Abstract
A cognitive challenge when imposed during a low-force isometric contraction will exacerbate sex- and age-related decreases in force steadiness, but the mechanism is not known. We determined the role of oscillations in the common synaptic input to motor units on force steadiness during a muscle contraction with a concurrent cognitive challenge. Forty-nine young adults (19–30 yr; 25 women, 24 men) and 36 old adults (60–85 yr; 19 women, 17 men) performed a cognitive challenge (counting backward by 13) during an isometric elbow flexion task at 5% of maximal voluntary contraction. Single-motor units were decomposed from high-density surface EMG recordings. For a subgroup of participants, motor units were matched during control and cognitive challenge trials, so the same motor unit was analyzed across conditions. Reduced force steadiness was associated with greater oscillations in the synaptic input to motor units during both control and cognitive challenge trials (r = 0.45–0.47, P < 0.01). Old adults and young women showed greater oscillations in the common synaptic input to motor units and decreased force steadiness when the cognitive challenge was imposed, but young men showed no change across conditions (session × age × sex, P < 0.05). Oscillations in the common synaptic input to motor units is a potential mechanism for altered force steadiness when a cognitive challenge is imposed during low-force contractions in young women and old adults.
NEW & NOTEWORTHY We found that oscillations in the common synaptic input to motor units were associated with a reduction in force steadiness when a cognitive challenge was imposed during low-force contractions of the elbow flexor muscles in young women and old men and women but not young men. Age- and sex-related muscle weakness was associated with these changes.
Keywords: aging, arousal, force fluctuations, sex differences
INTRODUCTION
Old adults are less steady than young adults during isometric contractions for several different muscle groups, including the first dorsal interosseous, hip and knee extensors, and the elbow flexor muscles (22, 25, 58, 72). The lower force steadiness that occurs with aging can be quantified as larger fluctuations in force output when normalized to the mean target force [coefficient of variation (CV)] (15). Furthermore, an age difference in force steadiness was reported to be greatest at very low forces, such as 5% of maximal voluntary contraction (MVC) compared with high intensities of contraction (22, 70, 72). A difficult cognitive challenge imposed during a contraction will exacerbate the age-related increase in CV of force (i.e., reduce force steadiness) during low-force contractions (58, 76, 77). This suggests that altering the cortical inputs to motor units through a cognitive challenge can alter the mechanisms contributing to the age-related differences in CV of force.
Women were observed to have greater CV of force during low-force isometric tasks for both the upper and lower limb muscles compared with men (31). Furthermore, the increase in the CV of force with either imposition of a cognitive challenge during a motor task or after a stressful event is larger for women than for men for the upper limb muscles (8, 54, 58). However, this was not observed for the lower limb (76) where men and women were more comparable in strength (21, 48). The mechanisms for the sex differences in CV of force with imposition of a cognitive challenge in the upper limb and for any potential interactions between sex and age are yet to be determined. Understanding such mechanisms is important because greater CV of force is associated with poor performance in laboratory-based functional tests of the upper extremity for both young and old adults (26, 38, 43) that are similar to activities of daily living.
In young and older adults, force steadiness is modulated primarily by oscillations in the common synaptic input to motor units (6, 12, 17). However, the contribution of the common modulation of synaptic input to motor units on force fluctuations with increased age in both men and women is not known. The mechanisms leading to oscillations in the common synaptic input to motor units can arise from several sources such as variations in the descending commands (79) and sensory feedback (41, 42). Cortical oscillations can modulate the synaptic input to motor units, as shown in simulated and experimental data with young adults (50, 51). However, there is minimal information regarding the influence of various levels of cognitive demand on the synaptic input to motor units in young or old men and women (10, 30). This has functional implications because motor tasks are frequently performed simultaneously with a cognitive task. In young women, motor unit activity (i.e., recruitment threshold, discharge rate at recruitment or de-recruitment, and variability of discharge rate) of the trapezius muscle did not change with imposition of cognitive challenge (67). Yet in the wrist extensors of young men and women there was a reduction in the variability of discharge rate of single-motor units (3), suggesting the possibility of muscle specificity on the effects of cognitive challenge during motor tasks. At higher oscillations frequencies that have minimum influence in force steadiness (i.e., α: ∼8- to 14-Hz bands; β: ∼15- to 32-Hz bands), a cognitive challenge will increase the synchronized oscillatory activation between corticospinal cells and motor units in old but not young adults (32). Whether the oscillations in the common modulation of the synaptic input to motor units is altered more among old adults and women and whether there are associations with the typical increase in CV of force that occurs with the imposition of a cognitive challenge are not known.
Thus, the aim of this study was twofold: 1) to compare the oscillations in the synaptic input to motor units between young and old adults and men and women during a low-intensity isometric contraction with and without imposition of a cognitive challenge and 2) to determine whether the low-force steadiness with a cognitive challenge imposed was associated with greater oscillations in the common synaptic input to motor units in young and old men and women. We hypothesized that a cognitive challenge imposed during a low-intensity isometric contraction would increase the oscillations in the common synaptic input. These alterations in the common synaptic input to motor units will contribute to reduced force steadiness. We also hypothesized that the changes in the common synaptic input to motor units and altered force steadiness with the cognitive challenge would be greater in old adults and women compared with the young men because of age and sex differences in motor unit properties (7, 30). To accomplish these aims, we used high-density surface EMG to record and decompose single-motor unit activity and discharge rates during a low-intensity contraction with the elbow flexor muscles. We also recorded indices of arousal and anxiety, including cardiovascular measures and self-reported anxiety during the experiments.
METHODS
Participants
Forty-nine young adults (19–30 yr) and 36 old adults (60–85 yr) volunteered to participate in the study (Table 1). All participants were without any known or reported neurological, neuropsychological, orthopedic, or cardiovascular conditions. Physical activity levels for each participant were assessed with a questionnaire that estimated the relative kilocalorie expenditure of energy per week in routinely performed physically engaging activities (39). All participants provided written, informed consent to participate in the study, and compensation was provided. The protocol was approved by the Institutional Review Board at Marquette University (protocol no. HR-1466).
Table 1.
