Abstract
To longitudinally monitor progressive fibrosis in the transforming growth factor-α (TGF-α) transgenic mouse model of lung fibrosis, we used retrospective self-gating ultrashort echo time (UTE) magnetic resonance imaging (MRI) to image mouse lung at baseline and after 4 and 8 wk of fibrosis initiation via doxycycline administration. Only bitransgenic mice were used in this study and divided into two cohorts: six mice were fed doxycycline food to induce lung fibrosis (referred to as Dox cohort), and five other mice were fed normal food (referred to as control cohort). Lung mechanics, histology, and hydroxyproline were assessed after the final MRI. A linear mixed-effects model was used to analyze MRI-derived longitudinal lung-function parameters. Tidal volume decreased at a rate of −0.016 ± 0.002 ml/week [χ2(1) = 16.48, P < 0.001] for Dox cohort and increased at a rate of 0.010 ± 0.003 ml/week [χ2(1) = 6.37, P = 0.01] for control cohort. Minute ventilation decreased at a rate of −1.71 ± 0.26 ml·min-1·wk-1 [χ2(1) = 14.04, P < 0.001] for Dox cohort but did not change significantly over time for control cohort. High-density lung volume percentage increased at a rate of 3.9 ± 0.7%/wk for Dox cohort [χ2(1) = 11.47, P < 0.001] but did not change significantly over time for control cohort. MRI-derived lung structure and function parameters were strongly correlated with pleural thickness, hydroxyproline content, lung compliance, airway resistance, and airway elastance. We conclude that self-gating UTE MRI could be used to longitudinally monitor lung fibrosis in the TGF-α transgenic mouse model.
NEW & NOTEWORTHY Self-gating UTE MRI was used to monitor morphology and physiology in lung fibrosis in a transforming growth factor-α transgenic mouse model. Tidal volume was shown for the first time to correlate strongly with conventional metrics of fibrosis such as hydroxyproline and pleural thickness.
Keywords: pulmonary fibrosis, self-gating, ultrashort echo time magnetic resonance imaging
INTRODUCTION
Lung fibrosis is a significant contributor to morbidity and mortality in a diverse spectrum of interstitial lung diseases, with idiopathic pulmonary fibrosis (IPF) being the most common and perhaps the most enigmatic and pernicious form of the disorder (15). Lung fibrosis claims more lives annually than many types of cancer, and in the United States, ~100,000 people are affected by IPF alone, resulting in 30,000–40,000 deaths each year (28). Although the United States Food and Drug Administration (FDA) approved the first drugs to treat IPF (pirfenidone and nintedanib) in 2014, no treatments have been developed to reverse or even halt fibrotic progression (20, 40). Furthermore, the FDA has yet to approve drugs for fibrotic lung diseases beyond IPF. This lack of effective and tolerable treatments for lung fibrosis underscores the need to better understand the mechanisms that initiate lung fibrosis and contribute to its progression.
Because the underlying etiology of pulmonary fibrosis is difficult to study in patients, animal models, particularly mice, are the primary means used by investigators to investigate the molecular cellular details underlying disease progression (32a) and preclinically to test novel therapies. However, to make the fullest use of these models, two key factors are essential: 1) selecting animal models that develop progressive pulmonary fibrosis like that seen in IPF patients, and 2) developing nonlethal methods that allow the structural, mechanical, and functional aspects of lung fibrosis progression to be assessed longitudinally in the same animals. For example, lung compliance correlates negatively with the severity of pulmonary fibrosis in lung fibrosis patients (10, 42, 56), and tidal volume decreases in animals with pulmonary fibrosis (48). While important physiological data such as these (e.g., lung mechanics) can be obtained via FlexiVent or similar experimental protocols, most investigators treat these interventions as terminal procedures in mice. Moreover, these protocols provide only global information and thus may mask the inherent spatial heterogeneity of lung fibrosis.
In contrast, noninvasive imaging can provide spatially resolved and longitudinal data in these models (58). The three main tomographic modalities are used to assess changes in experimental models of lung disease: positron emission tomography (PET), X-ray computed tomography (CT), and magnetic resonance imaging (MRI). While PET is useful for acquiring molecular and functional information, it requires the synthesis of specialized and expensive radio tracers, and the spatial resolution is relatively poor, particularly in the lungs (7). CT has become a gold standard for lung imaging in humans, but it has increased cancer risk due to ionizing radiation (22, 39) and introduces potentially confounding factors due to radiation damage in longitudinal studies. Moreover, it is difficult to assess functional changes with CT. While lung MRI has not yet been widely adopted in the clinic due to the complications of respiratory motion and short T2* relaxation (~1 ms or less), it is a promising imaging modality due to its nonionizing nature and the rich tissue-specific information that can be obtained through a wide range of contrast mechanisms. Moreover, complications resulting from short T2* can be largely mitigated through the use of center-out, ultrashort echo time (UTE) sequences (3, 12), which can begin to encode information within tens of microseconds.
Relative to T2*-induced signal loss, the complications resulting from respiratory motion are more challenging to address. Specifically, motion degrades spatial resolution of MRI and can introduce pronounced artifacts that often make the resulting images unusable. Motion artifacts are particularly pronounced in mice, which have respiratory rates that are 10–20 times higher than those of humans. While motional complications can largely be overcome by synchronizing MRI acquisitions to the respiratory cycle via mechanical ventilation or prospective respiratory gating, these approaches completely obscure the mechanics of breathing. However, both lung shape and ventilation are altered as a result of lesion growth and decreased compliance during disease progression, making respiratory motion a potentially powerful biomarker of fibrosis severity (33, 48). Moreover, it has been suggested that mechanical coupling between ventilatory work and the fibrotic matrix may be a driver of fibrotic progression (29, 30, 59), making respiratory motion of intrinsic interest in pulmonary fibrosis research. In this work, we overcome the technical problems related to T2* relaxation and motional artifacts, while preserving physiological information about breathing patterns using retrospectively gated UTE MRI (5, 9, 16, 17, 26, 49, 57).
In our approach, each line of k-space is encoded as radially sampled, free induction decay (FID), and the center of k-space (i.e., the first point on each FID or k-zero) is acquired in each radial view. Respiratory motion generates small, but readily detected, oscillations in the phase and magnitude of k-zero due to changes in lung susceptibility and tissue movement in the presence of inhomogeneous magnetic fields (5). These small signal amplitude variations were used to define the respiratory waveform in free-breathing mice. With the use of this known respiratory waveform, k-space data were retrospectively binned to generate images at full inspiration and end-expiration, allowing the percent of high-density lung tissue, tidal volume (TV), and minute ventilation to be calculated directly from the raw MRI data. These metrics were measured longitudinally in transforming growth factor- α (TGF-α)-induced transgenic mice that develop progressive adventitial and subpleural lung fibrosis characterized by severe restrictive changes in lung mechanics (15, 21, 27, 32), and thus provide an ideal means to assess the sensitivity of retrospectively gated UTE MRI to the structural and functional changes resulting from fibrosis progression.
MATERIALS AND METHODS
Disease model.
Transgenic mouse lines were generated with the reverse tetracycline-responsive transactivator (rtTA) fusion protein under control of the CCSP gene promoter (52). A separate transgenic mouse line was generated using a microinjection of the transgene (TetO)7-cmv-TGF-α, and bitransgenic mice were generated by mating CCSP rtTA mice to (TetO)7-cmv-TGF-α mice (15). Mice were housed under pathogen-free conditions and handled in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Cincinnati Children's Hospital Research Foundation. To induce TGF-α expression in the bitransgenic mice, doxycycline (Dox) (Sigma, St. Louis, MO) was administered in food (62.5 mg/kg).
Animal group.
Only bitransgenic mice were used in this study and were randomly divided into two cohorts. One cohort of mice (n = 6, 3 male/3 female, 17 wk old at baseline) was fed Dox food for 8 wk, thereby activating the transgene and inducing pulmonary fibrosis (thereafter referred to as Dox cohort), while the other cohort (n = 5, 2 male/3 female, 17 wk old at baseline) was fed normal, non-Dox-treated food (thereafter referred to as control cohort). To monitor the progression of pulmonary fibrosis, both cohorts were imaged by MRI at the same time points: baseline (the day just before Dox treatment) and at 4 and 8 wk of Dox treatment (thereafter referred to as Dox week 4 and Dox week 8, respectively). After the final MRI session, lung mechanics were analyzed via the FlexiVent system (SCIREQ, Montreal, Quebec, Canada). Mice were then euthanized by an overdose of pentobarbital sodium (65 mg/ml, Fort Dodge Animal Health, Fort Dodge, IA), and the lungs were excised and processed for histology and hydroxyproline assessment. A 6-wk-old C57BL/6J mouse with body mass of 23.0 g was imaged using the self-gating MRI stated here for demonstration purpose only.
