Abstract
Obesity, characterized by increased adiposity that develops when energy intake outweighs expenditure, is rapidly becoming a serious health crisis that affects millions of people worldwide and is associated with severe comorbid disorders including hypertension, cardiovascular disease, and type II diabetes. Obesity is also associated with the dysregulation of central neurocircuits involved in the control of autonomic, metabolic, and cognitive functions. Systemic inflammation associated with diet-induced obesity (DIO) has been proposed to be responsible for the development of these comorbidities as well as the dysregulation of central neurocircuits. A growing body of evidence suggests, however, that exposure to a high-fat diet (HFD) may cause neuroinflammation and astroglial activation even before systemic inflammation develops, which may be sufficient to cause dysregulation of central neurocircuits involved in energy homeostasis before the development of obesity. The purpose of this review is to summarize the current literature exploring astroglial-dependent modulation of central circuits following exposure to HFD and DIO, including not only dysregulation of neurocircuits involved in energy homeostasis and feeding behavior, but also the dysregulation of learning, memory, mood, and reward pathways.
Keywords: astroglia, diet, neuroplasticity, obesity
INTRODUCTION
Over the past two decades, the rates of obesity and associated metabolic disorders have increased dramatically, both in the United States and worldwide (Hammond and Levine 2010). Obesity and its comorbid disorders, including hypertension, type II diabetes, heart disease, stroke, and osteoarthritis, represent a serious health risk and imposes a significant strain on the economy, costing the United States an estimated $147 billion annually in healthcare and associated productivity costs (Hammond and Levine 2010; Paeratakul et al. 2002). While obesity is recognized as a multifactorial disorder with strong genetic and environmental components, it ultimately results from disordered and dysregulated energy balance, where caloric intake exceeds energy expenditure (Levin 2006, 2010a, 2010b). Prolonged exposure to a high-fat diet (HFD) increases food and caloric intake per meal, resulting in weight gain and increased adiposity in both humans and animal models alike (Daly et al. 2011; de Lartigue et al. 2011). Interestingly, prolonged HFD exposure and diet-induced obesity (DIO) are associated with not only dysregulation of autonomic and metabolic functions related to energy homeostasis (Chaar et al. 2016; Kentish et al. 2016; Little and Feinle-Bisset 2011; Little et al. 2007; McMenamin et al. 2018; Troy et al. 2016), but also the disruption of higher order functions such as learning, memory, mood, reward processes, and hippocampal activity (Cano et al. 2014; Hao et al. 2016; Spencer et al. 2017; Wu et al. 2018). One common thread between DIO and its wide range of comorbid disorders is the inflammation associated with increased adiposity (Ávalos et al. 2018; Belegri et al. 2018; Dalvi et al. 2017; Guillemot-Legris et al. 2016; Spencer et al. 2017). Whether inflammation plays a significant role in the development of obesity, however, appears to be a more challenging and unanswered question—one that is rapidly becoming an area of great interest.
DIO has a strong association with systemic inflammation, which has been assumed to lead to the development of neuroinflammation and the dysregulation of autonomic and metabolic functions (Ávalos et al. 2018; Bastard et al. 2006; Belegri et al. 2018; Purkayastha and Cai 2013; Tilg and Moschen 2006; Trayhurn 2005). Several groups have demonstrated recently, however, that neuroinflammation is detectable in central regions responsible for the regulation of food intake and energy homeostasis after only 1 day of HFD exposure, long before systemic inflammation is detected (Belegri et al. 2018; Waise et al. 2015), and well in advance of dysregulation of brainstem and hypothalamic neurocircuits (Astiz et al. 2017; Belegri et al. 2018, Buckman et al. 2015; Clyburn et al. 2018). This raises the question whether diet-associated neuroinflammation can occur independently of increased adiposity and its associated systemic inflammation, and whether neuroinflammation-induced neurocircuit dysregulation may also contribute directly toward the development of obesity and its associated comorbid disorders. The mechanisms by which HFD exposure can induce central neuroinflammation in the absence of increased adiposity and circulating proinflammatory cytokines have not been examined thoroughly, however. The primary immunoregulatory cells of the central nervous system (CNS), astrocytes and microglia, are able to modulate synaptic strength and neuronal excitability when they are phenotypically active, and usually observed during neuroinflammation, suggesting that astroglial activation following HFD exposure may be involved in the dysregulation of central neurocircuits associated with food intake and energy homeostasis following HFD and DIO exposure (Bonansco et al. 2011; Clasadonte and Prevot 2018; Fellin et al. 2004).
Understanding how altered diet composition and increased caloric intake affects neurosignaling to promote the development of obesity is critically important to elucidating novel therapeutic strategies to limit food intake and weight gain. The purpose of this review is to summarize the current literature exploring astroglial-dependent modulation of central circuits following exposure to HFD and DIO, in addition to highlighting evidence that suggests acute alterations in diet cause neuroinflammation that result not only in the dysregulation of energy homeostasis and feeding behavior, but also in the dysregulation of learning, memory, mood, and reward pathways. While, to date, only a few studies have examined the direct involvement of astroglia in the regulation of food intake during HFD exposure, there is a strong body of literature detailing the effects of HFD on astroglial modulation as well as the corresponding downstream effects of astroglia on local neurocircuits suggesting that astroglia may, at the very least, play a prominent role in diet-induced central neuroplasticity.
NEUROINFLAMMATION AND ASTROGLIAL FUNCTION
Canonically, DIO has a strong association with systemic inflammation in adipose and hepatic tissue (Bastard et al. 2006; Das 2010; Trayhurn 2005). While adipose tissue is responsible for the storage of energy in the form of lipid droplets, adipocytes are closely related to macrophages and participate in the inflammatory cascade (Grant and Dixit 2015; Tilg and Moschen 2006) and several other adipose tissue cell types, including lymphocytes and fibroblasts, serve as modulators of the immune system (Grant and Dixit 2015; Schäffler et al. 2007). Because of its secondary function as an immunoregulatory tissue, the level of adiposity has been assumed to correlate directly with serum levels of inflammatory cytokines (Bastard et al. 2006; Naznin et al. 2015). Following chronic HFD exposure and the development of DIO, levels of critical circulating immunoregulatory proteins, including the proinflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-6 and -1 (IL-6 and IL-1, respectively), increase (Fain 2006; Wisse 2004). Chronic adipose-associated systemic inflammation is associated with CNS neuroinflammation and with the consequent dysregulation of multiple neurocircuits, including those involved in caloric intake and energy homeostasis (Ávalos et al. 2018; Belegri et al. 2018; Dalvi et al. 2017; Guillemot-Legris et al. 2016; Li et al. 2018; Purkayastha and Cai 2013; Wu et al. 2014). While circulating cytokines have been shown to enter the central parenchyma through saturable transport mechanisms or via selective uptake in discrete brain regions such as the septum, emerging evidence suggests that neuroinflammation in response to HFD per se may actually appear long before the increase in circulating cytokines and the development of systemic inflammation associated with DIO (Banks and Kastin 1991; Bauer et al. 2007; Gutierrez et al. 1993).
Immune responses within the CNS involve a specialized group of inflammatory cells, composed primarily of microglia and astrocytes (Barone and Kilgore 2006; Cartier et al. 2005). In healthy, uninflamed tissue, astroglia (i.e., astrocytes and microglia) were described originally as being a merely passive and supportive cell type responsible primarily for maintaining a healthy neuronal population (Kimelberg 2004, 2007; Wang and Bordey 2008); they have since been shown to fulfill multiple critical roles in CNS function (Chowen et al. 2013; Pérez-Alvarez and Araque 2013; Pérez-Alvarez et al. 2014; Um 2017). Astrocytes, named for their star-shaped morphology, are derived from neural progenitor cells in the neuroectoderm and are responsible for maintaining the blood-brain barrier (BBB), promoting neuronal survival, and formation and maintenance of the synapse (Kimelberg 2007; Wang and Bordey 2008). Astrocytes that come in contact with and maintain the BBB, for example, referred to as tanycytes, play an important role in controlling BBB permeability to peptides and proteins in addition to acting as important biosensors, modulating neuronal activity in response to circulating factors (Langlet 2014). In contrast, microglia originate from hematopoietic stem cells and are the innate immune cells of the CNS (Eglitis and Mezey 1997; Wang and Bordey 2008).