Demographic and physical characteristics of young and old individuals
| Variable | Young Men (n = 24) | Young Women (n = 25) | Old Men (n = 15) | Old Women (n = 19) |
|---|---|---|---|---|
| Age, yr | 22.1 ± 3.1 | 21.6 ± 2.5 | 68.5 ± 5.6 | 66.8 ± 5.9 |
| Height, cm | 179.5 ± 0.1 | 166.6 ± 0.1 | 163.8 ± 0.1 | 160.1 ± 0.1 |
| Mass, kg | 78.9 ± 14.1 | 66.7 ± 9.9 | 79.8 ± 10.7 | 72.9 ± 15.2 |
| Physical activity level, MET-h/wk | 65.0 ± 54.1 | 54.1 ± 47.4 | 30.4 ± 21.9 | 31.5 ± 20.3 |
| Traits of anxiety, AU | 35.0 ± 8.1 | 30.9 ± 6.2 | 30.4 ± 8.4 | 29.5 ± 6.1 |
| SDMT oral (correct) | 74.9 ± 14.7 | 75.5 ± 12.5 | 56.1 ± 8.7 | 55.5 ± 10.6 |
| SDMT written (correct) | 60.7 ± 11.9 | 67.5 ± 8.5 | 44.8 ± 7.6 | 53.8 ± 11.4 |
| LNST, total raw score | 11.2 ± 3.1 | 11.6 ± 2.2 | 9.4 ± 1.7 | 8.9 ± 2.5 |
| AVLT, immediate recall | 11.4 ± 2.7 | 12.8 ± 1.7 | 7.0 ± 4.0 | 10.5 ± 3.3 |
| AVLT, delayed recall | 11.3 ± 2.5 | 12.6 ± 1.8 | 7.1 ± 3.9 | 10.6 ± 3.0 |
| MVC, Nm | ||||
| Control session | 69.4 ± 18.5 | 40.6 ± 9.9* | 53.5 ± 11.1 | 30.3 ± 4.7† |
| CC session | 68.5 ± 18.3 | 40.1 ± 10.5* | 54.1 ± 10.6 | 30.1 ± 3.4† |
| MDR, pulse/s | ||||
| Control session | 11.5 ± 2.0 | 11.7 ± 2.6 | 11.1 ± 1.9 | 10.8 ± 1.0 |
| CC session | 11.3 ± 1.5 | 12.5 ± 2.4 | 10.7 ± 1.6 | 12.0 ± 2.3 |
| ISI, ms | ||||
| Control session | 85.6 ± 16.6 | 90.2 ± 17.5 | 90.9 ± 20.1 | 87.7 ± 14.1 |
| CC session | 85.4 ± 14.6 | 87.9 ± 16.3 | 92.1 ± 17.6 | 86.5 ± 17.3 |
Values are means ± SD. AU, arbitrary units; AVLT, auditory verbal learning test; CC, cognitive challenge; ISI, interspike interval; LNST, letter number sequencing test; MDR, mean discharge rate; MET, metabolic equivalents; MMSE, mini-mental state examination; MVC, maximal voluntary contraction; SDMT, symbol digit modalities test. Traits of anxiety were estimated with State-Trait Anxiety Inventory.
Sex difference for young adults (P < 0.001);
sex difference for old adults (P < 0.001).
Force
The elbow flexor muscles were tested because large age- and sex-related differences in force steadiness are observed during low-force tasks in this muscle group (5, 23, 58). Each participant was seated upright in an adjustable chair, with the nondominant arm abducted slightly to the participant’s side and the elbow resting on a padded support with the elbow joint flexed to 90°. The setup was similar to that described previously (57, 58). In brief, the forearm was placed in a modified wrist/hand/thumb orthosis (Orthomerica, Newport Beach, CA), and the forearm was placed midway between pronation and supination. Elbow flexion force was measured with a linear force transducer (MLP; resolution: 0.10 N; 150 Transducer Techniques, Temecula, CA,) for one set of experiments and a JR-3 Force-Moment Sensor (resolution: 0.10 N; JR-3, Woodland, CA) for a second set of experiments securing that recorded force signal was linear and similar between force transducers. The force output was digitized at 500 samples/s by a Power 1401 analog-to-digital converter with Spike 2 software [Cambridge Electronic Design (CED), Cambridge, UK]. A 22-inch monitor placed 1.5 m in front each participant was used to display the force output. Force feedback of the MVC was provided with a constant y-axis in a 10-s window. During submaximal contractions, the force feedback consisted of a line moving from left to right and a constantly moving x-axis window of 20 s. Visual gain had minimal influence on the increase in CV of force with the imposition of a cognitive challenge (56).
Electromyography
Motor unit behavior was extracted from surface electromyography (EMG) recordings of the biceps brachii using a multichannel linear array of 64 surface EMG electrodes (13 rows, 5 columns, and 8 mm of inter-electrode distance, ELSCH064R3S; OT Bioelettronica, Turin, Italy) (18, 52). Previous reports indicated reliable motor unit decompositions in this muscle using two-source validation (28). The EMG array was placed over the muscle, and the 13 rows were oriented with the typically known direction of the muscle fibers over the biceps brachii muscle. The propagation of the action potentials was determined by visualization using a probe. The array electrode was then positioned and slightly adjusted to maximize the amplitude and propagation of the action potentials (4, 46). The reference strap electrode was placed on the ulnar styloid process on the contralateral arm. The EMG signal was amplified (×1,000–5,000) to maximize the amplitude of the recorded action potentials in each participant and band pass filtered (10–500 Hz) before being recorded at 2,048 Hz with an EMG-USB2 amplifier (OT Bioelettronica).
Cardiovascular Measurements
Heart rate and blood pressure were monitored with an automated beat-by-beat blood pressure monitor (Finapres 2300; Ohmeda, Englewood, CO). The blood pressure cuff was placed around the middle finger of the relaxed dominant hand with the arm placed on a table adjacent to the subject at heart level. The blood pressure signal was digitized at 500 samples/s with a Power 1401 analog-to-digital converter with Spike 2 software (CED).
Assessments of Anxiety
Individual differences in anxiety proneness were assessed during the familiarization session with the trait portion of the State-Trait Anxiety Inventory (STAI; 20 questions in a four-point Likert-type scale) (65). Acute changes in anxiety were assessed during the contraction with a visual analog scale (VAS; 0 to 10) anchored at the far left by “not at all anxious” and at the far right by “very anxious” (35, 81). The right anchor corresponded to the most anxious moment in the life of the subject. Anxiety was defined as the negative feelings regarding the immediate future. The VAS for anxiety was recorded immediately after each contraction.
Cognitive Function Assessment
Neuropsychological tests were administered to screen for normal ranges of memory and executive function. All the participants were within the normal range of cognitive function for their age, sex, and level of education. Dementia screening was conducted with the Mini-Mental State Examination (all scores >24 of 30) (19). Depression was screened for with the Geriatric Depression Scale (all scores <5 and within normal range of 0–9 of 30) (61). Episodic memory was assessed using the Auditory Verbal Learning Test (60, 75). Tests assessing executive function (processing speed, attention, working memory) included the Letter-Number Sequencing test (80) and the Symbol-Digit Modalities test (34). All these tests were administered by a trained investigator (B. Schlinder-DeLap) and supervised by a neuropsychologist (K. A. Nielson).
Cognitive Challenge (Mental Math Task)
Mental math was used as the cognitive challenge to increase cognitive demand and arousal (33). Each participant was asked to perform serial subtraction from a four-digit number by 13, with one response required every 3 s (54). If the participant made an error in serial subtraction or was unable to provide the correct answer within 3 s, the mental math procedure was restarted with a new four-digit number (35, 76). In the cognitive challenge trial, mental math was performed continuously for 4 min before the submaximal contraction and also during the submaximal contraction (indicated by the horizontal arrows in Fig. 1). All participants were able to perform the cognitive challenge. There was no difference in error rates between groups (group effect, P > 0.05).
Fig. 1.

Experimental protocol. Top: elbow flexor muscle force is represented. Bottom: horizontal arrows indicating when the cognitive challenge (CC; mental math) was performed during the protocol. During the cognitive challenge trials, mental math was performed without muscle contraction for 4 min and also continuously during contraction at 5% of maximal voluntary contraction (MVC). In the control trials, each individual sat quietly for the 4 min and performed the isometric contraction with no cognitive challenge. Horizontal arrows also indicate when visual analog scale (VAS) for anxiety, mean arterial pressure (MAP), electromyography (EMG) collected with high-density multiple array surface electrodes, and force steadiness were recorded. Results of different time points for anxiety (A1 and A2) and MAP (MAP1, MAP2, and MAP3) are further indicated in the text. The schematic is not to scale for time or force.