Lung mechanics.
Lung mechanics were assessed using a computerized FlexiVent system, as previously described (14, 43). Mice were anesthetized by intraperitoneal injection of 0.1 ml/10 g body wt phosphate-buffered saline (PBS) solution containing 178 mM (40 mg/ml) ketamine and 7.8 mM (2 mg/ml) xylazine. Mice were then tracheostomized and ventilated with a tidal volume of 8 ml/kg at rate of 450 breaths/min and positive end-expiratory pressure of 2 cmH2O. The ventilation mode was changed to forced oscillation (0.5–19.6 Hz) to determine respiratory impedance. The volume, pressure, and flow signals were fit to the classic single-compartment model using linear regression to yield airway resistance, airway elastance, and lung compliance.
Histology and morphometrics.
Lung histology was performed as previously described (15, 52). Following euthanasia, the lungs and heart were removed, and the left and right lungs were separated. The left lung was inflation fixed via trachea using 10% neutral buffered formalin at 25 cmH2O pressure, fixed overnight at 4°C, washed with PBS, dehydrated through a graded series of ethanol washes, and paraffin embedded for hematoxylin and eosin staining (H & E). Histomorphometric measurements of subpleural thickness were obtained from lung sections using MetaMorph (Molecular Devices, Sunnyvale, CA) as previously described (31). Five measurements per lung section were obtained using a Leica DM2700 M bright-field microscope (Leica Microsystems, Buffalo Grove, IL) and 3CCD color video camera.
Total lung collagen.
A hydroxyproline assay was performed using hydroxyproline standard solutions (0–800 µg/ml¸ Sigma) as previously described (15). One lobe from the right lung was lyophilized overnight, and 10 mg of dried tissue were incubated in 500 µl of 6 N HCl. Five microliters of the samples and standards were applied to an enzyme-linked immunosorbent assay plate, and 50 µl citric acetate buffer (5% citric acid, 7.2% sodium acetate, 3.4% NaOH, and 1.2% glacial acetic acid, pH 6.0) and 100 µl chloramine-T solution (564 mg chloramine-T, 4 ml H2O, 4 ml n-propanol, and 32 ml citrate acetate buffer) were added and incubated for 20 min at room temperature. Then 100 µl Ehrlich's solution were added (4.5 g 4-dimethylaminobenzaldehyde, 18.6 ml n-propanol, and 7.8 ml sulfuric acid) and incubated for 15 min at 100°C. Reaction product was read at optical density of 525 nm.
MR imaging.
Imaging was performed on a Bruker 7 T scanner (Bruker, Billerica, MA) with a home-built quadrature birdcage transmitter/receiver coil (inner diameter = 35 mm, length = 50 mm). Free-breathing animals were anesthetized by 2% isoflurane (in air), with one exception of 3% isoflurane needed to maintain unconsciousness, and were placed supine with the lung in the center of radiofrequency (RF) coil. A small-animal monitoring system (SA Instruments, Stony Brook, NY) was used to monitor the respiratory rate via a small pneumonic sensor secured on the animal's abdomen and maintain the body temperature at 34°C. Body mass of animals was collected immediately before MRI.
For radial UTE MRI, a hard, 4.3-µs RF pulse was used for excitation, and the readout gradient was ramped to the predetermined direction and amplitude and then remained constant until the end of data acquisition, with data acquisition beginning simultaneously with the readout gradient (13). A golden mean strategy for three-dimensional sampling was used to obtain a uniform distribution of projection end points on a unit sphere for spherical k-space coverage, as described previously (6, 13). To reduce in-plane field of view (FOV) and increase resolution within the same scanning time, the k-space coverage was expanded into an ellipsoid (13), in which the ratio of the kx, ky, and kz axes is 2:2:1 (i.e., the FOV was reduced in the x-y plane). In total, 154,440 projections were acquired in each imaging session with 64 points/projection. Additional parameters included the following: receiver bandwidth = 200 kHz, echo time (TE) = 0.108 ms, repetition time (TR) = 6 ms, flip angle = 6.3°, FOV = 30 mm × 30 mm × 60 mm, and voxel size = 0.23 mm × 0.23 mm × 0.47 mm. Total acquisition time for each mouse was ~15.5 min, including 1,000 dummy scans to establish steady-state magnetization.
Retrospective self-gating.
The magnitude of FID signal amplitude at k-zero (S) as a function of time (t) or projection number was smoothed by a moving-average filter. Then, numerical first- and second-order derivatives of the smoothed respiratory waveform were combined to extract data at end-expiration and end-inspiration, respectively. The conditions for extracting data at end-expiration and end-inspiration are described by Eq. 1 and Eq. 2 respectively.
| (1) |
Where εE (~5) and δE (0–0.0001) are thresholds that are user selected and adjustable to optimize self-gating for end-expiration.
| (2) |
where εI (~5) and δI (0.0001–0.03) are thresholds that are also user selected and adjustable to optimize self-gating for end-inspiration.
Depending on the respiratory rate, 30–40% and 4–8% of the total projections were binned to end-expiration and end-inspiration, respectively. Following retrospective gating, radial k-space data were resampled onto a Cartesian grid using established methods (18, 19, 34, 37, 38, 60) and reconstructed using fast Fourier transform. The entire reconstruction process was performed in MATLAB (MathWorks, Natick, MA) and C.
Respiratory rate measurement.
Smoothed respiratory waveforms were divided into a series of specified time intervals (for example, 2,000 projections correspond to a time interval of 12 s with TR = 6 ms used in this study). The data subset in each time interval was then analyzed by fast Fourier transform, with the largest peak in the frequency domain corresponding to the respiratory rate within each time interval. The average respiratory rate of each animal during the imaging period was calculated by averaging over all time intervals. With TR = 7 ms, data were acquired on one mouse breathing at three different respiratory rates (~110/min, 80/min, and 60/min by external monitoring) via adjusting isoflurane level to verify the feasibility and reliability of respiratory waveforms.
Image analysis.
The lung was segmented from the major bronchi, large blood vessels, and other soft tissues using the graphic processing software package Amira (FEI, Hillsboro, OR), with blinding over cohorts. Lung volume was calculated by multiplying the total number of voxels in lung by voxel volume. Tidal volume was calculated by subtracting the lung volume at end-tidal expiration from the lung volume measured at end-tidal inspiration. Minute ventilation volume was calculated by multiplying tidal volume by the respiratory rate of each animal. Magnetic resonance image intensity can differ between experiments due to technical factors, including receiver gain and magnet shimming. To mitigate this complication, image intensity was normalized by the average soft-tissue (heart, muscle, etc.) signal outside the lungs. High-density lung volume percentage was calculated from the percentage of voxels in the lung with normalized signal intensity larger than a threshold of 0.8.
Statistics.
Correlation between MRI-derived parameters and conventional metrics of fibrosis severity was analyzed using linear regression in R. A Mann-Whitney U test was used to examine differences in histology, lung mechanics, hydroxyproline content, tidal volume, and minute ventilation between the Dox and control cohorts at Dox week 8. The R language (39a) and lme4 package (2) were used to perform a linear mixed-effects analysis of the relationship between MRI-derived lung function parameters and time. As a fixed effect, time was entered into the model. As random effects, intercepts for subjects (individual mice) and by-subject random slopes for the effect of time were entered into the model. P values were obtained by likelihood ratio test (using ANOVA) of the full model with the effect of time in question against the model without the effect of time in question. Differences were considered significant when P < 0.05.
RESULTS
Retrospective gating.
