Abstract
The purpose of this study was to evaluate the application of multimodal intraoperative monitoring (MIOM) system in patients with congenital scoliosis (CS) and adolescent idiopathic scoliosis (AIS).
Twelve patients who underwent posterior surgical correction of scoliosis for CS and AIS from June 2014 to July 2018 were enrolled in this study. During the operation, we monitored the functional status of the spinal cord by MIOM. An abnormal somatosensory evoked potential was defined as a prolonged latency of more than 10% or a peak-to-peak amplitude decline of more than 50% when compared to baseline. An abnormal transcranial motor evoked potential (TcMEP) was defined as a TcMEP amplitude decrease of more than 50%. A normal triggered electromyography response, which presented with the absence of an electrical response on stimulation at 8.2 mA, indicated that the pedicle screw was not in contact with the spinal cord or nerve root.
A total of 12 patients underwent MIOM surgery, of which 9 patients with negative MIOM had no significant deterioration of neurological function postoperatively, and exhibited satisfactory surgical correction of scoliosis during follow-ups. However, the remaining 3 patients suffered from MIOM events, 2 patients had normal neurological function, and 1 patient had deteriorated neurological function postoperatively.
Using MIOM in CS and AIS surgery could promptly detect iatrogenic neurological injury at the early stage. Therefore, rapid response by appropriate intraoperative interventions can be taken to minimize the injury. Besides, stable MIOM recordings encourage surgeons to correct scoliosis even when the Cobb angle of scoliosis was extremely large.
Keywords: intraoperative monitoring, neurological lesion, scoliosis, spinal cord
1. Introduction
Congenital scoliosis (CS) and adolescent idiopathic scoliosis (AIS) are a 3-dimensional anatomical deformity of the spine, mainly including variations in alignment of the sagittal plane, deviations in coronal plane, and vertebral rotation in axial plane.[1–3] At present, screw-rod system fixation, osteotomy, and scoliosis correction in posterior approach have become the primary measure for CS and AIS.[3,4] We need to avoid iatrogenic nerve injury intraoperatively.
However, screw implantation, osteotomy, and scoliosis correction are the 3 major high-risk manipulations which may result in neurological deterioration during operation. Mechanical damage due to stretching of the nerve fibers during the correction of the scoliosis may lead to catastrophic neurological impairment.[5] In addition, literatures have reported that the incidence of pedicle screw misplacement ranged within 20% to 30%, and 1% of which suffered from neurological damage that could bring about serious consequences such as paralysis.[6,7]
Therefore, it is relatively indispensable for surgeons to clearly understand the real-time feedback of never-function during critical phases of surgical procedures. Hu et al,[8] Deletis et al,[9] and Yu et al[10] reported that intraoperative monitoring could be perfectly applied in spinal surgery, such as intramedullary tumor resection, reduction of spinal fracture dislocation, scoliosis correction, and lumbosacral spinal canal surgery. Previous studies have rarely introduced the detailed application of multimodal intraoperative monitoring (MIOM) in CS and AIS surgery. In my study, MIOM technology was applied to scoliosis patients to avoid spinal cord compromise, such as the monitoring of the integrity of the spinal cord pathway, especially when screw implantation, osteotomy, and scoliosis correction are carried out. The purpose of this study was to estimate the feasibility of MIOM in surgical treatment for CS and AIS, and provide clinical experience.
2. Material and methods
2.1. Ethics
The Ethics Committee of Second Hospital of Jilin University, Changchun, China approved this report. The patients provided written informed consent for this study, and the information has been anonymized.
