Abstract
Advanced glycation end-products (AGEs) are formed in vivo from the nonenzymatic reaction between sugars and proteins. AGEs accumulate in long-lived tissues like tendons, cross-linking neighboring collagen molecules, and are in part complicit in connective tissue pathologies experienced in aging and with diabetes. We have previously described discrete plasticity: a characteristic form of nanoscale collagen fibril damage consisting of serial fibril kinking and collagen denaturation that occurs in some mechanically overloaded tendons. We suspect that this failure mechanism may be an adaptive trait of collagen fibrils and have published evidence that inflammatory cells may be able to recognize and digest the denatured collagen produced by overload. In this study, we treated bovine tail tendons with ribose to simulate long-term AGE cross-linking in vitro. We hypothesized that a high degree of cross-linking would inhibit the intermolecular sliding thought to be necessary for discrete plasticity to occur. Tendons were mechanically overloaded, and properties were investigated by differential scanning calorimetry and scanning election microscopy. Ribose cross-linking treatment altered the mechanical response of tendons after the yield point, significantly decreasing postyield extensibility and strain energy capacity before rupture. Coincident with altered mechanics, ribose cross-linking completely inhibited the discrete plasticity failure mechanism of tendon. Our results suggest that discrete plasticity, which may be an important physiological mechanism, becomes pathologically disabled by the formation of AGE cross-links in aging and diabetes.
NEW & NOTEWORTHY We have previously shown that mechanically overloaded collagen fibrils in mammalian tendons accrue nanoscaled damage. This includes development of a characteristic kinking morphology within a shell of denatured collagen: discrete plasticity. Here, using a ribose-incubation model, we show that advanced glycation end-product cross-linking associated with aging and diabetes completely inhibits this mechanism. Since discrete plasticity appears to cue cellular remodeling, this result has important implications for diabetic tendinopathy.
Keywords: advanced glycation end-products cross-linking, diabetes, discrete plasticity, mechanical damage, tendon overload and injury
INTRODUCTION
The World Health Organization reports that 1.5 million deaths per year are directly attributable to diabetes and forecasts that this disease will become the 7th leading cause of death by the year 2030 (45a). The current overall disease burden is estimated to be a staggering 89 million lost person-years of productivity and health due to pathology and early death (45a, 31). Global prevalence of diabetes is now over 9% in adults, 90% of which is attributable to type 2 diabetes (4). A defining characteristic of type 2 diabetes is insulin resistance, in which the ability of cells to uptake glucose is impaired, resulting in chronic hyperglycemia. This exposes tissues to abnormally high and protracted glucose concentrations (11).
A major structural consequence of hyperglycemia is an increase in the accumulation of advanced glycation end-products (AGEs) in collagenous tissues (3). AGEs are hydrolytically stable linkages that begin with a nonenzymatic reaction between reducing sugars and free amines on proteins. Whereas enzymatic cross-links must bridge between the telopeptide and helical regions of neighboring collagen molecules, the nonspecific reactions involving sugars occur haphazardly and therefore can form physiologically abnormal helix-to-helix AGE cross-links (30). Modeling studies have identified 14 lysine-arginine pairs that are susceptible to glycation cross-linking (17). Interestingly, some of the most predisposed sites are associated with regions of the collagen molecule that are involved in cellular, enzymatic, and glycosaminoglycan binding sites. It is known that connective tissue pathologies may arise from type 2 diabetes (6) and that the elderly suffer increased risk of soft tissue injuries (9, 13, 14). However, the mechanistic connection between AGE accumulation on collagen and an elevated risk of soft tissue injury has not been fully elucidated.
In previous work, we have shown that both rupture and repeated subrupture overload of physiologically normal tendons produce a characteristic nanoscale damage motif in collagen fibrils termed “discrete plasticity.” Discrete plasticity is characterized by serial kinking along the length of collagen fibrils, accompanied by local denaturation of helical collagen (27, 38, 40, 41, 43). We suspect that the overload-induced kinks occur at specific sites of heterogeneity in fibril structure, either stochastic or systematic, where local, noncatastrophic failure may sacrificially absorb strain energy and help to prevent complete fibril rupture.
In addition to discrete plasticity’s role in preventing overload-induced rupture of collagen fibrils, we have also shown that cells may recognize this damage motif. Veres et al. (37) found that macrophage-like U937 cells cultured on fibrils with discrete plasticity were morphologically distinct from those on undamaged fibrils. On damaged collagen, the cells displayed ruffled membranes, indicative of active phagocytosis (42), and flattened out, increasing their contact area with the substrate (37). High-magnification electron micrographs showed pericellular enzymatic fibril degradation of only the damaged fibrils. These findings suggest that discrete plasticity may play an important role in the identification of damaged collagen fibrils, initiation of degradation, and perhaps subsequent repair or replacement. Since discrete plasticity may be important to both mechanical integrity and healing ability, inhibition of this mechanism by chemical and structural alternations like the accumulation of AGEs may have consequent impact on tissue function and health.
In this study, tendons from adult cattle were cross-linked by AGEs in vitro to simulate tissue affected by hyperglycemia. Previous work has shown that an elevated concentration of AGE cross-links affects mechanical behavior of tendons, rendering them stiffer and more brittle (6). We suspect that, at the nanoscale, cross-links may lock neighboring helices together and thereby impede intermolecular sliding. Since molecular slippage is likely required for the discrete plasticity failure mechanism, we hypothesized that AGE cross-linking would inhibit this mechanism. In this paper we show that the mechanism is suppressed entirely.
METHODS
All chemicals were purchased from Sigma-Aldrich (Oakville, ON or Milwaukee, WI).
Sample sourcing and preparation.
Bovine tails from steers killed for food, and aged between 18 and 30 mo (as determined by dentition in consultation with the provincially appointed inspector), were collected immediately after slaughter at a local abattoir. Tendons were dissected from the dorsal, proximal region of each tail, laid straight between sheets of gauze moistened with phosphate-buffered saline (PBS; pH 7.4), double bagged, and stored at −86°C for up to 4 wk. When ready for use, tendons were allowed to thaw in the sealed bags at room temperature. Previous work has shown that this method of storage does not alter the response of tendon collagen fibrils to overload (7, 38).
Tendons underwent a decellularization procedure as described by Ariganello et al. (5) to produce sterile samples of nearly pure collagen. Briefly, tendons were exposed to a hypotonic solution to lyse cells. This solution contained protease inhibitors (phenylmethylsulfonyl fluoride and ethylenediaminetetraacetic acid) and an antibiotic/antimycotic mixture consisting of 100 units/ml of penicillin, 0.1 mg/ml streptomycin, and 0.25 μg/ml of amphotericin B. After lysis, cellular debris was extracted with surfactant washes using Triton X-100. Samples then underwent a DNase/RNase treatment with subsequent surfactant washes performed in a biosafety cabinet. The decellularized, sterile samples were then stored in PBS with 1% antibiotic at 4°C for a maximum of 1 wk.
In total, 20 tendons dissected from 6 steer tails were used for the current study. All tendons underwent storage and decellularization treatment as described above. The tendons were cut into 4-cm-long samples and used in four experimental groups. Group 1 was ribose-cross-linked and overloaded samples (n = 8): these samples underwent ribose cross-linking treatment and then cyclic subrupture overload. They were taken from eight tendons, four each from animals one and two. Group 2 was untreated and overloaded samples (n = 8): these samples underwent cyclic subrupture overload without having undergone cross-linking treatment. They were taken from the same eight tendons used for the ribose-cross-linked and overloaded samples in group 1. Group 3 was ribose-cross-linked and unloaded samples (n = 4): these samples underwent ribose cross-linking but remained unloaded. They were taken from four tendons, two each from animals 3 and 4. Group 4 was untreated and unloaded samples (n = 8): these samples did not undergo cross-linking treatment, and remained unloaded. They were taken from eight tendons, four each from animals 5 and 6.
Ribose glycation cross-linking.
Tendon samples in the ribose cross-linked groups were blotted dry to remove surface water, weighed, and incubated for 28 days in 0.2 M ribose solution in PBS at 35°C. This technique was similar to previously described methods (22, 25, 35). Samples in the untreated, overloaded group were incubated under the same conditions but in the absence of ribose. To prevent the tendon samples from curling during this time, narrow incubation vessels were used, and the solutions were not stirred during incubation. No precipitate, crystallization, or other substance growth was observed on the tendons or vessel walls. After incubation, samples were rinsed with deionized water, blotted dry, and reweighed.
