Abstract
Atrial remodeling plays a significant role during the progression of atrial fibrillation (AF). Left atrial wall thickness (LAT) is a subjective and easily acquirable indicator referring to structural remodeling. Therefore, we aimed to investigate the association between LAT and atrial remodeling substrate, and to explore the predictive role of LAT about strong maintenance substrate and poor response to catheter ablation.
LAT was measured by cardiac computed tomography in 2 selected locations (roof and floor) in 100 persistent AF patients. Then the low-dose-ibutilide-facilitated catheter ablation was performed and atrial maintenance substrate was categorized as weak, mild, and strong, based on the response to circumferential pulmonary vein isolation or complex fractionated atrial electrograms ablation. During follow-up, the success rate was evaluated. LAT showed a progressive thickening tendency from weak, mild, to strong maintenance substrate (roof: 2.2 mm vs. 2.6 mm vs. 3.9 mm, P < .0001; floor: 1.7 mm vs. 2.0 mm vs. 2.5 mm, P < .0001). During follow-up, the success rate of ablation was decreased with the maintenance substrate strengthening (weak 80%, mild 64.53%, strong 31.43%, P = .009). LA roof thickness >3.10 mm might be the predictor to strong atrial maintenance substrate and poor response to ablation.
LAT was associated with the remodeling extent of atrial maintenance substrate and might predict the response to catheter ablation. These findings could help the clinicians to select the appropriate ablative strategy and predict the complexity and prognosis before catheter ablation.
Keywords: atrial fibrillation, atrial remodeling, ibutilide-facilitated catheter ablation, LA wall thickness, substrate
1. Introduction
Atrial fibrillation (AF) is the most common arrhythmia with worldwide prevalence, increased disability, and morbidity.[1–3] Although catheter ablation has been successfully used for alleviating the symptom of palpitation,[4] AF continues to perplex researchers with its incompletely understood mechanisms and poor response to ablation in non-paroxysmal AF patients. The heterogeneity of underlying atrial substrate, extent of atrial fibrosis, and the discrepancies between interindividual electrophysiological characteristics contribute to unpredictable responses to drug or ablation therapy.[5] Atrial remodeling or atrial maintenance substrate plays a significant role during AF progression.[6] Therefore, adequate assessment about atrial remodeling is crucial to select an appropriate therapeutic strategy. Low-voltage area, complex fractionated atrial electrograms (CFAEs) and rotors are some of the popular targets observed in the current mapping process, but are often affected by objective and subjective factors, such as the mapping density. Meanwhile, some popular indicators, such as atrial fibrosis [7] and left atrial wall thickness (LAT), could reflect the remodeling substrate subjectively. However, the quantification of atrial fibrosis was impeded mostly by the longer examination period of delayed enhancement magnetic resonance imaging and non-widespread analyzing software. Therefore, the present study was conducted to explore the potential association between LAT and atrial maintenance substrate, based on the response to low-dose-ibutilide-facilitated catheter ablation. In addition, we evaluated whether a thickened left atrial wall was associated with a poor response to catheter ablation.
2. Methods
2.1. Study population
This prospective study included 100 patients who underwent a first-time ablation procedure for symptomatic, persistent nonvalvular AF (54 men, 46 women; mean age 63.03 ± 9.78 years) in Beijing Chao-Yang Hospital. Persistent AF was defined as a sustained episode lasting >7 days.[8] Exclusion criteria included: New York Heart Association (NYHA) grading III or IV; severe hypertrophic cardiomyopathy (>20 mm); baseline QTc >500 ms; left atrial appendage thrombosis.[9] Demographic and clinical characteristics were obtained by completing face-to-face surveys and checking hospital or medical examination records. The study was conducted in accordance with the principles specified for research on human subjects in the Declaration of Helsinki and the research protocol was approved by the ethics committee of Beijing Chaoyang Hospital. All of the participants signed informed consents.
2.2. Cardiac CT imaging
Contrast-enhanced cardiac computed tomography (CT) imaging was performed with a dual-source CT scanner (SOMATOM Definition; Siemens Healthcare, Erlangen, Germany) within 3 days preceding the ablation. A mechanical injector was used to intravenously inject a bolus of 370 mg/mL iopromide (Ultravist; Bayer Healthcare Pharmaceuticals, Berlin, Germany) at a flow rate of 5.0 mL/s followed by a 50 mL saline flush. Parameters for imaging were: detector collimation, 2 × 32 × 0.6 mm; slice acquisition, 2 × 64 × 0.6 mm by the means of a z-flying focal spot; and gantry rotation time, 330 ms.