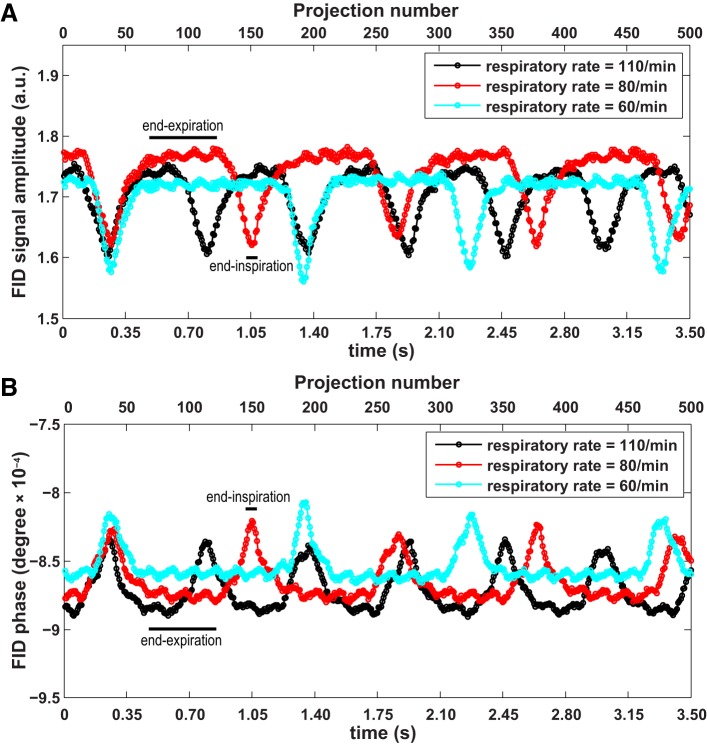
The representative respiratory waveforms generated from the FID signal amplitude at different respiratory rates (~110/min, 80/min, and 60/min obtained by applying 3 different levels of anesthesia) are shown in Fig. 1A, with peaks and troughs corresponding to end-expiration and end-inspiration, respectively. For comparison, respiratory waveforms generated by FID phase are shown in Fig. 1B, with peaks and troughs corresponding to end-inspiration and end-expiration, respectively. The respiratory rates calculated from the amplitude and phase plots are 109/min, 75/min, and 64/min, respectively, consistent with external monitoring (110/min, 80/min, 60/min). Contrast-to-noise ratio, which is defined as the peak-trough difference divided by standard deviation in plateau as indicated by end-expiration and black bar in Fig. 1, is 18~22 for FID signal amplitude and 14~18 for FID phase. These findings indicate that signal amplitude is more sensitive to motion than phase under the specific conditions used in this study, and therefore, amplitude was used to define respiratory waveform for all subsequent analyses in this study.
Fig. 1.
A: free induction decay (FID) signal amplitude as a function of projection number at a series of respiratory rates (~110/min, 80/min, and 60/min, read from small animal monitoring system). As indicated by the black bars, peaks and troughs correspond to end-expiration and end-inspiration, respectively. B: FID phase (phase of the leading FID point) as a function of projection number. Peaks and troughs correspond to end-inspiration and end-expiration, respectively, as indicated by the black bars.
A representative smoothed, self-gating respiratory waveform obtained from signal amplitude is shown in Fig. 2, with thin solid lines indicating data extracted at end-expiration and thick solid lines indicating data extracted at end-inspiration. With the use of these binned data, high-quality and motion-free three-dimensional magnetic resonance images of the lungs were successfully acquired at end-expiration and inspiration, respectively. For demonstration, coronal and sagittal images of a 6-wk-old C57BL/6J mouse with body mass of 23.0 g are shown in Fig. 3. The black lines emphasize the diaphragm displacement between end-expiration and inspiration. The inspiratory and expiratory lung volumes measured from these images are 0.55 ml and 0.44 ml, respectively. This indicates an anesthetized tidal volume of 0.11 ml, which is consistent with previous plethysmographic measurements in (nonanesthetized) C57BL/6J mice of 0.13–0.21 ml (50).
Fig. 2.
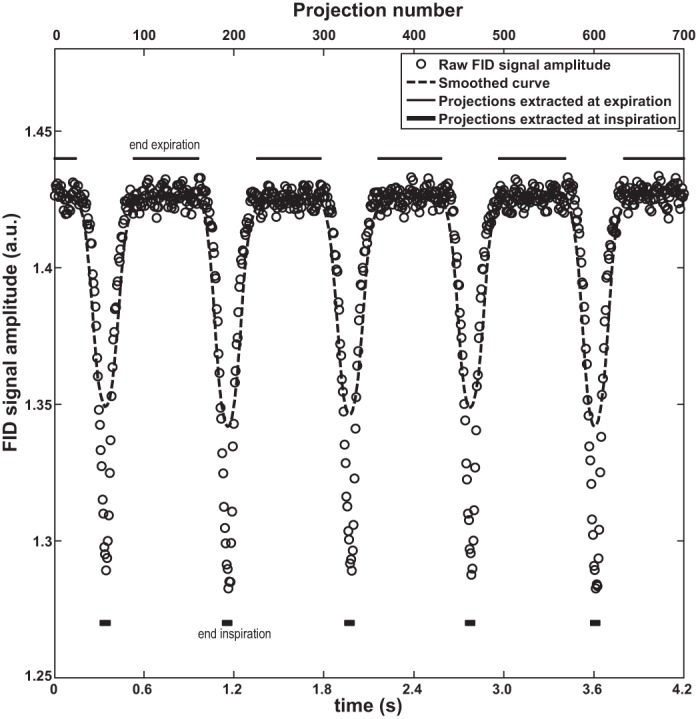
Projections extracted for end-expiration and end-inspiration, marked by thin and thick solid lines, respectively.
Fig. 3.
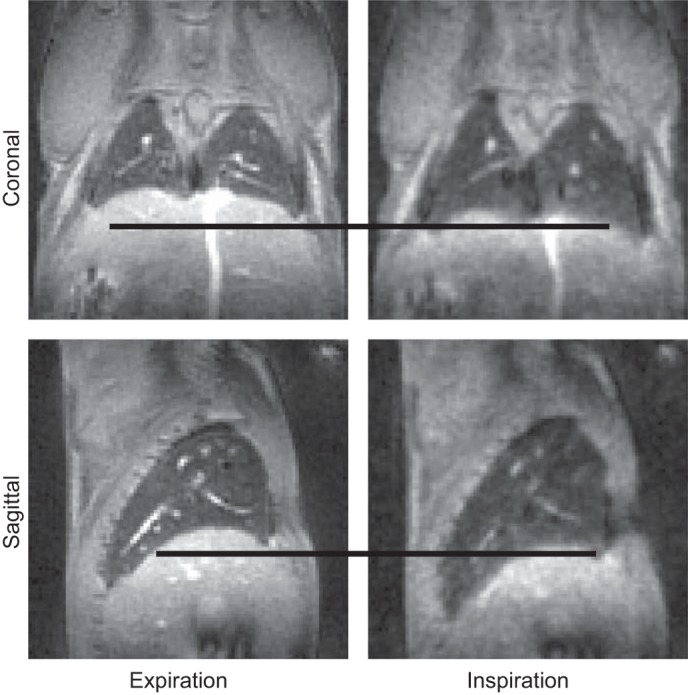
Representative lung images reconstructed from data extracted at end-expiration and end-inspiration. The black lines manifest the relative diaphragm displacement between end-expiration and end-inspiration.
Comparison of MRI with conventional lung fibrosis metrics.
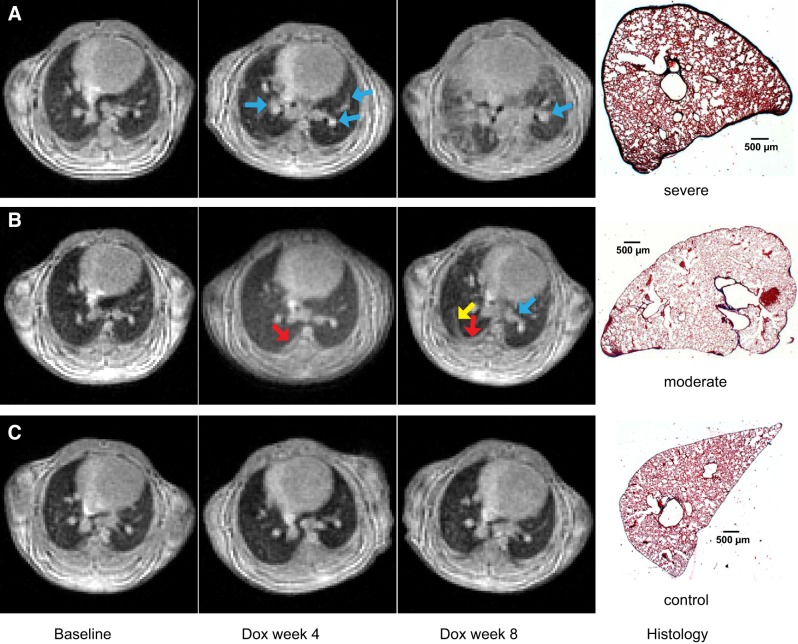
Representative expiratory images from a control mouse and two Dox mice at all three time points are shown in Fig. 4. Within the lung parenchyma (away from airways and vessels) of healthy control mice, signal intensity is relatively uniform (signal-to-noise ratio ~18). This general pattern, low MRI signal throughout the lung parenchyma and high signal only from vasculature, was observed at baseline in the Dox cohort and at all three time points in the control cohort. However, in Dox mice, increased high signal volume around blood vessels and bronchovascular bundles (blue arrows) demonstrated that perivascular fibrosis was already present at Dox week 4. The volume of these high-signal structures increased substantially by Dox week 8. High-signal fibrotic lesions are also observed extending from the subpleural region (Fig. 4B, red arrow) in the posterior right lung at Dox week 4. By Dox week 8, these structures have extended into the interstitium (yellow arrow). Similar progressive patterns of high-signal structures near the subpleural region of posterior right lung were seen in all Dox-treated mice.