Mechanical overload procedure.
Digital pictures of the ribose cross-linked and untreated tendon samples to be mechanically overloaded were captured for later calculation of their cross-sectional areas with an elliptical fit, as previously described (38, 41). This involved vertically suspending each tendon from one of its ends using a small binder clip, with the tendon hanging freely under its own weight in plane with a scale bar. When the clip was applied, the light clamping force and clip’s smooth surfaces caused tendons to rotate with major diameter parallel to the clip and the imaging plane. Tendon samples were photographed four times with a 12-megapixel digital camera at 90° intervals of axial rotation. In each photo, the diameter of the sample was measured at the midpoint of its length using ImageJ (version 1.46r; National Instittues of Health) by taking the distance between edges. The four photos gave two measurements of the major diameter, and two of the minor diameter, which were used to calculate each sample’s elliptical cross-sectional area. This was done for each of the 16 samples (8 untreated samples and 8 ribose-cross-linked samples).
With the use of a servohydraulic materials testing system (MTS 458-series; MTS, Eden Prairie, MN) and custom-written LabVIEW software (version 6.1, National Instruments), tendon samples were subjected repeated cycles of tensile subrupture overload (Fig. 1), a method previously demonstrated to cause significant discrete plasticity and molecular denaturation in native tendon collagen (40). Each tendon sample was mounted between two servohydraulic actuators using crush grips. One of the actuators was then retracted until a first increase in load was observed via a load cell positioned in series between the sample and the opposite actuator. The actuator was then extended very slightly to just restore zero load. The intergrip distance was measured with a digital caliper and recorded as the gauge length. Intergrip gauge lengths across the two sample groups ranged from 10 to 15 mm. Under software control, each sample was then elongated at a strain rate of 1%/s while the instantaneous slope of the sample’s load-deformation response was calculated in real time. Loading continued until the slope of the load-deformation curve reached zero, well into plastic deformation. The actuator then reversed direction, returning at the same rate to the zero extension position. The next loading cycle then began. Untreated samples each underwent 15 subrupture overload cycles. Untreated samples displayed a smooth decrease in modulus to zero slope during each overload cycle; however, ribose-treated samples showed little to no plasticity and abrupt drops in load occurred further into cyclic loading (Fig. 2). To avoid these samples rupturing, cyclic overload was restricted to 10 cycles for 7 of the 8 ribose-treated samples, with a single sample receiving 11 cycles. During the mechanical overload procedure samples were kept hydrated via regular application of PBS droplets.
Fig. 1.
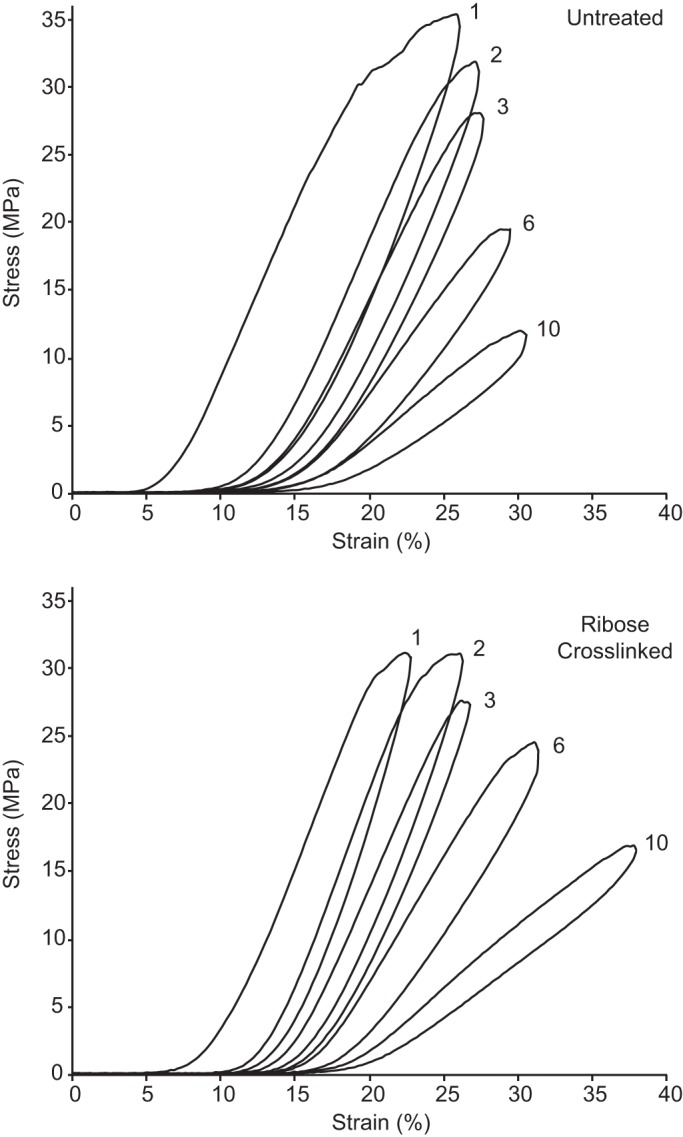
Representative subrupture overload stress-strain curves for an untreated tendon sample and matched-pair ribose cross-linked sample. For clarity, only cycles 1–3, 6, and 10 are shown. The untreated sample shows a characteristically longer postyield (plastic) region, with more energy dissipation after the yield point.
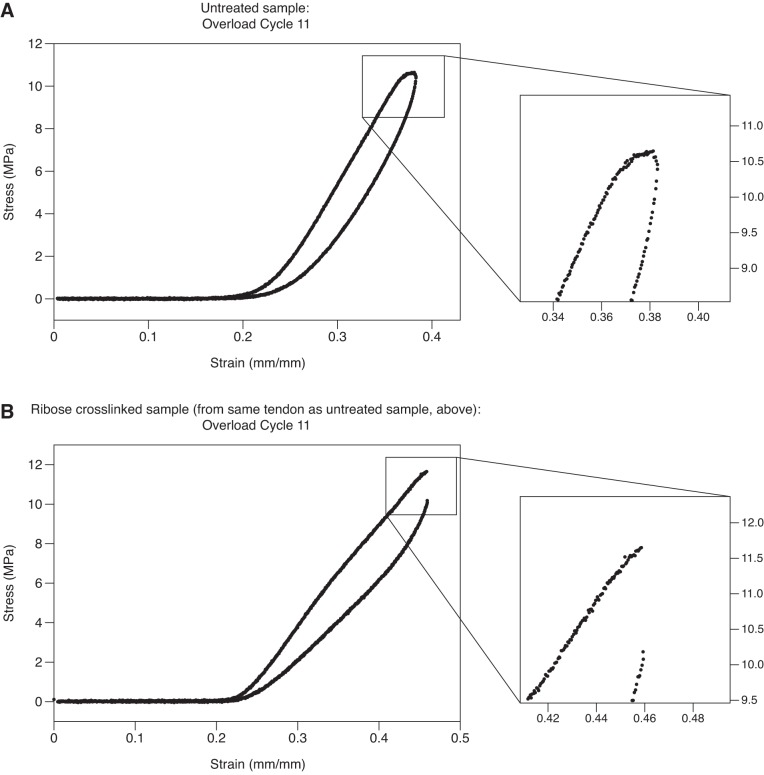
Fig. 2.
A and B: stress-strain curves for the 11th subrupture overload cycle are shown for 2 samples from the same tendon: one untreated (A) and the other after ribose cross-linking (B). A: in the untreated sample, the slope of the stress-strain curve gradually goes to zero. B: ribose cross-linking decreased plasticity. Instead of the stress-strain curve gradually going to zero, load suddenly drops, indicative of fiber rupture within the sample.
After mechanical overload, the gripped ends of each sample were cut off and discarded. Mechanical overloading thereby yielded: 1) n = 8 untreated, overloaded samples; and 2) n = 8 pair-matched, ribose cross-linked, overloaded samples. The overloaded (intergrip) portion of each sample was then bisected laterally with a razor blade, producing one sample for differential scanning calorimetry (DSC) and another for scanning election microscopy (SEM). Both DSC and SEM samples were stored overnight at 4°C in solution (PBS for the DSC sample and dH2O for the SEM sample).
Analysis of mechanical overload data.