2.3. CT-based measurement of LAT
Acquired images were transferred to a workstation (ADW 4.2; GE Healthcare, Boston) where epicardial fat was detected by assigning Hounsfield units from −50 to −200 to fat, to be excluded from the LAT measurement. To validate the difference between the LA wall and epicardial fat, we measured and compared the Hounsfield units at 3 randomly selected sites on the LA wall and at 3 other sites with fat in each AF patient. Wall thickness was measured at 2 preselected locations, LA roof (Fig. 1A) and LA floor (Fig. 1B), using the axial plane image. At each location, measurements were taken with electronic calipers at 3 sites, which were located within 5 mm of the thickest part of the LA, and the average was considered for analysis.
Figure 1.
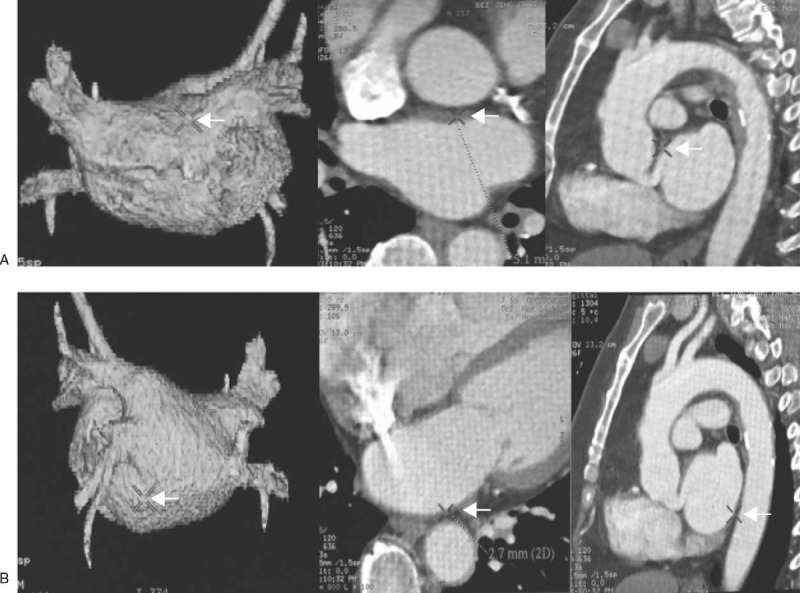
Measurement of the thickness of LA roof and floor. Left atrial wall thickness was measured at 2 preselected locations by using the axial plane image, roof (A) and floor (B). And measurements would be taken with electronic calipers at 3 sites, which were located within 5 mm of the thickest part of the LA, and the average was considered for further analysis. LA = left artery.
2.4. Low-dose-ibutilide-facilitated catheter ablation and category of atrial maintenance substrate
After double transseptal punctures guided by a three-dimensional (3D) electroanatomic mapping system (CARTO 3, Biosense Webster, Irvin, CA), a 3D reconstruction of the LA was obtained using a circular mapping catheter (NaviStar Thermocool, Biosense Webster) and this was merged with the 3D VR cardiac CT. After circumferential pulmonary vein isolation (CPVI), low-dose (0.25 mg) ibutilide was infused intravenously over 3 minutes in patients in whom persistent AF could not be terminated.
The following ablation strategy was depended on the response of ibutilide.[10] In cases where AF was terminated by ibutilide within 30 minutes, no further ablation would be taken. While in cases whose rhythm converted to atrial tachycardia (AT) within 30 minutes, the specific ablation would be taken until the sinus rhythm (SR) was turned. In contrast, in the patients with persisting AF, the 3D electroanatomic representation of the LA would be reconstructed and CFAEs would be mapped in the model. CFAEs were defined as the common criteria [11] and were mapped within LA and coronary sinus (CS). The destination of CFAEs’ ablation was either the restoration of SR or AT. And for AT, the specific ablation would be taken through the targeted mapping until the SR was turned. While the CFAEs of LA and CS had been eliminated or the CFAEs ablation time reached 30 minutes, electrical cardioversion was to be undertaken for SR restoration.
The block of ablation lines was identified in SR or pacing rhythm. If the isolation were not continued, a corresponding ablation would be delivered. After CPVI, adenosine triphosphate was injected intravenously to provoke dormant pulmonary vein conduction. Radiofrequency energy was applied, at 30 to 35 W with discharging time of 30 second for each point, to the dormant PV conduction sites until PV conduction disappeared (as shown in Fig. 2).
