Abstract
The technique of microneurography has advanced the field of neuroscience for the past 50 years. While there have been a number of reviews on microneurography, this paper takes an objective approach to exploring the impact of microneurography studies. Briefly, Web of Science (Thomson Reuters) was used to identify the highest citation articles over the past 50 years, and key findings are presented in a decade-by-decade highlight. This includes the establishment of microneurography in the 1960s, the acceleration of the technique by Gunnar Wallin in the 1970s, the international collaborations of the 1980s and 1990s, and finally the highest impact studies from 2000 to present. This journey through 50 years of microneurographic research related to peripheral sympathetic nerve activity includes a historical context for several of the laboratory interventions commonly used today (e.g., cold pressor test, mental stress, lower body negative pressure, isometric handgrip, etc.) and how these interventions and experimental approaches have advanced our knowledge of cardiovascular, cardiometabolic, and other human diseases and conditions.
Keywords: autonomic nervous system, cardiovascular disease, microneurographic, muscle sympathetic nerve activity, orthostatic intolerance
INTRODUCTION
The Journal of Neurophysiology (JN) recently celebrated 80 years of publishing ground-breaking studies related to the nervous system (Yates 2018). As outlined by Yates (2018), in 1938 JN became only the second journal devoted to the study of the nervous system in human and animal models, and over the past eight decades the journal has featured 122 publications from 15 recipients of the Nobel Prize of Physiology or Medicine.
Given the journal’s preeminent stature within the field of neuroscience, it was fitting that the Editorial Board approved a special call for papers entitled “50 Years of Microneurography: Insights into Neural Mechanisms in Humans.” In recent years, there have been a number of outstanding reviews that have focused on microneurography from historical (Vallbo et al. 2004), methodological (Charkoudian and Wallin 2014; Hart et al. 2017; Macefield 2013), and disease perspectives (Charkoudian and Wallin 2014; Macefield 2013), including two reviews in the current issue thus far (Macefield and Wallin 2018; Shoemaker et al. 2018). Moreover, a multidisciplinary team of scientists were recently commissioned to develop a Guidelines Paper on sympathetic neural recordings in conscious humans and other mammals that prominently featured the microneurographic technique and related analyses (Hart et al. 2017).
I have attempted to take the present review in a different direction by providing a decade-by-decade highlight on key accomplishments within the field of microneurography. To add a level of objectivity to the process, Web of Science (Thomson Reuters) was used to identify high citation articles within each decade. However, number of citations is not the only marker of impact; thus some subjectivity was necessary to include studies with a lower citation index when appropriate (particularly with more recent publications that represent emerging areas). As such, the studies highlighted within each decade are not meant to be all inclusive; instead, they are intended to represent a limited, yet appropriate, sampling of 50 years of progressive advancement within the field of microneurography as it relates to peripheral sympathetic nerve activity (and where the field may be headed over the next 50 years).
1960s: WHERE IT ALL BEGAN
The decade of the 1960s belonged to Karl-Erik Hagbarth and Ake Vallbo, who together established the technique of microneurography to record peripheral sympathetic neural recordings in humans (Vallbo and Hagbarth 1967). They systematically utilized microneurography to document muscle afferent (Hagbarth and Vallbo 1968a), muscle efferent (Hagbarth and Vallbo 1968b), skin afferent (Vallbo and Hagbarth 1968), and skin efferent (Delius et al. 1972c) fibers.
To appreciate how far the technique has advanced, one has to appreciate the risk these two investigators took back at a time when Institutional Review Boards did not exist as they do today. Hagbarth was the first to attempt the microneurographic procedure when he inserted a needle into his own ulnar nerve (Vallbo et al. 2004). At the time, there was considerable risk, as it was debated if it would cause nerve damage through mechanical trauma and/or intraneural bleeding. The two investigators continued to “self-experiment” and troubleshoot the technique with various electrode metals and designs and worked to improve the amplification process to visibly observe the neural recordings on an oscilloscope screen. Interestingly, the original aim of microneurography was to mechanistically study the fusimotor system, and initial recordings of sympathetic neural activity were actually thought to be recording artifact. There was also hope that microneurography would have direct clinical application, but it instead has primarily served as a valuable research methodology. Readers are referred to Vallbo et al. (2004) for detailed historical review of the early evolution of microneurography and the varied uses of microneurography (i.e., proprioception, sensorimotor control, pain, etc.). The remainder of this review will focus on the use of microneurography to examine peripheral sympathetic nerve activity.
1970s: THE DECADE OF GUNNAR WALLIN
As revered as Hagbarth and Vallbo are for their seminal contributions to microneurography in the late 1960s, it was their colleague Gunnar Wallin who led the 1970s with a number of impactful publications related to muscle and skin efferent activity. He also established a number of impactful external collaborations that challenged, deepened, and broadened the science. In many ways, Wallin was into “team science” before it was popular. While detailed more in later sections, he contributed to the expansion of microneurography in the United States and Australia through his impactful and direct collaborations with Dwain Eckberg in the 1970s, Allyn Mark in the 1980s, Murray Esler and Vaughan Macefield in the 1990s, and Michael Joyner and Nisha Charkoudian in the 2000s.
Table 1 depicts the most highly cited articles by “BG Wallin” in the 1970s. They also represent the top cited articles of the 1970s using alternative search terms of “microneurography,” “muscle sympathetic nerve activity,” and “skin sympathetic nerve activity.” Thus from a microneurographic perspective as it relates to peripheral sympathetic nerve activity, it is safe to term the 1970s the decade of Wallin.
Table 1.
Highly cited microneurographic studies by Wallin and colleagues from the 1970s
| Study | Citations | Key Finding(s) |
|---|---|---|
| Sundlöf and Wallin (1978b) | 472 | Marked interindividual differences in MSNA burst incidence that were not linked to resting BP; MSNA linked to spontaneous changes in diastolic blood pressure. |
| Sundlöf and Wallin (1977) | 412 | High intraindividual reliability of MSNA across laboratory sessions; simultaneous nerve recordings similar across two limbs. |
| Hagbarth et al. (1972) | 362 | Sympathetic ganglionic blocking agents and Lidocaine abolished SSNA; lack of pulse synchrony in SSNA recordings. |
| Delius et al. (1972c) | 360 | Mental stress, body cooling, and respiratory movements increased SSNA; maneuvers to elicit baroreceptor did not alter SSNA. |
| Delius et al. (1972b) | 347 | Maneuvers that caused forearm or calf vasoconstriction were associated with increases in MSNA; inverse changes of BP and MSNA were observed during Valsalva’s maneuver and mental stress. |
| Delius et al. (1972a) | 331 | Pulse rhythmicity of MSNA that was associated with transient reductions in BP; sympathetic ganglionic blockade abolished MSNA. |
| Sundlöf and Wallin (1978a) | 208 | Robust increase of MSNA with lower body negative pressure. |
| Burke et al. (1977) | 192 | Changes in posture modify MSNA; first recorded observation of quiescent MSNA during syncopal episode. |
| Wallin and Sundlöf (1979) | 165 | Taking age into consideration, there was no significant difference of resting MSNA in normotensive and hypertensive subjects. |
| Wallin et al. (1975) | 144 | Carotid sinus nerve stimulation inhibited MSNA but not SSNA. |
| Wallin et al. (1973) | 131 | No differences in MSNA or SSNA in normotensive and hypertensive subjects; wide variability of MSNA responsiveness to mental stress. |
SSNA, skin sympathetic nerve activity; MSNA, muscle sympathetic nerve activity; BP, blood pressure.
