Abstract
Background
This is an updated version of the original Cochrane review published in Issue 3, 2012. Caffeine has been added to common analgesics such as paracetamol, ibuprofen, and aspirin, in the belief that it enhances analgesic efficacy. Evidence to support this belief is limited and often based on invalid comparisons.
Objectives
To assess the relative efficacy of a single dose of an analgesic plus caffeine against the same dose of the analgesic alone, without restriction on the analgesic used or the pain condition studied. We also assessed serious adverse events.
Search methods
We searched CENTRAL, MEDLINE, and EMBASE from inception to 28 August 2014, the Oxford Pain Relief Database, and also carried out Internet searches and contacted pharmaceutical companies known to have carried out trials that have not been published.
Selection criteria
We included randomised, double‐blind studies that compared a single dose of analgesic plus caffeine with the same dose of the analgesic alone in the treatment of acute pain.
Data collection and analysis
Two review authors independently assessed the eligibility and quality of studies, and extracted data. Any disagreements or uncertainties were settled by discussion with a third review author. We sought any validated measure of analgesic efficacy, but particularly the number of participants experiencing at least 50% of the maximum possible pain relief over four to six hours, participants reporting a global evaluation of treatment of very good or excellent, or headache relief after two hours. We pooled comparable data to look for a statistically significant difference, and calculated numbers needed to treat to benefit (NNT) with caffeine. We also looked for any numerical superiority associated with the addition of caffeine, and information about any serious adverse events.
Main results
We identified no new studies with available results for this update. The earlier review included 20 studies (7238 participants) in valid comparisons, but because we used different outcomes for some headache studies, the number of participants in the analyses of the effects of caffeine is now 4262 when previously it was 5243. The studies were generally of good methodological quality, using standard designs and mostly standard scales of pain measurement, although many of those treating postoperative pain were small.
Most studies used paracetamol or ibuprofen, with 100 mg to 130 mg caffeine, and the most common pain conditions studied were postoperative dental pain, postpartum pain, and headache. There was a small but statistically significant benefit with caffeine used at doses of 100 mg or more, which was not dependent on the pain condition or type of analgesic. About 5% to 10% more participants achieve a good level of pain relief (at least 50% of the maximum over four to six hours) with the addition of caffeine, giving a NNT of about 14 (high quality evidence).
Most comparisons individually demonstrated numerical superiority with caffeine, but not statistical superiority. One serious adverse event was reported with caffeine, but was considered unrelated to any study medication.
We know of the existence of around 25 additional studies with almost 12,500 participants for which data for analysis were not obtainable. The additional analgesic effect of caffeine remained statistically significant but clinically less important even if all the known missing data had no effect; the bulk of the unobtainable data are reported to have similar results as this review.
Authors' conclusions
The addition of caffeine (≥ 100 mg) to a standard dose of commonly used analgesics provides a small but important increase in the proportion of participants who experience a good level of pain relief.
Keywords: Adolescent; Adult; Aged; Female; Humans; Male; Middle Aged; Pregnancy; Acetaminophen; Acetaminophen/therapeutic use; Acute Pain; Acute Pain/drug therapy; Analgesics; Analgesics/administration & dosage; Analgesics/therapeutic use; Caffeine; Caffeine/administration & dosage; Caffeine/therapeutic use; Chemotherapy, Adjuvant; Chemotherapy, Adjuvant/methods; Diclofenac; Diclofenac/therapeutic use; Drug Synergism; Dysmenorrhea; Dysmenorrhea/drug therapy; Headache; Headache/drug therapy; Ibuprofen; Ibuprofen/therapeutic use; Pain, Postoperative; Pain, Postoperative/drug therapy; Postpartum Period; Randomized Controlled Trials as Topic; ortho‐Aminobenzoates; ortho‐Aminobenzoates/therapeutic use
Plain language summary
Caffeine as an analgesic adjuvant for acute pain in adults
Caffeine is found in various plant products, and may be ingested in drinks like tea, coffee, and some soft drinks and energy drinks. Caffeine is a stimulant, and can improve alertness and prevent tiredness over short periods. It may disturb sleep in some people if taken before bed. Ordinary consumption of caffeine (less than 500 milligrams daily) is not harmful to health. Caffeine is commonly used in pain‐relieving medicines available from pharmacies without a prescription. An adjuvant is something that is added to a medicine to make it work better.
This review examined whether caffeine improves the pain‐relieving effects of such medicines. We searched for studies up to August 2014 and included twenty studies (7238 participants) examining several pain conditions, including headache, post‐dental pain, postoperative pain following childbirth, and menstrual period pain. The studies were generally of good methodological quality, using standard designs and mostly standard scales of pain measurement. Many of those in post‐dental and postoperative pain were small, and small studies can overestimate benefits.
A dose of caffeine equivalent to a mug of coffee added to a standard dose of common analgesics such as paracetamol or ibuprofen provided better pain relief. Analgesic plus caffeine increased the number of people who had a good level of pain relief by 5% to 10% compared with analgesic alone (high quality evidence).
No serious adverse events were reported that were related to either the analgesic or caffeine in these studies (low quality evidence). It is unlikely that adding caffeine to an analgesic will be harmful if the recommended dose is not exceeded.
Summary of findings
for the main comparison.
| Analgesic plus caffeine compared with analgesic alone for acute pain | ||||||
|
Patient or population: adults with acute pain Settings: community Intervention: analgesic plus caffeine Comparison: same dose of analgesic alone | ||||||
| Outcomes | Outcome with analgesic alone | Outcome with analgesic plus caffeine | RR and NNT (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Effective pain relief | 41% | 48% | RR 1.2 (1.1 to 1.3) NNT 14 (9.9 to 24) |
4262 (27 separate comparisons) |
High | Small effect size but large numbers of participants contributing. There is a large amount of data that cannot be incorporated into this review, but this result is robust to analysis assuming all missing data show no effect. In fact, the results of this review are consistent with an almost completely different analysis in 10,000 participants demonstrating the effect of caffeine to have a similar effect size |
| Serious adverse events | 1 event | 1 event | Not calculated | Not calculated | Very low | Neither event judged related to study medication. Single dose studies are not powered to assess serious adverse events |
| CI: confidence interval; NNT: number needed to treat to benefit; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
This is an updated version of the original Cochrane review, 'Caffeine as an analgesic adjuvant for acute pain in adults', published in Issue 3, 2012 (Derry 2012).
Description of the condition
Caffeine is added to a variety of basic analgesics that are used to treat a broad range of common painful conditions. We included information from any acute painful condition, with headache, postpartum pain, and postoperative pain the most commonly studied.
Description of the intervention
Caffeine is a naturally occurring compound found in the seeds, leaves, and fruit of many plants, where it is thought to function as a natural pesticide. It has a long (at least 5000 years) history of human consumption in the form of beverages such as tea and coffee, and foodstuffs such as chocolate. Caffeine intake varies widely among individuals and populations, but can be broadly divided into low (< 100 mg/day), moderate (100 mg to 400 mg/day), and high intake (> 400 mg/day), with the majority of people falling within the moderate intake range. Common sources of caffeine today include coffee (100 mg to 150 mg/mug), tea (75 mg/mug), cola drinks (up to 40 mg/drink), energy drinks (approximately 80 mg/drink), plain chocolate (up to 50 mg/bar), and caffeine tablets (100 mg/tablet). Some 'high‐energy' drinks have the caffeine content of five or six mugs of coffee.
Caffeine is a methylxanthine that is known to act as a central nervous system stimulant. It has a wide range of physiological effects in humans (Sawynok 1993), including increased wakefulness, alertness, endurance, heart rate, and blood pressure, and is regarded as a psychostimulant (enhances mood; Donovan 2001).
An adjuvant in this context is an agent that enhances the effects of a drug while having few if any direct effects when given by itself. There have been several reports of an intrinsic antinociceptive effect of caffeine from preclinical studies in rodents, but in general, only at very high doses of 50 mg/kg or more (Sawynok 2011a). A recent Cochrane review examined the use of high‐dose caffeine (300 mg) following post‐dural puncture headache; there were very few data (Basurto Ona 2013). Caffeine at dietary levels is not usually regarded as an analgesic in its own right in humans. Caffeine has been included as a constituent of both over‐the‐counter and prescription analgesic combinations for many years, based on the idea that it enhances analgesic efficacy.
The evidence supporting caffeine as a useful analgesic adjuvant has always been somewhat limited, with only a handful of often small studies providing any direct evidence of enhanced analgesia with a caffeine‐analgesic combination compared with the same analgesic alone. Randomised studies that have attempted to answer this question by comparing analgesic plus caffeine with the same dose of the analgesic alone have produced mixed results, with some showing a clear benefit for addition of caffeine (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; Migliardi 1994 Study 1; Migliardi 1994 Study 2), and others showing no significant analgesic effect (Forbes 1990; McQuay 1996). An ongoing problem is that a large number of clinical trials have only been published in part, in reviews, without full publication or clinical trial reports made available; in one review only four of 30 studies had previously been published (Laska 1984). With only a relatively small benefit shown in some studies, this makes any estimate of adjuvant efficacy particularly susceptible to publication bias from unpublished studies with no effect.
It is possible that part of the reason for these mixed results is differing efficacy in different clinical pain models: for example it is suggested that caffeine may be a useful adjuvant in headache, but not postsurgical pain (Sawynok 2011a; Sawynok 2011b). Another complication is the fact that several studies, including some that are often cited as supporting the addition of caffeine to analgesics, attempt to draw their conclusions from comparisons of an analgesic plus caffeine with a different dose of the analgesic, or even a completely different analgesic (Jain 1978; Schachtel 1991 (in a trial of headache)).
A small number of reviews have attempted to investigate systematically the effect of adding caffeine to individual commonly used analgesics, including paracetamol (Palmer 2010; Zhang 1996), aspirin (Zhang 1997), and ibuprofen (Li Wan Po 1998). The three reviews published in the 1990s failed to provide any conclusive evidence for an analgesic adjuvant effect with any of these three drugs. The most recent review did demonstrate a marginal benefit of paracetamol and caffeine over paracetamol alone (Palmer 2010).
To add to the confusion, several preclinical studies have reported that very low doses of caffeine (lower than those that may exhibit adjuvant analgesic effects) actually inhibit antinociception by several agents (Sawynok 2011b), particularly paracetamol (Sawynok 2011c)), raising the possibility that dietary caffeine might interfere with the analgesic efficacy of some treatments. In the case of headache, another complication is that abrupt caffeine withdrawal is associated with the onset of headache, and this can be reversed by caffeine administration (Juliano 2004). Both of these issues have the potential to complicate any assessment of caffeine as an analgesic adjuvant.
How the intervention might work
The mechanisms by which caffeine may contribute to, or enhance the efficacy of other analgesics are not well understood. It is known to be a competitive antagonist of adenosine A1 and A2 receptors at plasma concentrations observed through normal dietary caffeine intake (in the 10 to 100 μM range). Many of the putative mechanisms of action are thought of in terms of this disruption of normal adenosine signalling. Proposed mechanisms of action include the following (Renner 2007; Sawynok 1993; Zhang 2001).
Improved drug absorption through lower gastric pH and increased gastric blood flow.
Reduced metabolic clearance of drugs through reduced hepatic blood flow.
Blockade of peripheral pro‐nociceptive adenosine signalling, and activation of the central noradenosine pathway (pain‐suppressing systems).
Transcriptional down‐regulation of cyclo‐oxygenase‐2 (COX‐2), via blockade of the adenosine A2a receptor.
Relief of inhibitor adenosine actions on central cholinergic nerve terminals.
Changes in mood and emotional state contributing to changes in the perception of pain.
Why it is important to do this review
Caffeine has been added to a large number of analgesics for years on the basis of a kind of inherited wisdom from a small number of trials showing an enhanced analgesic effect of combinations including caffeine. However, this is not the full story as some of these studies do not compare like with like, and there have been at least as many studies published suggesting no additional effect when caffeine is added. It is important to try to resolve this confusion to inform best clinical practice.
One of the major problems faced in trying to demonstrate an analgesic adjuvant effect of caffeine is the relatively small magnitude of this effect compared with normal variation in the course of an individual patient's pain and in the responses of different patients to the same analgesic, as noted 30 years ago (Beaver 1984). Many of the studies carried out have simply been underpowered to expose a statistically significant difference in treatment effects of analgesic plus caffeine versus the analgesic alone (Moore 1998). Meta‐analyses, in which data from individual comparisons are pooled, are an important tool for showing up these small effects that individual trials, on the whole, are unable to demonstrate. This methodology has been used successfully in the past to demonstrate a statistical superiority of higher dose aspirin, ibuprofen, or paracetamol over lower doses of the same drug (McQuay 2007).
This review provides an opportunity to apply systematically the same methodology, pooling individual comparison data wherever possible, to investigate the possible analgesic adjuvant effect of caffeine.
Objectives
To assess the relative efficacy of a single dose of an analgesic plus caffeine against the same dose of the analgesic alone, without restriction on the analgesic used or the pain condition studied. We also assessed serious adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included double‐blind studies comparing a single dose of oral analgesic plus caffeine with the same dose of the analgesic alone for the treatment of acute pain in adults; the caffeine had to be administered at the same time as the analgesic. Studies had to have a minimum of 10 participants randomly allocated to each treatment group and report some measure of patient‐reported pain relief.
We included studies using multiple doses to treat a single episode only if appropriate data from the first dose were available. We included cross‐over studies and studies reporting treatment of consecutive episodes (for example, consecutive migraine attacks) in pain conditions that result in comparable, recurrent, acute pain episodes, such as migraine. We used first dose only data where possible, but we accepted data from both phases of a cross‐over, or consecutive phases for recurrent conditions, if there was adequate washout (at least 48 hours pain‐ and medication‐free between phases).
Types of participants
Studies included adult participants (at least 16 years of age) with any acute painful condition. We did not include studies of experimental pain in healthy volunteers because these do not accurately correlate with clinical pain. Ideally participants had moderate to severe pain, to ensure sensitivity to detect a change in pain intensity, but mild to moderate pain was accepted.
