Abstract
During adaptation from dim to bright environments, changes in retinal signaling are mediated, in part, by dopamine. Dopamine is released with light and can modulate retinal receptive fields, neuronal coupling, inhibitory receptors, and rod pathway inhibition. However, it is unclear how dopamine affects inner retinal inhibition to cone bipolar cells, which relay visual information from photoreceptors to ganglion cells and are important signal processing sites. We tested the hypothesis that dopamine (D)1 receptor activation is sufficient to elicit light-adapted inhibitory changes. Local light-evoked inhibition and spontaneous activity were measured from OFF cone bipolar cells in dark-adapted mouse retinas while stimulating D1 receptors, which are located on bipolar, horizontal, and inhibitory amacrine cells. The D1 agonist SKF38393 reduced local inhibitory light-evoked response magnitude and increased response transience, which mimicked changes measured with light adaptation. D1-mediated reductions in local inhibition were more pronounced for glycinergic than GABAergic inputs, comparable with light adaptation. The effects of D1 receptors on light-evoked input were similar to the effects on spontaneous input. D1 receptor activation primarily decreased glycinergic spontaneous current frequency, similar to light adaptation, suggesting mainly a presynaptic amacrine cell site of action. These results expand the role of dopamine to include signal modulation of cone bipolar cell local inhibition. In this role, D1 receptor activation, acting primarily through glycinergic amacrine cells, may be an important mechanism for the light-adapted reduction in OFF bipolar cell inhibition since the actions are similar and dopamine is released during light adaptation.
NEW & NOTEWORTHY Retinal adaptation to different luminance conditions requires the adjustment of local circuits for accurate signaling of visual scenes. Understanding mechanisms behind luminance adaptation at different retinal levels is important for understanding how the retina functions in a dynamic environment. In the mouse, we show that dopamine pathways reduce inner retinal inhibition similar to increased background luminance, suggesting the two are linked and highlighting a possible mechanism for light adaptation at an early retinal processing center.
Keywords: γ-aminobutyric acid (GABA), amacrine cell, bipolar cell, glycine, luminance
INTRODUCTION
The retina adjusts visual signaling by adapting to increased background luminance at multiple levels including at the photoreceptors (Tamura et al. 1991; Woodruff et al. 2008), the horizontal cells (Xin and Bloomfield 1999b), the inner retinal network of excitatory bipolar and inhibitory amacrine cells (Dunn et al. 2006, 2007; Flood et al. 2018; Green et al. 1975; Green and Powers 1982; Mazade and Eggers 2013, 2016; Naka et al. 1979; Page-McCaw et al. 2004; Shapley and Enroth-Cugell 1984), and the ganglion cells, the output neurons of the retina. Ganglion cell spatial sensitivity and signal strength increase with ambient light (Barlow et al. 1957; Dedek et al. 2008; Farrow et al. 2013), which may arise from modulation of inner retinal inhibition (Dedek et al. 2008; Mazade and Eggers 2013, 2016). A recent study found that local inhibition to inner retinal OFF bipolar cells, which respond to the offset of light, was reduced with light adaptation (Mazade and Eggers 2016). One likely mechanism for the reduction in local inhibition with increased luminance is through dopamine.
Dopamine, released from dopaminergic amacrine cells in the light (Bauer et al. 1980; Godley and Wurtman 1988; Witkovsky 2004), plays a key role in visual processing and light adaptation by modulating multiple retinal pathways (Boatright et al. 1989; Doyle et al. 2002; Godley and Wurtman 1988). Dopamine and/or dopamine D1 receptors, located on horizontal, bipolar, amacrine, and ganglion cells (Farshi et al. 2016; Nguyen-Legros et al. 1997; Veruki and Wässle 1996), can alter inhibitory inputs by modulating GABAA (Feigenspan and Bormann 1994a) and GABAC (Dong and Werblin 1994; Feigenspan and Bormann 1994b; Wellis and Werblin 1995) receptor currents as well as amacrine cell neurotransmitter release (Calaza et al. 2001; Kato et al. 1985; O’Brien and Dowling 1985; Pycock and Smith 1983). Additionally, D1 receptor activation reduced both light-evoked responses and spontaneous current frequency in retinal rod bipolar cells (Flood et al. 2018). Dopamine signaling also heavily regulates amacrine cell coupling (Hampson et al. 1992; Kothmann et al. 2009; Xia and Mills 2004) similar to background luminance (Bloomfield et al. 1997) as well as modulates the strength of the horizontal cell-mediated surround of bipolar cells (Chaffiol et al. 2017). However, the contribution of dopamine in shaping evoked and spontaneous inner retinal local inhibition to cone pathways and whether it is similar to light-adapted changes is unknown.
Based on the known roles of dopamine in retinal signaling, we hypothesized that activation of D1 dopaminergic pathways could mimic light-adapted inhibitory changes. We studied OFF cone bipolar cells because they receive inhibition from both rod and cone pathways and are sites for unique luminance-induced inhibitory changes (Mazade and Eggers 2013, 2016). Inhibitory inputs to OFF bipolar cells come from glycinergic and GABAergic amacrine cells (Eggers et al. 2007) and possibly GABAergic inputs from horizontal cells (Guo et al. 2009, 2010), although a previous paper suggested that there is limited direct horizontal cell-OFF bipolar cell inhibition (Zhang and Wu 2009). Although the contribution of horizontal cell inhibition to OFF bipolar cells is unclear, GABA- and especially glycine-mediated inhibition from amacrine cells may be differentially modulated post- and/or presynaptically by dopamine (Farshi et al. 2016; Nguyen-Legros et al. 1997; Veruki and Wässle 1996). We found that activation of D1 receptors mimicked almost all of the changes in light-adapted OFF bipolar cell inhibitory evoked and spontaneous activity. Local glycinergic inputs were more affected than local GABAergic inputs, and our results suggest a presynaptic site of action. We conclude that dopamine is an important modulator of local inhibition to OFF bipolar cells and that the actions of dopamine and increased background luminance are correlated, could be linked, and may be one inner retinal mechanism for light adaptation.
METHODS
Preparation of mouse retinal slices.
The University of Arizona Institutional Animal Care and Use Committee approved all animal protocols for this study. As described previously (Eggers and Lukasiewicz 2006; Eggers et al. 2013; Mazade and Eggers 2013), male mice, fed ad libitum (C57BL/6J strain; Jackson, Bar Harbor, ME) 35–60 days of age were euthanized using carbon dioxide, and their eyes were enucleated. The retina was removed and trimmed into one large flat rectangle leaving only the central retina surrounding the optic disk. A nitrocellulose membrane filter paper (0.45-μm pore size; Millipore) was placed on the retinal section that was transferred to a hand chopper. An average of six 250-μm slices were cut, rotated 90°, and mounted onto glass coverslips using vacuum grease. Cells used from these slices were selected using the same criteria as the previous study (Mazade and Eggers 2016). The tissue was maintained in oxygenated extracellular solution at room temperature. All dissection and storage procedures were performed under infrared illumination to preserve retinal light sensitivity. A total of 41 mice were used in this study.
Solutions and drugs.
The extracellular recording solution used for dissection and to examine light-evoked and spontaneous currents contained, in mM, 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 2 CaCl2, 20 glucose, and 26 NaHCO3. For voltage-clamp recordings, the intracellular solution contained, in mM, 120 CsOH, 120 gluconic acid, 1 MgCl2, 10 HEPES, 10 TEA-Cl, 10 phosphocreatine-Na2, 4 Mg-ATP, 0.5 Na-GTP, and 10 EGTA and 50 µM Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and was adjusted to pH 7.2 with CsOH. Extracellular solutions were bubbled with 95% O2-5% CO2. To isolate the inhibitory receptor inputs, SR-95531 (SR; 20 μM) to block GABAA receptors, (1,2,5,6-tetrahydropyridine-4-yl)methylphosphinic acid (TPMPA; 50 μM) to block GABAC receptors, and strychnine (1 μM) to block glycine receptors were used (Mazade and Eggers 2013, 2016). To activate dopamine D1 receptors, the D1 receptor agonist SKF38393 (SKF; 20 µM; Tocris) was used with a concentration similar to that used in previous studies to activate D1 receptors in isolated mouse horizontal cells (Liu et al. 2016) and salamander (Ichinose and Lukasiewicz 2007) and mouse (Hu et al. 2010) retinas and to get a saturating dose in fish retinas (Harsanyi and Mangel 1992). Although 20 µM may also activate D2 receptors on photoreceptors, SKF is 150 times more potent for D1 than D2-like receptors. Additionally, D2 receptors are primarily on dopaminergic amacrine cells (Nguyen-Legros et al. 1999), so the main effect of D2 receptor activation would be to reduce dopamine release. Since dopaminergic amacrine cells are not active in the dark-adapted retina we used here, this would likely have a limited impact. To isolate miniature postsynaptic currents (mIPSCs), CdCl2 (100 μM) and TTX (500 nM) were used. All drug solutions were applied to the slice for 5 min before recordings began using a gravity-driven superfusion system (Cell MicroControls, Norfolk, VA) at a rate of approximately 1–2 ml/min, with continuous perfusion throughout the experiment. Unless otherwise indicated, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Whole cell recordings.
Whole cell patch-clamp recordings, sampled at 10 kHz, were made from OFF bipolar cells in retinal slices. Light-evoked inhibitory postsynaptic currents (L-IPSCs) and spontaneous/miniature (s/m)IPSCs were recorded from retinal bipolar cells voltage-clamped to 0 mV, the reversal potential of nonselective cation channels. Bipolar cell recordings were stable, and no rundown of the light response was observed over the recording period. Liquid junction potentials of 20 mV, calculated with Clampex software (Molecular Devices, Sunnyvale, CA), were corrected at the beginning of each recording. Electrodes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) on a P-97 Flaming/Brown puller (Sutter Instrument, Novato, CA) and had resistances of 5–7 MΩ. Mice were dark-adapted overnight, and all recording procedures were performed in the dark under infrared illumination to preserve retinal light sensitivity. Recordings were made in extracellular solution heated to 32°C using thin stage and inline heaters (Cell MicroControls). Responses were filtered at 6 kHz with the four-pole Bessel filter on a MultiClamp 700B patch-clamp amplifier (Molecular Devices) and digitized with a Digidata 1140 data acquisition system (Molecular Devices).