Mechanical overload data were analyzed using Microsoft Excel (Excel for Mac, version 16.12). Deformation data were converted to strain by dividing by each sample’s intergrip length measured before the start of testing. Load data were converted to stress by dividing by each sample’s initial cross-sectional area. A variety of parameters were extracted from the cyclic stress-strain response of each overloaded tendon (Fig. 3). These included: linear modulus, yield stress and strain, maximum stress and corresponding strain, total strain after the yield point (εPY, where PY stands for “postyield”), resilience (area under the loading curve up to the yield point), strain energy density (area under the whole loading curve), hysteresis (area between loading and unloading curves), strain energy density after the yield point (“PY Energy”), and the portion of the hysteresis loop occurring after the yield point (“PY Hysteresis”).
Fig. 3.
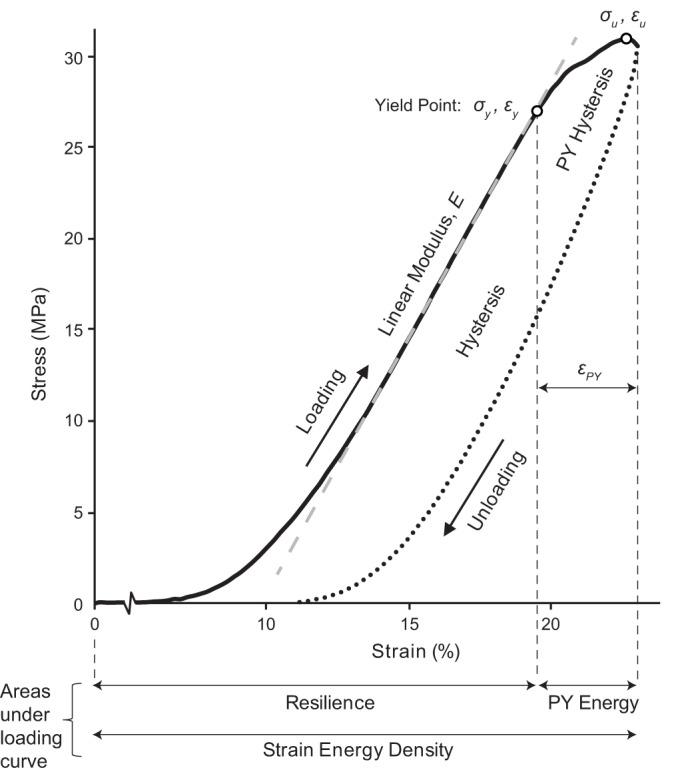
Mechanical properties calculated for each overload cycle included: linear modulus (E), yield stress and strain (σy, εy), maximum stress and corresponding strain (σu, εu), postyield strain (εPY), resilience, strain energy density, postyield strain energy density (PY Energy), hysteresis (full area between loading and unloading curves), and postyield hysteresis (PY Hysteresis; portion of the hysteresis loop after the yield point). The curve shown is the first overload cycle for a ribose cross-linked tendon.
Differential scanning calorimetry.
Samples were assessed using DSC to provide a quantitative description of collagen denaturation. A small length of each tendon sample was cut off and then longitudinally bisected to produce an ~5-mg subsample that incorporated half of the tendon’s cross section. These subsamples were blotted on weigh paper to remove excess surface water and weighed. With the use of blunt forceps, the longitudinal cut face of each subsample was gently pressed into the bottom of an aluminum DSC pan to maximize the contact area between pan and sample. Sample pans were hermetically sealed and run against an empty sealed pan. Calorimetry was performed using a Q200 differential scanning calorimeter with accuracy of 0.1°C (TA Instruments, New Castle, DE). In the instrument, samples were equilibrated at 20°C, and then ramped to 90°C at 10°C/min. Data were recorded at 10 Hz.
DSC endotherms were analyzed using Universal Analysis 2000 software (version 4.5A; TA Instruments) for the following descriptors of collagen denaturation: onset temperature (Tonset), peak temperature (Tpeak), and full-width at half-maximum (FWHM). Under the analysis software, a flat baseline was drawn across each sample’s endotherm. Tonset, calculated as the intersection between the baseline and a tangent line drawn at the endotherm’s inflection point (Fig. 4), describes the temperature at which thermal denaturation of the tendon collagen begins. Tpeak, the location of the endotherm’s peak, describes the temperature at which the heat flow producing collagen denaturation is maximal. FWHM, calculated as the endotherm’s width at half the distance from baseline to peak, is a descriptor for the heterogeneity of molecular thermal stabilities in the sample.
Fig. 4.
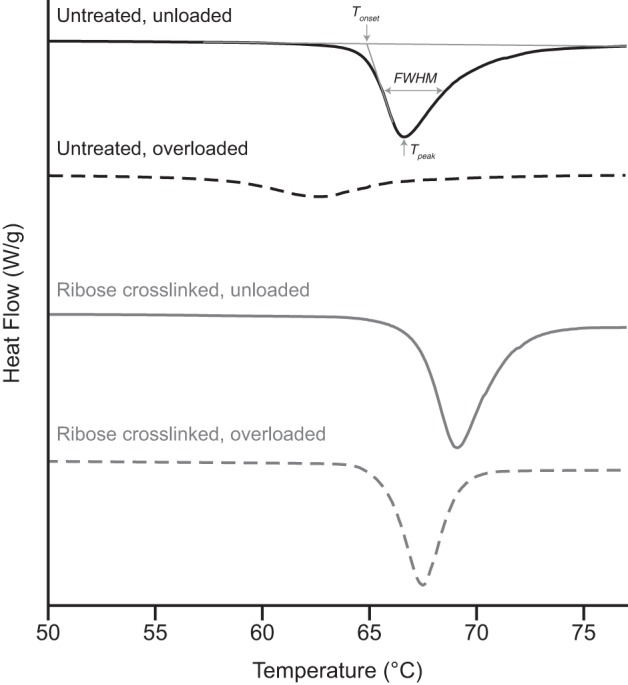
Representative differential scanning calorimetry endotherms for the study’s 4 sample groups. For untreated bovine tail tendon, mechanical overload caused substantial molecular packing disruption, shifting the endotherm to the left and broadening it significantly. While unloaded tendon showed marginal stabilization following ribose cross-linking, ribose cross-linking substantially altered the tendon response to overload, eliminating most of the change seen in untreated tendon.
Scanning electron microscopy.
Nanoscale assessment of collagen fibril morphology was conducted under SEM using a Hitachi S-4700 (Hitachi, Chula Vista, CA) operating at 3 kV, 15 μA, and magnifications up to 130,000×. Samples for SEM were fixed in SEM-grade glutaraldehyde, dehydrated in graded ethanol, and then cut longitudinally to expose the internal structure. The samples were critical point dried and mounted on SEM stubs using carbon tape with each sample’s longitudinally cut exposed interior surface facing upward for imaging. Mounted samples were coated with gold-palladium for 80 s at 50 mA (Leica EM ACE200, Wetzlar, Germany).
Statistical methods.
Values are presented as means ± SD. Statistical analyses were conducted using JMP software (version 13.2.0, SAS, NC). SDs on differences between sample groups were calculated using the method of Ku (23). Data were checked for normality using Shapiro-Wilk tests. Mechanical data for the untreated versus ribose cross-linked samples were compared using matched-pair t-tests, except for the analysis of PY Energy where some data were not well fit using a normal distribution and a matched-pair Wilcoxon signed rank test was therefore used instead. DSC data, for which there were two factors, loading history (unloaded vs. overload) and treatment group (untreated vs. ribose cross-linked), were first analyzed using a full-factorial two-way ANOVA, followed by t-tests between individual sample groups and matched-pair t-tests between the untreated overload and ribose cross-linked overload groups. For SEM data, the effect of treatment on collagen fibril response to mechanical overload was determined using Fisher’s exact test for the presence or absence of discrete plasticity. Statistical significance is reported for P ≤ 0.05; NS indicates comparisons that were not significant.
RESULTS
Effect of AGE cross-linking on the molecular stability of tendon collagen.
The ribose treatment induced a striking color change: the originally pearl-white tendons had yellowed notably by 14 days and were a golden yellow color by the end of the 28-day incubation (Fig. 5), consistent with clinical experience with tissues in diabetic patients (33).
Fig. 5.