Fig. 4.
Representative magnetic resonance images (repetition time = 6 ms, echo time = 0.108 ms, flip angle = 6.3°) of one doxycycline-fed (Dox) mouse with severe fibrosis (A), one Dox mouse with moderate fibrosis (B), and one control mouse (C) at baseline, Dox week 4, and Dox week 8, plus corresponding histological slides (hematoxylin and eosin stain; original magnification, × 1) at Dox week 8. No pulmonary fibrosis was visually seen at baseline in the Dox cohort and at all 3 time points in the control cohort. Perivascular fibrosis appeared in both Dox mice at Dox week 4 and Dox week 8 (indicated by blue arrows) in A and B. Fibrosis appeared near the pleural region at Dox week 4 (indicated by a red arrow) and extended into the interstitium at Dox week 8 (indicated by a yellow arrow), as seen in B. Apparent interstitial fibrosis also appeared in the Dox mouse in A at Dox week 8. Fibrosis severity varied among different mice as demonstrated in A and B at Dox week 8.
These observations are consistent with previous gradient echo MRI studies of this model (8) and with histology from the same animals at Dox week 8, which shows thickened pleura in the Dox-treated animals, and fibrotic lesions extending into the lung parenchyma. However, MRI also reveals that fibrosis severity varied significantly between Dox mice as seen by the volume of high-signal structures at Dox week 8 and the histological slides. These observations highlight the advantage of obtaining longitudinal data through noninvasive imaging.
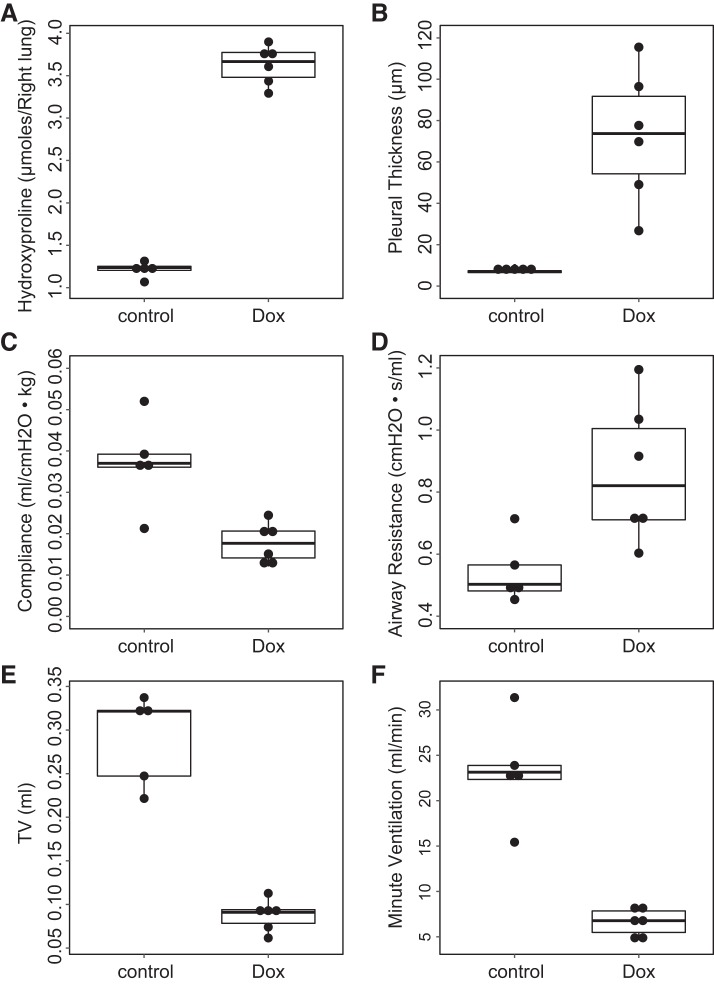
Beyond qualitative agreement, MRI-derived metrics were readily able to differentiate Dox cohorts from control cohorts at a level of significance comparable to conventional metrics. Group-wise comparisons by part of non-MRI measurements (airway resistance, compliance, pleural thickness, and hydroxyproline content) and part of MRI measurements (tidal volume and minute ventilation) are summarized in Fig. 5, and comparisons by all MRI and non-MRI measurements are summarized in Table 1. Except for body mass, differences between control and Dox cohorts were all statistically significant via Mann-Whitney U test. Specifically in non-MRI measurements, hydroxyproline content and pleural thickness were most sensitive to distinguish between the two cohorts (P = 0.004). Airway resistance, airway elastance, and compliance were also able to distinguish between the Dox and control cohorts (P = 0.02, P = 0.009, and P = 0.009, respectively). All MRI measurements including high-density lung volume percentage, tidal volume, and minute ventilation have the same significance as hydroxyproline and pleural thickness. No sex-based differences were seen in the data.
Fig. 5.
Histological, physiological, and magnetic resonance imaging measurements of fibrosis: hydroxyproline content (A), pleural thickness (B), compliance (C), airway resistance (D), tidal volume (E), and minute ventilation (F) for control (n = 5) and doxycycline-fed (Dox) (n = 6) cohorts. The graphs display box-whisker plot with individual data points. The hydroxyproline, airway resistance, and pleural thickness in the control cohort were all significantly smaller than in the Dox cohort (U = 0, P = 0.004, hydroxyproline; U = 2.0, P = 0.02, airway resistance; and U = 0, P = 0.004, pleural thickness) via Mann-Whitney U test, while the compliance, tidal volume, and minute ventilation in the control cohort were significantly bigger than that in the Dox cohort (U = 1.0, P = 0.009, compliance; U = 30, P = 0.004, tidal volume; U = 30, P = 0.004, minute ventilation).
Table 1.
Comparisons between Dox and control cohorts via Mann-Whitney U test
| Dox (n = 6) | Control (n = 5) | U | P | |
|---|---|---|---|---|
| Hydroxyproline, µmol/right lung | 3.67 (3.48, 3.77) | 1.24 (1.20, 1.25) | 0 | 0.004* |
| Airway resistance, cmH2O·s/ml | 0.82 (0.71, 1.00) | 0.50 (0.48, 0.56) | 2 | 0.02* |
| Airway elastance, cmH2O/ml | 57.81 (48.45, 70.90) | 27.02 (25.50, 27.71) | 1 | 0.009* |
| Compliance, ml/cmH2O·kg | 0.018 (0.014, 0.021) | 0.037 (0.036, 0.039) | 1 | 0.009* |
| Pleural thickness, µm | 73.77 (54.26, 91.77) | 7.04 (6.54, 7.38) | 0 | 0.004* |
| High-density lung volume percentage, % | 35.25 (24.41, 48.38) | 5.16 (5.06, 5.28) | 0 | 0.004* |
| Tidal volume, ml | 0.091 (0.078, 0.094) | 0.322 (0.247, 0.322) | 30 | 0.004* |
| Respiratory rate, breaths/min | 74.07 (65.60, 86.43) | 70.80 (69.46, 93.63) | 15 | 1 |
| Minute ventilation, ml/min | 6.78 (5.49, 7.85) | 23.15 (22.34, 23.88) | 30 | 0.004* |
| Body mass, g | 23.95 (23.30, 25.95) | 23.30 (23.00, 26.70) | 14 | 0.93 |
All quantities are displayed as median (1st quartile, 3rd quartile).
