Abstract
Skillful manipulation requires forming and recalling memories of the dynamics of objects linking applied force to motion. It has been assumed that such memories are associated with entire objects. However, we often control different locations on an object, and these locations may be associated with different dynamics. We have previously demonstrated that multiple memories can be formed when participants are explicitly instructed to control different visual points marked on an object. A key question is whether this novel finding generalizes to more natural situations in which control points are implicitly defined by the task. To answer this question, we used objects with no explicit control points and tasks designed to encourage the use of distinct implicit control points. Participants moved a handle, attached to a robotic interface, to control the position of a rectangular object (“eraser”) in the horizontal plane. Participants were required to move the eraser straight ahead to wipe away a column of dots (“dust”), located to either the left or right. We found that participants adapted to opposing dynamics when linked to the left and right dust locations, even though the movements required for these two contexts were the same. Control conditions showed this learning could not be accounted for by contextual cues or the fact that the task goal required moving in a straight line. These results suggest that people naturally control different locations on manipulated objects depending on the task context and that doing so affords the formation of separate motor memories.
NEW & NOTEWORTHY Skilled manipulation requires forming motor memories of object dynamics, which have been assumed to be associated with entire objects. However, we recently demonstrated that people can form multiple memories when explicitly instructed to control different visual points on an object. In this article we show that this novel finding generalizes to more natural situations in which control points are implicitly defined by the task.
Keywords: dynamics, human, motor control, motor learning, object manipulation
INTRODUCTION
Numerous studies of motor learning have examined adaptation of reaching movements to novel loads, or force fields, applied to the hand via a handle attached to a robotic interface (Shadmehr et al. 2010; Wolpert et al. 2011). Many of these studies have used a “viscous curl field” where the load depends on hand speed and acts perpendicular to hand direction. Although this unusual load initially perturbs the hand movement, over trials people adapt such that they can make roughly straight line movements to the target—learning that is thought to involve the formation of a motor memory, or internal model, of the load (Flanagan and Wing 1997; Shadmehr and Mussa-Ivaldi 1994; Wolpert and Flanagan 2010; Wolpert and Ghahramani 2000). Previous studies have also shown that subsequent adaptation to an opposing load (e.g., a viscous curl field that acts in the opposite direction) largely overwrites the initial learning such that people must readapt when the original load is experienced again following the opposing load (Caithness et al. 2004; Shadmehr and Mussa-Ivaldi 1994).
A number of studies have asked whether learning of opposing loads (or dynamics) can be facilitated by the provision of contextual information. Perhaps not surprisingly, it is well established that people can learn different loads if they are linked to different movements; for example, movement in different directions or in different regions of space (Howard et al. 2013; Thoroughman and Shadmehr 2000). However, when the parameters of the required movement are held constant, it has been shown that contextual cues, including arbitrary color cues, are not effective in allowing people to form separate motor memories for opposing loads (Gandolfo et al. 1996; Howard et al. 2013). Interesting, when visuomotor rotations are gradually applied such that participants unwittingly generate similar hand movements when moving a cursor to two different targets, they can form separate motor memories of dynamics for these identical hand movements (Hirashima and Nozaki 2012). However, in this case, distinct visuomotor transformations are involved in planning movements to the two targets.
In studies of force-field adaptation, such as those described above, the viewed “object” being moved is typically a small circular disk linked to the position of the handle, and the task involves controlling the center of the disk. However, most of the objects we grasp and move in real-world tasks have more complex geometry, and we can control different locations, or control points, on the object. Indeed, many objects, such as a pencil or a hammer, can serve more than one function, and these functions are often related to different control points. For example, we control opposite ends of a pencil for writing and erasing, and the middle when placing it behind our ear. Moreover, control can rapidly shift between different points on a single object within a single task. For example, we may control the lip of a glass as we bring it to our mouth and then the base of the glass when replacing it on a table. Importantly, there may be different dynamics experienced when controlling these different control points. Thus, when using a broom, we can control the left or right edge when that edge moves along and contacts a wall, and the dynamics will depend on which edge contacts the wall.
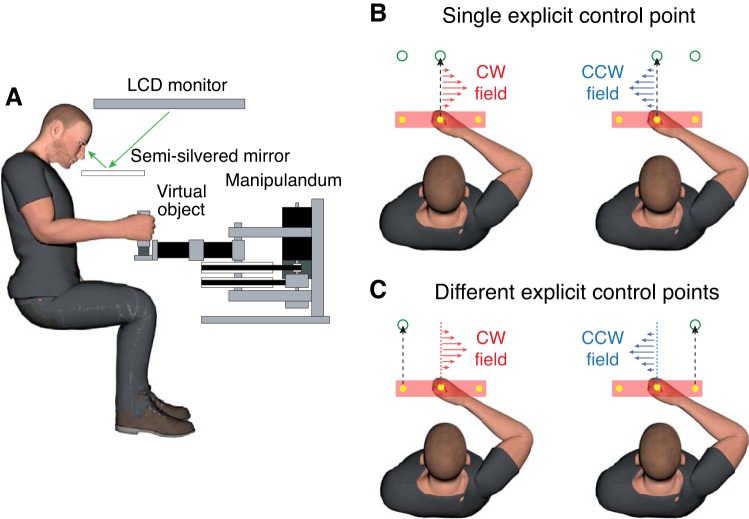
A recent study showed that people can form distinct motor memories of opposing loads when controlling different points on an object, even when making identical movements for the two loads (Heald et al. 2018). In this previous experiment, participants grasped the handle of a robotic manipulandum (Fig. 1A), which was aligned to the center of a virtual rectangular object (see Fig. 1, B and C). In the “Single Explicit Control Point” condition (Fig. 1B), the participant was required to move a central control point to the central target. A second, irrelevant “target” was visible on the left or right, and its position was linked to the load, either a clockwise (CW) or counterclockwise (CCW) viscous curl field, experienced during the movement. Thus the irrelevant target provided an arbitrary visual cue about the direction of the field. In the “Different Explicit Control Points” condition (Fig. 1C), participants moved either the left or right control point to the (now relevant) left or right target, respectively. The left and right targets were again linked to opposing viscous curl fields. Heald et al. (2018) found that participants could not form separate memories for the two fields in the Single Explicit Control Point condition. That is, no adaptation was observed for either field indicating complete interference. This result is consistent with previous work showing that arbitrary visual cues do not facilitate the formation of separate motor memories (Howard et al. 2013). In contrast, participants could form distinct motor memories in the Different Explicit Control Points condition, even though the movements were identical for the different loads.