Figure 2.
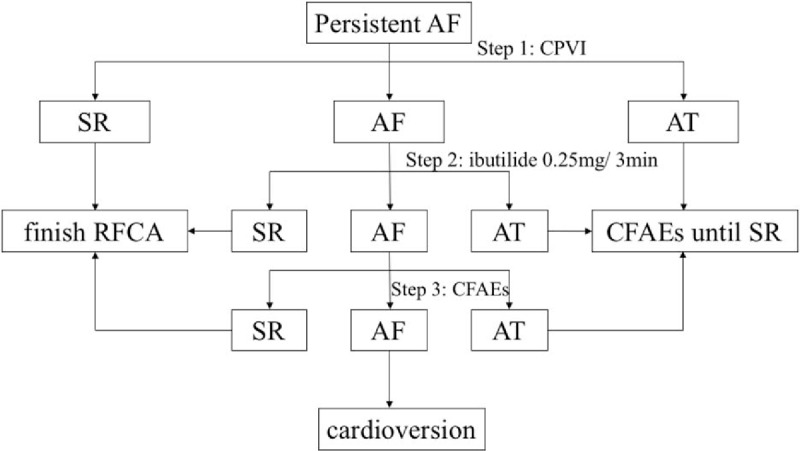
Flowchart of low-dose-ibutilide-facilitated RFCA. In cases where AF was terminated by ibutilide within 30 minutes, no further ablation would be taken. While in cases whose rhythm converted to atrial tachycardia (AT) within 30 minutes, the specific ablation would be taken until the sinus rhythm (SR) was turned. In patients with persisting AF, the 3D electroanatomic representation of the LA would be reconstructed and CFAEs would be mapped and ablated until the restoration of SR or AT. While the CFAEs of LA and CS had been eliminated or the CFAEs ablation time reached 30 minutes, electrical cardioversion was to be undertaken for SR restoration. AF = atrial fibrillation, CFAEs = complex fractionated atrial electrograms, LA = left artery.
Based on the response of low-dose-ibutilide-facilitated catheter ablation, the extent of atrial maintenance substrate was categorized as 3 group, weak (responsive to CPVI), mild (responsive to CPVI+CFAEs), and strong (unresponsive to CPVI+CFAEs).
2.5. Postoperative treatment and follow-up
Following catheter ablation, antiarrhythmic medications would be discontinued at the 3rd month, and warfarin at the 6th month, respectively, with INR maintained between 2 and 3. At 1, 3, 6, 9, 12, 18, and 24 months, patients underwent 72-hour Holter monitoring; an electrocardiogram was recorded if any patient complained of discomfort to detect AF recurrence or LA re-entry tachycardia after catheter ablation. Successful ablation was determined as the absence of episodes of AF or AT (>30 seconds) without antiarrhythmic treatment after the blanking period (3 months).
2.6. Statistical analysis
Normal continuous variables were presented as mean ± standard deviation. Continuous data were analyzed by 1-way ANOVA to test for significant differences. Abnormal continuous variables were shown as median and were analyzed by the Kruskal–Wallis test. Differences in categorical variables were analyzed by Fisher's exact test. Multivariate analysis was conducted with the ordered logistic regression model reporting coefficient (Coef.). AF recurrence was analyzed in a Kaplan–Meier survival analysis. The area under the receiver operating characteristic (ROC) curve was used to evaluate the prognostic value of the LAT. Differences were considered statistically significant at values of P < .05. Statistical analysis was performed with the SPSS v.22.0 statistical package (SPSS Inc., Chicago, IL), STATA 14 (Stata Corp, College Station, TX), and Microsoft Excel 2011 (Microsoft Corp, Redmond, WA).
