Abstract
To assess the effectiveness of the treatment with high dosage of corticosteroids (CCSs), as first-line therapy, in inducing remission in naïve Adult-onset Still's disease (AOSD) patients compared with low dosage of CCSs, after 6 months. To further evaluate the rate of patients maintaining the remission and the rate of CCSs discontinuation, after additional 12 months of follow-up.
A retrospective evaluation of patients prospectively followed was designed to compare the rate of clinical remission in naïve AOSD patients treated with high dosages of CCSs (0.8–1 mg/kg/day of prednisone-equivalent) or low dosage of CCSs (0.2–0.3 mg/kg/day of prednisone-equivalent), after 6 months. An additional analysis was performed to compare the rate of monocyclic pattern between these groups, after further 12 months of follow-up.
The clinical remission was achieved in a higher percentage of patients treated with the first-line treatment with high dosage of CCSs than treated the first-line treatment with low dosage of CCSs. At the end of 18 months of follow-up, a larger percentage of patients treated the first-line treatment with high dosage of CCSs was classified as monocyclic pattern and discontinued CCSs when compared with patients treated the first-line treatment with low dosage of CCSs. Patients defined as CCSs non-responder were treated with methotrexate (MTX)+CCSs or with combination therapy CCSs+MTX+biologic drug. The clinical remission was observed in a percentage of these patients.
We showed the effectiveness of the first-line treatment with high dosage of CCSs in inducing clinical remission in naïve AOSD patients when compared with the first-line treatment with low dosage of CCSs. The first-line treatment with high dosage of CCSs was also associated with the achievement of monocyclic pattern and CCSs discontinuation, after 18 months of follow-up.
Keywords: Adult-onset Still's disease, corticosteroids, first-line therapy, monocyclic pattern, remission
1. Introduction
Adult-onset Still's disease (AOSD) is a rare, systemic, inflammatory disorder of unknown etiology characterized by high daily spiking fever, arthritis, and evanescent rash.[1] AOSD patients are affected by life-threatening complications, mainly macrophage activation syndrome (MAS), influencing prognosis.[2–4] Although the pathogenic mechanisms are not entirely elucidated yet, AOSD is categorized as a complex multigenic autoinflammatory disease at the “crossroad” between autoinflammatory and autoimmune disorders.[1] During AOSD, laboratory tests reflect the systemic inflammatory process and high levels of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are observed.[1–4] Characteristically, ferritin levels are higher than those observed in other autoimmune, inflammatory, infectious, or neoplastic diseases.[5,6] Analyzing AOSD course, 3 different clinical patterns of AOSD are identified:
-
1.
monocyclic pattern, characterized by a systemic single episode;
-
2.
polycyclic pattern, characterized by multiple flares, alternating with remissions;
-
3.
chronic pattern, related to a persistently active disease with associated polyarthritis.[7]
Usually, 30% of AOSD patients develop a monocyclic pattern, 30% a polycyclic pattern and 40% a chronic pattern.[8,9] Concerning therapeutic strategy, despite the growing body of evidence in AOSD management, the therapy is largely empirical deriving from case series and lacking specific guidelines.[10–13] High dosages of corticosteroids (CCSs) are usually the first-line treatment when the systemic symptoms predominate.[12] Second-line therapies, including synthetic disease modifying anti-rheumatic drugs (sDMARD) and biologic agents, are administered after the lack of clinical response to first-line CCSs.[14,15] Despite these therapies, a large percentage of patients experiences several flares with an evolution toward the chronic disease course.[9,11,15,16] In fact, the optimal management of AOSD patients is not adequately clarified and the effect of first-line therapies on long-term outcomes, as observed in rheumatoid arthritis,[17–19] it is not fully elucidated yet.
In this study, we aimed to assess the effectiveness of high dosage of CCSs, as first-line treatment, in inducing clinical remission in naïve AOSD patients when compared with low dosage of CCSs after 6 months. Furthermore, we aimed to evaluate the rate of patients maintaining the remission after further 12 months of follow-up and the rate of CCSs discontinuation. Finally, we assessed additional endpoints, the MAS occurrence and if the combination therapy with other immunosuppressive drugs could increase the achievement of remission in non-responder patients.
2. Methods
2.1. Study design
We designed a retrospective evaluation of patients prospectively followed to compare the rate of clinical remission in naïve AOSD patients treated with high dosages of CCSs or low dosage of CCSs after 6 months. Following this first evaluation, we performed an additional analysis to compare the rate of monocyclic pattern and the rate of CCSs discontinuation in patients treated with high dosages of CCSs or low dosage of CCSs, after further 12 months of follow up. The local Ethics Committee approved the study protocol (ASL1 Avezzano-Sulmona-L’Aquila, L’Aquila, Italy; protocol number 0139815/16) and it was performed according to the good clinical practice guidelines and declaration of Helsinki. Informed consent was obtained from each patient for the use of clinical and laboratory data for study purposes. In reporting the results, we followed the STROBE checklist (Additional Table 1).