Fig. 1.
A: Robotic interface and virtual reality system used to simulate objects and force fields. B: Single Explicit Control Point experiment from Heald et al. (2018). Participants were required to move a central control point, on the object, to the central target. The location of the lateral target was linked to the direction of the force field. C: Different Explicit Control Points experiment from Heald et al. (2018). Participants were required to move either the left or right control point, on the object, to the left or right target, respectively. The location of the target was linked to the direction of the force field. CW, clockwise; CCW, counterclockwise.
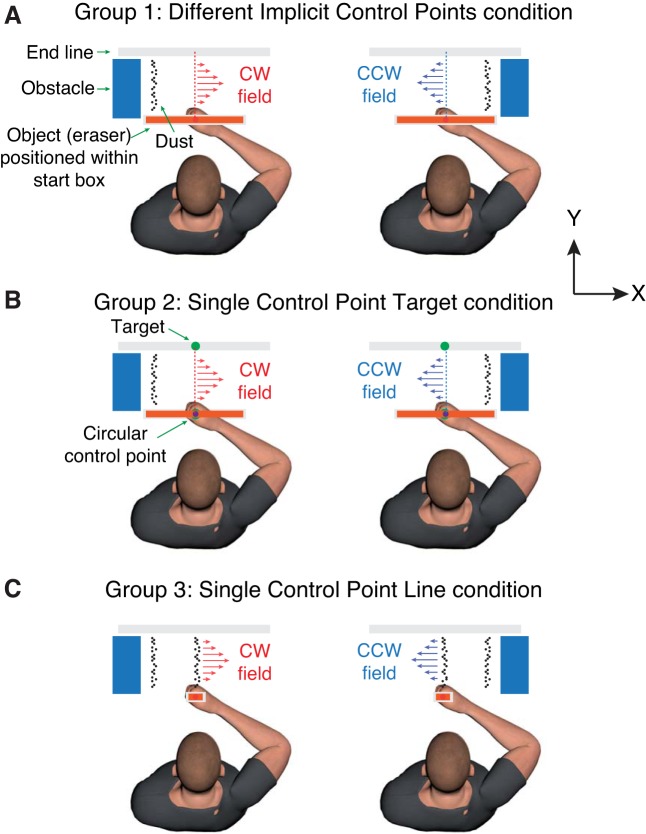
Whereas Heald et al. (2018) provided participants with visible, discrete control points that they were explicitly instructed to control, in many real-world manipulation tasks, the control points are implicitly specified by the demands of the task. Thus, in the broom example cited above, the controlled location (e.g., the edge closest to the wall) is implicitly specified by the task environment. The aim of the current study was to assess whether the formation of distinct motor memories for opposing dynamics, recently established for explicit control points, also occurs for implicitly specified control points. Our basic approach was similar to that employed by Heald and colleagues. That is, participants controlled the movement of a rectangular object by moving a handle attached to a robotic device. In our main condition (Different Implicit Control Points condition), participants were required to “erase” a column of dots (“dust”) while avoiding an obstacle (Fig. 2A). The dust and obstacle were located on either the left or right and positioned such that, for both locations, participants were required to make an approximately straight line movement to remove the dust while avoiding the obstacle. CW and CCW viscous curl fields were linked to the left and right dust/obstacle locations such that the load tended to perturb the hand away from the obstacle. We hypothesized that participants would control the side of the object where the dust and obstacle were located and that this would allow them to form distinct motor memories of the opposing force fields.
Fig. 2.
Three experimental groups. A: group 1, Different Implicit Control Points condition. Participants were required to move the object (“eraser”) straight ahead to remove (“erase”) a column of dots (“dust”) located on either the left or right while avoiding an obstacle. In all groups, clockwise (CW) and counterclockwise (CCW) viscous curl fields were linked to the location of the obstacle. B: group 2, Single Control Point Target condition. Participants were required to move a circle (explicit control point), located at the center of the object, from a circular start position to a circular target located straight ahead. C: group 3, Single Control Point Line condition. Participants were required to move a narrow object to remove a central column of dots.
Two single control point conditions were also run as control experiments. In the Single Control Point Target condition, participants were required to move a circle, located at the center of the object, to a circular target located straight ahead (Fig. 2B). As in the main condition, a column of dust and an obstacle were located on either the left or right and linked to CW and CCW fields, respectively. The aim of this control was to rule out the possibility that purely contextual information provided by the dust and obstacle (and the wiping away of the dust) can account for learning of opposing fields. In the Single Control Point Line condition, participants were required to move a narrow object to remove a central column of dust (Fig. 2C). As in the other conditions, a column of dust and an obstacle were located on either the left or right and linked to CW and CCW fields, respectively. The aim of this control was to rule out the possibility that learning occurs when the goal of the reaching movement is to remove a column of dust, as opposed to when the goal is simply to move the hand to a single target.
METHODS
Participants.
Thirty-two participants (19 women) between 18 and 23 yr of age were recruited from the student population at Queen’s University through the Queen’s Paid Research Study page on Facebook and advertisements. Participants received $15 an hour for their participation. All participants were right-handed and had normal or corrected-to-normal vision. After providing written informed consent, participants were assigned to one of three groups. Group 1 (n = 12) completed the Different Implicit Control Points condition, group 2 (n = 10) completed the Single Implicit Control Point Target condition, and group 3 (n = 10) completed the Single Implicit Control Point Line condition. The study was approved by the Queen’s General Research Ethics Board and complied with the Declaration of Helsinki.
Materials.
All tasks were performed using the wBOT planar robotic manipulandum and virtual reality system (Howard et al. 2009; see Fig. 1A). Torque motors allow forces to be generated on the handle. A monitor mounted above the wBOT projected virtual images into the plane of movement through an opaque horizontal mirror. Note that in our previous study (Heald et al. 2018), participants could see their actual hand through the mirror, whereas in the current study, they only saw a circle, or cursor, representing the position of their hand.