Experimental Protocol
The protocols for each experimental condition (i.e., control and cognitive challenge trials) are shown in Fig. 1 and involved the following procedures: 1) assessment of the MVC of the elbow flexor and VAS for anxiety at baseline (A1 in Fig. 1), 2) 4 min without muscle contraction of either quiet sitting (control) or mental math (cognitive challenge), 3) contractions at 5% MVC with and without mental math, and 4) assessment of anxiety (VAS) during the submaximal contractions (A2).
The control trial (contractions without mental math task) and cognitive challenge trial (contractions with mental math) were performed either in different sessions separated by 1 wk for 23 young and 12 old adults or in the same session separated by 10 min for 26 young and 24 old adults. The same-session experiments allowed tracking of the same motor unit across the trials because the EMG electrode remained in the same position during the session. Tracking motor units across trials minimized the confounding factor of decomposing a set of motor units with greater inherent discharge rate variability (i.e., discharge rate not associated with the increased cognitive load) for the cognitive challenge trial compared with control trial or vice versa. No individual participated in both experimental protocols (i.e., the experimental protocol over 2 sessions versus the experimental protocol over 1 session). The order of the control and cognitive challenge trials were randomized and counterbalanced between groups. All participants were naive to the protocol. However, a familiarization session was performed 1 wk before the experimental trials to minimize any learning effect. The experimental setup and equipment used were similar in both experimental protocols and trials, except for the force transducer that differed across the experimental protocols. The JR-3 force transducer was used when tracking motor units with similar force recordings to the linear force transducer.
MVC task.
Each participant performed three to four MVCs with the elbow flexor muscles, with 60 s of rest between each trial. If the force outputs achieved for two of the first three trials were not within 5% of each other, additional trials were performed until this criterion was met. The greatest force achieved with the elbow flexor muscles was taken as the MVC and used to calculate the target line for the submaximal contractions.
Submaximal contractions.
Isometric contractions were performed with individuals tracing a target line at 5% MVC for ∼45 s each. During the cognitive challenge trials, participants began the subtraction by 13 from a four-digit number once they achieved the required target line. During the control trials, each participant performed the submaximal contraction without mental math imposed. A low intensity of 5% MVC was chosen, as previous research indicated that age differences in steadiness with a cognitive challenge occurred at lower contraction intensities (58).
Data Analysis
Steadiness was calculated as the amplitude of the force fluctuations using the coefficient of variation of the force (CV = standard deviation of the force/mean of force × 100) (15). The CV of force was calculated over the middle 30-s period of each ∼45-s submaximal contraction (58). Torque was calculated as the product of force and the distance between the elbow joint and the point at which the wrist was attached to the force transducer.
Single motor unit activity was decomposed from high-density surface EMG using a convolutive blind source separation technique, as previously validated (27, 53). Each motor unit recording with an interspike interval of >250 or <20 ms was excluded from the analysis (16, 52). Such strict criteria resulted in a relatively small but reliable subset of motor units for each contraction (see results).
After motor units were identified, the instantaneous discharge rates of each motor unit were smoothed with a Hann window (duration of 400 ms) and a high-pass filter (cutoff frequency of 0.75 Hz) to remove offsets and trends (13). The average of all smoothed lines of discharge rate was then calculated for each participant to represent the combined variability of all motor units decomposed in each contraction (see Fig. 2). The common low-frequency oscillation in the neural drive was calculated with the first principal component, as previously described (52), and referred to as the first common component. The CV was calculated as the ratio of the standard deviation of the first common component and its mean value. This metric is a reliable estimator of the oscillations in the common synaptic input to motor units (17, 52) and explains a large portion of the CV of force that is influenced by descending commands (50, 51). At very low-force contractions, the CV of force can also be influenced by random fluctuations in membrane potentials (i.e., synaptic noise) (14). To determine the influence of the cognitive challenge on synaptic noise, we calculated the CV of interspike interval without smoothing the spike trains. The CV of interspike interval in the full range of spike intervals (i.e., 20–250 ms) without smoothing the spike trains was calculated using the ratio between the standard deviation of discharges (11) and the number of discharges in the time interval, as previously proposed (66).
Fig. 2.
A and B: smoothed instantaneous motor unit discharge rate during the control (A) and cognitive challenge (CC) trials (B). Three motor units were decomposed and matched across the control and CC trials. C and D: the 3 components from the smoothed discharge rates for control (C) and CC (D); the bottom component is the common component. The first common component (FCC) increased from control to the CC motor tasks. E and F: smoothed force records at 5% MVC for control (E) and CC (F) trials. Coefficient of variation of force increased from 1.8 to 5.1% from control to CC trial. PPS, pulse per second.
To match motor units between the control and cognitive challenge trials, the two-dimensional cross-correlation between each pair of motor unit action potentials was calculated. The motor unit action potentials were estimated from the instantaneous discharge times using spike-triggered averaging on the EMG array in differential mode. Correlations >0.8 were considered a reliable indication of the same motor unit across trials. In the cases where two motor units had a high correlation with one motor unit, the pair with the highest correlation was chosen. This method was shown to be sensitive and reliable to track motor units across sessions with high-density surface EMG (44). Moreover, visual inspection of each matched motor unit potential pair was performed to avoid false-positive matching that can occur, for example, with common artifacts in both motor unit potentials.
The blood pressure signal was analyzed for the mean peaks [systolic blood pressure (SBP)] and mean troughs [diastolic blood pressure (DBP)]. Mean arterial pressure (MAP) was calculated for each epoch with the following equation: MAP = DBP + 1/3 (SBP − DBP) (20). Heart rate was also recorded during each trial, but the results are not shown because they were similar to the findings for MAP.
Statistical Analysis
Repeated-measures ANOVA was used to first determine whether the time between the control and cognitive challenge experiment (i.e., protocol interval of 1 wk for 35 participants vs. 10 min in the same session for 50 participants) produced different results for each dependent variable. In this case, the protocol intervals and groups (men vs. women, young vs. old) were between factors, and the condition (control vs. cognitive challenge trial) was the within factor. Because there was no main effect of protocol interval (see results), they were pooled, and a repeated-measures analysis of variance (ANOVA) with sex and age as between-subject factors was used to determine changes in the dependent variables (CV of force, mean discharge rate, CV of interspike interval, CV of the first common component, MAP, anxiety, MVC) across the conditions (control vs. cognitive challenge trials). Trait-anxiety was compared between groups using a two-factor ANOVA, with age and sex as independent variables. For each ANOVA, the sphericity of data was verified with Mauchly’s test. In cases where the F-test was significant, pairwise comparisons with Bonferroni corrections were performed to detect differences among pairs. When necessary, additional interpretation was performed by calculating the effect size with the partial eta squared (9, 36). Several associations were performed with the Spearman’s rank correlation coefficient due to non-normality of the MVC, increase in CV of force, MAP, and anxiety (tested with the Shapiro-Wilk test). The statistical significance was considered as P < 0.05, and all analyses were performed in IBM Statistical Package for Social Sciences (SPSS) version 23. Data are reported in means ± SD in the text and means ± SE in the figures.
