Abstract
It is still controversial whether associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) or traditional staged hepatectomy such as portal vein embolization (PVE) and 2-staged hepatectomy (TSH) is better. The aim of this study was to compare these 3 available strategies in extended hepatectomy.
Trials were identified by searching MEDLINE, PubMed, the Cochrane Library, and Embase and additional articles were identified by hand searching. Comparative clinical studies reporting volumetric changes, mortality, morbidity, and feasibility of the second stage about ALPPS versus PVE or ALPPS versus TSH were included.
Nine studies involving 557 patients met the inclusion criteria. Five studies reported on comparison of ALPPS and PVE, and the other 4 reported about ALPPS and TSH. In the comparison of ALPPS versus traditional staged hepatectomy (PVE and TSH), ALPPS was associated with a greater increase in the future liver remnant (FLR) (RR: 4.87; 95%CI, 3.41–6.33) and more frequent completion of stage 2 resection (RR: 1.32; 95%CI, 1.21–1.44). Compared with the traditional staged hepatectomy, ALPPS had a trend toward higher morbidity (RR: 1.19, 95%CI, 0.96–1.47) and mortality (RR: 2.11, 95%CI, 1.02–4.33) after stage 2 resection.
ALPPS is associated with greater future liver remnant hypertrophy and a higher rate of completion of stage 2, but this may be at the price of greater morbidity and mortality.
Keywords: ALPPS, complication, feasibility, PVE, TSH
1. Introduction
The safe removal of extensive tumor load in the liver has been one of the main focuses of laboratory and clinical research for hepato-biliary surgeons over the past 3 decades.[1] The main reason for poor postoperative outcomes is inadequate volume of the future liver remnant (FLR), which leads to posthepatectomy liver failure.[2] Several strategies have been developed over the past 3 decades to induce compensatory hypertrophy of the FLR, thereby increasing the chance of resectability and lowering the risk of postoperative complications.[3–5] The first breakthrough is credited to Masatoshi Makuuchi, who in the 1980s, introduced the concept of the portal vein embolization (PVE) of the right portal branch to induce hypertrophy of the left side of the liver, enabling a safer removal of large or multiple tumors, mostly located in the right hemiliver and segment IV.[6] This technique was rapidly adopted by many to prevent liver failure after a variety of extensive right-sided hepatectomies.[2,7] Today preoperative PVE is considered standard therapy for patients with an insufficient FLR before extended liver resection.[8] However, insufficient hypertrophy of the FLR or disease progression after PVE may prevent curative liver resection in up to 20% of patients.[9,10]
Two-stage hepatectomy was introduced in the year 2000 for patients with bilateral multinodular colorectal liver metastases.[11] The liver grows in the interval between sequential resection, and the risk of postoperative liver failure (PLF) is presumably reduced due to the staged approach.[12,13] Although 2-staged hepatectomy (TSH) is well established, failure to proceed to stage 2 is reported to be a problem in up to one-third of patients (8%–31% of cases depending on the series) and occurs due to tumor progression during the period of liver regeneration or due to insufficient FLR hypertrophy.[12]
Recently, a novel technique of 2-stage liver resection was introduced, combining portal vein ligation (PVL) and transection of the liver between the FLR, and the deportalized part of the liver,[4,14] which was associating liver partition with PVL for staged hepatectomy. ALPPS has been reported to induce hypertrophy of the FLR of up to 80% in a shorter time than PVL or PVE.[14,15] However, this procedure has triggered serious concerns owing to the associated high morbidity and mortality rates of up to 40 and 15% respectively.[16] The safety of ALPPS compared with traditional strategies to induce hypertrophy of the FLR, such as PVE and TSH, is still controversial.
The purpose of the present article was to assess the efficacy of the ALPPS and PVE/TSH strategies used to increase FLR volume before extended liver resection in patients with primary or secondary liver resections. The main endpoints were comparison of volumetric changes, feasibility of the second stage, as well as postoperative morbidity and mortality after ALPPS and PVE/TSH.