While each of the studies in Table 1 have clearly impacted the field, there are some particularly noteworthy findings. Sundlöf and Wallin (1978b) is a highly cited manuscript for three key contributions. First, it reemphasized the marked interindividual differences of muscle sympathetic nerve activity (MSNA) burst frequency/incidence and highlighted that MSNA levels were not linked to resting levels of blood pressure. This had been noted in prior studies, but with reduced number of subjects and different study objectives (Delius et al. 1972a, 1972b). Second, there was a tendency for increased MSNA with increased age that has since been confirmed and emphasized in subsequent studies (Narkiewicz et al. 2005; Ng et al. 1993) and is now a well-recognized characteristic of MSNA. Third, and perhaps what has been driving citations in more recent years, was the reported inverse relationship between spontaneous fluctuations of MSNA and spontaneous fluctuations in diastolic arterial blood pressure (Sundlöf and Wallin 1978b). This finding served as the foundation for the development of various baroreflex analyses, including the spontaneous sympathetic baroreflex sensitivity analyses that several laboratories frequently employ (Holwerda et al. 2015; Kienbaum et al. 2001; Yang and Carter 2013).
One of the hallmarks of MSNA is the incredibly high intraindividual reliability/reproducibility of the measurement across laboratory sessions. Sundlöf and Wallin (1977) demonstrated this when they reported consistent baseline MSNA levels across two to three laboratory visits that spanned a minimum of 3 wk and a maximum of 21 mo. The robust reliability of MSNA across recording sessions within a single day, as well as across laboratory visits spanning days to years, has since been reported in numerous studies (Fagius and Wallin 1993; Fonkoue and Carter 2015; Grassi et al. 1997). Additionally, Sundlöf and Wallin (1977) is also highly cited because it was the first to systematically compare simultaneous recordings of MSNA in the right and left peroneal nerve and documented remarkable consistency in MSNA burst incidence.
Wallin et al. (1973) was the first to report the wide variability of MSNA responsiveness to mental stress, demonstrating that some subjects responded with a reduction of MSNA and others responded with an increase of MSNA. This wide variability of MSNA reactivity to mental stress was confirmed in a larger, retrospective study that classified subjects into negative responders, nonresponders, and positive responders and documented that the MSNA responsiveness was not associated with blood pressure responsiveness (Carter and Ray 2009). It has been suggested that differences in MSNA reactivity to stress may be related to interindividual differences in response to arousal (Donadio et al. 2012). More recently, the MSNA reactivity to mental stress was reported to be highly reproducible within a single laboratory visit and across laboratory visits separated by one month (Fonkoue and Carter 2015), and that family history of hypertension is associated with higher MSNA reactivity to mental stress (Fonkoue et al. 2016). The extent of the MSNA variability to mental stress is unique among the various laboratory stressors used in microneurographic studies, and the potential clinical implications of this wide variability have been highlighted in a recent review by Carter and Goldstein (2015).
In addition to mental stress, Wallin and colleagues performed the seminal microneurographic studies on body cooling (Delius et al. 1972c), changes in respiration (Delius et al. 1972c), lower body negative pressure (Sundlöf and Wallin 1978a), Valsalva’s maneuver (Delius et al. 1972b), and carotid sinus nerve stimulation (Wallin et al. 1975) throughout the 1970s. Moreover, the impact of these laboratory stressors were examined for both MSNA and skin sympathetic nerve activity (SSNA). Table 1 includes some of the key findings in these studies, with the overarching concept that maneuvers that influence baroreceptor function (i.e., lower body negative pressure, Valsalva strain, carotid sinus nerve stimulation) influence MSNA but not SSNA. Maneuvers that impacted emotion (i.e., mental stress) or thermal (i.e., body cooling) influenced SSNA (Delius et al. 1972c), but as previously outlined, some of these maneuvers also alter MSNA (Wallin et al. 1973). Finally, these studies also characterized/emphasized the pulse rhythmicity of MSNA (Delius et al. 1972a), as well as the lack of pulse synchrony with SSNA (Delius et al. 1972c).
1980s AND 1990s: THE GOLDEN YEARS OF COLLABORATION
Several impactful collaborations were formed between Wallin and key scientists and physicians throughout the United States and Australia in the 1980s and 1990s. Dwain Eckberg visited Wallin for microneurographic training and collaboration in 1979 and was the first to bring the approach to the United States through his laboratory at Virginia Commonwealth University. Shortly thereafter, Allyn Mark was trained by Wallin and established a microneurographic laboratory at the University of Iowa that led to some of the most highly cited microneurographic studies (see Table 2). My own microneurography “lineage” extends through both the Eckberg and Mark laboratories, being trained as a Ph.D. student with William Cooke (an Eckberg postdoctoral fellow) and receiving additional pre- and postdoctoral microneurographic training from Chester Ray (a Mark postdoctoral fellow). Indeed, most microneurographers within the United States can somehow trace their trainings back to the Eckberg or Mark laboratories. The trainings I experienced that trace to these two laboratories has been advantageous as both laboratories emphasized different aspects of the technique (i.e., internal stimulation vs. auditory confirmation, popliteal vs. fibular sites, etc.).
Table 2.
Highly cited microneurographic studies in the 1980s and 1990s
| Study | Citations | Key Finding(s) |
|---|---|---|
| Somers et al. (1995) | 1,383 | Elevated MSNA and nocturnal BP in patients with obstructive sleep apnea; continuous positive airway pressure decrease MSNA and BP during sleep. |
| Anderson et al. (1991a) | 928 | Infusion of low and high dose of insulin increased MSNA but led to vasodilation of the forearm. |
| Somers et al. (1993) | 840 | Non-REM sleep reduced MSNA from wakefulness, while REM sleep increased MSNA. |
| Converse et al. (1992) | 768 | Elevated MSNA in patients with chronic renal failure. |
| Leimbach et al. (1986) | 674 | Increased MSNA in patients with heart failure. |
| Mark et al. (1985) | 631 | Static handgrip exercise and postexercise muscle ischemia increased MSNA; central command influences MSNA. |
| Pagani et al. (1997) | 605 | Increased level of MSNA were associated with a shift of spectral power toward low-frequency component. |
| Carlson et al. (1993) | 467 | Elevated MSNA, as well as plasma norepinephrine, in subjects with obstructive sleep apnea. |
| Grassi et al. (1995a) | 412 | Elevated MSNA in obese normotensive subjects. |
| Victor et al. (1987a) | 374 | Cold pressor test increased MSNA in graded fashion. |
| Somers et al. (1989) | 371 | Hyperoxic hypercapnia elicits greater increase of MSNA compared with isocapnic hypoxia; combined hypoxia and hypercapnia had additive effect on MSNA. |
| Schobel et al. (1996) | 358 | Preeclampsia was associated with a threefold increase of MSNA compared with normotensive pregnancy. |
| Grassi et al. (1998a) | 352 | Elevated MSNA in subjects with essential hypertension. |
| Narkiewicz et al. (1998) | 338 | Increased MSNA in patients with moderate-to-severe obstructive sleep apnea. |
| Narkiewicz et al. (1999) | 334 | MSNA increase during hypoxia was similar in patients with obstructive sleep apnea and controls. |
| Bini et al. (1980) | 327 | Selective activation of sudomotor or vasoconstrictor SSNA through exposure to cold and warm environments. |