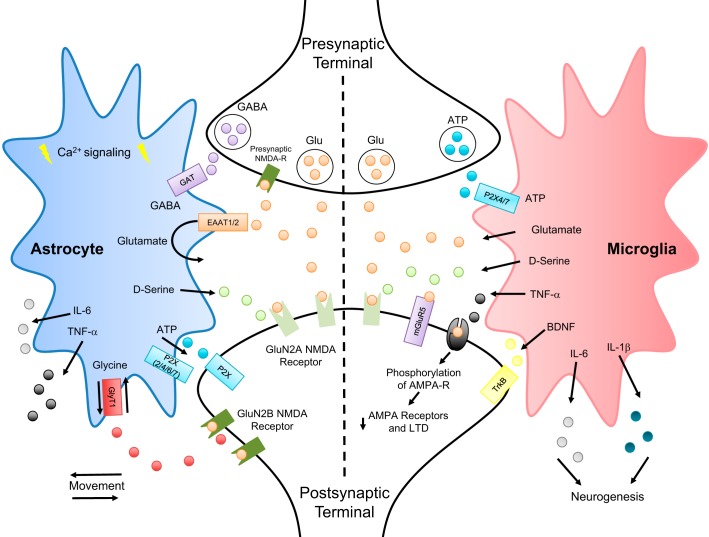
While astrocytes and microglia have been considered to fulfill distinct and separate primary functions, their roles are closely intertwined (Pascual et al. 2012; Rothhammer and Quintana 2015). Recent evidence suggests that each cell type has multiple subtypes and activity states, making their functional classification more complex (Kimelberg 2004; Nakajima and Kohsaka 2001). Microglial activation via LPS administration, for example, can trigger astrocyte activation through the release of ATP (Pascual et al. 2012) and, once activated, astrocytes are then able to modulate excitatory neurotransmission (Bonansco et al. 2011; Fellin et al. 2004; Jourdain et al. 2007; Pascual et al. 2012). Astrocytes also form regional specific networks through gap junction coupling (Contreras et al. 2002; Lee et al. 1994), which also allow for direct cytoplasmic coupling through the docking of hemichannels composed of membrane-located connexin 43 dodecamers (Contreras et al. 2002). This unique feature allows not only for the transmission of ionic and metabolic signals within a local network, but the amplification and coordination of astrocytic responses, including the modulation of excitatory neurotransmission (Contreras et al. 2002; Lee et al. 1994). Other studies have indicated that microglia may modulate synaptic strength both directly, through the release of ATP and glutamate, to activate purinergic and both ionotropic and metabotropic glutamate receptors, respectively (Angulo et al. 2004; Bessis et al. 2007; Roumier et al. 2004), as well as indirectly, through the release of d-serine (which acts as a coagonist of NMDA receptors) and brain-derived neurotrophic factor (BDNF) which has multiple actions to modulate both excitatory and inhibitory synaptic transmission (Bessis et al. 2007; Rose et al. 2018). BDNF, for example, which acts on the tyrosine kinase B (TrkB) receptor, can increase neuronal excitability not only by downregulating the K+-Cl− cotransporter (KCC2), thus altering chloride homeostasis and inducing a depolarizing shift in the reversal potential for fast GABAergic transmission (Coull et al. 2005; Gomes et al. 2013), but also by phosphorylation and activation of NR1 subunit of NMDA receptors (Fig. 1) (Liu et al. 2015).
Fig. 1.
Astroglial modulation of synaptic strength. A representative diagram illustrating key mechanisms by which astrocytes and microglia may modulate synaptic strength. Astrocytes are able to modulate synaptic strength through both neurotransmitter reuptake as well as by gliotransmitter release. By physically moving closer or further away from the synapse, thereby increasing or decreasing the rate of neurotransmitter reuptake of GABA through GAT, glutamate through EAAT1/2, and glycine through GlyT1, astrocytes are able to alter the neurotransmitter concentration in the synaptic cleft. Astrocytes are also responsible for the nonvesicular release of gliotransmitters such as glutamate, ATP, TNF-α, d-serine (coagonist for the synaptic NMDA receptors), and glycine (coagonist for the extrasynaptic NMDA receptors) through the functional reversal of the GlyT1 transporter. Astrocytes can also communicate to nearby glia through gap junctions and calcium signaling to coordinate responses in distinct networks. Microglia modulate synaptic strength through the release of proinflammatory cytokines and other neuroactive substances, including glutamate, d-serine, TNF-α, BDNF, IL-6, and IL-1. Indeed, microglial release of TNF-α has been shown to phosphorylate AMPA receptors resulting in their internalization, while BDNF release activates neuronal TrkB receptors hence modulates neuronal excitability. Cytokine release from microglia can also modulate neuronal networks by promoting neurogenesis, and may be responsible for the activation of local astrocytes, leading to further adaptations in synaptic plasticity. While further investigations are required to determine the exact cascade of events that results in diet-induced astroglial modulation of synaptic strength, several lines of evidence suggest that high-fat diet exposure leads to microglial activation and acute inflammation, which then induces astrocyte activation. Once activated, both astrocytes and microglia are able to modulate their control over synaptic strength through the mechanisms described above. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDNF, brain-derived neurotrophic factor; EAAT1/2, excitatory amino acid transporters; GlyT1, glycine transporter; GAT, GABA transporter; LTD, long-term depression; NMDA, N-methyl-d-aspartate; P2X, puringergic P2X receptor; -R, receptor; TrkB, tyrosine kinase B.
While astrocytic modulation of excitatory neurotransmission can modulate the gain and efficacy of nearby neuronal synapses, and subsequently modulate and alter physiological output, microglial release of glutamate and d-serine can also induce excitotoxicity and neurodegeneration throughout the CNS, including the hippocampus and cortex (Bessis et al. 2007; Bonansco et al. 2011; Carson et al. 2006; Hao et al. 2016; Nakajima and Kohsaka 2001). Inhibition of microglial glutamate and d-serine release following ischemic stroke rescues the observed loss of cortical neuron viability, while stimulation of astroglia in the hippocampus with soluble amyloid precursor protein (sAPP) results in hemichannel-dependent release of glutamate, and subsequent neuronal excitotoxicity (Barger and Basile 2001; Kim et al. 2007; Yrjänheikki et al. 1998). While astroglial modulation of synaptic strength will be discussed in more detail below; it is important to note that both activated astrocytes and microglia are responsible for gliotransmitter release and the modulation of neuronal activity, suggesting that both cell types share some similar functions, further complicating their functional classification.
The importance of both astrocytes and microglia in central inflammation is well recognized (Hanisch 2002; Norden et al. 2016). While many central proinflammatory cytokines may be derived from infiltrating blood-borne macrophages, studies using cultured astrocytes and microglia have found that they exhibit a stimulation method-dependent and cell-type specific release of cytokines (Aloisi et al. 1992; Chung and Benveniste 1990; Lee et al. 1993); astroglial networks, therefore, may themselves also induce and support central neuroinflammation (Colonna and Butovsky 2017, Hanisch 2002; Norden et al. 2016). Stimulation of astrocytes with interleukin 1β (IL-1β), for example, induces the release of tumor necrosis factor-α (TNF-α) and IL-6 in protein kinase C (PKC)-dependent manner (Aloisi et al. 1992, Chung and Benveniste 1990; Lee et al. 1993). Stimulation of microglia with lipopolysaccharide (LPS), commonly observed during ischemia (Lee et al. 1993), in contrast, increases release of TNF-α through a mechanism dependent on the transcription and translation of p38 mitogen-activated protein (MAP) kinase. Interestingly, TNF-α has both neuroprotective and deleterious effects in regards to local neuronal health (Czeh et al. 2011), having been shown to reduce neuronal oxidative stress in the cortex following traumatic brain injury (Sullivan et al. 1999), but also being responsible for neuronal apoptosis and inhibiting neurite growth in the hippocampus (Hallenbeck 2002; Neumann et al. 2002). Microglia have been shown to be capable of releasing an array of inflammatory cytokines including, but not limited to, IL-1β, IL-3 IL-6, IL-8, gamma interferon inducible protein-10 (IP-10) and transforming growth factor (TGFβ), all of which may have unique effects on neuronal function (Hanisch 2002; Lee et al. 1993). Thus, astrocyte- and microglia-dependent release of proinflammatory cytokines is a dynamic and responsive system with many potential functions and outcomes (Fig. 1). Defining whether neuroinflammation-induced neuroplasticity is caused by activation of microglia or astrocytes, however, is complex and difficult to separate into discrete cell-type specific mechanisms.
Regardless of the cell type involved, however, it is clear that central neuroinflammation can occur independently of peripheral inflammation, including activation of astroglia, although the mechanism(s) responsible are not well understood. Astroglia are capable of sensing and responding to the excitability of nearby neurons (Clasadonte and Prevot 2018; Fellin and Carmignoto 2004; Pérez-Alvarez and Araque 2013), suggesting that altered neuronal activity may be responsible, least in part, for alterations in astroglial function and subsequent release of gliotransmitter (Guillemot-Legris et al. 2016; Gutiérrez-Martos et al. 2018).
ASTROGLIAL MODULATION OF SYNAPTIC STRENGTH
Mechanisms by Which Astroglia Alter Synaptic Efficacy
It has become clear that astroglia play critical roles in several areas of CNS function, and their role in the maintenance and modulation of synaptic strength has profound implications on our understanding of neurophysiology and neurocircuit plasticity in response to different sensory inputs, including dietary modulation. As discussed earlier, astroglia are themselves able to release neurotransmitters (gliotransmission), including glutamate and adenosine triphosphate (ATP), which can modulate the excitability of nearby synapses directly, in addition to being capable of releasing receptor coagonists such as glycine and d-serine that are required for NMDA receptor activation (Araque et al. 2014; Beltrán-Castillo et al. 2017; Bonansco et al. 2011; Choe et al. 2012; Clasadonte and Prevot 2018; Covelo and Araque 2018; Rose et al. 2018; Savtchouk and Volterra 2018; Stern and Filosa 2013; Zorec et al. 2012). Using dual whole cell patch-clamp electrophysiology and calcium imaging, work from several groups has demonstrated that selective stimulation of astrocytes causes glutamate exocytosis and the activation of presynaptic NMDA receptors; this increases presynaptic terminal excitability and subsequently increases the probability of neurotransmitter release, providing direct evidence for astroglial modulation of neuronal excitability and transmission, and illustrating one mechanism by which this modulation may occur (Fig. 1) (Angulo et al. 2004; Jourdain et al. 2007).