Morphological identification of cells.
Alexa Fluor 488 included in the recording pipette was used to label OFF bipolar cells. They were classified as OFF bipolar cells based on their axonal morphologies, stratification within the inner plexiform layer, and the position of their somas in the inner nuclear layer (Ghosh et al. 2004). Cells were imaged with a Nikon Digital Sight camera with Elements software using a Nikon Intensilight C-HGFIE fluorescent lamp (Nikon Instruments, Tokyo, Japan). Analysis of axon terminal morphology and response properties was performed previously on OFF bipolar cell subtypes (Mazade and Eggers 2013), and these same criteria were used to identify OFF bipolar cells in the current study. Since no differences were found in inhibitory responses to narrow bars between OFF bipolar cell subtypes (Mazade and Eggers 2016), all subtypes were grouped together. Recordings from 45 cells (10 OFF type 1/2, 22 OFF type 3, and 13 OFF type 4) were included in the analyses.
Light stimulus.
Bar stimuli (25 µm wide, centered on the cell) were presented using a white organic light-emitting diode microdisplay (EMA-100503 SXGA XL Monochrome White; eMagin, Bellevue, WA) projected through the camera port of the microscope, which elicited strong and reproducible responses in both dark- and light-adapted conditions (Mazade and Eggers 2016). Recordings were from cells located within the regions of mixed green/UV cone opsin input (Applebury et al. 2000; Haverkamp et al. 2005) to ensure that all possible pathways were present, although UV cones were unlikely to be strongly activated. The stimulus was presented and controlled with custom MATLAB (MathWorks, Natick, MA) code with Psychtoolbox-3 (Brainard 1997) extensions by controlling the intensity (7.83 × 104 photons·μm−2·s−1), size, location, and duration of the stimulus. A 1-s stimulus was used to determine the type of inhibition to all recorded bipolar cells as well as to elicit robust responses using a small stimulus. For glycinergic spontaneous recordings in light-adapted conditions, the background luminance (1,150 photons·μm−2·s−1) was set either using the organic light-emitting diode screen or with a white light-emitting diode (30045 CIE; coordinates: x: 0.3, y: 0.31; NTE Electronics, Bloomfield, NJ).
Data analysis and statistics.
Clampfit software (Molecular Devices) was used to measure the charge transfer and peak amplitude of L-IPSC traces averaged from ≥2 stimulus repeats. Because of the significant amount of spontaneous activity, the peak amplitude of OFF bipolar cell L-IPSCs was estimated by reducing the sampling rate of averaged raw traces (50-fold) with each point replaced with the average of those data points, after which the maximum value was measured. To determine changes in total current charge transfer, the area of the response was measured in Clampfit over the length of the response, typically 1–2 s, using the same time parameters in each condition for the same cell. To limit any portion of the response that was due to baseline or spontaneous events, the mean of the baseline current, matching the same time window of the measured response, was added to the standard deviation of the baseline, and the new value was subtracted from the evoked response. All example light-response traces in the figures were additionally filtered with a low-pass Gaussian filter (1,000 Hz) to limit noise for display purposes. The light gray bar under the example trace notes the start and end of the light stimulus. A disconnected bar indicates that the light stimulus started before or proceeded after the example trace shown.
OFF bipolar neurons were tested first in dark-adapted conditions and then with either application of the D1 receptor agonist SKF or light adaptation but not both. The charge transfers and peak amplitudes of OFF bipolar cell L-IPSCs were normalized to their dark-adapted responses individually for each cell to control for variability between cells and to standardize the response magnitude. This was accomplished by dividing the measured response parameter of a cell after treatment (SKF or light adaptation) by the measured response value of the cell in the dark-adapted condition. To measure timing differences between conditions, the transient and sustained components of center L-IPSCs were measured. The transient L-IPSC component was measured as the 1st 20% charge transfer of the response to the 1-s light stimulus (200 ms), similar to previous studies (Mazade and Eggers 2016; Nobles et al. 2012). The sustained L-IPSC component was calculated as the difference between the total response and the transient response within each light or drug condition. Normalized percentage increase in transience was calculated by dividing the transient components by the total response and normalizing the value to the dark-adapted condition.
s/mIPSCs were analyzed using Clampfit and Mini Analysis (Synaptosoft, Decatur, GA) software. In Clampfit, an event template was created for each data file, using the average of >10 prototypical events from the recording. The software used this template to detect automatically spontaneous events that were manually accepted or rejected. Events used to calculate the frequency were rejected if they did not have the characteristic shape (rapid rise and smooth decay), and events used to calculate the average peak amplitude were further rejected if consisting of two or more overlapping events. In Mini Analysis, events were automatically selected by the program based on manually chosen fitting parameters, such as event peak, duration, and decay time, using the built-in curve-fitting application. Frequency was calculated by dividing the number of events by the recording time window. If there were no events after drug application, the value was set at 0. The average sIPSC peak amplitude and frequency for each individual cell were normalized to the dark-adapted condition by dividing the value measured in SKF or light adaptation by the dark-adapted value. Peak amplitude and interevent interval histogram distributions were normalized to the number of events. Display traces in figures show raw recordings.
The distribution of s/mIPSC peak amplitude and interevent interval values were compared using the Kolmogorov-Smirnov test (K-S test). The nonparametric Wilcoxon rank sum test (Wilcoxon test) was used to compare before and after drug application/light adaptation and between drug application and light condition due to smaller sample sizes. Some light-adapted data were adapted from previous studies and are indicated in the figure legends. All sample sizes of different groups are stated in the figure legends. All error bars are reported as means ± SE, and all bar graphs show the individual data points. Significance is noted as exact P values in text (P value <0.001 noted as P < 0.001).
RESULTS
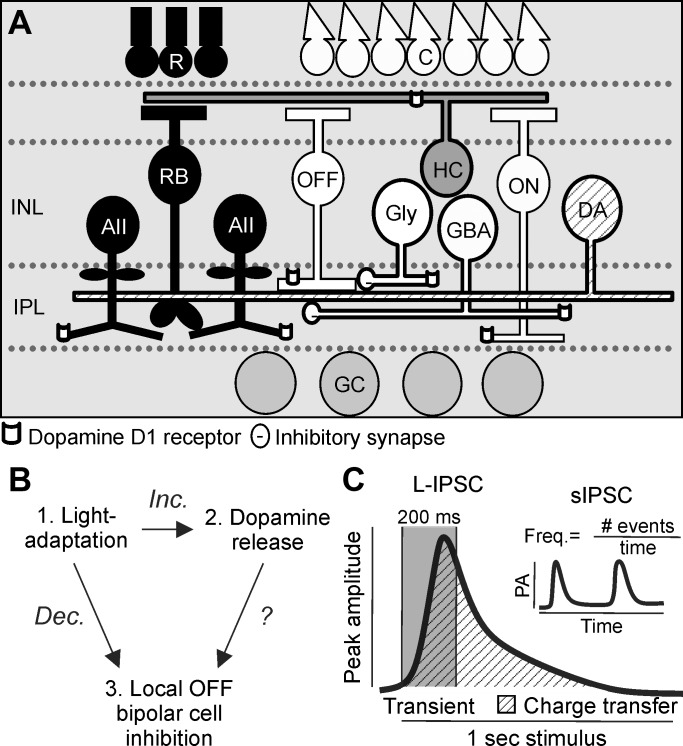
D1 receptors are a likely candidate for mediating light-adapted changes to OFF pathway inhibition since many populations of cells, including some OFF bipolar cells as well as horizontal and amacrine cells, express D1 receptors (Fig. 1A). However, it is unknown how dopamine receptor activation directly modulates inhibition to OFF bipolar cells (Fig. 1B). Light adaptation decreased the amplitude and frequency of OFF bipolar cell spontaneous (sIPSC) and light-evoked inhibitory currents (L-IPSCs; Mazade and Eggers 2013, 2016), and D1 receptor activation due to increased dopamine release, which occurs with increased luminance, could produce similar effects. To determine whether D1 receptor activation has similar effects on OFF bipolar cell inhibition as light adaptation, sIPSC peak amplitude and frequency and L-IPSC peak amplitude, charge transfer, and response transience were quantified (Fig. 1C) and compared among dark-adapted, D1 receptor-activated, and light-adapted conditions.
Fig. 1.
Schematic of inner retinal circuitry connections to OFF bipolar cells with dopaminergic receptors. A: dim-light conditions activate the rod pathway [black cells; rod photoreceptors (R)-rod bipolar cells (RB)-AII amacrine cells]. Glycinergic AII amacrine cells inhibit OFF cone bipolar cells (OFF) and are coupled to other AII amacrine cells. Bright-light conditions activate the cone pathway [white cells; cone photoreceptors (C)-OFF and ON cone (ON) bipolar cells-wide-field GABAergic (GBA) and narrow-field glycinergic (Gly) amacrine cells-inhibitory connections to OFF bipolar cells]. OFF bipolar cells bridge rod and cone circuits and activate OFF ganglion cells (GC). Dopaminergic amacrine cells (DA) have wide-field processes that activate dopamine D1 receptors on bipolar, horizontal (HC), and amacrine cells. INL, inner nuclear layer; IPL, inner plexiform layer. B: light adaptation increases (Inc.) dopamine release and decreases (Dec.) local inhibition to OFF bipolar cells, but it is unknown how dopamine release that activates dopamine receptors affects OFF bipolar cell inhibition. C: cartoons showing measurements of the peak amplitude, charge transfer, and transience of a light-evoked inhibitory postsynaptic current (L-IPSC) and the peak amplitude (PA) and frequency (Freq.) of spontaneous inhibitory postsynaptic currents (sIPSCs; inset). #, Number of.
D1 receptor activation mimics light-adapted reductions in spontaneous activity.