RESULTS
The cognitive challenge trial performed on the same day as the control trial produced similar findings to when the cognitive challenge trial was performed on a different day for all variables extracted (MVC, steadiness, motor unit activity, MAP and anxiety, all P > 0.05). Thus, cognitive challenge results from the two protocols were combined. There was also no difference between same day and different day protocols for the control trial, so they were combined before further analysis.
Baseline MVC and Physical Characteristics
Table 1 summarizes the physical characteristics of the participants. Young adults were stronger than old adults (age effect: P < 0.001), and men were stronger than women (sex effect: P < 0.001) with no interaction (age × sex: P = 0.66). Maximal torque was similar across control and cognitive challenge trials (trial effect: P = 0.45) for young and old adults (trial × age: P = 0.39) and men and women (trial × sex: P = 0.98) (Table 1).
Motor Unit Behavior
There were 317 single-motor units decomposed [191 motor units for young adults (men: 122; women: 69) and 126 motor units for old adults (men: 82; women: 44)]. Motor unit recordings (117 of the 317) were matched across the control and cognitive challenge trials performed in the same session, so that the same motor unit was recorded during each trial. Of the 117 matched, 69 motor units were matched in the young adults (44 for men and 25 for women) and 48 motor units in the old adults (29 for men and 19 for women).
Motor unit discharge rate.
The mean motor unit discharge rate during the 5% MVC was similar for the young and old adults (age effect: P = 0.16) and similar between men and women (sex effect: P = 0.31), with no interaction (age × sex: P = 0.97) (Table 1). Mean discharge rate did not differ across the control and cognitive challenge trials (cognitive challenge effect: P = 0.44), with no interaction for age group (cognitive challenge effect × age: P = 0.81) or the sex of the participant (cognitive challenge effect × sex: P = 0.11). There were no interactions between cognitive challenge, age, and sex (P = 0.99; Table 1).
Oscillations in the common synaptic input to motor units.
Old adults had a greater CV of the first common component than young adults (average of control and cognitive challenge trials: 17.4 ± 6.8 vs. 13.1 ± 6.8%, respectively, age effect: P = 0.03). Women had a greater CV of the first common component compared with men (average of control and cognitive challenge trials: 18.4 ± 6.9 vs. 11.9 ± 6.8%. respectively, sex effect: P = 0.01), with no interaction (age × sex: P = 0.85). The CV of the first common component was greater during contractions with cognitive challenge than control trials (average of all groups: 17.5 ± 9.8 vs. 12.9 ± 5.5%, respectively; cognitive challenge effect: P < 0.001; effect size = 0.27), except for young men (cognitive challenge × sex × age: P = 0.02) (Fig. 3A). Thus, the young women, old men, and old women had greater oscillations in the common synaptic input to motor units during the 5% MVC, with the cognitive challenge imposed compared with no cognitive challenge.
Fig. 3.
Coefficient of variation (CV) of the first common component (FCC; A) and CV of force (B) quantified during contractions at 5% of maximal voluntary contraction (MVC) for young and old men and women. All groups increased both the CV of discharge rate and CV of force, except for young men (session × age × sex: P < 0.05 for both variables). Values are means ± SE. CC, cognitive challenge.
The CV of interspike interval was greater during the cognitive challenge trial compared with the control (average of all groups: 7.9 ± 2.1 vs. 7.0 ± 1.8%, respectively; cognitive challenge effect: P = 0.01; effect size = 0.10), with no interactions (all P > 0.05).
Force Steadiness
The CV of force was greater (i.e., lower force steadiness) during the cognitive challenge trial than during the control trial (cognitive challenge effect: P < 0.001) in old adults and young women but not in the young men (cognitive challenge effect × age × sex: P = 0.03) (Fig. 3B). During the control trial, old adults had greater CV of force compared with the young adults (3.1 ± 1.3 vs. 1.9 ± 0.8% respectively; age effect: P < 0.001), with no sex differences for the young (P = 0.95) or old adults (P = 0.24) and no interaction (age × sex: P = 0.23). Thus, when the cognitive challenge was imposed during the 5% MVC task, the young women, old men, and old women had a greater CV of force than when no cognitive challenge was imposed.
Associations: Force Steadiness, Motor Unit Discharge Rate Variability, MVC, and Error Rates
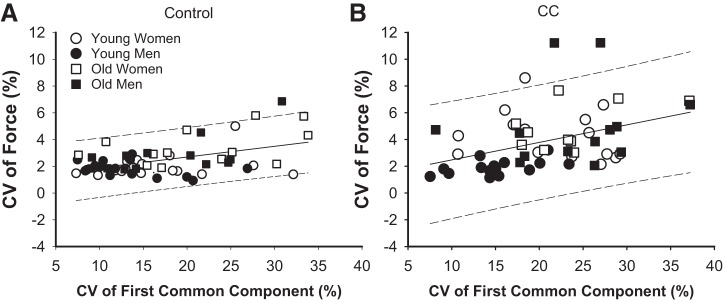
The CV of force was positively associated with the CV of the first common component during the control trial (r = 0.31, P < 0.01; Fig. 4A) and cognitive challenge trial (r = 0.50, P < 0.01; Fig. 4B). In addition, stronger individuals (greater MVC) had a lower CV of the first common component for both the control and cognitive challenge trials (r = −0.40, P < 0.01; and r = −0.44, P < 0.01, respectively). Similarly, stronger individuals also had a lower CV of force for both control and cognitive challenge trials (r = −0.40, P < 0.01; and r = −0.58; P < 0.01, respectively). There was no association between error rates in mental math and CV of force or CV of the first common component (P > 0.05 for all groups).
Fig. 4.
Associations between the coefficient of variation (CV) of force and CV of the first common component for the control trial (r =0.47, P < 0.05; A) and cognitive (CC) challenge trial (r = 0.45, P < 0.05; B). Solid lines, regression lines; dashed lines, 95% predicted interval.
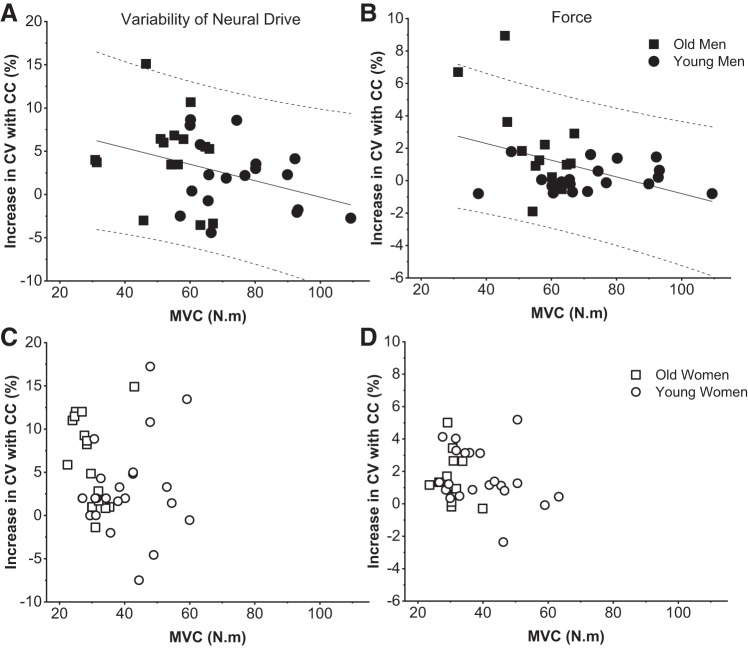
The difference between the control and cognitive challenge trial in the CV of the first common component was associated with baseline MVC for men but not women (r = −0.46, P = 0.01 vs. r = −0.25, P = 0.20, respectively) so that stronger men, but not women, had less change in the CV of the first common component with imposition of cognitive challenge (Fig. 5, A and C).
