Abstract
Preventing declines in cerebral blood flow is important for maintaining optimal brain health with aging. We compared the effects of a morning bout of moderate-intensity exercise, with and without subsequent light-intensity walking breaks from sitting, on cerebral blood velocity over 8 h in older adults. In a randomized crossover trial, overweight/obese older adults (n = 12, 70 ± 7 yr; 30.4 ± 4.3 kg/m2), completed three acute conditions (6-day washout); SIT: prolonged sitting (8 h, control); EX+SIT: sitting (1 h), moderate-intensity walking (30 min), followed by uninterrupted sitting (6.5 h); and EX + BR: sitting (1 h), moderate-intensity walking (30 min), followed by sitting (6.5 h) interrupted with 3 min of light-intensity walking every 30 min. Bilateral middle cerebral artery velocities (MCAv) were determined using transcranial Doppler at 13 time points across the day. The temporal pattern and average MCAv over 8 h was determined. The pattern of MCAv over 8 h was a negative linear trend in SIT (P < 0.001), but a positive quadratic trend in EX + SIT (P < 0.001) and EX + BR (P < 0.01). Afternoon time points in SIT were lower than baseline within condition (P ≤ 0.001 for all). A morning dip in MCAv was observed in EX + SIT and EX + BR (P < 0.05 relative to baseline), but afternoon time points were not significantly lower than baseline. The average MCAv over 8 h was higher in EX + SIT than SIT (P = 0.007) or EX + BR (P = 0.024). Uninterrupted sitting should be avoided, and moderate-intensity exercise should be encouraged for the daily maintenance of cerebral blood flow in older adults. The clinical implications of maintaining adequate cerebral blood flow include the delivery of vital oxygen and nutrients to the brain.
NEW & NOTEWORTHY This is the first study to measure the combined effects of an exercise bout with breaks in sitting on cerebral blood velocity in older adults. Using frequent recordings over an 8-h period, we have performed a novel analysis of the pattern of cerebral blood velocity, adjusting for concurrent measures of mean arterial pressure and other potential confounders in a linear mixed effects regression.
Keywords: acute exercise, brain health, older adults, sedentary behavior, transcranial Doppler
INTRODUCTION
The prevalence of stroke and dementia is increasing due to population aging (14). Aging is also associated with an increased prevalence of cardiovascular risk factors for cerebrovascular disease such as physical inactivity, obesity, hypertension, hyperlipidemia, and dysglycemia (43). Therefore, strategies to maintain cerebrovascular health among older adults with cardiovascular risk factors are a public health priority. Evidence demonstrates that exercise in particular is associated with a reduced incidence of stroke (24, 39) and may also delay the progression of dementia (19, 25). However, the mechanisms underlying these benefits in humans remain unclear. While exercise may exert some of its cardiovascular effects by modifying traditional risk factors (18, 22), there are also direct benefits of exercise on arterial function and health (17, 37). In addition, regular exercise can mitigate the decline in cerebral blood flow associated with aging (1). Insight from animal studies demonstrates the importance of exercise-induced increases in cerebral blood flow for neurogenesis, cerebral angiogenesis, and related growth factors (3, 28, 30, 35). To understand cerebrovascular regulation in response to exercise in humans, many studies focus on cerebral blood flow during or immediately after exercise (27). However, few experiments have characterized the cerebral blood flow response to different patterns of physical activity over the whole day, an imperative for the design of optimal exercise interventions.
Over a whole waking day, older adults spend ~5% of time engaged in exercise of moderate-to-vigorous intensity but spend a majority of time in sedentary behavior, which carries an increased risk for all-cause mortality (15, 20, 21). Recent evidence suggests that sedentary behaviors such as prolonged sitting may be negatively associated with aspects of brain health such as cognitive function and medial temporal lobe thickness (13, 33). In addition, laboratory studies that have investigated reducing and breaking up sitting with intermittent physical activity have reported beneficial impacts on multiple systems relevant to brain health, including carbohydrate and lipid metabolism (4, 16), blood pressure (5, 10), sympathetic function (10), and vascular function (7, 32, 36). In response to accumulating evidence, some government guidelines now recommend reducing sitting in addition to engaging in moderate-to-vigorous intensity exercise (2, 11). In the United States, the scientific report that informed the 2018 Physical Activity Guidelines for Americans highlighted a need for future studies to investigate different patterns of physical activity and sedentary behavior on brain health outcomes (29). However, it is currently unknown whether engaging in moderate-to-vigorous intensity exercise would mitigate any potential decline in cerebral blood flow during a subsequent period of prolonged sitting. It is also unknown whether combining a bout of moderate-to-vigorous exercise with subsequent breaks in sitting would further enhance the cerebral blood flow response.
The aim of this study was to assess the impact of a moderate-intensity exercise bout, with or without subsequent breaks in sitting, on middle cerebral artery blood velocity (MCAv) in older adults. We hypothesized that an acute bout of exercise would enhance cerebrovascular responses over an 8-h period, relative to prolonged uninterrupted sitting. In addition, we hypothesized that cerebrovascular responses following acute exercise would be further enhanced by subsequent exposure to breaks in sitting.
MATERIALS AND METHODS
This experiment is a substudy of a larger randomized crossover trial (ACTRN12614000737639), and the detailed methods have been published independently (12).
Participants.
