Abstract
Aging is associated with altered water, electrolyte, and glucose handling. Alternative beverages to those containing carbohydrate (CHO) should be considered for older adults. We hypothesized that reduced sodium (CNa+) and/or water (CH2O) clearance would underlie greater beverage retention in older compared with young adults, secondary to reduced glomerular filtration rate (GFR). We further hypothesized that amino acid (AA)- and CHO-based beverages would promote retention better than water. Over five visits, 12 young (23 ± 3 yr; 7 men, 5 women) and 12 older (67 ± 6 yr; 5 men, 7 women) subjects consumed 1 liter of distilled water or beverages with 6% CHO, 0.46 g/l Na+ [Gatorade (GAT)]; 2.5% CHO, 0.74 g/l Na+ [Pedialyte (PED)]; 5 AA, 1.04 g/l Na+ [enterade (ENT)-5]; or 8 AA, 1.38 g/l Na+ (ENT-8) over 30 min. Blood and urine were collected every hour for 4 h after ingestion; retention, CH2O, and CNa+ were calculated at 2 and 4 h. Additional calculations adjusted CH2O and CNa+ for estimated GFR (eGFR). Water yielded the lowest retention in both groups (P ≤ 0.02). Retention was higher in older vs. young adults except for ENT-8 at 4 h (P = 0.73). CH2O was higher for older vs. young adults for GAT at 2 h (P < 0.01) and GAT and PED at 4 h (P < 0.01) after ingestion but was otherwise similar between groups. CNa+ was lower in older vs. young adults except for ENT-8 (P ≥ 0.19). Adjusting for eGFR resulted in higher CH2O for all beverages in older vs. young adults (P < 0.05) but did not influence CNa+. Older adults may better retain beverages with less Na+ than young adults because of reduced CNa+. AA- and CHO-based electrolyte-rich beverages may similarly promote beverage retention.
NEW & NOTEWORTHY Commercially available amino acid (AA)-containing beverages may provide an alternative to traditional carbohydrate (CHO)-containing beverages, particularly for older adults with attenuated water, electrolyte, and glucose handling. We compared beverage retention and free water and sodium clearance between young and older adults after ingestion of water, two CHO-based beverages, and two AA-based beverages. Our data suggest that older adults better retain beverages with less sodium compared with young adults and that AA-based and CHO-based electrolyte-containing beverages similarly promote retention.
Keywords: aging, beverage retention, hydration
INTRODUCTION
With increasing life spans and an increasingly larger aged population globally, the necessity to better understand the physiological and behavioral changes that occur with aging is growing. Maintenance of euhydration and renal handling of water (H2O) and electrolyte loads is one such physiological process that may be altered with aging (5, 9, 19) and is essential for normal cardiovascular, cognitive, and digestive function (22). Under ad libitum conditions in a natural environment, healthy, free-living older adults consume enough fluids with meals to maintain adequate hydration. However, under stressed conditions (i.e., fluid restriction, exercise in warm conditions, or illness) they are more likely to become dehydrated than their younger counterparts because of an attenuated thirst response and alterations in the renal response to fluid restriction (6, 14, 23). Therefore, mild to moderate dehydration is often suspected in a clinical setting (30), and commercially available carbohydrate (CHO)-based, electrolyte-containing oral rehydration solutions may be provided or recommended to promote rapid and sustained expansion of body fluids (16). However, little is known about renal handling of beverages differing in composition in this population in such settings.
Many commercially available hydration beverages are formulated with glucose and electrolytes, leveraging the glucose-sodium (Na+) cotransporter to increase water absorption in the small intestine (10). However, older adults may be less tolerant to glucose because of impairments in glucose handling and insulin action (2, 28) and are more susceptible to gastric distress because of age-related glucose malabsorption (8), and the additional calories may be unwarranted. Amino acid (AA)-based beverages may be similarly effective in promoting water and electrolyte transport into the blood, utilizing an AA-based Na+ cotransport mechanism to promote fluid absorption and retention (29). Indeed, we have recently demonstrated that the beverage hydration indexes (BHIs) of AA-based beverages containing sodium and potassium are similar to those of CHO-based beverages in older adults (3), suggesting that AA-based electrolyte beverages are retained as readily as CHO-based electrolyte beverages in healthy aged individuals. However, there has yet to be investigation into differences in renal handling of these differing beverage types.
Attenuated free water clearance (CH2O) has been demonstrated in older compared with young adults (5) after a standard oral water load of 20 ml/kg. Similarly, older adults exhibited delayed renal clearance of Na+ (CNa+) compared with their younger counterparts after infusions of hypotonic or hypertonic saline solutions (9). However, to date no research has been conducted to examine differences in CH2O and CNa+ in young and older adults after consumption of beverages varying in macronutrient and electrolyte content. Furthermore, it is unclear how age-related changes in CH2O and CNa+ may influence beverage retention after consumption.