Fig. 5.
Associations between maximal voluntary contraction (MVC) and the increase in the coefficient of variation (CV) of the first common component (A and C) and force signal (B and D) during the cognitive challenge (CC) trial. Regression lines show that stronger young and old men had less increase in the CV of the first common component (r = −0.45, P < 0.05; A) and less increase in the CV of force (r = −0.44, P < 0.05; B) with imposition of the cognitive challenge. MVC was not associated with the increase in the CV of the first common component (C) or CV of force (D) for young and old women. Solid lines, regression lines; dashed lines, 95% predicted interval.
Furthermore, the difference between the cognitive challenge trial and control trial in CV of force was also associated with baseline MVC in men but not women (r = −0.44, P = 0.01 vs. r = 0.04, P = 0.79, respectively) so that stronger individuals had less of an increase in CV of force with imposition of cognitive challenge (Fig. 5, B and D).
Imposition of cognitive challenge minimally altered the CV of interspike interval without smoothing the spike trains (see Motor Unit Behavior and Force Steadiness With Aging in Men and Women). Thus, calculated differences between control and cognitive challenge trials for this variable were not associated with MVC or the increase in CV of force (all groups with P > 0.05).
Anxiety
Table 1 shows the baseline anxiety proneness assessed with the trait portion of the STAI for all groups. Young and old men and women had similar traits of anxiety (age effect: P = 0.08; sex effect: P = 0.10; age × sex: P = 0.34).
Acute changes in anxiety within the experimental protocol were also assessed with a VAS (0–10). At baseline (A1 in Fig. 1), VAS was similar between groups (age effect: P = 0.29; sex effect: P = 0.50; age × sex: P = 0.24) and between control and cognitive challenge trials (1.1 ± 1.1 v.s 1.2 ± 1.3 arbitrary units, respectively; cognitive challenge effect: P = 0.37) for young and old adults (cognitive challenge × age: P = 0.46) or men and women (cognitive challenge × sex: P = 0.16). During the 5% MVC task (A2 in Fig. 1), VAS for anxiety was greater during the cognitive challenge trial compared with control trial (cognitive challenge effect: P < 0.001). Old adults had greater increases in VAS with imposition of cognitive challenge compared with young adults (5.1 ± 2.2 vs. 3.4 ± 2.3 arbitrary units respectively; cognitive challenge × age: P = 0.01), with no interaction of sex (cognitive challenge × sex: P = 0.45) in either age group (cognitive challenge ×age × sex: P = 0.38). The increase in VAS with cognitive challenge was not associated with CV of force or CV of the first common component.
Mean Arterial Pressure
At baseline, mean arterial pressure (MAP) was similar for all groups (age, sex, or age × sex, all with P > 0.05; MAP1 in Fig. 1). MAP increased when the cognitive challenge was performed without muscle contraction (MAP2 in Fig. 1) compared with control trials (87.5 ± 11.9 vs. 107.2 ± 14.4 mmHg, respectively, cognitive challenge effect: P < 0.001). This increase was similar for young and old adults (cognitive challenge × age: P = 0.47) and between men and women (cognitive challenge × sex: P = 0.27). with no other interaction (cognitive challenge effect × age × sex: P = 0.15).
During 5% MVC contractions (MAP3 in Fig. 1), the MAP was greater during the cognitive challenge trial compared with control trial (89.5 ± 13.7 vs. 106.3 ± 16.1 mmHg. respectively; cognitive challenge effect: P < 0.001). The increase in the MAP with cognitive challenge was similar in the young and old adults (cognitive challenge × age: P = 0.17). There was no sex effect or interactions (all with P > 0.05). There was no association between MAP and CV of force or CV of the first common component (all with P > 0.05).
DISCUSSION
A cognitive challenge performed during a low-intensity contraction with the elbow flexor muscles increased the oscillations in the common synaptic input to motor units of biceps brachii, which was represented by the greater CV of the first common component. The greater CV of the first common component was accompanied by a decrease in force steadiness (increased CV of force) in the young women and old men and women, but not in the young men. For both the control and cognitive challenge trials, a higher CV of force was associated with higher CV of the first common component. Furthermore, the change in CV of force between the control trial and cognitive challenge trial was associated with strength (MVC force) for men only (young and old pooled), with stronger individuals less likely to have an increased CV of force during the cognitive challenge trial.
Motor Unit Behavior and Force Steadiness with Aging in Men and Women
During a muscle contraction, a motor neuron receives tens of thousands of inputs that will drive the train of motor unit action potentials and the variability in the timing of their discharge (17). In young and older adults, these oscillations in the common input to motor units at low frequencies (∼1–2 Hz) have strong influence on force fluctuations (6, 17). To evaluate the oscillations in the common input to motor units, we used the CV of the first common component (52). The imposition of cognitive challenge elicited different magnitudes of change between the young and old men and women shown in Fig. 3. At very low forces, synaptic noise can also alter CV of force (14, 45). The CV of interspike interval, that is, a metric more sensitive to synaptic noise, was altered to a lesser magnitude with imposition of cognitive challenge (small effect size = 0.10) compared with the relatively larger oscillations in the common synaptic input to motor units (medium size = 0.27). Thus, synaptic noise and its effects on the time course of the postspike after the hyperpolarization phase of the motor neuron, had less influence on the increase in the CV of force with the imposed cognitive challenge.
Age and sex differences were evident in our findings. Imposition of the cognitive challenge led to a large increase in the CV of force and CV of the first common component for the old men and women (Fig. 3). Young women also had an increase in the CV of the first common component and CV of force during the cognitive challenge trial, but the young men showed no change in either variable. Thus, this sex difference was not strong in old adults compared with young adults. The reason for the lack of change in both force steadiness and CV of the first common component in the young men is not clear. However, strength differences between the groups may account for some of these findings. The initial strength (MVC) was associated with increase in the CV of force and CV of the first common component in men but not in the women (Fig. 5). The lack of association between MVC magnitude and CV of force or CV of the first common component in women could be due to a more narrow range of MVC among the women compared with men in our study (20–60 vs. 30–110 N/m, respectively). Because women frequently have lower MVC than men in the elbow flexor muscles (2), it is difficult to determine whether the larger increase in CV of force in women during cognitive challenge trial is truly a sex difference or associated with strength alone. Although the CV of force calculation already accounts for the mean force during the task (i.e., CV = standard deviation of force/mean of force), a negative association between MVC and CV of force during contraction without cognitive challenge was reported (5, 43, 62–64). The reasons for this association require further examination, with potential factors including mechanical properties such as tendon stress that was shown to be negatively associated with CV of force (62, 63).
