Abstract
Short-term muscle disuse induces significant muscle loss in older adults and in some reports may be more accelerated with aging. Identifying muscle transcriptional events in response to bed rest may help identify therapeutic targets to offset muscle loss. Therefore, we compared the muscle transcriptome between young and older adults after bed rest and identified candidate targets related to changes in muscle loss. RNA was sequenced (HiSeq, Illumina; DESeq, R) from muscle biopsies obtained from young [n = 9; 23 yr (SD 3)] and older [n = 18; 68 yr (SD 6)] adults before and after 5-day bed rest. Significantly altered pathways in both young and old subjects relating to mechanosensing and cell adhesion (Actin Cytoskeleton Signaling, ILK Signaling, RhoA Signaling, and Integrin Signaling) were altered (activation z score) to a greater extent in old subjects. Hepatic Fibrosis/Hepatic Stellate Cell Activation was the top regulated pathway significantly altered only in the old. Fifty-one differentially regulated genes were only altered in the young after bed rest and resembled a gene expression profile like that in the old at baseline. Inflammation and muscle wasting genes (CXCL2, GADD45A) were uniquely increased in the old after bed rest, and the macrophage gene MAFB decreased in the old and correlated with the change in leg lean mass. In summary, skeletal muscle dysregulation during bed rest in the old may be driven by alterations in molecules related to fibrosis, inflammation, and cell adhesion. This information may aid in the development of mechanistic-based therapies to combat muscle atrophy during short-term disuse.
NEW & NOTEWORTHY Using RNA sequencing and bioinformatics approaches, we identified that older adult skeletal muscle was characterized by dysregulated pathways associated with fibrosis, inflammation (upregulated), and cell adhesion and mechanosensing (downregulated) pathways, with a subset of genes differentially regulated in old and young muscle after bed rest that may describe predisposition to muscle loss. Unique upregulated genes only expressed in old muscle after bed rest indicated increased inflammation and muscle wasting (CXCL2, GADD45A) and decreased MAFB correlated with the change in leg lean mass.
Keywords: disuse atrophy, Ingenuity Pathway Analysis, RNA sequencing, sarcopenia
INTRODUCTION
Aging is concomitant with biochemical, structural, and functional alterations in skeletal muscle that result in loss of satellite cell activation, increased fat and collagen deposition, reduced strength, and myofiber atrophy (22). Today, ~45% of individuals over age 75 yr are diagnosed with sarcopenia, defined as the age-related loss of muscle mass and function. Studies using dual-energy X-ray absorptiometry and MRI have demonstrated sarcopenic losses ranging from ~20% loss of total leg lean mass to ~40% losses in the vastus lateralis (35, 36). The causes of sarcopenia are multifaceted and include a decrease in anabolic hormones and an increase in motoneuron death, which is compounded by lifestyle choices typically observed with aging such as decreased activity and decreased appetite (2).
Other potential causes of sarcopenia are the repeated periods of muscle disuse (e.g., injury, illness) and incomplete muscle recovery experienced in the elderly (31). Our laboratory has found that short-term bed rest induced a rapid loss of leg lean mass in older adults, whereas muscle mass was maintained in young counterparts (26, 27, 32). The increased sensitivity to muscle loss during short-term disuse experienced by older adults may have devastating consequences, especially when the reserve of muscle and functional capacity are already likely at a critical threshold because of aging. Loss of muscle mass, if not adequately restored upon reambulation, may lead to muscle weakness, decreased physical function, and eventually reduced life span (21). Therefore, understanding the mechanisms of muscle loss during the early periods of disuse, with particular focus on susceptibility in older adults, is of great importance and may lead to discovery of new therapeutic targets.
As a model to investigate the molecular mechanisms underpinning muscle dysfunction with disuse, our laboratory has conducted several published studies using simulated hospitalization comparing the muscle, strength, and physical function responses to 5 days of bed rest in young and old subjects (26, 27, 32). Large-scale gene studies utilizing microarray approaches have been performed in young adults in response to disuse (6, 25) and human aging (15, 30, 35). However, a comparison of the global transcriptome responses in muscle between young and older adults after disuse has not been performed. Likewise, global gene expression profiling in skeletal muscle using RNA sequencing has been recently conducted to examine the effects acute exercise (7) and pharmacological interventions (16) on skeletal muscle. However, this emerging technology has not been used to examine transcriptional events after muscle disuse in older adults. Therefore, the purpose of our study was to utilize RNA sequencing to identify key pathways and molecular regulators altered by bed rest that may explain loss of muscle experienced by older adults. We analyzed a large cohort of skeletal muscle vastus lateralis samples (n = 27) from our previous studies (27, 32) and performed RNA deep sequencing followed by bioinformatics analysis [Ingenuity Pathway Analysis (IPA)] on skeletal muscle from young and older adults before and after 5 days of bed rest.
METHODS
Subject characteristics.
The subject characteristics of healthy young and older adults [Young: n = 9, 23 yr (SD 3); Old: n = 18, 68 yr (SD 6)] before and after 5 days of bed rest (which include strength, leg lean mass, and myofiber cross-sectional area) were pooled together from two identical previously published 5-day bed rest studies (27, 32). These characteristics can be found in Table 1. Briefly, subjects were recruited within the Salt Lake City area and screened to ensure the absence of preexisting medical conditions. All subjects read and signed the informed consent form. The study was reviewed and approved by the University of Utah Institutional Review Board and conformed to the Declaration of Helsinki and Title 45, US Code of Federal Regulations, Part 46, “Protection of Human Subjects.”
Table 1.
Baseline characteristics
| Young | Old | |
|---|---|---|
| Age, yr | 23 (SD 3) | 68 (SD 6)* |
| Sample size (male/female) | 9 (2/7) | 18 (11/7) |
| Height, cm | 172 (SD 6) | 174 (SD 7) |
| Weight, kg | 66 (SD 14) | 76 (SD 9)* |
| Fat mass, kg | 20 (SD 5) | 24 (SD 6) |
| Whole body lean mass, kg | 44 (SD 9) | 49 (SD 8) |
| Leg lean mass, kg | 15 (SD 3) | 15 (SD 3) |
| Activity level, steps/day | 6,060 (SD 1,302) | 6,070 (SD 1,994) |
Values are means [standard deviation (SD)].
Different from Young (P < 0.05).
Bed rest.
Bed rest (5 days; Monday–Friday) took place at the University of Utah Center for Clinical and Translational Science according to protocol and safety guidelines and nutritional control thoroughly described in our previous studies (27, 32). During bed rest, caloric intake for each subject was evenly distributed across meals and days predetermined by a research dietitian. Bathroom and hygiene activities were performed in a wheelchair, whereas the remainder of time was spent in a bed. Nursing staff was available 24 h a day for care during the 5 days of bed rest.