In all trials, the participant moved a rectangular object, centered on the wBOT handle, by translating the handle. The orientation of the object was fixed such that rotating the handle had no effect on the object’s orientation. On each trial, the wBOT could generate no force (baseline trials), forces specified by a velocity-dependent (i.e., viscous) curl field (perturbation trials), or forces specified by a force channel (channel trials). For the curl field, the force generated on the hand was given by
where Fx, Fy, and are the x and y forces and velocities at the handle. The viscosity, or field gain, b was set to ±15 N·s·m−1, and the sign of b specified whether the curl field was CW or CCW. Note that to compensate for a CW or CCW curl field, the participant must generate a leftward or rightward force, respectively, while moving the handle. On channel trials, the hand was constrained to move along a straight-ahead line. This was achieved by simulating forces associated with a stiff, damped spring with the forces acting in the x direction. The stiffness was 5,000 N/m, and the damping coefficient was 5 N·s·m−1. Channel trials enable the measurement of feedforward or predictive forces generated by the participant orthogonal to the reach direction (Milner and Franklin 2005; Scheidt et al. 2000; Smith et al. 2006). These forces can be used to estimate the level of adaptation to the force field (see Data analysis). The wBOT was also used to simulate contact forces if the object controlled by the participant contacted the obstacle. The sides of the obstacle were modeled as a stiff, damped spring with a stiffness of 4,000 N/m and a damping coefficient of 1 N·s·m−1. Note that we did not apply forces to the object to simulate inertia.
Procedure.
At the start of all trials, the object and start box (center ~30 cm in front of the middle of the participant’s chest) were displayed, and the robot moved the rectangular object (attached to the handle of the robot, which was held by the participant) to the start box (Fig. 2). Once the participant held the object within 0.3 cm of the center of the start box and below a speed of 0.5 cm/s for 100 ms, the remaining items in the scene were displayed (e.g., obstacle, dust, end line, target). After a 0.2-s delay, a brief tone was delivered, which served as the go signal.
In the Different Implicit Control Points condition (Fig. 2A), participants were required to move the object (orange rectangle, width 160 mm, height 10 mm) from the start box (gray rectangle, width 164 mm, height 14 mm) to the end line (gray rectangle, width 240 mm, height 14 mm) while erasing a column of dust (50 1-mm × 1-mm dots forming a column 10 mm wide and 80 mm high) and avoiding an obstacle (width 40 mm, height 100 mm). Participants were instructed to “remove the dust while avoiding the obstacle,” but no priority was given to either of these task demands. No instructions were given about gaze or head orientation. The required movement distance (i.e., the y distance between the centers of the start box and end line) was 120 mm. The dust could be located on the left or right with the center positioned 70 mm laterally from midline (i.e., the center of the object when at the start location) and thus 10 mm closer to midline than the edge of the object. The obstacle was positioned on the same side as the dust with the near edge located 90 mm laterally from the midline, and thus 10 mm farther from the midline than the edge of the object. The bottom edge of the obstacle was aligned, in the y direction, with the top edge of the object when at the start position, and the bottom edge of the dust was 50 mm above the top edge of the object. Finally, a slightly darker orange circle (diameter 8 mm) was located at the center of the object and indicated the located of the handle.
The environment in the Single Control Point Target condition (Fig. 2B) was similar to the Different Implicit Control Points condition except that a start circle and an end target (green circles 10 mm in diameter) were also displayed and the center circle on the object was blue and thus more visually salient. The target was positioned straight ahead and located in the center of the end line. The participant was required to move the center circle on the object, which served as an explicit control point, from the start circle to the target. Participants were told to avoid hitting the obstacle, but no instructions were given about the dust. If a participant asked about the dust, they were told to just focus on moving the center circle to the target.
The environment in the Single Control Point Line condition (Fig. 2C) was similar to the Different Implicit Control Points condition except that the object was narrow (20 mm) and an additional, centrally located column of dust was displayed. Participants were instructed to erase the central column of dust.
Trial structure.
The trial structure was the same in all three conditions. Trials were organized in blocks of eight trials, with half of the trials (randomly selected) featuring the obstacle on the left and half featuring the obstacle on the right. The experiment began with a pre-exposure phase with no force fields applied (i.e., baseline trials). This phase included 4 blocks of 8 trials, making 32 trials in total. This phase was followed by the exposure phase in which opposing force fields were associated with the two contexts. Specifically, CW and CCW curl fields were associated with the left and right obstacle positions. This phase consisted of 52 blocks of 8 trials (4 per context), making 416 trials in total. Each block of eight trials included one channel trial that was pseudorandomly selected but could not be the first or last trial of the block to avoid consecutive channel trials. The context (i.e., obstacle location) of the channel trial alternated across blocks such that one channel trial for each context was included for every two blocks (16 trials). The exposure phase was followed by the postexposure phase, which consisted of 12 blocks of 8 trials (4 per context), making 96 trials in total, with the force fields turned off (i.e., baseline trials).
Data analysis.
The x and y positions of the hand (i.e., handle) and the x and y forces output to the robot handle were sampled at 1,000 Hz and smoothed offline using a Butterworth fourth-order, zero-phase lag, low-pass filter with a cutoff frequency of 14 Hz. For analysis, we selected the primary movement generated by the participant as follows. We first found the time of the peak resultant velocity of the hand and then searched backward in time to find the time at which the hand last exceeded 10 mm/s (start) and forward in time to find the time of the sample before the hand first dropped below 10 mm/s (end).
Two measures of performance were calculated on the basis of the primary movement, as defined above. In non-channel trials, we first computed the maximum perpendicular error (MPE), defined as the largest lateral (x) deviation, positive or negative, of the hand from the straight-ahead line. Note that the CW and CCW force fields, associated with the obstacle being on the left and right, tended to push the hand to the right and left, resulting in positive and negative MPEs, respectively. So that we could combine all trials, we then computed the adjusted MPE by flipping the sign (i.e., negating) MPE for trials in which the obstacle was on the right. (Note that this tended to result in positive adjusted MPE values when participants did not compensate for either the CW or CCW force field.) In channel trials, we estimated the proportion of the ideal lateral force generated by the participant, where the ideal force is the force that the participant would have had to apply to move perfectly straight had the expected force field been applied. Specifically, we determined the slope when regressing, with no intercept, the actual lateral force time series generated by the participant during the movement against the corresponding ideal force. We will refer to this measure as “adaptation.” A value of 1 indicates full compensation for the force field, a value of 0 indicates no compensation, and negative values indicate the participant pushed in the wrong direction given the expected force field.
An ANOVA was performed to measure changes in adjusted MPE and adaptation during the perturbation phase of the experiment. Specifically, the first and final blocks of the exposure phase for each condition were compared. The significance level was set to P < 0.05.
RESULTS
Representative hand paths.