3. Results
3.1. Clinical characteristics of study subjects
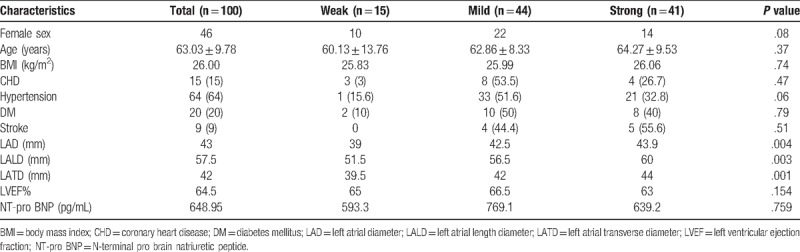
The clinical characteristics of all subjects are shown in Table 1. There was no significant difference between 3 groups in terms of age, sex category, body mass index, coronary heart disease, hypertension, diabetes mellitus, and stroke. Of note, the left atrial size, reflected by the left atrial diameter (LAD), left atrial length diameter (LALD), and left atrial transverse diameter (LATD) derived from echocardiogram, was significantly larger in the mild and strong group. Moreover, the decreased cardiac function reflected by left ventricular ejection fraction (LVEF) and N-terminal pro-brain natriuretic peptide (NT-proBNP) showed no specific correlation within the different maintenance substrate.
Table 1.
Baseline clinical characteristics of the study cohort.
3.2. Low-dose-ibutilide-facilitated catheter ablation and category of atrial maintenance substrate
In order to assess the extent of LA remodeling, we evaluated the atrial maintenance substrate based on the response to low-dose-ibutilide-facilitated catheter ablation and then categorized the atrial maintenance substrate as: weak (n = 15): responsive to CPVI, where persistent AF can be converted to SR or AT by CPVI or/and low-dose ibutilide. These included 14 patients converted to SR and 1 patient to AT after ibutilide injection. Mild (n = 44): CPVI and CFAEs ablation could restore the cardiac rhythm to SR (n = 23) and AT (n = 21). Strong (n = 41): unresponsive to CPVI+CFAEs, SR could be restored until electrical cardioversion performed. There was no complication in the electrophysiological examination and ablation. And the participants received antiarrhythmic therapy during the first 3 months after ablation, 79 of them took amiodarone, 7 of them took propafenone, and 3 of them received sotalol therapy. With a mean follow-up duration of 16.3months (range 3–50 months) after ablation, the success rate of ablation showed a declining trend with the maintenance substrate strengthening (weak 80%, mild 64.53%, strong 31.43%, P = .009). Furthermore, the recurrence rate among AF patients who received different types of antiarrhythmic therapy was similar (P = .186, amiodarone vs. propafenone; P = .367, amiodarone vs. sotalol; P = .266, propafenone vs. sotalol). Figure 3 showed the results of the Kaplan–Meier survival analysis of patients in normal SR after ablation. Significant differences in the AF-free survival rate were revealed among the 3 groups.
Figure 3.
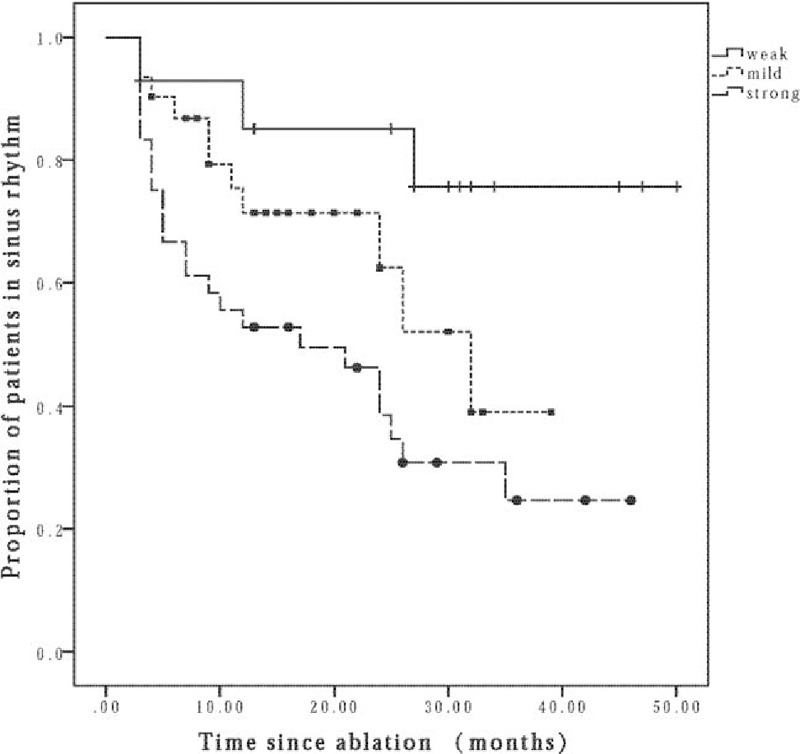
Kaplan–Meier survival analysis of patients in normal SR after ablation. The Kaplan–Meier survival analysis of patients in normal sinus rhythm after ablation. Significant differences in the AF-free survival rate were revealed among the 3 groups (weak 80%, mild 64.53%, strong 31.43%, P = .009). AF = atrial fibrillation.