Biopsies.
Muscle biopsies were obtained from the vastus lateralis after a 12-h fast with the Bergström needle approach with manual suction after anesthesia with 1% lidocaine. Biopsies from Young and Old subjects who underwent identical procedural treatments were obtained before bed rest (Pre; day 1) and after bed rest (Post; day 5).
Leg lean mass, strength, and fiber cross-sectional area.
Leg lean mass was determined with dual-energy X-ray absorptiometry, and leg isometric knee extension strength was measured on an isokinetic dynamometer with each leg tested individually and data reported as peak torque (Nm) from the average of both legs. Myofiber cross-sectional area was determined by immunohistochemical staining for laminin (Sigma-Aldrich catalog no. L9393) on frozen cross sections of tissue to delineate the sarcolemma of the myofiber (27). Fiber area was determined with the MATLAB plug-in SMASH as described previously (26).
RNA extraction.
Total RNA was isolated as described previously (32). Briefly, 10–15 mg of tissue was placed in TRI Reagent LS (Molecular Research Center, Cincinnati, OH) and disrupted with a handheld homogenizer (Bio-Gen PRO200; Pro Scientific, Oxford, CT). Chloroform was added to separate the RNA into an aqueous phase that was then precipitated with isopropanol. Extracted RNA was then washed with ethanol and suspended in nuclease-free water with EDTA. RNA purity and quantity were determined with a spectrophotometer (Epoch, Take3; BioTek). RNA integrity has been reported on some of these samples previously (~8.8) (32), while the remaining samples were recorded to have a RNA integrity number of ~8.7.
RNA sequencing and library construction.
RNA sequencing and library construction were performed by the University of Utah High-Throughput Genomics Core. Total RNA samples (100–500 ng) were hybridized with Ribo-Zero Gold to substantially deplete cytoplasmic and mitochondrial rRNA from the samples. Sequencing libraries (25 pM) were chemically denatured and applied to an Illumina HiSeq v4 single-read flow cell using an Illumina cBot. Hybridized molecules were clonally amplified and annealed to sequencing primers with reagents from an Illumina HiSeq SR Cluster Kit v4-cBot (GD-401-4001). After transfer of the flow cell to an Illumina HiSeq 2500 instrument (HCSv2.2.38 and RTA v1.18.61), a 50-cycle single-read sequence run was performed with HiSeq SBS Kit v4 sequencing reagents (FC-401-4002).
Library construction was performed with the Illumina TruSeq Stranded Total RNA Sample Preparation Kit with Ribo-Zero Human/Mouse/Rat (catalog nos. RS-122-2201, RS-122-2202). rRNA was removed from total RNA samples (100 ng to 4 μg) with biotinylated Ribo-Zero oligos attached to magnetic beads that are complementary to cytoplasmic rRNA. After purification, the rRNA-depleted sample was fragmented with divalent cations under elevated temperatures and primed with random hexamers in preparation for cDNA synthesis. First-strand reverse transcription was accomplished with Superscript II Reverse Transcriptase (Invitrogen catalog no. 18064-014). Second-strand cDNA synthesis was accomplished with DNA polymerase I and RNase H under conditions in which dUTP is substituted for dTTP, yielding blunt-ended cDNA fragments. An A base was added to the blunt ends as a means to prepare the cDNA fragments for adapter ligation and block concatemer formation during the ligation step. Adapters containing a T-base overhang were ligated to the A-tailed DNA fragments. Ligated fragments were PCR amplified (12–15 cycles) under conditions in which the PCR reaction enables amplification of the first-strand cDNA product, whereas attempted amplification of the second-strand product stalls at dUTP bases and therefore was not represented in the amplified library.
The PCR-amplified library was purified with Agencourt AMPure XP beads (Beckman Coulter Genomics catalog no. A63881). After amplification, the library was purified by bead-based methodologies. The concentration of the amplified library was measured with a NanoDrop spectrophotometer, and an aliquot of the library was resolved on an Agilent 2200 Tape Station using a D1K (catalog nos. 5067-5361 and 5067-5362) or a High Sensitivity D1K (catalog nos. 5067-5363 and 5067-5364) assay to define the size distribution of the sequencing library. Libraries were adjusted to a concentration of ~10 nM, and quantitative PCR was performed with the KapaBiosystems Kapa Library Quant Kit (catalog no. KK4824) to calculate the molarity of adapter-ligated library molecules. The concentration was further adjusted after qPCR to prepare the library for Illumina sequence analysis. These data have been submitted to the National Center for Biotechnology Information’s Gene Expression Omnibus (Accession No.: GSE113165).
Bioinformatics.
To analyze RNA sequencing data, human GRCh38 FASTA and GTF files were downloaded from Ensembl release 87 and the reference database was created with STAR version 2.5.2b with splice junctions optimized for 50-base pair reads (8, 38). Reads were aligned to the reference database with STAR in two-pass mode to output a BAM file sorted by coordinates. Mapped reads were assigned to annotated genes in the GTF file with featureCounts (v1.5.1) (19). The output files from FastQC, STAR, and featureCounts were summarized by using MultiQC to check for any sample outliers (12).
Differentially expressed genes were identified with a 5% false discovery rate with DESeq2 version 1.16.0 (20). An initial statistical model was performed only evaluating the response to bed rest irrespective of age. When assessing differences in gene expression with respect to age, since each subject had two observations [before bed rest (Pre) and after bed rest (Post)] and could only be in one group (Young or Old), the design formula was constructed by following the section on group-specific condition effects, individuals nested within groups in the DESeq2 vignette. The design included age + age:nested + age:time to test for differences in bed rest in old subjects, young subjects, and the interaction, in this case if bed rest effects are different between the two age groups (where age is Young or Old, nested is subject number nested by age, and time is Pre or Post). Fold changes were determined with maximum-likelihood estimates of the log2 fold change. An adjusted P value of P < 0.05 was required for a gene to be considered significantly altered. The significant results were processed by IPA to determine the gene expression pathways altered by bed rest within each age group.
Quantitative RT-PCR.
Gene expression was determined from a limited subset of Young and Old RNA samples to validate target genes that were the most highly correlated to leg lean mass. Five hundred nanograms of RNA was reverse-transcribed into cDNA (iScript cDNA synthesis kit; Bio-Rad). Primers (MAFB, catalog no. qHsaCED0002199; SMAD6, catalog no. qHsaCID0017103; THBS4, catalog no. qHsaCID0012105) were purchased from Bio-Rad (PrimePCR). Real-time qPCR was carried out with a CFX Connect real-time PCR cycler (Bio-Rad) in combination with SYBR Green fluorescence (SsoAdv Universal SYBR Green Supermix; Bio-Rad). Cycle threshold (Ct) values of target genes were normalized to β2-microglobulin, and then fold change values were calculated ().
