Abstract
Background
Dental caries (tooth decay) is one of the most common chronic childhood diseases. Caries prevalence in most industrialised countries has declined among children over the past few decades. The probable reasons for the decline are the widespread use of fluoride toothpaste, followed by artificial water fluoridation, oral health education and a slight decrease in sugar consumption overall. However, in regions without water fluoridation, fluoride supplementation for pregnant women may be an effective way to increase fluoride intake during pregnancy. If fluoride supplements taken by pregnant women improve neonatal outcomes, pregnant women with no access to a fluoridated drinking water supply can obtain the benefits of systemic fluoridation.
Objectives
To evaluate the effects of women taking fluoride supplements (tablets, drops, lozenges or chewing gum) compared with no fluoride supplementation during pregnancy to prevent caries in the primary teeth of their children.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 25 January 2017); the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11) in the Cochrane Library (searched 25 January 2017); MEDLINE Ovid (1946 to 25 January 2017); Embase Ovid (1980 to 25 January 2017); LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 25 January 2017); and CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 25 January 2017). We searched the US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform for ongoing trials to 25 January 2017. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
Randomised controlled trials (RCTs) of fluoride supplements (tablets, drops, lozenges or chewing gum) administered to women during pregnancy with the aim of preventing caries in the primary teeth of their children.
Data collection and analysis
Two review authors independently screened the titles and abstracts (when available) of all reports identified through electronic searches. Two review authors independently extracted data and assessed risk of bias, as well as evaluating overall quality of the evidence utilising the GRADE approach. We could not conduct data synthesis as only one study was included in the analysis.
Main results
Only one RCT met the inclusion criteria for this review. This RCT showed no statistical difference on decayed or filled primary tooth surfaces (dfs) and the percentage of children with caries at 3 years (risk ratio (RR) 1.46, 95% confidence interval (CI) 0.75 to 2.85; participants = 938, very low quality of evidence) and 5 years old (RR 0.84, 95% CI 0.53 to 1.33; participants = 798, very low quality of evidence). The incidence of fluorosis at 5 years was similar between the group taking fluoride supplements (tablets) during the last 6 months of pregnancy and the placebo group.
Authors' conclusions
There is no evidence that fluoride supplements taken by women during pregnancy are effective in preventing dental caries in their offspring.
Plain language summary
Fluoride supplements taken by pregnant women for preventing dental caries in the primary teeth of their children
Review question
How effective and safe is the use of fluoride supplementation (with tablets, drops, lozenges or chewing gum) in pregnant women for preventing tooth decay in the baby teeth of their children compared with placebo (tablets or other forms of supplements without fluoride) or no treatment?
Background
Tooth decay is one of the most common health problems among children. The condition has been decreasing among children in most parts of the world over the past few decades most likely due to the widespread use of fluoride toothpaste, followed by water fluoridation, oral health education and a slight decrease in sugar consumption. If fluoride supplements taken by pregnant women can prevent tooth decay in their children, pregnant women with no access to a fluoridated drinking water supply can obtain the benefits of systemic fluoridation. Fluoride tablets, drops, lozenges or chewing gums are sucked or chewed to provide topical fluoride and ingested to provide systemic fluoride.
Study characteristics
Authors from Cochrane Oral Health carried out this review of existing studies and the evidence is current up to 25 January 2017. It includes only one study in which 1400 pregnant women were randomly allocated to fluoride treatment or placebo. In this study, a daily dose of either 1 mg sodium fluoride tablets or placebo tablets were given to participants from the fourth month of pregnancy to delivery. Both groups were encouraged to use dietary fluoride supplements after delivery in the form of drops. A total of 1175 babies were born to participants in this study, and of this number, 938 children were followed up at 3 years (464 fluoride tablets versus 484 placebo tablets) and 798 children were followed up at 5 years (398 fluoride tablets versus 400 placebo tablets) of age. Published in 1997, this study took place in communities with unfluoridated drinking water in Southern Maine, USA.
Key results
Baby teeth decay measured in children aged 3 and 5 years old was very low in both the fluoride supplement group and the placebo group. At 5 years of age, 92% of children remained decay‐free in the fluoride supplement group and 91% remained decay‐free in the placebo group, showing no difference between the two groups. The incidence of fluorosis at 5 years was similar between the group taking fluoride supplements (tablets) during the last 6 months of pregnancy and the placebo group.
There is no evidence that fluoride supplements taken by women during pregnancy are effective in preventing dental caries in their offspring.
Quality of the evidence
The included study was assessed as being at high risk of bias and the evidence was of very low quality.
Summary of findings
Summary of findings for the main comparison. Fluoride supplementation (tablets) compared to placebo for pregnant women for preventing dental caries in the primary teeth of their children.
| Fluoride supplementation (tablets) compared to placebo for pregnant women for preventing dental caries in the primary teeth of their children | ||||||
| Population: pregnant women for preventing dental caries in the primary teeth of their children Setting: USA Intervention: fluoride supplementation (tablets) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with fluoride supplementation (tablets) | |||||
| Children with caries in the primary teeth at 3 years | Study population | RR 1.46 (0.75 to 2.85) | 938 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | At 5 years RR 0.84 (0.53 to 1.33) | |
| 30 per 1000 | 43 per 1000 (22 to 84) | |||||
| Decayed or filled primary tooth surfaces at 3 years | The mean decayed or filled surfaces at 3 years was 0.30 | MD 0.12 higher (0.05 lower to 0.29 higher) | ‐ | 938 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2, 3 | Not significant at 5 years |
| Fluorosis (maxillary teeth) at 5 years | Study population | RR 1.79 (0.95 to 3.40) | 798 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1, 2 | Fluorosis in mandibular teeth at 5 years RR 0.89 (0.35 to 2.29) | |
| 35 per 1000 | 63 per 1000 (33 to 119) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by 2 for imprecision: few events, and CI included appreciable benefits and harms. 2Downgraded by 1 for risk of bias: high attrition bias, and potential limitations in blinding of outcome assessment are likely to lower confidence in the estimate of effect 3Downgraded by 2 for imprecision: CI included appreciable benefits and harms.
Background
Description of the condition
Dental caries (tooth decay) is one of the most common chronic childhood diseases, and can have a negative impact on a child's growth, speech, self‐confidence, general health and quality of life (Anderson 2004; Casamassimo 2009; Jankauskiene 2010). Dental caries occurs because of long‐term exposure to a mixture of acid‐producing bacteria and fermentable carbohydrates, and many other factors that include saliva secretion rate and buffering capacity (Rozier 2010; Selwitz 2007). In particular, acid production from bacteria, especially mutans Streptococci and Lactobacilli, and the subsequent decrease in local pH, cause the demineralization of tooth tissue (Featherstone 2004). If this process is not reversed, carious lesion progresses. Dissolved calcium and phosphate mineral ions can be redeposited on the tooth surface even though they are provided from saliva. This demineralization/remineralization process occurs continuously in oral fluids. Dental caries is generated when the demineralization/remineralization process lose the balance.
Dental caries is widespread in all countries (WHO 2009). Dental caries affects 30% to 50% of children aged 5 to 6 years (Armfield 2009; CDC 2007; Public Health England 2012), 60% to 90% of school‐aged children and a large majority of adults (Petersen 2005). An increasing number of decayed, missing or filled teeth (dmft) is reported in some low‐income and middle‐income countries (Bagramian 2009). Although the mean dmft has declined in many high‐income countries, this disparity remains and some population groups have high dmft (Bagramian 2009; Dawkins 2013; Jones 2017). Prevalence of dental caries is associated with increased consumption of sugar in the diet including high‐sugar confectionery and sweet carbonated beverages (Ismail 1997), socioeconomic status, dental insurance coverage and residential locations (Campus 2009; Dawkins 2013; Hobdell 2003; Kolker 2007).