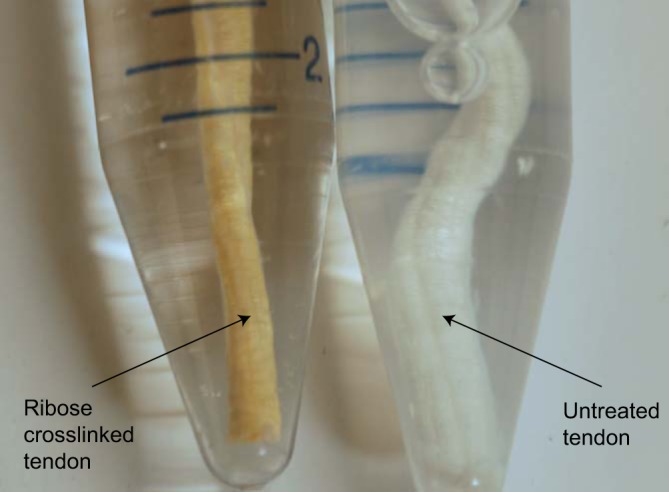
This representative image shows the color change caused in bovine tail tendon by the 28-day incubation with ribose used to induce advanced glycation end-product cross-links.
DSC analysis of the unloaded samples showed that ribose treatment had a slight but significant stabilizing effect on the collagen molecules in tendon (Fig. 4; Table 1). Ribose cross-linking increased the denaturation temperatures (Tonset and Tpeak) by ~1.5°C and narrowed the FWHM by ~1°C.
Table 1.
Effect of ribose cross-linking on the DSC response of unloaded bovine tail tendon
| DSC Parameters,°C | Change After Ribose Cross-Linking | P |
|---|---|---|
| Tonset | +1.7 ± 0.5 | <0.0001 |
| Tpeak | +1.6 ± 0.5 | <0.0001 |
| FWHM | −0.8 ± 0.5 | 0.013 |
Values are means ± SD. Ribose cross-linking had a small but significant effect on the thermal stability of tendon collagen when compared with untreated samples. DSC, differential scanning calorimetry; Tonset, onset temperature; Tpeak, peak temperature; FWHM, and full-width at half-maximum.
AGE cross-linking alters the postyield mechanical behavior of tendon.
Representative stress-strain curves for both untreated and ribose-cross-linked tendon are shown in Fig. 1. Mechanical parameters extracted from the first and tenth overload cycles for the two sample groups are presented in Table 2. Mean cross-sectional area for the eight untreated samples was 4.8 ± 1.1 mm2 and was unchanged for the eight matching ribose-cross-linked samples taken from the same tendons, which also measured 4.8 ± 1.1 mm2.
Table 2.
Mechanical data from overload cycles 1 and 10 for untreated and ribose cross-linked tendon
|
Cycle 1 |
Cycle 10 |
|||||
|---|---|---|---|---|---|---|
| Parameters | Untreated | Ribose Cross-linked | P | Untreated | Ribose Cross-linked | P |
| Tensile parameters | ||||||
| E, MPa | 239 ± 83 | 246 ± 70 | NS | 85 ± 12 | 64 ± 28 | NS |
| σy, MPa | 22.8 ± 6.8 | 25.6 ± 4.9 | NS | 12.2 ± 2.8 | 10.6 ± 5.3 | NS |
| εy, % | 18.1 ± 3.2 | 21.4 ± 5.4 | NS | 33.6 ± 6.8 | 39.1 ± 7.6 | NS |
| σu, MPa | 33.8 ± 10.1 | 30.6 ± 4.1 | NS | 13.1 ± 2.9 | 11.3 ± 5.5 | NS |
| εu, % | 26.6 ± 2.8 | 26.0 ± 5.9 | NS | 35.4 ± 6.7 | 40.7 ± 7.7 | NS |
| εPY, % | 8.9 ± 1.7 | 4.1 ± 1.7 | 0.0053 | 2.2 ± 0.7 | 1.0 ± 0.9 | 0.012 |
| Energy parameters, kJ/m3 | ||||||
| Resilience | 1,190 ± 380 | 1,510 ± 420 | NS | 860 ± 300 | 940 ± 510 | NS |
| Strain energy density | 3,890 ± 1360 | 2,880 ± 660 | NS | 1,150 ± 340 | 1150 ± 560 | NS |
| Hysteresis | 2,140 ± 620 | 1,540 ± 560 | NS | 420 ± 130 | 370 ± 200 | NS |
| PY Energy | 2,700 ± 1,100 | 1,170 ± 410 | 0.0061 | 290 ± 100 | 110 ± 130 | 0.031 |
| PY Hysteresis | 1,100 ± 430 | 320 ± 160 | 0.0035 | 55 ± 29 | 19 ± 17 | 0.017 |
Values are means ± SD. While not affecting any of the commonly measured mechanical properties (i.e., modulus, yield stress, etc.), ribose cross-linking significantly decreased the ability of tendon to absorb strain energy after the yield point. Significant reductions in extensibility after yield, strain energy after yield, and hysteresis after yield occurred following ribose cross-linking. E, linear modulus; σy and εy, yield stress and strain; σu and εu, maximum stress and corresponding strain; εPY, postyield strain; PY Energy, postyield strain energy density; PY Hysteresis, postyield hysteresis.
Ribose cross-linking did not significantly alter any of the commonly measured mechanical properties. Linear modulus, yield stress and strain, and maximum stress and strain were similar for untreated and ribose-cross-linked samples in both the 1st and 10th overload cycles. Ribose cross-linking did, however, significantly reduce the extensibility of tendons after the yield point: that is, it reduced the plasticity of the tendons. In the first overload cycle, postyield strain was reduced twofold for the ribose cross-linked tendons compared with the matched-pair untreated samples (εPY of 4.1 ± 1.7 vs. 8.9 ± 1.7%; P = 0.0053). A significant difference in postyield extensibility was still present at overload cycle 10, where εPY was 1.0 ± 0.9% for the ribose cross-linked samples and 2.2 ± 0.7% for the untreated samples (P = 0.012). The decreased postyield extensibility for the ribose-cross-linked tendons prevented them from absorbing and dissipating as much strain energy during overload. Postyield strain energy density was similarly significantly reduced for ribose cross-linked samples compared with untreated samples in the first loading cycle (1,170 ± 410 vs. 2,700 ± 1,100 kJ/m3; P = 0.0061) and remained significantly lower at the tenth loading cycle (110 ± 130 vs. 290 ± 100 kJ/m3; P = 0.031).
The greatest mechanical change caused by ribose cross-linking was seen in postyield hysteresis (strain energy dissipated per loading cycle after the yield point). For the first loading cycle, ribose cross-linking decreased postyield hysteresis by 71% compared with untreated tendon (320 ± 160 vs. 1100 ± 430 kJ/m3; P = 0.0035), and at overload cycle 10, the reduction was still 65% (19 ± 17 vs. 55 ± 29 kJ/m3; P = 0.017).
AGE cross-linking of tendon causes abnormal nanostructural response to overload.
DSC analysis of untreated tendons showed evidence that mechanical overload disrupted collagen structure in a manner consistent with altered molecular packing (Figs. 4 and 6; Table 3). The endotherm onset temperature dropped by 7.0 ± 1.0°C following overload (P < 0.0001) and the width of the endotherm broadened by 2.1 ± 1.0°C (P < 0.0001). These effects were markedly reduced after ribose treatment. The denaturation endotherm of ribose-cross-linked tendon shifted slightly with overload but did not change in shape (Fig. 4; Table 3). For instance, the onset temperature fell by only 1.3 ± 0.6°C with overload (Fig. 6).
Fig. 6.
