Abstract
Background:
Despite recent advances in gastric cancer surgery, open gastrectomy is often needed to treat gastric cancer. Due to the large incision in the abdomen, the amount of opioid required during surgery increases and postoperative pain becomes worse. It is well known that postoperative pain has a negative impact on the patient's immune system. Herein, we performed an ultrasound-guided bilateral rectus sheath block (RSB) in patients undergoing open gastrectomy under general anesthesia and analyzed the analgesic effectiveness of RSB in open gastrectomy.
Methods:
A total of 46 patients scheduled for open gastrectomy were randomly divided into 2 groups: Group A (n = 21) consisted of patients who received an RSB using 40 mL of 0.375% ropivacaine under ultrasound guidance and Group B (n = 20) consisted of patients who received an RSB using 40 mL of normal saline. An electronic injection pump was connected to each patient for patient-controlled analgesia (PCA) immediately after the skin closure. The amount of remifentanil required during the surgery was analyzed. After using PCA, data on the use of PCA bolus dose were extracted and analyzed using Excel.
Results:
Group A used significantly less remifentanil (1021.4 ± 172.0 μg) than group B (1415.0 ± 330.6 μg; P = .03). The number of PCA bolus dose provided to the patients after surgery was significantly lower in group A (1 h: 1.14 ± 0.9, 2 h: 0.85 ± 0.7) than in group B (1 h: 1.85 ± 0.7, 2 h: 1.45 ± 1.0) until 2 hours after the surgery (1 h, P = .008; 2 h, P = .03), but after 3 hours, there were no significant differences between the 2 groups.
Conclusions:
If ultrasound-guided bilateral RSB with 40 mL of 0.35% ropivacaine is performed precisely in patients undergoing open gastrectomy, the requirement for remifentanil during surgery can be reduced. In addition, it significantly reduces the use of PCA bolus dose for acute postoperative pain within 2 hours after surgery.
Keywords: Analgesia, opioid, pain, patient-controlled analgesia, rectus sheath block, regional anesthesia, ropivacaine
1. Introduction
Surgery is the only radical cure and the most important, effective treatment for stomach cancer.[1] Recently, laparoscopic surgery, which requires a minimal skin incision, has been gaining popularity as the preferred technique for gastric cancer surgery. However, open surgeries are still being performed depending on the cancer stage.[2] Due to the large skin incision in the abdomen required for open gastrectomy, the requirement of opioids to maintain anesthesia during the surgery is further increased and the pain experienced by the patient after surgery is also more severe. It is well known that the intensity of the pain experienced by the patient during or after the surgery itself has a considerably negative effect on the patient's immunity.[3] It is also known that the increased demand for opioids to control this severe pain also affects the immune system of the patients.[4] In cancer surgeries such as those for gastric cancer, maintaining or strengthening the patient's immunity during and after the surgery is crucial for preventing metastasis that may occur during the surgery or cancer recurrence that may occur after the surgery.[5] In addition, the age of patients undergoing gastrectomy is also increasing. These patients are more likely to have underlying medical conditions in major organs such as the heart or lungs, and the need for appropriate pain control after surgery is increasing.[6]
For these reasons, research is underway to reduce the use of opioids during surgery and to help control pain in patients after surgery by performing a rectus sheath block (RSB), which is a type of regional anesthesia used in various types of cancer surgeries under the guidance of general anesthesia.[7–10] Recently, the development and application of new regional anesthesia methods are becoming more interesting as it is possible to perform procedures in a safer, faster, and more accurate manner by administering regional anesthesia under ultrasound guidance.[11,12]
Introduced in 1899 by Schlech, the RSB was initially used to relax muscles of the anterior abdominal wall during surgery and served as an analgesic adjuvant.[13] Since then, as the number of procedures using ultrasound has increased in surgeries that require midline skin incisions of the abdomen, RSB is conducted with general anesthesia to reduce opioid use and postoperative pain.[8,9,14,15] If local anesthetics are injected accurately into the bilateral posterior rectus sheath with ultrasound guidance, the ventral rami of the 7th to 12th intercostal nerves are blocked, anesthetizing the middle portion of the anterior abdominal wall from the xiphoid process to the symphysis pubis in adults and providing an analgesic effect in the area.[12,15,16] Given these advantages, RSB can not only suppress excessive stimulation caused by the skin incision, but can also reduce the amount of anesthetic agent used during surgery (including opioids)[8] and reduce postoperative pain.