| Grassi et al. (1995b) | 325 | Increased MSNA in patients with mild and severe congestive heart failure; reduced sympathetic and cardiovagal baroreflex with congestive heart failure. |
| Wallin et al. (1981) | 321 | Positive correlation between MSNA and plasma norepinephrine in healthy subjects. |
| Ng et al. (1993) | 313 | Sex differences in resting MSNA, with lower levels in women compared with men. |
| Fagius and Wallin (1980) | 306 | Reported mean MSNA latencies in median and peroneal nerve, including some from simultaneous limb recordings. |
| Cooke et al. (1999) | 300 | Head-up tilt altered baroreflex control in a manner that reflected leftward movement of the subjects on the blood pressure and sympathetic/vagal response curves; head-up tilt modified respiratory gating of sympathetic and vagal responses using different strategies |
| Berne et al. (1992) | 289 | Hyperinsulinemia increased MSNA, but not SSNA. |
| Wallin and Sundlöf (1982) | 283 | Onset of syncope was marked by bradycardia and quiescence of MSNA. |
| Vollenweider et al. (1993) | 275 | Hyperinsulinemia euglycemic clamp increased MSNA; fructose infusion increased carbohydrate oxidation but had minor effect on insulinemia and did not alter MSNA. |
| Grassi et al. (1994) | 255 | Cigarette smoking decreased MSNA. |
| Ebert et al. (1992) | 255 | Propofol, a common anesthetic, reduced MSNA and impaired sympathetic and cardiovagal baroreflex sensitivity. |
| Somers et al. (1991) | 244 | Baroreflex activation selectively abolished MSNA reactivity to hypoxia but not to hypercapnia or the cold pressor test. |
| Grassi et al. (1998b) | 244 | Weight loss by hypocaloric diet reduced MSNA and plasma norepinephrine in obese normotensive subjects. |
| Spraul et al. (1993) | 229 | Pima Indians had lower MSNA than Caucasians, and MSNA was significantly related to body fat in Caucasians but not Pimas. |
| Wallin et al. (1992) | 230 | Significant positive correlation between MSNA and cardiac norepinephrine spillover. |
| Victor et al. (1987b) | 226 | Nonischemic rhythmic handgrip exercise did not alter MSNA, while ischemic handgrip and moderate arm cycling increased MSNA. |
| Halliwill et al. (1996) | 229 | Baroreflex control of MSNA and sympathetic vascular transduction were altered after dynamic exercise, which likely contributed to postexercise hypotension. |
| Eckberg et al. (1985) | 204 | Respiration altered MSNA and vagal cardiac control; neck pressure was used to modify carotid baroreceptor afferent traffic. |
| Hornyak et al. (1991) | 198 | Light and deep sleep led to reductions of MSNA, while high-amplitude K complexes and REM sleep increased MSNA. |
| Mosqueda-Garcia et al. (1997) | 187 | Patients with neurally medicated syncope had blunted increases of MSNA during tilt, followed by a decrease and disappearance of MSNA during syncope. |
| Dinenno et al. (1999) | 191 | Limb blood flow and vascular conductance were reduced in older adults, and these changes were associated with increased MSNA. |
| Eckberg et al. (1988) | 175 | Stepwise intravenous infusion of phenylephrine and nitroprusside altered MSNA. |
| Macefield et al. (1994) | 176 | Establishment of single-unit recordings of MSNA using modified tungsten microelectrode and analyses. |
| Anderson et al. (1987) | 171 | A dissociation between forearm and leg MSNA during mental stress. |
| Anderson et al. (1992) | 163 | Insulin increased MSNA, but not blood pressure, in borderline hypertensive adults. |
| Carlson et al. (1996) | 156 | Impaired sympathetic baroreflex in subjects with obstructive sleep apnea. |
| Biaggioni et al. (1991) | 151 | Adenosine increased MSNA in a dose-dependent manner, and the increase was greater than nitroprusside. |
SSNA, skin sympathetic nerve activity; MSNA, muscle sympathetic nerve activity; REM, rapid eye movement; BP, blood pressure.
The Allyn Mark Laboratory at the University of Iowa
As detailed in Table 2, it is clear that the collaboration between Mark and Wallin has been remarkably productive. The establishment of the University of Iowa microneurography laboratory by Mark has resulted, to this point, in four out of the top five cited publications (Anderson et al. 1991a; Converse et al. 1992; Leimbach et al. 1986; Somers et al. 1993, 1995). The top cited publication by Somers et al. (1995) documented elevated MSNA and blood pressure in patients with obstructive sleep apnea and a marked reduction of nocturnal MSNA and blood pressure with continuous positive airway pressure. In fact, this 1995 article has had such an impact that the Editors of the Journal of Clinical Investigation invited senior author Francois (Frank) Abboud to write a Hindsight article on the topic for the journal in 2014 (Abboud and Kumar 2014). Interestingly, Carlson et al. (1993), who was not affiliated with the Iowa laboratory, was actually the first to publish that obstructive sleep apnea was associated with elevated MSNA, but the study did not include a continuous positive airway pressure intervention; however, it is nevertheless still one of the top 10 cited microneurography studies (see Table 2).
In the third most cited microneurographic study, Somers et al. (1993) reported a marked increase of MSNA during rapid eye movement sleep and a decrease of MSNA during nonrapid eye movement sleep. An earlier study reported similar findings during sleep (Hornyak et al. 1991) but has not garnered the same number of citations as the New England Journal of Medicine citation by Somers et al. (1993). Nevertheless, these combined studies have been incredibly impactful within the field of sleep medicine, and the first author, Virend Somers, is the world’s leading expert on sleep and sympathetic activity. In fact, the early work of Somers and colleagues has shaped the direction of recent and ongoing work related to sleep deprivation in our laboratory. We have reported sex differences in MSNA responses to 24-h total sleep deprivation in which women were more sympathoexcitatory compared with men (Carter et al. 2012). More recently, we published the first study examining MSNA in patients with chronic insomnia and reported blunted sympathetic baroreflex function and heightened MSNA reactivity to the cold pressor test in subjects with insomnia compared with good-sleeper controls (Carter et al. 2018). When combined with the obstructive sleep apnea studies by Somers and others, there is growing evidence to suggest that sympathetic neural activity (and reactivity) is an important contributor to certain sleep disorders and insufficiencies.
While the sleep studies of Somers have garnered significant attention (Somers et al. 1993, 1995), another protégé of the Mark laboratory also fared quite well on citations. Earling Anderson first authored a number of impactful studies in the late 1980s and early 1990s with Mark. Anderson et al. (1991a) was the first to report that infusion of low- and high-dose insulin increased MSNA and forearm vascular conductance. These findings by Anderson et al. (1991a) have since been supported by other laboratories (Vollenweider et al. 1993; Young et al. 2010), and it is the second most cited microneurography article to date. Anderson et al. (1989) reported elevated MSNA in borderline hypertensive subjects, which remains a top 10 microneurographic citation. Lastly, Anderson et al. (1987) reported a dissociation of MSNA in the arm and leg during mental stress, which served as the foundational study for my own highest cited study to date in which we performed simultaneous recordings of MSNA and blood flow in both the forearm and leg (Carter et al. 2005). At a modest 75 citations (compared with many highlighted in Tables 1–3), we demonstrated a consistent vasodilation of the forearm and calf during mental stress that was not associated with any change in MSNA in either limb (Carter et al. 2005).
Table 3.