Statistically significant.
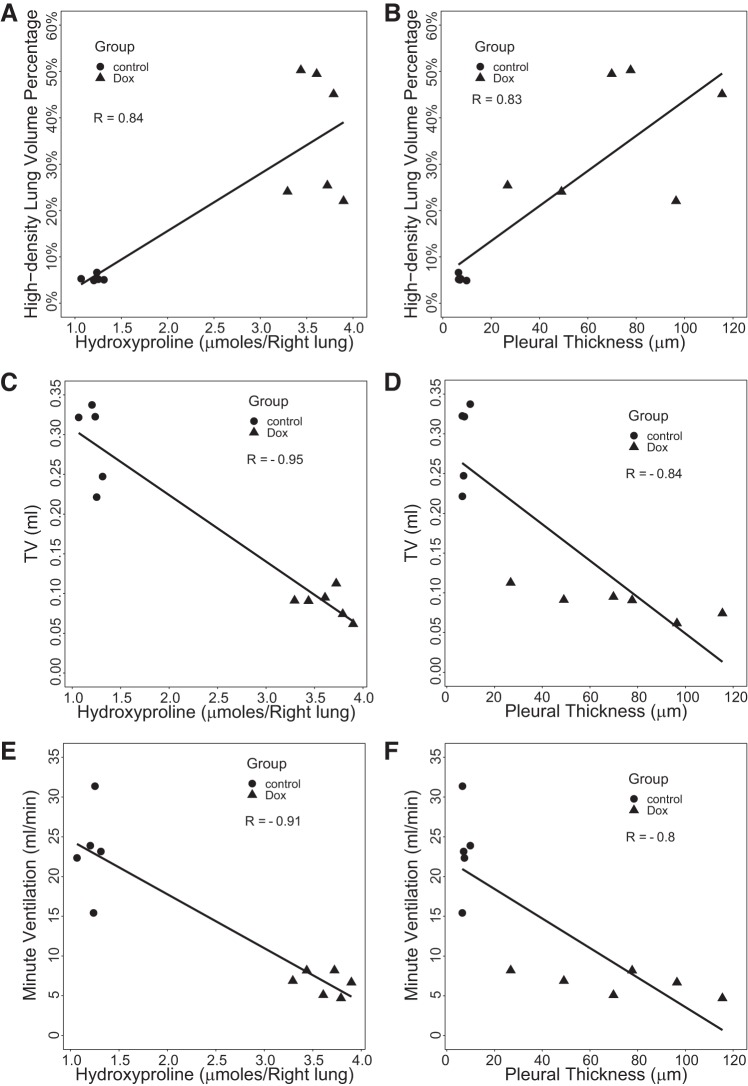
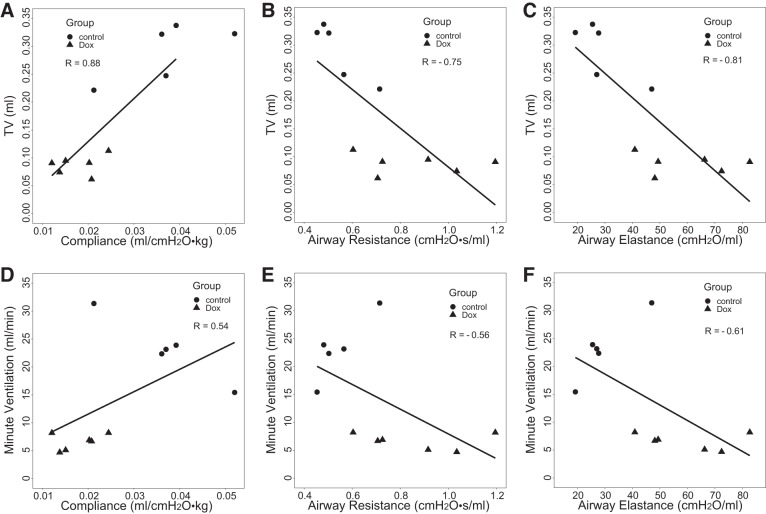
MRI measures also correlated quantitatively with conventional measures of fibrotic burden (summarized in Table 2). For example, high-density lung volume percentage (Fig. 6, A and B) was strongly and positively correlated with hydroxyproline (R = 0.84, P = 0.001) and pleural thickness (R = 0.83, P = 0.002). MRI-derived TV was negatively correlated with pleural thickness (R = −0.84, P = 0.001) and hydroxyproline content (R = −0.95, P < 0.001). In addition, MRI-derived minute ventilation was also negatively correlated with both hydroxyproline (R = −0.91, P < 0.001) and pleural thickness (R = −0.8, P = 0.003). Of greater physiological interest, Fig. 7 shows that TV was also strongly correlated with pulmonary mechanics parameters: compliance (R = 0.88, P < 0.001), airway resistance (R = −0.75, P = 0.008), and airway elastance (R = −0.81, P = 0.002). Compared with TV, minute ventilation showed smaller correlation with pulmonary mechanics parameters. Together, these data strongly indicate that these noninvasive MRI-derived parameters can detect fibrotic changes in the lungs, with sensitivity comparable to conventional metrics.
Table 2.
Correlation between MRI-derived metrics and conventional metrics
| Hydroxyproline, µmol/right lung | Pleural Thickness, µm | Compliance, ml/cmH2O·kg | Airway Resistance, cmH2O·s/ml | Airway Elastance, cmH2O/ml | |
|---|---|---|---|---|---|
| High-density lung volume percentage, % | 0.84 (0.001*) | 0.83 (0.002*) | −0.8 (0.003*) | 0.9 (<0.001*) | 0.92 (<0.001*) |
| Tidal volume, ml | −0.95 (<0.001*) | −0.84 (0.001*) | 0.88 (<0.001*) | −0.75 (0.008*) | −0.81 (0.002*) |
| Minute ventilation, ml/min | −0.91 (<0.001*) | −0.8 (0.003*) | 0.54 (0.09) | −0.56 (0.07) | −0.61 (0.05*) |
Correlation coefficients and P values are displayed as R (P).
Statistically significant.
Fig. 6.
Correlations of magnetic resonance imaging-derived parameters [high-density lung volume percentage, tidal volume (TV), and minute ventilation] with biochemical measurements (hydroxyproline content; A, C, E) and histological measurements (pleural thickness; B, D, F), with both doxycycline-fed (Dox) (n = 6) and control (n = 5) cohorts considered. TV demonstrated the strongest correlation with hydroxyproline as seen in C.
Fig. 7.
Correlations of magnetic resonance imaging (MRI)-derived tidal volume (TV) with pulmonary mechanical measurements: compliance (A), airway resistance (B), and airway elastance (C); correlations of MRI-derived minute ventilation with pulmonary mechanical measurements: compliance (D), airway resistance (E), and airway elastance (F), with both doxycycline-fed (Dox) (n = 6) and control (n = 5) cohorts considered.
Longitudinal MR imaging.