Types of interventions
Included studies had to use a single dose of oral analgesic plus caffeine to treat an acute painful episode. The analgesics we were particularly interested in were paracetamol, ibuprofen, aspirin, diclofenac, naproxen, oxycodone, ergotamine, and the triptans, although studies using other analgesics were not excluded. There was no restriction on dose of analgesic or caffeine.
To investigate the effect of caffeine on the efficacy of the analgesic with which it is combined, it was essential that the comparator was the same drug and dose as the combination, minus caffeine.
Types of outcome measures
We collected data on the type of painful condition and baseline pain intensity.
Primary outcomes
We considered the following primary outcomes.
The number of participants with at least 50% of maximum pain relief at four to six hours.
The number of participants rating their treatment as "very good" or "excellent" on a five‐point categorical patient global evaluation of treatment (PGE) scale with the wording "poor, fair, good, very good, excellent" (or equivalent).
The number of participants achieving a self defined clinically meaningful level of pain relief.
The number of participants with headache relief at two hours.
In many postsurgical studies the outcome of "at least 50% of maximum pain relief at four to six hours" had to be transformed from group‐mean pain measures, as described in the 'Data synthesis' section.
We report the pain measures used in the 'Characteristics of included studies' table and have been as explicit as possible about how we transformed data from the various scales to the dichotomous outcomes specified above.
We considered only data obtained directly from the participant (pain reported by a physician, nurse, or carer was not included in the analysis).
Secondary outcomes
Although single dose studies in acute pain are generally underpowered to assess safety and tolerability and cannot provide information on repeat dosing strategies, we sought information on serious adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL) via CRSO (to 28 August 2014);
MEDLINE via Ovid (1946 to 28 August 2014);
EMBASE via Ovid (1974 to 28 August 2014);
Oxford Pain Relief Database for the original review (Jadad 1996a). This database is no longer being updated.
See Appendix 1 for the search strategy for MEDLINE, Appendix 2 for EMBASE, and Appendix 3 for CENTRAL. We did not impose any language restrictions.
Searching other resources
We searched reference lists of retrieved studies and review articles for additional studies. We know of a number of unpublished trials using a caffeine analgesic combination, and we contacted relevant manufacturers to try to determine the extent of, and obtain, any unpublished data. We carried out Internet searches to identify any studies or study results that may have been reported to agencies such as the Food and Drug Administration (FDA).
For the update we searched two clinical trials databases (ClinicalTrials.gov (ClinicalTrials.gov)) and World Health Organization International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) to identify additional published or unpublished data.
We did not search grey literature and short abstracts (meeting reports).
Data collection and analysis
Selection of studies
Two review authors independently carried out the searches and selected studies for inclusion. We viewed the titles and abstracts of all studies identified by electronic searches on screen, and excluded any that clearly did not satisfy the inclusion criteria. We obtained full copies of the remaining studies to identify those suitable for inclusion, and settled any disagreements or uncertainty by discussion with the third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standard data extraction form. Disagreements and uncertainty were settled by discussion with the third review author. One review author entered data into RevMan 5.3 (RevMan 2014).
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum (Jadad 1996b).
Two authors independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study:
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, for example random number table; computer random number generator); or unclear risk of bias (when the method used to generate the sequence is not clearly stated). We excluded studies at a high risk of bias using a non‐random process (for example, odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (for example, telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); or unclear risk of bias (when the method is not clearly stated). We excluded studies that did not conceal allocation and are therefore at a high risk of bias (for example, open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (for example, study stated that it was blinded and described the method used to achieve blinding, for example, identical tablets, matched in appearance and smell); or unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies at a high risk of bias that were not double‐blind.
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
We used risk ratio (RR), calculated using a fixed‐effect model, to determine statistical difference between treatment groups, and number needed to treat to benefit (NNT) to provide an absolute measure of treatment effect. See 'Data synthesis' section for details.
Unit of analysis issues
We accepted randomisation of individual participants only. We did not, for example, accept studies where randomisation was by centre.
Dealing with missing data
The most likely source of missing data in single dose studies is cross‐over studies, in which multiple successive attacks are treated. In these studies, participants are excluded from the efficacy analyses after taking an initial dose simply because they do not have a sufficient number of qualifying pain episodes (for example, separate migraine attacks) to complete the cross‐over study. This is unlikely to introduce bias where it occurs equally in both treatment arms.
Assessment of heterogeneity
We assessed heterogeneity of studies visually using L'Abbé plots of the percentage of participants with the outcome with caffeine compared with those without caffeine (L'Abbé 1987), and with the use of the I2 statistic.
Assessment of reporting biases
Unpublished studies in this field are known to exist, as demonstrated in the review by Laska et al (Laska 1984), which analysed data from 30 clinical trials ‐ of which only four have been published in full. Obtaining unpublished studies, many of which were conducted 25 or more years ago, from the pharmaceutical companies sponsoring them was not possible. It is therefore difficult to make any meaningful assessment of reporting bias, and results must be interpreted with caution, with the knowledge that some degree of reporting bias is likely.
Approaches to pharmaceutical companies that may have had data were unsuccessful. In addition, although we know of data relating to three unpublished studies, we have been unable to obtain permission to use it. We identified further unpublished studies, with no available results, during this update.
We assessed the number of trials of average size amongst the included studies, with a RR of one (no effect), needed to reduce any statistically significant result to one that fails to meet statistical significance (following Moore 2008).
Data synthesis
Where possible, we used dichotomous data to calculate the RR with 95% confidence intervals (CIs) using a fixed‐effect model (Morris 1995). We calculated NNT with 95% CIs using the pooled number of events by the method of Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the RR did not include the number one.
Many studies provided data on pain measures using:
five‐point categorical pain relief (PR) scales with comparable wording to "none, slight, moderate, good or complete";
four‐point categorical pain intensity (PI) scales with comparable wording to "none, mild, moderate, severe";
visual analogue scale (VAS) for pain relief;
VAS for pain intensity.
We summed these pain measures over a four to six‐hour period to generate a measure of total pain relief (TOTPAR) or summed pain intensity difference (SPID) over this time period.
Where only non‐dichotomous, mean data are reported, we transformed them into dichotomous data using standardised methods. We converted any mean TOTPAR, SPID, VAS TOTPAR or VAS SPID values to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991). We calculated the proportion of participants in each treatment group achieving at least 50%maxTOTPAR using verified equations (Moore 1996; Moore 1997a; Moore 1997b), and converted these proportions into the number of participants achieving at least 50%maxTOTPAR by multiplying by the total number of participants in the treatment group. We then used information on the number of participants with at least 50%maxTOTPAR for the analgesic plus caffeine and the analgesic alone to calculate estimates of RR and NNT as before.
We tested for significant differences between groups in sensitivity analyses using the z test (Tramèr 1997).
Subgroup analysis and investigation of heterogeneity
We analysed data for different pain conditions and different analgesics separately where there were sufficient data (minimum of two studies and 200 participants).
Sensitivity analysis
We planned to carry out sensitivity analyses for dose of caffeine (< 70 mg, 70 mg to 150 mg, and > 150 mg), methodological quality (2 versus ≥ 3 on the Oxford Quality Scale), baseline pain intensity (mild versus ≥ moderate), and size (< 50 versus ≥ 50 participants in each treatment arm), where there were sufficient data (minimum of two studies and 200 participants). We also tried to ascertain whether any adjuvant effect of caffeine depended on the particular analgesic drug with which it was combined, and to investigate whether analgesic effect size with analgesic drug alone affects any adjuvant effects of caffeine through limiting the upside sensitivity of the analgesic assay (Cooper 1991).
Results
Description of studies
Results of the search
Searches of bibliographic databases for this update identified 279 potential studies in CENTRAL, 622 in MEDLINE, and 1216 in EMBASE; there were no new included or excluded studies. Searches of clinicaltrials.gov and the International Clinical Trials Registry Platform identified 42 and 21 potential studies, respectively. Of these, five appeared to satisfy the inclusion criteria, all of which have, or are likely to have, completed but none of which had any results available (IRCT201306121760N24; NCT00471952; NCT01172405; NCT01929031; NCT02183688) (Figure 1).
1.
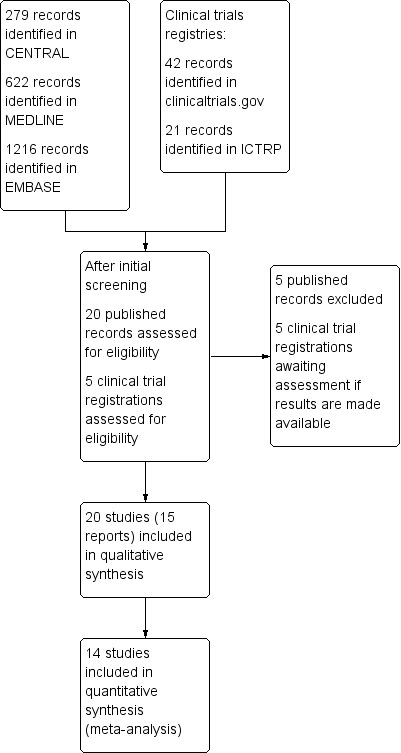
Study flow diagram.
The search for studies comparing a given dose of a given analgesic with the same dose of the same analgesic plus caffeine was complicated. The main reason was the large number of potentially relevant studies from which no data were available. The following narrative acts as a guide to the size the size of problem.
A 1994 review of caffeine as an analgesic adjuvant included 30 studies with over 10,600 participants (Laska 1984). Of these, it is likely that 12 studies with 4600 participants provided information regarding a suitable direct comparison. While the review provided no useful information for analysis, we believe that four studies (1206 participants) had been published previously and data from those were available (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4). However, the likelihood is that there are at least eight studies with 3394 participants for which we know data exist but which were unavailable.
We obtained from the Internet an undated document in the form of a Citizen Petition Summary from Bristol‐Myers Squibb relating to use of caffeine as an analgesic adjuvant (BMS summary). The document had information about 17 studies involving 8772 participants. Eight of these studies (3231 participants) were submitted to the FDA in 1982, and we believe that they were probably included in the 1984 review (Laska 1984); nine studies (5541 participants) had submission dates of 1986 or later, and were probably not included in the 1984 review.
We are aware of three studies with up to 850 participants conducted by McNeil Consumer that are likely to have useful comparison data. We have been unable to obtain permission to use these data.
Although 20 studies were eventually included with data on 7238 participants, we know or suspect of the existence of 20 studies with 9785 participants for which data for analysis were not obtainable. There were also an additional 2689 participants in the five studies identified in clinical trial registries, for whom no results are yet available. About 12,500 participants have contributed to relevant adjuvant caffeine studies without available information.
The search for this update did not identify any new studies with data.
Included studies
Twenty studies (15 publications) fulfilled the inclusion criteria for this review; all 20 were published in full peer‐reviewed journals (Ali 2007; Diamond 2000; Diener 2005; Forbes 1990; Forbes 1991; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; McQuay 1996; Migliardi 1994 Study 1; Migliardi 1994 Study 2; Peroutka 2004; Schachtel 1991a; Sunshine 1996; Tokola 1984; Ward 1991; Winter 1983; Wójcicki 1977 study 1; Wójcicki 1977 Study 2). These studies provided data on 7238 participants.
We identified no additional studies by contacting manufacturers or searching the Internet, but searches of clinical trials registries for this update identified five additional studies (estimated 2689 participants) that may satisfy the inclusion criteria (IRCT201306121760N24; NCT00471952; NCT01172405; NCT01929031; NCT02183688). Although all of these studies have probably been completed, none have been published, and no results are posted in the trial registries. We have placed them in 'Studies awaiting classification', and have provided as many relevant details as possible. We sent emails to the principal investigators or sponsors of the studies where this information was provided, but at the time of publication of this update none had responded.
The majority of included studies recruited participants aged 18 years or over, with some placing an upper age limit of 60 to 85 years of age. One study recruited participants aged 16 to 75 years of age (Winter 1983), while another two recruited participants aged 15 years or older (Forbes 1990; Forbes 1991). Five studies did not report the age range for recruiting participants, and overall mean ages ranged from 21 to 46 years (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; McQuay 1996). The majority of participants were female (58% to 100%) in 17 of the 19 included studies, and five of these had an exclusively female study population (Ali 2007; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; Sunshine 1996).
A number of different pain conditions were studied: postpartum pain (for example, episiotomy, uterine cramp), postoperative dental pain (third molar extraction), headache (tension, migraine, idiopathic), dysmenorrhoea, and sore throat. For analysis we combined postpartum pain and postoperative dental pain, since it was thought likely that most of the postpartum pain followed episiotomy.
The majority of studies prohibited participants from consuming any caffeine‐containing food, beverages, or medications within a defined period of treatment with study medication (ranging from three hours to midnight the night before), as well as during the study period. Three studies did not prohibit caffeine consumption before administration of study medication, but required that any caffeine consumed within four or 24 hours, respectively, of the study period was noted (Migliardi 1994 Study 1; Migliardi 1994 Study 2; Ward 1991). Seven studies did not comment on caffeine consumption before or during the study (Diener 2005; Forbes 1990; Peroutka 2004; Tokola 1984; Winter 1983; Wójcicki 1977 study 1; Wójcicki 1977 Study 2). Eight studies restricted participants from taking study medication within a defined time period of other analgesics and medications (Ali 2007; Diamond 2000; Diener 2005; Migliardi 1994 Study 1;Migliardi 1994 Study 2; Schachtel 1991a; Sunshine 1996; Winter 1983). The remaining 12 studies did not report on restricted use of other medications before administration of study medication.
Participants were generally excluded for: pregnancy or lactation, recent history of alcohol, analgesic or other substance abuse, allergy or intolerance to any of the study or rescue medications, or existing illness or medical condition that could compromise interpretation of the results. Reasons for exclusion specific to a particular study are described in the 'Characteristics of included studies' table.
The majority of studies required participants to have at least moderate pain (two or more on standard four‐point pain intensity scale, or equivalent) before treating with study medication. One study required only mild pain before treatment (Diener 2005), one study required a headache rated two or greater on the McGill Pain Questionnaire (Ward 1991), and two studies did not report baseline pain intensity (Diamond 2000; Tokola 1984).
Most of the included studies used a parallel‐group design (15/20), with the remaining five involving a treatment of multiple acute pain episodes (headache or migraine) in a cross‐over design (Ali 2007; Migliardi 1994 Study 1; Migliardi 1994 Study 2; Tokola 1984; Ward 1991).