Highly cited microneurographic studies from 2000 to present
| Study | Citations | Key Finding(s) |
|---|---|---|
| Alvarez et al. (2002) | 338 | MSNA was more closely associated with abdominal visceral fat when compared with subcutaneous fat. |
| Schlaich et al. (2004) | 324 | Elevated MSNA and reduced neuronal norepinephrine uptake in subjects with hypertension. |
| Minson et al. (2000) | 304 | Elevated baseline MSNA and sympathetic baroreflex sensitivity during the midluteal phase compared with the early follicular phase in young women. |
| Schlaich et al. (2003) | 263 | Hypertension with left ventricular hypertrophy was associated with elevated MSNA and cardiac norepinephrine spillover. |
| Heusser et al. (2010) | 238 | Bilateral electric baroreflex simulator at the carotid sinus (Rheos) led to reduced BP that was significantly correlated to reductions of MSNA. |
| Roveda et al. (2003) | 205 | Exercise training reduced MSNA in patients with advance heart failure. |
| Hering et al. (2013) | 200 | Renal denervation decreased multiunit and single-unit MSNA. |
| Grassi et al. (2004) | 199 | Central obesity was associated with higher levels of MSNA when compared to peripheral obesity and lean subjects. |
| Furlan et al. (2000) | 198 | The coupling between MSNA and HRV (as well as blood pressure variability) was enhanced during head-up tilt when compared with rest. |
| Grassi et al. (2005) | 193 | Elevated MSNA in subjects with metabolic syndrome. |
| Grassi et al. (2000) | 191 | MSNA was highest in obese, hypertensive subjects when compared with other obese normotensive and lean hypertensive subjects. |
| Kienbaum et al. (2001) | 152 | Use of natural BP fluctuations and MSNA to establish spontaneous baroreflex sensitivity analysis, including reproducibility analysis. |
| Charkoudian et al. (2005) | 145 | MSNA was negatively correlated with cardiac output, but not BP; established a baroreflex threshold analysis. |
| Hart et al. (2009) | 143 | Sex differences in the relationship between total peripheral resistance, cardiac output, and MSNA. |
| Shoemaker et al. (2001) | 133 | Blunted total MSNA response to head-up tilt and cold pressor test in women compared with men. |
| Levine et al. (2002) | 128 | Muscle sympathetic nerve activity was higher post-spaceflight in supine and tilted positions. |
| Kimmerly et al. (2005) | 118 | Cortical network contributed to the modulation of sympathetic baroreflex. |
| Fu et al. (2005) | 108 | Men and women demonstrated comparable MSNA responses to orthostatic stress under normovolemic and hypovolemic conditions. |
| Dinenno et al. (2000) | 105 | Aging was associated with femoral artery hypertrophy, and this was strongly correlated to increases in MSNA. |
SSNA, skin sympathetic nerve activity; MSNA, muscle sympathetic nerve activity; HRV, heart rate variability; BP, blood pressure.
Three additional high citation articles from the Mark laboratory warrant discussion. First, Mark et al. (1985) documented an increase of MSNA during static handgrip exercise and postexercise muscle ischemia, and demonstrated the important role of the muscle metaboreflex on MSNA. This work was published in collaboration with Doug Seals, who established microneurography at the University of Colorado–Boulder and has subsequently trained a number of active investigators (including a collaboration that helped establish microneurography at Mayo Clinic; detailed in The Dwain Eckberg Laboratory at Virginia Commonwealth University). Second, Leimbach et al. (1986) reported elevated MSNA in patients with heart failure. Third, Mark collaborated with Ronald Victor to demonstrate a graded increase of MSNA during the cold pressor test (Victor et al. 1987a). All of these seminal findings (Leimbach et al. 1986; Mark et al. 1985; Victor et al. 1987a) have been replicated by numerous laboratories since and continue to be key maneuvers/populations studied today.
Finally, Mark established a collaboration with Larry Sinoway in the early 1990s (Sinoway et al. 1992). This is relevant to the present review because Kevin Shoemaker began his microneurography career in the Sinoway laboratory, and Shoemaker has had several important contributions to the field of microneurography discussed in 2000 to present: continued expansion and impact of microneurography (and cited in Table 3).
The Dwain Eckberg Laboratory at Virginia Commonwealth University
The Eckberg and Wallin collaboration in the late 1970s and early 1980s focused heavily on baroreflex control and the importance of respiration on MSNA. Eckberg has published a number of reviews on these topics, highlighted by a highly cited paper in Circulation entitled “Sympathovagal balance: a critical appraisal” (Eckberg 1997). This review (Eckberg 1997) was in response to one of the high citation microneurography publications in Table 2 that examined the relations between heart rate variability (HRV) and MSNA (Pagani et al. 1997). In Eckberg’s review, he articulates the methods, mathematics, physiological principles, and dangers of using sympathovagal balance from HRV as an unchecked marker of sympathetic activity. The review by Eckberg (1997) does not attempt to devalue the use of HRV in stratifying risk in patients with cardiovascular disease or to better understand autonomic mechanisms; instead, it suggests that manipulation of HRV through spectral analysis should be done with caution and should not be used to quantify a weighted balance between the sympathetic and vagal neural outflows to the heart (or elsewhere). To this point, studies have shown a lack of reproducibility between HRV measurements and particularly the low frequency (or so-called “sympathetic”) domain of HRV (Goldstein et al. 2011; Lord et al. 2001).
Two highly cited Eckberg papers that have had a broader influence on the field are Saul et al. (1990) and Cooke et al. (1999). Saul et al. (1990) employed an experimental approach that examined HRV, MSNA, and plasma norepinephrine during graded stepwise infusions of nitroprusside and phenylephrine, which reduced and increased diastolic blood pressure by ~15 mmHg. The primary finding was that both parasympathetic and sympathetic activity contribute importantly to low frequency HRV (Saul et al. 1990).
Cooke et al. (1999) is the second highest cited microneurographic publication from the Eckberg laboratory. In this study, subjects performed controlled breathing (12 breaths/min) during a graded head-up tilt. The authors reported changes in baroreflex control during upright tilt in a manner that reflected leftward movement of the subjects on the blood pressure and sympathetic/vagal response curves. Moreover, head-up tilt modified respiratory gating of sympathetic and vagal responses via different strategies (Cooke et al. 1999).
In addition to Saul et al. (1990) and Cooke et al. (1999), three additional trainees from the Eckberg laboratory have been impactful to the field. First, Michael Smith was trained by Eckberg in the late 1980s and early 1990s. Smith was responsible for bringing microneurography to the laboratory of Peter Raven and subsequently the microneurographic training of Paul Fadel and others who are currently impacting the field. Second, J. Andrew Taylor was a postdoctoral fellow after initially learning the technique with Seals. Third, John Halliwill trained with Eckberg in the early-to-mid 1990s and contributed to the acceleration of microneurography within the laboratory of Michael Joyner at Mayo Clinic, who developed the technique in collaboration with Doug Seals in the early 1990s. This acceleration was also aided by Frank Dinenno, who initially trained in microneurography with Doug Seals. As such, the Joyner laboratory includes microneurgraphic lineages that have also been dually impacted by both the Eckberg and Mark laboratories. Additionally, the Joyner laboratory established a direct collaboration with Wallin in the 2000s that led to two high citation papers in Table 3 that demonstrate the relationships among cardiac output, MSNA, and peripheral blood flow are influenced by both sex and age (Charkoudian et al. 2005; Hart et al. 2009). Subsequent work has documented that α-adrenergic vasoconstriction is offset by β-adrenergic vasodilation in young women (Hart et al. 2011), a finding that may help explain why women are “cardioprotected” early in life before an aggressive increase of cardiovascular risk during postmenopausal years. Joyner has trained a number of microneurographers that have established successful laboratories and independent lines of research; this includes, but is not limited to, several investigators highlighted in Tables 2 and 3 (i.e., Christopher Minson, Nisha Charkoudian, and Emma Hart).
The Murray Esler and Vaughan Macefield Laboratories in Australia
There were two significant collaborations between Wallin and Australian investigators that have been impactful to the field of microneurography. The first collaboration to highlight is the work with Murray Esler of the Baker Heart Institute in Melbourne, Australia. Esler is the leading authority on the highly technical and invasive norepinephrine spillover technique. Briefly, this approach uses radiotracer-derived measurements of appearance rate of norepinephrine at various organs, including the heart and kidneys (Esler et al. 1990; Grassi and Esler 1999). This direct measure of cardiac and renal sympathetic activity in humans is important, because unlike animal models (Hart et al. 2017), direct recordings of renal and cardiac nerves in humans is not possible.
Wallin and Esler collaborated on two impactful studies that have been widely cited. Wallin et al. (1992) simultaneously measured MSNA via microneurography and cardiac norepinephrine spillover and reported significant correlation between the two measurements at rest. However, the relationship between MSNA and cardiac norepinephrine was altered during handgrip exercise and mental stress. Wallin et al. (1996) simultaneously performed microneurography and renal norepinephrine spillover measurements, and similar to the cardiac norepinephrine study (Wallin et al. 1992), the authors observed a significant correlation between MSNA and renal norepinephrine spillover during supine rest. Thus, while the sympathetic nervous system is highly regionalized, particularly during “stress,” there was at least a basal relationship between MSNA and norepinephrine spillover at the heart and kidney. While there were some additional collaborative studies between Esler and Wallin, these two studies on MSNA and cardiac/renal norepinephrine spillover were important in providing added confidence in both approaches, which together are considered the “gold-standard” measurements of sympathetic activity in humans.