2.2. Patients
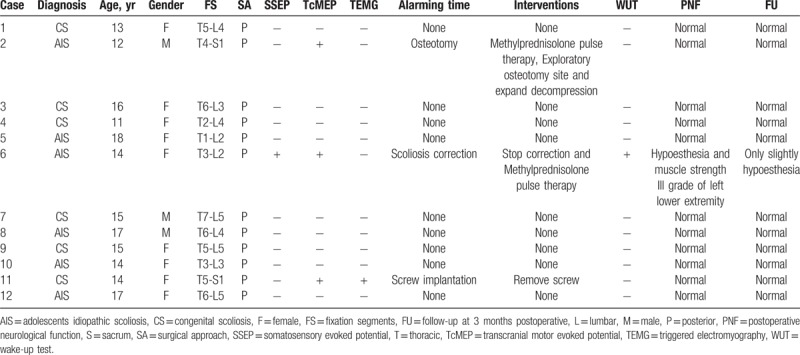
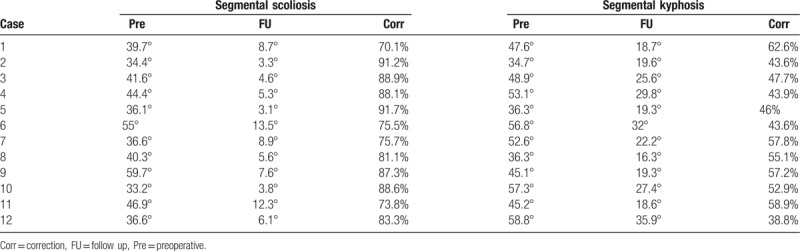
A total of 12 consecutive patients who underwent scoliosis correction surgery under MIOM at our institution between October 2014 and November 2018 were enrolled in this study (Table 1). Patients come to our outpatient department upon noticing the “razor back” deformity or unbalance of the trunk. All patients were diagnosed with CS or AIS. All patients underwent X-rays, computed tomography scans, and magnetic resonance imaging examinations preoperatively. Imaging examination demonstrated that the mean segmental scoliosis was 42° and the mean segmental kyphosis was 48.8°. The average distance between the C7 plumb line and the center sacral vertical line was 42.6 mm, and the mean sagittal imbalance was 39.1 mm.
Table 1.
Basic characteristics of patients.
2.3. Anesthesia
General anesthesia with intubation was achieved with propofol (200 μg/kg, Propofol Medium and Chain Fat Emulsion injection, Fresenius Kabi Deutschiand GmbH D-61346 Bad Homburg v.d.H., Germany), fentanyl (250 μg, Remifentanil Hydrochloride for injection, Yichang Ren Fu Pharmaceutical Co, Ltd, Yichang, Jiangsu, China), and midazolam (2 mg, Midazolam Injection, Yichang Ren Fu Pharmaceutical Co, Ltd, Yichang, Jiangsu, China) for all patients. Additionally, propofol (0.2–0.5 mg/kg per hour) was constantly infused for maintaining anesthesia. Muscle relaxants with cisatracurium besilate for injection (0.15 mg/kg, Jiangsu Hengrui Medicine Limited by Share Ltd, Jiangsu, China) were only provided during induction and intubation.
2.4. MIOM techniques
We used the Nicolet Endeavor CR IOM system, and disposable electrodes (Disposable Subdermal stainless Steel EEG-Needles, Natus Neurology), which are 12 mm in length, and 0.4 mm in diameter (27 gauge). MIOM is mainly composed of triggered electromyography (TEMG), somatosensory evoked potential (SSEP), and transcranial motor evoked potential (TcMEP).
TEMG was carried out with the stimulation of the head of screws previously implanted. Cathodic electrical stimulation was delivered through a prass electrode, which was referenced to a needle-type anode positioned in the paraspinal muscles, with a stimulation intensity of 8.2 mA,[11] a frequency of 1.1 Hz, and a duration of 0.3 ms.
The bilaterally posterior tibia nerves were stimulated by SSEP at the ankle with an intensity of 16 to 50 mA, a rate of 4.1 Hz, and a duration of 300 μs. The record needle electrodes of SSEP were placed on the scalp at locations C3 and C4; and Cz was referenced to Fpz (Fig. 1A and B).
Figure 1.