Statistical analysis.
We used a two-way ANOVA examining the interaction between time (Pre vs. Post) and age (Young vs. Old) for leg lean mass, leg strength, and fiber cross-sectional area. Post hoc analysis (Sidak’s multiple comparison test) was performed when a significant interaction was detected. To determine whether genes differentially regulated between Young and Old could predict muscle loss, these baseline gene expression values were correlated to the change in leg lean mass (fold change Post minus Pre). To identify specific gene targets that may regulate muscle loss during bed rest, the change in leg lean mass (log2 Post − log2 Pre) was correlated with the change in gene expression using the difference in regularized log-transformed values (rlog Post − rlog Pre). The rlog values in DESeq2 are similar to log2-normalized counts except that the variance in low-count genes is reduced. Bed rest-induced genes that correlated with the change in leg lean mass were screened by selecting genes that responded in Old (or Old and Young). A final list of genes was identified with a cutoff of P ≤ 0.015. All statistical analysis was performed with GraphPad Prism (v7.00 for Windows; GraphPad Software, La Jolla, CA).
RESULTS
Characteristics.
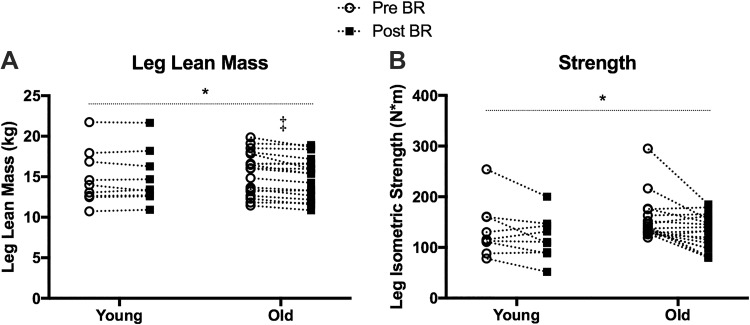
Baseline characteristics are presented in Table 1. As expected, the older adults were older than the young adults (P < 0.05). The older adults also weighed more than the young adults; however, fat mass, total lean mass, leg lean mass, and baseline activity level were not different between the age groups. In response to bed rest, there was a 3.4% loss of leg lean mass in Old {−525 g [95% confidence interval (CI): −792 to −258 g], P < 0.001} but not Young [−47 g (95% CI: −424 to 330 g), P = 0.9] (bed rest main effect, P = 0.007; interaction, P = 0.02) (Fig. 1). Similar observations were made for whole body lean mass [bed rest main effect, P < 0.001; interaction, P = 0.003; Old: −1,585 g (95% CI: −2,133 to −1,036 g), P < 0.001; Young: −291 g (95% CI: −1,067 to 485 g), P = 0.6] and myofiber cross-sectional area [interaction, P = 0.045; Old: −579 µm2 (95% CI: −1,162 to 5 µm2), P = 0.052; Young: 367 µm2 (95% CI: −494 to 1,228 µm2), P = 0.5] (data not shown). Leg strength decreased after bed rest; however, no difference between Young and Old subjects was detected [bed rest main effect, P = 0.02; Old: −25 Nm (95% CI: −43 to −6 Nm); Young: −11 Nm (95% CI: −40 to 18 Nm)] (Fig. 1).
Fig. 1.
Leg lean mass and strength before (Pre) and after (Post) bed rest (BR). Leg lean mass (A) and leg isometric strength (B) in young and older adults. n = 9 Young, 18 Old. ‡Pre and Post are different with interaction term (P ≤ 0.05). *Main effect for BR. Two-way ANOVA (age × time), repeated measures by time (Pre and Post).
Bed rest-induced changes in gene expression.
RNA sequencing revealed 2,965 genes that changed only in Young, only in Old, or commonly between the age groups. In the Old group 1,807 genes were altered (1,255 uniquely in Old, 589 up, 666 down), whereas 1,710 genes were altered in the Young group (1,159 uniquely in Young, 632 up, 527 down). Only 551 (224 increased, 327 decreased) of these gene changes occurred commonly between Young and Old. We also noted 3,931 gene differences between Old and Young subjects at baseline. Of the 3,931 age-related gene expression differences, 270 of these genes were altered in response to bed rest only in Old, 694 altered only in Young, and 223 were commonly altered in both age groups.
Pathway analysis.
The top 15 pathways identified by IPA as regulated by bed rest (ranked by P value significance) for Young and Old are presented in Table 2. Some of the top commonly pathways shared between Young and Old were related to Actin Cytoskeleton Signaling (activation z score: Young: −0.164; Old: −3.479), ILK Signaling (activation z score: Young: −1.061; Old: −1.897), RhoA Signaling (activation z score: Young: −2.132; Old: −2.558), Mitochondrial Dysfunction (no activation z score available), and Calcium Signaling (activation z score: Young: 1.213; Old: 1.291).
Table 2.
Top 15 pathways altered by 5-day bed rest
| Young |
Old |
||||
|---|---|---|---|---|---|
| Pathway | −log(P value) | z Score | Pathway | −log(P value) | z Score |
| Mitochondrial Dysfunction (72) | 36.6 | NaN | Hepatic Fibrosis/Hepatic Stellate Cell Activation (49) | 14.8 | NaN |
| Oxidative Phosphorylation (56) | 34.3 | NaN | Epithelial Adherens Junction Signaling (40) | 12.5 | NaN |
| Actin Cytoskeleton Signaling (42) | 7.85 | −0.164 | Actin Cytoskeleton Signaling (49) | 11 | −3.479 |
| Estrogen Receptor Signaling (28) | 7.01 | NaN | Calcium Signaling (41) | 10.1 | 1.291 |
| Epithelial Adherens Junction Signaling (29) | 6.29 | NaN | ILK Signaling (40) | 8.32 | −1.897 |
| Glucocorticoid Receptor Signaling (44) | 5.74 | NaN | Axonal Guidance Signaling (68) | 7.6 | NaN |
| Calcium Signaling (31) | 5.34 | 1.213 | Tight Junction Signaling (31) | 5.67 | NaN |
| RhoA Signaling (24) | 5.11 | −2.132 | Cellular Effects of Sildenafil (26) | 5.41 | NaN |
| ILK Signaling (32) | 4.94 | −1.061 | Germ Cell-Sertoli Cell Junction Signaling (31) | 5.34 | NaN |
| TR/RXR Activation (20) | 4.72 | NaN | Signaling by Rho Family GTPases (39) | 5.1 | −1.372 |
| Estrogen-Dependent Breast Cancer Signaling (17) | 4.56 | 1.000 | Sertoli Cell-Sertoli Cell Junction Signaling (31) | 5.07 | NaN |
| PAK Signaling (20) | 4.52 | −1.213 | Mitochondrial Dysfunction (30) | 5 | NaN |
| PPARα/RXRα Activation (29) | 4.43 | −2.502 | RhoGDI Signaling (30) | 4.89 | 1.732 |
| Integrin Signaling (33) | 4.34 | −0.898 | RhoA Signaling (24) | 4.85 | −2.558 |
| NRF2-Mediated Oxidative Stress Response (30) | 4.26 | −1.155 | Oxidative Phosphorylation (22) | 4.8 | NaN |
n = 9 Young, 18 Old. Number of genes regulated within each pathway in parentheses. Statistical design included age + age:nested + age:time. Fold changes were determined with maximum-likelihood estimates of the log2 fold change. Boldface indicates a pathway that was altered uniquely within that age group. NaN, z score could not be calculated. An adjusted P value of P < 0.05 was required for a gene to be considered significantly altered.