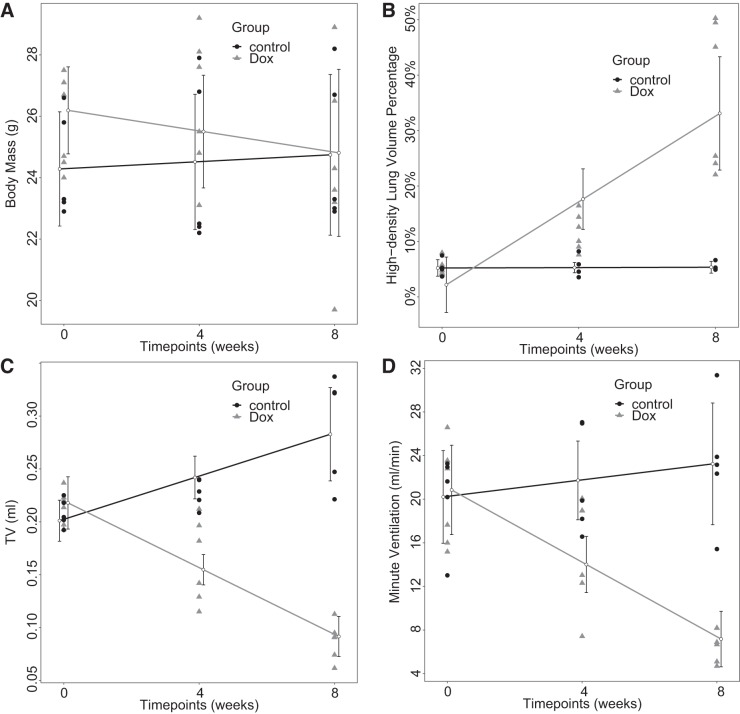
Linear mixed-effects analyses of MRI-derived longitudinal lung function parameters and body mass, a typical metric of disease severity in this model, as measures of pulmonary fibrotic burden are shown in Fig. 8 and summarized in Table 3. No significant body-mass changes over time were observed in either group (P = 0.19 and 0.34 for Dox and control cohorts, respectively). However, TV increased at a rate of 0.010 ± 0.003 ml/week [χ2(1) = 6.37, P = 0.01] for the control cohort and decreased significantly at a rate of −0.016 ± 0.002 ml/week [χ2(1) = 16.48, P < 0.001] for the Dox cohort. Similarly, minute ventilation did not change significantly over time for the control cohort (P = 0.31), whereas it significantly decreased at a rate of −1.71 ± 0.26 ml·min−1·wk−1 [χ2(1) = 14.04, P < 0.001] for the Dox cohort. High-density lung volume percentage increased at a rate of 3.9 ± 0.7%/wk for the Dox cohort [χ2(1) = 11.47, P < 0.001] but did not change significantly over time for the control cohort (P = 0.87).
Fig. 8.
Longitudinal analysis by linear mixed-effects model over body mass (A), high-density lung volume percentage (B), tidal volume (TV) (C), and minute ventilation (D) of control (n = 5) and doxycycline-fed (Dox) (n = 6) cohorts. All 3 MRI-derived parameters for Dox mice changed significantly with time. Contrary to Dox mice, TV for control mice increased significantly with time. Body mass in both control and Dox cohorts did not change significantly with time. Error bar displays 95% confidence interval.
Table 3.
Fibrosis progression assessed by MRI-derived parameters and body mass
| Dox (n = 6) |
Control (n = 5) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | P | Χ2(1) | Slope | Intercept | P | Χ2(1) | |
| Minute ventilation, ml/min | −1.71 ± 0.26 | 20.84 ± 1.93 | <0.001* | 14.04 | 0.38 ± 0.39 | 20.21 ± 1.97 | 0.31 | 1.04 |
| High-density lung volume percentage, % | 3.9 ± 0.7 | 2.2 ± 2.4 | <0.001* | 11.47 | 0.02 ± 0.10 | 5.21 ± 0.69 | 0.87 | 0.03 |
| Tidal volume, ml | −0.016 ± 0.002 | 0.218 ± 0.012 | <0.001* | 16.48 | 0.010 ± 0.003 | 0.201 ± 0.009 | 0.01* | 6.37 |
| Body mass, g | −0.17 ± 0.14 | 26.19 ± 0.69 | 0.19 | 1.68 | 0.058 ± 0.063 | 24.28 ± 0.86 | 0.34 | 0.89 |
Slopes and intercepts ± SE from linear mixed-effects model. P values were obtained by likelihood ratio test (using ANOVA) of the full model with the effect of time in question against the model without the effect of time in question.
Statistically significant.
DISCUSSION
To minimize T2* signal decay, various short TE magnetic resonance techniques including TE-minimized gradient-recalled echo imaging (GRE) (1), Shinnar-Le Roux pulse (36, 44–47) with minimum phase, UTE imaging (3, 12, 41), and zero TE imaging (4, 23–25, 53–55) have been developed and applied to lung imaging. GRE imaging with concurrent slice refocus gradient, phase-encoding gradient, and readout-dephase gradient can achieve sub-millisecond echo times (1), but this is still much longer than the T2* of lung at higher magnetic fields (13, 35). Similarly, Shinnar-Le Roux pulse with minimum phase yields sub-millisecond echo times still much longer than the T2* of lung at high fields due to the slice selection and slice refocus gradients. Moreover, these approaches do not sample the center of k-space with every view, making it impossible to implement the robust self-gating demonstrated in this work.
Zero TE sequences (53) employ no meaningful delay between the pulse and data acquisition (i.e., finite RF excitation pulse, T/R switch, and digital bandpass filtering are completed in microseconds) and are therefore almost immune to T2* effects. However, excitation is applied after imaging gradients that are ramped to a predetermined magnitude, so these sequences do not sample the true k-space center. While an algebraic approach can be used to extrapolate data to k-zero, noise is amplified in the central k-space data generated by the algebraic approach, leading to correlated artifacts and thus systematic errors in image quantification. Furthermore, the algebraic extrapolation requires two trajectories with opposite polarity, and this redundancy decreases the randomness of projection distribution in k-space. As such, ZTE is imperfectly suited for dynamic imaging applications, such as the self-gating MRI. UTE sequences (here, with TE much shorter than T2* of lung) are more straightforward to implement with present hardware limitations and are thus utilized in this study.
In contrast, we have demonstrated that self-gating UTE MRI can readily be implemented, even in rapidly breathing animals like mice, and the resulting images have minimal motion artifacts and high signal-to-noise ratio in lung parenchyma. Moreover, we have shown for the first time that a straightforward combination of first-order and second-order derivatives of k-zero signal can be applied during normal tidal breathing to extract the breathing pattern directly from unprocessed MRI data. Because the images acquired at end-inspiration and end-expiration are the focus of interest of many studies that can provide robust measures of not only lung structure, but also functional parameters including functional residual capacity and tidal volume, the retrospective self-gating MRI in this work provides a robust approach in quantifying lung diseases in animal models. The TV of a C57BL/6J mouse measured by self-gating UTE MRI in this study, being consistent with previous plethysmographic measurements in C57BL/6J mice (50), also speaks to the robustness of self-gating UTE MRI. While plethysmography has clear advantages in measuring physiological parameters like TV, the advantage of MRI is combining lung tomography with these physiological measurements during the same experiment.
With self-gating UTE MRI, we have successfully demonstrated the ability to longitudinally monitor the pulmonary fibrosis progression in a reproducible mouse model. In this TGF-α mouse model of pulmonary fibrosis, pulmonary fibrogenesis was induced by continuous administration of Dox, leading to fibrosis progressing with time (15). This fibrosis progression was visually seen in the magnetic resonance images of the Dox cohort as compared with that of the control cohort, with collagen and extracellular matrix deposition (manifested as high signal in magnetic resonance images) gradually appearing and increasing in the perivascular, subpleural, and interstitial regions (e.g., the structures indicated by red arrows in Fig. 4B). This was further verified by the photomicrographs of histological slides. It is worth noting that fibrotic structures tended to develop near the subplerual region in the posterior right lung in all Dox mice.
The formation of pulmonary fibrosis was also quantitatively and statistically verified by the significant correlations between MRI-derived lung function parameters and pleural thickness, hydroxyproline content, and pulmonary mechanical parameters (Figs. 6 and 7). However, the strength and direction of the correlations varied, depending on the tested parameters which reflect different aspects of lung properties. Among MRI-derived parameters, TV was observed to have the strongest correlation with other non-MRI-derived parameters. In particular, TV was negatively correlated with hydroxyproline content and positively correlated with compliance. This is reasonable, because TV can reflect compliance in the sense that they both reflect the ease of lung to inflate at a given pressure, while hydroxyproline content reflects the amount of collagen or fibrotic severity in the lung, the increase of which decreases the ease of inflation (14). Since airway elastance is the reciprocal of compliance, the reason why it correlates with TV negatively is simple: fibrosis near airways may decrease the airway diameter and increase airway resistance. TV was negatively correlated with pleural thickness as well, because the increase of pleural thickness in the TGF-α mouse model also results from the increase of fibrotic burden (14). For the similar reason, high-density lung volume percentage increased with fibrotic burden (i.e., collagen and extracellular matrix deposition). In addition to the significant correlations between MRI-derived lung function parameters and non-MRI-derived parameters, each of the non-MRI-derived parameters and part of the MRI-derived parameters (TV and minute ventilation) can individually distinguish between the Dox and control cohorts at Dox week 8 with statistically significant differences (Fig. 5), endorsing the reliability of this TGF-α mouse model in conditionally generating pulmonary fibrosis under control of doxycycline.