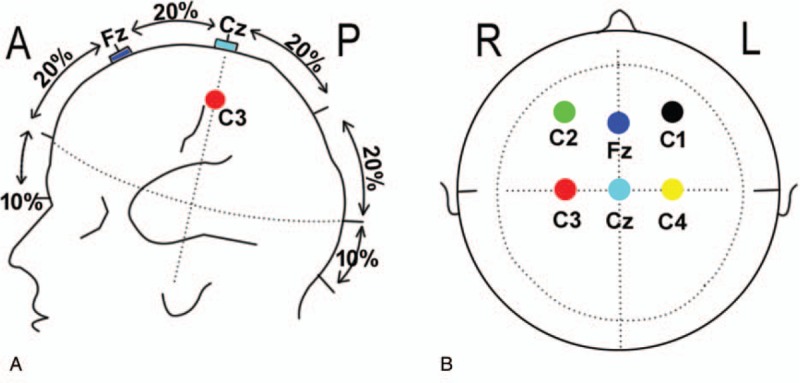
Schematic diagram of SSEP and TcMEP electrodes on the head. The side view (A) and overlooking (B) diagram for electrodes placement. C1 and C2 are the stimulating electrodes of TcMEP, C3 and C4 are the recording electrodes for upper extremity SSEP and Cz and Fz are the recording electrodes for lower extremity SSEP. SSEP = somatosensory evoked potential, TcMEP = transcranial motor evoked potential.
The TcMEP stimulating electrodes were placed on the scalp at locations C1 and C2 (Fig. 1A and B). The TcMEP was recorded from the needle electrodes placed on muscles, including the bilateral vastus lateralis, tibialis anterior, peroneal long muscle, gastrocnemius, and abductor halluces. A stimulation intensity that ranged within 100 to 400 V was presented to the scalp at an interstimulus interval of 2 ms for a duration of 300 μs.
2.5. Evaluations
The baselines of SSEP and TcMEP were measured after induction of anesthesia, and before the surgical operation. The latency and amplitude of SSEP were continuously monitored, and TEMG was generally performed after each screw insertion, and as required by the surgeon intraoperatively.
An abnormal SSEP was defined as a prolonged latency of more than 10% or a peak-to-peak amplitude decline of more than 50% when compared to baseline. An abnormal TcMEP was defined as a TcMEP amplitude decrease of more than 50%. A normal TEMG response, which presented with the absence of an electrical response on stimulation at 8.2 mA described by Isley et al,[11] indicated that the pedicle screw was not in contact with the spinal cord or nerve root. Otherwise, the screw was regarded as in contact with the adjacent neurological structure.
3. Results
MIOM was applied to 12 patients who received scoliosis correction surgery. Nine patients did not exhibit abnormal electrophysiological monitoring signals, while 3 patients (case 2, 6, and 11) encountered MIOM events, and 1 patient suffered from iatrogenic neurological impairment postoperatively (Table 1). Furthermore, all patients had satisfactory correction. The details are presented as follows (Table 2).
Table 2.
Surgical correction of scoliosis at follow up visit.
Case 2: A 12-year-old female presented with CS with intact neurological function. Imaging demonstrated that the left shoulder was 1.2 cm higher than the right shoulder, and revealed the presence of a right, single, fully segmented hemivertebra with a wedge shape at the T9 junction. At T9, the right rib was normal, and the left was absent; the segmental scoliosis (T8–T10) was 34.4° and the segmental kyphosis (T8–T10) was 34.7°. Scoliosis correction surgery was performed under MIOM. During the osteotomy, TcMEP amplitude reduced by 70% when osteotomy was performed, and SSEP signal had no abnormal change (Fig. 2A and B). The surgeon was informed, the operation was suspended and the spinal canal was immediately explored. Then, signal changes were resumed after the compression of the spinal cord was removed. Neurological function was normal postoperatively.
Figure 2.