We performed bilateral RSB with 40 mL of 0.375% ropivacaine and with 40 mL of normal saline under ultrasound guidance immediately after induction of general anesthesia in the operating room. The results were compared to verify 2 hypotheses. The first hypothesis was that bilateral RSB before surgery reduces the amount of remifentanil required during an open gastrectomy. The second hypothesis was that bilateral RSB before surgery reduces the requirement for patient-controlled analgesia (PCA) bolus doses after surgery.
2. Methods
2.1. Study design
This study was conducted at Kyungpook National University Chilgok Hospital, Daegu, South Korea, between December 2015 and April 2016. The study protocol was approved by the Research Ethics Committee of the Kyungpook National University Chilgok Hospital, Daegu, South Korea. The study received institutional approval (KNUMC_14-1011) and was conducted in accordance with the Declaration of Helsinki. All the subjects provided informed consent.
2.2. Patients selection
Forty-six out of 57 patients who underwent an open gastrectomy during this time period participated in the study. Only patients with American Society of Anesthesiologists (ASA) physical class I or II, who had undergone an open gastrectomy under general anesthesia in the General Surgery department of Kyungpook National University Chilgok Hospital and were aged between 20 and 80 years, were included.
Patients were excluded from this study if they: refused to participate, were above ASA class III, were younger than 19 or older than 81 years old, had difficulty in communicating due to an intellectual disability, had abdominal surgery in the past, had multiple surgeries on other parts or organs together, were excessively obese (BMI > 30 kg/m2), had a history of long-term opioid usage, had a hemorrhagic predisposition or hemorrhagic disorders, or had contraindications to regional anesthesia.
2.3. Randomization and blinding
Patients were randomly assigned to receive either RSB with 0.375% ropivacaine (A group, n = 23 patients) or RSB with normal saline (B group, n = 23 patients). The randomization was computer generated (www.randomization.com) and patient randomization numbers were concealed until the beginning of the anesthesia induction using the sealed opaque envelope method. Sealed opaque envelopes were opened only by the study investigator immediately before performing RSB. All patients, the anesthesiologist performing the block, and the staff involved in data collection and analyses were blinded to the group allocations. The group allocations were not revealed until the final statistical analysis was completed.
2.4. General anesthesia and monitoring
No premedication was given to the patients in this study. Routine monitoring was used during surgery: pulse oximetry, electrocardiogram, noninvasive arterial pressure measurement, capnography, bispectral index (BIS) measurement, and use of nasopharyngeal temperature probes. However, some elderly patients or those with cardiovascular disease were monitored using invasive radial artery blood pressure measurements. Total intravenous anesthesia using target-controlled infusion (Orchestra, Fresenius Vial, France) of propofol and remifentanil were initiated for the induction of general anesthesia. The initial effect-site concentrations of propofol were 6.0 μg/mL, and were gradually increased until BIS values reached 40 to 60. Then, effect-site target-controlled infusion of 3.0 ng/mL remifentanil was initiated. When the concentration of remifentanil reached the target value, 0.8 mg/kg rocuronium was administered to facilitate endotracheal intubation. The lungs were ventilated with a tidal volume of 8 mL/kg, and the breathing frequency was adjusted to maintain an end-tidal carbon dioxide partial pressure of 30 to 40 mm Hg. Target-controlled infusion of propofol and remifentanil was continued during surgery. In order to maintain BIS values between 40 to 60 and mean arterial pressure at baseline values ± 20%, concentrations of propofol and remifentanil were continuously adjusted. In cases where the concentration of remifentanil is kept below 2 ng/mL to maintain the patient's vital signs, or in cases where the patient's mean arterial blood pressure is kept lower than 80% of the baseline values, an injection of phenylephrine was given. Nicardipine was administered in cases where the concentration of remifentanil is kept higher than 8 ng/mL to maintain the patient's vital signs or in cases where the patient's mean arterial blood pressure is maintained above 120% of baseline values. Atropine and esmolol were administered separately if the patient's heart rate dropped to less than 50 beats per minute or increased to more than 90 beats per minute for more than 30 seconds. Repeated or continuous infusion was administered, if deemed necessary. Rocuronium was infused at 1 μg/kg/min to maintain intraoperative muscle relaxation and discontinued before closing the abdomen. In all patients, propofol and remifentanil infusions were stopped shortly before the end of the skin closure and the PCA was connected to the patient. During surgery, Lactated Ringer's solution was continuously infused at 8 mL/kg/h, and the volume of blood loss was supplemented with thrice the volume of Lactated Ringer's solution during the operation. A heating blanket, warm intravenous fluids, and operative flushing fluids were applied to maintain normothermia. After the operation was completed and the patient regained consciousness, the patient was successfully extubated and then transferred to the recovery room. Lactated Ringer's solution was infused at 2 mL/kg/h in the recovery room. Before surgery, the patient was instructed to press the bolus button of the PCA when he felt pain comparable to a visual analog scale (VAS) score of 3 or more in the recovery or patient room. If a pain VAS score of 3 or higher persisted for more than 15 minutes after pressing the bolus button, the same procedure was repeated. If the pain persisted, a rescue analgesic drug was used according to the existing manual set by the Department of General Surgery.