Men and postmenopausal women (n = 10 men and 2 women, age ≥55 to ≤80 yr; body mass index ≥25 to <45 kg/m2; English-speaking) were recruited from the local community via advertisement in Perth, WA, Australia. Full participant characteristics are found in Table 1. This study was approved by the Human Research Ethics Committee of The University of Western Australia (RA/4/1/6990). Participants provided written informed consent before testing. All participants were screened for cardiovascular risk and previous cardiovascular events. Exclusion criteria included self-reported sitting <5 h/day, self-reported engagement in moderate-intensity exercise ≥150 min/wk for >3 mo, probable dementia (Telephone Interview of Cognitive Status score of <19), cognitive impairment (Mini Mental State Exam <24), depressive symptoms of clinical relevance (Geriatric Depression Score >6 or Hospital Anxiety and Depression Scale Score: depression score >8), diagnosed diabetes, use of glucose/lipid-lowering medication, antidepressant medications, β-blockers, antianxiety medication, excessive alcohol consumption (>8 points on the Alcohol Use Disorders Identification Test), abnormal ECG (determined by study doctor), high resting blood pressure (office systolic >160 mmHg or diastolic >100 mmHg), or major illness/physical problems (acute or chronic) that would limit ability to perform moderate-intensity exercise.
Table 1.
Participant characteristics
| Demographic | Baseline |
|---|---|
| N | 12 |
| Sex (women/men) | 2/10 |
| Age, yr | 70 ± 7 |
| Body mass index, kg/m2 | 30.4 ± 4.3 |
| Waist circumference, cm | 103.4 ± 11.0 |
| Office SBP,* mmHg | 128 ± 13 |
| Office DBP,* mmHg | 76 ± 13 |
| Fasting glucose,† mmol/l | 5.0 ± 0.5 |
| Fasting insulin,† pmol/l | 30 ± 24.7 |
| Fasting cholesterol,† mmol/l | 5.1 ± 0.9 |
| Fasting triglycerides,† mmol/l | 1.1 ± 0.4 |
| Fasting HDL,† mmol/l | 1.3 ± 0.3 |
| Fasting LDL,† mmol/l | 3.3 ± 0.6 |
Data are means ± SD; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol.
Measured during familiarization visit.
Measured during first experimental visit.
Study design.
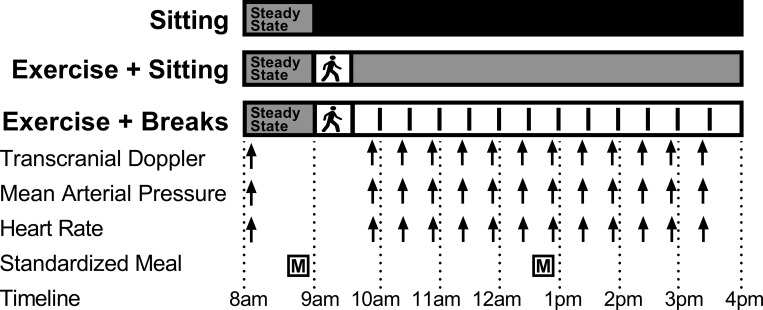
Participants were randomized to participate in three laboratory sessions, separated by a minimum of 6 days (Fig. 1). The order of conditions was block randomized and stratified by sex by an independent third party using a computer-generated random sequence and stored in sealed envelopes as previously outlined (12). Researchers were blinded to the order of conditions until familiarization was complete, and participants were blinded to the conditions until each day of testing. Experimental conditions included the following: sitting (SIT): uninterrupted sitting (8 h, control); exercise + sitting (EX + SIT): sitting (1 h), moderate-intensity treadmill walking (30 min) followed by uninterrupted sitting (6.5 h); and exercise + breaks (EX + BR): sitting (1 h), moderate-intensity treadmill walking (30 min) followed by sitting (6.5 h) interrupted every 30 min with 3 min of light-intensity treadmill walking. A familiarization session was completed three to five days before testing, in which participants were familiarized with all testing equipment and procedures, including treadmill walking. During the 48 h before testing, participants were instructed to avoid caffeine, alcohol and moderate-to-vigorous physical activity. In addition, food was controlled from the night before testing where participants consumed a standardized dinner at home between 7 PM and 9 PM in place of their regular dinner. This meal was tailored for each participant to meet 33% of estimated daily energy requirement with a macronutrient profile of 55–58% carbohydrate, 29–31% fat, and 12–15% protein as previously described (12).
Fig. 1.
Experimental design. Participants completed 3 conditions in a random order separated by a minimum of 6 days. Conditions were as follows: sitting (SIT); uninterrupted sitting (8 h, control); exercise + sitting (EX + SIT): sitting (1 h), moderate-intensity walking (30 min, denoted by walking figure) followed by uninterrupted sitting (6.5 h); and exercise + breaks (EX + BR): sitting (1 h), moderate-intensity walking (30 min) followed by sitting (6.5 h) interrupted every 30 min with 3 min of light-intensity walking. Walking breaks are denoted by vertical lines in the EX + BR condition. During each condition, participants consumed a standardized breakfast and lunch meal and transcranial Doppler, mean arterial pressure, and heart rate were recorded simultaneously across the day.
Exercise.