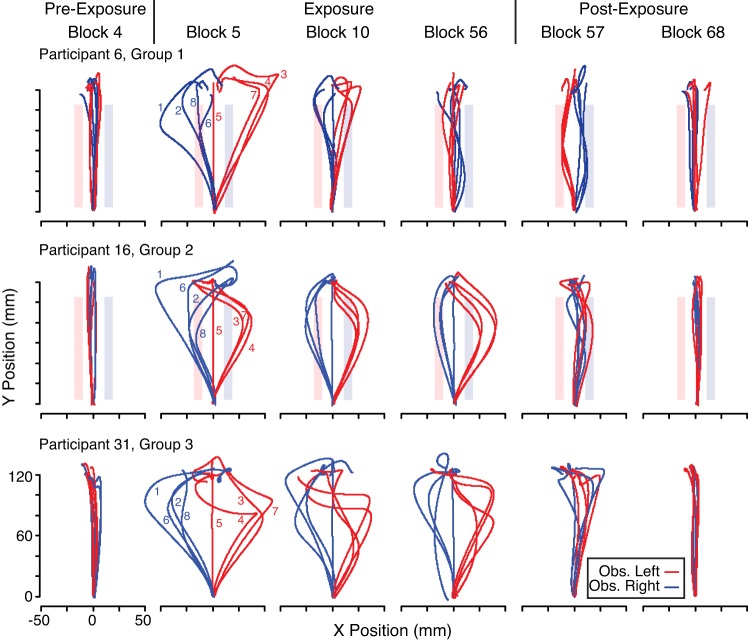
The top, middle, and bottom rows of Fig. 3 show hand paths from representative participants in group 1 (Different Implicit Control Points condition), group 2 (Single Control Point Target condition), and group 3 (Single Control Point Line condition), respectively. Paths from selected blocks are shown, including the last block of baseline trials in the pre-exposure phase (block 4), the first, sixth and last blocks of perturbation trials in the exposure phase (blocks 5, 10, and 57), and the first and last blocks of baseline trials in the postexposure phase (blocks 57 and 68). The red paths are from trials with the obstacle on the left, and the blue paths are from trials with the obstacle on the right. The red rectangles show the leftward limit of possible hand motion, due to the obstacle, in trials with the obstacle on the left (red paths). The blue rectangles show the rightward limit of possible hand motion, due to the obstacle, in trials with the obstacle on the right (blue paths). Note that rectangles are not displayed for the group 3 participant because these were ±80 mm away from the hand. Note that the force field tended to push the hand away from the obstacle. Individual trials are numbered for the first perturbation block, in which trial 5 was a channel trial.
Fig. 3.
Hand paths from representative participants in groups 1, 2, and 3 are shown in the top, middle, and bottom rows, respectively. Paths are from different blocks of trials including the last baseline block of the pre-exposure phase (block 4), the first (block 5), sixth (block 10), and last (block 56) perturbation blocks from the exposure phase, and the first (block 57) and last (block 68) baseline blocks from the postexposure phase. Individual trials are numbered for block 5; note that trial 5 is a channel trial. Red rectangles show the leftward limit of possible hand motion, due to the obstacle, in trials with the obstacle on the left (Obs. Left; red paths). Blue rectangles show the rightward limit of possible hand motion, due to the obstacle, in trials with the obstacle on the right (Obs. Right; blue paths). Rectangles are not displayed for the group 3 participant because they were ±80 mm away from the hand. Note that in perturbation trials, the force field tended to push the hand away from the obstacle. Whereas the participant in group 1 gradually adapted to the force fields, the participants in groups 2 and 3 did not.
Consider, first, the participant in group 1. In the last block of the pre-exposure phase (block 4), this participant generated approximately straight hand paths. When the force field was unexpectedly turned on in the first block of the exposure phase (block 5), hand paths were greatly perturbed away from the obstacle. However, the participant gradually adapted to the opposing force fields such that hand paths became increasing straight across blocks. Note that in later trials of the exposure phase, the object occasionally hit the obstacle. Thus, in block 56, the object hit the right obstacle in one of the trials (such that the blue hand path contacts the blue obstacle). In the first block of baseline trials after the force fields were turned off (block 57), clear aftereffects are observed where the hand is “perturbed” in the opposite direction, indicating that the participant was compensating for the expected, but unexpectedly removed, force field. In all of the trials in this block, the object contacted the obstacle. However, by the last block of the postexposure phase (block 68), the participant had fully de-adapted, and straight-line hand paths, similar to those observed before the exposure phase (block 4), were observed. These results indicate that this participant was able to form motor memories of the opposing force fields when implicitly controlling different ends of the object to remove the dust.
Now consider the hand paths for the representative participants in groups 2 and 3. In contrast to the representative participant in group 1, both of these participants failed to adapt to the opposing force fields such that their hand paths continued to be perturbed away from the obstacle throughout the exposure phase. Consistent with this failure to adapt, limited aftereffects were observed when the force fields were turned off at the start of the postexposure phase (block 57). These results indicate that these participants were not able to form memories of the opposing force fields when controlling a single control location to move to a target (group 2) or erase a line of dust (group 3).
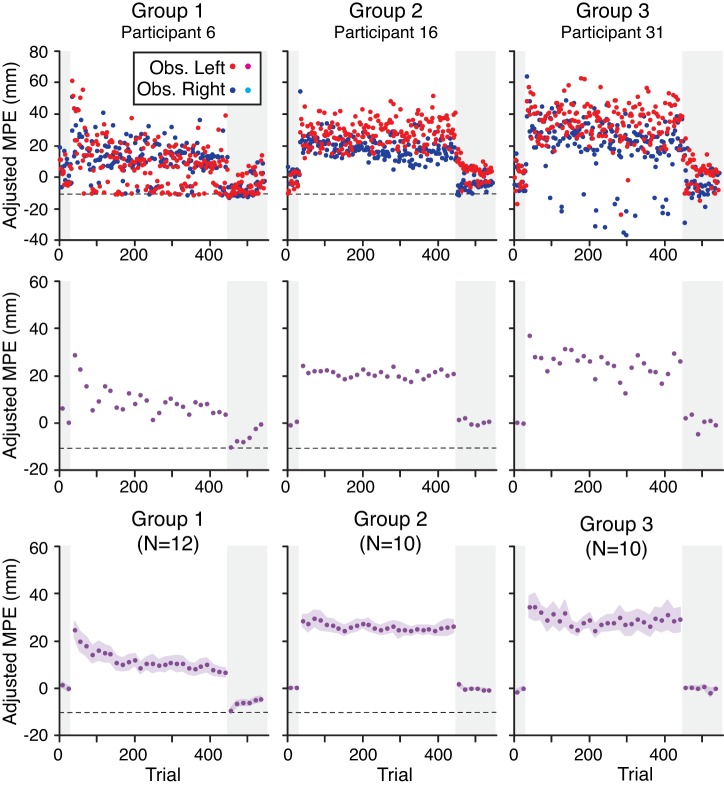
Adjusted maximum perpendicular error in non-channel trials.