2. Methods
2.1. Trial selection
A comprehensive systematic search of the databases MEDLINE, PubMed, the Cochrane Library, and Embase was conducted using the following terms: “portal vein embolization,” “portal vein ligation,” “portal vein occlusion,” “hepatic vein embolization,” “hepatic vein occlusion,” “staged hepatectomy,” “staged liver resection,” “2-stage hepatectomy,” “TSH,” and “ALPPS,” “hepatectomy (liver resection or hepatic resection or surgery or transection or partition).” Two reviewers scanned the abstract of identified studies to determine eligibility. Full articles were then selected for further assessment if the abstract suggested the study was relevant. Only comparative studies including PVE/TSH and ALPPS were identified. The last electronic search was on October 28, 2018. There were no language or time restrictions. Additional articles were identified by hand searching. Corresponding authors of included publications were asked for missing information. If the e-mail address provided in the publication was no longer valid, the first author was contacted by e-mail. The results of our search and selection of studies are shown in Figure 1.
Figure 1.
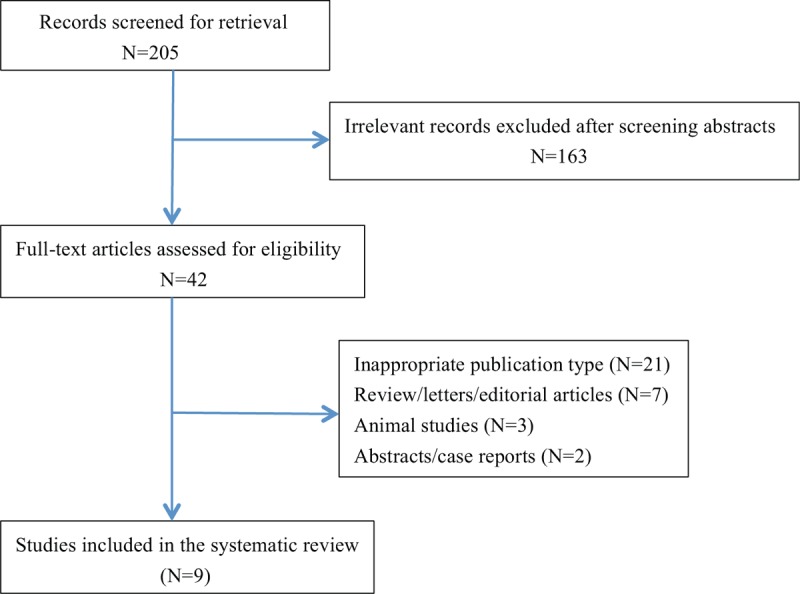
PRISMA diagram showing systematic search strategy.
2.2. Outcome measures
Volumetric data for the FLR were collected before stage 1 and stage 2, including volume of FLR (mL), FLR (percent; calculated as FLR/total liver volume × 100) or standardized FLR% and volumetric changes after stage 1 (including absolute increase in volume and hypertrophy ratio of FLR). The feasibility rate of stage 2 was recorded, where feasibility was defined as completion of liver resection at stage 2. Investigated reasons for not proceeding to stage 2 included intrahepatic and extrahepatic tumor progression and failure of the FLR to hypertrophy.
The total number of patients with complications was determined. In comparative studies between ALPPS and other strategies, morbidity after ALPPS was analyzed including both stage 1 and 2, as complications occur during the same hospital stay and information for each patient summarized the whole admission. Where complications after stages 1 and 2 of ALPPS had been reported separately, the highest rate of complications was used for analysis. For the other strategies, only complications after stage 2 were considered for comparison with ALPPS. Mortality was reported as 90-day mortality. Mortality after stage 1 and stage 2 was analyzed together for each strategy.
2.3. Quality assessment
To assess the overall strength/quality of evidence for the various outcome parameters, a quality assessment was carried out in the style of the Oxford Centre for Evidenc-based Medicine. Risk of bias in individual studies was assessed by means of the Newcastle–Ottawa quality assessment scale.
2.4. Statistics
Data were analyzed using the Comprehensive Meta-Analysis statistical software and were presented as proportions along with corresponding 95% confidence intervals (95% CIs), which were calculated by the Wilson score interval. To estimate pooled proportions we used random rather than fixed effects models in order to take into account the heterogeneity of the estimates. Statistical heterogeneity across studies was stated by using the Cochran's test, and quantified by I2 (percentage of total variation across studies that is attributable to heterogeneity rather than chance); values were considered statistically significant when P was <.1. Subgroup analyses were performed to identify possible sources of heterogeneity. This meta-analysis is exempt from ethical approval as the analysis involves only already published and anonymized data.