2.5. Rectus sheath block
After induction of general anesthesia, RSB was performed immediately on both sides of the linea alba from just below the xiphoid process to the lower part of the umbilicus, where the skin incision was made. RSB was performed by a single anesthesiologist bilaterally under ultrasound guidance (ProSound Alpha7 Premier, Hitachi Aloka Medical, Japan) with a broadband (4–13 MHz) linear array ultrasound probe. Patients in group A were given 40 mL 0.375% ropivacaine and patients in group B were given 40 mL normal saline. The procedure was divided into 3 or 4 injection sites on the left and right sides of the surgical site. Ultrasonography was performed to confirm that the drug was uniformly distributed throughout the skin incision.
2.6. PCA data extract and analysis
Patients were treated using an electronic intravenous (IV) PCA device (Accumate 1100, WOO YOUNG MEDICAL, South Korea) and the drug comprised only fentanyl and ramosetron, with a total dose of 60 mL. The bolus dose and basal dose were set at 0.5 mL and the lockout interval was set at 15 minutes. The IV PCA was connected into the patient's venous fluid line just before the end of the skin closure. After the IV PCA was completed, the number of bolus doses of the IV PCA used in each postoperative period was analyzed through the extraction of IV PCA excel data (Excel 2016, Microsoft, USA).
2.7. Study outcomes
The primary goal was to compare the difference in the requirement of remifentanil during the operation between group A, in which 40 mL of 0.375% ropivacaine was administered, and group B, which received 40 mL of normal saline. The secondary objective was to compare the numbers of PCA bolus dose used between the 2 groups at 1, 2, 3, 6, 12, 24, 48, and 72 hours after surgery. The tertiary objectives were to compare the frequency of rescue drug use based on the patient's chart review. The rescue analgesic drug was administered if the patient had a VAS score of 3 or higher continuously even after administering bolus dose twice to patient.
2.8. Sample size
The amount of remifentanil required during surgery was analyzed as a basis for judging the effectiveness of the RSB. In a preliminary study of 20 patients, the mean amount of remifentanil used during surgery in the RSB group with 40 mL of 0.375% ropivacaine was 1027 ug, the mean difference between the 2 groups was 370 ug, and the standard deviation (SD) was 314. Since we want to maintain 95% power and a 5% significance level, 19 patients per group were calculated. As a result, assuming a 20% dropout rate, a minimum of 23 patients per group would be required in order to achieve meaningful results in this study.
2.9. Statistical analysis
The data were entered and analyzed using IBM SPSS Statistics 25.0 (IBM Corp, New York). Statistical analysis of this study was performed by using the χ2 test for sex among the demographic data and the independent t test was used for other demographic data. In addition, the statistical significance between both groups of remifentanil usage and PCA data was verified by the independent t test. All values are expressed as means ± SD or medians (interquartile range). A P value < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
Of the 57 patients who had an open gastrectomy during the period of this study, 3 patients refused to participate, 3 patients were 81 years old or older, 1 patient who was treated for dementia, 1 patient had undergone abdominal surgery in the past, 2 patients had multiple surgeries, and 1 patient had a BMI above 31. Thus, 46 patients were randomly assigned to 2 groups. Three patients in Group B and 2 patients in Group A were excluded from the study because of IV PCA side effects such as nausea, vomiting, and urticaria. A total of 41 patients (A group; n = 21, B group; n = 20) were included in the final study sample (Fig. 1). Table 1 presents the results of the demographic and preoperative characteristics of the 2 groups. There was no significant difference between the 2 groups for these characteristics.
