1159/000164150; PUBMED: 18931525] [DOI] [PubMed] [Google Scholar]
Peltoniemi 2016
- Peltoniemi OM, Lano A, Yliherva A, Kari M A, Hallman M, Neonatal Hydrocortisone Working Group. Randomised trial of early neonatal hydrocortisone demonstrates potential undesired effects on neurodevelopment at preschool age. Acta Paediatrica 2016;105(2):159‐64. [DOI: 10.1111/apa.13074; PUBMED: 26058477] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romagnoli 2002
- Romagnoli C, Zecca E, Luciano R, Torrioli G, Tortorolo G. Controlled trial of early dexamethasone treatment for the prevention of chronic lung disease in preterm infants: a 3‐year follow‐up. Pediatrics 2002;109(6):e85. [PUBMED: 12042579] [DOI] [PubMed] [Google Scholar]
Ryan 1996
- Ryan SW, Nycyk J, Shaw NJ. Prediction of chronic neonatal lung disease on day 4 of life. European Journal of Pediatrics 1996;155(8):668‐71. [PUBMED: 8839722] [DOI] [PubMed] [Google Scholar]
Schmidt 2006
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. New England Journal of Medicine 2006;354(20):2112‐21. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Available from https://gdt.gradepro.org/app/handbook/handbook.html. Updated October 2013.
Shah 2007b
- Shah V, Ohlsson A, Halliday H, Dunn MS. Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001969.pub2] [DOI] [PubMed] [Google Scholar]
Shah 2012a
- Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD002058.pub2] [DOI] [PubMed] [Google Scholar]
Shah 2012b
- Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD002057.pub3] [DOI] [PubMed] [Google Scholar]
Shah 2017
- Shahh VS, Ohlsson A, Halliday HL, Dunn M. Early administration of inhaled corticosteroids for preventing chronic lung disease in very low birth weight preterm neonates. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD001969.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinwell 2002
- Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D, et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Archives of Disease in Childhood. Fetal and Neonatal Edition 2000;83(3):F177‐81. [PUBMED: 11040164] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanley 1982
- Stanley FJ. Using cerebral palsy data in the evaluation of neonatal intensive care: a warning. Developmental Medicine and Child Neurology 1982;24(1):93‐4. [PUBMED: 7106413] [DOI] [PubMed] [Google Scholar]
Stark 2014
- Stark AR, Carlo WA, Vohr BR, Papile LA, Saha S, Bauer CR, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Death or neurodevelopmental impairment at 18 to 22 months corrected age in a randomized trial of early dexamethasone to prevent death or chronic lung disease in extremely low birth weight infants. Journal of Pediatrics 2014;164(1):34‐9 e2. [DOI: 10.1016/j.jpeds.2013.07.027; PUBMED: 23992673] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarnow‐Mordi 1999
- Tarnow‐Mordi W, Mitra A. Postnatal dexamethasone in preterm infants is potentially life saving, but follow up studies are urgently needed. BMJ 1999;319(7222):1385‐6. [PUBMED: 10574836] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tschanz 1995
- Tschanz SA, Damke BM, Burri PH. Influence of postnatally administered glucocorticoids on rat lung growth. Biology of the Neonate 1995;68(4):229‐45. [PUBMED: 8580214] [DOI] [PubMed] [Google Scholar]
van Goudoever 1994
- Goudoever JB, Wattimena JD, Carnielli VP, Sulkers EJ, Degenhart HJ, Sauer PJ. Effect of dexamethasone on protein metabolism in infants with bronchopulmonary dysplasia. Journal of Pediatrics 1994;124(1):112‐8. [PUBMED: 8283359] [DOI] [PubMed] [Google Scholar]
Watterberg 2007
- Watterberg KL, Shaffer ML, Mishefske MJ, Leach CL, Mammel MC, Couser RJ, et al. Growth and developmental outcomes after early low‐dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 2007;120(1):40‐8. [DOI: 10.1542/peds.2006-3158; PUBMED: 17606560] [DOI] [PubMed] [Google Scholar]
Weichsel 1977
- Weichsel ME. The therapeutic use of glucocorticoid hormones in the perinatal period: potential neurologic hazards. Annals of Neurology 1977;2(5):364‐6. [DOI: 10.1002/ana.410020503; PUBMED: 617574] [DOI] [PubMed] [Google Scholar]
Werner 1992
- Werner JC, Sicard RE, Hansen TWR, Solomon E, Cowett RM, Oh W. Hypertrophic cardiomyopathy associated with dexamethasone therapy for bronchopulmonary dysplasia. Journal of Pediatrics 1992;120(2 Pt 1):286‐91. [PUBMED: 1735831] [DOI] [PubMed] [Google Scholar]
Yeh 1998
- Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow up study. Pediatrics 1998;101(5):E7. [PUBMED: 9565440] [DOI] [PubMed] [Google Scholar]
Yeh 2004
- Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. New England Journal of Medicine 2004;350(13):1304‐13. [DOI: 10.1056/NEJMoa032089; PUBMED: 15044641] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Doyle 2014a
- Doyle LW, Ehrenkranz RA, Halliday HL. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD001146.pub4] [DOI] [PubMed] [Google Scholar]
Halliday 2000
- Halliday HL, Ehrenkranz RA. Early postnatal (< 96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001146] [DOI] [PubMed] [Google Scholar]
Halliday 2001
- Halliday HL, Ehrenkranz RA. Early postnatal (< 96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001146] [DOI] [PubMed] [Google Scholar]
Halliday 2003b
- Halliday HL, Ehrenkranz RA, Doyle LW. Early (< 96 hours) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001146] [DOI] [PubMed] [Google Scholar]
Halliday 2010
- Halliday HL, Ehrenkranz RA, Doyle LW. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001146.pub3] [DOI] [PubMed] [Google Scholar]