Several studies have reported that caries in primary teeth are correlated with caries in permanent teeth (Helfenstein 1991; Li 2002; Seppa 1989). 'Early childhood caries', which is the presence of at least one carious lesion on a primary tooth in a child under the age of 6 years, is a serious problem in the world (Anil 2017). If the fluoride supplementation in pregnant woman is effective to prevent dental caries in the primary teeth of their children, the prevalence of dental caries can be prevented.
Description of the intervention
The benefits of topical fluorides, such as toothpastes, gels, varnishes and mouthrinses, for preventing dental caries in children and adolescents are well established (Marinho 2003a; Marinho 2003b; Marinho 2013; Marinho 2015; Marinho 2016). Topical fluoride plays an important role in preventing dental caries (Marinho 2003b) including the inhibition of demineralization of the crystal surfaces, the enhancement of remineralization in demineralized lesions (Featherstone 1990) and inhibition of bacterial metabolism (Featherstone 1999; Featherstone 2000; Fejerskov 2004). The American Dental Association (ADA) and Centers for Disease Control and Prevention (CDC) state that an appropriate concentration of fluoride, which varies depending on age, is effective to prevent dental caries (CDC 2001; Rozier 2010).
The upper limit of fluoride intake from all sources (fluoridated water, food, beverages, fluoride dental products and dietary fluoride supplements) is set at 0.10 mg/kg/day for infants, toddlers, and children through to 8 years old. For older children and adults, who are no longer at risk for dental fluorosis, the upper limit of fluoride is set at 10 mg/day regardless of weight (Levy 1999). CDC reported water fluoridation is especially beneficial for communities of low socioeconomic status (CDC 1999).
It should be noted that separation of pre‐eruptive, post‐eruptive, systemic or topical effects of fluoride is impossible. Fluoridated water may have all effects. Topically applied fluorides are not intended for ingestion but can be swallowed unintentionally. Fluoride drops, lozenges or chewing gums are sucked or chewed to provide topical fluoride and ingested to provide systemic fluoride. Fluoride supplements taken during pregnancy have the effect of prenatal fluoride and possibly reduce dental caries in offspring (Stephen 1993). However, risks associated with exposure to fluoride during pregnancy including miscarriage, premature delivery and premature birth were also reported (Diouf 2012; Sastry 2011).
How the intervention might work
The enamel formation process is composed of two principal stages: secretory stage and maturation stage. In the former stage, ameloblasts produce protein matrix (predominantly amelogenins) and crystallites are deposited in the protein matrix. In the latter stage, ameloblasts transport the substances used in enamel formation out of the enamel. Excess water and organic materials are removed and mineral is transported into the tissue in order to achieve full mineralization of enamel (Hiller 1975; Termine 1980).
Fluoride can be transported from maternal serum to the fetus and prenatal deciduous enamel through the placenta (Toyama 2001). The mechanism of fluoride placental transfer is controversial; there are some hypotheses that the placenta allows passive diffusion of fluoride from mother to fetus, while others suggest that the placenta acts as a barrier. It has been reported that the placenta allows passive diffusion of fluoride from mother to fetus when fluoride intake is low and that the placenta acts as a selective barrier when fluoride intake exceeds a particular level (Gupta 1993; Toyama 2001). In the development of enamel formation, fluoride is incorporated into the crystal lattice and binds to calcium, which is contained within the protein matrix. As a result, fluoride alters crystal formation in the enamel matrix and strengthens the properties of the enamel crystals (Tanimoto 2008).
All primary tooth enamel and some permanent tooth enamel start to grow and continue to develop in utero (Kraus 1965). Most of the permanent tooth enamel start to grow after birth. It has been reported that while the effect of fluoride supplements after birth was unclear on deciduous teeth, it was associated with a reduction in caries increment in permanent teeth (Tubert‐Jeannin 2011). Namely, it might be suggested that postnatal systemic fluoride did not attribute to caries prevention in primary teeth. In regions without water fluoridation, fluoride supplementation for pregnant women may be an effective way to increase fluoride intake during pregnancy. If fluoride supplements taken by pregnant women improve neonatal outcomes, pregnant women with no access to a fluoridated drinking water supply can obtain the benefits of systemic fluoridation.
Why it is important to do this review
Recent systematic reviews and Cochrane Reviews have evaluated evidence regarding the effectiveness and safety of fluoride treatment and the adverse effects of high fluoride exposure in children and adults (Choi 2012; Iheozor‐Ejiofor 2015; Ismail 2008; Marinho 2003a; Marinho 2003b; Marinho 2013; Marinho 2015; Marinho 2016; Tubert‐Jeannin 2011; Wong 2010). However, it remains uncertain whether fluoride supplementation in pregnant women is effective in preventing dental caries in their offspring. Currently, no systematic reviews have investigated the effectiveness and safety of this intervention. This Cochrane Review aims to address this gap in the literature and assess the current available evidence on fluoride supplementation during pregnancy.
Objectives
To evaluate the effects of women taking fluoride supplements (tablets, drops, lozenges or chewing gum) compared with no fluoride supplementation during pregnancy to prevent caries in the primary teeth of their children.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs), including quasi‐randomised and cluster‐randomised trials. We excluded cross‐over trials as they are an inappropriate study design (as the intervention may have a lasting effect that compromises entry to subsequent periods of the trial). We excluded observational studies (including cohort studies, case‐control studies, etc.). We included both clinical and community‐based trials.
Types of participants
We included pregnant women, regardless of their dental caries, exposure to fluorides, level of dental treatment, nationality or level of education. The women may or may not have had access to fluoridated water (naturally or artificially).
Types of interventions
We included studies of fluoride supplementation (tablets, drops, lozenges or chewing gum) of any dosage, frequency, duration and timing of delivery, which may or may have not included the use of topical fluorides such as fluoride dentifrice, fluoride rinse and topical fluoride application, compared with no fluoride supplementation.
Control group: no treatment or placebo.
Types of outcome measures
Primary outcomes
Primary outcomes (for deciduous teeth of children up to 6 years of age).
Number of children with caries in the primary teeth.
Decayed, missing and filled primary teeth (dmft) and components.
Decayed, missing and filled primary tooth surfaces (dmfs) and components.
Fluorosis.
Secondary outcomes
Adverse effects (apart from fluorosis), e.g. miscarriage, premature delivery, or dental and any other possible negative effects. A full investigation of adverse effects was not possible as we did not include observational or retrospective epidemiological studies.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 25 January 2017) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11) in the Cochrane Library (searched 25 January 2017) (Appendix 2);
MEDLINE Ovid (1946 to 25 January 2017) (Appendix 3);
Embase Ovid (1980 to 25 January 2017) (Appendix 4);
LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 25 January 2017) (Appendix 5);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 25 January 2017) (Appendix 6).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid.
Searching other resources
We searched the following trial registries for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 25 January 2017) (Appendix 7);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 25 January 2017) (Appendix 8).
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We did not perform a separate search for adverse effects of interventions used, and we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors (Rena Takahashi (RT) and Keika Hoshi (KH)) independently screened the titles and abstracts (when available) of all reports identified through the electronic searches and handsearching that were entered into EndNote X7 software. We obtained the full text of potentially relevant studies or studies where it was difficult to make a clear decision from only the title and abstract. The full‐text articles were assessed independently by two review authors to determine if they met the inclusion criteria. We resolved any disagreements by discussion. If this was not possible, we consulted a third review author. At the time of exclusion, we recorded reasons for exclusion in the 'Characteristics of excluded studies' tables.