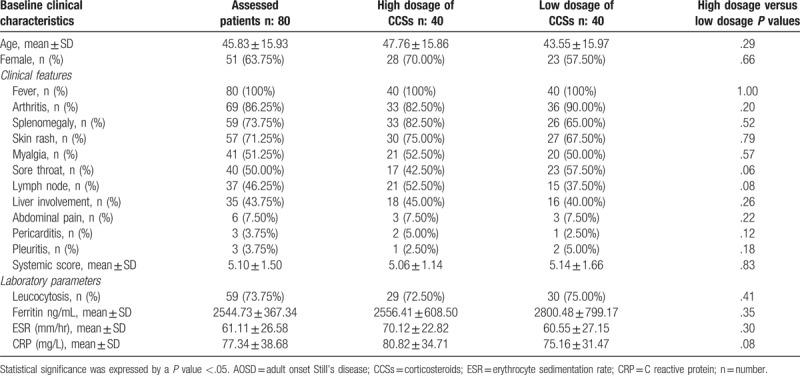
Table 1.
Baseline clinical characteristics of assessed AOSD patients.
2.2. Patients eligibility criteria
Consecutive naïve AOSD patients, who were visited at the recruiting centers, were assessed to be analyzed into the study. To be assessed in the study, all AOSD patients had to fulfill the following inclusion criteria:
-
1.
diagnostic criteria proposed by Yamaguchi;[20]
-
2.
naïve patients for high dosage of CCSs, sDMARD(s) and biologic drugs, at the time of diagnosis;
-
3.
at least 3 visits to be analyzed (baseline, after 6 and 18 months).
Exclusion criteria were:
-
1.
use of higher dosage of CCSs than 10 mg/day of prednisone before study evaluation;
-
2.
use of sDMARDs and any biologic drug before study evaluation;
-
3.
life-threating AOSD manifestations, at the time of diagnosis, requiring, that is, high dosage CCSs and/or cytotoxic drugs.
2.3. Settings and locations
This study was designed as retrospective analysis of patients prospectively followed, involving Rheumatologic Clinics at University of L’Aquila and University of Palermo, with extensive experience in AOSD diagnosis and management.[21,22] Consecutive AOSD patients, between January 2001 and December 2017, were evaluated and, if eligible according to aforementioned criteria, were included in the analysis.
2.4. Clinical management
During the clinical work-up before the diagnosis, NSAIDs and oral CCSs at low dosages (≤10 mg/daily of prednisone-equivalent[23]) were considered to be not-meaningful for the subsequent analyses. During the first phase of the evaluation, at the time of diagnosis, patients treated with CCSs at the dosage of 0.8 to 1 mg/kg/day of prednisone-equivalent (high dosage of CCSs) were included in this work. As comparator group, gender- and age-matched patients treated, at the time of diagnosis, with CCSs at the dosage of 0.2 to 0.3 mg/kg/day of prednisone-equivalent (low dosage of CCSs) were accurately selected and included in the analysis. All the patients included in the analysis did not present life-threating manifestations, defined according to available literature,[2] and the treatment with high dosage CCSs or low dosage CCSs was administered according to clinical judgment. In this retrospective evaluation, we recorded these data from the clinical chart of each patient thus designing a historical cohort study comparing 2 groups of AOSD patients, who were prospectively followed and treated with high dosage of CCSs or with low dosage of CCSs. If AOSD-complications occurred, patients were treated with iv high dosages of CCSs and cytotoxic drugs as rescue therapy,[22] the rate of patients who experienced complications was also assessed. Any increase of CCSs dosages to maintain clinical response was considered to be a flare of the disease identifying non-responder patients, who were consequently treated with sDMARD and/or biologic drugs, as suggested by available literature.[24] Specifically, the rates of patients treated with methotrexate (MTX) and treated with biologic drugs, anakinra, tumor necrosis factor inhibitor (TNFi) or tocilizumab, were registered. Furthermore, according to clinical judgment and patient's clinical picture, patients could be treated with the administration of medications to prevent the occurrence of predictable CCSs side effects (i.e., bisphosphonates for CCSs-induced osteoporosis) and/or the administration of prophylaxis for opportunistic infections. We did not record these therapies because considered to be not-meaningful for the analysis of study endpoints.
2.5. Endpoints
The primary endpoint of this study was the clinical remission in naïve AOSD patients, after 6 months of follow-up from the beginning. Clinical remission was defined as the absence of joint, systemic and laboratory evidence of disease activity, for at least 2 consecutive months, regardless of the initial therapeutic strategy, following the available literature.[25] AOSD flare was defined as the occurrence of systemic or joint features occurring after remission or the need of any additional treatment and/or any increase of medications dosage. Patient experiencing disease flare was defined as non-responder. The secondary endpoints were the percentage of patients maintaining the clinical remission, thus achieving the monocyclic pattern and the rate of CCSs discontinuation, after further 12 months of follow-up. According to the disease course, at the end of this phase, after 18 months of follow-up from the beginning, patients were categorized into 4 groups as described by Cush JJ et al[7]: 3 clinical patterns (monocyclic, polycyclic, and chronic) and death, whichever the course. A monocyclic course was defined as a single episode for more than 2 months but less than 1 year followed by sustained remission through the entire follow-up period. A polycyclic course was defined as recurrent systemic flares with remission between flares. A chronic course was defined as at least a single episode of persistent symptoms lasting longer than 1 year. AOSD-related death was defined as death correlated with the disease during the follow-up. Finally, a number of additional endpoints were assessed evaluating the percentage of patients discontinuing CCSs, the MAS occurrence, the achievement of remission in non-responder patients treated by CCSs+MTX or CCSs+MTX+biologic drugs. The discontinuation of CCSs was defined as the withdrawal of CCSs, regardless the initial dosage, during the follow-up. During first and second phases of the study, each patient was investigated, where appropriate, for MAS development, which was defined following the diagnostic criteria proposed by the Histiocyte Society.[26] The achievement of clinical remission in non-responder patients was defined as the percentage of patients achieving the primary endpoint with CCSs+MTX or CCSs+MTX+biologic drugs, after lack of response with first line CCSs.