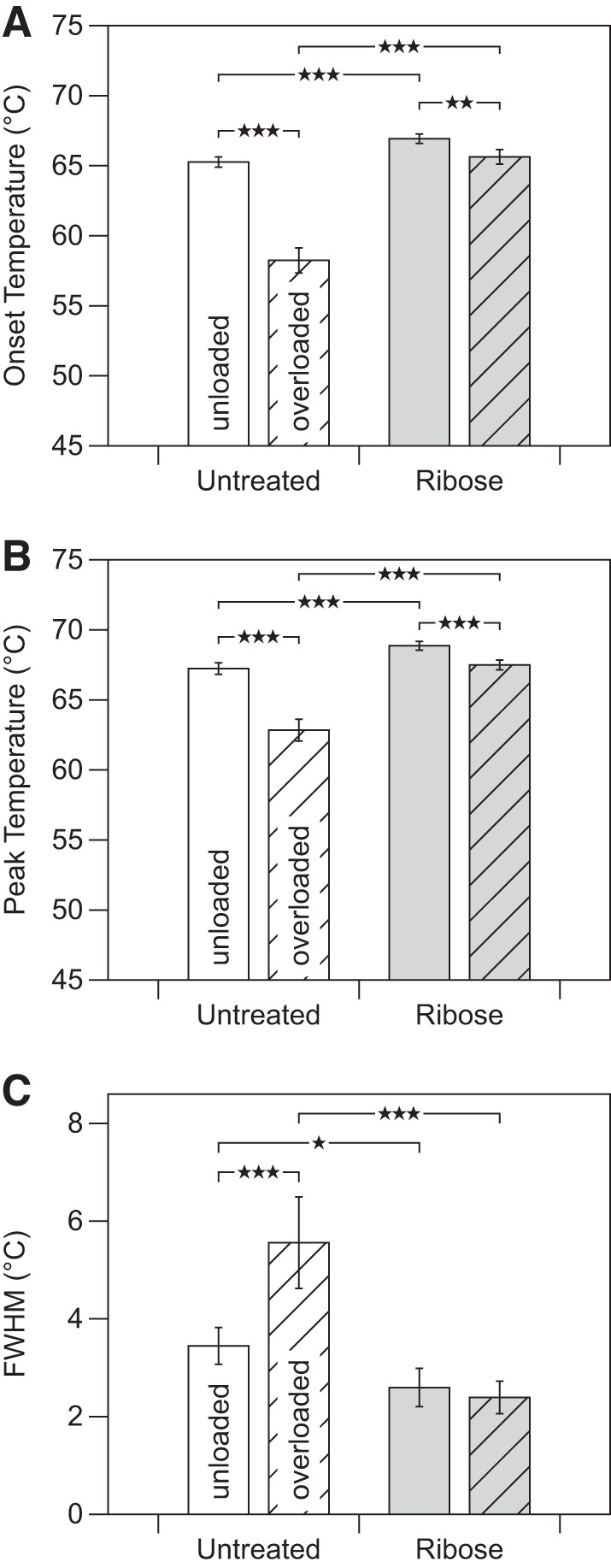
As assessed by differential scanning calorimetry, the effect of mechanical overload on the molecular packing structure of tendon was significantly altered by ribose cross-linking, with ribose cross-linking inhibiting much of the overload-induced destabilization seen in native tendon. A: onset temperature (Tonset). B: peak temperature (Tpeak). C: full-width at half-maximum (FWHM). *P < 0.05, **P < 0.005, ***P < 0.0005.
Table 3.
For untreated tendon, DSC showed that mechanical overload had a large destabilization effect on collagen molecules
| Change Caused by Overload |
||||
|---|---|---|---|---|
| DSC Parameters, °C | Untreated | P | Ribose cross-linked | P |
| Tonset | −7.0 ± 1.0 | <0.0001 | −1.3 ± 0.6 | 0.0006 |
| Tpeak | −4.4 ± 0.9 | <0.0001 | −1.4 ± 0.5 | 0.0003 |
| FWHM | +2.1 ± 1.0 | <0.0001 | −0.2 ± 0.5 | NS |
Values are means ± SD. For ribose cross-linked tendon, a significant effect was still seen but with greatly reduced magnitude. While shifted to slightly lower temperature, the endotherm of ribose cross-linked tendon did not experience overload-induced broadening like that seen for untreated tendon. Representative endotherms for untreated and ribose cross-linked tendon are shown in Fig. 4. DSC, differential scanning calorimetry; Tonset, onset temperature; Tpeak, peak temperature; FWHM, and full-width at half-maximum.
Under SEM, mechanical overload of untreated tendon resulted in easily identifiable and well-defined damage to collagen fibrils. The collagen fibrils of untreated tendon underwent discrete plasticity (38, 41): serial kinking along the length of fibrils with nanoscale repeat (Fig. 7B). Many of the serially kinked fibrils showed no D-banding, a sign that overload-induced molecular disruption had occurred over the entire fibril surface. Frequent sites of densified discrete plasticity were found, where repeated overload had evidently caused the interkink distances along fibrils to shorten substantially, leading to highly disrupted fibrils with visible substructure (Fig. 8, A and B).
Fig. 7.
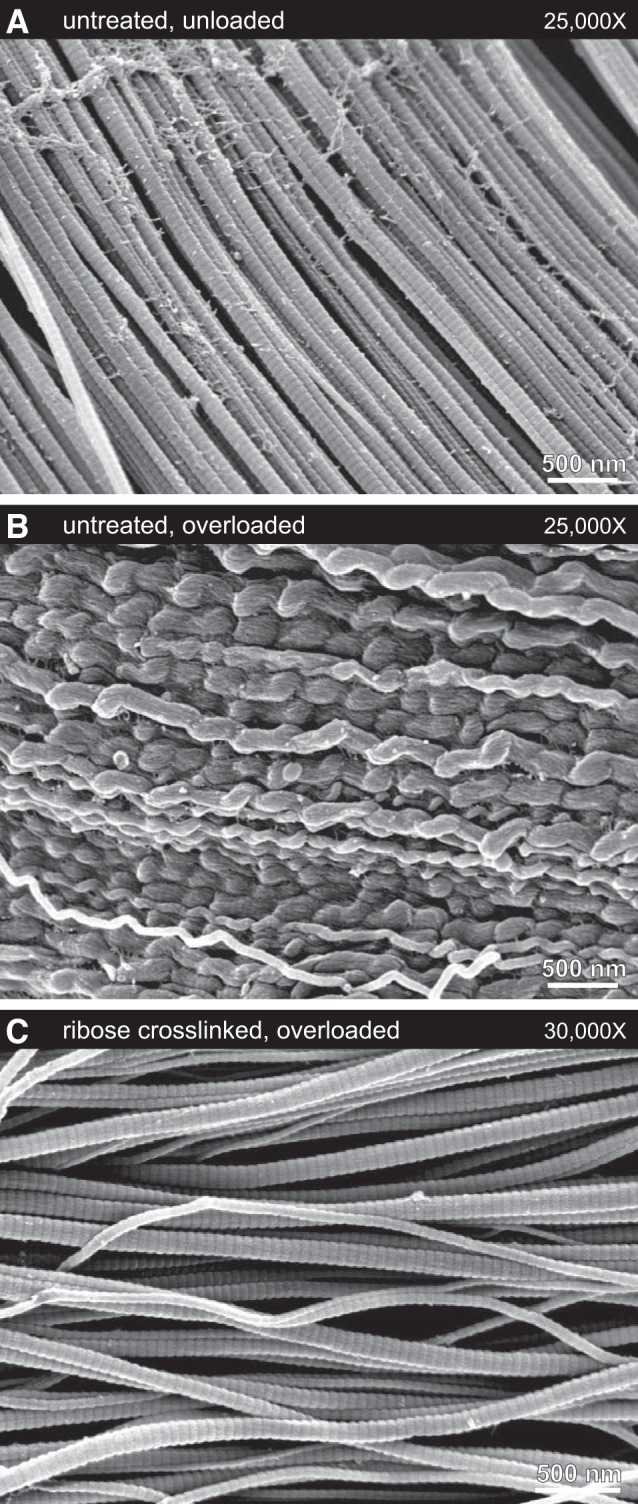
Typical collagen fibril ultrastructures under scanning election microscopy. A: untreated, unloaded tendon samples contained straight fibrils with clear D-banding. B: untreated, overloaded samples contained large regions of fibrils that had experienced discrete plasticity, with fibrils exhibiting the characteristic longitudinally repeating kinked morphology. The sample shown here underwent 15 cycles of subrupture overload. For the first overload cycle, the yield and maximum stresses were 20 and 26 MPa, respectively. C: ribose cross-linking totally inhibited overload-induced discrete plasticity. In tendon that had been ribose-cross-linked, overload had little effect on the fibrils. While occasional sites of fibril damage were identified (Fig. 8C), most fibrils retained the straight D-banded appearance typical of unloaded samples. The sample shown here underwent 10 cycles of subrupture overload. For the first overload cycle, the yield and maximum stresses were 29 and 31 MPa, respectively.
Fig. 8.