The moderate-intensity exercise bout was performed on a treadmill at the same predetermined speed and incline for both EX + SIT and EX + BR. The speed was set at 3.2 km/h and the incline was tailored for each participant during the familiarization session to induce a heart rate indicative of moderate-intensity, defined as 65–75% of age predicted maximum heart rate. Each 3-min light-intensity walking break performed during EX + BR was completed on a treadmill with 0% incline at a speed of 3.2 km/h, which was a walking speed for all participants. Heart rate (Polar Electro, Kempele, Finland) and ratings of perceived exertion (RPE; scale 6–20; light intensity 9–11 RPE; moderate-intensity 12–15 RPE) were collected at 5-min intervals during the 30-min bout of exercise and at the end of each 3-min walking break.
Experimental day protocol.
Participants reported to the laboratory at ~7 AM following an overnight fast (>10 h). Participants remained seated while equipment was set up and the bilateral middle cerebral arteries were located as detailed below, before the start of the experiment at ~8 AM (0 h). The experiment began with baseline recordings of MCAv, blood pressure, and heart rate, which were obtained before the administration of a standardized breakfast meal. Breakfast and lunch were administered at 40 and 280 min into the experiment and were consumed over a 20-min period. All meals were standardized according the same criteria as the evening meal and remained the same for a given participant during all conditions. After breakfast, the protocol was followed according to randomization and participants were instructed to remain seated apart from leaving the chair to void or to perform predetermined treadmill walking in the EX + SIT and EX + BR conditions. Study outcomes were measured at multiple time points across the day (Fig. 1). All measures of MCAv were taken during steady-state sitting periods, such that in the EX + BR condition measures were collected at least 25 min after the most recent activity break.
Cerebrovascular function.
Cerebral blood flow was indexed using transcranial Doppler (TCD; Spencer Technologies, Seattle, WA). Bilateral measures of MCAv were determined with a 2-MHz probe transfixed to the posterior aspect of the temporal window of the skull using the Mark 600 headframe (Spencer Technologies, Seattle, WA). The headframe was secured in place to negate movement effects on the insonation site, and participants remained instrumented for the entire experiment to avoid relocating the MCA. The location of the middle cerebral artery was determined by locating the trifurcation of the circle of Willis (~45–65 mm) in the anterior circulation of the brain, as previously outlined (42). The MCAv was continuously sampled for 5 min at baseline and for 30 s during subsequent time points, at 1,000 Hz via an analogue-to-digital converter (Powerlab; 16/30 AD Instruments, Colorado Springs, CO). Data were analyzed offline using a specialized analytical software package (LabChart 8; AD Instruments). The sum of bilateral velocities was calculated for statistical analyses. The sum of bilateral velocities represents a surrogate measure of the total amount of blood being delivered to the brain. Summing the bilateral velocities also accounts for expected anatomical differences between the left and right MCA, the detection of which would be diminished by averaging the bilateral velocities.
Assessment of hemodynamic variables.
Resting blood pressure and heart rate were measured in a seated position. A photoplethysmographic method was used for serial BP assessment (Finometer Pro; Finapres Medical Systems, Amsterdam, The Netherlands), and this was calibrated against automated brachial oscillometry (HEM-907; Omron, Kyoto, Japan). In all conditions, blood pressure and heart rate were measured contemporaneously with MCAv and at a time consistent with the period immediately preceding the 3-min walking break during the EX + BR condition.
Statistical analysis.
Based on previous evidence, we estimated the effect size (Cohen’s d for repeated measures) of exposure to intermittent light-intensity walking breaks relative to uninterrupted sitting to be ~1.1 for MCAv (6). With the assumpation of a within participant correlation of 0.6, the effective sample size to detect this difference with a power of 0.80 and a two-tailed probability of 0.05, is 9 participants. The order of conditions was block randomized and stratified by sex by an independent third party using a computer-generated random sequence and stored in sealed envelopes as previously outlined (12). Analysis was performed by technicians blinded to the study conditions. Following recent recommendations on data analysis of crossover trials (23), linear mixed models with random intercepts were used to evaluate the differential effects of the experimental conditions on the selected outcomes. Mixed models are appropriate for correlated data (repeated measures) with various distributional assumptions and can easily accommodate missing data (31). A treatment-by-time interaction term was included in regression models to examine between condition differences in temporal patterns of MCAv across the day. Marginal means (i.e., adjusted mean of the dependent variable when fixed effects are held at their mean) were calculated for individual time points and within condition comparisons, relative to baseline, and were performed for the sum of bilateral MCAv. Between condition comparisons of individual time points were performed on heart rate and mean arterial pressure variables. All models were adjusted for baseline, age, sex, waist circumference, and treatment order. Models with MCAv as the dependent variable were additionally adjusted for mean arterial pressure, which was recorded simultaneously with MCAv. Due to the large number of comparisons in the within and between condition analysis of individual time points, adjustment for multiple comparisons using a Šidák correction was performed. A probability level of 0.05 was adopted. Statistical analyses were performed using Stata 15 for Windows (StataCorp).
RESULTS
Exercise response.
The initial 30-min exercise bout induced similar (P > 0.05) heart rate and RPE responses (means ± SD) under each condition (EX + SIT: 104 ± 10 beats/min, 69 ± 7% HRmax, 11 ± 3 RPE; EX + BR: 108 ± 15 beats/min, 72 ± 11% HRmax, 11 ± 3 RPE). Average HR and RPE across all 12 walking breaks was 93 ± 14 beats/min, 62 ± 10% HRmax and 8 ± 2 RPE.