2.6. Clinical assessment and data sources
The assessment at baseline first included the exclusion of potential mimickers, including infections, cancers, and other autoimmune or autoinflammatory diseases, as previously detailed.[21,22] Briefly, blood tests and cultures, chest X-rays, abdominal echography were performed in all patients. Despite these exams, in the case of further suspicion of malignancy, we used CT and/or PET/CT exams, where appropriate bone marrow examination and lymph node biopsy were performed. After that, the presence of the following clinical features, at baseline, were recorded to assess the systemic score:[21,27] fever, typical skin rash, arthralgia or arthritis, myalgia, lymphadenopathy, sore throat, splenomegaly, hepatomegaly or abnormal liver function tests, abdominal pain, sore throat, weight loss, and gastrointestinal symptoms. The diagnosis of pleural effusion or pleuritis and lung parenchymal involvement was made by a chest radiograph or CT scan, patients with clinical suspicion of pericarditis also underwent echocardiography. In addition, at baseline and during the follow-up, each patient was assessed for the presence of AOSD-related complications. After 6 months of follow up, patients were assessed for achievement of clinical remission according to pre-specified criteria. After 18 months of follow-up from baseline, patients were newly assessed and stratified according to above mentioned disease patterns. During the study, the percentage of patients discontinuing CCSs, the MAS occurrence, the achievement of remission in non-responder patients treated by CCSs+MTX or CCSs+MTX+biologic drugs were assessed.
2.7. Sample size estimation
Despite the retrospective nature of our study, we calculated the sample size in order to maintain a conservative estimation on the basis of previous studies reporting the effectiveness of CCSs in AOSD. According to available information about high CCSs dosages and effectiveness in AOSD patients,[12] we expected remission percentage rates of 62.5% in patients treated with high CCSs dosages and of 29.4% in patients treated with low CCSs dosages. According to these expectations, we have provided the following sample size estimation for 2-sample comparison of proportions:
-
1.
test Ho: p1 = p2, where p1 is the proportion in population 1 and p2 is the proportion in population 2;
-
2.
assumptions: alpha = 0.05 (2-sided); power = 0.80; p1 = 0.625; p2 = 0.294; n2/n1 = 1.00;
-
3.
estimated required sample sizes: n1 = 34; n2 = 34.
This sample size was estimated choosing a type I error level (alpha) of 5% and a power (1-beta) of 80%. This choice was suggested by the clinical concern of reaching an adequate sample size in rare disease. According to the retrospective design of the study, we included in our evaluation 40 patients in each arm to minimize the missing data in endpoints.
2.8. Statistical analysis
Data generated from our study were analyzed adjusting for the longitudinal design. Due to the relatively simple design of the study, we had a very low percentage of missing data, these values, which were meaningful for analysis, were removed. Continuous variables were normally-distributed and were expressed as mean ± standard deviation (SD). To compare the clinical characteristics between groups of patients, the t test was used for all the continuous variables, the Chi square test was used for all the categorical variables. The statistical analysis of primary endpoint was planned considering that primary response variable was discretely distributed (“yes clinical remission”/”no clinical remission”). Cox regression analyses were thus performed assessing the statistical significance of the first-line treatment with high dosage of CCSs on the likelihood of the achievement of clinical remission after 6 months from the baseline. After univariate analysis, multivariate model was built adjusting for multiple features considered to be clinically relevant in primary endpoint achievement. The analysis of secondary endpoints was similarly performed considering that these variables were discretely distributed (“yes monocyclic pattern”/”no monocyclic pattern”; “yes CCSs discontinuation”/”no CCSs discontinuation”). Cox regression analyses were thus performed assessing the statistical significance of the first-line treatment with high dosage of CCSs on the likelihood of the achievement of monocyclic pattern and on the likelihood of the achievement of CCSs discontinuation after 18 months from the baseline. Multicollinearity between independent variables was evaluated by using the variance inflation factor (VIF) before entering each value in the regression model. After excluding collinearity, independent variables were added into the models. Additional endpoints were also investigated and compared between the 2 groups. Two-sided P values <.05 were considered statistically significant. The Statistics Package for Social Sciences (SPSS for Windows, version 17.0, SPSS Inc., Chicago, IL) was used for all analyses.