The response to study treatment was measured using a standard four‐point pain intensity scale or five‐point pain relief scale, or both, in the majority of studies (15 studies and 13 studies respectively). The remaining studies used alternative pain intensity or pain relief scales (for example, 100 mm VAS) or patient global evaluations which are described in the 'Characteristics of included studies' table. Some headache studies also used a patient global outcome (Diamond 2000; Diener 2005).
The 20 studies reported on 17 different treatment comparisons:
Paracetamol 500 mg + caffeine 65 mg versus paracetamol 500 mg (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3).
Paracetamol 648 mg + caffeine 65 mg versus paracetamol 648 mg (Ward 1991).
Paracetamol 648 mg + caffeine 130 mg versus paracetamol 648 mg (Ward 1991).
Paracetamol 1000 mg + caffeine 100 mg versus paracetamol 1000 mg (Wójcicki 1977 study 1; Wójcicki 1977 Study 2).
Paracetamol 1000 mg + caffeine 130 mg versus paracetamol 1000 mg (Ali 2007; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; Migliardi 1994 Study 1; Migliardi 1994 Study 2; Winter 1983).
Paracetamol 1500 mg + caffeine 195 mg versus paracetamol 1500 mg (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3).
Paracetamol 2000 mg + caffeine 260 mg versus paracetamol 2000 mg (Laska 1983 Study 4).
Ibuprofen 100 mg + caffeine 100 mg versus ibuprofen 100 mg (Forbes 1991; Sunshine 1996).
Ibuprofen 200 mg + caffeine 50 mg versus ibuprofen 200 mg (McQuay 1996).
Ibuprofen 200 mg + caffeine 100 mg versus ibuprofen 200 mg (Forbes 1991; McQuay 1996; Sunshine 1996).
Ibuprofen 200 mg + caffeine 200 mg versus ibuprofen 200 mg (McQuay 1996).
Ibuprofen 400 mg + caffeine 200 mg versus ibuprofen 400 mg (Diamond 2000).
Aspirin 650 mg + caffeine 65 mg versus aspirin 650 mg (Forbes 1990).
Aspirin 800 mg + caffeine 64 mg versus aspirin 800 mg (Schachtel 1991a).
Aspirin 500 mg + paracetamol 400 mg + caffeine 100 mg versus aspirin 500 mg + paracetamol 400 mg (Diener 2005).
Diclofenac sodium softgel 100 mg + caffeine 100 mg versus diclofenac sodium softgel 100 mg (Peroutka 2004).
Tolfenamic acid 200 mg + caffeine 100 mg versus tolfenamic acid 200 mg (Tokola 1984).
Full details of included studies are provided in the Characteristics of included studies table.
Excluded studies
For the original review we excluded six reports after reading them in full (BMS summary (17 studies); Jain 1988; Laska 1984 (probably 12 relevant studies); Migliardi 1994a; Mitchell 2008; Schachtel 1991b). The BMS summary had information on 17 potentially relevant studies in tension‐type headache (HPD‐H203; 170‐01‐88; 170‐02‐88), postoperative dental pain (HPD‐D104; HPD‐D105; 171‐01‐88; 2569; 2711; 2570; 2571), and postpartum pain (2255; 2576; 2577, 2578; 2579; 2580; 2581). Eight of these (2569; 2711; 2255; 2576; 2577, 2578; 2579; 2580) were probably also included in Laska 1984. Laska 1984 reported on 30 studies, of which 12 were probably relevant to this review, and four are included studies (published separately as Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4).
We did not exclude any additional studies identified for this update. The reasons for exclusions are provided in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Included studies were all randomised and double‐blind. Four studies scored five out of five on the Oxford Quality Scale (Ali 2007; Diener 2005; Forbes 1990; McQuay 1996), 11 studies scored four out of five (Forbes 1991; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; Migliardi 1994 Study 1; Migliardi 1994 Study 2; Schachtel 1991a; Sunshine 1996; Tokola 1984; Winter 1983), four studies scored three out of five (Diamond 2000; Peroutka 2004; Wójcicki 1977 study 1; Wójcicki 1977 Study 2), and one study scored two out of five (Ward 1991).
Comments on potential biases in individual studies related to random sequence generation, allocation concealment, blinding, and study size, are reported in the 'Risk of bias' section of the 'Characteristics of included studies' table. The findings are displayed in Figure 2 and Figure 3.
2.
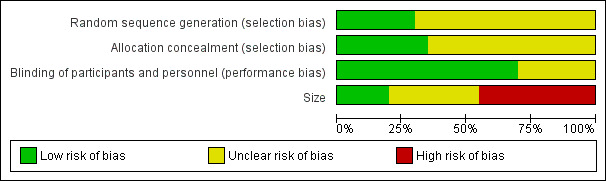
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
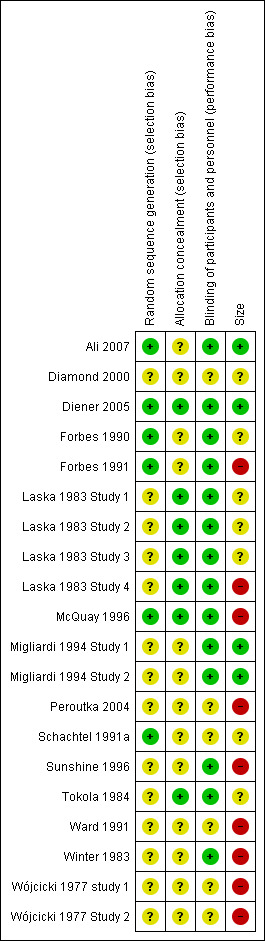
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
The large numbers of studies and participants excluded because of unavailability of data ‐ larger than the number with data for analysis ‐ provided a significant potential for publication bias.
Allocation
All studies stated that they were randomised but only six reported the method used to generate the random sequence, and only four described the method used to conceal the allocation of the sequence.
Blinding
All studies stated that they were double‐blind, but only 10 adequately described the method used to conceal the treatment allocation from the participants and study personnel.
Other potential sources of bias
We judged seven studies to be at high risk of bias because they randomised fewer than 50 participants to each treatment arm. We judged only three to be at low risk because they randomised at least 200 participants to each treatment arm.
Effects of interventions
See: Table 1
For all studies we report whether there was a simple numerical superiority for analgesic plus caffeine compared with analgesic alone using the primary outcomes (Appendix 4).
Most postoperative studies reported data that allowed calculation of the number of participants achieving at least 50% of the maximum possible pain relief over the duration of the study, and we used these studies for analyses in this review. One reported patients being pain‐free after four hours, and we used that (Wójcicki 1977 Study 2).
Headache studies reported various outcomes. One reported the number of participants rating their treatment as "very good" or "excellent" on a five‐point categorical patient global evaluation of treatment (Diamond 2000), while another reported a four‐point scale with the top value of "very good" (Diener 2005), which we used. Another reported the number of participants with headache relief at one hour: 41% (19/46) with diclofenac plus caffeine, and 27% (12/45) with diclofenac alone (Peroutka 2004). Tokola 1984 reported the number of participants with no pain or mild pain at 1.5 hours. Wójcicki 1977 study 1 reported the number of participants with headache relief at four hours with paracetamol + caffeine versus paracetamol alone. We did not convert average pain intensity and pain relief outcomes to dichotomous outcomes for two studies in tension headache (Migliardi 1994 Study 1; Migliardi 1994 Study 2), as the equations to do so were developed in postoperative pain and may not be appropriate. Ward 1991 also provided data on the mean pain intensity difference from baseline over two hours, but this could not be dichotomised as there is no valid method over this time period: the summed pain intensity difference (SPID) 2 for paracetamol 648 mg + caffeine 65 mg was 32.6, compared with 37.5 for paracetamol 648 mg + caffeine 130 mg, and 28.3 for paracetamol 648 mg alone.
Schachtel 1991a (sore throats) provided mean data for pain relief over two hours, but this could not be dichotomised as there is no valid method over this time period: the total pain relief (TOTPAR) at two hours for aspirin 800 mg + caffeine 64 mg was 6.3, compared with 4.7 for aspirin 800 mg alone.
Pain conditions
All pain conditions
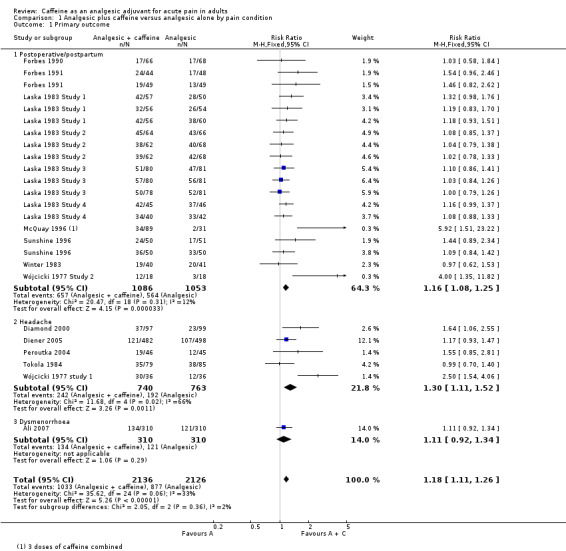
Twenty‐five comparisons (4262 participants) compared analgesic plus caffeine versus the same dose of analgesic alone (Figure 4). Caffeine provided additive analgesia irrespective of pain condition, dose of caffeine, analgesic, and proportion of responders with analgesic alone.
4.
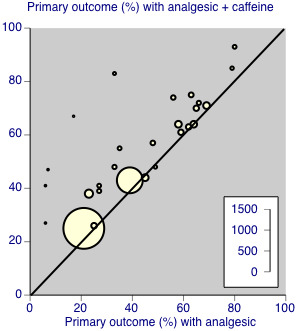
Individual studies comparing the primary outcome for analgesic + caffeine versus analgesic alone ‐ any pain condition
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 48% (1033/2136; range 26% to 83%).
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 41% (877/2126; range 6% to 66%).
The RR for the addition of caffeine was 1.2 (95% CI 1.1 to 1.3; Analysis 1.1), and the NNT was 14 (9.9 to 24).
1.1. Analysis.
Comparison 1 Analgesic plus caffeine versus analgesic alone by pain condition, Outcome 1 Primary outcome.
Postoperative/postpartum pain
There were 19 comparisons (2239 participants) of analgesic plus caffeine versus the same dose of analgesic alone for postoperative or postpartum pain (Analysis 1.1).
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 60% (657/1086; range 26% to 93%).
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 51% (568/1115; range 6% to 80%).
The RR for the addition of caffeine was 1.2 (1.1 to 1.3; Analysis 1.1), and the NNT was 10 (7.3 to 18).
Headache pain
Five studies (1503 participants) provided data in migraine or tension‐type headache (Diamond 2000; Diener 2005; Tokola 1984; Wójcicki 1977 study 1).
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 33% (242/740; range 25% to 83%).
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 25% (172/763; range 21% to 43%).
The RR for the addition of caffeine was 1.3 (1.1 to 1.5; Analysis 1.1), and the NNT was 13 (8.3 to 34).
Dysmenorrhoea
Only one study (620 participants) provided data in dysmenorrhoea; 134/310 (43%) of participants had at least 50% of the maximum pain relief after analgesic plus caffeine, and 121/310 (39%) after analgesic alone (Ali 2007).
Choice of analgesic
At least 50% of maximum pain relief
Studies combined caffeine with paracetamol, ibuprofen, or aspirin alone, or with aspirin plus paracetamol.
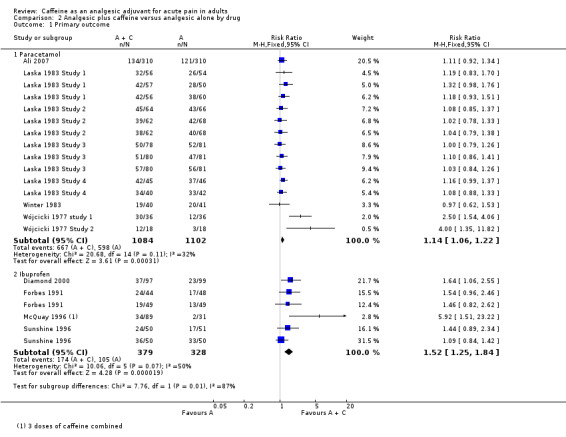
Paracetamol
Fifteen comparisons (2186 participants) compared paracetamol plus caffeine with paracetamol alone (Ali 2007; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; Winter 1983; Wójcicki 1977 study 1; Wójcicki 1977 Study 2). Doses of paracetamol ranged from 500 mg to 2000 mg.
The proportion of participants with at least 50% of the maximum pain relief after paracetamol plus caffeine was 62% (667/1084; range 43% to 93%).
The proportion of participants with at least 50% of the maximum pain relief after paracetamol alone was 54% (598/1102; range 39% to 80%).
The RR for the addition of caffeine was 1.1 (1.06 to 1.2; Analysis 2.1), and the NNT was 14 (8.8 to 32).
2.1. Analysis.
Comparison 2 Analgesic plus caffeine versus analgesic alone by drug, Outcome 1 Primary outcome.
Ibuprofen
Six comparisons (707 participants) compared ibuprofen plus caffeine with ibuprofen alone (Diamond 2000; Forbes 1991; McQuay 1996; Sunshine 1996). Doses of ibuprofen ranged from 100 mg to 400 mg.
The proportion of participants with at least 50% of the maximum pain relief after ibuprofen plus caffeine was 46% (174/379; range 38% to 72%).
The proportion of participants with at least 50% of the maximum pain relief after ibuprofen alone was 32% (105/328; range 6% to 66%).
The RR for the addition of caffeine was 1.5 (1.3 to 1.8; Analysis 2.1), and the NNT was 7.2 (4.8 to 15).
Aspirin
One study (134 participants) used aspirin 650 mg (Forbes 1990), in a comparison with the aspirin plus caffeine; 17/66 (26%) participants experienced at least 50% of the maximum pain relief with aspirin plus caffeine, and 17/68 (25%) with aspirin alone.