Temporal variation: 8-h pattern of cerebral blood velocity.
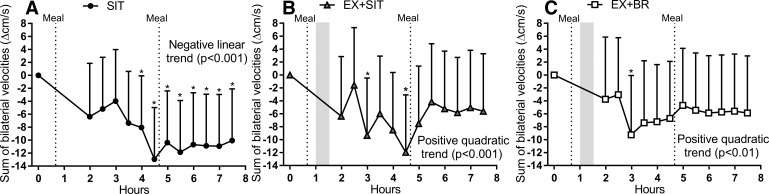
Recording the MCAv across an 8-h time period enabled the assessment of the pattern of cerebral blood velocity across the day. Observation of the response across time revealed a persistent decline in SIT (Fig. 2A). In the EX + SIT and EX + BR conditions, the initial decline of MCAv was followed by an afternoon recovery (Fig. 2, B and C). In support of these observations, a significant main effect of time was found for the sum of bilateral velocities (P < 0.001). Post hoc analysis revealed a negative linear trend in SIT (P < 0.001) but a positive quadratic trend for both EX + SIT (P < 0.001) and EX + BR (P < 0.01). A positive quadratic trend identifies the response as a convex curvilinear pattern. A significant main effect of time was also observed for left MCAv (P < 0.001) and right MCAv (P = 0.04). Left MCAv followed a negative linear trend in SIT (P < 0.001) but a positive quadratic trend in EX + SIT (P < 0.001) and EX + BR (P < .001). Right MCAv followed a negative linear trend for SIT (P < 0.001), a positive quadratic trend in EX + SIT (P = 0.02), and no significant trend for EX + BR (P > 0.05). Within condition analysis of the time course data in the SIT condition revealed a significant decline in the sum of bilateral MCAv during the morning period relative to baseline, which was sustained until the end of the condition (Fig. 2A). However, an initial decline in MCAv relative to baseline was followed by a recovery, which was sustained for the final 2.5 h of the EX + SIT condition and final 4 h of the EX + BR condition (Fig. 2, B and C).
Fig. 2.
The sum of bilateral velocities across the day. Conditions were as follows sitting (SIT): uninterrupted sitting (8 h, control); exercise + sitting (EX + SIT): sitting (1 h), moderate-intensity walking (30 min, denoted by walking figure) followed by uninterrupted sitting (6.5 h); and exercise + breaks (EX + BR): sitting (1 h), moderate-intensity walking (30 min) followed by sitting (6.5 h) interrupted every 30 min with 3 min of light-intensity walking. A–C: velocity trace displayed as a change from baseline during the SIT, EX + SIT, and EX + BR conditions, respectively. Baseline values (cm/s) in each condition are as follows: SIT 96 [90–101], EX + SIT 95 [89–101], and EX + BR 93 [87–99]. Dotted lines represent the timing of the standardized meals and the shaded area denotes the timing of the moderate-intensity exercise bout. Data are marginal means + 95% confidence interval, adjusted for baseline, age, sex, waist circumference, treatment order, and mean arterial pressure. *P < 0.05 within condition relative to baseline.
Average cerebral blood velocity across the day.
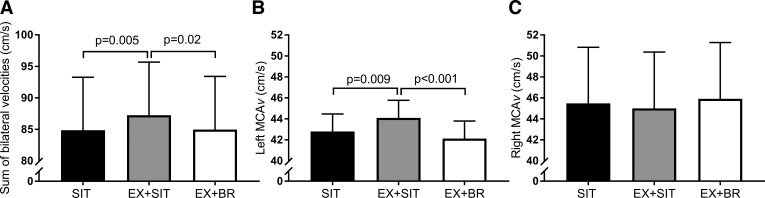
The sum of bilateral velocities (cm/s), averaged across the day (Fig. 3A), was higher in the EX + SIT condition 87 [95% confidence interval 79–96] relative to SIT 85 [76–93, P = 0.005] or EX + BR 85 [77–93, P = 0.02]. These between condition differences in MCAv (cm/s) were largely driven by the left MCA, which was higher in EX + SIT 44 [42–46] compared with SIT 43 [41–45, P = 0.009] or EX + BR 42 [40–44, P < 0.001] (Fig. 3B). However, no significant differences were observed between conditions in the average right MCAv (cm/s); SIT 45 [40–51], EX + SIT 45 [40–50], and EX + BR 46 [41–51] (Fig. 3C).
Fig. 3.
Between condition comparison of cerebral blood velocity. Conditions were as follows sitting (SIT): uninterrupted sitting (8 h, control); exercise + sitting (EX + SIT): sitting (1 h), moderate-intensity walking (30 min, denoted by walking figure) followed by uninterrupted sitting (6.5 h); and exercise + breaks (EX + BR): sitting (1 h), moderate-intensity walking (30 min) followed by sitting (6.5 h) interrupted every 30 min with 3 min of light-intensity walking. A–C: sum of bilateral velocities, left middle cerebral artery velocity (MCAv), and right MCAv respectively, displayed as an average across the day. Data are marginal means + 95% confidence interval, adjusted for baseline, age, sex, waist circumference, treatment order, and mean arterial pressure.