3. Results
3.1. Baseline characteristics
In this study, 80 patients were included and treated with high dosage of CCSs or low dosage of CCSs. All these patients were included at the time of AOSD diagnosis and were naïve for any treatment. All these patients experienced fever (100%), 86.76% of patients reported arthritis and 72.05% displayed typical skin rash, respectively. The systemic score resulted to be 5.10 ± 1.50. No patient was affected, at the time of diagnosis, by life-threatening AOSD manifestations. At baseline, inflammatory markers and ferritin were increased (ESR: 61.11 ± 26.58 mm/hour; CRP: 77.34 ± 38.68 mg/L; ferritin: 2544.73 ± 367.34 ng/mL). Comparing 40 patients receiving high dosage of CCSs and 40 patients receiving low dosage of CCSs, no significant difference was observed in baseline clinical characteristics. These findings are summarised in Table 1.
3.2. Primary endpoint
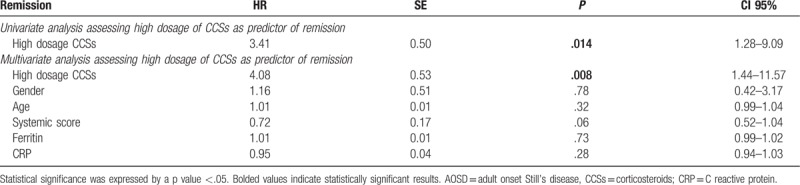
In this study, the primary endpoint was assessed on 73 patients, 7 patients were excluded from this analysis. Specifically, 4 patients developed MAS in the low dosage of CCSs group and 1 patient was lost to follow-up, during the first 6 months of observation. In the high dosage of CCSs group, 1 patient developed MAS and 1 patient was lost to follow-up, during the first 6 months of observation. The clinical remission was achieved in 33 (45.20%) patients in the first phase of the study, as reported in Table 2. Remarkably, 25 (64.79%) patients treated with the first-line treatment with high dosage of CCSs reached the primary endpoint, a significantly higher percentage when compared with 8 (22.85%) patients treated with the first-line treatment with low dosage of CCSs (P <.001). As detailed in Table 3, we also observed that the first-line treatment with high dosage of CCSs was a strong predictor of primary endpoint achievement. In fact, both univariate analysis (HR: 3.41; P: .014; CI 95%: 1.28–9.09) and multivariate analysis (HR: 4.08; P: .008; CI 95%: 1.44–11.57) showed that the first-line treatment with high dosage of CCSs was a significant predictor of the achievement of the clinical remission, during the first 6 months of observation. In multivariate analysis, selected clinical confounders (gender, age, systemic score, ferritin, and CRP) did not result to be significantly associated with the primary endpoint. No significant multicollinearity between the selected variables was observed.
Table 2.
Primary, secondary, and additional endpoints in assessed AOSD patients.
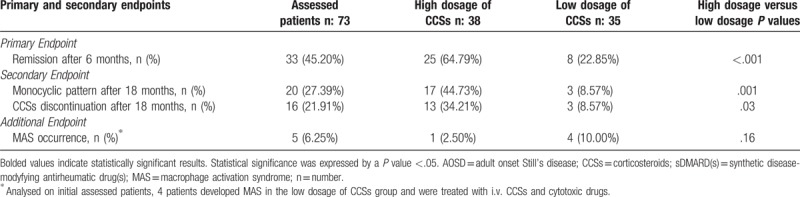
Table 3.
Cox regression analyses assessing the first-line therapy with high dosage of CCSs as predictor of the achievement of the remission in AOSD patients, after 6 months of follow-up.
3.3. Secondary endpoints
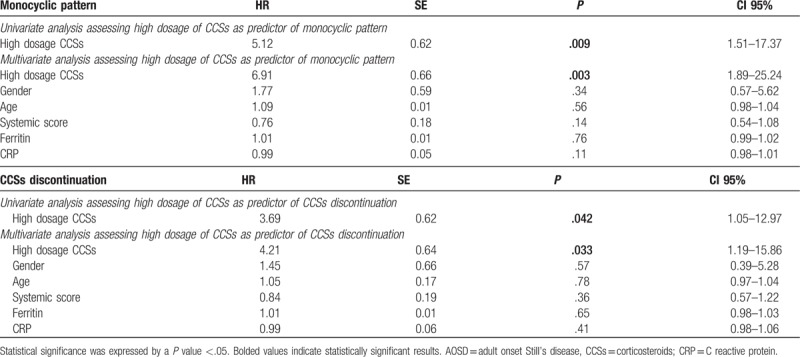
At the end of 18 months of follow-up, 17 (44.73%) patients treated with the first-line treatment with high dosage of CCSs maintained the clinical remission, being classified as monocyclic pattern, a significant higher percentage when compared with 3 (8.57%) patients treated with the first-line treatment with low dosage of CCSs (P: .001), as reported in Table 2. We also observed that the first-line treatment with high dosage of CCSs was a strong predictor of this secondary endpoint achievement. In fact, both univariate analysis (HR 5.12; P: .009; CI 95%: 1.51–17.37) and multivariate analysis (HR: 6.91; P: .003; CI 95%: 1.89–25.24) showed that the first-line treatment with high dosage of CCSs was a significant predictor of the achievement of the monocyclic pattern, after 18 months as shown in Table 4. In multivariate analysis, selected clinical confounders (gender, age, systemic score, ferritin, and CRP) did not result to be significantly associated with this secondary endpoint. Concerning CCSs discontinuation, in the high dosage of CCSs group, 13 (34.21%) patients discontinued CCSs treatment, a higher percentage when compared with 3 (10.35%) patients in the low dosage of CCSs group (P: .03). Furthermore, we observed that first-line treatment with high dosage of CCSs was a strong predictor of the achievement of the CCSs discontinuation. In fact, both univariate analysis (HR 3.69; P: .042; CI 95%: 1.05–12.97) and multivariate analysis (HR: 4.21; P: .033; CI 95%: 1.19–15.86) showed that the first-line treatment with high dosage of CCSs was a significant predictor of the achievement of the CCSs discontinuation, after 18 months. The analysis of CCSs tapering showed that, in our cohort, CCSs were usually tapered by the reduction of 5 mg prednisone-equivalent every 2 weeks until low-dose was reached (5–10 mg prednisone/day) or, where possible, CCSs were discontinued.