Figure 1.

Patient flowchart showing the patients included in enrollment, group allocation, follow-up, and analysis phases of the study.
Table 1.
Patient characteristics and intraoperative data.
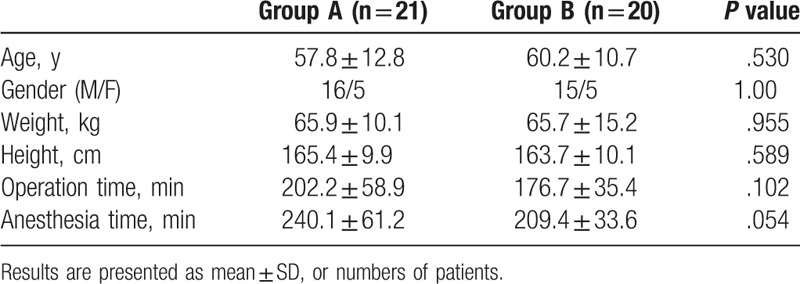
3.2. Requirement of remifentanil
The total amount of remifentanil required during surgery was significantly lower in the A group (1021.4 ± 172.0 ug) than in the B group (1415.0 ± 330.6 ug) (P = .03) (Fig. 2).
Figure 2.
Requirement of remifentanil. Data expressed as mean ± standard deviation. The amount of remifentanil required during surgery was significantly lower in group A (1021.4 ± 172.0 ug) than in group B (1415.0 ± 330.6 ug). (P = .003). ∗ P < .05 significant difference between groups.
3.3. PCA bolus dose
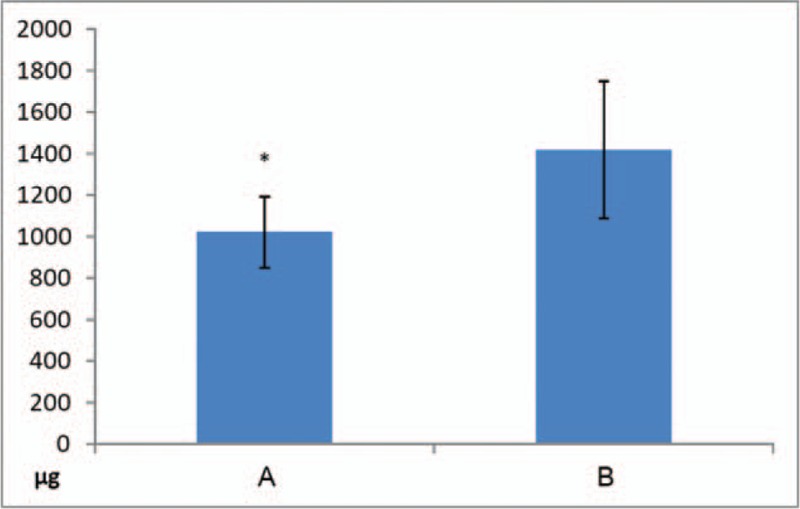
The number of bolus doses administered each time the patient experienced pain of VAS score 3 or higher as the time elapsed was compared between the 2 groups at 1, 2, 3, 6, 12, 24, 48, and 72 hours after surgery. The number of bolus doses used in group A (1 h: 1.14 ± 0.9, 2 h: 0.86 ± 0.7) was significantly lower than that in group B (1 h: 1.85 ± 0.7, 2 h: 1.45 ± 1.0) at 1 and 2 h after surgery (1 h: P = .008; 2 h: P = .03), but there was no significant difference between the 2 groups after 3 hours (Fig. 3).
Figure 3.
The number of bolus doses administered to a patient. The numbers of the bolus dose of PCA administered to the patient after surgery was significantly lower in group A (1 hour = 1.14 ± 0.9, 2 hours = 0.85 ± 0.7) than in group B (1 hour = 1.85 ± 0.7, 2 hours = 1.45 ± 1.0) from immediately after the operation until 2 hours after the operation (1 hour, P = .008; 2 hours, P = .03), but there was no significant difference between the 2 groups after 3 hours. ∗ P < .05 significant difference between groups.