Comparison between conditions in hemodynamic data.
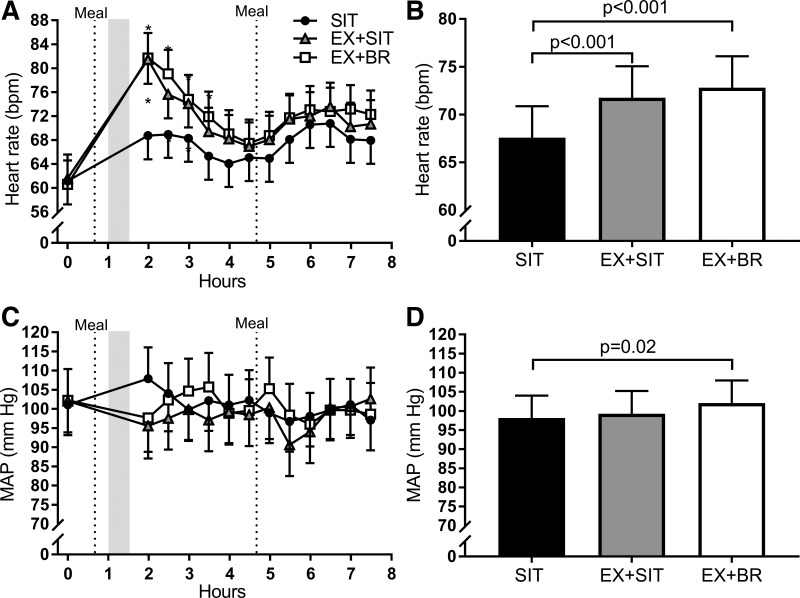
Heart rate, when averaged across the day, displayed a pattern of increase with increasing activity; SIT 68 [64–71], EX + SIT 72 [69–75, P < 0.001 vs. SIT], and EX + BR 73 [70–77, P < 0.001 vs. SIT]. This was predominantly due to increased heart rate following the 30-min bout of moderate-intensity exercise. In EX + SIT and EX + BR, heart rate remained elevated for ~2 h following the moderate-intensity exercise bout, relative to SIT (Fig. 4A). Despite a higher heart rate, mean arterial pressure was lower for ~2 h following the moderate-intensity exercise bout in EX + SIT and EX + BR, relative to SIT, although no significant differences between conditions were observed during this time. There was a small increase in the mean arterial pressure (mm Hg) averaged across the day in EX + BR 102 [96–107], compared with SIT 98 [92–104, P = 0.02], but no difference compared with EX + SIT 99 [93–105].
Fig. 4.
Between condition comparison of heart rate and mean arterial pressure. Conditions were as follows sitting (SIT): uninterrupted sitting (8 h, control); exercise + sitting (EX + SIT): sitting (1 h), moderate-intensity walking (30 min, denoted by walking figure) followed by uninterrupted sitting (6.5 h); and exercise + breaks (EX + BR): sitting (1 h), moderate-intensity walking (30 min) followed by sitting (6.5 h) interrupted every 30 min with 3 min of light-intensity walking. A and C: heart rate and mean arterial pressure (MAP), respectively, displayed as a time course across the day. B and D: the heart rate and MAP, respectively, displayed as an average across the day. Dotted lines represent the timing of the standardized meals, and the shaded area denotes the timing of the moderate-intensity exercise bout. bpm, Beats/min. Data are marginal means + 95% confidence interval, adjusted for baseline, age, sex, waist circumference, and treatment order. *P < 0.05 relative to SIT.
DISCUSSION
We observed that the pattern of MCAv during prolonged uninterrupted sitting was that of negative linear trend, with significant declines relative to baseline during the final 3.5 h of the experiment. In contrast, the pattern of MCAv following a morning bout of exercise with or without breaks in sitting, was that of a convex curvilinear response characterized by an initial decline followed by a subsequent recovery. Interestingly, the recovery of MCAv after the initial decline began earlier in the EX + BR condition, compared with EX + SIT, which may represent a benefit of intermittent walking on the temporal pattern of MCAv. The clinical implications of such a pattern of MCAv may be in avoiding sharp declines in the delivery of oxygen and nutrients to the brain (34). A decline in the delivery of glucose to the brain, for example, risks exposing the brain to hypoglycaemia, which can increase the risk of developing dementia (41). Previously, we hypothesized that fluctuations in glucose availability, more specifically than absolute concentrations, pose a risk to brain health and breaks in sitting may help mitigate this risk by maintaining a more stable supply of glucose to the brain (40). While the current data suggest that MCAv was most stable in the EX + BR condition, we did not measure glucose availability to the brain. Future studies to determine the effect of breaks in sitting on central glucose concentrations and oxygen delivery would be highly informative. To our knowledge, this is the first study to examine the 8-h pattern of MCAv in this way. This type of analysis involving frequent transcranial Doppler assessment offers unique insights into the temporal regulation of cerebral blood flow and may have implications for understanding cerebrovascular health.