Because of age-related alterations in renal fluid handling (5, 9, 19) and the paucity of research regarding optimization of oral rehydration solutions for older adults, the present study investigated differences in CH2O, CNa+, and beverage retention after consumption of 1 liter of water and four commercial CHO- or AA-based electrolyte rehydration beverages in young (18–30 yr) and older (60–85 yr) adults. We hypothesized that 1) increased fluid retention in older adults would be mediated by reduced CNa+ and/or CH2O, secondary to a reduced glomerular filtration rate (GFR), and 2) AA-based electrolyte beverages would be similarly efficacious to CHO-based electrolyte beverages in augmenting beverage retention.
METHODS
Study population.
This is a companion study to our paper examining BHI in young and older adults (3). On the basis of previous BHI data (20), we determined a priori (α = 0.05; power = 0.8) that 12 subjects per group would be sufficient to detect within- and between-group differences. Therefore, 24 healthy subjects participated in this study: 12 young subjects (7 men, 5 women) between the ages of 18 and 30 yr and 12 older subjects (5 men, 7 women) aged 60–85 yr. All subjects underwent a physical screening before participation that included anthropometric measurements, resting heart rate and blood pressure, and blood chemistry (Chem 24 panel). Subjects were excluded if they had existing or suspected renal, cardiovascular, metabolic, or prostate disease or if they were taking any medications that could alter fluid balance. Menstrual cycle phase is unlikely to influence urine output or fluid balance (25) and therefore was not controlled for. All procedures were approved by the Pennsylvania State University Institutional Review Board, and all subjects gave written and verbal informed consent before participation according to the Declaration of Helsinki. All testing was conducted at the Pennsylvania State University. The ClinicalTrials.gov Identifier for this study is NCT03559101.
Study design.
All 12 young subjects completed all trials. Ten older subjects completed all trials, one older subject completed all trials except enterade (ENT)-8, and one older subject completed all trials except Gatorade (GAT) and Pedialyte (PED). Subject groups were matched for weight and body mass index. The older group was significantly older by design (Table 1) and had a lower estimated GFR (eGFR; ml·min−1·1.73 m−2), calculated with the Modification of Diet in Renal Disease Study formula (17), compared with the young group.
Table 1.
Subject characteristics
| Young (7 men, 5 women) | Older (5 men, 7 women) | |
|---|---|---|
| Age, yr | 23 ± 3 | 68 ± 7* |
| Weight, kg | 73 ± 3 | 74 ± 4 |
| BMI, kg/m2 | 23 ± 1 | 26 ± 5 |
| eGFR, ml·min−1·1.73 m−2 | 94 ± 6 | 71 ± 3* |
| Serum Na+, mmol/l | 137 ± 0.2 | 138 ± 0.2 |
| Urine Na+, mmol/l | 70 ± 6.7 | 64 ± 4.1 |
| Sosm, mosmol/kg | 287 ± 0.5 | 293 ± 0.4 |
| Uosm, mosmol/kg | 569 ± 36 | 447 ± 26 |
Data are means ± SE. BMI, body mass index; eGFR, estimated glomerular filtration rate; Na+, sodium concentration; Sosm, serum osmolality; Uosm, urine osmolality. Serum values were calculated from pretest screening blood samples. Urine values were calculated from the “pre” time point urine samples of all trials.
P < 0.05 compared with young.
Subjects abstained from vigorous exercise for 24 h and from caffeine and alcohol intake for 12 h and underwent an overnight fast for at least 8 h before each experimental visit. All subjects provided a first morning urine sample in a sterile collection container. One hour before arriving at the laboratory, subjects drank 500 ml of spring water (Aquafina; PepsiCo, Purchase, NY) in a 15-min time period. Upon arrival at the laboratory, subjects voided their bladder into a 1-liter plastic urine container (“pre” time point for urine) and near-nude body mass was measured. Pretest euhydration was confirmed as urine specific gravity ≤ 1.020 (young: 1.015 ± 0.001; older: 1.012 ±0.001) (1). Subjects then sat for 10 min before an intravenous catheter was inserted into an antecubital vein. The subjects then began the 30-min drinking protocol, during which they drank 1 liter of a randomly assigned test beverage in four equal aliquots (0.25 liter every 7.5 min). After the subject consumed the test beverage, blood samples were collected 0, 30, 60, 120, 180, and 240 min after ingestion in two serum separator tubes (4 ml of blood each) to measure serum electrolytes and osmolality. Urine samples were collected in 1-liter urine containers after blood sampling at 0, 60, 120, 180, and 240 min after ingestion.
Test beverages.
Each participant completed the protocol five times, one for each test beverage, in a randomized study design with each trial separated by a minimum of 1 wk. The test beverages were distilled water, GAT (6% CHO, 0.460 g/l Na+, 0.125 g/l K+), ENT-5 (5 AAs, 0.736 g/l Na+, 0.390 g/l K+), PED (2.5% CHO, 1.035 g/l Na+, 0.780 g/l K+), and ENT-8 (8 AAs, 1.380 g/l Na+, 0.780 g/l K+). Beverage composition is presented in Table 2 and was recorded from the product labels. Beverage osmolality (in mosmol/kg: water 0 ± 0, GAT 326 ± 20, ENT-5 145 ± 1, PED 320 ± 1, ENT-8 221 ± 1) was measured in the laboratory. ENT-5 and ENT-8 are formulated with five (aspartic acid, serine, threonine, tyrosine, valine) and eight (aspartic acid, isoleucine, lysine, serine, threonine, tyrosine, valine, glycine) AAs, respectively, and both beverage formulas contain <7 g of AA/liter. All drinks were kept sealed and stored at 4–6°C until serving.