Similar to Mark and Eckberg in the United States, Esler expanded the microneurographic technique to a number of trainees within Australia. Four individuals associated with the Esler laboratory (Makus Schlaich, Elisabeth Lambert, Gavin Lambert, and Dagmara Hering) have authored/coauthored several highly cited publications listed in Table 3, and some of these works will be detailed in 2000 to present: continued expansion and impact of microneurography, which highlights impactful papers from 2000 to present.
The collaboration between Wallin and Macefield has proved to be equally impactful to the field. Macefield et al. (1994) introduced the methodology for single-unit recordings of MSNA. With this technically advanced approach, it is possible to tease out action potentials from one fiber through visual confirmation of amplitude height compared with other fibers. Macefield is the world’s leading authority on single unit microneurographic recordings, and this JN call for papers includes a review detailing the physiological and pathophysiological importance of this approach (Macefield and Wallin 2018).
It should be noted that while Esler and Macefield were critical to the advancement of microneurographic recordings of peripheral sympathetic nerve activity in Australia, the actual technique of microneurography was introduced in Australia by David Burke. Burke was trained by Hagbarth during a period that crossed over with Wallin, but diverged from Wallin by focusing on the fusimotor system (Burke et al. 1976a, 1976b; Hagbarth et al. 1975). Burke went on to train Simon Gandevia at the Prince Henry Hospital and School of Medicine at the University of New South Wales (Burke et al. 1982; Gandevia et al. 1983), and the two investigators have published a number of impactful studies related to sensorimotor control and proprioception (Burke et al. 1983, 1984, 1993; Fitzpatrick et al. 1994; Gandevia et al. 1983). In fact, Macefield published with Burke and Gandevia (Gandevia et al. 1993; Macefield et al. 1993) before his single unit microneurographic publication with Wallin (Macefield et al. 1994).
Microneurographic Laboratories in Europe, Japan, and Brazil
With the use of objective criteria from the Web of Science search, the standout European contributors to the field are clearly Guido Grassi and Giuseppe Mancia of Italy. There are several highly cited papers by Grassi and/or Mancia in Tables 2 and 3, many of them in collaboration with one another (Grassi et al. 1994, 1995a, 1998b 200, 2004, 2005). Their work primarily focused on populations with pathological conditions such as hypertension and congestive heart failure (Grassi 1998; Grassi et al. 1995b, 2000), but also include highly cited MSNA studies related to the impact of cigarette smoking (Grassi et al. 1994), obesity (Grassi et al. 2004; Grassi et al. 1995a), and metabolic syndrome (Grassi et al. 2005). In addition to Grassi and Mancia, Alberto Malliani and Massimo Pagani are two additional Italian scientists that have had a significant impact on the field (Pagani et al. 1997).
In Japan, Tadaaki Mano, Mitsuru Saito, and Satoshi Iwase collectively developed a microneurography laboratory at Nagoya University in the mid-1980s. The laboratory at Nagoya University has published a number of microneurographic studies over the past 25 years, and while they do not show up within Tables 1–3, it is important to trace some of the studies back to the establishment of this laboratory. Specifically, it was the collaborative work between Mano and Benjamin Levine during the NASA Neurolab mission that led to Qi Fu joining the Institute for Exercise and Environmental Medicine at the Presbyterian Hospital of Dallas. Fu was initially trained in the Nagoya University microneurography laboratory, and together with Levine they have published a number of impactful microneurography studies, including two that are listed in Table 3 (Fu et al. 2005; Levine et al. 2002).
In Brazil, Carlos Negrao of the University of Sao Paulo Medical School established a collaboration with Holly Middlekauff (who learned microneurography in the Mark laboratory at the University of Iowa in the early 1990s). Roveda et al. (2003) is the most highly cited microneurgraphic study from Negrao and Middlekauff, and demonstrated that exercise training reduced MSNA in patients with advanced heart failure. Brazilian microneurographers have also conducted a number of other MSNA exercise training studies as detailed in a recent review (Carter and Ray 2014).
2000 TO PRESENT: CONTINUED EXPANSION AND IMPACT OF MICRONEUROGRAPHY
Table 3 outlines the top cited microneurographic studies since 2000. There remains a healthy international flair to the microneurography field. As previously detailed, the Esler laboratory has been particularly impactful. Schlaich et al. (2004) reported simultaneous elevations of MSNA and neuronal norepinephrine uptake in subjects with hypertension. In a separate publication, Schlaich et al. (2003) demonstrated that hypertension with left ventricular hypertrophy was significantly associated with increased resting MSNA and cardiac norepinephrine spillover. Finally, Hering et al. (2013) demonstrated a reduction of multi- and single-unit MSNA with renal denervation.
Ng et al. (1993) were the first to present data on sex differences in resting MSNA, reporting reduced levels in women compared with men. However, it really was not until the 2000s that the number of microneurographic studies on women really accelerated. Minson et al. (2000) reported elevated baseline MSNA and sympathetic baroreflex sensitivity during the midluteal phase of the ovarian cycle. At first, there were a number of studies that seemed inconsistent with these initial findings; however, a multilaboratory effort to combine data sets led to a study that reported levels of sympathoexcitatory response during the midluteal phase might relate to the relative surges of estradiol and progesterone (Carter et al. 2013). Table 3 also includes studies by Hart et al. (2009), Shoemaker et al. (2001), and Fu et al. (2005) that reported MSNA sex differences at rest and during various stressors. Recent reviews have highlighted the impact of sex on sympathetic neural activity in humans (Hart et al. 2012; Joyner et al. 2016).
Table 3 also highlights a number of highly cited articles related to MSNA control in subjects with obesity and/or metabolic syndrome. Alvarez et al. (2002) claims the top-cited microneurographic article since 2000 in a study that documented a stronger association between MSNA and abdominal visceral fat (as opposed to subcutaneous fat). Grassi and colleagues reported higher levels of MSNA with obesity (Grassi et al. 2004), obesity with hypertension (Grassi et al. 2000), and metabolic syndrome (Grassi et al. 2005). As detailed in Table 2, Narkiewicz et al. (1998) presented evidence that the increased levels of MSNA with obesity are only observed in obese subjects with obstructive sleep apnea.
While >99% of microneurography studies have been performed on Earth, the procedure was conducted in space during the NASA Neurolab mission (Ertl et al. 2002). James Pawelczyk was the astronaut that physically conducted and oversaw the microneurography measurements taken in space. A number of studies were published on the Neurolab mission, and one of those publications has been cited enough to show up in Table 3. Levine et al. (2002) reported that MSNA was higher postspaceflight in both supine and tilted positions.
As noted several times throughout this review, the citation index is limited as a measure of impact. This is particularly true for studies published within the last 15–20 years. A good example of this is work being conducted by Kevin Shoemaker to better understand rate coding and sympathetic neural recruitment strategies via wavelet denoising and other signal processing analyses of both the raw and integrated MSNA signals (Shoemaker 2017). These novel analyses (Salmanpour et al. 2008a, 2008b, 2010), which build on the early work of Andre Diedrich and colleagues (Diedrich et al. 2003), have not yet been widely adopted by the microneurography field; as such, the studies did not meet inclusion criteria for Table 3. Nevertheless, the analyses are likely to become more heavily employed as the field becomes more familiar and comfortable with the analytical approach. Readers are referred to two recent reviews, including one that is part of this Special Call, for more details (Shoemaker 2017; Shoemaker et al. 2018).