3.3. LAT in different atrial maintenance substrate groups
As revealed by echocardiology, LA significantly enlarged with the LA maintenance substrate strengthening, and this seemed to be in accordance with our previous acknowledgment that LA enlargement is a traditional risk factor for AF. However, a complex remodeling process which may refer to inflammation[12] was involved in the duration from normal to enlarged left atrium. Therefore, we measured the LA wall thickness and found the potential association between LAT and different category of LA maintenance substrate. Overall, the thickness of LA presented a thicker tendency with the stronger substrate (roof: 2.2 mm vs. 2.6 mm vs. 3.9 mm, respectively, P < .0001; floor: 1.7 mm vs. 2.0 mm vs. 2.5 mm, respectively; P < .0001).
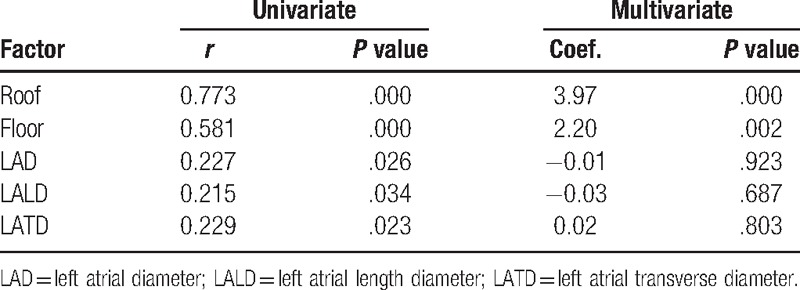
Correlation between the category of atrial substrate and potential covariates is shown in Table 2. A moderately positive correlation was found between the category of substrate and the wall thickness of LA roof (r = 0.773, P < .0001), floor (r = 0.581, P < .0001). Wall thickness of LA roof and floor was significantly associated with the category of atrial substrate (roof: Coef. 3.97, P = .000; floor: Coef. 2.20, P = .002).
Table 2.
Potential covariates related to the category of atrial maintenance substrate.
3.4. LAT as a predictor of poor response to ablation
Analysis of ROC curves (Fig. 4) with an area under the curve (AUC) of LA roof: 0.954 (95% confidence interval [CI] 0.909–0.999, P < .0001) and LA floor: 0.820 (95% CI 0.737–0.903, P < .0001) revealed that LAT of 3.10 mm (roof, sensitivity 90.2%, specificity 94.9%) and 2.35 mm (floor, sensitivity 58.5%, specificity 89.8%) were the optimal cutoff values predictive of strong atrial maintenance substrate, which might be the predictive indicator for poor prognosis of ablation. So far, we have identified the potential association between LAT and atrial remodeling substrate and explored the predictive value for LAT in detecting strong left atrial maintenance substrate and poor response to ablation in AF patients.
Figure 4.
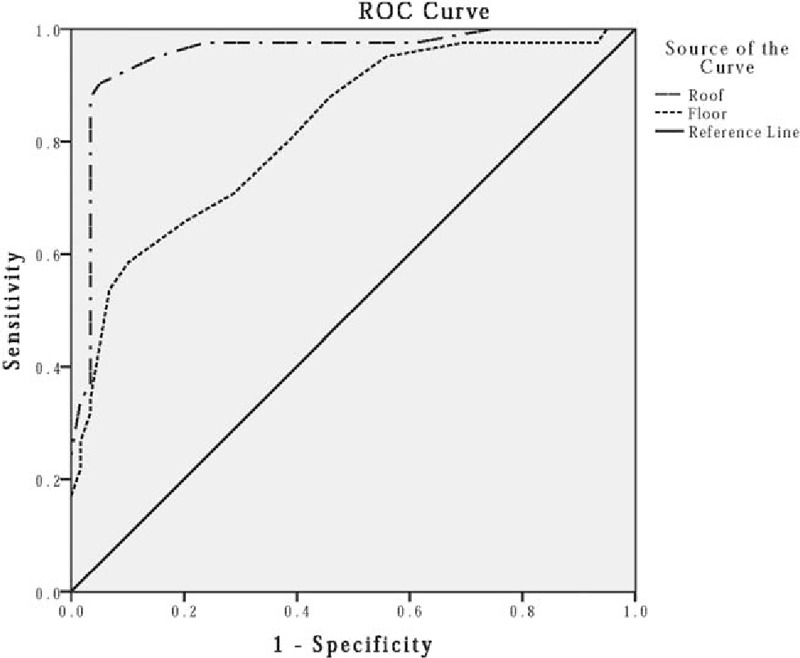
ROC curves of LAT for prediction of atrial substrate. The ROC curves of LAT for prediction of atrial substrate with an area under the curve (AUC) of LA roof: 0.954 (95% confidence interval [CI] 0.909–0.999, P < .0001) and LA floor: 0.820 (95% CI 0.737–0.903, P < .0001). ROC = receiver operating characteristic curve, LAT = left atrial wall thickness, LA = left artery.