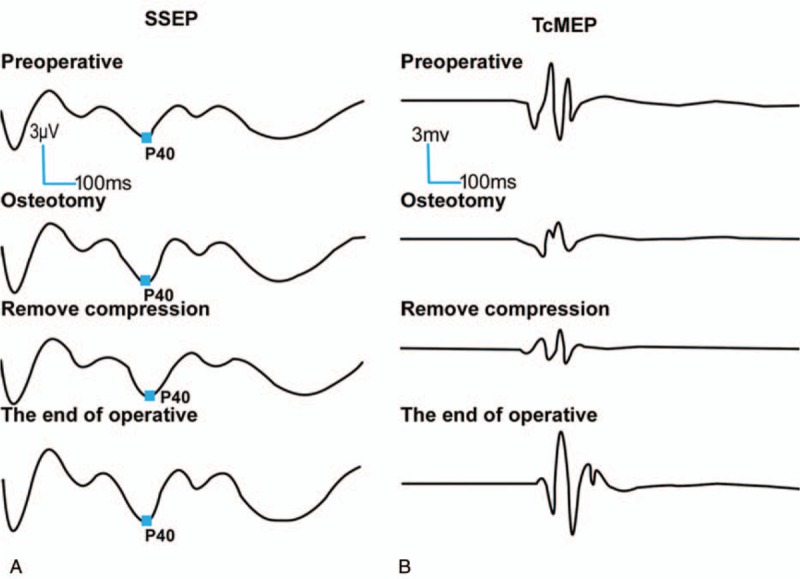
A 12-year-old female presented with CS received posterior scoliosis correction and fixation surgery. Surgery has led to abnormal neurological function. Electrophysiological abnormality was monitored by TcMEP (B) of MIOM, while SSEP (A) was normal. The change was reversed by appropriate measures and returned to baseline at the end of the surgery. No additional postoperative neurological injury was observed. CS = congenital scoliosis, MIOM = multimodal intraoperative monitoring, SSEP = somatosensory evoked potential, TcMEP = transcranial motor evoked potential.
Case 6: A 16-year-old female diagnosed as AIS. The preoperative muscle strength of both lower extremities was grade V. During the process of surgical correction of scoliosis, the SSEP amplitude decreased by 50% (Fig. 3A and B), and the TcMEP amplitude reduced by 75% of the baseline position. After excluding the interfering factors, the correction was suspended in time and methylprednisolone pulse therapy was performed. However, intraoperative wake-up test was positive. The SSEP returned to approximately 60% of the baseline, but the TcMEP continued to decline to more than 50% at the end of the surgery. After the operation, the patient was incapable of moving her left lower extremity, of which muscle strength was grade III, and she also had left lower extremity hypoesthesia. After 3 month of rehabilitation exercise, she had grade V muscle strength of the left lower extremity, and had a slight hypoesthesia of left lower extremity. Half a year later, her cutaneous sensation and muscle power were normal.
Figure 3.
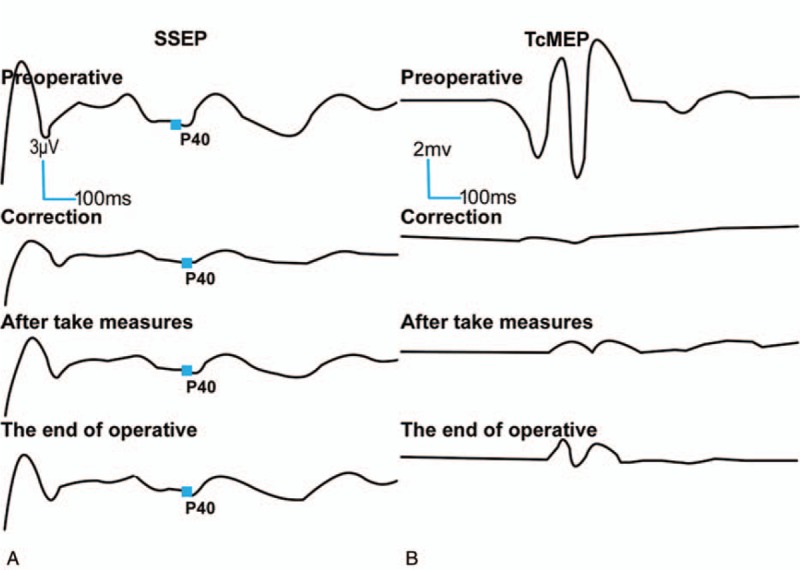
A 16-year-old female diagnosed as AIS. The SSEP (A) amplitude decreased by 50% and the TcMEP (B) amplitude decreased to 25% of the baseline position when scoliosis correction was performed. Although measures have been taken, the SSEP only returned to approximately 60% of the baseline, and the TcMEP continued to decline to more than 50% at the end of the surgery. Postoperative neurological impairment occurred. AIS = adolescent idiopathic scoliosis, SSEP = somatosensory evoked potential; TcMEP = transcranial motor evoked potential.