Table 2.
Beverage composition
| Beverage | CHO, g/l | Osmolality, mosmol/kg | Sodium, g/l | Potassium, g/l |
|---|---|---|---|---|
| Water | 0 | 0 ± 0 | 0 | 0 |
| GAT | 60 | 326 ± 20 | 0.460 | 0.125 |
| ENT-5 | 0 | 145 ± 1 | 0.736 | 0.390 |
| PED | 25 | 320 ± 1 | 1.035 | 0.780 |
| ENT-8 | 0 | 221 ± 1 | 1.380 | 0.780 |
Osmolality values are means ± SE. AA, amino acid; CHO, carbohydrates; ENT-5, enterade Advanced Oncology; ENT-8, enterade Anti-Diarrheal; GAT, Gatorade; PED, Pedialyte. AAs included in ENT-5: aspartic acid, serine, threonine, tyrosine, valine. AAs included in ENT-8: aspartic acid, isoleucine, lysine, serine, threonine, tyrosine, valine, glycine. Both AA-based beverages contain <7 g AA/l.
Urine and blood analyses.
Subjects voided completely into 1-liter plastic urine containers at each time point. If subjects needed to urinate between scheduled collection times, urine was collected and combined with the urine collection of the following hour. Urine mass was weighed to the nearest ±0.1 g with an electronic balance, and urine osmolality was measured at each time point in triplicate with a freezing-point osmometer (model 3320; Advanced Instruments, Norwood, MA; %CV = 1.1%). Serum separator tubes remained upright for 30 min so the serum had the opportunity to clot before centrifugation (10 min, 4°C, 4,000 rpm). One serum separator tube from each time point was analyzed (Quest Diagnostics, Pittsburgh, PA) for serum electrolyte concentrations, whereas the other remained in the laboratory for serum osmolality analysis (model 3320, Advanced Instruments).
Calculations.
Average plasma Na+ (PNa+; mmol/l), urine Na+ (UNa+; mmol/l), urine flow (V̇; ml/min), CNa+ (ml/min), CH2O (ml/min), and beverage retention (%) were calculated at 120 and 240 min after beverage consumption. For instances in which cumulative time point urine (TPU) is calculated, all urinary output up to and including that time point (i.e., 120 and 240 min) from 0 min is included. PNa+ was calculated as the average. UNa+ was calculated as the sum of the products of TPU Na+ concentration and TPU divided by 1,000, multiplied by cumulative urine divided by 1,000:
V̇ was calculated as cumulative TPU divided by the number of minutes after beverage consumption:
CNa+ was calculated at each time point as the product of UNa+ and V̇, divided by PNa+:
CH2O was calculated as the difference between V̇ and CNa+:
Additional calculations were made to adjust CNa+ and CH2O for eGFR at 120 and 240 min. CNa+ adjusted for eGFR (CNa+/eGFR; ml/min) was calculated for each time point by dividing CNa+ by the quotient of eGFR divided by 100:
Likewise, CH2O adjusted for eGFR (CH2O/eGFR; ml/min) was determined by dividing CH2O by the quotient of eGFR divided by 100:
Finally, beverage retention (%) was calculated for the 120 and 240 min time points as the difference between the initially ingested 1,000-ml fluid bolus and the cumulative urine volume (UV) at each respective time point, divided by 1,000, with the quotient being multiplied by 100:
Statistical analyses.
Student’s unpaired t-tests were used to compare subject characteristics. Data were analyzed with PROC MIXED three-way ANOVA (beverage × time × group) to detect within- and between-group differences (SAS 9.4, Cary, NC). Bonferroni post hoc corrections were performed to account for multiple comparisons in the ANOVA when necessary, and significance was set a priori and accepted at α = 0.05. Primary outcomes were CH2O, CNa+, and beverage retention. Data are presented as means ± SE.
RESULTS
ANOVA revealed significant group × beverage and group × time interactions for beverage retention, CH2O, CH2O adjusted for eGFR, and CNa+ (all P < 0.01). Additionally, time × beverage interaction effects were observed for beverage retention (P < 0.01), CH2O adjusted for eGFR (P = 0.03), and CNa+ (P = 0.04).