3.4. Rescue analgesic drug
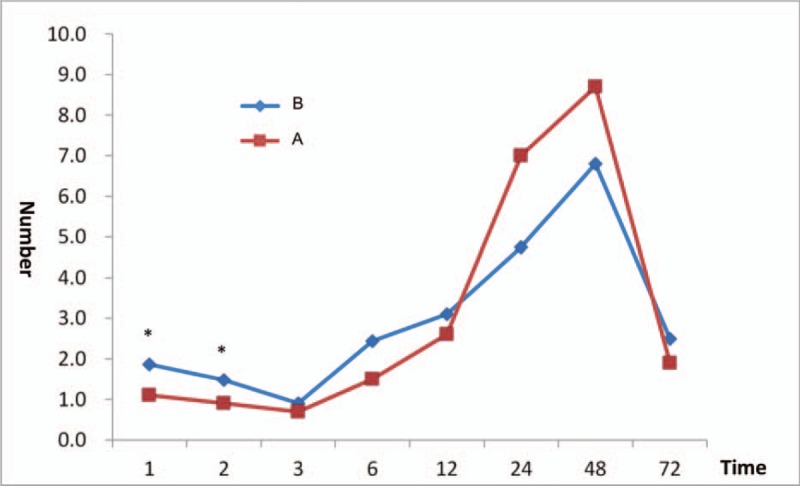
If a pain VAS score of 3 or more persisted for more than 15 minutes after pressing the bolus button, the same procedure was repeated. If the pain persisted, a rescue analgesic drug was used according to the existing manual set by the Department of Anesthesiology and Pain Medicine and the Department of General Surgery in the anesthesia recovery room and patient room, respectively. The rescue analgesic drug was injected in the anesthesia recovery room, and at 1, 2, 3, 6, 12, 24, 48, and 72 hours after the surgery, if necessary. The number of patients who required injections of rescue analgesic drug in the anesthesia recovery room was significantly lower in group A (n = 7) than in group B (n = 16; P = .01). However, at 6 and 12 hours after the surgery, this number was significantly higher in group A (6 h: n = 5; 12 h: n = 8) than in group B (6 h: n = 0; 12 h: n = 1; 6 h: P = .048; 12 h: P = .02). At all other times, there was no difference between the 2 groups (Fig. 4).
Figure 4.
Frequency of rescue analgesic drug administered to patients in each group. Group A had significantly lower rescue analgesic drug use in the anesthesia recovery room than group B (P = .01), but at 6 and 12 hours after the surgery, group A was higher than group B (6 h, P = .048; 12 h, P = .02). At other times, however, there was no difference between the 2 groups. ∗ P < .05 significant difference between groups.
4. Discussion
Open gastrectomy is a surgery performed by making a large midline skin incision in the abdomen. Rozen et al, through their cadaveric study and review of the literature,[17] reported that the anteromedial abdominal wall receives multiple segmental nerve supplies from the anterior division of the spinal nerve root from T6 to L1. These nerves travel between the internal oblique muscle and the transversus abdominis muscle within the transversus abdominis plane, while the branches extend extensively to communicate with each other. These nerves penetrate the posterior aspect of the rectus sheath, branching and communicating within the rectus sheath plexus that travels with the deep inferior epigastric artery. When these nerves enter the muscles, they branch and finally reach the skin. The skin from the xiphoid process level to the umbilicus level is dominated by cutaneous nerves from T6 to T9, T10 at the umbilicus level skin, and T11 to L1 below the umbilicus. Considering the pathways of the nerves that control the anteromedial abdominal wall, the target region of the RSB is the rectus sheath space between the rectus abdominis muscle and the posterior layer of the sheath through which the nerves within the rectus sheath plexus pass.[18] The ultimate goal of RSB is to achieve a anteromedial abdominal wall block by administering local anesthetics accurately to this site.