The top pathway regulated by bed rest in Old was related to Hepatic Fibrosis/Hepatic Stellate Cell Activation (no activation z score available), and this pathway was not represented in the top 15 pathways regulated in Young. Specific molecules in this pathway that mediate inflammatory signaling in skeletal muscle (NF-κB and regulatory cytokines) that changed in the Old group were p50 (NFKB1), ~13% increase, P = 0.043; p52 (NFKB2), ~36% increase, P = 0.011; IL-6 receptor (IL6R), ~73% increase, P = 0.0006; IL-6, 376% increase trend, P = 0.082; and IL-10 receptor (IL10RB), ~21% decrease, P = 0.011. Other factors included in this pathway related to fibrosis and tissue remodeling in the old are connective tissue growth factor (CTGF), which decreased by ~53% (P < 0.001), and vascular endothelial growth factor A (VEGFA), which decreased by ~28% (P < 0.0001).
Integrin Signaling appeared in the top 15 altered pathways only for the Young group and ranked as the 19th altered pathway in the Old group. However, when the activation z score was examined (Young: −0.898; Old: −3.053), the Integrin Signaling pathway was downregulated to a greater extent in Old, an important finding because of the role this pathway has in mechanosensing (1). Also noteworthy, bed rest induced a decrease in α7-integrin (ITGA7; ~28% decrease, log2 fold change: −0.46, adjusted P = 0.0009) in the old Group but not in the Young.
Top 10 genes upregulated and downregulated.
The top 10 genes upregulated and downregulated (significantly altered genes ranked by fold change) in Young and Old are presented in Table 3. In Old, five of the top upregulated genes (PNPLA3, KRT17P4, TMEM215, RGMB-AS1, CXCL2) and two of the top downregulated genes (ADAMTS8, METTL21C) were not significantly altered in Young. TNFRSF12A (codes for the Tweak receptor) decreased in Young, and although not on the top 10 list this transcript also decreased in Old (0.4 fold; P = 0.001) as a result of bed rest. GADD45A (growth arrest and DNA damage-inducible alpha) was the 18th highest upregulated gene in Old, increasing 2.2-fold (P = 0.001), with no bed rest-induced change in Young. None of these genes was correlated with the change in leg lean mass.
Table 3.
Top 10 genes upregulated and downregulated by 5 days of bed rest
| Young |
Old |
||||
|---|---|---|---|---|---|
| Gene name | Fold change | Adjusted P value | Gene name | Fold change | Adjusted P value |
| Upregulated | |||||
| ATP1A4 | 3.77 | 2.54E-04 | PFKFB3 | 3.34 | 9.34E-09 |
| RP11-680G24.4 | 2.99 | 2.22E-03 | RP11-369K17.1 | 3.00 | 1.27E-05 |
| FASN | 2.88 | 8.19E-03 | PNPLA3 | 2.89 | 2.45E-02 |
| MYH1 | 2.80 | 3.66E-02 | CPXM1 | 2.87 | 1.19E-02 |
| PFKFB3 | 2.79 | 2.36E-03 | KRT17P4 | 2.76 | 1.04E-02 |
| RPS4XP5 | 2.78 | 1.68E-04 | TMEM63C | 2.69 | 5.85E-03 |
| UNQ6494 | 2.66 | 1.69E-02 | TMEM215 | 2.56 | 1.15E-02 |
| USP6 | 2.53 | 2.21E-08 | HAS2-AS1 | 2.46 | 5.69E-03 |
| PDE11A | 2.44 | 8.36E-05 | RGMB-AS1 | 2.40 | 1.52E-02 |
| CTD-2095E4.3 | 2.43 | 2.43E-03 | CXCL2 | 2.39 | 4.33E-03 |
| Downregulated | |||||
| SLC26A9 | 0.14 | 2.29E-04 | RP11-145A3.1 | 0.09 | 9.12E-21 |
| RP11-145A3.1 | 0.18 | 6.18E-05 | LRRC52 | 0.17 | 2.12E-04 |
| THY1 | 0.23 | 5.35E-09 | SLC26A9 | 0.19 | 2.47E-06 |
| TNFRSF12A | 0.23 | 5.56E-04 | LBP | 0.20 | 3.19E-07 |
| HMGCS2 | 0.23 | 2.05E-03 | ADAMTS8 | 0.22 | 1.55E-09 |
| NOV | 0.27 | 7.69E-07 | ABCC12 | 0.24 | 3.50E-12 |
| LBP | 0.30 | 3.83E-02 | METTL21C | 0.25 | 1.52E-06 |
| TNC | 0.31 | 2.07E-02 | SEL1L2 | 0.26 | 4.05E-05 |
| APLN | 0.31 | 4.36E-05 | SLC5A1 | 0.27 | 1.75E-03 |
| MYL12A | 0.31 | 2.96E-11 | MUC22 | 0.28 | 5.09E-04 |
Genes are ordered by fold change.
Age-dependent differential regulation of genes.