Table 4.
Cox regression analyses assessing the first-line therapy with high dosage of CCSs as predictor of the achievement of monocyclic pattern in AOSD patients and CCSs discontinuation, after 18 months of follow-up.
3.4. Additional endpoints
In our study, additional endpoints were assessed. Patients, where appropriate, were assessed for MAS occurrence. We observed that 4 (10.00%) patients developed MAS in the low dosage of CCSs group, whereas 1 (2.50%) patient developed this complication in the high dosage of CCSs group. Despite a different trend we did not observe a significant difference between the 2 groups (P: .16). These 5 patients were treated with iv CCSs and cyclosporine with resolution of the complication. Finally, we analyzed the clinical response in non-responder patients. These were treated by the addition of MTX and/or biologic drugs to CCSs. Forty patients were classified as non-responder after the first-line treatment with CCSs and were treated with MTX at dosage of 10 to 15 mg/week, in both groups of patients. Administering therapeutic strategy with CCSs+MTX, 25 (62.50%) patients achieved the clinical remission, whereas 15 (37.50%) patients needed to be further treated with combination therapy CCSs+MTX+biologic drug. Specifically, 7 (46.67%) patients of these biologic drug-treated patients received anakinra, 5 (40%) patients received TNFi (3 etanercept and 2 infliximab) and 3 (20%) patients received tocilizumab. The clinical remission was observed in 10 (66.67%) patients treated with combination therapy CCSs+MTX+biologic, during the follow-up.
3.5. Safety
Concerning safety profile in these studies, we analyzed data reported in the clinical charts, 7% of enrolled patients’ experienced minor adverse events. No severe adverse events or deaths were observed. The pattern of adverse events was consistent with previous reports on CCSs, MTX, and biologic drugs with no new signal identified.
4. Discussion
In this study, we showed the effectiveness of the first-line treatment with high dosage of CCSs in inducing clinical remission in naïve AOSD patients when compared with the first-line treatment with low dosage of CCSs. Interestingly, the first-line treatment with high dosage of CCSs was also associated with achievement of monocyclic pattern and discontinuation of CCSs. Furthermore, we observed the effectiveness of combination therapies of CCSs with MTX and/or biologic drug in non-responder patients.
We observed that a significant percentage of patients treated with the first-line treatment with high dosage of CCSs achieved the clinical remission at the end of the first phase of evaluation, confirming that high dosages of CCSs could be more efficient in controlling the disease.[2,12] The effectiveness of CCSs is usually reported within few days and the tapering should be started after disappearance of symptoms and normalization of inflammatory parameters.[28,29] Interestingly, the first-line treatment with high dosage of CCSs was also associated with the maintaining of clinical remission, being a significant predictor of the achievement of the monocyclic pattern, after 18 months of follow-up. These data could highlight the effectiveness of the first-line treatment with high dosage of CCSs in AOSD patients, suggesting the clinical usefulness in inducing and maintaining remission in AOSD. In fact, differently from previous studies in which 30% of patients reached a clinical remission of the disease,[30–32] we observed almost 60% of patients treated with the first-line treatment with high dosage of CCSs achieved the clinical remission, after 6 months. Furthermore, we observed that 44% of these patients maintained the remission after 18 months of follow-up, an increased percentage compared with patients developing a chronic disease course in previous studies.[21,27,30–32] Taking together, the results of our study could improve AOSD management, the early administration of the treatment with high dosage of CCSs could increase the achievement of clinical remission and could reduce the development of chronic disease course. Furthermore, the early suppression of the inflammatory process could be also associated with the reduction of severe AOSD-related complications, associated with high mortality rate.[33–35] At the end of second phase of the study, we observed that a significant percentage of patients discontinued CCSs, after fist-line treatment with high dosage of CCSs. Considering that CCSs dependency may occur until up 45% of AOSD patients,[2,12,29] the possible discontinuation of CCSs, maintaining the clinical response, could be a further improvement in AOSD management.