Data extraction and management
Two review authors (RT and Erika Ota (EO)) independently extracted data using data extraction forms. We extracted data related to settings, participants (e.g. inclusion and exclusion criteria for pregnant women), interventions (e.g. type of intervention, comparison), outcomes (e.g. outcomes reported in the paper, duration and rates of follow‐up, adverse effects), methods (e.g. study designs, randomisation methods) and other information (e.g. pharmaceutical sponsorship data). We recorded if clinical trials reported the presence of calcium in the fluoride supplement. We noted the topical fluoride exposure of children (up to 6 years of age) in the follow‐up period of the studies. We included unpublished research data from recognised research groups and experts in the field, obtained via personal contact if the study was in the clinical register.
We resolved any disagreements by discussion and consultation with a third review author. In this review, we did not contact trial authors. In future updates, we will contact trial authors (if possible) asking for assistance with data transformation or raw data, if the data are not reported in a format suitable for analysis.
Assessment of risk of bias in included studies
Two review authors (KH and Yoshihiro Toyoshima (YT)) assessed the risk of bias independently for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third assessor (EO). We assessed the following domains of risk of bias: (1) random sequence generation (selection bias), (2) allocation concealment (selection bias), (3) blinding of participants and personnel (performance bias), (4) blinding of outcome assessors (detection bias), (5) incomplete outcome data (attrition bias), (6) selective reporting (reporting bias) and (7) other bias (recruitment bias, bias influenced by funding source, etc.).
Each domain was assessed as at either low, high or unclear risk of bias. We categorised the overall risk of bias of individual studies as follows:
low risk of bias (plausible bias unlikely to seriously alter the results) if all domains were at low risk of bias;
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more domains had an unclear risk of bias, but none at high risk of bias;
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more domains were at high risk of bias.
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratios for differences between the intervention and comparison groups, along with 95% confidence intervals (CI). For continuous outcomes, we calculated the mean difference (MD) and 95% CIs where means and standard deviations (SD) were presented or were calculable. We did not calculate the standardised mean difference (SMD) in this review. In future updates, where a continuous outcome is measured using different scales, we will calculate the SMD and SDs.
Unit of analysis issues
We did not include any cluster‐randomised trials in the analyses. In future updates, if we include cluster‐randomised trials, we will adjust their sample sizes using the methods described in Section 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using an estimate of the intracluster correlation coefficient (ICC) with either data from the trials (if possible), data from a similar trial or data from a study of a similar population. We will conduct sensitivity analyses to see the effect of variation in the ICC when we use ICCs from other sources. We plan to combine the relevant outcomes from both cluster‐randomised and individually randomised trials if there is no substantial heterogeneity between the two study designs.
Dealing with missing data
In this review we did not contact trial authors. In future updates, where possible, we will contact trial authors to provide missing data. We will note levels of attrition for included studies. We will conduct sensitivity analysis to check the impact of including studies with substantial levels (more than 20%) of missing data in the overall assessment of the intervention effect.
If possible, we will conduct analysis on an intention‐to‐treat basis. If there are missing data, the denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing in the analysis. If there are missing standard deviations of the continuous data, we will use the methods described in the Cochrane Handbook of Systematic Reviews of Interventions Section 7.7.3 (Higgins 2011) to estimate them.
Assessment of heterogeneity
We could not assess heterogeneity due to the inclusion of only one study. In future updates, we will assess heterogeneity by inspection of forest plots of the estimates and confidence intervals of treatment effects. We will assess statistical heterogeneity in each meta‐analysis using the I2 and Chi2 statistics. We defined substantial heterogeneity as I2 greater than 50%, or if there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
In future updates, if more than 10 trials are identified for any meta‐analysis, we will assess publication bias using visual assessment of funnel plots according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Should asymmetry be identified in the contour‐enhanced funnel plots, we will investigate possible causes by performing exploratory analyses.
Data synthesis
We could not conduct data synthesis as only one study was included in the review. In future updates, we will conduct meta‐analyses for studies with comparable analyses that report similar outcome measures using forest plots in Review Manager software (RevMan) (RevMan 2014). We will combine risk ratios for dichotomous data and mean differences for continuous data using random‐effects models, assuming that the identified studies allow this procedure. If random‐effects analyses are conducted, we will present the results as the average treatment effect with 95% CIs, and estimates of I2. If there are few studies or small sample sizes, it may be impossible to estimate between‐study variance with any precision. In that case, a random‐effects analysis would provide poor estimates of the distribution of intervention effects, therefore we would use a fixed‐effect model (Higgins 2011).
In future updates, we will analyse cluster‐RCTs at the individual level. We will meta‐analyse results from appropriately analysed cluster‐RCTs using the generic inverse variance method in RevMan. If original analyses do not account for clustering, we will conduct an adjusted analysis, provided that the necessary information (e.g. mean cluster size, proportion of individuals with events, ICCs) can be extracted (Higgins 2011). We will calculate the prevented fraction.
Subgroup analysis and investigation of heterogeneity
We could not assess heterogeneity by inspection of forest plots of the estimates and confidence intervals of treatment effects as only one study was included in the review. In future updates, if we identify substantial heterogeneity in the primary outcomes, we will conduct subgroup analyses for these relevant and clinically meaningful subgroups where sufficient data are available. In studies with more than one intervention group, such as those comparing different frequencies of application or different types of supplements, we will consider the results from all relevant experimental groups separately in the meta‐analyses.
Moreover, we could not conduct two different subgroup analyses:
low‐ and middle‐income countries versus high‐income countries (defined by World Bank criteria);
region of high‐level fluoride concentration in tap water or well water (1.5 mg/L or more, 0.3 mg/L or more) versus region of low‐ to medium‐level fluoride concentration.
Sensitivity analysis
In future updates, we will undertake sensitivity analysis for primary outcomes based on the risk of bias assessment for allocation concealment and attrition rates. We will redo analyses and remove studies that are at high risk of bias for these domains in order to assess whether this makes any difference to the overall result.
Summary of findings
We created 'Summary of findings' tables using GRADE profiler (GRADEpro GDT 2015), with data imported from RevMan. We used the GRADE approach (Schünemann 2009) to assess the quality of the body of evidence relating to primary outcomes for the main comparisons. The quality of the body of evidence for each outcome was assessed under five domains (study limitations, consistency of effect, imprecision, indirectness and publication bias) and judged to be of high, moderate, low or very low quality.
Results
Description of studies
Results of the search
A total of 173 references were identified by the above search strategy after duplicates were removed. Assessment of the titles and abstracts, where available, resulted in five references of potential relevance; all of which were obtained in full. Four full‐text articles were rejected since they were not randomised controlled trials (RCTs). We found one study suitable for inclusion in this review (Figure 1).
1.
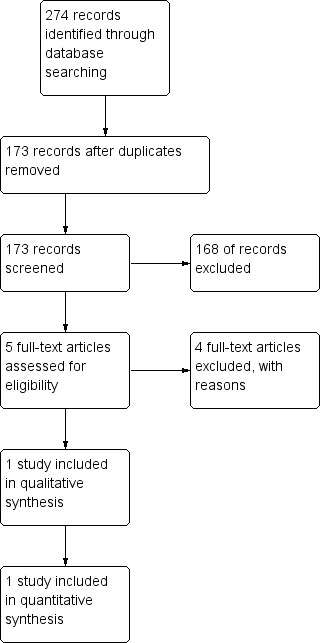
Study flow diagram.
Included studies
We included one individual RCT (Leverett 1997). See Characteristics of included studies table.
Participants
This trial recruited 1400 pregnant women in the first trimester residing in communities served by fluoride‐deficient drinking water. There were 1175 babies born to participants and of these, 938 children were followed up at 3 years (intervention 464 versus control 484) and 798 children were followed up at 5 years (intervention 398 versus control 400).