Case 11: A 14-year-old female diagnosed as CS. Preoperative lower extremity muscle strength was grade V and skin sensation was normal. After placing the last screw, TEMG response, which was elicited with extremely low current (<8.2 mA), was positive; and the amplitudes of SSEP and TcMEP dropped more than 50% rapidly (Fig. 4A and B). After excluding the interfering factors, the operation was suspended in time. Intraoperative radiography of the spine confirmed that the pedicle screw was involved in the internal wall of the pedicle. The responsible screw was immediately removed, the SSEP returned to approximately 90% of the baseline, and the TcMEP improved to 83.3% of the baseline at the end of surgery. After the operation, the patient presented with intact neurologic function.
Figure 4.
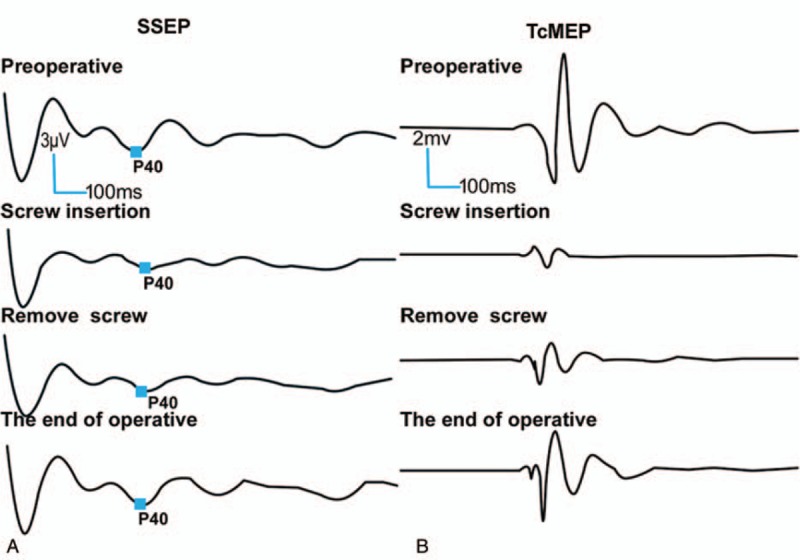
A 14-year-old female diagnosed as CS. After placing the last screw, TEMG response was positive, and the amplitudes of SSEP (A) and TcMEP (B) dropped rapidly. After the responsible screw was removed, the SSEP returned to approximately 90% of the baseline, and the TcMEP improved to 83.3% of the baseline at the end of the surgery. No additional postoperative neurological injury was observed. CS = congenital scoliosis, MIOM = multimodal intraoperative monitoring, SSEP = somatosensory evoked potential, TcMEP = transcranial motor evoked potential, TEMG = triggered electromyography.
4. Discussion
The aim of the scoliosis surgery is to partially correct scoliosis while preventing further development of scoliosis.[1,12,13] Surgery for CS and AIS remains technically demanding due to the potential risk of postoperative neurological deterioration such as sensory loss, lower limb weakness, and even paralysis. It is widely accepted that meticulous MIOM is of great value in spine surgery, and has been widely used to prevent neurological compromise in intramedullary tumor resection surgery.[14,15] The MIOM data has been trusted by various authors.[8,9,16–18] However, nerve function monitored by MIOM in the process of pedicle screw implantation and scoliosis correction in CS and AIS patients has been rarely reported. Therefore, we present a study to estimate the feasibility of MIOM on CS and AIS patients in scoliosis correction surgery.