The top row of Fig. 4 shows the adjusted MPE in non-channel trials as a function of trial for the same representative participants from each group shown in Fig. 3. The shaded zones on the left and right of each plot mark the pre- and postexposure phases, respectively. The red circles represent trials with the obstacle on the left, and the blue circles represent trials with the obstacle on the right. The black dashed horizontal line represents the limit of possible hand motion, imposed by the obstacle, in adjusted x coordinates. (Note that this limit was −80 mm in the Single Control Point Line condition and thus is off the scale for group 3.) The middle row of Fig. 4 shows, for each of these participants, corresponding data averaged across the 14 non-channel trials (7 per context) in each successive pair of trial blocks (or “block pair”). These plots provide a smoothed view of how adjusted MPE changes across the different phases of the experiment. Finally, the bottom row of Fig. 4 shows group mean data corresponding to the middle row. Participants in group 1 reduced adjusted MPE across trials during the exposure phase and exhibited aftereffects during the postexposure phase (negative adjusted MPE values). In contrast, for participants in groups 2 and 3, adjusted MPE remained elevated during the exposure phase and little or no aftereffects were observed. These results suggest that whereas participants in group 1 were able to adapt to the opposing CW and CCW force fields, participants in groups 2 and 3 were not.
Fig. 4.
Top row: adjusted maximum perpendicular error (MPE), in non-channel trials, as a function of trial for 3 representative participants from groups 1–3. The shaded areas on the left and right mark the pre- and postexposure phases, respectively. Red and blue points are from trials with the obstacle located on the left and right (Obs. Left and Obs. Right), respectively. Black dashed horizontal line represents the limit of possible hand motion, imposed by the obstacle, in adjusted x coordinates. (Note that this limit was −80 mm in the Single Control Point Line condition and thus is off the scale for group 3.) Middle row: corresponding data averaged across the 14 non-channel trials (7 per context) in perturbation trials, or the 16 non-channel trials (8 per context) in baseline trials, in every 2 blocks (16 trials). Bottom row: group mean data (n = no. of participants) corresponding to the middle row. Height of shaded regions represents ±SE.
An ANOVA with group (1–3) as a between-subjects factor and block pair (first and last block pairs of the exposure phase) as a within-subjects factor was carried out to assess changes in adjusted MPE during the exposure phase. A significant interaction (F2,29 = 3.99, P = 0.029) between block pair and group was observed. To follow up on this interaction, separate paired t-tests comparing the first and last block pairs were carried out. For group 1, adjusted MPE significantly decreased (t11 = 5.34, P < 0.001) from the first block pair (21.9 ± 3.3 mm, mean ± SE) to the last (7.2 ± 3.0 mm). In contrast, for groups 2 and 3, no significant difference (group 2: t9 = 0.58, P = 0.58; group 3: t9 = 1.36, P = 0.21) was observed between the first and last block pairs.
Adaptation measured in channel trials.
Adaptation involves learning to generate forces that counteract the force field, thus allowing the participant to move the object straight ahead and succeed at the task. This adaptation can be directly assessed by measuring the forces participants exert on randomly selected channel trials. Channel trials allows us to distinguish between adaptation and the use of a co-contraction strategy whereby the participant compensates for the force field by stiffening the limb. As outlined above (see methods), for channel trials we computed the slope of the relationship between the lateral force generated by the participant and the ideal lateral force that would fully compensate for the force field, had it been present (and as expected by the context). This slope provides a simple measure of the state of adaptation of the participant (Heald et al. 2018; Trewartha et al. 2014). We refer to this slope as the adaptation.
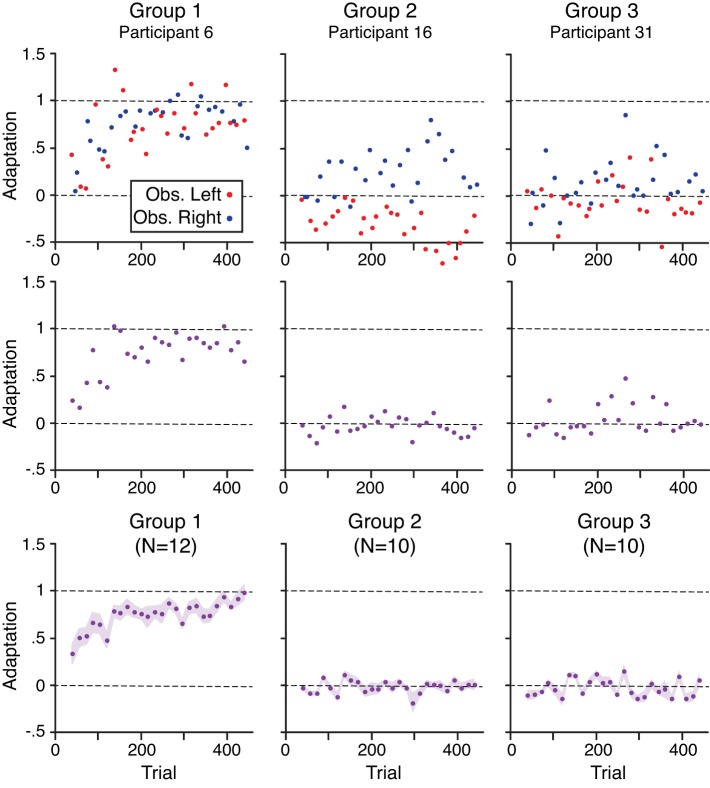
The top row of Fig. 5 shows adaptation, measured in channel trials, as a function of trial for the same representative participants from each group shown in Figs. 3 and 4. The red and blue circles represent trials with the obstacle on the left and right and associated with the CW and CCW force fields. (Note that channel trials were only included in the exposure phase.) Dashed horizontal lines indicate adaptation values of 0 (no adaptation) and 1 (full adaptation). For the participant from group 1, adaptation increases from close to 0 toward 1 across the exposure phase. For the participant from group 2, a reciprocal relationship between adaptation for the CW and CCW force fields was observed. That is, this participant, like several other participants in groups 2 and 3, could temporarily exhibit adaptation to one force field, but only at the expense of adaptation to the other force field. For the representative participant from group 3, little adaptation is observed for either force field. The middle row of Fig. 5 shows, for each of these participants, corresponding data averaged across the two channel trials (1 per context or force field) in each successive pair of trial blocks. These plots provide a smoothed view of how adaptation changes across the exposure phase and effectively remove reciprocal adaptation to the opposing fields. The bottom row of Fig. 5 shows group mean data corresponding to the middle row. Participants in group 1 began adapting early in the exposure phase and reached close to full adaptation by the end of the exposure phase. In contrast, participants in groups 2 and 3 failed to adapt to the opposing force fields.