We also observed that a morning bout of exercise sustained a higher average MCAv across a subsequent period of prolonged sitting. However, the finding that adding regular activity breaks to a morning bout of exercise abolished the increase in average MCAv was somewhat unexpected. There are some possible explanations worth exploring. First, day-to-day differences in the probe angle and location used when establishing the MCAv signal may have introduced measurement error into the between condition comparisons of average MCAv. Our within condition analysis of the pattern of MCAv helps mitigate this potential source of error as participants remained instrumented for the entire experiment to avoid relocating the MCA. Second, subtle changes in MCA diameter, undetectable by TCD, may have altered MCAv during intermittent walking. With the use of magnetic resonance imaging (MRI), both increases and decreases in MCA diameter have been observed following hypercapnia and rhythmic handgrip exercise respectively (8, 38). While it is unknown what effect, if any, intermittent walking would have on MCA diameter, an increased diameter would translate to a decrease in velocity and vice versa for a decreased diameter, assuming constant flow.
The effects of intermittent walking on MCAv have been documented in one previous study of lean healthy “desk workers” (6). The authors demonstrated an increase in MCAv pre to post a 4-h period involving breaks in sitting, compared with prolonged uninterrupted sitting (6). Although we observed an attenuation in average MCAv following intermittent walking, this was after an antecedent bout of morning exercise in a population of older overweight and obese adults (mean age 70 yr), compared with walking breaks alone in a younger healthy population (mean age 36 yr) in the study by Carter et al. (6). These differences between studies likely represented a different stimulus to a range of mechanisms responsible for regulating cerebral blood flow.
While our study was not designed to address the mechanisms responsible for effects on MCAv, several possibilities may exist. Brain blood flow is controlled by multiple redundant and integrative mechanisms and is highly protected by local and reflex pathways. Although differences existed between conditions in blood pressure responses (Fig. 4), MCAv data were statistically adjusted for contemporaneous blood pressure in regression analyses, and it is therefore unlikely that our cerebrovascular findings are primarily related to underlying changes in driving pressures. This type of correction avoids the need to meet the stringent assumptions required for ratio normalization, where one number is divided by another (9). A further mechanism that controls cerebral blood flow is the partial pressure of carbon dioxide in the circulating blood, and it is possible that the exercise bouts induced differences in this parameter. However, the major differences we observed between conditions occurred more than 4 h after the morning exercise bout and all of our MCAv data were obtained under quiet resting conditions. Furthermore, an impact of active breaks on carbon dioxide at the time of measurement seems unlikely, since there was ~25 min between these brief periods of walking and the subsequent resting measure of MCAv.
A strength of this study is the well-controlled randomized crossover design, which controls for person-specific factors and affords smaller sample sizes. Trial conditions were also standardized for potential confounders such as diet, physical activity, medications, and baseline values. There are also some limitations to our study. The experiment was designed as a superiority trial, and we did not include a fourth condition involving walking breaks alone. This was due to the general acceptance of the health benefits associated with continuous exercise bouts; we considered this the minimum standard for prescription. Our aim in the present study was to determine whether additive benefit was possible beyond that obtained from a morning bout of exercise. Our measure of cerebrovascular function, based on TCD ultrasound, is widely used, provides sensitive time course information, and has been shown to be a useful surrogate measure of cerebral blood flow between individuals (26). However, direct measures of intracranial diameters are not currently possible using ultrasound and velocity is therefore relied on as a surrogate measure of flow. This is less of an issue for within subject experimental designs because blood flow changes are heavily dependent on velocity change. However, we cannot rule out distinct effects on artery diameter responses that went undetected. Future experiments utilizing electroencephalography or near-infrared spectroscopy may help to better understand complementary and temporal patterns of change in cerebrovascular function in the future. Furthermore, positron emission tomography and MRI would provide information on spatial distribution of brain blood flow. In addition, it is unknown whether the changes observed simply represent a local effect on the brain vessels per se or an impact on cerebral activation that subsequently affected brain blood vessels. Future studies, perhaps including functional MRI, may be utilized to test how brain networks are affected by the combination of exercise and breaks in sitting. This is relevant since metabolic activity in the brain is known to also affect regional cerebral blood flow. Finally, given expected regional differences in cerebral blood flow, our findings are not generalizable to the posterior circulation.
Conclusion.
We have demonstrated in older overweight to obese adults, that the pattern of cerebral blood velocity over 8 h is improved following a morning bout of moderate-intensity exercise with or without subsequent breaks in sitting. In addition, a morning bout of exercise sustained a higher average MCAv during a period of subsequent sitting. Interestingly, adding intermittent walking breaks to a morning bout of exercise abolished the increase in average MCAv, which was unexpected. Future studies should seek to replicate these findings with more direct measures of cerebral blood flow using positron emission tomography or MRI. In addition, future studies using TCD should take advantage of the high temporal resolution this measure offers and collect frequent recordings to analyze the temporal pattern of cerebral blood velocity. Collecting and analyzing data in this way can also take advantage of current statistical techniques such as linear mixed effects modeling, which are particularly suited to repeated measures analysis and within subject study designs. In conclusion, our findings suggest that uninterrupted sitting should be avoided, and moderate-intensity exercise should be encouraged for the daily maintenance of cerebral blood flow.