In patients undergoing abdominal surgery, the performance of RSB with general anesthesia before the start of surgery can not only suppress excessive stimulation by skin incisions, but also reduce the use of anesthetics during the surgery, especially opioids.[7–10,19,20] Crosbie et al[18] also reported that patients undergoing major gynecological surgery were administered RSB after surgery to successfully control postoperative pain. Some reports have shown that the RSB can block a larger area when combined with other regional blocks. Abdelsalam and Mohamdin[7] reported that RSB was performed with a transversus abdominis block to show a better peri-operative analgesic effect in major upper abdominal surgeries. Yentis et al[21] reported that RSB was performed with an ilioinguinal block to successfully perform transverse incision surgery under the umbilicus.
In this study, we concluded that the requirement of remifentanil during surgery can be reduced when performing RSB precisely during an open gastrectomy. These results were consistent with the results of many previously mentioned studies. It is already known that opioids, such as remifentanil used in this study, have an immune modulatory effect in humans and experimental animals.[4,22] Results from human studies have shown that in some meta-analyses, regional anesthesia and analgesia may improve overall survival.[23] However, until very recently, this has not been validated with human studies.[23,24] Many researchers are waiting for the results of several well-conducted, large, randomized controlled trials on the impact of regional anesthesia and analgesia on cancer recurrence and survival.[25] If the results of these studies reveal that regional anesthesia and analgesia have positive effects on cancer metastasis, recurrence, and survival, the use of regional blocks such as RSB in cancer surgery is expected to be even higher.
The aim of this study was to evaluate the degree of postoperative pain felt by the patients through the amount of PCA bolus dose usage. We thought that it is impossible to express the degree of pain as objective data, since it is a subjective experience. In addition, we thought that there was a significant limitation in accurately communicating the degree of pain experienced by patients with VAS due to the patient not being fully awake after surgery or in severe pain after surgery. The electrical PCA pump can be used as an indicator of the degree of postoperative pain, taking advantage of its ability to identify the number of times the drug is injected. In the present study, the bolus dose injected when the patient experienced a pain of VAS 3 or higher was significantly lower in group A until 2 hours after the surgery. There was no difference between the 2 groups after that time period. The authors concluded that this was because RSB was administered only once before the start of surgery and a half-dose of ropivacaine (0.375%) was used for usual anesthesia. When open gastrectomy is performed, skin incisions range from just under the xiphoid process to below the umbilicus. Because of the wide range of incisions, a significant amount of ropivacaine is required to include the skin incisional range and there was difficulty in using high concentration drugs such as 0.75% ropivacaine. In addition, the requirement of remifentanil during surgery was significantly reduced by RSB before the onset of surgery, but it seems to be effective for only about 2 hours for postoperative pain control. This suggests that if the catheter is inserted into the rectus sheath for continuous infusion immediately before the operation and recovery from anesthesia is complete, the RSB will be more helpful in controlling postoperative pain for a longer period of time. Studies on this are actively underway.[26–28]
Comparisons of the number of rescue analgesic drug injections used by the patients in the anesthesia recovery room and at postoperative 1, 2, 3, 6, 12, 24, 48, and 72 h time points were interesting. In the anesthesia recovery room, the number of rescue analgesic drug use was significantly lower in group A. However, during the postoperative period, there was no significant difference between the two groups, except at 6 and 12 h after surgery, when rescue analgesic drugs were administered more frequently in group A. This means that the patients in group A felt relatively mild pain due to the effect of RSB until 2 h after the operation, but the pain increased gradually over time as the effect of RSB gradually disappeared after 3 h and the numbers of rescue analgesic drug administration became significantly more than that in group B.
There was no difference in the use of PCA bolus dose or rescue analgesic drug usage between the two groups after postoperative 12 h. This may be due to the fact that the postoperative pain experienced by patients most severely between 6 and 24 h after surgery is due to visceral pain.[29,30] RSB may reduce somatic pain from anteromedial abdominal wall structures superficial to the peritoneum, but it does not reduce visceral pain. Patients who underwent bowel resection under the peritoneum usually experience deeper visceral pain 6 to 24 hours postoperatively. Visceral pain is usually minimal at 24 h after surgery.[29] Therefore, almost all patients need additional doses of systemic opiates for visceral analgesia.