Regardless of the mechanisms involved, the association between MVC and increase in CV of force or CV of the first common component has potentially significant implications for old adults. For example, it was previously observed that CV of force was reduced in young and old adults after a strength training program (24, 29, 38, 40, 55, 71). Because our data show an association between baseline MVC and CV of force during the cognitive challenge trial, a strength training program has the potential to improve force steadiness not only during control contractions but also when old adults perform cognitively challenging tasks during a low-force motor task. This is important for old adults who are usually weaker (30) and have lower tendon stiffness (49) than young adults.
Cortical Interference on Motor Neuron Behavior with a Cognitive Challenge
The greater oscillations in neural drive during the cognitive challenge trial may be triggered by oscillations arising from the cortex. The mental math used in the current study taxed the working memory that is largely processed in the prefrontal cortex (1, 47). The prefrontal cortex is connected to multiple motor areas, including the lateral premotor cortex (area 6) (68, 69). Thus, heightened levels of cognitive demand induced by the mental math can potentially create activation signal interference from the prefrontal cortex to motor areas, producing disruption to the oscillations in effective neural drive to motor neuron pool. Accordingly, previous studies show that mental math imposed during contractions of the first dorsal interosseous increased CV of force and also increased the oscillatory activity between cortex and muscle revealed by corticomuscular coherence (32). The current study suggests that imposition of a cognitive challenge during a motor task may increase the oscillations in common synaptic input to the motor neuron.
The disruption of the oscillations in the common synaptic input to the motor neuron pool with imposition of cognitive challenge affected only old adults and the young women. Age and sex differences in cortical function may explain these findings. For example, Johnson and Shinohara (32) reported that old men and women had greater increases in the corticomuscular coherence than young adults when a cognitive challenge was performed simultaneously with a finger abduction task. Furthermore, although previous studies found no sex differences in the common oscillatory activity between cortex and muscle during control conditions (i.e., no cognitive challenge) (73, 74), young women had greater intra- and interhemispheric oscillatory activity during cognitive challenging tasks similar to the one used in the current study (37, 59, 78). Certainly, there is opportunity for further studies to understand the complex interactions between the disruption to neural drive and motor behavior in young and old men and women with imposition of a cognitive challenge.
To conclude, we showed that force steadiness decreased by imposing a cognitive challenge in the old men and women and young women but not in the young men. The primary mechanism for the decrease in force steadiness involved an increase in the oscillations of common synaptic input to motor units. The reduced force steadiness observed during a cognitively challenging task was lower in stronger individuals, which may have implications for old adults due to age-related decreases in strength.
GRANTS
This study was supported by a National Institute on Aging award (R15-AG-039697) to S. K. Hunter and the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 702491 (NeuralCon) to F. Negro and D. Farina.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.M.P., K.G.K., F.N., D.F., A.S.H., K.A.N., and S.K.H. conceived and designed research; H.M.P. and B.S.-D. performed experiments; H.M.P., F.N., and K.A.N. analyzed data; H.M.P., K.G.K., F.N., D.F., A.S.H., K.A.N., and S.K.H. interpreted results of experiments; H.M.P. prepared figures; H.M.P., K.G.K., F.N., D.F., A.S.H., K.A.N., and S.K.H. drafted manuscript; H.M.P., K.G.K., F.N., D.F., A.S.H., K.A.N., and S.K.H. edited and revised manuscript; H.M.P., B.S.-D., K.G.K., F.N., D.F., A.S.H., K.A.N., and S.K.H. approved final version of manuscript.
REFERENCES
- 1.Ashcraft MH, Krause JA. Working memory, math performance, and math anxiety. Psychon Bull Rev 14: 243–248, 2007. doi: 10.3758/BF03194059. [DOI] [PubMed] [Google Scholar]
- 2.Askew LJ, An KN, Morrey BF, Chao EY. Isometric elbow strength in normal individuals. Clin Orthop Relat Res (222): 261–266, 1987. [PubMed] [Google Scholar]
- 3.Bensoussan L, Duclos Y, Rossi-Durand C. Modulation of human motoneuron activity by a mental arithmetic task. Hum Mov Sci 31: 999–1013, 2012. doi: 10.1016/j.humov.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Beretta Piccoli M, Rainoldi A, Heitz C, Wüthrich M, Boccia G, Tomasoni E, Spirolazzi C, Egloff M, Barbero M. Innervation zone locations in 43 superficial muscles: toward a standardization of electrode positioning. Muscle Nerve 49: 413–421, 2014. doi: 10.1002/mus.23934. [DOI] [PubMed] [Google Scholar]
- 5.Brown RE, Edwards DL, Jakobi JM. Sex differences in force steadiness in three positions of the forearm. Eur J Appl Physiol 110: 1251–1257, 2010. doi: 10.1007/s00421-010-1600-x. [DOI] [PubMed] [Google Scholar]
- 6.Castronovo AM, Mrachacz-Kersting N, Stevenson AJT, Holobar A, Enoka RM, Farina D. Decrease in force steadiness with aging is associated with increased power of the common but not independent input to motor neurons. J Neurophysiol 120: 1616–1624, 2018. doi: 10.1152/jn.00093.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celichowski J, Drzymała H. Differences between properties of male and female motor units in the rat medial gastrocnemius muscle. J Physiol Pharmacol 57: 83–93, 2006. [PubMed] [Google Scholar]
- 8.Christou EA, Jakobi JM, Critchlow A, Fleshner M, Enoka RM. The 1- to 2-Hz oscillations in muscle force are exacerbated by stress, especially in older adults. J Appl Physiol (1985) 97: 225–235, 2004. doi: 10.1152/japplphysiol.00066.2004. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Mahwah, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 10.Corp DT, Drury HG, Young K, Do M, Perkins T, Pearce AJ. Corticomotor responses to attentionally demanding motor performance: a mini-review. Front Psychol 4: 165, 2013. doi: 10.3389/fpsyg.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox DR. Renewal Theory. London: Butle & Tanner, 1962. [Google Scholar]
- 12.De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends Neurosci 17: 299–305, 1994. doi: 10.1016/0166-2236(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 13.De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329: 129–142, 1982. doi: 10.1113/jphysiol.1982.sp014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dideriksen JL, Negro F, Enoka RM, Farina D. Motor unit recruitment strategies and muscle properties determine the influence of synaptic noise on force steadiness. J Neurophysiol 107: 3357–3369, 2012. doi: 10.1152/jn.00938.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol 13: 1–12, 2003. doi: 10.1016/S1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 16.Enoka RM, Robinson GA, Kossev AR. Task and fatigue effects on low-threshold motor units in human hand muscle. J Neurophysiol 62: 1344–1359, 1989. doi: 10.1152/jn.1989.62.6.1344. [DOI] [PubMed] [Google Scholar]