3. Results
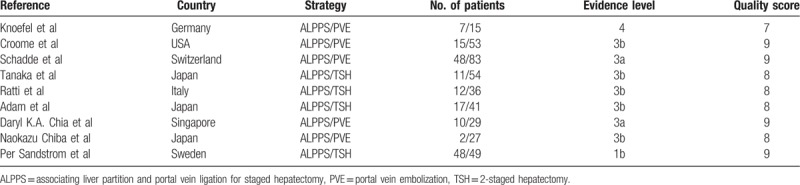
The strategies of the literature search and the selection of studies are summarized in Figure 1. Nine studies were included in the meta-analysis. Five studies comparing ALPPS with PVE and 4 studies comparing ALPPS with TSH met the inclusion criteria.[17–25] Eight studies were retrospective and one was randomized controlled trial.[23] The 9 studies involved 557 patients, of whom 207 were in the PVE group, 180 in the TSH group, and 170 in the ALPPS group. The characteristics of 9 studies included in this paper were summarized in Table 1. All of included studies were classified as evidence level 3 or 4, having a good quality score (Table 2). Pooled data were analyzed by combining the results of the 6 studies. A subgroup analysis was also performed to compare ALPPS with PVE[17–19,24,25] and TSH,[20–23] respectively.
Table 1.
Characteristics of comparative studies.
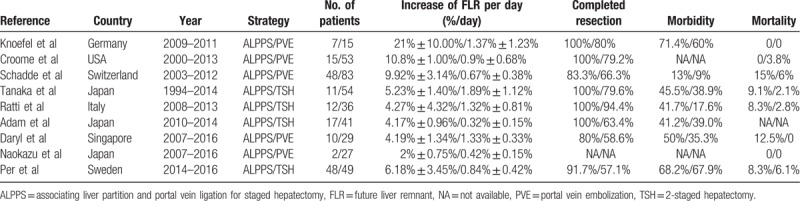
Table 2.
Characteristics of comparative studies with evidence level and bias evaluation.
3.1. Speed of future liver remnant hypertrophy before resection
The%age FLR increase was greater after ALPPS than PVE in 3 groups with 82 patients in the ALPPS group and 207 in the PVE group (RR: 6.30; 95%CI, 3.97–8.64). The same result was obtained when comparing ALPPS with TSH (RR: 3.27; 95%CI, 1.63–4.91). Patients receiving the operation of ALPPS had better FLR increase rate compared with other strategies, such as PVE and TSH (RR: 4.87; 95%CI, 3.41–6.33). Heterogeneity among studies of ALPPS vs PVE, ALPPS vs TSH and ALPPS vs PVE/TSH was significant (I2=93.4%, 94.4%, 95.3%, respectively). A random-effect model was used to estimate FLR increase rate between ALPPS, PVE, and TSH (Fig. 2).
Figure 2.
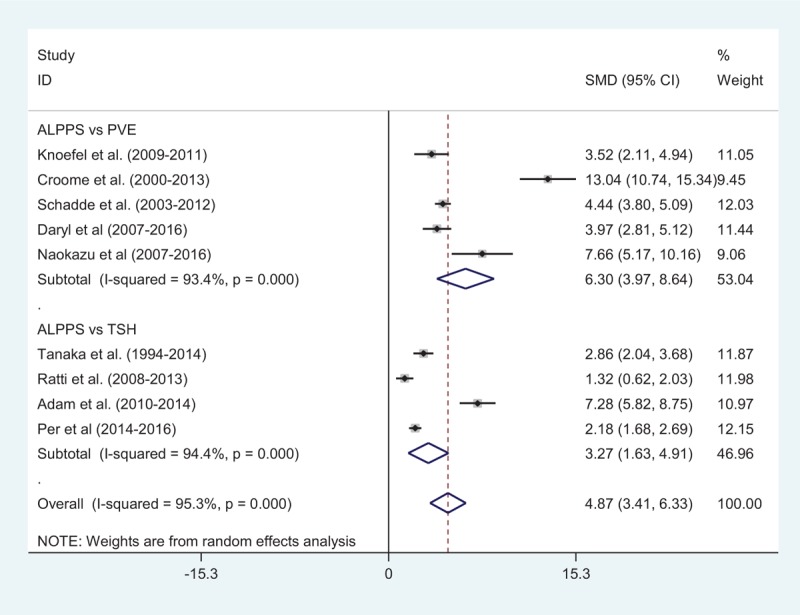
Comparison of FLR regeneration rate after first surgery between ALPPS and PVE/TSH. ALPPS = associating liver partition and portal vein ligation for staged hepatectomy, PVE = portal vein embolization, TSH = 2-staged hepatectomy.