4. Discussion
In the present study, we obtained seminal evidence delineating the association between thickness of left atrial wall and remodeling extent of maintenance substrate based on cardiac CT and response to low-dose-ibutilide-facilitated catheter ablation in a cohort of AF patients. The thickness of the left atrial wall tended to be thicker, not a stretch-related thinning tendency, when the atrial maintenance substrate got stronger. A thickened left atrial wall was associated with a poor response to ablation. LAT may be considered as a relatively objective indicator to predict the remodeling extent of atrial maintenance substrate and prognosis to catheter ablation.
4.1. LAT and AF
LAT has been shown thickened in a stepwise fashion, ranging from control subjects to patients with paroxysmal AF to those with persistent AF.[13] Other research has indicated that the LA wall was thicker at CFAEs sites.[14] Our findings further explored the thickening trend in correlation with strengthening of the atrial maintenance substrate. A thickened left atrial wall may induce structural remodeling. Histologic study of LA tissue in chronic AF patients [15] and rapid atrial pacing goat model [16] revealed the severe loss of myocytes, glycogen accumulation, changes in mitochondrial morphology, and cellular hypertrophy. Taken together, the thickened left atrial wall is associated with structural remodeling, which may accelerate AF progression. Furthermore, Nakatani et al[17] showed that thick left atrial wall contributes to AF recurrence after ablation, which was in accordance with the present study.
In addition, we selected roof and floor as representative of LA in the present study because they were supported by advantages of relatively accurate spatial orientation, unusual anatomic variants, and, particularly, common targets for AF substrate modification strategy. Other regions such as posterior, septal, or lateral wall of LA may associate with atrial remodeling either, but unsuitable to measure due to poor consistency or spatial orientation. Roof and floor could only represent part of LA, but it has been already identified with reasonable association in the present study.
Of note, the present study showed that thicker left atrial wall was associated with stronger atrial maintenance substrate, but with an enlarged left atrium measured by LAD from echocardiology. An enlarged LA and a thickened LA wall might seem to be contradicted. Actually, thickened left atrial wall may implicate the stage of inflammation, edema. With progression of AF to end-stage disease, remodeling may advance to atrial fibrosis, attendant to fractionated electrical activities with low voltage on the electroanatomic mapping that may be predictive of the high risk of AF initiation and persistence. Loss of adaptive atrial thickening may be the tipping point at which fibrosis and scar become irreversible and cardiac function is impaired.[18] We excluded heart failure patients with NYHA Class III, IV due to their poor tolerance of catheter ablation; therefore, our results may only reflect the adaptive reserve state before cardiac function is irreversibly impaired.
4.2. Ibutilide-facilitated AF catheter ablation
CPVI, which can interrupt abnormal electrical activities between the PVs and the LA, is the cornerstone of catheter ablation in AF. But for non-paroxysmal AF, the result of simple CPVI is generally unsatisfactory because of the presence of the complex substrate. Additional PV triggers were found frequently while mapping. Therefore, various strategies for atrial maintenance substrate modification are often applied in AF ablation procedures. The “roof line” and the “mitral isthmus line” showed an additive effect when compared with CPVI alone.[19] Another atrial maintenance substrate modification strategy is CFAEs ablation, which correlates with slowed conduction and pivot points of wavelets.[20] As many CFAEs sites are nonspecific and reflect passive atrial activation not critical to AF maintenance, extensive ablation may also be unnecessary.[21] A preclinical study identified that ibutilide, a class III antiarrhythmic drug, can limit atrial re-entry without impacting focal sources of AF in cases with persistent AF.[22–24] Meanwhile, some studies have also shown that the use of ibutilide in the context of a stepwise ablation could result in reduction of fractionation and higher rates of AF termination.[25] In our study, we performed the advantages of ibutilide to identify the sites that were critical for persistent AF maintenance to improve procedural efficacy and to categorize the left atrial maintenance substrate.