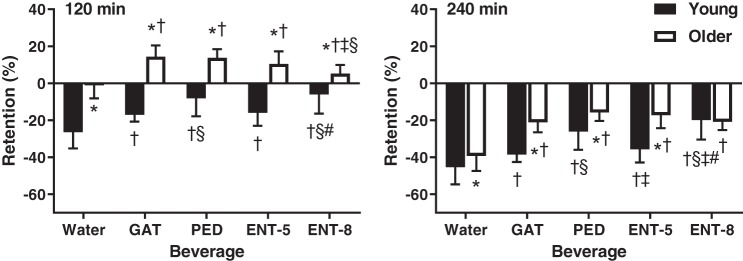
Figure 1 presents beverage retained as a percentage of the initial 1 liter consumed at the 120 and 240 min postconsumption time points. Beverage retention was higher in older compared with young adults 120 min after consumption of all five beverages (P < 0.01) and remained higher for all beverages (P < 0.01) except ENT-8 (P = 0.73) after 240 min. In young adults, fluid retention was lower for water compared with all four other beverages at 120 (P < 0.01) and 240 (P ≤ 0.02) min after consumption. ENT-8 and PED yielded similar retentions at 120 min (P = 0.45) after consumption, but beverage retention was greater for ENT-8 compared with PED at 240 min (P = 0.03). Beverage retention was augmented after consumption of ENT-8 or PED compared with all three other beverages at 120 and 240 min after consumption (P < 0.01). In older adults, beverage retention was attenuated for water compared with all four other beverages 120 min after consumption (P < 0.03) and remained lower at 240 min (P < 0.01). Beverage retention was lower 120 min after consumption of ENT-8 compared with GAT and PED (P < 0.01) but not ENT-5 (P = 0.06). Beverage retention was similar between GAT, PED, ENT-5, and ENT-8 (P > 0.05) at 240 min after consumption.
Fig. 1.
Beverage retention at 120 (left) and 240 (right) min after beverage consumption in young (n = 12; 7 men, 5 women) and older (n = 12; 5 men, 7 female) subjects. Values are means ± SE. Differences within and between groups were assessed by 3-way ANOVA, with Bonferroni post hoc analyses performed to correct for multiple comparisons. *P < 0.05 compared with young; †P < 0.05 compared with water; §P < 0.05 compared with Gatorade (GAT); ‡P < 0.05 compared with Pedialyte (PED); #P < 0.05 compared with enterade (ENT)-5.
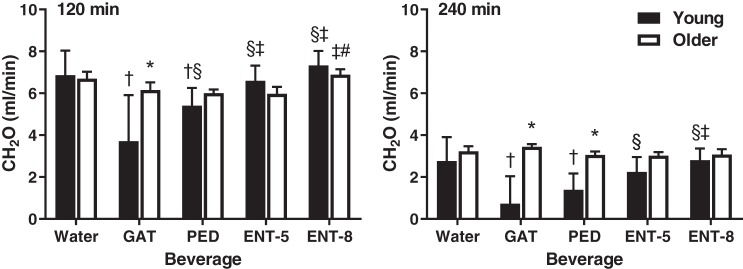
Figure 2 illustrates CH2O at 120 and 240 min after beverage consumption in young and older adults. CH2O was significantly higher in older compared with young adults at 120 (P < 0.01) and 240 (P < 0.01) min after consumption of GAT and at 240 min after consumption of PED (P < 0.01). Otherwise, no differences existed between young and older adults at 120 (P ≥ 0.16) or 240 (P ≥ 0.09) min. In young adults, GAT attenuated CH2O compared with water, PED, ENT-5, and ENT-8 at 120 min after consumption (all P < 0.01). CH2O remained lower 240 min after consumption of GAT compared with water, ENT-5, and ENT-8 (P < 0.01) but not PED (P = 0.14). PED resulted in a significantly lower CH2O compared with water, ENT-5, and ENT-8 (P < 0.01) at 120 min and water and ENT-8 (P < 0.01) at 240 min after consumption. CH2O was not different after consumption of water compared with ENT-5 or ENT-8 at either time point (P ≥ 0.25). In older adults, ENT-8 increased CH2O compared with PED (P = 0.05) and ENT-5 (P = 0.04) at 120 min after consumption, but there were otherwise no differences between beverages at either time point (P > 0.10).
Fig. 2.
Free water clearance (CH2O) at 120 (left) and 240 (right) min after beverage consumption in young (n = 12; 7 men, 5 women) and older (n = 12; 5 men, 7 women) subjects. Values are means ± SE. Differences within and between groups were assessed by 3-way ANOVA, with Bonferroni post hoc analyses performed to correct for multiple comparisons. *P < 0.05 compared with young; †P < 0.05 compared with water; §P < 0.05 compared with Gatorade (GAT); ‡P < 0.05 compared with Pedialyte (PED). #P < 0.05 compared with enterade (ENT)-5.
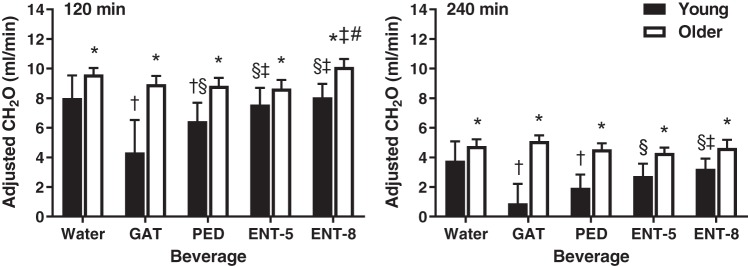
Figure 3 presents CH2O after adjustment for eGFR at 120 and 240 min after beverage consumption in young and older adults. Adjusting for eGFR resulted in significantly higher CH2O in older compared with young adults 120 and 240 min after consumption of all five beverages (P < 0.05). Otherwise, adjusting for eGFR did not influence the differences within groups that were observed before adjustment.