3.2. Feasibility of second stage
The feasibility rates of stage 2 after ALPPS (82 patients) and PVE (207 patients) were 94% versus 63%, respectively (RR: 1.26; 95%CI, 1.11–1.43). The feasibility rates between ALPPS and TSH were 95% versus 72%, respectively (RR: 1.38; 95%CI, 1.21–1.44). The overall feasibility rates were 94% versus 69%, respectively (RR: 1.32; 95%CI, 1.21–1.44) (Fig. 3). Heterogeneity among studies of ALPPS versus PVE, ALPPS vs TSH and ALPPS vs PVE/TSH was moderate (I2 = 0, 85.6%, 60.8%, respectively). A random-effect model was used to estimate feasibility rates between ALPPS, PVE, and TSH (Fig. 3).
Figure 3.
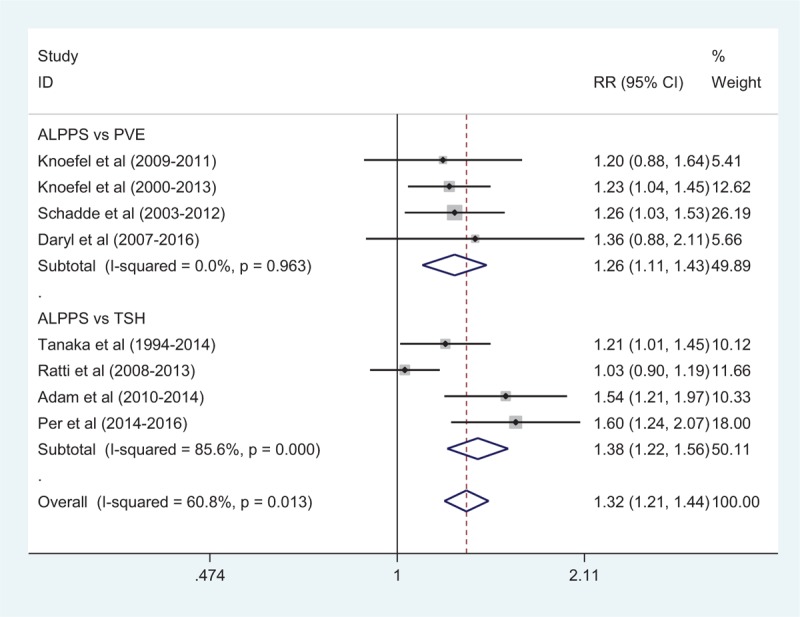
Comparison of resection rate after first surgery between ALPPS and PVE/TSH. ALPPS = associating liver partition and portal vein ligation for staged hepatectomy, PVE = portal vein embolization, TSH = 2-staged hepatectomy.
3.3. Safety
Comparison of morbidity between the ALPPS and PVE groups was available in 3 studies[17,19,24] with 65 patients in the ALPPS group and 127 patients in the PVE group. Complication rates were 25% after ALPPS and 21% after PVE (RR: 1.37, 95%CI, 0.84–2.21) (Fig. 4). There was no heterogeneity among included studies (I2 = 0). Adjusted mortality rates were 10% after ALPPS and 5% after PVE in 3 studies (RR: 2.26, 95%CI, 0.88–5.80). There was no heterogeneity among include studies (I2 = 0) (Fig. 5).
Figure 4.