In order to relieve postoperative pain effectively for a longer period of time, RSB may be administered one more time before emergence from anesthesia, or ropivacaine may be used at higher concentrations, as described above. However, in order to be more effective in controlling the pain after 6 h, another means of pain control may be needed. Since RSB has no effect on visceral pain, it probably does not seem to have much effect on the catheter, which is capable of continuous infusion. With the recent development of electrical infusion pump for PCA, it is possible to set different infusion programs for different intervals, so that it may be possible to control the patient's pain preemptively before the patient feels severe pain.[31]
In this study, RSB was performed by a single anesthesiologist who was proficient in procedures under ultrasound guidance for more than 10 years. Sandeman and Dilley[16] reported that tendinous intersections are not incorporated into the posterior rectus sheath, allowing local anesthetics to spread from the injected site across the compartments. They reported that the spread of local anesthetics can be observed with ultrasound, and if local anesthetics do not spread properly, they should inject local anesthetics into the nearby rectus sheaths. One of the many advantages of performing regional anesthesia under ultrasound guidance, as described by Webster,[29] is the ability to see in real time the spread of the injected local anesthetic. In this study, the skin incision was large and the injections were performed at least 3 or 4 sites on each side. During the procedure, the practitioner continued to use ultrasonography to confirm that the local anesthetic fully spread over the entire range of the skin incision.
In conclusion, if ultrasound-guided bilateral RSB with 40 mL of 0.35% ropivacaine is performed precisely in patients undergoing open gastrectomy, the requirement of remifentanil during surgery can be reduced. In addition, it significantly reduces the need of PCA bolus dose to reduce acute postoperative pain within 2 hours after surgery.
Author contributions
Conceptualization: Seongwook Hong, Junmo Park.
Data curation: Hyunjeong Kim.
Investigation: Hyunjeong Kim.
Methodology: Seongwook Hong.
Writing – original draft: Junmo Park.
Writing – review & editing: Seongwook Hong, Junmo Park.
Junmo Park orcid: 0000-0001-9234-5177.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BIS = bispectral index, IV = intravenous, PCA = patient-controlled analgesia, RSB = rectus sheath block, VAS = visual analog scale.
This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2014).
The authors have no conflicts of interest to disclose.
References
- [1].Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654–64. [DOI] [PubMed] [Google Scholar]
- [2].Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 2014;40:584–91. [DOI] [PubMed] [Google Scholar]
- [3].Juneja R. Opioids and cancer recurrence. Curr Opin Support Palliat Care 2014;8:91–101. [DOI] [PubMed] [Google Scholar]
- [4].Cruz FF, Rocco PR, Pelosi P. Anti-inflammatory properties of anesthetic agents. Crit Care 2017;21:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth 2010;105:106–15. [DOI] [PubMed] [Google Scholar]
- [6].Jin F, Chung F. Minimizing perioperative adverse events in the elderly. Br J Anaesth 2001;87:608–24. [DOI] [PubMed] [Google Scholar]
- [7].Abdelsalam K, Mohamdin OW. Ultrasound-guided rectus sheath and transversus abdominis plane blocks for perioperative analgesia in upper abdominal surgery: a randomized controlled study. Saudi J Anaesth 2016;10:25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jin F, Li Z, Tan WF, et al. Preoperative versus postoperative ultrasound-guided rectus sheath block for improving pain, sleep quality and cytokine levels in patients with open midline incisions undergoing transabdominal gynecological surgery: a randomized-controlled trial. BMC Anesthesiol 2018;18:19. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [9].Karaarslan E, Topal A, Avci O, et al. Research on the efficacy of the rectus sheath block method. Agri 2018;30:183–8. [DOI] [PubMed] [Google Scholar]
- [10].Cho S, Kim YJ, Jeong K, et al. Ultrasound-guided bilateral rectus sheath block reduces early postoperative pain after laparoscopic gynecologic surgery: a randomized study. J Anesth 2018;32:189–97. [DOI] [PubMed] [Google Scholar]