By linear mixed-effects model (Fig. 8 and Table 3), the body mass of both Dox and control cohorts did not change significantly over time. However, all three MRI-derived parameters could quantify fibrosis progression, with minute ventilation and TV having the highest statistical significance. In addition, TV of the Dox cohort showed a significant trend opposite to that of the control cohort, indicating that TV was potentially most sensitive to detecting fibrosis progression. Even though the control mice did not gain body mass significantly during the study, their increased TV possibly reflected lung growth. In the Dox cohort, the rate of increase for high-density lung volume percentage (3.9 ± 0.7%/wk, P < 0.001) in our study is similar to that (1.5 ± 0.3%/wk, P < 0.001) measured by the simple GRE MRI in the work of Cleveland et al. (8). Moreover, the rate of increase in our study is larger, suggesting that UTE MRI is more sensitive to short-T2* lung parenchyma remodeling than the simple GRE MRI.
Human pulmonary fibrosis is usually clinically diagnosed after significant lung function decline, and the disease is often progressive and fatal. There is an urgent need to identify novel therapies that can either prevent the progression or even reverse the existing fibrotic load particularly in early diseases. The data from this study are significant as they demonstrate MRI-derived parameters that are highly sensitive and consistent in detecting the longitudinal progression of pulmonary fibrosis in the TGF-α transgenic model over relatively short periods of time in the same mice. Therefore, these new MRI techniques are significant for future preclinical studies that will test the efficacy of novel and translational therapeutics in reversing progressive fibrotic disease.
Conclusions
We have demonstrated for the first time the application of self-gating UTE MRI to monitor morphology and physiology of lung fibrosis in a TGF-α transgenic mouse model. MRI-derived lung function parameters were well correlated with pulmonary-mechanical, histological, and biochemical measurements in quantifying fibrotic burden. These MRI-derived parameters were also able to differentiate cohorts receiving different treatments (with/without doxycycline administration) and track fibrosis progression in individual animals, with a novel finding that tidal volume and minute ventilation decrease in this pulmonary fibrosis model.
GRANTS
This study was carried out via support from National Institutes of Health Grants UL1TR001425, R00-HL-111217, and R01-HL-134801 and from the Cincinnati Children’s Hospital Medical Center, Center for Translational Fibrosis Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.G., C.R.D., and X.X. performed experiments; J.G., C.R.D., and J.C.W. analyzed data; J.G. and J.C.W. interpreted results of experiments; J.G., C.R.D., and X.X. prepared figures; J.G. drafted manuscript; J.G., W.D.H., Z.I.C., and J.C.W. edited and revised manuscript; J.G., W.D.H., Z.I.C., C.R.D., X.X., S.K.M., and J.C.W. approved final version of manuscript; J.C.W. conceived and designed research.
References
- 1.Alsop DC, Hatabu H, Bonnet M, Listerud J, Gefter W. Multi-slice, breathhold imaging of the lung with submillisecond echo times. Magn Reson Med 33: 678–682, 1995. doi: 10.1002/mrm.1910330513. [DOI] [PubMed] [Google Scholar]
- 2.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48, 2015. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 3.Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: projection reconstruction MR imaging. Radiology 179: 777–781, 1991. doi: 10.1148/radiology.179.3.2027991. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi A, Tibiletti M, Kjørstad Å, Birk G, Schad LR, Stierstorfer B, Rasche V, Stiller D. Three-dimensional accurate detection of lung emphysema in rats using ultra-short and zero echo time MRI. NMR Biomed 28: 1471–1479, 2015. doi: 10.1002/nbm.3417. [DOI] [PubMed] [Google Scholar]
- 5.Buehrer M, Curcic J, Boesiger P, Kozerke S. Prospective self-gating for simultaneous compensation of cardiac and respiratory motion. Magn Reson Med 60: 683–690, 2008. doi: 10.1002/mrm.21697. [DOI] [PubMed] [Google Scholar]
- 6.Chan RW, Ramsay EA, Cunningham CH, Plewes DB. Temporal stability of adaptive 3D radial MRI using multidimensional golden means. Magn Reson Med 61: 354–363, 2009. doi: 10.1002/mrm.21837. [DOI] [PubMed] [Google Scholar]
- 7.Chen DL, Rosenbluth DB, Mintun MA, Schuster DP. FDG-PET imaging of pulmonary inflammation in healthy volunteers after airway instillation of endotoxin. J Appl Physiol (1985) 100: 1602–1609, 2006. doi: 10.1152/japplphysiol.01429.2005. [DOI] [PubMed] [Google Scholar]
- 8.Cleveland ZI, Zhou YM, Akinyi TG, Dunn RS, Davidson CR, Guo J, Woods JC, Hardie WD. Magnetic resonance imaging of disease progression and resolution in a transgenic mouse model of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 312: L488–L499, 2017. doi: 10.1152/ajplung.00458.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe ME, Larson AC, Zhang Q, Carr J, White RD, Li D, Simonetti OP. Automated rectilinear self-gated cardiac cine imaging. Magn Reson Med 52: 782–788, 2004. doi: 10.1002/mrm.20212. [DOI] [PubMed] [Google Scholar]
- 10.Fulmer JD, Roberts WC, von Gal ER, Crystal RG. Morphologic-physiologic correlates of the severity of fibrosis and degree of cellularity in idiopathic pulmonary fibrosis. J Clin Invest 63: 665–676, 1979. doi: 10.1172/JCI109349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gewalt SL, Glover GH, Hedlund LW, Cofer GP, MacFall JR, Johnson GA. MR microscopy of the rat lung using projection reconstruction. Magn Reson Med 29: 99–106, 1993. doi: 10.1002/mrm.1910290117. [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Cao X, Cleveland ZI, Woods JC. Murine pulmonary imaging at 7T: T2* and T1 with anisotropic UTE. Magn Reson Med 79: 2254–2264, 2018. doi: 10.1002/mrm.26872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie WD, Korfhagen TR, Sartor MA, Prestridge A, Medvedovic M, Le Cras TD, Ikegami M, Wesselkamper SC, Davidson C, Dietsch M, Nichols W, Whitsett JA, Leikauf GD. Genomic profile of matrix and vasculature remodeling in TGF-α induced pulmonary fibrosis. Am J Respir Cell Mol Biol 37: 309–321, 2007. doi: 10.1165/rcmb.2006-0455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardie WD, Le Cras TD, Jiang K, Tichelaar JW, Azhar M, Korfhagen TR. Conditional expression of transforming growth factor-α in adult mouse lung causes pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 286: L741–L749, 2004. doi: 10.1152/ajplung.00208.2003. [DOI] [PubMed] [Google Scholar]
- 16.Heijman E, de Graaf W, Niessen P, Nauerth A, van Eys G, de Graaf L, Nicolay K, Strijkers GJ. Comparison between prospective and retrospective triggering for mouse cardiac MRI. NMR Biomed 20: 439–447, 2007. doi: 10.1002/nbm.1110. [DOI] [PubMed] [Google Scholar]
- 17.Hiba B, Richard N, Janier M, Croisille P. Cardiac and respiratory double self-gated cine MRI in the mouse at 7 T. Magn Reson Med 55: 506–513, 2006. doi: 10.1002/mrm.20815. [DOI] [PubMed] [Google Scholar]
- 18.Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a convolution function for Fourier inversion using gridding (computerised tomography application). IEEE Trans Med Imaging 10: 473–478, 1991. doi: 10.1109/42.97598. [DOI] [PubMed] [Google Scholar]
- 19.Johnson KO, Pipe JG. Convolution kernel design and efficient algorithm for sampling density correction. Magn Reson Med 61: 439–447, 2009. doi: 10.1002/mrm.21840. [DOI] [PubMed] [Google Scholar]
- 20.King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 21.Korfhagen TR, Le Cras TD, Davidson CR, Schmidt SM, Ikegami M, Whitsett JA, Hardie WD. Rapamycin prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 41: 562–572, 2009. doi: 10.1165/rcmb.2008-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krille L, Zeeb H, Jahnen A, Mildenberger P, Seidenbusch M, Schneider K, Weisser G, Hammer G, Scholz P, Blettner M. Computed tomographies and cancer risk in children: a literature overview of CT practices, risk estimations and an epidemiologic cohort study proposal. Radiat Environ Biophys 51: 103–111, 2012. doi: 10.1007/s00411-012-0405-1. [DOI] [PubMed] [Google Scholar]