Interventions and comparisons
The intervention group received one 2.2 mg dose of sodium fluoride (NaF) (1 mg active fluoride ion) in the form of one tablet to be taken daily from the fourth month of pregnancy. The control group received placebo tablets (no fluoride during pregnancy). Both the intervention and control groups received fluoride drops from birth to 2 years of age and one 0.5 mg tablet daily for children aged 2 to 3 years.
Participants were contacted in order to find out how many tablets remained in their supplied bottles. On the basis of that count, new supplies were mailed to coincide with the estimated date of exhaustion of the previous supply. Compliance during the prenatal period was very good. Of 938 subjects that were examined at the 3‐year interval, 88% had excellent adherence to the prenatal protocol with mean compliance scores (standard deviation (SD)) of 0.94 (0.10) and 0.95 (0.12) among the treatment and control groups respectively.
Outcomes
Caries experience between the two groups and the percentage of children with no caries in the primary teeth. Children's mean decayed, filled primary tooth surfaces (dfs) was taken at 3 and 5 years.
Settings
Communities with unfluoridated drinking water in Southern Maine, USA.
Excluded studies
We excluded four studies (Glenn 1979; Glenn 1982; Glenn 1984; Restrepo 1993) after full‐text assessment because these studies were not RCTs. See Characteristics of excluded studies table.
Risk of bias in included studies
We summarised risk of bias graphically using the plots available in RevMan (RevMan 2014). See Figure 2. The included study was at high overall risk of bias.
2.
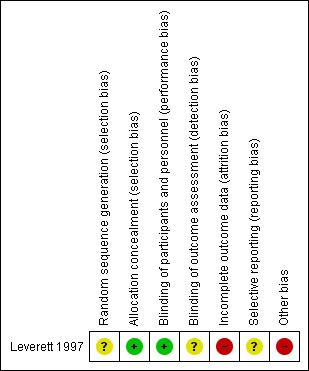
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed random sequence generation (selection bias) as being at unclear risk of bias (the method of randomisation was not described in detail), and allocation concealment (selection bias) as at low risk of bias.
Blinding
We assessed blinding of participants and personnel (performance bias) as at low risk of bias and blinding of outcome assessment (detection bias) as being at unclear risk of bias (no description provided).
Incomplete outcome data
We assessed incomplete outcome data (attrition bias) as at high risk of bias.
Selective reporting
We assessed selective reporting (reporting bias) as at unclear risk of bias (no protocol or registry was available for the study).
Other potential sources of bias
We assessed the other potential sources of bias as at high risk of bias, as administering fluoride supplementation to both groups after delivery might affect the results.
Effects of interventions
See: Table 1
Fluoride supplementation (tablets) versus placebo
One trial involving 938 women and their children was included (Leverett 1997).
Primary outcomes
Number of children with caries in the primary teeth
For this primary outcome, there was no difference in effect for children with caries in the primary teeth at 3 years in the fluoride supplementation group compared to the control group (risk ratio (RR) 1.46, 95% confidence interval (CI) 0.75 to 2.85; participants = 938; studies = 1) (Analysis 1.1).
1.1. Analysis.
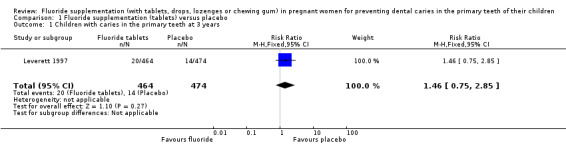
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 1 Children with caries in the primary teeth at 3 years.
There was no difference in effect at 5 years (RR 0.84, 95% CI 0.53 to 1.33; participants = 798; studies = 1) (Analysis 1.2).
1.2. Analysis.
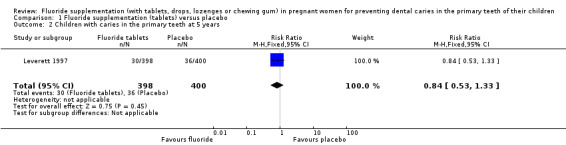
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 2 Children with caries in the primary teeth at 5 years.
Decayed, missing and filled primary teeth (dmft) and components
Outcome not assessed.
Decayed, missing and filled primary tooth surfaces (dmfs) and components
There was no difference in effect on decayed surfaces at 3 years (mean difference (MD) 0.05, 95% CI ‐0.02 to 0.12; participants = 938; studies = 1) (Analysis 1.3); filled surfaces at 3 years (MD 0.07, 95% CI ‐0.07 to 0.21; participants = 938; studies = 1) (Analysis 1.4); decayed or filled surfaces at 3 years (MD 0.12, 95% CI ‐0.05 to 0.29; participants = 938; studies = 1) (Analysis 1.5); decayed surfaces at 5 years (MD ‐0.06, 95% CI ‐0.17 to 0.05; participants = 798; studies = 1) (Analysis 1.6); filled surfaces at 5 years (MD 0.03, 95% CI ‐0.28 to 0.34; participants = 798; studies = 1) (Analysis 1.7); and decayed or filled surfaces at 5 years (MD ‐0.05, 95% CI ‐0.42 to 0.32; participants = 798; studies = 1) (Analysis 1.8). Leverett 1997 did not assess missing surfaces.
1.3. Analysis.
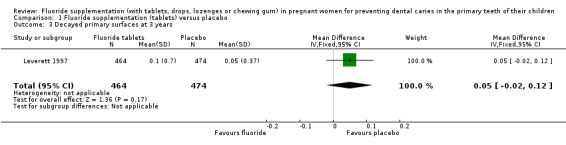
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 3 Decayed primary surfaces at 3 years.
1.4. Analysis.
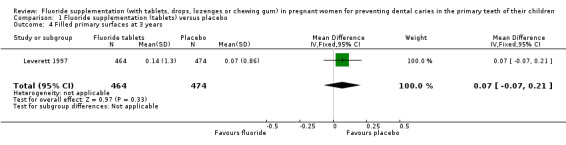
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 4 Filled primary surfaces at 3 years.
1.5. Analysis.
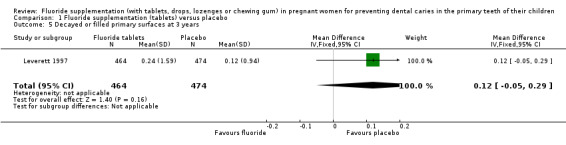
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 5 Decayed or filled primary surfaces at 3 years.
1.6. Analysis.
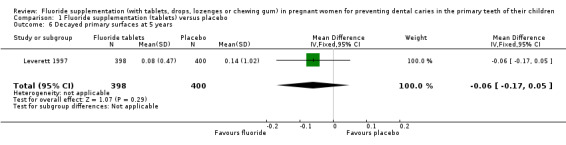
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 6 Decayed primary surfaces at 5 years.
1.7. Analysis.
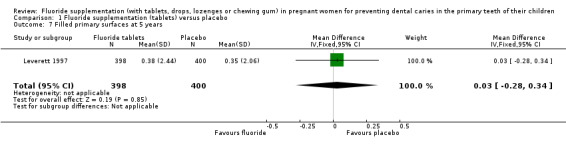
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 7 Filled primary surfaces at 5 years.
1.8. Analysis.
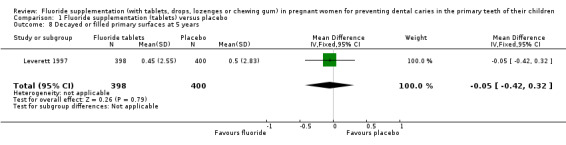
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 8 Decayed or filled primary surfaces at 5 years.
Fluorosis
Regarding side effects, there was no difference in effect for fluorosis (maxillary teeth) at 5 years (RR 1.79, 95% CI 0.95 to 3.40; participants = 798; studies = 1) (Analysis 1.9); and fluorosis (mandibular teeth) at 5 years (RR 0.89, 95% CI 0.35 to 2.29; participants = 798; studies = 1) (Analysis 1.10).