4.3. Atrial remodeling substrate in AF
Atrial remodeling, substrate plays a key role throughout the progression of AF. With the establishment of a novel therapeutic concept about fibrotic atrial cardiomyopathy (FACM),[26] atrial fibrosis has been valued. Extensive atrial fibrosis is always associated with poor outcomes following ablation.[5] Atrial fibrosis has been postulated as a potential cause of the abnormalities in the wave-front perpetuation and the formation of circuits necessary for re-entry, which may increase the underlying risk of initiation and maintenance of AF. Quantitative and qualitative assessments of FACM are often indicated by Late Gadolinium-Enhanced Cardiac MRI (LGE-CMRI). However, visualization and quantification of scar tissues have always been the difficulty during the clinical practice of LGE-CMRI. Recently, a novel fully automated multiview 2-task recursive attention mode has been designed, which can segment the LA and PV anatomy and the atrial scars directly, and generate a patient-specific anatomical and atrial scar assessment model.[27] Therefore, the development of technology about assessment of the atrial maintenance substrate helps the electrophysiologist to stratify patients, guide ablation therapy, and predict treatment success.
From the present study, LAT is an easy-acquired indicator which associated with atrial remodeling substrate and should be taken into consideration to perform an appropriately patient-tailored ablation strategy and predict the complexity and prognosis of ablation. In the future, it can potentially be extended to a multicenter study to establish a risk score system for identification of the left atrial substrate in AF patient population.
4.4. Potential limitations
Consideration of possible limitations is of relevance to our study and help to inform the design of future studies. We semi-quantitatively assess the remodeling extent of AF substrate based on the response to ibutilide during catheter ablation as weak, mild, and strong. The correlation between LAT and some other quantitative indicators, such as electrophysiologic characteristics involving low-voltage area or CFAE index, could provide more precise and valuable guidance in clinical practice. Furthermore, the histologic characteristics of LAT remain uncertain. Measurement of LAT, interstitial fibrosis, and inflammation in AF samples and analyzing their correlation with remodeling extent could provide stronger evidence. The present results provided preliminary clues and evidence for future investigations regarding the potential mechanisms between LAT and atrial remodeling in AF patients and further studies are still needed.
5. Conclusions
The present study provides the first description about the association between thickness of left atrial wall and remodeling extent of maintenance substrate in a cohort of AF patients. These novel findings are fundamental for further studies connecting an easily obtained indicator from cardiac CT with remodeling substrate while electrophysiologic mapping, but they are just the beginning. An extensive amount of research is still needed to explore the clinical values of LAT to reflect the remodeling extent of substrate precisely.
Author contributions
Conceptualization: Kun Zuo, Xinchun Yang.
Data curation: Kun Zuo, Kuibao Li.
Formal analysis: Kun Zuo, Kuibao Li, Jing Li.
Investigation: Kun Zuo.
Methodology: Kun Zuo, Kuibao Li, Min Liu.
Project administration: Xinchun Yang.
Software: Kuibao Li.
Supervision: Xinchun Yang.
Writing – original draft: Kun Zuo.
Writing – review & editing: Kuibao Li, Xiaoqing Liu, Xingpeng Liu, Jiuchang Zhong, Xinchun Yang.
Footnotes
Abbreviations: AF = atrial fibrillation, AT = atrial tachycardia, CFAEs = complex fractionated atrial electrograms, CPVI = circumferential pulmonary vein isolation, FACM = fibrotic atrial cardiomyopathy, LAD = left atrial diameter, LALD = left atrial length diameter, LAT = left atrial wall thickness, LATD = left atrial transverse diameter, LVEF = left ventricular ejection fraction, SR = sinus rhythm.
This work was supported by the National Natural Science Foundation of China (81500383, 81670214, 81870308, 81770253, 81370362), the Beijing Natural Science Foundation (7172080), the Beijing Municipal Administration of Hospitals’ Youth Programme (QML20170303), and the 1351 personnel training plan (CYMY-2017-03).
The authors have no conflicts of interest to disclose.