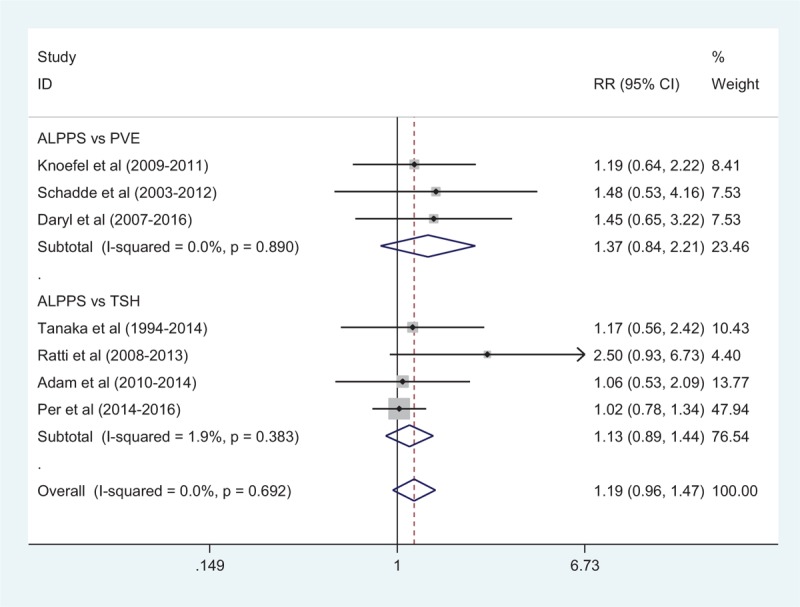
Comparison of morbidity rate after second surgery between ALPPS and PVE/TSH. ALPPS = associating liver partition and portal vein ligation for staged hepatectomy, PVE = portal vein embolization, TSH = 2-staged hepatectomy.
Figure 5.
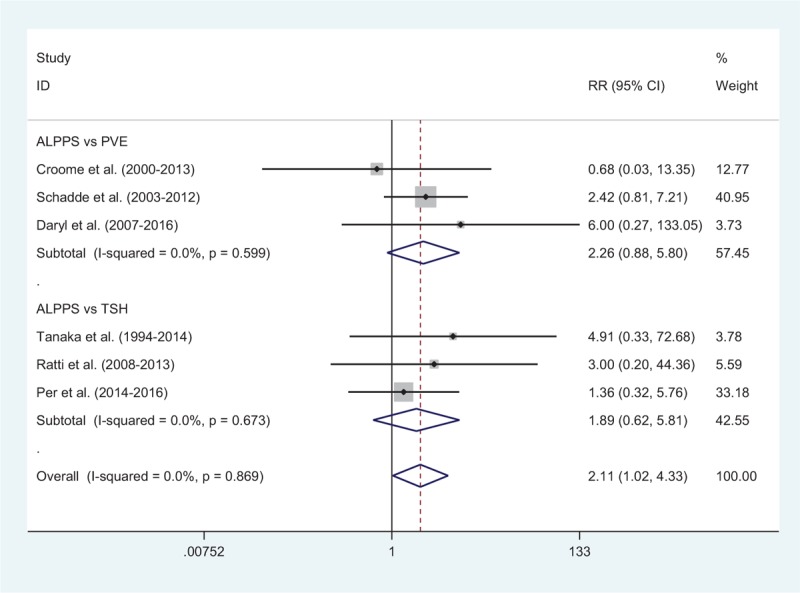
Comparison of mortality rate after second surgery between ALPPS and PVE/TSH. ALPPS = associating liver partition and portal vein ligation for staged hepatectomy, PVE = portal vein embolization, TSH = 2-staged hepatectomy.
Morbidity between ALPPS and TSH groups was available in 4 studies, with 42% after ALPPS and 33% after TSH (RR: 1.13, 95%CI, 0.89–1.44) (Fig. 4). The heterogeneity among include studies was very small (I2 = 1.9%). Adjusted mortality rates were available in 3 studies,[20,21,23] with 8.7% after ALPPS and 2.4% after TSH (RR: 1.89, 95%CI, 0.62–5.81). There was no heterogeneity among include studies (I2 = 0) (Fig. 5).
The overall morbidity between ALPPS and PVE/TSH groups was 29.7% and 26.1% (RR: 1.19, 95%CI, 0.96–1.47). There was no heterogeneity among include studies (I2 = 0). A fixed-effect model was used to estimate morbidity rates among ALPPS, PVE and TSH (Fig. 4). Adjusted mortality rates were 9.9% after ALPPS and 3.8% after PVE/TSH (RR: 2.11, 95%CI, 1.02–4.33). There was no heterogeneity among include studies (I2 = 0). A fixed-effect model was used to estimate mortality rates among ALPPS, PVE, and TSH (Fig. 5).