Fig. 3.
Free water clearance (CH2O) after adjusting for estimated glomerular filtration rate (eGFR) [CH2O/(eGFR/100)] at 120 (left) and 240 (right) min after beverage consumption in young (n = 12; 7 men, 5 women) and older (n = 12; 5 men, 7 women) subjects. Values are means ± SE. Differences within and between groups were assessed by 3-way ANOVA, with Bonferroni post hoc analyses performed to correct for multiple comparisons. *P < 0.05 compared with young; †P < 0.05 compared with water; §P < 0.05 compared with Gatorade (GAT); ‡P < 0.05 compared with Pedialyte (PED); #P < 0.05 compared with enterade (ENT)-5.
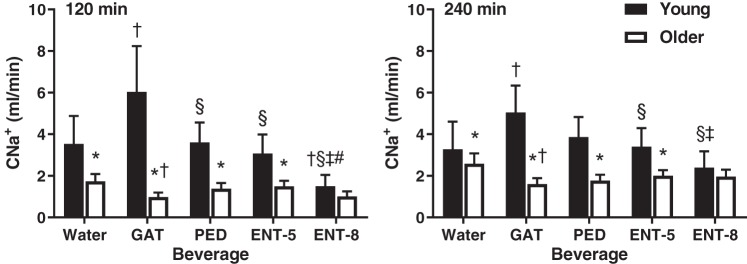
Figure 4 depicts CNa+ at 120 and 240 min after beverage consumption in young and older adults. CNa+ was lower in older compared with young adults 120 and 240 min after consumption of water, GAT, PED, and ENT-5 (P ≤ 0.01). CNa+ was not different between young and older adults 120 (P = 0.19) or 240 (P = 0.26) min after consumption of ENT-8. At 120 min, CNa+ in young adults was greater after consumption of GAT compared with all four other beverages (P < 0.01). Consumption of ENT-8 attenuated CNa+ compared with all other beverages (P < 0.01). There were no differences in CNa+ between water, PED, and ENT-5 (P ≥ 0.16). CNa+ in young adults remained higher 240 min after consumption of GAT compared with all other beverages (P < 0.01). Furthermore, CNa+ remained lower after consumption of ENT-8 compared with PED (P < 0.01) and ENT-5 (P < 0.01), whereas no differences were observed between water, PED, and ENT-5 (P ≥ 0.12). In older adults, CNa+ was lower after consumption of GAT at 120 (P = 0.05) and 240 (P = 0.01) min compared with water and at 240 min after consumption of PED compared with water (P = 0.03). Otherwise, there were no differences in CNa+ between beverages in older adults at either time point (P ≥ 0.06). Adjusting for eGFR did not have an impact on the differences observed between or within groups for CNa+ (not depicted). Importantly, serum Na+ was stable in both age groups across time and was similar between beverages (P = 0.88).
Fig. 4.
Sodium clearance (CNa+) at 120 (left) and 240 (right) min after beverage consumption in young (n = 12; 7 men, 5 women) and older (n = 12; 5 men, 7 women) subjects. Values are means ± SE. Differences within and between groups were assessed by 3-way ANOVA, with Bonferroni post hoc analyses performed to correct for multiple comparisons. *P < 0.05 compared with young; †P < 0.05 compared with water; §P < 0.05 compared with Gatorade (GAT); ‡P < 0.05 compared with Pedialyte (PED); #P < 0.05 compared with enterade (ENT)-5.
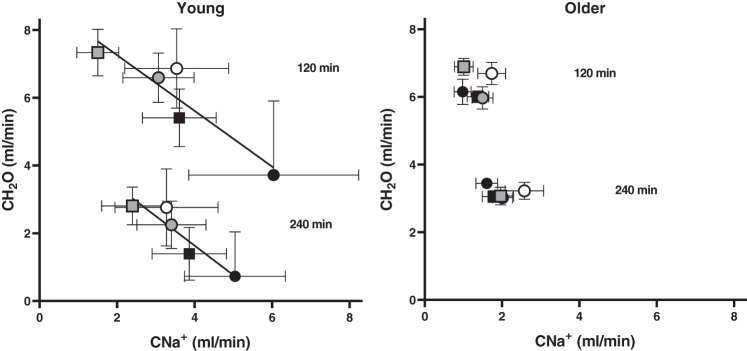
Figure 5 presents the association between CH2O and CNa+, an index of renal concentrating capacity, at 120 and 240 min in young and older adults. Younger participants displayed the classical inverse relation between CH2O and CNa+ at 120 min (R2 = 0.85, P = 0.03) and 240 min (R2 = 0.87, P = 0.02). However, CH2O and CNa+ were not related in the older cohort at either time point (120 min: R2 = 0.006, P = 0.90; 240 min: R2 = 0.03, P = 0.77), resulting in a dilute urine independent of the beverage solute load.
Fig. 5.