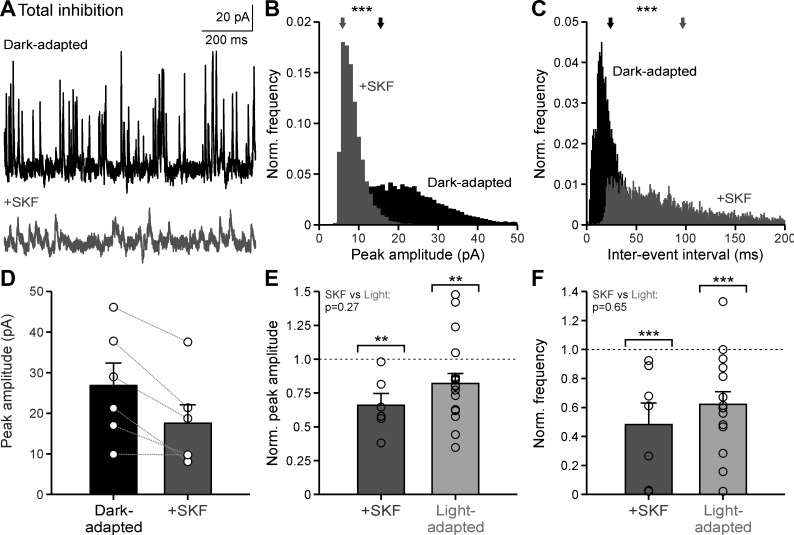
Dopamine could act on OFF bipolar cell inhibition through two pathways: D1 receptors could modulate the inhibitory receptors on the OFF bipolar cells themselves and/or modulate amacrine cell neurotransmitter release. If D1 receptors only affect inhibitory receptors on the OFF bipolar cells, then we would predict a change in sIPSC amplitude but not frequency after D1 receptor activation. Conversely, if D1 receptors only affect release from inhibitory amacrine cells, then we would predict a change in sIPSC frequency but not amplitude. To test where D1 receptors are acting, sIPSCs were recorded from OFF bipolar cells under dark-adapted conditions and the D1 receptor agonist SKF38393 (SKF) was applied (Fig. 2A). With SKF, the sIPSC peak amplitude distribution decreased significantly for all cells tested (Fig. 2B; example cell, P < 0.001; all individual cells, P < 0.01; K-S tests) and the sIPSC interevent interval distribution increased significantly for 5 of the 6 cells tested (Fig. 2C; example cell, P < 0.001; all significant cells, P < 0.01; K-S tests). Although the average sIPSC peak amplitude for each individual cell decreased with SKF, the average across cells was not significant, likely due to variability between values in dark-adapted cells (Fig. 2D, Table 1; P = 0.18, Wilcoxon test). However, when sIPSC peak amplitude after SKF application was normalized to the dark-adapted response, the amplitude was reduced by ~34% (Fig. 2E, Table 1; P = 0.002, Wilcoxon test), which was not different from the ~18% reduction in amplitude with light adaptation normalized to the dark-adapted condition (Fig. 2E, Table 1; P = 0.002; SKF vs. light-adapted, P = 0.271; Wilcoxon tests). Like sIPSC peak amplitude, sIPSC frequency with SKF normalized to the dark-adapted condition significantly decreased by ~52% (Fig. 2F, Table 1; P < 0.001, Wilcoxon test). Likewise, sIPSC frequency with light adaptation decreased by ~38% when normalized to the dark-adapted frequencies (Fig. 2F, Table 1; P < 0.001, Wilcoxon test), which was not significantly different from the reduction with SKF (P = 0.647, Wilcoxon test). Taken together, these results suggest that activation of D1 receptors is sufficient to elicit the magnitude of light-adapted changes in inhibitory noise to the OFF pathway. Additionally, these results demonstrate that D1 receptors may be affecting both the OFF bipolar cell inhibitory receptors themselves as well as the inhibitory neurotransmitter release from amacrine cells onto the OFF bipolar cell receptors.
Fig. 2.
Dopamine D1 receptor activation mimics light-adapted reductions in spontaneous inhibitory activity. A: example spontaneous inhibitory postsynaptic current (sIPSC) traces in dark-adapted conditions before (black) and after SKF38393 (SKF) application (dark gray). B: example sIPSC peak amplitude histogram, normalized (Norm.) to number of events, of the cell in A. Arrows show the average peak amplitude. C: same conditions as B for sIPSC interevent intervals of the cell seen in A. D: average peak amplitude in dark-adapted and SKF conditions (n = 6). E: average peak amplitude, normalized to the dark-adapted condition for each cell, compared between SKF (n = 6) and light-adapted (n = 18) conditions. Brackets indicate comparison with the dark-adapted condition (dotted line). F: same conditions as E for average frequency of SKF (n = 7) and light-adapted (n = 15) conditions. Light-adapted data were adapted from Mazade and Eggers (2013), Fig. 6, for comparison. Error bars are ±SE, and significance was calculated with the Wilcoxon rank sum test (**P < 0.01 and ***P < 0.001).
Table 1.
Average spontaneous (sIPSC) peak amplitudes and frequencies measured under different inhibitory conditions
| Inhibition | Dark-Adapted, pA | +SKF, pA | Dark-Adapted, pA | Light-Adapted, pA | Dark-Adapted, Hz | +SKF, Hz | Dark-Adapted, Hz | Light-Adapted, Hz |
|---|---|---|---|---|---|---|---|---|
| Total inhibition | 26.9 ± 5.5 | 17.5 ± 4.6* | 23.2 ± 3.8 | 17.1 ± 2.3* | 11.5 ± 5.8 | 3.2 ± 1.6* | 17.0 ± 5.5 | 10.4 ± 3.4* |
| Isolated GABA | 9.4 ± 1.3 | 9.4 ± 1.2 | 7.8 ± 1.2 | 0 ± 0 | 5.5 ± 4.3 | 0.8 ± 0.4* | 0.7 ± 0.5 | 0 ± 0 |
| Isolated glycine | 21.4 ± 4.7 | 9.8 ± 1.8* | 23.0 ± 5.3 | 17.9 ± 3.6* | 9.3 ± 4.9 | 6.0 ± 3.6* | 16.5 ± 7.3 | 13.3 ± 5.9* |
| Isolated glycine mIPSC | 8.4 ± 1.9 | 7.5 ± 1.7 | n.m. | n.m. | 3.3 ± 1.8 | 2.7 ± 1.7* | n.m. | n.m. |
Values are means ± SE. There were no significant differences between dark-adapted averages of SKF38393 (SKF) and light adaptation experiments. Italics indicate raw data that were adapted from Mazade and Eggers (2013) and used to compare SKF and light-adapted conditions. Value of 0 means that all spontaneous inhibitory postsynaptic currents (sIPSCs) were abolished in the given condition.
Values that are significantly different when normalized to dark-adapted values for each cell (Wilcoxon tests). Exact P values are given in the text. mIPSC, miniature inhibitory postsynaptic current; n.m., not measured.
D1 receptor activation mimics light-adapted reductions in local light-evoked inhibition.
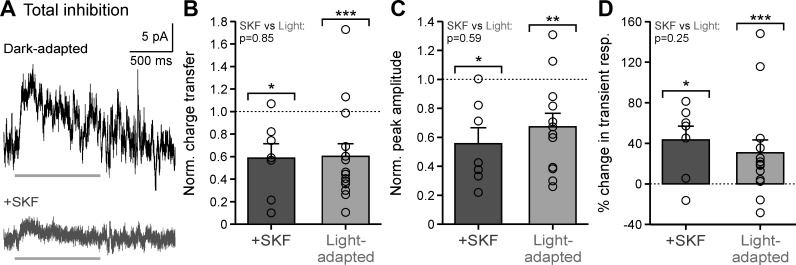
D1 receptor activation was sufficient to induce changes in spontaneous inhibition to OFF bipolar cells similar to increased background luminance. Although this suggests importance for D1 receptors in the synaptic mechanism for light adaptation, we sought to determine how D1 receptor activation affects physiological responses. Previously, we found that light adaptation had little effect on the magnitude of full-field evoked inhibition to OFF bipolar cells (Mazade and Eggers 2013) but reduced central and surround receptive field inhibition in all OFF bipolar cell subtypes regardless of the origin of their inhibition (Mazade and Eggers 2016). We predicted that activation of D1 receptors with SKF would also cause a decrease in evoked inhibition from local sources close to the bipolar cell.
To test this prediction, we presented a narrow light bar (25 µm, 1-s presentation time) directly over the cell to activate local inhibition. We found that OFF bipolar cell light-evoked inhibition was significantly reduced after SKF application (Fig. 3A). Responses were normalized to dark-adapted values to limit variability between cells and to compare between SKF and light-adapted conditions. The response charge transfer measured after SKF was ~40% smaller than the dark-adapted condition (Fig. 3B; P = 0.017, Wilcoxon test) and was not different from the reduction after light adaptation (Fig. 3B; P < 0.001; SKF vs. light-adapted, P = 0.852; Wilcoxon tests). The same was true for response peak amplitude. SKF and light-adapted conditions both reduced peak magnitude by ~45 and ~33%, respectively, to a similar level (Fig. 3C, Table 2; SKF, P = 0.017; light-adapted reduction, P = 0.003; SKF vs. light-adapted, P = 0.592; Wilcoxon tests). In a previous study, we (Mazade and Eggers 2016) found that light adaptation made the light-evoked local inhibitory responses more transient. We found that this was also the case when D1 receptors were activated in the dark with SKF (Fig. 3D; P = 0.017, Wilcoxon test). The ~43% increase in response transience with SKF was not different from the ~31% increase with light adaptation (Fig. 3D; P < 0.001; SKF vs. light-adapted, P = 0.248; Wilcoxon tests). This suggests that D1 receptor activation affects not only the magnitude, but also the time course of inhibition to OFF bipolar cells. Here, we demonstrate that D1 receptor activation decreases the local light-evoked inhibitory input of OFF bipolar cells to the same extent as increased background luminance.
Fig. 3.