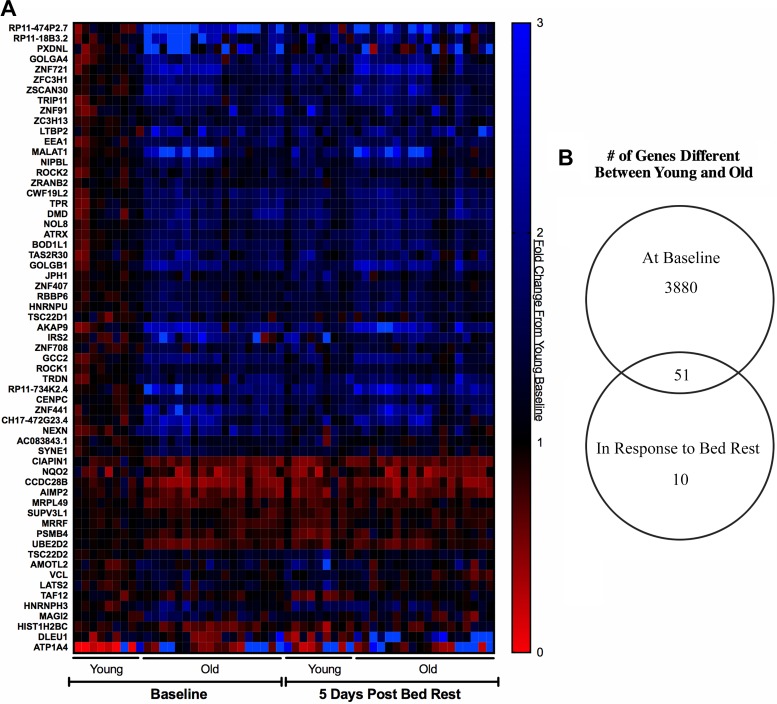
Sixty-one genes were differentially regulated in response to bed rest between Old and Young (Fig. 2) (representing the age × time interaction term of our statistical model). Fifty-one of sixty-one differentially regulated genes were altered in Young (42 increased, 9 decreased) but did not change in Old in response to bed rest. Nine of these genes were altered only in Old, of which five are protein coding (MRPL49, HIST1H2BC: increased; PXDNL, NEXN, MAGI2: decreased). Several of the 51 differentially regulated genes were also different at baseline between Young and Old, for 2 of which the baseline gene expression correlated with the change in leg lean mass (NOL8: R2 = 0.152, P = 0.044; HNRNPU: R2 = 0.147, P = 0.048).
Fig. 2.
Differential regulation between Young and Old subjects after bed rest. A: heat map values are expressed relative to the average of the Young baseline gene expression values within each gene. Blue indicates higher expression, and red indicates lower expression. B: Venn diagram illustrating overlap of genes different between Young and Old at baseline and in response to bed rest. Statistical design included age + age:nested + age:time. Fold changes were determined with maximum-likelihood estimates of the log2 fold change. An adjusted P value of P < 0.05 was required for a gene to be considered significantly altered. n = 9 Young, 18 Old.
Bed rest-induced genes and relationship to leg lean mass.
Next, we correlated bed rest-induced gene changes in the old with leg lean mass. To create an optimal spread in lean mass and gene expression [bed rest high responders (Old), bed rest low responders (Young)], we correlated both Young and Old leg lean mass and gene changes. This resulted in identification of 30 genes (Table 4). To narrow the list, these genes were further scrutinized by P value (P ≤ 0.015). The top five protein-coding genes most tightly associated with the change in leg lean mass were SPAG17 (R2 = 0.316, P = 0.003), KCTD10 (R2 = 0.265, P = 0.006), MAFB (R2 = 0.246, P = 0.009), SMAD6 (R2 = 0.231, P = 0.011), and THBS4 (R2 = 0.230, P = 0.013, after removal of 1 outlier from young), whereas CTD-2270P14.5 is a long intergenic noncoding RNA (lincRNA) and therefore not protein coding.
Table 4.
Genes altered by bed rest in Old subjects that correlate with change in leg lean mass
| LLM Correlation |
|||||
|---|---|---|---|---|---|
| Ensembl ID | Gene Name | Biotype | Ensembl Description | R2 coefficient | P value |
| ENSG00000155761 | SPAG17 | Protein_coding | Sperm associated antigen 17 | 0.297 | 0.003 |
| ENSG00000110906 | KCTD10 | Protein_coding | Potassium channel tetramerization domain containing 10 | 0.265 | 0.006 |
| ENSG00000276791 | CTD-2270P14.5 | lincRNA | 0.264 | 0.006 | |
| ENSG00000204103 | MAFB | Protein_coding | MAF bZIP transcription factor B | 0.246 | 0.009 |
| ENSG00000137834 | SMAD6 | Protein_coding | SMAD family member 6 | 0.231 | 0.011 |
| ENSG00000113296 | THBS4* | Protein_coding | Thrombospondin 4 | 0.230* | 0.013* |
| ENSG00000157538 | DSCR3 | Protein_coding | DSCR3 arrestin fold containing | 0.216 | 0.015 |
| ENSG00000129991 | TNNI3 | Protein_coding | Troponin I3, cardiac type | 0.207 | 0.017 |
| ENSG00000135469 | COQ10A | Protein_coding | Coenzyme Q10A | 0.205 | 0.018 |
| ENSG00000180447 | GAS1 | Protein_coding | Growth arrest specific 1 | 0.201 | 0.019 |
| ENSG00000100281 | HMGXB4 | Protein_coding | HMG-box containing 4 | 0.199 | 0.020 |
| ENSG00000169436 | COL22A1 | Protein_coding | Collagen type XXII α1 chain | 0.199 | 0.020 |
| ENSG00000182809 | CRIP2 | Protein_coding | Cysteine rich protein 2 | 0.187 | 0.024 |
| ENSG00000134243 | SORT1 | Protein_coding | Sortilin 1 | 0.180 | 0.027 |
| ENSG00000118298 | CA14 | Protein_coding | Carbonic anhydrase 14 | 0.180 | 0.027 |
| ENSG00000154767 | XPC | Protein_coding | XPC complex subunit, DNA damage recognition and repair factor | 0.174 | 0.030 |
| ENSG00000113273 | ARSB | Protein_coding | Arylsulfatase B | 0.165 | 0.035 |
| ENSG00000042445 | RETSAT | Protein_coding | Retinol saturase | 0.163 | 0.037 |
| ENSG00000261326 | LINC01355 | lincRNA | Long intergenic nonprotein coding RNA 1355 | 0.162 | 0.037 |
| ENSG00000166797 | FAM96A | Protein_coding | Family with sequence similarity 96 member A | 0.161 | 0.038 |
| ENSG00000156463 | SH3RF2 | Protein_coding | SH3 domain containing ring finger 2 | 0.159 | 0.040 |
| ENSG00000255874 | LINC00346 | lincRNA | Long intergenic nonprotein coding RNA 346 | 0.158 | 0.040 |
| ENSG00000166148 | AVPR1A | Protein_coding | Arginine vasopressin receptor 1A | 0.149 | 0.047 |
| ENSG00000151458 | ANKRD50 | Protein_coding | Ankyrin repeat domain 50 | 0.148 | 0.048 |
| ENSG00000163807 | KIAA1143 | Protein_coding | KIAA1143 | 0.148 | 0.048 |
| ENSG00000166780 | C16orf45 | Protein_coding | Chromosome 16 open reading frame 45 | 0.147 | 0.048 |
| ENSG00000248309 | MEF2C-AS1 | Antisense | MEF2C antisense RNA 1 | 0.147 | 0.048 |
| ENSG00000119669 | IRF2BPL | Protein_coding | Interferon regulatory factor 2 binding protein like | 0.146 | 0.049 |
| ENSG00000162377 | COA7 | Protein_coding | Cytochrome-c oxidase assembly factor 7 (putative) | 0.146 | 0.049 |
| ENSG00000163596 | ICA1L | Protein_coding | Islet cell autoantigen 1 like | 0.145 | 0.050 |
n = 9 Young, 18 Old. Statistical design included age + age:nested + age:time. Fold changes were determined with maximum-likelihood estimates of the log2 fold change. lincRNA, long intergenic noncoding RNA LLM, leg lean mass. An adjusted P value of P < 0.05 was required for a gene to be considered significantly altered.