One study (980 participants) used aspirin 500 mg plus paracetamol 400 mg in a comparison with the aspirin plus paracetamol plus caffeine; 429/482 (89%) participants experienced at least 50% of the maximum pain relief with aspirin plus paracetamol plus caffeine, and 435/566 (77%) with aspirin plus paracetamol only (Diener 2005).
Diclofenac
One study (91 participants) used diclofenac 100 mg in comparison with the diclofenac plus caffeine (Peroutka 2004); 19/46 (41%) had pain reduced to mild or none at one hour with diclofenac plus caffeine, and 12/45 with diclofenac alone.
Tolfenamic acid
One study (164 participants) used tolfenamic acid 200 mg in comparison with tolfenamic acid plus caffeine; 35/79 (44%) participants had no or mild pain at 1.5 hours with tolfenamic acid plus caffeine, and 38/85 (45%) with tolfenamic acid alone.
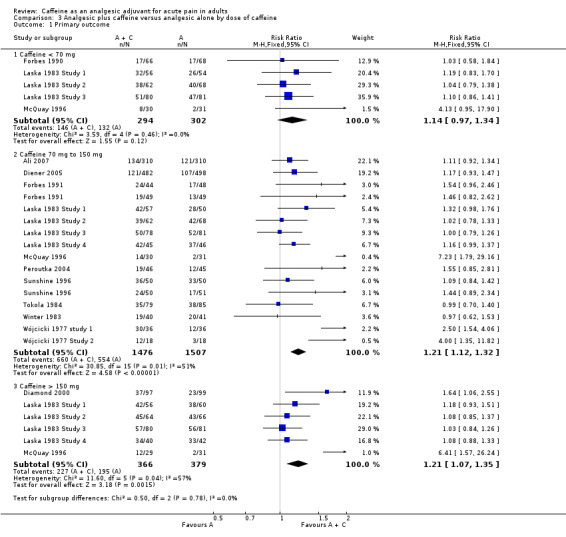
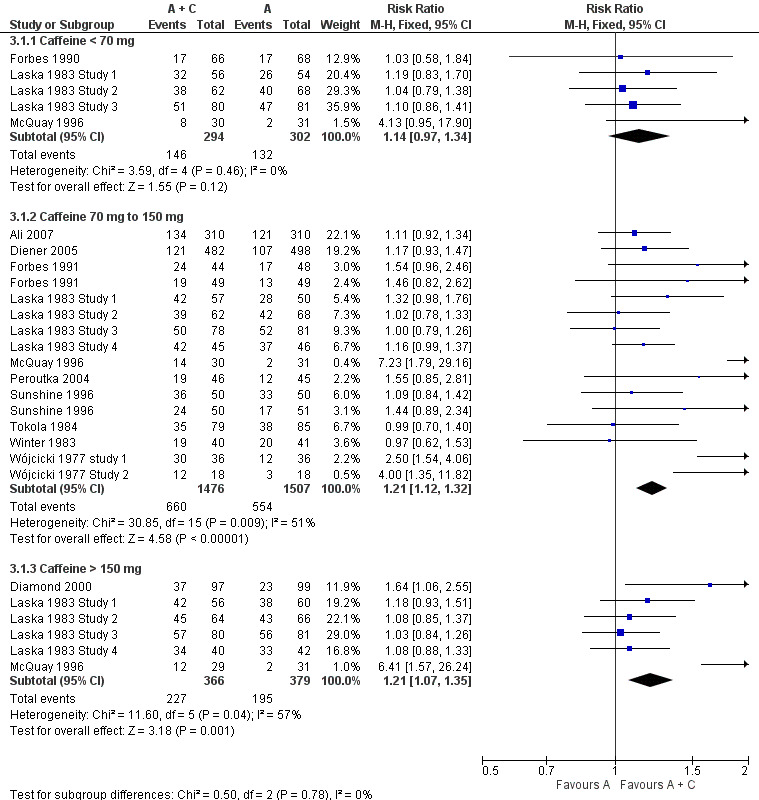
Dose of caffeine
Studies used doses of caffeine ranging from 50 mg to 260 mg, but typically they were 100 mg or 200 mg. We analysed all trials together to investigate whether there was a dose response for caffeine.
Caffeine < 70 mg
Five comparisons (596 participants) provided data (Forbes 1990; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; McQuay 1996). All were in postoperative pain.
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 50% (146/294; range 26% to 64%).
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 44% (132/302; range 6% to 59%).
The RR for the addition of caffeine was 1.1 (0.97 to 1.3; Analysis 3.1). There was no significant difference between treatment groups and the NNT was not calculated.
3.1. Analysis.
Comparison 3 Analgesic plus caffeine versus analgesic alone by dose of caffeine, Outcome 1 Primary outcome.
Caffeine 70 mg to 150 mg
Sixteen comparisons (2983 participants) provided data (Ali 2007; Diener 2005; Forbes 1991; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; McQuay 1996; Peroutka 2004; Sunshine 1996; Tokola 1984; Winter 1983; Wójcicki 1977 study 1; Wójcicki 1977 Study 2).
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 45% (660/1476; range 25% to 83%).
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 37% (554/1507; range 6% to 80%).
The RR for the addition of caffeine was 1.2 (1.1 to 1.3; Analysis 3.1), and the NNT was 13 (8.7 to 23).
Caffeine > 150 mg
Six comparisons (745 participants) provided data (Diamond 2000; Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4; McQuay 1996).
The proportion of participants with at least 50% of the maximum pain relief after analgesic plus caffeine was 62% (227/366; range 41% to 85%).
The proportion of participants with at least 50% of the maximum pain relief after analgesic alone was 51% (195/379; range 6% to 79%).
The RR for the addition of caffeine was 1.2 (1.1 to 1.4; Analysis 3.1), and the NNT was 9.5 (5.7 to 29).
There was no clear dose response (Figure 5).
5.
Forest plot of comparison: 3 Analgesic plus caffeine versus analgesic alone by dose of caffeine, outcome: 3.1 Primary outcome.
Sensitivity analyses
Methodological quality
Only one study had a quality score of two out of five (Ward 1991), so no sensitivity could be carried out for this criterion.
Baseline pain intensity
One study did not state baseline pain (Diamond 2000), one administered the first dose when pain was mild (Tokola 1984), two treated when pain was "at least mild" (Diener 2005; Ward 1991), while the remainder treated when pain was moderate or severe. Tokola 1984 and Ward 1991 did not provide data suitable for analysis. Comparing Diamond 2000 and Diener 2005 with studies in moderate or severe pain gave no statistically significant difference (z = 0.1995, P value = 0.905).
Size
Using data for all pain conditions, comparing the 13 comparisons with treatments arms of 50 participants or fewer (964 participants; NNT for analgesic + caffeine versus analgesic alone 7.0 (4.9 to 13)) with the 14 comparisons with treatment arms of more than 50 participants (3298 participants; NNT 21 (12 to 70)) gave a statistically significant difference (z = 2.6032, P value = 0.009). Larger studies produced a smaller effect size.
Serious adverse events
One study did not report any information about adverse events during the study (Ward 1991). The remaining studies all provided some information, and only one reported any serious adverse events (Diener 2005). One participant experienced acute enteritis after treatment with the aspirin plus paracetamol plus caffeine combination, and one experienced an attack of ulcerative colitis following paracetamol alone: neither event was considered by the investigators to be drug‐related.
Discussion
Since publication of the previous version of this review, we found no new studies.
Summary of main results
This review update was able to analyse 25 comparisons of analgesic plus caffeine versus the same dose of analgesic alone in 4262 participants. Most studies used paracetamol (500 mg to 1500 mg) or ibuprofen (100 mg to 400 mg), with two using aspirin (650 mg and 800 mg), one aspirin (500 mg) plus paracetamol (400 mg), one diclofenac (100 mg), and one tolfenamic acid (200 mg). Caffeine was added at doses of 50 mg to 260 mg, with most studies using between 100 mg and 200 mg. A number of different pain conditions were studied: headache, postoperative pain, postpartum pain, dysmenorrhoea, and sore throat.
Numerical superiority (all primary outcomes) was demonstrated in all but three out of 28 comparisons (Appendix 4):
Paracetamol 1000 mg + caffeine 130 mg versus paracetamol 1000 mg alone (Laska 1983 Study 3; 159 participants).
Tolfenamic acid 200 mg + caffeine 100 mg versus tolfenamic acid 100 mg alone (Tokola 1984; 164 participants).
Paracetamol 1000 mg + caffeine 130 mg versus paracetamol 1000 mg alone (Winter 1983; 81 participants).
We carried out pooled analyses for studies in which one primary outcome was reported or could be calculated. For all studies combined there was a small significant benefit for adjuvant caffeine (RR 1.2 (1.1 to 1.3) and NNT 14 (9.9 to 24)).
Analysis according to pain condition demonstrated a similar benefit in postoperative pain (NNT 10 (7.3 to 18)) and headache (NNT 13 (8.3 to 34)). The benefit was the same for both paracetamol and ibuprofen. Analysis according to dose of caffeine demonstrated a statistically significant effect for doses of 100 mg and more with NNT of about 10, at 65 mg and below the result was not significant (RR 1.2 (0.94 to 1.4)). The absolute proportion of additional participants achieving at least 50% maximum pain relief was 6% at doses of 65 mg caffeine or less, 8% with doses between 70 and 150 mg, and 11% with doses of 150 mg or more. Failure to achieve a statistically significant improvement with lowest doses of caffeine (generally 65 mg) may have reflected the small number of studies and participants with the lowest dose.
A particular feature of the finding of an adjuvant effect of caffeine was its consistency in terms of the pain condition, analgesic used, and level of pain relief with analgesic alone. Only one study used an effective analgesic (ibuprofen 200 mg) with groups treated with several different doses of caffeine in addition (McQuay 1996); this study had the same result as the overall finding, namely that doses of caffeine of 65 mg or below are ineffective as an analgesic adjuvant.
Caffeine is commonly consumed worldwide at doses similar to those used in these studies, and its side effect profile is well known; nervousness and dizziness are common (Zhang 2001). No unexpected events occurred and the one serious event in a participant treated with adjuvant caffeine was not considered related to the study medication.
Overall completeness and applicability of evidence
Caffeine was added to only a limited number of analgesics in these studies, and it is unclear whether the small additional benefit demonstrated overall and with paracetamol and ibuprofen individually will apply to any other analgesic. Several different pain conditions were studied; while other acute conditions such as non‐surgical trauma were not included, it is unlikely that the result would be different, given that analgesics do not appear to be condition‐specific. It should be noted, though, that the review was limited to acute pain only. Whether caffeine has any effect in chronic painful conditions has yet to be elucidated.
Over the dose range of 65 mg to 200 mg, no increase in adjuvant effect was noted with increasing caffeine dose. This may be a function of limited data combined with small effect size, or the limited dose range studied, but it may also reflect the mechanism by which caffeine achieves an adjuvant response. The information in this review can pose rather than answer these questions.
Quality of the evidence
Most of the studies were relatively old, with only three (in headache) published since 2000, but they were generally of good methodological quality, using standard designs and mostly standard scales of pain measurement, though studies in tension‐type headache are problematical as to outcomes (Moore 2014). Individual studies, especially those in postpartum and dental pain, were small and individually they rarely demonstrated a significant benefit for caffeine. Meta‐analyses of small trials are susceptible to overestimation of effects (Dechartres 2013; Nüesch 2010). The larger studies were predominantly in headache pain, and individually demonstrated statistical significance.
Potential biases in the review process
The biggest threat to the validity of the results arises from the large amount of data that is known to exist in unpublished studies with data unavailable for this review. The small, significant, and arguably clinically relevant 5% to 10% increased number of responders was derived from the 4262 participants providing data for one primary outcome. We calculated that the result would remain statistically significant (though probably clinically much less relevant) if we added 10,000 additional participants in studies where there was no difference between analgesic and the same dose of analgesic plus caffeine (RR = 1.0).
This is the approximate size of the amount of unpublished data about which we know. However, there is considerable evidence that a positive effect of caffeine occurs in these studies (BMS summary; Laska 1984; Sawynok 1993). In this circumstance it is unlikely that publication bias would play any role in changing either the direction or magnitude of the result.
There were two other possible interferences in these assays. One was a possible interference of caffeine in analgesic effects of paracetamol (Sawynok 2011c). A very large part of the data came from studies comparing paracetamol with paracetamol plus caffeine, therefore reducing any adjuvant analgesic effects of caffeine so that any bias would be a negative bias. Moreover, there was no difference between studies in which paracetamol was the analgesic and those in which ibuprofen was the analgesic used. The other possible interference was from caffeine withdrawal headache (Juliano 2004), where use of caffeine might have a possible positive bias; the effects of adjuvant caffeine in headache were virtually identical to the effects in other painful conditions.
Agreements and disagreements with other studies or reviews
This review is in agreement with several reviews that have reported a small, but significant, analgesic adjuvant effect of caffeine at doses in excess of 65 mg when combined with common analgesics such as paracetamol and ibuprofen (Laska 1984; Palmer 2010; Sawynok 2011b). Other reviews in postsurgical pain only have reported an inconsistent effect for caffeine combined with ibuprofen (Li Wan Po 1998), no effect for caffeine combined with aspirin (Zhang 1997), and no effect for caffeine combined with paracetamol (Zhang 1996).
This update has taken a somewhat different approach to outcomes in some headache studies, which led to modest differences in the numbers available for analysis, but the results are similar to the original review.
Authors' conclusions
Implications for practice.
Caffeine is an effective analgesic adjuvant in acute pain at doses of about 100 mg or above. It confers an additional benefit amounting to an extra 5% to 10% of patients achieving a good level of pain relief.
Implications for research.