Dopamine D1 receptor activation mimics light-adapted reductions in local light-evoked inhibition. A: example light-evoked inhibitory postsynaptic currents (L-IPSCs) in dark-adapted (black) and SKF38393 (SKF; dark gray) conditions in response to a 1-s, 25-µm bar, centered on the cell. Light gray bar indicates the stimulus presentation. B: average response charge transfer, normalized (Norm.) to the dark-adapted response, in SKF (n = 7) and light-adapted conditions (n = 14). Brackets indicate comparison with dark-adapted condition (dotted line). C: same conditions as B for average response peak amplitude in SKF and light-adapted conditions (n = 12). D: same conditions as B for average percentage change in the transient component of the L-IPSC in SKF and light-adapted (n = 13) conditions. resp., Response. Light-adapted data were adapted from Mazade and Eggers (2016), Fig. 2, for comparison. Error bars are ±SE, and significance was calculated with the Wilcoxon rank sum test (*P < 0.05, **P < 0.01, and ***P < 0.001).
Table 2.
Average light-evoked (L-IPSC) peak amplitudes measured under different inhibitory conditions
| Inhibition | Dark-Adapted, pA | +SKF, pA | Dark-Adapted, pA | Light-Adapted, pA |
|---|---|---|---|---|
| Total inhibition | 17.9 ± 6.6 | 7.8 ± 2.1* | 10.6 ± 6.3 | 6.4 ± 1.0* |
| Isolated GABA | 8.3 ± 1.3 | 8.6 ± 1.6 | 6.9 ± 1.4 | 5.0 ± 1.8 |
| Isolated GABAC | 17.4 ± 9.2 | 13.7 ± 6.6 | 6.1 ± 1.0 | 0 ± 0 |
| Isolated glycine | 18.2 ± 9.4 | 11.0 ± 3.9* | 8.2 ± 1.9 | 4.6 ± 0.9* |
Values are means ± SE. There were no significant differences between dark-adapted averages of SKF38393 (SKF) and light adaptation experiments. Italics indicate raw data that were adapted from Mazade and Eggers (2016) and used to compare SKF and light-adapted conditions.
Values that are significantly different when normalized to dark-adapted values for each cell (Wilcoxon tests). Exact P values are given in the text. L-IPSC, light-evoked inhibitory postsynaptic current.
D1 receptor activation reduces GABAergic spontaneous frequency but not peak amplitude.
The major components for light-evoked and synaptic inhibition to OFF bipolar cells in the dark consist of GABAergic and glycinergic amacrine cell input onto GABAA, GABAC, and glycine receptors. Each of these components may be independently modulated by D1 receptors, and we focused our investigation onto these specific inputs. The reduction of spontaneous and light-evoked inhibition (Figs. 2 and 3) could be due to reduced glycinergic or GABAergic inputs, but it is not known to what extent these pathways contribute to the D1 receptor effect. To understand the mechanisms that underlie the effect of D1 receptor activation, we analyzed the currents from different inputs separately, starting with the GABA receptors because dopamine has been shown to potentiate GABAA receptor currents in retinal horizontal cells (Feigenspan and Bormann 1994a).
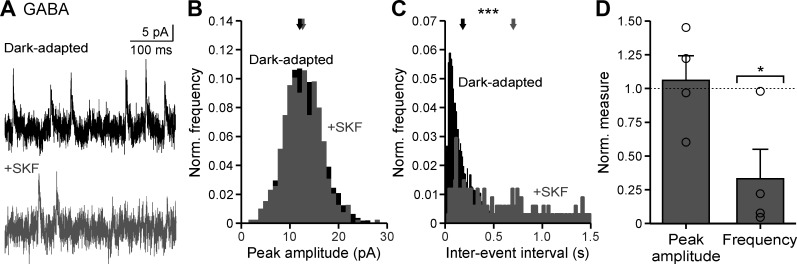
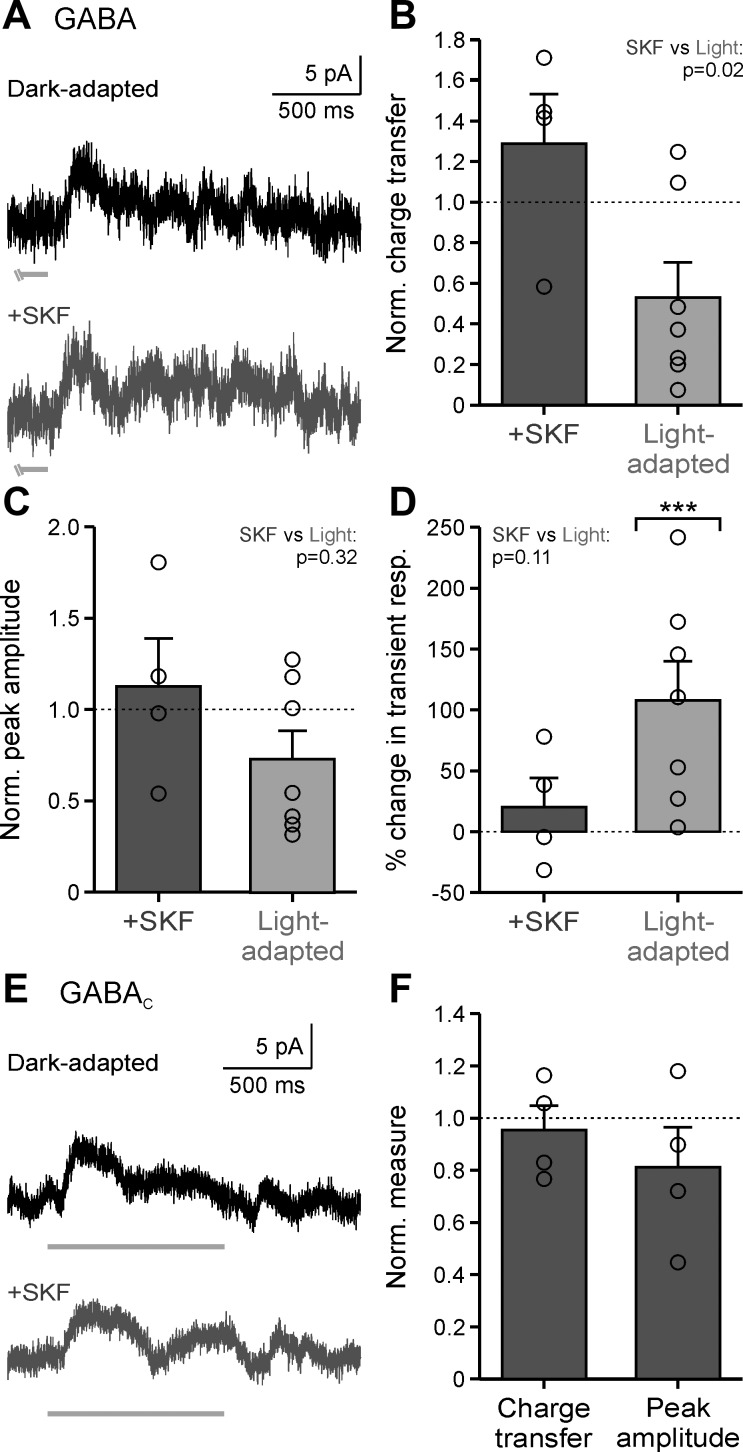
GABAergic sIPSCs were isolated (in strychnine to block glycine receptors) and measured under dark-adapted conditions before and after SKF application (Fig. 4A). sIPSC peak amplitude did not change significantly (Fig. 4B; P = 0.47, K-S test), and the average peak normalized to the dark-adapted condition was not significantly different (Fig. 4D, Table 1; P = 1.0, Wilcoxon test). This suggests that D1 receptor activation is unlikely modulating GABA receptors on OFF bipolar cells themselves. However, the sIPSC interevent intervals increased with SKF in all cells tested (Fig. 4C; example cell, P < 0.001; all individual cells, P < 0.001; K-S tests), and the sIPSC frequency, normalized to the dark-adapted condition, decreased by ~67% (Fig. 4D, Table 1; P = 0.029, Wilcoxon test).
Fig. 4.
Dopamine D1 receptor activation reduces GABAergic spontaneous frequency but not peak amplitude. A: example GABAergic spontaneous inhibitory postsynaptic current (sIPSC) traces in dark-adapted conditions before (black) and after SKF38393 (SKF) application (dark gray). B: example GABAergic sIPSC peak amplitude histogram, normalized (Norm.) to number of events, of the cell in A. Arrows show the average peak amplitude. C: same conditions as B for example GABAergic sIPSC interevent intervals of the cell seen in A. D: average sIPSC peak amplitude and frequency, normalized to the dark-adapted condition, with SKF application (n = 4). Brackets indicate comparison with the dark-adapted condition (dotted line). Error bars are ±SE, and significance was calculated with the Wilcoxon rank sum test (*P < 0.05 and ***P < 0.001).
The weak GABAergic input in dark-adapted OFF bipolar cells led to a relatively small number of cells where GABAergic inputs were successfully isolated (all but 1 cell had <2 spontaneous events per second). Additionally, since GABAergic sIPSCs were abolished in all cells tested with light adaptation (data not shown; n = 4), we could not compare the peak amplitude of sIPSCs between light-adapted and SKF conditions. Our findings suggest that D1 receptor-mediated reduction in GABAergic spontaneous inhibition acts through amacrine cell release. However, this reduction likely only makes a small contribution to the reduced spontaneous inhibitory input to OFF bipolar cells observed with SKF and increased background luminance because of the low baseline frequency of GABAergic events.
D1 receptor activation has no effect on local light-evoked GABAergic input to OFF bipolar cells.
Previous work found that the light-adapted reduction of OFF bipolar surrounds was due, in part, to a weaker GABAergic component limited to noncentral surround inputs (Mazade and Eggers 2016). Since activation of D1 receptors significantly decreased total central inhibitory input to OFF bipolar cells (Fig. 3) and decreased GABA release (Fig. 4), we wanted to determine whether D1 receptors could reduce local evoked GABAergic input. Pharmacologically isolated GABAergic responses (in strychnine to block glycine receptors) to a local bar stimulus were measured in the dark before and after SKF application. SKF application did not affect GABAergic local light-evoked inhibition (Fig. 5A). Response charge transfer, normalized to the dark-adapted condition, was unchanged with SKF (Fig. 5B; P = 0.314, Wilcoxon test). Although GABAergic response charge transfer was reduced in 5 of 7 cells with light adaptation, the average across cells was not significant (Fig. 5B; P = 0.18, Wilcoxon test). However, when the data were compared with SKF, response charge transfer was significantly higher than with light adaptation (Fig. 5B; P = 0.024, Wilcoxon test). Neither SKF nor light adaptation had a significant effect on GABAergic response peak amplitude (Fig. 5C, Table 2; SKF, P = 1.0; light-adapted, P = 0.69; SKF vs. light-adapted, P = 0.315; Wilcoxon tests). Although local GABAergic L-IPSCs became ~108% more transient with light adaptation (Fig. 5D; P < 0.001, Wilcoxon test), SKF did not change the temporal properties of light-evoked inhibition (Fig. 5D; P = 1.0, Wilcoxon test). The lack of any strong effects of D1 receptor activation on GABAergic L-IPSCs suggest that the dopaminergic pathway is not working through GABAergic amacrine cells to modulate local inner retinal inhibition similar to the effects of increased background luminance.