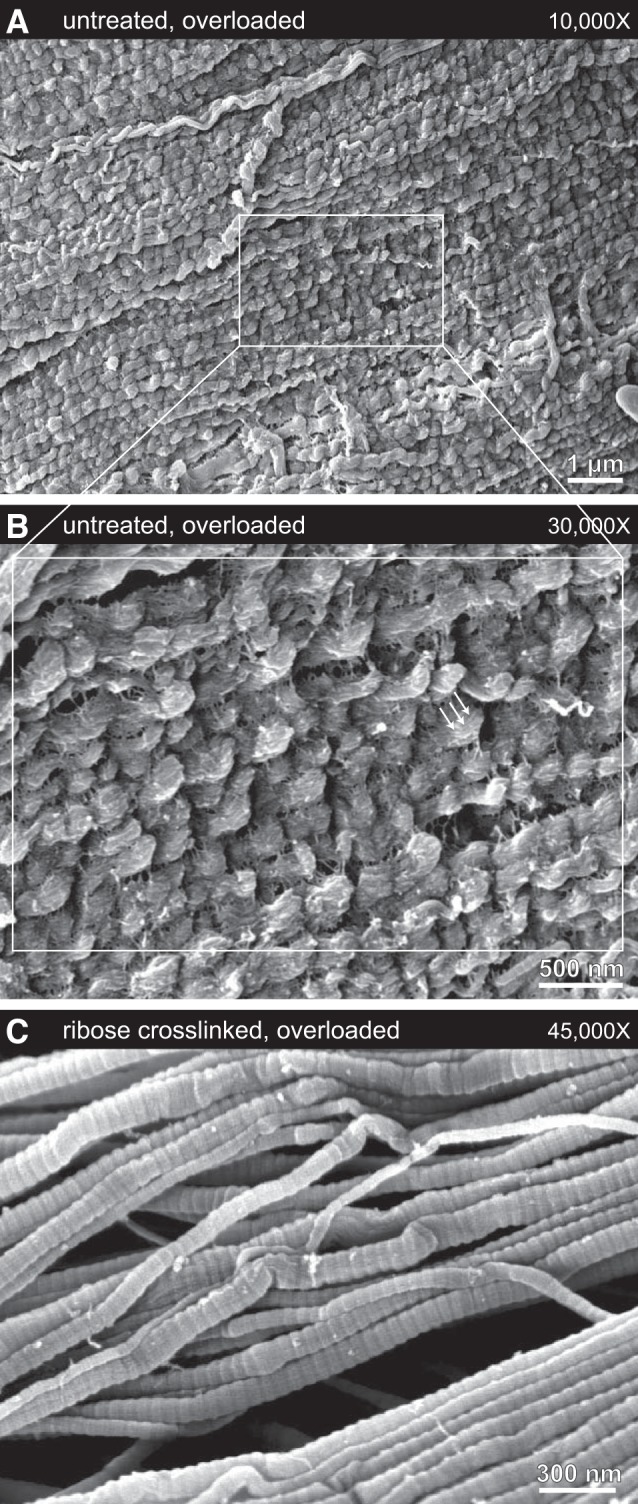
A–C: representative scanning election micrographs of the most-damaged fibrils found in untreated and overloaded (A and B) and ribose-cross-linked and overloaded (C) tendon collagen. A and B: sites of extreme fibril damage were easily found in untreated samples, with fibrils having a high linear density of discrete plasticity kinking, complete absence of D-banding, and visible subfibril structure (arrows). The sample shown here underwent 15 cycles of subrupture overload. For the first overload cycle, the yield and maximum stresses were 28 and 44 MPa, respectively. C: in contrast, the most severe fibril damage seen in tendon that was ribose-cross-linked before overload was some limited fibril buckling that was highly localized, not extending for more than a few hundred nanometers along the length of fibrils. Such fibrils retained their D-banding, indicating that the buckles had formed with minimal disruption to molecular packing, consistent with differential scanning calorimetry results. The sample shown here underwent 10 cycles of subrupture overload. For the first overload cycle, the yield and maximum stresses were 24 and 30 MPa, respectively.
Ribose cross-linking fully inhibited the formation of discrete plasticity damage, consistent with mechanical evidence of lost plasticity in treated tendons. While fibrils with discrete plasticity were easily found within all eight of the untreated and overloaded tendon samples, fibrils with discrete plasticity were not found in any of the eight tendon-matched ribose treated samples. Statistical analysis on the presence or absence of discrete plasticity following mechanical overload showed that the ability of the ribose cross-linking treatment to inhibit discrete plasticity was highly significant (P = 0.0002). Under SEM, the collagen fibrils of ribose-cross-linked tendon resembled those from untreated, unloaded tendon: most fibrils remained straight and clearly D-banded (Fig. 7C). While fibrils with localized buckling distortions were occasionally found (Fig. 8C), D-banding was not disrupted and the fibrils regained a normal, linear appearance within a few hundred nanometers. Discrete plasticity and its repeated serial kinking was never observed in the collagen fibrils of overloaded, ribose-cross-linked tendon.
DISCUSSION
We have previously reported the phenomenon of discrete plasticity: a characteristic nanoscale damage motif at the collagen fibril level in tendons that have been pulled to rupture (20, 41) or overloaded into plastic deformation without rupture (38–40). Discrete plasticity occurs as the result of excessive tensile strain, and the disruption morphology has been documented in single collagen fibrils pulled to rupture individually using atomic force microscopy (27, 28, 34). We suspect that this phenomenon may be an innate damage-control mechanism that decreases the risk of an outright rupture in mechanical overload. Furthermore, the resulting rebound kink structures may be an important cue in the cellular recognition and response to damaged collagen (37). In the current work we have explored ribose cross-linking as a simulation of the nonenzymatic glucose cross-linking that occurs with aging and diabetes and its consequent effect on the ability of collagen fibrils to undergo discrete plasticity. In confirmation of our hypothesis, electron microscopy showed that the introduction of these cross-links completely inhibited the formation of discrete plasticity in tendon.
The cross-linking agent employed in the current study was the reducing pentose sugar ribose, which generates pentosidine as a major product (30). Just as with the glucose-derived glucosepane, pentosidine forms a cross-link between lysine and arginine residues (30, 32, 45). This generates the same geometry around the arginyl imidazole motif, with one sugar-derived carbon bonded directly between an arginyl imine and lysyl amine nitrogen center. The result is a cross-link with the same structural backbone geometry and length, with the additional carbon from the hexose-derived glucosepane accounted for in the larger heterocycle. Both glucosepane and pentosidine should therefore have practically identical mechanical effects if compared at the same concentration.
Ribose reacts significantly faster than glucose due to the ~20-fold greater equilibrium concentration of the open chain aldehyde tautomer in solution (15, 25, 26, 29). This significantly faster rate of reaction facilitates in vitro work. For example, if tendons were exposed to the same concentration of glucose under otherwise comparable conditions, it is estimated that equivalent AGE cross-link density (to that reached in 28 days with ribose) would be achieved only after a year-and-a-half. For the present work, tendons were incubated in a 200 mM ribose solution: a concentration ~20 times greater than that of the diagnostic plasma glucose threshold for diabetes (4), and a factor of 10 greater than a concentration consistent with severe acute hyperglycemia (36). Considering both the selection of ribose and the chosen incubation concentration, these conditions allow for a simulation of over 20 yr of aging and diabetes in a matter of 4 wk. The drastically improved time frame, along with the retention of proper molecular geometry, make ribose cross-linking a very appealing and pragmatic in vitro technique for the introduction of AGE cross-links.
Normal levels of AGE cross-link accumulation do not appear to alter tendon mechanics during physiologic loading. Couppé et al. (12) studied the patellar tendons of young men (27 ± 2 yr) and older men (67 ± 3 yr). Despite the patellar tendons from the older men containing >6.5 times more pentosidine cross-links than those from the young men (73 vs. 11 mmol pentosidine/mol collagen), tendons from the two age groups were mechanically similar. For instance, during maximum isometric knee extension efforts, no differences in tendon modulus, stiffness, or maximum strain were observed. In vitro studies that have added AGE cross-links to tendons via ribose incubation have shown similar results. In the current study, we observed no change to the linear modulus of adult bovine tail tendon following 4 wk of ribose incubation. Similarly, Gautieri et al. (16) observed no change to the linear modulus of rat tail tendon fascicles after 12 days of incubation in ribose, despite the 12-day incubation significantly increasing AGE cross-link concentration. As well, Reddy et al. (29) observed no change to the linear modulus of rabbit Achilles tendon after 4 wk of ribose incubation.