Astrocytes also regulate synaptic strength by virtue of their ability to uptake neurotransmitters such as glutamate and GABA from the synaptic cleft via the excitatory amino acid (EAAT1 and EAAT2), GABA (GAT), and glycine (GlyT1) transporters (Schousboe 2003; Schousboe et al. 2013). Under normal physiological conditions, for example, GlyT1, a sodium- and chloride-dependent symporter (Huang et al. 2004; Shibasaki et al. 2017), allows astrocytes to transport glycine intracellularly from the synaptic cleft (Aroeira et al. 2014). Several studies in the brainstem and spinal cord have shown that increasing intracellular sodium concentrations can functionally reverse this transporter, allowing for nonvesicular release of glycine (Aubrey et al. 2005; Raiteri et al. 2008), as also demonstrated following dopamine stimulation in the neonatal prefrontal cortex (Shibasaki et al. 2017). Astrocytes may, therefore, be a major source of glycine and allow for the activation of neuronal glycine receptors, found in abundance throughout the neonatal cortex and brainstem. Because of its additional role as a coagonist, release of glycine from astroglia may also contribute to the activation of NMDA receptors and hence be involved in the modulation of excitatory neurotransmission (Fig. 1) (Shibasaki et al. 2017).
Because of their mobility, by physically moving closer or farther away from the synaptic cleft, astrocytes are able to modulate the rate of neurotransmitter uptake and, hence, the concentration of neurotransmitter within the synapse (Langle et al. 2002; Montagnese et al. 1987; Perlmutter et al. 1985). While the effects of diet per se on astrocytic control of synaptic glutamate concentration through physical mobility remains to be elucidated, studies have demonstrated that, within the supraoptic nucleus, lactation or water deprivation causes astrocytic processes to physically retract from the synaptic cleft, increasing the synaptic concentration of neurotransmitter, allowing greater diffusion, and the activation of presynaptic and extrasynaptic receptors (Miyata et al. 1994; Montagnese et al. 1987). Astrocytes, therefore, may exert a tight and dynamic control over the temporal pattern of neurotransmitter and, subsequently, synaptic strength and efficacy in an activity-dependent and on-demand manner (Fig. 1) (Aguado et al. 2002; Dani et al. 1992).
Although astrocytes do not exhibit the same canonical electrical signaling properties as their neighboring neurons and have not traditionally been considered electrically excitable (Perea and Araque 2005; Zorec et al. 2012), astrocytic processes do express ionotropic and metabotropic receptors as well as the signaling machinery required to detect and respond to local neuromodulators, such as acetylcholine and norepinephrine (Bekar et al. 2008; Ding et al. 2013; Paukert et al. 2014; Schipke and Kettenmann 2004; Takata et al. 2011; Zonta et al. 2003). Astrocytes are also able to buffer extracellular calcium and potassium levels, through calcium-dependent uptake of potassium, altering presynaptic excitability and the probability of neurotransmitter release (Lian and Stringer 2004; Wallraff et al. 2006). Alterations in intracellular calcium levels also regulates the production of transcription factors responsible for calcium-dependent gliotransmitter release (Scemes and Giaume 2006) in addition to signaling via gap junctions to neighboring astrocytes allowing for the communication and coordination of an astrocytic network through calcium waves (Evans and Martin 2002; Scemes and Giaume 2006). Astrocytes also express voltage-activated sodium currents, which results in outwardly rectifying current-voltage relationships (Akita et al. 2011; Bevan et al. 1985; Sontheimer et al. 1996) and a subset of astrocytes have also been shown to have a resting sodium conductance (O'Connor et al. 1994; Sontheimer et al. 1996), illustrating that subsets of astrocytes may be electrically excitable, and sensitive to not only the excitability of nearby neurons, but also able to communicate this signal within the astroglia network.
Thus, astrocytes can exert tight control over synaptic strength and efficacy and are able to modulate the gain of neuronal signaling through several passive and active mechanisms such as responding to released neurotransmitters and neuromodulators, releasing cofactors and gliotransmitters, and regulating network activity via gap junctions (Fig. 1). Astrocytes, therefore, should be considered dynamic components in the control, regulation, and modulation of neurocircuits, responsive to environmental and neuronal cues, and crucial components in the coordination of network responses over large distances.
Brainstem
Diet can have profound effects on synaptic strength in many areas of the brainstem, including those in the dorsal vagal complex (DVC), which includes the dorsal motor nucleus of the vagus (DMV), the nucleus tractus solitarius (NTS), and the area postrema (AP) (Bhagat et al. 2015; Clyburn et al. 2018; Kentish et al. 2012, 2016; McMenamin et al. 2018; Travagli and Anselmi 2016; Troy et al. 2016). DVC neurocircuits are responsible for integrating and responding to peripheral sensory information from cardiovascular, respiratory, and gastrointestinal (GI) systems (Lu and Bieger 1998; Travagli and Anselmi 2016). Astroglial modulation of synaptic strength has been observed in many autonomic brainstem neurocircuits, (Dallaporta et al. 2010). In the cardiovascular field, for example, the astrocytic buffering of extracellular glutamate via EAAT2 decreases NTS neuronal baseline activity and tonically decreases atrial pressure, suggesting that astrocytes are critical for the control of cardiovascular function under baseline conditions (Matott et al. 2017). In the respiratory field, activation of the proteinase-activated receptor 1 (PAR1) on NTS astrocytes potentiates neuronal synaptic activity (Beltrán-Castillo et al. 2017), increasing glutamate signaling to the NTS-rostral ventral respiratory group, hence regulating breathing patterns (Beltrán-Castillo et al. 2017). Astrocytes have also been implicated in the regulation of respiration under stress, during exercise, and involved in the determination of exercise capacity (Sheikhbahaei et al. 2018). Although the effects of diet on astroglial modulation of synaptic strength in the central respiratory and cardiovascular neurocircuits have not been examined directly, evidence from the GI field suggests that such dietary-induced changes in astroglial function are likely to occur (Balland and Cowley 2017; Buckman et al. 2015).
Extrinsic neural control of GI function, specifically motility of the stomach and upper GI tract that arises principally from parasympathetic inputs via the efferent vagus nerve, is primarily made up of vago-vagal reflexes (Travagli and Anselmi 2016). Briefly, vagal afferent (sensory) neurons, the cell bodies of which lie in the nodose ganglion, innervate the stomach and upper GI tract and relay this sensory signal to the NTS (Browning 2003; Travagli et al. 2006; Zhang et al. 1998). The NTS integrates this sensory signal with inputs from the brainstem and hypothalamus that are involved in energy homeostasis and sends GABAergic, glutamatergic, and catecholaminergic projections to the preganglionic motoneurons of the DMV (Travagli and Anselmi 2016; Travagli et al. 1991). The DMV sends cholinergic projections to the postganglionic neurons in the myenteric plexus, which makes up two distinct pathways, the excitatory cholinergic and inhibitory nonadrenergic, noncholinergic pathway (Travagli and Anselmi 2016). Diet-induced modulation of GI vagal pathways are apparent in response to both acute and chronic exposure (Bhagat et al. 2015; Clyburn et al. 2018; de Lartigue et al. 2011; Fox and Biddinger 2012; Kentish et al. 2012, 2016; Nefti et al. 2009). DIO is associated with a decreased intrinsic excitability of vagal afferent sensory neurons and fibers (Daly et al. 2011) as well as vagal efferent motoneurons, including decreasing the responsiveness to classical satiety peptides such as cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1), and leptin, as well as the glucose-dependent facilitation of serotonin (5-HT)-mediated responses (Bhagat et al. 2015; Covasa and Ritter 2000; Duca et al. 2013; Troy et al. 2016). Reduced afferent excitability decreases responsiveness to meal-induced mechanical and chemical stimulation, while decreased efferent motoneuron excitability reduces the tone of the stomach and increases gastric and fasting volume (Asakawa et al. 2003; Bhagat et al. 2015; Daly et al. 2011; de Lartigue et al. 2011; Fox and Biddinger 2012; Gallagher et al. 2007; Kentish et al. 2012). Together, these increase the volume of food required to signal satiation, ultimately increasing food intake and meal size, and contributing to the development and maintenance of obesity. Interestingly, the responsiveness of central vagal motoneurons is restored following Roux-en-Y gastric bypass surgery, suggesting that such neuroplasticity is transient and reversible (Browning et al. 2013). While such vagal neurocircuit plasticity may certainly be caused by the increase in adiposity and circulating proinflammatory cytokines associated with obesity, the rapid onset of some alterations, far in advance of weight gain or increased adiposity (Clyburn et al. 2018; Waise et al. 2015), suggests some effects are due to the diet itself, rather than obesity. One day of HFD exposure is sufficient to cause inflammation in the nodose ganglion and hypothalamus, for example, while vagal responsiveness to glucose-dependent facilitation of 5-HT signaling is reduced within 4 days (Troy et al. 2016; Waise et al. 2015). Interestingly, selective celiac branch vagotomy prevented the increase in proinflammatory cytokine expression in both the nodose ganglion and hypothalamus, suggesting that acute HFD-induced neuroinflammation may be vagally dependent and occurs long before peripheral inflammation is observed (Waise et al. 2015).