Fig. 5.
Dopamine D1 receptor activation has no effect on local light-evoked GABAergic input to OFF bipolar cells. A: example GABAergic light-evoked inhibitory postsynaptic currents (L-IPSCs) in dark-adapted (black) and SKF38393 (SKF; dark gray) conditions in response to a 1-s, 25-µm bar, centered on the cell. Light gray bar indicates the end of stimulus presentation. B: average response charge transfer normalized (Norm.) to the dark-adapted response in SKF (n = 4) and light-adapted conditions (n = 7). C: same conditions as B for average response peak amplitude in SKF (n = 4) and light-adapted conditions (n = 7). D: same conditions as B for average percentage change in the transient component of the L-IPSC in SKF (n = 4) and light-adapted (n = 7) conditions. Brackets indicate comparison with the dark-adapted condition (dotted line). resp., Response. E: same conditions as A for example GABAC L-IPSCs in dark-adapted (black) and SKF (dark gray) conditions. F: average response charge transfer and peak amplitude normalized to the dark-adapted response in SKF (n = 4) conditions. Light-adapted data were adapted from Mazade and Eggers (2016), Fig. 3, for comparison. Error bars are ±SE, and significance was calculated with the Wilcoxon rank sum test (***P < 0.001).
GABAergic spontaneous currents are mostly mediated through GABAA receptors (Eggers et al. 2007; Frech and Backus 2004), but evoked inhibition can occur through GABAA or GABAC receptors on the OFF bipolar cells. Although we found that only spontaneous frequency and not light-evoked inhibition was reduced with D1 receptor activation, we (Mazade and Eggers 2013, 2016) previously demonstrated that light adaptation significantly reduced GABAC receptor-mediated inputs. Since D1 receptor activation can also decrease GABAC receptor-mediated currents (Dong and Werblin 1994; Wellis and Werblin 1995), we investigated whether SKF had any effect on physiological light-evoked GABAC inhibition. We recorded isolated GABAC receptor-mediated local L-IPSCs (in SR, to block GABAA receptors, and strychnine, to block glycine receptors) and, as expected from GABAergic L-IPSCs (Fig. 5, A–D), observed no change in response with SKF (Fig. 5E). L-IPSC response charge transfer and peak amplitude were unchanged (Fig. 5F, Table 2; charge transfer, P = 1.0; peak amplitude, P = 0.314; Wilcoxon tests). In general, changes in GABAergic inhibition to OFF bipolar cells with light adaptation are not correlated with the activation of D1 receptors by dopamine, suggesting that D1 receptors play a very limited role in modulating GABAergic inhibition to OFF bipolar cells.
D1 receptor activation reduces glycinergic spontaneous activity to light-adapted levels.
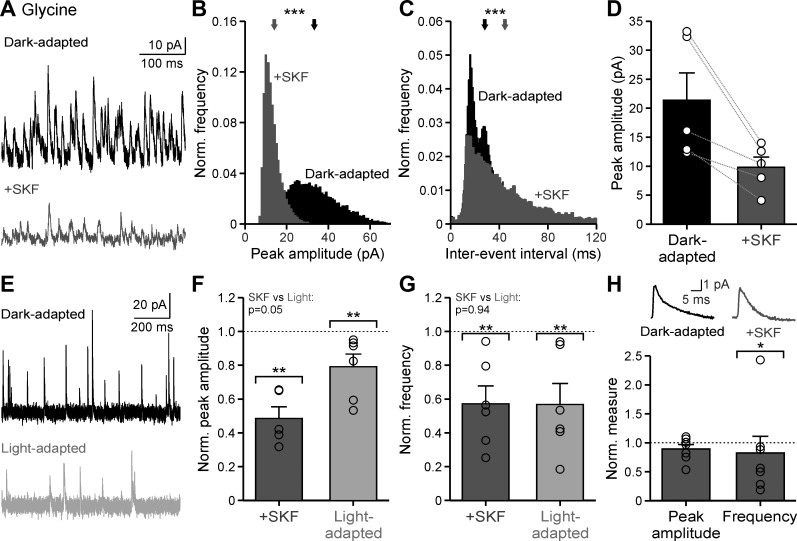
The results from Fig. 4 show that dopaminergic signaling has a limited effect on GABAergic sIPSCs, only reducing spontaneous frequency. However, since we observed a significant effect on both total sIPSC amplitude and frequency (Fig. 2), it is likely that D1 receptors are acting primarily through glycinergic pathways. Although GABAA receptors make a small contribution, OFF bipolar cell sIPSCs in the dark are dominated by glycine receptor-mediated currents (Eggers et al. 2007; Mazade and Eggers 2013) and the effect of dopamine on glycinergic currents is unknown. Therefore, we hypothesized that the light-adapted decrease in spontaneous currents is primarily due to a D1-mediated reduction in glycinergic input. Isolated glycinergic sIPSCs (in SR and TPMPA to block all GABA receptors) decreased significantly in dark-adapted conditions with application of SKF (Fig. 6A).
Fig. 6.
Dopamine D1 receptor activation reduces glycinergic spontaneous activity to light-adapted levels. A: example glycinergic spontaneous inhibitory postsynaptic current (sIPSC) traces in dark-adapted conditions before (black) and after SKF38393 (SKF) application (dark gray). B: example glycinergic sIPSC peak amplitude histogram, normalized (Norm.) to number of events, of the cell in A. Arrows show the average peak amplitude. C: same conditions as B for example glycinergic sIPSC interevent intervals of the cell seen in A. D: average peak amplitude in dark-adapted and SKF conditions (n = 5). E: example glycinergic sIPSC traces in dark- (black) and light-adapted (light gray) conditions. F: average peak amplitude, normalized to the dark-adapted condition, compared between SKF (n = 5) and light-adapted (n = 6) conditions. Brackets indicate comparison with the dark-adapted condition (dotted line). G: same conditions as F for average frequency compared between SKF (n = 5) and light-adapted (n = 6) conditions. H: average peak amplitude and frequency of glycinergic miniature (m)IPSCs with SKF application normalized to the dark-adapted condition (n = 7). Top inset shows example mIPSCs. Error bars are ±SE, and significance was calculated with the Wilcoxon rank sum test (*P < 0.05, **P < 0.01, ***P < 0.001).
Spontaneous current peak amplitude decreased in all cells with SKF (Fig. 6B; example cell, P < 0.001; all individual cells, P < 0.001; K-S tests), but the average reduction of raw peak amplitude was not significant (Fig. 6D, Table 1; P = 0.056, Wilcoxon test). Additionally, sIPSC interevent interval increased with SKF for all cells (Fig. 6C; example cell, P < 0.001; all individual cells, P < 0.01; K-S tests). Since isolated glycinergic spontaneous currents had not been measured under different luminance conditions, we recorded glycinergic currents in dark- and light-adapted conditions (Fig. 6E) and compared the effects with SKF application. Application of SKF reduced by ~52% the spontaneous peak amplitude of glycinergic events, when normalized to the dark-adapted condition (Fig. 6F; P = 0.008, Wilcoxon test), which was not significantly different from the ~21% reduction with light adaptation (Fig. 6F; P = 0.002; SKF vs. light-adapted, P = 0.052; Wilcoxon tests). Similarly, glycinergic spontaneous frequency, normalized to the dark-adapted condition, was reduced ~57% for both SKF (P = 0.002) and light-adapted (P = 0.002) conditions (Fig. 6G, Table 1; SKF vs. light-adapted, P = 0.937; Wilcoxon tests).
Similar to total inhibitory sIPSCs, glycinergic sIPSCs had reduced amplitude and frequency suggesting that both changes in glycine release from amacrine cells and modulation of glycine receptors on the OFF bipolar cells contribute to the effect of SKF. However, glycinergic sIPSCs in OFF bipolar cells have a high frequency and large amplitude, suggesting that at least some of these events could be due to release of multiple vesicles of neurotransmitter (multivesicular release). If multivesicular release contributes to the glycinergic sIPSCs, then the amplitude of these events would reflect both receptor properties on the postsynaptic cell and the number of vesicles of neurotransmitter released from the presynaptic cell. To differentiate between these possibilities, we instead determined the effect of SKF on glycinergic miniature (m)IPSCs (in the presence of SR, TPMPA, and TTX and Cd2+ to block voltage-gated Na+ and Ca2+ channels) that result from the spontaneous, non-Ca2+-mediated release of one vesicle of neurotransmitter. With the addition of TTX and Cd2+, the frequency and amplitude of spontaneous events were reduced (compare values between isolated glycinergic sIPSCs and mIPSCs in Table 1 and amplitude of s/mIPSC in Fig. 6). Because of this reduction, our suspicion that glycinergic spontaneous events were mediated in large part by multivesicular release was confirmed. For isolated mIPSCs, any changes in amplitude should reflect only postsynaptic changes and any changes in frequency should reflect only presynaptic changes to non-Ca2+-mediated release. Although the average peak amplitude of mIPSCs, normalized to the dark-adapted condition, did not change with SKF application (Fig. 6H, Table 1; P = 0.69, Wilcoxon test), mIPSC frequency was reduced by ~17% (Fig. 6H, Table 1; P = 0.017, Wilcoxon test). The small reduction in mIPSC frequency suggests some direct effects of D1 receptors on vesicle fusion and release. However, since there was no effect on mIPSC amplitude and much larger effects on sIPSC amplitude and frequency, our data suggest SKF is primarily changing Ca2+-mediated glycine release from amacrine cells and not modulating glycine receptors on OFF bipolar cells. We conclude that the main mechanism of D1 receptor action is through glycinergic amacrine cell release, which indicates that the reduction in inhibition to OFF bipolar cells with light adaptation is through a reduction in glycine release.