While tendon mechanics within the physiologic range of loading do not appear to be altered by ordinary AGE cross-link accumulation, addition of glycation cross-linking may have a profound effect on the response of tendons to mechanical overload, perhaps depending on the characteristics of the accompanying enzymatic cross-linking present. For instance, when rat tail tendon fascicles were incubated with ribose, linear modulus was not affected by addition of AGE cross-links but ultimate stress increased by 83% (16). The current study used adult bovine tail tendons in which collagen molecules are well connected via the divalent enzymatically produced aldimine cross-link hydroxylysinonorleucine (44). For such well-cross-linked tendons, addition of AGE cross-links did not alter the yield point or ultimate strength (Table 2). However, the addition of AGE cross-links did significantly alter postyield behavior. Both the extensibility εPY and energy absorption capacity PY Energy within the overload region decreased (Table 2).
The decrement in tendon overload mechanics brought on by increased AGE cross-linking in the current study was coincident with the inability of collagen fibrils within the AGE cross-linked tendons to undergo discrete plasticity (Fig. 7). It is suspected that molecular sliding is required for the formation of discrete plasticity damage under mechanical overload, taking the collagen molecules out of registry and producing both loss of normal fibril D-banding and partial denaturation. With the introduction of helical-domain glycation cross-links, the molecular slippage thought necessary for discrete plasticity was likely inhibited. Fibrils loaded beyond their yield point could not dissipate excess strain energy through discrete plasticity and therefore were more likely to fail by brittle fracture. Positive verification of collagen fibril fracture was not possible in our SEM samples since mechanical fractures cannot be definitively distinguished from cut ends arising from sample preparation. What can be confidently said is that glycation cross-linking inhibited development of plastic damage in the fibrils. As demonstrated in the stress-strain curves (Fig. 1), overload did cause significant damage to the ribose-cross-linked samples. In the absence of the discrete plasticity mechanism, and with the apparent retention of native D-banded fibril structure, the most likely fibril failure mechanism is brittle fracture.
Prior studies have shown that the mechanical response of collagen fibrils in tendon to overload depends on both the enzymatic cross-link profile and the loading rate. While the collagen fibrils of tendons that contain less hydrothermally stable enzymatic cross-linking readily undergo discrete plasticity in response to overload (20, 27, 39), those with more hydrothermally stable cross-linking only undergo discrete plasticity when overloaded at slow loading rates (10). The impact that AGE cross-linking may have on the overload response of tendons with significant levels of trivalent cross-linking, typically seen in high stress tendons such as the human patellar (12) or Achilles (19), remains to be determined.
Diabetics experience various tendon pathologies and abnormalities (1). Of particular interest here is evidence of substructural disorganization and tendon thickening. Abnormal fibril morphology, along with disorganization and disruption between fibrils has been reported (18). Grant and coworkers (8) described fibrils as “twisted, curved, and overlapping” in 11 of 12 Achilles tendons from diabetic individuals. Similarly, a large in vivo ultrasound study on the Achilles tendons of diabetic patients showed fiber disorganization in ~90% of diabetic individuals. In otherwise asymptomatic diabetic patients, tendon thickening was found concurrently with a significantly greater prevalence of supraspinatus tendon tears (1, 2). We speculate that the mechanisms of diabetic tendon thickening and fibril abnormalities involve the inability of inflammatory cells or tenocytes to properly recognize and remodel damaged collagen fibrils when the normal nanostructural response to overload has been inhibited. Previous work has shown that fibrils with overload-induced discrete plasticity are covered by a sheath of denatured collagen molecules (7, 27, 40, 41). It is known that inflammatory cells like macrophages can recognize and bind to denatured collagen (21), and previous work has shown that macrophage-like immortal cells respond specifically to discrete plasticity-damaged fibrils by removing the denatured collagen (37). While the three-dimensional structure of discrete plasticity kinks is the initially striking feature under SEM, it is possible that the associated sheath of denatured collagen is the primary cellular cue for damage. If excessive AGE cross-linking inhibits collagen denaturation on overload, cells may be unable to detect, and therefore remove, nonfunctional damaged fibrils. These “abandoned” fibrils would persist in the tissue, leading to tendon thickening and matrix disorganization as tenocytes attempt to restore a desirable stress state via new collagen deposition.
In conclusion, the introduction of glycation cross-links completely inhibited the formation of a characteristic collagen fibril damage motif, discrete plasticity, in response to tendon overload (41). It is reasonable to suspect that fibril kinking and the associated production of denatured collagen may be muted by the increased collagen cross-linking experienced with aging, especially with hyperglycemia. Inhibition of this damage mechanism and its cueing for healing (21) may contribute to the explanation of the increased risk of orthopedic injury with aging and soft tissue pathologies found in diabetes.
GRANTS
This work was supported by grants to JML and SPV from the Natural Sciences and Engineering Research Council of Canada (to J. M. Lee and S. Veres).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.L. conceived and designed research; J.M.L. and S.P.V. performed experiments; J.M.L. and S.P.V. analyzed data; J.M.L. and S.P.V. interpreted results of experiments; J.M.L. and S.P.V. prepared figures; J.M.L. and S.P.V. drafted manuscript; J.M.L. and S.P.V. edited and revised manuscript; J.M.L. and S.P.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the support of the Canada Foundation for Innovation, Atlantic Innovation Fund, and other partners that fund the Facilities for Materials Characterization, managed by the Clean Technologies Research Institute, Dalhousie University. Gratitude is expressed to Reid’s Meats, Wolfville, Nova Scotia for cooperation in obtaining the bovine tendon samples. We thank Adam Brown for assistance with some of the laboratory work and for contributions to the initial draft of this manuscript.
REFERENCES
- 1.Abate M, Schiavone C, Salini V, Andia I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology (Oxford) 52: 599–608, 2013. doi: 10.1093/rheumatology/kes395. [DOI] [PubMed] [Google Scholar]
- 2.Abate M, Schiavone C, Salini V. Sonographic evaluation of the shoulder in asymptomatic elderly subjects with diabetes. BMC Musculoskelet Disord 11: 278, 2010. doi: 10.1186/1471-2474-11-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin Pract 67: 3–21, 2005. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539–553, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 5.Ariganello MB, Labow RS, Lee JM. Response of macrophage-like U937 cells to decellularized tissue heart valve materials. J Heart Valve Dis 18: 187–197, 2009. [PubMed] [Google Scholar]
- 6.Avery NC, Bailey AJ. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scand J Med Sci Sports 15: 231–240, 2005. doi: 10.1111/j.1600-0838.2005.00464.x. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin SJ, Kreplak L, Lee JM. Characterization via atomic force microscopy of discrete plasticity in collagen fibrils from mechanically overloaded tendons: nano-scale structural changes mimic rope failure. J Mech Behav Biomed Mater 60: 356–366, 2016. doi: 10.1016/j.jmbbm.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Batista F, Nery C, Pinzur M, Monteiro AC, de Souza EF, Felippe FH, Alcântara MC, Campos RS. Achilles tendinopathy in diabetes mellitus. Foot Ankle Int 29: 498–501, 2008. doi: 10.3113/FAI.2008.0498. [DOI] [PubMed] [Google Scholar]