- [11].Marhofer P, Greher M, Kapral S. Ultrasound guidance in regional anaesthesia. Br J Anaesth 2005;94:7–17. [DOI] [PubMed] [Google Scholar]
- [12].Sites BD, Brull R. Ultrasound guidance in peripheral regional anesthesia: philosophy, evidence-based medicine, and techniques. Curr Opin Anaesthesiol 2006;19:630–9. [DOI] [PubMed] [Google Scholar]
- [13].Finnerty O, Carney J, McDonnell JG. Trunk blocks for abdominal surgery. Anaesthesia 2010;65suppl 1:76–83. [DOI] [PubMed] [Google Scholar]
- [14].Yassin HM, Abd Elmoneim AT, El Moutaz H. The analgesic efficiency of ultrasound-guided rectus sheath analgesia compared with low thoracic epidural analgesia after elective abdominal surgery with a midline incision: a prospective randomized controlled trial. Anesth Pain Med 2017;7:e14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Osaka Y, Kashiwagi M, Nagatsuka Y, et al. Ultrasound-guided rectus sheath block for upper abdominal surgery. Masui 2010;59:1039–41. [PubMed] [Google Scholar]
- [16].Sandeman DJ, Dilley AV. Ultrasound-guided rectus sheath block and catheter placement. ANZ J Surg 2008;78:621–3. [DOI] [PubMed] [Google Scholar]
- [17].Rozen WM, Tran TM, Ashton MW, et al. Refining the course of the thoracolumbar nerves: a new understanding of the innervation of the anterior abdominal wall. Clin Anat 2008;21:325–33. [DOI] [PubMed] [Google Scholar]
- [18].Crosbie EJ, Massiah NS, Achiampong JY, et al. The surgical rectus sheath block for post-operative analgesia: a modern approach to an established technique. Eur J Obstet Gynecol Reprod Biol 2012;160:196–200. [DOI] [PubMed] [Google Scholar]
- [19].Smith BE, MacPhearson GH, Jonge M, et al. Rectus sheath and mesosalpinx block for laparoscopic sterilization. Anesthesia 1991;46:875–7. [DOI] [PubMed] [Google Scholar]
- [20].Smith BE, Suchak M, Siggins D, et al. Rectus sheath block for diagnostic laparoscopy. Anesthesia 1988;43:947–8. [DOI] [PubMed] [Google Scholar]
- [21].Yentis SM, Hills-Wright P, Potparic O. Development and evaluation of combined rectus sheath and ilioinguinal blocks for abdominal gynaecological surgery. Anaesthesia 1999;54:475–9. [DOI] [PubMed] [Google Scholar]
- [22].Al-Hashimi M, Scott SW, Thompson JP, et al. Opioids and immune modulation: more questions than answers. Br J Anaesth 2013;111:80–8. [DOI] [PubMed] [Google Scholar]
- [23].Sun Y, Li T, Gan TJ. The effects of perioperative regional anesthesia and analgesia on cancer recurrence and survival after oncology surgery: a systematic review and meta-analysis. Reg Anesth Pain Med 2015;40:589–98. [DOI] [PubMed] [Google Scholar]
- [24].Grandhi RK, Lee S, Abd-Elsayed A. The relationship between regional anesthesia and cancer: a metaanalysis. Ochsner J 2017;17:345–61. [PMC free article] [PubMed] [Google Scholar]
- [25].Tedore T. Regional anaesthesia and analgesia: relationship to cancer recurrence and survival. Br J Anaesth 2015;115suppl 2:ii34–45. [DOI] [PubMed] [Google Scholar]
- [26].Dutton TJ, McGrath JS, Daugherty MO. Use of rectus sheath catheters for pain relief in patients undergoing major pelvic urologicalsurgery. BJU Int 2014;113:246–53. [DOI] [PubMed] [Google Scholar]
- [27].Bakshi SG, Mapari A, Shylasree TS. REctus Sheath block for postoperative analgesia in gynecological ONcology Surgery (RESONS): a randomized-controlled trial. Can J Anaesth 2016;63:1335–44. [DOI] [PubMed] [Google Scholar]
- [28].Purdy M, Kinnunen M, Kokki M, et al. A prospective, randomized, open label, controlled study investigating the efficiency and safety of 3 different methods of rectus sheath block analgesia following midline laparotomy. Medicine (Baltimore) 2018;97:e9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Webster K. Ultrasound guided rectus sheath block: analgesia for abdominal surgery. Update Anaesth 2010;26:12–7. [Google Scholar]
- [30].Wilkinson KM, Krige A, Brearley SG, et al. Thoracic Epidural analgesia versus Rectus Sheath Catheters for open midline incisions in major abdominal surgery within an enhanced recovery programme (TERSC): study protocol for a randomised controlled trial. Trials 2014;15:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Grass JA. Patient-controlled analgesia. Anesth Analg 2005;101(5 suppl):S44–61. [DOI] [PubMed] [Google Scholar]