Both humans and animal models alike respond to HFD exposure with a short (~24-h) period of hyperphagia, before energy homeostasis is restored and food intake returns to an isocaloric level within 3–5 days (Buckman et al. 2015). The mechanism(s) responsible for the homeostatic regulation of caloric intake following HFD exposure, and how this mechanism is lost following continued chronic HFD exposure when hyperphagia returns leading to development of DIO, is a growing area of interest. Recent studies from our laboratory have demonstrated that restoration of caloric balance occurs over the same time period (3–5 days) during which acute HFD exposure increases glutamatergic signaling to neurons of the DMV via increased activation of synaptic NMDA receptors, subsequently increasing DMV neuronal excitability, and vagal efferent control of gastric tone and motility (Clyburn et al. 2018). The timescale of this central vagal neuroplasticity corresponds with an observable increase in neuroinflammatory markers such as IL-1β and TNF-α within the pons and midbrain (Astiz et al. 2017; Balland and Cowley 2017; Dalvi et al. 2017). While the role of astroglia in the acute HFD modulation of brainstem synaptic strength remains to be elucidated, the time course of both the neuroplasticity as well as the increased neuroinflammatory markers suggests the involvement of astroglial-dependent compensatory mechanisms that restores caloric balance temporarily (Astiz et al. 2017; Buckman et al. 2015; Douglass et al. 2017). Indeed, several studies have suggests that acute neuroinflammation is protective against a wide range of insults, including hemorrhage, whereas chronic neuroinflammation induces significant damage and disruption to neurocircuit function (Astiz et al. 2017; Burda and Sofroniew 2014). Chronic HFD exposure and DIO, for example, decrease vagal motoneuron excitability, alter neuronal morphology, and inhibit the CCK-induced modulation of synaptic transmission (Bhagat et al. 2015; McMenamin et al. 2018).
Hypothalamus
While alterations in the brainstem neurocircuits that control gastrointestinal function have an obvious effect on gastric compliance, tone, motility, emptying rates and subsequently, meal size and energy homeostasis, HFD-induced astroglial modulation of hypothalamic signaling has also been observed (Ávalos et al. 2018; Balland and Cowley 2017; Buckman et al. 2015; Chowen et al. 2013; Douglass et al. 2017; Pfuhlmann et al. 2018). Given the critical role of the hypothalamus, especially the tuberal region, in controlling appetite, modulation of these circuits may have profound effects on feeding behaviors, caloric intake, and the development of obesity (King 2005; Velloso et al. 2008). As in the brainstem, modulation of hypothalamic circuits can occur rapidly; an increase in proinflammatory cytokines is observable within 24 h of HFD exposure, suggesting such neuroinflammation occurs via a mechanism independent of an increase in systemic cytokine level (Astiz et al. 2017; Waise et al. 2015). Astrocytes have important roles in the regulation of hypothalamic neurocircuitry, both in basal conditions as well as following HFD (Ávalos et al. 2018; Kälin et al. 2015; Langle et al. 2002). Within the basal medial hypothalamus, for example, astroglial activation decreases both basal and ghrelin-induced caloric intake through the activation of agouti-related protein (AgRP)-positive neurons (Yang et al. 2015). In the ventromedial hypothalamus, in contrast, astroglia release ketone bodies in response to HFD, altering neuronal excitability through the concurrent release of ATP (Le Foll et al. 2014). Thus, the diet-induced astroglial-dependent modulation of neuronal activity within the hypothalamus may even exhibit subtle regional, and nucleus-specific, variability.
Given the important role of leptin in food intake and energy balance, it is perhaps unsurprising that its involvement in the astroglial modulation of food intake has been proposed (Cheunsuang and Morris 2005; Kim et al. 2014; Pan et al. 2008; Yang et al. 2015). As a polypeptide produced by adipose tissue and the gastric mucosa, leptin enters the CNS via a selective, saturable transport system and acts within the hypothalamus to induce satiation and reduce food intake (Ahima and Lazar 2008; Berthoud 2005; Berthoud and Morrison 2008; Burguera et al. 2000; Kim et al. 2014). Hyperleptinemia, caused by an increase in adiposity, induces leptin insensitivity and resistance within the hypothalamus and brainstem, causing dysregulation of energy balance, resulting in increased meal size, weight gain, and the development of obesity (Berthoud 2005; Berthoud and Morrison 2008). While studies have traditionally focused on the neuronal effects of leptin, it should also be noted that astroglia, and hypothalamic astroglia in particular, express leptin receptors (Balland and Cowley 2017; Cheunsuang and Morris 2005; Kim et al. 2014). Indeed, astrocyte-specific deletion of the leptin receptor alters glial morphology as well as hypothalamic proopiomelanocortin and AgRP neuronal signaling, leading to the development of obesity (Jayaram et al. 2013). Interestingly, leptin resistance also decreases astrocytic release of ketone bodies in the ventromedial hypothalamus, attenuating the regulation of caloric intake and energy homeostasis (Le Foll and Levin 2016). Furthermore, in astrocyte-specific leptin receptor knockout mice, phosphorylated signal transducer and activator of transcription 3 (pSTAT3) signaling is decreased, and is accompanied by a mild reactive gliosis (Wang et al. 2015). Following exposure to HFD, these leptin receptor knockout mice increased body fat to a greater extent than mice fed either a control or HFD, suggesting that astrocytic leptin signaling is also important in regulating the hypothalamic response to HFD (Wang et al. 2015). Under both basal conditions as well as acute HFD exposure, therefore, astrocytes not only are sensitive to leptin levels, but they tonically regulate synaptic strength within feeding neurocircuits to regulate caloric intake.
Dorsal Striatum
While rapid neuroplasticity within the brainstem and hypothalamic neurocircuits and astroglial networks responsible for GI function, energy homeostasis, and feeding behavior could be considered to have an adaptive advantage, diet-induced astroglial plasticity can also be observed in other discrete areas of the brain. Modulation of activity within the dorsal striatum, involved with the refinement of motor activity and decision making and implicated in food seeking behaviors, occurs following a high-fat/high-carbohydrate diet (Fritz et al. 2018). Using electrophysiological recordings and fast-scan cyclic voltammetry techniques, the altered glutamatergic signaling apparent in neurons of the dorsal striatum following high-fat/high-carbohydrate diet exposure appears to be dependent on the expression of the glial glutamate transporter-1 (GLT-1 or EAAT2) and the subsequent slower reuptake of glutamate, which then increases neuronal excitability (Fritz et al. 2018). Thus, in addition to acting within autonomic networks to increase food intake, HFD may alter astrocytic signaling in the dorsal striatum to also alter food seeking, decision making, and motor activity.
Nucleus Accumbens
Diet-induced plasticity within astroglia networks may have also been identified within the nucleus accumbens, which plays a key role in the central reward circuits (Blancas-Velazquez et al. 2018; Gutiérrez-Martos et al. 2018). In studies where C57BL6/J mice were allowed to choose between a standard diet and a high-fat/high-carbohydrate food (chocolate bars; free choice model), or were allowed access only to the chocolate bars (binge model), the observed structural alterations in medium spiny neurons were prevented by the microglial inhibitor, minocycline (Gutiérrez-Martos et al. 2018), as were the increased expression of neuroinflammatory markers and the altered response to induced hyperlocomotion (Gutiérrez-Martos et al. 2018). While it is unclear whether these observations were due directly to activated microglia, or indirectly through their subsequent activation of astrocytes, it is evident that diet-induced astroglial activation affects reward pathways, not only through the modulation of neuronal function and signaling, but also through modulation of neuronal morphology. The physiological outcome of such diet-induced astroglial modulation in the nucleus accumbens remains unclear, however, but, given the prominent role of the nucleus accumbens in both reward-related as well as motivated behavior, this modulation may well have important implications in the hedonic control of food intake.
Amygdala and Hippocampus
Perhaps the most convincing evidence supporting HFD-dependent astroglial modulation of neuronal activity involves the amygdala and hippocampus. HFD exposure has long been associated with the disruption of cognitive performance, especially learning and memory, an area of increasing interest in populations already at risk for hippocampal impairment, such as the elderly and those with Alzheimer’s disease (Arcego et al. 2018; Hao et al. 2016; Koga et al. 2014; Spencer et al. 2017). Activated astroglia within the hippocampus become leptin-insensitive following HFD exposure, and leptin-induced modulation of hippocampal synaptic transmission is attenuated under the same conditions (Mainardi et al. 2017). HFD can also increase glutamatergic signaling within the hippocampus through altered regulation of astroglial GLT-1 activity and the inhibition of glutamine synthesis (Cano et al. 2014; Valladolid-Acebes et al. 2012). Other studies have shown that prolonged HFD exposure and DIO increases neuroinflammatory markers and levels of phosphorylated Tau protein (a marker for neurodegeneration) and potentially amyloid beta (Aβ) plaques (the hallmark sign of Alzheimer's disease) in the hippocampus, both of which have a detrimental effect on cognitive performance (Hawkes et al. 2015; Koga et al. 2014; Ledreux et al. 2016; Martino Adami et al. 2017). Interestingly, exposure to Aβ plaques appears to inhibit astroglial control of extracellular glutamate levels, promoting dysregulation within neurocircuits involved in learning and memory (Kawano et al. 2017), suggesting that HFD-induced disruption in learning and memory in pathophysiological conditions may be exacerbated by, and at least in part due to, astroglial network disruption. Interestingly, exercise, weight loss, and astroglial inhibition have all been shown to rescue DIO-associated cognitive decline, indicating that such effects are reversible (Koga et al. 2014). In addition, neuroinflammation and dysregulation of astroglia within the hippocampus are not observed until after the dysregulation of brainstem astroglia (Astiz et al. 2017), suggesting an ascending pattern of temporal diet-induced dysfunction. Further studies would be required to determine whether brainstem astroglial network disruption is responsible for hippocampal astroglial dysfunction, or whether there is a distinct temporal patterning and susceptibility of astroglia in different regions to the effects of HFD exposure.