One outlier removed from Young.
Finally, we attempted to validate (qPCR) the candidate genes with established links to skeletal muscle (13, 14, 29). Of these, MAFB validated our correlational findings. The correlational results from gene changes measured by qPCR with the change in leg lean mass were the following: MAFB (n = 14 Old, n = 8 Young, R2 = 0.248, P = 0.0184), SMAD6 (n = 10 Old, n = 7 Young, R2 = 0.094, P = 0.2320), and THBS4 (n = 14 Old, n = 8 Young, R2 = 0.011, P = 0.6440).
DISCUSSION
We conducted RNA sequencing on skeletal muscle biopsy tissue obtained from older and younger adults before and after 5 days of bed rest to identify key molecular regulators and pathways that may explain bed rest-induced changes in lean mass and differences between the age groups. Our primary findings were that bed rest resulted in alterations in a fibrotic and inflammatory pathway (Hepatic Fibrosis/Hepatic Stellate Cell Activation) and the downregulation of cell adhesion and mechanosensing pathways characterized specifically in older adult skeletal muscle. Additionally, we identified a subset of 51 genes that only changed in the young after bed rest and mirrored that of the old at baseline, suggesting that these genes may describe predisposition to muscle loss.
Ingenuity Pathway Analysis.
The top-ranking pathway in the Old group was the Hepatic Fibrosis/Hepatic Stellate Cell Activation Pathway, which was ranked as the 72nd pathway in IPA for the Young group, clearly identifying an age-related response to disuse. The molecules represented in this pathway demonstrate changes in fibrotic and proinflammatory processes within skeletal muscle after bed rest. The transcripts identified by this pathway include many cytokines, growth factors, collagen isoforms, and inflammatory response elements. In agreement, other clinical studies have noted alterations in these pathways, such as increased muscle fibrosis with aging and increased inflammatory signaling with both age and muscle disuse (23, 26). NF-κB, a central mediator of inflammatory signaling in skeletal muscle, has been shown to play a critical role in sarcopenia with aging as well as muscle atrophy with hindlimb suspension in rodents (17, 18, 24). Our study demonstrated that bed rest increased p50 (NFKB1) and p52 (NFKB2) only in muscle of older adults. An increase in muscle NFKB1 mRNA is a consistent finding that we also observe after 7 days of bed rest in older adults (10). Moreover, cytokines and related receptors that activate the NF-κB pathway were also induced, including an increase in the proinflammatory IL-6 receptor and a trend toward an increase in IL-6 (>300%, P = 0.08). Finally, and only in older adults, we observed a decrease in the transcription of the receptor for the anti-inflammatory cytokine IL-10 (IL10RB). While the ligand, IL-10, was not detected by RNA sequencing in this data set, we have previously shown a decrease in muscle IL-10 gene expression after 5-day bed rest (in a subset of these participants) and only in old subjects (26). These results demonstrate that short-term bed rest induced an inflammatory burden in aging skeletal muscle, a process that may partly explain the loss of muscle mass and possibly additional muscle loss if bed rest were to be continued.
The importance of cell adhesion signaling in mechanosensitive regulation of skeletal muscle mass has been rapidly gaining attention (34). Although precise regulation of mechanosensitive elements in muscle remains somewhat elusive, the focal adhesion complex at the costamere may play an important role. Signaling at the costamere involves integrin linked kinase (ILK), integrins, RhoA GTPases and various other cytoskeletal signaling dynamics (such as F actin and stress fiber formation), and the Hippo pathway (5, 34, 40). These pathways were similarly highlighted in our pathway analysis (Actin Cytoskeleton Signaling, ILK Signaling, RhoA Signaling) in both Young and Old skeletal muscle after bed rest. Although these pathways decreased in both age groups, the activation z scores for these pathways suggested an age-related susceptibility to decreased transcription of pathway-associated genes, as they were downregulated to a greater extent in older adults. Although Integrin Signaling appeared in the top 15 significantly altered pathways only for Young subjects, the integrin signaling pathway was downregulated (by activation z score) to a much larger extent in Old vs. Young subjects. The most abundantly expressed integrin in skeletal muscle, α7-integrin (ITGA7), was decreased only in older adults (~28%). α7-Integrin has been shown to increase expression in response to exercise in humans and mice, and genetic alteration of its expression in mice has identified its role in skeletal muscle growth (1, 40).
Differential skeletal muscle genes between Young and Old subjects after bed rest.
Although there were thousands of genes altered by bed rest, only 61 genes induced by bed rest were differentially regulated between Young and Old. On closer inspection of the heat map, the majority (51 genes) changed with bed rest in Young but were unresponsive in Old. Although these genes were different at baseline between Young and Old, after bed rest the gene responses in Young nearly matched the expression level of Old. For example, ROCK1, a RhoA-associated kinase and negative regulator of myogenesis (3, 39), was elevated 27% in Old compared with Young at baseline but increased 26% after bed rest only in Young. Interestingly, baseline expression of two of these genes predicted the change in leg lean mass [NOL8, also called NOP132, involved in cell growth and rRNA processing (28); HNRNPU, also called hnRNP U, involved in posttranscriptional modification of mRNA (37)]. These data may suggest a muscle atrophy susceptibility transcriptome in young subjects (like the old at baseline), perhaps predisposing individuals to future muscle losses should bed rest continue.
Highly regulated genes in response to bed rest.