Another example of impactful work that has not yet generated >100 citations are the ongoing studies to better understand the impact of MSNA on blood pressure and end-organ responsiveness. While the majority of MSNA and blood pressure studies have tended to focus on the classic negative feedback loop of the arterial baroreflex, studies examining the downstream impact of MSNA on blood pressure and end-organ responsiveness (i.e., sympathetic vascular transduction) have been increasing in recent years. For example, Paul Fadel and colleagues demonstrated that healthy aging was associated with attenuated “downstream” beat-to-beat increases of blood pressure after a spontaneous burst of MSNA, and that this attenuation was most dramatic in postmenopausal women (Vianna et al. 2012). Emma Hart and colleagues quantified and compared sympathetic vascular transduction among young men, young women, older men, and postmenopausal women (Briant et al. 2016). Using Doppler ultrasound, the laboratory of Fadel recently reported decreases in leg vascular conductance following spontaneous bursts of MSNA (Fairfax et al. 2013) and exaggerated MSNA-induced vasoconstriction in young black men when compared with young white men (Vranish et al. 2018). Our laboratory has reported sex differences in sympathetic vascular transduction during mental stress (Yang et al. 2013). In summary, studies utilizing microneurography to examine sympathetic vascular transduction are likely to increase in the future and have impact.
WHAT DO THE NEXT 50 YEARS LOOK LIKE?
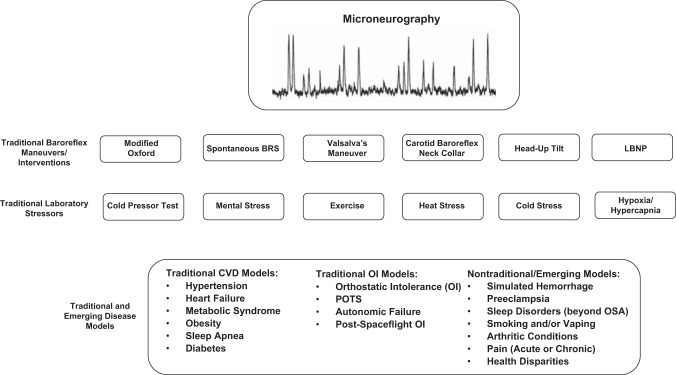
It is impossible to predict where we will be in the next 10 years, let alone 50 years. In the near future, microneurography will remain a niche procedure that can provide direct insight into the sympathetic nervous system. If the objective analysis of the highly cited studies outlined in this review tells a congruent story, it is that there are multiple diseases and disparities that can be studied using this procedure. If one looks to the most highly cited articles, which at this point come from the 1980s and 1990s, it is reasonable to draw the conclusion that hypertension, diabetes, sleep apnea, heart failure, and obesity will remain mainstays for the microneurography approach. However, conditions and interventions such as sleep, exercise, orthostatic stressors, thermal stressors, and other stressors appear to be equally important. Finally, there has been a marked increase in the interest of sex, race, and other heritable factors (i.e., family history of hypertension) on MSNA, and this is likely to continue with the current emphasis on health disparities. Figure 1 highlights some of the various diseases and interventions that one might consider in future microneurographic studies.
Fig. 1.
Conceptual overview of laboratory interventions and disease models that have been studied using microneurography. LBNP, lower body negative pressure; OSA, obstructive sleep apnea; POTS, postural orthostatic tachycardia syndrome; CVD, cardiovascular disease.
To date, the majority of microneurographic studies of peripheral sympathetic nerve activity have focused on cross-sectional or short-term interventional studies. While this is understandable given the specialty and cost of the technique, there remain a number of opportunities to better explore the relationship between MSNA and morbidity/mortality. For example, Barretto et al. (2009) utilized univariate, stepwise Cox proportional hazards analysis and Kaplan-Meier analysis to demonstrate that MSNA was a significant independent predictor of mortality in 122 heart failure patients followed up for 1 year, and that the survival rate was significantly lower in patients with >49 bursts/min. There is a need for more large-scale, prospective studies that examine the longitudinal relationship between MSNA and incident hypertension, incident diabetes, and other cardiometabolic conditions.
In summary, it is clear to see from the exponential growth of microneurographic studies that there remain plenty of unanswered questions. As the field moves forward, it will be important to maintain an appreciation for the early microneurography studies highlighted within this review and the meticulous care that was taken to ensure consistency in the acquisition and analytic approaches. There remains a human element to this procedure that requires significant face-to-face interaction and training. This does not suggest we should not explore novel ways to share the technique, but it will always require a high level of mentorship as that shown by Wallin and subsequent trainees. The recent Guidelines Paper by Hart et al. (Hart et al. 2017), and the classic paper on microneurography safety by Eckberg et al. (1989), are reminders that there are many procedural and analytical caveats that must be considered with the technique of microneurography. The high impact studies highlighted in this review, and the approaches highlighted in the methods of these studies, should be appreciated and carefully considered by any microneurographer.
GRANTS
This paper was supported, in part, by National Institutes of Health Grants HL-122919 and AA-024892 and the Portage Health Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
J.R.C. conceived and designed research; J.R.C. performed experiments; J.R.C. interpreted results of experiments; J.R.C. prepared figures; J.R.C. drafted manuscript; J.R.C. edited and revised manuscript; J.R.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank John Durocher, William Cooke, and Ian Greenlund for critical review of this manuscript.
REFERENCES
- Abboud F, Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest 124: 1454–1457, 2014. doi: 10.1172/JCI70420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 106: 2533–2536, 2002. doi: 10.1161/01.CIR.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Balon TW, Hoffman RP, Sinkey CA, Mark AL. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension 19: 621–627, 1992. doi: 10.1161/01.HYP.19.6.621. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 87: 2246–2252, 1991a. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 14: 177–183, 1989. doi: 10.1161/01.HYP.14.2.177. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Wallin BG, Mark AL. Dissociation of sympathetic nerve activity in arm and leg muscle during mental stress. Hypertension 9: III114–III119, 1987. doi: 10.1161/01.HYP.9.6_Pt_2.III114. [DOI] [PubMed] [Google Scholar]
- Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135: 302–307, 2009. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- Biaggioni I, Killian TJ, Mosqueda-Garcia R, Robertson RM, Robertson D. Adenosine increases sympathetic nerve traffic in humans. Circulation 83: 1668–1675, 1991. doi: 10.1161/01.CIR.83.5.1668. [DOI] [PubMed] [Google Scholar]
- Bini G, Hagbarth KE, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol 306: 537–552, 1980. doi: 10.1113/jphysiol.1980.sp013413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briant LJ, Burchell AE, Ratcliffe LE, Charkoudian N, Nightingale AK, Paton JF, Joyner MJ, Hart EC. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. J Physiol 594: 4753–4768, 2016. doi: 10.1113/JP272167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol 339: 535–552, 1983. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol 52: 435–448, 1984. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B, Skuse NF. Interactions between cutaneous and muscle afferent projections to cerebral cortex in man. Electroencephalogr Clin Neurophysiol 53: 349–360, 1982. doi: 10.1016/0013-4694(82)90001-3. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol 261: 695–711, 1976a. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L, Wallin BG. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol 261: 673–693, 1976b. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol 470: 383–393, 1993. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Sundlöf G, Wallin G. Postural effects on muscle nerve sympathetic activity in man. J Physiol 272: 399–414, 1977. doi: 10.1113/jphysiol.1977.sp012051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 103: 1763–1768, 1993. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Hedner JA, Sellgren J, Elam M, Wallin BG. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med 154: 1490–1496, 1996. doi: 10.1164/ajrccm.154.5.8912770. [DOI] [PubMed] [Google Scholar]