D1 receptor stimulation mirrors light-adapted reductions in local light-evoked glycinergic inhibition.
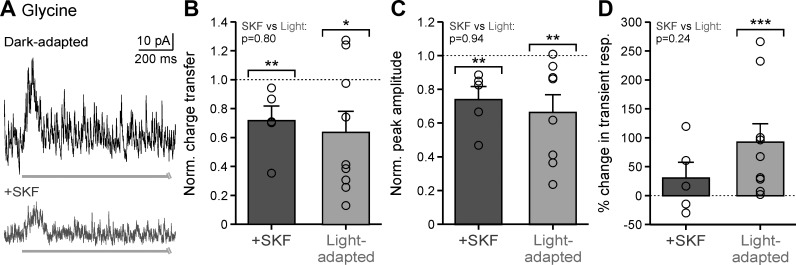
Light-evoked glycinergic input to OFF bipolar cells decreases with light adaptation (Mazade and Eggers 2013), and the glycinergic component of the receptive field surround becomes narrower and smaller (Mazade and Eggers 2016). However, modulation of light-evoked local glycinergic inputs by dopamine and how this compares with light-adapted changes has not been examined. Since activation of D1 receptors reduces total light-evoked (Fig. 3) and glycinergic spontaneous (Fig. 6) inhibition, and glycine is the dominant inhibitory signal in OFF bipolar cells (Eggers et al. 2007), we predicted that D1 receptor activation would elicit the same effects as increased background luminance.
With the use of the same local bar stimulus in Figs. 3 and 5, pharmacologically isolated glycinergic input (in SR and TPMPA to block all GABA receptors) was measured in dark-adapted conditions before and after SKF. OFF bipolar cell glycinergic L-IPSCs were significantly reduced with SKF application (Fig. 7A). The normalized charge transfer was significantly smaller by ~28% when D1 receptors were stimulated (Fig. 7B; P = 0.008, Wilcoxon test) and similar to the ~36% light-adapted reduction (Fig. 7B; P = 0.036; SKF vs. light-adapted, P = 0.797; Wilcoxon tests). Likewise, the peak amplitude of glycinergic-evoked input, normalized to dark-adapted responses, was reduced by ~26% for SKF and ~35% for light-adapted conditions (Fig. 7C, Table 2; SKF, P = 0.008; light-adapted, P = 0.006; SKF vs. light-adapted, P = 0.943; Wilcoxon tests). Whereas glycinergic L-IPSCs became ~92% more transient with light adaptation (Fig. 7D; P < 0.001, Wilcoxon test), D1 receptor activation had no significant effect on response time course (Fig. 7D; P = 0.683, Wilcoxon tests). These results suggest that D1 receptor activation is potentially correlated with light adaptation, where dopamine likely acts through glycinergic amacrine cells to reduce light-evoked responses in bright environments. However, the lack of increased response transience suggests that D1 receptors are not the only neuromodulator influencing light-adapted changes in inhibition.
Fig. 7.
Dopamine D1 receptor stimulation mirrors light-adapted reductions in local light-evoked glycinergic inhibition. A: example glycinergic light-evoked inhibitory postsynaptic currents (L-IPSCs) in dark-adapted (black) and SKF38393 (SKF; dark gray) conditions in response to a 1-s, 25-µm bar, centered on the cell. Light gray bar indicates the stimulus presentation. B: average response charge transfer, normalized (Norm.) to the dark-adapted response, in SKF (n = 5) and light-adapted conditions (n = 9). Brackets indicate comparison with dark-adapted condition (dotted line). C: same conditions as B for average response peak amplitude in SKF (n = 5) and light-adapted conditions (n = 8). D: same conditions as B for average percentage change in the transient component of the L-IPSC in SKF (n = 5) and light-adapted (n = 9) conditions. resp., Response. Light-adapted data were adapted from Mazade and Eggers (2016), Fig. 5, for comparison. Error bars are ±SE, and significance was calculated with the Wilcoxon rank sum test (*P < 0.05, **P < 0.01, ***P < 0.001).
DISCUSSION
We found that D1 receptor activation is sufficient to elicit the magnitude of light-adapted reductions in inhibition to OFF cone bipolar cells. This was likely mediated primarily by glycinergic inputs, as the D1 receptor- and light adaptation-mediated glycinergic reductions mirrored reductions of total inhibition. The site of action for these changes is most likely at the glycinergic amacrine cell terminals as the decrease in spontaneous events suggest reductions in presynaptic neurotransmitter release. However, light-adapted changes in GABAergic and glycinergic light-evoked response time course were not mimicked by D1 receptor activation, suggesting contributions of other mechanisms. Our findings in mouse OFF cone bipolar cells mirror recent findings in mouse rod bipolar cells showing that D1 receptor activation reduces light-evoked, electrically evoked, and spontaneous inhibition to rod bipolar cells in a similar manner as light adaptation (Flood et al. 2018). Since rod bipolar cells and OFF cone bipolar cells have very different inhibitory connections and roles in the retina, D1 receptor-mediated reductions in inhibition may be a common inner retinal mechanism that could contribute to light adaptation.
D1 receptor regulation of local inhibition may modulate ganglion cell responses.
Our results demonstrating that D1 receptor activation reduces bipolar cell inhibition add context to previous studies investigating how dopamine affects ganglion cell signals. Work in vitro and in vivo in rabbit OFF ganglion cells found that dopamine increased their spontaneous spiking activity, likely through D1 receptors (Jensen and Daw 1984, 1986). This is consistent with our results, as D1 receptor activation reduced both light-evoked and spontaneous OFF bipolar cell inhibition, which would increase bipolar cell glutamate release and ganglion cell responses. Although D1 receptor stimulation can explain dopamine-induced increases in ganglion cell activity, blocking dopamine should attenuate the inhibitory reduction and lead to a more inhibited bipolar cell output. This idea is supported by additional in vitro and in vivo studies in rabbit, cat, and mouse showing that blocking D1 receptors led to OFF ganglion cell hyperpolarization (Jensen 1992), reduced or abolished spontaneous activity (Jensen 1989, 1992; Jensen and Daw 1984, 1986; Maguire and Hamasaki 1994), and reduced light-evoked activity (Jensen and Daw 1984, 1986; Maguire and Hamasaki 1994; Yang et al. 2013). Furthermore, a recent study in bullfrog ON/OFF ganglion cells found that exogenous dopamine reduced the response latency of the OFF signal (Xiao et al. 2014). We find that D1 receptor activation made local light-evoked inhibition smaller and more transient, which could enable faster, more tuned inputs to ganglion cells. Our study, combined with previous reports, provides strong support for activation of dopaminergic pathways as a regulator of ganglion cell responses. Dopamine modulation of inner retinal inhibition could potentially regulate ganglion cell signals under different luminance conditions by changing bipolar cell release. In this way, dopamine control of the excitatory/inhibitory balance in the inner retina may explain increased ganglion cell signal strength with luminance (Barlow et al. 1957; Dedek et al. 2008; Farrow et al. 2013).
D1 receptors have a minimal effect on GABAergic local inhibition similar to light adaptation.
We report that D1 receptor activation has little effect on local and synaptic GABAergic inhibition to OFF bipolar cells. Although GABAergic spatial surrounds were reduced with light adaptation, local inhibition was not (Mazade and Eggers 2016), and we find that D1 receptor activation mirrors this lack of change. Whereas both dopamine and D1 receptors potentiated exogenous GABA-induced GABAA receptor currents in rat amacrine cells (Feigenspan and Bormann 1994a), the effects on synaptic or light-evoked GABAA receptor currents were unknown. We demonstrate that D1 receptors do not modulate postsynaptic GABAA receptors on OFF bipolar cells, unlike on rabbit ON bipolar cells (Chaffiol et al. 2017), since the peak amplitude of GABAergic spontaneous events did not change with D1 receptor activation. Instead, any effects on GABAergic currents were likely from decreased GABA release since we observed a reduction in spontaneous event frequency. However, the lack of effect of D1 receptors on GABAergic light-evoked inhibition suggests that reduced GABA release may play a minimal role in the total inhibitory reduction. This is supported by the relatively small GABAergic component compared with glycinergic component of inhibition to OFF bipolar cells in the dark (Eggers et al. 2007), which we also observed here (Tables 1 and 2). Nevertheless, reduced release is consistent with previous reports of dopamine decreasing GABA release in the chick (Calaza et al. 2001; Do Nascimento et al. 1998) and carp (Kato et al. 1985). However, others found that dopamine increased Ca2+-dependent and decreased Ca2+-independent GABA release in the goldfish (O’Brien and Dowling 1985).
Dopamine was also demonstrated to reduce GABA-evoked GABAC receptor-mediated currents in salamander bipolar cells (Wellis and Werblin 1995) and isolated catfish horizontal cells (Dong and Werblin 1994), and D1 receptor activation suppressed GABA release onto GABAC receptors in mouse ON bipolar cells (Smith et al. 2015). However, this was not shown in OFF bipolar cells. D1 receptor activation did not affect GABAC receptor-mediated input, unlike light adaptation (Mazade and Eggers 2013, 2016), indicating that D1 receptors are not responsible for modulating GABAC receptor currents in OFF bipolar cells. Together with previous findings, our results suggest that D1 receptors can affect GABAergic amacrine cell release but likely do not play a major role in the robust light-adapted reduction in total inhibition.
D1 receptors reduce glycinergic inhibition to light-adapted levels by modulating amacrine cell release.