SSEP, TcMEP, and TEMG are 3 common modalities of neurophysiological monitoring, and each has its advantages and shortcomings. The combination of these 3 models can enable the comprehensive intraoperative monitoring of spinal cord injury, and further, minimize the occurrence of false positive and false negative events.[19] Typically, SSEP monitoring has been used for continuous detection, while TcMEP and TEMG recordings were used intermittently. SSEP can only monitor the integrity of sensory pathways. Thus, injury to the motor pathways would be missed and may result in postoperative motor deficit.[20] On the other hand, TcMEP and TEMG could help in perfectly offsetting this shortage. Because SSEP and TcMEP are mediated by different nerve pathways in different vascular regions, monitoring both of them can provide a more complete assessment of spinal cord function. In case 2, there was spinal cord compression during operation, TcMEP showed abnormal signal changes during spinal cord monitoring, while SSEP signal was normal. No neurological damage was found after timely decompression. We owe this positive result to combined application of TcMEP and SSEP. Furthermore, TEMG plays a crucial role in identifying the position of the pedicle screw. Therefore, we chose these 3 modalities for spinal cord monitoring in CS and AIS surgery.
Instrumentation with the screw-rod system has been successfully applied in scoliosis patients for the stabilization of the spinal column.[21] However, instrumentation in CS and AIS patients remains challenging due to anatomical structure abnormality. Furthermore, the misplacement of pedicle screws can result in a high incidence of neurological complications.[22] Esses et al[23] reported that 4.7% of patients experienced nerve root lesions postoperatively. Jahangiri et al[24] suggested that the sensitivity of MIOM in detecting malpositioned screws depends on the frequency and accuracy of data collection. In our series, case 11 encountered MIOM events caused by pedicle screw misplacement, which was early detected by TEMG, SSEP, and TcMEP. The misplaced screw may lead to canal narrowing and spinal cord compression, and delayed intervention may result in serious consequences. It is paramount to collecting TcMEP and TEMG immediately after the implantation of each screw, in order to detect the spinal cord injury in a timely manner. In the study conducted by Dimar et al,[25] the spinal canals of 42 rats were placed with spacers. These rats were divided into 5 groups, and the decompression time was prolonged by 0, 2, 6, 24, and 72 hours. Neurological function recovery was evaluated by transcranial magnetic motor evoked potentials and serial BBB motor scores. The results of this study suggest that early decompression is beneficial to the recovery of spinal cord injury. Furthermore, neurological outcomes were related to the time length of spinal cord compression. The longer the compression was, the worse the result was. In addition, other studies[26,27] in humans also demonstrated that spinal cord contusion and canal narrowing may benefit from early decompression. In the present study, we concluded that MIOM can reflect the poor function of the spinal cord as soon as possible, alert surgeons to immediately take actions, and ultimately minimize the degree of the spinal cord lesion. In the present study, a patient (case 11) without function deficit was able to receive great help from MIOM. We attribute this early detection of neurological deficit to the utilization of MIOM.
CS and AIS can lead to trunk imbalance and impairment of heart and lung function that always need operative management. However, during the process of surgical correction, it may cause stretch injury of spinal cord and inferior clinical outcomes intraoperatively. It is critical for the surgical team to take measures to prevent the impending neurologic injury as soon as possible. In the present study, 2 (case 2 and 6) MIOM events were observed in the osteotomy and surgical correction process. The surgeon believed that the decrease in amplitudes was associated with the corresponding operation manipulation. After the exclusion of systemic or anesthetic factors, corresponding actions were immediately taken, including the suspension of further correction, flushing of the dura with warm normal saline to wash out the blocking potassium,[28] intravenous methylprednisolone pulse therapy, the elevation of mean arterial pressure to improve regional spinal cord perfusion, exploratory osteotomy site, and expanded decompression. No additional postoperative deficits were observed in case 2; however, neurological damage was detected in case 6 postoperatively. We attribute this early detection of neurological deficit to the utilization of MIOM. We suspect that if MIOM was not carried out during this procedure, more severe or irreversible spinal cord injury might have occurred.
In case 9, the Cobb angle of the patient was very large before operation. We were very worried about the nerve injury caused by overcorrection, so the operation of spinal correction was carried out under the monitoring of MIOM. Finally, the patient achieved a very perfect correction effect, and the correction rate of scoliosis was 87.3%. We attribute this satisfactory effect to MIOM monitoring.