The existing evidence is probably sufficient to support the use of caffeine with analgesics. Given the very large number of studies and participants for which data are not currently available, efforts should go into acquiring those data rather than performing additional studies. Additional research might explore the absence of an obvious dose response for caffeine over the range 65 mg to 200 mg, and whether this has implications for the mechanism of adjuvant actions of caffeine.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 11 October 2017 | Review declared as stable | No new studies likely to change the conclusions are expected. |
History
Protocol first published: Issue 8, 2011 Review first published: Issue 3, 2012
| Date | Event | Description |
|---|---|---|
| 10 December 2014 | Review declared as stable | This review will be assessed for further updating in 2024. |
| 22 October 2014 | New search has been performed | We ran new searches on 28 August 2014. No new studies for inclusion or exclusion, but identified five additional unpublished studies. We used a slightly different approach to outcomes from some headache studies, and corrected some minor errors. |
| 22 October 2014 | New citation required but conclusions have not changed | Conclusions unchanged. PRISMA flow chart and 'Summary of findings' table added |
| 27 June 2012 | Amended | Contact details updated. |
| 16 April 2012 | Amended | Minor correction to forest plot of Analysis 3.1. |
Acknowledgements
We wish to thank Prof Henry McQuay for his insight and useful discussions, and the peer reviewers and Cochrane PaPaS Group editorial team for their helpful comments on the original review.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is currently the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategy for MEDLINE (via OVID)
Caffeine/ or caffeine.mp. (26526)
exp Pain/ (311576)
(pain or painful or analgesi*).mp. (537685)
2 or 3 (613026)
randomized controlled trial.pt. (385941)
controlled clinical trial.pt. (89662)
randomized.ab. (282607)
drug therapy.fs. (1735361)
randomly.ab. (199356)
groups.ab. (1275406)
5 or 6 or 7 or 8 or 9 or 10 (3174781)
1 and 4 and 11 (622)
Appendix 2. Search strategy for EMBASE (via OVID)
caffeine/ or caffeine.mp. (43358)
exp pain/ (26526)
(pain or painful or analgesi*).mp. (913249)
2 or 3 (1137545)
clinical trial.sh. (837697)
controlled clinical trial.sh. (386524)
randomized controlled trial.sh. (351013)
double‐blind procedure.sh. (117557)
(clin* adj25 trial*).ab. (321203)
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab. (139362)
placebo*.ab. (199403)
random*.ab. (882264)
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (1697273)
1 and 4 and 13 (1216)
Appendix 3. Search strategy for CENTRAL (via CRSO)
MeSH descriptor Caffeine (1376)
caffeine:TI,AB,KY (2107)
1 or 2 (2107)
MeSH descriptor Pain explode all trees (29853)
(pain or painful or analgesi*):TI,AB,KY (71646)
4 or 5 (76877)
3 and 6 (279)
Appendix 4. Results for individual studies: efficacy and serious adverse events
| Study ID | Condition | Treatment | Efficacy outcome | Participants with outcome | Numerical superiority | Serious adverse events |
| Ali 2007 | Dysmenorrhoea | (1) Paracetamol 1000 mg + caffeine 140 mg, n = 310 (2) Paracetamol 1000 mg, n = 310 | TOTPAR 4 h: (1) 6.58 (2) 6.07 |
≥ 50% max PR: (1) 134/310 (2) 121/310 |
Yes | None |
| Diamond 2000 | Tension‐type headache | (1) Ibuprofen 400 mg + caffeine 200 mg, n = 97 (2) Ibuprofen 400 mg, n = 99 | Very good or excellent | (1) 37/97 (2) 23/99 |
Yes | None |
| Diener 2005 | Tension‐type headache and/or migraine | (1) Aspirin 500 mg + paracetamol 400 mg + caffeine 100 mg, n = 482 (2) Aspirin 500 mg + paracetamol 400 mg, n = 498 | Very good, good | Very good (1) 121/482 (2) 107/498 Very good or good (1) 353/482 (2) 328/498 |
Yes | (1) 1/482 (2) 0/498 Also 1 SAE following paracetamol only |
| Forbes 1990 | Dental | (1) Aspirin 650 mg + caffeine 65 mg, n = 66 (2) Aspirin 650 mg, n = 68 | TOTPAR 6 h: (1) 6.8 (2) 6.57 |
≥ 50% max PR: (1) 17/66 (2) 17/68 |
Yes Note: > 10% attrition |
None |
| Forbes 1991 | Dental | (1) Ibuprofen 100 mg + caffeine 100 mg, n = 49
(2) Ibuprofen 100 mg, n = 49 (3) Ibuprofen 200 mg + caffeine 100 mg, n = 44 (4) Ibuprofen 200 mg, n = 48 |
TOTPAR 6 h: (1) 8.95 (2) 6.67 (3) 12.1 (4) 8.65 |
≥ 50% max PR: (1) 19/49 (2) 13/49 (3) 24/44 (4) 17/48s |
Yes (100 mg) Yes (200 mg) Note: > 10% attrition |
None |
| Laska 1983 Study 1 | Postpartum pain and uterine cramping | (1) Paracetamol 500 mg + caffeine 65 mg, n = 56 (2) Paracetamol 500 mg, n = 54 (3) Paracetamol 1000 mg + caffeine 130 mg, n = 57 (4) Paracetamol 1000 mg, n = 50 (5) Paracetamol 1500 mg + caffeine 195 mg, n = 56 (6) Paracetamol 1500 mg, n = 60 |
TOTPAR 4 h: (1) 8.2 (2) 7.1 (3) 10.3 (4) 8.2 (5) 10.4 (6) 9.1 |
≥ 50% max PR: (1) 32/56 (2) 26/54 (3) 42/57 (4) 28/50 (5) 42/56 (6) 38/60 |
Yes (500 mg) Yes (1000 mg) Yes (1500 mg) Note: > 10% attrition |
None |
| Laska 1983 Study 2 | Postpartum pain and uterine cramping | (1) Paracetamol 500 mg + caffeine 65 mg, n = 62 (2) Paracetamol 500 mg, n = 68 (3) Paracetamol 1000 mg + caffeine 130 mg, n = 62 (4) Paracetamol 1000 mg, n = 68 (5) Paracetamol 1500 mg + caffeine 195 mg, n = 64 (6) Paracetamol 1500 mg, n = 66 |
TOTPAR 4 h: (1) 8.8 (2) 8.4 (3) 9.0 (4) 8.8 (5) 9.9 (6) 9.3 |
≥ 50% max PR: (1) 38/62 (2) 40/68 (3) 39/62 (4) 42/68 (5) 45/64 (6) 43/66 |
Yes (500 mg) Yes (1000 mg) Yes (1500 mg) Note: > 10% attrition |
None |
| Laska 1983 Study 3 | Postepisiotomy or postsurgical | (1) Paracetamol 500 mg + caffeine 65 mg, n = 80 (2) Paracetamol 500 mg, n = 81 (3) Paracetamol 1000 mg + caffeine 130 mg, n = 78 (4) Paracetamol 1000 mg, n = 81 (5) Paracetamol 1500 mg + caffeine 195 mg, n = 80 (6) Paracetamol 1500 mg, n = 81 |
TOTPAR 4 h: (1) 9.1 (2) 8.4 (3) 9.1 (4) 9.1 (5) 9.9 (6) 9.7 |
≥ 50% max PR: (1) 51/80 (2) 47/81 (3) 50/78 (4) 52/81 (5) 57/80 (6) 56/81 |
Yes (500 mg) No (1000 mg) Yes (1500 mg) Note: > 10% attrition |
None |
| Laska 1983 Study 4 | Dental | (1) Paracetamol 1000 mg + caffeine 130 mg, n = 45 (2) Paracetamol 1000 mg, n = 46 (3) Paracetamol 2000 mg + caffeine 260 mg, n = 40 (4) Paracetamol 2000 mg, n = 42 |
TOTPAR 4 h: (1) 12.6 (2) 11.0 (3) 11.6 (4) 10.8 |
≥ 50% max PR: (1) 42/45 (2) 37/46 (3) 34/40 (4) 33/42 |
Yes (1000 mg) Yes (2000 mg) |
None |
| McQuay 1996 | Dental | (1) Ibuprofen 200 mg + caffeine 50 mg, n = 30 (2) Ibuprofen 200 mg + caffeine 100 mg, n = 30 (3) Ibuprofen 200 mg + caffeine 200 mg, n = 29 (4) Ibuprofen 200 mg, n = 31 | TOTPAR 6 h: (1) 7.0 (2) 10.3 (3) 9.5 (4) 3.0 |
≥ 50% max PR: (1) 8/30 (2) 14/30 (3) 12/29 (4) 2/31 |
Yes (all doses of caffeine) | None |
| Migliardi 1994 Study 1 | Tension‐type headache | (1) Paracetamol 1000 mg + caffeine 130 mg, n = 336 (2) Paracetamol 1000 mg, n = 332 | No extractable data | No usable data | Yes | None |
| Migliardi 1994 Study 2 | Tension‐type headache | (1) Paracetamol 1000 mg + caffeine 130 mg, n = 339 (2) Paracetamol 1000 mg, n = 337 | No extractable data | No usable data | Yes | None |
| Peroutka 2004 | Migraine | (1) Diclofenac 100 mg + caffeine 100 mg, n = 46 (2) Diclofenac 100 mg, n = 45 | HR 1 h | No/mild pain (1) 19/46 (2) 12/45 |
Yes Note: > 10% attrition |
None |
| Schachtel 1991 | Tonsillopharyngitis | (1) Aspirin 800 mg + caffeine 64 mg, n = 70 (2) Aspirin 800 mg, n = 68 | TOTPAR 2: (1) 6.3 (2) 4.7 |
No usable data | Yes | None |
| Sunshine 1996 | Postepisiotomy | (1) Ibuprofen 100 mg + caffeine 100 mg, n = 50
(2) Ibuprofen 100 mg, n = 51 (3) Ibuprofen 200 mg + caffeine 100 mg, n = 50 (4) Ibuprofen 200 mg, n = 50 |
TOTPAR 4: (1) 8.41 (2) 6.65 (3) 10.6 (4) 10.3 |
≥ 50% max PR: (1) 24/50 (2) 17/51 (3) 36/50 (4) 33/50 |
Yes (100 mg) Yes (200 mg) |
None |
| Tokola 1984 | Migraine | (1) Tolfenamic acid 200 mg + caffeine 100 mg, n = 79 (2) Tolfenamic acid 200 mg, n = 200 mg, n = 85 |
PI 1.5 h | No/mild pain (1) 35/79 (2) 38/85 Baseline pain not reported |
No | None |
| Ward 1991 | Non‐migrainous headache | (1) Paracetamol 648 mg + caffeine 65 mg, n = 53 (2) Paracetamol 648 mg + caffeine 130 mg, n = 53 (3) Paracetamol 648 mg, n = 53 |
SPID 2 h: (1) 32.63 (2) 37.54 (3) 28.30 |
No usable data | Yes Note: > 10% attrition |
No data |
| Winter 1983 | Dental | (1) Paracetamol 1000 mg + caffeine 130 mg, n = 40 (2) Paracetamol 1000 mg, n = 41 | TOTPAR 4 h: (1) 7.1 (2) 7.4 |
≥ 50% max PR: (1) 19/40 (2) 20/41 |
No | None |
| Wójcicki 1977 study 1 | Idiopathic headache | (1) Paracetamol 500 mg + caffeine 50 mg, n = 36 (2) Paracetamol 500 mg, n = 36 | Pain‐free within 4 hours | No pain: (1) 26/36 (2) 13/36 |
Yes (headache) | None |
| Wójcicki 1977 Study 2 | Postoperative pain | (1) Paracetamol 500 mg + caffeine 50 mg, n = 18 (2) Paracetamol 500 mg, n = 18 | Pain‐free within 4 hours | (1) 12/18 (2) 3/19 |
Yes (postoperative) | None |
| Key: HR ‐ headache relief (moderate/severe to mild/none); PF ‐ pain free; PI ‐ pain intensity; PR ‐ pain relief; SAE ‐ serious adverse event; SPID ‐ summed pain intensity difference; TOTPAR ‐ total pain relief | ||||||
Data and analyses
Comparison 1. Analgesic plus caffeine versus analgesic alone by pain condition.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome | 16 | 4262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.11, 1.26] |
| 1.1 Postoperative/postpartum | 10 | 2139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.08, 1.25] |
| 1.2 Headache | 5 | 1503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.11, 1.52] |
| 1.3 Dysmenorrhoea | 1 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.34] |
Comparison 2. Analgesic plus caffeine versus analgesic alone by drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Paracetamol | 8 | 2186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.06, 1.22] |
| 1.2 Ibuprofen | 4 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.25, 1.84] |
Comparison 3. Analgesic plus caffeine versus analgesic alone by dose of caffeine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Caffeine < 70 mg | 5 | 596 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.97, 1.34] |
| 1.2 Caffeine 70 mg to 150 mg | 14 | 2983 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.12, 1.32] |
| 1.3 Caffeine > 150 mg | 6 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.07, 1.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ali 2007.