- 23.Kuethe DO, Adolphi NL, Fukushima E. Short data-acquisition times improve projection images of lung tissue. Magn Reson Med 57: 1058–1064, 2007. doi: 10.1002/mrm.21230. [DOI] [PubMed] [Google Scholar]
- 24.Kuethe DO, Caprihan A, Fukushima E, Waggoner RA. Imaging lungs using inert fluorinated gases. Magn Reson Med 39: 85–88, 1998. doi: 10.1002/mrm.1910390114. [DOI] [PubMed] [Google Scholar]
- 25.Kuethe DO, Filipczak PT, Hix JM, Gigliotti AP, Estépar RS, Washko GR, Baron RM, Fredenburgh LE. Magnetic resonance imaging provides sensitive in vivo assessment of experimental ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 311: L208–L218, 2016. doi: 10.1152/ajplung.00459.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med 51: 93–102, 2004. doi: 10.1002/mrm.10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Cras TD, Korfhagen TR, Davidson C, Schmidt S, Fenchel M, Ikegami M, Whitsett JA, Hardie WD. Inhibition of PI3K by PX-866 prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Pathol 176: 679–686, 2010. doi: 10.2353/ajpath.2010.090123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol 5: 483–492, 2013. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308: L344–L357, 2015. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706, 2010. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madala SK, Korfhagen TR, Schmidt S, Davidson C, Edukulla R, Ikegami M, Violette SM, Weinreb PH, Sheppard D, Hardie WD. Inhibition of the αvβ6 integrin leads to limited alteration of TGF-α-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 306: L726–L735, 2014. doi: 10.1152/ajplung.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madala SK, Schmidt S, Davidson C, Ikegami M, Wert S, Hardie WD. MEK-ERK pathway modulation ameliorates pulmonary fibrosis associated with epidermal growth factor receptor activation. Am J Respir Cell Mol Biol 46: 380–388, 2012. doi: 10.1165/rcmb.2011-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol 49: 167–179, 2013. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyilas S, Schreder T, Singer F, Poellinger A, Geiser TK, Latzin P, Funke M. Multiple breath washout: a new and promising lung function test for patients with idiopathic pulmonary fibrosis. Respirology 23: 764–770, 2018. doi: 10.1111/resp.13294. [DOI] [PubMed] [Google Scholar]
- 34.O’Sullivan JD. A fast sinc function gridding algorithm for fourier inversion in computer tomography. IEEE Trans Med Imaging 4: 200–207, 1985. doi: 10.1109/TMI.1985.4307723. [DOI] [PubMed] [Google Scholar]
- 35.Olsson LE, Lindahl M, Onnervik PO, Johansson LB, Palmér M, Reimer MK, Hultin L, Hockings PD. Measurement of MR signal and T2* in lung to characterize a tight skin mouse model of emphysema using single-point imaging. J Magn Reson Imaging 25: 488–494, 2007. doi: 10.1002/jmri.20840. [DOI] [PubMed] [Google Scholar]
- 36.Pauly J, Le Roux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm (NMR imaging). IEEE Trans Med Imaging 10: 53–65, 1991. doi: 10.1109/42.75611. [DOI] [PubMed] [Google Scholar]
- 37.Pauly JM. Gridding & the NUFFT for Non-Cartesian Image Reconstruction, 2012. [Google Scholar]
- 38.Pipe JG, Menon P. Sampling density compensation in MRI: rationale and an iterative numerical solution. Magn Reson Med 41: 179–186, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 39.Prokop M. Cancer screening with CT: dose controversy. Eur Radiol 15, Suppl 4: D55–D61, 2005. doi: 10.1007/s10406-005-0145-2. [DOI] [PubMed] [Google Scholar]
- 39a.R Core Team R A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 40.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR; INPULSIS Trial Investigators . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082, 2014. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 41.Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr 27: 825–846, 2003. doi: 10.1097/00004728-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Sansores RH, Ramirez-Venegas A, Pérez-Padilla R, Montaño M, Ramos C, Becerril C, Gaxiola M, Paré P, Selman M. Correlation between pulmonary fibrosis and the lung pressure-volume curve. Lung 174: 315–323, 1996. doi: 10.1007/BF00176190. [DOI] [PubMed] [Google Scholar]
- 43.Schuessler TF, Bates JH. A computer-controlled research ventilator for small animals: design and evaluation. IEEE Trans Biomed Eng 42: 860–866, 1995. doi: 10.1109/10.412653. [DOI] [PubMed] [Google Scholar]
- 44.Shinnar M, Bolinger L, Leigh JS. The synthesis of soft pulses with a specified frequency response. Magn Reson Med 12: 88–92, 1989. doi: 10.1002/mrm.1910120111. [DOI] [PubMed] [Google Scholar]
- 45.Shinnar M, Bolinger L, Leigh JS. The use of finite impulse response filters in pulse design. Magn Reson Med 12: 81–87, 1989. doi: 10.1002/mrm.1910120110. [DOI] [PubMed] [Google Scholar]
- 46.Shinnar M, Eleff S, Subramanian H, Leigh JS. The synthesis of pulse sequences yielding arbitrary magnetization vectors. Magn Reson Med 12: 74–80, 1989. doi: 10.1002/mrm.1910120109. [DOI] [PubMed] [Google Scholar]
- 47.Shinnar M, Leigh JS. The application of spinors to pulse synthesis and analysis. Magn Reson Med 12: 93–98, 1989. doi: 10.1002/mrm.1910120112. [DOI] [PubMed] [Google Scholar]
- 48.Snider GL, Celli BR, Goldstein RH, O’Brien JJ, Lucey EC. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin. Lung volumes, volume-pressure relations, carbon monoxide uptake, and arterial blood gas studied. Am Rev Respir Dis 117: 289–297, 1978. doi: 10.1164/arrd.1978.117.2.289. [DOI] [PubMed] [Google Scholar]
- 49.Spraggins TA. Wireless retrospective gating: application to cine cardiac imaging. Magn Reson Imaging 8: 675–681, 1990. doi: 10.1016/0730-725X(90)90001-I. [DOI] [PubMed] [Google Scholar]
- 50.Tankersley CG, Fitzgerald RS, Levitt RC, Mitzner WA, Ewart SL, Kleeberger SR. Genetic control of differential baseline breathing pattern. J Appl Physiol (1985) 82: 874–881, 1997. doi: 10.1152/jappl.1997.82.3.874. [DOI] [PubMed] [Google Scholar]
- 52.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 275: 11858–11864, 2000. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 53.Weiger M, Brunner DO, Dietrich BE, Müller CF, Pruessmann KP. ZTE imaging in humans. Magn Reson Med 70: 328–332, 2013. doi: 10.1002/mrm.24816. [DOI] [PubMed] [Google Scholar]
- 54.Weiger M, Pruessmann KP, Hennel F. MRI with zero echo time: hard versus sweep pulse excitation. Magn Reson Med 66: 379–389, 2011. doi: 10.1002/mrm.22799. [DOI] [PubMed] [Google Scholar]
- 55.Weiger M, Wu M, Wurnig MC, Kenkel D, Jungraithmayr W, Boss A, Pruessmann KP. Rapid and robust pulmonary proton ZTE imaging in the mouse. NMR Biomed 27: 1129–1134, 2014. doi: 10.1002/nbm.3161. [DOI] [PubMed] [Google Scholar]
- 56.West JR, Alexander JK. Studies on respiratory mechanics and the work of breathing in pulmonary fibrosis. Am J Med 27: 529–544, 1959. doi: 10.1016/0002-9343(59)90038-5. [DOI] [PubMed] [Google Scholar]
- 57.White RD, Paschal CB, Clampitt ME, Spraggins TA, Lenz GW. Electrocardiograph-independent, “wireless” cardiovascular cine MR imaging. J Magn Reson Imaging 1: 347–355, 1991. doi: 10.1002/jmri.1880010313. [DOI] [PubMed] [Google Scholar]
- 58.Zhou Y, Chen H, Ambalavanan N, Liu G, Antony VB, Ding Q, Nath H, Eary JF, Thannickal VJ. Noninvasive imaging of experimental lung fibrosis. Am J Respir Cell Mol Biol 53: 8–13, 2015. doi: 10.1165/rcmb.2015-0032TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123: 1096–1108, 2013. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zwart NR, Johnson KO, Pipe JG. Efficient sample density estimation by combining gridding and an optimized kernel. Magn Reson Med 67: 701–710, 2012. doi: 10.1002/mrm.23041. [DOI] [PubMed] [Google Scholar]