Although positive results were achieved in the present study, this study has a few limitations that should be mentioned. First, when using TEMG to determine the position of the screw, only the nerve dysfunction could be detected; and other injuries such as vascular injury could not be found. Therefore, it is necessary to verify the position of the pedicle screws by X-ray fluoroscopy intraoperatively. Further large-scale patients of spine injury should be included to evaluate the effectiveness of MIOM technology in scoliosis correction surgery for CS and AIS patients.
5. Conclusions
Our data demonstrates that using MIOM in CS and AIS surgery could promptly detect iatrogenic neurological injury at the early stage. Therefore, rapid response by appropriate intraoperative interventions can be taken to minimize the injury. Second, stable MIOM recordings encourage surgeons to correct scoliosis even when the anatomical situation was extremely difficult.
Acknowledgments
We are very grateful for the cooperation of anesthesiologists, so MIOM is hardly affected by muscle relaxants.
Author contributions
Conceptualization: Yao Wang, Jian-Wu Zhao.
Data curation: Qiu-Ju Li.
Investigation: Qi-Yao Jiang.
Methodology: Xi-Wen Zhang.
Project administration: Zhen-De Jiang.
Supervision: Jian-Wu Zhao.
Validation: Jian-Wu Zhao.
Visualization: Xiu-Jie Zhu.
Writing – original draft: Tong Yu, Qi-Yao Jiang.
Writing – review and editing: Jian-Wu Zhao.
Footnotes
Abbreviations: AIS = adolescent idiopathic scoliosis, CS = congenital scoliosis, MIOM = multimodal intraoperative monitoring, SSEP = somatosensory evoked potential, TcMEP = transcranial motor evoked potential, TEMG = triggered electromyography.
TY and Q-JL contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Chen Z, Qiu Y, Zhu Z, et al. Posterior-only hemivertebra resection for congenital cervicothoracic scoliosis: correcting neck tilt and balancing the shoulders. Spine (Phila Pa 1976) 2018;43:394–401. [DOI] [PubMed] [Google Scholar]
- [2].Zhu W, Liu Z, Sha S, et al. Postoperative changes in sagittal spinopelvic alignment in sitting position in adolescents with idiopathic thoracic scoliosis treated with posterior fusion: an initial analysis. J Neurosurg Pediatr 2018;22:74–80. [DOI] [PubMed] [Google Scholar]
- [3].Li QJ, Yu T, Liu LH, et al. Combined 3D rapid prototyping and computer navigation facilitate surgical treatment of congenital scoliosis: a case report and description of technique. Medicine (Baltimore) 2018;97:e11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kwan MK, Chiu CK, Hasan MS, et al. Perioperative outcome of single stage posterior spinal fusion for severe adolescent idiopathic scoliosis (AIS) (Cobb angle >/= 90 degrees): the role of a dual attending surgeon strategy. Spine (Phila Pa 1976) 2019;44:e348–56. [DOI] [PubMed] [Google Scholar]
- [5].Weiss HR, Goodall D. Rate of complications in scoliosis surgery – a systematic review of the Pub Med literature. Scoliosis 2008;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Upendra BN, Meena D, Chowdhury B, et al. Outcome-based classification for assessment of thoracic pedicular screw placement. Spine (Phila Pa 1976) 2008;33:384–90. [DOI] [PubMed] [Google Scholar]
- [7].Di Silvestre M, Parisini P, Lolli F, et al. Complications of thoracic pedicle screws in scoliosis treatment. Spine (Phila Pa 1976) 2007;32:1655–61. [DOI] [PubMed] [Google Scholar]
- [8].Hu Y, Luk KD, Lu WW, et al. Application of time-frequency analysis to somatosensory evoked potential for intraoperative spinal cord monitoring. J Neurol Neurosurg Psychiatry 2003;74:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kothbauer KF, Deletis V. Intraoperative neurophysiology of the conus medullaris and cauda equina. Childs Nerv Syst 2010;26:247–53. [DOI] [PubMed] [Google Scholar]