| Methods | Single‐centre, randomised, double‐blind, 3‐way cross‐over study, with a single oral dose administered at the onset of moderate or severe menstrual pain (and no later than 24 h after the onset of menstrual flow) Duration: 6 h, with assessments at baseline, 0.5, 1, 2, 3, 4, 5, and 6 h post dose |
|
| Participants | Primary dysmenorrhoea ‐ graded 2 or 3 on the Andersch and Milsom scoring system over 3 of the 4 previous menstrual cycles and requiring medication with OTC analgesics. Menstrual cycle duration between 21 and 35 days, and adequate past response to OTC analgesics for treatment of dysmenorrhoea Participants aged 18 years or older N = 320 (310 for efficacy) All F Mean age 21 years |
|
| Interventions | Paracetamol 1000 mg + caffeine 130 mg, n = 320 (310 for efficacy) Paracetamol 1000 mg, n = 320 (310 for efficacy) Caffeine 130 mg, n = 160 (155 for efficacy) Placebo, n = 160 (155 for efficacy) No alcohol or caffeine within the 6 h before and after dosing. No concomitant use of analgesics, psychoactive drugs, antispasmodics, natural treatments, or devices such as hot water bottles and heated pads within 6 h before or after dosing |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts Serious adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All treatments were supplied unmarked and blister packed. "caffeine and placebo tablets custom manufactured ... and matched the size and shape of the paracetamol‐containing caplets" |
| Size | Low risk | > 200 participants in relevant treatment arms |
Diamond 2000.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study, with a single oral dose administered (baseline pain intensity not reported) Duration: 6 h, with assessments at baseline, 15, 30, 45, 60, 90, and 120 minutes, then hourly through to 6 h post dose |
|
| Participants | Acute tension‐type headache ‐ in accordance with IHS criteria, with 3 to 15 tension‐type headaches every month for ≥ 1 year, and ≥ 75% of headaches responsive to non‐prescription‐strength analgesics Participants with occasional migraine headaches (< 2 per month) were not excluded provided they could differentiate the two types of headache No alcohol, caffeine‐containing foods/beverages, or any other analgesic within 4 h before dosing Participants at least 18 years of age N = 331 (301 for efficacy) M 57, F 244 Mean age 37 years |
|
| Interventions | Ibuprofen 400 mg + caffeine 200 mg, n = 97 for efficacy Ibuprofen 400 mg, n = 99 for efficacy Caffeine 200 mg, n = 57 for efficacy Placebo, n = 48 for efficacy |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: standard 5‐point scale Withdrawals and dropouts |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Method not described |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
Diener 2005.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study. Participants treated 2 headache episodes, each with a single oral dose administered when pain at least mild (≥ 30 mm on 100 mm VAS) Duration: 4 h, with assessments at baseline, 0.5, 1, 2, 3, and 4 h post dose |
|
| Participants | Episodic tension‐type headache (13%) or migraine with or without aura (84%) ‐ in accordance with IHS criteria, with a history of ≥ 12 months and ≥ 2 headache episodes in previous 3 months Participants were excluded if they: treated headaches with prescription analgesics or migraine drugs, required higher single doses of non‐prescription analgesics than indicated in the patient information leaflet, normally treated headaches with non‐prescription analgesics in effervescent tablet form, had > 10 days of headache per month, suffered possible menstrual migraine, or whose headaches normally spontaneously resolved within 4 h Participants aged 18 to 65 years N = 1889 (1743 for efficacy) M 453, F 1436 Mean age not reported Mean baseline pain intensity 64 mm on 100 mm VAS |
|
| Interventions | Aspirin 500 mg + paracetamol 400 mg + caffeine 100 mg, n = 521 (482 for efficacy) Aspirin 500 mg + paracetamol 400 mg, n = 538 (498 for efficacy) Aspirin 1000 mg, n = 276 (252 for efficacy) Paracetamol 1000 mg, n = 275 (251 for efficacy) Caffeine 100 mg, n = 141 (132 for efficacy) Placebo, n = 138 (128 for efficacy) No concomitant treatment with prescription or non‐prescription analgesics, antidepressants, or antipsychotic medication, or migraine prophylaxis |
|
| Outcomes | PI: 100 mm VAS PGE: 4‐point scale ('very good', 'good', 'less good', 'poor') Withdrawals and dropouts Serious adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Consecutive participants assigned in sequential order |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medication was "identical in colour, size, shape and taste" |
| Size | Low risk | > 200 participants in relevant treatment arms |
Forbes 1990.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study, with a single oral dose administered at the onset of steady moderate or severe pain Duration: 6 h, with assessments at baseline, 1, 2, 3, 4, 5, and 6 h post dose |
|
| Participants | Dental surgery: third molar removal Patients were ≥ 15 years of age N = 401 (350 for efficacy) M 147, F 203 Mean age 21 years |
|
| Interventions | Aspirin 650 mg + caffeine 65 mg, n = 66 for efficacy Aspirin 650 mg, n = 68 for efficacy Aspirin 1000 mg, n = 71 for efficacy Caffeine 65 mg, n = 70 for efficacy Placebo, n = 75 for efficacy |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: standard 5‐point scale Withdrawals and dropouts Serious adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "all tablets were identical in appearance" |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
Forbes 1991.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study, with a single oral dose administered at the onset of moderate or severe pain Duration: 8 h, with assessments at baseline, 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 h post dose |
|
| Participants | Dental surgery: third molar removal Patients were at least 15 years of age N = 362 (298 for efficacy) M 121, F 177 Mean age 22 years |
|
| Interventions | Ibuprofen 100 mg + caffeine 100 mg, n = 49 for efficacy Ibuprofen 100 mg, n = 49 for efficacy Ibuprofen 200 mg + caffeine 100 mg, n = 44 for efficacy Ibuprofen 200 mg, n = 48 for efficacy Ibuprofen 50 mg, n = 57 for efficacy Placebo, n = 51 for efficacy Caffeine‐containing foods and beverages were prohibited for 4 h before taking study medication and for the following 8‐h study period |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: standard 5‐point scale Withdrawals and dropouts Serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Gives reference to methods in earlier reports that are low risk |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "identically appearing capsules" |
| Size | High risk | < 50 participants in relevant treatment groups |
Laska 1983 Study 1.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after onset of moderate or severe pain Almost identical protocols for Studies 1, 2, 3 Duration: 4 h, with assessments baseline, 0.5, 1, 2, 3, and 4 h post‐dose |
|
| Participants | Postpartum pain: postepisiotomy, postsurgical, or uterine cramping N = 480 (373 in final analysed sample) No further participant characteristics reported |
|
| Interventions | Paracetamol 500 mg + caffeine 65 mg, n = 56 Paracetamol 500 mg, n = 54 Paracetamol 1000 mg + caffeine 130 mg, n = 57 Paracetamol 1000 mg, n = 50 Paracetamol 1500 mg + caffeine 195 mg, n = 56 Paracetamol 1500 mg, n = 60 Placebo, n = 40 Numbers of participants are those in final analysed sample Medications that might alter the response to the study analgesic during the study or in the 4 h preceding were prohibited. Participants who had taken caffeine during the 3 h before and after dosing were excluded |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Low risk | "sequentially assigned from individual packages prepared in random order" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Test medication prepared to look exactly like the standard paracetamol 500 mg tablet. Equal numbers of tablets given to each group in any study |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
Laska 1983 Study 2.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after onset of moderate or severe pain Almost identical protocols for Studies 1, 2, 3 Duration: 4 h, with assessments baseline, 0.5, 1, 2, 3, and 4 h post dose |
|
| Participants | Postpartum pain: postepisiotomy, postsurgical, or uterine cramping N = 577 (434 in final analysed sample) No further participant characteristics reported |
|
| Interventions | Paracetamol 500 mg + caffeine 65 mg, n = 62 Paracetamol 500 mg, n = 68 Paracetamol 1000 mg + caffeine 130 mg, n = 62 Paracetamol 1000 mg, n = 68 Paracetamol 1500 mg + caffeine 195 mg, n = 64 Paracetamol 1500 mg, n = 66 Placebo, n = 44 Numbers of participants are those in final analysed sample Medications that might alter the response to the study analgesic during the study or in the 4 h preceding were prohibited Participants who had taken caffeine during the 3 h before and after dosing were excluded |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Low risk | "sequentially assigned from individual packages prepared in random order" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Test medication prepared to look exactly like the standard paracetamol 500 mg tablet. Equal numbers of tablets given to each group in any study |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
Laska 1983 Study 3.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after onset of moderate or severe pain Almost identical protocols for Studies 1, 2, 3 Duration: 4 h, with assessments baseline, 0.5, 1, 2, 3, and 4 h post dose |
|
| Participants | Postpartum pain: postepisiotomy or postsurgical N = 552 (538 in final analysed sample) |
|
| Interventions | Paracetamol 500 mg + caffeine 65 mg, n = 80 Paracetamol 500 mg, n = 81 Paracetamol 1000 mg + caffeine 130 mg, n = 78 Paracetamol 1000 mg, n = 81 Paracetamol 1500 mg + caffeine 195 mg, n = 80 Paracetamol 1500 mg, n = 81 Placebo, n = 57 Numbers of participants are those in final analysed sample Medications that might alter the response to the study analgesic during the study or in the 4 h preceding were prohibited Participants who had taken caffeine during the 3 h before and after dosing were excluded |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Low risk | "sequentially assigned from individual packages prepared in random order" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Test medication prepared to look exactly like the standard paracetamol 500 mg tablet. Equal numbers of tablets given to each group in any study |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
Laska 1983 Study 4.
| Methods | Single‐centre, randomised, double‐blind, active‐controlled, parallel‐group study Single oral dose administered after onset of moderate or severe pain Duration 4 h, with assessments at baseline, 0.5, 1, 2, 3, and 4 h post‐dose |
|
| Participants | Dental surgery: third molar removal N = 200 (173 in final analysed sample) |
|
| Interventions | Paracetamol 1000 mg + caffeine 130 mg, n = 45 Paracetamol 1000 mg, n = 46 Paracetamol 2000 mg + caffeine 260 mg, n = 40 Paracetamol 2000 mg, n = 42 Numbers of participants are those in final analysed sample Medications that might alter the response to the study analgesic during the study or in the 4 h preceding were prohibited. Participants who had taken caffeine during the 3 h before and after dosing were excluded |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Low risk | "sequentially assigned from individual packages prepared in random order" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Test medication prepared to look exactly like the standard paracetamol 500 mg tablet. Equal numbers of tablets given to each group in any study |
| Size | High risk | < 50 participants in relevant treatment arms |
McQuay 1996.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after onset of moderate or severe pain Duration: 8 h, with first 2 h in hospital. Time points of individual assessments not reported |
|
| Participants | Dental surgery: third molar removal N = 164 (161 for efficacy) M 59, F 102 Mean age 25 years |
|
| Interventions | Ibuprofen 200 mg + caffeine 50 mg, n = 30 for efficacy Ibuprofen 200 mg + caffeine 100 mg, n = 30 for efficacy Ibuprofen 200 mg + caffeine 200 mg, n = 29 for efficacy Ibuprofen 200 mg, n = 31 for efficacy Ibuprofen 400 mg, n = 30 for efficacy Placebo, n = 11 for efficacy No caffeine‐containing products from midnight on the evening before surgery and no other analgesics in the 12 h before surgery |
|
| Outcomes | PI: standard 4‐point scale, an 8‐word scale (randomly placed words ranging from 'no pain' to 'excruciating', scored 0 to 7), and a 100 mm VAS PR: standard 5‐point scale and a 100 mm VAS PGE: standard 5‐point scale Withdrawals and dropouts Serious adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a random number computer program |
| Allocation concealment (selection bias) | Low risk | Remote packaging, labelled only with treatment number |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "identical matching capsules" |
| Size | High risk | < 50 participants in relevant treatment arms |
Migliardi 1994 Study 1.
| Methods | 6 individual studies reported, only the 2 'APAP/CAF' studies included Both multicentre, randomised, double‐blind, placebo‐controlled, 2‐period, cross‐over studies. Single oral dose taken at the onset of at least moderate headache pain to treat 2 separate attacks per treatment period (ie 4 attacks in total). At least 48 h between first and second treatment in each period, and at least 7 day washout period before cross‐over 4‐h study period, with assessments at baseline, 0.5, 1, 2, 3, and 4 h post‐dose |
|
| Participants | Tension headache ‐ in accordance with IHS criteria and criteria established by the Ad Hoc Committee on the Classification of Headache. 6 to 7 tension headaches per month for at least 1 year that usually responded to OTC analgesics Participants with histories of other types of headache (for example, chronic, recurrent, continuous, migraine, or post‐traumatic) were excluded No other analgesics in the 8 h before treatment, or alcoholic beverages in the 6 h before. Caffeine was allowed before treatment, but any caffeine consumed in the preceding 4 h was noted at baseline Participants aged 18 to 65 years APAP/CAF Study 1: N = 441 (415 for efficacy) M 79, F 362 Mean age 33 years |
|
| Interventions |
APAP/CAF Study 1: Paracetamol 1000 mg + caffeine 130 mg, n = 336 Paracetamol 1000 mg, n = 332 Placebo, n = 162 |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts Serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double dummy technique" |
| Size | Low risk | > 200 participants in relevant treatment arms |
Migliardi 1994 Study 2.
| Methods | 6 individual studies reported, only the 2 'APAP/CAF' studies included Both multicentre, randomised, double‐blind, placebo‐controlled, 2‐period cross‐over studies. Single oral dose taken at the onset of at least moderate headache pain to treat 2 separate attacks per treatment period (ie 4 attacks in total). At least 48 h between first and second treatment in each period, and at least a 7‐day washout period before cross‐over 4‐h study period, with assessments at baseline, 0.5, 1, 2, 3, and 4 h post‐dose |
|
| Participants | Tension headache ‐ in accordance with IHS criteria and criteria established by the Ad Hoc Committee on the Classification of Headache. 6 to 7 tension headaches per month for at least 1 year that usually responded to OTC analgesics Participants with histories of other types of headache (for example, chronic, recurrent, continuous, migraine, or post‐traumatic) were excluded No other analgesics in the 8 h before treatment, or alcoholic beverages in the 6 h before. Caffeine was allowed before treatment, but any caffeine consumed in the preceding 4 h was noted at baseline Participants aged 18 to 65 years APAP/CAF Study 2: N = 442 (423 for efficacy) M 75, F 367 Mean age 33 years |
|
| Interventions |
APAP/CAF Study 2: Paracetamol 1000 mg + caffeine 130 mg, n = 339 Paracetamol 1000 mg, n = 337 Placebo, n = 170 |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Withdrawals and dropouts Serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double dummy technique" |
| Size | Low risk | > 200 participants in relevant treatment arms |
Peroutka 2004.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, 3‐period cross‐over study Single oral dose administered at the onset of moderate or severe pain Duration: 24 h, with assessments at baseline, 1, 6, and 24 h post dose |
|
| Participants | Acute migraine attack without aura, in accordance with IHS criteria, with a history of ≥ 12 months Participants were 18 to 60 years of age N = 72 enrolled (52 treated first attack, 46 treated second attack, 39 treated the third attack); treated a total of 134 attacks M 10, F 62 Mean age 45 years |
|
| Interventions | Numbers of attacks treated: Diclofenac sodium softgel 100 mg + caffeine 100 mg, n = 43 Diclofenac sodium softgel 100 mg, n = 46 Placebo, n = 45 |
|
| Outcomes | Headache relief at 1 h Withdrawals and dropouts Serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Method not described |
| Size | High risk | < 50 participants in relevant treatment arms |
Schachtel 1991a.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose after onset of relatively severe throat pain (> 66 mm on 100 mm VAS). Duration: 2 h, with assessments at baseline, 15, 30, 45, 60, 90, and 120 minutes post dose |
|
| Participants | Acute sore throat, with a score of ≥ 4 on 12‐point tonsillopharyngitis assessment and pain intensity > 66/100 on VAS Participants at least 18 years of age N = 210 (207 for efficacy) M 69, F 138 Mean age 30 years |
|
| Interventions | Aspirin 800 mg + caffeine 64 mg, n = 70 for efficacy Aspirin 800 mg, n = 68 for efficacy Placebo, n = 69 for efficacy No "cold medication", mood‐altering drugs, or alcohol within 8 h, or caffeine‐containing medication or beverages within 12 h of dosing |
|
| Outcomes | PI: 100 mm VAS PR: 6‐point categorical scale (from 'no relief' to 'complete relief') Withdrawals and dropouts |
|
| Notes | Oxford Quality Score: R2, DB1, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Method not described |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
Sunshine 1996.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after the onset of severe pain Duration: 6 h, with assessments at baseline, 0.5, 1, 2, 3, 4, 5, and 6 h post dose |
|
| Participants | Postepisiotomy pain Participants aged 18 years or older N = 305 (302 for efficacy) All F Mean age 24 years |
|
| Interventions | Ibuprofen 100 mg + caffeine 100 mg, n = 50 Ibuprofen 100 mg, n = 51 Ibuprofen 200 mg + caffeine 100 mg, n = 50 Ibuprofen 200 mg, n = 50 Ibuprofen 50 mg, n = 51 Placebo, n = 50 No medications that might confound the interpretation of efficacy, or caffeine‐containing food and beverages were permitted during the 6 h before and after dosing |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: 4‐point categorical scale (0 = poor, 1 = fair, 2 = good, 3 = excellent) Withdrawals and dropouts Serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All medications were dispensed as capsules" |
| Size | High risk | All relevant treatment groups borderline at 50 or 51 participants each |
Tokola 1984.