- 17.Farina D, Negro F. Common synaptic input to motor neurons, motor unit synchronization, and force control. Exerc Sport Sci Rev 43: 23–33, 2015. doi: 10.1249/JES.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 18.Farina D, Negro F, Muceli S, Enoka RM. Principles of motor unit physiology evolve with advances in technology. Physiology (Bethesda) 31: 83–94, 2016. doi: 10.1152/physiol.00040.2015. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198, 1975. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Fox SI. Human Physiology. New York: MacGraw-Hill, 2003. [Google Scholar]
- 21.Frey-Law LA, Laake A, Avin KG, Heitsman J, Marler T, Abdel-Malek K. Knee and elbow 3D strength surfaces: peak torque-angle-velocity relationships. J Appl Biomech 28: 726–737, 2012. doi: 10.1123/jab.28.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol 69: 2108–2115, 1993. doi: 10.1152/jn.1993.69.6.2108. [DOI] [PubMed] [Google Scholar]
- 23.Graves AE, Kornatz KW, Enoka RM. Older adults use a unique strategy to lift inertial loads with the elbow flexor muscles. J Neurophysiol 83: 2030–2039, 2000. doi: 10.1152/jn.2000.83.4.2030. [DOI] [PubMed] [Google Scholar]
- 24.Griffin L, Painter PE, Wadhwa A, Spirduso WW. Motor unit firing variability and synchronization during short-term light-load training in older adults. Exp Brain Res 197: 337–345, 2009. doi: 10.1007/s00221-009-1920-4. [DOI] [PubMed] [Google Scholar]
- 25.Grunte I, Hunter GR, McCurry BD, Bolding MS, Roy JL, McCarthy JP. Age and gender differences in hip extension and flexion torque steadiness. Gerontology 56: 533–541, 2010. doi: 10.1159/000311935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton LD, Thomas E, Almuklass AM, Enoka RM. A framework for identifying the adaptations responsible for differences in pegboard times between middle-aged and older adults. Exp Gerontol 97: 9–16, 2017. doi: 10.1016/j.exger.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holobar A, Farina D. Blind source identification from the multichannel surface electromyogram. Physiol Meas 35: R143–R165, 2014. doi: 10.1088/0967-3334/35/7/R143. [DOI] [PubMed] [Google Scholar]
- 28.Holobar A, Minetto MA, Botter A, Negro F, Farina D. Experimental analysis of accuracy in the identification of motor unit spike trains from high-density surface EMG. IEEE Trans Neural Syst Rehabil Eng 18: 221–229, 2010. doi: 10.1109/TNSRE.2010.2041593. [DOI] [PubMed] [Google Scholar]
- 29.Hortobágyi T, Tunnel D, Moody J, Beam S, DeVita P. Low- or high-intensity strength training partially restores impaired quadriceps force accuracy and steadiness in aged adults. J Gerontol A Biol Sci Med Sci 56: B38–B47, 2001. doi: 10.1093/gerona/56.1.B38. [DOI] [PubMed] [Google Scholar]
- 30.Hunter SK, Pereira HM, Keenan KG. The aging neuromuscular system and motor performance. J Appl Physiol (1985) 121: 982–995, 2016. doi: 10.1152/japplphysiol.00475.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakobi JM, Haynes EMK, Smart RR. Is there sufficient evidence to explain the cause of sexually dimorphic behaviour in force steadiness? Appl Physiol Nutr Metab 43: 1207–1214, 2018. doi: 10.1139/apnm-2018-0196. [DOI] [PubMed] [Google Scholar]
- 32.Johnson AN, Shinohara M. Corticomuscular coherence with and without additional task in the elderly. J Appl Physiol (1985) 112: 970–981, 2012. doi: 10.1152/japplphysiol.01079.2011. [DOI] [PubMed] [Google Scholar]
- 33.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31: 151–178, 2006. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman AS, Lichtenberger E. Assessing Adolescent and Adult Intelligence (3rd ed.). Hoboken, NJ: Wiley, 2006. [Google Scholar]
- 35.Keller-Ross ML, Pereira HM, Pruse J, Yoon T, Schlinder-Delap B, Nielson KA, Hunter SK. Stressor-induced increase in muscle fatigability of young men and women is predicted by strength but not voluntary activation. J Appl Physiol (1985) 116: 767–778, 2014. doi: 10.1152/japplphysiol.01129.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keppel G. Design and Analysis: a Researcher’s Handbook. Englewood Cliffs, NJ: Prentice Hall, 1991. [Google Scholar]
- 37.Kober SE, Reichert JL, Neuper C, Wood G. Interactive effects of age and gender on EEG power and coherence during a short-term memory task in middle-aged adults. Neurobiol Aging 40: 127–137, 2016. doi: 10.1016/j.neurobiolaging.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Kornatz KW, Christou EA, Enoka RM. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol (1985) 98: 2072–2080, 2005. doi: 10.1152/japplphysiol.01149.2004. [DOI] [PubMed] [Google Scholar]
- 39.Kriska AM, Bennett PH. An epidemiological perspective of the relationship between physical activity and NIDDM: from activity assessment to intervention. Diabetes Metab Rev 8: 355–372, 1992. doi: 10.1002/dmr.5610080404. [DOI] [PubMed] [Google Scholar]
- 40.Laidlaw DH, Kornatz KW, Keen DA, Suzuki S, Enoka RM. Strength training improves the steadiness of slow lengthening contractions performed by old adults. J Appl Physiol (1985) 87: 1786–1795, 1999. doi: 10.1152/jappl.1999.87.5.1786. [DOI] [PubMed] [Google Scholar]
- 41.Laine CM, Yavuz SU, Farina D. Task-related changes in sensorimotor integration influence the common synaptic input to motor neurones. Acta Physiol (Oxf) 211: 229–239, 2014. doi: 10.1111/apha.12255. [DOI] [PubMed] [Google Scholar]
- 42.Lodha N, Christou EA. Low-frequency oscillations and control of the motor output. Front Physiol 8: 78, 2017. doi: 10.3389/fphys.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marmon AR, Pascoe MA, Schwartz RS, Enoka RM. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc 43: 560–567, 2011. doi: 10.1249/MSS.0b013e3181f3f3ab. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Valdes E, Negro F, Laine CM, Falla D, Mayer F, Farina D. Tracking motor units longitudinally across experimental sessions with high-density surface electromyography. J Physiol 595: 1479–1496, 2017. doi: 10.1113/JP273662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol 492: 597–628, 1996. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIntosh KC, Gabriel DA. Reliability of a simple method for determining muscle fiber conduction velocity. Muscle Nerve 45: 257–265, 2012. doi: 10.1002/mus.22268. [DOI] [PubMed] [Google Scholar]
- 47.Menon V, Mackenzie K, Rivera SM, Reiss AL. Prefrontal cortex involvement in processing incorrect arithmetic equations: evidence from event-related fMRI. Hum Brain Mapp 16: 119–130, 2002. doi: 10.1002/hbm.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller AE, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol 66: 254–262, 1993. doi: 10.1007/BF00235103. [DOI] [PubMed] [Google Scholar]
- 49.Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat 208: 433–443, 2006. doi: 10.1111/j.1469-7580.2006.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Negro F, Farina D. Decorrelation of cortical inputs and motoneuron output. J Neurophysiol 106: 2688–2697, 2011. doi: 10.1152/jn.00336.2011. [DOI] [PubMed] [Google Scholar]