Previous work has shown that light adaptation switches inhibition to OFF bipolar cells from glycinergic to GABAergic sources and decreases glycinergic local currents (Mazade and Eggers 2013, 2016). Our current results support these findings but add a correlation between the reduction in glycinergic local currents and D1 receptor activation. Additionally, the lack of large effects on GABAergic inhibition from D1 receptors or light adaptation supports that GABAergic inhibition is preserved in high-luminance conditions, whereas glycinergic inhibition is reduced. Our findings add to the relatively few studies investigating dopaminergic effects on glycinergic pathways. Work in the rabbit showed that the effects of D1 antagonists on the surround response of OFF ganglion cells (Falch et al. 1986; Jensen 1992) were blocked by inhibiting glycine receptors (Jensen 1989). This suggests dopamine is acting via glycine to increase ganglion cell firing, which is supported by our data. Although spontaneous glycine receptor currents were both smaller and less frequent after D1 receptor activation, miniature events evoked by single vesicles were only slightly less frequent. This suggests that glycine receptors on OFF bipolar cells are not directly modulated by D1 receptors. Thus D1 receptors appear to regulate multivesicular spontaneous events mainly by reducing glycine release from amacrine cells, possibly by D1 receptor reduction of Ca2+ currents as seen in mouse horizontal cells (Liu et al. 2016). This agrees with a previous study that showed dopamine application inhibited spontaneous release of [3H]glycine in the isolated rat retina (Pycock and Smith 1983). The reduction in total spontaneous inhibition is likely from reduced glycinergic spontaneous events, which would cause OFF bipolar cell depolarization and increase glutamate release. AII amacrine cells would be a large source of glycine since they provide significant inhibitory input to most OFF bipolar cells in the dark. D1 receptors (Hampson et al. 1992; Kothmann et al. 2009; Xia and Mills 2004) and increased background illumination (Bloomfield et al. 1997; Xin and Bloomfield 1997, 1999a) uncouple AII amacrine cell networks, which could lead to less activation and subsequently less glycinergic input to OFF bipolar cells. Our data provide a strong correlation between the reduction of local and spontaneous glycinergic input and the total inhibitory reduction seen with both D1 receptor activation and light adaptation.
Other factors may also contribute to light-adapted changes in inner retinal inhibition.
Overall, the results of this study implicate D1 receptor-mediated changes in glycinergic inhibition as being sufficient for most light-adapted changes in inner retinal inhibition. However, our results did not determine whether D1 receptors are the definitive mechanism. Future experiments using D1 receptor antagonists or D1 receptor/tyrosine hydroxylase (dopamine precursor enzyme) knockouts would test this question. These experiments, along with our work, would establish both sufficiency and necessity of D1 receptors for light adaptation.
Although we focused on D1 receptors as the most likely factor for replicating the effects of light adaptation, additional factors are likely involved. The rod pathway-to-cone pathway switch during light adaptation, horizontal cell inputs that do not involve D1 receptors, D4 receptors on photoreceptors (Cohen et al. 1992) as well as throughout the inner retinal layers (Li et al. 2013), and D2 receptors on the dopaminergic amacrine cells (Jackson et al. 2009; Nguyen-Legros et al. 1999; Pozdeyev et al. 2008) could all contribute to light-adapted changes. Finally, other neuromodulators, such as nitric oxide, which is released with light (Neal et al. 1998), may also play a role. It is reasonable that the retina would be influenced in a multifactorial manner during light adaptation to adjust retinal signaling, but more work is needed to determine how retinal inhibition is affected by these other pathways.
We provide evidence that bipolar cell inhibition is modulated by activation of D1 receptors. The correlations between the effects of D1 receptor activation and light adaptation suggest that D1 receptors are a likely candidate for the mechanism behind this process. Since dopamine release increases with light (Witkovsky 2004) and is necessary for high-contrast sensitivity and visual acuity (Jackson et al. 2012), we propose that D1 receptors are important for mediating retinal adaptation at the earlier level of inner retinal, bipolar cell signal processing. Our results could also have an impact on visual disease since dopamine dysfunction is linked with vision impairments in diabetic retinopathy (Aung et al. 2014; Gastinger et al. 2006; Nishimura and Kuriyama 1985), which may be partly due to changes in retinal inhibition (Kawasaki et al. 1987; Moore-Dotson et al. 2016; Ramsey et al. 2006; Shinoda et al. 2007) that could originate in the inner retina.
GRANTS
This study was supported by National Science Foundation Grant 1552184, Department of Defense Army Research Office Grant W911NF-15-1-0613, and National Eye Institute Grant R01-EY-026027 to E. D. Eggers, National Heart, Lung, and Blood Institute Grant 4-T32-HL-007249-40 to M. D. Flood, and Achievement Rewards for College Scientists Foundation and a University of Arizona Professional Student Council Research and Project Grant to R. E. Mazade.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.E.M. and E.D.E. conceived and designed research; R.E.M. and M.D.F. performed experiments; R.E.M. and M.D.F. analyzed data; R.E.M., M.D.F., and E.D.E. interpreted results of experiments; R.E.M. prepared figures; R.E.M. drafted manuscript; R.E.M., M.D.F., and E.D.E. edited and revised manuscript; R.E.M., M.D.F., and E.D.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Nicholas Brecha and Arlene Hirano for helpful comments on the manuscript.
Present address of R. E. Mazade: Dept. of Biological and Vision Sciences, Alonso Laboratory, State Univ. of New York College of Optometry, 33 W 42nd St., New York, NY 10036.
REFERENCES
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27: 513–523, 2000. doi: 10.1016/S0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Aung MH, Park HN, Han MK, Obertone TS, Abey J, Aseem F, Thule PM, Iuvone PM, Pardue MT. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci 34: 726–736, 2014. doi: 10.1523/JNEUROSCI.3483-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Fitzhugh R, Kuffler SW. Change of organization in the receptive fields of the cat’s retina during dark adaptation. J Physiol 137: 338–354, 1957. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Ehinger B, Åberg L. [3H]-Dopamine release from the rabbit retina. Albrecht Von Graefes Arch Klin Exp Ophthalmol 215: 71–78, 1980. doi: 10.1007/BF00414464. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Vis Neurosci 14: 565–576, 1997. doi: 10.1017/S0952523800012220. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Hoel MJ, Iuvone PM. Stimulation of endogenous dopamine release and metabolism in amphibian retina by light- and K+-evoked depolarization. Brain Res 482: 164–168, 1989. doi: 10.1016/0006-8993(89)90555-6. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Calaza KC, de Mello FG, Gardino PF. GABA release induced by aspartate-mediated activation of NMDA receptors is modulated by dopamine in a selective subpopulation of amacrine cells. J Neurocytol 30: 181–193, 2001. doi: 10.1023/A:1012764422711. [DOI] [PubMed] [Google Scholar]
- Chaffiol A, Ishii M, Cao Y, Mangel SC. Dopamine regulation of GABAA receptors contributes to light/dark modulation of the ON-cone bipolar cell receptive field surround in the retina. Curr Biol 27: 2600–2609.e4, 2017. doi: 10.1016/j.cub.2017.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O’Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci USA 89: 12093–12097, 1992. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek K, Pandarinath C, Alam NM, Wellershaus K, Schubert T, Willecke K, Prusky GT, Weiler R, Nirenberg S. Ganglion cell adaptability: does the coupling of horizontal cells play a role? PLoS One 3: e1714, 2008. doi: 10.1371/journal.pone.0001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Nascimento JL, Kubrusly RC, Reis RA, De Mello MC, De Mello FG. Atypical effect of dopamine in modulating the functional inhibition of NMDA receptors of cultured retina cells. Eur J Pharmacol 343: 103–110, 1998. doi: 10.1016/S0014-2999(97)01522-7. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Werblin FS. Dopamine modulation of GABAC receptor function in an isolated retinal neuron. J Neurophysiol 71: 1258–1260, 1994. doi: 10.1152/jn.1994.71.3.1258. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci 19: 593–601, 2002. doi: 10.1017/S0952523802195058. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the mammalian retina. J Neurosci 26: 3959–3970, 2006. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Lankheet MJ, Rieke F. Light adaptation in cone vision involves switching between receptor and post-receptor sites. Nature 449: 603–606, 2007. doi: 10.1038/nature06150. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABAA, GABAC and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Mazade RE, Klein JS. Inhibition to retinal rod bipolar cells is regulated by light levels. J Neurophysiol 110: 153–161, 2013. doi: 10.1152/jn.00872.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falch E, Hedegaard A, Nielsen L, Jensen BR, Hjeds H, Krogsgaard-Larsen P. Comparative stereostructure-activity studies on GABAA and GABAB receptor sites and GABA uptake using rat brain membrane preparations. J Neurochem 47: 898–903, 1986. doi: 10.1111/j.1471-4159.1986.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Farrow K, Teixeira M, Szikra T, Viney TJ, Balint K, Yonehara K, Roska B. Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron 78: 325–338, 2013. doi: 10.1016/j.neuron.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Farshi P, Fyk-Kolodziej B, Krolewski DM, Walker PD, Ichinose T. Dopamine D1 receptor expression is bipolar cell type-specific in the mouse retina. J Comp Neurol 524: 2059–2079, 2016. doi: 10.1002/cne.23932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proc Natl Acad Sci USA 91: 10893–10897, 1994a. doi: 10.1073/pnas.91.23.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Modulation of GABAC receptors in rat retinal bipolar cells by protein kinase C. J Physiol 481: 325–330, 1994b. doi: 10.1113/jphysiol.1994.sp020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood MD, Moore-Dotson JM, Eggers ED. Dopamine D1 receptor activation contributes to light-adapted changes in retinal inhibition to rod bipolar cells. J Neurophysiol 120: 867–879, 2018. doi: 10.1152/jn.00855.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech MJ, Backus KH. Characterization of inhibitory postsynaptic currents in rod bipolar cells of the mouse retina. Vis Neurosci 21: 645–652, 2004. doi: 10.1017/S0952523804214134. [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci 47: 3143–3150, 2006. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70–82, 2004. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Godley BF, Wurtman RJ. Release of endogenous dopamine from the superfused rabbit retina in vitro: effect of light stimulation. Brain Res 452: 393–395, 1988. doi: 10.1016/0006-8993(88)90046-7. [DOI] [PubMed] [Google Scholar]