CONCLUSIONS
Our understanding of the role of astroglia in CNS function has advanced dramatically and rapidly in recent years. From being considered responsible only for providing structural support to nearby neurons, to being recognized for their roles in central inflammation and immune responses, maintenance of the BBB, their responses to circulating factors, and role in the finely tuned regulation of synaptic strength to maintain homeostasis or control of neurocircuit function, it is clear that astroglia play critical roles in the physiology and pathophysiology of central neurocircuits. The effect that diet, particularly HFD, has on astroglial control of synaptic strength appears to be rapid, complex, and region specific. There is evidence for activation of astroglia and inflammatory processes in the hypothalamus and hippocampus within 24 h of HFD exposure, suggesting these may be the first, and most sensitive, responses (Table 1). While the acute modulation of some neurocircuits appears to be compensatory, other studies have shown adverse outcomes in response to chronic HFD exposure and DIO-induced astroglial activation including areas involved in higher functions such as learning and memory. Furthermore, studies in both the brainstem and the hippocampus suggest that diet-induced neuroplasticity may be reversed by reducing neuroinflammation or microglia activation (Table 1). Additional studies will be needed to define the roles of acute versus chronic neuroinflammation in the context of the food intake regulation and energy homeostasis, and whether neuroinflammation can spread from the early affected autonomic areas to higher areas affected by diet-induced inflammation, such as the amygdala, hippocampus, and dorsal striatum. While there is some evidence for the spread of inflammation through astroglial networks mediated via the gap junction-dependent spread of calcium currents, the role and mechanism of these effects in the context of diet-induced neuroinflammation still remains to be elucidated. It is also possible that altered neurocircuit activity per se is responsible for the modulation of astroglial activity in areas with reciprocal neuronal connections. While further investigation is needed to determine the temporal pattern of the event cascade that follows HFD exposure and results in the modulation of synaptic strength, it appears that microglial activation and central inflammation occurs first (within ~24 h), certainly long before the development of systemic inflammation. Such microglial responses may then induce astrocyte activation and the subsequent modulation of synaptic strength within neurocircuits involved feeding behaviors and gastric function. It is tempting to speculate, therefore, that astroglial modulation of central neurocircuits plays a far more significant role in the development of DIO, in contrast to the canonical model which considers that central inflammation and neurocircuit dysregulation occurs in response to obesity itself.
Table 1.
Evidence for diet-induced modulation of regional synaptic strength
| Region | Diet-Induced Modulation of Neuronal Function | Diet-Induced Modulation of Astroglial Function | Astroglial Modulation of Synaptic Strength | Diet-Induced Astroglial Synaptic Strength |
|---|---|---|---|---|
| Brainstem Vagal neurocircuits | Bhagat et al. 2015; Browning et al. 2013; Clyburn et al. 2018; McMenamin et al. 2017; Nefti et al. 2009; Troy and Browning 2016; Troy et al. 2016 | Buckman et al. 2015b; Waise et al. 2015 | Beltran-Castillo et al. 2017; Dallaporta et al. 2010; Matott et al. 2017; Sheikhbahaei et al. 2018 | Not Available |
| Hypothalamus Medial basal and ventromedial | Avalos et al. 2018; Dalvi et al. 2017 | Avalos et al. 2018; Balland and Cowley 2017; Belegri et al. 2018; Buckman et al. 2015b; Buckman et al. 2013; Dalvi et al. 2017; Douglass et al. 2017; Le Foll and Levin 2016; Waise et al. 2015 | Dalvi et al. 2017; Kim et al. 2014; Le Foll et al. 2014; Yang et al. 2015 | Dalvi et al. 2017; Douglass et al. 2017; Le Foll et al. 2014 |
| Dorsal striatum | Fritz et al. 2018 | Fritz et al. 2018 | Fritz et al. 2018 | Fritz et al. 2018 |
| Nucleus accumbens | Blancas-Velazquez et al. 2018; Gutierrez-Martos et al. 2018 | Blancas-Velazquez et al. 2018; Gutierrez-Martos et al. 2018 | Blancas-Velazquez et al. 2018; Gutierrez-Martos et al. 2018 | Blancas-Velazquez et al. 2018; Gutierrez-Martos et al. 2018 |
| Amygdala and hippocampus | Arcego et al. 2018; Ledreux et al. 2016; Mainardi et al. 2017; Martino Adami et al. 2017; Valladolid-Acebes et al. 2011 | Cano et al. 2014; Hao et al. 2016; Spencer et al. 2017a | Angulo et al. 2004; Hao et al. 2016; Rose et al. 2017 | Hao et al. 2016; Spencer et al. 2017a |
A brief overview of the evidence supporting diet-induced astroglial modulation of synaptic strength. Evidence for diet-induced modulation of neuronal activity, astroglial activity, astroglial modulation of synaptic strength, and diet-induced astroglial modulation of synaptic strength have been categorized by central nervous system (CNS) region. Not Available, CNS regions that do not have any supporting evidence available at this time.
Nevertheless, it is clear that astroglia exhibit rapid and complex responses to both acute and chronic alterations in diet and exert tight control over neuronal functions. Astroglia should be considered key players in the regulation of food intake and energy homeostasis, therefore making them an exciting area and target of interest in the study of diet-induced obesity.
GRANTS
This work was supported by NIH grants DK111667 (K. N. Browning) and DK118833 (C. Clyburn).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.C. and K.N.B. conceived and designed research; C.C. prepared figures; C.C. and K.N.B. drafted manuscript; C.C. and K.N.B. edited and revised manuscript; C.C. and K.N.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. R. Alberto Travagli for helpful critical comments on previous versions of this manuscript. We thank W. Nairn Browning for support and encouragement.
REFERENCES
- Aguado F, Espinosa-Parrilla JF, Carmona MA, Soriano E. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J Neurosci 22: 9430–9444, 2002. doi: 10.1523/JNEUROSCI.22-21-09430.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol 22: 1023–1031, 2008. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita T, Fedorovich SV, Okada Y. Ca2+ nanodomain-mediated component of swelling-induced volume-sensitive outwardly rectifying anion current triggered by autocrine action of ATP in mouse astrocytes. Cell Physiol Biochem 28: 1181–1190, 2011. doi: 10.1159/000335867. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Carè A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol 149: 2358–2366, 1992. [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci 24: 6920–6927, 2004. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 81: 728–739, 2014. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcego DM, Toniazzo AP, Krolow R, Lampert C, Berlitz C, Dos Santos Garcia E, do Couto Nicola F, Hoppe JB, Gaelzer MM, Klein CP, Lazzaretti C, Dalmaz C. Impact of high-fat diet and early stress on depressive-like behavior and hippocampal plasticity in adult male rats. Mol Neurobiol 55: 2740–2753, 2018. doi: 10.1007/s12035-017-0538-y. [DOI] [PubMed] [Google Scholar]
- Aroeira RI, Sebastião AM, Valente CA. GlyT1 and GlyT2 in brain astrocytes: expression, distribution and function. Brain Struct Funct 219: 817–830, 2014. doi: 10.1007/s00429-013-0537-3. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Ueno N, Makino S, Uemoto M, Fujino MA, Kasuga M. Ob/ob mice as a model of delayed gastric emptying. J Diabetes Complications 17: 27–28, 2003. doi: 10.1016/S1056-8727(02)00198-8. [DOI] [PubMed] [Google Scholar]
- Astiz M, Pernía O, Barrios V, Garcia-Segura LM, Diz-Chaves Y. Short-term high-fat diet feeding provides hypothalamic but not hippocampal protection against acute infection in male mice. Neuroendocrinology 104: 40–50, 2017. doi: 10.1159/000444527. [DOI] [PubMed] [Google Scholar]
- Aubrey KR, Vandenberg RJ, Clements JD. Dynamics of forward and reverse transport by the glial glycine transporter, glyt1b. Biophys J 89: 1657–1668, 2005. doi: 10.1529/biophysj.105.061572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávalos Y, Kerr B, Maliqueo M, Dorfman M. Cell and molecular mechanisms behind diet-induced hypothalamic inflammation and obesity. J Neuroendocrinol 30: e12598, 2018. doi: 10.1111/jne.12598. [DOI] [PubMed] [Google Scholar]
- Balland E, Cowley MA. Short-term high-fat diet increases the presence of astrocytes in the hypothalamus of C57BL6 mice without altering leptin sensitivity. J Neuroendocrinol 29: e12504, 2017. doi: 10.1111/jne.12504. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci 48: PL117–PL121, 1991. doi: 10.1016/0024-3205(91)90385-O. [DOI] [PubMed] [Google Scholar]
- Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem 76: 846–854, 2001. doi: 10.1046/j.1471-4159.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- Barone FC, Kilgore KS. Role of inflammation and cellular stress in brain injury and central nervous system diseases. Clin Neurosci Res 6: 329–356, 2006. doi: 10.1016/j.cnr.2006.09.010. [DOI] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17: 4–12, 2006. [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Miller DS. Tumor necrosis factor α and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 71: 667–675, 2007. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- Bekar LK, He W, Nedergaard M. Locus coeruleus α-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex 18: 2789–2795, 2008. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belegri E, Eggels L, Unmehopa UA, Mul JD, Boelen A, la Fleur SE. The effects of overnight nutrient intake on hypothalamic inflammation in a free-choice diet-induced obesity rat model. Appetite 120: 527–535, 2018. doi: 10.1016/j.appet.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Beltrán-Castillo S, Olivares MJ, Contreras RA, Zúñiga G, Llona I, von Bernhardi R, Eugenín JL. D-serine released by astrocytes in brainstem regulates breathing response to CO2 levels. Nat Commun 8: 838, 2017. doi: 10.1038/s41467-017-00960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R. A new role for leptin as a direct satiety signal from the stomach. Am J Physiol Regul Integr Comp Physiol 288: R796–R797, 2005. doi: 10.1152/ajpregu.00001.2005. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol 59: 55–92, 2008. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- Bessis A, Béchade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia 55: 233–238, 2007. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Bevan S, Chiu SY, Gray PT, Ritchie JM. The presence of voltage-gated sodium, potassium and chloride channels in rat cultured astrocytes. Proc R Soc Lond B Biol Sci 225: 299–313, 1985. doi: 10.1098/rspb.1985.0063. [DOI] [PubMed] [Google Scholar]
- Bhagat R, Fortna SR, Browning KN. Exposure to a high fat diet during the perinatal period alters vagal motoneurone excitability, even in the absence of obesity. J Physiol 593: 285–303, 2015. doi: 10.1113/jphysiol.2014.282806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancas-Velazquez AS, Unmehopa UA, Eggels L, Koekkoek L, Kalsbeek A, Mendoza J, la Fleur SE. A free-choice high-fat high-sugar diet alters day-night Per2 gene expression in reward-related brain areas in rats. Front Endocrinol (Lausanne) 9: 154, 2018. doi: 10.3389/fendo.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonansco C, Couve A, Perea G, Ferradas CA, Roncagliolo M, Fuenzalida M. Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity. Eur J Neurosci 33: 1483–1492, 2011. doi: 10.1111/j.1460-9568.2011.07631.x. [DOI] [PubMed] [Google Scholar]
- Browning KN. Excitability of nodose ganglion cells and their role in vago-vagal reflex control of gastrointestinal function. Curr Opin Pharmacol 3: 613–617, 2003. doi: 10.1016/j.coph.2003.06.011. [DOI] [PubMed] [Google Scholar]
- Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol 591: 2357–2372, 2013. doi: 10.1113/jphysiol.2012.249268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman LB, Thompson MM, Lippert RN, Blackwell TS, Yull FE, Ellacott KL. Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice. Mol Metab 4: 58–63, 2014. doi: 10.1016/j.molmet.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81: 229–248, 2014. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguera B, Couce ME, Curran GL, Jensen MD, Lloyd RV, Cleary MP, Poduslo JF. Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes 49: 1219–1223, 2000. doi: 10.2337/diabetes.49.7.1219. [DOI] [PubMed] [Google Scholar]
- Cano V, Valladolid-Acebes I, Hernández-Nuño F, Merino B, Del Olmo N, Chowen JA, Ruiz-Gayo M. Morphological changes in glial fibrillary acidic protein immunopositive astrocytes in the hippocampus of dietary-induced obese mice. Neuroreport 25: 819–822, 2014. doi: 10.1097/WNR.0000000000000180. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Thrash JC, Walter B. The cellular response in neuroinflammation: the role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin Neurosci Res 6: 237–245, 2006. doi: 10.1016/j.cnr.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev 48: 16–42, 2005. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Chaar LJ, Coelho A, Silva NM, Festuccia WL, Antunes VR. High-fat diet-induced hypertension and autonomic imbalance are associated with an upregulation of CART in the dorsomedial hypothalamus of mice. Physiol Rep 4: e12811, 2016. doi: 10.14814/phy2.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheunsuang O, Morris R. Astrocytes in the arcuate nucleus and median eminence that take up a fluorescent dye from the circulation express leptin receptors and neuropeptide Y Y1 receptors. Glia 52: 228–233, 2005. doi: 10.1002/glia.20239. [DOI] [PubMed] [Google Scholar]
- Choe KY, Olson JE, Bourque CW. Taurine release by astrocytes modulates osmosensitive glycine receptor tone and excitability in the adult supraoptic nucleus. J Neurosci 32: 12518–12527, 2012. doi: 10.1523/JNEUROSCI.1380-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowen JA, Argente J, Horvath TL. Uncovering novel roles of nonneuronal cells in body weight homeostasis and obesity. Endocrinology 154: 3001–3007, 2013. doi: 10.1210/en.2013-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol 144: 2999–3007, 1990. [PubMed] [Google Scholar]
- Clasadonte J, Prevot V. The special relationship: glia-neuron interactions in the neuroendocrine hypothalamus. Nat Rev Endocrinol 14: 25–44, 2018. doi: 10.1038/nrendo.2017.124. [DOI] [PubMed] [Google Scholar]
- Clyburn C, Travagli RA, Browning KN. Acute high-fat diet upregulates glutamatergic signaling in the dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 314: G623–G634, 2018. doi: 10.1152/ajpgi.00395.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 35: 441–468, 2017. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sánchez HA, Eugenín EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Sáez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA 99: 495–500, 2002. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438: 1017–1021, 2005. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Covasa M, Ritter RC. Adaptation to high-fat diet reduces inhibition of gastric emptying by CCK and intestinal oleate. Am J Physiol Regul Integr Comp Physiol 278: R166–R170, 2000. doi: 10.1152/ajpregu.2000.278.1.R166. [DOI] [PubMed] [Google Scholar]
- Covelo A, Araque A. Neuronal activity determines distinct gliotransmitter release from a single astrocyte. eLife 7: e32237, 2018. doi: 10.7554/eLife.32237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev Neurosci 33: 199–209, 2011. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- Dallaporta M, Bonnet MS, Horner K, Trouslard J, Jean A, Troadec JD. Glial cells of the nucleus tractus solitarius as partners of the dorsal hindbrain regulation of energy balance: a proposal for a working hypothesis. Brain Res 1350: 35–42, 2010. doi: 10.1016/j.brainres.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Dalvi PS, Chalmers JA, Luo V, Han D-Y, Wellhauser L, Liu Y, Tran DQ, Castel J, Luquet S, Wheeler MB, Belsham DD. High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int J Obes 41: 149–158, 2017. doi: 10.1038/ijo.2016.183. [DOI] [PubMed] [Google Scholar]
- Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol 589: 2857–2870, 2011. doi: 10.1113/jphysiol.2010.204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8: 429–440, 1992. doi: 10.1016/0896-6273(92)90271-E. [DOI] [PubMed] [Google Scholar]
- Das UN. Obesity: genes, brain, gut, and environment. Nutrition 26: 459–473, 2010. doi: 10.1016/j.nut.2009.09.020. [DOI] [PubMed] [Google Scholar]
- de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav 105: 100–105, 2011. doi: 10.1016/j.physbeh.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, O’Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54: 387–394, 2013. doi: 10.1016/j.ceca.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab 6: 366–373, 2017. doi: 10.1016/j.molmet.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Sakar Y, Covasa M. Combination of obesity and high-fat feeding diminishes sensitivity to GLP-1R agonist exendin-4. Diabetes 62: 2410–2415, 2013. doi: 10.2337/db12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA 94: 4080–4085, 1997. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WH, Martin PE. Gap junctions: structure and function (Review) Mol Membr Biol 19: 121–136, 2002. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm 74: 443–477, 2006. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol 559: 3–15, 2004. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43: 729–743, 2004. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fox EA, Biddinger JE. Early postnatal overnutrition: potential roles of gastrointestinal vagal afferents and brain-derived neurotrophic factor. Physiol Behav 106: 400–412, 2012. doi: 10.1016/j.physbeh.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BM, Muñoz B, Yin F, Bauchle C, Atwood BK. A high-fat, high-sugar ‘western’ diet alters dorsal striatal glutamate, opioid, and dopamine transmission in mice. Neuroscience 372: 1–15, 2018. doi: 10.1016/j.neuroscience.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher TK, Geoghegan JG, Baird AW, Winter DC. Implications of altered gastrointestinal motility in obesity. Obes Surg 17: 1399–1407, 2007. doi: 10.1007/s11695-007-9221-0. [DOI] [PubMed] [Google Scholar]