In our study, we also assessed the effectiveness of combination therapies in non-responder patients to the first-line therapy with CCSs. We reported clinical remission may be achieved adding MTX to CCSs treatments. In fact, MTX is the most frequently administered sDMARD in AOSD, for its CCSs-sparing effect, and for clinical effectiveness.[24,36,37] The lack of clinical response to first-line CCSs and second-line sDMARDs could identify refractory patients, although a validated definition of disease activity is still missing in AOSD. In these patients, biologic agents may be considered, as recently suggested by meta-analytic data reporting the efficacy of biologic drugs in AOSD.[38–40] In accordance with these data, we observed clinical remission could be achieved in non-responder patients by using a combination therapy including CCSs, MTX, and biologic drugs, as reported in available literature.[38–42]
Our work is affected by different limitations as observed in any retrospective study and the results should be cautiously interpreted. In general, a blinded randomized trial is regarded as being less subject to any bias analyzing therapeutic strategy, but it is not always feasible and difficult to organize in a rare disease in which carrying out prospective and adequately powered studies is quite difficult.[43–45] In this context, the clinical usefulness of small case series and retrospective studies could be suggested.[46–50] In addition, despite the achievement of adequate number of patients according to our sample size estimation, the retrospective selection of the comparator group could also limit the external validity of our findings and future studies are needed to entirely confirm our results.
In conclusions, in this study, we showed the effectiveness of the first-line treatment with high dosage of CCSs in inducing clinical remission in naïve AOSD patients when compared with the first-line treatment with low dosage of CCSs. Interestingly, the first-line treatment with high dosage of CCSs was also associated with achievement of monocyclic pattern and discontinuation of CCSs. Finally, we reported the effectiveness of combination therapies of CCSs with MTX and/or biologic drug in non-responder patients.
Acknowledgments
We thank Mrs Federica Sensini for her technical assistance.
Author contributions
Conceptualization: Piero Ruscitti, Paola Cipriani, Giuliana Guggino, Roberto Giacomelli.
Data curation: Piero Ruscitti, Paola Cipriani, Vasiliki Liakouli, Giuliana Guggino, Francesco Carubbi, Berardicurti Onorina, Francesco Ciccia, Roberto Giacomelli.
Formal analysis: Piero Ruscitti.
Investigation: Piero Ruscitti, Vasiliki Liakouli, Giuliana Guggino, Francesco Carubbi, Berardicurti Onorina, Francesco Ciccia, Roberto Giacomelli.
Methodology: Piero Ruscitti, Paola Cipriani, Roberto Giacomelli.
Project administration: Piero Ruscitti.
Resources: Piero Ruscitti.
Supervision: Piero Ruscitti, Paola Cipriani, Francesco Ciccia, Roberto Giacomelli.
Validation: Piero Ruscitti, Paola Cipriani, Vasiliki Liakouli, Giuliana Guggino, Francesco Carubbi, Berardicurti Onorina, Francesco Ciccia, Roberto Giacomelli.
Visualization: Piero Ruscitti, Paola Cipriani, Vasiliki Liakouli, Giuliana Guggino, Francesco Carubbi, Berardicurti Onorina, Francesco Ciccia, Roberto Giacomelli.
Writing – original draft: Piero Ruscitti, Roberto Giacomelli.
Writing – review & editing: Piero Ruscitti, Paola Cipriani, Vasiliki Liakouli, Giuliana Guggino, Francesco Carubbi, Berardicurti Onorina, Francesco Ciccia, Roberto Giacomelli.
Piero Ruscitti orcid: 0000-0003-3487-8551.
Footnotes
Abbreviations: AOSD = Adult-onset Still's disease, CCSs = corticosteroids, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, MAS = macrophage activation syndrome, MTX = methotrexate, sDMARD = synthetic drug modifying anti-rheumatic drugs, TNFi = tumor necrosis factor inhibitor.
The authors received no specific funding for this work.
The authors have no conflicts of interest to disclose.