4. Discussion
Since the original description by Schnitzbauer et al,[4] the ALPPS technique has taken many routes, sparking both intense enthusiasm as well as skepticism alike among the surgery community. It is still unclear where the technique should fit within the surgeon's armament. The most important contribution of ALPPS is the rapid hypertrophy of liver parenchyma FLR and, therefore, the acceptance of a decrease in the estimated size of the FLR required to avoid posthepatectomy liver failure. ALPPS has enabled larger resections, and even the acceptance of a monosegmental FLR because of its expected hypertrophy to become a possibility.[26,27] This increase in hypertrophy of the FLR raises the threshold for what is considered resectable, theoretically decreasing the proportion of patients who do not reach second stage.
Despite this enthusiasm, there is a lack of evidence to guide clinicians about the most appropriate role for ALPPS in liver surgery. To date, there is no Level 1 evidence that illustrates the benefit of ALPPS over PVE and 2-stage resections. Meaningful comparisons of clinical outcomes are difficult to make. To our knowledge, the only registered, randomized controlled trials recruiting currently are the Scandinavian multicenter Liver Growth Stimulation in Advanced Colorectal Liver Metastatic Disease (LIGRO) trial (ALPPS vs PVE) and the Regeneration of Liver: Portal Vein Embolization Versus Radiofrequency Assisted Ligation for Liver Hypertrophy (REBIRTH) trial from Imperial College London (RALPP vs PVE).
This systematic review and meta-analysis targeted available strategies aiming at increasing small FLR before extended liver resection in patients with primary or secondary liver malignancies. The main findings are that ALPPS induces a greater degree of hypertrophy of the FLR in a shorter time than PVE and TSH, that the likelihood of achieving a complete tumor-free resection is superior following ALPPS than after conventional 2-step procedures, and that there is a trend toward higher morbidity and mortality after ALPPS. The main reasons for not proceeding with the second step after PVE or TSH are intrahepatic or extrahepatic tumor progression.
This article has shown that ALPPS is associated with a trend toward higher morbidity and mortality compared with PVE and TSH. Different technical options in performing ALPPS (e.g., tourniquet, RFA, or microwave) were discussed including a lively debate about the use of partial versus complete transection of parenchyma during the first step to decrease morbidity and mortality rates. However, these variations are all at an early stage, without sufficient convincing data. The first analysis of the international ALPPS registry[28] showed that better selection of patients and indications decreased mortality and morbidity rates. With signs of liver failure such as a Model of End stage Liver Disease score >10 after step 1, step 2 should be postponed. Patients >60 years are at higher risk of poor outcome, and this factor should be included in the evaluation for suitability to ALPPS. The main limitation of this meta-analysis is only 9 studies included in the study, which makes the power of conclusion quite weak. Whether the patients could get benefit from ALPPS is still controversial, thus more studies about ALPPS are needed to confirm this.
5. Conclusion
ALPPS is associated with greater future liver remnant hypertrophy and a higher rate of completion of stage 2, but this may be at the price of greater morbidity and mortality. As the level of evidence to support the superiority of ALPPS over others is low, a randomized trial should be conducted for better assessment of ALPPS compared with other available strategies.
Author contributions
Conceptualization: Kezhong Tang.
Data curation: Yanmo Liu.
Formal analysis: Yanmo Liu.
Funding acquisition: Yingxin Yang.
Investigation: Yingxin Yang.
Methodology: Shenglong Gu.
Project administration: Shenglong Gu, Kezhong Tang.
Resources: Kezhong Tang.
Writing – original draft: Kezhong Tang.
Writing – review & editing: Kezhong Tang.
Footnotes
Abbreviations: ALPPS = associating liver partition and portal vein ligation for staged hepatectomy, FLR = future liver remnant, PLF = postoperative liver failure, PVE = portal vein embolization, PVL = portal vein ligation, TSH = 2-staged hepatectomy.
The authors have no conflicts of interest to disclose.