Upon examination of the top responding genes in older adults in response to bed rest, some modulate substrate use (PFKFB3, PNPLA3, SLC5A1) and some are ion exchangers (TMEM63C, LRRC52, SLC26A9), whereas others’ roles are not readily apparent or they are not protein-coding genes. Two genes highly elevated in Old, but not in Young, CXCL2 (upregulated ~140%) and GADD45A (upregulated ~120%), have been implicated in the process of skeletal muscle remodeling. CXCL2 codes for macrophage inflammatory protein-2 (Mip-2), a cytokine expressed by proinflammatory M1 inflammatory macrophages (33), and CXCL2 mRNA measured in blood has been shown to be increased in response to acute exercise (4). Although no information exists for the response of this gene to muscle disuse, it has been shown to increase in older individuals in conjunction with muscle atrophy associated with total knee arthroplasty (9). Although not in the top 10 upregulated list, GADD45A was upregulated ~120% in response to bed rest only in Old. GADD45A is induced by activation of the transcription factor activating transcription factor 4 (ATF4) in response to stresses such as hindlimb unloading and starvation in mice (11) and knee replacement in humans (9).
Finally, we validated MAFB expression because it was one of the top correlated genes with the change in leg lean mass following bed rest (Table 4). MAFB has been reported to be expressed specifically in macrophages and their precursors and decreased only in old subjects. The protein that codes for this transcript may play a role in maintenance of myelomonocytic cells by preventing erythroid-specific gene expression through inhibition of Ets-1 in precursor cells (29). This result would be in agreement with others and with our own data showing a decrease in total CD68+ (pan macrophage marker) skeletal muscle mRNA from old subjects after disuse (23, 26). Although in this study we report that M1 macrophage activity (CXCL2) may be increased in the old, total macrophage presence (CD68+) in older skeletal muscle is decreased with short-term bed rest.
Limitations.
Because of the vast transcriptome responses observed, it is possible that we have overlooked key regulators of muscle atrophy. Second, it is possible that we did not capture genes responses during the early time course of bed rest or posttranslational modification of proteins that may correlate more strongly with the change in leg lean mass. Third, although the Young group serves as a valuable comparator to the Old group (low responder vs. high responder), the low sample size of this group decreased the power of direct comparison because of interindividual variability. Our correlation analysis attempts to mitigate this fact by expressing change in gene expression within subjects, taking advantage of interindividual variation regardless of age category to associate the extent of muscle loss with the extent of a gene change. Finally, although gene expression was performed on muscle biopsies, it is not possible to decipher whether these changes are localized to the muscle fiber or other supporting cells (e.g., satellite cells, macrophages, fibroblasts).
This comprehensive muscle RNA sequencing profiling study demonstrated that alterations to fibrotic and inflammatory processes (CXCL2, GADD45A) and the downregulation of cell adhesion and mechanosensing pathways may underlie the rapid loss of muscle in older adults with short-term bed rest and identification of 51 differentially regulated genes may be related to predisposition of muscle loss with disuse. These data provide valuable information that may be used in the development of future therapeutics to preserve lean mass during disuse events in vulnerable patient populations at risk for physical function impairments.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01 AG-050781, T32 HL139451, and F32 AR-072481; the National Center for Advancing Translational Sciences (1ULTR001067); and the University of Utah’s Center on Aging.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.S.M., P.T.R., A.I.M., and M.J.D. conceived and designed research; A.I.M., M.T.H., and M.J.D. performed experiments; Z.S.M., C.S., and M.J.D. analyzed data; Z.S.M., P.T.R., A.I.M., C.S., M.T.H., and M.J.D. interpreted results of experiments; Z.S.M. and M.J.D. prepared figures; Z.S.M. and M.J.D. drafted manuscript; Z.S.M., P.T.R., A.I.M., M.T.H., and M.J.D. edited and revised manuscript; Z.S.M., P.T.R., A.I.M., C.S., M.T.H., and M.J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the University of Utah Center for Clinical and Translational Science nursing, dietary, and medical staff for assistance with the muscle biopsies, blood sampling, and patient care during the inpatient and outpatient visits. We also thank Dr. Mark Supiano for medical oversight of the bed rest studies.
REFERENCES
- 1.Boppart MD, Volker SE, Alexander N, Burkin DJ, Kaufman SJ. Exercise promotes alpha7 integrin gene transcription and protection of skeletal muscle. Am J Physiol Regul Integr Comp Physiol 295: R1623–R1630, 2008. doi: 10.1152/ajpregu.00089.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown WF. A method for estimating the number of motor units in thenar muscles and the changes in motor unit count with ageing. J Neurol Neurosurg Psychiatry 35: 845–852, 1972. doi: 10.1136/jnnp.35.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellani L, Salvati E, Alemà S, Falcone G. Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J Biol Chem 281: 15249–15257, 2006. doi: 10.1074/jbc.M601390200. [DOI] [PubMed] [Google Scholar]
- 4.Catoire M, Mensink M, Kalkhoven E, Schrauwen P, Kersten S. Identification of human exercise-induced myokines using secretome analysis. Physiol Genomics 46: 256–267, 2014. doi: 10.1152/physiolgenomics.00174.2013. [DOI] [PubMed] [Google Scholar]
- 5.Chaillou T, Lee JD, England JH, Esser KA, McCarthy JJ. Time course of gene expression during mouse skeletal muscle hypertrophy. J Appl Physiol (1985) 115: 1065–1074, 2013. doi: 10.1152/japplphysiol.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopard A, Lecunff M, Danger R, Lamirault G, Bihouee A, Teusan R, Jasmin BJ, Marini JF, Leger JJ. Large-scale mRNA analysis of female skeletal muscles during 60 days of bed rest with and without exercise or dietary protein supplementation as countermeasures. Physiol Genomics 38: 291–302, 2009. doi: 10.1152/physiolgenomics.00036.2009. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson JM, D’Lugos AC, Naymik MA, Siniard AL, Wolfe AJ, Curtis DR, Huentelman MJ, Carroll CC. Transcriptome response of human skeletal muscle to divergent exercise stimuli. J Appl Physiol (1985) 124: 1529–1540, 2018. doi: 10.1152/japplphysiol.00014.2018. [DOI] [PubMed] [Google Scholar]