1.9. Analysis.
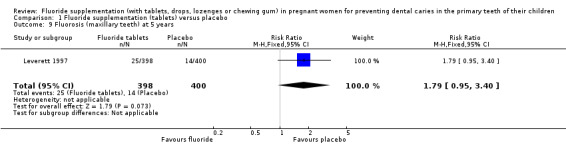
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 9 Fluorosis (maxillary teeth) at 5 years.
1.10. Analysis.
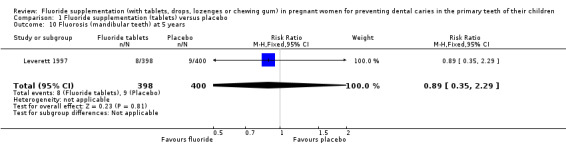
Comparison 1 Fluoride supplementation (tablets) versus placebo, Outcome 10 Fluorosis (mandibular teeth) at 5 years.
Secondary outcomes
Adverse effects (apart from fluorosis)
There were no other adverse events of interest for the review reported in this trial.
Discussion
Summary of main results
The main question addressed by this review was the efficacy of fluoride supplementation (with tablets, drops, lozenges or chewing gum) in women during pregnancy in preventing caries in the primary teeth of their children. Only one randomised controlled trial (RCT) (Leverett 1997) met the inclusion criteria for this review. This RCT had some limitations.
This RCT showed no statistical difference on decayed, filled primary tooth surfaces (dfs) and percentage of children with caries at 3 and 5 years. The incidence of fluorosis at 5 years was similar between the group taking fluoride supplements during the last 6 months of pregnancy and the placebo group. See Table 1 for the summary of the main results.
The trial authors stated that fluoride carried over into the postnatal period might well have contributed to the low level of dental caries in both groups.
Fluorosis was scored as very mild using Dean's score (Dean 1942). The number of all types of tooth fluorosis was counted. Fluorosis event rate was too low to detect a difference between the groups.
Overall completeness and applicability of evidence
No evidence was found on decayed, missing and filled primary teeth (dmft) or missing surfaces (ms). Limited information was available on adverse events, and only dental fluorosis at 5 years was reported. In addition, systemic side effects were not examined in the included trial. The different dosages of fluoride supplements (tablets, drops, lozenges or chewing gum) were not explored in this review. Furthermore in the included study, fluoride supplements were routinely given to both the intervention and control groups after birth. At the time that this RCT was conducted, ingestion of fluoride supplements until the age of 3 was common practice. Therefore, fluoride supplements were given to both the intervention and control groups. Whilst the effect of fluoride supplements on the primary teeth after birth is unclear (Tubert‐Jeannin 2011), children could obtain the benefits of fluoride supplements after birth (Limeback 1999). Thus, the effect of prenatal fluoride supplementation is unclear. In future research, a postnatal preventive approach would be needed equally between the groups. Such ethical consideration is necessary in a study to investigate the effect of prenatal fluoride supplementation in pregnant women for preventing dental caries in the primary teeth of their children.
Quality of the evidence
Overall, the included trial was at high risk of bias due mainly to high attrition bias. Due to the long‐term follow‐up, the rate of losses to follow‐up was quite high (20% at 3 years and 38% at 5 years). The study was affected by attrition bias, however most of the other biases such as selection bias (random sequence generation), blinding of outcome assessment (detection bias), and reporting bias were not evaluated or no information was available and were assessed as unclear. Participant compliance could also influence the result of the study.
The quality of the evidence as assessed using GRADE (Table 1) was very low for children with caries in the primary teeth at 3 years. This was downgraded due to potential limitations in the blinding of outcome assessment, which are likely to lower confidence in the estimate of effect, as well as imprecision due to few events, and the 95% confidence interval (CI) included appreciable benefits and harms. The quality of the evidence for children with caries in the primary teeth at 5 years was graded as very low due to high attrition bias and imprecision (few events and the CI included appreciable benefits and harms).
The evidence for the outcomes of decayed or filled primary tooth surfaces at 3 years and decayed or filled surfaces at 5 years was considered to be of very low quality due to high attrition bias, potential limitations in the blinding of outcome assessment, which were likely to lower confidence in the estimate of effect, and imprecision (CI included appreciable benefits and harms).
The evidence for fluorosis (maxillary teeth) at 5 years and fluorosis (mandibular teeth) at 5 years was also of very low quality, downgraded due to imprecision (few events and CI included appreciable benefits and harms), and risk of bias (high attrition bias and potential limitations in blinding of outcome assessment).
Potential biases in the review process
We tried to minimize the potential biases in this review following the guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For example, the search was conducted by the Cochrane Oral Health's Information Specialist. Two review authors screened and assessed studies for eligibility for inclusion and risk of bias independently. We described the reasons for each judgement of risk of bias. Data entry was checked by two review authors.
Agreements and disagreements with other studies or reviews
A previous review (Fassman 1993) of prenatal fluoridation also mentioned that the effect was inconclusive. However, the quality of this previous review is questionable. First, inclusion criteria were not clearly shown. Secondly, quality assessment for included studies was not implemented. Those drawbacks are possibly due to the fact that at the time of publication, the systematic review process was not well established. Our study examined relevant literature thoroughly and found that the effect of prenatal fluoride supplement was inconclusive.
We only included one RCT in this review, therefore, we conducted further searches of the literature for observational studies for discussion to obtain more information below.
Kailis et al (Kailis 1968) enrolled 374 children in Perth, Australia, at the age of 4 to 6 years old and implemented a questionnaire on prenatal fluoridation. Due to the study method, the dose, duration, and gestational age of ingestion were not reported. Participants were divided into three groups (non‐fluoride, postnatal fluoride, and prenatal and postnatal fluoride). Because our question was to evaluate the effect of prenatal fluoride supplementation, we extracted the results of postnatal fluoride, and prenatal and postnatal fluoride groups. The decayed, missing and filled primary teeth (dmft) rates and the percentage of caries‐free children among the prenatal and postnatal fluoride group were significantly lower than that of the postnatal fluoride group.
Glenn et al (Glenn 1982) examined 375 children in Miami, USA without a prenatal fluoride supplement (without PNF) and 117 children with a prenatal 2.2 mg sodium fluoride tablet (with PNF) in a fluoridated water area. Because of the study method, duration and gestational age of ingestion were not reported. The results were as follows: the mean birth weight of the group with PNF was heavier than that of the group without PNF. The mean birth length of the group with PNF was longer than that of the group without PNF group. The group without PNF included two children with Down's syndrome, two children with an intellectual disability, one child with an intraventricular septal defect, one child with minimal brain dysfunction syndrome, one child with clubfoot, one child with congenital hip dysplasia, one child with congenital epidermolysis bullosa, four children with congenitally missing teeth, and six children with supernumerary teeth. No medical and dental defects were found in the group with PNF, while no dental fluorosis were found in either group.
Accumulated fluoride to immature matrix proteins onto the apatite crystal surface of calcifying deciduous dentition seems to be the essential mechanism for preventing caries. Prichard examined the prenatal and postnatal effects of fluoride supplementation on Australian children. There were three groups: a non‐fluoride group, a prenatal and postnatal fluoride supplementation group, and a postnatal fluoride supplementation group. He reported caries reduction in primary teeth of 70% after prenatal and postnatal fluoride supplementation and 40% after postnatal fluoride supplementation only (Prichard 1969). From this result, prenatal fluoride supplementation might work if the placenta does not act as a barrier.