References
- [1].de Santibanes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg 2012;255:415–7. [DOI] [PubMed] [Google Scholar]
- [2].Clavien PA, Petrowsky H, DeOliveira ML, et al. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med 2007;356:1545–59. [DOI] [PubMed] [Google Scholar]
- [3].Honjo I, Suzuki T, Ozawa K, et al. Ligation of a branch of the portal vein for carcinoma of the liver. Am J Surg 1975;130:296–302. [DOI] [PubMed] [Google Scholar]
- [4].Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405–14. [DOI] [PubMed] [Google Scholar]
- [5].Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg 1986;10:803–8. [DOI] [PubMed] [Google Scholar]
- [6].Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521–7. [PubMed] [Google Scholar]
- [7].Clavien PA, Oberkofler CE, Raptis DA, et al. What is critical for liver surgery and partial liver transplantation: size or quality? Hepatology 2010;52:715–29. [DOI] [PubMed] [Google Scholar]
- [8].Pamecha V, Nedjat-Shokouhi B, Gurusamy K, et al. Prospective evaluation of two-stage hepatectomy combined with selective portal vein embolisation and systemic chemotherapy for patients with unresectable bilobar colorectal liver metastases. Dig Surg 2008;25:387–93. [DOI] [PubMed] [Google Scholar]
- [9].Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 2006;243:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 2008;247:49–57. [DOI] [PubMed] [Google Scholar]
- [11].Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lam VW, Laurence JM, Johnston E, et al. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB 2013;15:483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Giuliante F, Ardito F, Ferrero A, et al. Tumor progression during preoperative chemotherapy predicts failure to complete 2-stage hepatectomy for colorectal liver metastases: results of an Italian multicenter analysis of 130 patients. J Am Coll Surg 2014;219:285–94. [DOI] [PubMed] [Google Scholar]
- [14].Schadde E, Raptis DA, Schnitzbauer AA, et al. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the International ALPPS Registry. Ann Surg 2015;262:780–5. discussion 785–786. [DOI] [PubMed] [Google Scholar]
- [15].Schadde E, Schnitzbauer AA, Tschuor C, et al. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol 2015;22:3109–20. [DOI] [PubMed] [Google Scholar]
- [16].Shindoh J, Vauthey JN, Zimmitti G, et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg 2013;217:126–33. discussion 133-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Knoefel WT, Gabor I, Rehders A, et al. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Brit J Surg 2013;100:388–94. [DOI] [PubMed] [Google Scholar]
- [18].Croome KP, Hernandez-Alejandro R, Parker M, et al. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB 2015;17:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schadde E, Ardiles V, Slankamenac K, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg 2014;38:1510–9. [DOI] [PubMed] [Google Scholar]
- [20].Tanaka K, Matsuo K, Murakami T, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): short-term outcome, functional changes in the future liver remnant, and tumor growth activity. Eur J Surg Oncol 2015;41:506–12. [DOI] [PubMed] [Google Scholar]
- [21].Ratti F, Schadde E, Masetti M, et al. Strategies to increase the resectability of patients with colorectal liver metastases: a multi-center case-match analysis of ALPPS and conventional two-stage hepatectomy. Ann Surg Oncol 2015;22:1933–42. [DOI] [PubMed] [Google Scholar]
- [22].Adam R, Imai K, Castro Benitez C, et al. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Brit J Surg 2016;103:1521–9. [DOI] [PubMed] [Google Scholar]
- [23].Sandstrom P, Rosok BI, Sparrelid E, et al. ALPPS improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg 2018;267:833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chia DKA, Yeo Z, Loh SEK, et al. Greater hypertrophy can be achieved with associating liver partition with portal vein ligation for staged hepatectomy compared to conventional staged hepatectomy, but with a higher price to pay? Am J Surg 2018;215:131–7. [DOI] [PubMed] [Google Scholar]
- [25].Chiba N, Yokozuka K, Ochiai S, et al. The diagnostic value of 99m-Tc GSA scintigraphy for liver function and remnant liver volume in hepatic surgery: a retrospective observational cohort study in 27 patients. Patient Saf Surg 2018;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alvarez FA, Ardiles V, de Santibanes M, et al. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg 2015;261:723–32. [DOI] [PubMed] [Google Scholar]
- [27].Schadde E, Malago M, Hernandez-Alejandro R, et al. Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery 2015;157:676–89. [DOI] [PubMed] [Google Scholar]
- [28].Oldhafer KJ, Stavrou GA, van Gulik TM, et al. Ann Surg 2016;263:839–41. [DOI] [PubMed] [Google Scholar]