- 8.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreyer HC. Tourniquet use during knee replacement surgery may contribute to muscle atrophy in older adults. Exerc Sport Sci Rev 44: 61–70, 2016. doi: 10.1249/JES.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MJ, Timmerman KL, Markofski MM, Walker DK, Dickinson JM, Jamaluddin M, Brasier AR, Rasmussen BB, Volpi E. Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am J Physiol Regul Integr Comp Physiol 305: R216–R223, 2013. doi: 10.1152/ajpregu.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebert SM, Dyle MC, Kunkel SD, Bullard SA, Bongers KS, Fox DK, Dierdorff JM, Foster ED, Adams CM. Stress-induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy. J Biol Chem 287: 27290–27301, 2012. doi: 10.1074/jbc.M112.374777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32: 3047–3048, 2016. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frolova EG, Drazba J, Krukovets I, Kostenko V, Blech L, Harry C, Vasanji A, Drumm C, Sul P, Jenniskens GJ, Plow EF, Stenina-Adognravi O. Control of organization and function of muscle and tendon by thrombospondin-4. Matrix Biol 37: 35–48, 2014. doi: 10.1016/j.matbio.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hata A, Lagna G, Massagué J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev 12: 186–197, 1998. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jozsi AC, Dupont-Versteegden EE, Taylor-Jones JM, Evans WJ, Trappe TA, Campbell WW, Peterson CA. Aged human muscle demonstrates an altered gene expression profile consistent with an impaired response to exercise. Mech Ageing Dev 120: 45–56, 2000. doi: 10.1016/S0047-6374(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni AS, Brutsaert EF, Anghel V, Zhang K, Bloomgarden N, Pollak M, Mar JC, Hawkins M, Crandall JP, Barzilai N. Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell 17: e12723, 2018. doi: 10.1111/acel.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon OS, Tanner RE, Barrows KM, Runtsch M, Symons JD, Jalili T, Bikman BT, McClain DA, O’Connell RM, Drummond MJ. MyD88 regulates physical inactivity-induced skeletal muscle inflammation, ceramide biosynthesis signaling, and glucose intolerance. Am J Physiol Endocrinol Metab 309: E11–E21, 2015. doi: 10.1152/ajpendo.00124.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl) 86: 1113–1126, 2008. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 20.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch GS, Schertzer JD, Ryall JG. Therapeutic approaches for muscle wasting disorders. Pharmacol Ther 113: 461–487, 2007. doi: 10.1016/j.pharmthera.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 95: 139–159, 2010. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 23.Peake J, Della Gatta P, Cameron-Smith D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 298: R1485–R1495, 2010. doi: 10.1152/ajpregu.00467.2009. [DOI] [PubMed] [Google Scholar]
- 24.Peterson JM, Bakkar N, Guttridge DC. NF-κB signaling in skeletal muscle health and disease. Curr Top Dev Biol 96: 85–119, 2011. doi: 10.1016/B978-0-12-385940-2.00004-8. [DOI] [PubMed] [Google Scholar]
- 25.Reich KA, Chen YW, Thompson PD, Hoffman EP, Clarkson PM. Forty-eight hours of unloading and 24 h of reloading lead to changes in global gene expression patterns related to ubiquitination and oxidative stress in humans. J Appl Physiol (1985) 109: 1404–1415, 2010. doi: 10.1152/japplphysiol.00444.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reidy PT, Lindsay CC, McKenzie AI, Fry CS, Supiano MA, Marcus RL, LaStayo PC, Drummond MJ. Aging-related effects of bed rest followed by eccentric exercise rehabilitation on skeletal muscle macrophages and insulin sensitivity. Exp Gerontol 107: 37–49, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reidy PT, McKenzie AI, Brunker P, Nelson DS, Barrows KM, Supiano M, LaStayo PC, Drummond MJ. Neuromuscular electrical stimulation combined with protein ingestion preserves thigh muscle mass but not muscle function in healthy older adults during 5 days of bed rest. Rejuvenation Res 20: 449–461, 2017. doi: 10.1089/rej.2017.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekiguchi T, Hayano T, Yanagida M, Takahashi N, Nishimoto T. NOP132 is required for proper nucleolus localization of DEAD-box RNA helicase DDX47. Nucleic Acids Res 34: 4593–4608, 2006. doi: 10.1093/nar/gkl603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sieweke MH, Tekotte H, Frampton J, Graf T. MafB is an interaction partner and repressor of Ets-1 that inhibits erythroid differentiation. Cell 85: 49–60, 1996. doi: 10.1016/S0092-8674(00)81081-8. [DOI] [PubMed] [Google Scholar]
- 30.Su J, Ekman C, Oskolkov N, Lahti L, Ström K, Brazma A, Groop L, Rung J, Hansson O. A novel atlas of gene expression in human skeletal muscle reveals molecular changes associated with aging. Skelet Muscle 5: 35, 2015. doi: 10.1186/s13395-015-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985) 107: 1172–1180, 2009. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 32.Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, Young LM, Hopkins PN, Volpi E, Marcus RL, LaStayo PC, Drummond MJ. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol 593: 4259–4273, 2015. doi: 10.1113/JP270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Melton DW, Porter L, Sarwar ZU, McManus LM, Shireman PK. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol 184: 1167–1184, 2014. doi: 10.1016/j.ajpath.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watt KI, Goodman CA, Hornberger TA, Gregorevic P. The Hippo signaling pathway in the regulation of skeletal muscle mass and function. Exerc Sport Sci Rev 46: 92–96, 2018. doi: 10.1249/JES.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welle S, Brooks AI, Delehanty JM, Needler N, Bhatt K, Shah B, Thornton CA. Skeletal muscle gene expression profiles in 20–29 year old and 65–71 year old women. Exp Gerontol 39: 369–377, 2004. doi: 10.1016/j.exger.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics 14: 149–159, 2003. doi: 10.1152/physiolgenomics.00049.2003. [DOI] [PubMed] [Google Scholar]
- 37.Ye J, Beetz N, O’Keeffe S, Tapia JC, Macpherson L, Chen WV, Bassel-Duby R, Olson EN, Maniatis T. hnRNP U protein is required for normal pre-mRNA splicing and postnatal heart development and function. Proc Natl Acad Sci USA 112: E3020–E3029, 2015. doi: 10.1073/pnas.1508461112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, Laird MR, Lavidas I, Liu Z, Loveland JE, Maurel T, McLaren W, Moore B, Mudge J, Murphy DN, Newman V, Nuhn M, Ogeh D, Ong CK, Parker A, Patricio M, Riat HS, Schuilenburg H, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Zadissa A, Frankish A, Hunt SE, Kostadima M, Langridge N, Martin FJ, Muffato M, Perry E, Ruffier M, Staines DM, Trevanion SJ, Aken BL, Cunningham F, Yates A, Flicek P. Ensembl 2018. Nucleic Acids Res 46: D754–D761, 2018. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Ying ZZ, Tang ZL, Long LQ, Li K. MicroRNA-148a promotes myogenic differentiation by targeting the ROCK1 gene. J Biol Chem 287: 21093–21101, 2012. doi: 10.1074/jbc.M111.330381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou K, Meador BM, Johnson B, Huntsman HD, Mahmassani Z, Valero MC, Huey KA, Boppart MD. The α7β1-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol (1985) 111: 1134–1141, 2011. doi: 10.1152/japplphysiol.00081.2011. [DOI] [PubMed] [Google Scholar]