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over study Each participant treated up to 12 consecutive migraine attacks with up to 2 oral doses of medication; first dose taken at first warning of pain, second dose available after 1.5 h if the response to the first was not good enough Assessment at baseline and 1.5 h, but total study duration and other assessment time points not reported |
|
| Participants | Migraine as defined by the Ad Hoc Committee on the Classification of Headache Participants at least 18 years of age N = 49 (with a total of 482 attacks treated) M 3, F 46 Mean age 37 years |
|
| Interventions | Numbers of attacks treated: Tolfenamic acid 200 mg + caffeine 100 mg, n = 79 Tolfenamic acid 200 mg, n = 200 mg, n = 85 Tolfenamic acid 200 mg + metoclopramide 10 mg, n = 80 Caffeine 100 mg, n = 81 Metoclopramide, n = 75 Placebo, n = 82 |
|
| Outcomes | Severity of attack at 1.5 h: 4‐point scale (no symptoms, slight, moderate, severe) Baseline pain intensity not reported so unable to determine level of pain relief from reported pain intensity data at 1.5 h Serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Low risk | "assigned sequentially from individual packages prepared in random order" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Capsules of identical appearance" |
| Size | Unclear risk | 50 to 200 participants in relevant treatment arms |
Ward 1991.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, 6‐period cross‐over study Single oral dose administered to treat a headache rated ≥ 2 on the McGill Pain Questionnaire Only 1 test dose could be taken on any given day Duration: 2 h, with assessments at baseline, 0.5, 1, 2 h |
|
| Participants | Headache: participants with no history of migraines and with headaches that had no migrainous features ≥ 6 headaches per month during the past 3 months with pain severity averaging ≥ 2 on a 5‐point scale Participants were 18 to 60 years of age N = 60 completed the study (53 for efficacy) M 17, F 36 Mean age 37 years |
|
| Interventions | Paracetamol 648 mg + caffeine 65 mg, n = 53 Paracetamol 648 mg + caffeine 130 mg, n = 53 Paracetamol 648 mg, n = 53 Caffeine 65 mg, n = 53 Caffeine 130 mg, n = 53 Placebo, n = 53 No caffeine or other analgesics during the 2‐h study period. Participants documented any caffeine consumed in the 24 h before dosing |
|
| Outcomes | PI: 100 mm VAS ('no pain' at left end, 'pain as bad as it could be' at right end' and 'mild, moderate, severe' in sequence below the line) | |
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Method not described |
| Size | High risk | All relevant treatment groups borderline at 53 participants each |
Winter 1983.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study Single oral dose administered after the onset of moderate or severe pain Duration: 4 h, with assessments at baseline, 0.5, 1, 2, 3, and 4 h post dose |
|
| Participants | Oral surgery, including multiple bony impactions, single bony impaction, single tissue impaction, multiple tissue impactions, multiple extractions, alveolectomy, and difficult (complicated) extraction Participants were 16 to 75 years of age N = 167 (164 for efficacy) M 67, 97 Mean age 27 years |
|
| Interventions | Paracetamol 1000 mg + caffeine 130 mg, n = 40 for efficacy Paracetamol 1000 mg, n = 41 for efficacy Caffeine 130 mg, n = 42 for efficacy Placebo, n = 41 for efficacy No analgesic agent for ≥ 4 h before taking test medication |
|
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: standard 5‐point scale Withdrawals and dropouts Serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "identically appearing 2‐capsule doses" |
| Size | High risk | < 50 participants in relevant treatment arms |
Wójcicki 1977 study 1.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study If, 4 h after administration of a single dose of study medication, participants required additional analgesia, they were crossed‐over to receive one of the other study medications (only data from the first dose useable) Duration: 4 h if only first dose taken, or 8 h if both doses taken, with assessments at baseline, 4 h and, if required, 8 h |
|
| Participants | Idiopathic headache: severe and frequently occurring Participants were 19 to 85 years of age N = 144 Mean age 46 years |
|
| Interventions | Paracetamol 1000 mg + caffeine 100 mg, n = 36 Paracetamol 1000 mg, n = 36 Aspirin 1000 mg, n = 36 Placebo, n = 36 No narcotic analgesics in the 24 h before dosing |
|
| Outcomes | PR: 4‐point non‐standard scale ('no more pain', 'pain greatly improved', 'pain slightly improved', and 'pain unchanged') Serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Inadequate description of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Method not described |
| Size | High risk | < 50 participants in relevant treatment arms |
Wójcicki 1977 Study 2.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study If, 4 h after administration of a single dose of study medication, participants required additional analgesia, they were crossed‐over to receive one of the other study medications (only data from the first dose useable) Duration: 4 h if only first dose taken, or 8 h if both doses taken, with assessments at baseline, 4 h and, if required, 8 h |
|
| Participants | Orthopedic surgery: ≥ 24 h after completion of surgery, suffering at least moderate pain and for whom an analgesic would normally be prescribed Participants were 18 to 91 years of age N = 72 Mean age 44 years |
|
| Interventions | Paracetamol 1000 mg + caffeine 100 mg, n = 18 Paracetamol 1000 mg, n = 18 Aspirin 1000 mg, n = 18 Placebo, n = 18 No narcotic analgesics in the 24 h before dosing |
|
| Outcomes | PR: 3‐point non‐standard scale ('no more pain', 'pain improved', and 'pain unchanged') Serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Inadequate description of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Method not described |
| Size | High risk | < 50 participants in relevant treatment arms |
APAP: paracetamol (American); CAF: caffeine; DB: double‐blinding; F: female; h: hours; IHS: International Headache Society; M: male; OTC: over‐the‐counter; PGE: patient global evaluation; PI: pain intensity; PR: pain relief; R: randomisation; VAS: visual analogue scale; W: withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| BMS summary | 170‐01‐88, 170‐02‐88, and 171‐01‐88: number of participants in each treatment arm not reported Remaining studies: no usable data, only summary statistics reported |
| Jain 1988 | Invalid comparison: ibuprofen 400 mg compared with ibuprofen 200 mg + caffeine 100 mg |
| Laska 1984 | Studies 1 to 17 and 30 excluded due to invalid comparison, for example aspirin compared with aspirin + acetaminophen + caffeine. Studies 18 to 29 make a valid comparison, but fail to report the number of participants in each treatment arm. Four of these are believed to have been reported in full (Laska 1983 Study 1; Laska 1983 Study 2; Laska 1983 Study 3; Laska 1983 Study 4) |
| Migliardi 1994a | Only the 4 APAP/ASA/CAF studies excluded. Invalid comparison: paracetamol 1000 mg compared with paracetamol 500 mg + aspirin 500 mg + caffeine 130 mg |
| Mitchell 2008 | Invalid comparison: paracetamol 300 mg + caffeine 15 mg + codeine 30 mg compared with paracetamol 325 mg + ibuprofen 400 mg. In addition, no single‐dose outcome data |
| Schachtel 1991b | Invalid comparison ‐ paracetamol 1000 mg compared with aspirin 1000 mg + caffeine 64 mg |
APAP: paracetamol (American); ASA: aspirin (acetylsalicylic acid); CAF: caffeine;
Characteristics of studies awaiting assessment [ordered by study ID]
IRCT201306121760N24.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study Single dose Study medication provided in capsules, placed in envelope |
| Participants | Root canal treatment (acute apical periodontitis of pulpal origin) N = 45 Age 18 to 65 years M and F Pain intensity ≥ moderate (≥ 4/10) |
| Interventions | Paracetamol 325 mg + ibuprofen 200 mg + caffeine 40 mg, n = 15 Paracetamol 325 mg + ibuprofen 200 mg, n = 15 Placebo |
| Outcomes | Pain intensity (VAS) at 4, 6, 12, 24, 48, 72 h |
| Notes | Recruitment due to end April 2008 Email sent to contact person (Dr Ali Bijani) on 28 August 2014. No response by date of submission of update Sponsor: Babol University of Medical Sciences, Iran |
NCT00471952.
| Methods | Randomised, double‐blind (double‐dummy), placebo‐controlled, cross‐over study |
| Participants | Migraine, with or without aura. History ≥ 1 year, with 1 to 6 attacks per month in previous 3 months, and successfully treated a migraine attack with a triptan N = 50 Age 18 to 65 years M and F |
| Interventions | Rizatriptan 10 mg + caffeine 75 mg Rizatriptan 10 mg + placebo Placebo + placebo Rizatriptan given as orally disintegrating tablets Any preventive medication stable for ≥ 1 month |
| Outcomes | Headache relief at 2 h Pain‐free at 2 h and remaining pain‐free up to 24 h Resolution of migraine‐associated symptoms Adverse events Patient Global Evaluation |
| Notes | Study completed; final collection date for primary outcome scheduled February 2008 Sponsor: Diamond Headache Clinic Collaborator: Merck Sharp & Dohme Corp |
NCT01172405.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study Probably single dose |
| Participants | Headache (migraine or tension). Pain intensity mild to moderate, 2 to 5 headache attacks in previous 30 days Estimated N = 144 Age 18 to 65 years M and F |
| Interventions | Ibuprofen 400 mg + caffeine 200 mg Ibuprofen 400 mg 1 or 2 tablets when presenting headache (sic) |
| Outcomes | Intensity of headache before and after initiation of treatment, using Functional Disabling Scale Tolerability ‐ assessed by investigator |
| Notes | Unclear if completed; estimated final collection date for primary outcome October 2012 Email to Claudia Domingues (contact person) on 28 August 2014 bounced: "address failed" Sponsor: Mantecorp Industria Quimica e Farmaceutica Ltd. |
NCT01929031.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study Single and multiple dose phases |
| Participants | Third molar extraction (3 to 4 molars, ≥ 2 mandibular) N = 561 Age 18 to 55 years M and F Pain intensity moderate (≥ 5/10) |
| Interventions | Ibuprofen 400 mg + caffeine 100 mg Ibuprofen 400 mg Caffeine 100 mg Placebo |
| Outcomes | SPRID 0 to 2 h Time to rescue medication |
| Notes | Completed; estimated final collection date for primary outcome March 2014 Sponsor: Boehringer Ingelheim |
NCT02183688.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study |
| Participants | Migraine or tension‐type headache (IHS). History ≥ 1 year, ≥ 2 episodes per month in previous 3 months, usually treated successfully with non‐prescription analgesics N = 1889 Age 18 to 65 years M and F |
| Interventions | Low dose aspirin + low dose paracetamol + caffeine Low dose aspirin + low dose paracetamol High dose aspirin High dose paracetamol Caffeine Placebo |
| Outcomes | 50% pain relief at 0.5, 1, 2, 3, and 4 h %max SPID Impairment of daily activities (4‐point scale) Patient global assessment of efficacy (4‐point scale) Adverse events |
| Notes | Completed; estimated final collection date for primary outcome January 2003 Sponsor: Boehringer Ingelheim |
F: female; IHS: International Headache Society; M: male; N: number of participants in study; SPID: weighted sum of pain intensity difference; SPRID: weighted sum of pain relief and pain intensity difference
Contributions of authors
All authors contributed to writing the protocol.
For the original review CD and SD carried out searches and data extraction. RAM contacted pharmaceutical companies and conduct Internet searches for otherwise unpublished data. All authors were involved with data analysis and preparation of the manuscript. RAM will be responsible for any update, though the paucity of recent studies with caffeine make any update unlikely in the near future.
For this update SD and RAM carried out the searches. All authors contributed to updating the text.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
No sources of support supplied
Declarations of interest
CD has no conflicts relating to this review or any similar product.
SD has no conflicts relating to this review or any similar product.
RAM has no conflicts relating to this review or any similar product.