Fig. 5.
Top row: adaptation, in channel trials, as a function of trial for 3 representative participants from groups 1–3. Red and blue points are from trials with the obstacle located on the left and right (Obs. Left and Obs. Right), respectively. Middle row: corresponding data averaged across the 2 non-channel trials (1 per context) in every 2 blocks (16 trials). Bottom row: group mean data (n = no. of participants) corresponding to the middle row. Height of the shaded regions represents ±SE.
A group (1–3) by block pair (first and last block pairs of the exposure phase) ANOVA was carried out to examine changes in adaptation during the exposure phase. A significant interaction (F2,29 = 10.88, P < 0.001) between group and block pair was observed. To follow up on this interaction, separate paired t-tests comparing the first and last block pairs were carried out. For group 1, adaptation significantly increased (t11 = −5.65, P < 0.001) from the first block pair (0.34 ± 0.07) to the last (0.98 ± 0.07). In contrast, for groups 2 and 3, no significant difference (group 2: t9 = −0.48, P = 0.64; group 3: t9 = −1.58, P = 0.15) was observed between the first and last block pairs. These results confirm that whereas participants in group 1 adapted to the opposing force fields, participants in groups 2 and 3 did not.
Note that although adaptation at the end of the exposure phase was, on average, close to 1 for participants in group 1, the corresponding adjusted MPE measure did not return to its baseline (i.e., pre-exposure) level. This apparent discrepancy is due to the fact that the slope of the relationship between the actual force and the ideal force (i.e., “adaptation”) can be ~1 without there being a perfect correspondence between these two forces. Thus an adaptation value of 1 does not imply perfect adaptation.
DISCUSSION
The aim of the current study was to test the hypotheses that 1) people implicitly control different locations on a tool depending on the task environment and that 2) this flexible control affords the formation of separate motor memories of dynamics linked to these locations. In support of these hypotheses, we found that participants could adapt to opposing force fields linked to erasing a line of target dots with either the left or right end of a rectangular object. This adaptation occurred even though the movement kinematics associated with these two contexts were similar. Control conditions showed this learning could not be accounted for by contextual cues associated with the location of the obstacle and dust or by the fact that the task goal (i.e., erasing the dust) required moving in a straight line. These results suggest that participants implicitly exerted control over different locations on the object and that this allowed them to form separate motor memories for each control location. This finding extends our previous work showing that multiple memory formation is possible when controlling different explicitly defined and visually marked control points on an object (Heald et al. 2018).
Previous studies of motor learning have shown that people can simultaneously adapt to different (typically opposing) dynamics when these are applied to reach movements with different kinematics (Howard et al. 2013; Thoroughman and Shadmehr 2000). Moreover, under certain conditions people can, at least partially, adapt to opposing dynamics applied to reaching movements with the same kinematics. Thus adaptation is seen when one force field is applied during unimanual reaching and the opposing force field is applied (to the same hand) during bimanual reaching (Nozaki et al. 2006; see also Yokoi et al. 2011). Adaptation is also observed when the common reach movement to which the opposing force fields are applied is preceded by (or followed by) different “lead in” (or “follow through”) movements linked to the force fields (Howard et al. 2012, 2015; Sheahan et al. 2016). Finally, it has been shown that, following gradual adaptation to opposing visuomotor rotations that make participants unwittingly believe they are reaching to different targets even though the same hand movement is generated, participants can adapt to opposing dynamics linked to the two visually distinct, but physically identical, reaching movements (Hirashima and Nozaki 2012). In all of these cases, the movements to which the opposing dynamics are applied differ in the either the sensorimotor transformation or the overall movements required to perform the task. However, when different dynamics are applied to the same (physical and visually perceived) isolated movement, previous work has found that people are generally unable to adapt despite a variety of contextual cues (Gandolfo et al. 1996; Heald et al. 2018; Howard et al. 2013). In all of the previous work, participants controlled a small circular object (or “cursor”) linked to the position of the hand (or handle grasped by the hand). However, in real-world manipulation tasks, we often manipulate objects with more complex geometry and may control different locations on the object depending on the task at hand. The current study, together with our recent study (Heald et al. 2018), demonstrates that when controlling, either implicitly or explicitly, different parts of the object, people can learn different dynamics for movements with the same kinematics.
The idea that control (and motor memories) can be flexibly assigned to different locations on an object can be related to the “sensorimotor control point” framework for understanding the control of object manipulation tasks (Flanagan et al. 2006; Johansson and Flanagan 2009). This framework views manipulation tasks as a series of action phases demarcated by contact events (or potential contact events) that give rise to distinct, and often discrete, multisensory signals. Consider the simple task, examined by Johansson et al. (2001), in which participants grasped a bar from the near end, lifted and moved it around an obstacle to contact a button with the far end, and then replaced it. In this example, contact between the fingers and bar marks the end of the reach phase, the breaking of contact between the object and surface marks the end of the load phase, the clearance of the far end of the bar around the obstacle (a potential contact event) marks the end of the first movement phase, and so on. These contact events (or potential contact events) give rise to distinct tactile signals, as well as visual, proprioceptive, and even auditory signals, that indicate whether the goal of the action phase has been achieved. Thus they serve as key sensorimotor control points in the task: by comparing predicted and actual sensory signals linked to these points, the brain can monitor task progress and launch appropriate corrective actions if necessary (Johansson and Flanagan 2009). Critically, these corrective actions depend on the phase of the task (Johansson and Westling 1987, 1988), and thus manipulation tasks involve switching between different sensorimotor control policies that govern motor responses to sensed errors (Flanagan et al. 2006). Note that sensorimotor control points are both spatial and temporal in nature; they occur at specific times during the unfolding task and are also associated with contact locations (e.g., between the tip of the object and the target button or between the bottom of the object and the landing surface). Thus sensorimotor control points can be linked to locations on manipulated objects. Finally, across sequential phases of the task, the dynamics experienced by the actor can vary due to changing interactions between the objects in the environment, and this may necessitate changes in the underlying control (Chib et al. 2009). Given these aspects of the sensorimotor control of manipulation tasks, the ability to flexibly assign distinct memories of dynamics to different locations on an object is highly advantageous.