Relation between free water (CH2O) and sodium (CNa+) clearance at 120 and 240 min after beverage consumption in young (n = 12; 7 men, 5 women; left) and older (n = 12; 5 men, 7 women; right) subjects. Separate ordinary least-squares regression analyses assessed the correlation between CH2O and CNa+ at each time point for each group. CH2O and CNa+ were significantly related in young subjects at 120 min (R2 = 0.85, P = 0.03) and 240 min (R2 = 0.87, P = 0.02) but were not related in older subjects at either time point (120 min: R2 = 0.006, P = 0.90; 240 min: R2 = 0.03, P = 0.77). Open circles, water; black circles, Gatorade; black squares, Pedialyte; gray circles, enterade-5; gray squares, enterade-8.
DISCUSSION
Age-related differences.
The results of the present study indicate that older adults possess greater beverage retention compared with their younger counterparts. Augmented beverage retention in our older participants can be attributed to attenuated CNa+ observed in all beverages except ENT-8, independent of eGFR. CH2O was similar between young and older adults at 2 and 4 h after beverage consumption except for CHO-containing beverages, which elicited significantly lower CH2O in young compared with older subjects. When adjusted for eGFR, however, CH2O was significantly greater in older compared with young adults for all five beverages. Furthermore, CH2O and CNa+ were inversely related in young, but not older, adults. Taken together, these findings suggest that augmented beverage retention observed in older compared with young adults is likely due to the contribution of GFR and reduced Na+ excretion for any given beverage Na+ load.
CH2O has previously been reported to be lower at 2 h, but higher at 4 h, after ingestion of an oral water load of 20 ml/kg in older compared with young adults (5). Furthermore, the results of that study demonstrated that CH2O was greatest in the first 2 h after ingestion in young subjects but was delayed until after the first 2 h in older subjects. Our results differ in that CH2O was similar between older and young adults at 2 and 4 h after consumption, except for CHO-containing beverages, and that CH2O was lower at 4 h compared with 2 h after consumption in both groups for all beverages. The differences between our findings and those reported in the previous study may be explained by methodological differences; participants in the present study drank 1 liter of each test beverage, whereas subjects in the previous study consumed a bolus of water normalized to body mass. The weight range for young and older subjects in that study was 53–86 kg, and therefore all subjects drank a larger fluid volume (1,060–1,720 ml) compared with the present study. Therefore, it may be that the contrasting results are explained by differences in fluid loads between studies. After adjusting CH2O for eGFR, however, we observed greater CH2O in older subjects compared with their younger counterparts across all beverages because of reductions in eGFR in the older group. The reductions observed in eGFR in the present study are consistent with those previously reported (12, 18). Consistent with the reductions observed in eGFR in our aging subjects, CNa+ was blunted in older compared with young adults. Additionally, CNa+ was inversely related to CH2O in young subjects, but this relation was not observed in the older age group. These findings are in alignment with previous data (9) that demonstrated reductions in CNa+ in older compared with young subjects after infusion of either hypotonic (0.45%) or hypertonic (0.5%) saline solutions. Furthermore, older adults in the present study demonstrated augmented beverage retention compared with the young cohort, similar to previously reported findings (26). Because total urine volume is closely approximated by the sum of CH2O and CNa+, the greater fluid retention observed in older adults in the first 2 h after ingestion appears to be explained by reduced eGFR and CNa+. These results indicate that older adults may be better able to retain beverages with less salt compared with young adults.
Beverage differences in young adults.
In young adults, CNa+ was greater, whereas CH2O was reduced, after consumption of GAT compared with water. In contrast, CNa+ was lower, but CH2O was similar, after consumption of ENT-8 compared with water. Both CHO-containing beverages resulted in blunted CH2O compared with water, whereas CH2O was similar between water and the two AA-based beverages. Consistent with previous findings (20), these results demonstrate that beverage retention in young adults increases with increasing Na+ content, likely because of increased plasma Na+ concentration, resulting in augmented H2O retention in the vascular space (13, 24).
The increased CNa+ after ingestion of CHO-containing beverages in young adults in our study is in contrast to past data that have demonstrated a Na+-sparing effect of insulin (7, 21). The disparate findings in our study may be due to the fact that CNa+ was examined only at 120 and 240 min after ingestion, and therefore there may have been a period of antinatriuresis immediately following ingestion of CHO-containing beverages that was not observable within the time course we examined. Furthermore, because we do not have data for the insulin responses after ingestion of the five test beverages, the role of insulin in CNa+ or CH2O in our study is unclear. Future studies may seek to examine age-related differences in insulin-mediated renal Na+ handling after ingestion of macronutrient-containing beverages.
Beverage differences in older adults.
In older adults, CH2O was similar after water consumption compared with all four other beverages across the 4-h postconsumption time period. Beverage retention, however, was augmented compared with water in the first 2 h after beverage consumption, likely because of reductions in CNa+ (but P > 0.05) (27), after consumption of all CHO- and AA-based beverages. Furthermore, beverage retention was not different between the four macronutrient-containing beverages, despite differences in beverage Na+ load. Taken together, these results suggest that beverage retention requires less salt in older compared with young adults and that AA-based beverages are equally as effective as those containing CHO for this population.
Limitations.