- Green DG, Dowling JE, Siegel IM, Ripps H. Retinal mechanisms of visual adaptation in the skate. J Gen Physiol 65: 483–502, 1975. doi: 10.1085/jgp.65.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DG, Powers MK. Mechanisms of light adaptation in rat retina. Vision Res 22: 209–216, 1982. doi: 10.1016/0042-6989(82)90120-1. [DOI] [PubMed] [Google Scholar]
- Guo C, Hirano AA, Stella SL Jr, Bitzer M, Brecha NC. Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD65, and the GABA vesicular transporter. J Comp Neurol 518: 1647–1669, 2010. doi: 10.1002/cne.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Stella SL Jr, Hirano AA, Brecha NC. Plasmalemmal and vesicular γ-aminobutyric acid transporter expression in the developing mouse retina. J Comp Neurol 512: 6–26, 2009. doi: 10.1002/cne.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson EC, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci 12: 4911–4922, 1992. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsanyi K, Mangel SC. Activation of a D2 receptor increases electrical coupling between retinal horizontal cells by inhibiting dopamine release. Proc Natl Acad Sci USA 89: 9220–9224, 1992. doi: 10.1073/pnas.89.19.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci 25: 5438–5445, 2005. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu EH, Pan F, Völgyi B, Bloomfield SA. Light increases the gap junctional coupling of retinal ganglion cells. J Physiol 588: 4145–4163, 2010. doi: 10.1113/jphysiol.2010.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. Ambient light regulates sodium channel activity to dynamically control retinal signaling. J Neurosci 27: 4756–4764, 2007. doi: 10.1523/JNEUROSCI.0183-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Chaurasia SS, Zhou H, Haque R, Storm DR, Iuvone PM. Essential roles of dopamine D4 receptors and the type 1 adenylyl cyclase in photic control of cyclic AMP in photoreceptor cells. J Neurochem 109: 148–157, 2009. doi: 10.1111/j.1471-4159.2009.05920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, McMahon DG. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci 32: 9359–9368, 2012. doi: 10.1523/JNEUROSCI.0711-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RJ. Effects of the dopamine antagonist (+)-SCH 23390 on intracellularly recorded responses of ganglion cells in the rabbit retina. Vis Neurosci 8: 463–467, 1992. doi: 10.1017/S095252380000496X. [DOI] [PubMed] [Google Scholar]
- Jensen RJ. Mechanism and site of action of a dopamine D1 antagonist in the rabbit retina. Vis Neurosci 3: 573–585, 1989. doi: 10.1017/S0952523800009901. [DOI] [PubMed] [Google Scholar]
- Jensen RJ, Daw NW. Effects of dopamine and its agonists and antagonists on the receptive field properties of ganglion cells in the rabbit retina. Neuroscience 17: 837–855, 1986. doi: 10.1016/0306-4522(86)90049-7. [DOI] [PubMed] [Google Scholar]
- Jensen RJ, Daw NW. Effects of dopamine antagonists on receptive fields of brisk cells and directionally selective cells in the rabbit retina. J Neurosci 4: 2972–2985, 1984. doi: 10.1523/JNEUROSCI.04-12-02972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Negishi K, Teranishi T. Dopamine inhibits calcium-independent γ-[3H]aminobutyric acid release induced by kainate and high K+ in the fish retina. J Neurochem 44: 893–899, 1985. doi: 10.1111/j.1471-4159.1985.tb12900.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Yonemura K, Yokogawa Y, Saito N, Kawakita S. Correlation between ERG oscillatory potential and psychophysical contrast sensitivity in diabetes. Doc Ophthalmol 64: 209–215, 1987. doi: 10.1007/BF00159995. [DOI] [PubMed] [Google Scholar]
- Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci 29: 14903–14911, 2009. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O’Brien J. Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. J Neurosci 33: 3135–3150, 2013. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Grove JC, Hirano AA, Brecha NC, Barnes S. Dopamine D1 receptor modulation of calcium channel currents in horizontal cells of mouse retina. J Neurophysiol 116: 686–697, 2016. doi: 10.1152/jn.00990.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G, Hamasaki DI. The retinal dopamine network alters the adaptational properties of retinal ganglion cells in the cat. J Neurophysiol 72: 730–741, 1994. doi: 10.1152/jn.1994.72.2.730. [DOI] [PubMed] [Google Scholar]
- Mazade RE, Eggers ED. Light adaptation alters inner retinal inhibition to shape OFF retinal pathway signaling. J Neurophysiol 115: 2761–2778, 2016. doi: 10.1152/jn.00948.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazade RE, Eggers ED. Light adaptation alters the source of inhibition to the mouse retinal OFF pathway. J Neurophysiol 110: 2113–2128, 2013. doi: 10.1152/jn.00384.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Dotson JM, Beckman JJ, Mazade RE, Hoon M, Bernstein AS, Romero-Aleshire MJ, Brooks HL, Eggers ED. Early retinal neuronal dysfunction in diabetic mice: reduced light-evoked inhibition increases rod pathway signaling. Invest Ophthalmol Vis Sci 57: 1418–1430, 2016. doi: 10.1167/iovs.15-17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka KI, Chan RY, Yasui S. Adaptation in catfish retina. J Neurophysiol 42: 441–454, 1979. doi: 10.1152/jn.1979.42.2.441. [DOI] [PubMed] [Google Scholar]
- Neal M, Cunningham J, Matthews K. Selective release of nitric oxide from retinal amacrine and bipolar cells. Invest Ophthalmol Vis Sci 39: 850–853, 1998. [PubMed] [Google Scholar]
- Nguyen-Legros J, Simon A, Caillé I, Bloch B. Immunocytochemical localization of dopamine D1 receptors in the retina of mammals. Vis Neurosci 14: 545–551, 1997. doi: 10.1017/S0952523800012207. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Vernier P. Dopamine receptor localization in the mammalian retina. Mol Neurobiol 19: 181–204, 1999. doi: 10.1007/BF02821713. [DOI] [PubMed] [Google Scholar]
- Nishimura C, Kuriyama K. Alterations in the retinal dopaminergic neuronal system in rats with streptozotocin-induced diabetes. J Neurochem 45: 448–455, 1985. doi: 10.1111/j.1471-4159.1985.tb04008.x. [DOI] [PubMed] [Google Scholar]
- Nobles RD, Zhang C, Müller U, Betz H, McCall MA. Selective glycine receptor α2 subunit control of crossover inhibition between the on and off retinal pathways. J Neurosci 32: 3321–3332, 2012. doi: 10.1523/JNEUROSCI.5341-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien DR, Dowling JE. Dopaminergic regulation of GABA release from the intact goldfish retina. Brain Res 360: 41–50, 1985. doi: 10.1016/0006-8993(85)91218-1. [DOI] [PubMed] [Google Scholar]
- Page-McCaw PS, Chung SC, Muto A, Roeser T, Staub W, Finger-Baier KC, Korenbrot JI, Baier H. Retinal network adaptation to bright light requires tyrosinase. Nat Neurosci 7: 1329–1336, 2004. doi: 10.1038/nn1344. [DOI] [PubMed] [Google Scholar]
- Pozdeyev N, Tosini G, Li L, Ali F, Rozov S, Lee RH, Iuvone PM. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur J Neurosci 27: 2691–2700, 2008. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycock CJ, Smith LF. Interactions of dopamine and the release of [3H]-taurine and [3H]-glycine from the isolated retina of the rat. Br J Pharmacol 78: 395–404, 1983. doi: 10.1111/j.1476-5381.1983.tb09404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey DJ, Ripps H, Qian H. An electrophysiological study of retinal function in the diabetic female rat. Invest Ophthalmol Vis Sci 47: 5116–5124, 2006. doi: 10.1167/iovs.06-0364. [DOI] [PubMed] [Google Scholar]
- Shapley RM, Enroth-Cugell C. Visual adaptation and retinal gain controls. Prog Retin Eye Res 3: 263–346, 1984. doi: 10.1016/0278-4327(84)90011-7. [DOI] [Google Scholar]
- Shinoda K, Rejdak R, Schuettauf F, Blatsios G, Völker M, Tanimoto N, Olcay T, Gekeler F, Lehaci C, Naskar R, Zagorski Z, Zrenner E. Early electroretinographic features of streptozotocin-induced diabetic retinopathy. Clin Exp Ophthalmol 35: 847–854, 2007. doi: 10.1111/j.1442-9071.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Côté PD, Tremblay F. Dopamine modulation of rod pathway signaling by suppression of GABAC feedback to rod-driven depolarizing bipolar cells. Eur J Neurosci 42: 2258–2270, 2015. doi: 10.1111/ejn.12993. [DOI] [PubMed] [Google Scholar]
- Tamura T, Nakatani K, Yau KW. Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol 98: 95–130, 1991. doi: 10.1085/jgp.98.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Wässle H. Immunohistochemical localization of dopamine D1 receptors in rat retina. Eur J Neurosci 8: 2286–2297, 1996. doi: 10.1111/j.1460-9568.1996.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Wellis DP, Werblin FS. Dopamine modulates GABAC receptors mediating inhibition of calcium entry into and transmitter release from bipolar cell terminals in tiger salamander retina. J Neurosci 15: 4748–4761, 1995. doi: 10.1523/JNEUROSCI.15-07-04748.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol 108: 17–39, 2004. doi: 10.1023/B:DOOP.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Janisch KM, Peshenko IV, Dizhoor AM, Tsang SH, Fain GL. Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J Neurosci 28: 2064–2074, 2008. doi: 10.1523/JNEUROSCI.2973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XB, Mills SL. Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina. Vis Neurosci 21: 791–805, 2004. doi: 10.1017/S0952523804215127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Zhang PM, Gong HQ, Liang PJ. Effects of dopamine on response properties of ON-OFF RGCs in encoding stimulus durations. Front Neural Circuits 8: 72, 2014. doi: 10.3389/fncir.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis Neurosci 16: 653–665, 1999a. doi: 10.1017/S0952523899164058. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Dark- and light-induced changes in coupling between horizontal cells in mammalian retina. J Comp Neurol 405: 75–87, 1999b. doi:. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. J Comp Neurol 383: 512–528, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Yang J, Pahng J, Wang GY. Dopamine modulates the off pathway in light-adapted mouse retina. J Neurosci Res 91: 138–150, 2013. doi: 10.1002/jnr.23137. [DOI] [PubMed] [Google Scholar]
- Zhang AJ, Wu SM. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci 29: 789–797, 2009. doi: 10.1523/JNEUROSCI.4984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]