When reaching to a single target with the hand, a cursor controlled by the hand, or an object held in the hand, people fixate the target and almost never fixate the hand, cursor, or the object in the hand (Flanagan and Johansson 2003; Johansson et al. 2001). When the target of action is a line, as in our erasing task (which is effectively a tracing task), gaze is directed along the line, ahead of the hand (Gowen and Miall 2006; Ketcham et al. 2006; Reina and Schwartz 2003). This raises the question whether the learning we observed in our main experiment is due to different eye movements being generated for the opposing force fields. Importantly, in our previous study (Heald et al. 2018), we showed that when controlling different explicit locations on the object, participants could still adapt to opposing force fields when required to fixate a central point throughout each trial. Of course, even when fixating a central location, it is obvious that participants attend to different locations when controlling different parts of the object. However, this “attention” is not some abstract cognitive resource that is distinct from motor control. Rather, as outlined in the sensorimotor control point framework (Johansson and Flanagan 2009), it is part and parcel of controlling movement (e.g., providing retinal and extraretinal information about target locations, monitoring task performance, and detecting and responding to errors) and can reasonably be referred to as “sensorimotor attention” centered on control points. Indeed, for us, sensorimotor attention and control points are not really separable, because sensorimotor attention is fundamentally linked to the point being controlled and control points imply not only a location but also the processes involved in control.
We recognize that our interpretation of our results is based on inference. Ultimately, we cannot “know” that participants are controlling a particular location. However, given the correspondence between the current results and those from our previous study (Heald et al. 2018), we feel it is reasonable to suggest that participants controlled separate locations on the object in our main (Different Implicit Control Points) condition and a single location in our two control conditions.
The final level of adaptation we observed in the main experimental task was close to 1, suggesting that participants, on average, strongly compensated for the force field. This adaptation is greater than the level we observed in our previous study (Heald et al. 2018), which was ~0.8 (i.e., 80% compensation), as well as previous studies of force-field learning, which have reported adaptation values ranging from 0.6 to 0.8 (Smith et al. 2006; Trewartha et al. 2014). This more complete compensation is presumably due to the task requirements, specifically, the fact that participants needed to generate approximately straight line hand paths to remove all of the dust while avoiding the obstacle. In contrast, previous studies have used standard target reaching tasks in which the goal is to move the hand to a small circular target. Whereas participants tend to generate roughly straight hand paths, following adaptation, when reaching to such targets in the presence of a force field, perfectly straight hand paths are not required by the task. Importantly, as we demonstrated in the Single Control Point Line condition, the requirement of moving in a straight line, per se, does not necessarily result in adaptation. That is, participants in this condition failed to form separate memories for the opposing force fields.
A number of studies have provided evidence for the idea, dating back over a century (Head and Holmes 1911), that tool use can dynamically change somatosensory and visual representations. Thus psychophysical studies have shown that tool use can change the perceptual representation of peripersonal space (Berti and Frassinetti 2000; Farnè et al. 2005; Witt et al. 2005) and the body schema (Cardinali et al. 2009), and neurophysiological studies have found that tool use can lead to neural activity changes in premotor, primary somatosensory, and parietal regions (Hihara et al. 2006; Inoue et al. 2001; Iriki et al. 1996; Obayashi et al. 2001; Maravita and Iriki 2004; Schaefer et al. 2004). It is plausible that controlling different locations on a tool may result in distinct activity changes in sensorimotor regions, which in turn may provide a neural basis for representing different dynamics (Nozaki and Scott 2009; Yokoi et al. 2011).
In summary, we have provided evidence that people naturally control different locations on manipulated objects depending on the functional task they are performing and that distinct motor memories of dynamics can be linked to these controlled locations. This ability is important, because in natural manipulatory tasks, different dynamics can be associated with controlling different parts of the object during the unfolding task. Our results, which both confirm and extend our recent study on explicit control points (Heald et al. 2018), suggest that our ability to allocate multiple motor memories to a single object, even when making the same movement, is quite general and can be exploited in a number of contexts.
GRANTS
This work was supported by Natural Sciences and Engineering Research Council of Canada Grant RGPIN/04837-2014 (to J. R. Flanagan), Canadian Institutes of Health Research Grant 126158 (J. R. Flanagan), the Wellcome Trust (D. M. Wolpert), and the Royal Society (D. M. Wolpert).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.P., J.B.H., J.N.I., J.P.G., D.M.W., and J.R.F. conceived and designed research; K.P. performed experiments; K.P. and J.R.F. analyzed data; K.P., J.B.H., J.N.I., J.P.G., D.M.W., and J.R.F. interpreted results of experiments; K.P. and J.R.F. prepared figures; K.P. and J.R.F. drafted manuscript; K.P., J.B.H., J.N.I., J.P.G., D.M.W., and J.R.F. edited and revised manuscript; K.P., J.B.H., J.N.I., J.P.G., D.M.W., and J.R.F. approved final version of manuscript.