- Gomes C, Ferreira R, George J, Sanches R, Rodrigues DI, Gonçalves N, Cunha RA. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflammation 10: 780, 2013. doi: 10.1186/1742-2094-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring) 23: 512–518, 2015. doi: 10.1002/oby.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot-Legris O, Masquelier J, Everard A, Cani PD, Alhouayek M, Muccioli GG. High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J Neuroinflammation 13: 206, 2016. doi: 10.1186/s12974-016-0666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol 47: 169–176, 1993. doi: 10.1016/0165-5728(93)90027-V. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Martos M, Girard B, Mendonça-Netto S, Perroy J, Valjent E, Maldonado R, Martin M. Cafeteria diet induces neuroplastic modifications in the nucleus accumbens mediated by microglia activation. Addict Biol 23: 735–749, 2018. doi: 10.1111/adb.12541. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med 8: 1363–1368, 2002. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes 3: 285–295, 2010. doi: 10.2147/DMSO.S7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia 40: 140–155, 2002. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hao S, Dey A, Yu X, Stranahan AM. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun 51: 230–239, 2016. doi: 10.1016/j.bbi.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CA, Gentleman SM, Nicoll JA, Carare RO. Prenatal high-fat diet alters the cerebrovasculature and clearance of β-amyloid in adult offspring. J Pathol 235: 619–631, 2015. doi: 10.1002/path.4468. [DOI] [PubMed] [Google Scholar]
- Huang H, Barakat L, Wang D, Bordey A. Bergmann glial GlyT1 mediates glycine uptake and release in mouse cerebellar slices. J Physiol 560: 721–736, 2004. doi: 10.1113/jphysiol.2004.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram B, Pan W, Wang Y, Hsuchou H, Mace A, Cornelissen-Guillaume GG, Mishra PK, Koza RA, Kastin AJ. Astrocytic leptin-receptor knockout mice show partial rescue of leptin resistance in diet-induced obesity. J Appl Physiol (1985) 114: 734–741, 2013. doi: 10.1152/japplphysiol.01499.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10: 331–339, 2007. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kälin S, Heppner FL, Bechmann I, Prinz M, Tschöp MH, Yi CX. Hypothalamic innate immune reaction in obesity. Nat Rev Endocrinol 11: 339–351, 2015. doi: 10.1038/nrendo.2015.48. [DOI] [PubMed] [Google Scholar]
- Kawano H, Oyabu K, Yamamoto H, Eto K, Adaniya Y, Kubota K, Watanabe T, Hirano-Iwata A, Nabekura J, Katsurabayashi S, Iwasaki K. Astrocytes with previous chronic exposure to amyloid β-peptide fragment 1-40 suppress excitatory synaptic transmission. J Neurochem 143: 624–634, 2017. doi: 10.1111/jnc.14247. [DOI] [PubMed] [Google Scholar]
- Kentish S, Li H, Philp LK, O’Donnell TA, Isaacs NJ, Young RL, Wittert GA, Blackshaw LA, Page AJ. Diet-induced adaptation of vagal afferent function. J Physiol 590: 209–221, 2012a. doi: 10.1113/jphysiol.2011.222158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish S, Li H, Philp LK, O’Donnell TA, Isaacs NJ, Young RL, Wittert GA, Blackshaw LA, Page AJ. Diet-induced adaptation of vagal afferent function. J Physiol 590: 209–221, 2012b. doi: 10.1113/jphysiol.2011.222158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish SJ, Vincent AD, Kennaway DJ, Wittert GA, Page AJ. High-fat diet-induced obesity ablates gastric vagal afferent circadian rhythms. J Neurosci 36: 3199–3207, 2016. doi: 10.1523/JNEUROSCI.2710-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong J-S, Chen P-S, Chuang D-M. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther 321: 892–901, 2007. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu ZW, Zimmer MR, Jeong JK, Szigeti-Buck K, Gao Y, Garcia-Caceres C, Yi CX, Salmaso N, Vaccarino FM, Chowen J, Diano S, Dietrich MO, Tschöp MH, Horvath TL. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci 17: 908–910, 2014. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. The problem of astrocyte identity. Neurochem Int 45: 191–202, 2004. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Supportive or information-processing functions of the mature protoplasmic astrocyte in the mammalian CNS? A critical appraisal. Neuron Glia Biol 3: 181–189, 2007. doi: 10.1017/S1740925X08000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King PJ. The hypothalamus and obesity. Curr Drug Targets 6: 225–240, 2005. doi: 10.2174/1389450053174587. [DOI] [PubMed] [Google Scholar]
- Koga S, Kojima A, Ishikawa C, Kuwabara S, Arai K, Yoshiyama Y. Effects of diet-induced obesity and voluntary exercise in a tauopathy mouse model: implications of persistent hyperleptinemia and enhanced astrocytic leptin receptor expression. Neurobiol Dis 71: 180–192, 2014. doi: 10.1016/j.nbd.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Langle SL, Poulain DA, Theodosis DT. Neuronal-glial remodeling: a structural basis for neuronal-glial interactions in the adult hypothalamus. J Physiol Paris 96: 169–175, 2002. doi: 10.1016/S0928-4257(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Langlet F. Tanycytes: a gateway to the metabolic hypothalamus. J Neuroendocrinol 26: 753–760, 2014. doi: 10.1111/jne.12191. [DOI] [PubMed] [Google Scholar]
- Le Foll C, Dunn-Meynell AA, Miziorko HM, Levin BE. Regulation of hypothalamic neuronal sensing and food intake by ketone bodies and fatty acids. Diabetes 63: 1259–1269, 2014. doi: 10.2337/db13-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll C, Levin BE. Fatty acid-induced astrocyte ketone production and the control of food intake. Am J Physiol Regul Integr Comp Physiol 310: R1186–R1192, 2016. doi: 10.1152/ajpregu.00113.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledreux A, Wang X, Schultzberg M, Granholm AC, Freeman LR. Detrimental effects of a high fat/high cholesterol diet on memory and hippocampal markers in aged rats. Behav Brain Res 312: 294–304, 2016. doi: 10.1016/j.bbr.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150: 2659–2667, 1993. [PubMed] [Google Scholar]
- Lee SH, Kim WT, Cornell-Bell AH, Sontheimer H. Astrocytes exhibit regional specificity in gap-junction coupling. Glia 11: 315–325, 1994. doi: 10.1002/glia.440110404. [DOI] [PubMed] [Google Scholar]
- Levin BE. Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Philos Trans R Soc Lond B Biol Sci 361: 1107–1121, 2006. doi: 10.1098/rstb.2006.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE. Developmental gene x environment interactions affecting systems regulating energy homeostasis and obesity. Front Neuroendocrinol 31: 270–283, 2010a. doi: 10.1016/j.yfrne.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE. Interaction of perinatal and pre-pubertal factors with genetic predisposition in the development of neural pathways involved in the regulation of energy homeostasis. Brain Res 1350: 10–17, 2010b. doi: 10.1016/j.brainres.2009.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Wang B, Kodali MC, Chen C, Kim E, Patters BJ, Lan L, Kumar S, Wang X, Yue J, Liao FF. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J Neuroinflammation 15: 8, 2018. doi: 10.1186/s12974-017-1038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X-Y, Stringer JL. Astrocytes contribute to regulation of extracellular calcium and potassium in the rat cerebral cortex during spreading depression. Brain Res 1012: 177–184, 2004. doi: 10.1016/j.brainres.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Little TJ, Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity—oral and gastrointestinal sensory contributions. Physiol Behav 104: 613–620, 2011. doi: 10.1016/j.physbeh.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Little TJ, Horowitz M, Feinle-Bisset C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: implications for the pathophysiology of obesity. Am J Clin Nutr 86: 531–541, 2007. doi: 10.1093/ajcn/86.3.531. [DOI] [PubMed] [Google Scholar]
- Liu M, Kay JC, Shen S, Qiao L-Y. Endogenous BDNF augments NMDA receptor phosphorylation in the spinal cord via PLCγ, PKC, and PI3K/Akt pathways during colitis. J Neuroinflammation 12: 151, 2015. doi: 10.1186/s12974-015-0371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WY, Bieger D. Vagovagal reflex motility patterns of the rat esophagus. Am J Physiol Regul Integr Comp Physiol 274: R1425–R1435, 1998. doi: 10.1152/ajpregu.1998.274.5.R1425. [DOI] [PubMed] [Google Scholar]
- Mainardi M, Spinelli M, Scala F, Mattera A, Fusco S, D’Ascenzo M, Grassi C. Loss of leptin-induced modulation of hippocampal synaptic trasmission and signal transduction in high-fat diet-fed mice. Front Cell Neurosci 11: 225, 2017. doi: 10.3389/fncel.2017.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino Adami PV, Galeano P, Wallinger ML, Quijano C, Rabossi A, Pagano ES, Olivar N, Reyes Toso C, Cardinali D, Brusco LI, Do Carmo S, Radi R, Gevorkian G, Castaño EM, Cuello AC, Morelli L. Worsening of memory deficit induced by energy-dense diet in a rat model of early Alzheimer’s disease is associated to neurotoxic Aβ species and independent of neuroinflammation. Biochim Biophys Acta Mol Basis Dis 1863: 731–743, 2017. doi: 10.1016/j.bbadis.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Matott MP, Kline DD, Hasser EM. Glial EAAT2 regulation of extracellular nTS glutamate critically controls neuronal activity and cardiorespiratory reflexes. J Physiol 595: 6045–6063, 2017. doi: 10.1113/JP274620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin CA, Travagli RA, Browning KN. Perinatal high fat diet increases inhibition of dorsal motor nucleus of the vagus neurons regulating gastric functions. Neurogastroenterol Motil 30: e13150, 2018. doi: 10.1111/nmo.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Nakashima T, Kiyohara T. Structural dynamics of neural plasticity in the supraoptic nucleus of the rat hypothalamus during dehydration and rehydration. Brain Res Bull 34: 169–175, 1994. doi: 10.1016/0361-9230(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Montagnese CM, Poulain DA, Vincent J-D, Theodosis DT. Structural plasticity in the rat supraoptic nucleus during gestation, post-partum lactation and suckling-induced pseudogestation and lactation J Endocrinol 115: 97–105, 1987. doi: 10.1677/joe.0.1150097. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem 130: 169–175, 2001. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- Naznin F, Toshinai K, Waise TM, NamKoong C, Md Moin AS, Sakoda H, Nakazato M. Diet-induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J Endocrinol 226: 81–92, 2015. doi: 10.1530/JOE-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefti W, Chaumontet C, Fromentin G, Tomé D, Darcel N. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. Am J Physiol Regul Integr Comp Physiol 296: R1681–R1686, 2009. doi: 10.1152/ajpregu.90733.2008. [DOI] [PubMed] [Google Scholar]