- Carter JR, Durocher JJ, Larson RA, DellaValla JP, Yang H. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol 302: H1991–H1997, 2012. doi: 10.1152/ajpheart.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension 61: 395–399, 2013. doi: 10.1161/HYPERTENSIONAHA.112.202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Goldstein DS. Sympathoneural and adrenomedullary responses to mental stress. Compr Physiol 5: 119–146, 2015. doi: 10.1002/cphy.c140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Grimaldi D, Fonkoue IT, Medalie L, Mokhlesi B, Cauter EV. Assessment of sympathetic neural activity in chronic insomnia: evidence for elevated cardiovascular risk. Sleep (Basel) 42: zsy048, 2018. doi: 10.1093/sleep/zsy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Kupiers NT, Ray CA. Neurovascular responses to mental stress. J Physiol 564: 321–327, 2005. doi: 10.1113/jphysiol.2004.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 296: H847–H853, 2009. doi: 10.1152/ajpheart.01234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Ray CA. Sympathetic neural adaptations to exercise training in humans. Auton Neurosci 188: 36–43, 2015. doi: 10.1016/j.autneu.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 568: 315–321, 2005. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Wallin BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 4: 825–850, 2014. doi: 10.1002/cphy.c130038 [DOI] [PubMed] [Google Scholar]
- Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327: 1912–1918, 1992. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. J Physiol 517: 617–628, 1999. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972a. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand 84: 82–94, 1972b. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84: 177–186, 1972c. doi: 10.1111/j.1748-1716.1972.tb05168.x. [DOI] [PubMed] [Google Scholar]
- Diedrich A, Charoensuk W, Brychta RJ, Ertl AC, Shiavi R. Analysis of raw microneurographic recordings based on wavelet de-noising technique and classification algorithm: wavelet analysis in microneurography. IEEE Trans Biomed Eng 50: 41–50, 2003. doi: 10.1109/TBME.2002.807323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999. doi: 10.1161/01.CIR.100.2.164. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol 278: H1205–H1210, 2000. doi: 10.1152/ajpheart.2000.278.4.H1205. [DOI] [PubMed] [Google Scholar]
- Donadio V, Liguori R, Elam M, Karlsson T, Giannoccaro MP, Pegenius G, Giambattistelli F, Wallin BG. Muscle sympathetic response to arousal predicts neurovascular reactivity during mental stress. J Physiol 590: 2885–2896, 2012. doi: 10.1113/jphysiol.2012.228981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert TJ, Muzi M, Berens R, Goff D, Kampine JP. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology 76: 725–733, 1992. doi: 10.1097/00000542-199205000-00010. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation 96: 3224–3232, 1997. doi: 10.1161/01.CIR.96.9.3224. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J Physiol 365: 181–196, 1985. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Rea RF, Andersson OK, Hedner T, Pernow J, Lundberg JM, Wallin BG. Baroreflex modulation of sympathetic activity and sympathetic neurotransmitters in humans. Acta Physiol Scand 133: 221–231, 1988. doi: 10.1111/j.1748-1716.1988.tb08401.x. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Wallin BG, Fagius J, Lundberg L, Torebjörk HE. Prospective study of symptoms after human microneurography. Acta Physiol Scand 137: 567–569, 1989. doi: 10.1111/j.1748-1716.1989.tb08804.x. [DOI] [PubMed] [Google Scholar]
- Ertl AC, Diedrich A, Biaggioni I, Levine BD, Robertson RM, Cox JF, Zuckerman JH, Pawelczyk JA, Ray CA, Buckey JC Jr, Lane LD, Shiavi R, Gaffney FA, Costa F, Holt C, Blomqvist CG, Eckberg DL, Baisch FJ, Robertson D. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. J Physiol 538: 321–329, 2002. doi: 10.1113/jphysiol.2001.012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev 70: 963–985, 1990. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci 47: 433–448, 1980. doi: 10.1016/0022-510X(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res 3: 201–205, 1993. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol 478: 363–372, 1994. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonkoue IT, Carter JR. Sympathetic neural reactivity to mental stress in humans: test-retest reproducibility. Am J Physiol Regul Integr Comp Physiol 309: R1380–R1386, 2015. doi: 10.1152/ajpregu.00344.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonkoue IT, Wang M, Carter JR. Sympathetic neural reactivity to mental stress in offspring of hypertensive parents: 20 years revisited. Am J Physiol Heart Circ Physiol 311: H426–H432, 2016. doi: 10.1152/ajpheart.00378.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol 289: R109–R116, 2005. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- Furlan R, Porta A, Costa F, Tank J, Baker L, Schiavi R, Robertson D, Malliani A, Mosqueda-Garcia R. Oscillatory patterns in sympathetic neural discharge and cardiovascular variables during orthostatic stimulus. Circulation 101: 886–892, 2000. doi: 10.1161/01.CIR.101.8.886. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Burke D, McKeon BB. Convergence in the somatosensory pathway between cutaneous afferents from the index and middle fingers in man. Exp Brain Res 50: 415–425, 1983. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Macefield VG, Bigland-Ritchie B, Gorman RB, Burke D. Motoneuronal output and gradation of effort in attempts to contract acutely paralysed leg muscles in man. J Physiol 471: 411–427, 1993. doi: 10.1113/jphysiol.1993.sp019907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol 96: 1255–1261, 2011. doi: 10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens 16, Suppl: 1979–1987, 1998. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- Grassi G, Bolla G, Seravalle G, Turri C, Lanfranchi A, Mancia G. Comparison between reproducibility and sensitivity of muscle sympathetic nerve traffic and plasma noradrenaline in man. Clin Sci (Lond) 92: 285–289, 1997. doi: 10.1042/cs0920285. [DOI] [PubMed] [Google Scholar]
- Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 31: 68–72, 1998a. doi: 10.1161/01.HYP.31.1.68. [DOI] [PubMed] [Google Scholar]
- Grassi G, Dell’Oro R, Facchini A, Quarti Trevano F, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens 22: 2363–2369, 2004. doi: 10.1097/00004872-200412000-00019. [DOI] [PubMed] [Google Scholar]
- Grassi G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, Gamba PL, Mancia G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia 48: 1359–1365, 2005. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens 17: 719–734, 1999. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Calhoun DA, Bolla GB, Giannattasio C, Marabini M, Del Bo A, Mancia G. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation 90: 248–253, 1994. doi: 10.1161/01.CIR.90.1.248. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension 25: 560–563, 1995a. doi: 10.1161/01.HYP.25.4.560. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M, Mancia G. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 92: 3206–3211, 1995b. doi: 10.1161/01.CIR.92.11.3206. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 97: 2037–2042, 1998b. doi: 10.1161/01.CIR.97.20.2037. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension 36: 538–542, 2000. doi: 10.1161/01.HYP.36.4.538. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjörk HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand 84: 164–176, 1972. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Vallbo AB. Discharge characteristics of human muscle afferents during muscle stretch and contraction. Exp Neurol 22: 674–694, 1968a. doi: 10.1016/0014-4886(68)90156-8. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand 74: 96–108, 1968b. doi: 10.1111/j.1365-201X.1968.tb10904.x. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Wallin G, Burke D, Löfstedt L. Effects of the Jendrassik manoeuvre on muscle spindle activity in man. J Neurol Neurosurg Psychiatry 38: 1143–1153, 1975. doi: 10.1136/jnnp.38.12.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwill JR, Taylor JA, Eckberg DL. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol 495: 279–288, 1996. doi: 10.1113/jphysiol.1996.sp021592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53: 571–576, 2009. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol 312: H1031–H1051, 2017. doi: 10.1152/ajpheart.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol 590: 2069–2079, 2012. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 61: 457–464, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, Peters T, Sweep FC, Haller H, Pichlmaier AM, Luft FC, Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 55: 619–626, 2010. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- Holwerda SW, Reynolds LJ, Restaino RM, Credeur DP, Leidy HJ, Thyfault JP, Fadel PJ. The influence of reduced insulin sensitivity via short-term reductions in physical activity on cardiac baroreflex sensitivity during acute hyperglycemia. J Appl Physiol (1985) 119: 1383–1392, 2015. doi: 10.1152/japplphysiol.00584.2015. [DOI] [PubMed] [Google Scholar]