REFERENCES
- Berti A, Frassinetti F. When far becomes near: remapping of space by tool use. J Cogn Neurosci 12: 415–420, 2000. doi: 10.1162/089892900562237. [DOI] [PubMed] [Google Scholar]
- Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR. Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci 24: 8662–8671, 2004. doi: 10.1523/JNEUROSCI.2214-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinali L, Frassinetti F, Brozzoli C, Urquizar C, Roy AC, Farnè A. Tool-use induces morphological updating of the body schema. Curr Biol 19: R478–R479, 2009. doi: 10.1016/j.cub.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Chib VS, Krutky MA, Lynch KM, Mussa-Ivaldi FA. The separate neural control of hand movements and contact forces. J Neurosci 29: 3939–3947, 2009. doi: 10.1523/JNEUROSCI.5856-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnè A, Iriki A, Làdavas E. Shaping multisensory action-space with tools: evidence from patients with cross-modal extinction. Neuropsychologia 43: 238–248, 2005. doi: 10.1016/j.neuropsychologia.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Bowman MC, Johansson RS. Control strategies in object manipulation tasks. Curr Opin Neurobiol 16: 650–659, 2006. doi: 10.1016/j.conb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Johansson RS. Action plans used in action observation. Nature 424: 769–771, 2003. doi: 10.1038/nature01861. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci 17: 1519–1528, 1997. doi: 10.1523/JNEUROSCI.17-04-01519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo F, Mussa-Ivaldi FA, Bizzi E. Motor learning by field approximation. Proc Natl Acad Sci USA 93: 3843–3846, 1996. doi: 10.1073/pnas.93.9.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen E, Miall RC. Eye-hand interactions in tracing and drawing tasks. Hum Mov Sci 25: 568–585, 2006. doi: 10.1016/j.humov.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain 34: 102–254, 1911. doi: 10.1093/brain/34.2-3.102. [DOI] [Google Scholar]
- Heald JB, Ingram JN, Flanagan JR, Wolpert DM. Multiple motor memories are learned to control different points on a tool. Nat Hum Behav 2: 300–311, 2018. doi: 10.1038/s41562-018-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hihara S, Notoya T, Tanaka M, Ichinose S, Ojima H, Obayashi S, Fujii N, Iriki A. Extension of corticocortical afferents into the anterior bank of the intraparietal sulcus by tool-use training in adult monkeys. Neuropsychologia 44: 2636–2646, 2006. doi: 10.1016/j.neuropsychologia.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Hirashima M, Nozaki D. Distinct motor plans form and retrieve distinct motor memories for physically identical movements. Curr Biol 22: 432–436, 2012. doi: 10.1016/j.cub.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Franklin DW, Wolpert DM. Gone in 0.6 seconds: the encoding of motor memories depends on recent sensorimotor states. J Neurosci 32: 12756–12768, 2012. doi: 10.1523/JNEUROSCI.5909-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Wolpert DM. A modular planar robotic manipulandum with end-point torque control. J Neurosci Methods 181: 199–211, 2009. doi: 10.1016/j.jneumeth.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Howard IS, Wolpert DM, Franklin DW. The effect of contextual cues on the encoding of motor memories. J Neurophysiol 109: 2632–2644, 2013. doi: 10.1152/jn.00773.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Wolpert DM, Franklin DW. The value of the follow-through derives from motor learning depending on future actions. Curr Biol 25: 397–401, 2015. doi: 10.1016/j.cub.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Kawashima R, Sugiura M, Ogawa A, Schormann T, Zilles K, Fukuda H. Activation in the ipsilateral posterior parietal cortex during tool use: a PET study. Neuroimage 14: 1469–1475, 2001. doi: 10.1006/nimg.2001.0942. [DOI] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport 7: 2325–2330, 1996. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Flanagan JR. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci 10: 345–359, 2009. doi: 10.1038/nrn2621. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp Brain Res 66: 141–154, 1987. doi: 10.1007/BF00236210. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res 71: 59–71, 1988. doi: 10.1007/BF00247522. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G, Bäckström A, Flanagan JR. Eye-hand coordination in object manipulation. J Neurosci 21: 6917–6932, 2001. doi: 10.1523/JNEUROSCI.21-17-06917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcham CJ, Dounskaia NV, Stelmach GE. The role of vision in the control of continuous multijoint movements. J Mot Behav 38: 29–44, 2006. doi: 10.3200/JMBR.38.1.29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravita A, Iriki A. Tools for the body (schema). Trends Cogn Sci 8: 79–86, 2004. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Milner TE, Franklin DW. Impedance control and internal model use during the initial stage of adaptation to novel dynamics in humans. J Physiol 567: 651–664, 2005. doi: 10.1113/jphysiol.2005.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki D, Kurtzer I, Scott SH. Limited transfer of learning between unimanual and bimanual skills within the same limb. Nat Neurosci 9: 1364–1366, 2006. doi: 10.1038/nn1785. [DOI] [PubMed] [Google Scholar]
- Nozaki D, Scott SH. Multi-compartment model can explain partial transfer of learning within the same limb between unimanual and bimanual reaching. Exp Brain Res 194: 451–463, 2009. doi: 10.1007/s00221-009-1720-x. [DOI] [PubMed] [Google Scholar]
- Obayashi S, Suhara T, Kawabe K, Okauchi T, Maeda J, Akine Y, Onoe H, Iriki A. Functional brain mapping of monkey tool use. Neuroimage 14: 853–861, 2001. doi: 10.1006/nimg.2001.0878. [DOI] [PubMed] [Google Scholar]
- Reina GA, Schwartz AB. Eye-hand coupling during closed-loop drawing: evidence of shared motor planning? Hum Mov Sci 22: 137–152, 2003. doi: 10.1016/S0167-9457(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Rothemund Y, Heinze HJ, Rotte M. Short-term plasticity of the primary somatosensory cortex during tool use. Neuroreport 15: 1293–1297, 2004. doi: 10.1097/01.wnr.0000129573.36301.db. [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA. Persistence of motor adaptation during constrained, multi-joint, arm movements. J Neurophysiol 84: 853–862, 2000. doi: 10.1152/jn.2000.84.2.853. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108, 2010. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Sheahan HR, Franklin DW, Wolpert DM. Motor planning, not execution, separates motor memories. Neuron 92: 773–779, 2016. doi: 10.1016/j.neuron.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4: e179, 2006. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature 407: 742–747, 2000. doi: 10.1038/35037588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewartha KM, Garcia A, Wolpert DM, Flanagan JR. Fast but fleeting: adaptive motor learning processes associated with aging and cognitive decline. J Neurosci 34: 13411–13421, 2014. doi: 10.1523/JNEUROSCI.1489-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt JK, Proffitt DR, Epstein W. Tool use affects perceived distance, but only when you intend to use it. J Exp Psychol Hum Percept Perform 31: 880–888, 2005. doi: 10.1037/0096-1523.31.5.880. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci 12: 739–751, 2011. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor learning. Curr Biol 20: R467–R472, 2010. doi: 10.1016/j.cub.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci 3, Suppl: 1212–1217, 2000. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- Yokoi A, Hirashima M, Nozaki D. Gain field encoding of the kinematics of both arms in the internal model enables flexible bimanual action. J Neurosci 31: 17058–17068, 2011. doi: 10.1523/JNEUROSCI.2982-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]