- [10].Yu T, Wang Y, Zhang XW, et al. Multimodal intraoperative monitoring during reduction of spine burst fracture and dislocation prevents neurologic injury. Medicine (Baltimore) 2018;97:e0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Isley MR, Pearlman RC, Wadsworth##JS Recent advances in intraoperative neuromonitoring of spinal cord function: pedicle screw stimulation techniques. Am J Electroneurodiagn Technol 1997;37:93–126. [Google Scholar]
- [12].Yu T, Wang GS, Qu Y, et al. Posterior hemivertebra resection with a navigated drilling method for congenital scoliosis: a case report and description of surgical technique. Int J Clin Exp Med 2017;10:15562–8. [Google Scholar]
- [13].Dufvenberg M, Adeyemi F, Rajendran I, et al. Does postural stability differ between adolescents with idiopathic scoliosis and typically developed? A systematic literature review and meta-analysis. Scoliosis Spinal Disord 2018;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Novak K, Widhalm G, de Camargo AB, et al. The value of intraoperative motor evoked potential monitoring during surgical intervention for thoracic idiopathic spinal cord herniation. J Neurosurg Spine 2012;16:114–26. [DOI] [PubMed] [Google Scholar]
- [15].Forster MT, Marquardt G, Seifert V, et al. Spinal cord tumor surgery – importance of continuous intraoperative neurophysiological monitoring after tumor resection. Spine (Phila Pa 1976) 2012;37:E1001–1008. [DOI] [PubMed] [Google Scholar]
- [16].Thirumala PD, Huang J, Thiagarajan K, et al. Diagnostic accuracy of combined multimodality somatosensory evoked potential and transcranial motor evoked potential intraoperative monitoring in patients with idiopathic scoliosis. Spine (Phila Pa 1976) 2016;41:E1177–1184. [DOI] [PubMed] [Google Scholar]
- [17].Park JH, Lee SH, Kim ES, et al. Analysis of multimodal intraoperative monitoring during intramedullary spinal ependymoma surgery. World Neurosurg 2018;120:e169–80. [DOI] [PubMed] [Google Scholar]
- [18].Jin SH, Chung CK, Kim CH, et al. Multimodal intraoperative monitoring during intramedullary spinal cord tumor surgery. Acta Neurochir (Wien) 2015;157:2149–55. [DOI] [PubMed] [Google Scholar]
- [19].Pencovich N, Korn A, Constantini S. Intraoperative neurophysiologic monitoring during syringomyelia surgery: lessons from a series of 13 patients. Acta Neurochir (Wien) 2013;155:785–91. [DOI] [PubMed] [Google Scholar]
- [20].Kothbauer KF, Deletis V, Epstein FJ. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus 1998;4:e1. [DOI] [PubMed] [Google Scholar]
- [21].Okten AI, Gezercan Y, Ozsoy KM, et al. Results of treatment of unstable thoracolumbar burst fractures using pedicle instrumentation with and without fracture-level screws. Acta Neurochir (Wien) 2015;157:831–6. [DOI] [PubMed] [Google Scholar]
- [22].Matsuzaki H, Tokuhashi Y, Matsumoto F, et al. Problems and solutions of pedicle screw plate fixation of lumbar spine. Spine (Phila Pa 1976) 1990;15:1159–65. [DOI] [PubMed] [Google Scholar]
- [23].Esses SI, Sachs BL, Dreyzin V. Complications associated with the technique of pedicle screw fixation. A selected survey of ABS members. Spine (Phila Pa 1976) 1993;18:2231–8. [DOI] [PubMed] [Google Scholar]
- [24].Jahangiri FR, Sheryar M, Al Behairy Y. Early detection of pedicle screw-related spinal cord injury by continuous intraoperative neurophysiological monitoring (IONM). Neurodiagn J 2014;54:323–37. [DOI] [PubMed] [Google Scholar]
- [25].Dimar JR, 2nd, Glassman SD, Raque GH, et al. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976) 1999;24:1623–33. [DOI] [PubMed] [Google Scholar]
- [26].Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am 1995;77:1042–9. [DOI] [PubMed] [Google Scholar]
- [27].Marshall LF, Knowlton S, Garfin SR, et al. Deterioration following spinal cord injury. A multicenter study. J Neurosurg 1987;66:400–4. [DOI] [PubMed] [Google Scholar]
- [28].Kothbauer KF. Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin 2007;37:407–14. [DOI] [PubMed] [Google Scholar]