For transparency we have received research support from charities, government, and industry sources at various times, but none relate to this review. We are funded by the NIHR for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Ali 2007 {published data only}
- Ali Z, Burnett I, Eccles R, North M, Jawad M, Jawad S, et al. Efficacy of a paracetamol and caffeine combination in the treatment of the key symptoms of primary dysmenorrhoea. Current Medical Research and Opinion 2007;23(4):841‐51. [DOI] [PubMed] [Google Scholar]
Diamond 2000 {published data only}
- Diamond S, Balm TK, Freitag FG. Ibuprofen plus caffeine in the treatment of tension‐type headache. Clinical Pharmacology and Therapeutics 2000;68(3):312‐9. [DOI] [PubMed] [Google Scholar]
- Diamond S, Freitag FG. The use of ibuprofen plus caffeine to treat tension‐type headache. Current Pain and Headache Reports 2001;5(5):472‐8. [DOI] [PubMed] [Google Scholar]
Diener 2005 {published data only}
- Diener HC, Pfaffenrath V, Pageler L, Peil H, Aicher B. The fixed combination of acetylsalicylic acid, paracetamol and caffeine is more effective than single substances and dual combination for the treatment of headache: a multicentre, randomized, double‐blind, single‐dose, placebo‐controlled parallel group study. Cephalalgia 2005;25(10):776‐87. [DOI] [PubMed] [Google Scholar]
- Pfaffenrath V, Diener HC, Pageler L, Peil H, Aicher B. OTC analgesics in headache treatment: open‐label phase vs randomized double‐blind phase of a large clinical trial. Headache 2009;49(5):638‐45. [DOI] [PubMed] [Google Scholar]
Forbes 1990 {published data only}
- Forbes JA, Jones KF, Kehm CJ, Smith WK, Gongloff CM, Zeleznock JR, et al. Evaluation of aspirin, caffeine, and their combination in postoperative oral surgery pain. Pharmacotherapy 1990;10(6):387‐93. [PubMed] [Google Scholar]
Forbes 1991 {published data only}
- Forbes JA, Beaver WT, Jones KF, Kehm CJ, Smith WK, Gongloff CM, et al. Effect of caffeine on ibuprofen analgesia in postoperative oral surgery pain. Clinical Pharmacology and Therapeutics 1991;49(6):674‐84. [DOI] [PubMed] [Google Scholar]
Laska 1983 Study 1 {published data only}
- Laska EM, Sunshine A, Zighelboim I, Roure C, Marrero I, Wanderling J, et al. Effect of caffeine on acetaminophen analgesia. Clinical Pharmacology and Therapeutics 1983;33(4):498‐509. [DOI] [PubMed] [Google Scholar]
Laska 1983 Study 2 {published data only}
- Laska EM, Sunshine A, Zighelboim I, Roure C, Marrero I, Wanderling J, et al. Effect of caffeine on acetaminophen analgesia. Clinical Pharmacology and Therapeutics 1983;33(4):498‐509. [DOI] [PubMed] [Google Scholar]
Laska 1983 Study 3 {published data only}
- Laska EM, Sunshine A, Zighelboim I, Roure C, Marrero I, Wanderling J, et al. Effect of caffeine on acetaminophen analgesia. Clinical Pharmacology and Therapeutics 1983;33(4):498‐509. [DOI] [PubMed] [Google Scholar]
Laska 1983 Study 4 {published data only}
- Laska EM, Sunshine A, Zighelboim I, Roure C, Marrero I, Wanderling J, et al. Effect of caffeine on acetaminophen analgesia. Clinical Pharmacology and Therapeutics 1983;33(4):498‐509. [DOI] [PubMed] [Google Scholar]
McQuay 1996 {published data only}
- McQuay HJ, Angell K, Carroll D, Moore RA, Juniper RP. Ibuprofen compared with ibuprofen plus caffeine after third molar surgery. Pain 1996;66(2‐3):247‐51. [DOI] [PubMed] [Google Scholar]
Migliardi 1994 Study 1 {published data only}
- Migliardi JR, Armellino JJ, Friedman M, Gillings DB, Beaver WT. Caffeine as an analgesic adjuvant in tension headache. Clinical Pharmacology and Therapeutics 1994;56(5):576‐86. [DOI] [PubMed] [Google Scholar]
Migliardi 1994 Study 2 {published data only}
- Migliardi JR, Armellino JJ, Friedman M, Gillings DB, Beaver WT. Caffeine as an analgesic adjuvant in tension headache. Clinical Pharmacology and Therapeutics 1994;56(5):576‐86. [DOI] [PubMed] [Google Scholar]
Peroutka 2004 {published data only}
- Peroutka SJ, Lyon JA, Swarbrick J, Lipton RB, Kolodner K, Goldstein J. Efficacy of diclofenac sodium softgel 100 mg with or without caffeine 100 mg in migraine without aura: a randomized, double‐blind, crossover study. Headache 2004;44(2):136‐41. [DOI] [PubMed] [Google Scholar]
Schachtel 1991a {published data only}
- Schachtel BP, Fillingim JM, Lane AC, Thoden WR, Baybutt RI. Caffeine as an analgesic adjuvant. A double‐blind study comparing aspirin with caffeine to aspirin and placebo in patients with sore throat. Archives of Internal Medicine 1991;151(4):733‐7. [DOI] [PubMed] [Google Scholar]
Sunshine 1996 {published data only}
- Sunshine A, Zighelboim I, Bartizek RD. A double‐blind, placebo‐controlled, single‐dose comparison study of ibuprofen, and ibuprofen in combination with caffeine, in the treatment of postepisiotomy pain. Royal Society of Medicine International Congress and Symposium Series 218 1996;218:105‐88. [Google Scholar]
Tokola 1984 {published data only}
- Tokola RA, Kangasniemi P, Neuvonen PJ, Tokola O. Tolfenamic acid, metoclopramide, caffeine and their combinations in the treatment of migraine attacks. Cephalalgia 1984;4(4):253‐63. [DOI] [PubMed] [Google Scholar]
Ward 1991 {published data only}
- Ward N, Whitney C, Avery D, Dunner D. The analgesic effects of caffeine in headache. Pain 1991;44(2):151‐5. [DOI] [PubMed] [Google Scholar]
Winter 1983 {published data only}
- Winter L Jr, Appleby F, Ciccone PE, Pigeon JG. A double‐blind, comparative evaluation of acetaminophen, caffeine, and the combination of acetaminophen and caffeine in outpatients with post‐operative oral surgery pain. Current Therapeutic Research 1983;33(1):115‐22. [Google Scholar]
Wójcicki 1977 study 1 {published data only}
- Wójcicki J, Samochowiec L, Lawczyński L, Szwed G, Olszewska M. A double‐blind comparative evaluation of aspirin, paracetamol and paracetamol + caffeine (Finimal) for their analgesic effectiveness. Archivum Immunologiae et Therapiae Experimentalis 1977;25(2):175‐9. [PubMed] [Google Scholar]
Wójcicki 1977 Study 2 {published data only}
- Wójcicki J, Samochowiec L, Lawczyński L, Szwed G, Olszewska M. A double‐blind comparative evaluation of aspirin, paracetamol and paracetamol + caffeine (Finimal) for their analgesic effectiveness. Archivum Immunologiae et Therapiae Experimentalis 1977;25(2):175‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
BMS summary {unpublished data only}
- Anon. Citizen Petition Summary. http://www.fda.gov/ohrms/dockets/dockets/77n0094/77n‐0094‐cp00015_01.pdf (accessed 5 December 2014).
Jain 1988 {published data only}
- Jain AK, McMahon FG, Ryan JR, Narcisse C. A double‐blind study of ibuprofen 200 mg in combination with caffeine 100 mg, ibuprofen 400 mg, and placebo in episiotomy pain. Current Therapeutic Research 1988;43(4):762‐9. [Google Scholar]
Laska 1984 {published data only}
- Laska EM, Sunshine A, Mueller F, Elvers WB, Siegel C, Rubin A. Caffeine as an analgesic adjuvant. JAMA 1984;251(13):1711‐8. [PubMed] [Google Scholar]
Migliardi 1994a {published data only}
- Migliardi JR, Armellino JJ, Friedman M, Gillings DB, Beaver WT. Caffeine as an analgesic adjuvant in tension headache. Clinical Pharmacology and Therapeutics 1994;56(5):576‐86. [DOI] [PubMed] [Google Scholar]
Mitchell 2008 {published data only}
- Mitchell A, Zanten SV, Inglis K, Porter G. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. Journal of the American College of Surgeons 2008;206(3):472‐9. [DOI] [PubMed] [Google Scholar]
Schachtel 1991b {published data only}
- Schachtel BP, Thoden WR, Konerman JP, Brown A, Chaing DS. Headache pain model for assessing and comparing the efficacy of over‐the‐counter analgesic agents. Clinical Pharmacology & Therapeutics 1991;50(3):322‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
IRCT201306121760N24 {published data only}
- Comparison of analgesic effect ‐ the combination of acetaminophen and ibuprofen in the presence and absence of caffeine on pain after root canal treatment. who.int/trialsearch/Trial2.aspx?TrialID=IRCT201306121760N24 2014 (accessed 28 August 2014). [IRCT: IRCT201306121760N24]
NCT00471952 {published data only}
- Maxalt 10 mg plus caffeine 75 mg in the acute treatment of migraine headache. clinicaltrials.gov/ct2/results?term=NCT00471952&Search=Search 2014 (accessed 28 August 2014). [NCT: NCT00471952]
NCT01172405 {published data only}
- Efficacy and safety study to compare ibuprofen + caffeine with ibuprofen alone in the treatment of headache. clinicaltrials.gov/ct2/results?term=NCT01172405&Search=Search 2014 (accessed 28 August 2014). [NCT: NCT01172405]
NCT01929031 {published data only}
- A single‐centre, double‐blind, randomised, two‐stage, parallel‐group study to assess the efficacy and safety of the fixed dose combination of ibuprofen 400 mg and caffeine 100 mg versus ibuprofen 400 mg, caffeine 100 mg and placebo in patients with postoperative dental pain. clinicaltrials.gov/ct2/results?term=NCT01929031&Search=Search 2014 (accessed 28 August 2014). [NCT: NCT01929031]
NCT02183688 {published data only}
- Acetylsalicylic acid (ASA) + paracetamol + caffeine combination compared with ASA + paracetamol as well as ASA, paracetamol, and caffeine in headache patients. clinicaltrials.gov/ct2/show/NCT02183688?term=NCT02183688&rank=1 2014 (accessed 28 August 2014). [NCT: NCT02183688]
Additional references
Basurto Ona 2013
- Basurto Ona X, Uriona Tuma SM, Martínez García L, Solà I, Bonfill Cosp X. Drug therapy for preventing post‐dural puncture headache. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD001792.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beaver 1984
- Beaver WT. Caffeine revisited. JAMA 1984;251(13):1732‐3. [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1991
- Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity. The Design of Analgesic Clinical Trials. Advances in Pain Research Therapy 1991;18:117‐24. [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donovan 2001
- Donovan JL, DeVane CL. A primer on caffeine pharmacology and its drug interactions in clinical psychopharmacology. Psychopharmacology Bulletin 2001;35(3):30‐48. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jadad 1996a
- Jadad AR, Carroll D, Moore RA, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jain 1978
- Jain AK, McMahon FG, Ryan JR, Unger D, Richard W. Aspirin and aspirin‐caffeine in postpartum pain relief. Clinical Pharmacology and Therapeutics 1978;24(1):69‐75. [DOI] [PubMed] [Google Scholar]
Juliano 2004
- Juliano LM, Griffiths RR. A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology (Berl) 2004;176(1):1‐29. [DOI: 10.1007/s00213-004-2000-x] [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
Li Wan Po 1998
- Li Wan Po A, Zhang WY. Analgesic efficacy of ibuprofen alone and in combination with codeine or caffeine in post‐surgical pain: a meta‐analysis. European Journal of Clinical Pharmacology 1998;53:303‐11. [DOI] [PubMed] [Google Scholar]
McQuay 2007
- McQuay HJ, Moore RA. Dose‐response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. British Journal of Clinical Pharmacology 2007;63(3):271‐8. [DOI: 10.1111/j.1365-2125.2006.02723.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2‐3):229‐37. [DOI: 10.1016/0304-3959(96)03032-1] [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain 1997;69(3):311‐5. [DOI: 10.1016/S0304-3959(96)03306-4] [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 1997;69(1‐2):127‐30. [DOI: 10.1016/S0304-3959(96)03251-4] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2014
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratio and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with Confidence ‐ Confidence Intervals and Statistical Guidelines. London: BMJ Books, 1995:50‐63. [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2010
- Palmer H, Graham G, Williams K, Day R. A risk‐benefit assessment of paracetamol (acetaminophen) combined with caffeine. Pain Medicine 2010;11(6):951‐65. [DOI: 10.1111/j.1526-4637.2010.00867.x] [DOI] [PubMed] [Google Scholar]
Renner 2007
- Renner B, Clarke G, Grattan T, Beisel A, Mueller C, Werner U, et al. Caffeine accelerates absorption and enhances the analgesic effect of acetaminophen. Journal of Clinical Pharmacology 2007;47(6):715‐26. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sawynok 1993
- Sawynok J, Yaksh TL. Caffeine as an analgesic adjuvant: a review of pharmacology and mechanisms of action. Pharmacological Reviews 1993;45(1):43‐85. [PubMed] [Google Scholar]
Sawynok 2011a
- Sawynok J. Methylxanthines and pain. Handbook of Experimental Pharmacology 2011;200:311‐29. [DOI: 10.1007/978-3-642-13443-2_11] [DOI] [PubMed] [Google Scholar]
Sawynok 2011b
- Sawynok J. Caffeine and pain. Pain 2011;152(4):726‐9. [DOI: 10.1016/j.pain.2010.10.011] [DOI] [PubMed] [Google Scholar]
Sawynok 2011c
Schachtel 1991
- Schachtel BP, Fillingim JM, Lane AC, Thoden WR, Baybutt RI. Caffeine as an analgesic adjuvant. A double‐blind study comparing aspirin with caffeine to aspirin and placebo in patients with sore throat. Archives of Internal Medicine 1991;151(4):733‐7. [DOI] [PubMed] [Google Scholar]
Tramèr 1997
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate results on meta‐analysis: a case study. BMJ 1997;315(7109):635‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 1996
- Zhang WY, Li Wan Po A. Analgesic efficacy of paracetamol and its combination with codeine and caffeine in surgical pain ‐ a meta‐analysis. Journal of Clinical Pharmacology and Therapeutics 1996;21(4):261‐82. [DOI] [PubMed] [Google Scholar]
Zhang 1997
- Zhang WY, Po AL. Do codeine and caffeine enhance the analgesic effect of aspirin? A systematic overview. Journal of Clinical Pharmacology and Therapeutics 1997;22(2):79‐97. [DOI] [PubMed] [Google Scholar]
Zhang 2001
- Zhang WY. A benefit‐risk assessment of caffeine as an analgesic adjuvant. Drug Safety 2001;24(15):1127‐42. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Derry 2012
- Derry CJ, Derry S, Moore RA. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009281.pub2] [DOI] [PubMed] [Google Scholar]