The AA-based beverages included in this study consisted of proprietary blends of AAs. Although the AA concentrations are presently undisclosed, the total quantity of AAs is <7 g/l in both ENT formulations. It may be that varying AA concentrations between beverages influence their hydrating properties (i.e., 5 vs. 8 AAs). Indeed, varying concentrations of glucose have been demonstrated to impact rates of gastric emptying and intestinal absorption, and therefore to influence the fluid uptake kinetics between CHO-containing beverages (4, 15). Although the AA concentrations are unknown in the AA-based beverages in the present study, the electrolyte concentration and osmolality, known to play important roles in fluid absorption (15), are known and presented for each beverage. Furthermore, the specific contributions of CHO, AA, and electrolytes to the observed physiological responses are unclear, as we were unable to control individual beverage components. However, because the test beverages are available for consumer use, these findings are practically and clinically relevant. Future studies may be warranted to elucidate whether differences in efficacy between AA-based beverages are associated with AA concentrations per se.
Summary and conclusions.
In summary, age-related changes in renal handling of ingested fluids resulted in greater fluid retention in older adults in the first 2 h after beverage consumption due to attenuated GFR and CNa+. The two CHO-based beverages and beverages with higher Na+ concentrations resulted in reduced CH2O and improved fluid retention (i.e., less net fluid excretion) compared with water in young adults, likely a function of increased plasma Na+ and CNa+ that attenuated free H2O losses. AA- and CHO-based beverages similarly augmented beverage retention in older adults compared with water, likely because of reduced CNa+ despite differences in beverage Na+ content. Taken together, these results suggest that older adults require less salt compared with young adults to retain fluids and that AA-based beverages are as effective as those containing CHO. Further investigation is warranted to elucidate the influence of beverage composition on fluid uptake kinetics from stomach, to intestine, to kidney with a tracer such as deuterium (11).
GRANTS
This research was supported by National Institute on Aging Grant T32 AG-049676-03 (S. T. Wolf).
DISCLAIMERS
The opinions or assertions contained herein are the private views of the authors and should not be construed as official or reflecting the views of the Army or the Department of Defense. Any citations of commercial products, organizations, and trade names in this report do not constitute an official Department of the Army endorsement of approval of the products or services of these organizations. Approved for public release: distribution unlimited.
DISCLOSURES
This study was partially funded by an unrestricted gift from Entrinsic Health Solutions, Inc.
AUTHOR CONTRIBUTIONS
S.N.C., R.W.K., and W.L.K. conceived and designed research; S.T.W. and M.M.C. performed experiments; S.T.W., A.E.S., M.M.C., S.N.C., R.W.K., and W.L.K. analyzed data; S.T.W., A.E.S., S.N.C., R.W.K., and W.L.K. interpreted results of experiments; S.T.W. prepared figures; S.T.W. drafted manuscript; S.T.W., A.E.S., M.M.C., S.N.C., R.W.K., and W.L.K. edited and revised manuscript; S.T.W., A.E.S., M.M.C., S.N.C., R.W.K., and W.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are thankful for the subjects’ participation and for the assistance of Susan Slimak and Jane Pierzga.
REFERENCES
- 1.American College of Sports Medicine, Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39: 377–390, 2007. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 2.Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 52: 1738–1748, 2003. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 3.Clarke MM, Stanhewicz AE, Wolf ST, Cheuvront SN, Kenefick RW, Kenney WL. A randomized trial to assess beverage hydration index in healthy older adults. Am J Clin Nutr. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costill DL, Saltin B. Factors limiting gastric emptying during rest and exercise. J Appl Physiol 37: 679–683, 1974. doi: 10.1152/jappl.1974.37.5.679. [DOI] [PubMed] [Google Scholar]
- 5.Crowe MJ, Forsling ML, Rolls BJ, Phillips PA, Ledingham JG, Smith RF. Altered water excretion in healthy elderly men. Age Ageing 16: 285–293, 1987. doi: 10.1093/ageing/16.5.285. [DOI] [PubMed] [Google Scholar]
- 6.de Castro JM. Age-related changes in natural spontaneous fluid ingestion and thirst in humans. J Gerontol 47: P321–P330, 1992. doi: 10.1093/geronj/47.5.P321. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 55: 845–855, 1975. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drozdowski L, Woudstra T, Wild G, Clandindin MT, Thomson AB. The age-associated decline in the intestinal uptake of glucose is not accompanied by changes in the mRNA or protein abundance of SGLT1. Mech Ageing Dev 124: 1035–1045, 2003. doi: 10.1016/j.mad.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Fish LC, Murphy DJ, Elahi D, Minaker KL. Renal sodium excretion in normal aging: Decreased excretion rates lead to delayed handling of sodium loads. Geriatr Nephrol Urol 4: 145–151, 1994. doi: 10.1007/BF01523974. [DOI] [Google Scholar]