As we mentioned in the introduction, fluoride placental transfer is questionable and no new investigation has been undertaken on this topic in 20 years. This theoretical weakness discourages investigators from administering prenatal fluoride supplementation. In contrast, topical fluoride administration after birth has a growing evidence base of efficacy, and topical fluoride is currently considered to have a caries‐inhibiting effect (Marinho 2003b). As Glenn 1982 suggested, the probability of side effects of prenatal fluoride ingestion is another point to consider. A lack of evidence for prenatal fluoride treatment, the establishment of an alternative method, and the probability of side effects means that limited resources are available for RCTs as well as observational studies. Overall, only poor quality studies were published.
Authors' conclusions
Implications for practice.
There is no evidence that fluoride supplements taken by women during pregnancy are effective in preventing dental caries in their offspring.
Implications for research.
Careful consideration should be given to whether or not this is a priority topic for future research. The caries levels in the included study are far below those which cause concern. If future studies are undertaken they should focus on areas where young children are at high risk of developing caries.
Acknowledgements
We would like to thank Cochrane Oral Health for their help in developing this review. We thank Valeria Marinho, Helen Worthington, Annetta Tsang and Derek Richards for their comments on the draft. We would also like to thank Emma Barber for her editorial assistance in preparing the protocol for this review, and Anne Littlewood for developing the search strategy.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
1 (pregnan* or prenatal:ti,ab) AND (INREGISTER) 2 ((expect* and mother*):ti,ab) AND (INREGISTER) 3 (#1 or #2) AND (INREGISTER) 4 ((fluorid* and (tablet* or drop* or lozenge* or pill* or gum* or supplement*))) AND (INREGISTER) 5 (#3 and #4) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 [mh Pregnancy] #2 (pregnan* or prenatal) #3 (expect* near/3 mother*) #4 #1 or #2 or #3 #5 [mh Fluorides] #6 fluorid* #7 #5 or #6 #8 (tablet* or drop* or lozenge* or pill* or gum* or supplement*) #9 #7 and #8 #10 #4 and #9
Appendix 3. MEDLINE Ovid search strategy
1 exp Pregnancy/ 2 (pregnan$ or prenatal).ti,ab. 3 (expect$ adj3 mother$).ti,ab. 4 or/1‐3 5 exp Fluorides/ 6 fluorid$.ti,ab. 7 or/5‐6 8 (tablet$ or drop$ or lozenge$ or pill$ or gum$ or supplement$).ti,ab. 9 7 and 8 10 4 and 9
Appendix 4. Embase Ovid search strategy
1 exp Pregnancy/ 2 (pregnan$ or prenatal).ti,ab. 3 (expect$ adj3 mother$).ti,ab. 4 or/1‐3 5 exp Fluorides/ 6 fluorid$.ti,ab. 7 or/5‐6 8 (tablet$ or drop$ or lozenge$ or pill$ or gum$ or supplement$).ti,ab. 9 7 and 8 10 4 and 9
Appendix 5. LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database) search strategy
((Mh pregnancy or pregnan$ or embarazo or gravidez) AND (Mh Fluorides or fluor$))
AND
(tablet$ or pill$ or drop$ or supplement$ or gum$ or lozenge$ or comprimido$ or pilula$ or suplemento$ or goma$ or pastilha$ or pildora$ or pastilla$ or chicle$)
Appendix 6. CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature)
S10 S4 and S9 S9 S7 and S8 S8 (tablet* or drop* or lozenge* or pill* or gum* or supplement*) S7 S5 or S6 S6 fluorid* S5 (MH "Fluorides+") S4 S1 or S2 or S3 S3 (expect* N3 mother*) S2 (pregnan* or prenatal) S1 (MH "Pregnancy+")
Appendix 7. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
pregnancy and fluoride
Appendix 8. World Health Organization International Clinical Trials Registry Platform search strategy
pregnancy and fluoride
Data and analyses
Comparison 1. Fluoride supplementation (tablets) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Children with caries in the primary teeth at 3 years | 1 | 938 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.75, 2.85] |
| 2 Children with caries in the primary teeth at 5 years | 1 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 3 Decayed primary surfaces at 3 years | 1 | 938 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.02, 0.12] |
| 4 Filled primary surfaces at 3 years | 1 | 938 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| 5 Decayed or filled primary surfaces at 3 years | 1 | 938 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.05, 0.29] |
| 6 Decayed primary surfaces at 5 years | 1 | 798 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.17, 0.05] |
| 7 Filled primary surfaces at 5 years | 1 | 798 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.28, 0.34] |
| 8 Decayed or filled primary surfaces at 5 years | 1 | 798 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.42, 0.32] |
| 9 Fluorosis (maxillary teeth) at 5 years | 1 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.95, 3.40] |
| 10 Fluorosis (mandibular teeth) at 5 years | 1 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.35, 2.29] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Leverett 1997.
| Methods | Trial design: RCT Location: Southern Maine, USA Duration: September 1983 to May 1985 |
|
| Participants | 1400 pregnant women in the first trimester recruited from communities served by fluoride‐deficient drinking water 1175 born babies 938 children followed up at 3 years (intervention 464 versus control 484) 798 children followed up at 5 years (Intervention 398 versus control 400) |
|
| Interventions | Fluoride supplementation (tablets) versus placebo Group A: 1 dose of 2.2 mg sodium fluoride (NaF) (1 mg active fluoride ion), 1 tablet to be taken daily from 4th month of pregnancy Group B: placebo tablets (no fluoride) Both the intervention and control groups received fluoride drops from birth to 2 years of age and 1 0.5 mg tablet daily for children aged 2 to 3 years Follow‐up: 5.5 years (6 months prenatal ‐ 5 years after birth) |
|
| Outcomes | Caries experience between 2 groups, prevalence of "caries‐free" children, mean dfs | |
| Notes | Funding: National Institutes of Health/National Institute of Dental Research (NIH/NIDR) grants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized on ID number into one‐half containing fluoride and one‐half containing placebo. The bottle labels did not distinguish between active drug and the placebo" Comment: the method of randomisation is not described in detail |
| Allocation concealment (selection bias) | Low risk | Quote: "The manufacturer of the fluoride products provided...." Comment: similar to central allocation, participants and personnel did not know their allocation before the intervention started |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The bottle labels did not distinguish between active drug and the placebo" Comment: participants and personnel did not know their allocation during the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 years follow‐up: 464/585 (79%) for the intervention group, 474/590 (80%) for placebo group 5 years follow‐up: 398/585 (68%) for the intervention group, 400/590 (68%) for placebo group High attrition bias existed due to long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registry available |
| Other bias | High risk | Giving fluoride supplementation to both groups after delivery might affect the results |
dfs: decayed, filled primary tooth surfaces; RCT: randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Glenn 1979 | 771 participants were divided into 3 groups (control, 1.0 mg tablet per day, and 2.2 mg tablet per day). Allocation method was not described |
| Glenn 1982 | 33% of participants were randomly selected from 1374 private practice patients. Group 1: control, Group 2a: sibling of Group 1: no tablet, Group 2b: sibling of Group 1: with prenatal fluoride tablet, Group 3a: no prenatal intervention and no postnatal fluoride tablet, Group 3b: no prenatal intervention with postnatal fluoride tablet, Group 4a: twins with no prenatal intervention and no postnatal fluoride tablet, Group 4b: twins with prenatal fluoride tablet. Groups 3a and 3b are families with only 1 child. All participated patients were observed. It is cohort design |
| Glenn 1984 | Participants were divided into 3 groups (control, postnatal fluoride tablet, and prenatal fluoride tablet). Allocation method was not described |
| Restrepo 1993 | Narrative review |
Differences between protocol and review
Fluorosis added as a primary outcome of the review. Adverse effects other than fluorosis added as secondary outcomes in the review.