References
- [1].Ruscitti P, Giacomelli R. Pathogenesis of adult onset still's disease: current understanding and new insights. Expert Rev Clin Immunol 2018;14:965–76. [DOI] [PubMed] [Google Scholar]
- [2].Efthimiou P, Kadavath S, Mehta B. Life-threatening complications of adult-onset Still's disease. Clin Rheumatol 2014;33:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ruscitti P, Rago C, Breda L, et al. Macrophage activation syndrome in Still's disease: analysis of clinical characteristics and survival in paediatric and adult patients. Clin Rheumatol 2017;36:2839–45. [DOI] [PubMed] [Google Scholar]
- [4].Ruscitti P, Cipriani P, Ciccia F, et al. Prognostic factors of macrophage activation syndrome, at the time of diagnosis, in adult patients affected by autoimmune disease: analysis of 41 cases collected in 2 rheumatologic centers. Autoimmun Rev 2017;16:16–21. [DOI] [PubMed] [Google Scholar]
- [5].Ruscitti P, Cipriani P, Ciccia F, et al. H-ferritin and CD68(+) /H-ferritin(+) monocytes/macrophages are increased in the skin of adult-onset Still's disease patients and correlate with the multi-visceral involvement of the disease. Clin Exp Immunol 2016;186:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ruscitti P, Ciccia F, Cipriani P, et al. The CD68(+)/H-ferritin(+) cells colonize the lymph nodes of the patients with adult onset Still's disease and are associated with increased extracellular level of H-ferritin in the same tissue: correlation with disease severity and implication for pathogenesis. Clin Exp Immunol 2016;183:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cush JJ, Medsger TA, Jr, Christy WC, et al. Adult-onset Still's disease: clinical course and outcome Arthritis Rheum 1987;30:186–94. [DOI] [PubMed] [Google Scholar]
- [8].Lebrun D, Mestrallet S, Dehoux M, et al. Validation of the Fautrel classification criteria for adult-onset Still's disease. Semin Arthritis Rheum 2018;47:578–85. [DOI] [PubMed] [Google Scholar]
- [9].Sfriso P, Priori R, Valesini G, et al. Adult-onset Still's disease: an Italian multicentre retrospective observational study of manifestations and treatments in 245 patients. Clin Rheumatol 2016;35:1683–9. [DOI] [PubMed] [Google Scholar]
- [10].Castañeda S, Blanco R, González-Gay MA. Adult-onset Still's disease: advances in the treatment. Best Pract Res Clin Rheumatol 2016;30:222–38. [DOI] [PubMed] [Google Scholar]
- [11].Colafrancesco S, Priori R, Valesini G, et al. Response to interleukin-1 inhibitors in 140 Italian patients with Adult-onset Still's disease: a multicentre retrospective observational study. Front Pharmacol 2017;8:369doi: 10.3389/fphar.2017.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kong X-D, Xu D, Zhang W, et al. Clinical features and prognosis in adult-onset Still's disease: a study of 104 cases. Clin Rheumatol 2010;29:1015–114. [DOI] [PubMed] [Google Scholar]
- [13].Cipriani P, Ruscitti P, Carubbi F, et al. Tocilizumab for the treatment of adult-onset Still's disease: results from a case series. Clin Rheumatol 2014;33:49–55. [DOI] [PubMed] [Google Scholar]
- [14].Cipriani P, Ruscitti P, Carubbi F, et al. Methotrexate in rheumatoid arthritis: optimizing therapy among different formulations. Current and emerging paradigms. Clin Ther 2014;36:427–35. [DOI] [PubMed] [Google Scholar]
- [15].Maria AT, Le Quellec A, Jorgensen C, et al. Adult onset Still's disease (AOSD) in the era of biologic therapies: dichotomous view for cytokine and clinical expressions. Autoimmun Rev 2014;13:1149–59. [DOI] [PubMed] [Google Scholar]
- [16].Kalyoncu U, Solmaz D, Emmungil H, et al. Response rate of initial conventional treatments, disease course, and related factors of patients with adult-onset Still's disease: data from a large multicenter cohort. J Autoimmun 2016;69:59–63. [DOI] [PubMed] [Google Scholar]
- [17].Landewé RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum 2002;46:347–56. [DOI] [PubMed] [Google Scholar]
- [18].van Tuyl LH, Boers M, Lems WF, et al. Survival, comorbidities and joint damage 11 years after the COBRA combination therapy trial in early rheumatoid arthritis. Ann Rheum Dis 2010;69:807–12. [DOI] [PubMed] [Google Scholar]
- [19].Markusse IM, Dirven L, Gerards AH, et al. Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the BeSt study. Arthritis Res Ther 2015;17:232doi: 10.1186/s13075-015-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol 1992;19:424–30. [PubMed] [Google Scholar]
- [21].Ruscitti P, Cipriani P, Masedu F, et al. Adult-onset Still's disease: evaluation of prognostic tools and validation of the systemic score by analysis of 100 cases from three centers. BMC Med 2016;14:194doi. 10.1186/s12916-016-0738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ruscitti P, Iacono D, Ciccia F, et al. Macrophage activation syndrome in patients affected by adult onset Still's disease: analysis of survival rate and predictive factors in GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) cohort. J Rheumatol J 2018;45:864–72. [DOI] [PubMed] [Google Scholar]
- [23].Chatzidionysiou K, Emamikia S, Nam J, et al. Efficacy of glucocorticoids, conventional and targeted synthetic disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2017;76:1102–7. [DOI] [PubMed] [Google Scholar]
- [24].Fautrel B, Borget C, Rozenberg S, et al. Corticosteroid sparing effect of low dose methotrexate treatment in adult Still's disease. J Rheumatol 1999;26:373–8. [PubMed] [Google Scholar]
- [25].Pay S, Türkçapar N, Kalyoncu M, et al. A multicenter study of patients with adult-onset Still's disease compared with systemic juvenile idiopathic arthritis. Clin Rheumatol 2006;25:639–44. [DOI] [PubMed] [Google Scholar]