- 10.Fordtran JS. Stimulation of active and passive sodium absorption by sugars in the human jejunum. J Clin Invest 55: 728–737, 1975. doi: 10.1172/JCI107983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill RJ, Bluck LJ, Davies PS. The hydration ability of three commercially available sports drinks and water. J Sci Med Sport 11: 116–123, 2008. doi: 10.1016/j.jsams.2006.12.117. [DOI] [PubMed] [Google Scholar]
- 12.Hoang K, Tan JC, Derby G, Blouch KL, Masek M, Ma I, Lemley KV, Myers BD. Determinants of glomerular hypofiltration in aging humans. Kidney Int 64: 1417–1424, 2003. doi: 10.1046/j.1523-1755.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 13.Kamijo Y, Ikegawa S, Okada Y, Masuki S, Okazaki K, Uchida K, Sakurai M, Nose H. Enhanced renal Na+ reabsorption by carbohydrate in beverages during restitution from thermal and exercise-induced dehydration in men. Am J Physiol Regul Integr Comp Physiol 303: R824–R833, 2012. doi: 10.1152/ajpregu.00588.2011. [DOI] [PubMed] [Google Scholar]
- 14.Kenney WL, Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exerc 33: 1524–1532, 2001. doi: 10.1097/00005768-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Leiper JB. Fate of ingested fluids: factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr Rev 73, Suppl 2: 57–72, 2015. doi: 10.1093/nutrit/nuv032. [DOI] [PubMed] [Google Scholar]
- 16.Leiper JB. Intestinal water absorption—implications for the formulation of rehydration solutions. Int J Sports Med 19, Suppl 2: S129–S132, 1998. doi: 10.1055/s-2007-971977. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130: 461–470, 1999. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 19.Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol (1985) 76: 1615–1623, 1994. doi: 10.1152/jappl.1994.76.4.1615. [DOI] [PubMed] [Google Scholar]
- 20.Maughan RJ, Watson P, Cordery PA, Walsh NP, Oliver SJ, Dolci A, Rodriguez-Sanchez N, Galloway SD. A randomized trial to assess the potential of different beverages to affect hydration status: development of a beverage hydration index. Am J Clin Nutr 103: 717–723, 2016. doi: 10.3945/ajcn.115.114769. [DOI] [PubMed] [Google Scholar]
- 21.Miller JH, Bogdonoff MD. Antidiuresis associated with administration of insulin. J Appl Physiol 6: 509–512, 1954. doi: 10.1152/jappl.1954.6.8.509. [DOI] [PubMed] [Google Scholar]
- 22.Ritz P, Berrut G. The importance of good hydration for day-to-day health. Nutr Rev 63: S6–S13, 2005. doi: 10.1111/j.1753-4887.2005.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 23.Rowe JW, Shock NW, DeFronzo RA. The influence of age on the renal response to water deprivation in man. Nephron 17: 270–278, 1976. doi: 10.1159/000180731. [DOI] [PubMed] [Google Scholar]
- 24.Shi X, Summers RW, Schedl HP, Chang RT, Lambert GP, Gisolfi CV. Effects of solution osmolality on absorption of select fluid replacement solutions in human duodenojejunum. J Appl Physiol (1985) 77: 1178–1184, 1994. doi: 10.1152/jappl.1994.77.3.1178. [DOI] [PubMed] [Google Scholar]
- 25.Sollanek KJ, Tsurumoto M, Vidyasagar S, Kenefick RW, Cheuvront SN. Neither body mass nor sex influences beverage hydration index outcomes during randomized trial when comparing 3 commercial beverages. Am J Clin Nutr 107: 544–549, 2018. doi: 10.1093/ajcn/nqy005. [DOI] [PubMed] [Google Scholar]
- 26.Stachenfeld NS, Mack GW, Takamata A, DiPietro L, Nadel ER. Thirst and fluid regulatory responses to hypertonicity in older adults. Am J Physiol Regul Integr Comp Physiol 271: R757–R765, 1996. doi: 10.1152/ajpregu.1996.271.3.R757. [DOI] [PubMed] [Google Scholar]
- 27.Stanhewicz AE, Kenney WL. Determinants of water and sodium intake and output. Nutr Rev 73, Suppl 2: 73–82, 2015. doi: 10.1093/nutrit/nuv033. [DOI] [PubMed] [Google Scholar]
- 28.Stolk RP, Pols HA, Lamberts SW, de Jong PT, Hofman A, Grobbee DE. Diabetes mellitus, impaired glucose tolerance, and hyperinsulinemia in an elderly population. The Rotterdam Study. Am J Epidemiol 145: 24–32, 1997. doi: 10.1093/oxfordjournals.aje.a009028. [DOI] [PubMed] [Google Scholar]
- 29.Tai CY, Joy JM, Falcone PH, Carson LR, Mosman MM, Straight JL, Oury SL, Mendez C Jr, Loveridge NJ, Kim MP, Moon JR. An amino acid-electrolyte beverage may increase cellular rehydration relative to carbohydrate-electrolyte and flavored water beverages. Nutr J 13: 47, 2014. doi: 10.1186/1475-2891-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas DR, Cote TR, Lawhorne L, Levenson SA, Rubenstein LZ, Smith DA, Stefanacci RG, Tangalos EG, Morley JE; Dehydration Council . Understanding clinical dehydration and its treatment. J Am Med Dir Assoc 9: 292–301, 2008. doi: 10.1016/j.jamda.2008.03.006. [DOI] [PubMed] [Google Scholar]