Contributions of authors
Erika Ota (EO) conceived and drafted the full review. Rena Takahashi (RT) and Keika Hoshi (KH) were responsible for the selection of studies. RT and EO were responsible for data extraction and management. KH, Yoshihiro Toyoshima (YT) and EO were responsible for the risk of bias assessment. KH, Hidemichi Yuasa (HY), YT and Rintaro Mori (RM) commented on and supervised the protocol and review. All review authors read and approved the final version.
Sources of support
Internal sources
-
National Center for Child Health and Development, Japan.
Grant 26A‐5
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland
-
Ministry of Health Labour and Welfare, Japan.
Health Labour Sciences Research Grant (No. 13800128)
School of Dentistry, The University of Manchester, UK.
The Clinical Research Program for Child Health and Development from the Japan Agency for Medical Research and Development (AMED), Japan.
Declarations of interest
Rena Takahashi: none known. Erika Ota: none known. Keika Hoshi: none known. Toru Naito: none known. Yoshihiro Toyoshima: none known. Hidemichi Yuasa: none known. Rintaro Mori: none known. Eishu Nango: none known.
New
References
References to studies included in this review
Leverett 1997 {published data only}
- Leverett DH, Adair SM, Vaughan BW, Proskin HM, Moss ME. Randomized clinical trial of the effect of prenatal fluoride supplements in preventing dental caries. Caries Research 1997;31(3):174‐9. [PUBMED: 9165186] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Glenn 1979 {published data only}
- Glenn FB. Immunity conveyed by sodium‐fluoride supplement during pregnancy: part II. ASDC Journal of Dentistry for Children 1979;46(1):17‐24. [PUBMED: 283075] [PubMed] [Google Scholar]
Glenn 1982 {published data only}
- Glenn FB, Glenn WD 3rd, Duncan RC. Fluoride tablet supplementation during pregnancy for caries immunity: a study of the offspring produced. American Journal of Obstetrics and Gynecology 1982;143(5):560‐4. [PUBMED: 7091227] [DOI] [PubMed] [Google Scholar]
Glenn 1984 {published data only}
- Glenn FB, Glenn WD 3rd, Duncan RC. Prenatal fluoride tablet supplementation and the fluoride content of teeth: Part VII. ASDC Journal of Dentistry for Children 1984;51(5):344‐51. [PUBMED: 6592186] [PubMed] [Google Scholar]
Restrepo 1993 {published data only}
- Restrepo Baena EA. Vitamins and minerals supplements during pregnancy [Suplemento de vitaminas y minerales durante el periódico de gestión]. Iatreia 1993;6(3):144‐9. [Google Scholar]
Additional references
Anderson 2004
- Anderson HK, Drummond BK, Thomson WM. Changes in aspects of children's oral‐health‐related quality of life following dental treatment under general anaesthesia. International Journal of Paediatric Dentistry 2004;14(5):317‐25. [DOI] [PubMed] [Google Scholar]
Anil 2017
- Anil S, Anand PS. Early childhood caries: prevalence, risk factors, and prevention. Frontiers in Pediatrics 2017;5:157. [PUBMED: 28770188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Armfield 2009
- Armfield JM, Spencer AJ, Brennan DS. Dental health of Australia's teenagers and pre‐teen children: the Child Dental Health Survey, Australia 2003‐04. Canberra: Australian Institute of Health and Welfare; 2009. Dental Statistics and Research Series No. 52 Cat. No. DEN 199.
Bagramian 2009
- Bagramian RA, Garcia‐Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. American Journal of Dentistry 2009;22(1):3‐8. [PUBMED: 19281105] [PubMed] [Google Scholar]
Campus 2009
- Campus G, Solinas G, Strohmenger L, Cagetti MG, Senna A, Minelli L, et al. National pathfinder survey on children's oral health in Italy: pattern and severity of caries disease in 4‐year‐olds. Caries Research 2009;43(2):155‐62. [DOI] [PubMed] [Google Scholar]
Casamassimo 2009
- Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E. Beyond the dmft: the human and economic cost of early childhood caries. Journal of the American Dental Association 2009;140(6):650‐7. [DOI] [PubMed] [Google Scholar]
CDC 1999
- CDC. Achievements in public health, 1900‐1999: fluoridation of drinking water to prevent dental caries. MMWR Weekly 1999;48(41):933‐40. [Google Scholar]
CDC 2001
- CDC. Recommendations for using fluoride to prevent and control dental caries in the United States. MMWR Recommendations and Reports 2001;50(RR14):1‐42. [PubMed] [Google Scholar]
CDC 2007
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton‐Evans G, et al. Trends in oral health status: United States, 1988‐1994 and 1999‐2004. Vital and Health Statistics. Series 11, Data from the National Health Survey 2007;(248):1‐92. [PubMed] [Google Scholar]
Choi 2012
- Choi AL, Sun G, Zhang Y, Grandjean P. Developmental fluoride neurotoxicity: a systematic review and meta‐analysis. Environmental Health Perspectives 2012;120(10):1362‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawkins 2013
- Dawkins E, Michimi A, Ellis‐Griffith G, Peterson T, Carter D, English G. Dental caries among children visiting a mobile dental clinic in South Central Kentucky: a pooled cross‐sectional study. BMC Oral Health 2013;13:19. [PUBMED: 23639250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dean 1942
- Dean HT. The investigation of physiological effects by the epidemiological method. In: Moulton FR editor(s). Fluorine and Dental Health. Washington DC: American Association for the Advancement of Science, 1942:23‐31. [Google Scholar]
Diouf 2012
- Diouf M, Cisse D, Lo CM, Ly M, Faye D, Ndiaye O. Pregnant women living in areas of endemic fluorosis in Senegal and low birthweight newborns: case‐control study [Femme enceinte vivant en zone de fluorose endémique au Sénégal et faible poids du nouveau‐né à la naissance: étude cas‐témoins]. Revue d'Epidémiologie et de Santé Publique 2012;60(2):103‐8. [DOI] [PubMed] [Google Scholar]
Fassman 1993
- Fassman DK. Prenatal fluoridation. A literature review. New York State Dental Journal 1993;59(6):47‐51. [PubMed] [Google Scholar]
Featherstone 1990
- Featherstone JD, Glena R, Shariati M, Shields CP. Dependence of in vitro demineralization of apatite and remineralization of dental enamel on fluoride concentration. Journal of Dental Research 1990;69 Spec No:620‐5; discussion 634‐6. [PUBMED: 2312892] [DOI] [PubMed] [Google Scholar]
Featherstone 1999
- Featherstone JD. Prevention and reversal of dental caries: role of low level fluoride. Community Dentistry and Oral Epidemiology 1999;27(1):31‐40. [PUBMED: 10086924] [DOI] [PubMed] [Google Scholar]
Featherstone 2000
- Featherstone JD. The science and practice of caries prevention. Journal of the American Dental Association 2000;131(7):887‐99. [PUBMED: 10916327] [DOI] [PubMed] [Google Scholar]
Featherstone 2004
- Featherstone JD. The continuum of dental caries ‐ evidence for a dynamic disease process. Journal of Dental Research 2004;83 Spec No C:C39‐42. [DOI] [PubMed] [Google Scholar]
Fejerskov 2004
- Fejerskov O. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Research 2004;38(3):182‐91. [PUBMED: 15153687] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 11 May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gupta 1993
- Gupta S, Seth AK, Gupta A, Gavane AG. Transplacental passage of fluorides. Journal of Pediatrics 1993;123(1):139‐41. [DOI] [PubMed] [Google Scholar]
Helfenstein 1991
- Helfenstein U, Steiner M, Marthaler TM. Caries prediction on the basis of past caries including precavity lesions. Caries Research 1991;25(5):372‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hiller 1975