References
- [1].Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Oldgren J, Healey JS, Ezekowitz M, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation 2014;129:1568–76. [DOI] [PubMed] [Google Scholar]
- [3].Kotecha D, Holmes J, Krum H, et al. Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet 2014;384:2235–43. [DOI] [PubMed] [Google Scholar]
- [4].Mont L, Bisbal F, Hernandez-Madrid A, et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J 2014;35:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- [6].Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 2008;51:802–9. [DOI] [PubMed] [Google Scholar]
- [7].Zghaib T, Nazarian S. New insights into the use of cardiac magnetic resonance imaging to guide decision making in atrial fibrillation management. Can J Cardiol 2018;34:1461–70. [DOI] [PubMed] [Google Scholar]
- [8].Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- [9].Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017;33:369–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].King GS, McGuigan JJ. Antiarrhythmic Medications. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-.2018 Feb 11.
- [11].Nademanee K, McKenzie J, Kosar E, Schwab M. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044–53. [DOI] [PubMed] [Google Scholar]
- [12].Scott L, Jr, Li N, Dobrev D. Role of inflammatory signaling in atrial fibrillation. Int J Cardiol 2018;pii: S0167-5273 (18)35864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Keiko T, Yasuo O, Ichiro W, et al. Relation between left atrial wall thickness in patients with atrial fibrillation and intracardiac electrogram characteristics and ATP-provoked dormant pulmonary vein conduction. J Cardiovasc Electrophysiol 2015;26:597–605. [DOI] [PubMed] [Google Scholar]
- [14].Jin WI, Hye-Jeong LEE, Jae-Sun UHM, et al. Complex fractionated atrial electrograms related to left atrial wall thickness. J Cardiovasc Electrophysiol 2014;25:1141–9. [DOI] [PubMed] [Google Scholar]
- [15].Corradi D, Callegari S, Benussi S, et al. Myocyte changes and their left atrial distribution in patients with chronic atrial fibrillation related to mitral valve disease. Hum Pathol 2005;36:1080–9. [DOI] [PubMed] [Google Scholar]
- [16].Ausma J, Wijffels M, Thone F, et al. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation 1997;96:3157–63. [DOI] [PubMed] [Google Scholar]
- [17].Nakatani Y, Sakamoto T, Yamaguchi Y, et al. Heterogeneity in the left atrial wall thickness contributes to atrial fibrillation recurrence after catheter ablation. Heart Vessels 2018;33:1549–58. [DOI] [PubMed] [Google Scholar]
- [18].Bunch TJ, Day JD. Adverse remodeling of the left atrium in patients with atrial fibrillation: when is the tipping point in which structural changes become permanent? J Cardiovasc Electrophysiol 2015;26:606–7. [DOI] [PubMed] [Google Scholar]
- [19].Hocini M1, Jaïs P, Sanders P, et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation. A prospective randomized study. Circulation 2005;112:3688–96. [DOI] [PubMed] [Google Scholar]
- [20].Konings KT, Smeets JL, Penn OC, et al. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation 1997;95:1231–41. [DOI] [PubMed] [Google Scholar]
- [21].Rostock T, Rotter M, Sanders P, et al. High-density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm 2006;3:27–34. [DOI] [PubMed] [Google Scholar]
- [22].Chou CC, Zhou S, Tan AY, et al. High-density mapping of pulmonary veins and left atrium during ibutilide administration in a canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol 2005;299:2704–13. [DOI] [PubMed] [Google Scholar]
- [23].Wang M, Zhao Q, Ding W, et al. Comparison of direct current synchronized cardioversion to ibutilide-guided catheter ablation for long-term sinus rhythm maintenance after isolated pulmonary vein isolation of persistent atrial fibrillation. Am J Cardiol 2017;119:1997–2002. [DOI] [PubMed] [Google Scholar]
- [24].Singh SM, d’Avila A, Kim YH, et al. The modified stepwise ablation guided by low-dose ibutilide in chronic atrial fibrillation trial (The MAGIC-AF Study). Eur Heart J 2016;37:1614–21. [DOI] [PubMed] [Google Scholar]
- [25].Enriquez A, Hashemi J, Michael K, et al. Use of intraprocedural ibutilide during stepwise ablation of long-standing persistent atrial fibrillation. J Atr Fibrillation 2018;10:1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hans K. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J 2013;34:2731–8. [DOI] [PubMed] [Google Scholar]
- [27].Chen J, Yang G, Gao Z, et al. Multiview two-task recursive attention model for left atrium and atrial scars segmentation. MICCAI 2018 Lecture Notes in Computer Science 2018;11071:455–63. [Google Scholar]