GRANTS
This work was funded by a project grant from the National Health and Medical Research Council (NHMRC) of Australia (1062338). M. J. Wheeler was supported by the University of Western Australia and the Baker Heart and Diabetes Institute. D. W. Dunstan was supported by a NHMRC Senior Research Fellowship (NHMRC APP1078360). I. Heinonen was supported by the University of Turku, Hospital District of South-West Finland and the Juho Vainio Foundation. E. Cerin was supported by an Australin Research Council Future Fellowship (ARC FT140100085). P. N. Ainslie was supported by a Canadian Research Chair. D. J. Green was supported a NHMRC Principal Research Fellowship (APP1080914).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.W., D.W.D., L.H.N., K.A.E., E.C., P.N.A., and D.J.G. conceived and designed research; B.S., K.J.S., A.S., J.L., and I.H. performed experiments; M.J.W. and B.S. analyzed data; M.J.W., D.W.D., K.J.S., L.H.N., I.H., E.C., P.N.A., and D.J.G. interpreted results of experiments; M.J.W. prepared figures; M.J.W., D.W.D., and D.J.G. drafted the manuscript; M.J.W., D.W.D., B.S., K.J.S., A.S., J.L., L.H.N., I.H., K.A.E., E.C., P.N.A., and D.J.G. edited and revised the manuscript; M.J.W., D.W.D., B.S., K.J.S., A.S., J.L., L.H.N., I.H., K.A.E., E.C., P.N.A., and D.J.G. approved final version of the manuscript.
REFERENCES
- 1.Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJA, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586: 4005–4010, 2008. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australian Government Department of Health Australia’s Physical Activity and Sedentary Behaviour Guidelines for Adults (18–64 years) (Government Report). Canberra, Australia: Department of Health, 2014. [Google Scholar]
- 3.Bayod S, Del Valle J, Canudas AM, Lalanza JF, Sanchez-Roige S, Camins A, Escorihuela RM, Pallàs M. Long-term treadmill exercise induces neuroprotective molecular changes in rat brain. J Appl Physiol (1985) 111: 1380–1390, 2011. doi: 10.1152/japplphysiol.00425.2011. [DOI] [PubMed] [Google Scholar]
- 4.Benatti FB, Ried-Larsen M. The effects of breaking up prolonged sitting time: a review of experimental studies. Med Sci Sports Exerc 47: 2053–2061, 2015. doi: 10.1249/MSS.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 5.Bhammar DM, Sawyer BJ, Tucker WJ, Gaesser GA. Breaks in sitting time: effects on continuously monitored glucose and blood pressure. Med Sci Sports Exerc 49: 2119–2130, 2017. doi: 10.1249/MSS.0000000000001315. [DOI] [PubMed] [Google Scholar]
- 6.Carter SE, Draijer R, Holder SM, Brown L, Thijssen DHJ, Hopkins ND. Regular walking breaks prevent the decline in cerebral blood flow associated with prolonged sitting. J Appl Physiol (1985) 125: 790–798, 2018. doi: 10.1152/japplphysiol.00310.2018. [DOI] [PubMed] [Google Scholar]
- 7.Climie RE, Wheeler MJ, Grace M, Lambert E, Cohen N, Owen N, Kingwell B, Dunstan DW, Green DJ. Simple intermittent resistance activity mitigates the detrimental effect of prolonged unbroken sitting on arterial function in overweight and obese adults. J Appl Physiol (1985) 125: 1787–1794, 2018. doi: 10.1152/japplphysiol.00544.2018. [DOI] [PubMed] [Google Scholar]
- 8.Coverdale NS, Badrov MB, Shoemaker JK. Impact of age on cerebrovascular dilation versus reactivity to hypercapnia. J Cereb Blood Flow Metab 37: 344–355, 2017. doi: 10.1177/0271678X15626156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran-Everett D. Explorations in statistics: the analysis of ratios and normalized data. Adv Physiol Educ 37: 213–219, 2013. doi: 10.1152/advan.00053.2013. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey PC, Sacre JW, Larsen RN, Straznicky NE, Sethi P, Cohen ND, Cerin E, Lambert GW, Owen N, Kingwell BA, Dunstan DW. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens 34: 2376–2382, 2016. doi: 10.1097/HJH.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health Start Active, Stay Active: a Report on Physical Activity for Health from the Four Home Countries’ Chief Medical Officers (Government Report). London: Department of Health, United Kingdom, 2011. [Google Scholar]
- 12.Dunstan DW, Wheeler MJ, Ellis KA, Cerin E, Green DJ. Interacting effects of exercise with breaks in sitting time on cognitive and metabolic function in older adults: Rationale and design of a randomised crossover trial. Ment Health Phys Act 15: 11–16, 2018. doi: 10.1016/j.mhpa.2018.05.003. [DOI] [Google Scholar]
- 13.Falck RS, Davis JC, Liu-Ambrose T. What is the association between sedentary behaviour and cognitive function? A systematic review. Br J Sports Med 51: 800–811, 2017. doi: 10.1136/bjsports-2015-095551. [DOI] [PubMed] [Google Scholar]
- 14.GBD 2015 Neurological Disorders Collaborator Group; Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF, Abyu GY, Ahmed MB, Aichour AN, Aichour I, Aichour MT, Akinyemi RO, Alabed S, Al-Raddadi R, Alvis-Guzman N, Amare AT, Ansari H, Anwari P, Ärnlöv J, Asayesh H, Asgedom SW, Atey TM, Avila-Burgos L, Frinel E, Avokpaho GA, Azarpazhooh MR, Barac A, Barboza M, Barker-Collo SL, Bärnighausen T, Bedi N, Beghi E, Bennett DA, Bensenor IM, Berhane A, et al.. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16: 877–897, 2017. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorman E, Hanson HM, Yang PH, Khan KM, Liu-Ambrose T, Ashe MC. Accelerometry analysis of physical activity and sedentary behavior in older adults: a systematic review and data analysis. Eur Rev Aging Phys Act 11: 35–49, 2014. doi: 10.1007/s11556-013-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grace MS, Dempsey PC, Sethi P, Mundra PA, Mellett NA, Weir JM, Owen N, Dunstan DW, Meikle PJ, Kingwell BA. Breaking up prolonged sitting alters the postprandial plasma lipidomic profile of adults with type 2 diabetes. J Clin Endocrinol Metab 102: 1991–1999, 2017. doi: 10.1210/jc.2016-3926. [DOI] [PubMed] [Google Scholar]