- Hiller CR, Robinson C, Weatherell JA. Variations in the composition of developing rat incisor enamel. Calcified Tissue Research 1975;18(1):1‐12. [PUBMED: 1148889] [DOI] [PubMed] [Google Scholar]
Hobdell 2003
- Hobdell MH, Oliveira ER, Bautista R, Myburgh NG, Lalloo R, Narendran S, et al. Oral diseases and socio‐economic status (SES). British Dental Journal 2003;194(2):91‐6; discussion 88. [DOI] [PubMed] [Google Scholar]
Iheozor‐Ejiofor 2015
- Iheozor‐Ejiofor Z, Worthington HV, Walsh T, O'Malley L, Clarkson JE, Macey R, et al. Water fluoridation for the prevention of dental caries. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD010856.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismail 1997
- Ismail AI, Tanzer JM, Dingle JL. Current trends of sugar consumption in developing societies. Community Dentistry and Oral Epidemiology 1997;25(6):438‐43. [DOI] [PubMed] [Google Scholar]
Ismail 2008
- Ismail AI, Hasson H. Fluoride supplements, dental caries and fluorosis: a systematic review. Journal of the American Dental Association 2008;139(11):1457‐68. [DOI] [PubMed] [Google Scholar]
Jankauskiene 2010
- Jankauskiene B, Narbutaite J. Changes in oral health‐related quality of life among children following dental treatment under general anaesthesia. A systematic review. Stomatologija 2010;12(2):60‐4. [PubMed] [Google Scholar]
Jones 2017
- Jones CM, Davies GM, Monaghan N, Morgan MZ, Neville JS, Pitts NB. The caries experience of 5 year‐old children in Scotland in 2013‐2014, and in England and Wales in 2014‐2015. Reports of cross‐sectional dental surveys using BASCD criteria. Community Dental Health 2017;34(3):157‐62. [PUBMED: 28872810] [DOI] [PubMed] [Google Scholar]
Kailis 1968
- Kailis DG, Taylor SR, Davis GB, Bartlett LG, Fitzgerald DJ, Grose IJ, et al. Fluoride and caries: observations on the effects of prenatal and postnatal fluoride on some Perth pre‐school children. Medical Journal of Australia 1968;2(23):1037‐1040. [DOI] [PubMed] [Google Scholar]
Kolker 2007
- Kolker JL, Yuan Y, Burt BA, Sandretto AM, Sohn W, Lang SW, et al. Dental caries and dietary patterns in low‐income African American children. Pediatric Dentistry 2007;29(6):457‐64. [PubMed] [Google Scholar]
Kraus 1965
- Kraus BS, Jordan RE. The Human Dentition Before Birth. 1st Edition. Philadelphia: Lea & Febiger, 1965. [Google Scholar]
Levy 1999
- Levy SM, Guha‐Chowdhury N. Total fluoride intake and implications for dietary fluoride supplementation. Journal of Public Health Dentistry 1999;59(4):211‐23. [PUBMED: 10682326] [DOI] [PubMed] [Google Scholar]
Li 2002
- Li Y, Wang W. Predicting caries in permanent teeth from caries in primary teeth: an eight‐year cohort study. Journal of Dental Research 2002;81(8):561‐6. [DOI] [PubMed] [Google Scholar]
Limeback 1999
- Limeback H. A re‐examination of the pre‐eruptive and post‐eruptive mechanism of the anti‐caries effects of fluoride: is there any anti‐caries benefit from swallowing fluoride?. Community Dentistry and Oral Epidemiology 1999;27(1):62‐71. [DOI] [PubMed] [Google Scholar]
Marinho 2003a
- Marinho VCC, Higgins J, Logan S, Sheiham A (deceased). Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2003b
- Marinho VCC, Higgins JPT, Logan S, Sheiham A (deceased). Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD002782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2013
- Marinho VCC, Worthington HV, Walsh T, Clarkson JE. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD002279.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2015
- Marinho VCC, Worthington HV, Walsh T, Chong LY. Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD002280.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2016
- Marinho VCC, Chong LY, Worthington HV, Walsh T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD002284.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersen 2005
- Petersen PE, Bourgeois D, Ogawa H, Estupinan‐Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bulletin of the World Health Organization 2005;83(9):661‐9. [PMC free article] [PubMed] [Google Scholar]
Prichard 1969
- Prichard JL. The pre‐natal and post‐natal effects of fluoride supplements on West Australian schoolchildren, aged 6, 7, and 8, Perth, 1967. Australian Dental Journal 1969;14(5):335‐8. [PUBMED: 5264547] [DOI] [PubMed] [Google Scholar]
Public Health England 2012
- Public Health England. National Dental Epidemiology Programme for England: oral health survey of five‐year‐old children 2012. A report on the prevalence and severity of dental decay. www.nwph.net/dentalhealth/Oral%20Health%205yr%20old%20children%202012%20final%20report%20gateway%20approved.pdf (accessed 11 May 2017).
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rozier 2010
- Rozier RG, Adair S, Graham F, Iafolla T, Kingman A, Kohn W, et al. Evidence‐based clinical recommendations on the prescription of dietary fluoride supplements for caries prevention: a report of the American Dental Association Council on Scientific Affairs. Journal of the American Dental Association 2010;141(12):1480‐9. [DOI] [PubMed] [Google Scholar]
Sastry 2011
- Sastry GM, Mohanty S, Bhongir AV, Mishra AK, Rao P. Association of higher maternal serum fluoride with adverse fetal outcomes. International Journal of Medicine and Public Health 2011;1(2):13‐7. [Google Scholar]
Schünemann 2009
- Schünemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Selwitz 2007
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369(9555):51‐9. [DOI] [PubMed] [Google Scholar]
Seppa 1989
- Seppa L, Hausen H, Pöllänen L, Helasharju K, Kärkkäinen S. Past caries recordings made in Public Dental Clinics as predictors of caries prevalence in early adolescence. Community Dentistry and Oral Epidemiology 1989;17(6):277‐81. [DOI] [PubMed] [Google Scholar]
Stephen 1993
- Stephen KW. Systemic fluorides: drops and tablets. Caries Research 1993;27 Suppl 1:9‐15. [DOI] [PubMed] [Google Scholar]
Tanimoto 2008
- Tanimoto K, Le T, Zhu L, Chen J, Featherstone JD, Li W, et al. Effects of fluoride on the interactions between amelogenin and apatite crystals. Journal of Dental Research 2008;87(1):39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Termine 1980
- Termine JD, Belcourt AB, Miyamoto MS, Conn KM. Properties of dissociatively extracted fetal tooth matrix proteins. II. Separation and purification of fetal bovine dentin phosphoprotein. Journal of Biological Chemistry 1980;255(20):9769‐72. [PUBMED: 7430100] [PubMed] [Google Scholar]
Toyama 2001
- Toyama Y, Nakagaki H, Kato S, Huang S, Mizutani Y, Kojima S, et al. Fluoride concentrations at and near the neonatal line in human deciduous tooth enamel obtained from a naturally fluoridated and a non‐fluoridated area. Archives of Oral Biology 2001;46(2):147‐53. [DOI] [PubMed] [Google Scholar]
Tubert‐Jeannin 2011
- Tubert‐Jeannin S, Auclair C, Amsallem E, Tramini P, Gerbaud L, Ruffieux C, et al. Fluoride supplements (tablets, drops, lozenges or chewing gums) for preventing dental caries in children. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD007592.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization. Milk fluoridation for the prevention of dental caries. www.who.int/oral_health/publications/milk_fluoridation_2009_en.pdf?ua=1 (accessed 11 May 2017).
Wong 2010
- Wong MCM, Glenny AM, Tsang BWK, Lo ECM, Worthington HV, Marinho VCC. Topical fluoride as a cause of dental fluorosis in children. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007693.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