- Hornyak M, Cejnar M, Elam M, Matousek M, Wallin BG. Sympathetic muscle nerve activity during sleep in man. Brain 114: 1281–1295, 1991. doi: 10.1093/brain/114.3.1281. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Wallin BG, Charkoudian N. Sex differences and blood pressure regulation in humans. Exp Physiol 101: 349–355, 2016. doi: 10.1113/EP085146. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly DS, O’Leary DD, Menon RS, Gati JS, Shoemaker JK. Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol 569: 331–345, 2005. doi: 10.1113/jphysiol.2005.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimbach WN Jr, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986. doi: 10.1161/01.CIR.73.5.913. [DOI] [PubMed] [Google Scholar]
- Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC Jr, Cooke WH, Baisch FJ, Eckberg DL, Blomqvist CG. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol 538: 331–340, 2002. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SW, Senior RR, Das M, Whittam AM, Murray A, McComb JM. Low-frequency heart rate variability: reproducibility in cardiac transplant recipients and normal subjects. Clin Sci (Lond) 100: 43–46, 2001. doi: 10.1042/cs1000043. [DOI] [PubMed] [Google Scholar]
- Macefield VG. Sympathetic microneurography. Handb Clin Neurol 117: 353–364, 2013. doi: 10.1016/B978-0-444-53491-0.00028-6. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman RB, Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol 471: 429–443, 1993. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Physiological and pathophysiological firing properties of single postganglionic sympathetic neurons in humans. J Neurophysiol 119: 944–956, 2018. doi: 10.1152/jn.00004.2017. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol 481: 799–809, 1994. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. doi: 10.1161/01.RES.57.3.461. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. doi: 10.1161/01.CIR.101.8.862. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, Desia T, Snell M, Jarai Z, Robertson RM, Robertson D. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest 99: 2736–2744, 1997. doi: 10.1172/JCI119463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 98: 772–776, 1998. doi: 10.1161/01.CIR.98.8.772. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99: 1183–1189, 1999. doi: 10.1161/01.CIR.99.9.1183. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498–503, 1993. doi: 10.1161/01.HYP.21.4.498. [DOI] [PubMed] [Google Scholar]
- Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 95: 1441–1448, 1997. doi: 10.1161/01.CIR.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrão CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42: 854–860, 2003. doi: 10.1016/S0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- Salmanpour A, Brown LJ, Shoemaker JK. Detection and classification of raw action potential patterns in human muscle sympathetic nerve activity. Conf Proc IEEE Eng Med Biol Soc 2008: 2928–2931, 2008a. doi: 10.1109/IEMBS.2008.4649816 [DOI] [PubMed] [Google Scholar]
- Salmanpour A, Brown LJ, Shoemaker JK. Performance analysis of stationary and discrete wavelet transform for action potential detection from sympathetic nerve recordings in humans. Conf Proc IEEE Eng Med Biol Soc 2008: 2932–2935, 2008b. doi: 10.1109/IEMBS.2008.4649817. [DOI] [PubMed] [Google Scholar]
- Salmanpour A, Brown LJ, Shoemaker JK. Spike detection in human muscle sympathetic nerve activity using a matched wavelet approach. J Neurosci Methods 193: 343–355, 2010. doi: 10.1016/j.jneumeth.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Saul JP, Rea RF, Eckberg DL, Berger RD, Cohen RJ. Heart rate and muscle sympathetic nerve variability during reflex changes of autonomic activity. Am J Physiol Heart Circ Physiol 258: H713–H721, 1990. doi: 10.1152/ajpheart.1990.258.3.H713 [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation 108: 560–565, 2003. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension 43: 169–175, 2004. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia–a state of sympathetic overactivity. N Engl J Med 335: 1480–1485, 1996. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK. Recruitment strategies in efferent sympathetic nerve activity. Clin Auton Res 27: 369–378, 2017. doi: 10.1007/s10286-017-0459-x. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281: H2028–H2035, 2001. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Klassen SA, Badrov MB, Fadel PJ. Fifty years of microneurography: learning the language of the peripheral sympathetic nervous system in humans. J Neurophysiol 119: 1731–1744, 2018. doi: 10.1152/jn.00841.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinoway LI, Rea RF, Mosher TJ, Smith MB, Mark AL. Hydrogen ion concentration is not the sole determinant of muscle metaboreceptor responses in humans. J Clin Invest 89: 1875–1884, 1992. doi: 10.1172/JCI115792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328: 303–307, 1993. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest 87: 1953–1957, 1991. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol (1985) 67: 2101–2106, 1989. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J Clin Invest 92: 1730–1735, 1993. doi: 10.1172/JCI116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol 272: 383–397, 1977. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol 278: 525–532, 1978a. doi: 10.1113/jphysiol.1978.sp012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978b. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE. Impulses recorded with micro-electrodes in human muscle nerves during stimulation of mechanoreceptors and voluntary contractions. Electroencephalogr Clin Neurophysiol 23: 392, 1967. [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol 21: 270–289, 1968. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol (1985) 96: 1262–1269, 2004. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012. doi: 10.1152/ajpheart.01105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Leimbach WN Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987a. doi: 10.1161/01.HYP.9.5.429. [DOI] [PubMed] [Google Scholar]
- Victor RG, Seals DR, Mark AL. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. Insight from intraneural recordings in humans. J Clin Invest 79: 508–516, 1987b. doi: 10.1172/JCI112841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider P, Tappy L, Randin D, Schneiter P, Jéquier E, Nicod P, Scherrer U. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest 92: 147–154, 1993. doi: 10.1172/JCI116542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranish JR, Holwerda SW, Young BE, Credeur DP, Patik JC, Barbosa TC, Keller DM, Fadel PJ. Exaggerated vasoconstriction to spontaneous bursts of muscle sympathetic nerve activity in healthy young black men. Hypertension 71: 192–198, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Delius W, Hagbarth KE. Comparison of sympathetic nerve activity in normotensive and hypertensive subjects. Circ Res 33: 9–21, 1973. doi: 10.1161/01.RES.33.1.9. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol 453: 45–58, 1992. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Sundlöf G. A quantitative study of muscle nerve sympathetic activity in resting normotensive and hypertensive subjects. Hypertension 1: 67–77, 1979. doi: 10.1161/01.HYP.1.2.67. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst 6: 287–291, 1982. doi: 10.1016/0165-1838(82)90001-7. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlöf G, Delius W. The effect of carotid sinus nerve stimulation on muscle and skin nerve sympathetic activity in man. Pflugers Arch 358: 101–110, 1975. doi: 10.1007/BF00583921. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlöf G, Eriksson BM, Dominiak P, Grobecker H, Lindblad LE. Plasma noradrenaline correlates to sympathetic muscle nerve activity in normotensive man. Acta Physiol Scand 111: 69–73, 1981. doi: 10.1111/j.1748-1716.1981.tb06706.x. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol 491: 881–887, 1996. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Carter JR. Baroreflex sensitivity analysis: spontaneous methodology vs. Valsalva’s maneuver. Clin Auton Res 23: 133–139, 2013. doi: 10.1007/s10286-013-0195-9. [DOI] [PubMed] [Google Scholar]
- Yang H, Drummer TD, Carter JR. Sex differences in sympathetic neural and limb vascular reactivity to mental stress in humans. Am J Physiol Heart Circ Physiol 304: H436–H443, 2013. doi: 10.1152/ajpheart.00688.2012. [DOI] [PubMed] [Google Scholar]
- Yates BJ. Happy 80th birthday to the Journal of Neurophysiology! J Neurophysiol 119: 1589–1591, 2018. doi: 10.1152/jn.00230.2018. [DOI] [PubMed] [Google Scholar]
- Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 588: 3593–3603, 2010. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]