- Neumann H, Schweigreiter R, Yamashita T, Rosenkranz K, Wekerle H, Barde Y-A. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J Neurosci 22: 854–862, 2002. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 64: 300–316, 2016. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor ER, Sontheimer H, Ransom BR. Rat hippocampal astrocytes exhibit electrogenic sodium-bicarbonate co-transport. J Neurophysiol 72: 2580–2589, 1994. doi: 10.1152/jn.1994.72.6.2580. [DOI] [PubMed] [Google Scholar]
- Paeratakul S, Lovejoy JC, Ryan DH, Bray GA. The relation of gender, race and socioeconomic status to obesity and obesity comorbidities in a sample of US adults. Int J Obes Relat Metab Disord 26: 1205–1210, 2002. doi: 10.1038/sj.ijo.0802026. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology 149: 2798–2806, 2008. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci USA 109: E197–E205, 2012. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82: 1263–1270, 2014. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci 25: 2192–2203, 2005. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Alvarez A, Araque A. Astrocyte-neuron interaction at tripartite synapses. Curr Drug Targets 14: 1220–1224, 2013. doi: 10.2174/13894501113149990203. [DOI] [PubMed] [Google Scholar]
- Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci 34: 12738–12744, 2014. doi: 10.1523/JNEUROSCI.2401-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter LS, Tweedle CD, Hatton GI. Neuronal/glial plasticity in the supraoptic dendritic zone in response to acute and chronic dehydration. Brain Res 361: 225–232, 1985. doi: 10.1016/0006-8993(85)91293-4. [DOI] [PubMed] [Google Scholar]
- Pfuhlmann K, Schriever SC, Legutko B, Baumann P, Harrison L, Kabra DG, Baumgart EV, Tschöp MH, Garcia-Caceres C, Pfluger PT. Calcineurin A beta deficiency ameliorates HFD-induced hypothalamic astrocytosis in mice. J Neuroinflammation 15: 35, 2018. doi: 10.1186/s12974-018-1076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab 2: 356–363, 2013. doi: 10.1016/j.molmet.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri L, Stigliani S, Usai C, Diaspro A, Paluzzi S, Milanese M, Raiteri M, Bonanno G. Functional expression of release-regulating glycine transporters GLYT1 on GABAergic neurons and GLYT2 on astrocytes in mouse spinal cord. Neurochem Int 52: 103–112, 2008. doi: 10.1016/j.neuint.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Rose CR, Felix L, Zeug A, Dietrich D, Reiner A, Henneberger C. Astroglial glutamate signaling and uptake in the hippocampus. Front Mol Neurosci 10: 451, 2018. doi: 10.3389/fnmol.2017.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Quintana FJ. Role of astrocytes and microglia in central nervous system inflammation. Semin Immunopathol 37: 575–576, 2015. doi: 10.1007/s00281-015-0521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier A, Béchade C, Poncer J-C, Smalla K-H, Tomasello E, Vivier E, Gundelfinger ED, Triller A, Bessis A. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J Neurosci 24: 11421–11428, 2004. doi: 10.1523/JNEUROSCI.2251-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savtchouk I, Volterra A. Gliotransmission: beyond black-and-white. J Neurosci 38: 14–25, 2018. doi: 10.1523/JNEUROSCI.0017-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia 54: 716–725, 2006. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffler A, Schölmerich J, Salzberger B. Adipose tissue as an immunological organ: Toll-like receptors, C1q/TNFs and CTRPs. Trends Immunol 28: 393–399, 2007. doi: 10.1016/j.it.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Schipke CG, Kettenmann H. Astrocyte responses to neuronal activity. Glia 47: 226–232, 2004. doi: 10.1002/glia.20029. [DOI] [PubMed] [Google Scholar]
- Schousboe A. Role of astrocytes in the maintenance and modulation of glutamatergic and GABAergic neurotransmission. Neurochem Res 28: 347–352, 2003. doi: 10.1023/A:1022397704922. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Bak LK, Waagepetersen HS. Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol (Lausanne) 4: 102, 2013. doi: 10.3389/fendo.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhbahaei S, Turovsky EA, Hosford PS, Hadjihambi A, Theparambil SM, Liu B, Marina N, Teschemacher AG, Kasparov S, Smith JC, Gourine AV. Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nat Commun 9: 370, 2018. doi: 10.1038/s41467-017-02723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Hosoi N, Kaneko R, Tominaga M, Yamada K. Glycine release from astrocytes via functional reversal of GlyT1. J Neurochem 140: 395–403, 2017. doi: 10.1111/jnc.13741. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Black JA, Waxman SG. Voltage-gated Na+ channels in glia: properties and possible functions. Trends Neurosci 19: 325–331, 1996. doi: 10.1016/0166-2236(96)10039-4. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, D’Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol Aging 58: 88–101, 2017b. doi: 10.1016/j.neurobiolaging.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Filosa JA. Bidirectional neuro-glial signaling modalities in the hypothalamus: role in neurohumoral regulation. Auton Neurosci 175: 51–60, 2013. doi: 10.1016/j.autneu.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DKS, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci 19: 6248–6256, 1999. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 31: 18155–18165, 2011. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772–783, 2006. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol 13: 389–401, 2016. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 260: G531–G536, 1991. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P. The biology of obesity. Proc Nutr Soc 64: 31–38, 2005. doi: 10.1079/PNS2004406. [DOI] [PubMed] [Google Scholar]
- Troy AE, Simmonds SS, Stocker SD, Browning KN. High fat diet attenuates glucose-dependent facilitation of 5-HT3-mediated responses in rat gastric vagal afferents. J Physiol 594: 99–114, 2016. doi: 10.1113/JP271558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW. Roles of glial cells in sculpting inhibitory synapses and neural circuits. Front Mol Neurosci 10: 381, 2017. doi: 10.3389/fnmol.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladolid-Acebes I, Merino B, Principato A, Fole A, Barbas C, Lorenzo MP, García A, Del Olmo N, Ruiz-Gayo M, Cano V. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am J Physiol Endocrinol Metab 302: E396–E402, 2012. doi: 10.1152/ajpendo.00343.2011. [DOI] [PubMed] [Google Scholar]
- Velloso LA, Araújo EP, de Souza CT. Diet-induced inflammation of the hypothalamus in obesity. Neuroimmunomodulation 15: 189–193, 2008. doi: 10.1159/000153423. [DOI] [PubMed] [Google Scholar]
- Waise TMZ, Toshinai K, Naznin F, NamKoong C, Md Moin AS, Sakoda H, Nakazato M. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun 464: 1157–1162, 2015. doi: 10.1016/j.bbrc.2015.07.097. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Köhling R, Heinemann U, Theis M, Willecke K, Steinhäuser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26: 5438–5447, 2006. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol 86: 342–367, 2008. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hsuchou H, He Y, Kastin AJ, Pan W. Role of astrocytes in leptin signaling. J Mol Neurosci 56: 829–839, 2015. doi: 10.1007/s12031-015-0518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 15: 2792–2800, 2004. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- Wu H, Liu Q, Kalavagunta PK, Huang Q, Lv W, An X, Chen H, Wang T, Heriniaina RM, Qiao T, Shang J. Normal diet vs high fat diet—a comparative study: behavioral and neuroimmunological changes in adolescent male mice. Metab Brain Dis 33: 177–190, 2018. doi: 10.1007/s11011-017-0140-z. [DOI] [PubMed] [Google Scholar]
- Wu Y, Yu Y, Szabo A, Han M, Huang XF. Central inflammation and leptin resistance are attenuated by ginsenoside Rb1 treatment in obese mice fed a high-fat diet. PLoS One 9: e92618, 2014. doi: 10.1371/journal.pone.0092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Qi Y, Yang Y. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Reports 11: 798–807, 2015. doi: 10.1016/j.celrep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 95: 15769–15774, 1998. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Renehan WE, Fogel R. Neurons in the vagal complex of the rat respond to mechanical and chemical stimulation of the GI tract. Am J Physiol Gastrointest Liver Physiol 274: G331–G341, 1998. doi: 10.1152/ajpgi.1995.268.5.G780. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann K-A, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6: 43–50, 2003. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro 4: e00080, 2012. doi: 10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]