- 17.Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97: 495–528, 2017. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green DJ, O’Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol (1985) 105: 766–768, 2008. doi: 10.1152/japplphysiol.01028.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 9: 58–65, 2008. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 20.Jefferis BJ, Parsons TJ, Sartini C, Ash S, Lennon LT, Papacosta O, Morris RW, Wannamethee SG, Lee IM, Whincup PH. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med bjsports-2017-098733, 2018. doi: 10.1136/bjsports-2017-098733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jefferis BJ, Sartini C, Lee I-M, Choi M, Amuzu A, Gutierrez C, Casas JP, Ash S, Lennnon LT, Wannamethee SG, Whincup PH. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health 14: 382, 2014. doi: 10.1186/1471-2458-14-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587: 5551–5558, 2009. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenward MG, Roger JH. The use of baseline covariates in crossover studies. Biostatistics 11: 1–17, 2010. doi: 10.1093/biostatistics/kxp046. [DOI] [PubMed] [Google Scholar]
- 24.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, Cercy K, Vos T, Murray CJL, Forouzanfar MH. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ 354: i3857, 2016. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lautenschlager NT, Cox K, Cyarto EV. The influence of exercise on brain aging and dementia. Biochim Biophys Acta 1822: 474–481, 2012. doi: 10.1016/j.bbadis.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Miyazawa T, Shibata S, Nagai K, Hirasawa A, Kobayashi Y, Koshiba H, Kozaki K. Relationship between cerebral blood flow estimated by transcranial Doppler ultrasound and single-photon emission computed tomography in elderly people with dementia. J Appl Physiol (1985) 125: 1576–1584, 2018. doi: 10.1152/japplphysiol.00118.2018. [DOI] [PubMed] [Google Scholar]
- 27.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol (1985) 107: 1370–1380, 2009. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- 28.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 104: 5638–5643, 2007. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Physical Activity Guidelines Advisory Committee 2018 Physical Activity Guidelines Advisory Committee Scientific Report (Scientific Report). Washington, DC: Department of Health and Human Services, 2018. [Google Scholar]
- 30.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96: 13427–13431, 1999. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabe-Hesketh S, Yang S, Pickles A. Multilevel models for censored and latent responses. Stat Methods Med Res 10: 409–427, 2001. doi: 10.1177/096228020101000604. [DOI] [PubMed] [Google Scholar]
- 32.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol 100: 829–838, 2015. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddarth P, Burggren AC, Eyre HA, Small GW, Merrill DA. Sedentary behavior associated with reduced medial temporal lobe thickness in middle-aged and older adults. PLoS One 13: e0195549, 2018. doi: 10.1371/journal.pone.0195549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith KJ, MacLeod D, Willie CK, Lewis NC, Hoiland RL, Ikeda K, Tymko MM, Donnelly J, Day TA, MacLeod N, Lucas SJE, Ainslie PN. Influence of high altitude on cerebral blood flow and fuel utilization during exercise and recovery. J Physiol 592: 5507–5527, 2014. doi: 10.1113/jphysiol.2014.281212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM. Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol (1985) 111: 1066–1071, 2011. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- 36.Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc 47: 843–849, 2015. doi: 10.1249/MSS.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 37.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 38.Verbree J, Bronzwaer A, van Buchem MA, Daemen M, van Lieshout JJ, van Osch M. Middle cerebral artery diameter changes during rhythmic handgrip exercise in humans. J Cereb Blood Flow Metab 37: 2921–2927, 2017. doi: 10.1177/0271678X16679419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wendel-Vos GC, Schuit AJ, Feskens EJ, Boshuizen HC, Verschuren WM, Saris WH, Kromhout D. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol 33: 787–798, 2004. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler MJ, Dempsey PC, Grace MS, Ellis KA, Gardiner PA, Green DJ, Dunstan DW. Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimers Dement (N Y) 3: 291–300, 2017. doi: 10.1016/j.trci.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 301: 1565–1572, 2009. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011. doi: 10.1016/j.jneumeth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D, Feldman H, Fratiglioni L, Frisoni GB, Gauthier S, Georges J, Graff C, Iqbal K, Jessen F, Johansson G, Jönsson L, Kivipelto M, Knapp M, Mangialasche F, Melis R, Nordberg A, Rikkert MO, Qiu C, Sakmar TP, Scheltens P, Schneider LS, Sperling R, Tjernberg LO, Waldemar G, Wimo A, Zetterberg H. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol 15: 455–532, 2016. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]