- 51.Negro F, Farina D. Linear transmission of cortical oscillations to the neural drive to muscles is mediated by common projections to populations of motoneurons in humans. J Physiol 589: 629–637, 2011. doi: 10.1113/jphysiol.2010.202473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Negro F, Holobar A, Farina D. Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol 587: 5925–5938, 2009. doi: 10.1113/jphysiol.2009.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Negro F, Muceli S, Castronovo AM, Holobar A, Farina D. Multi-channel intramuscular and surface EMG decomposition by convolutive blind source separation. J Neural Eng 13: 026027, 2016. doi: 10.1088/1741-2560/13/2/026027. [DOI] [PubMed] [Google Scholar]
- 54.Noteboom JT, Fleshner M, Enoka RM. Activation of the arousal response can impair performance on a simple motor task. J Appl Physiol (1985) 91: 821–831, 2001. doi: 10.1152/jappl.2001.91.2.821. [DOI] [PubMed] [Google Scholar]
- 55.Onushko T, Baweja HS, Christou EA. Practice improves motor control in older adults by increasing the motor unit modulation from 13 to 30 Hz. J Neurophysiol 110: 2393–2401, 2013. doi: 10.1152/jn.00345.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pereira HM, Schlinder Delap B, Yoon T, Keenan KG, Hunter SK. Cognitive stress and visual gain affects force fluctuations at low forces. Med Sci Sports Exerc 46: S521, 2014. doi: 10.1249/01.mss.0000495501.30221.61. [DOI] [Google Scholar]
- 57.Pereira HM, Spears VC, Schlinder-Delap B, Yoon T, Harkins A, Nielson KA, Hoeger Bement M, Hunter SK. Sex Differences in Arm Muscle Fatigability With Cognitive Demand in Older Adults. Clin Orthop Relat Res 473: 2568–2577, 2015. doi: 10.1007/s11999-015-4205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pereira HM, Spears VC, Schlinder-Delap B, Yoon T, Nielson KA, Hunter SK. Age and sex differences in steadiness of elbow flexor muscles with imposed cognitive demand. Eur J Appl Physiol 115: 1367–1379, 2015. doi: 10.1007/s00421-015-3113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Razumnikova OM. Gender differences in hemispheric organization during divergent thinking: an EEG investigation in human subjects. Neurosci Lett 362: 193–195, 2004. doi: 10.1016/j.neulet.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 60.Rey A. L’examen Clinique en Psychologie. Paris: Press Universitaire de France, 1958. [Google Scholar]
- 61.Sheikh J, Yesavage J.. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontology: A Guide to Assessment and Intervention. Philadelphia, PA: Haworth Press, 1986. [Google Scholar]
- 62.Smart RR, Baudry S, Fedorov A, Kuzyk SL, Jakobi JM. Influence of biceps brachii tendon mechanical properties on elbow flexor force steadiness in young and old males. Scand J Med Sci Sports 28: 983–991, 2018. doi: 10.1111/sms.13024. [DOI] [PubMed] [Google Scholar]
- 63.Smart RR, Kohn S, Richardson CM, Jakobi JM. Influence of forearm orientation on biceps brachii tendon mechanics and elbow flexor force steadiness. J Biomech 76: 129–135, 2018. doi: 10.1016/j.jbiomech.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 64.Sosnoff JJ, Newell KM. Are age-related increases in force variability due to decrements in strength? Exp Brain Res 174: 86–94, 2006. doi: 10.1007/s00221-006-0422-x. [DOI] [PubMed] [Google Scholar]
- 65.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory (Self-evaluation questionnaire). Palo Alto, CA: Consulting Psychologists Press, 1970. [Google Scholar]
- 66.Stein RB, Gossen ER, Jones KE. Neuronal variability: noise or part of the signal? Nat Rev Neurosci 6: 389–397, 2005. doi: 10.1038/nrn1668. [DOI] [PubMed] [Google Scholar]
- 67.Stephenson JL, Maluf KS. Discharge behaviors of trapezius motor units during exposure to low and high levels of acute psychosocial stress. J Clin Neurophysiol 27: 52–61, 2010. doi: 10.1097/WNP.0b013e3181cb81d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahara D, Inoue K, Hirata Y, Miyachi S, Nambu A, Takada M, Hoshi E. Multisynaptic projections from the ventrolateral prefrontal cortex to the dorsal premotor cortex in macaques - anatomical substrate for conditional visuomotor behavior. Eur J Neurosci 36: 3365–3375, 2012. doi: 10.1111/j.1460-9568.2012.08251.x. [DOI] [PubMed] [Google Scholar]
- 69.Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev 88: 37–57, 2008. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- 70.Taylor AM, Christou EA, Enoka RM. Multiple features of motor-unit activity influence force fluctuations during isometric contractions. J Neurophysiol 90: 1350–1361, 2003. doi: 10.1152/jn.00056.2003. [DOI] [PubMed] [Google Scholar]
- 71.Tracy BL, Byrnes WC, Enoka RM. Strength training reduces force fluctuations during anisometric contractions of the quadriceps femoris muscles in old adults. J Appl Physiol (1985) 96: 1530–1540, 2004. doi: 10.1152/japplphysiol.00861.2003. [DOI] [PubMed] [Google Scholar]
- 72.Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol (1985) 92: 1004–1012, 2002. doi: 10.1152/japplphysiol.00954.2001. [DOI] [PubMed] [Google Scholar]
- 73.Ushiyama J, Suzuki T, Masakado Y, Hase K, Kimura A, Liu M, Ushiba J. Between-subject variance in the magnitude of corticomuscular coherence during tonic isometric contraction of the tibialis anterior muscle in healthy young adults. J Neurophysiol 106: 1379–1388, 2011. doi: 10.1152/jn.00193.2011. [DOI] [PubMed] [Google Scholar]
- 74.Ushiyama J, Takahashi Y, Ushiba J. Muscle dependency of corticomuscular coherence in upper and lower limb muscles and training-related alterations in ballet dancers and weightlifters. J Appl Physiol (1985) 109: 1086–1095, 2010. doi: 10.1152/japplphysiol.00869.2009. [DOI] [PubMed] [Google Scholar]
- 75.Van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. Rey’s verbal learning test: normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc 11: 290–302, 2005. doi: 10.1017/S1355617705050344. [DOI] [PubMed] [Google Scholar]
- 76.Vanden Noven ML, Pereira HM, Yoon T, Stevens AA, Nielson KA, Hunter SK. Motor Variability during Sustained Contractions Increases with Cognitive Demand in Older Adults. Front Aging Neurosci 6: 97, 2014. doi: 10.3389/fnagi.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Voelcker-Rehage C, Stronge AJ, Alberts JL. Age-related differences in working memory and force control under dual-task conditions. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 13: 366–384, 2006. doi: 10.1080/138255890969339. [DOI] [PubMed] [Google Scholar]
- 78.Volf NV, Razumnikova OM. Sex differences in EEG coherence during a verbal memory task in normal adults. Int J Psychophysiol 34: 113–122, 1999. doi: 10.1016/S0167-8760(99)00067-7. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe RN, Magalhães FH, Elias LA, Chaud VM, Mello EM, Kohn AF. Influences of premotoneuronal command statistics on the scaling of motor output variability during isometric plantar flexion. J Neurophysiol 110: 2592–2606, 2013. doi: 10.1152/jn.00073.2013. [DOI] [PubMed] [Google Scholar]
- 80.Wechsler D. WAIS-III WMS-III Technical ManualPsychological Corporation. San Antonio, TX: Psychological Corp., 1997. [Google Scholar]
- 81.Yoon T, Keller ML, De-Lap BS, Harkins A, Lepers R, Hunter SK. Sex differences in response to cognitive stress during a fatiguing contraction. J Appl Physiol (1985) 107: 1486–1496, 2009. doi: 10.1152/japplphysiol.00238.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]