- [26].Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. [DOI] [PubMed] [Google Scholar]
- [27].Pouchot J, Sampalis JS, Beaudet F, et al. Adult Still's disease: manifestations, disease course, and outcome in 62 patients. Medicine (Baltimore) 1991;70:118–36. [PubMed] [Google Scholar]
- [28].Jamilloux Y, Gerfaud-Valentin M, Henry T, et al. Treatment of adult-onset Still's disease: a review. Ther Clin Risk Manag 2014;11:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Franchini S, Dagna L, Salvo F, et al. Efficacy of traditional and biologic agents in different clinical phenotypes of adult-onset Still's disease. Arthritis Rheum 2010;62:2530–5. [DOI] [PubMed] [Google Scholar]
- [30].Gerfaud-Valentin M, Maucort-Boulch D, Hot A, et al. Adult-onset still disease: manifestations, treatment, outcome, and prognostic factors in 57 patients. Medicine (Baltimore) 2014;93:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim YJ, Koo BS, Kim Y-G, et al. Clinical features and prognosis in 82 patients with adult-onset Still's disease. Clin Exp Rheumatol 2013;32:28–33. [PubMed] [Google Scholar]
- [32].Colina M, Zucchini W, Ciancio G, et al. The evolution of adult-onset Still disease: an observational and comparative study in a cohort of 76 Italian patients. Semin Arthritis Rheum 2011;41:279–85. [DOI] [PubMed] [Google Scholar]
- [33].Ruscitti P, Cipriani P, Di Benedetto P, et al. Advances in immunopathogenesis of macrophage activation syndrome during rheumatic inflammatory diseases: toward new therapeutic targets. Expert Rev Clin Immunol 2017;13:1041–104. [DOI] [PubMed] [Google Scholar]
- [34].Ruscitti P, Cipriani P, Di Benedetto P, et al. H-ferritin and proinflammatory cytokines are increased in the bone marrow of patients affected by macrophage activation syndrome. Clin Exp Immunol 2018;19108:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ruscitti P, Cipriani P, Di Benedetto P, et al. Increased level of H-ferritin and its imbalance with L-ferritin, in bone marrow and liver of patients with adult onset Still's disease, developing macrophage activation syndrome, correlate with the severity of the disease. Autoimmun Rev 2015;14:429–37. [DOI] [PubMed] [Google Scholar]
- [36].Yoo DH. Treatment of adult-onset still's disease: up to date. Expert Rev Clin Immunol 2017;13:849–66. [DOI] [PubMed] [Google Scholar]
- [37].Zhou S, Qiao J, Bai J, et al. Biological therapy of traditional therapy-resistant adult-onset Still's disease: an evidence-based review. Ther Clin Risk Manag 2018;14:167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ruscitti P, Ursini F, Cipriani P, et al. Biologic drugs in adult onset Still's disease: a systematic review and meta-analysis of observational studies. Expert Rev Clin Immunol 2017;13:1089–97. [DOI] [PubMed] [Google Scholar]
- [39].Hong D, Yang Z, Han S, et al. Interleukin 1 inhibition with anakinra in adult-onset Still disease: a meta-analysis of its efficacy and safety. Drug Des Devel Ther 2014;8:2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ma Y, Wu M, Zhang X, et al. Efficacy and safety of tocilizumab with inhibition of interleukin-6 in adult-onset Still's disease: A meta-analysis. Mod Rheumatol 2018;1–9. [DOI] [PubMed] [Google Scholar]
- [41].Junge G, Mason J, Feist E. Adult onset Still's disease-the evidence that anti-interleukin-1 treatment is effective and well-tolerated (a comprehensive literature review). Semin Arthritis Rheum 2017;47:295–302. [DOI] [PubMed] [Google Scholar]
- [42].Kontzias A, Efthimiou P. The use of Canakinumab, a novel IL-1β long-acting inhibitor, in refractory adult-onset Still's disease. Semin Arthritis Rheum 2012;42:201–5. [DOI] [PubMed] [Google Scholar]
- [43].Sun P, Garrison LP. Retrospective outcomes studies for orphan diseases: challenges and opportunities. Curr Med Res Opin 2012;28:665–7. [DOI] [PubMed] [Google Scholar]
- [44].Guilpain P, Le Quellec A. About the complexity of adult onset Still's disease…and advances still required for its management. BMC Med 2017;15:5doi: 10.1186/s12916-016-0769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Giacomelli R, Afeltra A, Alunno A, et al. International consensus: what else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren's syndrome)?: the unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun Rev 2017;16:911–24. [DOI] [PubMed] [Google Scholar]
- [46].Giampietro C, Ridene M, Lequerre T, et al. Anakinra in adult-onset Still's disease: long-term treatment in patients resistant to conventional therapy. Arthritis Care Res (Hoboken) 2013;65:822–6. [DOI] [PubMed] [Google Scholar]
- [47].Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still's disease. J Autoimmun 2018;93:24–36. [DOI] [PubMed] [Google Scholar]
- [48].Néel A, Wahbi A, Tessoulin B, et al. Diagnostic and management of life-threatening Adult-onset Still disease: a French nationwide multicenter study and systematic literature review. Crit Care 2018;22:88doi: 10.1186/s13054-018-2012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kadavath S, Zapantis E, Zolty R, et al. A novel therapeutic approach in pulmonary arterial hypertension as a complication of adult-onset Still's disease: targeting IL-6. Int J Rheum Dis 2014;17:336–409. [DOI] [PubMed] [Google Scholar]
- [50].Giacomelli R, Gorla R, Trotta F, et al. Quality of life and unmet needs in patients with inflammatory arthropathies: results from the multicentre, observational RAPSODIA study. Rheumatology (Oxf) 2015;54:792–7. [DOI] [PubMed] [Google Scholar]