- 9.Buckwalter JA, Woo SL, Goldberg VM, Hadley EC, Booth F, Oegema TR, Eyre DR. Soft-tissue aging and musculoskeletal function. J Bone Joint Surg Am 75: 1533–1548, 1993. doi: 10.2106/00004623-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Chambers NC, Herod TW, Veres SP. Ultrastructure of tendon rupture depends on strain rate and tendon type. J Orthop Res 36: 2842–2850, 2018. doi: 10.1002/jor.24067. [DOI] [PubMed] [Google Scholar]
- 11.Chiu HK, Tsai EC, Juneja R, Stoever J, Brooks-Worrell B, Goel A, Palmer JP. Equivalent insulin resistance in latent autoimmune diabetes in adults (LADA) and type 2 diabetic patients. Diabetes Res Clin Pract 77: 237–244, 2007. doi: 10.1016/j.diabres.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Couppé C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol (1985) 107: 880–886, 2009. doi: 10.1152/japplphysiol.00291.2009. [DOI] [PubMed] [Google Scholar]
- 13.Doran DM, Hirdes JP, Blais R, Baker GR, Poss JW, Li X, Dill D, Gruneir A, Heckman G, Lacroix H, Mitchell L, O’Beirne M, White N, Droppo L, Foebel AD, Qian G, Nahm S-M, Yim O, McIsaac C, Jantzi M. Adverse events among Ontario home care clients associated with emergency room visit or hospitalization: a retrospective cohort study. BMC Health Serv Res 13: 227, 2013. doi: 10.1186/1472-6963-13-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP. A potential mechanism for age-related declines in patellar tendon biomechanics. J Orthop Res 20: 1315–1322, 2002. doi: 10.1016/S0736-0266(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 15.Drew KN, Zajicek J, Bondo G, Bose B, Serianni AS. 13C-labeled aldopentoses: detection and quantitation of cyclic and acyclic forms by heteronuclear 1D and 2D NMR spectroscopy. Carbohydr Res 307: 199–209, 1998. doi: 10.1016/S0008-6215(98)00040-8. [DOI] [Google Scholar]
- 16.Gautieri A, Passini FS, Silván U, Guizar-Sicairos M, Carimati G, Volpi P, Moretti M, Schoenhuber H, Redaelli A, Berli M, Snedeker JG. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol 59: 95–108, 2017. doi: 10.1016/j.matbio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Gautieri A, Redaelli A, Buehler MJ, Vesentini S. Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: candidate amino acids involved in advanced glycation end-products. Matrix Biol 34: 89–95, 2014. doi: 10.1016/j.matbio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Grant WP, Sullivan R, Sonenshine DE, Adam M, Slusser JH, Carson KA, Vinik AI. Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg 36: 272–278, 1997. doi: 10.1016/S1067-2516(97)80072-5. [DOI] [PubMed] [Google Scholar]
- 19.Hansen P, Kovanen V, Hölmich P, Krogsgaard M, Hansson P, Dahl M, Hald M, Aagaard P, Kjaer M, Magnusson SP. Micromechanical properties and collagen composition of ruptured human achilles tendon. Am J Sports Med 41: 437–443, 2013. doi: 10.1177/0363546512470617. [DOI] [PubMed] [Google Scholar]
- 20.Herod TW, Chambers NC, Veres SP. Collagen fibrils in functionally distinct tendons have differing structural responses to tendon rupture and fatigue loading. Acta Biomater 42: 296–307, 2016. doi: 10.1016/j.actbio.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Hopper KE, Adelmann BC, Gentner G, Gay S. Recognition by guinea-pig peritoneal exudate cells of conformationally different states of the collagen molecule. Immunology 30: 249–259, 1976. [PMC free article] [PubMed] [Google Scholar]
- 22.Kohn RR, Cerami A, Monnier VM. Collagen aging in vitro by nonenzymatic glycosylation and browning. Diabetes 33: 57–59, 1984. doi: 10.2337/diab.33.1.57. [DOI] [PubMed] [Google Scholar]
- 23.Ku HH. Notes on the use of propagation of error formulas. J Res Natl Bur Stand 70C: 263–273, 1966. doi: 10.6028/jres.070C.025. [DOI] [Google Scholar]
- 25.Mentink CJ, Hendriks M, Levels AA, Wolffenbuttel BH. Glucose-mediated cross-linking of collagen in rat tendon and skin. Clin Chim Acta 321: 69–76, 2002. doi: 10.1016/S0009-8981(02)00097-9. [DOI] [PubMed] [Google Scholar]
- 26.Monnier VM. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J Gerontol 45: B105–B111, 1990. doi: 10.1093/geronj/45.4.B105. [DOI] [PubMed] [Google Scholar]
- 27.Quigley AS, Bancelin S, Deska-Gauthier D, Légaré F, Kreplak L, Veres SP. In tendons, differing physiological requirements lead to functionally distinct nanostructures. Sci Rep 8: 4409, 2018. doi: 10.1038/s41598-018-22741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quigley AS, Bancelin S, Deska-Gauthier D, Légaré F, Veres SP, Kreplak L. Combining tensile testing and structural analysis at the single collagen fibril level. Sci Data 5: 180229, 2018. doi: 10.1038/sdata.2018.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy GK, Stehno-Bittel L, Enwemeka CS. Glycation-induced matrix stability in the rabbit achilles tendon. Arch Biochem Biophys 399: 174–180, 2002. doi: 10.1006/abbi.2001.2747. [DOI] [PubMed] [Google Scholar]
- 30.Robins SP. Biochemistry and functional significance of collagen cross-linking. Biochem Soc Trans 35: 849–852, 2007. doi: 10.1042/BST0350849. [DOI] [PubMed] [Google Scholar]
- 31.Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, Connolly V, King H. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 28: 2130–2135, 2005. doi: 10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- 32.Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem 264: 21597–21602, 1989. [PubMed] [Google Scholar]
- 33.Snedeker JG, Gautieri A. The role of collagen crosslinks in ageing and diabetes - the good, the bad, and the ugly. Muscles Ligaments Tendons J 4: 303–308, 2014. doi: 10.11138/mltj/2014.4.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svensson RB, Mulder H, Kovanen V, Magnusson SP. Fracture mechanics of collagen fibrils: influence of natural cross-links. Biophys J 104: 2476–2484, 2013. doi: 10.1016/j.bpj.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka S, Avigad G, Eikenberry EF, Brodsky B. Isolation and partial characterization of collagen chains dimerized by sugar-derived cross-links. J Biol Chem 263: 17650–17657, 1988. [PubMed] [Google Scholar]
- 36.Umpierrez G, Korytkowski M. Diabetic emergencies–ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol 12: 222–232, 2016. doi: 10.1038/nrendo.2016.15. [DOI] [PubMed] [Google Scholar]
- 37.Veres SP, Brennan-Pierce EP, Lee JM. Macrophage-like U937 cells recognize collagen fibrils with strain-induced discrete plasticity damage. J Biomed Mater Res A 103: 397–408, 2015. doi: 10.1002/jbm.a.35156. [DOI] [PubMed] [Google Scholar]
- 38.Veres SP, Harrison JM, Lee JM. Repeated subrupture overload causes progression of nanoscaled discrete plasticity damage in tendon collagen fibrils. J Orthop Res 31: 731–737, 2013. doi: 10.1002/jor.22292. [DOI] [PubMed] [Google Scholar]
- 39.Veres SP, Harrison JM, Lee JM. Cross-link stabilization does not affect the response of collagen molecules, fibrils, or tendons to tensile overload. J Orthop Res 31: 1907–1913, 2013. doi: 10.1002/jor.22460. [DOI] [PubMed] [Google Scholar]
- 40.Veres SP, Harrison JM, Lee JM. Mechanically overloading collagen fibrils uncoils collagen molecules, placing them in a stable, denatured state. Matrix Biol 33: 54–59, 2014. doi: 10.1016/j.matbio.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Veres SP, Lee JM. Designed to fail: a novel mode of collagen fibril disruption and its relevance to tissue toughness. Biophys J 102: 2876–2884, 2012. doi: 10.1016/j.bpj.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warheit DB, Hill LH, Brody AR. Surface morphology and correlated phagocytic capacity of pulmonary macrophages lavaged from the lungs of rats. Exp Lung Res 6: 71–82, 1984. doi: 10.3109/01902148409087896. [DOI] [PubMed] [Google Scholar]
- 43.Wiens R, Findlay CR, Baldwin SG, Kreplak L, Lee JM, Veres SP, Gough KM. High spatial resolution (1.1 μm and 20 nm) FTIR polarization contrast imaging reveals pre-rupture disorder in damaged tendon. Faraday Discuss 187: 555–573, 2016. doi: 10.1039/C5FD00168D. [DOI] [PubMed] [Google Scholar]
- 44.Willett TL, Labow RS, Avery NC, Lee JM. Increased proteolysis of collagen in an in vitro tensile overload tendon model. Ann Biomed Eng 35: 1961–1972, 2007. doi: 10.1007/s10439-007-9375-x. [DOI] [PubMed] [Google Scholar]
- 45.Willett TL, Sutty S, Gaspar A, Avery N, Grynpas M. In vitro non-enzymatic ribation reduces post-yield strain accommodation in cortical bone. Bone 52: 611–622, 2013. doi: 10.1016/j.bone.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 45a.World Health Organization Global Status Report on Noncommunicable Diseases 2014. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]